Abstract
Pseudotumor cerebri syndrome (PTCS) is defined by the presence of elevated intracranial pressure in the setting of normal brain parenchyma and cerebrospinal fluid. PTCS can occur in the pediatric and adult populations and, if untreated, may lead to permanent visual loss. In this review, discussion will focus on PTCS in the pediatric population and will outline its distinct epidemiology and key elements of diagnosis, evaluation and management. Finally, although the precise mechanisms are unclear, the underlying pathophysiology will be considered.
Pseudotumor cerebri syndrome (PTCS) encompasses the constellation of symptoms caused by elevated intracranial pressure of unclear etiology with normal brain parenchyma and cerebrospinal fluid constituents.1 PTCS may be considered as primary or secondary. Although secondary PTCS has been attributed to a number of medications and medical conditions, when there is no identifiable secondary cause of PTCS, the condition is termed primary PTCS or idiopathic intracranial hypertension (IIH).1 PTCS, either IIH or secondary PTCS, can occur in both pediatric and adult populations. PTCS, in the pediatric population, shares some, but not all, features of adult PTCS. In this review, discussion will focus on PTCS in the pediatric population and will examine the following aspects: (1) epidemiology, (2) diagnosis and evaluation, (3) management, and (4) pathophysiology.
Epidemiology of Pediatric PTCS
Estimated annual incidence rates of PTCS are 0.9 per 100,000 in both the symptomatic pediatric and general adult populations.2–4 Previous studies have illustrated an influence of age, sex, and pubertal status on the epidemiology of pediatric PTCS.5–9 Indeed, both female sex and obesity appear to be more strongly associated with PTCS in older, but not younger, pediatric patients.3,5 In a collaborative, multicenter study using pediatric-specific measurements of weight status, there was an increasing prevalence of obesity seen in older children with IIH, whereas normal height and weight was observed in female children younger than 7 years and in male children younger than 8.5 years.10 Thus, multiple subgroups of pediatric IIH may be identified and defined by pediatric-specific thresholds of overweight and obesity. In addition to weight status, height and linear growth acceleration also appear to help differentiate these subgroups.10 In this study, the early adolescent population of children with IIH tended to be overweight (and not obese) and were typically taller than their age- and sex-matched peers.10 The anthropometrics of secondary pediatric PTCS may be similar to those of pediatric IIH11; however, this has not been observed in previous studies in which greater than 50% of pediatric patients with minocycline-associated secondary PTCS were not obese at presentation.12 Indeed, pediatric secondary PTCS may occur in nonobese children, under conditions ranging from exposure to recombinant growth hormone (rGH)13,14 to children with secondary aldosteronism.15 The clinical presentation of pediatric PTCS may also vary with age, with a greater number of young children presenting on routine examination without symptoms as compared to adolescents.5,8,16 These observations highlight the need for vigilance by the medical provider not only for overweight and obese children but also for young, thin children who may present with atypical or no symptoms.
Diagnosis and Evaluation of Pediatric PTCS
The diagnosis of PTCS was initially based on the modified Dandy criteria.17,18 Subsequent changes have been adopted, first based on increasing recognition of typical signs and symptoms of this disorder.1,19 It has been illustrated, however, that 29% of pediatric patients with PTCS (ages 3 – 11 years of age) are asymptomatic, 5% develop sixth nerve palsies while 57% have headaches.5 The most recent updated diagnostic criteria were published in 20131 and incorporated timely insights into common neuroimaging characteristics of raised intracranial pressure (ICP) and reference ranges of normal cerebrospinal fluid (CSF) opening pressure in children.20 These criteria also created common diagnostic criteria for the adult and pediatric populations and distinguished between primary PTCS and secondary (eg, medication-related) causes of PTCS.1
The accurate diagnosis of PTCS is first based on the presence or absence of papilledema. Optic disk edema is observed in many conditions, however, papilledema is a term specifically reserved for optic nerve head swelling due to raised ICP. A careful dilated fundus examination is typically sufficient to identify papilledema; however, orbital ultrasound, including a 30° test by an experienced ultrasonographer, may be a useful adjunctive test. There is increasing evidence surrounding the use of optical coherence tomography (OCT) imaging of the optic nerve to aid the diagnosis of papilledema in the pediatric population.21,22 OCT provides high-resolution imaging of the retina and optic nerve structures. As papilledema develops, stasis of axoplasmic flow causes swelling of the peripapillary retinal nerve fiber layer (RNFL) and optic nerve head. OCT may be able to identify and quantify this process. Indeed, one prospective study involving 13 pediatric patients found that objective measurements of the RNFL correlated with the severity of papilledema.22 Another study also illustrated that RNFL thickness was significantly more elevated in pediatric eyes evaluated in the setting of raised ICP and papilledema compared with eyes with pseudopapilledema.23
In the presence of papilledema, or if clinical suspicion remains high in the absence of papilledema, the diagnosis of PTCS requires the presence of normal brain parenchyma on contrast-enhanced neuroimaging (magnetic resonance imaging [MRI] or computed tomography [CT]). In patients with clinical characteristics typical of most PTCS cases (eg, postpubertal, female, and obese; discussed later), a MRI venogram is not routinely necessary, but it should be considered for those patients at high risk for cerebral venous sinus thrombosis. Venous imaging is required for all atypical patients (males and thin females). Next, a lumbar puncture is necessary to document elevated ICP. Most often, opening pressure greater than 28 cm H2O in children is considered elevated; although, greater than 25 cm H2O is considered elevated in those not sedated during the lumbar puncture and nonobese children.20 CSF composition should be normal, with CSF analyses including cell count, cytology, and concentrations of glucose and protein. Interestingly, decreases in CSF protein have been measured in prepubertal, but not pubertal, children with IIH, an observation felt to be related to a relative CSF overproduction in this younger population.24,25
Thus, in the presence of papilledema, key diagnostic requirements include (1) normal brain parenchyma on neuroimaging with contrast-enhanced MRI or CT, (2) normal venous imaging with MRI or CT venogram in select cases, and (3) normal CSF composition on lumbar puncture. With elevated ICP, a diagnosis of definite PTCS can be made.1 If a normal ICP is measured, but the criteria above are otherwise satisfied, a diagnosis of probable PTCS can be made.
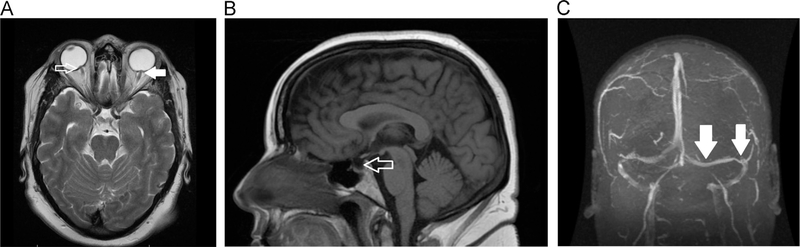
In the absence of papilledema, key diagnostic requirements include (1) normal brain parenchyma on neuroimaging with contrast-enhanced MRI or CT and (2) elevated ICP and normal CSF composition on lumbar puncture. With these features, in the added presence of unilateral or bilateral sixth nerve palsy (ies), the diagnosis of definite PTCS can be made.1 However, without a sixth nerve palsy, the diagnosis of suggested PTCS can be made if key neuroimaging features are observed.1 These neuroimaging features include distension of the perioptic subarachnoid space with or without optic nerve tortuosity (Fig. A), flattening of the posterior aspect of the globe (Fig. A), an empty sella (Fig. B), and transverse venous sinus stenosis (Fig. C).1 These neuroimaging features are highly suggestive of pediatric PTCS.21,26,27 There are no clear guidelines for closing pressure in the pediatric population, although a recent study illustrated that for every 0.91 mL of CSF removed ICP declined by 1 cm H2O if the pressure change (opening pressure or closing pressure) is less than 15 cm H2O.28 Symptom changes after a lumbar puncture and headache characteristics are not diagnostic of PTCS under the revised criteria.
Figure.
Radiographic features of primary pseudotumor cerebri syndrome. (A) As illustrated on T2-weighted axial image, there are radiographic features of raised intracranial pressure, namely flattening of the posterior aspect of globes (open arrow) and perioptic dilation of the optic nerve sheaths (solid arrow). (B) As illustrated on T2-weighted sagittal image, an empty sella is observed, a feature of raised intracranial pressure. (C) In this MRV, transverse sinus stenosis is observed, another feature of raised intracranial pressure. In the presence of clinical observed disk swelling, these radiographic features (A-C) are not required to confirm a diagnosis of definite or probable PTCS. They are required to establish a diagnosis of suggested PTCS. MRV, magnetic resonance venogram.
Management of Pediatric PTCS
There are no randomized clinical trials to allow for evidence-based recommendations in the treatment of PTCS in children. A recent multicenter, randomized, placebo-controlled study of adult subjects with IIH with mild visual loss showed that patients managed with acetazolamide and a low sodium weight-reduction diet had improved visual field function compared with patients managed with diet alone.29
The primary goals of treatment of PTCS are to prevent vision loss and to relieve symptoms of elevated ICP (eg, headache). The 2 mainstays of treatment are (1) medications to lower ICP and (2) weight loss. Surgical interventions are typically reserved for progressive vision loss despite maximal medical therapy or when there is severe visual loss at presentation. In these situations, the management of acute, vision-threatening PTCS may also include a short course of high-dose methylprednisolone,30 following which, the lack of clear visual improvement is an indication for surgical intervention. The 2 most commonly used surgical procedures are optic nerve sheath fenestration and CSF shunting (ie, lumboperitoneal or ventriculoperitoneal). Serial lumbar punctures are no longer commonly used. In less fulminant presentations, carbonic anhydrase inhibitors reduce CSF production and provide effective management of PTCS.31 The recommended starting dose in children for acetazolamide is 15–25 mg/kg/d divided into 2–3 doses; and may be gradually increased up to 100 mg/kg/d (maximum 2 g/d in children and 4 g/d in adolescents) as needed. Common side effects include paresthesia, fatigue, metallic taste, gastrointestinal upset, and loss of appetite. Metabolic acidosis is a well-recognized adverse effect but is typically asymptomatic, well tolerated, and generally does not require treatment.29 Alternatives to acetazolamide include furosemide (a loop diuretic), topiramate (an antiepileptic drug32), and spironolactone (an aldosterone antagonist; discussed later).
The duration of medical treatment is based on resolution of papilledema. The optic nerve head appearance is most often followed clinically, although there is an increasing use of OCT to monitor optic nerve swelling over time.21,33 When associated with being overweight or obese, weight loss is an additional key treatment in the management of PTCS. Based on studies in adult patients,34,35 patients are advised to lose 6%−10% body weight to manage the disease acutely and prevent its recurrence. In children, addressing excess adiposity is also an important step in management. Many pediatric patients are still growing, thus weight management recommendations should take this into account. A full review of the treatment of pediatric obesity is beyond the scope of this article36; however, weight management goals depend on patient age, current body mass index, and the presence or absence of other comorbidities. These goals can be addressed initially by primary care pediatricians and if necessary, by subspecialists. In general, healthful, family-centered lifestyle modifications, including increased vegetable intake, decreased concentrated sweets, increased physical activity, and attention toward adequate sleep are important first steps.
Pathophysiology of Pediatric PTCS
The pathophysiology of pediatric PTCS is complex. This section highlights common themes and ideas that exist in the understanding of the pathophysiology of this condition and is not meant to be an exhaustive review of all endocrine and structural factors contributing to the development of pediatric PTCS.37–40 In its most fundamental level, PTCS is related to abnormal CSF dynamics, either impaired CSF outflow, or aberrant CSF production, or both.41 With respect to impaired CSF outflow, increased resistance of CSF flow through arachnoid granulations may contribute to raised ICP. Impaired CSF lymphatic outflow (the “glymphatic pathway”), a CSF drainage pathway recently appreciated in the CNS, may also be contributing.41 In the pediatric population, IIH is characterized by a multifaceted relationship between age, obesity, pubertal status, and sex, factors which, in truth, may be acting to influence CSF production or outflow; however, many studies (discussed later) have focused on their influence on CSF production. The interaction of these factors may change with age, producing age-dependent phenotypes likely driven by distinct factors with possibly different pathophysiology. For example, adiposity clearly contributes to the presentation of disease in early and late adolescents, who are more frequently obese and female, likely similar to those in adults,42 Whereas factors other than adiposity likely contribute to the phenotype of young, prepubertal children with IIH who are less likely to be obese, are equally male and female, and may present without symptoms of headache or visual blurring.5,8 Pediatric adiposity has complex pathophysiology and includes potential contributions from alterations in growth hormone and gonadal hormones, factors also known to play a role in secondary PTCS (discussed later).37,39
Secondary PTCS refers to a clinical diagnosis of PTCS attributable to one or more of a variety of identifiable causes, including venous sinus thrombosis, medications, and medical conditions other than obesity alone (Table). In the pediatric population, some of the more common causes of secondary PTCS include withdrawal from chronic corticosteroids, exposure to tetracycline-related antibiotics, and synthetic growth hormone.11 By understanding the mechanism of these triggers, it may provide opportunities to identify unifying pathophysiologies. In the setting of secondary PTCS from withdrawal of chronic corticosteroids, multiple lines of evidence provide support for the hypothesis that the pathophysiology of PTCS involves aberrant glucocorticoid metabolism. Case reports have illustrated that PTCS can occur following surgical resection of pituitary tumors for Cushing disease,43,44 as an initial presentation of Addison disease45 or after withdrawal or tapering of chronic steroid medications.46,47 In a large retrospective review of more than 700 adults and 200 children who underwent surgical resection of adrenocorticotropic hormone–secreting pituitary tumors, no adult developed PTCS following resection whereas 3% of the pediatric group did.43 Thus, in the pediatric population, relative reductions in cortisol levels may be an inciting factor for PTCS. This is further supported by the fact that, as discussed earlier, in the acute management of vision-threatening PTCS, a short course of high-dose methylprednisolone is a treatment option.30 Although the exact mechanism of how a relative cortisol deficiency can lead to PTCS in the pediatric population is unclear, recent studies have suggested that this may involve the enzyme complex of 11-β-hydroxysteroid dehydrogenase type 1 and 2 (HSD1 and HSD2) that, together, modulate local availability of cortisol within the choroid plexus.48–50
Table.
Classification of Pseudotumor Cerebri Syndrome1
| Primary pseudotumor cerebri |
| Idiopathic intracranial hypertension |
| Secondary pseudotumor cerebri (most common causes) |
| Cerebral venous abnormalities |
| Sinus venous thrombosis |
| Medications |
| Tetracycline derivatives |
| Vitamin A and retinoids |
| Lithium |
| Hormones |
| Corticosteroids (withdrawal of chronic use) |
| Human growth hormone |
| Progestin |
| Medical conditions |
| Addison disease |
| Hypoparathyroidism |
| Anemia (severe) |
| Renal failure |
| Down syndrome |
| Turner syndrome |
Further in the pediatric population, the renin-angiotensin-aldosterone pathways seem important in regulating CSF dynamics. A recent retrospective series and literature review described 12 patients with PTCS and aldosteronism15 and 2 demographic groups emerged. There was a middle-aged, overweight adult population with primary aldosteronism and a pediatric population with secondary aldosteronism, seen in the setting of kidney disease. Activation of the renin-aldosterone system may be enhanced by PTCS-associated risk factors, including obesity, hypervitaminosis A, and corticosteroids.51 Providing further support for the role of aldosterone in PTCS, spironolactone, an aldosterone receptor antagonist, can assist in the management of PTCS.15 Salpietro et al39 have developed a neuroendocrine model of PTCS and suggest that mineralcorticoid pathway, acting through aldosterone, may help explain how hypervitaminosis A, pediatric obesity, and rGH contribute to PTCS. Interestingly, this mineralcorticoid pathway appears to parallel cellular mechanisms modulated by cortisol and HSD1/HSD2 activities.
The first reports of rGH or insulin-like growth factor-1 therapy causing PTCS were published in the early 1990s.52 Since then, a number of studies have suggested a causal relationship between the administration of rGH and PTCS.13,53 Genetech and Pfizer have both compiled the frequency of PTCS in children being treated with rGH (Nutropin and Genotropin, respectively) and found that it is increased by 23–100 times above than that seen in the general pediatric population.13,14 The presentation of PTCS seen following rGH treatment may differ from pediatric IIH. Namely, obesity and female sex are less convincingly risk factors. For example, obesity appears associated with an increased risk of rGH-related PTCS in children with renal failure and Prader-Willi syndrome. How rGH leads to PTCS is unclear but studies have implicated both elevations in serum aldosterone54 and a relative cortisol deficiency.55 Thus, through these examples, one may appreciate how pediatric PTCS may have common pathologic “nodes”, one of which may involve mineralcoticoid and glucocorticoid signaling pathways within the brain.
Conclusions
Patients with pediatric PTCS have elevated ICP of unclear etiology. This diagnosis requires the presence of normal brain parenchyma on neuroimaging and normal CSF composition without evidence of infection or neoplasm. PTCS can be a highly morbid complication of obesity; however, the diverse clinical spectrum of pediatric PTCS is becoming increasingly recognized. Accurate diagnosis follows clear guidelines based on clinical examination, neuroimaging, and CSF analysis.
References
- 1.Friedman DI, Liu GT, Digre KB: Revised diagnostic criteria for the pseudotumor cerebri syndrome in adults and children. Neurology 81: 1–7, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Durcan FJ, Corbett JJ, Wall M: The incidence of pseudotumor cerebri. Population studies in Iowa and Louisiana. Arch Neurol 45:875–877, 1988 [DOI] [PubMed] [Google Scholar]
- 3.Gordon K: Pediatric pseudotumor cerebri: Descriptive epidemiology. Can J Neurol Sci 24:219–221, 1997 [DOI] [PubMed] [Google Scholar]
- 4.Bursztyn LL, Sharan S, Walsh L, et al. : Has rising pediatric obesity increased the incidence of idiopathic intracranial hypertension in children? Can J Ophthalmol 49:87–91, 2014 [DOI] [PubMed] [Google Scholar]
- 5.Balcer LJ, Liu GT, Forman S, et al. : Idiopathic intracranial hypertension: Relation of age and obesity in children. Neurology 52:870–872, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Ko MW, Liu GT: Pediatric idiopathic intracranial hypertension (pseudo-tumor cerebri). Horm Res Paediatr 74:381–389, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Rangwala LM, Liu GT: Pediatric idiopathic intracranial hypertension. Surv Ophthalmol 52:597–617, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Cinciripini GS, Donahue S, Borchert MS: Idiopathic intracranial hypertension in prepubertal pediatric patients: Characteristics, treatment, and outcome. Am J Ophthalmol 127:178–182, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Stiebel-Kalish H, Kalish Y, Lusky M, et al. : Puberty as a risk factor for less favorable visual outcome in idiopathic intracranial hypertension. Am J Ophthalmol 142:279–283, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Sheldon CA, Paley GL, Xiao R, et al. : Pediatric idiopathic intracranial hypertension: Age, gender, and anthropometric features at diagnosis in a large, retrospective, multisite cohort. Ophthalmology 123:2424–2431, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paley GL, Sheldon CA, Burrows EK, et al. : Overweight and obesity in pediatric secondary pseudotumor cerebri syndrome e1. Am J Ophthalmol 159:344–352, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiu AM, Chuenkongkaew WL, Cornblath WT, et al. : Minocycline treatment and pseudotumor cerebri syndrome. Am J Ophthalmol 126:116–121, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Reeves GD, Doyle DA: Growth hormone treatment and pseudotumor cerebri: Coincidence or close relationship? J Pediatr Endocrinol Metab 15:723–730, 2002. (suppl 2) [DOI] [PubMed] [Google Scholar]
- 14.Darendeliler F, Karagiannis G, Wilton P: Headache, idiopathic intracranial hypertension and slipped capital femoral epiphysis during growth hormone treatment: A safety update from the KIGS database [Epub 2007 Dec 10]. Horm Res 68:41–47, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Khan MU, Khalid H, Salpietro V, Weber KT: Idiopathic intracranial hypertension associated with either primary or secondary aldosteronism. Am J Med Sci 346:194–198, 2013 [DOI] [PubMed] [Google Scholar]
- 16.Bassan H, Berkner L, Stolovitch C, Kesler A: Asymptomatic idiopathic intracranial hypertension in children. Acta Neurol Scand 118:251–255, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Dandy WE: Intracranial pressure without brain tumor: Diagnosis and treatment. Ann Surg 106:492–513, 1937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith JL: Whence pseudotumor cerebri? J Clin Neuroophthalmol 5: 55–56, 1985 [PubMed] [Google Scholar]
- 19.Friedman DI, Jacobson DM: Diagnostic criteria for idiopathic intracranial hypertension. Neurology 59:1492–1495, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Avery RA, Shah SS, Licht DJ, et al. : Reference range for cerebrospinal fluid opening pressure in children. N Engl J Med 363:891–893, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gilbert AL, Heidary G: Update on the evaluation of pediatric idiopathic intracranial hypertension. Curr Opin Ophthalmol 27:493–497, 2016 [DOI] [PubMed] [Google Scholar]
- 22.Lee YA, Tomsak RL, Sadikovic Z, et al. : Use of ocular coherence tomography in children with idiopathic intracranial hypertension: A single-center experience e1. Pediatr Neurol 58:101–106, 2016 [DOI] [PubMed] [Google Scholar]
- 23.Martinez MR, Ophir A: Optical coherence tomography as an adjunctive tool for diagnosing papilledema in young patients. J Pediatr Ophthalmol Strabismus 48:174–181, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Margeta MA, Buckley EG, El-Dairi MA: Low cerebrospinal fluid protein in prepubertal children with idiopathic intracranial hypertension. J AAPOS 19:135–139, 2015 [DOI] [PubMed] [Google Scholar]
- 25.Chandra V, Bellur SN, Anderson RJ: Low CSF protein concentration in idiopathic pseudotumor cerebri. Ann Neurol 19:80–82, 1986 [DOI] [PubMed] [Google Scholar]
- 26.Hirfanoglu T, Aydin K, Serdaroglu A, Havali C: Novel magnetic resonance imaging findings in children with intracranial hypertension. Pediatr Neurol 53:151–156, 2015 [DOI] [PubMed] [Google Scholar]
- 27.Gorkem SB, Doganay S, Canpolat M, et al. : MR imaging findings in children with pseudotumor cerebri and comparison with healthy controls. Childs Nerv Syst 31:373–380, 2015 [DOI] [PubMed] [Google Scholar]
- 28.McLaren SH, Monuteaux MC, Delaney AC, et al. : How much cerebrospinal fluid should we remove prior to measuring a closing pressure? J Child Neurolt 32:356–359, 2017 [DOI] [PubMed] [Google Scholar]
- 29.Wall M, McDermott MP, Kieburtz KD, et al. : Effect of acetazolamide on visual function in patients with idiopathic intracranial hypertension and mild visual loss. J Am Med Assoc 311:1641–1651, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu GT, Glaser JS, Schatz NJ: High-dose methylprednisolone and acetazolamide for visual loss in pseudotumor cerebri. Am J Ophthalmol 118:88–96, 1994 [DOI] [PubMed] [Google Scholar]
- 31.Melby JM, Miner LC, Reed DJ: Effect of acetazolamide and furosemide on the production and composition of cerebrospinal fluid from the cat choroid plexus. Can J Physiol Pharmacol 60:405–409, 1982 [DOI] [PubMed] [Google Scholar]
- 32.Celebisoy N, Gokcay F, Sirin H, Akyurekli O: Treatment of idiopathic intracranial hypertension: Topiramate vs acetazolamide, an open-label study. Acta Neurol Scand 116:322–327, 2007 [DOI] [PubMed] [Google Scholar]
- 33.El-Dairi MA, Holgado S, O’Donnell T, et al. : Optical coherence tomography as a tool for monitoring pediatric pseudotumor cerebri. J AAPOS 11:564–570, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Wong R, Madill SA, Pandey P, Riordan-Eva P: Idiopathic intracranial hypertension: The association between weight loss and the requirement for systemic treatment. BMC Ophthalmol 7:15, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ko MW, Chang SC, Ridha MA, et al. : Weight gain and recurrence in idiopathic intracranial hypertension: A case-control study. Neurology 76:1564–1567, 2011 [DOI] [PubMed] [Google Scholar]
- 36.Spear BA, Barlow SE, Ervin C, et al. : Recommendations for treatment of child and adolescent overweight and obesity. Pediatrics 120:S254–S288, 2007. (suppl 4) [DOI] [PubMed] [Google Scholar]
- 37.Sheldon CA, Kwon YJ, Liu GT, McCormack SE: An integrated mechanism of pediatric pseudotumor cerebri syndrome: Evidence of bioenergetic and hormonal regulation of cerebrospinal fluid dynamics. Pediatr Res 77:282–289, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Andrews LE, Liu GT, Ko MW: Idiopathic intracranial hypertension and obesity. Horm Res Paediatr 81:217–225, 2014 [DOI] [PubMed] [Google Scholar]
- 39.Salpietro V, Polizzi A, Berte LF, et al. : Idiopathic intracranial hypertension: A unifying neuroendocrine hypothesis through the adrenal-brain axis. Neuro Endocrinol Lett 33:569–573, 2012 [PubMed] [Google Scholar]
- 40.Markey KA, Mollan SP, Jensen RH, Sinclair AJ: Understanding idiopathic intracranial hypertension: Mechanisms, management, and future directions. Lancet Neurol 15:78–91, 2016 [DOI] [PubMed] [Google Scholar]
- 41.Mollan SP, Ali F, Hassan-Smith G, et al. : Evolving evidence in adult idiopathic intracranial hypertension: Pathophysiology and management. J Neurol Neurosurg Psychiatry 87:982–992, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brara SM, Koebnick C, Porter AH, Langer-Gould A: Pediatric idiopathic intracranial hypertension and extreme childhood obesity. J Pediatr 161:602–607, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kiehna EN, Keil M, Lodish M, et al. : Pseudotumor cerebri after surgical remission of Cushing’s disease [Epub 10 Feb 17]. J Clin Endocrinol Metab 95:1528–1532, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zada G, Tirosh A, Kaiser UB, et al. : Cushing’s disease and idiopathic intracranial hypertension: Case report and review of underlying pathophysiological mechanisms. J Clin Endocrinol Metab 95:4850–4854, 2010 [DOI] [PubMed] [Google Scholar]
- 45.Condulis N, Germain G, Charest N, et al. : Pseudotumor cerebri: A presenting manifestation of Addison’s disease. Clin Pediatr (Phila) 36:711–713, 1997 [DOI] [PubMed] [Google Scholar]
- 46.Liu GT, Kay MD, Bienfang DC, Schatz NJ: Pseudotumor cerebri associated with corticosteroid withdrawal in inflammatory bowel disease. Am J Ophthalmol 117:352–357, 1994 [DOI] [PubMed] [Google Scholar]
- 47.Kwon YJ, Allen JL, Liu GT, McCormack SE: Presumed pseudotumor cerebri syndrome after withdrawal of inhaled glucocorticoids. Pediatrics 137, 2016. pii: e20152091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sinclair AJ, Onyimba CU, Khosla P, et al. : Corticosteroids, 11beta-hydroxysteroid dehydrogenase isozymes and the rabbit choroid plexus. J Neuroendocrinol 19:614–620, 2007 [DOI] [PubMed] [Google Scholar]
- 49.Sinclair AJ, Walker EA, Burdon MA, et al. : Cerebrospinal fluid corticosteroid levels and cortisol metabolism in patients with idiopathic intracranial hypertension: A link between 11beta-HSD1 and intracranial pressure regulation? J Clin Endocrinol Metab 95:5348–5356, 2010 [DOI] [PubMed] [Google Scholar]
- 50.Markey KA, Uldall M, Botfield H, et al. : Idiopathic intracranial hypertension, hormones, and 11beta-hydroxysteroid dehydrogenases. J Pain Res 9:223–232, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rossi GP, Seccia TM: Changes in aldosterone and obesity-related cardiometabolic risk factors with a 1-yearweight loss intervention in normotensive overweight and obese young adults. Hypertens Res 36:856–858, 2013 [DOI] [PubMed] [Google Scholar]
- 52.Malozowski S, Tanner LA, Wysowski D, Fleming GA: Growth hormone, insulin-like growth factor I, and benign intracranial hypertension. N Engl J Med 329:665–666, 1993 [DOI] [PubMed] [Google Scholar]
- 53.Rogers AH, Rogers GL, Bremer DL, McGregor ML: Pseudotumor cerebri in children receiving recombinant human growth hormone [discussion 9–90]. Ophthalmology 106:1186–1189, 1999 [DOI] [PubMed] [Google Scholar]
- 54.Lampit M, Nave T, Hochberg Z: Water and sodium retention during short-term administration of growth hormone to short normal children. Horm Res 50:83–88, 1998 [DOI] [PubMed] [Google Scholar]
- 55.Zuckerman-Levin N, Tsivlin L, Knopf C, et al. : 11beta-Hydroxysteroid dehydrogenase type 1 activity in short small-for-GA children and in response to GH therapy. Pediatr Res 70:208–212, 2011 [DOI] [PubMed] [Google Scholar]