Abstract
The West African country Nigeria features highly diverse vegetation and climatic conditions that range from rain forest bordering the Atlantic Ocean in the South to the Desert (Sahara) at the Northern extreme. Based on data from the World Conservation Monitoring Center of the United Nations Environmental Protection, Nigeria, with ~5,000 documented vascular plants, ranks amongst the top 50 countries in terms of biodiversity. Such a rich biodiversity implies that the country is rich in diverse secondary metabolites—natural products/unique chemicals produced by the plant kingdom to confer selective advantages to them. Like many tropical countries, Nigeria is also endemic to numerous infectious diseases particularly those caused by parasitic pathogens. These phytochemicals have been exploited for the treatment of diseases and as a result, a new branch of chemistry, natural product chemistry, has evolved, to try to reproduce and improve the therapeutic qualities of particular phytochemicals. In this review, we have compiled a compendium of natural products, isolated from Nigerian flora, that have been reported to be effective against certain protozoan parasites with the aim that it will stimulate interests for further investigations, and give impetus to the development of the natural products into registered drugs. In total 93 structurally characterized natural compounds have been identified with various levels of anti-parasite activity mainly from Nigerian plants. The synthesis protocol and molecular target for some of these natural anti-parasite agents have been established. For instance, the anti-plasmodial compound fagaronine (7), a benzophenanthridine alkaloid from Fagara zanthoxyloides has been successfully synthesized in the laboratory, and the anti-trypanosomal compound azaanthraquinone (55) elicits its effect by inhibiting mitochondrial electron transfer in trypanosomes. This review also discusses the barriers to developing approved drugs from phytochemicals, and the steps that should be taken in order to accelerate the development of new antiparasitics from the highlighted compounds.
Keywords: anti-parasitics, neglected tropical disease (NTD), phytomedicine, Nigeria, drug development
Introduction
Natural products are broadly defined as chemical entities with pharmacological properties, produced by naturally occurring living organisms such as plants, fungi, bacteria, protists, sponges, including other invertebrates that are present in diverse environments. The prospects of using natural product-based remedies for treating and managing infections can be understood from the perspective of traditional medicines. Historically, natural products have played a key role in fighting infectious diseases across the globe. Archives for such evidence were found in cuneiform engraved on clay tablets from Mesopotamia, which date back to around 2600 BC (Cragg and Newman, 2005).
People living in resource-poor communities in Nigeria and elsewhere continue to depend largely on natural product-derived medicines (particularly those of plant origin) to combat many pathological conditions, notwithstanding the dearth of pharmacological elucidation of their mechanisms of action and of standard clinical trials. This is often due to personal beliefs, economic reasons, or difficulty in accessing pharmaceutical products. In contrast to combinatorial chemistry, natural products provide enormous structural diversity, creating the opportunity to discover novel lead compounds. It is estimated that about 75,000 species of flowering plants are known to exist on earth, out of which only about 10% have been investigated for possible therapeutic value against any condition. Out of this 10%, only 1–5% have been scientifically researched for any bioactivity (Amit Koparde et al., 2019). Thus, countless important natural lead compounds, with a vast range of structures and pharmacological properties, await discovery in the Earth's biodiversity.
Many protozoan diseases are endemic in tropical countries, affecting millions of humans and animals, and causing serious economic losses annually, especially to developing economies. Most of these parasitic diseases attract little attention with regard to the development of new drugs, mainly because of poor profitability prospects. In addition, the development of resistance by these parasites to nearly all currently used chemotherapies, in addition to toxicity issues and the increasing cost of unrelated drugs, has led to an ever-increasing need to explore cheaper, accessible sources of safe new antiprotozoal agents (de Koning, 2017).
To date, a large number of Nigerian medicinal plant extracts has been successfully tested and found to show antiprotozoal activity (Lifongo et al., 2014). Several studies have reported remarkable in vitro and in vivo activity of extracts and fractions of Nigerian plants against Plasmodium spp. (Adebayo and Krettli, 2011), Trypanosoma spp. (Abiodun et al., 2012; Nwodo et al., 2015a), and Leishmania spp. (Bello et al., 2017). Indeed, promising lead compounds have been identified from some of these plants (Amoa Onguéné et al., 2013; Ntie-Kang et al., 2014; Bekono et al., 2020). However, due to research and resource limitations, the active principles of too many extracts from Nigerian medicinal plants remain unknown, and further studies that would facilitate the translation of the few identified compounds into drug candidates are limited by a lack of funding for such work (Ebiloma et al., 2018b). It should be noted that previous reviews have provided a compilation of evidence of antiprotozoal activity in materials collected from Nigerian flora. Other works have also documented bioactive compounds from plants that grow in Nigeria and the wider African continent, but not necessarily isolated from materials collected in the country or the region (Adebayo and Krettli, 2011; Nwodo et al., 2015a). With detailed analytical chemistry and bioactivity screening of plant materials collected specifically from Nigeria increasing, it has become opportune to document the compounds isolated from these plants, and their utility for development as antiprotozoal drugs. This would provide a guide for a focused and evidence-based approach for advancement and development of natural compounds from Nigeria and hence, from Africa in general.
In this review, we present an overview of some of the most important antimalarial, antitrypanosomal, and/or antileishmanial compounds that have been isolated from medicinal plant materials collected in Nigeria. An extensive internet-based search was carried out on PubMed, Web of Science, Science Direct, and Google Scholar using appropriate combinations of the keywords “antimalarial, antitrypanosomal, antileishmanial, plant, natural product, extract, compound, and Nigeria.” The last search was carried out on October 10, 2020 and only compounds with elucidated structures and reported half maximal inhibitory concentration (IC50), half maximal effective concentration (EC50) or minimum inhibitory concentration (MIC) of ≤50 μg/mL or μM were included. References lists of included and related studies were also screened visually to identify further relevant studies. Beyond evaluating the antiprotozoal utility of these natural drugs, the aim is to identify the current limitations in natural product drug discovery in Nigeria and elsewhere, recommend a way forward, and encourage further studies that would identify the actual translation of the best leads into new antiprotozoal chemotherapies, which are sorely needed.
Plants as a Source of Antiparasitic Drugs
The annual global drug market is worth about one trillion USD (Calixto, 2019). A comprehensive analysis of drugs approved by the United States' Food and Drug Administration (FDA) from 1981 to 2010 shows that ~35% of these drugs were directly or indirectly of natural products origin, including plants (25% of total new pharmaceuticals) (Newman and Cragg, 2012, 2016). Over the last two centuries, the global pharmaceutical industry has significantly profited from the biodiversity of several countries when it comes to identifying new therapeutics against important pathogens, especially the development of new chemotypes for managing protozoal diseases such as malaria and leishmaniasis (Calixto, 2019).
Some of the natural compounds that have been isolated from plants include alkaloids, phenolics, terpenes, saponins, and quinones, and their derivatives (Table 1). They are valuable in combating diseases because of their anti-parasitic efficacies and their selective mode of action (Ebiloma et al., 2017). In addition to the direct usage of natural products from plants (phytomedicines or pure drugs), the scaffolds of many of these compounds have been successfully modified to produce pharmacologically more complex or semi-synthetic active molecules. They can also be used as taxonomic indicators for the discovery of new drugs; others can be used as models for designing lead molecules. Antique wisdom is the basis of modern medicine and a significant base of future medicines and therapeutics. Examples of well-established plant-based anti-protozoal drugs currently in use today are quinine and artemisinin, which are used for the treatment of malaria.
Table 1.
Compounds isolated from Nigerian medicinal plants with antimalarial, antitrypanosomal, and antileishmanial activity.
| Compound | Compound class/ subclass | Part of plant studied | Species name | Plant family | Place of collection | Ethnomedicinal use of the plant | Activity of the compound | References |
|---|---|---|---|---|---|---|---|---|
| 1–6 | Indole alkaloid | Fruits | Picralima nitida | Apocynaceae | Nnewi | Fever, jaundice, gastrointestinal disorders, and malaria | Antiplasmodial | Okunji et al., 2005 |
| 7 | Benzophenanthridine alkaloid | Root | Fagara zanthoxyloides | Rutaceae | Ile-Ife, Osun State | Chewing sticks | Antiplasmodial | Kassim et al., 2005 |
| 8 | Beta carboline Alkaloid | Bark | Nauclea pobeguinii | Rubiaceae | Oju LGA Benue State | Fever, jaundice, malaria, diarrhea | Antitrypanosomal | Igoli et al., 2011 |
| 9 and 10 | Alkaloid | Seed | Monodora myristica | Annonaceae | Oju LGA Benue State | Fever, sepsis, stomach-ache, and constipation | Antitrypanosomal | Igoli et al., 2011 |
| 11–14 | Isoquinoline alkaloid | Stembark | Enantia chlorantha Oliv. | Annonaceae | Ore, Ondo state | Stomach problems, rickettsia, typhoid fever, jaundice, malaria, and tuberculosis, some | Antiplasmodial and antitrypanosomal | Imieje et al., 2017 |
| 15–24 | Steroid alkaloid | Leaves and stembark | Holarrhena africana (syn. Holarrhena floribunda) | Apocynaceae | Nsukka LGA Enugu State | Convulsion, Fever, malaria, snake venom antidote | Antitrypanosomal | Nnadi et al., 2017, 2019 |
| 25 | Diterpenoid | Leaves | Hyptis suaveolens | Lamiaceae | South-Eastern Nigeria | Respiratory tract infections, colds, pain, fever, cramps, and skin diseases | Antiplasmodial | Chukwujekwu et al., 2005 |
| 26–28 | Diterpenoid | Stembark | Jatropha multifida | Euphorbiaceae | Edo State | Hepatitis and leishmaniasis | Antiplasmodial and antileishmanial | Falodun et al., 2014 |
| 29–32 | Diterpenes/ sesquiterpenes | Rhizomes | Siphonochilus aethiopicus | Zingiberaceae | Makurdi, Benue State | Colds, coughs, hysteria, infections, wound dressing, fevers, and pain | Antitrypanosomal | Igoli N. et al., 2011 |
| 33–35 | Diterpenoid | Leaves | Polyalthia longifolia | Annonaceae | Anyigba, Kogi State | In the treatment of trypanosomiasis, leishmaniasis, and malaria | Trypanosomiasis | Ebiloma et al., 2017 |
| 36 | Diterpenoid | Roots | Jatropha gossypiifolia | Euphorbiaceae | Benin City, Edo State | Leprosy, as an antidote for snakebite and in urinary problems | Antiplasmodial, antileishmanial | Ogbonna et al., 2017 |
| 37 | Sesquiterpenes | Leaves | Piliostigma thonningii | Leguminosae | Abuja | Treatment of ulcers, wounds, heart pain, arthritis, malaria, fever, leprosy, sore throat, diarrhea, toothache, and cough | Antitrypanosomal | Afolayan et al., 2018 |
| 38 and 39 | Labdane diterpenes | Leaves | Piliostigma thonningii | Leguminosae | Abuja | Treatment of ulcers, wounds, heart pain, arthritis, malaria, fever, leprosy, sore throat, diarrhea, toothache, and cough | Antitrypanosomal and antileishmanial | Afolayan et al., 2018 |
| 40 | Cassane diterpene | Roots | Calliandra portoricensis | Fabaceae | Jos, Plateau State | Treatment of tuberculosis, constipation, and helminthiasis | Antitrypanosomal and antileishmanial | Nvau et al., 2020 |
| 41 | Terpenoid/ diterpenoid | Leaves | Eucalyptus maculata | Myrtaceae | Anyigba, Kogi State | In the treatment of trypanosomiasis, leishmaniasis, and malaria | Antitrypanosomal | Ebiloma et al., 2017 |
| 42–44 | Triterpenoid | stem bark | Spathodea campanulata | Bignoniaciaciae | – | Malaria | Antiplasmodial | Amusan et al., 1996 |
| 45 and 46 | Triterpenoid | stem bark | Khaya grandifoliola | Meliaceae | – | Malaria and other febrile conditions | Antiplasmodial | Agbedahunsi and Elujoba, 1998 |
| 47 | Triterpenoid | Cassia siamea L. | Fabaceae | Otu, Oyo State | As laxative and in treatment of insomnia, diabetes, and hypertension | Antiplasmodial | Ajaiyeoba et al., 2008 | |
| 48 | Triterpene | Leaves | Combretum racemosum | Combretaceae | Ibadan, Oyo State | Parasitic diseases and fever | Antiplasmodial | Oluyemi et al., 2020 |
| 49 | Tetraterpenoids/ carotenoids | Leaves | Bridelia ferruginea | Euphorbiaceae | Eruwa, Oyo State | Purgative and vermifuge | Antitrypanosomal | Afolayan et al., 2019 |
| 50 | Flavonol | Roots | Spondias mombim | Anacardiaceae | Oju LGA Benue State | Cough, sore throat, antiseptic soap, and malaria | Antitrypanosomal | Igoli et al., 2011 |
| 51 | Flavonol | Bark | Alcornea cordifolia | Euphorbiaceae | Oju LGA Benue State | Fever, rheumatic pains, urinary tract infections, ringworm, and dysentery | Antitrypanosomal | Igoli et al., 2011 |
| 52 | Flavonoid | Leaves | Chromolaena odorata | Asteraceae | Lagos State | Malaria | Antiplasmodial | Ezenyi et al., 2014 |
| 53 and 54 | Flavonoid | Leaves | Vitex simplicifolia | Verbenaceae | Nsukka LGA, Enugu State | To treat edema, gout, malaria, skin diseases, toothache, and dermatitis | Antitrypanosomal | Nwodo et al., 2015a |
| 55 | Chalcone | Leaves | Cajanus cajan | Fabaceae | Otu, Oyo State | Laxative, antimalarial | Antiplasmodial | Ajaiyeoba et al., 2013 |
| 56 | Anthraquinone | Leaves | Mitracarpus scaber | Rubiaceae | Zaria, Kaduna State | Headaches, skin and venereal diseases, toothaches, leprosy, amenorrhoea | Antitrypanosomal | Nok, 2002 |
| 57 | Quinone | Whole plant | Cassia nigricans | Caesalpinaceae | Kaduna State | Stomach ulcers | Antiplasmodial | Obodozie et al., 2004 |
| 57 | Quinone | Stem bark | Cassia siamea | Fabaceae | Otu in Oyo State | For insomnia, as laxative and antidiabetic | Antiplasmodial | Ajaiyeoba et al., 2008 |
| 58 | Anthraquinone | Leaves | Crateva adansonii | Capparaceae | Nsukka, Enugu State | Treatment of headaches, ear, and parasitic infections | Antitrypanosomal | Igoli et al., 2014 |
| 59 and 60 | Isoflavanquinones | Roots | Abrus precatorius | Fabaceae | Umuoriehi Umuahia, Abia State | Diabetes, hemoglobinuria, sore throat, rheumatism, skin infections, and jaundice | Antileishmanial | Okoro et al., 2020 |
| 61–65 | Tannins | stem bark | Terminalia avicennoides and Anogeissus leiocarpus | Combretaceae | Bauchi State | TA: cure dental carries and skin infections AL: diarrhea, dysentery, and malaria, |
Antiplasmodial, antitrypanosomal and antileishmanial | Shuaibu et al., 2008a,b,c |
| 66 | Coumarin | Stem | Euphorbia poisonii | Euphorbiaceae | Oju LGA Benue State | Pain killer, laxative, pesticide, arrow-poison | Antitrypanosomal | Igoli et al., 2011 |
| 67 | Saponins | Seed | Dracaena mannii (DM) and D. arborea (DA) | Dracaenaceae | Isi-Enu, Nsukka, Enugu State |
DM: Stomach-ache, mental illness gonorrhea, and chest pains DA: epilepsy, measles, smallpox, venereal diseases |
Antiplasmodial | Okunji et al., 1996 |
| 68–70 | Glycoside/glucoside | Leaves | Stachytarpheta cayennensis | Verbenaceae | – | Malaria, helminthiasis fever and constipation | Antiplasmodial | Ifeoma Chinwude et al., 2016 |
| 71 and 72 | Irodid glucoside | Leaves | Vitex grandifolia | Lamiaceae | Ilorin, Kwara State | Colic, umbilical cord infections, toothache, rheumatism, and orchitis | Antileishmaial | Bello et al., 2018 |
| 73 | Peptide | Leaves | Crateva adansonii DC | Nsukka, Enugu State | Treatment of headaches, ear-aches, and parasitic infections | Antitrypanosomal | Igoli et al., 2014 | |
| 74 and 75 | Amine (dipeptides) | Roots | Zapoteca portoricensis | Fabaceae | Nsukka, Enugu State | For wound healing, toothache, tonsilitis, diarrhea, and as an anticonvulsant and antispasmodic | Antitrypanosomal | Nwodo et al., 2014 |
| 76 and 77 | Fatty acids | Leaves | Carica papaya | Caricaceae | Abia State | Asthma, rheumatism, fever, diarrhea, boils, and hypertension and as lactogenic | Antiplasmodial | Melariri et al., 2011 |
| 78 and 79 | Taccalonolide | Tubers | Tacca leontopetaloides | Taccaceae | Benue State | Stomach disorders, gastric ulcers, tooth ache, high blood pressure, hepatitis, enteritis, and sexual dysfunction | Antitrypanosomal | Dike et al., 2016 |
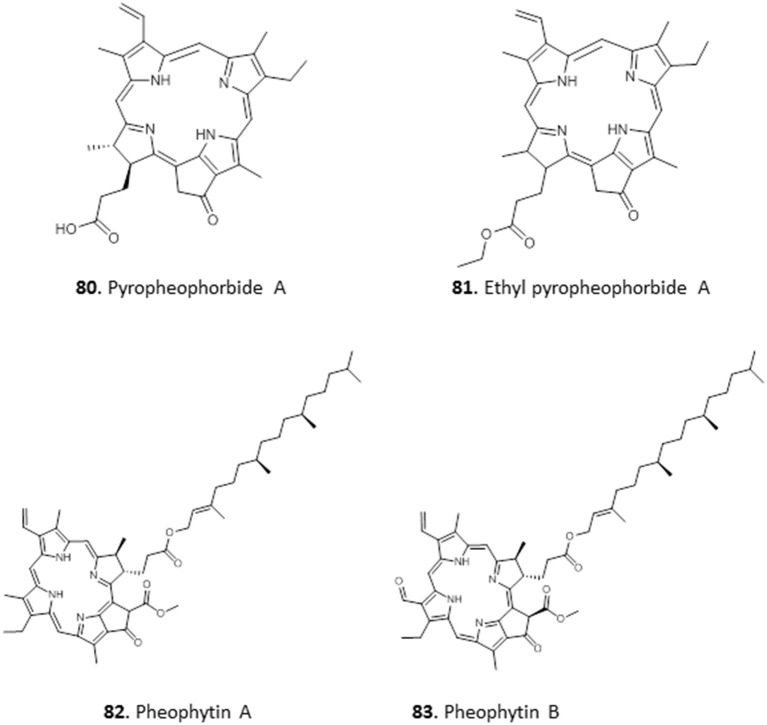
| 80 and 81 | Pheophorbide | Leaves | Crateva adansonii DC | Capparaceae | Nsukka, Enugu State | Treatment of headaches, ear, and parasitic infections. | Antitrypanosomal | Igoli et al., 2014 |
| 82 and 83 | Pheophytins | Leaves | Newbouldia laevis | Bignoniaceae | Anyigba, Kogi State | To hasten parturition and placenta delivery | Antitrypanosomal | Ebiloma et al., 2017 |
For centuries, the bark of Cinchona species was used by the natives living in the Amazon area for the treatment of malaria caused by Plasmodium. Interestingly, as early as 1820, Pelletier and Caventou successfully isolated quinine, the bioactive principle, from the bark of Cinchona officinalis (Dias et al., 2012). Consequently, the curative agent for malaria in those early days was quinine. Subsequently, several synthetic derivatives including chloroquine, mefloquine, amodiaquine, and primaquine were developed. The long history of usage notwithstanding, i.e., first as extract preparations and later as pure compound, malaria chemotherapy is still to an extent dependent on quinine; this is especially true for the most lethal form, cerebral malaria. Interestingly, while resistance to the synthetic derivatives such as chloroquine developed rapidly, resistance to quinine itself is relatively rare (Achan et al., 2011; Okombo et al., 2011).
The remarkable contributions of plants to the discovery of antiprotozoal drugs would not be complete without mentioning the plant Artemisia annua. A. annua is another plant that was long used for the treatment of fevers, in this case in Chinese folkloric medicine. The successful isolation of the bioactive agent, Artemether [a strong anti-malarial agent that was derived from artemisinin (qinghaosu), a sesquiterpene lactone], from A. annua represents one of the most significant discoveries in the fight against diseases caused by a protozoan parasite (Tu, 2011). Since then, several semi-synthetic artemisinin-based derivatives have been approved and registered for use: artesunate, dihydroartemisinin, and artemether (Paddon and Keasling, 2014).
Active Compounds From Nigerian Plants
Alkaloids
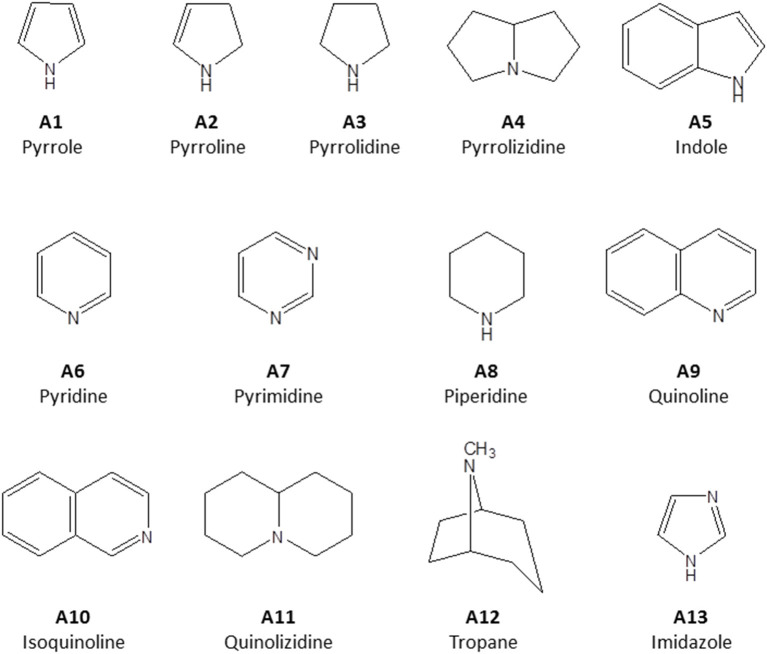
Alkaloids are a structurally diverse class of secondary metabolites whose key feature is a basic nitrogen (nitrogen in a negative oxidation state) in a carbon ring. They are classified according to their principal C-N skeleton into pyrroles (A1), pyrrolines (A2), pyrrolidines (A3), pyrrolizidines (A4) indoles (A5), pyridines (A6), pyrimidines (A7), piperidines (A8) quinolines (A9), isoquinolines (A10), quinolizidines (A11), tropanes (A12), imidazoles (A13) (Figure 1). They can also be classified according to their biological origin. Alkaloids are widely distributed in plants and some animal species. For many years, plant alkaloids have been utilized in the treatment of various health conditions (Bribi, 2018).
Figure 1.
Structure of principal C-N skeleton of alkaloid sub-groups.
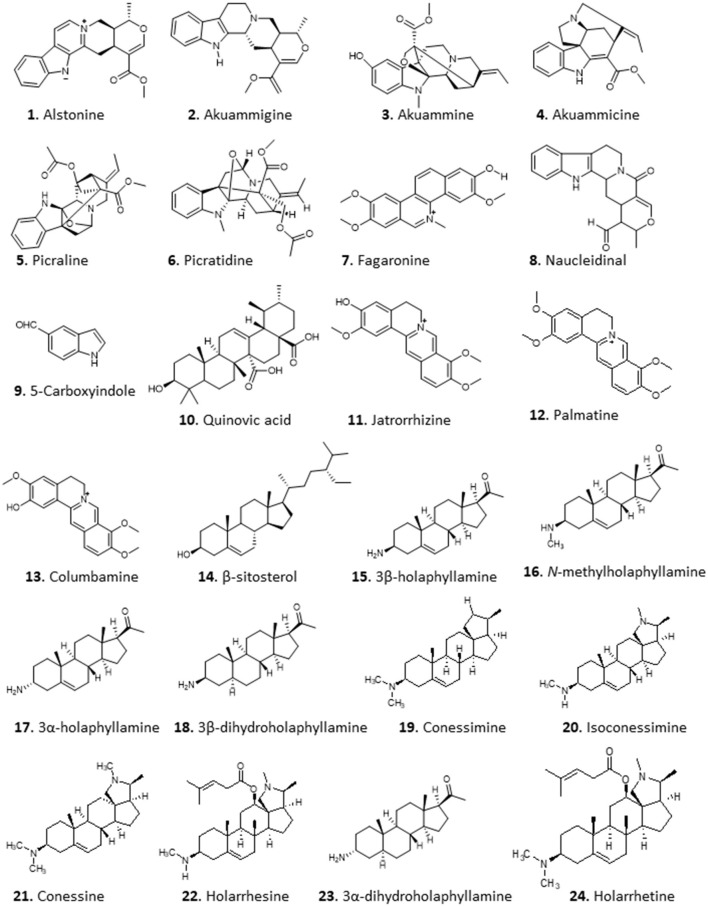
An excellent example is the isolation of anti-malarial alkaloids from the fruit rind of Picralima nitida. The crude dichloromethane extract displayed in vitro antiplasmodial activity with half maximal inhibitory concentration (IC50) values of 1.6–2.4 μg/mL. Moreover, the methanolic extract of the plant's stem bark, root, and fruit rind yielded active alkaloid fractions with IC50s of 0.54–2.16 and 0.79–1.59 μg/mL against P. falciparum chloroquine-resistant (W2) and chloroquine-sensitive (D6) clones, respectively (Iwu and Kiayman, 1992). Using pH-zone-refining counter-current chromatography, Okunji et al. (2005) isolated indole alkaloids from the rind of the plant. The purified compounds, alstonine (1), akuammigine (2), akuammine (3), akuammicine (4), picraline (5), and picratidine (6), showed remarkable activity against both P. falciparum clones (D6 and W2; IC50 0.01–0.9 μg/mL) (Okunji et al., 2005) (for the structures of compounds 1–24 see Figure 2).
Figure 2.
Structure of alkaloids isolated from Nigerian plants with selected antiprotozoal activity.
The crude, semi-purified and purified aqueous extracts of the roots of Fagara zanthoxyloides, known as Senegal prickly-ash, displayed highly promising antiplasmodial activity with IC50 values of 4.90, 1.00, and 0.13 μg/mL, respectively, using the [3H]-hypoxanthine incorporation assay. Further separation yielded a very potent antiplasmodial benzophenanthridine alkaloid, fagaronine (7) with an IC50 of 0.018 μg/mL against P. falciparum strain 3D7 (Kassim et al., 2005). A short and efficient synthesis for this compound was developed by Rivaud et al. (2012), who also confirmed the compound's activity against chloroquine-sensitive and -resistant P. falciparum isolates superior to chloroquine with half maximal effective concentration (EC50) of ~10 nM in vitro and comparable to chloroquine against P. vinckei in vivo [median effective dose (ED50) of 6 mg/Kg/day for 4 days]. Moreover, the compound, displayed no toxicity against Vero cells in vitro (Rivaud et al., 2012) or by single injection in the mouse at up to 50 mg/Kg (Nakanishi et al., 1999). The methanol extract of F. zanthoxyloides also displayed >90% inhibition of Plasmodium berghei in vivo, with an estimated EC50 of 235 mg/Kg b.w. for the extract while the lethal dose 50 (LD50) was >5,000 mg/Kg b.w. in mice (Enechi et al., 2019), providing independent confirmation of the antimalarial potential of this plant.
A preparation of 80% ethanolic extract of the stem bark of Nauclea pobeguinii, containing 5.6% of the beta-carboline alkaloid strictosamide as the presumed active agent, displayed highly promising activity against falciparum malaria in a Phase IIB clinical trial as a herbal medicine (Mesia et al., 2012). A beta carboline alkaloid, 8, was isolated from the ethyl acetate extract of the bark of the same plant and showed moderate activity against the causative agent of sleeping sickness, Trypanosoma brucei, with a Minimum Inhibitory Concentration (MIC) of 12.5 μg/mL and low cytotoxicity against PNT2A cells with IC50 >100 μg/mL (Igoli et al., 2011). Two other alkaloids, compounds 9 and 10, with similar moderate trypanocidal activity were obtained from the ethyl acetate extract of Monodora myristica, showing MICs of 12.5 and 25 μg/mL, respectively, for T. brucei (Igoli et al., 2011).
Imieje et al. (2017) screened the crude methanolic extract and different fractions of Enantia chlorantha (African Whitewood, family Annonaceae), which is used in Nigerian ethnomedicine for the treatment of wounds, ulcers, and fevers including malaria, for antiprotozoal activity and reported that the extract indeed displayed very high activity against P. falciparum, with very good IC50 values against chloroquine-sensitive D6 (<0.37 μg/mL) and chloroquine-resistant W2 (<0.32 μg/mL) clones as well as against Leishmania donovani (IC50 <0.8 μg/mL), but only moderate activity against T. brucei (IC50 of 15.2 μg/mL). The butanol and ethanol fractions of the extract yielded three closely related isoquinoline alkaloids: jatrorrhizine (11), palmatine (12), and columbamine (13). The steroid β-sitosterol (14) was also obtained from the extracts. Compound 11 displayed good but only moderately specific activity against chloroquine-sensitive P. falciparum clone D6 (IC50 2.2 μM), but activity against L. donovani and T. brucei was low (IC50s >29.6 and 18.3 μM, respectively), and IC50s >14.1 and >29.6 to VERO and human acute monocytic leukemia (THP1) cells, respectively. Compounds 12 and 13 showed essentially identical activity as 11 against P. falciparum D6 with IC50 of 1.90 and 2.16 μM, respectively, showing that the methylation of the isoquinoline hydroxy groups is not essential for activity, whereas 14 displayed only moderate activity (Pf IC50 >11.5). Compounds 12 and 14 were also tested against L. donovani and T. brucei, with IC50 values ≥23.1 μM (Imieje et al., 2017).
The in vitro and in vivo antitrypanosomal activity of the aqueous extract of young leaves of Holarrhena africana from Nigeria has been reported by multiple authors (Nwodo et al., 2007; Alhaji et al., 2014; Nnadi et al., 2016). Nnadi et al. investigated the antitrypanosomal, antileishmanial and antiplasmodial activity of this plant in more detail, using extracts and fractions from both stem bark and leaves (Nnadi et al., 2016, 2017). The crude extract and alkaloid fraction both displayed potent antitrypanosomal activity. Bioactivity guided fractionation of the active alkaloid fraction led to the isolation of 19 compounds, 17 of which were steroid alkaloids. Remarkable activities (EC50 in μM ± absolute deviation) against T. brucei rhodesiense were recorded for some aminosteroids from the leaves: 3β-holaphyllamine (0.40 ± 0.28) 15, N-methylholaphyllamine (0.08 ± 0.01) 16, 3α-holaphyllamine (0.37 ± 0.16) 17 and 3β-dihydroholaphyllamine (0.67 ± 0.03) 18. Related compounds from the stembark, Conessimine (0.17 ± 0.08) 19; isoconessimine (0.17 ± 0.11) 20; conessine (0.42 ± 0.09) 21; and holarrhesine (0.12 ± 0.08) 22 also showed very high antitrypanosomal activity, with high selectivity to the parasites [selectivity index (SI) >100] compared to mammalian L6 cells (Nnadi et al., 2017). A 3D-Quantitave Structure Activity Relationship (QSAR) study revealed that steric activity around the C-3 amino group tends to increase activity, while steric activity in the vicinity of the amino group of the pyrroline/pyrrolidine rings and the C-17β-acetyl or C-20 methyl groups tended to decrease activity (Nnadi et al., 2018). Following on from this, the active aminosteroids together with 22, 3α- dihydroholaphyllamine (23), and holarrhetine (24) were also tested on animal trypanosome species using resazurin-based in vitro screening assay in our lab (Nnadi et al., 2019). Interestingly, most of these compounds displayed no cross-resistance with pentamidine, isometamidium, diamidines, and melaminophenyl arsenicals in T. b. brucei. The activity of these compounds varies between T. b. brucei and T. congolense, suggesting possible differences in the mode of action of these compounds in the 2 species as seen with some trypanocides. The compounds with highest activity (IC50 ± standard deviation) and selectivity [represented as Selectivity Index (SI)] against T. congolense were 20 (IC50 = 0.22 ± 0.35 μM; SI = 123.5), 21 (IC50 = 1.65 ± 0.92 μM; SI = 37.2), 22 (IC50 = 0.22 ± 0.13 μM; SI = 65.9), and 23 (IC50 = 0.045 ± 0.03 μM; SI = 2,130) (Nnadi et al., 2019). Further exploration into the mechanism of action revealed that the compounds caused a slow but irreversible trypanocidal effect by targeting the mitochondrion, preventing kinetoplast division (Nnadi et al., 2019).
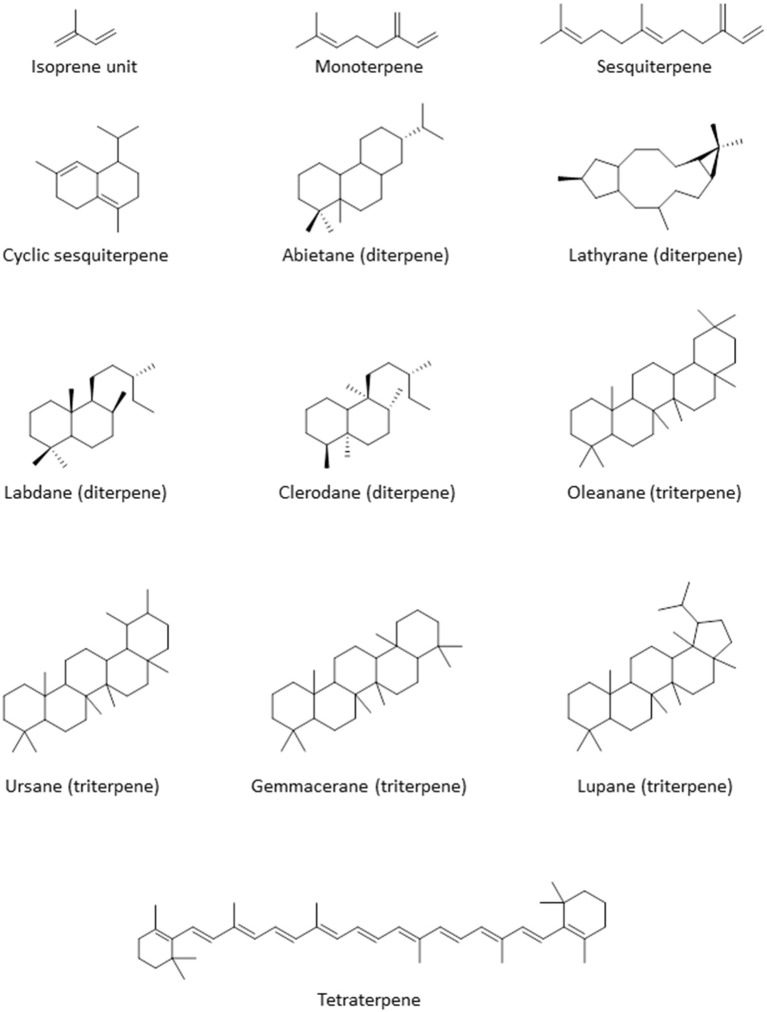
Terpenoids
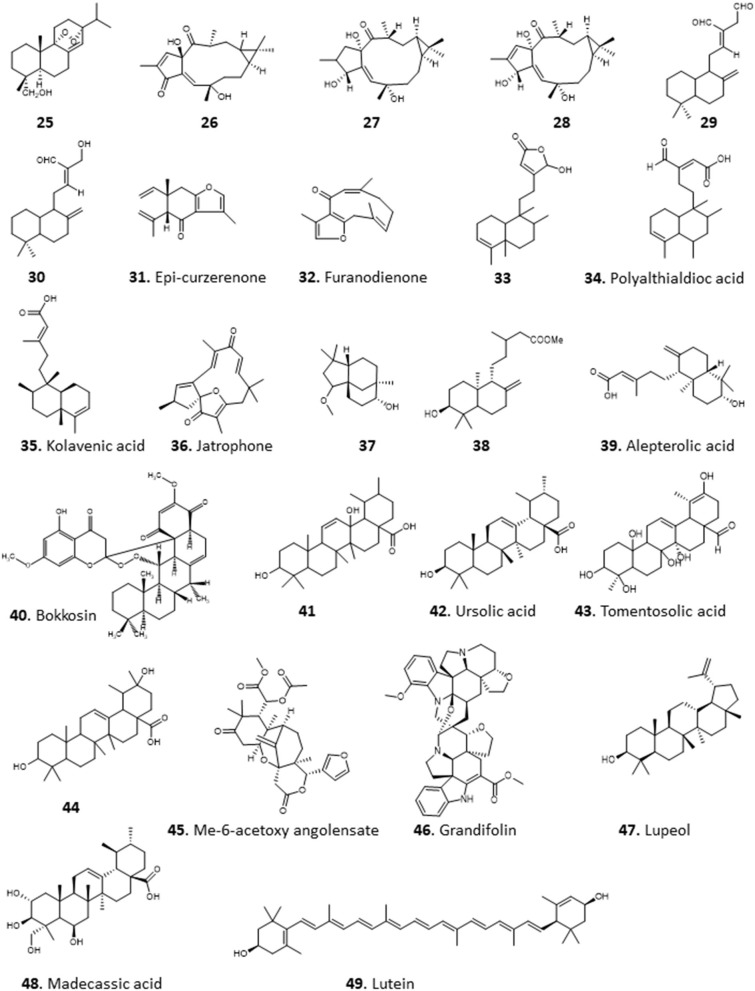
Terpenoids are commonly found in plants and are biosynthesized from isoprene units (CH2=C(CH3)-CH=CH2). They are classified based on the number of units in the compound; monoterpenes consist of 2 units, while sesquiterpenes, diterpenes, triterpenes, and tetraterpenes consist of 3, 4, 6, and 8 units, respectively (Figure 3). The number of carbon atoms in the compounds are thus 10, 15, 20, 30, and 40 depending on the number of isoprene units. Terpenoids have shown good activity in various assay screens and constitute the active entities in many drugs. Compound 25 (9α, 13α-epi-dioxiabiet-8(14)-en-18-ol) was obtained through a bioassay-guided fractionation of the petroleum ether extract of the leaves of Hyptis suaveolens, a common shrub in the tropics, used traditionally for the treatment of many ailments including fever, colds, and respiratory tract infections (Iwu, 2014). The compound demonstrated high antiplasmodial activity with an IC50 of 0.1 μg/mL against chloroquine-sensitive P. falciparum clone D10 (Chukwujekwu et al., 2005).
Figure 3.
Structure of the basic skeleton of terpene sub-groups.
The methanol extract of Jatropha multifidi, known as coral bush, which is in fact used in Nigerian folk medicine for the treatment of parasitic infections as well as cancer (Gill, 1992), yielded 3 closely related macrocyclic lathyrane diterpenoids: 14-deoxy-1β-hydroxy-4(4E)-jatrogrossidentadione (26), the saturated 15-deoxy-1β-hydroxy-4(4E)-jatrogrossidentadione (27) and the unsaturated 15-deoxy-1β-hydroxy-4(4E)-jatrogrossidentadione (28) (Falodun et al., 2014). The compounds showed particular promise for their antileishmanial activity, with IC50 of 11.9, 4.69, and 4.56 μg/mL, respectively, showing that the oxidation state of the oxygen on the cyclopentene ring is likely to be important for high activity. The authors state that traditional healers have used the plant successfully against leishmaniasis. The compounds possessed virtually no activity against chloroquine-sensitive P. falciparum D6, with IC50s >7 mg/mL, and poor activity against a panel of bacterial and fungal pathogens, thus confirming specific activity against Leishmania species and perhaps other kinetoplastid parasites (Falodun et al., 2014).
The crude hexane extracts of the rhizomes of wild ginger, Siphonochilus aethiopicus, showed promising activity against T. brucei with an MIC of 6.5 μg/mL and low toxicity against several mammalian cells (MIC of 53.3–114.2 μg/mL). Two active diterpenes, 8(17),12E-labdadiene15,16-dial (29), 15-hydroxy-8(17),12E-labdadiene-16-al (30) and two sesquiterpenes, epi-curzerenone (31), furanodienone (32) were isolated from the extract (Igoli N. et al., 2011). 29, 31, 32 showed a MIC of 1.55 μg/mL while 30 displayed an MIC of 6.25 μg/mL against T. brucei. The compounds appeared to be selective for T. brucei as cytotoxicity tests with the mammalian cells showed MICs of 35.9–116 μg/mL, except for 29, which was toxic to Jurkat and SH-SY5Y cells (MIC 4.1 and 9.0 μg/mL, respectively) (Igoli N. et al., 2011).
The Indian-native ornamental plant Polyalthia longifolia, commonly found in Nigeria is an important source of traditional remedies for malaria and fevers (Bankole et al., 2016). The hexane extract of the leaves of P. longifolia showed potent activity against T. b. brucei in our lab, with EC50 ± SEM of 2.4 ± 0.1 μg/mL. Bioactivity-guided fractionation led to the isolation of a diterpenoid identified as clerodane (16-α-hyroxy-cleroda-3-13(-14)-Z-dien-15,16-olide; compound 33), which displayed an EC50 ± SEM of 0.38 ± 0.05 μg/mL against T. b. brucei. Other isolated compounds from the fraction were polyalthialdioc acid (34) and kolavenic acid (35), which also showed significant antitrypanosomal activity, with EC50 ± SEM values of 3.57 ± 0.16 and 12.3 ± 0.5 μg/mL, respectively. Interestingly, all the 3 compounds also showed activity against T. congolense and L. mexicana, presented no cross-resistance to diamidines and arsenicals, and were not toxic to Human Embryonic Kidney (HEK) cells at up to 200 μg/mL (Ebiloma et al., 2017). Compound 33 has also been previously described as an oral antileishmanial agent with in vivo activity (Misra et al., 2010). We further investigated the effect of 33 and reported a multi-target mechanism of action for the compound on T. brucei, including severe cell cycle defects, DNA fragmentation, ATP depletion, and a marked depolarisation of the mitochondrial membrane potential (Ebiloma et al., 2018b).
Chromatographic fractionation and separation of fractions of the methanol extract of the root bark of Jatropha gossypifolia (“bellyache bush”), a pantropical plant with many ethnopharmacological applications for its various parts (Félix-Silva et al., 2014), yielded a macrocyclic diterpenoid compound, jatrophone (36), with antiprotozoal activity. The compound displayed broad antiparasitic activity, with low EC50 values against chloroquine-sensitive P. falciparum clone D6 (0.55 μg/mL), chloroquine-resistant P. falciparum clone W2 (<0.52 μg/mL), L. donovani (<0.4 μg/mL), and T. brucei (<0.4 μg/mL). However, 36 was also found to be similarly active against VERO cells (0.43 μg/mL), and its antiprotozoal effects thus reflect a more general toxicity (Ogbonna et al., 2017).
Several compounds including a bioactive sesquiterpene, 2β-methoxyclovan-9α-ol (37), two active labdane diterpenes, methyl-ent-3β-hydroxylabd-8(17)-en-15-oate (38) and alepterolic acid (39) were obtained from the methanol extract of the leaves of Piliostigma thonningii, a plant of the subfamily Caesalpinioideae in the legume family with myriad ethnopharmacological uses. Compounds 37 and 39 were antitrypanosomal with IC50s of 7.89 and 3.42 μM against T. brucei, respectively. Compound 38 displayed a broader antikinetoplastid activity with IC50 of 3.84 and 7.82 μM in T. brucei and L. donovani, respectively. The authors suggested that hydroxylation of the sesquiterpenes at C-2 position improves the antileishmanial activity, while hydroxylation at C-3 enhances the antitrypanosomal activity of the labdane diterpenes (Afolayan et al., 2018).
Column chromatography of the ethyl acetate extract of the roots of Calliandra portericensis yielded a novel diterpene-substituted chromanyl benzoquinone, bokkosin, 40. The compound showed potent activity against the kinetoplastid parasites, T. brucei (0.69 μg/mL), T. congolense (21.6 μg/mL), and L. mexicana (5.8 μg/mL). In addition, it exhibited very low prospects of cross-resistance to antitrypanosomal drugs, pentamidine and diminazene, and low toxicity to mammalian cell lines (Nvau et al., 2020). The ethyl acetate extracts of the leaves of Eucalyptus maculata also exhibited antitrypanosomal activity (12.3 ± 0.3 μg/mL, EC50 ± SEM). An ursane type triterpenoid, 3β,13β-dihydroxy-urs-11-en-28-oic acid (41) isolated from this extract appeared to be the active ingredient, with EC50 ± SEM of 1.58 ± 0.03 μg/mL against T. b. brucei. Compound 41 showed no cross-resistance to diamidines and arsenicals, and was not toxic to HEK cells at concentrations up to 200 μg/mL (Ebiloma et al., 2017).
Amusan et al. (1996) reported the isolation of antimalarial triterpenoids from the chloroform extract of the stem bark of one of the plants that is tradionally used for the treatment of malaria, Spathodea campanulata (Makinde et al., 1987, 1988a). The triterpenoids, 3β-hydroxyurs-12-en-28-oic acid (ursolic acid; 42) and two of its derivatives, 3β-hydroxyurs-12,19-dien-28-oic acid (tomentosolic acid; 43) and 3β,20 β-dihydroxyurs-12-en-28-oic (44) resulted in a significant (P < 0.05) reduction in parasitaemia and enhanced survival of mice infected with P. berghei. Notably, the effect of 60 mg/Kg/day of 42 on parasitaemia and survival was comparable to 10 mg/Kg/day chloroquine (Amusan et al., 1996).
The same group reported that aqueous extracts of another Nigerian antimalarial plant, Khaya grandifoliola (African mahogany), also displayed activity against P. berghei infection in mice (Makinde et al., 1988b), but this extract only suppressed early infections and was ineffective against established infections of the parasite. However, Agbedahunsi et al. re-tested the antimalarial activity of K. grandifoliola stem bark extracts using different solvents (Agbedahunsi et al., 1998). Among the extracts tested, the n-hexane crude extract and purified fractions displayed the highest activities, comparable to the reference drug chloroquine diphosphate, with EC50 values of 1.4 μg/mL (for a multi-drug resistant clone) or 0.84 μg/mL (for Nigerian P. falciparum isolates). Further studies on the active n-hexane fraction yielded a tetranortriterpenoid, methyl-6-acetoxy angolensate (45), and a novel compound, grandifolin (46) (Agbedahunsi and Elujoba, 1998). Another triterpenoid, lupeol (47), isolated from methanol of Cassia siamea stem bark extract showed an IC50 of 5 μg/mL against P. falciparum using a parasite lactate dehydrogenase (pLDH) assay (Ajaiyeoba et al., 2008). Phytochemical investigation of the methanolic leaf extract of Combretum racemosum led to the identification of ursane-type triterpenes, with the most active antiplasmodial compound being madecassic acid (48) with mean EC50 ± SD values of 28 ± 12 and 17 ± 4 μg/mL against chloroquine-sensitive (D10) and chloroquine-resistant (W2) P. falciparum, respectively (Oluyemi et al., 2020).
The crude methanolic extract of the leaves of Bridelia ferruginea displayed potent activity against T. brucei with >90% inhibition and EC50 of 8.48 μM. A tetraterpenoid/carotenoid, lutein (49), was isolated from the extract among other compound and showed activity against T. brucei (EC50 4.16 μM) and L. donovani (EC50 9.3 μM) (Afolayan et al., 2019) (for the structures of compounds 25–49, see Figure 4).
Figure 4.
Structure of terpenoids isolated from Nigerian plants with selected antiprotozoal activity.
Flavonoids and Chalcones
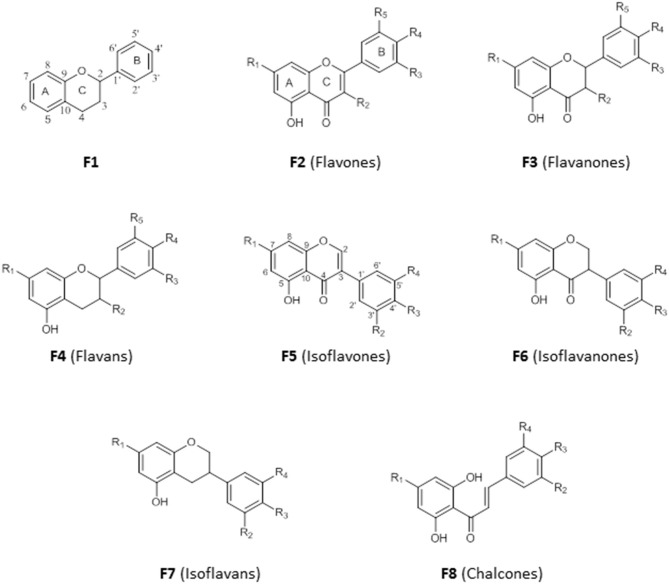
Flavonoids are secondary metabolites with diverse medicinal properties and are commonly found in fruits, leaves, and flowers of plants. They are phenolics, with structures based on a 15-carbon skeleton made of two benzene rings (A and B) connected by a pyran ring (C) (F1). The saturation (F3) or unsaturation (F2) of the pyran ring, absence of the carbonyl group at C-4 (F4), connection of ring B to ring C at C-3 (F5, F6, F7) and the opening of ring C (F8) is the basis for their classification into flavones, flavanones, flavans, isoflavone, isoflavanones, isoflavans, and chalcones (Figure 5). An –OH substituent at C-3 on the flavones skeleton (F2) leads to flavonols, while on flavanone skeleton (F3) leads to flavanonols.
Figure 5.
Structure of basic skeleton of flavonoid sub-groups.
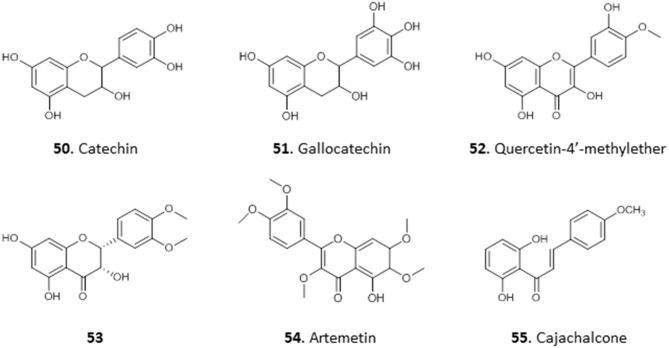
We have reported the isolation of two flavonols, 50 and 51, from the combined hexane and ethyl acetate extracts of the roots of Spondias mombim and the methanolic extract of the bark of Alcornea cordifolia, respectively. Compound 50 displayed poor activity against T. brucei (EC50 25 μg/mL) whereas 51, different only by a single hydroxy substituent on the 5′ position, showed quite remarkable activity against this parasite (EC50 <0.2 μg/mL). However, 51 also displayed toxicity against PNT2A prostatic cells with an EC50 of 1.5 μg/mL and would thus be too toxic for therapeutic use (Igoli et al., 2011). Nonetheless, it might be worth exploring structural variations with the 5′-OH substitution in a SAR study.
The methanol extract of the leaves of the common tropical shrub Chromolaena odorata, extracts of which are used to treat malaria in South-Eastern Nigeria, suppressed parasitaemia in mice infected with P. berghei by 99.2 and 97.8% at 200 and 400 mg/Kg/day, respectively. The extract yielded a flavonoid identified as 3, 5, 7, 3′-tetrahydroxy-4′-methoxyflavone (52), which displayed high antiplasmodial activity, with 81.5% suppression of parasitaemia in mice infected with P. berghei at 2.5 mg/Kg/day, even better than the control chloroquine and artemether (Ezenyi et al., 2014). Nwodo et al. (2015b) isolated seven flavonoid derivatives from the leaves of Vitex simplicifolia (family Verbenaceae), which is used to treat trypanosomiasis in Nigeria, and evaluated their activity against T. b. rhodesiense as well as cytotoxicity in L6 cells. The most active and least toxic of the compounds were 2-(5′-methoxyphenyl)-3,4′,5,7,8-trihydroxychroman-4-one (53) with IC50 = 10.2 μg/mL and SI = 9.8, as well as artemetin (54) with IC50 = 4.7 μg/mL and SI = 9.8 (Nwodo et al., 2015b). This level of selectivity seems insufficient for any clinical development.
The crude methanol extract of the leaves of Cajanus cajan, a member of the family Fabaceae used for its antimalarial properties, nevertheless had an IC50 value of just 53.5 μg/mL against the multidrug-resistant P. falciparum K1 strain in vitro; however, its ethyl acetate fraction yielded cajachalcone, 2′,6′-dihydroxy-4-methoxy chalcone (55) with IC50 of 2.0 μg/mL (7.4 μM) (Ajaiyeoba et al., 2013). The relatively simple structures of these flavonoids and chalcones, which lack chiral centers, should enable a systematic exploration of their structure-activity relationships (for the structures of compounds 50–55, see Figure 6).
Figure 6.
Structure of flavonoids isolated from Nigerian plants with selected antiprotozoal activity.
Quinones
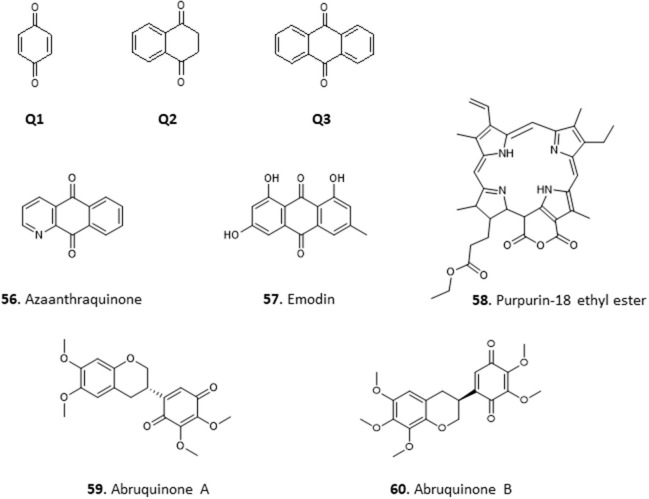
The common feature among quinones is the presence of two carbonyl groups in a six-membered unsaturated ring (carbonyl groups can also be on adjacent rings). They are classified into three main groups: benzoquinones which have two carbonyl groups in a benzene ring (Q1), naphthoquinones, which have two carbonyl groups on one of the rings in naphthalene (Q2) and anthraquinones, which are derivatives of anthracene (Q3) (Figure 7). They are found in the leaves, seeds, and woody parts of higher plants, in some fungi and bacteria but rarely in higher animals. Quinones have been reported to have antioxidant, anti-inflammatory, antibiotic, antimicrobial, and anticancer activities (El-Najjar et al., 2011).
Figure 7.
Structure of basic skeleton of quinone sub-groups (Q1–Q3) and quinones isolated from Nigerian plants with selected antiprotozoal activity (56–60).
Treatment of Trypanosoma congolense-infected mice with the crude ethanol extract of the leaves of Mitracarpus scaber, a popular medicinal plant with known antifungal and antimicrobial properties (Irobi and Daramola, 1993; Ekpendu et al., 1994), at 50 and 150 mg/Kg/day cleared parasitaemia within 6 and 2 days, respectively, with no relapse for about 2 months. Benz(g)isoquinoline 5,10 dione (Azaanthraquinone; 56) was isolated from the extract. It completely lysed T. congolense cells within 60 min at a concentration of 5 μM, and dose-dependently inhibited their motility and respiration (Nok, 2002). Nok investigated the mechanism of action further and concluded that this M. scaber quinone (56) interferes with the essential function of coenzyme Q (ubiquinone) in the trypanosomes, which carries electrons for aerobic respiration from mitochondrial glycerol-3-phosphate dehydrogenase to the Trypanosome Alternative Oxidase (TAO) (Ebiloma et al., 2018b). This is a rare example where the mechanism of the antiparasite action of a natural compound is well-understood. Considering that T. brucei spp. are even more susceptible to TAO inhibitors than T. congolense (Ebiloma et al., 2018a), due to differences in their mitochondrial pathways, it would be worth revisiting the wider trypanocidal activities of 56 (for the structures of compounds 56–60, see Figure 7).
Cassia nigricans is a herbal plant used to treat various fevers in Nigeria. The methanol extract of the whole plant yielded an antiplasmodial compound, emodin (57), an anthraquinone with an IC50 of 10.8 μg/mL in chloroquine-resistant P. falciparum strain K1 (Obodozie et al., 2004). In another study 57 was isolated from a methanol stem bark extract of another plant from the same genus, C. siamea, and confirmed to have strong activity against multidrug-resistant P. falciparum K1 strain (IC50 of 5 μg/mL) (Ajaiyeoba et al., 2008). Such independent confirmation of the activity is obviously important but unfortunately rare, in part because such data are harder to publish.
The crude hexane and ethyl acetate extracts of the leaves of Crateva adansonii showed moderate anti-trypanosomal activity with a MIC of 12.5 μg/mL (Igoli et al., 2012). In a further study, purpurin-18 ethyl ester (58), an anthraquinone derivative, was isolated from the extracts of the leaves of the related species C. adansonii and displayed a MIC of 6.25 μM against T. brucei. Molecular docking studies of 58 with some trypanosomal proteins revealed glutathione synthetase as its most likely target, followed by sterol-14α-demethylase and riboflavin kinase, with riboflavin kinase showing highest affinity (Igoli et al., 2014), although these models need experimental verification.
The methanol extract of the roots of Abrus precatorius was recently reported to possess antileishmanial activity with IC50 ± SD of 22.2 ± 0.54 μg/mL. The ethyl acetate fraction of the extract yielded two antileishmanial isoflavanquinones identified as abruquinone A (59) and abruquinone B (60), which displayed identical activity against L. major (IC50 ± SD 6.35 ± 0.005 and 6.32 ± 0.008 μg/mL, respectively) and L. tropica (IC50 6.29 ± 0.015 and 6.31 ± 0.005 μg/mL, respectively) (Okoro et al., 2020).
Phenolics
Phenolics represent a diverse class of compounds whose structures contain at least one hydroxyl substituent on an aromatic ring. They include phenyl propanoids, benzoquinones, phenolic acids, acetophenones, phenylacetic acids, hydroxycinnamic acids, phenylpropenes, coumarins and isocoumarins, chromones, naphtoquinones, xanthones, stilbenes, anthraquinones, lignans, neolignans, lignins, and condensed tannins; and have been reported to have various medicinal properties including antioxidant, anticancer, anti-inflammatory, boosting immunity, etc. Phenolics are widely distributed in the plant kingdom.
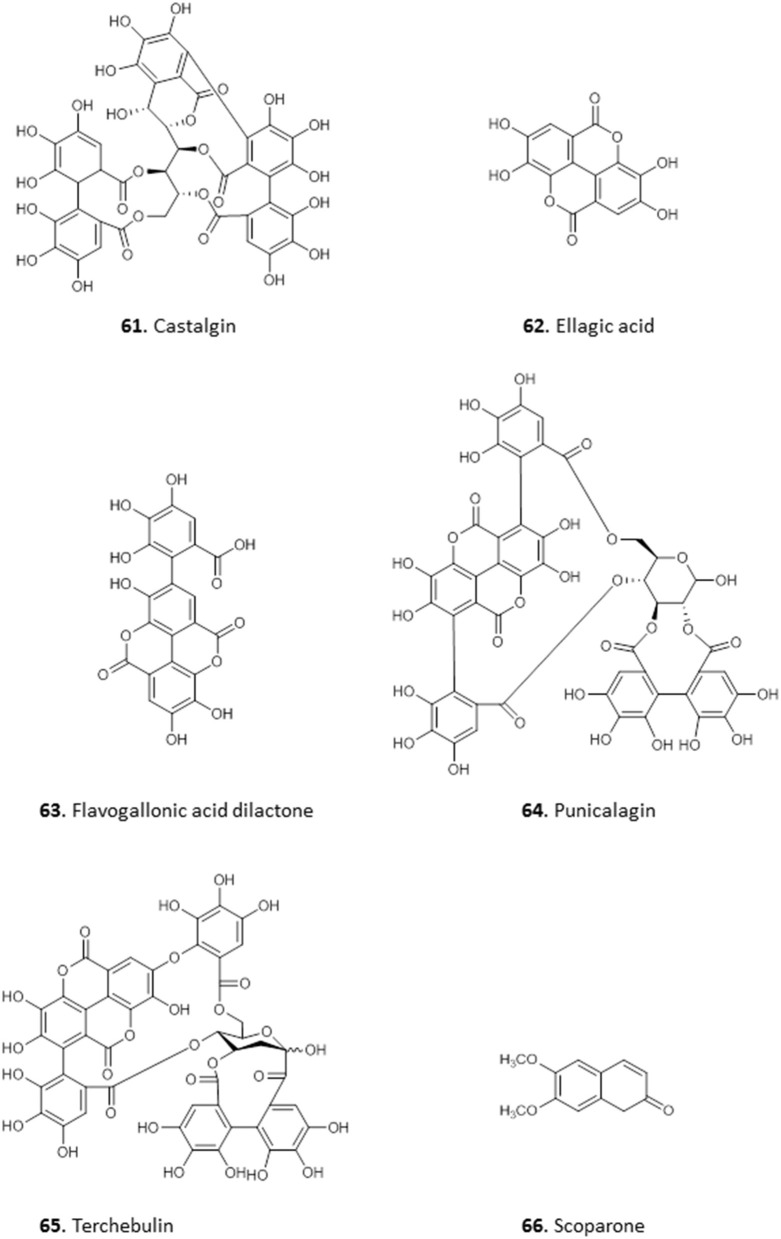
The antiprotozoal activity of the crude extract fractions and isolated tannins of Terminalia avicennoides and Anogeissus leiocarpus have been investigated along other Nigerian medicinal plants (Shuaibu et al., 2008a,b,c). Using PicoGreen as flourimetric monitor, the IC50 of the compounds against chloroquine-sensitive P. falciparum 3D7 was determined and all showed moderate activity: castalagin, 61 (10.57 μg/mL); ellagic acid, 62 (12.14 μg/mL); flavogallonic acid, 63 (8.89 μg/mL); punicalagin, 64 (9.42 μg/mL) and terchebulin, 65 (8.89 μg/mL). None of the compounds showed cross resistance to chloroquine (Shuaibu et al., 2008b). The compounds were also tested against trypanosome species T. brucei brucei, T. b. ambience, T. b. rhodesiense, and T. evansi and showed slightly higher activity against T. b. brucei with MICs of 22.5, 7.5, and 27.5 μg/mL for 61, 63, and 65, respectively. However, the compounds displayed low antileishmanial activity with 61, isolated from Anogeissus leiocarpus, being the most active with MIC of 55 μg/mL against L. aethiopica. Further morphological examination and electron microscopy revealed cell swelling and changes in the ultrastructure of organelles in L. aethiopica promastigotes exposed to 61 (Shuaibu et al., 2008a). All the tannins showed an MIC of ≥1,500 μg/mL against Newborn Mouse Heart Fibroblast cells in a cytotoxicity assay, suggesting good antiparasitic selectivity (Shuaibu et al., 2008a). The stem extract of Euphorbia poisonii yielded scoparone 66, a coumarin with strong activity against T. brucei (MIC = 1.56 μg/mL) (Igoli et al., 2011) (for the structures of compounds 61–66, see Figure 8).
Figure 8.
Structure of phenolic compounds isolated from Nigerian plants with selected antiprotozoal activity.
Glycosides
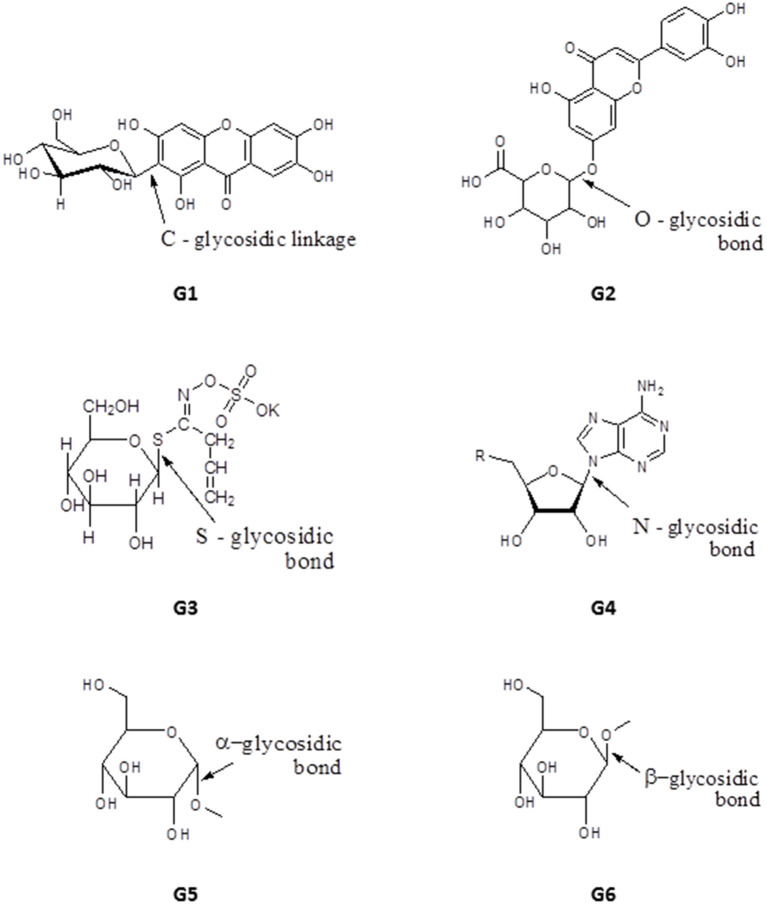
Glycosides have one or more sugar moieties bonded through the anomeric carbon to a non-sugar group. The glycosidic bond can be carbon (C-glycosides, e.g., mangiferin, G1), oxygen (e.g., Luteolin-7-O-glucuronide, G2), sulfur (in thioglycosides e.g., Sinigrin, G3) or nitrogen (in glycosylamines e.g., adenosine, G4) (Figure 9). The sugar moiety is called the glycone and may contain one or more sugar groups, while the non-sugar group is the aglycone or genin part of the glycoside. According to their glycone moieties, glycosides are grouped into glucosides, fructosides, ribosides, glucuronides (glucuronnoside). Classification can also be based on the orientation of the glycosidic bond, α-glycosides have their glycosidic bond below the plane of the cyclic sugar moiety (glycone) e.g., methyl-α-Dglucopyranoside, G5, whereas when the glycosidic bond lies above the plane of the glycone a β-glycoside is formed e.g., methyl-β-D-glucopyranoside, G6 (Figure 9). Classification of glycosides can also be based on their aglycone moieties e.g., steroid (cardiac) glycosides, coumarin glycosides, anthraquinone glycosides, saponins etc. The medicinal properties of glycosides have been exploited extensively for the treatment of heart diseases and as antibiotics, for example streptomycin, kanamycin and neomycin (Kren and Martinkova, 2001).
Figure 9.
Structure of basic skeleton of glycoside sub-groups showing different glycosidic bonds.
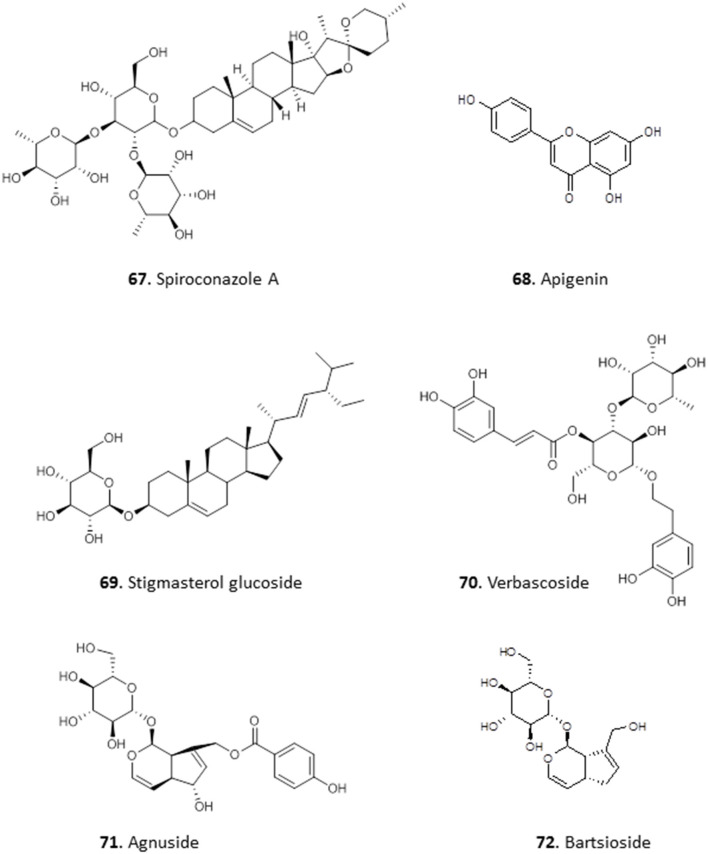
Okunji et al. isolated a bioactive saponin, identified as Spiroconazole A (67), from the methanol extracts of the seed pulp of two “soap tree” species, Dracaena mannii and D. arborea. The compound showed significant antileishmanial and antimalarial activity (Okunji et al., 1996). Three glycosides were obtained from the methanol extract of the leaves of Stachytarpheta cayennensis, which is used in Central and West Africa to treat malaria. The compounds were identified as apigenin (68), stigmasterol glucoside (69) and verbascoside (70), and displayed significant (P < 0.05) antimalarial activity in vitro and in vivo, with a dose of 2.5 mg/Kg lowering P. falciparum parasitaemia in mice by up to 89% (Ifeoma Chinwude et al., 2016). Two antikinetoplastid irodid glucosides were isolated from the methanol leaf extracts of Vitex grandifolia. Agnuside (71) showed an IC50 of 5.38 and 13.7 μg/mL against L. donovani amastigotes in THP1 cells and against T. brucei, respectively. However, the other compound, bartsioside (72), was essentially inactive, with IC50 >25 μg/mL against both parasites, suggesting that the hydroxyl group on C-6 and/or the p-hydroxy benzoic acid moiety at C-8 are probably essential for activity (Bello et al., 2018) (for the structures of compounds 67–72, see Figure 10).
Figure 10.
Structure of glycosides isolated from Nigerian plants with selected antiprotozoal activity.
Peptides
Peptides are organic polymers made of amino acid monomers in which the alpha amino group (-NH) of one acid is linked to the alpha carboxylic acid group (-CO2H) of another through an amide bond. Peptides that contain more than 100 amino acid monomers are called proteins. Peptides have been isolated from roots, seeds, flowers, stems, and leaves of plants (Nawrot et al., 2014).
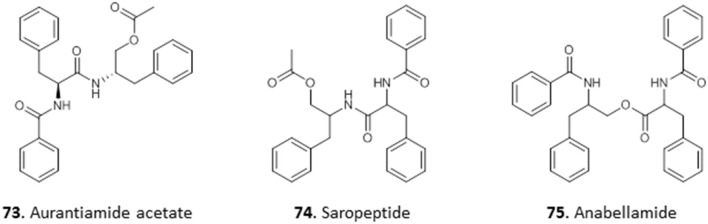
One antitrypanosomal peptide from Nigerian plants is aurantiamide acetate (73). It was isolated from the leaves of Crateva adansonii, and displayed an MIC of 25 μM against T. brucei. Further investigation into its possible mechanism of action through docking studies revealed strong binding interactions between 73 and trypanosomal enzymes sterol-14α-demethylase and trypanothione reductase (Igoli et al., 2014). Nwodo et al. (2015a) isolated two dipeptides from the methanol extract of the roots of Zapoteca portoricensis elucidated as saropeptide (74) and anabellamide (75). Compound 74 was selectively active against T. brucei with IC50 of 3.63 μM, but had only low activity against Trypanosoma cruzi (IC50 = 41.6 μM), which is the causative agent of Chagas disease. On the other hand, 75 displayed moderate activity against both species (T. brucei IC50 = 12.2 μM; T. cruzi IC50 = 16.1 μM). Although both compounds displayed relatively low toxicity against mammalian L6 cells with IC50 values of 92.1 μM and 71.2 μM for 74 and 75, respectively, this does not seem to leave sufficient selectivity for further development (Nwodo et al., 2014) (for the structures of compounds 73–75, see Figure 11).
Figure 11.
Structure of peptides isolated from Nigerian plants with selected antiprotozoal activity.
Fatty Acid and Steroids
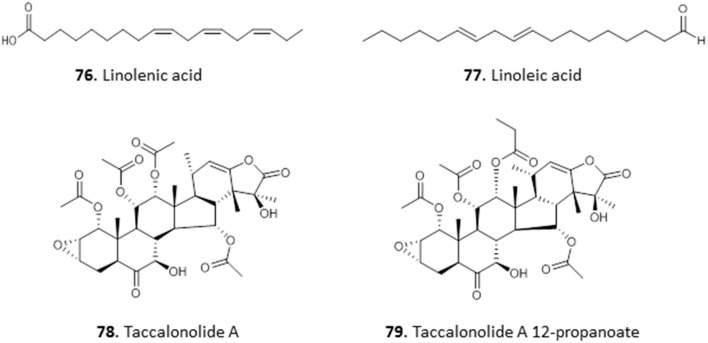
The ethyl acetate and methanol extracts of the leaves of Carica papaya exhibited promising antiplasmodial activity with IC50 values of 2.6 and 12.8 μg/mL in chloroquine-sensitive P. falciparum D10 strain, respectively. Bioassay-guided fractionation of the ethyl acetate extracts yielded two fatty acids, 9,12,15-octadecatrienoic acid (linolenic acid; 76) and 9,12-octadecadienoic acid (linoleic acid; 77) with IC50 values of 3.58 ± 0.22 and 6.88 ± 0.02 μg/mL in P. falciparum. The compounds were not cross-resistant to chloroquine but the level of selectivity for the parasite over Chinese hamster ovarian cells is quite low (SI = 7.43–15.27) and does not invite extensive in vivo follow-on (Melariri et al., 2011). In our own investigations of antiprotozoal natural products from Nigeria and beyond, we have reported the isolation of Taccalonolide A (78) and its novel derivative, Taccalonolide A 12-propanoate (79), from the tubers of Tacca leontopetaloides. Both compounds showed activity against T. brucei, with EC50 ± SEM of 11.4 ± 0.39 and 3.1 ± 0.09 μg/mL. However, we have observed remarkable antitrypanosomal activity in one of the impure fractions (EC50 = 0.76 ± 0.03 μg/mL), suggesting a need for further investigation of T. leontopetaloides as a source of more potent antiparasitic agents (Dike et al., 2016) (for the structures of compounds 76–79, see Figure 12).
Figure 12.
Structures of fatty acids and steroids isolated from Nigerian plants with selected antiprotozoal activity.
Chlorophyll Metabolites
Extracts of the leaves of Crateva adansonii has yielded two pheophorbides, Pyropheophorbide A (80) and Ethyl pyropheophorbide A (81). Both 80 and 81 showed potent antitrypanosomal activity with an MIC of 6.5 μM. Both compounds showed strong binding interactions with trypanosomal riboflavin kinase and trypanothione reductase in molecular docking studies (Igoli et al., 2014). The ethyl acetate leaf extracts of Newbouldia laevis showed interesting activity against T. b. brucei (EC50 ± SEM = 4.2 ± 0.7 μg/mL). Two similar antitrypanosomal compounds, pheophytin A (82) and pheophytin B (83), with EC50 ± SEM values of 25.0 ± 2.8 and 1.58 ± 0.09 μg/mL, respectively, were identified from the extract of Newbouldia laevis (Family Bignoniaceae); both compounds were non-toxic to HEK cells (Ebiloma et al., 2017) (for the structures of compounds 80–83, see Figure 13).
Figure 13.
Structures of chlorophyll metabolites isolated from Nigerian plants with selected antiprotozoal activity.
Compounds From Other Nigerian Natural Sources
Lichen
A depside, 4-(1-hydroxylprop-1-en-1-yl) atranorin-1-carboxylic acid (84), was obtained from Dirinaria picta, a lichen epiphyte of Elaeis guineensis palm trees, collected in Port Harcourt, Rivers State. It showed some antiplasmodial activity with IC50 value of 37 μg/mL, and low toxicity to mammalian HeLa cells (IC50 = 100 μg/mL) (Afieroho et al., 2018).
Nigerian Red Propolis
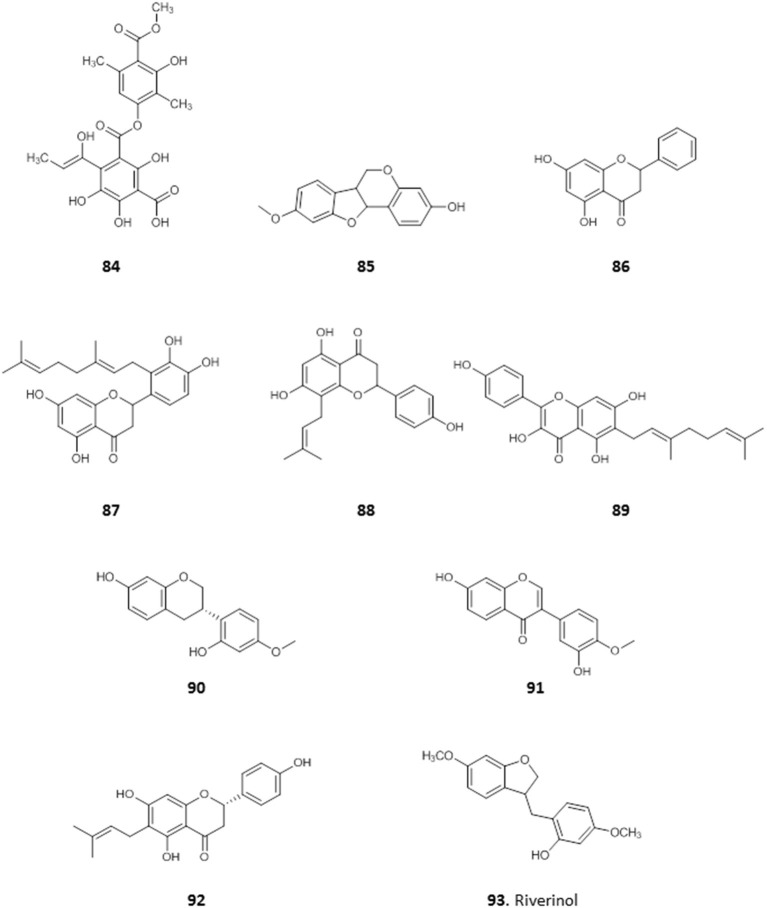
We have previously reported on the efficacy of Nigerian red propolis (NRP) in rats experimentally infected with T. brucei. Treatment with NRP at 400 and 600 mg/Kg/day resulted in significantly (P < 0.05) reduced parasitaemia and improved blood parameters (higher PCV and HBC) and weight gain compared to a DMSO treated control group (Nweze et al., 2017). This prompted further investigation into the active compounds in NRP. The ethanol extract of NRP yielded antitrypanosomal compounds, most of which were flavones and flavonoids (Omar et al., 2016). The MIC against T. brucei was determined for three of the compounds: medicarpin, 85 (3.12 μg/mL); pinocembrin, 86 (12.5 μg/mL), and propolin D, 87 (3.12 μg/mL) and EC50 values were determined for the remaining compounds. The most active of these were 8-prenylnarigenin (88), macarangin (89), and vestitol (90), with EC50 ± SEM of 6.1 ± 0.1, 7.8 ± 0.1, 8.3 ± 0.1 μg/mL, respectively. Other compounds with significant antitrypanosomal activity included calycosin (91; EC50 ± SEM = 10.0 ± 0.44 μg/mL), 6-prenylnaringenin (92; EC50 ± SEM = 11.4 ± 0.34 μg/mL), and riverinol (93; EC50 ± SEM = 16.24 ± 0.24 μg/mL). Interestingly, none of the compounds showed cross-resistance to pentamidine and melarsoprol but 93 was significantly more active against two multi-drug resistant strains (3.6 and 2.5-fold, respectively (p < 0.001) (Omar et al., 2016), from which either the TbAT1/P2 (Carter et al., 1999) or the HAPT1/AQP2 drug transporter (Alghamdi et al., 2020) had been deleted (for the structures of compounds 84–93, see Figure 14).
Figure 14.
Structures of natural compounds isolated from Nigerian red propolis and lichen with selected antiprotozoal activity.
Barriers to Natural Product-Based Research and Drug Discovery
The challenges facing the development of new natural products-derived drugs discussed in this review are not peculiar to Nigeria alone but to many developing countries with richer natural resources than scientific infrastructure and funding. The identification of a pharmacologically active compound from a medicinal plant in a systematic and efficient manner is not an easy task. However, there is no gainsaying that specialized molecules of plant origin are the spine of our modern-day pharmacopeia, or that traditional herbal remedies continue to be successful in the treatment of many ailments. Notwithstanding this historic significance in drug discovery and development, there has been a steady drop in the number of new natural product-based drugs entering into clinical use in the past 30 years (Wright, 2019). The modern-day pharmaceutical industry has largely shifted from the traditional natural product-based drug discovery strategy to one that could easily be created with a synthetic approach such as combinatorial chemistry combined with high-throughput screening. Consequently, synthetic compounds are currently being favored by the global pharmaceutical industries over the natural product-based compounds as a more tractable alternative in their use to develop new drugs. This is a consequence of a combination of many factors.
Complexity of Isolation Processes
Perhaps the most challenging impediments to natural product-based drug discovery over the past few decades are the resource intensiveness and the tedious purification protocols involved in the identification of bioactive molecules from the highly complex mixtures that are the initial extracts. When purified, the isolated bioactive compound is often obtained in tiny amounts (usually a few milligrams) that are unlikely to go the full length of the drug discovery process, especially in protocols involving the use of large cultures such as required for metabolomic studies and other assessments of drug action, or for in vivo studies. This is compounded by the fact that this tedious process frequently yields subversive compounds with multiple behavior in assays such as covalent bond formation, chelation, membrane perturbation, and redox activity, which are collectively known as Pan Assay Interference compoundS (PAINS) (Baell and Holloway, 2010; Baell, 2016). Prominent natural (plant) products that frequently contain PAINS include catechols, quinones, phenolic manic bases, and hydroxyphenylhydrazones (Baell, 2016). PAINS and PAINS-like, despite a possible nano- or micro-molar potency, lack a distinct biological mechanism, exhibit poor SAR or optimisability, and thus have very low prospects for clinical development (Baell, 2015). Thus, it is important to utilize assay techniques that will screen-out PAINS and to carry out structural studies of complexes of hits-target molecules and structure-activity optimization studies of the hits (Baell and Nissink, 2018; Balogun et al., 2019).
Lack of Precision and Mechanism
In addition to the challenge of the tedious process flow from the screening of medicinal plants to the isolation of new bioactive principle(s) with low yield, the subsequent determination of the exact mechanism of action of the isolated compound requires expertise, experience, the appropriate research environment (infrastructure and equipment) and substantial amounts of funding. But due to research and resource limitations, finding a robust and viable lead candidate for the drug discovery pipeline has become a challenging scientific task. Hence, the majority of the research efforts aimed at identifying drug leads end up at the level of crude extract testing stage, which is not very informative and fails to give a usable measure of activity as the concentration of the active molecule(s) is unknown. Moreover, the amount of active compound in the extract is likely to be different between preparations.
In addition, the therapeutic effect of natural remedies may be through immuno-modulation rather than a direct antimicrobial activity. Assaying for antimicrobial properties, a compound with immunomodulatory properties will not show bioactivity in vitro during the traditional bioassay-guided screening of the complex extract mixture and will be ruled out. This is a serious impediment to determining the exact active principle in a medicinal plant or its extract unless the primary screen is based on an infectious animal model. Furthermore, some natural compounds may exhibit a combined or synergistic effect in their mode of action. Hence, such compounds, when isolated in their pure form, may not appear to have promising activity in the bioassay screening protocol (Druilhe et al., 1988; Williamson, 2001; Wagner, 2011). A prominent example is the case of the presently leading anti-malarial plant, Artemisia annua. Herbal teas prepared from the dried leaves of A. annua are found to be 6–18 folds more effective at killing the Plasmodium parasites than the isolated active anti-malarial agent, artemisinin (Wan et al., 1992; Wright et al., 2010). These possible scenarios can frustrate efforts toward identifying the bioactive compound(s) from some plants/extracts that show initial promise.
Novelty of Isolated Natural Compounds
The majority of compounds isolated from plants will have been previously reported, and such compounds lacking novelty are unlikely to be advanced into drug development and less likely to lead to academic publications. In fact, over 200,000 isolated natural compounds have so far been reported in the scientific literature using the traditional natural product extraction and purification methods (Walsh and Yang, 2017). This means that there is a high probability of identifying known rather than novel molecules using the standard bioassays guided fractionation and identification of lead compounds, thus often giving a poor return on investment.
A range of processes known as “dereplication,” based on hyphenated techniques are standard procedures that are applied in plant metabolomics to avoid the re-isolation and re-characterization of known compounds by eliminating known entities (Carnevale Neto et al., 2016; Hubert et al., 2017; Kildgaard et al., 2017). However, to systematically extract vital pieces of information from acquired data, chemometric tools are needed for the dereplication process. This is because biological samples such as plant extracts are complex in nature, with a very large concentration range. This notwithstanding, the GC-MS-based technique for identifying non-targeted metabolites is one of the emerging techniques to improve the dereplication method (Carnevale Neto et al., 2016), but the procedures involved can be time-consuming and resource-intensive.
Undesirable Physical Properties
Another fundamental challenge is that in the drug discovery setting, it is predicted that poor absorption becomes probable when there are more than 5 H-bond donors, 5 H-bond acceptors, the molecular weight of the compound is above 500, or the calculated partition coefficient (cLog P) is >5 (Lipinski et al., 1997). This is known as Lipinski's rule of five. Unfortunately, most natural compounds do not obey this rule; hence, in the eye of the pharmaceutical companies, many natural compounds do not have the required drug-like characteristics, based on their structure. This is in addition to the solubility issues associated with some natural compounds; many are not completely soluble in the assay's aqueous environment, which can interfere with the reproducibility of research results. Many of these issues require increased troubleshooting in standardized assays, leading to higher costs and a high rejection rate for otherwise active compounds.
Other undesirable physical properties of natural compounds like colouration make them more difficult to assay using contemporary drug discovery strategies such as the standardized high throughput screening of compound libraries, compared with synthetic compounds. For instance, deep colouration of some plant compounds may interfere with the assay methods involving color change in spectroscopic/colorimetric determinations.
Finally, it is imperative to highlight the huge structural complexities of many natural compounds, making synthesizing them and their derivatives very difficult (Harvey, 2000; Strohl, 2000; Li and Vederas, 2009), and the construction of a large series of related compounds for SAR or pharmacodynamic optimization almost impossible. The combination of these problems diminishes the prospects of using compounds of natural origins for drug discovery.
Accessibility of Plant Materials and Poor Documentation of Herbal Remedies
Another significant challenge is the absence of specific government legislation and international agreements regulating the access to plant resources in the biodiversity-rich flora. Some of the indigenous plants used in folklore are rapidly disappearing due to indiscriminate use, deforestation, and deliberate bush-burning for farming activities and hunting. This makes subsequent access to these important medicinal plants for follow-up studies increasingly difficult, which in turn discourage further natural products research. This is a serious problem particularly in developing countries where the implementation of the Nagoya protocol is yet to be fully implemented. The Nagoya Protocol on Access and Benefit Sharing (ABS) is a build-up on the Convention on Biological Diversity (CBD), which is a key regulatory document for sustainable development aimed at developing national strategies for the conservation and sustainable use of biological diversity in all countries (Convention on Biological Diversity, 2010; Buck and Hamilton, 2011). Contrary to the opinions of critics of ABS who think it will create bottlenecks for scientific research and impedes new discoveries, the implementation of the Nagoya Protocol in Nigeria will prevent the extinction of plants from which useful medicinal products can be discovered. There can also be difficulty in physically gaining access to the natural habitats of these essential medicinal plants. This challenge is due to the absence of accessible roads to the often remote rural areas where the majority of these important medicinal plants are located.
Furthermore, traditional herbal remedies, which are justly regarded as the basis for modern medicine, are based on oral tradition (folklore) and much of that is usually shrouded in secrecy and a reluctance to divulge vital information regarding the preparation or use of the key medicinal plants. To keep the traditional healers in business, the pharmacologically active plants are often used in combination with other plants to prepare the herbal remedies, in order to obfuscate the true source of the medication. Unfortunately, but understandably, permission for the associated extracts/concoctions to be used for scientific research and innovation is frequently denied.
Limited Research Funding
As explained above, the processes involved in the isolation, purification, and chemical characterization of the bioactive agents are expensive and time-consuming. Together with potential worries about continuous access to sufficient quantities of the natural compound, this makes the pharmaceutical industries less interested in investing in such ventures. This lack of investment is further compounded by the problem of reduced funding of academic research institutions, which hinders the provision of modern state-of-the-art facilities including such that enable long-term storage and (high-throughput) screening of natural products-based compound libraries. Scale-up of the production of natural compounds with complex (stereo)-chemistry that elude viable commercial synthesis can be another major hurdle, especially for compounds from relatively rare and/or slow-growing plants, or where very low or variable concentrations of active compounds are found in the primary plant source.
Climate Change
Climate change has also already negatively impacted the natural product-based drug discovery effort. Environmental issues, like desert encroachment due to global warming, have led to the extinction of many valuable land species including key medicinal plants. In addition, there is the disappearance of marine organisms of high medicinal value due to water pollution and temperature increases.
These challenges can be overcome when there is a huge national, multidisciplinary approach to drug discovery efforts, and synergy between academia and pharmaceutical companies. This requires concerted efforts from the appropriate government ministries and parastatals, academia and research institutes, and the pharmaceutical companies. When the lack of resources is factored into this challenge, it is imperative to establish a close partnership between local research institutions and local or foreign pharmaceutical companies.
Recommendations and Future Perspective
In this review we described and listed scores of phytochemicals isolated from Nigerian medicinal plants and, importantly, they have been reported to possess therapeutic capabilities against important Africa-endemic parasitic infectious diseases. The focus of this paper has been on the protozoan infections- malaria, African trypanosomiasis, and leishmaniasis. Apart from malaria, these diseases are popularly termed neglected tropical diseases (NTDs) because they disproportionately affect the economically bottom billion (world's poorest) people; consequently, drug discovery for such diseases is not of interest to the pharmaceutical industry due to projected lack of profitable return on the investments. Since the majority of the people affected by these diseases are Africans, it would be fulfilling, and indeed transformational, to see a few of the novel natural compounds that are highlighted in this review make their ways to the market as drugs of choice for malaria or some of the NTDs. It should be noted that, according to the World Health Organization, Nigeria is the country most afflicted by malaria, shouldering 25% of the global malaria burden (World Health Organization, 2019). In order words, the goal of this review is not only to provide a compendium of antiparasitic natural compounds from Nigerian bioresources, but also to stimulate and channel research focus toward further development of the compounds to marketable drugs. If successful, this will become a leading example of solutions to African problems from Africa, and by Africans. However, at this moment, every single compound listed here is still experimental and in the early stages of drug development. The subsequent paragraphs provide perspectives and steps to be taken toward genuine clinical development of African natural compounds for African diseases.
Advancement of the Compounds Through the Drug Development Pipeline (DDP)
The process of drug development takes 7–15 years of hard work, huge funding, and is comprised of four stages each having multiple steps: (1) the discovery stage, involving successful demonstration of the compound as being potent against the target parasites; (2) the pre-clinical development stage where the compounds are tried in various laboratory animals to understand their safety, pharmacology, in vivo potency, and possible mechanism of action; (3) the clinical development stage, which is comprised of five phases of clinical trials; and (4) the approval stage where regulatory bodies evaluate the accumulated data of stages 1–3 and take a decision to approve or reject their use as medicines for specific applications. Certainly, some of the 93 compounds highlighted fulfill the prima facie criteria to move from stage 1 to stage 2, and considerations such as compound availability, ease of synthesis, selectivity, solubility, stability etc. would aid in prioritization. It is logical to explore a shortlist further and progress them through the DDP with the overall aim to get new approved medicine(s) for malaria and some NTDs, from the current collection of compounds, although further discovery efforts, coupled to innovative dereplication, remain important. To achieve this, and in order to enhance success within shorter time, it is recommendable that Nigerian scientists form interdisciplinary networks internally, with collaborators within as well as outside the continent, and with pharmaceutical companies and/or international non-profit organizations. One highly encouraging and very recent development is the partnership between the Drugs for Neglected Diseases initiative (DNDi), Institut Pasteur Korea (IPK) and Fundación MEDINA, funded by La Caixa Health Research [Drugs for Neglected Diseases initiative (DNDi), 2020]. This will allow the screening of the large natural compounds library of Fundación MEDINA against Leishmania and T. cruzi by IPK, using state-of-the art high-throughput imaging technologies. This development shows the added value of large compound libraries held and curated centrally, over the dispersed efforts of many small-scale efforts, when it comes to securing the needed funding and infrastructure. A national or, better, regional repository for medicinal plant species, extracts, and natural compounds should be established in Nigeria as a partner for international funders and consortia. While much excellent work has been done by the individual laboratories, results are not necessarily comparable between them, due to differences in test strains and procedures, among other factors. Nor are compounds produced this way, on a necessarily small scale and often sub-optimally stored, easily available for further experimentation. The proposed facility should be curated by a Society of Herbal Medicine, with State funding.
Exploration of Microbes and Marine Organisms for More Novel Compounds
In addition to length of time needed, the attrition rate in DDP is very high. The number of starting compounds that enters the DDP drastically thins out after each step and stage- from available records only 2.5% of stage-1 compounds make it to stage 2, of which just 2% pass to stage 3, and 5% of the stage-3 candidates may eventually end up getting approved to be in the market (Kola and Landis, 2004; Tonkens, 2005). The implication is that only 1 out of 250 candidate compounds that enters stage 2 may become an approved medicine. With limited funding for even stage 2 studies, critical selection of what enters this stage is essential to decrease the attrition rate. Bearing in mind the aforementioned, it is advisable to continue natural compound discovery and screening against target pathogens, in a coordinated fashion, in order to expand this collection of compounds. Since most of the compounds at hand thus far are from plants, we are recommending that we expand our efforts to less-explored sources of medicinal natural products such as the microbial and marine organisms. Nigeria, and Africa at large, is blessed with diverse ecosystems that are habitats to myriad aquatic and non-aquatic macro- and micro-organisms. While the antibiotic penicillin is a leading example of drugs from microorganisms, the analgesic drug, Ziconotide was the first approved medicine from marine source. After these, numerous drugs from microbial and marine sources have been successfully brought to market (Molinski et al., 2009; Lobanovska and Pilla, 2017).
Formulate a Virtual Library of the Compounds
The recent advances in the fields of medicinal chemistry, and computer and information technology have resulted in a synergy that birthed an emerging field known as computational medicinal chemistry, which basically entails in silico approaches for the curation, design, development, testing and synthesis of pharmacologically active compounds. In silico curation is simply the act of keeping a collection of information in computers and making it accessible to interested people remotely, this is simply a database or virtual library. This can be organized relatively easily and even after the establishment of a national repository as described above, would retain its relevance if actively curated and updated by the research community. Numerous relevant virtual libraries/databases are available containing information on parasites—e.g., for genomes PlasmoDB (https://plasmodb.org/plasmo/app), TriTrypDB (https://tritrypdb.org/tritrypdb/app); metabolism (http://vm-trypanocyc.toulouse.inra.fr/; Shameer et al., 2015) and small molecules, e.g., ChEMBL (https://www.ebi.ac.uk/chembl/), PubChem (https://pubchem.ncbi.nlm.nih.gov/), and the recently curated TrypInDB (http://trypindb.biomedinformri.com/; Vijayakumar et al., 2020). Likewise, we propose the formulation of a virtual library/database to contain the 93 highlighted antiparasitic natural products of Nigerian origin, as well as information on all tested but inactive natural compounds in order to minimize needless replication, and continue to expand the library as new pharmacologically active compounds are being discovered. This database should further be expanded in collaboration, first to include natural compounds reported from other African nations, and potentially from other continents. As the database will be freely accessible, it will promote collaborations with scientists within and outside the continent and accelerate the process of drug discovery. As it grows, it should be explored whether this database could be linked to other resources such as TrypInDB or CHEMBL in order to promote its visibility.
Setting Up Synthesis Programs for the Various Compounds
Except for compounds 84–93, which were isolated from Nigerian propolis, the remaining compounds (1–83) enumerated were isolated from plants (Table 1) and as expected, the final yields are very low. Environmental concerns and low yield are major hurdles for drug development efforts using natural products from plants because large amounts of compounds are usually required, particularly at stages 2 and 3. Moreover, even after approval of a final drug, it would be hard to envisage a good business model built on a product whose supply is reliant on its isolation from plants in very small quantities—especially since the cost per treatment for NTDs must necessarily be low. Thus, in many cases the crux of a profitable and sustainable drug development plan for plant products may be to be able to establish a process for its large-scale production independent of the native plant. Above, we have already highlighted the ease of synthesis as one of the shortlisting criteria for progressing natural compounds down the DDP, and efforts should be initiated to establish economically viable synthesis routes for the selected candidates. To the best of our knowledge, so far only compound 7 (fagaronine) has an established chemical synthesis procedure (Rivaud et al., 2012) and funding of natural compounds synthesis research should be part of the national scientific strategy of Nigeria.
Most of the natural compounds are structurally and stereochemically complex, making their chemical synthesis routes difficult, and often non-feasible in terms of cost/yield. For such compounds, the alternative to chemical synthesis will be synthetic biology. The use of the synthetic biology approach requires: (1) identification of the biosynthesis pathways and the genes encoding the enzymes for every step in the biosynthesis of the secondary metabolite (natural compound) in the native organism, (2) heterologous assemblage of the pathway in a suitable microorganism, which is achieved by cloning and transformation of all the genes into the surrogate microorganism, (3) establishment of a fermentation technology for the large-scale culture and production of the product, and (4) a standardized large-scale purification method for the produced secondary metabolite. Recently, the antimalarial terpenoid artemisinin, which is natively produced by the plant Artemisia annua, has been partially produced in yeast and Escherichia coli through synthetic biology, yielding quantities as high as 25 g/L of culture (Tsuruta et al., 2009; Paddon et al., 2013). Another interesting example of synthetic biology is the complete biosynthesis of noscapine in Saccharomyces cerevisiae. Noscapine is a potential anticancer drug that is natively derived from the opium poppy plant, Papaver somniferum. Engineering S. cerevisiae by introducing 10-gene clusters from opium poppy, which encodes 30 enzymes in the biosynthetic pathway of noscapine resulted in the heterologous production of the compound with a yield 2.2 mg/L (Li et al., 2018).
Conclusion
Over the years, the bioactivity of extracts from Nigerian flora has been established, thus validating the widespread use of herbal medicine in that part of the world. Bioactivity-driven fractionation efforts have now identified candidate drug leads in some of these plants, including potential antiprotozoal agents. However, the number of pure compounds isolated and investigated is still low, despite the thousands of preliminary studies showing promising activity of plant extracts. Furthermore, the translation of in vitro anti-protozoal activity of the already identified phytochemicals into in vivo preclinical studies has also been slow. While the challenges affecting the development of these natural compounds are numerous and complex, they can be surmounted by concerted collaborative and multi-disciplinary efforts, especially when underpinned by a national strategy and the establishment of a natural compounds repository to enable it. This will facilitate competitive bids for international funding, enable synergy between academia and pharmaceutical companies and not-for-profit organizations, and would bridge many gaps in scientific infrastructure. Thus, while investment in research in developing countries like Nigeria is indispensable, coordination within the country is essential for the development of international collaborations. Nigeria has the raw materials and resources as well as the drive and the talent in their scientific community, now is the time to translate these into valuable pharmaceutics that benefit its population.
Author Contributions
MU, JI, and GE conceptualized the project. MU, GE, HK, NI, and EB contributed to the development and writing of the manuscript. HK, JI, and EB contributed in validating, reviewing, and supervising the project. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. MU was in receipt of a PhD studentship from the Petroleum Technology Development Fund of Nigeria. EB was a Fulbright Fellow and also supported by an Africa Center of Excellence for Development (ACE Impacts) project through funding to the Africa Center of Excellence for Neglected Tropical Disease and Forensic Biotechnology (ACENTDFB), Ahmadu Bello University, Nigeria.
References
- Abiodun O. O., Gbotosho G. O., Ajaiyeoba E. O., Brun R., Oduola A. M. (2012). Antitrypanosomal activity of some medicinal plants from Nigerian ethnomedicine. Parasitol. Res. 110, 521–526. 10.1007/s00436-011-2516-z [DOI] [PubMed] [Google Scholar]
- Achan J., Talisuna A. O., Erhart A., Yeka A., Tibenderana J. K., Baliraine F. N., et al. (2011). Quinine, an old anti-malarial drug in a modern world: role in the treatment of malaria. Malar. J. 10:144. 10.1186/1475-2875-10-144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adebayo J. O., Krettli A. U. (2011). Potential antimalarials from Nigerian plants: a review. J. Ethnopharmacol. 133, 289–302. 10.1016/j.jep.2010.11.024 [DOI] [PubMed] [Google Scholar]
- Afieroho O. E., Noundou X. S., Krause R. W., Isaacs M., Olley L., Hoppe H. C., et al. (2018). An antiplasmodial depside from a nigerian lichen dirinaria picta, epiphytic on the oil palm elaeis guineense. Rev. Boliv. Química 35 Available online at: http://www.redalyc.org/articulo.oa?id=426355610005
- Afolayan M., Srivedavyasasri R., Asekun O. T., Familoni O. B., Orishadipe A., Zulfiqar F., et al. (2018). Phytochemical study of Piliostigma thonningii, a medicinal plant grown in Nigeria. Med. Chem. Res. 27, 2325–2330. 10.1007/s00044-018-2238-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afolayan M., Srivedavyasasri R., Asekun O. T., Familoni O. B., Ross S. A. (2019). Chemical and biological studies on Bridelia ferruginea grown in Nigeria. Nat. Prod. Res. 33, 287–291. 10.1080/14786419.2018.1440225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agbedahunsi J., Elujoba A. (1998). Grandifolin from khaya gradifoliola stem bark. Niger. J. Nat. Prod. Med. 2, 34–36. 10.4314/njnpm.v2i1.11779 [DOI] [Google Scholar]
- Agbedahunsi J. M., Elujoba A. A., Makinde J. M., Oduda A. M. J. (1998). Antimalarial activity of Khaya grandifoliola stem-bark. Pharm. Biol. 36, 8–12. 10.1076/phbi.36.1.8.4613 [DOI] [Google Scholar]
- Ajaiyeoba E. O., Ashidi J. S., Okpako L. C., Houghton P. J., Wright C. W. (2008). Antiplasmodial compounds from Cassia siamea stem bark extract. Phyther. Res. 22, 254–255. 10.1002/ptr.2254 [DOI] [PubMed] [Google Scholar]
- Ajaiyeoba E. O., Ogbole O. O., Abiodun O. O., Ashidi J. S., Houghton P. J., Wright C. W. (2013). Cajachalcone: an antimalarial compound from cajanus cajan leaf extract. J. Parasitol. Res. 2013:703781. 10.1155/2013/703781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alghamdi A. H., Munday J. C., Campagnaro G. D., Gurvic D., Svensson F., Okpara C. E., et al. (2020). Positively selected modifications in the pore of TBAQP2 allow pentamidine to enter trypanosoma brucei. Elife 9:e56416. 10.7554/ELIFE.56416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhaji U. I., Samuel N. U., Aminu M., Chidi A. V., Umar Z. U., Umar U. A., et al. (2014). In vitro antitrypanosomal activity, antioxidant property and phytochemical constituents of aqueous extracts of nine Nigerian medicinal plants. Asian Pacific J. Trop. Dis. 4, 348–355. 10.1016/S2222-1808(14)60586-7 [DOI] [Google Scholar]
- Amit Koparde A., Chandrashekar Doijad R., Shripal Magdum C. (2019). Natural products in drug discovery, in Pharmacognosy - Medicinal Plants, eds Perveen S., Al-Taweel A. (IntechOpen; ). 10.5772/intechopen.82860 [DOI] [Google Scholar]
- Amoa Onguéné P., Ntie-Kang F., Lifongo L., Ndom J., Sippl W., Mbaze L. (2013). The potential of anti-malarial compounds derived from African medicinal plants, part I: a pharmacological evaluation of alkaloids and terpenoids. Malar. J. 12:449. 10.1186/1475-2875-12-449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amusan O. O. G., Adesogan E. K., Makinde J. M. (1996). Antimalarial active principles of Spathodea campanulata stem bark. Phyther. Res. 10, 692–693. [DOI] [Google Scholar]
- Baell J. B. (2015). Screening-based translation of public research encounters painful problems. ACS Med. Chem. Lett. 6, 229–234. 10.1021/acsmedchemlett.5b00032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baell J. B. (2016). Feeling nature's PAINS: natural products, natural product drugs, and Pan Assay Interference Compounds (PAINS). J. Nat. Prod. 79, 616–628. 10.1021/acs.jnatprod.5b00947 [DOI] [PubMed] [Google Scholar]
- Baell J. B., Holloway G. A. (2010). New substructure filters for removal of Pan Assay Interference Compounds (PAINS) from screening libraries and for their exclusion in bioassays. J. Med. Chem. 53, 2719–2740. 10.1021/jm901137j [DOI] [PubMed] [Google Scholar]
- Baell J. B., Nissink J. W. M. (2018). Seven year itch: Pan-Assay Interference Compounds (PAINS) in 2017—utility and limitations. ACS Chem. Biol. 13, 36–44. 10.1021/acschembio.7b00903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balogun E. O., Inaoka D. K., Shiba T., Tsuge C., May B., Sato T., et al. (2019). Discovery of trypanocidal coumarins with dual inhibition of both the glycerol kinase and alternative oxidase of Trypanosoma brucei brucei. FASEB J. 33, 13002–13013. 10.1096/fj.201901342R [DOI] [PubMed] [Google Scholar]
- Bankole A. E., Adekunle A. A., Sowemimo A. A., Umebese C. E., Abiodun O., Gbotosho G. O. (2016). Phytochemical screening and in vivo antimalarial activity of extracts from three medicinal plants used in malaria treatment in Nigeria. Parasitol. Res. 115, 299–305. 10.1007/s00436-015-4747-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekono B. D., Ntie-Kang F., Onguéné P. A., Lifongo L. L., Sippl W., Fester K., et al. (2020). The potential of anti-malarial compounds derived from African medicinal plants: a review of pharmacological evaluations from 2013 to 2019. Malar. J. 19:183. 10.1186/s12936-020-03231-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello O., Ogbesejana A., Osibemhe M. (2018). Iridoid glucosides from Vitex grandifolia displayed anti-inflammatory and antileishmania effects and structure activity relationship. J. Appl. Sci. Environ. Manag. 22:373 10.4314/jasem.v22i3.14 [DOI] [Google Scholar]
- Bello O. M., Zaki A. A., Khan S. I., Fasinu P. S., Ali Z., Khan I. A., et al. (2017). Assessment of selected medicinal plants indigenous to West Africa for antiprotozoal activity. South African J. Bot. 113, 200–211. 10.1016/j.sajb.2017.08.002 [DOI] [Google Scholar]
- Bribi N. (2018). Pharmacological activity of alkaloids: a review. Asian J. Bot. 1, 1–6. 10.1016/S2221-1691(12)60116-6 [DOI] [Google Scholar]
- Buck M., Hamilton C. (2011). The nagoya protocol on access to genetic resources and the fair and equitable sharing of benefits arising from their utilization to the convention on biological diversity. Rev. Eur. Community Int. Environ. Law 20, 47–61. 10.1111/j.1467-9388.2011.00703.x [DOI] [Google Scholar]
- Calixto J. B. (2019). The role of natural products in modern drug discovery. An. Acad. Bras. Cienc. 91(Suppl. 3):e20190105. 10.1590/0001-3765201920190105 [DOI] [PubMed] [Google Scholar]
- Carnevale Neto F., Pilon A. C., Selegato D. M., Freire R. T., Gu H., Raftery D., et al. (2016). Dereplication of natural products using GC-TOF mass spectrometry: improved metabolite identification by spectral deconvolution ratio analysis. Front. Mol. Biosci. 3:59. 10.3389/fmolb.2016.00059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter N. S., Barrett M. P., De Koning H. P. (1999). A drug resistance determinant in Trypanosoma brucei. Trends Microbiol. 7, 469–471. 10.1016/S0966-842X(99)01643-1 [DOI] [PubMed] [Google Scholar]
- Chukwujekwu J. C., Smith P., Coombes P. H., Mulholland D. A., van Staden J. (2005). Antiplasmodial diterpenoid from the leaves of Hyptis suaveolens. J. Ethnopharmacol. 102, 295–297. 10.1016/j.jep.2005.08.018 [DOI] [PubMed] [Google Scholar]
- Convention on Biological Diversity (2010). About The Nagoya Protocol on Access and Benefit Sharing. Available online at: https://www.cbd.int/abs/about/ (accessed November 21, 2020).
- Cragg G. M., Newman D. J. (2005). Biodiversity: a continuing source of novel drug leads. Pure Appl. Chem. 77, 7–24. 10.1351/pac20057701000723428572 [DOI] [Google Scholar]
- de Koning H. P. (2017). Drug resistance in protozoan parasites. Emerg. Top. Life Sci. 1, 627–632. 10.1042/etls20170113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias D. A., Urban S., Roessner U. (2012). A historical overview of natural products in drug discovery. Metabolites 2, 303–336. 10.3390/metabo2020303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dike V. T., Vihiior B., Bosha J. A., Yin T. M., Ebiloma G. U., de Koning H. P., et al. (2016). Antitrypanosomal activity of a novel taccalonolide from the tubers of tacca leontopetaloides. Phytochem. Anal. 27, 217–221. 10.1002/pca.2619 [DOI] [PubMed] [Google Scholar]
- Drugs for Neglected Diseases initiative (DNDi) (2020). Fundación MEDINA, DNDi, and Institut Pasteur Korea Announce New Funding from “la Caixa” Health Research for Research Partnership to Discover New Natural Products against Leishmaniasis and Chagas Disease. Available online at: https://dndi.org/press-releases/2020/fundacion-medina-dndi-institut-pasteur-korea-announce-new-funding-from-la-caixa-health-research-to-discover-new-natural-products-against-leishmaniasis-chagas-disease/ (accessed October 11, 2020).
- Druilhe P., Brandicourt O., Chongsuphajaisiddhi T., Berthe J. (1988). Activity of a combination of three cinchona bark alkaloids against Plasmodium falciparum in vitro. Antimicrob. Agents Chemother. 32, 250–254. 10.1128/AAC.32.2.250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebiloma G. U., Ayuga T. D., Balogun E. O., Gil L. A., Donachie A., Kaiser M., et al. (2018a). Inhibition of trypanosome alternative oxidase without its N-terminal mitochondrial targeting signal (ΔMTS-TAO) by cationic and non-cationic 4-hydroxybenzoate and 4-alkoxybenzaldehyde derivatives active against T. brucei and T. congolense. Eur. J. Med. Chem. 150, 385–402. 10.1016/j.ejmech.2018.02.075 [DOI] [PubMed] [Google Scholar]
- Ebiloma G. U., Igoli J. O., Katsoulis E., Donachie A. M., Eze A., Gray A. I., et al. (2017). Bioassay-guided isolation of active principles from Nigerian medicinal plants identifies new trypanocides with low toxicity and no cross-resistance to diamidines and arsenicals. J. Ethnopharmacol. 202, 256–264. 10.1016/j.jep.2017.03.028 [DOI] [PubMed] [Google Scholar]
- Ebiloma G. U., Katsoulis E., Igoli J. O., Gray A. I., De Koning H. P. (2018b). Multi-target mode of action of a Clerodane-type diterpenoid from Polyalthia longifolia targeting African trypanosomes. Sci. Rep. 8:4613. 10.1038/s41598-018-22908-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekpendu T. O., Akah P. A., Adesomoju A. A., Okogun J. I. (1994). Antiinflammatory and antimicrobial activities of mitracarpus scaber extracts. Pharm. Biol. 32, 191–196. 10.3109/13880209409082992 [DOI] [Google Scholar]
- El-Najjar N., Gali-Muhtasib H., Ketola R. A., Vuorela P., Urtti A., Vuorela H. (2011). The chemical and biological activities of quinones: overview and implications in analytical detection. Phytochem. Rev. 10, 353–370. 10.1007/s11101-011-9209-1 [DOI] [Google Scholar]
- Enechi O. C., Amah C. C., Okagu I. U., Ononiwu C. P., Azidiegwu V. C., Ugwuoke E. O., et al. (2019). Methanol extracts of Fagara zanthoxyloides leaves possess antimalarial effects and normalizes haematological and biochemical status of Plasmodium berghei-passaged mice. Pharm. Biol. 57, 577–585. 10.1080/13880209.2019.1656753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezenyi I. C., Salawu O. A., Kulkarni R., Emeje M. (2014). Antiplasmodial activity-aided isolation and identification of quercetin-4'-methyl ether in Chromolaena odorata leaf fraction with high activity against chloroquine-resistant Plasmodium falciparum. Parasitol. Res. 113, 4415–4422. 10.1007/s00436-014-4119-y [DOI] [PubMed] [Google Scholar]
- Falodun A., Imieje V., Erharuyi O., Joy A., Langer P., Jacob M., et al. (2014). Isolation of antileishmanial, antimalarial and antimicrobial metabolites from Jatropha multifida. Asian Pac. J. Trop. Biomed. 4, 374–378. 10.12980/APJTB.4.2014C1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Félix-Silva J., Giordani R. B., Silva-Jr A. A, Da Zucolotto S. M., Fernandes-Pedrosa M. D. F. (2014). Jatropha gossypiifolia L. (Euphorbiaceae): a review of traditional uses, phytochemistry, pharmacology, and toxicology of this medicinal plant. Evidence-based complement. Altern. Med. 2014, 1–32. 10.1155/2014/369204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill L. S. (1992). Ethnomedical Uses of Plants in Nigeria. Nigeria: University of Benin Press. [Google Scholar]
- Harvey A. (2000). Strategies for discovering drugs from previously unexplored natural products. Drug Discov. Today 5, 294–300. 10.1016/S1359-6446(00)01511-7 [DOI] [PubMed] [Google Scholar]
- Hubert J., Nuzillard J.-M., Renault J.-H. (2017). Dereplication strategies in natural product research: How many tools and methodologies behind the same concept? Phytochem. Rev. 16, 55–95. 10.1007/s11101-015-9448-7 [DOI] [Google Scholar]
- Ifeoma Chinwude E., Peter Achunike A., Charles Ogbonnaya O. (2016). Antiplasmodial Activity and some active compounds from Stachytarpheta cayennensis Vahl. (Verbenaceae) leaf fractions. Anti Infective Agents 14, 132–138. 10.2174/2211352514666160725130125 [DOI] [Google Scholar]
- Igoli J. O., Grey A. I., Clements C. J., Mouad H. A. (2011). Anti-trypanosomal activity and cytotoxicity of some compounds and extracts from nigerian medicinal plants, in Phytochemicals - Bioactivities and Impact on Health (London: IntechOpen; ), 375–388. 10.5772/26203 [DOI] [Google Scholar]
- Igoli N., Clements C., Singla R., Igoli J., Uche N., Gray A. (2014). Antitrypanosomal activity & docking studies of components of Crateva adansonii DC leaves: novel multifunctional scaffolds. Curr. Top. Med. Chem. 14, 981–990. 10.2174/1568026614666140324120006 [DOI] [PubMed] [Google Scholar]
- Igoli N., Obanu Z., Gray A., Clements C. (2011). Bioactive diterpenes and sesquiterpenes from the rhizomes of wild ginger (Siphonochilus aethiopicus (Schweinf) B.L Burtt). Afr. J. Tradit. Complement. Altern. Med. 9, 88–93. 10.4314/ajtcam.v9i1.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igoli N. P., Gray A. I., Clements C. J., Igoli J. O., Nzekwe U., Singla R. K. (2012). Scientific investigation of antitrypanosomal activity of Crateva adansonii DC leaves extracts. Indo Glob. J. 2, 226–229. http://www.iglobaljournal.com/wp-content/uploads/2012/11/1.-Ngozichukwuka-Peace-Igoli-et-al-2012.pdf [Google Scholar]
- Imieje V., Falodun A., Zaki A., Ali Z., Imieje V., Khan I., et al. (2017). Antiprotozoal and cytotoxicity studies of fractions and compounds from enantia chlorantha. Trop. J. Nat. Prod. Res. 1, 89–94. 10.26538/tjnpr/v1i2.8 [DOI] [Google Scholar]
- Irobi O. N., Daramola S. O. (1993). Antifungal activities of crude extracts of Mitracarpus villosus (Rubiaceae). J. Ethnopharmacol. 40, 137–140. 10.1016/0378-8741(93)90059-E [DOI] [PubMed] [Google Scholar]
- Iwu M. M. (2014). Handbook of African Medicinal Plants, 2nd Edn. Boca Raton, FL: CRC Press. [Google Scholar]
- Iwu M. M., Kiayman D. L. (1992). Evaluation of the in vitro antimalarial activity of Picralima nitida extracts. J. Ethnopharmacol. 36, 133–135. 10.1016/0378-8741(92)90012-G [DOI] [PubMed] [Google Scholar]
- Kassim O. O., Loyevsky M., Elliott B., Geall A., Amonoo H., Gordeuk V. R. (2005). Effects of root extracts of Fagara zanthoxyloides on the in vitro growth and stage distribution of Plasmodium falciparum. Antimicrob. Agents Chemother. 49, 264–268. 10.1128/AAC.49.1.264-268.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kildgaard S., Subko K., Phillips E., Goidts V., de la Cruz M., Díaz C., et al. (2017). A dereplication and bioguided discovery approach to reveal new compounds from a marine-derived fungus Stilbella fimetaria. Mar. Drugs 15:253. 10.3390/md15080253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kola I., Landis J. (2004). Can the pharmaceutical industry reduce attrition rates? Nat. Rev. Drug Discov. 3, 711–716. 10.1038/nrd1470 [DOI] [PubMed] [Google Scholar]
- Kren V., Martinkova L. (2001). Glycosides in medicine: “the role of glycosidic residue in biological activity”. Curr. Med. Chem. 8, 1303–1328. 10.2174/0929867013372193 [DOI] [PubMed] [Google Scholar]
- Li J. W. H., Vederas J. C. (2009). Drug discovery and natural products: end of an era or an endless frontier? Science 325, 161–165. 10.1126/science.1168243 [DOI] [PubMed] [Google Scholar]
- Li Y., Li S., Thodey K., Trenchard I., Cravens A., Smolke C. D. (2018). Complete biosynthesis of noscapine and halogenated alkaloids in yeast. Proc. Natl. Acad. Sci. U.S.A. 115, E3922–E3931. 10.1073/pnas.1721469115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lifongo L. L., Simoben C. V., Ntie-Kang F., Babiaka S. B., Judson P. N. (2014). A bioactivity versus ethnobotanical survey of medicinal plants from Nigeria, West Africa. Nat. Products Bioprospect. 4, 1–19. 10.1007/s13659-014-0005-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinski C. A., Lombardo F., Dominy B. W., Feeney P. J. (1997). Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 23, 3–25. 10.1016/S0169-409X(96)00423-1 [DOI] [PubMed] [Google Scholar]
- Lobanovska M., Pilla G. (2017). Penicillin's discovery and antibiotic resistance: lessons for the future? Yale J. Biol. Med. 90, 135–145. [PMC free article] [PubMed] [Google Scholar]
- Makinde J. M., Adesogan E. K., Amusan O. O. G. (1987). The schizontocidal activity of Spathodea campanulata leaf extract on Plasmodium berghei berghei in mice. Phyther. Res. 1, 65–68. 10.1002/ptr.2650010205 [DOI] [PubMed] [Google Scholar]
- Makinde J. M., Amusan O. O. G., Adesogan E. K. (1988a). The antimalarial activity of Spathodea campanulata stem bark extract on Plasmodium berghei berghei in mice. Planta Med. 54, 122–125. 10.1055/s-2006-962367 [DOI] [PubMed] [Google Scholar]
- Makinde J. M., Awe S. O., Agbedahunsi J. M. (1988b). Effect of Khaya grandifoliola extract on Plasmodium berghei berghei in mice. Phyther. Res. 2, 30–32. 10.1002/ptr.2650020104 [DOI] [Google Scholar]
- Melariri P., Campbell W., Etusim P., Smith P. (2011). Antiplasmodial properties and bioassay-guided fractionation of ethyl acetate extracts from Carica papaya leaves. J. Parasitol. Res. 2011:104954. 10.1155/2011/104954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesia K., Tona L., Mampunza M., Ntamabyaliro N., Muanda T., Muyembe T., et al. (2012). Antimalarial efficacy of a quantified extract of Nauclea Pobeguinii stem bark in human adult volunteers with diagnosed uncomplicated falciparum malaria. Part 2: a clinical phase IIB trial. Planta Med. 78, 853–860. 10.1055/s-0031-1298488 [DOI] [PubMed] [Google Scholar]
- Misra P., Sashidhara K. V., Singh S. P., Kumar A., Gupta R., Chaudhaery S. S., et al. (2010). 16α-hydroxycleroda-3,13 (14)Z-dien-15,16-olide from Polyalthia longifolia: a safe and orally active antileishmanial agent. Br. J. Pharmacol. 159, 1143–1150. 10.1111/j.1476-5381.2009.00609.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinski T. F., Dalisay D. S., Lievens S. L., Saludes J. P. (2009). Drug development from marine natural products. Nat. Rev. Drug Discov. 8, 69–85. 10.1038/nrd2487 [DOI] [PubMed] [Google Scholar]
- Nakanishi T., Suzuki M., Saimoto A., Kabasawa T. (1999). Structural considerations of NK109, an antitumor benzo[c]phenanthridine alkaloid. J. Nat. Prod. 62, 864–867. 10.1021/np990005d [DOI] [PubMed] [Google Scholar]
- Nawrot R., Barylski J., Nowicki G., Broniarczyk J., Buchwald W., Gozdzicka-Józefiak A. (2014). Plant antimicrobial peptides. Folia Microbiol. 59, 181–196. 10.1007/s12223-013-0280-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman D. J., Cragg G. M. (2012). Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 75, 311–335. 10.1021/np200906s [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman D. J., Cragg G. M. (2016). Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 79, 629–661. 10.1021/acs.jnatprod.5b01055 [DOI] [PubMed] [Google Scholar]
- Nnadi C., Althaus J., Nwodo N., Schmidt T. (2018). A 3D-QSAR study on the antitrypanosomal and cytotoxic activities of steroid alkaloids by comparative molecular field analysis. Molecules 23:1113. 10.3390/molecules23051113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nnadi C., Ebiloma G., Black J., Nwodo N., Lemgruber L., Schmidt T., et al. (2019). Potent antitrypanosomal activities of 3-aminosteroids against African trypanosomes: investigation of cellular effects and of cross-resistance with existing drugs. Molecules 24:268. 10.3390/molecules24020268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nnadi C., Nwodo Ngozi J., Brun R., Kaiser M., Schmidt T. (2016). Antitrypanosomal alkaloids from Holarrhena africana. Planta Med. 81, S1–S381. 10.1055/s-0036-159686628684718 [DOI] [Google Scholar]
- Nnadi C. O., Nwodo N. J., Kaiser M., Brun R., Schmidt T. J. (2017). Steroid alkaloids from Holarrhena africana with strong activity against Trypanosoma Brucei rhodesiense. Molecules 22:1129. 10.3390/molecules22071129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nok A. J. (2002). Azaanthraquinone inhibits respiration and in vitro growth of long slender bloodstream forms of Trypanosoma congolense. Cell Biochem. Funct. 20, 205–212. 10.1002/cbf.948 [DOI] [PubMed] [Google Scholar]
- Ntie-Kang F., Onguéné P., Lifongo L. L., Ndom J., Sippl W., Mbaze L. (2014). The potential of anti-malarial compounds derived from African medicinal plants, part II: a pharmacological evaluation of non-alkaloids and non-terpenoids. Malar. J. 13:81 10.1186/1475-2875-13-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nvau J. B., Alenezi S., Ungogo M. A., Alfayez I. A. M., Natto M. J., Igoli J. O., et al. (2020). Antiparasitic and cytotoxic activity of bokkosin, a novel diterpene-substituted chromanyl benzoquinone from Calliandra portoricensis. Front. Chem. 8:574103. 10.3389/fchem.2020.574103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nweze N. E., Okoro H. O., Al Robaian M., Omar R. M. K., Tor-Anyiin T. A., Watson D. G., et al. (2017). Effects of nigerian red propolis in rats infected with Trypanosoma brucei brucei. Comp. Clin. Path. 26, 1129–1133. 10.1007/s00580-017-2497-0 [DOI] [Google Scholar]
- Nwodo N., Ibezim A., Ntie-Kang F., Adikwu M., Mbah C. (2015a). Anti-trypanosomal activity of Nigerian plants and their constituents. Molecules 20, 7750–7771. 10.3390/molecules20057750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwodo N., Okoye F., Lai D., Debbab A., Kaiser M., Brun R., et al. (2015b). Evaluation of the in vitro trypanocidal activity of methylated flavonoid constituents of Vitex simplicifolia leaves. BMC Complement. Altern. Med. 15:82. 10.1186/s12906-015-0562-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwodo N. J., Brun R., Osadebe P. O. (2007). In vitro and in vivo evaluation of the antitrypanosomal activity of fractions of Holarrhena africana. J. Ethnopharmacol. 113, 556–559. 10.1016/j.jep.2007.07.018 [DOI] [PubMed] [Google Scholar]
- Nwodo N. J., Okoye F. B. C., Lai D., Debbab A., Brun R., Proksch P. (2014). Two trypanocidal dipeptides from the roots of Zapoteca portoricensis (fabaceae). Molecules 19, 5470–5477. 10.3390/molecules19055470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obodozie O. O., Okpako L. C., Tarfa F. D., Orisadipe A. T., Okogun J. I., Inyang U. S., et al. (2004). Antiplasmodial principles from Cassia nigricans. Pharm. Biol. 42, 626–628. 10.1080/13880200490902545 [DOI] [Google Scholar]
- Ogbonna J. C., Igbe I., Erharuyi O., Imieje V. O., Falodun A. (2017). Biological activities of a macrocyclic diterpenoid isolated from the roots of Jatropha gossypiifolia. J. African Assoc. Physiol. Sci. 5, 111–120. https://www.ajol.info/index.php/jaaps/article/view/164946 [Google Scholar]
- Okombo J., Ohuma E., Picot S., Nzila A. (2011). Update on genetic markers of quinine resistance in Plasmodium falciparum. Mol. Biochem. Parasitol. 177, 77–82. 10.1016/j.molbiopara.2011.01.012 [DOI] [PubMed] [Google Scholar]
- Okoro E. E., Ahmad M. S., Osoniyi O. R., Onajobi F. D. (2020). Antifungal and antileishmanial activities of fractions and isolated isoflavanquinones from the roots of Abrus precatorius. Comp. Clin. Path. 29, 391–396. 10.1007/s00580-019-03073-z [DOI] [Google Scholar]
- Okunji C. O., Iwu M. M., Ito Y., Smith P. L. (2005). Preparative separation of indole alkaloids from the rind of Picralima nitida (Stapf) T. Durand & H. Durand by pH-zone-refining countercurrent chromatography. J. Liq. Chromatogr. Relat. Technol. 28, 775–783. 10.1081/JLC-200048915 [DOI] [Google Scholar]
- Okunji C. O., Iwu M. M., Jackson J. E., Tally J. D. (1996). Biological activity of saponins from two dracaena species, in Advances in Experimental Medicine and Biology, (Boston, MA: Springer; ), 415–428. 10.1007/978-1-4899-1367-8_33 [DOI] [PubMed] [Google Scholar]
- Oluyemi W. M., Samuel B. B., Kaehlig H., Zehl M., Parapini S., D'Alessandro S., et al. (2020). Antiplasmodial activity of triterpenes isolated from the methanolic leaf extract of Combretum racemosum P. Beauv. J. Ethnopharmacol. 247:112203. 10.1016/j.jep.2019.112203 [DOI] [PubMed] [Google Scholar]
- Omar R. M. K., Igoli J., Gray A. I., Ebiloma G. U., Clements C., Fearnley J., et al. (2016). Chemical characterisation of Nigerian red propolis and its biological activity against Trypanosoma Brucei. Phytochem. Anal. 27, 107–115. 10.1002/pca.2605 [DOI] [PubMed] [Google Scholar]
- Paddon C. J., Keasling J. D. (2014). Semi-synthetic artemisinin: a model for the use of synthetic biology in pharmaceutical development. Nat. Rev. Microbiol. 12, 355–367. 10.1038/nrmicro3240 [DOI] [PubMed] [Google Scholar]
- Paddon C. J., Westfall P. J., Pitera D. J., Benjamin K., Fisher K., McPhee D., et al. (2013). High-level semi-synthetic production of the potent antimalarial artemisinin. Nature 496, 528–532. 10.1038/nature12051 [DOI] [PubMed] [Google Scholar]
- Rivaud M., Mendoza A., Sauvain M., Valentin A., Jullian V. (2012). Short synthesis and antimalarial activity of fagaronine. Bioorg. Med. Chem. 20, 4856–4861. 10.1016/j.bmc.2012.05.061 [DOI] [PubMed] [Google Scholar]
- Shameer S., Logan-Klumpler F. J., Vinson F., Cottret L., Merlet B., Achcar F., et al. (2015). TrypanoCyc: a community-led biochemical pathways database for Trypanosoma brucei. Nuc. Acids Res. 43, D637–D644. 10.1093/nar/gku944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuaibu M. N., Pandey K., Wuyep P. A., Yanagi T., Hirayama K., Ichinose A., et al. (2008a). Castalagin from Anogeissus leiocarpus mediates the killing of leishmania in vitro. Parasitol. Res. 103, 1333–1338. 10.1007/s00436-008-1137-7 [DOI] [PubMed] [Google Scholar]
- Shuaibu M. N., Wuyep P. A., Yanagi T., Hirayama K., Tanaka T., Kouno I. (2008b). The use of microfluorometric method for activity-guided isolation of antiplasmodial compound from plant extracts. Parasitol. Res. 102, 1119–1127. 10.1007/s00436-008-0879-6 [DOI] [PubMed] [Google Scholar]
- Shuaibu M. N., Wuyep P. T. A., Yanagi T., Hirayama K., Ichinose A., Tanaka T., et al. (2008c). Trypanocidal activity of extracts and compounds from the stem bark of Anogeissus leiocarpus and Terminalia avicennoides. Parasitol. Res. 102, 697–703. 10.1007/s00436-007-0815-1 [DOI] [PubMed] [Google Scholar]
- Strohl W. R. (2000). The role of natural products in a modern drug discovery program. Drug Discov. Today 5, 39–41. 10.1016/S1359-6446(99)01443-9 [DOI] [PubMed] [Google Scholar]
- Tonkens R. (2005). An overview of the drug development process. Physician Exec. 31, 48–52. [PubMed] [Google Scholar]
- Tsuruta H., Paddon C. J., Eng D., Lenihan J. R., Horning T., Anthony L. C., et al. (2009). High-level production of amorpha-4,11-diene, a precursor of the antimalarial agent artemisinin, in Escherichia coli. PLoS ONE 4:e4489. 10.1371/journal.pone.0004489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu Y. (2011). The discovery of artemisinin (qinghaosu) and gifts from Chinese medicine. Nat. Med. 17, 1217–1220. 10.1038/nm.2471 [DOI] [PubMed] [Google Scholar]
- Vijayakumar S., Ranjan R., Kumar R., Das P. (2020). TrypInDB: a searchable online resource of small molecule inhibitors against Trypanosoma sp. Parasitol. Int. 78:102131. 10.1016/j.parint.2020.102131 [DOI] [PubMed] [Google Scholar]
- Wagner H. (2011). Synergy research: approaching a new generation of phytopharmaceuticals. Fitoterapia 82, 34–37. 10.1016/j.fitote.2010.11.016 [DOI] [PubMed] [Google Scholar]
- Walsh C. T., Yang T. (2017). Natural Product Biosynthesis: Chemical Logic and Enzymatic Machinery. London: Royal Society of Chemistry. [Google Scholar]
- Wan Y. D., Zang Q. Z., Wang J. S. (1992). Studies on the antimalarial action of gelatin capsule of Artemisia annua. Chinese J. Parasitol. Parasit. Dis. 10, 290–294. [PubMed] [Google Scholar]
- Williamson E. (2001). Synergy and other interactions in phytomedicines. Phytomedicine 8, 401–409. 10.1078/0944-7113-00060 [DOI] [PubMed] [Google Scholar]
- World Health Organization (2019). World Malaria Report 2019. Available online at: https://www.who.int/publications/i/item/9789241565721; https://www.who.int/news-room/fact-sheets/detail/malaria
- Wright C. W., Linley P. A., Brun R., Wittlin S., Hsu E. (2010). Ancient Chinese methods are remarkably effective for the preparation of artemisinin-rich extracts of Qing Hao with potent antimalarial activity. Molecules 15, 804–812. 10.3390/molecules15020804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright G. D. (2019). Unlocking the potential of natural products in drug discovery. Microb. Biotechnol. 12, 55–57. 10.1111/1751-7915.13351 [DOI] [PMC free article] [PubMed] [Google Scholar]