Abstract
Chronic inflammatory diseases cause profound alterations in tissue homeostasis, including unchecked activation of immune and nonimmune cells leading to disease complications such as aberrant tissue repair and fibrosis. Current anti-inflammatory therapies are often insufficient in preventing or reversing these complications. Remodeling of the intracellular cytoskeleton is critical for cell activation in inflamed and fibrotic tissues; however, the cytoskeleton has not been adequately explored as a therapeutic target in inflammation. Septins are GTP-binding proteins that self-assemble into higher order cytoskeletal structures. The septin cytoskeleton exhibits a number of critical cellular functions, including regulation of cell shape and polarity, cytokinesis, cell migration, vesicle trafficking, and receptor signaling. Surprisingly, little is known about the role of the septin cytoskeleton in inflammation. This article reviews emerging evidence implicating different septins in the regulation of host-pathogen interactions, immune cell functions, and tissue fibrosis. Targeting of the septin cytoskeleton as a potential future therapeutic intervention in human inflammatory and fibrotic diseases is also discussed.
Acute and chronic inflammation result in dramatic changes in tissue homeostasis. These changes include altered cellular composition and interactions between immune and nonimmune cells in affected tissues, as well as the perturbed biochemical environment driven by the release of various pro- and anti-inflammatory mediators.1,2 Similarly, the physical properties of affected tissues change due to acute and chronic inflammation-induced development of edema, aberrant tissue remodeling, and fibrosis.3,4 On a cellular level, inflammatory states result in functional adaptations toward accelerated recognition and elimination of the invaded pathogens, as well as enhanced production of extracellular matrix and tissue turnover.2,5,6 These alterations of cellular function in inflamed tissues are mediated by reprograming of the fundamental molecular processes, including gene expression, protein synthesis, vesicle trafficking, and cytoskeletal assembly.
The cytoskeleton is a critical regulator of the architecture and function of eukaryotic cells. It comprises various filamentous structures formed via self-assembly and the polymerization of specialized proteins.7 The four components of the cytoskeleton include: actin filaments, microtubules, intermediate filaments, and septin polymers. These cytoskeletal elements play crucial roles in mediating housekeeping and specialized functions in multiple cell types. Examples include regulation of cell shape and size, cell division, migration, cell–cell interactions, protein uptake and secretion, receptor signaling, etc.7, 8, 9 Defects in the assembly and remodeling of different cytoskeletal elements play major roles in the development of various diseases, which is exemplified by tumor progression and metastasis.7,10,11 The cytoskeletal regulation of tissue inflammation has also been extensively investigated. For example, the actin cytoskeleton controls the inflammatory response by regulating activation of immune cells and permeability of epithelial and endothelial barriers.12, 13, 14 Microtubules regulate pathogens sensing by inflammasomes,15 assembly of the immune synapse,16 and vascular leakage in the inflamed tissues.17 Finally, intermediate filaments have been implicated in glial cell activation during neural inflammation18 and development of inflammatory skin disorders.19 Although the roles of actin filaments, microtubules, and intermediate filament in tissue inflammation has attracted significant attention, the role of the fourth cytoskeletal element, the septin cytoskeleton, in modulating the inflammatory response remains poorly understood. This review addresses this knowledge gap by summarizing existing evidence for the involvement of the septin cytoskeleton in inflammation and tissue fibrosis and outlining possible mechanisms of such involvement.
Diversity of the Septin Protein Family
Septins (SEPT) are small GTP-binding proteins abundantly expressed in all eukaryotic organisms except for higher plants. In mammals, there are 13 septin genes (SEPT1 to SEPT12 and SEPT14) and at least 11 pseudogenes, with extensive post-transcriptional alternative splicing in many septin genes.20,21 Existence of multiple members of the septin family (paralogs) along with expression of their differentially spliced isoforms gives rise to a complex array of different septin proteins playing either redundant or unique functional roles in different tissues. Bioinformatics analysis of the amino acid sequence homology separates mammalian septins into the following four groups named by their founding members: SEPT2 (SEPT1, -2, -4, and -5), SEPT3 (SEPT3, -9, and -12), SEPT6 (SEPT6, -8, -10, -11, and -14), and SEPT7 (SEPT7).20 All members of these groups share a GTP-binding domain flanked by an N-terminal polybasic domain and a C-terminal septin unique element.22 The groups diverge in their N-terminal extensions and C-terminal parts that contain different number of coiled-coil repeats.
Although septins are widely expressed in human tissues, some septin paralogs (SEPT2, -4, -7, -8, -9, and -10) appear to be ubiquitous, whereas others are enriched in specific tissues.23,24 For example, high expression of SEPT3, -4, -5, and -8 is observed in the central nervous system, SEPT1, -6, and -9 are enriched in hematopoietic cells, whereas SEPT12 is especially abundant in testes.23,24 A recent correlative analysis indicated consistent co-expression of subsets of septin paralogs in various tissues. For example, SEPT3 and SEPT5 expression displays high correlation in different brain regions, whereas a subset of septins including SEPT2, -7, -10, and to a lesser extent, SEPT11, are consistently co-expressed in non-neural tissues.24 It has been suggested that septin family members with highly correlated tissue expression could be parts of the same protein complexes.
Assembly and Interactions of Septin Proteins
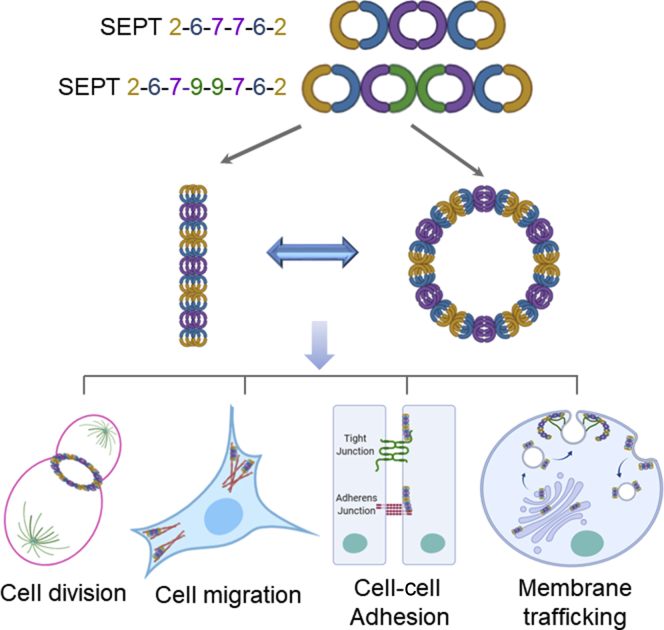
One of the most characteristic features of septins is their ability to self-associate, forming nonpolar rod-like hetero-oligomers and higher-order polymeric structures. Although the existence of septin rod complexes is well-documented from yeasts to mammals, their exact molecular composition is a subject of continuous debates and revisions.25 Thus, recent studies have challenged a decade-old model of the organization of mammalian septin oligomers by suggesting a novel subunit arrangement in the rod complexes. According to these studies, the simplest building block of the septin cytoskeleton is composed of either six or eight protein subunits. A typical hexamer and octamer have the following subunit order: SEPT2-SEPT6-SEPT7-SEPT7-SEPT6-SEPT2 and SEPT2-SEPT6-SEPT7-SEPT9-SEPT-9-SEPT7-SEPT6-SEPT2, respectively.25,26 Such oligomeric composition suggests that members of all four septin groups participate in the complex formation. Furthermore, according to the Kinoshita rule, members of the same septin group are interchangeable in the oligomers. For example, SEPT2 may be replaced by either SEPT4 or SEPT5, whereas SEPT9 may be replaced by either SEPT3 or SEPT12. This enables the assembly of functional septin complexes in different tissues that express distinct repertoires of septin genes. Based on this, SEPT7, the only member in its group, is predicted to be the most common and important component of the septin cytoskeleton. Indeed, several studies have demonstrated that loss of SEPT7 destabilizes other subunits of the septin oligomers and triggers their degradation.27,28 The septin rod oligomers spontaneously collide to form short filaments that can further assemble by end-to-end joining or lateral interactions into bundles, rings, gauzes, and sheer-like structures (Figure 1).8,29 It is noteworthy that the composition of septin oligomers is primarily based on the in vitro reconstitution studies that utilized purified recombinant septin proteins.25,26 The composition of such oligomers could be much more complex in vivo. Septin complexes isolated from different mammalian cells belong to either the canonical hetero-oligomers (SEPT2, -6, -7, -9; SEPT3, -5, -7; or SEPT7, -9, -11), or have unusual subunit composition inconsistent with the canonical oligomerization (SEPT2, -5, -6, -7; SEPT4, -5, -8; SEPT2, -5, etc).30 It is also possible that the hetero-oligomerization is not an obligate mechanism of septin assembly and activity, and some septin family members can function as monomers or homo-oligomers. For example, loss of SEPT1, but not SEPT2, SEPT6, SEPT7, or SEPT9, has been shown to disrupt Golgi morphology in cervical cancer cells,31 which selectively implicates SEPT1 in the regulation of Golgi integrity and trafficking. Furthermore, a specific SEPT4 splice isoform, SEPT4_i2, alias apoptosis-related protein in the transforming growth factor (TGF)-β signaling pathway (ARTS), was shown to selectively localize to mitochondria in kidney epithelial cells and regulate cell apoptosis.32 Such peculiar functional activity of SEPT4 may be linked to its ability to homo-oligomerize and form amyloid-like filaments in vitro.33 The unique cellular activities of certain septin paralogs and splice isoforms could add another level of complexity to the septin functions and regulation in different mammalian cells and tissues.
Figure 1.
Organization and cellular function of the septin cytoskeleton. The assembly of the septin cytoskeleton involves initial formation of hetero-oligomers with their subsequent polymerization into higher order structures. The most well-characterized functions of septin polymers include the regulation of cell division, migration, cell–cell adhesions, and intracellular vesicle trafficking.
Importantly, a current paradigm considers the septin cytoskeleton as a universal molecular scaffold mediating the organization of other key cytoskeletal elements and their interactions with lipid membranes.8,29,30 This puts the septin cytoskeleton into the center of cytoskeletal regulation. Several important molecular features of septin polymers enable such a scaffolding role. First, due to a slow rate of the GTP hydrolysis, septin filaments are more stable compared with actin filaments and microtubules.34 Hence, they can serve as templates and provide a structural memory for the assembly of more labile cytoskeletal elements. Second, septins are known to physically interact with and regulate the organization of actin filaments and microtubules.35,36 Indeed, Drosophila SEPT1/SEPT2/SEPT7 and human SEPT2/SEPT6/SEPT7 complexes, as well as human SEPT9 cross-link actin filaments into long, curved bundles.37,38 Furthermore, SEPT9 suppresses F-actin disassembly caused by the actin-depolymerizing protein cofilin.39 SEPT2 and SEPT9 are known to interact with a key actin motor, nonmuscle myosin II (NM II).40,41 Interestingly, such interactions have opposite effects on the NM II activity, with SEPT2 activating and SEPT9 inhibiting this motor protein.39,41 In addition to their association with actomyosin structures, septins also interact with microtubules.36 SEPT9_i1 splice isoform directly binds to β-tubulin and induces microtubule bundling,42 whereas the SEPT2/6/7 complex preferentially associates with microtubule plus ends and modulates their dynamics.43 The functional relevance of these septin-microtubule interactions was highlighted by the findings that either SEPT2, or SEPT7 depletion disrupts different microtubule populations in polarized and migrating epithelial cells.44,45 It should be noted that the ability of septins to form multiprotein complexes is not limited to their oligomerization and interactions with actin filaments and microtubules. Published interactomes of different septin family members show a large variety of additional binding partners that include actin-binding proteins, regulators of vesicle trafficking, and signaling molecules such as kinases, phosphatases, and small GTPases.40,46 However, functional significance of the majority of these interactions remains to be established.
Another key molecular feature of the septin oligomers is their affinity to lipid membranes. Mammalian septins preferentially bind to phosphoinositides on different cellular membranes.47,48 Such binding could be essential for the annealing of short septin oligomers and assembly of higher order polymeric structures.49 Interestingly, septins have been identified among the very few eukaryotic proteins that can sense a micron-scale membrane curvature.50, 51, 52 Because of this, septin polymers preferentially assemble at the curvature-associated cellular structures such as cytokinetic furrows, bases of dendritic spines in neurons, branches in filamentous fungi, and intracellular lipid droplets.47,51,52 Septin–membrane interactions not only promote assembly of higher-order septin cytoskeletal structures, but also significantly affect the membrane organization. For example, septin binding can induce membrane deformation, thereby controlling the shape of cellular organelles and vesicles.48,50 Furthermore, membrane-associated septin polymers create physical barriers for the lateral diffusion of lipids and transmembrane proteins, enabling formation of distinct membrane compartments.53 Finally, due to their dual affinity to membrane lipids and other cytoskeletal elements, septins physically link cellular membranes to actin filaments and microtubules, thereby creating a higher order cellular architecture.
Cellular Functions of the Septin Cytoskeleton
Given the unique ability of septins to engage in multiple interactions at different cellular compartments, it is not surprising that the septin cytoskeleton has been implicated in the regulation of a variety of cellular functions (Figure 1). Because cellular roles of septins have been described in several excellent reviews,29,30 this review briefly summarizes their role in tissue inflammation and fibrosis. However, the canonical activity of the septin cytoskeleton in regulating cell division, also essential for septin functions in inflamed tissues has been extensively reviewed elsewhere and not discussed here.29,30
Intracellular Vesicular Trafficking
The prominent lipid-binding ability of septins allows them to act as important regulators of intracellular vesicle trafficking. At the plasma membrane, SEPT2 participates in the formation of macropinosomes that mediate bulk uptake of the surrounding liquids.54 SEPT2, SEPT6, and SEPT7 are essential for the intracellular trafficking of internalized liquid cargo by regulating its transit from early to late endosomes and delivery to lysosomes.54,55 A different line of evidence has implicated mammalian septins in the control of internalization and intracellular fate of plasma membrane receptors, most notably members of the epidermal growth factor receptor family.56,57 Specifically, binding with SEPT2 or SEPT9 stabilizes these receptors at the plasma membrane and attenuates their ubiquitination and degradation. Modulation of endocytosis and endosomal transit of the internalized molecules represents only one aspect of septin-dependent regulation of vesicle trafficking. In addition, septins are important for the formation of Golgi-derived vesicles and regulation of both constitutive and stimulated protein exocytosis.48,58 The diverse septin-dependent trafficking events indicate that the septin cytoskeleton may play important roles in regulating receptor signaling on the plasma membrane, controlling transmembrane fluxes of ions and metabolites, and regulating protein secretion. The ability of septins to control multiple steps of vesicle trafficking underlies the important functions of the septin cytoskeleton in regulating host–pathogen interactions and deposition of the extracellular matrix in fibrotic tissues as discussed below.
Cell Migration and Matrix Adhesion
Another important function of the septin cytoskeleton involves regulation of cell migration. Several studies demonstrate that loss of SEPT2, SEPT7, and SEPT9 consistently attenuates wound healing in cultured epithelial and cancer cell monolayers, as well as in Xenopus embryo.37,45,59,60 The observed promigratory activity of the septin cytoskeleton may be mediated by different mechanisms such as control of cortical microtubule assembly at the migrating cell edge,45 formation of basal actin-based stress fibers,37 stabilization of focal adhesions,37,59 and activation of mitogen-activated protein (MAP) kinase signaling.60 Interestingly, SEPT2 and SEPT9 are not only essential for focal adhesion maturation, but also regulate the assembly of other adhesion structures (such as podosomes), that mediate matrix degradation.61,62 As discussed below, septin-dependent regulation of cell migration could be an important contributor to leukocyte infiltration in the inflamed tissues.
Regulation of Cellular Signaling
In addition to interacting with other cytoskeletal structures, septins participate in additional protein–protein interactions, thereby regulating different intracellular signaling pathways. Thus, the plasma membrane–endoplasmic reticulum contact sites are important places for the septin activity in mammalian cancer cells and Drosophila neurons, where SEPT2, SEPT4, SEPT5, and SEPT7 control assembly of STIM1-ORAI1 calcium channels and regulate store-operated calcium entry.63,64 SEPT4 has recently been identified as a novel regulator of a signal transducer and activator of transcription (STAT) 3 activity in vascular smooth muscle cells.65 SEPT4 binds to STAT3 and promotes its interaction with SIRT1 deacetylase, which results in inhibition of STAT3-mediated signaling.65 In contrast to STAT3 inhibition, septin binding may promote MAP kinase-dependent signaling, as supported by the direct interaction of the SEPT9_v1 isoform with c-Jun N-terminal kinase (JNK) in human mammary epithelial cells, protecting it from degradation and increasing kinase activity.66 This results in a marked enhancement of JNK-dependent gene expression. Similarly, the protective effects of septin binding have significant functional consequences for hypoxia-inducible factor-1 (HIF-1) signaling. SEPT9_v1 is an important binding partner for HIF-1α in human cancer cell lines.67,68 This interaction protects HIF-1α protein from ubiquitination and degradation under normoxic and hypoxic conditions and markedly enhances its transcriptional activity.68 The septin-dependent regulation of STAT3, JNK, and HIF-1α signaling could be particularly important during mucosal inflammation where these signaling events are markedly up-regulated by proinflammatory cytokines and tissue hypoxia.5,69
Septin-Dependent Regulation of Inflammation and Tissue Fibrosis
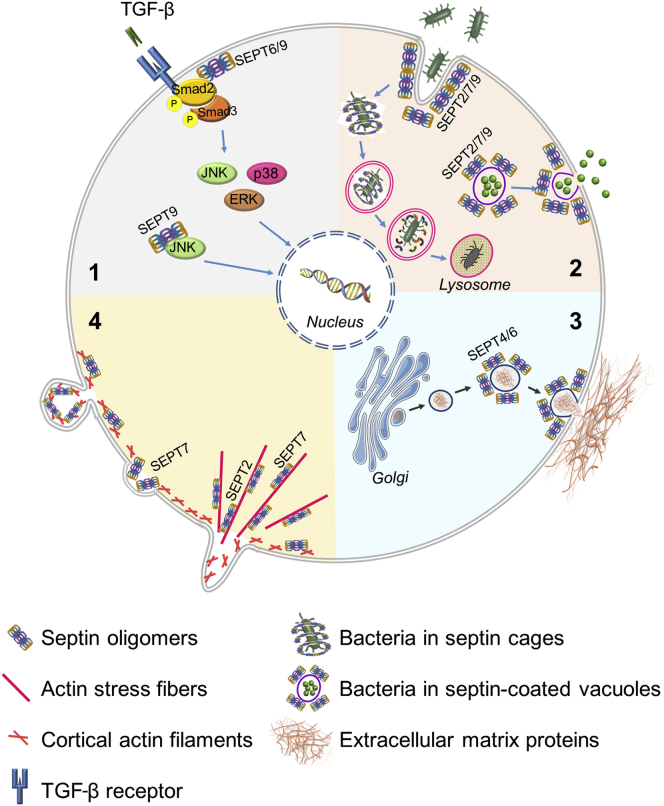
Since the discovery of the first septins in yeast cells more than 30 years ago, these proteins have been extensively studied by cell and developmental biologists. Recently, it became clear that abnormal organization and regulation of the septin cytoskeleton may contribute to the pathophysiology of different human diseases. The main focus in these studies is on understanding the roles of septin dysfunction in the development of neurodegenerative diseases and cancer.10,70,71 Although several lines of evidence strongly suggest roles of septins in inflammatory disorders, very little is known about the specfics of those roles. The most plausible functions of the septin cytoskeleton during tissue inflammation are discussed below. They involve regulation of pathogen uptake, control of immune cell infiltration and activity, regulation of tissue barrier permeability, and contribution to tissue fibrosis. A summary of these functions can be found in Table 1 and Figure 2.
Table 1.
Functional Effects of Targeting Different Septins on Experimental Infection, Inflammation, and Fibrosis Models
| Septin paralog | Interference with the septin cytoskeleton | Experimental system | Effects of the septin cytoskeleton perturbation | Reference |
|---|---|---|---|---|
| Host–pathogen interactions | ||||
| SEPT2 | siRNA-mediated knockdown | HeLa cervical epithelial cells, JEG-3 trophoblast cells | Inhibited Listeria and Shigella invasion | 72 |
| SEPT2, SEPT7, SEPT9 | siRNA-mediated knockdown | HeLa cells | Inhibited Salmonella invasion | 73 |
| SEPT7 | siRNA-mediated knockdown | Human endothelial cells | Inhibited Candida endocytosis | 74 |
| SEPT9 | siRNA-mediated knockdown | HeLa cells | Inhibited enteropathogenic Escherichia coli attachment | 75 |
| SEPT2, SEPT7 | siRNA-mediated knockdown | HeLa cells | Increased survival and proliferation of intracellular Shigella | 76,77 |
| SEPT2, SEPT7, SEPT9 | siRNA-mediated knockdown | HeLa cells | Inhibited Chlamydia extrusion by host cells | 78 |
| SEPT7 | siRNA-mediated knockdown | A549 lung epithelial cells | Accelerated vaccinia virus release from host cells | 79 |
| SEPT9 | siRNA-mediated knockdown | Huh7 liver epithelial cells | Inhibited hepatitis C virus (HCV) replication | 47 |
| SEPT7 | Morpholino-mediated knockdown | Zebrafish larvae | Increased susceptibility to Shigella infection | 80 |
| Immune cell development and tissue barrier integrity | ||||
| SEPT2 | small harpin (sh)RNA-mediated knockdown | HeLa cells, RAW264.7 macrophages | Inhibited Fc receptor–mediated phagocytosis | 81 |
| SEPT7 | shRNA-mediated knockdown | D10.G4 T-cell line | Decreased spontaneous crawling velocity. Increased transmigration through porous membrane | 82 |
| SEPT7 | T-cell–specific knockout | Mice | Declines T-cell population in vivo. Inhibited proliferation of stimulated T cells | 83 |
| SEPT9 | T-cell–specific knockout | Mice | Decreased mature T-cell number in vivo | 84 |
| SEPT2 | shRNA-mediated knockdown | Primary human bronchial epithelial cells | Increased epithelial permeability | 85 |
| SEPT7 | Clustered regularly interspaced short palindromic repeats (CRISPR)-Cas9–mediated knockout | HT-29 human colonic epithelial cells | Increased epithelial permeability | 28 |
| SEPT2 | shRNA-mediated knockdown | Primary human endothelial cells | Increased endothelial permeability. Accelerated leukocyte transmigration | 86 |
| SEPT2 | siRNA-mediated knockdown | Primary human endothelial cells | Exaggeration of thrombin-induced barrier breakdown | 87 |
| Tissue fibrosis | ||||
| SEPT4 | Whole-body knockout | Mice | More severe liver fibrosis in 3 in vivo models | 88 |
| SEPT4 | Adenovirus-mediated overexpression | Mice | Suppression of Schistosoma-induced liver fibrosis | 89 |
| SEPT6 | shRNA-mediated knockdown | Mice | Suppression of thioacetamide-induced liver fibrosis | 90 |
| SEPT8 | Whole-body knockout | Mice | No effect on the unilateral ureteral obstruction model of kidney fibrosis | 91 |
Figure 2.
Mechanisms of regulation of inflammation and fibrosis through septins. This figure depicts the known or proposed impact of septins on: TGF-β and MAP kinase signaling (1); pathogen invasion, processing, and dissemination (2); exocytosis and assembly of extracellular matrix proteins (3); and immune cell migration (4). SEPT, septin; TGF-β, transforming growth factor-β.
Host–Pathogen Interactions
Septin-dependent regulation of host–pathogen interactions have been initially alluded to by a study that identified SEPT9 as a major protein enriched in phagosomes responsible for the cellular entry of Listeria monocytogenes.92 Subsequent studies showed crucial regulatory roles of the septin cytoskeleton during infection of cultured mammalian cells with different bacterial, fungal, or viral pathogens.8,93 Interestingly, septins appear to have a dual role in controlling pathogen invasion and spread in the host cells. On the one hand, pathogens hijack the septin cytoskeleton to invade the host cells, as supported by septin accumulation at the pathogen entry sites during L. monocytogenes, Shigella flexneri, Salmonella typhimurium, and Candida albicans invasion.72, 73, 74 On the other hand, depletion of key septin paralogs, such as SEPT2, SEPT7, and SEPT9, markedly inhibits internalization of these bacterial pathogens.72, 73, 74 Although mechanisms underlying septin-dependent regulation of bacterial entry remain poorly investigated, they likely involve remodeling of the cortical actin cytoskeleton. Thus, SEPT7 mediates recruitment of an important actin cytoskeletal regulator, Rho-associated kinase 2, to the S. typhimurium entry sites and promotes Salmonella-induced phosphorylation of the actin polymerizing protein, formin homology domain containing-1.73 Additionally, atomic force microscopy indicates decreased viscosity and elasticity of the cell cortex following SEPT2 or SEPT11 depletion, which is consistent with altered organization of the submembranous actin cytoskeleton.94 Altered organization of the cortical actin cytoskeleton in septin-deficient cells can also explain the inhibitory effects of SEPT9 depletion on the attachment of enteropathogenic Escherichia coli, which requires extensive cytoskeletal remodeling of the host cell.75
Despite accelerating pathogen entry, septin cytoskeleton also acts as a negative regulator of infection by limiting survival and spread of intracellular pathogens. Septin filament assembly entraps a significant fraction of intracytosolic Shigella, including actively dividing bacteria.95,96 This septin caging inhibits bacterial metabolism, proliferation, and motility inside the host cells.76,77,96 More importantly, assembled septin cages recruit components of the autophagosomal machinery that accelerates the delivery of entrapped Shigella into lysosomes for degradation.95,96 Formation of septin cages is not a unique event of Shigella infection, and also occurs during invasion of other intracellular pathogens. For example, septin filament network assembles around intracellular vacuoles containing Chlamydia trachomatis in human epithelial cells78 and internalized enteropathogenic E. coli in macrophages.97 Furthermore, during vaccinia virus infection, septin filaments wrap around newly assembled virions attached to the host cell surface, forming cage-like structures.79 Interestingly, encaging of Chlamydia and vaccinia virus by the host septin cytoskeleton has opposite effects on the pathogen release from the host cells. Specifically, the septin cytoskeleton prevents vaccinia virus release and cell-to-cell dissemination while promoting Chlamydia extrusion.78,79 Finally, a different mechanism exploiting the lipid-binding ability of the septin cytoskeleton was shown to drive hepatitis C virus (HCV) infection of liver cells.47 HCV infection markedly accelerates SEPT9 expression and septin filament assembly. This results in accumulation of intracellular lipid droplets, which is dependent on septin filament interactions with phosphoinositide and microtubules. Such septin-driven formation of lipid droplets is crucial for efficient HCV replication.47
While a growing number of studies focus on the septin-dependent regulation of pathogen interactions with cultured cells in vitro, almost nothing is known about the ability of the septin cytoskeleton to regulate pathogen infection in vivo. The only study addressing this reported that septins restrict Shigella infection and the associated inflammatory response in zebrafish larvae.80 In this study, loss of SEPT7 increased bacterial burden, which was linked to overactivation of IL-1β signaling and increase in bacteria-induced neutrophil death.80 Interestingly, pharmacologic inhibition of the inflammatory response using IL-1 receptor antagonist rescued neutrophil survival and decreased bacterial burden in septin-deficient zebrafish. Together, these studies strongly suggest that the septin cytoskeleton could serve as an important regulator of host–pathogen interactions and inflammatory responses triggered by bacterial or viral infections.
Immune Cell Development and Functions
Different septin paralogs are abundantly expressed in human macrophages, lymphocytes, and dendritic cells.81,82,98 However, the role of the septin cytoskeleton in regulation of immune cell development and functions remains poorly understood (Table 1). In macrophages, SEPT2 and SEPT11 accumulate at the phagosomes in actin cytoskeleton-dependent fashion, and SEPT2 depletion was shown to inhibit Fc receptor–mediated phagocytosis.81 Disruption of the septin cytoskeleton by SEPT7 depletion causes profound alterations in T lymphocyte cell morphology manifested by the excessive membrane blebbing and increased uropod length.82,99 The disruption of the septin cytoskeleton also has a complex effect on T-cell motility such that SEPT7-deficient T cells exhibit decreased crawling velocity during random planar migration, but are able to more efficiently transmigrate through confined spaces, such as the small pores in the Transwell membrane filters.82 It has been suggested that decreased rigidity of the cell cortex in septin-deficient T cells increases the ability of the cell membrane to form protrusions, which impairs the processive and directional movement of the cell in open spaces. By contrast, such cortical destabilization allows for higher deformability of septin-depleted cells that accelerates their passing through narrow spaces.82,100 Disruption of the septin cytoskeleton negatively impacts other aspects of T-cell development and functions. Thus, transgenic mice with T-cell–specific ablation of SEPT9 are characterized by a decreased number of mature peripheral T cells, especially the CD8+ T-cell population.84 These maturation defects are associated with the decreased proliferation capacity of SEPT9-deficient CD8+ T cells. Similarly, conditional knockout of SEPT7 in T lymphocytes results in the diminished CD8+ cell population in aged mice.83 Additionally, isolated SEPT7-deficient CD8+ T cells show markedly reduced proliferation and cytokinesis failure after stimulation with soluble immune activators such as cytokines, phorbol ester, or ionomycin.83
Regulation of Tissue Barriers
In addition to playing important roles in regulating pathogen engagement and immune cell responses, septin cytoskeleton serves as essential regulator of the integrity and permeability of tissue barriers (Table 1). Indeed, disruption of epithelial and vascular endothelial barriers is a common feature of different inflammatory processes.101,102 Limited and transient increased barrier permeability has beneficial effects by allowing tissue influx of immune cells and efficient pathogen clearance. However, poorly controlled and prolonged disruption of epithelial and endothelial barriers is detrimental because it increases body exposure to environmental pathogens and stressors, and exaggerates the inflammatory response.101,102 Permeability of epithelial and endothelial barriers is regulated by specialized adhesive structures, adherens and tight junctions, which are regulated by the underlying cortical actomyosin cytoskeleton.13,14,103 Emerging evidence suggests that the septin cytoskeleton acts as an important regulator of epithelial and endothelial junctions. For example, SEPT2 is enriched at intercellular junctions in cultured human endothelial86 and bronchial epithelial cells.85 Down-regulation of SEPT2 and SEPT7 expression increases permeability of model bronchial and intestinal epithelial cell monolayers, respectively.28,85 In vascular endothelium, loss of SEPT2 accelerates leakiness of a resting endothelial barrier,86 as well as exaggerates barrier disruption and attenuates barrier recovery following endothelial stimulation with thrombin.87 Interestingly, increased vascular permeability of SEPT2-depleted endothelium promotes transendothelial migration of natural killer cells.86 Furthermore, loss of SEPT2 sensitizes bronchial epithelial cells to the barrier-disruptive action of an environmental hazard, particulate matter.85 These examples demonstrate that septin-dependent stabilization of epithelial and endothelial barriers could serve as a protective mechanism limiting immune cell exposure to environmental pathogens during tissue inflammation.
Tissue Fibrosis
Limited evidence, primarily derived from the liver and kidney, implicates the septin cytoskeleton in the regulation of aberrant tissue repair and fibrosis (Table 1). The only comprehensive characterization of septin expression in fibrotic tissues was performed in a mouse model of kidney fibrosis.91 A gene expression analysis for different septins (SEPT1 and SEPT4 through SEPT11) demonstrates a significant up-regulation of all septin transcripts in the fibrotic kidney compared with controls. Immunohistologic experiments detect SEPT7 and SEPT8 protein up-regulation primarily in the tubulointerstitial region of the fibrotic kidney. Myofibroblasts are considered the chief profibrotic cell type across organs. Interestingly, septins were shown to significantly colocalize with the myofibroblast marker α-smooth muscle actin in the diseased kidneys.91 SEPT4 and SEPT6 were also found up-regulated in different rodent models of liver fibrosis, where they were associated with hepatic stellate cells (HSC).90,104 However, given a recent report that shows down-regulation of SEPT9 expression in carbon tetrachloride–induced liver fibrosis, septin expression could be a subject of more complex regulation in fibrotic tissues.105
Functional effects of selected septins on fibrogenesis were examined in a limited number of studies that utilized knockout mice models and adenovirus-mediated manipulation of septin expression in vivo. Mice with total knockout of SEPT4 display exaggerated hepatic fibrosis in three different experimental models (carbon tetrachloride, and cholestasis- and steatohepatitis-induced fibrosis).88 These profibrotic effects of SEPT4 deletion are linked to the increased activation and collagen-producing ability of HSC, the major myofibroblast precursor cell type in the liver. Supporting this loss of function data, adenoviral-mediated overexpression of SEPT4 suppresses hepatic fibrosis induced by Schistosoma japonicum infection in mice.89 Antifibrotic effects of SEPT4 overexpression are associated with inhibition of HSC activity and their increased apoptosis. It is noteworthy that SEPT4 was shown to be a potent regulator of spontaneous and TGF-β–induced apoptosis in different tissues.32,106 In contrast to the reported antifibrotic functions of SEPT4, a recent study describes a profibrotic activity of SEPT6.90 Indeed, shRNA-mediated depletion of SEPT6 in vivo attenuates thioacetamide-induced hepatic fibrosis in rats by inhibiting HSC activation and decreasing the excessive extracellular matrix deposition seen in fibrosis.90 Finally, SEPT8-null mice do not show any differences in the development of kidney fibrosis as compared with wild-type controls, thereby suggesting dispensability of SEPT8 in this disease model.91 At present, it is unclear whether the conflicting evidence reflects peculiar roles of different members of the septin family in fibrosis, or tissue-specific effects of the septin cytoskeleton on fibrogenesis. In addition, a temporal role of the septin cytoskeleton in early versus late tissue damage has not been evaluated yet.
On a cellular level, septin-dependent regulation of tissue fibrosis is likely linked to the central profibrotic signaling pathway involving TGF-β. TGF-β is known to modulate septin expression and localization by up-regulating SEPT690,107 and down-regulating SEPT9 levels in the liver,105 as well as triggering nuclear translocation of SEPT4/ARTS in epithelial cells.32 Septins themselves could act as positive regulators of TGF-β signaling by stimulating expression of this growth factor and promoting TGF-β–driven Smad signaling, as was shown for SEPT6 and SEPT9 actions in HSC90 and glioma cells,108 respectively. These data indicate that the septin cytoskeleton could be part of an important fibrogenic positive feedback loop mechanism involving TGF-β–induced up-regulation of septins, which in turn triggers further increase in TGF-β level and activity. Septins modulate other signaling pathways important for fibrogenesis in HSC. For example, SEPT6 up-regulates the activity of all three major MAP kinases: ERK, JNK, and p38,90 all of which have been implicated in organ fibrosis.109 Furthermore, SEPT4 can limit HSC activation by stimulating expression of a Wnt antagonist, Dickkopf2, thereby inhibiting the canonical Wnt signaling.110
The Septin Cytoskeleton as a Potential Target for Therapeutic Interventions
Emerging functions of the septin cytoskeleton in regulating various aspects of the immune response and tissue remodeling during inflammation and fibrosis raise the important question of whether septins could be successfully targeted to develop novel anti-inflammatory and antifibrotic therapies. In general, functional activity of different cytoskeletal structures such as actin filaments and microtubules could be modulated by cell-permeable small molecules that cause either stabilization or disassembly of targeted cytoskeletal structures. The feasibility of clinical application of such compounds is illustrated by the successful use of microtubule-stabilizing taxane class drugs as anticancer agents. However, pharmacologic modulation of the septin cytoskeleton, while possible, remains at the very early stage of exploration. The experimental toolbox of septin-modulating compounds is very narrow with only one small molecule, forchlorfenuron (FCF), being extensively used to target the septin cytoskeleton in different experimental systems.111 FCF was shown to block septin filament turnover, resulting in accumulation of thick septin bundles and aggregates.112 Interestingly, such FCF-induced stabilization of the septin cytoskeleton mimics functional effects of the depletion of key septin paralogs by inhibiting cell division, migration, vesicle trafficking, calcium entry, and cell–cell adhesions.57,58,60,64,85,112 Despite diverse effects on cell function, FCF itself may not be suitable for clinical use in its current form. It has a relatively low affinity for septin filaments and hence is only active at high, submillimolar concentrations. Furthermore, possible off-target effects and animal tissue toxicity of FCF have been reported.28,111,113 Nevertheless, studies utilizing FCF have laid the ground for the development of next-generation septin inhibitors. We expect that these inhibitors will be first developed as anticancer drugs, given the substantial body of evidence about mutations or altered expression of different septin paralogs being associated with tumorigenesis.10 Indeed, two recent studies report synthesis of different chemical derivatives of FCF and their effects on cancer cells.113,114 While one study characterizes cytotoxic effects of FCF derivatives in malignant mesothelioma cells, 113 the other compares effects of FCF and its analogs on viability of ovarian and endometrial cancer cells.114 Not much is known about the possibility of pharmacologic inhibition of the septin cytoskeleton during inflammation and tissue fibrosis. Further studies are required to examine potential utilization of FCF and its derivatives for the development of novel anti-inflammatory and antifibrotic therapies.
Conclusion
The septin cytoskeleton represents a critical component of cellular architecture that is known to mediate a variety of housekeeping and specialized functions. Most known for their roles in normal tissue development and homeostasis, septins are increasingly recognized as important contributors to human diseases. A significant body of literature links the altered expression and mutations of different septins with the development of neurological diseases and cancer. Much less is known about the role of the septin cytoskeleton in regulating tissue inflammation and fibrosis. As demonstrated in this review article, exciting recent developments in this field include discovering the roles of septins in the regulation of host interactions with bacterial and viral pathogens, modulation of lymphocytes differentiation, and profibrotic signaling (Table 1 and Figure 2). However, a number of important questions remain to be answered. For example, it is crucial to understand whether, as part of the same cytockeletal structure, different septins similarly regulate inflammatory and fibrotic responses, or whether members of the septin protein family have unique functional roles. It would be important to examine how septins regulate signaling by pattern recognition receptors that sense different pathogens, as well as signaling by different inflammatory cytokines and growth factors. Given the known roles of septins in the regulation of vesicle trafficking and the Golgi, it should be a research priority to determine whether the septin cytoskeleton controls secretion of different inflammatory factors by activated immune cells and regulates deposition of the extracellular matrix in fibrotic tissues. There is obvious need for extensive in vivo studies involving mouse models with tissue-specific knockout of different septins. From a therapeutic standpoint, it is critical to understand whether the septin cytoskeleton could be a druggable target during inflammation and tissue fibrosis, and to develop specific pharmacologic modulators of septin cytoskeletal assembly and dynamics. We are still at the very beginning of the exploration in this exciting and potentially fruitful research field, and future studies will pave the way for the modulation of septins to treat human inflammatory and fibrotic diseases.
Footnotes
Supported by NIH-NIDDK grants RO1 DK108278 (A.I.I.), K08 DK110415, and R01 DK123233 (F.R.); and the Kenneth Rainin Foundation Synergy Award (A.I.I. and F.R.).
Disclosures: F.R. is a consultant or on an advisory board for Agomab, Allergan, AbbVie, BMS, Boehringer Ingelheim, Celgene, CDISC, Cowen, Falk Pharma, Genentech, Gilead, Gossamer, Guidepoint, Helmsley, Index Pharma, Janssen, Koutif, Metacrine, Morphic, Pfizer, Pliant, Prometheus Biosciences, Receptos, RedX, Roche, Samsung, Takeda, Techlab, Theravance, Thetis, and UCB.
Contributor Information
Andrei I. Ivanov, Email: ivanova2@ccf.org.
Florian Rieder, Email: riederf@ccf.org.
References
- 1.Barnig C., Bezema T., Calder P.C., Charloux A., Frossard N., Garssen J., Haworth O., Dilevskaya K., Levi-Schaffer F., Lonsdorfer E., Wauben M., Kraneveld A.D., Te Velde A.A. Activation of resolution pathways to prevent and fight chronic inflammation: lessons from asthma and inflammatory bowel disease. Front Immunol. 2019;10:1699. doi: 10.3389/fimmu.2019.01699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brazil J.C., Quiros M., Nusrat A., Parkos C.A. Innate immune cell-epithelial crosstalk during wound repair. J Clin Invest. 2019;129:2983–2993. doi: 10.1172/JCI124618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li J., Mao R., Kurada S., Wang J., Lin S., Chandra J., Rieder F. Pathogenesis of fibrostenosing Crohn's disease. Transl Res. 2019;209:39–54. doi: 10.1016/j.trsl.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 4.Van Damme N., Van Hecke A., Remue E., Van den Bussche K., Moore Z., Gefen A., Verhaeghe S., Beeckman D. Physiological processes of inflammation and edema initiated by sustained mechanical loading in subcutaneous tissues: a scoping review. Wound Repair Regen. 2020;28:242–265. doi: 10.1111/wrr.12777. [DOI] [PubMed] [Google Scholar]
- 5.López-Posadas R., Neurath M.F., Atreya I. Molecular pathways driving disease-specific alterations of intestinal epithelial cells. Cell Mol Life Sci. 2017;74:803–826. doi: 10.1007/s00018-016-2363-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mortensen J.H., Lindholm M., Langholm L.L., Kjeldsen J., Bay-Jensen A.C., Karsdal M.A., Manon-Jensen T. The intestinal tissue homeostasis - the role of extracellular matrix remodeling in inflammatory bowel disease. Expert Rev Gastroenterol Hepatol. 2019;13:977–993. doi: 10.1080/17474124.2019.1673729. [DOI] [PubMed] [Google Scholar]
- 7.Hohmann T., Dehghani F. The cytoskeleton-a complex interacting meshwork. Cells. 2019;8:362. doi: 10.3390/cells8040362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mostowy S., Cossart P. Septins: the fourth component of the cytoskeleton. Nat Rev Mol Cell Biol. 2012;13:183–194. doi: 10.1038/nrm3284. [DOI] [PubMed] [Google Scholar]
- 9.Tang D.D., Gerlach B.D. The roles and regulation of the actin cytoskeleton, intermediate filaments and microtubules in smooth muscle cell migration. Respir Res. 2017;18:54. doi: 10.1186/s12931-017-0544-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Angelis D., Spiliotis E.T. Septin mutations in human cancers. Front Cell Dev Biol. 2016;4:122. doi: 10.3389/fcell.2016.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fife C.M., McCarroll J.A., Kavallaris M. Movers and shakers: cell cytoskeleton in cancer metastasis. Br J Pharmacol. 2014;171:5507–5523. doi: 10.1111/bph.12704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lagrue K., Carisey A., Oszmiana A., Kennedy P.R., Williamson D.J., Cartwright A., Barthen C., Davis D.M. The central role of the cytoskeleton in mechanisms and functions of the NK cell immune synapse. Immunol Rev. 2013;256:203–221. doi: 10.1111/imr.12107. [DOI] [PubMed] [Google Scholar]
- 13.Lechuga S., Ivanov A.I. Disruption of the epithelial barrier during intestinal inflammation: quest for new molecules and mechanisms. Biochim Biophys Acta Mol Cell Res. 2017;1864:1183–1194. doi: 10.1016/j.bbamcr.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schnoor M., Garcia Ponce A., Vadillo E., Pelayo R., Rossaint J., Zarbock A. Actin dynamics in the regulation of endothelial barrier functions and neutrophil recruitment during endotoxemia and sepsis. Cell Mol Life Sci. 2017;74:1985–1997. doi: 10.1007/s00018-016-2449-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loeven N.A., Medici N.P., Bliska J.B. The pyrin inflammasome in host-microbe interactions. Curr Opin Microbiol. 2020;54:77–86. doi: 10.1016/j.mib.2020.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin-Cofreces N.B., Baixauli F., Sanchez-Madrid F. Immune synapse: conductor of orchestrated organelle movement. Trends Cell Biol. 2014;24:61–72. doi: 10.1016/j.tcb.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karki P., Birukova A.A. Microtubules-associated Rac regulation of endothelial barrier: a role of Asef in acute lung injury. J Investig Med. 2017;65:1089–1092. doi: 10.1136/jim-2017-000571. [DOI] [PubMed] [Google Scholar]
- 18.Pekny M., Wilhelmsson U., Bogestal Y.R., Pekna M. The role of astrocytes and complement system in neural plasticity. Int Rev Neurobiol. 2007;82:95–111. doi: 10.1016/S0074-7742(07)82005-8. [DOI] [PubMed] [Google Scholar]
- 19.Coulombe P.A., Kerns M.L., Fuchs E. Epidermolysis bullosa simplex: a paradigm for disorders of tissue fragility. J Clin Invest. 2009;119:1784–1793. doi: 10.1172/JCI38177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kinoshita M. Assembly of mammalian septins. J Biochem. 2003;134:491–496. doi: 10.1093/jb/mvg182. [DOI] [PubMed] [Google Scholar]
- 21.Russell S.E., Hall P.A. Septin genomics: a road less travelled. Biol Chem. 2011;392:763–767. doi: 10.1515/BC.2011.079. [DOI] [PubMed] [Google Scholar]
- 22.Valadares N.F., d' Muniz Pereira H., Ulian Araujo A.P., Garratt R.C. Septin structure and filament assembly. Biophys Rev. 2017;9:481–500. doi: 10.1007/s12551-017-0320-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hall P.A., Jung K., Hillan K.J., Russell S.E. Expression profiling the human septin gene family. J Pathol. 2005;206:269–278. doi: 10.1002/path.1789. [DOI] [PubMed] [Google Scholar]
- 24.Zuvanov L., Mota D.M.D., Araujo A.P.U., DeMarco R. A blueprint of septin expression in human tissues. Funct Integr Genomics. 2019;19:787–797. doi: 10.1007/s10142-019-00690-3. [DOI] [PubMed] [Google Scholar]
- 25.McMurray M.A., Thorner J. Turning it inside out: the organization of human septin heterooligomers. Cytoskeleton. 2019;76:449–456. doi: 10.1002/cm.21571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mendonca D.C., Macedo J.N., Guimaraes S.L., Barroso da Silva F.L., Cassago A., Garratt R.C., Portugal R.V., Araujo A.P.U. A revised order of subunits in mammalian septin complexes. Cytoskeleton. 2019;76:457–466. doi: 10.1002/cm.21569. [DOI] [PubMed] [Google Scholar]
- 27.Menon M.B., Sawada A., Chaturvedi A., Mishra P., Schuster-Gossler K., Galla M., Schambach A., Gossler A., Forster R., Heuser M., Kotlyarov A., Kinoshita M., Gaestel M. Genetic deletion of SEPT7 reveals a cell type-specific role of septins in microtubule destabilization for the completion of cytokinesis. PLoS Genet. 2014;10:e1004558. doi: 10.1371/journal.pgen.1004558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun L., Cao X., Lechuga S., Feygin A., Naydenov N.G., Ivanov A.I. A septin cytoskeleton-targeting small molecule, forchlorfenuron, inhibits epithelial migration via septin-independent perturbation of cellular signaling. Cells. 2019;9:84. doi: 10.3390/cells9010084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bridges A.A., Gladfelter A.S. Septin form and function at the cell cortex. J Biol Chem. 2015;290:17173–17180. doi: 10.1074/jbc.R114.634444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dolat L., Hu Q., Spiliotis E.T. Septin functions in organ system physiology and pathology. Biol Chem. 2014;395:123–141. doi: 10.1515/hsz-2013-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song K., Gras C., Capin G., Gimber N., Lehmann M., Mohd S., Puchkov D., Rödiger M., Wilhelmi I., Daumke O., Schmoranzer J., Schürmann A., Krauss M. A SEPT1-based scaffold is required for Golgi integrity and function. J Cell Sci. 2019;132:jcs225557. doi: 10.1242/jcs.225557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Larisch S., Yi Y., Lotan R., Kerner H., Eimerl S., Tony Parks W., Gottfried Y., Birkey Reffey S., de Caestecker M.P., Danielpour D., Book-Melamed N., Timberg R., Duckett C.S., Lechleider R.J., Steller H., Orly J., Kim S.J., Roberts A.B. A novel mitochondrial septin-like protein, ARTS, mediates apoptosis dependent on its P-loop motif. Nat Cell Biol. 2000;2:915–921. doi: 10.1038/35046566. [DOI] [PubMed] [Google Scholar]
- 33.Garcia W., de Araújo A.P., Lara F., Foguel D., Tanaka M., Tanaka T., Garratt R.C. An intermediate structure in the thermal unfolding of the GTPase domain of human septin 4 (SEPT4/bradeion-beta) forms amyloid-like filaments in vitro. Biochemistry. 2007;46:11101–11109. doi: 10.1021/bi700702w. [DOI] [PubMed] [Google Scholar]
- 34.Hagiwara A., Tanaka Y., Hikawa R., Morone N., Kusumi A., Kimura H., Kinoshita M. Submembranous septins as relatively stable components of actin-based membrane skeleton. Cytoskeleton. 2011;68:512–525. doi: 10.1002/cm.20528. [DOI] [PubMed] [Google Scholar]
- 35.Lam M., Calvo F. Regulation of mechanotransduction: emerging roles for septins. Cytoskeleton. 2019;76:115–122. doi: 10.1002/cm.21485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spiliotis E.T. Spatial effects - site-specific regulation of actin and microtubule organization by septin GTPases. J Cell Sci. 2018;131:jcs207555. doi: 10.1242/jcs.207555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dolat L., Hunyara J.L., Bowen J.R., Karasmanis E.P., Elgawly M., Galkin V.E., Spiliotis E.T. Septins promote stress fiber-mediated maturation of focal adhesions and renal epithelial motility. J Cell Biol. 2014;207:225–235. doi: 10.1083/jcb.201405050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mavrakis M., Azou-Gros Y., Tsai F.-C., Alvarado J., Bertin A., Iv F., Kress A., Brasselet S., Koenderink G.H., Lecuit T. Septins promote F-actin ring formation by crosslinking actin filaments into curved bundles. Nat Cell Biol. 2014;16:322–334. doi: 10.1038/ncb2921. [DOI] [PubMed] [Google Scholar]
- 39.Smith C., Dolat L., Angelis D., Forgacs E., Spiliotis E.T., Galkin V.E. Septin 9 exhibits polymorphic binding to f-actin and inhibits myosin and cofilin activity. J Mol Biol. 2015;427:3273–3284. doi: 10.1016/j.jmb.2015.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hecht M., Rosler R., Wiese S., Johnsson N., Gronemeyer T. An interaction network of the human SEPT9 established by quantitative mass spectrometry. G3 (Bethesda) 2019;9:1869–1880. doi: 10.1534/g3.119.400197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Joo E., Surka M.C., Trimble W.S. Mammalian SEPT2 is required for scaffolding nonmuscle myosin II and its kinases. Dev Cell. 2007;13:677–690. doi: 10.1016/j.devcel.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 42.Bai X., Bowen J.R., Knox T.K., Zhou K., Pendziwiat M., Kuhlenbaumer G., Sindelar C.V., Spiliotis E.T. Novel septin 9 repeat motifs altered in neuralgic amyotrophy bind and bundle microtubules. J Cell Biol. 2013;203:895–905. doi: 10.1083/jcb.201308068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakos K., Radler M.R., Spiliotis E.T. Septin 2/6/7 complexes tune microtubule plus-end growth and EB1 binding in a concentration- and filament-dependent manner. Mol Biol Cell. 2019;30:2913–2928. doi: 10.1091/mbc.E19-07-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bowen J.R., Hwang D., Bai X., Roy D., Spiliotis E.T. Septin GTPases spatially guide microtubule organization and plus end dynamics in polarizing epithelia. J Cell Biol. 2011;194:187–197. doi: 10.1083/jcb.201102076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shindo A., Audrey A., Takagishi M., Takahashi M., Wallingford J.B., Kinoshita M. Septin-dependent remodeling of cortical microtubule drives cell reshaping during epithelial wound healing. J Cell Sci. 2018;131:jcs212647. doi: 10.1242/jcs.212647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Neubauer K., Zieger B. The mammalian septin interactome. Front Cell Dev Biol. 2017;5:3. doi: 10.3389/fcell.2017.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Akil A., Peng J., Omrane M., Gondeau C., Desterke C., Marin M., Tronchère H., Taveneau C., Sar S., Briolotti P., Benjelloun S., Benjouad A., Maurel P., Thiers V., Bressanelli S., Samuel D., Bréchot C., Gassama-Diagne A. Septin 9 induces lipid droplets growth by a phosphatidylinositol-5-phosphate and microtubule-dependent mechanism hijacked by HCV. Nat Commun. 2016;7:12203. doi: 10.1038/ncomms12203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Omrane M., Camara A.S., Taveneau C., Benzoubir N., Tubiana T., Yu J., Guérois R., Samuel D., Goud B., Poüs C., Bressanelli S., Garratt R.C., Thiam A.R., Gassama-Diagne A. Septin 9 has two polybasic domains critical to septin filament assembly and Golgi integrity. iScience. 2019;13:138–153. doi: 10.1016/j.isci.2019.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bridges A.A., Zhang H., Mehta S.B., Occhipinti P., Tani T., Gladfelter A.S. Septin assemblies form by diffusion-driven annealing on membranes. Proc Natl Acad Sci U S A. 2014;111:2146–2151. doi: 10.1073/pnas.1314138111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beber A., Taveneau C., Nania M., Tsai F.-C., Di Cicco A., Bassereau P., Lévy D., Cabral J.T., Isambert H., Mangenot S., Bertin A. Membrane reshaping by micrometric curvature sensitive septin filaments. Nat Commun. 2019;10:420. doi: 10.1038/s41467-019-08344-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bridges A.A., Jentzsch M.S., Oakes P.W., Occhipinti P., Gladfelter A.S. Micron-scale plasma membrane curvature is recognized by the septin cytoskeleton. J Cell Biol. 2016;213:23–32. doi: 10.1083/jcb.201512029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cannon K.S., Woods B.L., Crutchley J.M., Gladfelter A.S. An amphipathic helix enables septins to sense micrometer-scale membrane curvature. J Cell Biol. 2019;218:1128–1137. doi: 10.1083/jcb.201807211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Caudron F., Barral Y. Septins and the lateral compartmentalization of eukaryotic membranes. Dev Cell. 2009;16:493–506. doi: 10.1016/j.devcel.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 54.Dolat L., Spiliotis E.T. Septins promote macropinosome maturation and traffic to the lysosome by facilitating membrane fusion. J Cell Biol. 2016;214:517–527. doi: 10.1083/jcb.201603030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Traikov S., Stange C., Wassmer T., Paul-Gilloteaux P., Salamero J., Raposo G., Hoflack B. Septin6 and septin7 GTP binding proteins regulate AP-3- and ESCRT-dependent multivesicular body biogenesis. PLoS One. 2014;9:e109372. doi: 10.1371/journal.pone.0109372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Diesenberg K., Beerbaum M., Fink U., Schmieder P., Krauss M. SEPT9 negatively regulates ubiquitin-dependent downregulation of EGFR. J Cell Sci. 2015;128:397–407. doi: 10.1242/jcs.162206. [DOI] [PubMed] [Google Scholar]
- 57.Marcus E.A., Tokhtaeva E., Turdikulova S., Capri J., Whitelegge J.P., Scott D.R., Sachs G., Berditchevski F., Vagin O. Septin oligomerization regulates persistent expression of ErbB2/HER2 in gastric cancer cells. Biochem J. 2016;473:1703–1718. doi: 10.1042/BCJ20160203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tokhtaeva E., Capri J., Marcus E.A., Whitelegge J.P., Khuzakhmetova V., Bukharaeva E., Deiss-Yehiely N., Dada L.A., Sachs G., Fernandez-Salas E., Vagin O. Septin dynamics are essential for exocytosis. J Biol Chem. 2015;290:5280–5297. doi: 10.1074/jbc.M114.616201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zeng Y., Cao Y., Liu L., Zhao J., Zhang T., Xiao L., Jia M., Tian Q., Yu H., Chen S., Cai Y. SEPT9_i1 regulates human breast cancer cell motility through cytoskeletal and RhoA/FAK signaling pathway regulation. Cell Death Dis. 2019;10:720. doi: 10.1038/s41419-019-1947-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang N., Liu L., Fan N., Zhang Q., Wang W., Zheng M., Ma L., Li Y., Shi L. The requirement of SEPT2 and SEPT7 for migration and invasion in human breast cancer via MEK/ERK activation. Oncotarget. 2016;7:61587–61600. doi: 10.18632/oncotarget.11402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Collins K.B., Kang H., Matsche J., Klomp J.E., Rehman J., Malik A.B., Karginov A.V. Septin2 mediates podosome maturation and endothelial cell invasion associated with angiogenesis. J Cell Biol. 2020;219:e201903023. doi: 10.1083/jcb.201903023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Møller A.M.J., Füchtbauer E.-M., Brüel A., Andersen T.L., Borggaard X.G., Pavlos N.J., Thomsen J.S., Pedersen F.S., Delaisse J.M., Søe K. Septins are critical regulators of osteoclastic bone resorption. Sci Rep. 2018;8:13016. doi: 10.1038/s41598-018-31159-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Deb B.K., Pathak T., Hasan G. Store-independent modulation of Ca(2+) entry through Orai by septin 7. Nat Commun. 2016;7:11751. doi: 10.1038/ncomms11751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sharma S., Quintana A., Findlay G.M., Mettlen M., Baust B., Jain M., Nilsson R., Rao A., Hogan P.G. An siRNA screen for NFAT activation identifies septins as coordinators of store-operated Ca2+ entry. Nature. 2013;499:238–242. doi: 10.1038/nature12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang N., Zhang Y., You S., Tian Y., Lu S., Cao L., Sun Y. Septin4 prevents PDGF-BB-induced HAVSMC phenotypic transformation, proliferation and migration by promoting SIRT1-STAT3 deacetylation and dephosphorylation. Int J Biol Sci. 2020;16:708–718. doi: 10.7150/ijbs.39843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gonzalez M.E., Makarova O., Peterson E.A., Privette L.M., Petty E.M. Up-regulation of SEPT9_v1 stabilizes c-Jun-N-terminal kinase and contributes to its pro-proliferative activity in mammary epithelial cells. Cell Signal. 2009;21:477–487. doi: 10.1016/j.cellsig.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Amir S., Golan M., Mabjeesh N.J. Targeted knockdown of SEPT9_v1 inhibits tumor growth and angiogenesis of human prostate cancer cells concomitant with disruption of hypoxia-inducible factor-1 pathway. Mol Cancer Res. 2010;8:643–652. doi: 10.1158/1541-7786.MCR-09-0497. [DOI] [PubMed] [Google Scholar]
- 68.Amir S., Wang R., Matzkin H., Simons J.W., Mabjeesh N.J. MSF-A interacts with hypoxia-inducible factor-1alpha and augments hypoxia-inducible factor transcriptional activation to affect tumorigenicity and angiogenesis. Cancer Res. 2006;66:856–866. doi: 10.1158/0008-5472.CAN-05-2738. [DOI] [PubMed] [Google Scholar]
- 69.Taylor C.T., Colgan S.P. Regulation of immunity and inflammation by hypoxia in immunological niches. Nat Rev Immunol. 2017;17:774–785. doi: 10.1038/nri.2017.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Marttinen M., Kurkinen K.M., Soininen H., Haapasalo A., Hiltunen M. Synaptic dysfunction and septin protein family members in neurodegenerative diseases. Mol Neurodegener. 2015;10:16. doi: 10.1186/s13024-015-0013-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Poüs C., Klipfel L., Baillet A. Cancer-related functions and subcellular localizations of septins. Front Cell Dev Biol. 2016;4:126. doi: 10.3389/fcell.2016.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mostowy S., Nam Tham T., Danckaert A., Guadagnini S., Boisson-Dupuis S., Pizarro-Cerdá J., Cossart P. Septins regulate bacterial entry into host cells. PLoS One. 2009;4:e4196. doi: 10.1371/journal.pone.0004196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Boddy K.C., Gao A.D., Truong D., Kim M.S., Froese C.D., Trimble W.S., Brumell J.H. Septin-regulated actin dynamics promote Salmonella invasion of host cells. Cell Microbiol. 2018;20:e12866. doi: 10.1111/cmi.12866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Phan Q.T., Eng D.K., Mostowy S., Park H., Cossart P., Filler S.G. Role of endothelial cell septin 7 in the endocytosis of Candida albicans. mBio. 2013;4:e00542-1–3. doi: 10.1128/mBio.00542-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Scholz R., Imami K., Scott N.E., Trimble W.S., Foster L.J., Finlay B.B. Novel host proteins and signaling pathways in enteropathogenic E. coli pathogenesis identified by global phosphoproteome analysis. Mol Cell Proteomics. 2015;14:1927–1945. doi: 10.1074/mcp.M114.046847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lobato-Márquez D., Krokowski S., Sirianni A., Larrouy-Maumus G., Mostowy S. A requirement for septins and the autophagy receptor p62 in the proliferation of intracellular Shigella. Cytoskeleton (Hoboken) 2019;76:163–172. doi: 10.1002/cm.21453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sirianni A., Krokowski S., Lobato-Márquez D., Buranyi S., Pfanzelter J., Galea D., Willis A., Culley S., Henriques R., Larrouy-Maumus G., Hollinshead M., Sancho-Shimizu V., Way M., Mostowy S. Mitochondria mediate septin cage assembly to promote autophagy of Shigella. EMBO Rep. 2016;17:1029–1043. doi: 10.15252/embr.201541832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Volceanov L., Herbst K., Biniossek M., Schilling O., Haller D., Nölke T., Subbarayal P., Rudel T., Zieger B., Häcker G. Septins arrange F-actin-containing fibers on the Chlamydia trachomatis inclusion and are required for normal release of the inclusion by extrusion. mBio. 2014;5:e01802–e01814. doi: 10.1128/mBio.01802-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pfanzelter J., Mostowy S., Way M. Septins suppress the release of vaccinia virus from infected cells. J Cell Biol. 2018;217:2911–2929. doi: 10.1083/jcb.201708091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mazon-Moya M.J., Willis A.R., Torraca V., Boucontet L., Shenoy A.R., Colucci-Guyon E., Mostowy S. Septins restrict inflammation and protect zebrafish larvae from Shigella infection. PLoS Pathog. 2017;13:e1006467. doi: 10.1371/journal.ppat.1006467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huang Y.W., Yan M., Collins R.F., Diciccio J.E., Grinstein S., Trimble W.S. Mammalian septins are required for phagosome formation. Mol Biol Cell. 2008;19:1717–1726. doi: 10.1091/mbc.E07-07-0641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tooley A.J., Gilden J., Jacobelli J., Beemiller P., Trimble W.S., Kinoshita M., Krummel M.F. Amoeboid T lymphocytes require the septin cytoskeleton for cortical integrity and persistent motility. Nat Cell Biol. 2009;11:17–26. doi: 10.1038/ncb1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mujal A.M., Gilden J.K., Gerard A., Kinoshita M., Krummel M.F. A septin requirement differentiates autonomous and contact-facilitated T cell proliferation. Nat Immunol. 2016;17:315–322. doi: 10.1038/ni.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lassen L.B., Füchtbauer A., Schmitz A., Sørensen A.B., Pedersen F.S., Füchtbauer E.-M. Septin9 is involved in T-cell development and CD8+ T-cell homeostasis. Cell Tissue Res. 2013;352:695–705. doi: 10.1007/s00441-013-1618-6. [DOI] [PubMed] [Google Scholar]
- 85.Sidhaye V.K., Chau E., Breysse P.N., King L.S. Septin-2 mediates airway epithelial barrier function in physiologic and pathologic conditions. Am J Respir Cell Mol Biol. 2011;45:120–126. doi: 10.1165/rcmb.2010-0235OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim J., Cooper J.A. Septins regulate junctional integrity of endothelial monolayers. Mol Biol Cell. 2018;29:1693–1703. doi: 10.1091/mbc.E18-02-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Amado-Azevedo J., de Menezes R.X., van Nieuw Amerongen G.P., van Hinsbergh V.W.M., Hordijk P.L. A functional siRNA screen identifies RhoGTPase-associated genes involved in thrombin-induced endothelial permeability. PLoS One. 2018;13:e0201231. doi: 10.1371/journal.pone.0201231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Iwaisako K., Hatano E., Taura K., Nakajima A., Tada M., Seo S., Tamaki N., Sato F., Ikai I., Uemoto S., Kinoshita M. Loss of Sept4 exacerbates liver fibrosis through the dysregulation of hepatic stellate cells. J Hepatol. 2008;49:768–778. doi: 10.1016/j.jhep.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 89.He X., Bao J., Chen J., Sun X., Wang J., Zhu D., Song K., Peng W., Xu T., Duan Y. Adenovirus-mediated over-expression of Septin4 ameliorates hepatic fibrosis in mouse livers infected with Schistosoma japonicum. Parasitol Int. 2015;64:487–492. doi: 10.1016/j.parint.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 90.Fan Y., Du Z., Steib C.J., Ding Q., Lu P., Tian D., Liu M. Effect of SEPT6 on the biological behavior of hepatic stellate cells and liver fibrosis in rats and its mechanism. Lab Invest. 2019;99:17–36. doi: 10.1038/s41374-018-0133-5. [DOI] [PubMed] [Google Scholar]
- 91.Neubauer K., Neubauer B., Seidl M., Zieger B. Characterization of septin expression in normal and fibrotic kidneys. Cytoskeleton. 2019;76:143–153. doi: 10.1002/cm.21473. [DOI] [PubMed] [Google Scholar]
- 92.Pizarro-Cerdá J., Jonquières R., Gouin E., Vandekerckhove J., Garin J., Cossart P. Distinct protein patterns associated with Listeria monocytogenes InlA- or InlB-phagosomes. Cell Microbiol. 2002;4:101–115. doi: 10.1046/j.1462-5822.2002.00169.x. [DOI] [PubMed] [Google Scholar]
- 93.Torraca V., Mostowy S. Septins and bacterial infection. Front Cell Dev Biol. 2016;4:127. doi: 10.3389/fcell.2016.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mostowy S., Janel S., Forestier C., Roduit C., Kasas S., Pizarro-Cerdá J., Cossart P., Lafont F. A role for septins in the interaction between the Listeria monocytogenes invasion protein InlB and the Met receptor. Biophys J. 2011;100:1949–1959. doi: 10.1016/j.bpj.2011.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Krokowski S., Lobato-Márquez D., Chastanet A., Pereira P.M., Angelis D., Galea D., Larrouy-Maumus G., Henriques R., Spiliotis E.T., Carballido-López R., Mostowy S. Septins recognize and entrap dividing bacterial cells for delivery to lysosomes. Cell Host Microbe. 2018;24:866–874.e4. doi: 10.1016/j.chom.2018.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mostowy S., Bonazzi M., Hamon M.A., Tham T.N., Mallet A., Lelek M., Gouin E., Demangel C., Brosch R., Zimmer C., Sartori A., Kinoshita M., Lecuit M., Cossart P. Entrapment of intracytosolic bacteria by septin cage-like structures. Cell Host Microbe. 2010;8:433–444. doi: 10.1016/j.chom.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 97.Lee P.P., Lobato-Márquez D., Pramanik N., Sirianni A., Daza-Cajigal V., Rivers E., Cavazza A., Bouma G., Moulding D., Hultenby K., Westerberg L.S., Hollinshead M., Lau Y.-L., Burns S.O., Mostowy S., Bajaj-Elliott M., Thrasher A.J. Wiskott-Aldrich syndrome protein regulates autophagy and inflammasome activity in innate immune cells. Nat Commun. 2017;8:1576. doi: 10.1038/s41467-017-01676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sui L., Zhang W., Liu Q., Chen T., Li N., Wan T., Yu M., Cao X. Cloning and functional characterization of human septin 10, a novel member of septin family cloned from dendritic cells. Biochem Biophys Res Commun. 2003;304:393–398. doi: 10.1016/s0006-291x(03)00601-6. [DOI] [PubMed] [Google Scholar]
- 99.Gilden J.K., Peck S., Chen Y.C., Krummel M.F. The septin cytoskeleton facilitates membrane retraction during motility and blebbing. J Cell Biol. 2012;196:103–114. doi: 10.1083/jcb.201105127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gilden J., Krummel M.F. Control of cortical rigidity by the cytoskeleton: emerging roles for septins. Cytoskeleton. 2010;67:477–486. doi: 10.1002/cm.20461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Peng Z., Shu B., Zhang Y., Wang M. Endothelial response to pathophysiological stress. Arterioscler Thromb Vasc Biol. 2019;39:e233–e243. doi: 10.1161/ATVBAHA.119.312580. [DOI] [PubMed] [Google Scholar]
- 102.Zuo L., Kuo W.T., Turner J.R. Tight junctions as targets and effectors of mucosal immune homeostasis. Cell Mol Gastroenterol Hepatol. 2020;10:327–340. doi: 10.1016/j.jcmgh.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ivanov A.I., Parkos C.A., Nusrat A. Cytoskeletal regulation of epithelial barrier function during inflammation. Am J Pathol. 2010;177:512–524. doi: 10.2353/ajpath.2010.100168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Duan Y.N., Qian H.Y., Qin Y.W., Zhu D.D., He X.X., Zhou Q., Yang Y.N., Bao J., Feng J.R., Sun W., Chen J.L. Dynamics of Sept4 expression in fibrotic livers of mice infected with Schistosoma japonicum. Parasitology. 2011;138:1003–1010. doi: 10.1017/S0031182011000667. [DOI] [PubMed] [Google Scholar]
- 105.Wu Y., Bu F., Yu H., Li W., Huang C., Meng X., Zhang L., Ma T., Li J. Methylation of Septin9 mediated by DNMT3a enhances hepatic stellate cells activation and liver fibrogenesis. Toxicol Appl Pharmacol. 2017;315:35–49. doi: 10.1016/j.taap.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 106.Koren E., Yosefzon Y., Ankawa R., Soteriou D., Jacob A., Nevelsky A., Ben-Yosef R., Bar-Sela G., Fuchs Y. ARTS mediates apoptosis and regeneration of the intestinal stem cell niche. Nat Commun. 2018;9:4582. doi: 10.1038/s41467-018-06941-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Simi A.K., Anlaş A.A., Stallings-Mann M., Zhang S., Hsia T., Cichon M., Radisky D.C., Nelson C.M. A soft microenvironment protects from failure of midbody abscission and multinucleation downstream of the EMT-promoting transcription factor Snail. Cancer Res. 2018;78:2277–2289. doi: 10.1158/0008-5472.CAN-17-2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang G., Feng W., Wu J. Down-regulation of SEPT9 inhibits glioma progression through suppressing TGF-beta-induced epithelial-mesenchymal transition (EMT) Biomed Pharmacother. 2020;125:109768. doi: 10.1016/j.biopha.2019.109768. [DOI] [PubMed] [Google Scholar]
- 109.Ma F.Y., Sachchithananthan M., Flanc R.S., Nikolic-Paterson D.J. Mitogen activated protein kinases in renal fibrosis. Front Biosci. 2009;1:171–187. doi: 10.2741/s17. [DOI] [PubMed] [Google Scholar]
- 110.Yanagida A., Iwaisako K., Hatano E., Taura K., Sato F., Narita M., Nagata H., Asechi H., Uemoto S., Kinoshita M. Downregulation of the Wnt antagonist Dkk2 links the loss of Sept4 and myofibroblastic transformation of hepatic stellate cells. Biochim Biophys Acta. 2011;1812:1403–1411. doi: 10.1016/j.bbadis.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 111.Heasley L.R., McMurray M.A. Small molecule perturbations of septins. Methods Cell Biol. 2016;136:311–319. doi: 10.1016/bs.mcb.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 112.Hu Q., Nelson W.J., Spiliotis E.T. Forchlorfenuron alters mammalian septin assembly, organization, and dynamics. J Biol Chem. 2008;283:29563–29571. doi: 10.1074/jbc.M804962200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Blum W., Henzi T., Pecze L., Diep K.L., Bochet C.G., Schwaller B. The phytohormone forchlorfenuron decreases viability and proliferation of malignant mesothelioma cells in vitro and in vivo. Oncotarget. 2019;10:6944–6956. doi: 10.18632/oncotarget.27341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kim K.K., Singh R.K., Khazan N., Kodza A., Singh N.A., Jones A., Sivagnanalingam U., Towner M., Itamochi H., Turner R., Moore R.G. Development of potent forchlorfenuron analogs and their cytotoxic effect in cancer cell lines. Sci Rep. 2020;10:3241. doi: 10.1038/s41598-020-59824-4. [DOI] [PMC free article] [PubMed] [Google Scholar]