Abstract
Background: JWH-018 was the first synthetic cannabinoid introduced as a legal high and the first of the new generation of novel psychoactive substances that flooded worldwide drug markets. JWH-018 was marketed as “spice,” “herbal incense,” or “herbal blend,” as a popular and legal (at the time) alternative to cannabis (marijuana). JWH-018 is a potent synthetic cannabinoid with considerable toxicity associated with its use. JWH-018 has qualitatively similar but quantitatively greater pharmacological effects than cannabis, leading to intoxications and even deaths. The mechanisms of action of the drug’s toxicity require research, and thus, the aim of the present study was to investigate the toxicological profile of JWH-018 in human SH-SY5Y neuronal cells. Methods: SH-SY5Y neuronal cells were exposed to increasing concentrations from 5 to 150 μM JWH-018 over 24 h. Cytotoxicity, DNA damage, the apoptotic/necrotic rate, and oxidative stress were assessed following SH-SY5Y exposure. Results: JWH-018 did not produce a significant decrease in SH-SY5Y cell viability, did not alter apoptotic/necrotic rate, and did not cause genotoxicity in SH-SY5Y cells with 24-h exposure. Glutathione reductase and catalase activities were significantly reduced; however, there was no significant change in glutathione peroxidase activity. Also, JWH-018 treatment significantly decreased glutathione concentrations, significantly increased protein carbonylation, and significantly increased malondialdehyde (MDA) concentrations. For significance, all P < 0.05. Discussion/Conclusion: JWH-018 produced oxidative stress in SH-SY5Y cells that could be an underlying mechanism of JWH-018 neurotoxicity. Additional in vivo animal and human-based studies are needed to confirm our findings.
Keywords: JWH-018, synthetic cannabinoid, toxicity, in vitro, oxidative stress
Introduction
Synthetic cannabinoids (SCs), one of the largest classes of new psychoactive substances (NPS), mimic the effects of cannabis or marijuana, and their use is an important global public health concern. Legal authorities constantly update their regulations to include SC as scheduled or controlled substances. The term NPS refers to SCs, synthetic cathinones, synthetic opioids, synthetic benzodiazepines, tryptamines, hallucinogens, and other drugs producing qualitatively similar but usually more potent drugs with minor structural changes to avoid drug scheduling. SCs were first developed in the 1970’s as pharmacological tools for studying the endogenous cannabinoid neurotransmitter system, and to investigate cannabinoids as pharmacotherapies for cancer treatment and pain management [1, 2]. In 2008, SCs were first reported in Germany in the analysis of smoking blends named “Spice,” “K2,” “Yucatan,” “Chill,” “Black Mamba,” and many other names. They are marketed as “herbal incense” and “herbal blends” and are labeled as “not smokable” and “not for human consumption,” providing popular and legal alternatives to cannabis [3, 4].
SCs are agonists at CB1 and/or CB2 cannabinoid receptors. CB1 receptors are located primarily in the central nervous system, but also in the periphery, while CB2 receptors are found primarily in the periphery in the immune system, although they are found in low density in the brain. Stimulation of CB1 cannabinoid receptors produces cannabis’ euphoric and cognitive effects, while the stimulation of CB2 receptors is involved in the body’s defence against infectious agents and other less understood functions. The ability of a ligand to bind and act as an agonist at the CB1 receptor provides an alternative to cannabis to produce the “psychoactive high” [5–11]. There are few data from controlled human SC administration studies for safety and ethical reasons, with most of the pharmacological data about their toxicity being generated from emergency department and drug treatment reports and forensic case studies. SC users frequently enter the hospital with severe adverse effects including hypertension, tachycardia, dysrhythmias, chest pain, minor elevation of blood glucose, hypokalaemia, hallucinations, seizures, myoclonia agitation, acute psychosis, and even death [12–19]. In addition, there is evidence that chronic SC use leads to the development of dependence, tolerance, and withdrawal [20–22].
JWH-018 [1-pentyl-3-(1-naphthoyl) indole] was the first SC identified in illicit herbal mixtures [4, 8]. It is a full cannabinoid agonist indole, first synthesized by John W. Huffman in the early 1990s for research purposes to investigate the endogenous cannabinoid system [20]. JWH-018 binds to CB1 and CB2 receptors with a Ki of 9.0 and 2.9 nM affinities, respectively, compared with a THC’s affinities of 40.7 and 36.4 nM, respectively. This demonstrates that JWH-018 has a 5-fold increased affinity for the CB1-cannabinoid receptor compared with THC [23, 24]. JWH-018’s primary phase I human metabolites are formed by the oxidation of the indole ring or the N-alkyl side-chain to form mono-hydroxylated metabolites, which exhibit equal or greater affinity to CB1 receptors than THC [25–31].
Poklis et al. [32] studied the JWH-018 disposition in blood and brain of mice after controlled exposure to smoke from an herbal incense product. They found high concentrations of JWH-018 in the brain compared with the blood due to its highly lipophilic character. In JWH-018 intoxication cases, significant neurobehavioral symptoms were described including anxiety, extreme agitation, generalized or local seizures, psychosis, paranoia, delirium, and hallucinations [13, 33–36].
There are few data on the toxicology of JWH-018. According to Koller et al. [37], JWH-018 showed in vitro cytotoxicity at the highest 100 μM concentration in the mammary line (MCF-7) and buccal epithelial cells (TR146). Tomiyama and Funada [38] documented JWH-018-induced cytotoxicity on primary neuronal cells of the forebrain in a concentration-dependent manner. Couceiro et al. [39] showed that a JWH-018 phase I N-(3-hydroxypentyl metabolite is toxic for the human embryonic kidney (HEK283T) and human neuroblastoma (SH-SY5Y) cell lines, in contrast to its parent. Thus, the aim of this research was to investigate the in vitro neurotoxicity of JWH-018 in SH-SY5Y cells.
Materials and Methods
Chemicals
Minimum Essential Medium (MEM), F-12 Nutrient Medium, fetal bovine serum (FBS), phosphate buffer saline (PBS), penicillin–streptomycin (10 000 units/mL) solution, and trypsin–EDTA solution were purchased from Gibco Invitrogen Corp. (UK). JWH-018 purity was ≥98.5% and was obtained from Lipomed AG (Switzerland). All other chemicals were analytical grade and acquired from Sigma-Aldrich (USA) unless otherwise specified.
Cell culture and treatments
The human neuroblastoma SH-SY5Y cell line is a suitable human neuronal cell model for studying neuronal toxicity. The American Tissue Culture Collection was cultured in a 1:1 solution of Eagle’s MEM and Ham’s F12 nutrient containing 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin. Cells were maintained in a humidified atmosphere with 95% air and 5% CO2 at 37°C, and passaged every 3 days by trypsinization. For all experiments, cells were treated with JWH-018 at 5, 10, 25, 50, 100, or 150 μM concentration for 24 h.
Cell viability assays
Cell viability change was determined by using 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), Neutral Red Uptake (NRU), and Lactate Dehydrogenase (LDH) assays. In the MTT assay, the tetrazolium salt was reduced by viable cells [40]. Cells were seeded at 1 × 104 per well in 96-well plates and allowed to adhere for 24 h before JWH-018 treatment. After JWH-018 exposure for 24 h, cells were incubated with 5 mg/mL MTT-medium for 3 h at 37°C in the dark. After 3 h, the medium was removed and 100 μL DMSO was added to dissolve the purple formazan crystals and absorbance measured at 590 nm using a microplate reader (Biotek, Epoch, USA).
In the NRU assay, neutral red dye uptake by functional lysosomes of viable cells was measured [41]. Briefly, 1 × 104 cells/well were seeded in 96 well plates and grown for 24 h. The cells were treated with JWH-018 for 24 h, the medium in each well was removed and replaced with 150 μL medium containing 50 μg/mL neutral red dye, and incubated for 3 h at 37°C. After incubation, wells were washed with PBS and neutral red dye was solubilized with 150 μL glacial acetic acid:ethanol:water [1:49:50] per well. After 20 min of gentle shaking, optical density was determined at 540 nm with a microplate reader.
LDH is a cytoplasmic enzyme that is rapidly released into the supernate when cell membranes are damaged. Extracellular LDH concentrations were evaluated to determine the integrity of cellular membranes. SH-SY5Y cells were seeded at 1 × 104 per well in 96-well plates and allowed to adhere for 24 h before JWH-018 treatment for 24 h. The medium was collected and cellular membrane integrity evaluated using a commercially available kit (RayBiotech, Inc. USA) according to the instructions provided by the manufacturer. Absorbance was measured at 495 nm with a microplate reader and the percentage of total LDH release was determined from the following equation:
LDH release (%) = [(Test Sample − Negative Control)/(Positive Control − Negative Control)] × 100.
For all cytotoxicity assays, 1% DMSO-treated cells were the negative control and 0.1% Triton X-100-treated cells were the positive control.
Assessment of genotoxicity
The comet assay examined JWH-018 genotoxic activity as previously described with minor modifications [42, 43]. Migration of DNA strand breaks was visualized after cells were embedded and lysed in agarose on a microscope slide in response to an electric field. For this purpose, SH-SY5Y cells were grown at 5 × 105 cells in 6-well plates and treated with JWH-018 for 24 h. Also, positive and negative controls, 50 μM hydrogen peroxide and 1% DMSO, respectively, were included. Assessment of the DNA damage was performed with a fluorescence microscope (Olympus BX53, Olympus, Japan) at 400X magnification, equipped with the imaging system comet Assay IV (Perceptive Instruments, UK). For every group, 100 cells were scored and DNA damage was expressed as the percentage of the DNA in the comet tail (% tail intensity).
Assessment of apoptosis and necrosis
To determine whether the JWH-018 treatments induced apoptosis, the Annexin V and propidium iodide (PI) assays were completed. Cells were seeded into 6-well plates in a density of 5 × 105 cells/well and treated with JWH-018 for 24 h. Cells were moved by trypsinization and centrifuged at 1500 rpm for 5 min. Cell pellets were mixed with assay buffer and Annexin V and PI added. After incubation for 5 min in the dark, cell suspensions were placed on glass slides, covered with coverslips, and immediately observed under a fluorescent microscope (Olympus BX53, Olympus, Japan). Annexin V positive/PI negative cells were early apoptotic cells, Annexin V/PI double positive cells were late apoptotic cells, and Annexin V negative/PI positive cells were necrotic cells. About 500 cells were counted for each concentration. Results were expressed as the percent of the total number of cells.
Oxidative damage parameters
To evaluate oxidative damage, SH-SY5Y cells were cultured in 25-cm2 flasks [2]. After treatment with 5–150 μM JWH-018, cells were suspended in ice-cold PBS, homogenized by sonication, centrifuged at 14 000 g for 10 min at 4°C, and the supernatant evaluated for oxidative damage.
Glutathione peroxidase (GPx) and Glutathione reductase (GR) activities were measured by EnzyChrom™ Glutathione Peroxidase Assay Kit and EnzyChrom™ Glutathione Reductase Assay Kit (BioAssay systems, USA), respectively, according to the manufacturer’s instructions. The absorbance was read in a microplate reader at 340 nm for GPx activity and 412 nm for GR activity assay. Catalase activity was assayed with CAT100 Catalase assay kit (Sigma-Aldrich, USA) following the manufacturer’s protocol. Absorbance was measured at 520 nm in a Shimadzu UV 1800 spectrophotometer (Shimadzu, Japan).
GSH and protein carbonyl (PC) contents of SH-SY5Y cells were determined with an enzyme-linked immunosorbent assay (ELISA) (Bioassay Technology Laboratory, China) based on the biotin double antibody sandwich technology following the manufacturer’s procedure. Absorbance was measured at 450 nm with a microplate reader.
Lipid peroxidation was estimated by the thiobarbituric acid (TBA) reaction with malondialdehyde (MDA), which was an end product of peroxidation of membrane lipids. For this purpose, the commercially available QuantiChrom™ TBARS assay (BioAssay Systems, USA) was run according to the manufacturer’s protocol. Absorbance was measured at 535 nm with a microplate reader.
Statistical analysis
All experiments were performed in triplicate as a minimum, and results were presented as the mean ± SD. After confirming normal data distribution with the Shapiro–Wilk normality test, statistical analysis of the data employed one-way analysis of variance followed by Tukey’s test and statistical significance was set at P < 0.05.
Results
The effects of JWH-018 on SH-SY5Y cell viability were assessed with the MTT, NRU, and LDH assays. Treatments with JWH-018 did not cause a significant decrease in SH-SY5Y cell viability or an increase in mitochondrial and lysosomal dysfunction, even at the highest JWH-018 concentration (150 μM). The results of LDH concentration in the supernate showed that JWH-018 treatment did not cause significant damage to cell membrane integrity (Fig. 1).
Figure 1.
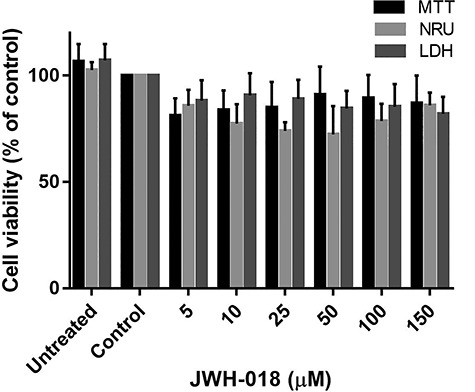
Effects of JWH-018 treatment on SH-SY5Y cells viability. Data are shown as the means ± SD (n:3); *p < 0.05 versus control cells; MTT: 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyl tetrazolium bromide; LDH: Lactate dehydrogenase; NRU: Neutral red uptake SD: standard deviation
Genotoxicity was evaluated with the comet assay that identifies damaged DNA fragments as they migrate out of the nucleus in an electrical field. The intensity of the DNA comet tail indexes DNA damage expressed as tail intensity percentage. After 24-h JWH-018 exposure, there was no increase in tail intensity percentage compared with the negative control, as illustrated in Fig. 2.
Figure 2.
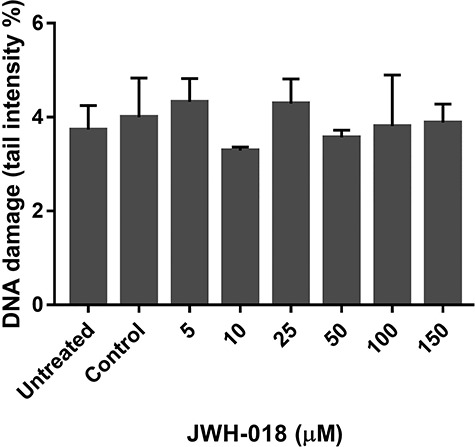
Genotoxicity assessment of JWH-018 in SH-SY5Y cells with comet assay. Data are shown as the means ± SD (n:3); *p < 0.05 versus control cells; SD: standard deviation
The type of cell death was analyzed by double staining with Annexin V and PI under a fluorescent microscope. After 24-h exposure to JWH-018, we did not observe upregulated apoptosis and/or necrosis (Fig. 3).
Figure 3.
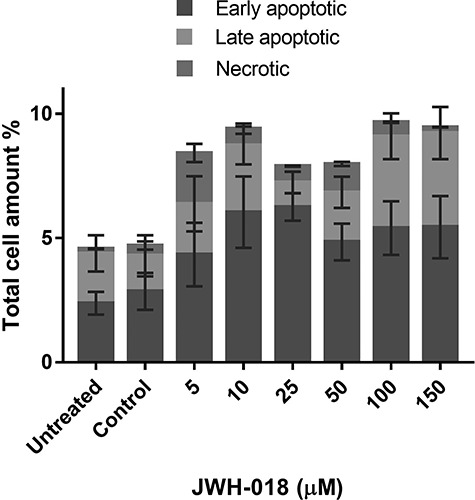
Evaluation of apoptosis and necrosis inducing potentials of JWH-018 in SH-SY5Y cells with Annexin V/PI double staining under fluorescent microscope. Results are presented as percentage of the total cell amount
The effect of JWH-018 GR, GPx, catalase, and GSH is shown in Fig. 4. GR and catalase activities were decreased compared with the positive control and significant at the two highest JWH-018 concentrations. GSH gradually decreased and no significant alteration in GPx activity was observed.
Figure 4.
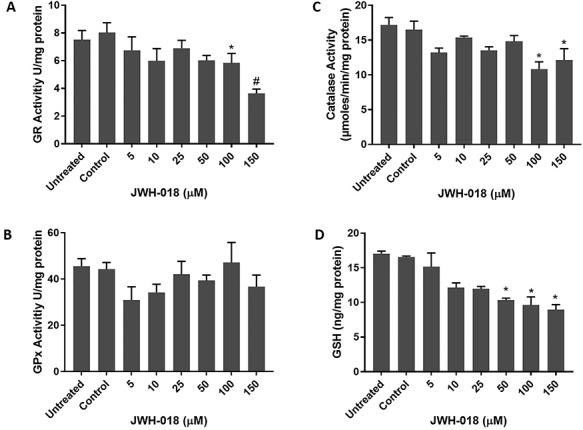
Effects of JWH-018 treatment on antioxidant enzyme activities; GR (A), GPx (B), Catalase (C) and glutathione level (D) in SH-SY5Y cells. Data are shown as the means ± SD (n:3); *p < 0.05 versus control cells; #p < 0.01 versus control cells; GR: Glutathione reductase; GPx: Glutathione peroxidase; GSH: Glutathione
Changes in PC and MDA concentrations are displayed in Fig. 5. Carbonylated protein and MDA concentrations gradually increased with JWH-018 treatments for 24 h.
Figure 5.
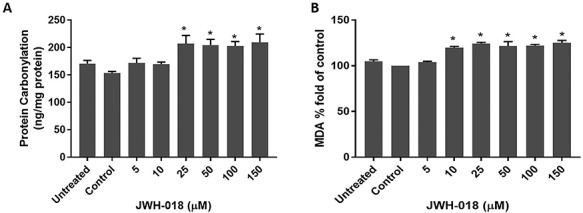
Effects of JWH-018 treatment on protein carbonylation (A) and lipid peroxidation (B) in SH-SY5Y cells. Data are shown as the means ± SD (n:3); *p < 0.05 versus control cells; MDA: Malondialdehyde
Discussion
JWH-018, an aminoalkylindole SC, produces cannabimimetic effects at lower doses than those of natural THC. Due to JWH-018’s full agonism at the CB1 receptor, JWH-018 effects can be more severe and life-threatening than following THC. Limited data are currently available on the mechanisms of JWH-018 toxicity. In the present study, we characterized the toxicological properties of JWH-018 as a neurotoxicant to human neuroblastoma, SH-SY5Y cells, which are known to express CB1 but not CB2 receptors on the surface of their membranes [44]. JWH-018 (0.1–199 ng/mL) concentrations were reported in post-mortem blood of various origins (cardiac, femoral, and other sources) [45, 46]. In line with the current literature, 5–150 μM JWH-018 concentrations were used for these experiments because by conversion, these concentrations represent an exposure of 1.7–51.2 ng/mL. Koller et al. [37] reported LDH release in MCF-7 and TR146 cells, but not HepG2 cells, after exposure to 50, 75, and 100 μM JWH-018 for 24 h. Their XTT results showed reduced cell viability only in HepG2 cells; however, the neutral red assay was negative in all three cell types. None of the cell lines they tested possessed CB1 receptors, while our cell lines did include the appropriate cannabinoid receptors for JWH-018 binding. Our MTT and NR results did not show a significant change in SH-SY5Y cell viability over the concentration range of 5–150 μM JWH-018. Similarly, Couceiro et al. [39] employed the MTT assay to study the cytotoxic effects of JWH-018 in HEK293T and SH-SY5Y cells and showed no statistically significant decrease in cell viability with exposure to 5–150 μM JWH-018. However, JWH-018’s phase I metabolite N-(3-hydroxypentyl) was cytotoxic for both cell lines. In the present study, the LDH assay evaluated JWH-018 effects on membrane integrity in SH-SY5Y cells and showed that JWH-018 did not increase LDH levels, indicating no significant damage to cell membrane integrity, concordant with Couceiro et al. [39] findings in the same cell line. In contrast, Tomiyama and Funada [38] documented that JWH-018 (30 μM) induced cytotoxicity in mouse forebrain culture, which is more sensitive than immortalized cell lines such as SH-SY5Y.
JWH-018 is a full agonist at the CB1 receptor and has greater adverse effects than THC, the primary psychoactive component of cannabis that is only a partial agonist of the CB1 receptor. Also, others observed that five hydroxylated metabolites of JWH-018 retained their affinity and activity at CB1 receptors in vitro and in vivo [26]. According to Seely et al. [47], the major glucuronic acid conjugate of an omega-hydroxyl metabolite of JWH-018 retains affinity for CB1 receptors and can act as a neutral antagonist. However, pharmacokinetic and pharmacodynamic profiles of JWH-018 and other SC in humans are largely unknown.
Oxidative stress is considered a common mechanism in neurodegeneration. Drugs, pesticides, and many industrial chemicals induce oxidative stress. Oxidative stress emerges when the oxidative insult supersedes the cells’ ability to neutralize the threat. To defend against oxidative stress, cells have an antioxidant system, including enzymatic and non-enzymatic antioxidants. The overproduction of oxygen free radicals and other reactive species results in oxidative stress. Lipid peroxidation is a free-radical-mediated chain of reactions that, once initiated, results in an oxidative deterioration of polyunsaturated lipids, the components of biological membranes. There are controversial reports about the relationship between the CB1 endocannabinoid system and oxidative stress [48–52]. According to Mukhopadhyay et al. [49], CB1 activation may amplify the reactive oxygen/nitrogen species-MAPK activation-cell death pathway by excessive inflammation and/or oxidative/nitrosative stress, which may contribute to the pathophysiology of cardiovascular diseases. Zhuang et al. [52] demonstrated that CB1 is likely to provide an appropriate antioxidant balance by increasing endogenous free radical scavengers for neuroprotection.
The oxidative stress potential of JWH-018 was evaluated by measuring cellular MDA, GSH, and protein carbonylation concentrations, and GPx, GR, and catalase activities. MDA concentrations are proportional to the extent of lipid peroxidation. In our study, we observed a dose-dependent increment of MDA production, as a marker of oxidative stress induced by JWH-018 in SH-SY5Y cells [53]. Glutathione is a well-known intrinsic antioxidant detoxifying oxygen-free radicals and other reactive species, and its regeneration from its oxidized form is dependent on GR activity [54]. JWH-018 caused reduction in GR activity at higher doses and significant GSH depletion in a dose-dependent manner. However, GPx activity did not change significantly. Although GR activity reduced at higher doses, GSH depletion may be explained by general cellular response to oxidative stress. Exposure to JWH-018 also significantly decreased catalase activity in SH-SY5Y cells, indicating that JWH-018 impaired the ability of catalase to detoxify H2O2. Proteins are also major targets of oxygen-free radicals and other reactive species. Oxidative protein damage can modulate the biochemical characteristics of proteins, including their structures [55]. The concentration of carbonyl groups is a widely employed the measure of protein oxidation [56]. Under our experimental conditions, carbonyl groups in SH-SY5Y cells exposed to JWH-018 were significantly increased, providing evidence that JWH-018 damages cellular proteins by oxidative reactions.
To investigate cell death through necrosis or apoptosis, dual staining with fluorescent Annexin V-FITC and PI showed no significant results; however, Couceiro et al. [39] noted that the JWH-018 hydroxylated phase I metabolite, JWH-018 N-(3-hydroxypentyl), promoted cellular death through necrosis by the same technique. JWH-018 did not induce genotoxicity in SH-SY5Y cells based on the comet assay findings. Koller et al. [37] treated MCF-7, TR146, and HepG2 cells with different concentrations of JWH-018 along with other SC and evaluated DNA migration. At the highest concentration (100 μM), JWH-018 induced DNA migration significantly in TR146 and HepG2 cells. However, they retested positive compounds in a second experiment in which only the highest doses were used and JWH-018 was not included. However, there are modifications of the basic comet assay to detect specifically oxidative DNA damage [57] which were unfortunately a limitation of this study that was a budgetary restriction that did not permit the inclusion of these tests. In the future, evaluation of this aspect of oxidative stress would be useful.
According to our knowledge, this is the first comprehensive characterization of the toxicological mechanisms of JWH-018 toxicity including oxidative stress parameters and the comet assay for genotoxicity. These results suggest that JWH-018 toxicity occurs due to oxidative stress. Additional studies are needed to assess the in vivo effects of JWH-018 and metabolites in animal models.
Acknowledgement
The authors would like to thank the Research Fund of Istanbul University for the financial support toward this study.
Contributor Information
Yigit Sezer, Council of Forensic Medicine, Ministry of Justice, Istanbul 34197, Turkey.
Ayse Tarbin Jannuzzi, Faculty of Pharmacy, Department of Pharmaceutical Toxicology, Istanbul University, Istanbul 34126, Turkey.
Marilyn A Huestis, Institute of Emerging Health Professions, Thomas Jefferson University, Philadelphia, PA 19107, USA.
Buket Alpertunga, Faculty of Pharmacy, Department of Pharmaceutical Toxicology, Istanbul University, Istanbul 34126, Turkey.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Scientific Research Projects Coordination Unit of Istanbul University (Grant number 55841).
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1. Langer N, Lindigkeit R, Schiebel HM et al. Identification and quantification of synthetic cannabinoids in “spice-like” herbal mixtures: a snapshot of the German situation in the autumn of 2012. Drug Test Anal. 2015;6(1–2):59–71. doi: 10.1002/dta.1499. [DOI] [PubMed] [Google Scholar]
- 2. Debruyne D, Boisselier R. Emerging drugs of abuse: current perspectives on synthetic cannabinoids. Subst Abuse Rehabil. 2015;6:113–29. doi: 10.2147/sar.s73586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. European Monitoring Centre for Drugs and Drug Addiction Understanding the spice phenomenon. Themat Pap. 2009. Retrieved from https://www.emcdda.europa.eu/publications/thematic-papers/understanding-spice-phenomenon_en. doi: 10.2810/27063. [DOI] [Google Scholar]
- 4. European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) Synthetic cannabinoids in Europe - update 2017. Perspect Drugs. 2017. Retrieved from https://www.emcdda.europa.eu/publications/pods/synthetic-cannabinoids_en. doi: 10.2810/32306. [DOI] [Google Scholar]
- 5. Herkenham M, Lynn AB, Little MD et al. Cannabinoid receptor localization in brain. Proc Natl Acad Sci. 1990;87(5):1932–36. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Poso A, Huffman JW. Targeting the cannabinoid CB2 receptor: modelling and structural determinants of CB2 selective ligands. Br J Pharmacol. 2008;153:335–46. doi: 10.1038/sj.bjp.0707567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Svíženská I, Dubový P, Šulcová A. Cannabinoid receptors 1 and 2 (CB1 and CB2), their distribution, ligands and functional involvement in nervous system structures - a short review. Pharmacol Biochem Behav. 2008;90(4):501–11. doi: 10.1016/j.pbb.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 8. Lindigkeit R, Boehme A, Eiserloh I et al. Spice: a never ending story? Forensic Sci Int. 2009;191:58–63. doi: 10.1016/j.forsciint.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 9. Wells DL, Ott CA. The “new” marijuana. Ann Pharmacother. 2011;45(3):414–17. doi: 10.1345/aph.1P580. [DOI] [PubMed] [Google Scholar]
- 10. Carter GT, Flanagan AM, Earleywine M et al. Cannabis in palliative medicine: improving care and reducing opioid-related morbidity. Am J Hosp Palliat Med. 2011;28(5):297–303. doi: 10.1177/1049909111402318. [DOI] [PubMed] [Google Scholar]
- 11. Mark C. Prescribing cannabis for harm reduction. Harm Reduct J. 2012;9:1. doi: 10.1186/1477-7517-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Müller H, Sperling W, Köhrmann M et al. The synthetic cannabinoid spice as a trigger for an acute exacerbation of cannabis induced recurrent psychotic episodes. Schizophr. Res. 2010;118(1–3):309–10. doi: 10.1016/j.schres.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 13. Lapoint J, James LP, Moran CL et al. Severe toxicity following synthetic cannabinoid ingestion. Clin Toxicol. 2011;49(8):760–64. doi: 10.3109/15563650.2011.609822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gunderson EW, Haughey HM, Ait-Daoud N et al. “Spice” and “k2” herbal highs: a case series and systematic review of the clinical effects and biopsychosocial implications of synthetic cannabinoid use in humans. Am J Addict. 2012;1–7. doi: 10.1111/j.1521-0391.2012.00240.x. [DOI] [PubMed] [Google Scholar]
- 15. Young AC, Schwarz E, Medina G et al. Cardiotoxicity associated with the synthetic cannabinoid, K9, with laboratory confirmation. Am J Emerg Med. 2012;30(7):1320.E5–1320.E7. doi: 10.1016/j.ajem.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 16. Pant S, Deshmukh A, Dholaria B et al. Spicy seizure. Am J Med Sci. 2012;344(1):67–68. doi: 10.1097/MAJ.0b013e31824cf5c2. [DOI] [PubMed] [Google Scholar]
- 17. Papanti D, Schifano F, Botteon G et al. “spiceophrenia”: a systematic overview of “spice”- related psychopathological issues and a case report. Hum Psychopharmacol. 2013;28(4):379–89. doi: 10.1002/hup.2312. [DOI] [PubMed] [Google Scholar]
- 18. Brewer TL, Collins M. A review of clinical manifestations in adolescent and young adults after use of synthetic cannabinoids. J. Pediatr. Nurs. 2014;19(2):119–26. doi: 10.1111/jspn.12057. [DOI] [PubMed] [Google Scholar]
- 19. Santacroce R, Corazza O, Martinotti G et al. Psyclones: a roller coaster of life? Hidden synthetic cannabinoids and stimulants in apparently harmless products. Hum Psychopharmacol. 2015;30(4):265–71. doi: 10.1002/hup.2410. [DOI] [PubMed] [Google Scholar]
- 20. Zimmermann US, Winkelmann PR, Pilhatsch M et al. Withdrawal phenomena and dependence syndrome after the consumption of spice gold. Dtsch Aerzteblatt Online. 2009;106(27):464–67. doi: 10.3238/arztebl.2009.0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Besli GE, Ikiz MA, Yildirim S et al. Synthetic cannabinoid abuse in adolescents: a case series. J Emerg Med. 2015;49(5):644–50. doi: 10.1016/j.jemermed.2015.06.053. [DOI] [PubMed] [Google Scholar]
- 22. Macfarlane V, Christie G. Synthetic cannabinoid withdrawal: a new demand on detoxification services. Drug Alcohol Rev. 2015;34(2):147–53. doi: 10.1111/dar.12225. [DOI] [PubMed] [Google Scholar]
- 23. Wiley JL, Marusich JA, Huffman JW. Moving around the molecule: relationship between chemical structure and in vivo activity of synthetic cannabinoids. Life Sci. 2014;97(1):55–63. doi: 10.1016/j.lfs.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wiley JL, Marusich JA, Martin BR et al. 1-Pentyl-3-phenylacetylindoles and JWH-018 share in vivo cannabinoid profiles in mice. Drug Alcohol Depend. 2012;123(1–3):148–53. doi: 10.1016/j.drugalcdep.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sobolevsky T, Prasolov I, Rodchenkov G. Detection of JWH-018 metabolites in smoking mixture post-administration urine. Forensic Sci Int. 2010;200(1–3):141–47. doi: 10.1016/j.forsciint.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 26. Brents LK, Reichard EE, Zimmerman SM et al. Phase i hydroxylated metabolites of the k2 synthetic cannabinoid jwh-018 retain in vitro and in vivo cannabinoid 1 receptor affinity and activity. PLoS One. 2011;6(7):e21917. doi: 10.1371/journal.pone.0021917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chimalakonda KC, Seely KA, Bratton SM et al. Cytochrome P 450-mediated oxidative metabolism of abused synthetic cannabinoids found in K2/spice: identification of novel cannabinoid receptor ligands. Drug Metab Dispos. 2012;40(11):2174–84. doi: 10.1124/dmd.112.047530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chimalakonda KC, Bratton SM, Le VH et al. Conjugation of synthetic cannabinoids JWH-018 and JWH-073, metabolites by human UDP-glucuronosyltransferases. Drug Metab Dispos. 2011;39(10):1967–76. doi: 10.1124/dmd.111.040709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tai S, Fantegrossi WE. Synthetic cannabinoids: pharmacology, Behavioral effects, and abuse potential. Curr Addict Reports. 2014;1:129–36. doi: 10.1007/s40429-014-0014-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hegstad S, Westin AA, Spigset O. Detection times of carboxylic acid metabolites of the synthetic cannabinoids JWH-018 and JWH-073 in human urine. J Anal Toxicol. 2015;39(4):280–86. doi: 10.1093/jat/bkv013. [DOI] [PubMed] [Google Scholar]
- 31. Erol Öztürk Y, Yeter O, Alpertunga B. Validation of JWH-018 and its metabolites in blood and urine by UPLC-MS/MS: monitoring in forensic cases. Forensic Sci Int. 2015;248:88–93. doi: 10.1016/j.forsciint.2014.12.029. [DOI] [PubMed] [Google Scholar]
- 32. Poklis JL, Amira D, Wise LE et al. Determination of naphthalen-1-yl-(1-pentylindol-3-yl) methanone (JWH-018) in mouse blood and tissue after inhalation exposure to “buzz” smoke by HPLC/MS/MS. Biomed Chromatogr. 2012;26(11):1393–98. doi: 10.1002/bmc.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vearrier D, Osterhoudt KC. A teenager with agitation: higher than she should have climbed. Pediatr Emerg Care. 2010;26(6):462–65. doi: 10.1097/PEC.0b013e3181e4f416. [DOI] [PubMed] [Google Scholar]
- 34. Every-Palmer S. Synthetic cannabinoid JWH-018 and psychosis: an explorative study. Drug Alcohol Depend. 2011;117(2–3):152–57. doi: 10.1016/j.drugalcdep.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 35. Simmons J, Cookman L, Kang C et al. Three cases of spice exposure. Clin Toxicol. 2011;49(5):431–33. doi: 10.3109/15563650.2011.584316. [DOI] [PubMed] [Google Scholar]
- 36. Hermanns-Clausen M, Kneisel S, Szabo B et al. Acute toxicity due to the confirmed consumption of synthetic cannabinoids: clinical and laboratory findings. Addiction. 2013;108(3):534–44. doi: 10.1111/j.1360-0443.2012.04078.x. [DOI] [PubMed] [Google Scholar]
- 37. Koller VJ, Zlabinger GJ, Auwärter V et al. Toxicological profiles of selected synthetic cannabinoids showing high binding affinities to the cannabinoid receptor subtype CB1. Arch Toxicol. 2013;87:1287–97. doi: 10.1007/s00204-013-1029-1. [DOI] [PubMed] [Google Scholar]
- 38. Tomiyama KI, Funada M. Cytotoxicity of synthetic cannabinoids on primary neuronal cells of the forebrain: the involvement of cannabinoid CB1 receptors and apoptotic cell death. Toxicol Appl Pharmacol. 2014;274(1):17–23. doi: 10.1016/j.taap.2013.10.028. [DOI] [PubMed] [Google Scholar]
- 39. Couceiro J, Bandarra S, Sultan H et al. Toxicological impact of JWH-018 and its phase I metabolite N-(3-hydroxypentyl) on human cell lines. Forensic Sci Int. 2016;264:100–105. doi: 10.1016/j.forsciint.2016.03.024. [DOI] [PubMed] [Google Scholar]
- 40. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 1983;65:55–63. [DOI] [PubMed] [Google Scholar]
- 41. Borenfreund E, Puerner JA. Toxicity determined in vitro by morphological alterations and neutral red absorption. Toxicol Lett. 1985;24(2–3):119–24. doi: 10.1016/0378-4274(85)90046-3. [DOI] [PubMed] [Google Scholar]
- 42. Tice RR, Agurell E, Anderson D, et al. Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ Mol Mutagen. 2000;35(3):206–21. doi: . [DOI] [PubMed] [Google Scholar]
- 43. Singh NP, McCoy MT, Tice RR et al. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988;175(1):184–91. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- 44. Callén L, Moreno E, Barroso-Chinea P et al. Cannabinoid receptors CB 1 and CB 2 form functional heteromers in brain. J Biol Chem. 2012;287(25):20851–65. doi: 10.1074/jbc.M111.335273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shanks KG, Dahn T, Terrell AR. Detection of JWH-018 and JWH-073 by UPLC-MS-MS in postmortem whole blood casework. J Anal Toxicol. 2012;36(3):145–52. doi: 10.1093/jat/bks013. [DOI] [PubMed] [Google Scholar]
- 46. Yeakel JK, Logan BK. Blood synthetic cannabinoid concentrations in cases of suspected impaired driving. J Anal Toxicol. 2013;37(8):547–51. doi: 10.1093/jat/bkt065. [DOI] [PubMed] [Google Scholar]
- 47. Seely KA, Lapoint J, Moran JH et al. Spice drugs are more than harmless herbal blends: a review of the pharmacology and toxicology of synthetic cannabinoids. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2012;39(2):234–43. doi: 10.1016/j.pnpbp.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Han KH, Lim S, Ryu J et al. CB1 and CB2 cannabinoid receptors differentially regulate the production of reactive oxygen species by macrophages. Cardiovasc Res. 2009;84(3):378–86. doi: 10.1093/cvr/cvp240. [DOI] [PubMed] [Google Scholar]
- 49. Mukhopadhyay P, Rajesh M, Bátkai S et al. CB1 cannabinoid receptors promote oxidative stress and cell death in murine models of doxorubicin-induced cardiomyopathy and in human cardiomyocytes. Cardiovasc Res. 2010;85(4):773–84. doi: 10.1093/cvr/cvp369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rajesh M, Bátkai S, Kechrid M et al. Cannabinoid 1 receptor promotes cardiac dysfunction, oxidative stress, inflammation, and fibrosis in diabetic cardiomyopathy. Diabetes. 2012;61(3):716–27. doi: 10.2337/db11-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Vendel E, De Lange ECM. Functions of the CB1 and CB2 receptors in neuroprotection at the level of the blood-brain barrier. Neuro Molecular Med. 2014;16:620–42. doi: 10.1007/s12017-014-8314-x. [DOI] [PubMed] [Google Scholar]
- 52. Zhuang Q, Dai C, Yang L et al. Stimulated CB1 cannabinoid receptor inducing ischemic tolerance and protecting neuron from cerebral ischemia. Cent Nerv Syst Agents Med Chem. 2017;17(2):141–50. doi: 10.2174/1871524916666160504104624. [DOI] [PubMed] [Google Scholar]
- 53. Ayala A, Muñoz MF, Argüelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev. 2014;2014:1–31. doi: 10.1155/2014/360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sies H. Oxidative stress: oxidants and antioxidants. Exp Physiol. 1997;82(2); 291–95. [DOI] [PubMed] [Google Scholar]
- 55. Dahl JU, Gray MJ, Jakob U. Protein quality control under oxidative stress conditions. J Mol Biol. 2015;427(7):1549–63. doi: 10.1016/j.jmb.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fedorova M, Bollineni RC, Hoffmann R. Protein carbonylation as a major hallmark of oxidative damage: update of analytical strategies. Mass Spectrom Rev 2014;33:79–97. [DOI] [PubMed] [Google Scholar]
- 57. Collins AR. Measuring oxidative damage to DNA and its repair with the comet assay. Biochim Biophys Acta Biomembr. 2014;1840(2):794–800. doi: 10.1016/j.bbagen.2013.04.022. [DOI] [PubMed] [Google Scholar]


