Abstract
The ubiquitin-proteasome system plays diverse regulatory and homeostatic roles in mammalian reproduction. Ubiquitin ligases are the substrate-specific mediators of ubiquitin-binding to its substrate proteins. The NEDD4-like ubiquitin ligase 2 (aliases NEDL2, HECW2) is a HECT-type ubiquitin ligase that contains one N-terminal HECW ubiquitin ligase domain, one C-terminal HECT ubiquitin ligase domain, one C2 domain, and two WW protein‐protein interaction modules. Beyond its predicted ubiquitin-ligase activity, its cellular functions are largely unknown. Current studies were designed to investigate the content and distribution of NEDL2 in porcine spermatozoa, oocytes, zygotes, and early preimplantation embryos, and in cumulus cells before and after in vitro maturation with oocytes, and fibroblast cells as positive control by western blot and immunocytochemistry, and to examine its roles during oocyte fertilization. Multiple isoforms of NEDL2 were identified by WB. One at approximately 52 kDa was detected only in the germinal vesicle (GV) stage and metaphase II oocytes, and in early preimplantation embryos. Other isoforms were high mass bands at 91, 136, and 155 kDa, which were only detected in somatic cells. Interestingly, ejaculated spermatozoa prominently displayed the same 52 kDa band as oocytes; they also had two minor bands of 74 and 129 kDa, which were not detected in somatic cells or oocytes. By immunofluorescence, NEDL2 showed a diffused cytoplasmic localization in all cell types and accumulated in distinct foci in the germinal vesicles (GVs) of immature oocytes, in maternal and paternal pronuclei of zygotes and nuclei of embryo blastomeres and somatic cells. In blastocysts, the labeling intensity of NEDL2 was stronger in the inner cell mass than in trophoblast, indicating higher NEDL2 content in the ICM cells than in trophectoderm. NEDL2 abundance was 10 times higher in post-maturation oocyte-surrounding cumulus cells than that of cumulus cells before in vitro maturation with hormones, indicating that NEDL2 may have a unique role in cumulus cells after ovulation. Microinjection of anti-NEDL2 antibody into oocyte before IVF did not affect the percentage of oocytes fertilized, percentage of oocytes cleaved, or blastocyst formation. However, the anti-NEDL2 antibody decreased the number of pronuclei, accelerated the formation of nuclear precursor bodies at 6 h postfertilization, inhibited sperm DNA decondensation, and resulted in more fertilized oocytes without male pronuclear formation. In summary, NEDL2 may play a key role during fertilization, especially during sperm DNA decondensation.
Keywords: fertilization, oocyte, sperm, ubiquitin, HECW2, NEDL2
NEDL2 is present in the mammalian gametes, zygotes, and preimplantation embryos and is implied in the sperm DNA decondensation and formation of male pronucleus.
Introduction
The ubiquitin-proteasome system (UPS) plays diverse regulatory and homeostatic roles in many basic cellular processes including differentiation, cell cycle control, apoptosis, and immune response [1, 2] and mammalian reproduction such as spermatogenesis, capacitation, and fertilization [3, 4]. Ubiquitin is a highly conserved protein of 76 amino acid residues, which is attached covalently to substrate proteins via an energy-dependent three-step process, involving an E1 ubiquitin-activating enzyme, an E2 ubiquitin-conjugating enzyme, and an E3 ubiquitin-protein ligase. The E3 ubiquitin ligase largely determines the substrate specificity of the system and in mammals, there are hundreds of ubiquitin-protein ligases [2]. They can be grouped into two main classes: the RING (Really Interesting New Gene) E3s, which mediate the direct transfer of ubiquitin to the substrate [5], and the HECT (Homologous to E6-AP C-Terminus) E3s, which are involved in the transfer of activated ubiquitin from the E2 to the substrate by forming an intermediate complex with the C-terminus of the E3 [6].
The NEDD4-like ubiquitin ligase 2 (NEDL2, also called HECW2) are the members of NEDD4 subfamily of E3 ubiquitin ligases, which share a similar domain composition: an N-terminal HECW domain of which the function is not clearly understood, and might determine the substrate specificity of the ligase or even posses E3 ligase activity, a C2 domain that mediates anchoring of E3s to intracellular membranes, two WW modules that mediate protein–protein interaction, and a catalytic HECT domain at the C-terminus. It was demonstrated that NEDL2 protein interacts with two isoforms of p73, α, and β (p73 a tumor protein related to the p53); it binds to p73, and ubiquitinates p73. This ubiquitination leads to the stabilization of p73α/β and increased p73α transcriptional activities [7]. NEDL2 is involved in the regulation of cell cycles, specifically transition from metaphase to anaphase. It has the potential to interact with many other functional enzymes/proteins as indicated by immunoprecipitation (IP) and mass spectrometry study [8]. Thus, the homozygote mutated NEDL2−/− mice died within 2 weeks after birth [9].
WW domains, named after its two highly conserved tryptophan (Trp) residues are small functional domains, found in signaling proteins, which mediate protein‐protein interactions in a similar but distinct manner from Src homology three domains [10]. WW domain-containing proteins and their cognate ligands have been demonstrated to be involved in a wide spectrum of cellular events including cell cycle control (Pin1/Ess1), ubiquitin ligation (Nedd4/Rsp5 and smurf1), and transcriptional activation (YAP65) [10, 11]. Relevant to this study, the group I WW domain-containing proteins (i.e., YAP65, Nedd4, and dystrophin) recognize proline-rich effector molecules with a minimal consensus sequence of PY motif [12].
Beyond its predicted ubiquitin-ligase activity, its exact cellular functions in mammalian fertilization and embryo development are not known and have never been studied before. To our knowledge, there is also no detailed study describing the distribution and expression of NEDL2 in an animal oocyte and embryo. Because NEDL2 contains two WW domains, NEDL2 could be engaged during fertilization by the WW-binding motifs of the sperm-released postacrosomal sheath WW domain-binding protein (PAWP). A candidate component of the sperm-borne oocyte-activating factor, PAWP elicits calcium oscillations, activates oocytes, and promotes pronuclear development following microinjection of PAWP protein or human/porcine Pawp cRNA [13–16].
The objective of the present study is to investigate the content and distribution of NEDL2 protein in porcine spermatozoa, oocytes zygotes, and early preimplantation embryos, as well as in somatic cell controls (cumulus and day 35 fetal fibroblast cells). Furthermore, we aim to determine its roles during oocyte fertilization and early embryo development by using a function-blocking method after microinjection of anti-NEDL2 antibody into matured oocytes and followed by in vitro fertilization (IVF). We postulate that NEDL2 plays a role during oocyte fertilization and embryo development. For the very first time, we demonstrate that: (1) gametic and somatic NEDL2 isoforms are present in the pig; (2) NEDL2 is predominantly present in embryo blastomere and somatic cellular nucleus; and (3) NEDL2 plays an important role in fertilization, especially during sperm DNA decondensation.
Materials and methods
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures involving vertebrate animals were completed under the strict guidance of an Animal Care and Use protocol approved by the Animal Care and Use Committee of the University of Missouri. This article does not contain any studies with human participants performed by any of the authors.
Chemicals, antibody, and recombinant protein
All chemicals used in this study were purchased from Sigma Chemical Co. (St. Louis, MO) unless otherwise stated. The main anti-NEDL2 antibody (ab154888, Abcam, Cambridge, MA) used in this study was a rabbit polyclonal antibody raised against a synthetic peptide, corresponding to a region within N terminal amino acids 1–44 of human NEDL2. The human amino acid 1–44 sequence is 100% homologous with porcine sequence (while the full sequence was 91.6% homologous). This N-terminal portion is immediately adjacent to a HECW domain, and thus the said antibody is predicted to be function-blocking. The second anti-NEDL2 primary antibody used to confirm and validate experimental results was also a rabbit polyclonal antibody raised against synthetic peptide derived from an internal region of human NEDL2 (ab92711, Abcam). Agarose-immobilized, p62-derived UBA domain for affinity purification of ubiquitinated proteins [17] was purchased from Biomol (cat. # BML-UW9010-0500, Biomol, Plymouth Meeting, PA). Affinity-purified goat anti-rabbit IgG FITC secondary antibody (cat. # 65–6111) and goat anti-rabbit IgG (H + L) cross-absorbed TRITC (cat. # T-2769) secondary antibodies were purchased from Invitrogen— ThermoFisher (Rockford, IL).
Porcine oocyte maturation, antibody preparation, and microinjection
Detailed procedures for oocyte collection and in vitro maturation have been described previously [18]. Briefly, ovaries from prepubertal gilts were collected at a local slaughterhouse and transported to the laboratory in a warm box (25–30°C). Cumulus oocyte complexes (COCs) were aspirated from antral follicles (3–6 mm in diameter). Oocytes with uniform ooplasm and compact cumulus were collected and washed three times in HEPES-buffered Tyrode lactate (TL-HEPES, pH 7.4) medium containing 0.1% (w/v) polyvinyl alcohol (PVA) and one-time wash with the maturation medium [18]. A total of 50 COCs were transferred to 500 μL of the maturation medium that had been covered with mineral oil in a 4-well plate (Nunc) and equilibrated at 38.5°C, 5% CO2 in the air. Oocyte maturation medium was tissue culture medium (TCM) 199 (Mediatech, Manassas, VA) supplemented with 0.1% PVA, 3.05 mM D-glucose, 0.91 mM sodium pyruvate, 0.57 mM cysteine, 0.5 μg/mL LH (L 5269, Sigma), 0.5 μg/mL FSH (F 2293, Sigma), 10 ng/mL epidermal growth factor (E 4127; Sigma), 10% porcine follicular fluid, 75 μg/mL penicillin G, and 50 μg/mL streptomycin. After 40 h in vitro maturation, cumulus cells were removed with 0.1% hyaluronidase in TL-HEPES-PVA medium and the oocytes were washed three times with TL-HEPES-PVA medium and transferred into HEPES-buffered manipulation medium TCM-199 supplemented with 0.6 mM NaHCO3, 2.9 mM HEPES, 30 mM NaCl, 0.3% (wt/v) BSA, and 0.01 mg/mL gentamicin (pH 7.4) for microinjections.
Before microinjection, the anti-NEDL2 polyclonal antibody was desalted using Zeba spin desalting columns (cat # 89890, ThermoFischer Scientific). The final antibody concentration was 0.5 mg/mL, with approximately 10 pL injected per oocyte. Microinjection was performed on the stage of a Zeiss Axiovert-35 inverted microscope (Jena, Germany) fitted with Eppendorf micromanipulators and Femtojet 5247 injector (Eppendorf, Hauppauge, NY). Through a glass micropipette, the antibody was microinjected to 3–5% of oocyte volume, as estimated from the displacement caused by injections. Surviving oocytes were washed three times in the fertilization medium and used for IVF.
IVF, culture of putative zygotes, and assessment of developmental ability
According to the experimental design, both non-manipulated (samples collected for immunocytochemistry (ICC) and Western blot analysis) and sham/antibody injected (for functional blocking analysis) MII oocytes were used for IVF. Thereafter, 30–35 oocytes were placed into each of 100-μl drops of a modified Tris-buffered medium (mTBM) containing 2 mM caffeine and 0.2% BSA, covered with mineral oil, in a 35-mm polystyrene petri dish, which had been equilibrated for 48 h at 38.5 8C in 5% CO2 in the air. The dishes were kept in a CO2 incubator until spermatozoa were added for fertilization.
In the current study, to eliminate variation in IVF experiments among different boars, the sperm-rich fraction of ejaculate, from a known fertile boar, was collected on a weekly basis and used for IVF 24 and 48 h later. The semen was immediately checked for motility, and only those ejaculates with 80–90% were used for further experiments. Sperm samples used for WB were washed three times in TBS right after collection and stored at −20°C until further use. For IVF, semen was cooled to ambient temperature and centrifuged at 600 g for 10 min to remove the seminal plasma. The sperm pellets were resuspended with Beltsville Thawing Solution extender (Minitube, Delavan, WI) to the final concentration of 1 × 108 cell/mL. On fertilization day, semen was diluted 10 times in mTBM. Then 1 μL of diluted semen was added into oocyte-containing fertilization droplets with the final sperm concentration of 1 × 105 cells/mL. Oocytes were co-incubated with spermatozoa for up to 6 h at 38.5°C, 5% CO2.
At the end of spermatozoa‐oocyte co-incubation, putative embryos were washed three times and transferred to four-well plates containing 500 mL of porcine zygote culture medium 3 (PZM3) (108.0 mmol/L NaCl, 10.0 mmol/L KCl, 0.35 mmol/L KH2PO4, 0.4 mmol/L MgSO4.7H2O, 25.07 mmol/L NaHCO3, 0.2 mmol/L sodium pyruvate, 2.0 mmol/L Calcium lactate (ThermoFisher Scientific, Pittsburg, PA), 1.0 mmol/L glutamine, 5.0 mmol/L hypotaurine, 20 mL/L Eagle basal medium amino acid solution, 10 mL/L modified Eagle medium amino acid solution, 0.01 mg/mL gentamicin, and 3 mg/mL bovine serum albumin (BSA, pH 7.3)) and incubated at 38.5 °C, 5% CO2. At 48 and 168 h after insemination, cleavage rate and blastocyst formation rate were evaluated under a stereomicroscope, respectively. Only embryos with blastomeres of equal size were counted to determine the cleavage rate.
Immunofluorescence
A standard immunofluorescence procedure, as described previously [19], was performed for ICC of samples. GV stage oocytes before in vitro maturation were treated with hyaluronidase to disassociate cumulus cells. Metaphase II (MII) oocytes and cumulus cells before and after 40 h maturation and different-stage embryos, from zygotes to 2-, 4-, 8-cell, and d7 blastocysts were collected at different time points after IVF (6–168 h) according to the embryo development stage. Zona pellucida was removed by short (5 s) incubation in acidic PBS (pH = 2). Oocytes, embryos, and cell samples were fixed in 2% formaldehyde for 40 min at room temperature, washed, permeabilized in PBS with 0.1% (v/v) Triton X-100 (PBST), and blocked for 25 min in PBST containing 5% normal goat serum in 9-well plates. Spermatozoa were allowed to settle onto polylysine-coated coverslips, fixed in 2% formaldehyde, and all the following steps were carried out on coverslips. Samples were incubated overnight at 4°C with primary antibody (Ab154888 diluted at 1:200 in PBST containing 1% normal goat serum. On the following day, after a wash in PBST, the samples we incubated with the corresponding secondary antibody, goat anti-rabbit IgG-FITC diluted at 1:100 and DNA counter-stain 4,6-diamidino-2-phenylindole (DAPI, 2.5 μg/mL) at room temperature for 40 min.
Negative controls were obtained by the replacement of the anti-NEDL2 antibody with normal rabbit serum at the concentration identical to that of the specific antibody. Image acquisition was performed on a Nikon Eclipse 800 microscope (Nikon Instruments, Melville, NY) with a Cool Snap camera (Roper Scientific, Tucson, AZ, USA) and MetaMorph software (v7.1, Universal Imaging, Downington, PA).
To detect injected anti-NEDL2 antibody and endogenous oocyte NEDL2 protein, a double-labeling procedure with two secondary antibodies conjugated with FITC or TRITC was performed. At 10 h after IVF, anti-NEDL2 antibody injected, and normal serum injected control oocytes were collected, zona removed, fixed in 2% formaldehyde in PBS, permeabilized in 0.1% Triton X-100 in PBS, and incubated with goat-anti-rabbit (GAR)-IgG-FITC for 40 min at room temperature to detect injected antibody. Then, the samples were incubated with the primary antibody as above. On the second day of ICC, the goat-anti-rabbit secondary antibody conjugated with TRITC was used to detect both injected and oocyte endogenous NEDL2 (in yellow after merge green with red). The remaining steps of ICC were the same as described above.
Multiple trials were performed with non-manipulated normal GV/metaphase II stage ova, IVF-generated zygotes, embryos, and normal rabbit serum injected sham control and antibody-injected oocytes and zygotes. Immunofluorescence intensity data were analyzed with Image Studio Lite (v5.2, Li-Cor, Lincoln, NE), and images were edited by using Adobe Photoshop CC (Adobe Systems, Mountain View, CA).
SDS/PAGE and western blotting analysis
Western blotting (WB) as described previously [19] was used for the current study with a little modification. Briefly, sperm samples were collected from a proven fertile boar and washed three times in TBS by centrifuging at 700 g for 10 min. A total of 50 million spermatozoa were loaded per lane. For oocyte and embryo samples, the zona pellucida was removed in acidic PBS for 5 s and washed in PBS three times. Oocyte-surrounding cumulus cells were collected after oocyte in vitro maturation and washed in PBS three times. Fibroblast cells from day 35 fetal skin was a kind gift from Dr. Randall Prather, University of Missouri, Columbia, MO. After washing, all the experimental samples were boiled in SDS loading buffer (50 mM TRIS (pH 6.8), 150 mM NaCl, 2% SDS, 20% glycerol, 0.02% bromophenol blue). To achieve reducing conditions, 5% β-mercaptoethanol was added to the samples. Gel electrophoresis was performed on 4–20% gradient gels (PAGEr Gels; Lonza Rockland Inc., Rockland, ME) by loading sperm/oocyte/embryo/somatic cell extracts, followed by transfer to PVDF membranes (Millipore, Bedford, MA) using an Owl wet transfer system (Fisher Scientific, Houston, TX) at a constant 50 V for 4 h. The membranes were incubated sequentially with 10% non-fat milk for 30 min at room temperature, rabbit anti-NEDL2 antibody (ab154888, 1:10,000 dil./ab92711, 1:20,000 dil.; Abcam) overnight at 4°C, and HRP-conjugated goat anti-rabbit antibody (1:10 000 dilution) for 40 min at room temperature. Membranes were reacted with chemiluminescent substrate (SuperSignal, Pierce, Rockford, IL) and exposed to Kodak BioMax Light Film (Kodak, New Haven, CT) for various times using a Kodak M35A X-OMAT Processor (Kodak). Densitometry quantification of the bands of interest was performed by scanning the film (300 dpi) with Epson Perfection V500 photo color scanner (Epson America, Inc., Long Beach, CA) and determined by Image Studio software (v5.2, LI-COR, Lincoln, NE). Negative control was obtained by the replacement of the anti-NEDL2 primary antibody with non-immune rabbit serum.
The specificity of both NEDL2 antibodies (ab154888, ab97211) was confirmed by WB with NEDL2 overexpression lysate (cat. # NBP2–06601, Novus Biologicals, Centennial, CO). Briefly, NEDL2 overexpression lysate and the empty vector lysate (negative control) with approximately the same total endogenous vector protein load were resolved in parallel on SDS-PAGE, transferred onto PVDF membrane, and probed with both NEDL2 antibodies, as described above. Alternatively, spermatozoa and MII oocytes were run in parallel on SDS-PAGE, transferred onto PVDF membranes and NEDL2 antibodies were preincubated with NEDL2 overexpression vector lysate and empty control vector in parallel with approximately the same total endogenous vector protein load.
IP and isolation of polyubiquitinated proteins
For NEDL2 IP, Pierce™ Cross-link IP Kit (cat# 26147, ThermoFisher Scientific) was used, closely following the manufacturer’s protocol. Briefly, 10 μg of anti-NEDL2 antibody (ab154888) was adsorbed to Protein A/G agarose following cross-linking with disuccinimidyl suberate. Protein extract from 500 MII oocytes (0.5 mg of total protein, estimated by Pierce™ BCA Proteins Assay Kit, cat# 23225) was applied to the column with the cross-linked antibody and left to incubate at 4°C with end-over-end rotation overnight. The following day, the supernatant (non-binding/flow-through fraction) was saved for further WB analysis, and the column was thoroughly washed. The bound antigen was eluted by a pH drop (pH 2.8) in three successive 5-min incubations at ambient temperature. Those three elution fractions were pooled, proteins were precipitated using a 2-D clean-up kit (cat # GE80-6484-51, GE Healthcare), and redissolved in the SDS loading buffer for WB analysis. Following the low pH elution, the beads were washed and incubated with the SDS loading buffer (10 min, 70°C). Conventional IP, omitting the cross-linking step, was performed in parallel, wherein the epitope was eluted directly by the SDS loading buffer. For WB analysis, instead of HRP-conjugated secondary antibody, Clean-Blot™ IP Detection Reagent (HRP, cat # 21230, ThermoFisher Scientific) was used to avoid binding to fragments of IP antibody, since the expected molar mass of the immunoprecipitated protein was 52 kDa, overlapping with the mass of antibody heavy chain (50 kDa).
The method as described by Sutovsky et al. [19] was used for the isolation of polyubiquitinated sperm proteins. Briefly, spermatozoa were lysed with RIPA extraction buffer (50 mM TRIS∙HCl pH 7.4, 150 mM NaCl, 1% TrX-100, 1% sodium deoxycholate, 0.1% SDS, 1 mM DTT, 2 mM EDTA), and incubated with recombinant agarose-matrix-bound UBA domain of ubiquitin-binding protein p62/SQSTM1 for 20 min at room temperature. Secondary interactions with the column were disrupted by three washes with RIPA buffer, and bound polyubiquitinated sperm proteins were eluted with SDS-PAGE loading buffer, resolved on a 4–20% reducing gel under non-reducing conditions, transferred onto a PVDF membrane, and probed with the polyclonal anti-NEDL2 antibody, as described above.
Statistical analysis
Dependent variables were analyzed for normality by using the Wilk–Shapiro test [20]. Data for dependent variables, fertilization rate, percentage of oocytes cleaved at day 2 of culture (cleavage rate), and percentage of oocytes forming apparent blastocysts (blastocyst formation rate) were arcsine-transformed and analyzed by the general linear model procedure of SAS [20]. For all variables, sources of variation were experimental day, treatment, and day by treatment interaction. Regression analysis was performed to determine the correlation between the number of blastomeres and the WB band intensity of NEDL2 by the CORR procedure of SAS (SAS, 2014). A value of P < 0.05 was considered statistically significant. In the results, least-squares means and standard errors of means are presented.
Results
Specificity of the NEDL2 antibodies
The main anti-NEDL2 antibody (ab154888) used for WB, ICC, and microinjection of function block experiments was raised against immunogen, a synthetic peptide corresponding to a region within N terminal amino acids 1–44 (MASSAREHLLFVRRRNPQMRYTLSPENLQSLAAQSSMPENMTLQ) of NEDL2 protein. When this protein sequence was blasted against known proteins in the whole animal kingdom and porcine species with two independent online programs, NCBI online program (https://blast.ncbi.nlm.nih.gov/Blast.cgi) and BLASTP (v2.2.31) online program (http://web.expasy.org/tmp/1week/blastf4984.html), only one protein matched the 44 amino acid sequence, which was a NEDL2 (HECW2) protein in pig, human, mouse, and other species. Because there are no other proteins that have the identical sequence used as the immunogen sequence, we anticipated that the antibody would recognize the NEDL2 epitope only. To further support its unique specificity, the anti-NEDL2 antibody from the same company did not detect any signal by western blot using NEDL2-/- mutation mouse brain tissue, while NEDL2 is highly translated in the normal animal brain [9]. We further confirmed antibody specificity by using NEDL2 overexpression lysate for WB analysis and immunosaturation prior to the incubation with the primary NEDL2 antibodies (Figure 1). Both NEDL2 antibodies were able to recognize the full-length 175.8 kDa NEDL2 recombinant protein (Figure 1A) in NEDL2 expression vector lysate, as well as the other, lower molecular isoforms, representing processed forms, and/or degradation products of the full-length, recombinant protein. Immunosaturation of the primary antibodies completely removed the 52 kDa band detected by ab92711, and the majority of the 52 kDa signal was also removed in WB with ab154888 (Figure 1B). The 52 kDa isoform was not detected in spermatozoa with ab154888 antibody, most likely because of steric hindrance caused by proteins that co-migrated with NEDL2.
Figure 1.
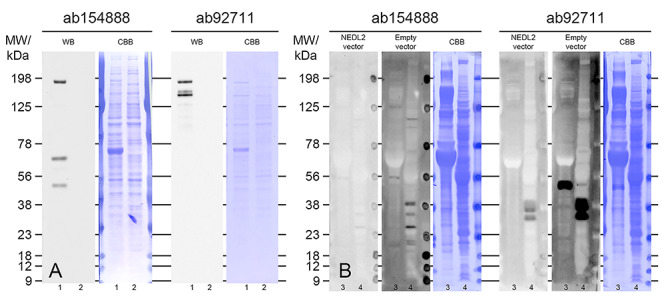
Confirmation of NEDL2 antibody specificity. (A) WB detection of recombinant NEDL2 protein in NEDL2 expression vector lysate (lanes “1”) and empty vector lysate (lanes “2”); cDNA based, predicted molecular weight of NEDL2 protein is 175.7 kDa. Comparable amounts of total vector’s endogenous proteins were loaded per lane, as shown by CBB staining. (B) Alternative validation by immune-saturation approach; WB detection of NEDL2 in MII oocytes (lanes “3”) and spermatozoa (lanes “4”) with NEDL2 antibodies saturated with NEDL2 expression vector lysate or empty vector lysate prior to incubation. Comparable amounts of total vector’s endogenous proteins were used for immunosaturation. Extracts from 100 million spermatozoa and 150 MII oocytes were loaded per lanes “3” and “4,” respectively. The blots in (B) represent the same exposure time.
Two distinct forms of NEDL2 are present in gametic vs somatic cells
WB with a commercial, well-characterized, and specificity-confirmed (Figure 1) rabbit polyclonal antibody (ab154888, Abcam) was used to detect NEDL2 in oocytes, preimplantation embryos, ejaculated spermatozoa, and somatic cells. As shown in Figure 2A, a single unique band at approximately 52 kDa was detected in the germinal vesicle (GV) stage oocytes, metaphase II (MII) oocytes, zygotes (30 h after IVF), 2-, 4-, 8-cell, and blastocyst stage embryos. Oocyte-surrounding cumulus cells and fibroblast cells, both used as somatic controls displayed higher mass bands (91 kDa and 136 kDa for cumulus cells, 155 kDa for fibroblast cells); however, they lacked the 52 kDa form detected in oocytes and embryos. Ejaculated spermatozoa prominently displayed the same 52 kDa band as oocytes and embryos; as well as other higher mass bands at 74 and 129 kDa, which were not detected in somatic cells or oocytes. To further validate the observations of the single 52 kDa band in pig oocytes and embryo embryos, the PVDF membrane was probed with a different anti-NEDL2 antibody (ab92711, Abcam). WB with the ab92711 antibody again revealed a single band with the same molecular weight (at approximately 52 kDa) immune-reactive to NEDL2 in the GV- and MII-stage oocytes, zygote to the blastocyst stage embryos (Figure 2B). Then, different GV-stage and MII stage oocyte samples were collected, and WB was repeated two more times on different samples, including different loading volume of fibroblast cell lysate. As expected, GV and MII oocytes had the same sized single band at approximately 52 kDa (Figure 2C) across different exposure times (data not shown). Somatic cell control (fibroblast cells) showed the band at 155 kDa and protein load-dependent band density (Figure 2C’).
Figure 2.
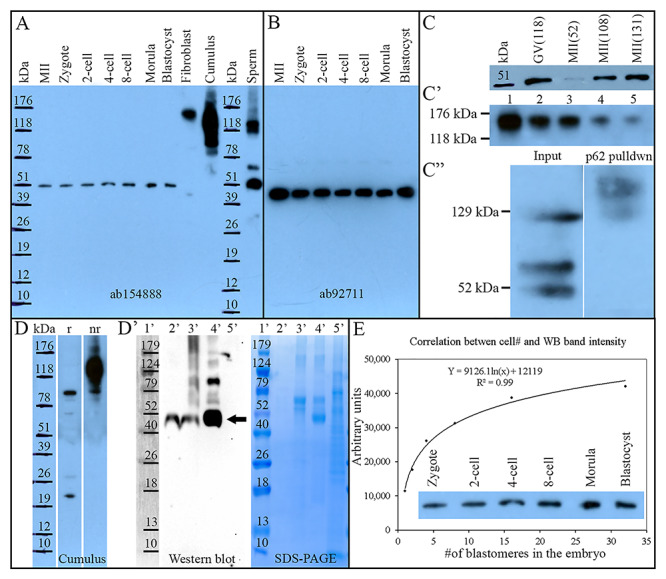
Proteomic characterization of NEDL2 in the porcine oocytes, preimplantation embryos, ejaculated spermatozoa, and somatic cells. (A) Western blot analysis of NEDL2 in porcine ova, embryos (30 per lane), somatic cells (fibroblast and cumulus cells), and spermatozoa, probed with rabbit polyclonal anti-NEDL2 antibody (ab154888, Abcam; the same antibody was used in C, D, and E). A single unique band at 52 kDa was detected in oocytes and embryos. Both somatic controls displayed multiple high mass bands; cumulus cells at 91 and 136 kDa (this band disappears under reducing conditions, see D), and fibroblast cells at 155 kDa. Ejaculated sperm extracts prominently displayed the same 52 kDa band and two other bands at 74 and 129 kDa. (B) Western blot analysis of NEDL2 in porcine ova and embryos (30 per lane) by using a different rabbit polyclonal anti-NEDL2 antibody (ab92711, Abcam). A single band in these tissue samples confirmed the observation in (A). (C) Repeated WB of NEDL2 by using different oocyte samples (52 kDa), (C’) series dilution of fibroblast cell lysate (Lane 1, undiluted; Lanes 2 to 5: 1:1, 1:3, 1:7, and 1:15 dilution in loading buffer), and (C”) NEDL2 detection in polyubiquitinated protein fraction, affinity-purified from ejaculated sperm sample including input control. (D) NEDL2 detection in cumulus cell under reducing (r) and non-reducing (nr) SDS-PAGE conditions, (D’) WB analysis of NEDL2 IP from 500 metaphase-II-arrested ova with SDS-PAGE gel run in parallel. Lane 1′: molecular mass standards (Marker); Lane 2′: IP with cross-linked antibody, epitope eluted with low pH; Lane 3′: SDS loading buffer eluted epitope followed after the low pH elution; Lane 4′: conventional IP, epitope eluted with SDS loading buffer; Lane 5′: antibody non-binding/flow-through fraction. The 52 kDa band of expected gametic NEDL2 is labeled with an arrow. (E) Correlation between cell number in developing embryos and NEDL2 abundancy detected by WB and densitometric quantification (r = 0.99, P < 0.01).
For spermatozoa, another prominent band besides the 52 kDa prominent band was observed at approximately 129 kDa. To determine whether NEDL2 protein may be (auto) ubiquitinated, a common posttranslational modification seen in sperm proteins, the recombinant ubiquitin-binding UBA domain protein SQSTM1/p62 [17], was used to pull down ubiquitinated proteins from ejaculated sperm extracts. The SQSTM1 bound protein fraction was resolved by 1-D PAGE and screened for NEDL2 by WB. The 129 kDa form was detected in the polyubiquitinated protein fraction, prepared by affinity purification from ejaculated sperm lysate using a recombinant ubiquitin-binding protein, p62 (Figure 2C”). The presence of 129 kDa isoform and additional higher molecular forms in the p62 pulldown fraction suggested that the 129 kDa isoform may be a result of polyubiquitination of the lower mass NEDL2 species. Again, WB of ejaculate sperm extract revealed the presence of the 52 kDa band, but an absence of 136 and 155 kDa bands observed in cumulus oophorus and fibroblast cells, respectively, confirmed the observation described above. Furthermore, the presence/absence of the 155 kDa band in cumulus oophorus cells depended on reducing conditions of SDS-PAGE (Figure 2D).
IP following WB was performed to further confirm that the single 52 kDa band is NEDL2, and the NEDL2 antibody directly binds to the intended target. Five hundred MII ova were lysed and immunoprecipitated with the anti-NEDL2 antibody (ab154888). The immunoprecipitate was resolved by 1D-PAGE and probed by the NEDL2 antibody using the WB technique. Again, WB displayed a prominent band at 52 kDa in the immunoprecipitates (Figure 2D’, lanes 2–4), indicating that this protein band is indeed an isoform or fragment of NEDL2. Collectively, NEDL2 protein was present in all examined cell types, including embryos. Multiple NEDL2 forms with different molecular weight were observed by WB, a gametic isoform at approximately 52 kDa, and somatic isoforms at 91 kDa and 136 kDa in cumulus oophorus cells (136 kDa form was not present under reducing conditions, Figure 2D), and 155 kDa in fibroblast cells.
When WB band intensity was plotted against cell number per embryo based on the developmental stage (1-cell zygotes to average 34 of nuclei in day 7 blastocyst embryo), there was a significant logarithmic correlation between them (R2 = 0.99), which indicates that the NEDL2 protein content within each blastomere at different stages of development was unchanged (Figure 2E). Also, interestingly, NEDL2 protein content in the cumulus cells increased significantly after in vitro maturation with oocytes in the LH- and FSH-containing medium (P < 0.01), compared to the cumulus cells before maturation, detected by both WB and immunofluorescence, as described below (Figure 3).
Figure 3.
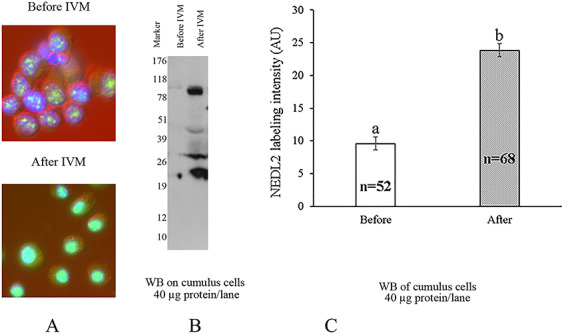
Assessment of NEDL2 content (labeling intensity) in cumulus oophorus cells before and after 40 h of in vitro maturation with oocytes, by ICC (A) and WB analysis (B), NEDL2 is detected at 91 kDa under reducing conditions and the lower mass forms are assumed to be its degradation products. (C) Densitometric quantification before vs. after IVM. Ab154888 antibody was used in both ICC and WB. a,b: means of labeling intensity by ICC with different letters differ (P < 0.01).
NEDL2 protein localizes to cytoplasm, pronuclei, and blastomere nuclei
By immunofluorescence (Figure 4), NEDL2 showed a diffused cytoplasmic localization at all stages of oocytes and embryos and in fibroblast and cumulus cells. Additionally, NEDL2 protein accumulated and formed granular particles in the GVs of immature oocytes, in maternal and paternal pronuclei of zygotes, and the blastomere nuclei of 2-, 4-, 8-cell, and blastocyst stage embryos (Figure 4A’–G’) and the nucleus of cumulus and fibroblast cells (Figure 4H’ and I’). During oocyte maturation, GV broke down, the visible nucleus disappeared, and thus NEDL2 protein in MII oocyte was in a diffused format. Furthermore, NEDL2 was not present on the metaphase plate in MII oocyte (Figure 4B), which is different from the somatic cell [8]. The presence of NEDL2 diffused in metaphase II oocyte was confirmed by WB analysis as described above (refer to Figure 2). Interestingly, for the differentiating multicell blastocyst embryo, the labeling intensity of NEDL2 protein was different between the outside trophectoderm cells and the inner cell mass (Figure 4G). In general, there was stronger NEDL2 labeling inside the inner cell mass than in trophoblast. The labeling pattern was different as well, more NEDL2 in the cytoplasm and some in nuclei in trophoblast cells. In ICM, the labeling of NEDL2 was very strong both in cytoplasm and nuclei, indicating higher NEDL2 protein content in ICM cells compared to trophoblast cells (Figure 4G). For ejaculated spermatozoa, the NEDL2 protein was present in the postacrosomal region and tail (Figure 4J).
Figure 4.
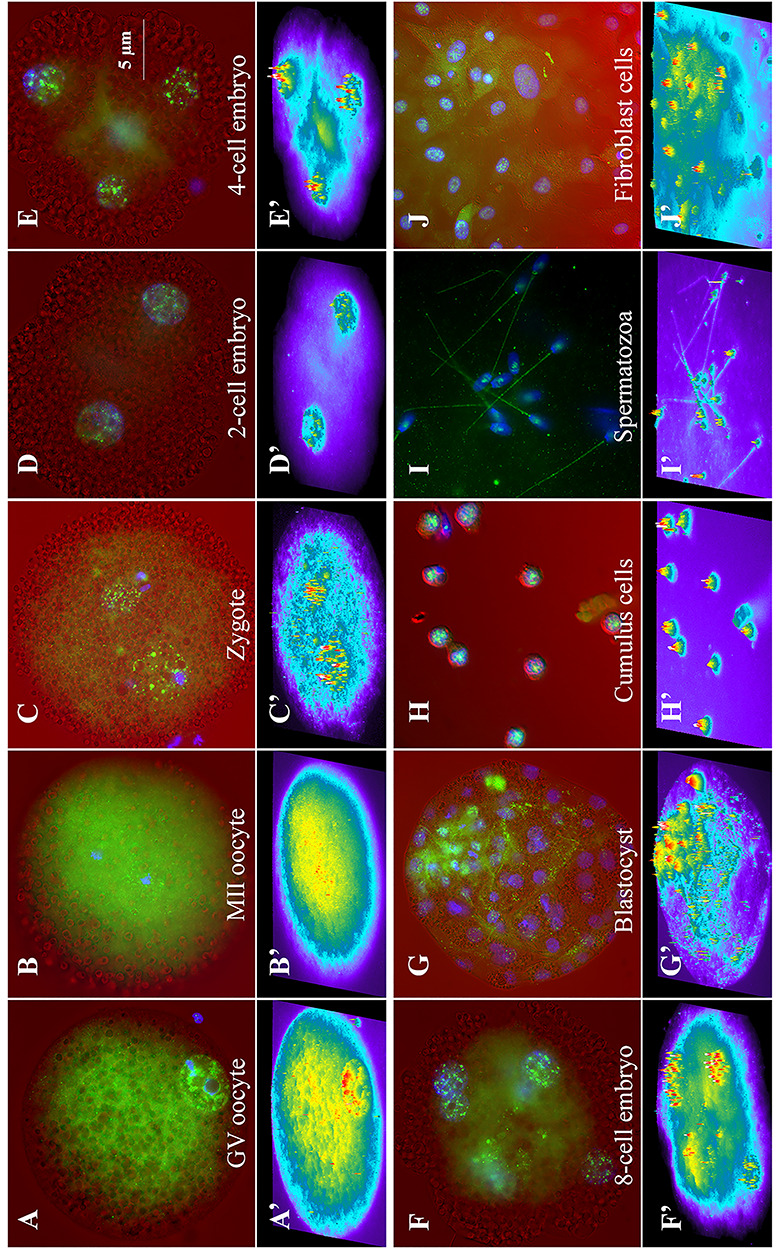
Representative immunofluorescence images of NEDL2 (green) in porcine ova, zygote, and embryos generated by IVF. NEDL2 labeled in green, and DNA counterstained with DAPI in blue were superimposed over the corresponding light-microscopic images in red acquired with differential interference-contrast (DIC) optics (on top, A–J). The intensity of NEDL2 expression was further analyzed by MetaMorph software (v7.1) and presented as intensity profiles in the bottom panels (A” to J”) using heat map pseudocoloring. The following oocyte, embryos, and cell types are shown: (A) GV-stage oocyte, (B) metaphase II oocyte, (C) 30 h post-IVF zygote, (D) 2-cell stage, (E) 4-cell stage, (F) 8-cell stage, (G) day 7 blastocyst stage embryo, (H) surrounding GV-stage-oocyte cumulus cells, (I) d35 fetal fibroblast cells, and (J) ejaculated spermatozoa. Typical, representative patterns of NEDL2 labeling are shown (color merged A’–J’). NEDL2 showed both a diffused cytoplasmic localization and accumulation in distinct foci in the GVs, pronuclei, or nuclei at all stages of oocytes and embryos and in fibroblast and cumulus cells. Ab154888 antibody was used in ICC analysis.
Microinjection of anti-NEDL2 antibody into MII oocytes prevents the formation of male pronucleus afterfertilization
The objective of the current experiment was to determine whether microinjection of anti-NEDL2 antibody into MII oocytes before IVF would block the function of NEDL2 protein and affect fertilization, pronuclear formation, and subsequent embryo development. The experiment was repeated seven times (seven replicates) for 21 h post-IVF fertilization study. The experiment was repeated five times for embryo culture. The percentage of fertilized oocytes was calculated as the number of oocytes fertilized by sperm penetration divided by total oocytes in fertilization droplets. The percentage of pronuclear formation was computed as the number of oocytes with one or more pronuclei divided by the number of fertilized oocytes. Oocyte fertilization and embryo development data are presented in Table 1. Overall, anti-NEDL2 antibody microinjection into oocytes before IVF did not affect the fertilization rate, the percentage of oocytes that formed pronucleus, the number of spermatozoa that penetrated zona pellucida or the percentage of embryo cleaved at day 2 or percent blastocyst formation at day 7 of culture after IVF. However, more oocytes were found to be penetrated by spermatozoa, but the sperm head did not undergo decondensation in the antibody-injected group, compared to the normal serum injected sham controls as shown at 6, 10, and 21 h post-IVF in Figure 5. The average number of pronuclei formed inside fertilized oocytes was significantly lower, but monospermic fertilization percent was higher in the antibody-injected group than in controls (Table 1 and Figure 5E and F at 21 h after IVF). Such findings indicate that anti-NEDL2 antibody may have interfered with fertilization by inhibiting sperm nucleus decondensation and paternal pronucleus formation (referring to still condensed sperm nuclei in Figure 5B, D, and F).
Table 1.
Effects of anti-NEDL2 antibody microinjection into oocytes on fertilization, pronuclear formation, and embryo development in vitro.
| Hours post-IVF | Treatment | ||
|---|---|---|---|
| Parameters | Control (n) | NEDL2 AB (n) | |
| 21 h | % of oocytes fertilized | 92.8 ± 1.9 (219) | 91.5 ± 1.9 (232) |
| % of oocytes formed pronucleus | 96.0 ± 2.2 | 91.2 ± 2.2 | |
| % of oocytes fertilized but no pronucleus formed | 4 ± 1.8a | 12.9 ± 1.8b | |
| # of spermatozoa penetrated oocyte | 4.2 ± 0.2 | 4.1 ± 0.2 | |
| # of pronuclei in oocyte | 4.2 ± 0.1a | 2.8 ± 0.1b | |
| % of monospermic fertilization | 5.6 ± 1.6a | 12.9 ± 1.6b | |
| 48 h | % of oocytes cleaved | 52.2 ± 8.4 (172) | 55.7 ± 8.4 (154) |
| 168 h | % of blastocysts formed | 14.2 ± 4.3 | 7.9 ± 4.3 |
a,bMeans with the column with different superscripts differ (P < 0.05).
Figure 5.
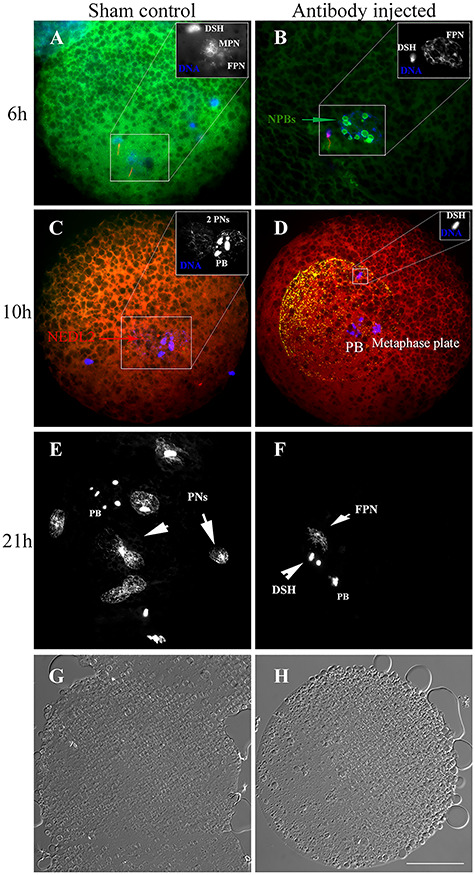
Representative images of oocytes at 6, 10, and 21 h after IVF in normal serum (sham) and anti-NEDL2 antibody (ab154888) injected groups. DNA was counterstained with DAPI (blue). (A, B) Immunofluorescence of NEDL2 (green) in the sham and anti-NEDL2 antibody-injected oocytes at 6 h post-IVF. It also illustrates that the anti-NEDL2 antibody injection accelerated the formation of nucleolus precursor bodies. Spermatozoa were labeled with MitoTracker in red. (C, D) Immunofluorescence of injected anti-NEDL2 antibody (green) and oocyte endogenous NEDL2 (red) at 10 h post-IVF. The injected antibody was detected and labeled in green and red, and shown in orange in (D) after merging the two colors. (E, F) Comparison of pronuclei formed at 21 h after IVF. Multiple pronuclei formed in the sham control, while only female pronucleus formed at this time, and sperm DNA was still not decondensed. (G, H) The corresponding light-microscopic images acquired with differential interference-contrast (DIC) of sham and antibody injected zygotes. Note: the sperm DNA did not decondense in the antibody injected group at 6, 10, and 21 h after IVF. DSH: decondensing sperm heard; NPB: nucleolus precursor body; PB: polar body; PN(s): pronucleus (pronuclei); FPN: female pronucleus; MPN: male pronucleus. Bar = 25 μm.
As demonstrated by the immunofluorescence of NEDL2, this protein was accumulated in the pronuclei of zygote at 30 h post-IVF (Figure 4C). We were interested in knowing whether NEDL2 protein was present at the very beginning of pronuclear formation both in male and female pronuclei; its early presence may indicate its importance for early fertilization events and development. We also wanted to know whether injected anti-NEDL2 antibody could still be detected at 10 h after IVF and possible interactions with pronuclear formation. We harvested in vitro fertilized oocytes at 6 and 10 h after IVF and examined NEDL2 protein by ICC. As expected, this protein was present in both male and female pronuclei regardless of treatment, as soon as DNA started to decondense and form pronucleus, as shown in Figure 5A and B, and Figure 6. NEDL2 had been accumulating before the pronucleus developed fully (Figure 6). Moreover, the injected antibody could be detected at 10 h after IVF (Figure 5D). As shown in Figure 5D, spermatozoon could penetrate and fertilize the oocyte in the antibody-injected area. However, neither the sperm head nor the oocyte metaphase plate decondensed, indicating a possible interaction between the antibody and DNA decondensation in both spermatozoon and oocyte. The lower number of pronuclei in the antibody-injected group further supports this possibility. Interestingly, and unexpectedly, at 6 and 10 h after IVF, zygotes from the antibody-injected group formed nucleolar precursor bodies (NPBs) while none of the control zygotes had any NPB formation (Figure 5A and B). The average number of NPBs was 2.6 ± 0.5 vs. 0.1 ± 0.4 for the NEDL2 antibody group and the sham control group, respectively. These results suggested that the anti-NEDL2 antibody also accelerated the NPB formation.
Figure 6.
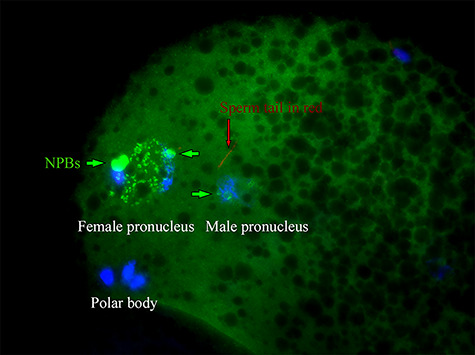
NEDL2 protein presents in both male and female pronuclei at the beginning of pronuclear formation (6 h after IVF). Note: NEDL2 accumulated in the male pronucleus as soon as it started to develop. Ab154888 antibody was used. Bar = 25 μm.
Discussion
As a part of our ongoing effort to evaluate the role of the ubiquitin-proteasome pathway in mammalian fertilization and early embryo development, we determined the expression pattern of NEDL2 protein in gametes, preimplantation embryos, fibroblast cells, in cumulus cells before and after oocyte maturation with LH/FSH, and its roles in fertilization. To our knowledge, this is the first report of multiple distinct isoforms of NEDL2 protein, the gametic isoform found exclusively in spermatozoa, oocytes, early preimplantation embryos; and the somatic isoforms present in porcine cumulus oophorus and fibroblast cells. During early embryo development, the consistent protein level in the blastomere of different stage embryos from 1-cell zygote to multicell blastocyst, like a housekeeping gene, indicated that this ligase is needed for fertilization and embryo development. In order to study the NEDL2 function, an attempt was undertaken to ablate NEDL2 by morpholino and CRISPR/Cas9 approaches, but unlike in the case of the zygotic ubiquitin-tail ribonucleoprotein UBA52 ablation [21], this approach was unsuccessful. This lack of effect is likely due to the fact that the NEDL2 protein is abundant in the oocyte both before and after meiotic maturation, and involved in very early events of zygotic development that occur before translational (from stored mRNA) and transcriptional activation (de novo) of the zygote. Never the less, an alternative approach of the microinjection of anti-NEDL2 antibody into oocytes before fertilization decreased the number of pronuclei formed at 21 h after IVF and there were more oocytes fertilized, but sperm DNA did not decondense and no male pronucleus was formed compared to the sham control group. Moreover, antibody injection accelerated the formation of NPBs. Taken together, the current study suggests that NEDL2 is important during fertilization, specifically during sperm DNA decondensation and early stage of pronuclear development, perhaps participating in the recycling of sperm-nuclear protamines.
The ubiquitin-proteasome pathway (UPS) plays diverse regulatory and homeostatic roles in many basic cellular processes including spermatogenesis and fertilization [3, 4]. This omnipresent proteolytic system ensures rapid, substrate-specific degradation of various proteins in the cell cytoplasm and nucleus (see reviews [1, 4, 22]). This pathway involves multiple steps and enzymes. Single ubiquitin molecules are activated by ubiquitin-activating enzymes (E1), and the activated ubiquitin is covalently ligated to the substrate’s internal lysine residue with the help of ubiquitin carrier protein (E2) and ubiquitin-protein ligases (E3s). High NEDL2 levels in gametes and a stable protein level in blastomeres at all stages of pre-embryo development from one-cell zygote to blastocyst indicates that NEDL2, one of the E3 enzymes, might be pivotal to the final step in the ubiquitination cascade in fertilization and early embryonic development. Through recognition of particular substrates, this enzyme defines the exquisite spatiotemporal nature and confers specificity to the pathway. Canonical NEDL2 has an N-terminal HECW domain with not clearly understood function, a C2 domain that mediates anchoring of E3s to intracellular membranes, the catalytic HECT domain at the C-terminus that catalyzes the attachment of ubiquitin molecule onto the substrate, and two internal WW domains that mediate protein‐protein interaction (https://www.uniprot.org/uniprot/Q9P2P5). These WW domains are group 1 domains and recognize proline-rich ligands with a minimal consensus sequence, PPXY [12]. The epithelial Na+ channel ENaC is the best-studied example of a Nedd4/Nedd4-like substrate. Its cell surface expression is regulated by the ubiquitin-protein ligase Nedd4‐2 via direct PY motif/WW domain interaction. This regulatory mechanism is impaired in Liddle’s disease [23]. Similar to this PY motif/WW domain interaction mechanism, the interaction between WW-containing NEDL2 protein and postacrosomal sheath WW domain-binding protein (PAWP) in sperm could be possible. PAWP is a novel sperm protein identified as a candidate sperm-born oocyte-activating factor [14–16]. PAWP elicits calcium oscillations, activates oocytes, and promotes pronuclear development following fertilization. Therefore, WW domains in NEDL2 could be engaged during fertilization by WW-binding motifs PAWP. Interestingly, when Zheng [24] tried to identify the protein that could interact with PAWP, the author found that PAWP bound to a 52 kDa protein, which had the characteristics of Nedd4 but was unable to identify the protein. Current findings suggest that NEDL2 meets all these criteria (size, Nedd4 family member, and WW-containing domains), thus making it a candidate protein for PAWP interactions.
Multiple isoforms of NEDL2 have been reported in other species. At least two NEDL2 isoforms were reported and four other predicted to this date in the human as the products of an alternative RNA splicing (Table 2). Three isoforms of NEDL2 have been reported in the mouse. The canonical, full-length NEDL2 has a molecular weight of 176 kDa and 1578 aa [25]. The isoform CRA-b has a 294 aa [26], and the third isoform only has 240 aa [27]. According to the porcine NEDL2 gene sequence data [28], the corresponding pig NEDL2 protein contains 1574 AA and has a 96% homology to the human sequence and 95% homology to the mouse sequence. There was no report on different porcine NEDL2 isoforms before. We discovered a small size NEDL2 isoform of approximately 52 kDa in pig oocytes and embryos. Since this small NEDL2 species was recognized by the antibody raised against amino acid sequence 1–44 of the N- terminal portion as well as by the antibody raised against synthetic peptide derived from an internal region of NEDL2, it is highly likely that the catalytic C-terminal HECT domain (338 amino acids, 39.6 kDa) is missing in this small NEDL2 isoform. The gametic isoform might therefore perform its functions independently of its C-terminal E3 ligase domain. The small-sized gamete isoform of NEDL2 could be the result of alternative splicing, alternative promoter usage, and alternative initiation sites of translation. For the exact splicing mechanisms of small NEDL2 in oocytes and preimplantation embryo, its regulation of isoform expression and gene structure requires future studies. Different isoforms in different cells as observed in the present study could explain its diverse roles in reproduction and somatic cells in general as reported as in other proteins [29]. The second possible mechanism for protein isoforms was protein ubiquitination as one of the fundamental regulatory posttranslational modifications controlling cellular protein turnover and homeostasis, which may be the case of sperm samples examined in the current study. To determine if the higher band at 129 kDa was the ubiquitination of the lower 52 kDa band, we used recombinant ubiquitin-binding UBA domain protein SQSTM1/p62 [17] to pull down ubiquitinated proteins from ejaculated sperm extracts. The SQSTM1 bound protein fraction was resolved by 1-D PAGE and screened for NEDL2. The presence of 129 kDa isoform and another higher band in this p62 pulldown fraction suggests the ubiquitination of lower mass NEDL2 species. Thus, NEDL2 protein, as an E3 ligase, can be modified by other enzymes [8], and it also modifies other proteins as reported in many other enzymes [30].
Table 2.
Reported and computationally predicted splicing variants of human NEDL2 mRNA.
| Splice variant | UniProt reference | Protein class | Length (AA) | Mass (Da) |
|---|---|---|---|---|
| HECW2-201 | Q9P2P5-1 | Enzymes Predicted intracellular proteins Plasma proteins Disease related genes | 1572 | 175 769 |
| HECW2-201 | Q9P2P5-2 | 82 | 9041 | |
| HECW2-202 | A0A2C9F2N4a | Computationally predicted | 1216 | 135 904 |
| HECW2-203 | C9JHL2 | Computationally predicted | 120 | 13 673 |
| HECW2-209 | A0A2R8Y6F3 | Computationally predicted | 1579 | 176 601 |
| HECW2-212 | A0A2R8YE75 | Computationally predicted | 814 | 93 786 |
aThis entry became obsolete on 12–05–2018 and the predicted sequence is available at UniParc as UPI0000F0A541.
The roles of NEDL2 during final oocyte maturation and cumulus expansion after LH surge are not yet known. Based on published studies in other cells and current observations of a significant increase in NEDL2 protein level after 40 h maturation with gonadotrophins in vitro, we proposed two possible roles of NEDL2 protein in the final cumulus cell expansion. The first possible role of NEDL2 involved in non-proteolytic roles in histone regulation, and signal transduction in cumulus cells after LH surge. During COC expansion, cumulus cells started to differentiate and produce many cytokines and inflammatory factors and are released from the ovary along with the oocyte at the time of ovulation [31, 32]. Thus, the oocyte is the center of a pronounced inflammatory reaction and is essentially treated as a foreign body. This dramatic function shift of cumulus cells in synthesizing different proteins may be explained by histone modification via the ubiquitin-proteasome pathway. Ubiquitination is one main posttranslational modification at the histone NH2-terminus [33]. The second possible role of NEDL2 in cumulus cells was their distinct fate involving the apoptosis pathway. As discussed above, ubiquitin binds to histones and may regulate chromatin structure during transcription [2], and thus has been immunochemically detected in both cytoplasm and the nucleus [34] as observed in the current study. Ubiquitin upregulation has been associated with apoptosis [35]. In the present study, NEDL2 contents in cumulus cells were increased two and a half times by ICC and 10 times by WB. Moreover, tumor suppressor protein p73 is involved in cell cycle regulation and induction of apoptosis. NEDL2 interacts with two isoforms of p73, α, and β. It binds to p73 and ubiquitinates p73. This ubiquitination leads to the stabilization of p73α/β and increased p73α transcriptional activities [7], further supporting the idea of NEDL2 involving the cell apoptosis pathway after maturation in an LH-containing medium. Otherwise, similar to its sister protein NEDL1, which is associated with the C-terminus of tumor protein p53, NEDL2 may increase its proapoptotic functions independently of its E3 ligase activity [36].
At fertilization, the inactive sperm nucleus must be rapidly transformed into a DNA replication-competent male pronucleus before the formation of the zygote. The sequential events of this crucial process are well conserved among animals and are controlled by molecules present in the egg. Because ubiquitin-dependent proteolysis is required for cell cycle progression, it has been demonstrated that proteasome is activated during egg activation in the Xenopus [37]. It was concluded that such proteasome activation is due to the calcium-induced assembly of 26S proteasome from the 20S proteasome. Microinjection of anti-NEDL2 antibody reduced the number of pronuclei in oocytes, and accelerated the formation of nuclear precursor bodies; both may be caused by the same mechanism of high MAPK and MPF activities. In mouse, small NPBs appeared about 4 h after ethanol activation and took about 1.5 h to fuse into a large NPB, which persisted for about 10 h before the disappearance. MAPK and MPF activities at the initial stage of activation regulate NPB fusion after pronuclear formation [38]. Therefore, it was possible that in antibody-injected oocytes, the ubiquitin-proteasome pathway was interrupted by antibody neutralizing NEDL2 activities, resulting in higher MAPK and MPF activities and NPBs formed sooner than the controls. For the very same reason and pathway, higher MAPK activities also may explain the low number of pronuclei formed in NEDL2 antibody-injected oocytes. It is known that completion of meiosis and entry into interphase depends upon a fall in the activities of MAPK (principally ERK1 and ERK 2), since preventing its decline using phosphatase inhibitors, or by injecting constitutively activate MEK, prevents pronuclear formation [39]. Therefore, high activities of MAPK and MPF may impair sperm DNA decondensation and pronuclear formation. In any case, the effect of the NEDL2 antibody injection on MAPK and MPF activities and quantities needs to be addressed by future studies. On the other hand, very recently it was found that NEDL2 protein can interact with many other proteins/enzymes such as nucleoplasmin (NPM) 3 [8]. Mammalian homologs NPM1–3 are expressed in oocytes, and recent in vitro studies showed that NPM1 and the complex NPM1–3 efficiently promoted the sperm nuclear decondensation and nucleosome organization [40]. NEDL2 is also shown to be the substrate of APC/C-Cdh1 [8], which regulates metaphase exit. Therefore, there are many possibilities for the anti-NEDL2 antibody to disrupt sperm decondensation pathways and inhibit this process.
In summary, we have demonstrated different isoforms of NEDL2 in porcine oocytes, embryos, spermatozoa, and somatic cells. NEDL2 protein was detected in both the nucleus and cytoplasm. Its protein content in oocytes and blastomere of different stage preimplantation embryos was constant but significantly increased in cumulus cells after maturation in the LH-containing medium in vitro. Microinjection of anti-NEDL2 antibody in metaphase II oocytes increased the number of oocytes fertilized without male pronucleus formation, decreased the number of pronuclei, and accelerated the formation of nuclear precursor bodies. These data suggest that NEDL2 plays a key role in oocyte fertilization, especially in sperm DNA decondensation and during the early stages of pronuclear development.
Acknowledgment
Clerical and editorial assistance by Ms. Kathy Craighead is sincerely appreciated.
Footnotes
† Grant Support: This work was in part funded by Agriculture and Food Research Initiative Competitive Grant no. 2015–67015-23231 and 2020-67015-31017 from U.S. Department of Agriculture, The National Institute of Food and Agriculture USDA NIFA, and seed funding from the Food for the 21st Century F21C program of the University of Missouri-Columbia. Porcine gametes and fibroblast cells were provided by National Swine Resource & Research Center at The University of Missouri, supported by grant U42 OD011140 from National Institutes of Health (NIH) Office of Research Infrastructure Programs (ORIP). Financial support provided by grants #2011-67015-20025 and 2015-67015-23231 from USDA-NIFA and by MU F21C Program funding to P.S.
Contributor Information
Jiude Mao, Division of Animal Sciences, University of Missouri, Columbia, MO, USA.
Michal Zigo, Division of Animal Sciences, University of Missouri, Columbia, MO, USA.
Dalen Zuidema, Division of Animal Sciences, University of Missouri, Columbia, MO, USA.
Miriam Sutovsky, Division of Animal Sciences, University of Missouri, Columbia, MO, USA.
Peter Sutovsky, Division of Animal Sciences, University of Missouri, Columbia, MO, USA; Department of Obstetrics, Gynecology and Women’s Health, University of Missouri, Columbia, MO, USA.
Authors contributions
J.M. and P.S. designed the study; J.M., M.Z., D.Z., and M.S. performed the experiments; J.M. and M.Z. performed the analyses, J.M., M.Z., and P.S. wrote the manuscript.
Conflict of interest
The authors have declared that no conflict of interest exists.
References
- 1. Glickman MH, Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev 2002; 82:373–428. [DOI] [PubMed] [Google Scholar]
- 2. Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem 1998; 67:425–479. [DOI] [PubMed] [Google Scholar]
- 3. Yi YJ, Manandhar G, Sutovsky M, Zimmerman SW, Jonakova V, van Leeuwen FW, Oko R, Park CS, Sutovsky P. Interference with the 19S proteasomal regulatory complex subunit PSMD4 on the sperm surface inhibits sperm-zona pellucida penetration during porcine fertilization. Cell Tissue Res 2010; 341:325–340. [DOI] [PubMed] [Google Scholar]
- 4. Sutovsky P. Ubiquitin-dependent proteolysis in mammalian spermatogenesis, fertilization, and sperm quality control: killing three birds with one stone. Microsc Res Tech 2003; 61:88–102. [DOI] [PubMed] [Google Scholar]
- 5. Deshaies RJ, Joazeiro CA. RING domain E3 ubiquitin ligases. Annu Rev Biochem 2009; 78:399–434. [DOI] [PubMed] [Google Scholar]
- 6. Rotin D, Kumar S. Physiological functions of the HECT family of ubiquitin ligases. Nat Rev Mol Cell Biol 2009; 10:398–409. [DOI] [PubMed] [Google Scholar]
- 7. Miyazaki K, Ozaki T, Kato C, Hanamoto T, Fujita T, Irino S, Watanabe K, Nakagawa T, Nakagawara A. A novel HECT-type E3 ubiquitin ligase, NEDL2, stabilizes p73 and enhances its transcriptional activity. Biochem Biophys Res Commun 2003; 308:106–113. [DOI] [PubMed] [Google Scholar]
- 8. Lu L, Hu S, Wei R, Qiu X, Lu K, Fu Y, Li H, Xing G, Li D, Peng R, He F, Zhang L. The HECT type ubiquitin ligase NEDL2 is degraded by anaphase-promoting complex/cyclosome (APC/C)-Cdh1, and its tight regulation maintains the metaphase to anaphase transition. J Biol Chem 2013; 288:35637–35650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wei R, Qiu X, Wang S, Li Y, Wang Y, Lu K, Fu Y, Xing G, He F, Zhang L. NEDL2 is an essential regulator of enteric neural development and GDNF/ret signaling. Cell Signal 2015; 27:578–586. [DOI] [PubMed] [Google Scholar]
- 10. Macias MJ, Wiesner S, Sudol M. WW and SH3 domains, two different scaffolds to recognize proline-rich ligands. FEBS Lett 2002; 513:30–37. [DOI] [PubMed] [Google Scholar]
- 11. Sudol M, Hunter T. NeW wrinkles for an old domain. Cell 2000; 103:1001–1004. [DOI] [PubMed] [Google Scholar]
- 12. Sudol M, Chen HI, Bougeret C, Einbond A, Bork P. Characterization of a novel protein-binding module—the WW domain. FEBS Lett 1995; 369:67–71. [DOI] [PubMed] [Google Scholar]
- 13. Aarabi M, Balakier H, Bashar S, Moskovtsev SI, Sutovsky P, Librach CL, Oko R. Sperm-derived WW domain-binding protein, PAWP, elicits calcium oscillations and oocyte activation in humans and mice. FASEB J 2014; 28:4434–4440. [DOI] [PubMed] [Google Scholar]
- 14. Oko R, Sutovsky P. Biogenesis of sperm perinuclear theca and its role in sperm functional competence and fertilization. J Reprod Immunol 2009; 83:2–7. [DOI] [PubMed] [Google Scholar]
- 15. Sutovsky P, Manandhar G, Wu A, Oko R. Interactions of sperm perinuclear theca with the oocyte: Implications for oocyte activation, anti-polyspermy defense, and assisted reproduction. Microsc Res Tech 2003; 61:362–378. [DOI] [PubMed] [Google Scholar]
- 16. Wu AT, Sutovsky P, Manandhar G, Xu W, Katayama M, Day BN, Park KW, Yi YJ, Xi YW, Prather RS, Oko R. PAWP, a sperm-specific WW domain-binding protein, promotes meiotic resumption and pronuclear development during fertilization. J Biol Chem 2007; 282:12164–12175. [DOI] [PubMed] [Google Scholar]
- 17. Vadlamudi RK, Joung I, Strominger JL, Shin J. p62, a phosphotyrosine-independent ligand of the SH2 domain of p56lck, belongs to a new class of ubiquitin-binding proteins. J Biol Chem 1996; 271:20235–20237. [DOI] [PubMed] [Google Scholar]
- 18. Abeydeera LR, Wang WH, Prather RS, Day BN. Maturation in vitro of pig oocytes in protein-free culture media: Fertilization and subsequent embryo development in vitro. Biol Reprod 1998; 58:1316–1320. [DOI] [PubMed] [Google Scholar]
- 19. Sutovsky P, Manandhar G, Laurincik J, Letko J, Caamano JN, Day BN, Lai L, Prather RS, Sharpe-Timms KL, Zimmer R, Sutovsky M. Expression and proteasomal degradation of the major vault protein (MVP) in mammalian oocytes and zygotes. Reproduction 2005; 129:269–282. [DOI] [PubMed] [Google Scholar]
- 20. SAS . SAS/STAT User’s Guide, 9.4 ed. Cary, NC: Statistical Analysis Systems Institute, Inc.; 2014. [Google Scholar]
- 21. Mao J, O'Gorman C, Sutovsky M, Zigo M, Wells KD, Sutovsky P. Ubiquitin A-52 residue ribosomal protein fusion product 1 (Uba52) is essential for preimplantation embryo development. Biol Open 2018; 7:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kerns K, Morales P, Sutovsky P. Regulation of sperm capacitation by the 26S proteasome: an emerging new paradigm in Spermatology. Biol Reprod 2016; 94:117. [DOI] [PubMed] [Google Scholar]
- 23. Flores SY, Debonneville C, Staub O. The role of Nedd4/Nedd4-like dependant ubiquitylation in epithelial transport processes. Pflugers Arch 2003; 446:334–338. [DOI] [PubMed] [Google Scholar]
- 24. Zheng Q. Contribution of a sperm protein, pawp, to the signal transduct pathway during vertebrate fertilization. Kingston: Queen’s University; 2008. [Google Scholar]
- 25. Okazaki N, Kikuno R, Ohara R, Inamoto S, Koseki H, Hiraoka S, Saga Y, Nagase T, Ohara O, Koga H. Prediction of the coding sequences of mouse homologues of KIAA gene: III. The complete nucleotide sequences of 500 mouse KIAA-homologous cDNAs identified by screening of terminal sequences of cDNA clones randomly sampled from size-fractionated libraries. DNA Res 2003; 10:167–180. [DOI] [PubMed] [Google Scholar]
- 26. Mural RJ, Adams MD, Myers EW, Smith HO, Miklos GL, Wides R, Halpern A, Li PW, Sutton GG, Nadeau J, Salzberg SL, Holt RA et al. A comparison of whole-genome shotgun-derived mouse chromosome 16 and the human genome. Science 2002; 296:1661–1671. [DOI] [PubMed] [Google Scholar]
- 27. Kawai J, Shinagawa A, Shibata K, Yoshino M, Itoh M, Ishii Y, Arakawa T, Hara A, Fukunishi Y, Konno H, Adachi J, Fukuda S et al. Functional annotation of a full-length mouse cDNA collection. Nature 2001; 409:685–690. [DOI] [PubMed] [Google Scholar]
- 28. Sironen A, Uimari P, Nagy S, Paku S, Andersson M, Vilkki J. Knobbed acrosome defect is associated with a region containing the genes STK17b and HECW2 on porcine chromosome 15. BMC Genomics 2010; 11:699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Norskov-Lauritsen L, Brauner-Osborne H. Role of post-translational modifications on structure, function and pharmacology of class C G protein-coupled receptors. Eur J Pharmacol 2015; 763:233–240. [DOI] [PubMed] [Google Scholar]
- 30. Benkirane M, Sardet C, Coux O. Lessons from interconnected ubiquitylation and acetylation of p53: Think metastable networks. Biochem Soc Trans 2010; 38:98–103. [DOI] [PubMed] [Google Scholar]
- 31. Hernandez-Gonzalez I, Gonzalez-Robayna I, Shimada M, Wayne CM, Ochsner SA, White L, Richards JS. Gene expression profiles of cumulus cell oocyte complexes during ovulation reveal cumulus cells express neuronal and immune-related genes: Does this expand their role in the ovulation process? Mol Endocrinol 2006; 20:1300–1321. [DOI] [PubMed] [Google Scholar]
- 32. Richards JS, Liu Z, Shimada M. Immune-like mechanisms in ovulation. Trends Endocrinol Metab 2008; 19:191–196. [DOI] [PubMed] [Google Scholar]
- 33. Pavri R, Zhu B, Li G, Trojer P, Mandal S, Shilatifard A, Reinberg D. Histone H2B monoubiquitination functions cooperatively with FACT to regulate elongation by RNA polymerase II. Cell 2006; 125:703–717. [DOI] [PubMed] [Google Scholar]
- 34. Haas AL, Bright PM. The immunochemical detection and quantitation of intracellular ubiquitin-protein conjugates. J Biol Chem 1985; 260:12464–12473. [PubMed] [Google Scholar]
- 35. Sandri M, Podhorska-Okolow M, Geromel V, Rizzi C, Arslan P, Franceschi C, Carraro U. Exercise induces myonuclear ubiquitination and apoptosis in dystrophin-deficient muscle of mice. J Neuropathol Exp Neurol 1997; 56:45–57. [DOI] [PubMed] [Google Scholar]
- 36. Li Y, Ozaki T, Kikuchi H, Yamamoto H, Ohira M, Nakagawara A. A novel HECT-type E3 ubiquitin protein ligase NEDL1 enhances the p53-mediated apoptotic cell death in its catalytic activity-independent manner. Oncogene 2008; 27:3700–3709. [DOI] [PubMed] [Google Scholar]
- 37. Aizawa H, Kawahara H, Tanaka K, Yokosawa H. Activation of the proteasome during Xenopus egg activation implies a link between proteasome activation and intracellular calcium release. Biochem Biophys Res Commun 1996; 218:224–228. [DOI] [PubMed] [Google Scholar]
- 38. Li JJ, Lian HY, Zhang SY, Cui W, Sui HS, Han D, Liu N, Tan JH. Regulation of fusion of the nucleolar precursor bodies following activation of mouse oocytes: roles of the maturation-promoting factors and mitogen-activated protein kinases. Zygote 2012; 20:291–303. [DOI] [PubMed] [Google Scholar]
- 39. Moos J, Xu Z, Schultz RM, Kopf GS. Regulation of nuclear envelope assembly/disassembly by MAP kinase. Dev Biol 1996; 175:358–361. [DOI] [PubMed] [Google Scholar]
- 40. Okuwaki M, Sumi A, Hisaoka M, Saotome-Nakamura A, Akashi S, Nishimura Y, Nagata K. Function of homo- and hetero-oligomers of human nucleoplasmin/nucleophosmin family proteins NPM1, NPM2 and NPM3 during sperm chromatin remodeling. Nucleic Acids Res 2012; 40:4861–4878. [DOI] [PMC free article] [PubMed] [Google Scholar]


