Abstract
Lake Van fish (Alburnus tarichi Guldenstadt 1814) is the only fish that can adapt to the extreme conditions (pH 9.8 salinity 0.2% and alkalinity 151.2 meq/L) of Lake Van. In this study, it was aimed to determine the cytotoxic and genotoxic effects of chlorpyrifos (CPF) on Lake Van fish primary gill cell culture. Gill epithelium from Lake Van fish was isolated enzymatically and grown in primary culture on Leibovitz’s L-15 medium. After different doses (0.01, 0.1, 1, and 10 μM) of CPF were applied to the gill cells, the total oxidant status (TOS), total antioxidant status (TAS), malondialdehyde (MDA), and DNA damage levels (8-hydroxyguanine (8-OHdG)) were examined at the end of 24 and 48 h. It was determined that the TOS, MDA, and 8-OHdG levels increased in the cells exposed to high doses (1 and 10 μM) of CPF and the TAS was decreased (P < 0.05). It was revealed from this study that CPF administered at a dose higher than 1 μM can cause oxidative stress and DNA damage in the primary gill cell culture of Lake Van fish. In addition, the findings showed that Lake Van fish primary gill cell culture was useful in determining the effects of toxic substances likely to be the contaminants of a lake.
Keywords: soda lake, pesticide, oxidative stress, MDA, DNA damage
Graphical Abstract
Graphical Abstract.
Introduction
Pesticide pollution increases day by day around the world. As with other anthropogenic pollutants, pesticides can accumulate in soil and aquatic ecosystems when they are overused [1]. Chlorpyrifos (CPF) (O, O-diethyl-O-3,5,6-trichlor-2-pyridyl phosphorothioate) is one of the most commonly used organic phosphorus insecticides and it is used extensively in the fight against insects all over the world. It has been used in domestic areas in addition to agricultural areas. CPF is used to control agricultural pests, including termites and mosquitoes, which are associated with vegetable, and fruit fields in Turkey [2]. The target organ of insects affected by CPF is nerve tissue. Like other organic phosphorous insecticides, it inhibits acetylcholinesterase and converts it into choline and acetate [3]. Although CPF is used to fight against insects, it also affects nontarget animals, such as fish, which are the group most affected by pesticides [4–6]. CPF causes histopathology and dysfunction, and neurobehavioral and neurochemical changes in vital organs, such as the gills, brain, and liver [3, 7–9]. In previous studies, it has been stated that CPF causes an increase in reactive oxygen species and a decrease in antioxidant enzyme levels in fish [8, 10–12].
Gills of fish are an important organ in which important physiological events, such as osmoregulation, nitrogenous waste disposal, ionoregulation, and acid–base balance, occur. This organ consists of mucus, respiratory, mitochondria rich, pilar, and neuroepithelial cells [13]. Gills are in contact with the external environment, they are directly affected by many stress factors and toxic substances in aquatic areas [14]. Gill surfaces make up 50% of the total surface in fish [15]. Therefore, biochemical and enzymatic changes in the gills can serve as useful biomarkers to assess pollution in aquatic environments.
Lake Van is one of the world’s largest soda lakes, and it is also the largest lake in Turkey [16]. Lake Van fish is the only fish species that lives in Lake Van, and it has great economic importance for the people of the region. Fish is a cheap source of protein in Van and approximately 10 000 tons of fish is caught there every year. Many pollutants leech into Lake Van from the surrounding settlements and agricultural areas [17, 18]. In addition, it was determined in a study by Aksoy et al. [19] that Lake Van fish were contaminated with organochlorine pesticides and polychlorinated biphenyls, although at a low level. In recent years, a decrease in the fish population has been observed; hence, Lake Van fish has been included on the IUCN red list [20].
One of the most commonly used organic phosphorus pesticides in the Lake Van basin is CPF. No research could be found regarding the level of CPF in the lake and its effects on Lake Van fish. The objective of this study was to evaluate the effects of CPF on the oxidative stress biomarkers in the primary gill cell culture of Lake Van fish. Since the number of Lake Van fish has decreased substantially, primary gill cell culture was used as a tool to evaluate the effects of CPF on the gills, instead of conducting experiments directly on the fish. This made it possible to save a great number of fish.
Material and methods
Fish
In this study, fish of an average length (17.5–19.5 cm) and weight (about 80–110.5 g) were caught using casting nets from streams during the spawning season. The fish were acclimated for 1 week prior to starting the experiment. During acclimation, the fish were kept in chlorinated and air stone-bound tanks under 24°C static conditions with filtered water, and a light/dark photoperiod of 12:12. The fish were fed commercial trout feed once a day during the experiment.
All of the animal experimental procedures were performed in accordance with the animal study protocols approved by the Animal Researchers Local Ethics Committee of Van Yüzüncü Yıl University (2018/08).
The effects of different concentrations (0.01, 0.1, 1, and 10 μM) of CPF were examined for 24 and 48 h on the oxidative stress biomarkers in the primary gill cell culture of Lake Van fish.
Primary fish gill cell culture
Lake Van fish gill epithelial cells were isolated using the method described by Pärt et al. [21] with minor modifications. Euthanasia was performed by cutting off the fish heads in such a way that the anesthetic materials did not affect the study material. The gill archs were removed and placed into phosphate buffered saline (PBS) (136.9 mM of NaCl, 8.06 mM of Na2PO4, 2.68 mM of KCl, and 1.47 mM of KH2PO4). Gill filaments were cut into small pieces with a sharp scalpel and treated with PBS that contained antibiotic-antimycotic solution (A5955, Sigma-Aldrich, St. Louis, MO, USA) at 18°C. The filaments were placed into a solution that contained trypsin enzyme and kept in a shaker for 20 min, and this process was repeated twice. The cell suspension was passed through an 80-μm filter and then placed into PBS that contained fetal bovine serum (FBS). The suspension was centrifuged at 250X g. The cells were washed with PBS that contained 2% FBS and centrifuged at 250X g for 8 min. The cells were suspended with medium that contained 5% FBS and antibiotic-antimycotic, and then cultured in 48-well cell culture dishes (Greiner Bio-One, Germany). After staining with the trypan blue from the isolated cell suspension, a dead-live cell count was performed under a light microscope. The pH change and microbial contamination were determined via microscopic observations in the cell culture vessels until the end of the experiment. CPF was added to the cells at does of 0.01, 0.1, 1, and 10 μM. The proportion of acetone used as a solvent did not exceed 1% in the medium.
Gill cells exposed to different concentrations of CPF were trypsinized after 24 and 48 h. The cells were then centrifuged at 2000 g for 5 min. At the end of centrifugation, the supernatant was discarded and the cell pellet was washed with 1 ml of PBS. The gill cell was homogenized and the cell pellet was again centrifuged at 2000 g for 5 min. The supernatants were stored at −80°C in a deep freezer until the biochemical analyses were performed.
Measurement of the total oxidant and antioxidant status
Total oxidant status (TOS) and total antioxidant status (TAS) assays were performed on the cell culture extracts using commercially available kits (Rel Assay Diagnostics, Gaziantep, Turkey). The TOS assay was calibrated with hydrogen peroxide and the results were expressed in terms of micromolar hydrogen peroxide equivalent per liter (μmol H2O2 Equiv./L). The TAS was expressed as mmol Trolox equiv./L [22, 23].
Malondialdehyde analysis
The method of Jain et al. [24], which was based on thiobarbituric acid reactivity, was used to determine the malondialdehyde (MDA) levels within the gill culture extracts. In Eppendorf tubes, from each of the gill cell extracts, 200 μl of supernatant was added to 800 μl of phosphate buffer at a pH of 7.4, followed by 500 μl of trichloroacetic acid (30%) and 25 μl of butylated hydroxytoluene, and then mixed. After 2 h of incubation at −20°C, centrifugation of the mixture at 400 g was performed for 15 min. Next, 1 ml of the supernatant was transferred into a different Eppendorf tube, and to that, 250 μl of TBA (1%) and 75 μl of 0.1 M EDTA were added. The tubes, which had screw caps lined with Teflon, were then incubated in a water bath at 90°C for 15 min, followed by cooling until they reached room temperature. For the gill cells, measurement of the optical density was performed using a Shimadzu UV-1800 spectrophotometer (Tokyo, Japan) at 532 and 600 nm.
Determination of 8-hydroxy deoxyguanosine
Preparation of all of the standard solutions, reagents, and samples was performed following the manufacturer’s instructions (Bioassay Technology Laboratory, Shanghai, China). Before being used, all of the reagents were allowed to equilibrate at room temperature. Room temperate was also used to perform the essays. First, into each well, 50 μl of the standard solutions were added. After that, 50 μl of streptavidin-HRP (as the standard) and 10 μl of anti-8-hydroxyguanine (8-OHdG) antibody were added to samples that had been transferred into individual sample wells. Following 1 h of incubation at 37°C, the seals were removed, and the plates were washed five times with washing buffer. After blotting the plates onto paper towels, to each well, 50 μl of substrate solutions A and B were added. Following resealing, the plates were incubated at 37°C for 10 min in the dark. Next, into each well, stop solution was added, followed by an immediate change of color from blue to yellow in the samples. Within 30 min of adding the stop solution, in each sample, a microplate reader (Dasitaly, Rome, Italy) was used to determine the optical density at 450 nm.
Statistical analysis
The data were expressed as the mean ± standard error of the mean (SEM). The data were analyzed using one-way ANOVA, followed by Duncan post hoc test comparison to determine differences between the groups. Statistical analyses were performed using IBM SPSS Statistics for Windows 21.0 (IBM Corp., Armonk, NY, USA). Statistical significance was accepted as P < 0.05.
Results
Gill cell isolation and culture
Morphological characteristics of the gill cells of Lake Van fish were visualized under inverted light microscopy. The results showed that, after cell isolation, erythrocytes were also found in the gill cell culture. It was observed that the cells placed into the Petri dishes were round in shape and kept their shape during the culture. The cells also adhered to the base of the poly-L-Lysine-coated Petri dishes within hours, and the dead cells and cell wastes were suspended in the medium.
TAS and TOS analysis
The TOS and TAS are shown in Fig. 1A and B, respectively. The TAS levels were significantly higher in the gill cells at the end of 24 and 48 h when compared to the control group (P < 0.05). A decrease in the TAS was observed with the highest dose of CPF (10 μM) in contrast to the TOS (P < 0.05). However, there were no significant differences between the other CPF dosages after 24 and 48 h (P > 0.05).
Figure 1.
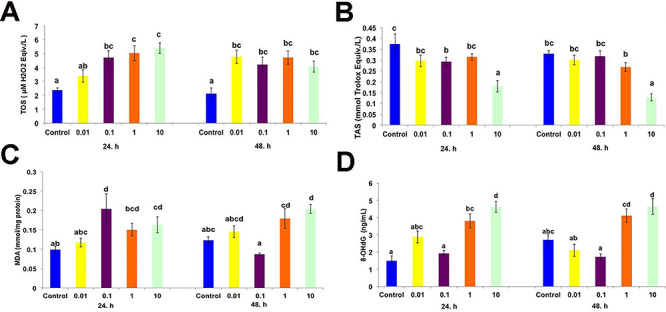
(A) Effects of different doses of chlorpyrifos on the TOS (µM H2O2 Eqiv./L), (B) TAS (mmol Trolox Eqiv./L), (C) malondialdehyde (mmol/mg protein), and (D) 8-OHdG (ng/mL) in primary gill cell culture after 24 and 48 h. Each value is the mean ± SEM (n = 6). Different letters indicate statistically significant differences between the groups.
Lipid peroxidation and DNA damage
MDA and 8-OHdG levels in the gill cells of Lake Van fish with different doses of CPF are shown in Fig. 1C and D, respectively. The MDA level increased only with the highest dose of CPF (10 μM) after 24 and 48 h (P < 0.05). DNA damage also increased with high doses of CPF (P < 0.05).
Discussion
Free radical production causes cell damage when it exceeds the endogenous antioxidant level. This damage occurs on large biomolecules, such as lipids, proteins and nucleic acids, in the cell. In many fish species, organic phosphorous pesticides have been shown to increase reactive oxygen species in tissues and cause cell damage [8, 25]. In this study, it was observed that the total oxidant level (Fig. 1A) in primary gill cell culture increased in the groups treated with different doses of CPF, while the total antioxidant level (Fig. 1B) decreased. The decrease in the total antioxidant level may have been due to the prevention of toxicity caused by reactive oxygen species. In biological systems, antioxidant enzymes, such as superoxide dismutases, catalases, and glutathione peroxidases, protect cells from oxidative damage [26, 27]. In this study, similar to previous in vivo and in vitro studies with fish, CPF induced oxidative stress by causing an increase in reactive oxygen species.
One of the indicators of cellular damage observed as a result of oxidative stress is the peroxidation of unsaturated fatty acids. Lipids are probably the most sensitive when compared to other macromolecules. An increased level of, MDA, a lipid peroxidation product, in cells exposed to CPF has been reported in in vivo and in vitro studies [11, 28]. Contrary to these results, while glutathione S-transferase activity from antioxidant enzymes increased in tilapia gonads exposed to CPF, there was no change in the level of lipid peroxidation [10]. In the present study, it was clearly shown that CPF increased the levels of MDA, depending on the dose, by inducing oxidative stress in the gill cells (Fig. 1C). The reason for the increase in the MDA level can be explained as the decrease of antioxidant enzymes (Fig. 1B) and the protective effect of these enzymes in the cell.
Mitkovska and Chassovnikarova [29] stated that CPF causes DNA and nuclear damage in fish, even below the concentrations considered as acceptable by the European legislation. 8-OHdG is a biomarker of oxidative damage caused by free radicals on nuclear and mitochondrial DNA [30]. High doses of CPF (1–10 μM) resulted in gill cell DNA damage, while other doses had no effect (P < 0.05) (Fig. 1D). Ali et al. [31] demonstrated that CPF induced a significantly higher number of micronuclei formation, which increased with the concentrations and durations.
The decrease in the Lake Van fish population and the reason for its red listing were stated as the result of the destruction of their habitats and overfishing [20]. The effects of pollutants in the lake and agricultural chemicals carried by the streams pouring into the lake on Lake Van fish have been ignored. Analyses performed on the lake surface water and sediments over recent years have been minimal when compared to those in developed countries [17, 18]. There are no current data on the pollution of the lake. In this study, it was observed that CPF had a highly toxic effect on the gill cells, which are vital for fish. It is necessary to determine the accumulation of CPF in the water, sediments, and fish in the Lake Van basin to evaluate the influx of CPF from the adjoining agricultural fields. In addition, limiting the use of CPF is important for the fish population.
Acknowledgments
The author kindly thanks Burcu Ergöz and Ayşenur Kıraççakalı for their help with the laboratory studies. This study was supported by Van Yüzüncü Yıl University Scientific Research Projects Coordination Unit (Project number: FBA-2019-7491).
Contributor Information
Ahmet Regaib Oğuz, Department of Science Faculty, Department of Biology, Van Yüzüncü Yıl University, Van, Turkey.
Elif Kaval Oğuz, Department of Science Education, Van Yüzüncü Yıl University, Van, Turkey.
Necati Özok, Department of Science Faculty, Department of Biology, Van Yüzüncü Yıl University, Van, Turkey.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- 1. Diez MC. Biological aspects involved in the degradation of organic pollutants. J Soil Sci Plant Nutr 2010;10:244–67. [Google Scholar]
- 2. Yücel Ü, Ylim M, Gözek K et al. Chlorpyrifos degradation in Turkish soil. J Environ Sci Health B 1999;34:75–95. [DOI] [PubMed] [Google Scholar]
- 3. Eaton DL, Daroff RB, Autrup H et al. Review of the toxicology of chlorpyrifos with an emphasis on human exposure and neurodevelopment. Crit Rev Toxicol 2008;38:1–125. [DOI] [PubMed] [Google Scholar]
- 4. Deb N, Das S. Chlorpyrifos toxicity in fish: a review. Curr World Environ 2013;8:77. [Google Scholar]
- 5. Wang L, Wang L, Shi X et al. Chlorpyrifos induces the apoptosis and necroptosis of L8824 cells through the ROS/PTEN/PI3K/AKT axis. J Hazard Mater 2020;398:122905. [DOI] [PubMed] [Google Scholar]
- 6. Wang S, Zheng S, Zhang Q et al. Atrazine hinders PMA-induced neutrophil extracellular traps in carp via the promotion of apoptosis and inhibition of ROS burst, autophagy and glycolysis. Environ Pollut 2018;243:282–91. [DOI] [PubMed] [Google Scholar]
- 7. Rao JV, Begum G, Pallela R et al. Changes in behavior and brain acetylcholinesterase activity in mosquito fish, Gambusia affinis in response to the sub-lethal exposure to chlorpyrifos. Int J Environ Res Public Health 2005;2:478–483. [DOI] [PubMed] [Google Scholar]
- 8. Kavitha P, Rao JV. Toxic effects of chlorpyrifos on antioxidant enzymes and target enzyme acetylcholinesterase interaction in mosquito fish, Gambusia affinis. Environ Toxicol Pharmacol 2008;26:192–198. [DOI] [PubMed] [Google Scholar]
- 9.. Altun S, Özdemir S, Arslan H. Histopathological effects, responses of oxidative stress, inflammation, apoptosis biomarkers and alteration of gene expressions related to apoptosis, oxidative stress, and reproductive system in chlorpyrifos-exposed common carp (Cyprinus carpio L.). Environ Pollut 2017;230:432–43. [DOI] [PubMed] [Google Scholar]
- 10.. Oruc E. Oxidative stress responses and recovery patterns in the liver of Oreochromis niloticus exposed to chlorpyrifos-ethyl. Bull Environ Contam Toxicol 2012;88:678–684. [DOI] [PubMed] [Google Scholar]
- 11.. Özkan F, Gündüz SG, Berköz M et al. The protective role of ascorbic acid (vitamin C) against chlorpyrifos-induced oxidative stress in Oreochromis niloticus. Fish Physiol 2012;38:635–643. [DOI] [PubMed] [Google Scholar]
- 12.. Palanikumar L, Kumaraguru AK, Ramakritinan CM et al. Toxicity, biochemical and clastogenic response of chlorpyrifos and carbendazim in milkfish Chanos chanos. Int J Environ Sci Technol 2014;11:765–774. [Google Scholar]
- 13.. Wilson JM, Laurent P. Fish gill morphology: inside out. J Exp Zool 2002;293:192–213. [DOI] [PubMed] [Google Scholar]
- 14.. Bury NR, Schnell S, Hogstrand C. Gill cell culture systems as models for aquatic environmental monitoring. J Exp Biol 2014;217:639–650. [DOI] [PubMed] [Google Scholar]
- 15.. Wood CM. Toxic responses of the gill In: Schlenk D, Benson WH (eds). Target Organ Toxicity in Marine and Freshwater Teleosts. London: Taylor and Francis, 2001, 1–89. [Google Scholar]
- 16.. Danulat E, Selçuk B. Life history and environmental conditions of the anadromous Chalcalburnus tarichi (Cyprinidae) in the highly alkaline Lake Van, eastern Anatolia, Turkey. Arch Hydrobiol 1992;126:105–125. [Google Scholar]
- 17.. Ünal G, Türkoğlu V, Oğuz AR et al. Gonadal histology and some biochemical characteristics of Chalcalburnus tarichi (Pallas, 1811) having abnormal gonads. Fish Physiol Biochem 2007;33:153–165. [Google Scholar]
- 18.. Oğuz AR, Kankaya E. Determination of selected endocrine disrupting chemicals in Lake Van, Turkey. Bull Environ Contam Toxicol 2013;91:283–286. [DOI] [PubMed] [Google Scholar]
- 19.. Aksoy A, Das YK, Yavuz O et al. Organochlorine pesticide and polychlorinated biphenyls levels in fish and mussel in Van region, Turkey. Bull Environ Contam Toxicol 2011;87:65–69. [DOI] [PubMed] [Google Scholar]
- 20.. Freyhof J. Alburnus tarichi. The IUCN Red List of Threatened Species 2014. https://dx.doi.org/10.2305/IUCN.UK.2014-1.RLTS.T4375A19222678.en (10 September 2020, date last accessed).
- 21.. Part P, Norrgren L, Bergstrom E et al. Primary cultures of epithelial cells from rainbow trout gills. J Exp Biol 1993;175:219–32. [Google Scholar]
- 22.. Erel O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem 2004;37:277–285. [DOI] [PubMed] [Google Scholar]
- 23.. Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem 2005;38:1103–1111. [DOI] [PubMed] [Google Scholar]
- 24.. Jain SK, McVie R, Duett J et al. Erythrocyte membrane lipid peroxidation and glycosylated hemoglobin in diabetes. Diabetes 1989;38:1539–43. [DOI] [PubMed] [Google Scholar]
- 25.. Monteiro DA, De Almeida JA, Rantin FT et al. Oxidative stress biomarkers in the freshwater characid fish, Brycon cephalus, exposed to organophosphorus insecticide Folisuper 600 (methyl parathion). Comp Biochem Physiol C Toxicol Pharmacol 2006;143:141–149. [DOI] [PubMed] [Google Scholar]
- 26.. Davies KJ. Oxidative stress, antioxidant defenses, and damage removal, repair, and replacement systems. IUBMB Life 2000;50:279–289. [DOI] [PubMed] [Google Scholar]
- 27.. Slaninova A, Smutna M, Modra H et al. A review: oxidative stress in fish induced by pesticides. Neuro Endocrinol Lett 2009;30:2. [PubMed] [Google Scholar]
- 28.. Gultekin F, Ozturk M, Akdogan M. The effect of organophosphate insecticide chlorpyrifos-ethyl on lipid peroxidation and antioxidant enzymes (in vitro). Arch Toxicol 2000;74:533–538. [DOI] [PubMed] [Google Scholar]
- 29.. Mitkovska V, Chassovnikarova T. Chlorpyrifos levels within permitted limits induce nuclear abnormalities and DNA damage in the erythrocytes of the common carp. Environ Sci Pollut Res Int 2020;27:7166–7176. [DOI] [PubMed] [Google Scholar]
- 30.. Valavanidis A, Vlachogianni T, Fiotakis C. 8-hydroxy-2′-deoxyguanosine (8-OHdG): a critical biomarker of oxidative stress and carcinogenesis. J Environ Sci Health C 2009;27:120–139. [DOI] [PubMed] [Google Scholar]
- 31.. Ali D, Nagpure NS, Kumar S et al. Assessment of genotoxic and mutagenic effects of chlorpyrifos in freshwater fish Channa punctatus (Bloch) using micronucleus assay and alkaline single-cell gel electrophoresis. Food Chem Toxicol 2009;47:650–656. [DOI] [PubMed] [Google Scholar]


