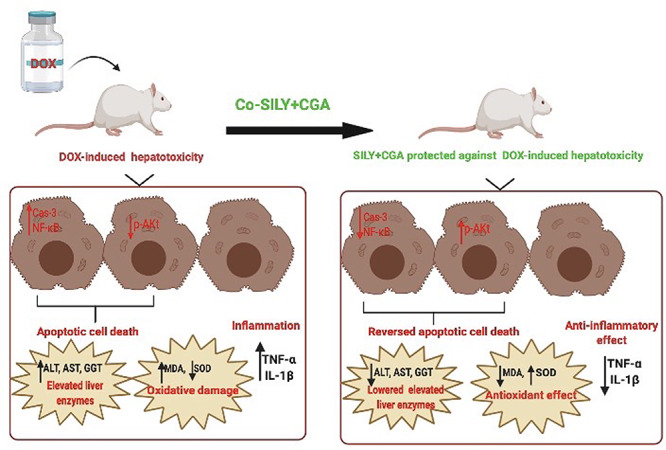
Abstract
Many xenobiotics are known to cause hepatic damage with subsequent significant morbidity and mortality. Doxorubicin (DOX) is a broad-spectrum antineoplastic agent. DOX is reported to cause hepatocellular damage. Previous studies verified the promising role of many natural antioxidant products against various models of hepatic dysfunction. We conducted this study to evaluate the possible hepatoprotective effect of silymarin (SILY) and/or chlorogenic acid (CGA) in a rat model of DOX-induced hepatotoxicity. For this purpose, we randomly divided 30 adult male rats into five equal groups as control, DOX, co-treated DOX with SILY, co-treated DOX with GCA and co-treated DOX with SILY and CGA groups. All treatments were administered every second day for 4 weeks. Our results showed that simultaneous SILY and CGA administration caused a significant decrease in hepatic apoptosis biomarkers (hepatic caspase-3 and nuclear factor-κB levels), a significant improvement in hepatic oxidant/antioxidant status (malondialdehyde and superoxide dismutase) and significant decrease in hepatic pro-inflammatory biomarkers (tumor necrosis factor-alpha and interlukin-1β) compared with DOX treatment. We concluded that adding CGA to SILY acts as a hepatoprotective agent against DOX-induced liver injury through inhibiting apoptosis biomarkers, maintaining antioxidant enzyme levels, decreasing pro-inflammatory cytokines as well as regulating liver adenosine monophosphate-activated protein kinase signaling.
Keywords: apoptosis, inflammation, chlorogenic acid, doxorubicin, oxidative stress, silymarin
Graphical abstract
Introduction
Doxorubicin (DOX) is a broad-spectrum anticancer drug, used to manage a large-scale of malignancies. However, use of DOX is linked to acute and chronic dose-dependent effects and a wide range of organ toxicities. Additionally, up till now, there are no suitable protective strategies to prevent drug-induced hepatotoxicity; therefore, searching for an alternative hepatoprotective drugs, herbs and dietary antioxidant products may play a good way to elicit a new source for discovery of hepatoprotective agents. The formation of free radicals is the most important mechanism by which DOX induces toxicity to many tissues and organs, but cardiotoxicity and hepatotoxicity are the most frequent reported adverse effects to DOX [1–3].
The liver is the primarily involved organ in detoxification of harmful drugs, xenobiotics and natural toxins [4, 5]. Oxidative stress represents a major event in the pathophysiology of numerous hepatic disorders [6]. Various precipitating factors, including drugs, chemicals, viral infections and environmental pollutants, may prompt hepatic oxidative stress, which in turn causes liver insults. Reactive oxygen species (ROS) are principally produced in the mitochondria and the endoplasmic reticulum of hepatocytes by the cytochrome P-450 enzymes. Under the normal physiological conditions, liver cells are able to maintain the level of oxidative stress and to keep a balance between oxidant and antioxidant molecules. While during stressful conditions, hepatic ability to maintain this balance is disturbed and oxidative stress settled [7, 8].
Silymarin (SILY) is widely used as a supportive treatment for many liver diseases of different etiology. SILY is extracted from Silybum marianum plant, or milk thistle, and its major active constituent is silybin, which has a significant biological effect. It acts mainly through its antioxidant, anti-inflammatory and anti-fibrotic potential. Many Studies validated its role in different liver disorders, such as cirrhosis and hepatocellular carcinoma [9, 10].
Chlorogenic acid (CGA) is an ester form of caffeic acid. It is a powerful antioxidant in many body systems. It can be naturally found in green coffee extracts and tea. CGA has several beneficial health effects such as antioxidant activity, anti-microbial, hepatoprotective, anti-inflammatory, anti-obesity and a central nervous system stimulator [11, 12].
We conducted this study to evaluate the possible hepatoprotective effect of SILY and/or CGA in a rat model of DOX-induced hepatotoxicity. To our knowledge, this is the first study to demonstrate the combined effects of SILM and CGA on DOX-mediated hepatotoxic effect in rats and to compare such effects to their respective individual effects.
Accordingly, we hypothesized that simultaneous SILY and CGA administration exerts better hepatoprotective effects than individual agents in a rat model of DOX-induced hepatotoxicity.
Materials and Methods
Materials
We obtained DOX (Adriamycin ® a vial contains 2 mg/mL of Doxorubicin HCl, Pfizer, Egypt), CGA (CAS Number 327-97-9, with 98% purity, Sigma-Aldrich, St. Louis, USA) and SILY (sachet dissolved in distilled water, a sachet contains 140 mg of SILY, Sedico Company, Egypt) for use in this study.
Animals
Thirty adult male albino rats weighting 180–200 gm were obtained from the Faculty of Veterinary Medicine, Zagazig University, Egypt. They were kept under the full hygienic conditions and free access to well-balanced water and diet, under standard humidity. All animals received humane care in compliance with the Animal Care Guidelines of the National Institutes of Health (NIH), and the Research Ethics Committee of the Faculty approved the design of the experiment.
Experimental design
We randomly assigned animals into five groups of six rats each as follows.
Group I: Control group: Rats received 0.5 mL/kg body weight of normal saline, intraperitoneal (ip) injection.
Group II (DOX): DOX-intoxicated rats. Rats received oral dose of 1.5 mg/kg body weight of DOX dissolved in 0.5 mL of normal saline (ip) every second day during the 4 weeks of the experiment. The cumulative dose here is 21 mg/kg. The cumulative dose of DOX to induce hepatotoxicity is ~20–25 mg/kg [13–15].
Group III (SILY): DOX-intoxicated rats and treated with SILY. The rats received DOX as in group II, and then rats were orally administrated a dose of 206.7 mg/kg body weight of SILY every second day during the 4 weeks of the experiment.
Group IV (CGA): DOX-intoxicated rats and treated with CGA. Rats received DOX as in group II, and then rats were orally administrated a dose of 124 mg/kg body weight of CGA every second day during the 4 weeks of the experiment.
Group V: DOX-intoxicated rats and treated concomitantly with SILY and CGA. Rats received DOX as in group II, and then SILY and CGA as in groups III and IV, respectively.
The doses of SILY and CGA are equivalent to the human dosing regimen of 2 and 1.2 g/day, respectively, based on body surface area conversion [animal dose (mg/kg) = human equivalent dose (mg/kg) × 6.2], assuming that an adult person weighs 60 kg [16–18].
Blood and tissue preparation
After 24 hours of the last dose of treatment, rats of all groups were subjected to ip injection of sodium-thiopental anesthesia (40 mg/kg/body weight). Blood samples were collected and centrifuged at 3000 rpm for 10 minutes, and the serum activity for alanine aminotransferase (ALT), aspartate aminotransferase (AST) and gamma-glutamyl transferase (GGT) were estimated. Then, rats were sacrificed by cervical dislocation. After that, liver was removed and homogenized, and then the homogenate was frozen rapidly at −80°C for the determination of malondialdehyde (MDA) and superoxide dismutase (SOD) activity, as well as the determination of tumor necrosis factor-alpha (TNFα), interlukin-1β (IL-1β) and caspase-3 activity, adenosine monophosphate-activated protein kinase (AMPK) and protein kinase B (PKB, also known as Akt).
Biochemical analysis
Serum enzyme activities determination
According to the method of Reitman and Frankel [19], AST and ALT activities were measured, while GGT assay was measured according to Szasz [20] by (Greiner) a test reagent kit.
Assessment of hepatic lipid peroxidation (MDA) and antioxidant status (SOD enzyme activity)
Both liver MDA and enzymatic activity of SOD were colorimetrically assayed according to Ohkawa et al. [21] and Nishikimi et al., [22], respectively.
Measurement of nuclear factor-κB concentrations in the liver homogenates
Nuclear factor-κB (NF-κB) concentrations were measured using commercially available kits (CUSABIO-Biotech. Co, China) according to manufacturer’s instructions.
Measurements of TNFα and IL-β concentrations in the liver homogenates
Hepatic TNFα concentrations were measured according to Wei et al. [23] by using quantitative enzyme-linked immunosorbent assay (Ray Bio rat TNFα ELISA kits). Hepatic IL-β concentration was measured using RayBio® (ELISA) kit (Ray Biotech Inc., GA, USA).
Assessment of caspase-3 activity in the liver homogenates
Hepatic activity of caspase-3 was assayed by the commercially available assay ELISA kits (Rat Casp-3, My Biosource, USA).
Measurement of AMPK
Hepatic cellular AMP-activated protein kinase (AMPK) activities were estimated by colorimetric reaction using an AMPK kinase assay kit (Cyclex, Ina, Nagano, Japan).
Measurement of phosphorylated-Akt/protein kinase B concentrations in the liver homogenates
Phosphorylated-Akt (p-Akt)/protein kinase B activities in liver homogenates were determined by using a solid-phase sandwich enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions (Kit-3997, DRG International, Inc., USA).
Statistical analysis
Continuous data were expressed as mean ± SEM as data were normally distributed. Normality was checked by Shapiro–Wilk test. A one-way ANOVA was used to detect statistical differences between groups. Post hoc Tukey’s test was performed for multiple comparisons between groups. The differences were considered significant at P < 0.05. All statistical comparisons were two-tailed. All statistical calculations were carried out using Graphpad Prism, Version 8.0 Software (GraphPad Software, SanDiego, CA, USA).
Results
Death rates
We recorded zero deaths in all groups.
SILY and/or CGA lowered the elevated liver enzymes-induced by DOX in male rats
DOX-treated rats exhibited statistically significantly higher mean liver enzymes activity (AST, ALT and GGT) (119 ± 2.9, 73.6 ± 1.6 and 4.9 ± 0.12 U/L, respectively) compared with control (27 ± 1, 14 ± 0.7 and 1.6 ± 0.11 U/L, respectively) and other treated groups (P < 0.05). SILY had statistically significantly lower mean liver enzymes activity (55 ± 1.6, 36.8 ± 1.2 and 2.9 ± 0.1 U/L, respectively) than CGA treatment (83 ± 3.1, 57.4 ± 2.7 and 3.5 ± 0.2 U/L, respectively) (P < 0.05), but more statistically significant improvement was evident in combined SILY and CGA treatment compared with the use of each agent individually (32.4 ± 0.7, 19.6 ± 0.5 and 1.8 ± 0.1 U/L, respectively) (P < 0.05), as shown in Fig. 1.
Figure 1.
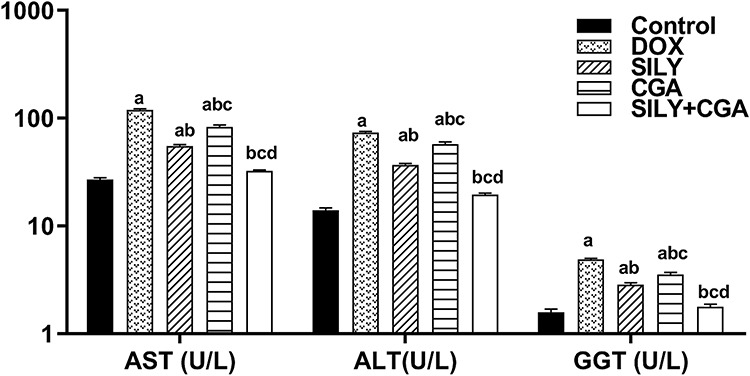
Effects of SILY, CGA, and SILY plus CGA on serum liver enzymes activity of DOX-treated rats. (a) Significant vs. control group. (b) Significant vs. DOX. (c) Significant vs. SILY. (d) Significant vs. CGA. (P < 0.05). All values are expressed as mean ± SEM, n = 6.
SILY and/or CGA antagonized hepatic apoptotic cell death induced by DOX in male rats
DOX-induced apoptosis in the hepatocytes was proved by the statistically significant increase in caspase-3 level (17.2 ± 0.31 ng/g) and NF-κB (4.56 ± 0.18 ng/mL) as well as the statistically significant decrease in p-AKt level (1.07 ± 0.06 u/g) compared with the control group (8.9 ± 0.30, 1.28 ± 0.5 and 2.1 ± 0.07 u/g), respectively) (P < 0.05). Treatment with SILY, CGA and their combination statistically significantly decreased caspase-3 level (12.4 ± 0.15, 13.7 ± 0.44 and 10 ± 0.91 ng/g, respectively) (P < 0.05) compared with DOX treatment. Caspase-3 level and NF-κB were similar between SILY- and CGA-treated groups (P > 0.05), but more statistically significant improvement was evident with their combination (P < 0.05). There was no statistically significant difference between DOX (1.07 ± 0.06 u/g)- and CGA-treated rats regarding p-AKt level (1.2 ± 0.09 u/g) (P > 0.05). Administration of SILY alone (1.9 ± 0.05) was comparable with co-administration with CGA (2 ± 0.07 u/g) for increasing p-AKt level (P > 0.05), as shown in Table 1.
Table 1.
Effects of SILY, chlorogenic acid, and SILY plus chlorogenic acid on caspase-3, p-AKt, and NF-κB in liver homogenates of DOX-treated rats
| Variables | Groups | ||||
|---|---|---|---|---|---|
| Control | DOX | SILY | CGA | SILY+CGA | |
| Caspase-3 (ng/g) | 8.9 ± 0.30 | 17.2 ± 0.31a | 12.4 ± 0.15ab | 13.7 ± 0.44ab | 10 ± 0.91bcd |
| p-AKt (u/g) | 2.1 ± 0.07 | 1.07 ± 0.06a | 1.9 ± 0.05b | 1.2 ± 0.09ac | 2 ± 0.07bd |
| NF-κB (ng/mL) | 1.28 ± 0.5 | 4.56 ± 0.18a | 3.32 ± 0.14a | 3.23 ± 0.24a | 1.67 ± 0.58abc |
All values are expressed as mean ± SEM, n = 6. A one-way ANOVA followed by post hoc Tukey test for multiple comparisons between groups. Abbreviation: p-Akt/protein kinase B.
aSignificant vs. control group.
bSignificant vs. DOX.
cSignificant vs. SILY.
dSignificant vs. CGA (P < 0.05).
SILY and/or CGA reversed hepatic oxidative stress damage induced by DOX in male rats
DOX-induced oxidative stress in the hepatocytes was proved by the statistically significant increase in MDA level (18.8 ± 0.2 nmol/g) and the statistically significant decrease in SOD activity (64.7 ± 2.8 U/g) and AMPK (9.5 ± 0.14 ng/mg) level compared with the control group (9.4 ± 0.3 nmol/g, 139.6 ± 1.6 U/g and 16.5 ± 0.18 ng/mg, respectively) (P < 0.05). Treatment with SILY, CGA and their combination showed a statistically significant decrease in MDA level (11.7 ± 0.6, 12.2 ± 0.5 and 9.9 ± 0.4 nmol/g, respectively) and a statistically significant increase in SOD (113.5 ± 2.1, 99.2 ± 0.5 and 136.4 ± 1.2 U/g, respectively) and AMPK (12.1 ± 0.29, 10.5 ± 0.18 and 15 ± 0.3 ng/mg, respectively) levels compared with DOX treatment (P < 0.05). The level of MDA was similar between SILY- and CGA-treated groups (P > 0.05), but more statistically significant improvement was evident with their combination (P < 0.05). SILY had statistically significantly higher mean SOD and AMPK levels than CGA treatment (P < 0.05), but their combination elicited more statistically significant improvement than each agent individually (P < 0.05).
SILY and/or CGA alleviated hepatic inflammatory reaction induced by DOX in male rats
CGA treatment induced inflammation in the hepatocytes as evidenced by the statistically significant increase in TNFα and IL-1β concentrations (21.3 ± 0.4 and 60 ± 2.3 pg/g, respectively) compared with the control group (9.9 ± 0.1 and 12.5 ± 0.8 pg/g, respectively) (P < 0.05). SILY treatment statistically significantly decreased the level of the inflammatory markers (TNFα and IL-1β, 13.9 ± 0.36 and 33.5 ± 1.7 pg/g, respectively) than CGA treatment (15.5 ± 0.39 and 24.5 ± 2 pg/g, respectively) (P < 0.05), but more statistically significant reduction was obvious in SILY+CGA group (10.9 ± 0.37 and 16.9 ± 1.96 pg/g, respectively) compared with the use of each agent individually (P < 0.05), as shown in Table 2.
Table 2.
Effects of SILY, chlorogenic acid, and SILY plus chlorogenic acid oxidative stress and inflammatory markers of DOX-treated rats
| Variables | Groups | ||||
|---|---|---|---|---|---|
| Control | DOX | SILY | CGA | SILY+CGA | |
| MDA (nmol/g) | 9.4 ± 0.3 | 18.8 ± 0.2a | 11.7 ± 0.6ab | 12.2 ± 0.5ab | 9.9 ± 0.4bcd |
| SOD (U/g) | 139.6 ± 1.6 | 64.7 ± 2.8a | 113.5 ± 2.1ab | 99.2 ± 0.5abc | 136.4 ± 1.2bcd |
| AMPK (ng/mg) | 16.5 ± 0.18 | 9.5 ± 0.14a | 12.1 ± 0.29ab | 10.5 ± 0.18abc | 15 ± 0.3abcd |
| TNFα (pg/g) | 9.9 ± 0.1 | 21.3 ± 0.4a | 13.9 ± 0.36ab | 15.5 ± 0.39abc | 10.9 ± 0.37bcd |
| IL-1β (pg/g) | 12.5 ± 0.8 | 60 ± 2.3a | 33.5 ± 1.7ab | 24.5±2abc | 16.9 ± 1.96bcd |
All values are expressed as mean ± SEM, n = 6. A one-way ANOVA followed by post hoc Tukey test for multiple comparisons between groups.
aSignificant vs. control group.
bSignificant vs. DOX.
cSignificant vs. SILY.
dSignificant vs. CGA (P < 0.05).
Discussion
The present work showed that the administration of DOX induced an increase in the ALT, AST and GGT serum levels. Increased serum level of ALT, AST and GGT are important biomarkers for the diagnosis of liver affection and diseases [24].
According to a study conducted by Damodar and coworkers [25] in 2014, 40% of DOX receiving breast cancer patients developed DOX-induced hepatoxicity.
Treatment with SILY and CGA significantly decreased the activity of the liver enzymes suggesting the hepatoprotective effect of this combination through the ability of this combination to preserve the cells. In the current study, SOD activity was significantly increased in the rat group treated with SILY and CGA compared with DOX intoxicated group. Moreover, hepatic MDA level significantly decreased in rats treated with CGA and SILY. It is noteworthy that both SILY and CGA were recognized to exert antioxidant properties and down regulating ROS with CGA protect against methotrexate induced increase in MDA content in DOX-induced cardiotoxicity in rat models [26, 27]. Similarly, SILY alleviated the elevation of MDA level in liver tissues [28, 29]. Furthermore, SILY has increased hepatic SOD activity in a model of DOX-induced cardiotoxicity, and hepatotoxicity in rats and CGA is shown to up regulating SOD activity in the blood and the liver of albino rats in a model of methotrexate-induced hepatotoxicity [11, 30].
Caspase-dependent apoptotic pathway is responsible for DNA fragmentation, and caspase-3 is the final mediator of this apoptotic cascade [31, 32]. DOX-induced apoptosis appears to involve mitochondrial pathway as DOX increases oxidative stress in the mitochondria and raises mitochondrial calcium load. The calcium overload enhances mitochondrial swelling and structural damage of the mitochondria [33].
The results in the present study revealed that DOX induced apoptosis in liver cells as shown by marked and significant increased activity of apoptotic marker caspase-3 in liver tissue of rat intoxicated with DOX. Intake of SILY in combination with CGA to DOX intoxicated rats pronouncedly down regulated increase in liver caspase-3 similar observation was supported by Vaughan et al. [34] who reported that caspase-3-dependent pathway activated deoxyribonuclease leading to DNA fragmentation.
TNFα is a pro-inflammatory cytokine produced mainly by macrophage. A variety of effects are mediated by binding of TNFα to its TNF-R1 receptor, for example, TNFα induces many of the proteins involved in the apoptotic pathways such as caspase-3 and cytochrome-c which leads to subsequent liver cell injury and apoptosis [35].
Likewise, a study of Al-Rasheed and coworkers [36] indicated that the combination of SILY and CGA elicited a reduction in the inflammatory biomarker (TNFα) level in a rat model of CCl4-induced hepatic injury.
In the current study, rats subjected to DOX administration developed a significant liver inflammation as evidenced by marked increase in the serum pro-inflammatory cytokines TNFα and IL-β levels. The study also showed that combining SILY with CGA have prominent anti-inflammatory effects demonstrated by their ability to reduce the elevated TNFα and IL-β levels. Andrés and coworkers [37] demonstrated that profound directly lethal drug-induced hepatotoxicity is due to promotion and activation of many inflammatory cytokines such as TNFα and IL-β.
These pro-inflammatory cytokines (TNFα and IL-β levels) can induce apoptosis and worsen liver cell damage by amplifying the inflammatory process. In addition, TNFα and IL-β levels can induce transactivation of NF-κB and can also induce the expression of ICAM-1 (intercellular adhesion molecule-1) an important adhesion molecule on the surface of hepatic endothelial cells. ICAM-1 can induce adhesion between the hepatic endothelial cells and circulating mononuclear cells. Both pathways resulting in the induction of more cytokines expression and more inflammatory reaction [38, 39].
Lettéron et al. [40] have reported that SILY exhibited hepato-protection against the acute damage of the liver cells by carbon tetrachloride (CCL4) via decreasing the level of inflammatory cytokines including TNFα.
The importance of NF-κB in inflammation is based mainly on its ability to regulate the transcription of multiple inflammatory genes including TNFα and IL-β levels; our results showed marked increased in NF-κB tissue level after DOX in intoxication and SILY in combination with CGA attenuated this increase. Our results are inconsistent with Ye et al. [41] who reported that CGA attenuated lipopolysaccharide induced acute kidney injury by inhibiting TLR4/NF-κB signal pathway.
The Akt pathway controls and regulates diverse cellular function such as protein synthesis, metabolism, growth and proliferation [42]. Akt also enhances cell survival via phosphorylation and inactivation of Gsk3β, which is responsible mainly for the activation of oxidative stress mediated cell death [43]. In addition, Akt targets mitochondria to regulate apoptosis and it has been reported to protect the liver via enhancement of cell survival and mitigation of apoptosis [42]. In the present work, SILY and CGA increased Akt activation; in parallel to these results, Guo et al. [44] found that Akt pathway plays a significant role in protection of the liver against oxidative injury. Many studies have shown that AMPK plays a critical role in transition between cell survival and death reported that the up regulation of AMPK enhanced hepatocyte survival and significantly decreased H2O2-induced hepatic necrosis [45]. In our study, DOX induced a significant suppression of AMPK compared with the normal control group. These results are inconsistent with Abbas and Kabil [43] who reported that DOX induced cardiotoxic effects via significant suppression of AMPK.
Zhao et al. [46] have indicated that AMPK phosphorylation inhibited the oxidative stress and gastric epithelial cell apoptosis. Peralta et al. [47] showed that an AMPK activator was useful in reducing liver ischemia reperfusion injury. Additionally, another crucial process enhanced by AMPK is mitochondrial biogenesis. AMPK protects hepatic damage by regulating inflammatory, oxidative stress and mitochondrial dysfunction [48]. Furthermore, AMPK can suppress the production of TNFα and IL6 and inhibit the activation of NF-κB; in addition, AMPK activation can protect hepatocytes from CCL4-induced acute liver damage [49, 50].
Conclusions
We concluded that adding CGA to SILY acts as a protective agent against DOX-induced liver injury through inhibiting apoptosis, maintaining antioxidant enzyme levels, decreasing pro-inflammatory cytokines as well as regulating liver AMPK signaling. This will open novel perspectives for the utilization of this combination as hepatoprotectant agent on DOX receiving cancer patients.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors’ contributions
N.A.T.A. and M.M.A. designed the experiment. All authors contributed to data analysis, drafting, and revising the article, approved the final version, and agreed to be accountable for all aspects of the study.
Conflict of interest statement
There are no conflicts of interest to declare.
Contributor Information
Noha A T Abbas, Faculty of Medicine, Department of Clinical Pharmacology, Zagazig University, Zagazig 44519, Egypt.
Mohammed M Awad, Endocrinology Division, Faculty of Medicine, Department of Internal Medicine, Zagazig University, Zagazig 44519, Egypt.
Ola E Nafea, Faculty of Medicine, Department of Forensic Medicine and Clinical Toxicology, Zagazig University, Zagazig 44519, Egypt; Department of Clinical Pharmacy, College of Pharmacy, Taif University, Taif 11099, Saudi Arabia.
References
- 1. Injac R, Perse M, Cerne M et al. Protective effects of fullerenol C60(OH)24 against doxorubicin-induced cardiotoxicity and hepatotoxicity in rats with colorectal cancer. Biomaterials 2009;30:1184–96. [DOI] [PubMed] [Google Scholar]
- 2. Buraimoh AA, Bako IG, Ibrahim FB. Hepatoprotective effect of ethanolic leave extract of Moringa oleifera on the histology of paracetamol induced liver damage in Wistar rats. Int J Anim Vet Adv 2011;3:10–3. [Google Scholar]
- 3. Wali AF, Rashid S, Rashid SM et al. Naringenin regulates doxorubicin-induced liver dysfunction: impact on oxidative stress and inflammation. Plants 2020;9:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shirani K, Yousefsani BS, Shirani M et al. Protective effects of naringin against drugs and chemical toxins induced hepatotoxicity: a review. Phyther Res 2020;34:1734–44. [DOI] [PubMed] [Google Scholar]
- 5. Prasanna PL, Renu K, Valsala Gopalakrishnan A. New molecular and biochemical insights of doxorubicin-induced hepatotoxicity. Life Sci 2020;250:117599. [DOI] [PubMed] [Google Scholar]
- 6. Arauz J, Ramos-Tovar E, Muriel P. Redox state and methods to evaluate oxidative stress in liver damage: from bench to bedside. Ann Hepatol 2016;15:160–73. [DOI] [PubMed] [Google Scholar]
- 7. Cichoż-Lach H. Oxidative stress as a crucial factor in liver diseases. World J Gastroenterol 2014;20:8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McGill MR, Jaeschke H. Oxidant stress, antioxidant defense, and liver injury In: Kaplowitz N, DeLeve L (eds.), Drug-Induced Liver Dis, 3rd edn. Boston: Academic Press, 2013, 71–84. [Google Scholar]
- 9. Fukumoto LR, Mazza G. Assessing antioxidant and prooxidant activities of phenolic compounds. J Agric Food Chem 2000;48:3597–604. [DOI] [PubMed] [Google Scholar]
- 10. Federico A, Dallio M, Loguercio C. Silymarin/silybin and chronic liver disease: a marriage of many years. Molecules 2017;22:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rašković A, Stilinović N, Kolarović J et al. The protective effects of silymarin against doxorubicin-induced cardiotoxicity and hepatotoxicity in rats. Molecules 2011;16:8601–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Naveed M, Hejazi V, Abbas M et al. Chlorogenic acid (CGA): a pharmacological review and call for further research. Biomed Pharmacother 2018;97:67–74. [DOI] [PubMed] [Google Scholar]
- 13. Singla S, Kumar NR, Kaur J. In vivo studies on the protective effect of propolis on doxorubicin-induced toxicity in liver of male rats. Toxicol Int 2014;21:191–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yagmurca M, Bas O, Mollaoglu H et al. Protective effects of erdosteine on doxorubicin-induced hepatotoxicity in rats. Arch Med Res 2007;38:380–5. [DOI] [PubMed] [Google Scholar]
- 15. Dewanjee S, Joardar S, Bhattacharjee N et al. Edible leaf extract of Ipomoea aquatica Forssk. (Convolvulaceae) attenuates doxorubicin-induced liver injury via inhibiting oxidative impairment, MAPK activation and intrinsic pathway of apoptosis. Food Chem Toxicol 2017;105:322–36. [DOI] [PubMed] [Google Scholar]
- 16. Peredy TR. Clinical manifestations, diagnosis, and treatment In: Wiley JF. (ed.). Amatoxin-Containing Mushroom Poisoning (eg, Amanita phalloides). UpToDate, 2019. [Google Scholar]
- 17. Zuñiga LY, Aceves-De La Mora MCAD, González-Ortiz M et al. Effect of chlorogenic acid administration on glycemic control, insulin secretion, and insulin sensitivity in patients with impaired glucose tolerance. J Med Food 2018;21:469–73. [DOI] [PubMed] [Google Scholar]
- 18. Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J 2008;22:659–61. [DOI] [PubMed] [Google Scholar]
- 19. Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol 1957;28:56–63. [DOI] [PubMed] [Google Scholar]
- 20. Szasz G. A kinetic photometric method for serum gamma-glutamyl transpeptidase. Clin Chem 1969;15:124–36. [PubMed] [Google Scholar]
- 21. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979;95:351–8. [DOI] [PubMed] [Google Scholar]
- 22. Nishikimi M, Appaji N, Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun 1972;46:849–54. [DOI] [PubMed] [Google Scholar]
- 23. Wei Q, Costanzi S, Liu Q-Z et al. Activation of the P2Y1 receptor induces apoptosis and inhibits proliferation of prostate cancer cells. Biochem Pharmacol 2011;82:418–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. David C, Rodrigues G, Bona S et al. Role of quercetin in preventing thioacetamide-induced liver injury in rats. Toxicol Pathol 2011;39:949–57. [DOI] [PubMed] [Google Scholar]
- 25. Damodar G, Smitha T, Gopinath S et al. An evaluation of hepatotoxicity in breast cancer patients receiving injection doxorubicin. Ann Med Health Sci Res 2014;4:74–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Abd Elrazik NA, El-Mesery M, El-Karef A et al. Chlorogenic acid potentiates antitumor effect of doxorubicin through upregulation of death receptors in solid Ehrlich carcinoma model in mice. Egypt J Basic Appl Sci 2019;6:158–72. [Google Scholar]
- 27. Ali N, Rashid S, Nafees S et al. Protective effect of Chlorogenic acid against methotrexate induced oxidative stress, inflammation and apoptosis in rat liver: an experimental approach. Chem Biol Interact 2017;272:80–91. [DOI] [PubMed] [Google Scholar]
- 28. Zimmermann AK, Loucks FA, Schroeder EK et al. Glutathione binding to the Bcl-2 homology-3 domain groove: a molecular basis for Bcl-2 antioxidant function at mitochondria. J Biol Chem 2007;282:29296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Patel N, Joseph C, Corcoran GB et al. Silymarin modulates doxorubicin-induced oxidative stress, Bcl-xL and p53 expression while preventing apoptotic and necrotic cell death in the liver. Toxicol Appl Pharmacol 2010;245:143–52. [DOI] [PubMed] [Google Scholar]
- 30. Koriem KMM, Soliman RE. Chlorogenic and caftaric acids in liver toxicity and oxidative stress induced by methamphetamine. J Toxicol 2014;2014:583494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Karakus E, Karadeniz A, Simsek N et al. Protective effect of Panax ginseng against serum biochemical changes and apoptosis in liver of rats treated with carbon tetrachloride (CCl4). J Hazard Mater 2011;195:208–13. [DOI] [PubMed] [Google Scholar]
- 32. Zhang YW, Shi J, Li YJ et al. Cardiomyocyte death in doxorubicin-induced cardiotoxicity. Arch Immunol Ther Exp (Warsz) 2009;57:435–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ueno M, Kakinuma Y, Yuhki K et al. Doxorubicin induces apoptosis by activation of caspase-3 in cultured cardiomyocytes in vitro and rat cardiac ventricles in vivo. J Pharmacol Sci 2006;101:151–8. [DOI] [PubMed] [Google Scholar]
- 34. Vaughan ATM, Betti CJ, Villalobos MJ. Surviving apoptosis. Apoptosis 2002;7:173–7. [DOI] [PubMed] [Google Scholar]
- 35. Rüdiger HA, Clavien P-A. Tumor necrosis factor alpha, but not Fas, mediates hepatocellular apoptosis in the murine ischemic liver. Gastroenterology 2002;122:202–10. [DOI] [PubMed] [Google Scholar]
- 36. Al-Rasheed N, Faddah L, Al-Rasheed N et al. Protective effects of silymarin, alone or in combination with chlorogenic acid and/or melatonin, against carbon tetrachloride-induced hepatotoxicity. Pharmacogn Mag 2016;12:337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Andrés D, Sánchez-Reus I, Bautista M et al. Depletion of Kupffer cell function by gadolinium chloride attenuates thioacetamide-induced hepatotoxicity. Biochem Pharmacol 2003;66:917–26. [DOI] [PubMed] [Google Scholar]
- 38. Liu SQ, Ye X, Malik AB. Inhibition of NF-κB activation by pyrrolidine dithiocarbamate prevents in vivo expression of proinflammatory genes. Circulation 1999;100:1330–7. [DOI] [PubMed] [Google Scholar]
- 39. Lukacs NW, Strieter RM, Elner VM et al. Intercellular adhesion molecule-1 mediates the expression of monocyte- derived MIP-1α during monocyte-endothelial cell interactions. Blood 1994;83:1174–8. [PubMed] [Google Scholar]
- 40. Lettéron P, Labbe G, Degott C et al. Mechanism for the protective effects of silymarin against carbon tetrachloride-induced lipid peroxidation and hepatotoxicity in mice. Evidence that silymarin acts both as an inhibitor of metabolic activation and as a chain-breaking antioxidant. Biochem Pharmacol 1990;39:2027–34. [DOI] [PubMed] [Google Scholar]
- 41. Ye H-Y, Jin J, Jin L-W et al. Chlorogenic acid attenuates lipopolysaccharide-induced acute kidney injury by inhibiting TLR4/NF-κB signal pathway. Inflammation 2017;40:523–9. [DOI] [PubMed] [Google Scholar]
- 42. Abdelsameea AA, Abbas NAT, Abdel Raouf SM. Liraglutide attenuates partial warm ischemia-reperfusion injury in rat livers. Naunyn Schmiedebergs Arch Pharmacol 2017;390:311–9. [DOI] [PubMed] [Google Scholar]
- 43. Abbas NAT, Kabil SL. Liraglutide ameliorates cardiotoxicity induced by doxorubicin in rats through the Akt/GSK-3β signaling pathway. Naunyn Schmiedebergs Arch Pharmacol 2017;390:1145–53. [DOI] [PubMed] [Google Scholar]
- 44. Guo JY, Yang T, Sun XG et al. Ischemic postconditioning attenuates liver warm ischemia-reperfusion injury through Akt-eNOS-NO-HIF pathway. J Biomed Sci 2011;18:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Saberi B, Shinohara M, Ybanez MD et al. Regulation of H 2 O 2 -induced necrosis by PKC and AMP-activated kinase signaling in primary cultured hepatocytes. Am J Physiol Physiol 2008;295:C50–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhao H, Zhu H, Lin Z et al. Compound 13, an α1-selective small molecule activator of AMPK, inhibits helicobacter pylori -induced oxidative stresses and gastric epithelial cell apoptosis. Biochem Biophys Res Commun 2015;463:510–7. [DOI] [PubMed] [Google Scholar]
- 47. Peralta C. Adenosine monophosphate–activated protein kinase mediates the protective effects of ischemic preconditioning on hepatic ischemia-reperfusion injury in the rat. Hepatology 2001;34:1164–73. [DOI] [PubMed] [Google Scholar]
- 48. Zheng L, Yin L, Xu L et al. Protective effect of dioscin against thioacetamide-induced acute liver injury via FXR/AMPK signaling pathway in vivo. Biomed Pharmacother 2018;97:481–8. [DOI] [PubMed] [Google Scholar]
- 49. Cacicedo JM, Yagihashi N, Keaney JF et al. AMPK inhibits fatty acid-induced increases in NF-κB transactivation in cultured human umbilical vein endothelial cells. Biochem Biophys Res Commun 2004;324:1204–9. [DOI] [PubMed] [Google Scholar]
- 50. Jung JY, Lee CW, Park SM et al. Activation of AMPK by Buddleja officinalis maxim. Flower extract contributes to protecting hepatocytes from oxidative stress. Evidence-Based Complement Altern Med 2017;2017:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]


