Abstract
Monitoring the health of a pregnancy is of utmost importance to both the fetus and the mother. The diagnosis of pregnancy complications typically occurs after the manifestation of symptoms, and limited preventative measures or effective treatments are available. Traditionally, pregnancy health is evaluated by analyzing maternal serum hormone levels, genetic testing, ultrasonographic imaging, and monitoring maternal symptoms. However, researchers have reported a difference in extracellular vesicle (EV) quantity and cargo between healthy and at-risk pregnancies. Thus, placental EVs (PEVs) may help to understand normal and aberrant placental development, monitor pregnancy health in terms of developing placental pathologies, and assess the impact of environmental influences, such as infection, on pregnancy. The diagnostic potential of PEVs could allow for earlier detection of pregnancy complications via noninvasive sampling and frequent monitoring. Understanding how PEVs serve as a means of communication with maternal cells and recognizing their potential utility as a readout of placental health have sparked a growing interest in basic and translational research. However, to date, PEV research with animal models lags behind human studies. The strength of animal pregnancy models is that they can be used to assess placental pathologies in conjunction with isolation of PEVs from fluid samples at different time points throughout gestation. Assessing PEV cargo in animals within normal and complicated pregnancies will accelerate the translation of PEV analysis into the clinic for potential use in prognostics. We propose that appropriate animal models of human pregnancy complications must be established in the PEV field.
Keywords: extracellular vesicle, exosome, placenta, animal models, adverse pregnancy outcomes
Summary sentence Experimental animal models will be essential for defining the opportunity that placental extracellular vesicles may provide for monitoring placental health and function and understanding the pathophysiology of adverse pregnancy outcomes.
Introduction
Placental complications arise in approximately 15% of pregnancies, and due to pregnancy and childbirth, maternal deaths occur in approximately 275,000 cases worldwide annually [1]. In 2010, preterm birth (PTB) was the most common cause of infant mortality and morbidity affecting approximately 15 million babies; Southeastern Asia, South Asia, and sub-Saharan Africa had the highest rates [2]. PTB causes approximately 1 million neonatal deaths each year across the world [3], with surviving infants displaying elevated risks of cardiovascular and respiratory diseases, neurological deficits, and developmental disabilities [4]. Fetal growth restriction (FGR) is the second most common pregnancy complication (impacts ~8% of pregnancies) [5]. FGR increases the risks of intrauterine demise, neonatal morbidity, cognitive delay, and adult onset disease later in life [6, 7]. Women with pre-gestational diabetes (pre-GD) (having type 1 or type 2 diabetes before becoming pregnant) also have a greater risk of exacerbated symptoms during pregnancy, such as diabetic ketoacidosis, myocardial infarctions, retinopathy, and nephropathy, as well as obstetric complications including preeclampsia (PE), uteroplacental insufficiency, preterm labor, shoulder dystocia, and stillbirth [8–11]. Pregnancy complications not only cause emotional stress and trauma on the parents but also create an extreme financial burden for the family and the healthcare system. The annual financial costs are estimated to be $26.2 billion for PTB, $2.18 billion for PE, and $1.8 billion for pregnancy-acquired diabetes (gestational diabetes mellitus; GDM) in the United States [12–14]. Earlier detection of complications, preventative strategies or therapeutics, and placenta-targeted treatments are imperative to improving the in utero environment of the fetus to benefit the long-term health of both the mother and the child.
Diagnosis of a pregnancy complication relies on close monitoring of maternal and fetal health. Obstetricians monitor maternal health by measuring blood pressure, checking vital organs (e.g., renal function), and monitoring fetal health by measuring uterine growth via fundal height and fetal growth directly with ultrasound. Clinicians can also complete a biophysical profile and use Doppler velocimetry to monitor fetal blood flow within the umbilical cord and fetal middle cerebral artery [15]. Although the placenta is essential in pregnancy, no minimally invasive techniques to directly monitor placental health are available. If biomarkers of abnormal placentation could be identified before the pathophysiological complication manifests itself, they may reveal the underlying mechanism(s) that contributes to the insult and provide a targeted approach to develop therapies/treatments.
Pregnancy complications that arise due to a malfunctioning or maldeveloped placenta include PE, early/recurrent pregnancy loss (EPL/RPL), PTB, FGR/intrauterine growth restriction (IUGR), and pre-GD/GDM (Table 1, Supplemental Table 1) [16]. An overview of complications is provided in Table 1 and includes the frequency of the complication within the population, the diagnostic measure to identify the pregnancy complication, and the known pathophysiology for the condition. While GDM is not thought to be a placental disease per se, it may impact fetal well-being as structural and functional alterations can occur in the placenta [17–19]. In addition, maternal infection with vertically transmitted pathogens also gives rise to adverse pregnancy outcomes and may impact placental function or fetal development [20, 21]. Among the implicated pathogens, the TORCHZ group is of particular neonatal concern and consists of the following: Toxoplasma gondii, Other (Listeria monocytogenes, Treponema pallidum, varicella zoster virus, human immunodeficiency virus, enteroviruses and parvovirus B19), Rubella virus, Cytomegalovirus, Herpes simplex virus, and Zika virus [20, 22]. Thus, it is necessary to monitor placental health during and after maternal infection to determine if vertical transmission has occurred and to assess the risk of developing an adverse pregnancy outcome following infection.
Table 1.
Human pregnancy complications.
| Pregnancy complication | Percent impact | Current means of identification | Underlying pathology |
|---|---|---|---|
| PE | • 3–5% of pregnancies [177] • Accounts for 14% of pregnancy-associated maternal deaths [177, 178] | • High blood pressure [354] • Proteinuria [354] • Possible renal, liver, pulmonary, and neurological sequelae [177] • PE is sometimes split into two categories with separate pathologies: early onset PE (EPE), <34 weeks gestation and late onset PE (LPE), ≥34 weeks gestation | • Abnormal trophoblast invasion of the maternal decidua and incomplete remodeling of maternal spiral arteries leads to placental ischemia and a pro-inflammatory environment [179] • Often associated with IUGR [177] • EPE: considered a fetal disease with associated placental dysfunction • LPE: a maternal disorder; placenta usually functions properly and is associated with better maternal and fetal outcomes, at least in part based on later delivery (obviating prematurity as a contributor to pathology) [180] |
| EPL | • 15–25% of all clinical pregnancies [181–183] | • Fetal chromosomal abnormalities are the major cause [184, 185]; however, in cases of normal fetal karyotype, the cause is typically unknown [186–189] | |
| RPL | • ~1% of all women [190] | • RPL is identified by two or more clinical EPLs (pregnancy diagnosis based on ultrasound or tissue/pathology, not chorionic gonadotropin detection alone) [181, 191] | • When uterine anatomical anomalies, genetic factors, antiphospholipid syndrome, or hormonal pathologies are ruled out, more than 50% of patients suffer from unexplained RPL [181, 182, 192] |
| FGR/IUGR | • 10% of pregnancies [193] | • Ultrasound-estimated fetal weight less than the 10th percentile for that gestational age or a fetus at <10th percentile at birth [193] | • Origins stem from errors in early placental development [194] • Caused by placental insufficiency, genetic syndromes, maternal malnutrition, multiple gestation, teratogens, and oxygen deprivation [134, 195] |
| • Maternal vascular malperfusion is considered the leading cause of pathology in FGR placentas and can be accompanied with placental hypoplasia, infarction, and hemorrhage [196] | |||
| • Often associated with PE [177] | |||
| Pre-GD | • ~7% of all pregnancies [11] • High risk of maternal morbidity [197] | • Having type 1 or type 2 diabetes prior to start of pregnancy | • Hyperglycemia can impair and disrupt fetal organ development [8] • Placentas from women with diabetes generally have greater surface area, Hofbauer cells, vasculature, and diffusion distance [199] |
| GDM | • Leading cause of fetal macrosomia [198] | • Glucose intolerance and insulin resistance development during pregnancy | • Placental pathologies include chorionic villus immaturity, high villus density, stromal edema, thicker than average collagen fibers, and diffuse villous stromal calcifications [17], potentially impacting function |
| PTB | • ~11% of all pregnancies [170] • 75% of perinatal mortality is due to PTB [200] | • Birth before 37 weeks of gestation [170] | • Oxidative stress and inflammation leading to early rupture of membranes are known to be leading causes of PTB, in addition to retroplacental abruption, chronic villitis, and twin gestations [201] • Other causes hypothesized to be involved include vertically transmitted infections, maternal environmental stress, and intra-amniotic inflammation [170, 200] |
PEVs may provide an excellent tool with which to monitor placental health and function in human patients. Isolation from fluids is minimally invasive, repeated sampling is feasible, and the ability to monitor the same patient over time provides valuable information as to how the placenta matures, develops, and responds to insult. Most importantly, PEVs contain placenta-specific proteins, which may be used to selectively isolate them from more complex samples, such as blood [23, 24]. However, the roles of PEVs in cellular communication and maternal physiology are not well understood. PEVs are present in maternal blood as early as 6 weeks of gestation [25], and their presence early on makes them attractive molecular packages that may contain a readout of placental health from the early stages of placental development through delivery of the newborn.
The purposes of this review are to (1) introduce EVs, or more specifically PEVs, as a molecular readout of placental health, (2) provide an overview of current information known about human PEVs, (3) discuss animal models used to study pregnancy complications, and (4) discuss future expansion of animal model PEV research to address critical challenges in human PEV research. Although extensive information from human EVs has been obtained from cell cultures and maternal blood sampling, there is a limited understanding of in vivo PEV function in humans and animal pregnancy models. Implementing animal pregnancy models will extend our understanding of trophoblast physiology during pregnancy complications, define PEV cargo and function, and explore the diagnostic and therapeutic potential of PEVs. In vivo studies in experimental pregnancy models are essential to make PEV research translational to a human clinical setting. Due to the breadth of topics this review covers, we apologize for any publications not discussed and refer to more specific reviews wherever possible.
What are extracellular vesicles?
The three main subtypes of extracellular vesicles (EVs) are exosomes (small, 60–80 nm in diameter [26] and large 90–120 nm [26]), microvesicles approximately 100–1000 nm [27], and apoptotic bodies approximately <5 μm [27]. These are distinguished not only by size but also by their route of cellular release (Figure 1) [27–31]. Figure 1 also shows EV release and highlights some unique cargo among the different subtypes. These naming conventions have been greatly debated since it is now well understood that there is overlap in the size and cargo across EV classes [32–34]. The size criteria do not consider the mechanism(s) by which the EV is derived or secreted from the cell. For instance, an EV population isolated based on EV size may encompass both large exosomes and microvesicles; however, these vesicles may differ in their cargo, composition, and biological role. The lipid content may be a more definitive criterion to classify vesicles, as Ouyang et al. [35] demonstrated that phospholipid composition varies between EV classes. Standardized nomenclature across studies regarding the vesicle of interest would greatly strengthen this field of research, as inconsistent nomenclature makes it difficult to compare data across studies. When referencing the work of others in this review, we have used the terminology stated in that publication.
Figure 1.
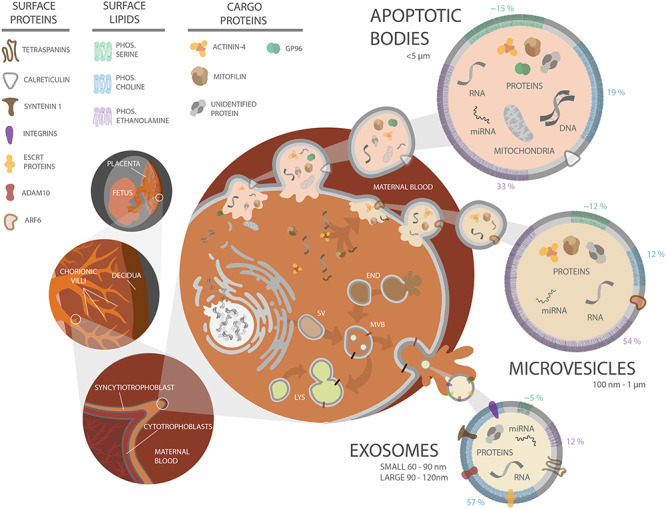
Schematic diagram of human placental villous structure and PEV biogenesis. Placental structure with increasing magnification (organ level, tissue level, cellular level) is depicted at the left, along with apoptotic body, microvesicle, and exosome biogenesis in a trophoblast cell. A key for molecular elements is at the upper left. Top right: Apoptotic bodies form when a cell is undergoing apoptosis, as depicted by the breakdown of the nucleus and packaging of DNA and organelles. Middle right: Microvesicles are formed via pinching off of the plasma membrane, entrapping molecular cargo. Bottom right: Exosomes are assembled within the secretory pathway. Secretory vesicles (SV) are released by the golgi body and can fuse with endosomes (END). Endosomes can also fuse together or fuse with the lysosome (LYS) for cargo degradation. Late stage endosomes are also referred to as MVBs if they contain intraluminal vesicles (depicted as small light yellow vesicles inside the MVB). All of these vesicles are then released into the maternal bloodstream. Some cargo and membrane proteins specific to the different EV classes are depicted. Lipid composition is depicted in a simplified, grouped manner on the border of the vesicles (green, phosphatidylserine; blue, phosphatidylcholine; purple, phosphatidylethanolamine), with the percent of each lipid content indicated adjacent to the membrane region. The gray membrane of the vesicles represents other lipids that have been identified including sphingomyelin, phosphatidylglycerol, phosphatidylinositol, phosphatidic acid, bis-monoacylglycerophosphate, cardiolipin, lysophosphatidylcholine, and lysophosphatidylethanolamine [35].
A range of isolation techniques have been used to isolate exosomes, including differential ultracentrifugation, size exclusion chromatography, and polyethylene glycol precipitation [33, 36–42]. A major goal of these techniques is to obtain a homogenous population of EVs (i.e., only exosomes), but this remains a challenge given the overlap in size across EV subtypes. It is therefore difficult to compare data across studies, as sample types and isolation techniques vary greatly, especially if the EV populations being analyzed are not homogenous. While there is great scientific interest in exosomes due to their potentially bioactive cargo and ability to be taken up by target cells, recipient cells can also take up microvesicles [43]. Moreover, the quantity of both exosomes and microvesicles present in maternal blood increases throughout gestation [44]. Given the current limitations in isolating a homogeneous population of an EV subtype, it may be more appropriate to globally assess all PEVs to develop diagnostic tools for pregnancy complications. Once techniques are developed to isolate vesicles of a specific EV class, the approach to evaluating PEVs can be modified to assess specific EV subtypes.
EV formation, cargo packaging, and function
Exosomes form within an endosome that is also referred to as a multivesicular body (MVB). As depicted in Figure 1, MVBs can either fuse with the lysosome or the plasma membrane for cellular release. Exosomal surface proteins, such as the tetraspanins, are widely conserved across mammals, as shown in Table 2. Microvesicles form on the cell’s surface where they bleb off from the plasma membrane, and therefore, phosphatidylserine is commonly a component within their membranes [27]. Apoptotic bodies form when a cell undergoes apoptosis, where the cell’s organelles organize into these noninflammatory packages [27]. Microvesicles and apoptotic bodies commonly contain heat shock protein 96 (GP96), actinin-4, and mitofilin [34]. Although there is evidence for conservation of mammalian EV surface markers across EV subtypes, packaging of cargo still remains poorly understood. For more details on packaging of EV cargo and EV secretion pathways, the reader is directed to the following reviews [27, 29, 42, 45–47].
Table 2.
Summary of known EV markers and placenta-specific markers across species.
| Species | General EV markers | Placental markers | References |
|---|---|---|---|
| Human | CD9 | PLAP | [27, 34, 202–208] |
| CD63 | PP13 | ||
| CD81 | Syncytin-1 and -2 | ||
| syntenin-1 | PAPP-A | ||
| EHD4 | HLA-G | ||
| ADAM10 | PSG-1 | ||
| ESCRT proteins | C19MC | ||
| NHP | CD63 | PP13 | [168, 209–214] |
| CD81 | Syncytin-1 and -2 | ||
| Flotillin-2 | PAPP-A | ||
| Mamu-AG | |||
| C19MC | |||
| Guinea pig | PLAP | [215, 216] | |
| Env-cav1 | |||
| Rabbit | CD9 | PLAP | [215, 217, 218] |
| CD63 | Syncytin-ory1 | ||
| CD81 | |||
| HSP101 | |||
| Mouse | CD9 | Syncytin-A and -B | [89, 215, 219–222] |
| Alix | PLAP | ||
| CD63 | |||
| CD81 | |||
| Rat | CD63 | PLAP* | [215, 223–226] |
| TSG101 | |||
| Sheep | CD63 | Syn-Rum1 | [227–229] |
| HSP70 | |||
| Cattle | CD9 | PLAP | [103, 229–231] |
| CD63 | Syn-Rum1 | ||
| Pig | CD63 | [232] |
CD, cluster of differentiation; EHD4, EH domain containing protein 4; ADAM10, a disintegrin and metalloproteinase domain-containing protein 10; ESCRT, endosomal-sorting complexes required for transport.
*Also expressed in other tissues.
Biologically active nucleic acids, proteins, carbohydrates, and lipids can be packaged into EVs and secreted as a means of intercellular communication [28–30, 48–56]. Extensive research supports the concept that EVs have roles in diverse biological processes including the immune response [57, 58], inflammation [28–30, 59], and transmission of viral infection [28, 48–51, 60]. For example, researchers have shown that EV uptake by recipient cells induces cytokine release [61], inhibits protein translation [53, 62], influences cell proliferation and migration [63], and protects cells from oxidative stress [64]. Additional information on the impact of EVs on recipient cells and EV tropism and therapeutic potential can be found in the following papers [65–70].
PEVs: sample sources, interactions with immune cells, and clinical potential
To highlight the potential information available from EV analysis, scientists have used the terms “circulating biopsy” [71], “fingerprint” [72], and “liquid biopsies” [72, 73]. EVs have received great attention as it has been shown that EV cargo may be altered under diseased and infection states [62, 64, 74], and they can be isolated from minimally invasive fluid samples. PEVs have been detected as early as 6 weeks of gestation [25] (see Table 3 for an overview of the outcomes from human PEV research); however, trophoblasts secrete chorionic gonadotropin into maternal blood shortly after embryo implantation, suggesting that PEVs may encounter maternal cells just as soon.
Table 3.
PEV studies in humans, rodents, cows, pigs, and sheep.
| Experimental model | Sources of EVs (experimental and control) | Pregnancy complication | Findings | References | |
|---|---|---|---|---|---|
| Human | • Plasma from healthy first, second, and third trimester women | • Plasma from healthy, nonpregnant women | — | • Increased quantity of exosomes and PLAP+ exosomes with gestation • Exosomes had a positive impact on endothelial cell wound healing, with early gestation having the greatest impact | [102] |
| • Plasma from women with PE | • Control pregnant plasma | PE | • Higher quantities of EVs, ST-derived EVs, and phosphatidylserine/annexin-V-positive EVs in PE patients | [87, 91, 233, 234] | |
| • HUVEC-conditioned media | • Exosomes isolated from plasma | PE | • Decreased expression of eNOS in HUVECs exposed to exosomes from PE patients • More miR-155 within EVs from PE patients • miR-155 downregulates eNOS expression | [62] | |
| • Plasma from women with PE | • Plasma from healthy women, gestation-matched | PE | • Elevated levels of exosomes and PLAP+ exosomes, throughout gestation in PE patients | [92] | |
| • Exosomal miRNA profiles differed significantly | |||||
| • Plasma from women with EPE & LPE | • Plasma from gestation age-matched, normotensive women | PE | • Greater exosome quantity in EPE or LPE compared to control • Placental vesicle quantity was elevated in EPE and significantly decreased in LPE, which indicates that the etiology between them is different | [90] | |
| • PE placentas that underwent either mechanical disruption or perfusion | • Healthy placentas that underwent either mechanical disruption or perfusion | PE | • Increased Flt-1 in PE ST-derived EVs • Decreased endoglin in PE ST-derived EVs | [74] | |
| • PE placental explant-conditioned media | • Healthy, gestation age-matched placental explant-conditioned media | PE | • Decreased presence of integrins in ST microvesicles from PE placentas may be associated with reduced trophoblast invasion and defective placental vascularization | [235] | |
| • PE placental explant-conditioned media | • Healthy, gestation age-matched placental explant-conditioned media | PE | • Isolated macro, micro, and nanovesicles with differential ultracentrifugation • Microvesicles from PE placentas were larger in size • PE placentas extruded more micro- and nanovesicles, which contained more Flt-1 than control vesicles • More VEGF was detected in EVs secreted by PE placentas | [93] | |
| • Sera from women with PE | • Primary term trophoblast cell-conditioned media | PE | • Syncytin-1 and -2 and PLAP are on the membrane of exosomes released by primary trophoblast cells | [145] | |
| • Sera from gestation age-matched, healthy women | • Less syncytin-2 in exosomes from women with PE compared to control • Decreased internalization of exosomes released by trophoblast cells deficient in syncytin-1 and -2 (via siRNA transfection) compared to controls | ||||
| • Plasma from women throughout gestation that later developed LPE | • Plasma from healthy, gestation age-matched women | PE | • Nonsignificant increase in total number of MP over gestation • Over gestation in healthy samples, the quantity of HLA-G+ MPs that contained dsDNA decreased and minimal change in the quantity of PLAP+ MPs with dsDNA was observed • LPE samples contained significantly more total MP than healthy; however, the quantity of HLA-G+ or PLAP+ MPs was not different. This suggests that the ontology of LPE is different than EPE | [236] | |
| • Placental explant-conditioned media from women with RPL or PE | • Placental explant-conditioned media from healthy, gestation age-matched, women | RPL and PE | • Altered lipid composition in ST microvesicles from RPL and PE compared to controls | [237] | |
| • Plasma from women with either SGA or FGR fetuses | • Plasma from healthy, gestation age-matched women | FGR | • No difference in the total number of exosomes or PLAP+ exosomes in fetal plasma • Lower percentage of placental exosomes in maternal and fetal plasma from FGR and SGA pregnancies compared to controls • More placental exosomes in fetal than maternal plasma | [238] | |
| • Plasma from women with either EPE, LPE, or normotensive IUGR | • Plasma from healthy, gestation age-matched women and nonpregnant women | PE and IUGR | • Significantly higher quantities of ST microparticles in EPE plasma, but not LPE or normotensive IUGR, compared to controls | [91] | |
| • Plasma from women with GDM | • Plasma from healthy, gestation age-matched controls | GDM | • Increase in total exosomes and PLAP+ exosomes from women with GDM compared to controls • Total number of exosomes detected in the plasma of GDM patients at later stages of gestation was negatively correlated with placental weight, while the amount of exosomal PLAP was positively correlated | [56] | |
| • Increased cytokine release from HUVECs exposed to GDM exosomes than healthy exosomes or baseline | |||||
| • Term perfused placenta from women with GDM | • Term perfused placenta from healthy term women | GDM | • 56% of medium/large EVs and 36% of small EVs were PLAP+ • DPPIV, an enzyme that is inhibited in type 2 diabetes mellitus therapy, was co-expressed on PLAP+ EVs. DPPIV retained enzymatic activity on EVs | [239] | |
| • EVs from GDM samples had greater quantities of DPPIV+ EVs and greater DPPIV activity than controls | |||||
| • Plasma from second trimester women with GDM | • Plasma from healthy, gestation age-matched women | GDM | • 78 proteins were differentially expressed in exosomes from women with GDM compared to controls, including proteins involved in metabolic processes and biological regulation | [240] | |
| • CAMK2beta was more abundant and PAPP-A was less abundant in exosomes from women with GDM compared to controls • Selective enrichment of proteins from the placenta in GDM exosomes compared to controls | |||||
| • Serum from first trimester women with GDM | • Serum from healthy, gestation age-matched women | GDM | • PLAP+ EVs were not detected until the 8th week of pregnancy • 17 miRNAs were analyzed by qPCR in total serum EVs | [241] | |
| • Differential miRNA content between GDM and control EVs. Pathway analysis showed that increased quantities of miRNAs in EVs from GDM were involved in placental development, fetal growth, and insulin and glucose regulation | |||||
| • Placental explant-conditioned media from women with GDM | • Placental explant-conditioned media from healthy women | GDM | • Explants from GDM placentas secreted more EVs • Exosomes from controls increased skeletal muscle cell proliferation and migration, in vitro, compared to GDM. Potentially due to differential miRNA expression in exosomes from GDM versus control | [63] | |
| • miRNA content in exosomes differed from miRNAs in the cells of origin | |||||
| • Plasma from women obese or overweight | • Plasma from lean women | Obese/overweight | • There is an increase in exosome and PLAP+ exosome quantity over gestation, which is independent of BMI | [142] | |
| • There is a correlation between BMI and exosome quantity | |||||
| • No significant increase (12–20%) in PLAP+ exosomes throughout gestation | |||||
| • Exosomes were taken up by HUVECs and altered HUVEC cytokine release. Cytokine release changed based on the trimester and health status of the woman. Exosomes from obese plasma resulted in significantly more IL-6, TNF-alpha, and IL-8 release. No change in IL-10 was observed. | |||||
| • First trimester trophoblast cell-conditioned media incubated in high and low levels of glucose to mimic maternal hyperglycemia | • First trimester trophoblast cell-conditioned media from cells grown under varying oxygen concentrations | Hyperglycemia and hypoxia | • Exposure to hypoxia and high quantities of glucose resulted in increased exosome release • HUVECs released higher quantities of cytokines when exposed to exosomes isolated from cells grown under hypoxia and incubated in high quantities of glucose | [242] | |
| • HTR8/SVneo-conditioned media grown under normoxia and hypoxia | • Plasma from patients with normal pregnancies, PTB, or PE | PTB and PE | • Exosomes from cells grown under hypoxia negatively impacted endothelial cell wound healing • Unique miRNAs were identified in each condition and three miRNAs were only detected in exosomes from pathological conditions (hypoxic cells, PTB and PE) | [115] | |
| • Similar exosome miRNA cargo between cells grown under hypoxia and exosome miRNA cargo in early gestation maternal blood from PTB | |||||
| • Plasma collected throughout gestation from women who had a PTB | • Plasma of gestation age-matched, healthy women who had a term birth | PTB | • Altered exosomal miRNA cargo between term and PTB (throughout gestation) • Shows that alterations in miRNA expression are present early in gestation, which supports possible use of EVs as a screening tool for risk of PTB | [243] | |
| • Plasma from women who were undergoing preterm labor | • Plasma from healthy, gestation-age matched women | Preterm labor | • Small RNAseq was performed on whole plasma, EV-depleted plasma, and EVs • Pregnancy-associated miRNA cluster expression, including C14MC and C19MC miRNAs, were expressed at lower levels in preterm labor EV samples compared to healthy pregnancies | [116] | |
| • Plasma from women who experienced PTB or PPROM | • Plasma from healthy, gestation-age matched women | PTB | • No change in exosome quantity between the three groups • Significantly decreased levels of placental exosomes and different protein cargo in instances of PPROM compared to women with PTB or controls | [244] | |
| Rodent | • Mouse PEVs (syncytin-positive) obtained from freeze–thaw injured placentas | — | PE | • Pregnant mice injected with PEVs had proteinuria and vascular leakage • Nonpregnant mice injected with PEVs resulted in hypertension and proteinuria • Enhancing EV clearance by treatment with the microvesicle-scavenging factor lactadherin prevented the development of this PE-like phenotype | [89] |
| • Lactadherin −/− mice had elevated blood pressure, proteinuria, and fewer litters | |||||
| • HTR8/SVneo cells grown under hypoxic oxygen | • HTR8/SVneo cells grown under normoxic oxygen | — | • Hypoxia induces increased secretion of small EVs • Protein cargo was different between small EVs from hypoxic and normoxic cells | [226] | |
| • Injection of the small EVs from cells grown under hypoxic conditions into rats resulted in elevated blood pressure, but had no impact on fetal survival or placental size | |||||
| • Human first trimester placenta explant conditioned media | — | — | • Nanovesicles (vesicles smaller than 100 nm) altered the sensitivity of mesenteric artery dilation in pregnant, but not nonpregnant, mice • Vesicles did not alter uterine artery dilation or constriction | [245] | |
| • Human vesicles preferentially end up in mouse maternal lungs, liver, and kidneys while the feto/placental unit was negative | |||||
| • The uterus and cervix were not analyzed | |||||
| • Pregnant mouse plasma | — | PTB | • Quantity of exosomes increased with gestation | [221] | |
| • Mouse plasma exosomes trafficked to the cervix, uterus, fetal membranes, and placenta, and no exosomes were detected in other maternal or fetal tissues | |||||
| • Late gestation exosomes had increased quantities of proinflammatory proteins compared to nonpregnant or early gestation exosomes | |||||
| • Injection of late gestation exosomes into early gestation mice resulted in PTB | |||||
| Cow | • Pregnant cow plasma | • Nonpregnant cow plasma | — | • Pregnant plasma exosomes suppressed the expression of the inflammatory cytokines TNF-alpha and IL-6 in BEND cells | [231] |
| • Preferential location of miR-499 is in exosomes | |||||
| • Inhibition of miR-499 expression in mice resulted in increased uterine inflammation, EPL, and FGR | |||||
| Pig | • Porcine trophectoderm cell line (PTr2) | • Porcine aortic endothelial cells (PAOEC) | — | • EVs from PTr2 influenced the proliferation of PAOECs • Uptake of heterologous EVs was more likely that autologous EV uptake | [148] |
| Sheep | • Pregnant sheep sera | • Nonpregnant sheep sera | — | • Exosomal miRNA cargo changed throughout gestation | [228] |
| • C14 miRNAs decreased in quantity as gestation continued | |||||
MP, microparticle; eNOS, endothelial nitric oxide synthase; HUVEC, human umbilical vein endothelial cells; SGA, small for gestational age; PPROM, preterm premature rupture of membranes; C14MC, chromosome 14 microRNA cluster; C19MC, chromosome 19 microRNA cluster; TNF-alpha, tumor necrosis factor-alpha; IL-6, interleukin-6; HTR8/SVneo, extravillous trophoblast cell line, BEND cells, bovine endometrial epithelial cells; ST, syncytiotrophoblast; DPPIV, dipeptidyl peptidase IV; qPCR, quantitative real time polymerase chain reaction; CAMK2beta, calcium/calmodulin-dependent protein kinase II beta.
The cellular and molecular bases of pregnancy complications are difficult to address within the complexities of an in vivo pregnancy setting, owing to variability in genetics, lifestyle influences, physiology, and differing environmental exposure. Researchers examining human PEVs have focused extensively on characterizing EVs isolated from placental cell cultures (e.g., primary cells and immortalized trophoblast cell lines), placental explants, the perfusate of intact placentas, and maternal peripheral blood. PEVs isolated from placental cell cultures and explants can interact with maternal immune cells [59, 75–77], suggesting that PEVs may modulate maternal immune responses during pregnancy.
Current in vivo and in vitro sources for isolating PEVs
In vitro placental cell cultures provide a means to isolate an enriched PEV population. Stable cell lines can be expanded quickly to generate a homogenous cell population, allowing investigators to define a cell type-specific readout or secretory profile in response to an experimental manipulation (e.g., genetic mutation, infection, hypoxia, drug treatment). However, because some trophoblast cell lines are derived from spontaneously arising tumors or have been immortalized by molecular tools, their gene and protein expression profiles differ from each other and with primary trophoblast cultures [78, 79].
Primary cells provide a more accurate representation of in vivo trophoblasts and can be isolated from gestationally age-matched healthy and maldeveloped placentas for direct comparison. The ability to obtain trophoblasts from first and second trimester placentas, however, may be limited due to constraints surrounding human samples. Until recently, primary term villous cytotrophoblast cells were used only for short-term experiments as they do not proliferate and spontaneously syncytialize [80]. Recent optimization of primate trophoblast cell culture conditions now supports long-term cell proliferation and culture of trophoblast stem (TS) cells derived from primary placental cells or embryos that can be directed toward cell type-specific differentiation [81–84].
Placental explant cultures contain all cell types within the placenta (placental macrophages, cytotrophoblasts, and syncytiotrophoblasts) and therefore provide a more complete system compared to primary cell cultures. Limitations of this culture system include a limited duration of tissue viability [85, 86] and difficulty obtaining placentas from early pregnancies. It also is well accepted that pregnancy complications often stem from errors in early placentation. Therefore, it cannot be known if a complication would have arisen later in gestation when a placenta sample is obtained early in pregnancy. In addition, the large volume of medium used to culture explants can dilute the EV sample [86]. Alternative approaches include obtaining EVs by mechanical scraping the placental villi [61, 87] or perfusing fully intact term placentas [85]. Notably, vaginally delivered term placentas may be confounded by the process of labor.
Overall, in vitro models are useful because they can allow for the rapid generation of PEV-enriched samples, be used to validate antibodies, and provide physiological readout in response to trophoblast insult (i.e., exposure to toxins, hypoxia, or pathogens). These in vitro models also predominantly contain EVs secreted by trophoblasts, whereas maternal blood contains the totality of EVs released by all maternal as well as placental cell types. It is likely that the changes in plasma-derived EVs in pregnancy reflect the maternal adaptation to pregnancy by all maternal organ systems and not specifically placental development. While EVs from nonplacental sources during pregnancy may provide novel biological information, they do not specifically provide direct feedback about placental health per se. Previous studies have demonstrated that the source (bodily fluids, culture media, tissue homogenization) and sample preparation method influence EV function [87, 88]. Therefore, when establishing a PEV model or interpreting the study results, it is important to consider the origin of the PEV population being analyzed.
PEVs isolated from adverse human pregnancies
The analysis of human PEVs isolated from in vitro sources and maternal blood has revealed alterations in PEV cargo between healthy and unhealthy placentas. The isolation methods vary greatly across studies, thus limiting the ability to identify consensus biomarkers associated with an abnormal placental condition. A summary of the findings from studies that have evaluated EV cargo in human and animal pregnancy models in relation to a pregnancy complication is provided in Table 3, and here, we focus on the highlights of quantity and cargo of EVs associated with human pregnancy complications.
Elevated PEV levels offer potential as a way to distinguish complicated from healthy pregnancies. Increased quantities of EVs in maternal blood have been associated with various pregnancy complications as summarized in Table 3 [56, 89–93]. However, there are two concerns with these reports. First, it is unclear whether the research findings are specific to one complication or if they are applicable across pregnancy complications. If it is the latter, elevated EVs would be a general reflection of abnormal placental development and/or function. To determine the diagnostic value of EVs, a comprehensive analysis of EVs isolated from healthy pregnant women and women with a range of pregnancy complications is needed. Second, the techniques used to isolate and quantify (different instruments and/or different setting for the same instrument) EV samples vary widely, which dramatically hinders interstudy comparisons. Until standardized isolation and sample analysis techniques are implemented, variation in EV quantity will continue to be an uncertain indicator of a pregnancy’s health status.
Although human PEV cargo is not well characterized, EVs may contain evidence of placental infection and may serve as means of modulating immune responses. For instance, EVs isolated from trophoblast-conditioned media protected nonplacental cells from viral infection [94–96]. Viral proteins and genomes also have been detected within exosomes and infection was found to alter exosome cargo [60, 97]. As such, clinicians could examine PEVs to learn if a pathogen has breached the maternal–fetal interface as they could directly monitor placental response and health status.
PEVs interact with immune cells during pregnancy
Maternal immune systems inappropriately adapted to pregnancy are associated with pregnancy complications and pregnancy loss [98]. PEVs contain a range of immunoregulatory molecules [99, 100] and interact with maternal immune cells in vitro, which suggests that PEVs may be involved in maternal immune adaptation in pregnancy. EVs are involved in the recruitment of monocytes and macrophages as well as in cytokine and chemokine regulation [76]. Syncytiotrophoblast-derived EVs from healthy placentas suppress and/or promote immunological pathways [77]. Thus, understanding the interaction between the maternal immune system, the fetoplacental unit, and EVs is important. In addition, EVs from pregnant women impact immune cells differently than EVs from nonpregnant women [59, 75]. The dynamic complexities between PEVs and the immune system in a healthy and diseased state support the importance of in vivo models.
Differences in EV tropism for immune cells appear to depend on the source of the EV sample, that is, peripheral blood versus placental tissue. Germain et al. [87] observed strong binding of syncytiotrophoblast microvesicles from term placentas to monocytes in first trimester blood with decreased binding throughout gestation, as determined by enzyme-linked immunosorbent assay (ELISA). However, microvesicles from third trimester human placentas bound preferentially to monocytes and B-cells versus T and NK cells as assessed by Image Stream technology [61]. In contrast, microvesicles isolated from third trimester blood bound to T cells and not B or NK cells via fluorescence-activated cell sorting [59]. These studies co-incubated peripheral blood mononuclear cells (PBMCs) with EV samples in vitro. Since the methodologies used to isolate these EVs impacted tropism and downstream function, in vivo experiments will be important to study the interplay between PEVs and the immune system. EVs obtained from mechanical scraping of term placental villi did not stimulate PBMCs, whereas EV samples obtained from placental perfusate were more stimulatory [61, 87]. Notably, these EVs were obtained from term placentas and represent the end point of pregnancy. Pap et al. [59] found that 50% of microvesicles positive for human leukocyte antigen G (HLA-G) were also positive for Fas ligand (FasL). The authors hypothesized that these two molecules present on the EV surface are involved in maternal immune tolerance [59].
Surveying PEV markers and cargo to identify pregnancy complications
Classic EV markers, such as tetraspanins and ESCRT proteins [27], have been routinely identified in human and animal EVs, as listed in Table 2. As an adjunct to monitoring maternal systemic physiological changes (e.g., blood pressure or proteinuria), clinicians could directly monitor placental health and development by surveying PEVs. The application of PEVs as a prognostic molecular tool, however, is hindered by the current lack of validated placental biomarkers associated with a pregnancy complication in humans or animals. Furthermore, there are few validated placenta-specific EV surface markers to isolate PEVs. The most widely used placenta-specific marker for PEV isolation is placenta alkaline phosphatase (PLAP) [23, 24, 87, 101]. A PLAP ELISA has been used to quantify PEVs from human [25, 90, 102] and bovine samples [103]; however, validation and information regarding cross-reactivity of this antibody are lacking. Despite its use by several labs, there are challenges with specificity as other alkaline phosphatases have been detected in various healthy tissues that can be recognized by PLAP antibodies [104–106]. Validating these PLAP antibodies and making other in-house antibodies, such as NDOG2 and ED822 [74, 87, 107], commercially available will increase reproducibility across studies. HLA-G, another placenta-specific protein, has also been used to isolate and detect PEVs [24, 59], recognizing that this would be an extravillous trophoblast marker. Other placenta-specific protein candidates include syncytin-2, placental protein 13 (PP13), pregnancy-specific glycoprotein 1 (PSG1), and pappalysin-1 (PAPP-A) (Table 2).
Changes in PEV cargo highlight a difference between healthy and pathological placentas, as shown in Table 3. Cuffe et al. [108] discussed the presence of two classes of molecules: “passive” and “bioactive”. Passive molecules are hypothesized to have high predictive potential, whereas bioactive molecules are constitutively secreted by the placenta [108]. This further supports the use of PEVs to monitor placental health. PE is perhaps one of the more well-studied pregnancy complications in which PEV quantities and cargo have been evaluated [109]. Nair and Salomon’s recent review on human GDM [110] discussed systemic and placental changes in EVs. Several studies have focused on RPL and procoagulant microparticles/EVs secreted by platelets and endothelial cells [111–114]; however, we were unable to find any reports regarding the association of PEVs and EPL/RPL. Researchers have observed alterations in PEV cargo between women experiencing PTB compared to controls; however, they have not identified a consistent biomarker [115, 116]. For instance, one study only detected miR-525-5p in EVs from a pathological condition (PTB, PE, or cells grown under hypoxia) [115]. Another study found miR-525-5p to be significantly lower in PTB EVs compared to controls [116]. To understand the changes in PEV cargo associated with the cell’s physiological state, it is necessary to first understand whether EV cargo is selectively packaged. The survey of PEV cargo upon experimental knockout of lysosomal enzymes would provide insight into the critical question of cargo selection that spans all disciplines of the EV field.
The dearth of placenta-specific markers for PEV isolation and the lack of agreement upon biomarkers of a pregnancy complication highlight the need for consistent methodologies and nomenclature in studies that isolate PEVs from these various pregnancy complications. Identification of biomarkers resulting from aberrant placental development may allow for earlier diagnosis and intervention or earlier application of a placental therapy. PEV analysis could also enable better categorization of adverse pregnancy outcomes via specific molecular changes in the placenta rather than by less specific maternal symptoms and fetal measurements.
Animal models of human pregnancy complications
Most PEV research has been performed using human fluid samples and in vitro trophoblast cultures, with relatively few studies in animal models. Due to the complexities of obtaining and working with human samples and the limitations of vitro systems discussed thus far, other systems are needed to advance the study of PEVs. Animal models can provide the rigor and reproducibility that are difficult to achieve with human samples, due to uncontrolled external factors and genetic diversity among clinical patients. Advantages of collecting PEV data from animal models include access to large cohorts raised in controlled environments, rigorous sampling (i.e., the ability to collect samples early in pregnancy and at precise time points), control of the factor(s) causing the pregnancy complication (in some instances), the ability to utilize an animal as its own control during the same or subsequent pregnancy, the potential for longitudinal studies spanning preconception to the delivery of the offspring, and the opportunity for transgenerational studies.
Despite the differences in placentation across animal models, each animal model has strengths and shares similarities to humans that are useful when disentangling the mechanisms underlying pregnancy complications. When selecting an animal model to study a pregnancy complication, the following considerations should be addressed: placentation (i.e., depth of invasion, trophoblast cell organization, immune cell presence), animal husbandry for maintaining a study cohort (without/or without manipulation of an environmental factor), and the specific questions asked during the study (i.e., the importance of the fetus being born precocial; monotocous versus polytocous species). For example, the lack of endometrial trophoblast invasion by the pig placenta and minimal invasion in sheep has limited their utility in modeling PE. Although the pig placenta may not ideally model PE, its similarities in fetal development with humans could be beneficial to study FGR [117]. Furthermore, FGR studies in sheep and rabbit models provide an opportunity for the animal to serve as its own control by evaluating the unmanipulated, opposite uterine horn [5, 118–120]. An overview of placentation is depicted in Figure 2, and the details regarding pregnancy and translatability of each animal model discussed in this review are listed in Table 4 [121–125].
Figure 2.
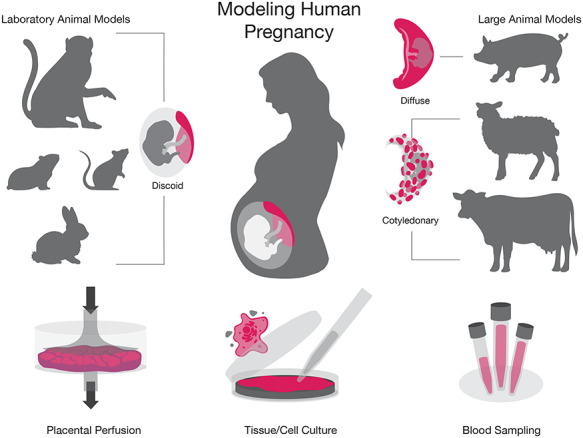
Experimental models of human pregnancy. Laboratory animal models (NHP, guinea pig, rodent, and rabbit), large animal models (pig, sheep, and cow), and in vitro systems (placental perfusion, tissue/cell culture, and blood) are depicted to represent the experimental models of human pregnancy.
Table 4.
Comparison of placentation across different research models.
| Model | Type of placentation | Gestation length (days) | Advantages | Limitations |
|---|---|---|---|---|
| Human | • Hemochorial | 280 | • Maternal plasma readily accessible | • Difficult to control environment factors |
| • Discoid • Villous organization and extensive spiral artery remodeling • Interstitial extravillous trophoblast invasion | • Placenta samples available from a broad spectrum of adverse pregnancy outcomes • Diverse and extensive literature database • Well established in vitro systems (e.g., cell culture, explants, placental perfusion) | • Highly variable genetics • Restrictions to testing treatments/therapeutics • Precise timing of start of pregnancy can be uncertain • Delay in pregnancy detection limits the ability to obtain samples within first few weeks of pregnancy | ||
| • TS cells available [81–83] | ||||
| Macaque monkey | • Hemochorial • Bi-discoid placenta • Extensive endovascular extravillous trophoblast remodeling of decidual spiral arteries | 165 | • Ability to test treatments/therapeutics • Most appropriate model available for human placental physiology, immunology, and endocrine function at the maternal–fetal interface [121, 246, 247] | • Trophoblast interstitial extravillous invasion is superficial in comparison to human • Limited transgenic models • Specialized veterinary expertise and housing required |
| • Placental architecture is highly translational [121] • Offspring are born precocial | ||||
| • Placental transfer of passive immunity | ||||
| • Placental expression of C19MC [203] and nonclassical MHC class I [247] | ||||
| Guinea Pig | • Hemomonochorial • Discoid • Labyrinthine, invasive [141, 169, 248, 249] | 67 | • Offspring born precocial [141, 169, 249] • Blastocyst is completely encapsulated within the decidua, similar to human [248] | • Dearth of available antibodies • Lack of transgenic models |
| • Passive immunity in late term [250] | ||||
| • Substantial trophoblast invasion [5] | ||||
| • Similar steroid production and metabolism in the decidua and fetal membranes as the human [169] | ||||
| Rabbit | • Hemodichorial • Discoid • Labyrinth organization [125, 169] | 32 | • Fully sequenced genome [122] • Induced ovulator allowing for timed matings [122] • Offspring born precocial [172] | • Placental endocrinology is different than human [169] • Dearth of available antibodies |
| • Passive immunity in late term [250] • Housing facilities are readily available | ||||
| Mouse | • Hemotrichorial | 20 | • Facile manipulation of genetics [122] | • Offspring not born precocial |
| • Discoid • Labyrinthine organization and some spiral artery remodeling [125] | • Ability to test treatments/therapeutics • Timed mating • NK cells present at MFI as with human [251] • Ability to perfuse mid and late gestation placentas [85] • TS cells available [85, 252] | • Placental organization, cell types, and endocrine profile differ compared to humans [253–255] • PLAP is not expressed • Blood flow to the placenta is more limited than in human [121] • Lack of nonclassical MHC expression [251, 256] | ||
| • Shallow implantation compared to rat or human [251] | ||||
| • Placental expression of C19MC miRNA is not conserved [203] | ||||
| • TS cell isolation and propagation differ from primate [81–84, 257] | ||||
| • Murine cytotrophoblast cells are in direct contact with maternal blood, whereas syncytiotrophoblasts are in direct contact in the human [98] | ||||
| Rat | • Hemotrichorial | 22 | • Nonclassical MHC expression [251, 256] | • Offspring not born precocial |
| • Discoid • Labyrinthine organization • Deep placental implantation [251, 258] | • NK cells at MFI as human [251] • larger size compared to mouse allows practical advantages (tissue availability, surgical procedures) • TS cells available [85, 259] | • Different placental organization and cell types compared to humans [258] • Placental expression of C19MC miRNA is not conserved [203] | ||
| • Less extensive genetic technology and antibody development compared to the mouse [122] | ||||
| • Different endocrine profile than humans [255] | ||||
| Sheep | • Epitheliochorial (synepitheliochorial) | 150 | • Relatively few offspring per liter [5, 260] | • Minimal trophoblast invasion [260] |
| • Cotyledonary [250] | • Offspring born precocial [122] • Large blood samples and surgical manipulations including chronic instrumentation of the fetus are feasible | • Practical limitations on housing of research animals and length of gestation • Different placental cell types and endocrine profile than humans [255] | ||
| Cattle | • Epitheliochorial (synepitheliochorial) | 280 | • Sites of nutrient and waste exchange are villous [124] | • Minimal trophoblast invasion [260] |
| • Cotyledonary • Partially nondeciduate [103, 261, 262] | • Macrophages located at the maternal–fetal interface at low levels during the first two-thirds of pregnancy and increase substantially by term [261] • Nonclassical MHC expression towards the end of pregnancy [263] | • Practical limitations on housing of research animals and length of gestation • Different placental cell types and endocrine profile than humans [255] | ||
| • TS cells available [264] | ||||
| • Large blood samples and surgical manipulations are feasible | ||||
| Pig | • Epitheliochorial • Diffuse [262] | 114 | • Fully sequenced genome [260] • Placental attachment is superficial and interdigitates with the highly folded maternal endometrium • TS cells available [265] • Large blood samples and surgical manipulations are feasible | • Fetal nutrition is predominantly acquired through uterine gland secretions • Passive immunity does not occur until after birth [250] • Unlike humans, there is no syncytiotrophoblast cell type [124] • Practical limitations on housing of research animals and length of gestation |
Animal models allow for longitudinal study to identify early biomarkers prior to or at the onset of a complication, which is not possible in humans due to the delays in confirming pregnancy and the need to avoid perturbing early gestation. Identifying changes or biomarkers at the onset of a complication, even before placentation is complete, may be important for diagnosing or preventing a further adverse outcome. Once biomarkers have been identified in an animal model, they can then be validated for tissue specificity and reproducibility within and across species for subsequent translation to humans. For example, miR-210 is more highly expressed in the placentas of mice with PE compared to healthy placentas. It is also expressed in human and ovine placentas [126, 127] with aberrant regulation in human PE and upregulation of the miRNA in hypoxic human placentas. Thus, animal models have led to the identification of a putative biomarker of PE, where upon further refinement of the timing of aberrant expression and identification of target mRNAs may reveal the biological processes contributing to the manifestation of the placental complication.
Animal studies will have an essential role in showing proof of principle for the potential of a therapeutic intervention or diagnostic assay prior to translation into human clinical studies. For example, pregnant guinea pigs and sheep were used to test an experimental placental treatment, in which injection of an adenoviral vector overexpressing VEGF reduced FGR [128, 129]. Due to the positive outcomes in these animal models, this therapy subsequently was transitioned into a clinical trial in Europe (EVERREST project) [130]. Further use of animal models will not only enable the development of improved diagnostics, but they also can provide a platform for developing and evaluating the efficacy of placental therapies [120]. In these animal models, a thorough understanding of the complication as well as the safety of a therapeutic can be appropriately evaluated prior to transition into a clinical trial; however, there must be a thorough understanding of the etiology of that pregnancy complication. The guinea pig and macaque, for example, are both ideal for placenta-targeted therapies as they similarly share a hemochorial type of placentation, bypassing additional maternal layers present in livestock species. Thus, a common workflow in placental research, and other fields, is to identify in rodents, verify in nonhuman primates (NHPs), and then translate to humans. This approach can be implemented in the PEV field by broadly utilizing various experimental animal pregnancy models.
Spontaneous versus induced animal models of adverse pregnancies
While pregnancy complications can develop spontaneously in animal models, they are frequently induced. Similar to the selection of the animal model, there are distinct advantages and limitations for choosing between a spontaneous or induced model to study adverse pregnancy outcomes in animals. For the sake of brevity, we have collated the induction methods of a complication for the various species that are listed in Table 5.
Table 5.
Induced animal models of human pregnancy complications.
| Induction mechanism | NHP | Rabbit | Mouse/rat | Guinea pig | Pig | ||
| Artery ligation | • Abdominal aortic constriction and uterine artery ligation led to hypertension and proteinuria with renal damage and/or impaired function [153, 154, 266] | • Constriction of the maternal aorta below the renal arteries led to proteinuria, kidney damage, and fetal demise [267] | • RUPP led to elevated TNF-alpha levels, hypertension, proteinuria, and FGR [268] | ||||
| PE | Other | • TNF-alpha injection resulted in proteinuria, hypertension, and elevated sFLT-1 levels [269] | • Fetal hemoglobin injection resulted in proteinuria, fetal demise, and increased apoptosis in the kidneys and placentas [173] | • sFlt-1 injection resulted in hypertension and proteinuria [270] • TNF-alpha infusions resulted in hypertension [271] • Injection of sEng and sFlt-1 [272] • Modified nitric oxide production resulted in hypertension and FGR [273, 274] | • Diet restriction led to ketosis, and minor PE symptoms and pathology [131] | • Fasting resulted in HELLP syndrome, hypertension, and proteinuria [275–277] • Glucose restriction induced hemolysis and increased levels of free heme [278] | |
| Induction mechanism | NHP | Sheep | Pig | Cow | |||
| EPL/RPL | Other | • Low protein diet resulted in poor placental perfusion, miscarriage, and FGR [155] | • Lower insulin and progesterone levels were reported in pregnancy loss during days 18–40 of gestation [279] | • 20–45% implantation failure [136, 138, 139] • Defects in vascular remodeling, inflammation, and altered miRNA expression have been associated with porcine embryonic death [138, 139] | • ~40% implantation failure [135–137] • Genotyping of fetuses that died in utero, and genetic selection of sperm donors provided insight as to genes associated with EPL [135, 137] • EPL is also associated with infection and uterine inflammation [136] • Progesterone levels prior to implantation may contribute to the establishment and maintenance of pregnancy [135] | ||
| Induction mechanism | NHP | Guinea Pig | Rabbit | Mouse/Rat | Sheep | Pig | |
| Uteroplacental vascular modification | • Ligation of the vessels connecting the primary and secondary discs led to placental insufficiency, fetal death, and FGR [156] | • Uterine artery ligation did not result in fetal demise, but did result in smaller fetuses and impaired organ function [280] • Ablation of uterine artery branches leading to individual placentas [281] and gradual ligation techniques [282] can remain in place for long periods of time and do not result in high fetal mortality | • Uterine artery ligation negatively impacted kidney development in the fetuses [283], which is also seen in human cases of IUGR [284] • Artery ligation resulted in a high prevalence of fetal mortality [118, 119] and negatively impacted renal development [119] • A limitation of uteroplacental vessel ligation is the necessity of a short study duration due to high fetal mortality [285] | • Bilateral uterine artery ligation resulted in asymmetrical IUGR and negatively impacted kidney development in the fetuses [283] • Has been shown to result in altered insulin levels, IGF and IGF binding protein levels, decreased placental weight, hypertension, and delayed growth [120] • A limitation of uteroplacental vessel ligation is the necessity of a short study duration due to high fetal mortality [285] | • Uteroplacental embolization [134, 286] • Single umbilical artery ligation [134, 286] | ||
| FGR/IUGR | Other | • Experience placental insufficiency and IUGR similar to humans [121, 155] | • Hypoxia impaired placental development and invasion and resulted in hypertension [287] | • Natural model due to the large litter size (embryo crowding within the uterus can impact growth) [5] • See review [285] | • Injection of sEng and sFlt-1 resulted in FGR [272] • See reviews [120, 288] | • Heat stress decreased placental blood flow and led to IUGR [134, 286] • Sheep living at high elevations develop IUGR [134] • See reviews [117, 289] | • Duration of nutrient restriction is correlated to the severity of IUGR [117] • A limitation of nutrient restriction is lack of hyperleptinemia, as is observed in humans and rats [290] • See review [117] |
| Induction mechanism | NHP | Guinea Pig | Rabbit | Mouse/Rat | Sheep | Pig | |
| Pre-GD/GDM | Chemical induced | • Alloxan and STZ [157, 158] | • STZ [291] | • Alloxan and STZ [171, 292] | • Alloxan and STZ [293–296] | • STZ [297, 298] | • STZ [299] did not significantly alter fetal size at birth |
| Other | • Useful to model GDM: as the humans, the placenta and fetus are reliant on maternal supply of glucose until late in gestation, which is also the case in humans [249] • Low preconception vitamin D status increases the risk of developing GDM [300] | • Stress, nutritional, and drug manipulations accurately recapitulate insulin resistance, hyperlipidemia, increased inflammation, fetal death, and macrosomia [301] • Limitation: dams had decreased body weight during pregnancy, which is not seen in human GDM [301] • A transgenerational mouse model resulted in metabolic dysfunction, high glucose levels, and lower beta-cell mass in late gestation [302] • Rats fed a high fat, high sugar diet instigated increased glucose and insulin levels but decreased insulin sensitivity. Offspring were not born macrosomic, potentially due to the immature state of rats at birth [303] | • Useful to model GDM, as the placenta and fetus are reliant on maternal supply of glucose until late in gestation, which is also the case in humans [249] • A high fat diet resulted in increased levels of insulin, glucose, and cortisol in the ewe and fetus compared to controls [304] | ||||
| Induction mechanism | NHP | Guinea pig | Rabbit | Mouse/rat | Sheep | Cow | |
| PTB | Infection/Inflammation | • Intra-amniotic injection of bacterial proteins, IL-1beta, and TNF-alpha resulted in PTB [169] | • Intrauterine administration of bacterial agents and inflammatory cytokines resulted in PTB [305, 306] | • Inoculation of rodents with inflammatory cytokines and alarmins present during human PTB resulted in PTB [307–310] • Intrauterine administration of bacterial agents and inflammatory cytokines led to PTB in mice [311–313], which had comparable inflammatory cytokine profiles to those observed in human infection associated PTB [311, 314] | • The ability for intra-amniotic modeling of infection as opposed to intrauterine is of great utility, [316, 317] • Intrauterine administration of bacterial agents and inflammatory cytokines [318–320] | • Candida infection resulted in severe placental pathology and is associated with abortion in cows [321] and PTB in women [322–324] | |
| • Intrauterine inflammation can promote myometrial increases of contraction-associated proteins and trigger cervical remodeling in rats [315] | |||||||
| Parturition | • Progesterone withdrawal is not a prerequisite for labor, similar to humans [122, 169, 325] | • Increasing progesterone levels in late pregnancy similar to humans [169, 326, 327] | • Labor is dependent on progesterone withdrawal [169, 327] | • Labor is dependent on progesterone withdrawal [169, 326, 327] | • Labor is dependent on progesterone withdrawal [169, 326, 327] | ||
| Other | • Naturally experience preterm birth (~7% compared to 5–11% in human) [169, 170] | • Genetic contributions to PTB in humans have been elucidated through mutant mouse models, such as Calmus et al.’s work on Ehlers–Danlos syndrome [328] • Additional noninflammatory molecules induce PTB in mice [329, 330] • Increased hyaluronan expression has been observed in human and mouse PTB regardless of infection [329, 331] | • Glucocorticoids have been shown to induce preterm labor [318–320]. However, in humans, glucocorticoids are actually used as a treatment in PTB pregnancies to accelerate fetal lung development [332] | ||||
Acronyms: nonhuman primate (NHP), soluble Endoglin (sEng), soluble fms-like tyrosine kinase-1 (sFlt-1), hemolysis, elevated liver enzymes, and low platelets (HELLP), insulin growth factor (IGF), streptozotocin (STZ), Reduced uterine perfusion pressure (RUPP).
Spontaneous development of a pregnancy complication in animals is valuable as it suggests there may be mechanistic overlap with humans. Although PE is observed primarily in humans, spontaneous cases have been documented in mice, rats, rabbits, guinea pigs, and monkeys [122, 131, 132]. Here, we briefly describe a few examples that support the use of an animal model for various complications. In general, litter-bearing species can serve as natural models of FGR and IUGR [5, 117, 133]. For example, spontaneous IUGR occurs in 15–20% of swine [134]. Likewise, spontaneous pregnancy loss is common in cattle (~40%) [135–137], pigs (20–45%) [136, 138, 139], and marmoset monkeys (26%) [140]. In a cohort of guinea pigs, 20% of pregnancies spontaneously developed toxemia, and the observations from those animals validated their induced toxemia model [131]. Decreased reproductive efficiency has been observed in both humans and sheep at high altitudes; however, this environmental factor would limit the ability to study FGR in sheep to specific locations and might limit the relevance of such studies to the general human population. While spontaneous instances of these pregnancy complications can be used for experimental modeling, by definition, spontaneous complications are difficult to predict. Events preceding the adverse outcome cannot be efficiently studied and may require larger animal cohorts or specific conditions than is practical for many investigators.
In contrast to the uncertainty in occurrence and timing of a spontaneous complication, researchers can administer precise insults or experimental treatments to control the induction of a pregnancy complication (Table 5). Pregnancy complications may be induced by drug treatment, diet, surgery, or genetic manipulation. Genetic manipulation is commonly used in rodent models to induce a pregnancy complication, where a gene knockin or knockout can aid in further investigating causative genes underlying the development of a complication. Information derived from these genetic mutations then can be translated to other animal models, such as NHPs, that more closely model human pregnancy. The biological relevance of the induced complication must be determined on a species and approach basis. As with any laboratory study, there are limitations to the comparisons that can be made to natural cases of disease. Data gleaned from induced models should be compared to data obtained from spontaneous cases of pregnancy complications whenever possible.
Etiology is important when selecting an approach to induce a pregnancy complication, as the mechanisms impacted may not be translatable to humans. For example, diet-restricted guinea pigs displayed similar symptoms and pathology as those with spontaneous PE [141]; however, the etiologies appear different. Spontaneous PE resulted from uteroplacental ischemia induced by aortic compression caudal to the renal arteries. In contrast, fasting-induced PE led to ketosis and resulted in less severe symptoms and pathology [131]. Notably, similar symptoms of varying severity were observed in this study, and it is important to consider the mechanistic differences underlying the PE symptoms observed as they may relate or differ from the human pathogenesis of the complication.
Animal models of experimental infections during pregnancy
Pathogen infection of a host is species restricted, so ensuring that a pathogen can induce similar pathophysiology in an animal model of human pregnancy is essential to better understanding the downstream implications. Other important considerations for congenital infection models include the route of infection, maternal symptoms, fetal/congenital symptoms, and the role the maternal immune system plays in fighting the infection. Researchers have used in vitro animal and human placental cell culture systems to identify the cell types most susceptible to vertically transmitted pathogens and to unravel the mechanisms behind infection [20]. While in vitro systems have aided in understanding the cellular mechanisms of vertical transmission (e.g., receptors that mediate pathogen trophoblast entry), these mechanisms largely remain elusive. The various animal models that have been used to model TORCHZ infections during pregnancy are broadly summarized in Table 6.
Table 6.
Pathogens in animal models that cause APOs.
| Pathogen | Animal model | Observed APO |
|---|---|---|
| Brucella | Cattle [333] | Pregnancy loss |
| Sheep [333] | ||
| Chlamydia | Sheep [334] | Pregnancy loss |
| Mice [334, 335] | ||
| Guinea pigs [336] | ||
| Pigs [337] | ||
| Cytomegalovirus | Mice [338] | Neurological sequelae, pregnancy loss |
| Guinea pigs [338, 339] | ||
| NHP [159, 160] | ||
| Hepatitis E virus | Rabbit [340, 341] | Pregnancy loss |
| Group B Streptococcus | Mice [342] NHP [343] | Pregnancy loss, meningitis, pneumonia, neurological developmental disabilities |
| Listeria monocytogenes | NHP [161–163] | Pregnancy loss |
| Guinea pigs [254, 344] | ||
| Rubella | Rats [345, 346] | Pregnancy loss, neonatal demise, ocular abnormalities |
| Toxoplasma gondii | Sheep [347, 348] | Pregnancy loss |
| Mice [349] | ||
| NHP [350] | ||
| Zika virus | NHP [164–166] | Pregnancy loss, fetal malformations |
| Mice [351] | ||
| Guinea pigs [352] | ||
| Pigs [353] |
APOs, adverse pregnancy outcomes.
Current knowledge of PEVs in animal pregnancy models
This section provides a brief overview of PEV studies that have been performed using mouse and livestock pregnancy models, with more details presented in Table 3. Similar to humans [102, 142], the total number of exosomes and PLAP-positive vesicles isolated from blood increased throughout bovine gestation (Table 3) [103]. Sequencing of miRNAs from bovine exosomes revealed unique expression profiles across trimesters [103]. The placental miRNA profile also changes throughout gestation in humans [143] and rhesus macaques [144]. These data suggest that despite minimal placental invasion in the cow, placental exosomes similarly circulate in the maternal bloodstream as observed in humans. Thus, there may be conservation in marker expression and function of cargo given the similarities in EV miRNA profiles during gestation.
Data collected from mouse and human studies support that EV clearance may impact pregnancy health status. Excess vesicles in lactadherin−/− pregnant mice resulted in elevated blood pressure, proteinuria, and fewer litters, suggesting that EV clearance opposes the development of PE-like symptoms (Table 3) [89]. Moreover, elevated levels of PEVs or PEVs from injured murine placentas can induce PE symptoms when injected into pregnant mice [89]. This impact supports and expands upon human data. An in vitro human trophoblast culture study found that less syncytin-1 and -2 cellular expression resulted in decreased exosome uptake and, thus, an excess of released EVs [145]. Interestingly, placentas from women with PE expressed less syncytin-1 and -2 than controls [145]. Germain et al. [87] also reported elevated levels of circulating free and fewer bound syncytiotrophoblast microvesicles in patients with PE than healthy subjects. The overlap in findings support the use rodent models can offer in terms of unraveling the impact of EVs.
Observations from rodent pregnancy models have revealed that PEVs may serve as a means of communication at the maternal–fetal interface. In mice, fetal and maternal exosomes trafficked across the maternal–fetal interface and fetal exosomes impacted maternal cell function [146]. Similarly, PEV trafficking to maternal cells also has been shown in large animal pregnancy models. A recent study showed that the binucleate trophoblast cells of the ruminant placenta also secrete exosome-like vesicles to the maternal uterine epithelium and connective tissue [147]. Similarly, in vitro study showed that porcine trophectoderm cell lines secreted EVs that influenced the proliferation of porcine aortic endothelial cells (Table 3). This supports fetomaternal cross-talk in the pig [148], a phenomenon similarly observed in sheep [149]. In addition, EVs isolated from ovine uterine fluid are taken up by embryos/trophectodermal cells and vice versa [149, 150]. EV uptake by these cells suggests that maternal–fetal communication occurs very early in pregnancy and shows the potential to assess embryo-derived PEVs as early as the pre/peri-implantation stages. Investigation of the earliest stages of development could reveal how these EVs may be altered in EPL.
While the results of in vitro PEV studies are intriguing, the prevalence of PEVs in ovine and porcine maternal blood remains unclear. PEVs are expected to be present in ovine maternal circulation as they have been detected in bovine maternal blood, and these species share similar placental architecture. Further isolation of PEVs from all stages of pregnancy, including embryo-derived EVs, across livestock species will help to understand complications that arise from errors in the establishment of pregnancy as well as maldeveloped placentation. Although there are few published animal PEV studies, the similarities in findings between animal models and human in vitro data further support the need for additional in vivo animal studies.
Future perspectives: expansion of PEV research in animal pregnancy models
The studies discussed in the previous section represent all current, but limited, publications on PEVs in animal pregnancy models. Studies with animal models provide an opportunity to improve our understanding of the consequences of placental complications through comprehensive study of PEVs and their cargo. Additional studies in the animal models discussed above, especially in those with a hemochorial placenta, are needed to identify biomarkers and expand our knowledge of PEV cargo and function. The development of PEV animal models is especially important to elucidate the impact EVs have on the maternal immune system and maternal physiology in healthy and complicated pregnancies, as this cannot be studied in vitro.
The use of NHPs in PEV research would be particularly valuable as there are extensive similarities between humans and NHPs as listed in Table 4. Macaques share a similar hemochorial type of placentation with extensive remodeling of decidual spiral arteries by endovascular trophoblasts [121, 123]. Unlike rodent models but similar to humans, NHPs express miRNAs from the primate-specific chromosome 19 microRNA cluster (C19MC) (Table 4). miRNAs of the C19MC are almost exclusively expressed in the placenta and have been detected within human EVs [35]. The C19MC miRNAs have roles in placental function and are aberrantly expressed in pregnancy complications [151, 152]. Several pregnancy complication paradigms are already in place with NHPs [121, 153–167]. Applying the study of PEVs to these established models will enable advances not feasible in human pregnancy research—for example, monitoring vertical pathogen transmission by PEV analysis with timed infection studies. Investigators have recently characterized and validated rhesus and cynomolgus macaque TS cell lines that can be differentiated into syncytiotrophoblasts and extravillous trophoblasts [84, 168]. These cell lines may have tremendous value in terms of identifying PEV biomarkers of infection and disease.
Guinea pigs may also offer utility in PEV research as they have a discoid, hemomonochorial, labyrinthine placenta and are relatively low cost compared to NHPs. Their longer gestation (~68 days) compared to the mouse and rat (~20 and ~22 days, respectively) allows for enhanced longitudinal sampling. They also naturally experience PTB at approximately 7% rate (the human rate is 5–11%) [169, 170]. Hence, guinea pigs may be a useful model for biomarker identification as well as drug development as an intermediate model between rodents and NHPs.
The use of rabbits could be beneficial as they are induced ovulators [122, 171], which allows for early and precisely timed pregnancy sample collection. Rabbits, like humans, have a syncytial trophoblast layer [122], and their genome has been fully sequenced. Their relatively short gestation (~32 days) allows for short studies that can assess the impact of pregnancy on the fetus as the offspring are born precocial [172], a feature more similar to humans than rodents. A representative example of the rabbit model being used to understand a pregnancy complication is a study in which injection of fetal hemoglobin resulted in proteinuria, fetal demise, and increased apoptosis in the kidneys and placentas [173]. This study helped elucidate the impact fetal hemoglobin may have on PE in humans and showed the efficacy of alpha1-microglobulin (A1M) as a therapy to alleviate PE-like symptoms [173].
There are additional advantages and limitations of a model that are particularly relevant to PEV biomarker identification. Animal models with smaller litter sizes, such as the macaque or the sheep, allow for more focused biomarker detection for singleton pregnancies as in humans. In animals with large litters, some fetuses may normally develop, while others are resorbed. If healthy fetuses are present, it may be difficult to parse out and identify a biomarker of the pregnancy complication. Animals that mature more quickly typically have shorter gestations and allow for transgenerational study design. Moreover, animal pregnancy models are advantageous as they provide the ability to survey PEVs in relation to fetal growth and development over time, as well as in association with offspring physiology throughout their lifespan in a manageable time frame.
Next steps in establishing animal pregnancy models for PEV research
A major limitation in the use of PEVs to diagnose pregnancy complications is the lack of information regarding early, predictive markers [108], which makes the identification of longitudinal markers difficult. As illustrated in Table 2, there is overlap between general human EV markers and those of the animal models; however, only a few studies have specifically looked at PEVs in animal models despite similarity in some placental markers. For PEV research to be translational, we propose the following goals:
Rigorous assessment of placenta-specific markers in longitudinal in vivo studies, in additional cohorts for repeatability, and across species to ensure translatability.
Validation of antibodies that are subsequently made commercially available for use across labs and species (when applicable).
Thorough assessment of the prognostic potential of a biomarker associated with a pregnancy complication in various animal models.
Development of a database for placenta-specific markers and biomarkers of pregnancy complications using high-throughput techniques (i.e., next-generation RNA sequencing, mass spectrometry for proteomics, and lipidomics).
Preclinical evaluation of a biomarker associated with a human pregnancy condition in human samples (retrospective and prospective studies).
Animal models will enable the development of datasets with predictive markers because researchers will have control over sample collection and the timing of the insult. Researchers can also use the established animal models to determine the predictive power of potential biomarkers that were identified in humans [108]. The harrows of identifying a single biomarker in humans also support the need for induced pregnancy complication studies because a marker consistently identified across species with varied disease severity has translational potential. Overall, animal models can greatly strengthen the PEV field in terms of studying and prospectively identifying pregnancy complications.
Current questions and future opportunities in the PEV field
Having discussed the opportunities in pregnancy complication research with a range of animal models, there remain questions in PEV research that are cross-cutting, regardless of the species used, including research with human clinical samples.
▪ Is PEV cargo selectively packaged?—If so, how?
▪ How are the presence of PEV membrane proteins and cargos altered when EVs are derived from a diseased placenta?
▪ Do the embryo and the placenta use PEVs to communicate to maternal cells before and during implantation?
▪ What role(s) do PEVs have in regulating maternal immune adaptation to pregnancy? Are PEVs essential for the successful establishment of pregnancy?
▪ Do PEVs from a maldeveloped placenta cause or contribute to a pregnancy complication by triggering a maternal physiological or immune response, or is their presence a manifestation of the impact of the pregnancy complication on placental function?
PEVs hold the promise of future prognostic and diagnostic development as they can provide high “clinical predictive power” [101] for pregnancy complications. Unraveling the mechanisms of cargo packaging is crucial to understand how EV cargo of a malfunctioning placenta may be altered in comparison with those derived from a healthy placenta. There is currently a debate in the EV literature as to what “exosomes” are, and whether these truly can be isolated from a complex EV population [32]. Consistent nomenclature and standard techniques to isolate EVs would allow comparison among studies. There are currently three EV databases, EVpedia [174], ExoCarta [175], and Vesiclepedia [176]; however, a database specifically for PEV research would enable meta-analysis of results, allow for marker identification reported across pregnancy complication research, and enable the field to quickly advance. Since placental development is continuous and gene expression changes throughout pregnancy, it is important that investigators develop a database of EV cargo from all stages of pregnancy. The NIH Human Placenta Project would be an excellent platform to support such a database.
In conclusion, representative in vitro and in vivo animal models are necessary to identify biomarkers of pregnancy complications. A better understanding of PEV biology will allow deeper insights into placental function and development throughout gestation, help to identify maldeveloped and/or infected placentas, and potentially underpin development of placental therapeutics. We propose that in order to achieve these advances, appropriate animal models of human pregnancy complications must be established.
Supplementary data
Supplementary data are available at BIOLRE online.
Conflict of interest
The authors have declared that no conflict of interest exists.
Supplementary Material
Acknowledgments
We thank Dr. Sathish Kumar for thoughtful comments on the manuscript draft. We also thank all those who helped edit the manuscript.
Footnotes
† Grant support: This research was funded by NIH grants F31 HD100057 to LNB, K99 HD099154 to JKS, R01 AI132519 and R21 HD091163 to TGG, and P51 OD011106 to the Wisconsin National Primate Research Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Contributor Information
Lindsey N Block, Wisconsin National Primate Research Center, University of Wisconsin-Madison, Madison, WI, USA; Department of Pathology and Laboratory Medicine, University of Wisconsin-Madison, Madison, WI, USA.
Brittany D Bowman, Wisconsin National Primate Research Center, University of Wisconsin-Madison, Madison, WI, USA.
Jenna Kropp Schmidt, Wisconsin National Primate Research Center, University of Wisconsin-Madison, Madison, WI, USA.
Logan T Keding, Wisconsin National Primate Research Center, University of Wisconsin-Madison, Madison, WI, USA.
Aleksandar K Stanic, Department of Obstetrics and Gynecology, University of Wisconsin-Madison, Madison, WI, USA.
Thaddeus G Golos, Wisconsin National Primate Research Center, University of Wisconsin-Madison, Madison, WI, USA; Department of Obstetrics and Gynecology, University of Wisconsin-Madison, Madison, WI, USA; Department of Comparative Biosciences, University of Wisconsin-Madison, Madison, WI, USA.
References
- 1. Koblinsky M, Chowdhury ME, Moran A, Ronsmans C. Maternal morbidity and disability and their consequences: neglected agenda in maternal health. J Health Popul Nutr 2012; 30:124–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, Adler A, Vera Garcia C, Rohde S, Say L, Lawn JE. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet 2012; 379:2162–2172. [DOI] [PubMed] [Google Scholar]
- 3. Lawn JE, Kerber K, Enweronu-Laryea C, Cousens S. 3.6 million neonatal deaths--what is progressing and what is not? Semin Perinatol 2010; 34:371–386. [DOI] [PubMed] [Google Scholar]
- 4. Harrison MS, Goldenberg RL. Global burden of prematurity. Semin Fetal Neonatal Med 2016; 21:74–79. [DOI] [PubMed] [Google Scholar]
- 5. Swanson AM, David AL. Animal models of fetal growth restriction: considerations for translational medicine. Placenta 2015; 36:623–630. [DOI] [PubMed] [Google Scholar]
- 6. American College of Obstetricians and Gynecologists' Committee on Practice Bulletins–Obstetrics and the Society for Maternal–Fetal Medicin. ACOG Practice Bulletin No. 204: fetal growth restriction. Obstet Gynecol 2019; 133:e97–e109. [DOI] [PubMed] [Google Scholar]
- 7. Nardozza LM, Caetano AC, Zamarian AC, Mazzola JB, Silva CP, Marcal VM, Lobo TF, Peixoto AB, Araujo Junior E. Fetal growth restriction: current knowledge. Arch Gynecol Obstet 2017; 295:1061–1077. [DOI] [PubMed] [Google Scholar]
- 8. American College of Obstetricians and Gynecologists' Committee on Practice Bulletins–Obstetrics and the Society for Maternal–Fetal Medicin. ACOG Practice Bulletin No. 201 summary: pregestational diabetes mellitus. Obstet Gynecol 2018; 132:1514–1516. [DOI] [PubMed] [Google Scholar]
- 9. Mackin ST, Nelson SM, Kerssens JJ, Wood R, Wild S, Colhoun HM, Leese GP, Philip S, Lindsay RS, Group SE . Diabetes and pregnancy: national trends over a 15 year period. Diabetologia 2018; 61:1081–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rackham O, Paize F, Weindling AM. Cause of death in infants of women with pregestational diabetes mellitus and the relationship with glycemic control. Postgrad Med 2009; 121:26–32. [DOI] [PubMed] [Google Scholar]
- 11. Committee on Practice B-O ACOG Practice Bulletin No. 190: gestational diabetes mellitus. Obstet Gynecol 2018; 131:e49–e64. [DOI] [PubMed] [Google Scholar]
- 12. Stevens W, Shih T, Incerti D, Ton TGN, Lee HC, Peneva D, Macones GA, Sibai BM, Jena AB. Short-term costs of preeclampsia to the United States health care system. Am J Obstet Gynecol 2017; 217:237–248 e216. [DOI] [PubMed] [Google Scholar]
- 13. Behrman RE, Butler AS (eds.). Preterm Birth: Causes, Consequences, and Prevention. Washington (DC): National Academies Press; 2007. [PubMed] [Google Scholar]
- 14. Lenoir-Wijnkoop I, Beek EM, Garssen J, Nuijten MJ, Uauy RD. Health economic modeling to assess short-term costs of maternal overweight, gestational diabetes, and related macrosomia - a pilot evaluation. Front Pharmacol 2015; 6:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Signore C, Spong C. Overview of Antepartum Fetal Surveillance. Waltham, MA: Wolters Kluwer; UpToDate; 2020. [Google Scholar]
- 16. Brosens I, Pijnenborg R, Vercruysse L, Romero R. The "great obstetrical syndromes" are associated with disorders of deep placentation. Am J Obstet Gynecol 2011; 204:193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Edu A, Teodorescu C, Dobjanschi CG, Socol ZZ, Teodorescu V, Matei A, Albu DF, Radulian G. Placenta changes in pregnancy with gestational diabetes. Romanian J Morphol Embryol 2016; 57:507–512. [PubMed] [Google Scholar]
- 18. Nguyen-Ngo C, Jayabalan N, Salomon C, Lappas M. Molecular pathways disrupted by gestational diabetes mellitus. J Mol Endocrinol 2019; 63:R51–R72. [DOI] [PubMed] [Google Scholar]
- 19. Whittington JR, Cummings KF, Ounpraseuth ST, Aughenbaugh AL, Quick CM, Dajani NK. Placental changes in diabetic pregnancies and the contribution of hypertension. J Matern Fetal Neonatal Med 2020; 1–9. [DOI] [PubMed] [Google Scholar]
- 20. Delorme-Axford E, Sadovsky Y, Coyne CB. The placenta as a barrier to viral infections. Annu Rev Virol 2014; 1:133–146. [DOI] [PubMed] [Google Scholar]
- 21. Leeper C, Lutzkanin A. 3rd. Infections during pregnancy. Prim Care 2018; 45:567–586. [DOI] [PubMed] [Google Scholar]
- 22. Coyne CB, Lazear HM. Zika virus - reigniting the TORCH. Nat Rev Microbiol 2016; 14:707–715. [DOI] [PubMed] [Google Scholar]
- 23. Sabapatha A, Gercel-Taylor C, Taylor DD. Specific isolation of placenta-derived exosomes from the circulation of pregnant women and their immunoregulatory consequences. Am J Reprod Immunol 2006; 56:345–355. [DOI] [PubMed] [Google Scholar]
- 24. Lai A, Elfeky O, Rice GE, Salomon C. Optimized specific isolation of placenta-derived exosomes from maternal circulation. Methods Mol Biol 2018; 1710:131–138. [DOI] [PubMed] [Google Scholar]
- 25. Sarker S, Scholz-Romero K, Perez A, Illanes SE, Mitchell MD, Rice GE, Salomon C. Placenta-derived exosomes continuously increase in maternal circulation over the first trimester of pregnancy. J Transl Med 2014; 12:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang H, Freitas D, Kim HS, Fabijanic K, Li Z, Chen H, Mark MT, Molina H, Martin AB, Bojmar L, Fang J, Rampersaud S et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat Cell Biol 2018; 20:332–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pocsfalvi G, Stanly C, Vilasi A, Fiume I, Capasso G, Turiak L, Buzas EI, Vekey K. Mass spectrometry of extracellular vesicles. Mass Spectrom Rev 2016; 35:3–21. [DOI] [PubMed] [Google Scholar]
- 28. Schorey JS, Cheng Y, Singh PP, Smith VL. Exosomes and other extracellular vesicles in host-pathogen interactions. EMBO Rep 2015; 16:24–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yuana Y, Sturk A, Nieuwland R. Extracellular vesicles in physiological and pathological conditions. Blood Rev 2013; 27:31–39. [DOI] [PubMed] [Google Scholar]
- 30. Gyorgy B, Szabo TG, Pasztoi M, Pal Z, Misjak P, Aradi B, Laszlo V, Pallinger E, Pap E, Kittel A, Nagy G, Falus A et al. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci 2011; 68:2667–2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kalra H, Drummen GP, Mathivanan S. Focus on extracellular vesicles: introducing the next small big thing. Int J Mol Sci 2016; 17:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Thery C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK, Ayre DC, Bach JM et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 2018; 7:1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Coumans FAW, Brisson AR, Buzas EI, Dignat-George F, Drees EEE, El-Andaloussi S, Emanueli C, Gasecka A, Hendrix A, Hill AF, Lacroix R, Lee Y et al. Methodological guidelines to study extracellular vesicles. Circ Res 2017; 120:1632–1648. [DOI] [PubMed] [Google Scholar]
- 34. Kowal J, Arras G, Colombo M, Jouve M, Morath JP, Primdal-Bengtson B, Dingli F, Loew D, Tkach M, Thery C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci U S A 2016; 113:E968–E977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ouyang Y, Bayer A, Chu T, Tyurin VA, Kagan VE, Morelli AE, Coyne CB, Sadovsky Y. Isolation of human trophoblastic extracellular vesicles and characterization of their cargo and antiviral activity. Placenta 2016; 47:86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang W, Luo J, Wang S. Recent progress in isolation and detection of extracellular vesicles for cancer diagnostics. Adv Healthc Mater 2018; 7:e1800484. [DOI] [PubMed] [Google Scholar]
- 37. Lobb RJ, Becker M, Wen SW, Wong CS, Wiegmans AP, Leimgruber A, Moller A. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J Extracell Vesicles 2015; 4:27031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li P, Kaslan M, Lee SH, Yao J, Gao Z. Progress in exosome isolation techniques. Theranostics 2017; 7:789–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kalra H, Adda CG, Liem M, Ang CS, Mechler A, Simpson RJ, Hulett MD, Mathivanan S. Comparative proteomics evaluation of plasma exosome isolation techniques and assessment of the stability of exosomes in normal human blood plasma. Proteomics 2013; 13:3354–3364. [DOI] [PubMed] [Google Scholar]
- 40. Van Deun J, Mestdagh P, Sormunen R, Cocquyt V, Vermaelen K, Vandesompele J, Bracke M, De Wever O, Hendrix A. The impact of disparate isolation methods for extracellular vesicles on downstream RNA profiling. J Extracell Vesicles 2014; 3:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gardiner C, Di Vizio D, Sahoo S, Thery C, Witwer KW, Wauben M, Hill AF. Techniques used for the isolation and characterization of extracellular vesicles: results of a worldwide survey. J Extracell Vesicles 2016; 5:32945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Simon C, Greening DW, Bolumar D, Balaguer N, Salamonsen LA, Vilella F. Extracellular vesicles in human reproduction in health and disease. Endocr Rev 2018; 39:292–332. [DOI] [PubMed] [Google Scholar]
- 43. Tong M, Kleffmann T, Pradhan S, Johansson CL, DeSousa J, Stone PR, James JL, Chen Q, Chamley LW. Proteomic characterization of macro-, micro- and nano-extracellular vesicles derived from the same first trimester placenta: relevance for feto-maternal communication. Hum Reprod 2016; 31:687–699. [DOI] [PubMed] [Google Scholar]
- 44. Lok CA, Van Der Post JA, Sargent IL, Hau CM, Sturk A, Boer K, Nieuwland R. Changes in microparticle numbers and cellular origin during pregnancy and preeclampsia. Hypertens Pregnancy 2008; 27:344–360. [DOI] [PubMed] [Google Scholar]
- 45. Gruenberg J, Stenmark H. The biogenesis of multivesicular endosomes. Nat Rev Mol Cell Biol 2004; 5:317–323. [DOI] [PubMed] [Google Scholar]
- 46. Villarroya-Beltri C, Baixauli F, Gutierrez-Vazquez C, Sanchez-Madrid F, Mittelbrunn M. Sorting it out: regulation of exosome loading. Semin Cancer Biol 2014; 28:3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mincheva-Nilsson L, Baranov V. Placenta-derived exosomes and syncytiotrophoblast microparticles and their role in human reproduction: immune modulation for pregnancy success. Am J Reprod Immunol 2014; 72:440–457. [DOI] [PubMed] [Google Scholar]
- 48. Ahsan NA, Sampey GC, Lepene B, Akpamagbo Y, Barclay RA, Iordanskiy S, Hakami RM, Kashanchi F. Presence of viral RNA and proteins in exosomes from cellular clones resistant to Rift Valley fever virus infection. Front Microbiol 2016; 7:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Longatti A, Boyd B, Chisari FV. Virion-independent transfer of replication-competent hepatitis C virus RNA between permissive cells. J Virol 2015; 89:2956–2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Saadeldin IM, Oh HJ, Lee BC. Embryonic-maternal cross-talk via exosomes: potential implications. Stem Cells Cloning 2015; 8:103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chahar HS, Bao X, Casola A. Exosomes and their role in the life cycle and pathogenesis of RNA viruses. Viruses 2015; 7:3204–3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 2007; 9:654–659. [DOI] [PubMed] [Google Scholar]
- 53. Kambe S, Yoshitake H, Yuge K, Ishida Y, Ali MM, Takizawa T, Kuwata T, Ohkuchi A, Matsubara S, Suzuki M, Takeshita T, Saito S et al. Human exosomal placenta-associated miR-517a-3p modulates the expression of PRKG1 mRNA in Jurkat cells. Biol Reprod 2014; 91:129. [DOI] [PubMed] [Google Scholar]
- 54. Kahlert C, Melo SA, Protopopov A, Tang J, Seth S, Koch M, Zhang J, Weitz J, Chin L, Futreal A, Kalluri R. Identification of double-stranded genomic DNA spanning all chromosomes with mutated KRAS and p53 DNA in the serum exosomes of patients with pancreatic cancer. J Biol Chem 2014; 289:3869–3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sansone P, Savini C, Kurelac I, Chang Q, Amato LB, Strillacci A, Stepanova A, Iommarini L, Mastroleo C, Daly L, Galkin A, Thakur BK et al. Packaging and transfer of mitochondrial DNA via exosomes regulate escape from dormancy in hormonal therapy-resistant breast cancer. Proc Natl Acad Sci U S A 2017; 114:E9066–E9075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Salomon C, Scholz-Romero K, Sarker S, Sweeney E, Kobayashi M, Correa P, Longo S, Duncombe G, Mitchell MD, Rice GE, Illanes SE. Gestational diabetes mellitus is associated with changes in the concentration and bioactivity of placenta-derived exosomes in maternal circulation across gestation. Diabetes 2016; 65:598–609. [DOI] [PubMed] [Google Scholar]
- 57. Gohner C, Plosch T, Faas MM. Immune-modulatory effects of syncytiotrophoblast extracellular vesicles in pregnancy and preeclampsia. Placenta 2017; 60 Suppl 1:S41-S51. [DOI] [PubMed] [Google Scholar]
- 58. Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol 2014; 14:195–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pap E, Pallinger E, Falus A, Kiss AA, Kittel A, Kovacs P, Buzas EI. T lymphocytes are targets for platelet- and trophoblast-derived microvesicles during pregnancy. Placenta 2008; 29:826–832. [DOI] [PubMed] [Google Scholar]
- 60. Altan-Bonnet N. Extracellular vesicles are the Trojan horses of viral infection. Curr Opin Microbiol 2016; 32:77–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Southcombe J, Tannetta D, Redman C, Sargent I. The immunomodulatory role of syncytiotrophoblast microvesicles. PLoS One 2011; 6:e20245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Shen L, Li Y, Li R, Diao Z, Yany M, Wu M, Sun H, Yan G, Hu Y. Placentaassociated serum exosomal miR155 derived from patients with preeclampsia inhibits eNOS expression in human umbilical vein endothelial cells. Int J Mol Med 2018; 41:1731–1739. [DOI] [PubMed] [Google Scholar]
- 63. Nair S, Jayabalan N, Guanzon D, Palma C, Scholz-Romero K, Elfeky O, Zuniga F, Ormazabal V, Diaz E, Rice GE, Duncombe G, Jansson T et al. Human placental exosomes in gestational diabetes mellitus carry a specific set of miRNAs associated with skeletal muscle insulin sensitivity. Clin Sci (Lond) 2018; 132:2451–2467. [DOI] [PubMed] [Google Scholar]
- 64. Eldh M, Ekstrom K, Valadi H, Sjostrand M, Olsson B, Jernas M, Lotvall J. Exosomes communicate protective messages during oxidative stress; possible role of exosomal shuttle RNA. PLoS One 2010; 5:e15353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Smyth T, Kullberg M, Malik N, Smith-Jones P, Graner MW, Anchordoquy TJ. Biodistribution and delivery efficiency of unmodified tumor-derived exosomes. J Control Release 2015; 199:145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ohno S, Takanashi M, Sudo K, Ueda S, Ishikawa A, Matsuyama N, Fujita K, Mizutani T, Ohgi T, Ochiya T, Gotoh N, Kuroda M. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol Ther 2013; 21:185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. AY-T W, Sung Y-C, Chou ST-Y, Guo V, Chien JC-Y, Ko JJ-S, Yang AL, Chuang J-C, Huang H-C, Wu Y, Ho M-R, Ericsson M et al. Redirected tropisms of extracellular vesicles and exomeres yield distinct biodistribution profiles. bioRxiv 2020:1–56. doi: 10.1101/2020.03.27.012625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wiklander OP, Nordin JZ, O'Loughlin A, Gustafsson Y, Corso G, Mager I, Vader P, Lee Y, Sork H, Seow Y, Heldring N, Alvarez-Erviti L et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J Extracell Vesicles 2015; 4:26316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kooijmans SAA, Schiffelers RM, Zarovni N, Vago R. Modulation of tissue tropism and biological activity of exosomes and other extracellular vesicles: new nanotools for cancer treatment. Pharmacol Res 2016; 111:487–500. [DOI] [PubMed] [Google Scholar]
- 70. Di Rocco G, Baldari S, Toietta G. Towards therapeutic delivery of extracellular vesicles: strategies for in vivo tracking and biodistribution analysis. Stem Cells Int 2016; 2016:5029619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Tannetta D, Collett G, Vatish M, Redman C, Sargent I. Syncytiotrophoblast extracellular vesicles - circulating biopsies reflecting placental health. Placenta 2017; 52:134–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Salomon C, Nuzhat Z, Dixon CL, Menon R. Placental exosomes during gestation: liquid biopsies carrying signals for the regulation of human parturition. Curr Pharm Des 2018; 24:974–982. [DOI] [PubMed] [Google Scholar]
- 73. Homer H, Rice GE, Salomon C. Review: embryo- and endometrium-derived exosomes and their potential role in assisted reproductive treatments-liquid biopsies for endometrial receptivity. Placenta 2017; 54:89–94. [DOI] [PubMed] [Google Scholar]
- 74. Tannetta DS, Dragovic RA, Gardiner C, Redman CW, Sargent IL. Characterisation of syncytiotrophoblast vesicles in normal pregnancy and pre-eclampsia: expression of Flt-1 and endoglin. PLoS One 2013; 8:e56754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Nardi Fda S, Michelon TF, Neumann J, Manvailer LF, Wagner B, Horn PA, Bicalho Mda G, Rebmann V. High levels of circulating extracellular vesicles with altered expression and function during pregnancy. Immunobiology 2016; 221:753–760. [DOI] [PubMed] [Google Scholar]
- 76. Atay S, Gercel-Taylor C, Suttles J, Mor G, Taylor DD. Trophoblast-derived exosomes mediate monocyte recruitment and differentiation. Am J Reprod Immunol 2011; 65:65–77. [DOI] [PubMed] [Google Scholar]
- 77. Mincheva-Nilsson L, Baranov V. The role of placental exosomes in reproduction. Am J Reprod Immunol 2010; 63:520–533. [DOI] [PubMed] [Google Scholar]
- 78. Hannan NJ, Paiva P, Dimitriadis E, Salamonsen LA. Models for study of human embryo implantation: choice of cell lines? Biol Reprod 2010; 82:235–245. [DOI] [PubMed] [Google Scholar]
- 79. Gierman LM, Stodle GS, Tangeras LH, Austdal M, Olsen GD, Follestad T, Skei B, Rian K, Gundersen AS, Austgulen R, Iversen AC. Toll-like receptor profiling of seven trophoblast cell lines warrants caution for translation to primary trophoblasts. Placenta 2015; 36:1246–1253. [DOI] [PubMed] [Google Scholar]
- 80. Orendi K, Kivity V, Sammar M, Grimpel Y, Gonen R, Meiri H, Lubzens E, Huppertz B. Placental and trophoblastic in vitro models to study preventive and therapeutic agents for preeclampsia. Placenta 2011; 32 Suppl:S49–54. [DOI] [PubMed] [Google Scholar]
- 81. Okae H, Toh H, Sato T, Hiura H, Takahashi S, Shirane K, Kabayama Y, Suyama M, Sasaki H, Arima T. Derivation of human trophoblast stem cells. Cell Stem Cell 2018; 22:50–63.e56. [DOI] [PubMed] [Google Scholar]
- 82. Haider S, Meinhardt G, Saleh L, Kunihs V, Gamperl M, Kaindl U, Ellinger A, Burkard TR, Fiala C, Pollheimer J, Mendjan S, Latos PA et al. Self-renewing trophoblast organoids recapitulate the developmental program of the early human placenta. Stem Cell Reports 2018; 11:537–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Turco MY, Gardner L, Kay RG, Hamilton RS, Prater M, Hollinshead MS, McWhinnie A, Esposito L, Fernando R, Skelton H, Reimann F, Gribble FM et al. Trophoblast organoids as a model for maternal-fetal interactions during human placentation. Nature 2018; 564:263–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kropp Schmidt J, Meyer MG, Wiepz GJ, Block LN, Dusek BM, Kroner KM, Bertogliat MJ, Keding LT, Koenig MR, Mean KD, Golos TG. Derivation of macaque trophoblast stem cells. bioRxiv 2020:1–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Renaud SJ. Strategies for Investigating Hemochorial Placentation. Amsterdam, Netherlands: Elsevier Inc.; 2017: 1259. [Google Scholar]
- 86. Di Santo S, Malek A, Sager R, Andres AC, Schneider H. Trophoblast viability in perfused term placental tissue and explant cultures limited to 7-24 hours. Placenta 2003; 24:882–894. [DOI] [PubMed] [Google Scholar]
- 87. Germain SJ, Sacks GP, Sooranna SR, Sargent IL, Redman CW. Systemic inflammatory priming in normal pregnancy and preeclampsia: the role of circulating syncytiotrophoblast microparticles. J Immunol 2007; 178:5949–5956. [DOI] [PubMed] [Google Scholar]
- 88. Rice TF, Donaldson B, Bouqueau M, Kampmann B, Holder B. Macrophage- but not monocyte-derived extracellular vesicles induce placental pro-inflammatory responses. Placenta 2018; 69:92–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Han C, Wang C, Chen Y, Wang J, Xu X, Hilton T, Cai W, Zhao Z, Wu Y, Li K, Houck K, Liu L et al. Placenta-derived extracellular vesicles induce preeclampsia in mouse models. Haematologica 2019; 105:1686–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Pillay P, Maharaj N, Moodley J, Mackraj I. Placental exosomes and pre-eclampsia: maternal circulating levels in normal pregnancies and, early and late onset pre-eclamptic pregnancies. Placenta 2016; 46:18–25. [DOI] [PubMed] [Google Scholar]
- 91. Goswami D, Tannetta DS, Magee LA, Fuchisawa A, Redman CW, Sargent IL, Dadelszen P. Excess syncytiotrophoblast microparticle shedding is a feature of early-onset pre-eclampsia, but not normotensive intrauterine growth restriction. Placenta 2006; 27:56–61. [DOI] [PubMed] [Google Scholar]
- 92. Salomon C, Guanzon D, Scholz-Romero K, Longo S, Correa P, Illanes SE, Rice GE. Placental exosomes as early biomarker of preeclampsia: potential role of exosomal MicroRNAs across gestation. J Clin Endocrinol Metab 2017; 102:3182–3194. [DOI] [PubMed] [Google Scholar]
- 93. Tong M, Chen Q, James JL, Stone PR, Chamley LW. Micro- and nano-vesicles from first trimester human placentae carry Flt-1 and levels are increased in severe preeclampsia. Front Endocrinol (Lausanne) 2017; 8:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Delorme-Axford E, Donker RB, Mouillet JF, Chu T, Bayer A, Ouyang Y, Wang T, Stolz DB, Sarkar SN, Morelli AE, Sadovsky Y, Coyne CB. Human placental trophoblasts confer viral resistance to recipient cells. Proc Natl Acad Sci U S A 2013; 110:12048–12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Bayer A, Delorme-Axford E, Sleigher C, Frey TK, Trobaugh DW, Klimstra WB, Emert-Sedlak LA, Smithgall TE, Kinchington PR, Vadia S, Seveau S, Boyle JP et al. Human trophoblasts confer resistance to viruses implicated in perinatal infection. Am J Obstet Gynecol 2015; 212:71.e71–71.e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Bayer A, Lennemann NJ, Ouyang Y, Bramley JC, Morosky S, Marques ET Jr, Cherry S, Sadovsky Y, Coyne CB. Type III interferons produced by human placental Trophoblasts confer protection against Zika virus infection. Cell Host Microbe 2016; 19:705–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Kaminski VL, Ellwanger JH, Chies JAB. Extracellular vesicles in host-pathogen interactions and immune regulation - exosomes as emerging actors in the immunological theater of pregnancy. Heliyon 2019; 5:e02355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Ander SE, Diamond MS, Coyne CB. Immune responses at the maternal-fetal interface. Sci Immunol 2019; 4:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Kshirsagar SK, Alam SM, Jasti S, Hodes H, Nauser T, Gilliam M, Billstrand C, Hunt JS, Petroff MG. Immunomodulatory molecules are released from the first trimester and term placenta via exosomes. Placenta 2012; 33:982–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Alam SMK, Jasti S, Kshirsagar SK, Tannetta DS, Dragovic RA, Redman CW, Sargent IL, Hodes HC, Nauser TL, Fortes T, Filler AM, Behan K et al. Trophoblast glycoprotein (TPGB/5T4) in human placenta: expression, regulation, and presence in extracellular microvesicles and exosomes. Reprod Sci 2018; 25:185–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Morgan TK. Cell- and size-specific analysis of placental extracellular vesicles in maternal plasma and pre-eclampsia. Transl Res 2018; 201:40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Salomon C, Torres MJ, Kobayashi M, Scholz-Romero K, Sobrevia L, Dobierzewska A, Illanes SE, Mitchell MD, Rice GE. A gestational profile of placental exosomes in maternal plasma and their effects on endothelial cell migration. PLoS One 2014; 9:e98667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Zhao G, Guo S, Jiang K, Zhang T, Wu H, Qiu C, Deng G. MiRNA profiling of plasma-derived exosomes from dairy cows during gestation. Theriogenology 2019; 130:89–98. [DOI] [PubMed] [Google Scholar]
- 104. Hamilton-Dutoit SJ, Lou H, Pallesen G. The expression of placental alkaline phosphatase (PLAP) and PLAP-like enzymes in normal and neoplastic human tissues. An immunohistological survey using monoclonal antibodies. APMIS 1990; 98:797–811. [DOI] [PubMed] [Google Scholar]
- 105. Garattini E, Margolis J, Heimer E, Felix A, Udenfriend S. Human placental alkaline phosphatase in liver and intestine. Proc Natl Acad Sci U S A 1985; 82:6080–6084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Galski H, Weinstein D, Abraham K, Groot N, Segal S, Folman R, Hochberg AA. The in vitro synthesis and secretion of alkaline phosphatase from first trimester human decidua. Eur J Obstet Gynecol Reprod Biol 1982; 14:1–11. [DOI] [PubMed] [Google Scholar]
- 107. Alijotas-Reig J, Palacio-Garcia C, Llurba E, Vilardell-Tarres M. Cell-derived microparticles and vascular pregnancy complications: a systematic and comprehensive review. Fertil Steril 2013; 99:441–449. [DOI] [PubMed] [Google Scholar]
- 108. Cuffe JSM, Holland O, Salomon C, Rice GE, Perkins AV. Review: placental derived biomarkers of pregnancy disorders. Placenta 2017; 54:104–110. [DOI] [PubMed] [Google Scholar]
- 109. Dutta S, Kumar S, Hyett J, Salomon C. Molecular targets of aspirin and prevention of preeclampsia and their potential association with circulating extracellular vesicles during pregnancy. Int J Mol Sci 2019; 20:1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Nair S, Salomon C. Extracellular vesicles as critical mediators of maternal-fetal communication during pregnancy and their potential role in maternal metabolism. Placenta 2020; 28:1–9. [DOI] [PubMed] [Google Scholar]
- 111. Carp H, Dardik R, Lubetsky A, Salomon O, Eskaraev R, Rosenthal E, Inbal A. Prevalence of circulating procoagulant microparticles in women with recurrent miscarriage: a case-controlled study. Hum Reprod 2004; 19:191–195. [DOI] [PubMed] [Google Scholar]
- 112. Kaptan K, Beyan C, Ifran A, Pekel A. Platelet-derived microparticle levels in women with recurrent spontaneous abortion. Int J Gynaecol Obstet 2008; 102:271–274. [DOI] [PubMed] [Google Scholar]
- 113. Alijotas-Reig J, Palacio-Garcia C, Farran-Codina I, Zarzoso C, Cabero-Roura L, Vilardell-Tarres M. Circulating cell-derived microparticles in women with pregnancy loss. Am J Reprod Immunol 2011; 66:199–208. [DOI] [PubMed] [Google Scholar]
- 114. Toth B, Nieuwland R, Kern M, Rogenhofer N, Berkmans R, Rank A, Lohse P, Friese K, Thaler CJ. Systemic changes in haemostatic balance are not associated with increased levels of circulating microparticles in women with recurrent spontaneous abortion. Am J Reprod Immunol 2008; 59:159–166. [DOI] [PubMed] [Google Scholar]
- 115. Truong G, Guanzon D, Kinhal V, Elfeky O, Lai A, Longo S, Nuzhat Z, Palma C, Scholz-Romero K, Menon R, Mol BW, Rice GE et al. Oxygen tension regulates the miRNA profile and bioactivity of exosomes released from extravillous trophoblast cells - liquid biopsies for monitoring complications of pregnancy. PLoS One 2017; 12:e0174514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Fallen S, Baxter D, Wu X, Kim TK, Shynlova O, Lee MY, Scherler K, Lye S, Hood L, Wang K. Extracellular vesicle RNAs reflect placenta dysfunction and are a biomarker source for preterm labour. J Cell Mol Med 2018; 22:2760–2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Wu G, Bazer FW, Wallace JM, Spencer TE. Board-invited review: intrauterine growth retardation: implications for the animal sciences. J Anim Sci 2006; 84:2316–2337. [DOI] [PubMed] [Google Scholar]
- 118. Eixarch E, Hernandez-Andrade E, Crispi F, Illa M, Torre I, Figueras F, Gratacos E. Impact on fetal mortality and cardiovascular Doppler of selective ligature of uteroplacental vessels compared with undernutrition in a rabbit model of intrauterine growth restriction. Placenta 2011; 32:304–309. [DOI] [PubMed] [Google Scholar]
- 119. Bassan H, Trejo LL, Kariv N, Bassan M, Berger E, Fattal A, Gozes I, Harel S. Experimental intrauterine growth retardation alters renal development. Pediatr Nephrol 2000; 15:192–195. [DOI] [PubMed] [Google Scholar]
- 120. Haugaard C, Bauer M. Rodent models of intrauterine growth restriction. Scand J Lab Anim Sci 2001; 28:10–22. [Google Scholar]
- 121. Grigsby PL. Animal models to Study placental development and function throughout normal and dysfunctional human pregnancy. Semin Reprod Med 2016; 34:11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Bonney EA. Demystifying animal models of adverse pregnancy outcomes: touching bench and bedside. Am J Reprod Immunol 2013; 69:567–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Carter AM, Pijnenborg R. Evolution of invasive placentation with special reference to non-human primates. Best Pract Res Clin Obstet Gynaecol 2011; 25:249–257. [DOI] [PubMed] [Google Scholar]
- 124. Leiser R, Kaufmann P. Placental structure: in a comparative aspect. Exp Clin Endocrinol 1994; 102:122–134. [DOI] [PubMed] [Google Scholar]
- 125. Furukawa S, Kuroda Y, Sugiyama A. A comparison of the histological structure of the placenta in experimental animals. J Toxicol Pathol 2014; 27:11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Frazier S, McBride MW, Mulvana H, Graham D. From animal models to patients: the role of placental microRNAs, miR-210, miR-126, and miR-148a/152 in preeclampsia. Clin Sci (Lond) 2020; 134:1001–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Hu XQ, Dasgupta C, Xiao D, Huang X, Yang S, Zhang L. MicroRNA-210 targets ten-eleven translocation methylcytosine dioxygenase 1 and suppresses pregnancy-mediated adaptation of large conductance Ca(2+)-activated K(+) channel expression and function in ovine uterine arteries. Hypertension 2017; 70:601–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Swanson AM, Rossi CA, Ofir K, Mehta V, Boyd M, Barker H, Ledwozyw A, Vaughan O, Martin J, Zachary I, Sebire N, Peebles DM et al. Maternal therapy with ad.VEGF-A165 increases fetal weight at term in a guinea-pig model of fetal growth restriction. Hum Gene Ther 2016; 27:997–1007. [DOI] [PubMed] [Google Scholar]
- 129. Carr DJ, Wallace JM, Aitken RP, Milne JS, Mehta V, Martin JF, Zachary IC, Peebles DM, David AL. Uteroplacental adenovirus vascular endothelial growth factor gene therapy increases fetal growth velocity in growth-restricted sheep pregnancies. Hum Gene Ther 2014; 25:375–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Spencer R, Ambler G, Brodszki J, Diemert A, Figueras F, Gratacos E, Hansson SR, Hecher K, Huertas-Ceballos A, Marlow N, Marsal K, Morsing E et al. EVERREST prospective study: a 6-year prospective study to define the clinical and biological characteristics of pregnancies affected by severe early onset fetal growth restriction. BMC Pregnancy Childbirth 2017; 17:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Seidl DC, Hughes HC, Bertolet R, Lang CM. True pregnancy toxemia (preeclampsia) in the guinea pig (Cavia porcellus). Lab Anim Sci 1979; 29:472–478. [PubMed] [Google Scholar]
- 132. Krugner-Higby L, Luck M, Hartley D, Crispen HM, Lubach GR, Coe CL. High-risk pregnancy in rhesus monkeys (Macaca mulatta): a case of ectopic, abdominal pregnancy with birth of a live, term infant, and a case of gestational diabetes complicated by pre-eclampsia. J Med Primatol 2009; 38:252–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Wang J, Feng C, Liu T, Shi M, Wu G, Bazer FW. Physiological alterations associated with intrauterine growth restriction in fetal pigs: causes and insights for nutritional optimization. Mol Reprod Dev 2017; 84:897–904. [DOI] [PubMed] [Google Scholar]
- 134. Gonzalez-Bulnes A, Astiz S, Parraguez VH, Garcia-Contreras C, Vazquez-Gomez M. Empowering translational research in fetal growth restriction: sheep and swine animal models. Curr Pharm Biotechnol 2016; 17:848–855. [DOI] [PubMed] [Google Scholar]
- 135. Diskin MG, Waters SM, Parr MH, Kenny DA. Pregnancy losses in cattle: potential for improvement. Reprod Fertil Dev 2016; 28:83–93. [DOI] [PubMed] [Google Scholar]
- 136. Spencer TE. Early pregnancy: concepts, challenges, and potential solutions. Anim Front 2013; 3:48–55. [Google Scholar]
- 137. Kropp J, Penagaricano F, Salih SM, Khatib H. Invited review: genetic contributions underlying the development of preimplantation bovine embryos. J Dairy Sci 2014; 97:1187–1201. [DOI] [PubMed] [Google Scholar]
- 138. Bidarimath M, Tayade C. Pregnancy and spontaneous fetal loss: a pig perspective. Mol Reprod Dev 2017; 84:856–869. [DOI] [PubMed] [Google Scholar]
- 139. Kridli RT, Khalaj K, Bidarimath M, Tayade C. Placentation, maternal-fetal interface, and conceptus loss in swine. Theriogenology 2016; 85:135–144. [DOI] [PubMed] [Google Scholar]
- 140. Rutherford JN, deMartelly VA, Layne Colon DG, Ross CN, Tardif SD. Developmental origins of pregnancy loss in the adult female common marmoset monkey (Callithrix jacchus). PLoS One 2014; 9:e96845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Manning PJ, Wagner JP, Harkness JE. Laboratory Animal Medicine. Cambridge, MA: Academic Press; 1984: 149–181. [Google Scholar]
- 142. Elfeky O, Longo S, Lai A, Rice GE, Salomon C. Influence of maternal BMI on the exosomal profile during gestation and their role on maternal systemic inflammation. Placenta 2017; 50:60–69. [DOI] [PubMed] [Google Scholar]
- 143. Morales-Prieto DM, Chaiwangyen W, Ospina-Prieto S, Schneider U, Herrmann J, Gruhn B, Markert UR. MicroRNA expression profiles of trophoblastic cells. Placenta 2012; 33:725–734. [DOI] [PubMed] [Google Scholar]
- 144. Schmidt JK, Block LN, Golos TG. Defining the rhesus macaque placental miRNAome: conservation of expression of placental miRNA clusters between the macaque and human. Placenta 2018; 65:55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Vargas A, Zhou S, Ethier-Chiasson M, Flipo D, Lafond J, Gilbert C, Barbeau B. Syncytin proteins incorporated in placenta exosomes are important for cell uptake and show variation in abundance in serum exosomes from patients with preeclampsia. FASEB J 2014; 28:3703–3719. [DOI] [PubMed] [Google Scholar]
- 146. Sheller-Miller S, Choi K, Choi C, Menon R. Cyclic-recombinase-reporter mouse model to determine exosome communication and function during pregnancy. Am J Obstet Gynecol 2019; 221:502.e501–502.e512. [DOI] [PubMed] [Google Scholar]
- 147. Klisch K, Schraner EM. Intraluminal vesicles of binucleate trophoblast cell granules are a possible source of placental exosomes in ruminants. Placenta 2020; 90:58–61. [DOI] [PubMed] [Google Scholar]
- 148. Bidarimath M, Khalaj K, Kridli RT, Kan FW, Koti M, Tayade C. Extracellular vesicle mediated intercellular communication at the porcine maternal-fetal interface: a new paradigm for conceptus-endometrial cross-talk. Sci Rep 2017; 7:40476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Burns GW, Brooks KE, Spencer TE. Extracellular vesicles originate from the conceptus and uterus during early pregnancy in sheep. Biol Reprod 2016; 94:56. [DOI] [PubMed] [Google Scholar]
- 150. Ruiz-Gonzalez I, Xu J, Wang X, Burghardt RC, Dunlap KA, Bazer FW. Exosomes, endogenous retroviruses and toll-like receptors: pregnancy recognition in ewes. Reproduction 2015; 149:281–291. [DOI] [PubMed] [Google Scholar]
- 151. Anton L, Olarerin-George AO, Hogenesch JB, Elovitz MA. Placental expression of miR-517a/b and miR-517c contributes to trophoblast dysfunction and preeclampsia. PLoS One 2015; 10:e0122707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Hromadnikova I, Kotlabova K, Ondrackova M, Pirkova P, Kestlerova A, Novotna V, Hympanova L, Krofta L. Expression profile of C19MC microRNAs in placental tissue in pregnancy-related complications. DNA Cell Biol 2015; 34:437–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Abitbol MM, Ober MB, Gallo GR, Driscoll SG, Pirani CL. Experimental toxemia of pregnancy in the monkey, with a preliminary report on renin and aldosterone. Am J Pathol 1977; 86:573–590. [PMC free article] [PubMed] [Google Scholar]
- 154. Makris A, Thornton C, Thompson J, Thomson S, Martin R, Ogle R, Waugh R, McKenzie P, Kirwan P, Hennessy A. Uteroplacental ischemia results in proteinuric hypertension and elevated sFLT-1. Kidney Int 2007; 71:977–984. [DOI] [PubMed] [Google Scholar]
- 155. Roberts VHJ, Lo JO, Lewandowski KS, Blundell P, Grove KL, Kroenke CD, Sullivan EL, Roberts CT Jr, Frias AE. Adverse placental perfusion and pregnancy outcomes in a new nonhuman primate model of gestational protein restriction. Reprod Sci 2018; 25:110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Myers RE, Hill DE, Holt AB, Scott RE, Mellits ED, Cheek DB. Fetal growth retardation produced by experimental placental insufficiency in the rhesus monkey. I. Body weight, organ size. Biol Neonate 1971; 18:379–394. [DOI] [PubMed] [Google Scholar]
- 157. Kemnitz JW, Eisele SG, Lindsay KA, Engle MJ, Perelman RH, Farrell PM. Changes in food intake during menstrual cycles and pregnancy of normal and diabetic rhesus monkeys. Diabetologia 1984; 26:60–64. [DOI] [PubMed] [Google Scholar]
- 158. Reynolds WA, Chez RA, Bhuyan BK, Neil GL. Placental transfer of streptozotocin in the rhesus monkey. Diabetes 1974; 23:777–782. [DOI] [PubMed] [Google Scholar]
- 159. Barry PA, Lockridge KM, Salamat S, Tinling SP, Yue Y, Zhou SS, Gospe SM Jr, Britt WJ, Tarantal AF. Nonhuman primate models of intrauterine cytomegalovirus infection. ILAR J 2006; 47:49–64. [DOI] [PubMed] [Google Scholar]
- 160. Itell HL, Kaur A, Deere JD, Barry PA, Permar SR. Rhesus monkeys for a nonhuman primate model of cytomegalovirus infections. Curr Opin Virol 2017; 25:126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Smith MA, Takeuchi K, Brackett RE, McClure HM, Raybourne RB, Williams KM, Babu US, Ware GO, Broderson JR, Doyle MP. Nonhuman primate model for listeria monocytogenes-induced stillbirths. Infect Immun 2003; 71:1574–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Wolfe B, Kerr AR, Mejia A, Simmons HA, Czuprynski CJ, Golos TG. Sequelae of fetal infection in a non-human primate model of listeriosis. Front Microbiol 2019; 10:2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Wolfe B, Wiepz GJ, Schotzko M, Bondarenko GI, Durning M, Simmons HA, Mejia A, Faith NG, Sampene E, Suresh M, Kathariou S, Czuprynski CJ et al. Acute fetal demise with first trimester maternal infection resulting from listeria monocytogenes in a nonhuman primate model. MBio 2017; 8:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Mohr EL, Block LN, Newman CM, Stewart LM, Koenig M, Semler M, Breitbach ME, Teixeira LBC, Zeng X, Weiler AM, Barry GL, Thoong TH et al. Ocular and uteroplacental pathology in a macaque pregnancy with congenital Zika virus infection. PLoS One 2018; 13:e0190617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Nguyen SM, Antony KM, Dudley DM, Kohn S, Simmons HA, Wolfe B, Salamat MS, Teixeira LBC, Wiepz GJ, Thoong TH, Aliota MT, Weiler AM et al. Highly efficient maternal-fetal Zika virus transmission in pregnant rhesus macaques. PLoS Pathog 2017; 13:e1006378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Hirsch AJ, Roberts VHJ, Grigsby PL, Haese N, Schabel MC, Wang X, Lo JO, Liu Z, Kroenke CD, Smith JL, Kelleher M, Broeckel R et al. Zika virus infection in pregnant rhesus macaques causes placental dysfunction and immunopathology. Nat Commun 2018; 9:263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Sever JL, Meier GW, Windle WF, Schiff GM, Monif GR, Fabiyi A. Experimental rubella in pregnant rhesus monkeys. J Infect Dis 1966; 116:21–26. [DOI] [PubMed] [Google Scholar]
- 168. Matsumoto S, Porter CJ, Ogasawara N, Iwatani C, Tsuchiya H, Seita Y, Chang YW, Okamoto I, Saitou M, Ema M, Perkins TJ, Stanford WL et al. Establishment of macaque trophoblast stem cell lines derived from cynomolgus monkey blastocysts. Sci Rep 2020; 10:6827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Mitchell BF, Taggart MJ. Are animal models relevant to key aspects of human parturition? Am J Physiol Regul Integr Comp Physiol 2009; 297:R525–R545. [DOI] [PubMed] [Google Scholar]
- 170. Vogel JP, Chawanpaiboon S, Moller AB, Watananirun K, Bonet M, Lumbiganon P. The global epidemiology of preterm birth. Best Pract Res Clin Obstet Gynaecol 2018; 52:3–12. [DOI] [PubMed] [Google Scholar]
- 171. Fischer B, Chavatte-Palmer P, Viebahn C, Navarrete Santos A, Duranthon V. Rabbit as a reproductive model for human health. Reproduction 2012; 144:1–10. [DOI] [PubMed] [Google Scholar]
- 172. Carter AM. Animal models of human placentation--a review. Placenta 2007; 28 Suppl A:S41–47. [DOI] [PubMed] [Google Scholar]
- 173. Naav A, Erlandsson L, Axelsson J, Larsson I, Johansson M, Wester-Rosenlof L, Morgelin M, Casslen V, Gram M, Akerstrom B, Hansson SR. A1M ameliorates preeclampsia-like symptoms in placenta and kidney induced by cell-free fetal hemoglobin in rabbit. PLoS One 2015; 10:e0125499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Kim DK, Lee J, Kim SR, Choi DS, Yoon YJ, Kim JH, Go G, Nhung D, Hong K, Jang SC, Kim SH, Park KS et al. EVpedia: a community web portal for extracellular vesicles research. Bioinformatics 2015; 31:933–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Keerthikumar S, Chisanga D, Ariyaratne D, Al Saffar H, Anand S, Zhao K, Samuel M, Pathan M, Jois M, Chilamkurti N, Gangoda L, Mathivanan S. ExoCarta: a web-based compendium of exosomal cargo. J Mol Biol 2016; 428:688–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Pathan M, Fonseka P, Chitti SV, Kang T, Sanwlani R, Van Deun J, Hendrix A, Mathivanan S. Vesiclepedia 2019: a compendium of RNA, proteins, lipids and metabolites in extracellular vesicles. Nucleic Acids Res 2019; 47:D516–D519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Ramos JGL, Sass N, Costa SHM. Preeclampsia. Rev Bras Ginecol Obstet 2017; 39:496–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Possomato-Vieira JS, Khalil RA. Mechanisms of endothelial dysfunction in hypertensive pregnancy and preeclampsia. Adv Pharmacol 2016; 77:361–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Rana S, Lemoine E, Granger JP, Karumanchi SA. Preeclampsia: pathophysiology, challenges, and perspectives. Circ Res 2019; 124:1094–1112. [DOI] [PubMed] [Google Scholar]
- 180. Raymond D, Peterson E. A critical review of early-onset and late-onset preeclampsia. Obstet Gynecol Surv 2011; 66:497–506. [DOI] [PubMed] [Google Scholar]
- 181. Practice Committee of the American Society for Reproductive M Evaluation and treatment of recurrent pregnancy loss: a committee opinion. Fertil Steril 2012; 98:1103–1111. [DOI] [PubMed] [Google Scholar]
- 182. ACOG Practice Bulletin No. 200: early pregnancy loss. Obstet Gynecol 2018; 132:e197–e207. [DOI] [PubMed] [Google Scholar]
- 183. Hosseini MK, Gunel T, Gumusoglu E, Benian A, Aydinli K. MicroRNA expression profiling in placenta and maternal plasma in early pregnancy loss. Mol Med Rep 2018; 17:4941–4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Menasha J, Levy B, Hirschhorn K, Kardon NB. Incidence and spectrum of chromosome abnormalities in spontaneous abortions: new insights from a 12-year study. Genet Med 2005; 7:251–263. [DOI] [PubMed] [Google Scholar]
- 185. Pylyp LY, Spynenko LO, Verhoglyad NV, Mishenko AO, Mykytenko DO, Zukin VD. Chromosomal abnormalities in products of conception of first-trimester miscarriages detected by conventional cytogenetic analysis: a review of 1000 cases. J Assist Reprod Genet 2018; 35:265–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Goddijn M, Leschot NJ. Genetic aspects of miscarriage. Baillieres Best Pract Res Clin Obstet Gynaecol 2000; 14:855–865. [DOI] [PubMed] [Google Scholar]
- 187. Jia CW, Wang L, Lan YL, Song R, Zhou LY, Yu L, Yang Y, Liang Y, Li Y, Ma YM, Wang SY. Aneuploidy in early miscarriage and its related factors. Chin Med J 2015; 128:2772–2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Petracchi F, Colaci DS, Igarzabal L, Gadow E. Cytogenetic analysis of first trimester pregnancy loss. Int J Gynaecol Obstet 2009; 104:243–244. [DOI] [PubMed] [Google Scholar]
- 189. Massalska D, Zimowski JG, Bijok J, Pawelec M, Czubak-Barlik M, Jakiel G, Roszkowski T. First trimester pregnancy loss: clinical implications of genetic testing. J Obstet Gynaecol Res 2017; 43:23–29. [DOI] [PubMed] [Google Scholar]
- 190. Shahine L, Lathi R. Recurrent pregnancy loss: evaluation and treatment. Obstet Gynecol Clin N Am 2015; 42:117–134. [DOI] [PubMed] [Google Scholar]
- 191. Practice Committee of the American Society for Reproductive Medicine. Electronic address aao Definitions of infertility and recurrent pregnancy loss: a committee opinion. Fertil Steril 2020; 113:533–535. [DOI] [PubMed] [Google Scholar]
- 192. Hong Li Y, Marren A. Recurrent pregnancy loss: a summary of international evidence-based guidelines and practice. Aust J Gen Pract 2018; 47:432–436. [DOI] [PubMed] [Google Scholar]
- 193. Gagnon R. Placental insufficiency and its consequences. Eur J Obstet Gynecol Reprod Biol 2003; 110 Suppl 1:S99–107. [DOI] [PubMed] [Google Scholar]
- 194. Burton GJ, Jauniaux E. Pathophysiology of placental-derived fetal growth restriction. Am J Obstet Gynecol 2018; 218:S745–S761. [DOI] [PubMed] [Google Scholar]
- 195. Darby JRT, Varcoe TJ, Orgeig S, Morrison JL. Cardiorespiratory consequences of intrauterine growth restriction: influence of timing, severity and duration of hypoxaemia. Theriogenology 2020; 150:84–95. [DOI] [PubMed] [Google Scholar]
- 196. Zur RL, Kingdom JC, Parks WT, Hobson SR. The placental basis of fetal growth restriction. Obstet Gynecol Clin N Am 2020; 47:81–98. [DOI] [PubMed] [Google Scholar]
- 197. Langer O, Yogev Y, Most O, Xenakis EM. Gestational diabetes: the consequences of not treating. Am J Obstet Gynecol 2005; 192:989–997. [DOI] [PubMed] [Google Scholar]
- 198. Plows JF, Stanley JL, Baker PN, Reynolds CM, Vickers MH. The pathophysiology of gestational diabetes mellitus. Int J Mol Sci 2018; 19:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Vambergue A, Fajardy I. Consequences of gestational and pregestational diabetes on placental function and birth weight. World J Diabetes 2011; 2:196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet 2008; 371:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Morgan TK. Role of the placenta in preterm birth: a review. Am J Perinatol 2016; 33:258–266. [DOI] [PubMed] [Google Scholar]
- 202. Luo SS, Ishibashi O, Ishikawa G, Ishikawa T, Katayama A, Mishima T, Takizawa T, Shigihara T, Goto T, Izumi A, Ohkuchi A, Matsubara S et al. Human villous trophoblasts express and secrete placenta-specific microRNAs into maternal circulation via exosomes. Biol Reprod 2009; 81:717–729. [DOI] [PubMed] [Google Scholar]
- 203. Morales-Prieto DM, Ospina-Prieto S, Chaiwangyen W, Schoenleben M, Markert UR. Pregnancy-associated miRNA-clusters. J Reprod Immunol 2013; 97:51–61. [DOI] [PubMed] [Google Scholar]
- 204. Holder BS, Tower CL, Abrahams VM, Aplin JD. Syncytin 1 in the human placenta. Placenta 2012; 33:460–466. [DOI] [PubMed] [Google Scholar]
- 205. Soygur B, Sati L, Demir R. Altered expression of human endogenous retroviruses syncytin-1, syncytin-2 and their receptors in human normal and gestational diabetic placenta. Histol Histopathol 2016; 31:1037–1047. [DOI] [PubMed] [Google Scholar]
- 206. Masoura S, Kalogiannidis IA, Gitas G, Goutsioulis A, Koiou E, Athanasiadis A, Vavatsi N. Biomarkers in pre-eclampsia: a novel approach to early detection of the disease. J Obstet Gynaecol 2012; 32:609–616. [DOI] [PubMed] [Google Scholar]
- 207. Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol 2013; 200:373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Jin J, Menon R. Placental exosomes: a proxy to understand pregnancy complications. Am J Reprod Immunol 2018; 79:e12788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Hong X, Schouest B, Xu H. Effects of exosome on the activation of CD4+ T cells in rhesus macaques: a potential application for HIV latency reactivation. Sci Rep 2017; 7:15611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. McNamara RP, Costantini LM, Myers TA, Schouest B, Maness NJ, Griffith JD, Damania BA, MacLean AG, Dittmer DP. Nef secretion into extracellular vesicles or exosomes is conserved across human and simian immunodeficiency viruses. MBio 2018; 9:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Dupressoir A, Lavialle C, Heidmann T. From ancestral infectious retroviruses to bona fide cellular genes: role of the captured syncytins in placentation. Placenta 2012; 33:663–671. [DOI] [PubMed] [Google Scholar]
- 212. Lin TM, Halbert SP. Immunological relationships of human and subhuman primate pregnancy-associated plasma proteins. Int Arch Allergy Appl Immunol 1978; 56:207–223. [DOI] [PubMed] [Google Scholar]
- 213. Sammar M, Drobnjak T, Mandala M, Gizurarson S, Huppertz B, Meiri H. Galectin 13 (PP13) facilitates Remodeling and structural stabilization of maternal vessels during pregnancy. Int J Mol Sci 2019; 20:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Boyson JE, Iwanaga KK, Golos TG, Watkins DI. Identification of a novel MHC class I gene, Mamu-AG, expressed in the placenta of a primate with an inactivated G locus. J Immunol 1997; 159:3311–3321. [PubMed] [Google Scholar]
- 215. Manning JP, Inglis NR, Green S, Fishman WH. Characterization of placental alkaline phosphatase from the rabbit, guinea pig mouse and hamster. Enzymologia 1970; 39:307–318. [PubMed] [Google Scholar]
- 216. Vernochet C, Heidmann O, Dupressoir A, Cornelis G, Dessen P, Catzeflis F, Heidmann T. A syncytin-like endogenous retrovirus envelope gene of the guinea pig specifically expressed in the placenta junctional zone and conserved in Caviomorpha. Placenta 2011; 32:885–892. [DOI] [PubMed] [Google Scholar]
- 217. Liu X, Wang L, Ma C, Wang G, Zhang Y, Sun S. Exosomes derived from platelet-rich plasma present a novel potential in alleviating knee osteoarthritis by promoting proliferation and inhibiting apoptosis of chondrocyte via Wnt/beta-catenin signaling pathway. J Orthop Surg Res 2019; 14:470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Black SG, Arnaud F, Palmarini M, Spencer TE. Endogenous retroviruses in trophoblast differentiation and placental development. Am J Reprod Immunol 2010; 64:255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Bobrie A, Colombo M, Krumeich S, Raposo G, Thery C. Diverse subpopulations of vesicles secreted by different intracellular mechanisms are present in exosome preparations obtained by differential ultracentrifugation. J Extracell Vesicles 2012; 12:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Luga V, Zhang L, Viloria-Petit AM, Ogunjimi AA, Inanlou MR, Chiu E, Buchanan M, Hosein AN, Basik M, Wrana JL. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell 2012; 151:1542–1556. [DOI] [PubMed] [Google Scholar]
- 221. Sheller-Miller S, Trivedi J, Yellon SM, Menon R. Exosomes cause preterm birth in mice: evidence for paracrine signaling in pregnancy. Sci Rep 2019; 9:608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Gong R, Huang L, Shi J, Luo K, Qiu G, Feng H, Tien P, Xiao G. Syncytin-a mediates the formation of syncytiotrophoblast involved in mouse placental development. Cell Physiol Biochem 2007; 20:517–526. [DOI] [PubMed] [Google Scholar]
- 223. Otero-Ortega L, Gomez de Frutos MC, Laso-Garcia F, Rodriguez-Frutos B, Medina-Gutierrez E, Lopez JA, Vazquez J, Diez-Tejedor E, Gutierrez-Fernandez M. Exosomes promote restoration after an experimental animal model of intracerebral hemorrhage. J Cereb Blood Flow Metab 2018; 38:767–779. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 224. Campbell WJ, Larsen D, Deb S, Kwok SC, Soares MJ. Expression of alkaline phosphatase in differentiated rat labyrinthine trophoblast tissue. Placenta 1991; 12:227–237. [DOI] [PubMed] [Google Scholar]
- 225. Hunt JS, Soares MJ. Expression of histocompatibility antigens, transferrin receptors, intermediate filaments, and alkaline phosphatase by in vitro cultured rat placental cells and rat placental cells in situ. Placenta 1988; 9:159–171. [DOI] [PubMed] [Google Scholar]
- 226. Dutta S, Lai A, Scholz-Romero K, Shiddiky MJA, Yamauchi Y, Mishra JS, Rice GE, Hyett J, Kumar S, Salomon C. Hypoxia-induced small extracellular vesicle proteins regulate proinflammatory cytokines and systemic blood pressure in pregnant rats. Clin Sci (Lond) 2020; 134:593–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Burns G, Brooks K, Wildung M, Navakanitworakul R, Christenson LK, Spencer TE. Extracellular vesicles in luminal fluid of the ovine uterus. PLoS One 2014; 9:e90913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Cleys ER, Halleran JL, McWhorter E, Hergenreder J, Enriquez VA, Silveira JC, Bruemmer JE, Winger QA, Bouma GJ. Identification of microRNAs in exosomes isolated from serum and umbilical cord blood, as well as placentomes of gestational day 90 pregnant sheep. Mol Reprod Dev 2014; 81:983–993. [DOI] [PubMed] [Google Scholar]
- 229. Cornelis G, Heidmann O, Degrelle SA, Vernochet C, Lavialle C, Letzelter C, Bernard-Stoecklin S, Hassanin A, Mulot B, Guillomot M, Hue I, Heidmann T et al. Captured retroviral envelope syncytin gene associated with the unique placental structure of higher ruminants. Proc Natl Acad Sci U S A 2013; 110:E828–E837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Caballero JN, Frenette G, Belleannee C, Sullivan R. CD9-positive microvesicles mediate the transfer of molecules to bovine spermatozoa during epididymal maturation. PLoS One 2013; 8:e65364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Zhao G, Yang C, Yang J, Liu P, Jiang K, Shaukat A, Wu H, Deng G. Placental exosome-mediated Bta-miR-499-Lin28B/let-7 axis regulates inflammatory bias during early pregnancy. Cell Death Dis 2018; 9:704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Eirin A, Zhu XY, Puranik AS, Tang H, McGurren KA, Wijnen AJ, Lerman A, Lerman LO. Mesenchymal stem cell-derived extracellular vesicles attenuate kidney inflammation. Kidney Int 2017; 92:114–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Guller S, Tang Z, Ma YY, Di Santo S, Sager R, Schneider H. Protein composition of microparticles shed from human placenta during placental perfusion: potential role in angiogenesis and fibrinolysis in preeclampsia. Placenta 2011; 32:63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Gilani SI, Anderson UD, Jayachandran M, Weissgerber TL, Zand L, White WM, Milic N, Suarez MLG, Vallapureddy RR, Naav A, Erlandsson L, Lieske JC et al. Urinary extracellular vesicles of podocyte origin and renal injury in preeclampsia. J Am Soc Nephrol 2017; 28:3363–3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Baig S, Kothandaraman N, Manikandan J, Rong L, Ee KH, Hill J, Lai CW, Tan WY, Yeoh F, Kale A, Su LL, Biswas A et al. Proteomic analysis of human placental syncytiotrophoblast microvesicles in preeclampsia. Clin Proteomics 2014; 11:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Orozco AF, Jorgez CJ, Ramos-Perez WD, Popek EJ, Yu X, Kozinetz CA, Bischoff FZ, Lewis DE. Placental release of distinct DNA-associated micro-particles into maternal circulation: reflective of gestation time and preeclampsia. Placenta 2009; 30:891–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Baig S, Lim JY, Fernandis AZ, Wenk MR, Kale A, Su LL, Biswas A, Vasoo S, Shui G, Choolani M. Lipidomic analysis of human placental syncytiotrophoblast microvesicles in adverse pregnancy outcomes. Placenta 2013; 34:436–442. [DOI] [PubMed] [Google Scholar]
- 238. Miranda J, Paules C, Nair S, Lai A, Palma C, Scholz-Romero K, Rice GE, Gratacos E, Crispi F, Salomon C. Placental exosomes profile in maternal and fetal circulation in intrauterine growth restriction - liquid biopsies to monitoring fetal growth. Placenta 2018; 64:34–43. [DOI] [PubMed] [Google Scholar]
- 239. Kandzija N, Zhang W, Motta-Mejia C, Mhlomi V, McGowan-Downey J, James T, Cerdeira AS, Tannetta D, Sargent I, Redman CW, Bastie CC, Vatish M. Placental extracellular vesicles express active dipeptidyl peptidase IV; levels are increased in gestational diabetes mellitus. J Extracell Vesicles 2019; 8:1617000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Jayabalan N, Lai A, Nair S, Guanzon D, Scholz-Romero K, Palma C, McIntyre HD, Lappas M, Salomon C. Quantitative proteomics by SWATH-MS suggest an association between circulating exosomes and maternal metabolic changes in gestational diabetes mellitus. Proteomics 2019; 19:e1800164. [DOI] [PubMed] [Google Scholar]
- 241. Gillet V, Ouellet A, Stepanov Y, Rodosthenous RS, Croft EK, Brennan K, Abdelouahab N, Baccarelli A, Takser L. miRNA profiles in extracellular vesicles from serum early in pregnancies complicated by gestational diabetes mellitus. J Clin Endocrinol Metab 2019; 104:5157–5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Rice GE, Scholz-Romero K, Sweeney E, Peiris H, Kobayashi M, Duncombe G, Mitchell MD, Salomon C. The effect of glucose on the release and bioactivity of exosomes from first trimester trophoblast cells. J Clin Endocrinol Metab 2015; 100:E1280–E1288. [DOI] [PubMed] [Google Scholar]
- 243. Menon R, Debnath C, Lai A, Guanzon D, Bhatnagar S, Kshetrapal PK, Sheller-Miller S, Salomon C, Garbhini Study T. Circulating exosomal miRNA profile during term and preterm birth pregnancies: a longitudinal Study. Endocrinology 2019; 160:249–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Menon R, Dixon CL, Sheller-Miller S, Fortunato SJ, Saade GR, Palma C, Lai A, Guanzon D, Salomon C. Quantitative proteomics by SWATH-MS of maternal plasma exosomes determine pathways associated with term and preterm birth. Endocrinology 2019; 160:639–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Tong M, Stanley JL, Chen Q, James JL, Stone PR, Chamley LW. Placental nano-vesicles target to specific organs and modulate vascular tone in vivo. Hum Reprod 2017; 32:2188–2198. [DOI] [PubMed] [Google Scholar]
- 246. Terasawa E, Keen KL, Grendell RL, Golos TG. Possible role of 5′-adenosine triphosphate in synchronization of Ca2+ oscillations in primate luteinizing hormone-releasing hormone neurons. Mol Endocrinol 2005; 19:2736–2747. [DOI] [PubMed] [Google Scholar]
- 247. Golos TG, Bondarenko GI, Dambaeva SV, Breburda EE, Durning M. On the role of placental major histocompatibility complex and decidual leukocytes in implantation and pregnancy success using non-human primate models. Int J Dev Biol 2010; 54:431–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Telugu BP, Green JA. Comparative placentation Heide Schatten, Gheorghe M. Constantinescu (eds.) In: Comparative Reproductive Biology. Ames, IA, USA: Blackwell Publishing; 2008:271–319.
- 249. Morrison JL, Botting KJ, Darby JRT, David AL, Dyson RM, Gatford KL, Gray C, Herrera EA, Hirst JJ, Kim B, Kind KL, Krause BJ et al. Guinea pig models for translation of the developmental origins of health and disease hypothesis into the clinic. J Physiol 2018; 596:5535–5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Enders AC, Blankenship TN. Comparative placental structure. Adv Drug Deliv Rev 1999; 38:3–15. [DOI] [PubMed] [Google Scholar]
- 251. Clark DA. The use and misuse of animal analog models of human pregnancy disorders. J Reprod Immunol 2014; 103:1–8. [DOI] [PubMed] [Google Scholar]
- 252. Tanaka S, Kunath T, Hadjantonakis AK, Nagy A, Rossant J. Promotion of trophoblast stem cell proliferation by FGF4. Science 1998; 282:2072–2075. [DOI] [PubMed] [Google Scholar]
- 253. Soares MJ, Varberg KM, Iqbal K. Hemochorial placentation: development, function, and adaptations. Biol Reprod 2018; 99:196–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Lowe DE, Robbins JR, Bakardjiev AI. Animal and human tissue models of vertical listeria monocytogenes transmission and implications for other pregnancy-associated infections. Infect Immun 2018; 86:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Soares MJ. The prolactin and growth hormone families: pregnancy-specific hormones/cytokines at the maternal-fetal interface. Reprod Biol Endocrinol 2004; 2:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Bainbridge DR. Evolution of mammalian pregnancy in the presence of the maternal immune system. Rev Reprod 2000; 5:67–74. [DOI] [PubMed] [Google Scholar]
- 257. Niakan KK, Eggan K. Analysis of human embryos from zygote to blastocyst reveals distinct gene expression patterns relative to the mouse. Dev Biol 2013; 375:54–64. [DOI] [PubMed] [Google Scholar]
- 258. Soares MJ, Chakraborty D, Karim Rumi MA, Konno T, Renaud SJ. Rat placentation: an experimental model for investigating the hemochorial maternal-fetal interface. Placenta 2012; 33:233–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Verstuyf A, Sobis H, Goebels J, Fonteyn E, Cassiman JJ, Vandeputte M. Establishment and characterization of a continuous in vitro line from a rat choriocarcinoma. Int J Cancer 1990; 45:752–756. [DOI] [PubMed] [Google Scholar]
- 260. Andersen MD, Alstrup AKO, Duvald CS, Mikkelsen EFR, Vendelbo MH, Ovesen PG, Pedersen M. Animal models of Fetal medicine and obstetrics. 2018.
- 261. Peter AT. Bovine placenta: a review on morphology, components, and defects from terminology and clinical perspectives. Theriogenology 2013; 80:693–705. [DOI] [PubMed] [Google Scholar]
- 262. Rosenfeld CS. Introduction to comparative placentation Heide Schatten, Gheorghe M. Constantinescu (eds.) In: Comparative Reproductive Biology. Ames, IA, USA: Blackwell Publishing; 2008:263–270.
- 263. Rapacz-Leonard A, Dabrowska M, Janowski T. Major histocompatibility complex I mediates immunological tolerance of the trophoblast during pregnancy and may mediate rejection during parturition. Mediat Inflamm 2014; 2014:579279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Hashizume K, Shimada A, Nakano H, Takahashi T. Bovine trophoblast cell culture systems: a technique to culture bovine trophoblast cells without feeder cells. Methods Mol Med 2006; 121:179–188. [PubMed] [Google Scholar]
- 265. Flechon JE, Laurie S, Notarianni E. Isolation and characterization of a feeder-dependent, porcine trophectoderm cell line obtained from a 9-day blastocyst. Placenta 1995; 16:643–658. [DOI] [PubMed] [Google Scholar]
- 266. Cavanagh D, Rao PS, Tsai CC, O'Connor TC. Experimental toxemia in the pregnant primate. Am J Obstet Gynecol 1977; 128:75–85. [DOI] [PubMed] [Google Scholar]
- 267. Abitbol MM, Gallo GR, Pirani CL, Ober WB. Production of experimental toxemia in the pregnant rabbit. Am J Obstet Gynecol 1976; 124:460–470. [DOI] [PubMed] [Google Scholar]
- 268. Fushima T, Sekimoto A, Minato T, Ito T, Oe Y, Kisu K, Sato E, Funamoto K, Hayase T, Kimura Y, Ito S, Sato H et al. Reduced uterine perfusion pressure (RUPP) model of preeclampsia in mice. PLoS One 2016; 11:e0155426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Sunderland NS, Thomson SE, Heffernan SJ, Lim S, Thompson J, Ogle R, McKenzie P, Kirwan PJ, Makris A, Hennessy A. Tumor necrosis factor alpha induces a model of preeclampsia in pregnant baboons (Papio hamadryas). Cytokine 2011; 56:192–199. [DOI] [PubMed] [Google Scholar]
- 270. Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 2003; 111:649–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. LaMarca BB, Bennett WA, Alexander BT, Cockrell K, Granger JP. Hypertension produced by reductions in uterine perfusion in the pregnant rat: role of tumor necrosis factor-alpha. Hypertension 2005; 46:1022–1025. [DOI] [PubMed] [Google Scholar]
- 272. Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, Bdolah Y, Lim KH, Yuan HT, Libermann TA, Stillman IE, Roberts D et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med 2006; 12:642–649. [DOI] [PubMed] [Google Scholar]
- 273. Huang PL, Huang Z, Mashimo H, Bloch KD, Moskowitz MA, Bevan JA, Fishman MC. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature 1995; 377:239–242. [DOI] [PubMed] [Google Scholar]
- 274. Salas SP, Altermatt F, Campos M, Giacaman A, Rosso P. Effects of long-term nitric oxide synthesis inhibition on plasma volume expansion and fetal growth in the pregnant rat. Hypertension 1995; 26:1019–1023. [DOI] [PubMed] [Google Scholar]
- 275. Talosi G, Nemeth I, Nagy E, Pinter S. The pathogenetic role of heme in pregnancy-induced hypertension-like disease in ewes. Biochem Mol Med 1997; 62:58–64. [DOI] [PubMed] [Google Scholar]
- 276. Wester-Rosenlof L, Casslen V, Axelsson J, Edstrom-Hagerwall A, Gram M, Holmqvist M, Johansson ME, Larsson I, Ley D, Marsal K, Morgelin M, Rippe B et al. A1M/alpha1-microglobulin protects from heme-induced placental and renal damage in a pregnant sheep model of preeclampsia. PLoS One 2014; e86353:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Thatcher CD, Keith JC Jr. Pregnancy-induced hypertension: development of a model in the pregnant sheep. Am J Obstet Gynecol 1986; 155:201–207. [DOI] [PubMed] [Google Scholar]
- 278. Buehler PW, D'Agnillo F. Toxicological consequences of extracellular hemoglobin: biochemical and physiological perspectives. Antioxid Redox Signal 2010; 12:275–291. [DOI] [PubMed] [Google Scholar]
- 279. Brun V, Meikle A, Fernandez-Foren A, Forcada F, Palacin I, Menchaca A, Sosa C, Abecia JA. Failure to establish and maintain a pregnancy in undernourished recipient ewes is associated with a poor endocrine milieu in the early luteal phase. Anim Reprod Sci 2016; 173:80–86. [DOI] [PubMed] [Google Scholar]
- 280. Briscoe TA, Rehn AE, Dieni S, Duncan JR, Wlodek ME, Owens JA, Rees SM. Cardiovascular and renal disease in the adolescent guinea pig after chronic placental insufficiency. Am J Obstet Gynecol 2004; 191:847–855. [DOI] [PubMed] [Google Scholar]
- 281. Turner AJ, Trudinger BJ. A modification of the uterine artery restriction technique in the guinea pig fetus produces asymmetrical ultrasound growth. Placenta 2009; 30:236–240. [DOI] [PubMed] [Google Scholar]
- 282. Lopez-Tello J, Jimenez-Martinez MA, Herrera EA, Krause BJ, Sferruzzi-Perri AN. Progressive uterine artery occlusion in the guinea pig leads to defects in placental structure that relate to fetal growth. Placenta 2018; 72-73:36–40. [DOI] [PubMed] [Google Scholar]
- 283. Pham TD, MacLennan NK, Chiu CT, Laksana GS, Hsu JL, Lane RH. Uteroplacental insufficiency increases apoptosis and alters p53 gene methylation in the full-term IUGR rat kidney. Am J Physiol Regul Integr Comp Physiol 2003; 285:R962–R970. [DOI] [PubMed] [Google Scholar]
- 284. Wang YP, Chen X, Zhang ZK, Cui HY, Wang P, Wang Y. Increased renal apoptosis and reduced renin-angiotensin system in fetal growth restriction. J Renin-Angiotensin-Aldosterone Syst 2016; 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Lopez-Tello J, Arias-Alvarez M, Gonzalez-Bulnes A, Sferuzzi-Perri AN. Models of intrauterine growth restriction and fetal programming in rabbits. Mol Reprod Dev 2019; 86:1781–1809. [DOI] [PubMed] [Google Scholar]
- 286. Vuguin PM. Animal models for small for gestational age and fetal programming of adult disease. Horm Res 2007; 68:113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Thompson LP, Pence L, Pinkas G, Song H, Telugu BP. Placental hypoxia during early pregnancy causes maternal hypertension and placental insufficiency in the hypoxic guinea pig model. Biol Reprod 2016; 95:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Sunderland N, Hennessy A, Makris A. Animal models of pre-eclampsia. Am J Reprod Immunol 2011; 65:533–541. [DOI] [PubMed] [Google Scholar]
- 289. Morrison JL. Sheep models of intrauterine growth restriction: fetal adaptations and consequences. Clin Exp Pharmacol Physiol 2008; 35:730–743. [DOI] [PubMed] [Google Scholar]
- 290. Metges CC, Lang IS, Hennig U, Brussow KP, Kanitz E, Tuchscherer M, Schneider F, Weitzel JM, Steinhoff-Ooster A, Sauerwein H, Bellmann O, Nurnberg G et al. Intrauterine growth retarded progeny of pregnant sows fed high protein:low carbohydrate diet is related to metabolic energy deficit. PLoS One 2012; 7:e31390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Saintonge J, Cote R. Fetal brain development in diabetic guinea pigs. Pediatr Res 1984; 18:650–653. [DOI] [PubMed] [Google Scholar]
- 292. Merritt TA, Curbelo V, Gluck L, Clements RS Jr. Alterations in fetal lung phosphatidylinositol metabolism associated with maternal glucose intolerance. Biol Neonate 1981; 39:217–224. [DOI] [PubMed] [Google Scholar]
- 293. Caluwaerts S, Holemans K, Bree R, Verhaeghe J, Van Assche FA. Is low-dose streptozotocin in rats an adequate model for gestational diabetes mellitus? J Soc Gynecol Investig 2003; 10:216–221. [DOI] [PubMed] [Google Scholar]
- 294. Hellerstrom C, Swenne I, Eriksson UJ. Is there an animal model for gestational diabetes? Diabetes 1985; 34 Suppl 2:28–31. [DOI] [PubMed] [Google Scholar]
- 295. Giachini FR, Carriel V, Capelo LP, Tostes RC, Carvalho MH, Fortes ZB, Zorn TM, San Martin S. Maternal diabetes affects specific extracellular matrix components during placentation. J Anat 2008; 212:31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296. Vajnerova O, Kafka P, Kratzerova T, Chalupsky K, Hampl V. Pregestational diabetes increases fetoplacental vascular resistance in rats. Placenta 2018; 63:32–38. [DOI] [PubMed] [Google Scholar]
- 297. Dickinson JE, Meyer BA, Brath PC, Chmielowiec S, Walsh SW, Parisi VM, Palmer SM. Placental thromboxane and prostacyclin production in an ovine diabetic model. Am J Obstet Gynecol 1990; 163:1831–1835. [DOI] [PubMed] [Google Scholar]
- 298. Dickinson JE, Meyer BA, Chmielowiec S, Palmer SM. Streptozocin-induced diabetes mellitus in the pregnant ewe. Am J Obstet Gynecol 1991; 165:1673–1677. [DOI] [PubMed] [Google Scholar]
- 299. Ezekwe MO, Ezekwe EI, Sen DK, Ogolla F. Effects of maternal streptozotocin-diabetes on fetal growth, energy reserves and body composition of newborn pigs. J Anim Sci 1984; 59:974–980. [DOI] [PubMed] [Google Scholar]
- 300. Tabatabaei N, Rodd CJ, Kremer R, Weiler HA. High vitamin D status before conception, but not during pregnancy, is inversely associated with maternal gestational diabetes mellitus in guinea pigs. J Nutr 2014; 144:1994–2001. [DOI] [PubMed] [Google Scholar]
- 301. Abdul Aziz SH, John CM, Mohamed Yusof NI, Nordin M, Ramasamy R, Adam A, Mohd Fauzi F. Animal model of gestational diabetes mellitus with pathophysiological resemblance to the human condition induced by multiple factors (nutritional, pharmacological, and stress) in rats. Biomed Res Int 2016; 2016:9704607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Szlapinski SK, King RT, Retta G, Yeo E, Strutt BJ, Hill DJ. A mouse model of gestational glucose intolerance through exposure to a low protein diet during fetal and neonatal development. J Physiol 2019; 597:4237–4250. [DOI] [PubMed] [Google Scholar]
- 303. Holemans K, Caluwaerts S, Poston L, Van Assche FA. Diet-induced obesity in the rat: a model for gestational diabetes mellitus. Am J Obstet Gynecol 2004; 190:858–865. [DOI] [PubMed] [Google Scholar]
- 304. Pasek RC, Gannon M. Advancements and challenges in generating accurate animal models of gestational diabetes mellitus. Am J Physiol Endocrinol Metab 2013; 305:E1327–E1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Dombroski RA, Woodard DS, Harper MJ, Gibbs RS. A rabbit model for bacteria-induced preterm pregnancy loss. Am J Obstet Gynecol 1990; 163:1938–1943. [DOI] [PubMed] [Google Scholar]
- 306. Bry K, Hallman M. Transforming growth factor-beta 2 prevents preterm delivery induced by interleukin-1 alpha and tumor necrosis factor-alpha in the rabbit. Am J Obstet Gynecol 1993; 168:1318–1322. [DOI] [PubMed] [Google Scholar]
- 307. Heng YJ, Liong S, Permezel M, Rice GE, Di Quinzio MK, Georgiou HM. The interplay of the interleukin 1 system in pregnancy and labor. Reprod Sci 2014; 21:122–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Romero R, Chaiworapongsa T, Alpay Savasan Z, Xu Y, Hussein Y, Dong Z, Kusanovic JP, Kim CJ, Hassan SS. Damage-associated molecular patterns (DAMPs) in preterm labor with intact membranes and preterm PROM: a study of the alarmin HMGB1. J Matern Fetal Neonatal Med 2011; 24:1444–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Romero R, Mazor M, Tartakovsky B. Systemic administration of interleukin-1 induces preterm parturition in mice. Am J Obstet Gynecol 1991; 165:969–971. [DOI] [PubMed] [Google Scholar]
- 310. Gomez-Lopez N, Romero R, Plazyo O, Panaitescu B, Furcron AE, Miller D, Roumayah T, Flom E, Hassan SS. Intra-amniotic administration of HMGB1 induces spontaneous preterm labor and birth. Am J Reprod Immunol 2016; 75:3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Hirsch E, Saotome I, Hirsh D. A model of intrauterine infection and preterm delivery in mice. Am J Obstet Gynecol 1995; 172:1598–1603. [DOI] [PubMed] [Google Scholar]
- 312. Kaga N, Katsuki Y, Obata M, Shibutani Y. Repeated administration of low-dose lipopolysaccharide induces preterm delivery in mice: a model for human preterm parturition and for assessment of the therapeutic ability of drugs against preterm delivery. Am J Obstet Gynecol 1996; 174:754–759. [DOI] [PubMed] [Google Scholar]
- 313. Fidel PL Jr, Romero R, Wolf N, Cutright J, Ramirez M, Araneda H, Cotton DB. Systemic and local cytokine profiles in endotoxin-induced preterm parturition in mice. Am J Obstet Gynecol 1994; 170:1467–1475. [DOI] [PubMed] [Google Scholar]
- 314. Romero R, Brody DT, Oyarzun E, Mazor M, Wu YK, Hobbins JC, Durum SK. Infection and labor. III. Interleukin-1: a signal for the onset of parturition. Am J Obstet Gynecol 1989; 160:1117–1123. [DOI] [PubMed] [Google Scholar]
- 315. Okawa T, Suzuki H, Yaanagida K, Sato A, Vedernikov Y, Saade G, Garfield R. Effect of lipopolysaccharide on uterine contractions and prostaglandin production in pregnant rats. Am J Obstet Gynecol 2001; 184:84–89. [DOI] [PubMed] [Google Scholar]
- 316. Gibbs RS, Blanco JD, St Clair PJ, Castaneda YS. Quantitative bacteriology of amniotic fluid from women with clinical intraamniotic infection at term. J Infect Dis 1982; 145:1–8. [DOI] [PubMed] [Google Scholar]
- 317. Kemp MW. Infection-Associated Preterm Birth: Advances From the Use of Animal Models. Cambridge, MA, USA: Academic Press; 2017: 769–804. [Google Scholar]
- 318. Liggins GC. Premature delivery of foetal lambs infused with glucocorticoids. J Endocrinol 1969; 45:515–523. [DOI] [PubMed] [Google Scholar]
- 319. Liggins GC. Premature parturition after infusion of corticotrophin or cortisol into foetal lambs. J Endocrinol 1968; 42:323–329. [DOI] [PubMed] [Google Scholar]
- 320. Schlafer DH, Yuh B, Foley GL, Elssaser TH, Sadowsky D, Nathanielsz PW. Effect of Salmonella endotoxin administered to the pregnant sheep at 133-142 days gestation on fetal oxygenation, maternal and fetal adrenocorticotropic hormone and cortisol, and maternal plasma tumor necrosis factor alpha concentrations. Biol Reprod 1994; 50:1297–1302. [DOI] [PubMed] [Google Scholar]
- 321. Foley GL, Schlafer DH. Candida abortion in cattle. Vet Pathol 1987; 24:532–536. [DOI] [PubMed] [Google Scholar]
- 322. Rasti S, Asadi MA, Taghriri A, Behrashi M, Mousavie G. Vaginal candidiasis complications on pregnant women. Jundishapur J Microbiol 2014; 7:e10078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323. Holzer I, Farr A, Kiss H, Hagmann M, Petricevic L. The colonization with Candida species is more harmful in the second trimester of pregnancy. Arch Gynecol Obstet 2017; 295:891–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324. Maki Y, Fujisaki M, Sato Y, Sameshima H. Candida chorioamnionitis leads to preterm birth and adverse Fetal-neonatal outcome. Infect Dis Obstet Gynecol 2017; 2017:9060138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325. The Physiology of Reproduction. Ely, MN, USA: Raven Press; 1988: 2183–2186. [Google Scholar]
- 326. Hirst JJ, Palliser HK, Shaw JC, Crombie G, Walker DW, Zakar T. Birth and neonatal transition in the guinea pig: experimental approaches to prevent preterm birth and protect the premature Fetus. Front Physiol 2018; 9:1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327. Csapo AI, Puri CP, Tarro S. Relationship between timing of ovariectomy and maintenance of pregnancy in the guinea-pig. Prostaglandins 1981; 22:131–140. [DOI] [PubMed] [Google Scholar]
- 328. Calmus ML, Macksoud EE, Tucker R, Iozzo RV, Lechner BE. A mouse model of spontaneous preterm birth based on the genetic ablation of biglycan and decorin. Reproduction 2011; 142:183–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329. Akgul Y, Holt R, Mummert M, Word A, Mahendroo M. Dynamic changes in cervical glycosaminoglycan composition during normal pregnancy and preterm birth. Endocrinology 2012; 153:3493–3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330. Elovitz MA, Wang Z, Chien EK, Rychlik DF, Phillippe M. A new model for inflammation-induced preterm birth: the role of platelet-activating factor and toll-like receptor-4. Am J Pathol 2003; 163:2103–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331. Osmers R, Rath W, Pflanz MA, Kuhn W, Stuhlsatz HW, Szeverenyi M. Glycosaminoglycans in cervical connective tissue during pregnancy and parturition. Obstet Gynecol 1993; 81:88–92. [PubMed] [Google Scholar]
- 332. Brownfoot FC, Gagliardi DI, Bain E, Middleton P, Crowther CA. Different corticosteroids and regimens for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev 2013; CD006764. [DOI] [PubMed] [Google Scholar]
- 333. Giakoumelou S, Wheelhouse N, Cuschieri K, Entrican G, Howie SE, Horne AW. The role of infection in miscarriage. Hum Reprod Update 2016; 22:116–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334. Rodolakis A, Salinas J, Papp J. Recent advances on ovine chlamydial abortion. Vet Res 1998; 29:275–288. [PubMed] [Google Scholar]
- 335. De Clercq E, Kalmar I, Vanrompay D. Animal models for studying female genital tract infection with Chlamydia trachomatis. Infect Immun 2013; 81:3060–3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336. Poonia A, Purohit VD. Abortion in guinea pigs by Chlamydia psittaci isolates from natural sheep abortion. Indian J Exp Biol 1998; 36:411–413. [PubMed] [Google Scholar]
- 337. Thoma R, Guscetti F, Schiller I, Schmeer N, Corboz L, Pospischil A. Chlamydiae in porcine abortion. Vet Pathol 1997; 34:467–469. [DOI] [PubMed] [Google Scholar]
- 338. Weisblum Y, Panet A, Haimov-Kochman R, Wolf DG. Models of vertical cytomegalovirus (CMV) transmission and pathogenesis. Semin Immunopathol 2014; 36:615–625. [DOI] [PubMed] [Google Scholar]
- 339. Harrison CJ, Caruso N. Correlation of maternal and pup NK-like activity and TNF responses against cytomegalovirus to pregnancy outcome in inbred guinea pigs. J Med Virol 2000; 60:230–236. [PubMed] [Google Scholar]
- 340. Ahn HS, Han SH, Kim YH, Park BJ, Kim DH, Lee JB, Park SY, Song CS, Lee SW, Choi C, Myoung J, Choi IS. Adverse fetal outcomes in pregnant rabbits experimentally infected with rabbit hepatitis E virus. Virology 2017; 512:187–193. [DOI] [PubMed] [Google Scholar]
- 341. Xia J, Liu L, Wang L, Zhang Y, Zeng H, Liu P, Zou Q, Wang L, Zhuang H. Experimental infection of pregnant rabbits with hepatitis E virus demonstrating high mortality and vertical transmission. J Viral Hepat 2015; 22:850–857. [DOI] [PubMed] [Google Scholar]
- 342. Andrade EB, Magalhaes A, Puga A, Costa M, Bravo J, Portugal CC, Ribeiro A, Correia-Neves M, Faustino A, Firon A, Trieu-Cuot P, Summavielle T et al. A mouse model reproducing the pathophysiology of neonatal group B streptococcal infection. Nat Commun 2018; 9:3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343. Rubens CE, Raff HV, Jackson JC, Chi EY, Bielitzki JT, Hillier SL. Pathophysiology and histopathology of group B streptococcal sepsis in Macaca nemestrina primates induced after intraamniotic inoculation: evidence for bacterial cellular invasion. J Infect Dis 1991; 164:320–330. [DOI] [PubMed] [Google Scholar]
- 344. Bakardjiev AI, Stacy BA, Fisher SJ, Portnoy DA. Listeriosis in the pregnant guinea pig: a model of vertical transmission. Infect Immun 2004; 72:489–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345. Avila L, Rawls WE, Dent PB. Experimental infection with rubella virus. I. Acquired and congenital infection in rats. J Infect Dis 1972; 126:585–592. [DOI] [PubMed] [Google Scholar]
- 346. Cotlier E, Fox J, Bohigian G, Beaty C, Du Pree A. Pathogenic effects of rubella virus on embryos and newborn rats. Nature 1968; 217:38–40. [DOI] [PubMed] [Google Scholar]
- 347. Owen MR, Clarkson MJ, Trees AJ. Acute phase toxoplasma abortions in sheep. Vet Rec 1998; 142:480–482. [DOI] [PubMed] [Google Scholar]
- 348. Buxton D. Protozoan infections (Toxoplasma gondii, Neospora caninum and Sarcocystis spp.) in sheep and goats: recent advances. Vet Res 1998; 29:289–310. [PubMed] [Google Scholar]
- 349. Menzies FM, Henriquez FL, Roberts CW. Immunological control of congenital toxoplasmosis in the murine model. Immunol Lett 2008; 115:83–89. [DOI] [PubMed] [Google Scholar]
- 350. Schoondermark-Van de Ven E, Melchers W, Galama J, Camps W, Eskes T, Meuwissen J. Congenital toxoplasmosis: an experimental study in rhesus monkeys for transmission and prenatal diagnosis. Exp Parasitol 1993; 77:200–211. [DOI] [PubMed] [Google Scholar]
- 351. Morrison TE, Diamond MS. Animal models of Zika virus infection, pathogenesis, and immunity. J Virol 2017; 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352. Bierle CJ, Fernandez-Alarcon C, Hernandez-Alvarado N, Zabeli JC, Janus BC, Putri DS, Schleiss MR. Assessing Zika virus replication and the development of Zika-specific antibodies after a mid-gestation viral challenge in guinea pigs. PLoS One 2017; 12:e0187720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353. Udenze D, Trus I, Berube N, Gerdts V, Karniychuk U. The African strain of Zika virus causes more severe in utero infection than Asian strain in a porcine fetal transmission model. Emerg Microbes Infect 2019; 8:1098–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354. Burton GJ, Redman CW, Roberts JM, Moffett A. Pre-eclampsia: pathophysiology and clinical implications. BMJ 2019; 366:l2381. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


