Highlights
-
•
Episodic memory function in MND is impaired but does not worsen over time.
-
•
The PhG but not the ERC is subject to substance loss with disease progression.
-
•
Hippocampal atrophy is related to poorer performance in all memory sub functions.
-
•
Memory deficits and medial temporal lobe atrophy are independent of MND phenotype.
Keywords: ALS, MND, Longitudinal episodic memory, Entorhinal cortex, Hippocampus, MRI
Abstract
Memory impairment in motor neuron disease (MND) is still an underrecognized feature and has traditionally been attributed to executive dysfunction. Here, we investigate the rate of memory impairment in a longitudinal cohort of MND patients, its relationship to other cognitive functions and the underlying neuroanatomical correlates. 142 patients with MND and 99 healthy controls (HC) underwent comprehensive neuropsychological testing and structural MRI at 3T up to four times over a period of 18 months. Linear-mixed effects models were fitted to identify changes at baseline and over time in episodic memory function (learning, immediate and delayed recall, recognition), composed cognitive scores (memory, verbal fluency, executive function), and memory-related structural brain regions (hippocampus, entorhinal cortex, parahippocampal gyrus). Associations between episodic memory performance and volumetric or cortical thickness changes of these regions were computed using Pearson's r. Learning, immediate and delayed recall, as well as recognition performance were significantly reduced in MND when compared to controls at baseline. Performances in these subtests improved over time although MND showed less improvement than controls. This relationship did not change when only “classical” ALS patients were considered. Patients with MND showed thinning of the right parahippocampal gyrus (PhG) in comparison to controls that was progressing over time. Bilateral hippocampal atrophy was observed in MND patients with memory impairment after splitting the group according to their overall episodic memory performance, with the right hippocampus shrinking over time. In MND patients, the bilateral hippocampal atrophy was associated with impairment in learning, recall, and recognition at baseline. In contrast, left PhG thinning was associated with a poorer learning performance. These results show that episodic memory impairment in MND is a frequent cognitive dysfunction. Since deficits are not clearly declining with disease course, an early involvement of this cognitive domain in the disease seems probable. The memory performance-dependent atrophy of the hippocampus and PhG provide evidence for a widespread involvement of these non-motor cortical areas in disease pathology.
1. Introduction
Motor neuron disease (MND) is a neurodegenerative condition affecting both the upper and lower motor neurons in the cortex, brainstem, and spinal cord. The term motor neuron disease encompasses a spectrum of different motor neuron syndromes, including the phenotypes of amyotrophic lateral sclerosis (ALS), progressive muscular atrophy (PMA), primary lateral sclerosis (PLS), and upper and lower motor neuron dominance (Swinnen and Robberecht, 2014). There is growing evidence of significant extra-motor involvement including cognitive and behavioral impairment, not only in classical ALS (Phukan et al., 2012) but also in the more “restricted phenotypes” such as PMA and PLS (de Vries et al., 2019). Current consensus criteria define the co-occurrence of cognitive impairment if patients present with executive or language dysfunction (Strong et al., 2017), although other cognitive domains can be affected as well. The recognition of substantial cognitive and behavioral impairment contributing to disease severity and prognosis (Bersano et al., 2020, Elamin et al., 2011) in MND has gained further relevance as genetic (DeJesus-Hernandez et al., 2011, Renton et al., 2011), histopathologic (Neumann et al., 2006), and clinical (Lomen-Hoerth et al., 2002) similarities with frontotemporal dementias (FTD) have been observed. The hallmark feature of FTD includes cognitive and behavioral dysfunction associated with frontal and temporal brain regions. While the former one has been extensively studied in MND, only few studies investigated temporal lobe dysfunction, and particularly memory function, as one of the core cognitive domains associated with this region. Only recently, substantial temporal lobe pathology including hippocampal volume loss (Abdulla et al., 2014) and shape alterations (Machts et al., 2018b) have been elucidated in MND. Findings are related to individual verbal memory performance (Christidi et al., 2019) and differ between different cognitive phenotypes of MND (Machts et al., 2015) and other disorders, such as Alzheimer’s disease (Christidi et al., 2019, Machts et al., 2014).
Although the presence of cognitive and behavioral impairment has been associated with higher disease burden (Crockford et al., 2018) and more rapid motor function decline (Westeneng et al., 2018), little is known about the progression of these deficits during the disease course and their associated neuroanatomical correlates. Few longitudinal studies have investigated the development of cognitive and behavioral deficits in ALS, revealing either a decline (Bersano et al., 2020, Elamin et al., 2013), no decline (Woolley et al., 2018), or even improvement (Poletti et al., 2018) of cognitive functions over time. While the majority of studies focused on the evolution of executive deficits, the progression of MND-related memory dysfunction has not been of primary interest.
This study aims to investigate 1) memory function at baseline and its evolution over a time period of up to 18 months in a large consecutive sample of MND patients, 2) its relationship to other cognitive functions and 3) the underlying structural correlates of memory impairment, i.e., hippocampal volume as well as entorhinal and parahippocampal thickness at baseline and over time.
2. Methods
2.1. Participant characteristics
Between April 2011 and July 2013, 178 patients diagnosed with motor neuron disease (MND) and 99 healthy controls (HC) were enrolled in this prospective, longitudinal study. The study was conducted at two sites, Rostock and Magdeburg, and patients were recruited through the outpatient’s department of the university hospitals in Rostock and Magdeburg. After the baseline visit, up to three visits took place after 3- to 6-months in the patient cohort, while the HC were followed-up once after six months. Exclusion criteria for all participants were a history of psychiatric or neurological illness (other than MND), traumatic brain injury, and cerebrovascular disease. HC were recruited via local advertisement, screened to match the patient cohort in education, gender, and age, and only included if they scored within the normal range (≥26/30 points) of the Montreal Cognitive Assessment (MoCA)(Nasreddine et al., 2005), a brief screening instrument to assess mild cognitive impairment. In total, 132 healthy controls were screened, of which 99 were included for further neuropsychological testing. Of the 33 controls, that were excluded after screening, n=13 scored below the cut-off in the MoCA (Median: 25, range: 22–25), n=1 was suffering from microangiopathy, n=15 were female and their level of education was too high to fit the MND population, and n=4 had MR contraindications.
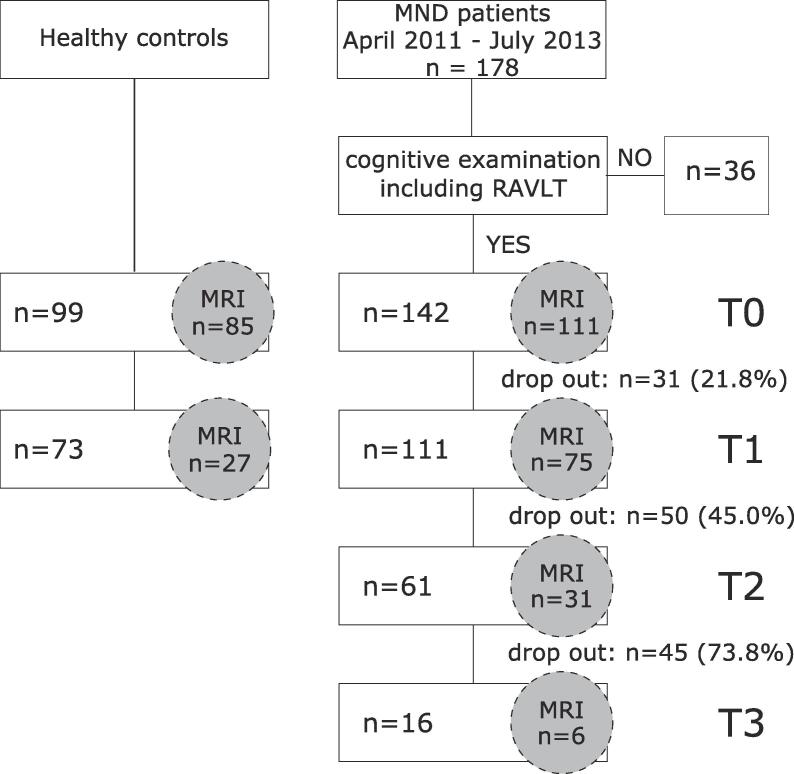
As we were particularly interested in MND-related memory function, patients who did not complete the Rey Auditory Verbal Learning Test (RAVLT) during cognitive examination were not included in the analysis. At baseline, a subgroup of 111 patients and 99 controls underwent structural MRI at 3T; longitudinal 3T MRI conducted at follow-up was available in 75 patients and 73 controls. Fig. 1 provides a detailed flow diagram including drop-out rates.
Fig. 1.
Flow diagram of recruitment scheme. MND: Motor neuron disease, RAVLT: Rey Auditory Verbal Learning Test, HC: Healthy controls, MRI: Magnetic resonance imaging, T: Timepoint.
Patients were classified according to the revised El-Escorial criteria (Brooks et al., 2000) and physical disability was rated using the ALS functional rating scale revised (ALSFRS-R)(Cedarbaum et al., 1999) at each visit by a senior ALS specialist (SV and JP). ALSFRS-R values were used to further stage the patients according to the King's staging system (Balendra et al., 2014, Roche et al., 2012). In order to get the full range of impairment across the MND spectrum, a broad range of phenotypes with varying degrees of upper and lower motor neuron involvement were included in this study. Clinical phenotypes of motor neuron disease were classified following current phenotypic classifications (Chio et al., 2011, Swinnen and Robberecht, 2014). In detail, MND phenotypes included classical ALS, Upper motor neuron dominant ALS (UMN), flail limb and flail arm (flail limb), primary lateral sclerosis (PLS), and primary muscular atrophy (PMA). The phenotypic classification followed closely previous descriptions (Schreiber et al., 2018). A comorbid diagnosis of FTD was made based on current consensus criteria (Gorno-Tempini et al., 2011, Rascovsky et al., 2011) by interviewing the caregiver and observing the patient during the clinical and neuropsychological assessment. Basic demographic data can be found in Table 1.
Table 1.
Demographic data of baseline and follow-up visits.
| Baseline (T0) |
First follow-up (T1) |
Second follow-up (T2) |
Third follow-up (T3) |
|||||
|---|---|---|---|---|---|---|---|---|
| MND | HC | p | MND | HC | p | MND | MND | |
| No. | 142 | 99 | 111 | 73 | 61 | 16 | ||
| Median age, y (range) | 60.5 (26–85) | 62.0 (33–83) | 0.59 | 60.1 (34–85) | 62.6 (34–83) | 0.20 | 60.4 (40–85) | 57.4 (42–76) |
| Median Education, y (range) | 13.0 (8–21) | 13.0 (10–21) | 0.03 | 13.0 (8–21) | 13.0 (10–21) | 0.07 | 13.0 (8–21) | 13.0 (10–20) |
| ISCED-2011 levels 2 (lower secondary) 3 (upper secondary) 4 (post-secondary, non-tertiary) 5 (short cycle tertiary) 6 (Bachelor or equivalent) 7 (Master or equivalent) 8 (Doctoral or equivalent) |
4 0 91 7 22 15 3 |
0 0 49 10 25 13 2 |
0.07 |
2 0 75 4 17 11 2 |
0 0 35 8 18 10 2 |
0.06 |
0 0 38 4 13 5 1 |
0 0 10 1 3 1 1 |
| Sex (Male/Female) | 92/50 | 63/36 | 0.85 | 74/37 | 43/30 | 0.28 | 42/19 | 9/7 |
| Median ALSFRS-R (range) | 39.0 (14–46) | na | 36.0 (13–46) | na | 35.0 (13–46) | 33.0 (12–45) | ||
| Median disease duration, m (range) | 17.0 (0.7–273) | na | 21.7 (5–280) | na | 26.3 (11–198) | 48.6 (21–124) | ||
| MND phenotype | na | na | ||||||
| ALS | 100 | 79 | 40 | 10 | ||||
| UMN | 12 | 10 | 6 | 2 | ||||
| PMA | 12 | 10 | 6 | 2 | ||||
| flail limb | 9 | 6 | 4 | 2 | ||||
| PLS | 9 | 6 | 5 | 0 | ||||
| King’s stage | ||||||||
| 1 | 47 | 28 | 14 | 3 | ||||
| 2 | 37 | na | 32 | na | 16 | 3 | ||
| 3 | 47 | 37 | 24 | 7 | ||||
| 4a | 1 | 2 | 2 | 0 | ||||
| 4b | 8 | 11 | 4 | 2 | ||||
| C9orf72 | 8 | na | 7 | na | 3 | 0 | ||
| SOD1 | 5 | na | 5 | na | 3 | 0 | ||
| Cognitive impairment* | ||||||||
| ci | 24 (17%) | 5 (5%) | 24 22%) | 5 (7%) | 13 (21%) | 3 (19%) | ||
| FTD | 5 (4%) | 0 | 4 (4%) | 0 | 2 (3%) | 0 | ||
| RAVLT | ||||||||
| Learning | 45.5 (16–53) | 46.0 (28–65) | 0.007 | 49.0 (18–70) | 52.0 (30–72) | 46.0 (15–73) | 48.0 (26–64) | |
| Immediate recall | 9.0 (0–15) | 10.0 (4–15) | <0.001 | 10.0 (1–15) | 11.0 (4–15) | 9.0 (0–15) | 11.0(2–15) | |
| Delayed recall | 9.0(0–15) | 10.0(3–15) | <0.001 | 10.0(0–15) | 11.0(5–15) | 8.0(0–15) | 10.0(4–15) | |
| Recognition | 11.0(-14–15) | 12.0(-1–15) | <0.001 | 11.0(-11–15) | 13.0(-2–15) | 10.0(-21–15) | 12.0(4–15) | |
MND: Motor neuron disease; HC: Healthy controls; ALSFRS-R: Amyotrophic Lateral Sclerosis Functional Rating Scale - revised; ALS: Amyotrophic Lateral Sclerosis; UMN: Upper motor neuron dominant ALS; PMA: Progressive Muscular Atrophy; PLS: Primary lateral sclerosis; ci: cognitive impairment; FTD: Frontotemporal dementia; ISCED: International standard classification of education; RAVLT: Rey Auditory Verbal Learning Test [raw values]; m: months; y: years; T: Timepoint
*cognitive impairment was rated according to the Strong criteria (Strong et al., 2017)
The study was approved by the local ethics committee of the Otto-von-Guericke University (No. 75/11) and all subjects gave written informed consent prior to their inclusion.
2.2. Measurement of cognition
2.2.1. Neuropsychological test battery
All participants underwent a detailed neuropsychological test battery within two days from their clinical examination, covering the domains of episodic memory, verbal fluency, and executive function. Episodic memory function was assessed using the German version of the RAVLT. The following scores were considered: sum of words learned, immediate recall, delayed recall, and recognition performance corrected for errors. Standardized parallel versions were used for follow-up visits. For the assessment of verbal fluency performance, motor-adjusted indices (Abrahams et al., 2000) were acquired for phonemic fluency (letter K/B in alternation between visits; index inverted) and flexibility (alternation between letters G and R/ H and T; index inverted), and semantic fluency (animals/ groceries; index inverted) and flexibility (alternation between sports and fruits/ clothing and flowers; index inverted). Executive function was measured using the digit span (forward and backward) from the Wechsler Memory Scale revised (WMS-r), Trail Making Test (TMT; time-adjusted quotient between part B and A, score inverted), and a computerized version of the Stroop test (score inverted). The Stroop test performance was calculated as the quotient of the inverse efficiency (Khng and Lee, 2014) between naming and reading to account for speed and accuracy: (1) Stroop-ratio. In order to evaluate deficits in memory function, participants’ RAVLT values for verbal learning, immediate and delayed recall, and recognition performance of all timepoints were standardized by the mean and standard deviation of healthy controls at baseline.
2.2.2. Principal component analysis
As memory deficits in MND have previously been attributed to executive dysfunction, we used a principal component analysis (PCA) to extract latent variables from all of the conducted neuropsychological tests in order to test the relationship between them. The PCA was fitted to the performance of the HC at baseline visit to preserve the factor structure typically found in age-, gender-, and education-matched controls. All variables were z-standardized before entering the PCA. Subsequently, the obtained component weights were used to calculate component scores for all participants and visits. The following variables were included into the PCA: RAVLT-Learning, RAVLT-Immediate recall, RAVLT-Delayed recall, RAVLT-Recognition, phonemic fluency index, phonemic flexibility index, semantic fluency index, semantic flexibility index, WMS-r digit span forward, WMS-r digit span backward, TMT ratio, Stroop ratio. Following the results of a Horn Parallel Analysis, we calculated three principal components that were subsequently Varimax rotated. The PCA revealed a clear one-to-one mapping between tests and components (Fig. 2), i.e., each component is a weighted average of four tests while the other eight tests enter with close-to-zero weights. The components corresponded well to the cognitive domains of episodic memory (comp1), verbal fluency (comp2), and executive function (comp3), therefore enabling the evaluation of memory deficits in the light of verbal fluency and executive deficits. Component scores for each participant at baseline and follow-up were calculated by first z-standardizing each variable using the mean and standard deviation (SD) of the HC at baseline, replacing missing values by single imputation, and subsequently multiplying these values with the regression weight for each component as derived from the PCA. The resulting standardized component scores can therefore be interpreted as z values, were individual scores below −1.5 SD were considered as impaired in one of the three cognitive domains. Following current consensus criteria (Strong et al., 2017), a patient was therefore categorized as ALSci if the z-standardized verbal fluency or executive component score fell below −1.5 (see Table 1).
Fig. 2.
Component weights of the PCA derived components (comp) from neuropsychological tests. The color bar indicates the individual normalized weights.
2.3. MRI data acquisition and analysis
To investigate structural changes related to memory function in MND, high-resolution, T1-weighted structural MRI scans were acquired at both study sites using 3T Siemens VERIO Magnetom scanners (Siemens, Erlangen/Germany) with an identical acquisition protocol (Machts et al., 2018a) at baseline and follow-up. MRI data were analyzed using FreeSurfer version 6.0.0 (http://surfer.nmr.mgh.harvard.edu/). Thickness and volumetric data from memory related regions, namely the entorhinal cortex (ERC) and parahippocampal gyrus (PhG) as well as the hippocampus, were extracted separately for each hemisphere at baseline and follow-up(s) using the longitudinal processing stream (Reuter et al., 2012). Entorhinal and parahippocampal voxels were labelled according to regions of the Desikan-Killiany probabilistic atlas and extracted thickness values were adjusted for age using the covariance method (Jack et al., 1989). Hippocampal total volume estimations were based on a Bayesian longitudinal segmentation using subject-specific atlases (Iglesias et al., 2016) and adjusted for total intracranial volume (TIV) using the covariance method (Jack et al., 1989).
2.4. Statistical analyses
All statistical analyses were done using R version 3.5.2. Between-group differences in demographic variables were tested using chi-square (sex) or Mann-Whitney-U (age, education) tests for nonparametric variables. To assess changes in episodic memory function between groups over time, linear mixed-effects (LME) models with random intercepts per subject were fitted to the data testing for main effects of time (in days from initial assessment), group (MND vs. HC), and the interaction between them while accounting for age at baseline, education, and sex: (2) LME(Y ~ time*group + age + education + sex, random=~1|id), where Y was defined as learning, immediate recall, delayed recall, or recognition. A similar model was computed to assess changes in the composed cognitive scores, derived from the PCA, namely episodic memory, verbal fluency, executive function.
To assess the relationship between episodic memory and memory-related regions, performances between memory sub functions and hippocampal volumes as well as ERC and PhG thickness, correlations were computed separately for MND and HC at baseline using Pearson’s r. Resulting p values were adjusted for multiple comparisons following Bonferroni correction (using p.adjust from the R Stats Package).
Changes in ERC and PhG thickness as well as hippocampal volume (D) were assessed for each hemisphere separately by fitting LME models with random intercepts per subject while accounting for sex [and age at baseline in the case of hippocampal volume], as well as for the three PCA-derived cognitive components: (3) LME(D ~ time* group + sex [+age] + comp1 + comp2 + comp3, random=~1 + time|id). Random slopes for time were added to the models if possible, i.e., if the model converged.
In order to determine if the overall memory performance was related to structural changes in memory-relevant brain regions over time, a subgroup analysis between patients with and without memory impairment was conducted. Patients “with memory impairment” had to score below −1.5SDs of the HCs mean baseline episodic memory score derived from the PCA at baseline or follow-up, whereas patients “without memory impairment” had to score better than −1.5SD. The fitted LME models were similar to the ones assessing structural changes in memory-related regions (D), without accounting for comp1 (corresponding to episodic memory).
To exclude the possibility that non-classical MND variants drive the results, we conducted a supplementary analysis where only classical ALS patients were included. Additionally, we checked if the exclusion of C9orf72 repeat expansion carriers did change the results, and if true, report that in the results section.
Next to effect sizes (including 95% confidence intervals) as estimated by the individual models and the test statistics (likelihood ratio/χ2), we report model comparisons based on Akaike’s (AIC) and Bayes’ information criterion (BIC).
3. Results
3.1. Episodic memory function
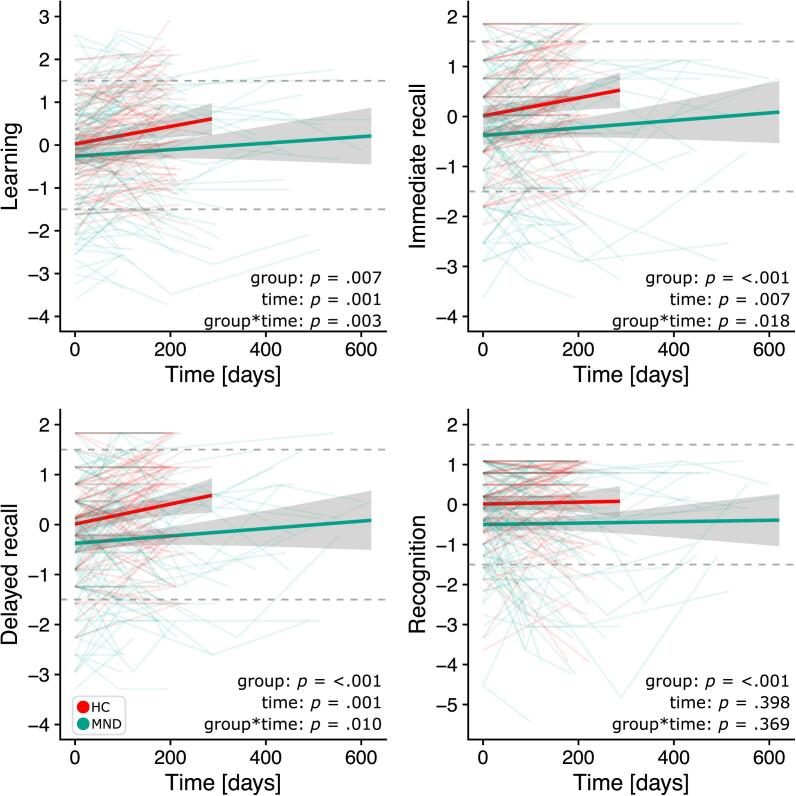
Verbal learning, immediate recall, delayed recall, and recognition performance were significantly reduced in MND when compared to HC (main effect of group). Over time, learning and recall performances improved in both MND and HC (main effect of time), although MND patients showed less improvement than HC (interaction effect, Fig. 3). There was no change in recognition performance over time, neither in HC nor in MND. The results did not change when patients with PMA, PLS, flail limb, and UMN dominant ALS (Supplementary Table 1, Supplementary Fig. 1) were excluded from the analysis. Effect sizes for fixed effects, the 95% confidence interval, as well as corresponding t and p values are reported in Table 2.
Fig. 3.
Individual evolution of episodic memory sub functions over time in HC (red) and MND patients (green). Dotted lines show the cut-off for < 1.5/ >1.5 standard deviations. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Table 2.
Test statistics and effect sizes for linear mixed effects models.
| Measurement | Fixed effects | Likelihood ratio χ2 | p | Estimate | 95% CI | t | df | p | |
|---|---|---|---|---|---|---|---|---|---|
| lower | upper | ||||||||
| Learning | time | 10.50 | 0.001 | 0.002 | 0.001 | 0.003 | 4.27 | 248 | <0.001 |
| group | 7.19 | 0.01 | −0.225 | −0.506 | 0.055 | −1.57 | 236 | 0.12 | |
| time*group | 8.86 | 0.003 | −0.002 | −0.003 | −0.001 | −2.98 | 248 | <0.01 | |
| Immediate recall | time | 7.41 | 0.01 | 0.002 | 0.001 | 0.003 | 3.44 | 247 | <0.001 |
| group | 13.66 | <0.001 | −0.377 | −0.651 | −0.103 | −2.69 | 236 | <0.01 | |
| time*group | 5.58 | 0.02 | −0.002 | −0.003 | −0.0003 | −2.36 | 247 | 0.02 | |
| Delayed recall | time | 10.81 | 0.001 | 0.002 | 0.001 | 0.003 | 3.94 | 248 | <0.001 |
| group | 14.07 | <0.001 | −0.354 | −0.618 | −0.091 | −2.63 | 236 | <0.01 | |
| time*group | 6.67 | 0.01 | −0.002 | −0.003 | −0.001 | −2.58 | 248 | 0.01 | |
| Recognition | time | 0.72 | 0.40 | 0.001 | −0.001 | 0.002 | 0.32 | 248 | 0.75 |
| group | 14.23 | <0.001 | −0.502 | −0.815 | −0.189 | −3.13 | 236 | <0.01 | |
| time*group | 0.81 | 0.37 | −0.001 | −0.002 | 0.001 | −0.89 | 248 | 0.37 | |
| Left hippocampus | time | 0.38 | 0.54 | −0.004 | −0.11 | −0.17 | −0.06 | 141 | 0.96 |
| group | 2.60 | 0.11 | −67.41 | −163.11 | 7.05 | −1.58 | 194 | 0.12 | |
| time*group | 0.06 | 0.80 | −0.021 | −0.22 | 0.09 | −0.25 | 144 | 0.80 | |
| Right hippocampus | time | 4.17 | 0.04 | −0.054 | −0.24 | 0.13 | −0.57 | 141 | 0.57 |
| group | 0.68 | 0.41 | −39.52 | −137.1 | 58.1 | −0.79 | 194 | 0.43 | |
| time*group | 0.17 | 0.68 | −0.043 | −0.25 | 0.16 | −0.41 | 141 | 0.68 | |
| Left parahippocampal cortex | time | 2.19 | 0.14 | 0.000 | −0.0001 | 0.0002 | 0.52 | 143 | 0.60 |
| group | 1.01 | 0.35 | −0.031 | −0.101 | 0.038 | −0.88 | 195 | 0.38 | |
| time*group | 1.76 | 0.19 | −0.0001 | −0.0003 | 0.000 | −1.32 | 143 | 0.19 | |
| Right parahippocampal cortex | time | 2.62 | 0.11 | 0.0001 | −0.000 | 0.0002 | 1.09 | 143 | 0.28 |
| group | 5.50 | 0.02 | −0.063 | −0.12 | −0.005 | −2.13 | 195 | 0.035 | |
| time*group | 4.06 | 0.04 | −0.0002 | −0.0003 | −0.000 | −2.02 | 143 | 0.045 | |
| Left entorhinal cortex | time | 1.51 | 0.22 | 0.000 | −0.0002 | 0.0002 | −0.09 | 143 | 0.93 |
| group | 0.42 | 0.52 | −0.024 | −0.105 | 0.057 | −0.58 | 195 | 0.56 | |
| time*group | 0.26 | 0.61 | −0.000 | −0.0003 | 0.0002 | −0.51 | 143 | 0.61 | |
| Right entorhinal cortex | time | 4.22 | 0.04 | −0.0002 | −0.0005 | −0.000 | −1.84 | 143 | 0.07 |
| group | 0.41 | 0.52 | −0.034 | −0.122 | 0.055 | −0.74 | 195 | 0.46 | |
| time*group | 1.10 | 0.29 | 0.0002 | −0.0001 | 0.0004 | 1.04 | 143 | 0.30 | |
Estimates for sex, comp1, comp2, comp3 [and age for hippocampal volume] are not displayed.
Regression weights (column Estimate) are to be interpreted as the difference in z-scores [z], volume [mm3], or thickness [mm] between groups, days, or the interaction between them. Bold values indicate significance at p < 0.05.
3.2. Relationship between episodic memory function and other cognitive domains
The analysis of the component scores derived from the PCA revealed a similar distribution (χ2 = 1.21, p = 0.27) of MND patients falling below −1.5 SD in episodic memory (MND < -1.5SD: 19.7%) and verbal fluency (MND < -1.5SD: 14.8%), while the proportion of patients with episodic memory impairment was significantly higher (χ2 = 6.4, p = 0.01) than the proportion of patients with executive dysfunction (MND < -1.5SD: 9.2%).
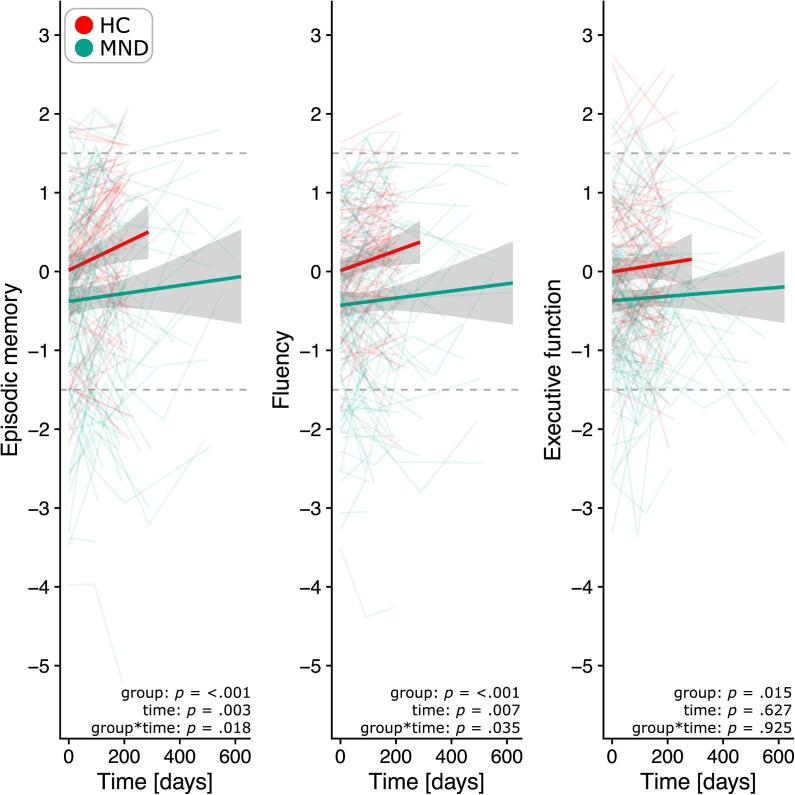
Over time, memory function and verbal fluency performance improved in HC and MND (main effect of time and group), while MND patients showed less improvement (interaction effect, Fig. 4). No change in performance was observed for executive function (for details see Supplementary Table 2). This was similar when only patients with classical ALS were included (Supplementary Fig. 2). At the individual level, a total of nine patients with no initial memory impairment showed memory deficits at follow-up (T1: n = 6; T2: n = 3). Similar numbers of additional impairment were observed for verbal fluency (T1: n = 8; T2: n = 1).
Fig. 4.
Distribution of z-standardized composite cognitive scores over time in HC (red) and MND patients (green). The y-axis displays the component density, dotted lines indicate the cut-off for < 1.5/ >1.5 standard deviations. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.3. Neuroanatomical correlates of memory function
3.3.1. Hippocampal volume
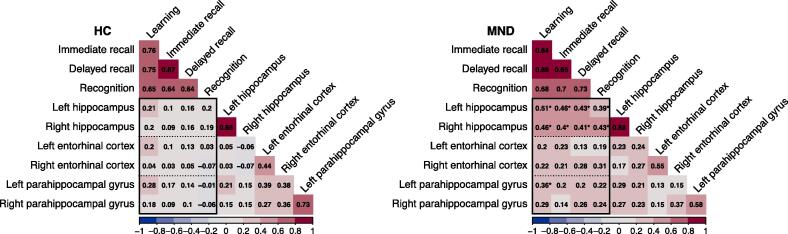
There was no difference in bilateral hippocampal volume between HC and MND patients, nor any change in volume over time (Table 2). Similarly, there was no significant interaction between group*time for hippocampal volume change (left: ΔAIC = 1.9, ΔBIC = 5.8; right: ΔAIC = 1.8, ΔBIC = 5.7). When MND patients were grouped according to their memory performance, patients “with memory impairment” had significantly lower bilateral hippocampal volumes than patients “without memory impairment” (left: χ2 = 4.95, p = 0.026; right: χ2 = 4.84, p = 0.028; Fig. 5, top). This relationship did not remain significant when C9orf72 repeat expansion carriers were excluded from the analysis (left: χ2 = 2.42, p = 0.12; right: χ2 = 2.82, p = 0.09). Bilateral hippocampal volumes of patients “with memory impairment” decreased over time although only the right hippocampal volume reduction reached the significance level of p < 0.05 (right: χ2 = 4.62, p = 0.03; left: χ2 = 0.64, p = 0.42). Importantly, patients “with memory impairment” did not have more hippocampal volume loss over time than patients “without memory impairment” (right: χ2 = 0.003, p = 0.95, ΔAIC = 2.0, ΔBIC = 5.4; left: χ2 = 2.90, p = 0.09; ΔAIC = -0.9, ΔBIC = 2.5). In detail, lower bilateral hippocampal volumes were associated with decreased performance in all assessed memory functions (all p < 0.001), namely learning, immediate recall, delayed recall, and recognition. This relationship was only observed in patients with MND but not in HC (Fig. 6). Results did not change when patients with non-classical MND variants were excluded (UMN, flail limb, PLS, PMA, see Supplementary Fig. 3), although the relationship between right hippocampal atrophy and poorer immediate delayed recall lost significance, similar to the association between bilateral hippocampal atrophy and poorer recognition performance (Supplementary Fig. 4).
Fig. 5.
Individual trajectories of hippocampal volume and entorhinal and parahippocampal thickness over time in HC (red), MND without (violett) and MND with memory impairment (turquoise). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 6.
Correlation matrices (diagonal omitted) for the association between PhG and ERC thickness, hippocampal volume and episodic memory sub functions in HC (left) and MND (right). *Indicate significance at p < 0.05 following Bonferroni correction.
3.3.2. Entorhinal thickness
Progressive thinning of the right ERC was observed in both, patients with MND and HC (main effect of time), but there were neither between-group differences in thickness (no main effect of group), nor did the ERC become thinner in MND over time (no group*time interaction; ΔAIC = 0.9, ΔBIC = 4.8). No differences between groups, change over time, or interactions between them (ΔAIC = 1.7, ΔBIC = 5.6) were observed for the left ERC. The subgroup analysis between MND patients with and without memory impairment did not reveal any specific thinning related to memory dysfunction over time, i.e., no significant group*time interaction (Fig. 5, middle), neither in the left nor right ERC (left: χ2 = 0.26, p = 0.61; ΔAIC = 1.7, ΔBIC = 5.2; right: χ2 = 1.86, p = 0.17; ΔAIC = 0.14, ΔBIC = 3.6). The results did not change when non-classical MND variants were excluded (Supplementary Fig. 3).
3.3.3. Parahippocampal thickness
Patients with MND showed right PhG thinning compared to HC (main effect of group), that was progressing over time (interaction effect, Table 2). This was supported by negative differences in the information criteria (ΔAIC = -2.1, ΔBIC = 1.8; Fig. 5, bottom). No changes in thickness between MND patients and HC could be observed for the left PhG. When patients were separated based on their memory performance, no differences were observed in neither left (χ2 = 0.82, p = 0.36; ΔAIC = 2.0, ΔBIC = 5.5) nor right (χ2 = 0.77, p = 0.38; ΔAIC = 0.5, ΔBIC = 4.0) PhG thickness (Fig. 5, bottom), although left PhG thinning was associated with lower learning (p = 0.008) in MND (Fig. 6). Results were the same when only classical ALS patients were considered (Supplementary Fig. 3).
4. Discussion
The evolution of cognitive dysfunction over time in patients with motor neuron disease is still under debate, given the difficulty of obtaining follow-up data from a patient cohort with relentlessly progressive motor symptoms. In this longitudinal study we assessed changes of episodic memory function and their associated neuroanatomical correlates. MND patients showed a substantial deficit in verbal learning, immediate and delayed recall, and recognition, when compared to HC. Performance in learning and recall improved over time although MND patients showed less improvement than controls. Structural change in memory-related regions in the medial temporal lobe became apparent as progressive thinning of the right parahippocampal gyrus in MND as well as bilateral hippocampal atrophy in MND patients with memory impairment. These changes were associated with varying degrees of performance loss, i.e.., the observed hippocampal atrophy was an indicator of poor function in all memory domains, whereas atrophy of the parahippocampal gyrus was only associated with poorer learning performance.
4.1. Episodic memory function
Baseline performance in verbal learning, immediate and delayed recall, as well as recognition performance was impaired in the MND group. Although these deficits were initially attributed to pure frontal lobe dysfunction (Mantovan et al., 2003, Raaphorst et al., 2011), there is a considerable body of literature (Beeldman et al., 2016) indicating that verbal memory impairment is a feature of cognitive dysfunction in MND that should be considered at neuropsychological assessment. With respect to other cognitive functions derived from a principal component analysis, memory impairment was as common as verbal fluency impairment, whereas this was not the case for executive function. Recent reports suggest language processing and not pure executive function to be the main factor modulating fluency performance (Whiteside et al., 2016). Interestingly, it is language dysfunction that is discussed to be even more frequent than executive dysfunction in MND (Taylor et al., 2013) and might be the underlying factor driving both verbal fluency and memory deficits in this study.
4.2. Cognitive change over time
Patients with MND showed less improvement in learning, immediate and delayed recall as well as in the overall cognitive components of memory and verbal fluency than HC, a fact which has previously been attributed to “a lack of practice effects” (Burkhardt et al., 2017, Poletti et al., 2018). This lack of practice effects is often interpreted as an early sign of cognitive decline, but could also be a result of insensitive test instruments, failing to detect smaller cognitive changes. Another possibility is the heterogeneity among individual performances, which average out to no observable change at the group level. Here, we observed an improvement in episodic memory performance and verbal fluency in both, HC and MND. Despite the carefully chosen longitudinal design in our study, i.e., the use of parallel versions of neuropsychological tests, as well as multiple timepoints for both, healthy controls and patients, we cannot exclude the possibility that there were some practice effects which for example have come about through familiarization with the test situation, the test material or less nervousness that might have influenced performance at baseline. Particularly the administration of the RAVLT at first time holds some surprise moments for the person being tested, e.g., the immediate delayed recall after the presentation of an interference list or the delayed recall after 30 min. This is difficult to control for in such a setting and therefore we cannot rule out that it might have been a confounding factor.
In this study we observed memory, verbal fluency, and executive impairment of MND patients with an average disease duration of 17 months at baseline but no further decline over time. Based on these results it is more likely to assume that cognition is affected earlier in the disease course, and remains more or less stable over time. An early involvement of cognition is supported by recent studies of ALS patients with shorter disease duration (<12 months), revealing a significant decline in episodic memory, verbal fluency, and social cognition over a period of six months in this group (Beeldman et al., 2020) and a high probability of conversion from cognitively normal to impaired in the first seven months after diagnosis (Bersano et al., 2020). Other population-based investigations including patients with longer disease duration showed that ALS patients without cognitive impairment at baseline remain stable over time, while patients with initial cognitive change are more likely to develop further cognitive impairment during the disease course (Elamin et al., 2013). Whether the progression of deficits is caused by a more aggressive form of MND pathology, e.g., a higher burden of accumulated TDP-43 pathology, needs to be addressed in future studies combining longitudinal data with post-mortem analysis. Besides the duration of the disease or TDP-43 accumulation, our results could have been have influenced by the time passing between baseline testing and follow-up, that might have been too short to see a substantial decline in cognition. Although we modeled memory decline across all four timepoints, we acknowledge that the regression model might have been underpowered for the later time points.
4.3. Structural change in the medial temporal lobe
Structures of the medial temporal lobe (MTL), such as the hippocampus, entorhinal, and parahippocampal cortices have an essential role in episodic memory. There is a significant body of literature providing evidence for their involvement in memory dysfunction in MND (Abdulla et al., 2014, Christidi et al., 2019, Machts et al., 2015), but only few studies have investigated this relationship over the disease course. This study revealed MTL structural changes as progressive thinning of the right parahippocampal gyrus, a region that is highly interconnected with frontal, parietal, and temporal cortices and therefore likely to be affected by disease spread. The PhG has been associated with contextual processing which is needed in a broad variety of different cognitive functions (Aminoff et al., 2013), including episodic memory and verbal fluency. Here, thinning of the PhG was associated with poorer learning performance in the MND group, highlighting its involvement in retrieving the most remote memories (Lux et al., 2016). It is also actively involved in spatial and non-spatial navigation, a function that has not been actively investigated in MND and could be of interest for further studies.
The progressive thinning of the PhG was independent of the patients' memory decline, pointing towards a more general susceptibility to pTDP-43 accumulation, the main proteinopathy associated with MND. A TDP-43 staging system reports the anteromedial areas of the temporal lobe to be affected in stage 4 in patients with ALS (Brettschneider et al., 2013), in contrast to Tau pathology, which is more likely to affect the posterior parts of the MTL (Llado et al., 2018). Along this line, it should be the entorhinal cortex that is more affected by disease pathology as opposed to the PhG, which, surprisingly, did not show any MND-related thinning at all, neither in this study nor in other studies investigating cortical thinning over time (van der Burgh et al., 2020). This problem can only be fully addressed once TDP-43 PET tracers are available for clinical use.
In contrast to the observed PhG thinning, bilateral hippocampal volume reductions were dependent of the patients’ memory performance and highly correlated with all assessed memory functions. In-vivo hippocampal involvement in MND has been reported as grey matter volume loss (Abdulla et al., 2014, Machts et al., 2015) and shape alterations (Machts et al., 2015, Machts et al., 2018b), that can be located to specific hippocampal subfields (Machts et al., 2018b) and differentiated from other pathologies, such as Alzheimer’s Disease (Christidi et al., 2019). Here, we report hippocampal volume reductions only for patients with memory impairment that fulfilled mostly, due to the strong correlation with verbal fluency, the criteria for MND-ci. Previous studies show that the involvement of the hippocampus is highly dependent on the patients’ cognitive performance (Machts et al., 2015), underlining the importance of assessing cognition in neuroimaging studies in MND. Interestingly, the observed hippocampal involvement is not progressive, once again raising the question if the proposed spreading of TDP-43 disease pathology from the motor cortices to prefrontal and temporal areas (Brettschneider et al., 2013) is already happening in a “preclinical phase” (Eisen et al., 2014), resulting in no progression of atrophy in these regions despite progression of clinical severity. This would have major implications for the design of future clinical trials.
5. Conclusions
Episodic memory impairment in MND is as frequent as verbal fluency impairment while deficits are not declining with disease course and suggest an early involvement of cognition in the disease. Performance-dependent atrophy and thinning of memory-related structures of the medial temporal lobe provide evidence for a widespread involvement of non-motor cortical areas in disease pathology.
6. Author disclosures
Dr. Machts has nothing to disclose.
Dr. Keute has nothing to disclose.
Dr. Kaufmann has nothing to disclose.
Prof. Dr. Schreiber has nothing to disclose.
Dr. Kasper has nothing to disclose.
Prof. Dr. Petri has nothing to disclose.
Prof. Dr. Prudlo has nothing to disclose.
Prof. Dr. Vielhaber has nothing to disclose.
Prof. Dr. Schoenfeld has nothing to disclose.
Funding
Research leading to this study was funded by the German Centre for Neurodegenerative Diseases (DZNE) to JP and SV, intersite project “cognition in motor neuron disease”. JM is funded by the federal state of Saxony-Anhalt and the European Regional Development Fund (ERDF) in the Center for Behavioral Brain Sciences (CBBS, ZS/2016/04/78113).
CRediT authorship contribution statement
Judith Machts: Conceptualization, Methodology, Formal analysis, Investigation, Writing - original draft, Writing - review & editing. Marius Keute: Formal analysis, Writing - review & editing. Joern Kaufmann: Conceptualization, Methodology, Investigation, Writing - review & editing. Stefanie Schreiber: Investigation, Resources, Writing - review & editing. Elisabeth Kasper: Conceptualization, Methodology, Investigation, Writing - review & editing. Susanne Petri: Investigation, Resources. Johannes Prudlo: Conceptualization, Methodology, Investigation, Resources, Funding acquisition. Stefan Vielhaber: Conceptualization, Methodology, Investigation, Resources, Funding acquisition. Mircea Ariel Schoenfeld: Conceptualization, Methodology, Resources, Supervision, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2020.102545.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Abdulla S., Machts J., Kaufmann J., Patrick K., Kollewe K., Dengler R., Heinze H.-J., Petri S., Vielhaber S., Nestor P.J. Hippocampal degeneration in patients with amyotrophic lateral sclerosis. Neurobiol. Aging. 2014;35(11):2639–2645. doi: 10.1016/j.neurobiolaging.2014.05.035. [DOI] [PubMed] [Google Scholar]
- Abrahams S., Leigh P.N., Harvey A., Vythelingum G.N., Grisé D., Goldstein L.H. Verbal fluency and executive dysfunction in amyotrophic lateral sclerosis (ALS) Neuropsychologia. 2000;38(6):734–747. doi: 10.1016/S0028-3932(99)00146-3. [DOI] [PubMed] [Google Scholar]
- Aminoff E.M., Kveraga K., Bar M. The role of the parahippocampal cortex in cognition. Trends in Cognitive Sciences. 2013;17(8):379–390. doi: 10.1016/j.tics.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balendra R., Jones A., Jivraj N., Knights C., Ellis C.M., Burman R., Turner M.R., Leigh P.N., Shaw C.E., Al-Chalabi A. Estimating clinical stage of amyotrophic lateral sclerosis from the ALS Functional Rating Scale. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration. 2014;15(3-4):279–284. doi: 10.3109/21678421.2014.897357. [DOI] [PubMed] [Google Scholar]
- Beeldman E., Govaarts R., de Visser M., Klein Twennaar M., van der Kooi A.J., van den Berg L.H., Veldink J.H., Pijnenburg Y.A.L., de Haan R.J., Schmand B.A., Raaphorst J. Progression of cognitive and behavioural impairment in early amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2020;91(7):779–780. doi: 10.1136/jnnp-2020-322992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeldman E., Raaphorst J., Klein Twennaar M., de Visser M., Schmand B.A., de Haan R.J. The cognitive profile of ALS: a systematic review and meta-analysis update. J Neurol Neurosurg Psychiatry. 2016;87(6):611–619. doi: 10.1136/jnnp-2015-310734. [DOI] [PubMed] [Google Scholar]
- Bersano E., Sarnelli M.F., Solara V., Iazzolino B., Peotta L., De Marchi F., Facchin A., Moglia C., Canosa A., Calvo A., Chiò A., Mazzini L. Decline of cognitive and behavioral functions in amyotrophic lateral sclerosis: a longitudinal study. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration. 2020;21(5-6):373–379. doi: 10.1080/21678421.2020.1771732. [DOI] [PubMed] [Google Scholar]
- Brettschneider J., Del Tredici K., Toledo J.B., Robinson J.L., Irwin D.J., Grossman M., Suh EunRan, Van Deerlin V.M., Wood E.M., Baek Y., Kwong L., Lee E.B., Elman L., McCluskey L., Fang L., Feldengut S., Ludolph A.C., Lee V.-Y., Braak H., Trojanowski J.Q. Stages of pTDP-43 pathology in amyotrophic lateral sclerosis: ALS Stages. Ann Neurol. 2013;74(1):20–38. doi: 10.1002/ana.23937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks B.R., Miller R.G., Swash M., Munsat T.L. El Escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis and Other Motor Neuron Disorders. 2000;1(5):293–299. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- Burkhardt C., Neuwirth C., Weber M. Longitudinal assessment of the Edinburgh Cognitive and Behavioural Amyotrophic Lateral Sclerosis Screen (ECAS): lack of practice effect in ALS patients? Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration. 2017;18(3-4):202–209. doi: 10.1080/21678421.2017.1283418. [DOI] [PubMed] [Google Scholar]
- Cedarbaum J.M., Stambler N., Malta E., Fuller C., Hilt D., Thurmond B., Nakanishi A. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III) J Neurol Sci. 1999;169:13–21. doi: 10.1016/s0022-510x(99)00210-5. [DOI] [PubMed] [Google Scholar]
- Chio A., Calvo A., Moglia C., Mazzini L., Mora G., Group P.S. Phenotypic heterogeneity of amyotrophic lateral sclerosis: a population based study. J Neurol Neurosurg Psychiatry. 2011;82:740–746. doi: 10.1136/jnnp.2010.235952. [DOI] [PubMed] [Google Scholar]
- Christidi F., Karavasilis E., Rentzos M., Velonakis G., Zouvelou V., Xirou S., Argyropoulos G., Papatriantafyllou I., Pantolewn V., Ferentinos P., Kelekis N., Seimenis I., Evdokimidis I., Bede P. Hippocampal pathology in amyotrophic lateral sclerosis: selective vulnerability of subfields and their associated projections. Neurobiol Aging. 2019;84:178–188. doi: 10.1016/j.neurobiolaging.2019.07.019. [DOI] [PubMed] [Google Scholar]
- Crockford C., Newton J., Lonergan K., Chiwera T., Booth T., Chandran S., Colville S., Heverin M., Mays I., Pal S., Pender N., Pinto-Grau M., Radakovic R., Shaw C.E., Stephenson L., Swingler R., Vajda A., Al-Chalabi A., Hardiman O., Abrahams S. ALS-specific cognitive and behavior changes associated with advancing disease stage in ALS. Neurology. 2018;91:e1370–e1380. doi: 10.1212/WNL.0000000000006317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries B.S., Rustemeijer L.M.M., Bakker L.A., Schroder C.D., Veldink J.H., van den Berg L.H., Nijboer T.C.W., van Es M.A. Cognitive and behavioural changes in PLS and PMA:challenging the concept of restricted phenotypes. J Neurol Neurosurg Psychiatry. 2019;90:141–147. doi: 10.1136/jnnp-2018-318788. [DOI] [PubMed] [Google Scholar]
- DeJesus-Hernandez M., Mackenzie I.R., Boeve B.F., Boxer A.L., Baker M., Rutherford N.J., Nicholson A.M., Finch N.A., Flynn H., Adamson J., Kouri N., Wojtas A., Sengdy P., Hsiung G.Y., Karydas A., Seeley W.W., Josephs K.A., Coppola G., Geschwind D.H., Wszolek Z.K., Feldman H., Knopman D.S., Petersen R.C., Miller B.L., Dickson D.W., Boylan K.B., Graff-Radford N.R., Rademakers R. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011;72:245–256. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen A., Kiernan M., Mitsumoto H., Swash M. Amyotrophic lateral sclerosis: a long preclinical period? J Neurol Neurosurg Psychiatry. 2014;85:1232–1238. doi: 10.1136/jnnp-2013-307135. [DOI] [PubMed] [Google Scholar]
- Elamin M., Bede P., Byrne S., Jordan N., Gallagher L., Wynne B., O'Brien C., Phukan J., Lynch C., Pender N., Hardiman O. Cognitive changes predict functional decline in ALS: a population-based longitudinal study. Neurology. 2013;80:1590–1597. doi: 10.1212/WNL.0b013e31828f18ac. [DOI] [PubMed] [Google Scholar]
- Elamin M., Phukan J., Bede P., Jordan N., Byrne S., Pender N., Hardiman O. Executive dysfunction is a negative prognostic indicator in patients with ALS without dementia. Neurology. 2011;76:1263–1269. doi: 10.1212/WNL.0b013e318214359f. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini M.L., Hillis A.E., Weintraub S., Kertesz A., Mendez M., Cappa S.F., Ogar J.M., Rohrer J.D., Black S., Boeve B.F., Manes F., Dronkers N.F., Vandenberghe R., Rascovsky K., Patterson K., Miller B.L., Knopman D.S., Hodges J.R., Mesulam M.M., Grossman M. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias, J.E., Van Leemput, K., Augustinack, J., Insausti, R., Fischl, B., Reuter, M., Alzheimer's Disease Neuroimaging, I., 2016. Bayesian longitudinal segmentation of hippocampal substructures in brain MRI using subject-specific atlases. Neuroimage 141, 542-555. [DOI] [PMC free article] [PubMed]
- Jack C.R., Jr., Twomey C.K., Zinsmeister A.R., Sharbrough F.W., Petersen R.C., Cascino G.D. Anterior temporal lobes and hippocampal formations: normative volumetric measurements from MR images in young adults. Radiology. 1989;172:549–554. doi: 10.1148/radiology.172.2.2748838. [DOI] [PubMed] [Google Scholar]
- Khng K.H., Lee K. The relationship between Stroop and stop-signal measures of inhibition in adolescents: influences from variations in context and measure estimation. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0101356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llado A., Tort-Merino A., Sanchez-Valle R., Falgas N., Balasa M., Bosch B., Castellvi M., Olives J., Antonell A., Hornberger M. The hippocampal longitudinal axis-relevance for underlying tau and TDP-43 pathology. Neurobiol Aging. 2018;70:1–9. doi: 10.1016/j.neurobiolaging.2018.05.035. [DOI] [PubMed] [Google Scholar]
- Lomen-Hoerth C., Anderson T., Miller B. The overlap of amyotrophic lateral sclerosis and frontotemporal dementia. Neurology. 2002;59:1077–1079. doi: 10.1212/wnl.59.7.1077. [DOI] [PubMed] [Google Scholar]
- Lux V., Atucha E., Kitsukawa T., Sauvage M.M. Imaging a memory trace over half a life-time in the medial temporal lobe reveals a time-limited role of CA3 neurons in retrieval. Elife. 2016;5 doi: 10.7554/eLife.11862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machts J., Bittner V., Kasper E., Schuster C., Prudlo J., Abdulla S., Kollewe K., Petri S., Dengler R., Heinze H.J., Vielhaber S., Schoenfeld M.A., Bittner D.M. Memory deficits in amyotrophic lateral sclerosis are not exclusively caused by executive dysfunction: a comparative neuropsychological study of amnestic mild cognitive impairment. BMC Neurosci. 2014;15:83. doi: 10.1186/1471-2202-15-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machts J., Cardenas-Blanco A., Acosta-Cabronero J., Kaufmann J., Loewe K., Kasper E., Schuster C., Prudlo J., Vielhaber S., Nestor P.J. Prefrontal cortical thickness in motor neuron disease. Neuroimage Clin. 2018;18:648–655. doi: 10.1016/j.nicl.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machts J., Loewe K., Kaufmann J., Jakubiczka S., Abdulla S., Petri S., Dengler R., Heinze H.J., Vielhaber S., Schoenfeld M.A., Bede P. Basal ganglia pathology in ALS is associated with neuropsychological deficits. Neurology. 2015;85:1301–1309. doi: 10.1212/WNL.0000000000002017. [DOI] [PubMed] [Google Scholar]
- Machts J., Vielhaber S., Kollewe K., Petri S., Kaufmann J., Schoenfeld M.A. Global Hippocampal Volume Reductions and Local CA1 Shape Deformations in Amyotrophic Lateral Sclerosis. Front Neurol. 2018;9:565. doi: 10.3389/fneur.2018.00565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovan M.C., Baggio L., Dalla Barba G., Smith P., Pegoraro E., Soraru G., Bonometto P., Angelini C. Memory deficits and retrieval processes in ALS. Eur J Neurol. 2003;10:221–227. doi: 10.1046/j.1468-1331.2003.00607.x. [DOI] [PubMed] [Google Scholar]
- Nasreddine Z.S., Phillips N.A., Bedirian V., Charbonneau S., Whitehead V., Collin I., Cummings J.L., Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- Neumann M., Sampathu D.M., Kwong L.K., Truax A.C., Micsenyi M.C., Chou T.T., Bruce J., Schuck T., Grossman M., Clark C.M., McCluskey L.F., Miller B.L., Masliah E., Mackenzie I.R., Feldman H., Feiden W., Kretzschmar H.A., Trojanowski J.Q., Lee V.M. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- Phukan J., Elamin M., Bede P., Jordan N., Gallagher L., Byrne S., Lynch C., Pender N., Hardiman O. The syndrome of cognitive impairment in amyotrophic lateral sclerosis: a population-based study. J Neurol Neurosurg Psychiatry. 2012;83:102–108. doi: 10.1136/jnnp-2011-300188. [DOI] [PubMed] [Google Scholar]
- Poletti B., Solca F., Carelli L., Faini A., Madotto F., Lafronza A., Monti A., Zago S., Ciammola A., Ratti A., Ticozzi N., Abrahams S., Silani V. Cognitive-behavioral longitudinal assessment in ALS: the Italian Edinburgh Cognitive and Behavioral ALS screen (ECAS) Amyotroph Lateral Scler Frontotemporal Degener. 2018;19:387–395. doi: 10.1080/21678421.2018.1473443. [DOI] [PubMed] [Google Scholar]
- Raaphorst J., de Visser M., van Tol M.J., Linssen W.H., van der Kooi A.J., de Haan R.J., van den Berg L.H., Schmand B. Cognitive dysfunction in lower motor neuron disease: executive and memory deficits in progressive muscular atrophy. J Neurol Neurosurg Psychiatry. 2011;82:170–175. doi: 10.1136/jnnp.2009.204446. [DOI] [PubMed] [Google Scholar]
- Rascovsky K., Hodges J.R., Knopman D., Mendez M.F., Kramer J.H., Neuhaus J., van Swieten J.C., Seelaar H., Dopper E.G., Onyike C.U., Hillis A.E., Josephs K.A., Boeve B.F., Kertesz A., Seeley W.W., Rankin K.P., Johnson J.K., Gorno-Tempini M.L., Rosen H., Prioleau-Latham C.E., Lee A., Kipps C.M., Lillo P., Piguet O., Rohrer J.D., Rossor M.N., Warren J.D., Fox N.C., Galasko D., Salmon D.P., Black S.E., Mesulam M., Weintraub S., Dickerson B.C., Diehl-Schmid J., Pasquier F., Deramecourt V., Lebert F., Pijnenburg Y., Chow T.W., Manes F., Grafman J., Cappa S.F., Freedman M., Grossman M., Miller B.L. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renton A.E., Majounie E., Waite A., Simon-Sanchez J., Rollinson S., Gibbs J.R., Schymick J.C., Laaksovirta H., van Swieten J.C., Myllykangas L., Kalimo H., Paetau A., Abramzon Y., Remes A.M., Kaganovich A., Scholz S.W., Duckworth J., Ding J., Harmer D.W., Hernandez D.G., Johnson J.O., Mok K., Ryten M., Trabzuni D., Guerreiro R.J., Orrell R.W., Neal J., Murray A., Pearson J., Jansen I.E., Sondervan D., Seelaar H., Blake D., Young K., Halliwell N., Callister J.B., Toulson G., Richardson A., Gerhard A., Snowden J., Mann D., Neary D., Nalls M.A., Peuralinna T., Jansson L., Isoviita V.M., Kaivorinne A.L., Holtta-Vuori M., Ikonen E., Sulkava R., Benatar M., Wuu J., Chio A., Restagno G., Borghero G., Sabatelli M., Consortium I., Heckerman D., Rogaeva E., Zinman L., Rothstein J.D., Sendtner M., Drepper C., Eichler E.E., Alkan C., Abdullaev Z., Pack S.D., Dutra A., Pak E., Hardy J., Singleton A., Williams N.M., Heutink P., Pickering-Brown S., Morris H.R., Tienari P.J., Traynor B.J. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011;72:257–268. doi: 10.1016/j.neuron.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M., Schmansky N.J., Rosas H.D., Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage. 2012;61:1402–1418. doi: 10.1016/j.neuroimage.2012.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche J.C., Rojas-Garcia R., Scott K.M., Scotton W., Ellis C.E., Burman R., Wijesekera L., Turner M.R., Leigh P.N., Shaw C.E., Al-Chalabi A. A proposed staging system for amyotrophic lateral sclerosis. Brain. 2012;135:847–852. doi: 10.1093/brain/awr351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber S., Spotorno N., Schreiber F., Acosta-Cabronero J., Kaufmann J., Machts J., Debska-Vielhaber G., Garz C., Bittner D., Hensiek N., Dengler R., Petri S., Nestor P.J., Vielhaber S. Significance of CSF NfL and tau in ALS. J Neurol. 2018;265:2633–2645. doi: 10.1007/s00415-018-9043-0. [DOI] [PubMed] [Google Scholar]
- Strong M.J., Abrahams S., Goldstein L.H., Woolley S., McLaughlin P., Snowden J., Mioshi E., Roberts-South A., Benatar M., HortobaGyi T., Rosenfeld J., Silani V., Ince P.G., Turner M.R. Amyotrophic lateral sclerosis - frontotemporal spectrum disorder (ALS-FTSD): Revised diagnostic criteria. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18:153–174. doi: 10.1080/21678421.2016.1267768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinnen B., Robberecht W. The phenotypic variability of amyotrophic lateral sclerosis. Nat Rev Neurol. 2014;10:661–670. doi: 10.1038/nrneurol.2014.184. [DOI] [PubMed] [Google Scholar]
- Taylor L.J., Brown R.G., Tsermentseli S., Al-Chalabi A., Shaw C.E., Ellis C.M., Leigh P.N., Goldstein L.H. Is language impairment more common than executive dysfunction in amyotrophic lateral sclerosis? J Neurol Neurosurg Psychiatry. 2013;84:494–498. doi: 10.1136/jnnp-2012-303526. [DOI] [PubMed] [Google Scholar]
- van der Burgh H.K., Westeneng H.J., Walhout R., van Veenhuijzen K., Tan H.H.G., Meier J.M., Bakker L.A., Hendrikse J., van Es M.A., Veldink J.H., van den Heuvel M.P., van den Berg L.H. Multimodal longitudinal study of structural brain involvement in amyotrophic lateral sclerosis. Neurology. 2020 doi: 10.1212/WNL.0000000000009498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westeneng H.J., Al-Chalabi A., Hardiman O., Debray T.P., van den Berg L.H. The life expectancy of Stephen Hawking, according to the ENCALS model. Lancet Neurol. 2018;17:662–663. doi: 10.1016/S1474-4422(18)30241-2. [DOI] [PubMed] [Google Scholar]
- Whiteside D.M., Kealey T., Semla M., Luu H., Rice L., Basso M.R., Roper B. Verbal Fluency: Language or Executive Function Measure? Appl Neuropsychol Adult. 2016;23:29–34. doi: 10.1080/23279095.2015.1004574. [DOI] [PubMed] [Google Scholar]
- Woolley S., Goetz R., Factor-Litvak P., Murphy J., Hupf J., Garofalo D.C., Lomen-Hoerth C., Andrews H., Heitzman D., Bedlack R., Katz J., Barohn R., Sorenson E., Oskarsson B., Filho A.F., Kasarskis E., Mozaffar T., Nations S., Swenson A., Koczon-Jaremko A., Christodoulou G., Mitsumoto H. Longitudinal Screening Detects Cognitive Stability and Behavioral Deterioration in ALS Patients. Behav Neurol. 2018;2018:5969137. doi: 10.1155/2018/5969137. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.