Key Points
Question
How common is pulmonary embolism among patients with chronic obstructive pulmonary disease who are admitted to the hospital with acutely worsening respiratory symptoms?
Findings
In this cross-sectional study with prospective follow-up that used a predefined pulmonary embolism diagnostic algorithm and included 740 consecutive patients with chronic obstructive pulmonary disease, pulmonary embolism was detected in 5.9% of patients.
Meaning
Among patients with chronic obstructive pulmonary disease admitted to the hospital with an acute worsening of respiratory symptoms, pulmonary embolism was detected in 5.9% patients using a predefined diagnostic algorithm.
Abstract
Importance
The prevalence of pulmonary embolism in patients with chronic obstructive pulmonary disease (COPD) and acutely worsening respiratory symptoms remains uncertain.
Objective
To determine the prevalence of pulmonary embolism in patients with COPD admitted to the hospital for acutely worsening respiratory symptoms.
Design, Setting, and Participants
Multicenter cross-sectional study with prospective follow-up conducted in 7 French hospitals. A predefined pulmonary embolism diagnostic algorithm based on Geneva score, D-dimer levels, and spiral computed tomographic pulmonary angiography plus leg compression ultrasound was applied within 48 hours of admission; all patients had 3-month follow-up. Patients were recruited from January 2014 to May 2017 and the final date of follow-up was August 22, 2017.
Exposures
Acutely worsening respiratory symptoms in patients with COPD.
Main Outcomes and Measures
The primary outcome was pulmonary embolism diagnosed within 48 hours of admission. Key secondary outcome was pulmonary embolism during a 3-month follow-up among patients deemed not to have venous thromboembolism at admission and who did not receive anticoagulant treatment. Other outcomes were venous thromboembolism (pulmonary embolism and/or deep vein thrombosis) at admission and during follow-up, and 3-month mortality, whether venous thromboembolism was clinically suspected or not.
Results
Among 740 included patients (mean age, 68.2 years [SD, 10.9 years]; 274 women [37.0%]), pulmonary embolism was confirmed within 48 hours of admission in 44 patients (5.9%; 95% CI, 4.5%-7.9%). Among the 670 patients deemed not to have venous thromboembolism at admission and who did not receive anticoagulation, pulmonary embolism occurred in 5 patients (0.7%; 95% CI, 0.3%-1.7%) during follow-up, including 3 deaths related to pulmonary embolism. The overall 3-month mortality rate was 6.8% (50 of 740; 95% CI, 5.2%-8.8%). The proportion of patients who died during follow-up was higher among those with venous thromboembolism at admission than the proportion of those without it at admission (14 [25.9%] of 54 patients vs 36 [5.2%] of 686; risk difference, 20.7%, 95% CI, 10.7%-33.8%; P < .001). The prevalence of venous thromboembolism was 11.7% (95% CI, 8.6%-15.9%) among patients in whom pulmonary embolism was suspected (n = 299) and was 4.3% (95% CI, 2.8%-6.6%) among those in whom pulmonary embolism was not suspected (n = 441).
Conclusions and Relevance
Among patients with chronic obstructive pulmonary disease admitted to the hospital with an acute worsening of respiratory symptoms, pulmonary embolism was detected in 5.9% of patients using a predefined diagnostic algorithm. Further research is needed to understand the possible role of systematic screening for pulmonary embolism in this patient population.
This cross-sectional study characterizes the prevalence of pulmonary embolism defined by a uniform diagnostic algorithm in patients in France with COPD hospitalized with acutely worsening respiratory symptoms.
Introduction
Chronic obstructive pulmonary disease (COPD) is a frequent disease with high morbidity and mortality worldwide.1 From 1969 to 2017, it was the third leading cause of death, and mortality is projected to increase in the next 20 years.2,3 Acute exacerbations of COPD, defined by an acute worsening of respiratory symptoms leading to a change in medication, contribute to this mortality.1
Bronchial infections or air pollution are major causes of acute exacerbation. However, several studies reported a high frequency of pulmonary embolism in these patients.4,5,6,7 In a meta-analysis, patients admitted to the hospital for an unexplained acute COPD exacerbation had a 16% prevalence of pulmonary embolism, albeit with a wide 95% CI (8.3% to 25.8%).8 In a retrospective postmortem analysis, pulmonary embolism was the main cause of death in 21% of patients admitted for acute COPD exacerbation, independently of the premortem suspected cause of exacerbation.9 In addition, patients with COPD more often develop pulmonary embolism than deep vein thrombosis.10
However, how and when patients admitted to the hospital for an acute COPD exacerbation should be screened for pulmonary embolism remains challenging. Well-established diagnostic management of suspected acute pulmonary embolism in the general population might be ineffective in the setting of COPD exacerbations, particularly when including a ventilation-prefusion lung scan.11,12,13,14 Furthermore, clinical presentations of acute pulmonary embolism and COPD exacerbation are similar, making it difficult to determine whether pulmonary embolism should be suspected in this context.15
In the present multicenter cross-sectional study with prospective follow-up, the main objective was to determine the prevalence of pulmonary embolism in patients with COPD admitted to the hospital for acutely worsening respiratory symptoms.
Methods
Ethical Review and Study Organization
The prevalence of symptomatic Pulmonary Embolism in Patients With an Acute Exacerbation of Chronic Obstructive Pulmonary Disease (PEP) study was conducted in accordance with the ethical principles stated in the Declaration of Helsinki, Good Clinical Practice, and relevant French regulations regarding ethics and data protection. The protocol and amendments were approved by a central independent ethics committee (Person Protection Committee Ouest-6, Brest, France), and written informed consent was obtained from all patients before inclusion. The protocol and statistical analysis plan can be found in Supplement 1.
The study was supervised by an academic steering committee. An independent data and safety monitoring committee periodically reviewed study outcomes (recurrent venous thromboembolism, bleeding events, and deaths).
Study Population
From January 8, 2014, to May 22, 2017, consecutive outpatients 18 years or older admitted for acutely worsening respiratory symptoms of COPD to 1 of the 7 French participating centers were eligible (ie, emergency department or respiratory care unit). Availability of previous pulmonary function tests showing a postbronchodilator forced expiratory volume in the first second of expiration:forced vital capacity ratio of less than 70% was required to validate the diagnosis of COPD or a prior diagnosis of COPD by a pulmonologist was required.16 Acutely worsening respiratory symptoms of COPD was defined by a sustained worsening of patients’ baseline symptoms (dyspnea, cough, sputum) beyond normal day-to-day variability and a required treatment modification.
The main exclusion criteria were contraindication to undergoing spiral computed tomographic pulmonary angiography (known allergy to iodine contrast agents); creatinine clearance lower than 30 mL/min/1.73 m2 (to convert to mL/s/m2, multiply by 0.0167); pregnancy; hospitalization for more than 48 hours before inclusion; anticoagulant treatment for a condition other than venous thromboembolism; pneumothorax at admission; severe COPD exacerbation making imaging tests unfeasible; a life expectancy of less than 3 months; and inability to give written informed consent (eMethods in Supplement 2).
Pulmonary Embolism Diagnostic Procedure
Patients were included within 48 hours following hospital admission and immediately classified as having clinically suspected or not suspected pulmonary embolism and as having a diagnosis other than pulmonary embolism more or less likely. These assessments were based on physicians’ judgment; no clinical scores were applied during this step. Thereafter, a predefined standardized algorithm was used to initiate the diagnostic work-up within 48 hours.
Clinical probability of pulmonary embolism was assessed in all patients using the revised Geneva score.17 Patients with a high clinical probability (ie, revised Geneva score ≥11) directly proceeded to spiral computed tomographic pulmonary angiography and legs ultrasound. For patients with a low or intermediate clinical probability (revised Geneva score <11), a D-dimer test was performed, which was interpreted according to the usual cutoff of 500 ng/mL threshold as Fibrinogen Equivalent Units. In patients with a negative D-dimer test result (ie, D-dimer value <500 ng/mL, to convert to nmol/L, multiply by 5476), pulmonary embolism was excluded and no additional testing was performed; these patients were left without anticoagulant therapy.
In case of confirmed venous thromboembolism, anticoagulation was administered according to current guidelines.14 The diagnostic strategy is depicted in the eFigure in Supplement 2. The study protocol had no influence on prophylactic anticoagulation, which was started as recommended within 24 hours following admission in the absence of venous thromboembolism.18
All patients were followed up at 3 months. Patients were instructed to report to the study center immediately if any symptoms or signs suggestive of venous thromboembolism or bleeding occurred between the inclusion and the 3-month follow-up visit. At the end follow-up, all included patients were interviewed at a visit or by phone call by a physician using a structured questionnaire. All events occurring after hospital discharge were collected and charts were reviewed in case of hospital readmission for any cause.
Outcome Measures
The primary outcome was pulmonary embolism diagnosed within 48 hours of admission. The diagnosis of pulmonary embolism was based on the presence of an intraluminal filling defect in a segmental or more proximal pulmonary artery on spiral computed tomographic pulmonary angiography.14 In case of inconclusive computed tomographic angiography (eg, isolated subsegmental pulmonary embolism19), pulmonary embolism diagnosis was based on (1) a segmental or larger perfusion defect with normal ventilation on ventilation-perfusion lung scan; (2) a proximal deep vein thrombosis on lower-limb ultrasonography showing noncompressibility of a proximal vein; or (3) documented pulmonary embolism on autopsy.14
The key secondary outcome was pulmonary embolism that occurred during the 3-month follow-up in patients deemed not to have had venous thromboembolism within 48 hours of admission and who did not receive anticoagulant treatment. Other secondary outcomes were (1) venous thromboembolism at admission, including pulmonary embolism and/or isolated proximal or distal deep vein thrombosis; (2) venous thromboembolism during 3-month follow-up in patients deemed not to have had venous thromboembolism at admission and who had not received anticoagulant treatment; (3) 3-month all-cause mortality in all patients; and (4), alternative diagnoses to venous thromboembolism at admission. Deep vein thrombosis diagnosis was based on lower-limb ultrasonography showing noncompressibility of a distal or a proximal vein.14 Diagnostic tests are described in the eMethods in Supplement 2. All outcomes were adjudicated by an independent clinical events committee.
Sample Size and Statistical Methods
The sample size was estimated to obtain an estimate of the primary outcome. For a prevalence of 10% (based on the outpatient subpopulation in the meta-analysis of Aleva et al8) and a 95% CI comprised between −25% and 25% of the observed percentage of pulmonary embolism at admission, 690 patients were required. This estimate was based on binomial distribution for the exact calculation of the bilateral 95% CI. Considering potential nonevaluable patients (incomplete diagnostic workup, consent withdrawal, etc), the final sample size was 750 patients.
For outcomes analyses, missing data were not replaced. Outcomes and case-fatality rates were calculated (1) in the whole population; (2) whether pulmonary embolism was suspected within 48 hours of admission; and (3) whether venous thromboembolism was diagnosed at admission. The upper limit of the unilateral 95% CI for the 3-month thromboembolic risk should not exceed 3% for a clinically appropriate diagnostic strategy.19 In a post hoc analysis, D-dimer was interpreted according to age-adjusted D-dimer threshold and subsequent absolute reduction of computed tomographic angiography was calculated.20
All tests were 2-sided, and a P value <.05 was considered statistically significant. Because of the potential for type I error due to multiple comparisons, findings for analyses of secondary end points should be interpreted as exploratory. Statistical analyses were performed using SAS version 9.4 software (SAS Institute Inc).
Results
From January 2014 to May 2017, 2268 consecutive patients with COPD and acutely worsened respiratory symptoms were admitted in the 7 participating hospitals and were immediately screened to participate in this study; of those, 1518 presented with exclusion criteria, mainly related to long-term anticoagulation or inability to give informed consent (Figure). Of the remaining 750 patients, 10 were not analyzed for the following reasons: 5 had been previously included at the time of a prior exacerbation, 3 were under guardianship, and 2 withdrew consent after inclusion. Among the remaining 740 patients, inclusion process and initiation of diagnostic workup was performed within 48 hours of admission.
Figure. Study Flow of Patients Hospitalized With COPD With Acutely Worsening Respiratory Symptoms and Prevalence of Pulmonary Embolism .
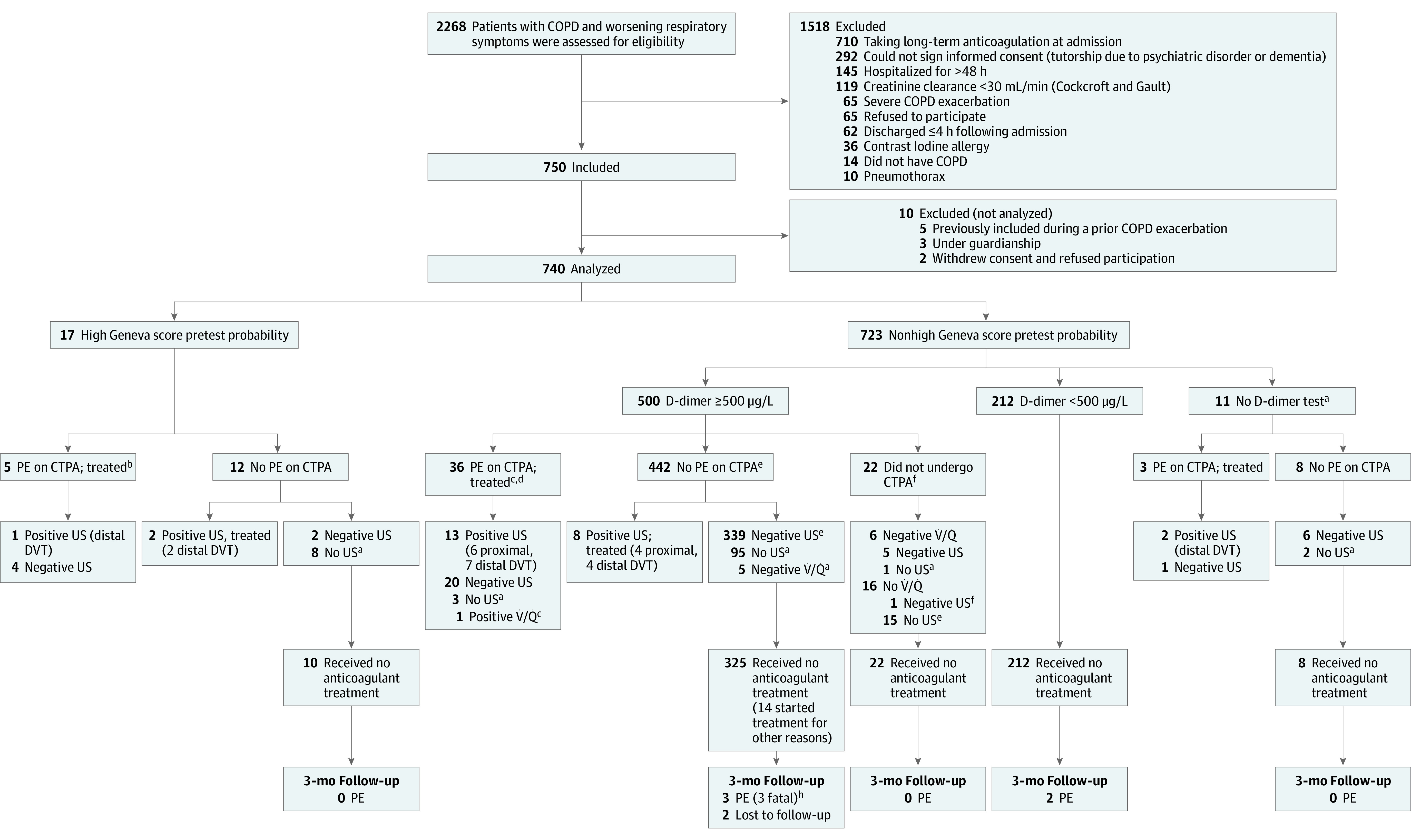
aMinor protocol violation. bThree patients with pulmonary embolism (PE) were not taking long-term anticoagulation before inclusion. cSpiral computed tomographic pulmonary angiography (CTPA) inconclusive (compression US, negative) for 1 patient; V̇/Q̇, high probability; CTPA, positive for PE. dTwo had a PE history but not receiving long-term anticoagulation before inclusion. eCTPA inconclusive for 4 patients: compression US negative; V̇/Q̇ not performed; CTPA, positive for PE. fMajor protocol violation. gThree patients, atrial fibrillation; 2, cancer, and 9, isolated PE not validated. hVenous thromboembolism (VTE) assessment at admission for all 3 patients.
The baseline characteristics of the 740 (mean age, 68.2 years [SD, 10.9 years]; 274 women [37.0%]) analyzed study patients are summarized in Table 1. The following data were missing at admission: tobacco smoking status for 4, prior spirometry for 63, and dyspnea scale for 15 patients. Overall, diagnostic protocol violations occurred among 139 of the 740 included patients (18.8%), 16 (11.5%) were considered major (Figure; eTable 1 in Supplement 2).
Table 1. Baseline Characteristics of Patients.
| No. (%) (N = 740) | |
|---|---|
| Age, mean (SD), y | 68.2 (10.9) |
| Age >65 y | 435 (58.8) |
| Women | 274 (37.0) |
| Men | 466 (63.0) |
| BMI, mean (SD) | 25.7 (6.7) |
| Spirometry, mean (SD), No. | 677a |
| FEV1, % predicted | 0.58 (0.96) |
| FEV1/FVC, % | 53.0 (16.7) |
| Active tobacco use, No. | 736 |
| Current active smokers | 263 (38.7) |
| Ex-smokers | 416 (61.3) |
| Pack-year, mean (SD) | 46.4 (26.4) |
| No. | 679 |
| GOLD stages, No.b | 675 |
| I (mild) | 86 (12.7) |
| II (moderate) | 239 (32.3) |
| III (severe) | 259 (38.4) |
| IV (very severe) | 91 (12.3) |
| No. of previous exacerbations in the past year, median (IQR) | 1.0 (0.0-3.0) |
| Venous thromboembolism risk factors | |
| Prolonged immobilization | 133 (18.0) |
| Familial history | 66 (9.5) |
| Cancer in the past 2 y | 59 (8.0) |
| Previous | 55 (7.4) |
| Surgery | 24 (3.3) |
| Trauma | 15 (2.0) |
| Clinical probability of pulmonary embolism (revised Geneva score)c | |
| Low | 139 (18.8) |
| Intermediate | 584 (78.9) |
| High | 17 (2.3) |
| Pulmonary embolism suspectedd | 299 (40.4) |
| Alternative diagnosis to pulmonary embolism more likelye | 567 (76.6) |
| Severity of COPD exacerbation at hospital admission | |
| MRC dyspnea scale during exacerbation, No.f | 725 |
| Normalg | 7 (0.9) |
| Stage | |
| I (dyspnea at strenuous exercise) | 15 (2.0) |
| II (dyspnea at moderate exercise) | 52 (7.0) |
| III (dyspnea at mild exercise) | 88 (11.9) |
| IV (dyspnea during short walk) | 179 (24.2) |
| V (dyspnea at rest) | 384 (51.9) |
| Heart rate >110/min | 158 (21.4) |
| Systolic blood pressure <100 mm Hg | 19 (2.6) |
| Use of accessory inspiratory muscles | 244 (33.0) |
| Expiratory use of abdominal muscles | 150 (20.3) |
| Cyanosis | 59 (8.0) |
| Neurological impairment | 35 (4.7) |
| Bilateral lower limb edema | 118 (15.9) |
| Oxygen therapy required | 401 (54.3) |
Abbreviations: BMI, body mass index, calculated as weight in kilograms divided by height in meters squared; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in the first second of expiration; FVC, forced vital capacity; GOLD, Global Initiative of Chronic Obstructive Lung Disease; IQR, interquartile range; MRC, Medical Research Council.
Spirometry data were not available for 63 patients; however, all of these patients had a previous assessment of COPD by a pulmonologist.
GOLD stages: stage I, FEV1, more than 80% predicted; stage II, 50% FEV1, less than 80% predicted; stage III, 30% FEV1, less than 50% predicted; and stage IV, FEV1 less than 30% predicted.
Revised Geneva score (range, 0-19): low probability (range, 0-3); intermediate probability (range, 4-10); high probability (≥11).
Suspicion of pulmonary embolism was assessed by a senior physician once the patient arrived at the emergency department based on medical history, physical examination, and physician’s judgment and before any diagnostic tests for pulmonary embolism were ordered.
Assessment of an alternative diagnosis to pulmonary embolism more or less likely was performed by a senior physician once the patient arrived at the emergency department based on medical history, physical examination, and physician’s judgment and before any diagnostic tests for pulmonary embolism were ordered.
MRC scale: grade 1, not troubled by breathlessness except on strenuous exercise; grade 2, short of breath when hurrying on a level surface or walking up a slight hill; grade 3, walks slower than most people on a level surface, stops after a mile or so, or stops after 15 minutes walking at own pace; grade 4, stop for breath after walking about 100 yards or after a few minutes on a level surface; and grade 5, too breathless to leave the house or breathless when undressing.
Five patients had productive cough, 1 had tachycardia, and 1 had chest pain.
Primary Outcome
Among the 740 included patients, 17 (2.3%) had a high Geneva pretest clinical probability: pulmonary embolism was confirmed in 5 patients on spiral computed tomographic pulmonary angiography; 2 were associated with a distal deep vein thrombosis (Figure). For the remaining 12 patients without pulmonary embolism following computed tomographic angiography, an isolated distal deep vein thrombosis was diagnosed in 2 patients, legs compression ultrasonography was negative for 2 patients and was not performed for 8 patients.
Among the 723 patients with a low or moderate pretest clinical probability, a D-dimer assay was performed in 712 patients. D-dimer levels were lower than 500 μg/mL in 212 patients and pulmonary embolism was considered excluded without further work up. Among the 500 patients with a positive D-dimer test result, 478 patients underwent spiral computed tomographic pulmonary angiography, 36 of whom had confirmed pulmonary embolism. Thirteen were associated with deep vein thrombosis (proximal, 6; distal, 7). Among the 442 patients with a negative computed tomographic angiographic result, 8 had an isolated deep vein thrombosis (proximal, 4; distal, 4), 339 had a negative leg compression ultrasonographic result, and 95 did not undergo leg compression ultrasonography. Among the 22 patients with positive D-dimer results who did not undergo computed tomographic angiography, ventilation-perfusion lung scans were performed for 6 patients and did not reveal pulmonary embolism (normal or low probability); of the 16 patients who did not have ventilation-prefusion lung scan, leg compression ultrasonography was negative in 1 patient and was not performed in the remaining 15 patients. Among the 11 patients with a low to moderate pretest clinical probability who did not have D-dimer testing, computed tomographic angiography confirmed pulmonary embolism among 3 patients and was negative in the remaining 8 patients.
Thus, among the 740 included patients, pulmonary embolism was confirmed in 44 patients (5.9%; 95% CI, 4.5%-7.9%) (Figure).
Secondary Outcomes
Within 48 hours of admission, an additional 10 patients had an isolated deep vein thrombosis (1.4%) (4 proximal; 6 distal) yielding an overall prevalence of venous thromboembolism of 7.3% (95% CI, 5.6%-9.4%) at admission (Figure, Table 2). Details regarding pulmonary emboli extent and deep venous thrombosis location within 48 hours of admission are shown in eTables 2 and 3 in Supplement 2. Final causes of acutely worsening respiratory symptoms in COPD patients are listed in Table 3.
Table 2. Causes of Acutely Worsening Respiratory Symptoms at Hospital Admission in Patients With Chronic Obstructive Pulmonary Disease After Pulmonary Embolism Assessment.
| Inclusion delay, h | No. of patients | Anticoagulation, No.a | Hospitalization length, median (IQR), d | At 3 mo, No. | ||
|---|---|---|---|---|---|---|
| Preventive | Curative | Pulmonary embolism follow-up | All-cause death | |||
| Pulmonary embolismb | ||||||
| Total No. | 44 | 9.0 (7.0-14.0) | ||||
| ≤24 | 43 | 0 | 0 | 0 | 12 | |
| >24-≤48 | 1 | 0 | 1 | 0 | 0 | |
| Deep vein thrombosisc | ||||||
| Total No. | 10 | 10.0 (7.0-38.0) | ||||
| ≤24 | 10 | 0 | 10 | 0 | 2 | |
| >24-≤48 | 0 | |||||
| Bacterial bronchial infectiond | ||||||
| Total No. | 462 | 7.0 (4.0-10.0) | ||||
| ≤24 | 452 | 108 | 6 | 3 | 19 | |
| >24-≤48 | 10 | 4 | 0 | 0 | 0 | |
| Pneumoniae | ||||||
| Total No. | 69 | 8.0 (5.0-10.5) | ||||
| ≤24 | 66 | 26 | 0 | 0 | 6 | |
| >24-≤48 | 3 | 3 | 0 | 0 | 0 | |
| Undetermined respiratory failure | ||||||
| Total No. | 69 | 6.0 (3.0-10.0) | ||||
| ≤24 | 67 | 24 | 1 | 10 | 3 | |
| >24-≤48 | 2 | 22 | 0 | 0 | 0 | |
| Pneumothoraxf | ||||||
| Total No. | 2 | 8.5 (3.0-14.0) | ||||
| ≤24 | 2 | 1 | 0 | 0 | 0 | |
| >24-≤48 | 0 | |||||
| Atrial fibrillation | ||||||
| Total No. | 4 | 9.0 (4.5-14.0) | ||||
| ≤24 | 4 | 0 | 4 | 0 | 0 | |
| >24-≤48 | 0 | |||||
| Left cardiac failure | ||||||
| Total No. | 42 | 8.0 (5.0-11.0) | ||||
| ≤24 | 42 | 6 | 2 | 0 | 4 | |
| >24-≤48 | 0 | |||||
| New cancer or worsening of previous cancer | ||||||
| Total No. | 10 | 10.5 (6.0-20.0) | ||||
| ≤24 | 10 | 2 | 1 | 0 | 3 | |
| >24-≤48 | 0 | |||||
| Iatrogenic | ||||||
| Total No. | 2 | 12.5 (9.0-16.0) | ||||
| ≤24 | 2 | 0 | 0 | 0 | 0 | |
| >24-≤48 | 0 | |||||
| Otherg | ||||||
| Total No. | 26 | 5.5 (3.0-15.0) | ||||
| ≤24 | 26 | 6 | 5 | 1 | 1 | |
| >24-≤48 | 0 | |||||
Abbreviation: IQR, interquartile range.
Anticoagulation administered before inclusion.
One patient was classified as having adjudicated pulmonary embolism with bacterial infection.
Nine patients were classified as having adjudicated deep vein thrombosis: 2 patients also had pneumonia; 4, bacterial infection; and 3 worsening respiratory symptoms without evidence for bacterial infection and not requiring antibiotics.
Bacterial infection was based on respiratory failure requiring antibiotics without evidence for pneumonia.
Worsening respiratory symptoms without evidence for bacterial infection and not requiring antibiotics.
Two incomplete pneumothorax, not detected on chest x-ray at hospital admission, were diagnosed on spiral computed tomographic pulmonary angiography performed at inclusion.
No detail was collected for patients classified as “other.”
Table 3. Prevalence of Pulmonary Embolism (With or Without Deep Vein Thrombosis) and Isolated Deep Vein Thrombosis at Inclusion and Thromboembolic Events Rates During 3-Month Follow-up.
| At admission | At 3 mo | ||||||
|---|---|---|---|---|---|---|---|
| No./total (%) [95% CI] | Pulmonary embolism case-fatality rate, No./total (%) | No./total (%) [95% CI] | Pulmonary embolism case-fatality rate, No./total (%) | ||||
| Pulmonary embolism | Isolated deep vein thrombosis | Venous thromboembolism (deep vein thrombosis and/or pulmonary embolism) | Symptomatic pulmonary embolisma | Overall mortality | |||
| All patients | 44/740 (5.9) [4.5-7.9] | 10/740 (1.4) [0.7-2.5] | 54/740 (7.3) [5.6-9.4] | 2/44 (5) | 5/670 (0.7) [0.3-1.7] | 50/740 (6.8) [5.2-8.8] | 3/5 (60) |
| Pulmonary embolismb | |||||||
| Suspected | 30/299 (10.0) [7.1-14.0] | 5/299 (1.7) [0.7-3.9] | 35/299 (11.7) [8.6-15.9] | 1/30 (3) | 2/257 (0.8) [0.2-2.7] | 25/299 (8.4) [5.7-12.1] | 1/2 (50) |
| Not suspected | 14/441 (3.2) [1.9-5.3] | 5/441 (1.1) [0.5-2.6] | 19/441 (4.3) [2.8-6.6] | 1/14 (7) | 3/413 (0.7) [0.2-2.1] | 25/441 (5.7) [3.9-8.3] | 2/3 (66) |
| Likelihood of an alternative diagnosis to pulmonary embolismc | |||||||
| Less likely | 22/173 (12.7) [8.6-18.5] | 4/173 (2.3) [0.9-6.8] | 26/173 (15.0) [10.5-21.4] | 1/22 (5) | 1/143 (0.7) [0.1-3.8] | 14/173 (8.1) [4.9-13.1] | 1/2 (50) |
| More likely | 22/567 (3.9) [2.6-5.8] | 6/567 (1.1) [0.5-2.3] | 28/567 (4.9) [3.4-7.1] | 1/22 (5) | 4/527 (0.8) [0.2-2.1] | 36/567 (6.4) [4.6-8.7] | 2/3 (66) |
Fourteen patients who were in the non–venous thromboembolism group who were receiving anticoagulant treatment for reasons other than adjudicated venous thromboembolism and 2 who were lost-to follow-up were excluded from analysis at 3 months.
Suspicion of pulmonary embolism was assessed by a senior physician once the patient arrived at the emergency department based on medical history, physical examination, and physician’s judgment and before any diagnostic tests for pulmonary embolism were ordered.
Assessment of an alternative diagnosis to pulmonary embolism more or less likely was performed by a senior physician once the patient arrived at the emergency department based on medical history, physical examination, and physician’s judgment and before any diagnostic tests for pulmonary embolism were ordered.
At the 3-month follow-up, among the 686 patients deemed not to have pulmonary embolism or deep vein thrombosis at admission, curative anticoagulation was started in 14 for reasons other than adjudicated venous thromboembolism and 2 were lost-to follow-up. Among the remaining 670 patients, pulmonary embolism was diagnosed during follow-up in 5 patients (0.7%; 95% CI, 0.3%-1.7%): 2 patients had objectively confirmed pulmonary embolism, and death was related to fatal pulmonary embolism in 3 patients (Figure, Table 2). No isolated cases of deep venous thrombosis were detected at 3 months.
The overall 3-month mortality rate was 6.8% (50 of 740; 95% CI, 5.2%-8.8%). The adjudicated causes of deaths are shown in Table 4. Three-month mortality rates among patients with venous thromboembolism at admission was 14 (25.9%) of 54 vs 36 (5.2%) of 686 among those without it (risk difference, 20.7%; 95% CI, 10.7%-33.8%; P < .001) (eTable 4 in Supplement 2).
Table 4. Causes of Death.
| Causes of deatha | No. (%) of patients | ||
|---|---|---|---|
| Total (n = 50) | Venous thromboembolism within 48 h of admission | ||
| No (n = 36) | Yes (n = 14) | ||
| Sepsis | 13 (26.0) | 9 (25.0) | 4 (28.6) |
| Acute exacerbation of COPD | 12 (24.0) | 10 (27.8) | 2 (14.3) |
| Cancer | 11 (22.0) | 5 (13.9) | 6 (42.9)b |
| Pulmonary embolism | 5 (10.0) | 3 (8.3)c | 2 (14.3)d |
| Aspiration pneumonia | 2 (4.0) | 2 (5.6) | 0 |
| End-stage COPD | 2 (4.0) | 2 (5.6) | 0 |
| Hemorrhagic stroke | 1 (2.0) | 1 (2.8) | 0 |
| Ischemic stroke | 1 (2.0) | 1 (2.8) | 0 |
| Acute cardiac failure | 1 (2.0) | 1 (2.8) | 0 |
| Digestive occlusion | 1 (2.0) | 1 (2.8) | 0 |
| Ischemic myocardial infarction | 1 (2.0) | 1 (2.8) | 0 |
Abbreviation: COPD, chronic obstructive pulmonary disease.
All outcomes were adjudicated by an independent clinical events committee. Causes of death were mutually exclusive. Patients with terminal illness (eg, cancer patients in palliative care) were considered as having died of the terminal illness, unless death circumstances indicated clearly that pulmonary embolism was most likely the immediate cause of death and significantly shortened life expectancy.
Of the 6 deaths related to cancer, 4 were related to newly diagnosed cancer during follow-up.
Fatal pulmonary embolism occurred at 23, 37, and 46 days.
Death related to pulmonary embolism diagnosed at admission occurred at 13 and 51 days.
Among the 299 patients suspected at admission of having pulmonary embolism, 30 (10%) were diagnosed vs 14 patients (3.2%) of 441 who were not suspected at admission were diagnosed with pulmonary embolism (risk difference, 6.9%; 95% CI, 3.3%-11.0%; P < .001). Twenty-two patients (12.7%) of 173 for whom pulmonary embolism was considered by the physician as the most likely diagnosis (more than any other diagnosis) had a pulmonary embolism vs 22 patients (3.9%) of 567 for whom pulmonary embolism was considered a less likely diagnosis had a pulmonary embolism (risk difference, 8.8%; 95% CI, 4.3%-14.8%; P < .001). Three-month mortality was similar among these subgroups (Table 2; eTable 4 in Supplement 2).
In post hoc analysis (eTable 5 in Supplement 2), applying the age-adjusted D-dimer threshold would have reduced the use of computed tomographic angiographies performed by 22%; however, among the 110 patients 50 years or older who had D-dimer concentrations higher than 500 ng/mL and less than the age-adjusted threshold, 4 patients had venous thromboembolism diagnosed at admission and 1 had fatal pulmonary embolism within 3 months (4.5%, 95% CI, 2.0%-10.2%).
Discussion
In this cross-sectional study with prospective follow-up including 740 patients with COPD admitted to the hospital for acutely worsening respiratory symptoms, systematic screening for pulmonary embolism within 48 hours of hospital admission showed a pulmonary embolism prevalence of 5.9%, and an additional 1.4% of patients had an isolated deep vein thrombosis. The prevalence of pulmonary embolism reached 10% when it was suspected, and remained at more than 3% among patients without a clinical suspicion.21 Among the 670 patients for whom the diagnosis of venous thromboembolism was ruled-out at inclusion and who did not receive anticoagulant therapy during the 3-month follow-up, 0.7% developed pulmonary embolism.
The overall prevalence of pulmonary embolism in patients with COPD admitted to the hospital for acutely worsening respiratory symptoms in this study (5.9%) is lower than the 16.1% reported in a meta-analysis involving 880 patients.8 However, the comparison with the 7 studies included in this meta-analysis is difficult due to low sample sizes (<200 patients in the largest study). In addition, the heterogeneity across studies in terms of pulmonary embolism diagnostic algorithms, COPD assessment and study populations, with some studies recruiting only patients with no evident cause of exacerbation other than suspected pulmonary embolism and others analyzing only patients with severe acutely worsening respiratory symptoms of COPD renders definitive conclusions difficult.5,6,7,8 Therefore, to our knowledge, this study is the largest prospective study assessing the prevalence of pulmonary embolism—whether the diagnosis was suspected or not—using a predefined and standardized diagnostic algorithm for pulmonary embolism within 48 hours following admission among patients with documented COPD (ie, using validated criteria16) who were admitted to the hospital for acutely worsening respiratory symptoms.14,16 Because all pulmonary embolism events were adjudicated and confirmed by an independent clinical events committee using predefined, standardized, and validated criteria,14 this study provides a valid and reliable prevalence estimate of pulmonary embolism in this setting.
Among patients in whom pulmonary embolism was suspected at admission, the prevalence of pulmonary embolism (10%) was close to that observed in recent diagnostic management studies performed in unselected outpatients with a suspicion of pulmonary embolism.22,23 However, in patients without a clinical suspicion of pulmonary embolism, the prevalence of venous thromboembolism (3.2%, Table 2) cannot be considered as negligible, which raises the question of whether performing a systematic search for pulmonary embolism even in this subpopulation should occur. The observation of additional patients with isolated proximal deep vein thrombosis further supports such consideration.
During the 3-month follow-up, only 0.7% of patients (95% CI, 0.3%-1.7%) considered not to have venous thromboembolism within 48 hours of admission and not treated with an anticoagulant agent developed pulmonary embolism. Among patients with clinically suspected pulmonary embolism, the upper limit of the 95% CI was also lower than the safety threshold of 3% proposed by the subcommittee of the International Society of Thrombosis and Hemostasis.21 It remains to be determined in larger studies if, after a negative computed tomographic angiographic scan, a leg compression ultrasound should be systematically added when patients with COPD and acutely worsening respiratory symptoms are admitted.24 It is an important question because this practice is no longer recommended for patients with suspected pulmonary embolism.14
Consistent with other studies, mortality was higher among patients with pulmonary embolism than it was for patients without it.10,25,26,27 As shown in Table 4, 42% (6 of 14) of adjudicated deaths among those in the venous thromboembolism group were related to cancer. It is also known that the prognosis of patients with cancer is poorer when associated with associated venous thromboembolism.28 In practice, clinicians should consider the probability of an underlying cancer when pulmonary embolism is diagnosed in patients with COPD exacerbation.
Limitations
This study has several limitations. First, patients with mild acutely worsening respiratory symptoms, as well as those with severe respiratory failure, were likely underrepresented, so that these finding may not apply to such patients. However, in this study, these criteria corresponded with usual practice in the setting of patients with COPD admitted to the hospital for acutely worsened respiratory symptoms. Second, among the 686 patients considered not to have venous thromboembolism within 48 hours of admission, 121 (17.6%) did not complete the full initial assessment for pulmonary embolism, predominantly due to limited access to leg ultrasound (Figure; eTable 1 in Supplement 2). These (mostly minor) protocol violations may have slightly underestimated venous thromboembolism prevalence at inclusion, but all these patients were followed-up and none had a symptomatic venous thromboembolic event within 3 months. Third, it remains possible that having a clinical suspicion of pulmonary embolism by emergency department physicians contributed to the decision to admit some patients, which potentially biased the sample toward a higher pulmonary embolism frequency in this study. Fourth, among patients with low to moderate pretest clinical probability, the age-adjusted D-dimer threshold was not applied, the study having started before the prospective validation of this threshold in unselected patients.20 Based on post hoc analysis, whether the age-adjusted D-dimer threshold algorithm excludes pulmonary embolism and is a cost-effective diagnostic test for patients with COPD remains to be confirmed in larger prospective study.
Conclusions
Among patients with chronic obstructive pulmonary disease admitted to the hospital with an acute worsening of respiratory symptoms, pulmonary embolism was detected in 5.9% of patients using a predefined diagnostic algorithm. Further research is needed to understand the possible role of systematic screening for pulmonary embolism in this patient population.
Trial Protocol and Statistical Analysis
eMethods
eFigure 1. Pulmonary embolism algorithm
eTable 1. Protocol violations (CUS, CT, lung scan) and outcome at 3 months
eTable 2. Clinical extent of venous thromboembolism at inclusion
eTable 3. Characteristics of segmental or more proximal pulmonary embolism and isolated proximal deep vein thrombosis at admission and adverse events during follow-up
eTable 4. Three-month mortality according to whether patients had venous thromboembolism at inclusion or not and according to whether pulmonary embolism was suspected or not
eTable 5. Prevalence of pulmonary embolism (with or without deep vein thrombosis) and isolated deep vein thrombosis at inclusion and thromboembolic events rates during 3-month follow-up according to age-adjusted D-dimer threshold
eReferences
References
- 1.Singh D, Agusti A, Anzueto A, et al. . Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease. Eur Respir J. 2019;53(5):1900164. doi: 10.1183/13993003.00164-2019 [DOI] [PubMed] [Google Scholar]
- 2.GBD 2017 Causes of Death Collaborators Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017. Lancet. 2018;392(10159):1736-1788. doi: 10.1016/S0140-6736(18)32203-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ma J, Ward EM, Siegel RL, Jemal A. Temporal trends in mortality in the United States, 1969-2013. JAMA. 2015;314(16):1731-1739. doi: 10.1001/jama.2015.12319 [DOI] [PubMed] [Google Scholar]
- 4.Sapey E, Stockley RA. COPD exacerbations, 2: aetiology. Thorax. 2006;61(3):250-258. doi: 10.1136/thx.2005.041822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tillie-Leblond I, Marquette CH, Perez T, et al. . Pulmonary embolism in patients with unexplained exacerbation of chronic obstructive pulmonary disease. Ann Intern Med. 2006;144(6):390-396. doi: 10.7326/0003-4819-144-6-200603210-00005 [DOI] [PubMed] [Google Scholar]
- 6.Akpinar EE, Hoşgün D, Akpinar S, Ataç GK, Doğanay B, Gülhan M. Incidence of pulmonary embolism during COPD exacerbation. J Bras Pneumol. 2014;40(1):38-45. doi: 10.1590/S1806-37132014000100006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rizkallah J, Man SFP, Sin DD. Prevalence of pulmonary embolism in acute exacerbations of COPD. Chest. 2009;135(3):786-793. doi: 10.1378/chest.08-1516 [DOI] [PubMed] [Google Scholar]
- 8.Aleva FE, Voets LWLM, Simons SO, de Mast Q, van der Ven AJAM, Heijdra YF. Prevalence and localization of pulmonary embolism in unexplained acute exacerbations of COPD. Chest. 2017;151(3):544-554. doi: 10.1016/j.chest.2016.07.034 [DOI] [PubMed] [Google Scholar]
- 9.Zvezdin B, Milutinov S, Kojicic M, et al. . A postmortem analysis of major causes of early death in patients hospitalized with COPD exacerbation. Chest. 2009;136(2):376-380. doi: 10.1378/chest.08-2918 [DOI] [PubMed] [Google Scholar]
- 10.Bertoletti L, Quenet S, Mismetti P, et al. ; RIETE Investigators . Clinical presentation and outcome of venous thromboembolism in COPD. Eur Respir J. 2012;39(4):862-868. doi: 10.1183/09031936.00058811 [DOI] [PubMed] [Google Scholar]
- 11.Hartmann IJ, Hagen PJ, Melissant CF, Postmus PE, Prins MH; ANTELOPE Study Group . Diagnosing acute pulmonary embolism. Am J Respir Crit Care Med. 2000;162(6):2232-2237. doi: 10.1164/ajrccm.162.6.2006030 [DOI] [PubMed] [Google Scholar]
- 12.Gotway MB, Edinburgh KJ, Feldstein VA, Lehman J, Reddy GP, Webb WR. Imaging evaluation of suspected pulmonary embolism. Curr Probl Diagn Radiol. 1999;28(5):129-184. doi: 10.1016/S0363-0188(99)90018-X [DOI] [PubMed] [Google Scholar]
- 13.Gleeson FV, Turner S, Scarsbrook AF. Improving the diagnostic performance of lung scintigraphy in suspected pulmonary embolic disease. Clin Radiol. 2006;61(12):1010-1015. doi: 10.1016/j.crad.2006.05.017 [DOI] [PubMed] [Google Scholar]
- 14.Konstantinides SV, Meyer G, Becattini C, et al. ; ESC Scientific Document Group . 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41(4)543-603. doi: 10.1093/eurheartj/ehz405 [DOI] [PubMed] [Google Scholar]
- 15.Moua T, Wood K. COPD and PE: a clinical dilemma. Int J Chron Obstruct Pulmon Dis. 2008;3(2):277-284. doi: 10.2147/copd.s1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Celli BR, MacNee W; ATS/ERS Task Force . Standards for the diagnosis and treatment of patients with COPD. Eur Respir J. 2004;23(6):932-946. doi: 10.1183/09031936.04.00014304 [DOI] [PubMed] [Google Scholar]
- 17.Le Gal G, Righini M, Roy PM, et al. . Prediction of pulmonary embolism in the emergency department. Ann Intern Med. 2006;144(3):165-171. doi: 10.7326/0003-4819-144-3-200602070-00004 [DOI] [PubMed] [Google Scholar]
- 18.Kahn SR, Lim W, Dunn AS, et al. . Prevention of VTE in nonsurgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl 2):e195S-e226S. doi: 10.1378/chest.11-2296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carrier M, Righini M, Wells PS, et al. . Subsegmental pulmonary embolism diagnosed by computed tomography. J Thromb Haemost. 2010;8(8):1716-1722. doi: 10.1111/j.1538-7836.2010.03938.x [DOI] [PubMed] [Google Scholar]
- 20.Righini M, Van Es J, Den Exter PL, et al. . Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism: the ADJUST-PE study. JAMA. 2014;311(11):1117-1124. doi: 10.1001/jama.2014.2135 [DOI] [PubMed] [Google Scholar]
- 21.Dronkers CEA, van der Hulle T, Le Gal G, et al. . Towards a tailored diagnostic standard for future diagnostic studies in pulmonary embolism. J Thromb Haemost. 2017;15(5):1040-1043. doi: 10.1111/jth.13654 [DOI] [PubMed] [Google Scholar]
- 22.Stein PD, Fowler SE, Goodman LR, et al. ; PIOPED II Investigators . Multidetector computed tomography for acute pulmonary embolism. N Engl J Med. 2006;354(22):2317-2327. doi: 10.1056/NEJMoa052367 [DOI] [PubMed] [Google Scholar]
- 23.Kearon C, de Wit K, Parpia S, et al. . Diagnosis of Pulmonary Embolism with D-dimer adjusted to clinical probability. N Engl J Med. 2019;381(22):2125-2134. doi: 10.1056/NEJMoa1909159 [DOI] [PubMed] [Google Scholar]
- 24.Jiménez D, Agustí A, Monreal M, et al. ; SLICE investigators . The rationale, design, and methods of a randomized, controlled trial to evaluate the efficacy and safety of an active strategy for the diagnosis and treatment of acute pulmonary embolism during exacerbations of chronic obstructive pulmonary disease. Clin Cardiol. 2019;42(3):346-351. doi: 10.1002/clc.23161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gunen H, Gulbas G, In E, Yetkin O, Hacievliyagil SS. Venous thromboemboli and exacerbations of COPD. Eur Respir J. 2010;35(6):1243-1248. doi: 10.1183/09031936.00120909 [DOI] [PubMed] [Google Scholar]
- 26.Carson JL, Terrin ML, Duff A, Kelley MA. Pulmonary embolism and mortality in patients with COPD. Chest. 1996;110(5):1212-1219. doi: 10.1378/chest.110.5.1212 [DOI] [PubMed] [Google Scholar]
- 27.Lee AY, Rickles FR, Julian JA, et al. . Randomized comparison of low molecular weight heparin and coumarin derivatives on the survival of patients with cancer and venous thromboembolism. J Clin Oncol. 2005;23(10):2123-2129. doi: 10.1200/JCO.2005.03.133 [DOI] [PubMed] [Google Scholar]
- 28.Chew HK, Wun T, Harvey D, Zhou H, White RH. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch Intern Med. 2006;166(4):458-464. doi: 10.1001/archinte.166.4.458 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol and Statistical Analysis
eMethods
eFigure 1. Pulmonary embolism algorithm
eTable 1. Protocol violations (CUS, CT, lung scan) and outcome at 3 months
eTable 2. Clinical extent of venous thromboembolism at inclusion
eTable 3. Characteristics of segmental or more proximal pulmonary embolism and isolated proximal deep vein thrombosis at admission and adverse events during follow-up
eTable 4. Three-month mortality according to whether patients had venous thromboembolism at inclusion or not and according to whether pulmonary embolism was suspected or not
eTable 5. Prevalence of pulmonary embolism (with or without deep vein thrombosis) and isolated deep vein thrombosis at inclusion and thromboembolic events rates during 3-month follow-up according to age-adjusted D-dimer threshold
eReferences


