Abstract
Melphalan at a dose of 200 mg/m2 (MEL200) remains the standard high dose therapy before autologous stem cell transplantation (ASCT) for multiple myeloma (MM). Intensifying the high dose regimen has shown promising results. We report here 7-year follow up of our novel high dose regimen of busulfan and melphalan followed by bortezomib (BuMelVel). Forty-three MM patients received BuMelVel high dose therapy with pharmacokinetic adjusted busulfan. Outcomes were compared to a matched control cohort from the CIBMTR database (n = 162) receiving MEL200. The primary endpoint was progression free survival. Five year PFS was 47% v 30% (95% CI; 32–62) in favor or the BuMelVel group (95% CI; 23–37) (p=0.05). In multivariate analysis for PFS, BuMelVel (HR 0.65; 95% CI 0.44–0.97)(p=0.036) was predictive. Similar to recent reports of double alkylator therapy, although depth of response was similar between the BuMelVel group and MEL200, the BUMELVEL group experienced an improved PFS.
Keywords: Multiple Myeloma, Autologous Stem Cell Transplantation, Melphalan, Busulfan, Bortezomib
Introduction:
Multiple myeloma (MM) is a malignant disorder of plasma cells primarily affecting elderly patients. The development and ultimately FDA approval of Lenalidomide in 2006 and Bortezomib in 2003 began a period of rapid myeloma therapy expansion including 2nd and 3rd generation proteasome inhibitors and immunomodulators, monoclonal antibodies, and more recently chimeric antigen receptor T-cell therapy (CAR-T). Median progression free survival (PFS) in up front autologous stem cell transplantation (ASCT) eligible patients now surpasses 4 years1 and with maintenance improved 5 year survival is noted for patients of any age and ethnic background2. Despite the incorporation of novel myeloma therapies, the disease remains incurable when treated with novel myeloma therapies in combination with high dose chemotherapy and autologous stem cell transplantation.
In contrast to the accelerated development and exploration of effective new drugs for induction, maintenance, and relapse, Mel200 remains the international standard for high dose therapy before ASCT for MM in both the upfront and second transplant setting3 since it was first evaluated by Gore et al in 19894. Despite the first BMT-CTN state of the science symposium having as one of its priorities to intensify and improve the preparative regimen for MM, until recently, little progress has been made5. In preclinical studies, both the combination of busulfan and melphalan6 as well the combination of bortezomib and melphalan7 have been shown to be synergistic. When pharmacologically adjusted8 to yield a four day total systemic plasma drug exposure of 20,000 uM-min, busulfan is safe and early concerns regarding sinusoidal obstructive syndrome (SOS) appear to be eliminated9,10.
This open label phase I/II trial prospectively evaluated a high dose regimen consisting of high-dose intravenous busulfan and melphalan followed by bortezomib (BuMelVel). After a phase 1 roll-in, we completed the phase II portion and conducted a planned comparison of patients who received the phase II doses against a contemporaneous matched cohort of patients receiving Mel200. This control cohort was prospectively reported to the Center for International Blood and Marrow Transplant Research (CIBMTR) with similar characteristics. We previously reported an improved short term one year PFS of 90% in the BuMelVel arm as compared to 77% in the Mel200 arm (p=0.02) indicating an improved early benefit by intensifying the preparative regimen9. Here we report our 7 year follow up comparative data for this novel high dose regimen to the same cohort of CIBMTR patients with the primary aim of long term PFS as well as its effect on overall survival (OS). We believe our experience when added to recent Phase III data by Bashir et al which showed a median progression-free survival of 64.7 months with busulfan plus melphalan versus 43.5 months with melphalan alone potentially establishes a new standard of care.
Methods:
This open label phase I/II study was conducted between July 2009 and May 2012 at Loyola University Medical Center (Maywood,IL). All patients with any disease response following induction therapy were enrolled at time of stem cell transplantation workup. General inclusion criteria included adults aged less than 70, a diagnosis of symptomatic MM per international myeloma working group criteria11, creatinine less than 2.5 mg/dL, no active infections, no severe obstructive and/or restrictive pulmonary disease determined by pulmonary function testing (ie, DLCO < 50% and/or FEV1 < 50% and/or FVC < 50%), and cardiac ejection fraction greater than 40%. In total, 43 patients received BUMELVEL high dose therapy followed by ASCT on the phase II portion of this study.
Procedures:
Standard response criteria12 were used and risk defined per the Mayo Risk Stratification and International Staging System13,14. Neutrophil and platelet engraftment were defined as the first of 3 days with a neutrophil count > 0.5 × 109/L and first date of 7 consecutive laboratory values with platelet count ≥ 20 × 109/L without platelet transfusion. Common Terminology Criteria for Adverse Events (CTCAE) were used to grade and monitor for AEs. The Baltimore diagnostic criteria was used to diagnosis SOS15. Busulfan was administered i.v. daily for a total of 4 days with the first 2 days (days −6 and −5) at fixed dose of 130 mg/m2 over 3 hours and the subsequent 2 doses (days −4 and −3) adjusted to achieve a target AUC total of 20,000 μM·min as determined by pharmacokinetic analysis after the first dose of i.v. busulfan. Melphalan at 140 mg/m2 and bortezomib at 1.6 mg/m2 were administered i.v. on days −2 and −1, respectively. There was no planned maintenance therapy.
Patients received prophylaxis for oral mucositis with palifermin. Two doses of 6.25 mg were administered by i.v. bolus injection for 2 consecutive days a minimum of 24 hours before the first busulfan dose (days −8 and −7), and a third dose of 6.25 mg was administered on day 0 after stem cell infusion. Patients received supportive care per institutional guidelines. The Loyola University Chicago Stritch School of Medicine Institutional Review Board approved this study and all patients voluntarily signed informed consent.
Data from this phase II clinical trial were compared against a matched control cohort of contemporaneous North American MM patients (n = 162) receiving high dose therapy with single agent intravenous melphalan at a dose of 200mg/m2. Only patients who received the phase II (maximum tolerated) dose were included in the comparison analysis. Fifty-four centers, not including the study center, contributed patients for the control group.
Statistical Analysis:
The primary outcome of this study was median progression free survival. Descriptive statistics were used to report results including demographics, disease related factors, transplant-related factors, incidence and severity of mucositis, incidence and severity of SOS, remission rates, and relapse rates. The entire cohort was re-examined to confirm eligibility and descriptive factors. To adjust for potential imbalances of patient and disease related risk factors between trial and matched comparison cohorts, a one-to-four matched paired analysis was performed. Variables used in matching include patient gender; Karnosfsky score (≤80 vs. 90–100%); international disease stage (I, II vs. III); cytogenetic or mayo risk (high vs. standard); patient age; and time from diagnosis to transplant. For each case a matched control is selected with the smallest age difference among all potential matched controls. The BuMelVel cohort consisted of 43 patients and the matched control cohort of 162 patients, in which 39 patients were with 1–4 matching, 1 with 1–3 matching and 3 pairs were 1–1 matched.
The probabilities of PFS and OS were calculated using the Kaplan Meier estimator and the probability of transplant related mortality (TRM) and relapse generated using cumulative incidence estimates to account for competing risks. Marginal Cox model was used to compare the two groups while adjusting for matched pairs. The variables further considered in multivariate models are listed above. The assumption of proportional hazards for each factor in the Cox model was tested using time-dependent covariates. A stepwise model selection approach was used to identify all significant risk factors. Each step of model building contained the main effect for treatment group. Factors which were significant at a 5% level were kept in the final model. The potential interactions between main effect and all significant risk factors were tested. Adjusted probabilities of PFS and OS and adjusted cumulative incidence curves for TRM and relapse were generated from the final regression models stratified on main treatment group and weighted averages of covariate values using the pooled sample proportion as the weight function. These adjusted probabilities estimate likelihood of outcomes in populations with similar prognostic factors.
Results:
Baseline patient characteristics are described in Table 1. The two cohorts were well balanced for age, gender, KPS, MM isotypes, time from diagnosis to transplantation, disease stage, and disease status before transplantation. Of note, the median age at transplant was identical between groups at 62 years and KPS was 90 or higher in 74% and 75% of patients in the BuMelVel and Mel200 groups respectively. More patients in the BuMelVel group received greater than one line of therapy prior to transplant (53 v 33%). Remission status at transplant was similar between the BuMelVel and Mel200 groups with very good partial remission (VGPR) or greater in 42% v 47% respectively. In particular, chemo-resistant disease at transplant was very low in both groups at 2%. There were more standard risk patients per Mayo Stratification (mSMART) in the Mel200 group (78%) v the BuMelVel group (40%). Median follow up of survivors was also similar between groups at 86 months (range 27–109) in the BuMelVel group and 85 months (range 12–121) in the Mel200 group. No patients in the BuMelVel group received planned maintenance therapy post-transplant whereas 112 (69.6%) of the Mel200 control arm were reported to have received planned post-transplant maintenance. Within the limitations of the CIBMTR database, maintenance choice and duration in the Mel200 group is not available.
Table 1.
Baseline characteristics
| Characteristic | BUMELVEL | MEL200 | P Value |
|---|---|---|---|
| Number of patients | 43 | 162 | |
| Number of centers | 1 | 54 | |
| Age at transplant - median (min-max) | 62 (46–69) | 62 (42–69) | 0.47 |
| Age at transplant | 0.17 | ||
| 40–59 | 9 (21) | 56 (35) | |
| 60–64 | 17 (40) | 61 (38) | |
| 65–70 | 17 (40) | 45 (28) | |
| Gender | 0.97 | ||
| Male | 24 (56) | 91 (56) | |
| Female | 19 (44) | 71 (44) | |
| Karnofsky Performance Score group | 0.90 | ||
| <90 | 11 (26) | 40 (25) | |
| ≥ 90 | 32 (74) | 122 (75) | |
| Isotype | 0.86 | ||
| IgG | 26 (60) | 100 (62) | |
| IgA | 8 (19) | 32 (20) | |
| Light chain | 7 (16) | 27 (17) | |
| IgD | 1 (2) | 1 (1) | |
| non-secretory | 1 (2) | 2 (1) | |
| International stage at transplant | 0.74 | ||
| Stage I | 16 (37) | 51 (31) | |
| Stage II/III | 20 (47) | 79 (49) | |
| Unknown | 7 (16) | 32 (20) | |
| Mayo risk stratification at diagnosis (mSMART) | < 0.001 | ||
| Standard risk | 17 (40) | 127 (78) | |
| High risk | 4 (9) | 18 (11) | |
| Unknown | 22 (51) | 17 (10) | |
| Lines of chemotherapy | 0.02 | ||
| 1 | 20 (47) | 108 (67) | |
| >1 | 23 (53) | 54 (33) | |
| Disease status at transplant | 0.19 | ||
| CR | 3 (7) | 33 (20) | |
| VGPR | 15 (35) | 43 (27) | |
| PR | 23 (53) | 72 (44) | |
| SD | 1 (2) | 11 (7) | |
| REL/PROG | 1 (2) | 3 (2) | |
| Time from Diagnosis to transplant | 0.61 | ||
| ≤ 12 months | 33 (77) | 130 (80) | |
| >12 months | 10 (23) | 32 (20) | |
| Median follow-up of survivors (range), months | 86 (5–109) | 85 (1–121) |
BuMelVel: Bulsulfan, Melphalan, Bortezomib; Mel200: Melphalan 200mg/m2; CR: complete response; VGPR: very good partial response; PR: partial response; SD: stable disease; REL/PROG: relapse or progressive disease
Response Rates:
The BuMelVel regimen resulted in an overall response rate of 98%. There was no difference in response rates at day 100 (p=0.48) but a trend toward improved response at 1 year in the BuMelVel arm with 77% achieving a VGPR or better versus 60% in the Mel200 group (p-0.09) (table 2).
Table 2:
Best Response Following Transplant
| Characteristic | BUMELVEL | MEL200 | P Value |
|---|---|---|---|
| Number of patients | 43 | 162 | |
| Best response @ 100 days | 0.48 | ||
| sCR/CR | 17 (40) | 53 (33) | |
| VGPR | 12 (28) | 44 (27) | |
| PR | 12 (28) | 40 (25) | |
| SD | 1 (2) | 20 (12) | |
| PD/Relapse | 0 | 2 (1) | |
| Unknown | 1 (2) | 3 (2) | |
| Best response @ 1 year | 0.09 | ||
| sCR/CR | 20 (47) | 79 (49) | |
| VGPR | 13 (30) | 27 (17) | |
| PR | 9 (21) | 27 (17) | |
| SD | 0 | 11 (7) | |
| PD/Relapse | 0 | 6 (4) | |
| Unknown | 1 (2) | 12 (7) |
Hypothesis testing:
Kruskal-Wallis test
Pearson chi-square test
BuMelVel: Bulsulfan, Melphalan, Bortezomib; Mel200: Melphalan 200mg/m2; sCR: stringent complete response; CR: complete response; VGPR: very good partial response; PR: partial response; SD: stable disease; REL/PROG: relapse or progressive disease
Overall and Progression Free Survival:
As of this report and a median of more than seven years of follow up, 14 patients (32%; 95% CI 18–48) were still alive without disease progression the BuMelVel group versus 38 (23%; 95% CI 16–31) in the Mel200 group (p=0.33). The five year PFS was 47% (95% CI; 32–62) in the BuMelVel group versus 30% (95% CI; 23–37) in the Mel200 group (p=0.05) (Figure 1). Overall survival was not different between the two groups with a 7 year survival of 64% (95% CI; 48–79) in the BuMelVel group versus 55% (95% CI; 46–64%) in the Mel200 group (multivariate p value =0.33) (figure 1).
Figure 1:
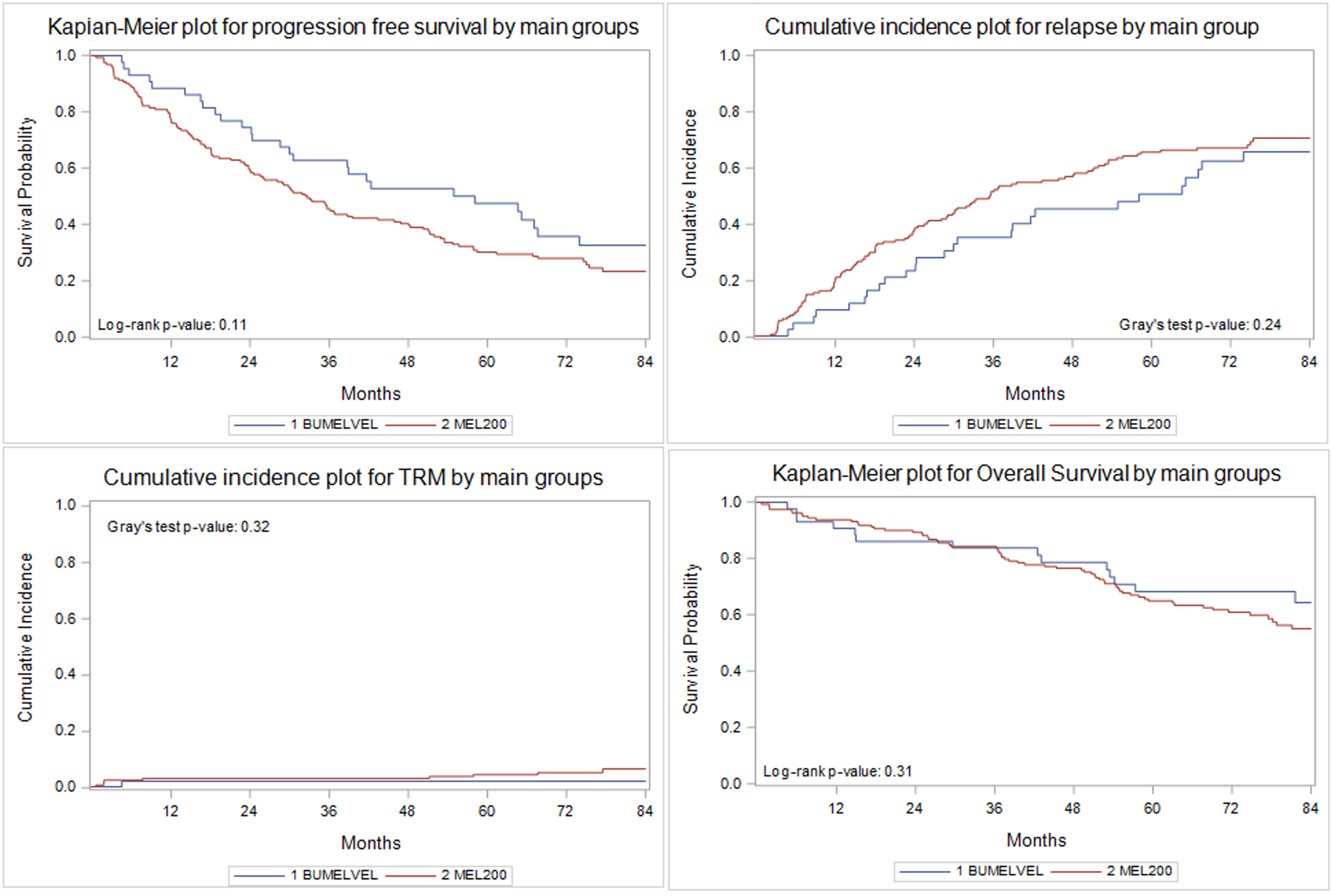
Progression free survival, relapse, transplant related mortality (TRM) and overall survival
In multivariate analysis, time from diagnosis to transplant of greater than versus less than 12 months was associated with an increased risk of relapse (HR 1.99; 95% CI 1.45 – 2.71)(p=0<0.01). In multivariate analysis for progression free survival, BuMelVel high dose therapy (HR 0.65; 95% CI 0.44–0.97)(p=0.036), shorter interval from diagnosis to transplant of less than 12 months (HR 1.64 for >12 months; 95% CI 1.18 – 2.27), and disease status at transplant (complete remission HR 1.00; p=0.006; see table 3 for individual response HR) were all associated with improved progression free survival (table 3).
Table 3:
Marginal-Cox model for Relapse and Progression Free Survival
| RELAPSE | |||||
|---|---|---|---|---|---|
| Parameter | Hazard | 95% Hazard Ratio Confidence Limits | p-value | ||
| Ratio | |||||
| Main groups | Control (MEL200) | 1.00 | 0.13 | ||
| Case (BUMELVEL) | 0.72 | 0.48 | 1.10 | ||
| Time from diagnosis to transplant | ≤12 | 1.00 | <0.01 | ||
| >12 | 1.99 | 1.45 | 2.71 | ||
| PROGRESSION FREE SURVIVAL | |||||
| Parameter | Hazard | 95% Hazard Ratio Confidence Limits | p-value | ||
| Ratio | |||||
| Main groups | Control (MEL200) | 1.00 | 0.036 | ||
| Case (BUMELVEL) | 0.65 | 0.44 | 0.97 | ||
| Time from diagnosis to transplant | ≤ 12 | 1.00 | 0.003 | ||
| >12 | 1.64 | 1.18 | 2.27 | ||
| Disease status prior to HCT | CR | 1.00 | 0.006 | ||
| VGPR | 1.42 | 0.82 | 2.44 | 0.21 | |
| PR | 2.01 | 1.20 | 3.37 | 0.008 | |
| SD | 1.87 | 0.74 | 4.74 | 0.19 | |
| REL/Prog | 3.96 | 1.47 | 10.65 | 0.007 | |
BuMelVel: Bulsulfan, Melphalan, Bortezomib; Mel200: Melphalan 200mg/m2; CR: complete response; VGPR: very good partial response; PR: partial response; SD: stable disease; REL/PROG: relapse or progressive disease
Regimen-Related Toxicity:
There was no difference in time to neutrophil or platelet engraftment between the two groups. Treatment related mortality was low in both groups. Only one patient (2%, range 0–9%) in the BuMelVel group died from transplant related causes compared to 10 (6%, range 3–11) in the Mel200 group but this was not statistically significant. PK targeted busulfan dosing was successful in all of the BuMelVel patients with only 23% of patients having a first dose AUC outside an acceptable range of 5000 μM·min ± 20% (<4000 or >6000 μM·min). There were no episodes of SOS in the BuMelVel group. The most common grade 3 adverse events included the expected febrile neutropenia and mucositis. In particular, 37% of patients experienced grade 3 mucositis and there was no grade 4 mucositis in the BuMelVel group. No enteric feeding or total parenteral nutrition was needed. The median hospital stay for the BuMelVel group was 19 days.
Discussion:
This study is a long term follow up of our earlier report of this novel Bu/Mel/Vel preparative regimen in advanced MM. Similar to recent reports in the literature comparing busulfan and melphalan to melphalan alone10, we show that although depth of response was similar between the BuMelVel group and the historical Mel200 comparator, the BuMelVel group experienced an improved PFS including an impressive 7 year PFS without maintenance therapy of 32%.
The combination of busulfan and melphalan has been explored in several settings (see table 4) including the phase III setting by the PETHEMA/GEM group16 and the MD-Anderson group10. Following a modern induction therapy, the MD-Anderson group randomized patients to receive either standard Mel200 or busulfan and melphalan (BuMel) high dose therapy with PK adjusted busulfan dosing to achieve an AUC total of 20,000 mmol-minute. With a shorter median follow up of approximately 20 months, they found similar results to ours with a median PFS of 64.7 versus 43.5 months (p=0.022) and a 3 year PFS of 72% versus 50% in the BuMel and Mel200 arms respectively. 84% of patients in the BUMEL arm and 86% of patients in the MEL200 arm initiated maintenance therapy post-transplantation. The Spanish Myeloma Group (PETHEMA/GEM) are currently conducting a randomized phase III study comparing BuMel to Mel200 following a modern induction of lenalidomide, bortezomib, and dexamethasone with preliminary safety data presented at ASH in 201716. This may validate the recently published MD-Anderson data.
Table 4:
Notable studies examining busulfan and melphalan plus/minus bortezomib in the literature
| Reference | Study Design | Comparator | Response | Progression free survival | Toxicity |
|---|---|---|---|---|---|
| Blanes et al26, Annals of hematology 2019 | Phase II multi-institution, Spain; 1:2 matched control analyses | BuMel* v Mel200 | No difference: BuMel v Mel200 ORR: 82 v 91% |
Median PFS: 33 v 24m (p=0.04) | TRM: 4% in BuMel v 2% in control No grade III/IV mucositis in BuMel |
| Park et al, BBMT 2019 | Phase I/II multicenter trial, Korea | No comparator BuMelVel** |
At day 90: ≥VGPR: 75% CR: 55% |
Median PFS: 26.8m | Grade 3 mucositis: 14.6% |
| Bashir et al, Lancet Haematol 2019 | Phase III single institution, MD Anderson Cancer Center | BuMel*** v Mel200 | No difference at day 100 BuMel v Mel: MRD negative: 58 v 61% CR: 52 v 49% |
Median PFS: BuMel 64.7m v Mel 43.5m (p=0.022) |
Grade 3–4 non-haematological: BuMel (84%) v Mel (33%) (p<0.0001) Grade 3 Mucositis: BuMel 14% v Mel 0% (p<0.0001) |
| Jung et al, BBMT 2018 | Phase II multicenter, Korea | No comparator BuMel# |
ORR: 94% 43.5% sCR 27.3% VGPR |
Median PFS: 27.2m | Grade 3–4 mucositis 15.2% 3 patients (3.2%) developed SOS |
| Byun et al27, Blood Research 2018 | Korean National Health Database, retrospective comparison | BuMel## v HDMel | Non-reported | Improved 3-year PFS in the BuMel group: 70.3 v 52.5% (P-0.043) | No differences (mucositis not specifically reported) |
| Rosinol et al, ASH 2017 | Phase III national multicenter, Spain | BuMel### v Mel200 | Per high dose arm, pending | Per high dose arm, pending | Per high dose arm, pending |
| Barta et al28, CLML 2017 | Phase II multi-institution, US | No comparator BuMelVel ŧ |
ORR 100% at day 100 | 2-year PFS 59% (95%CI 38–89%) | 53% grade 3 or 4 mucositis |
(Blanes et al): BuMel: intravenous Busulfan at 3.2mg/kg daily over days −5 to −3
(Park et al) BuMelVel: intravenous busulfan (i.v., 3.2 mg/kg/day from days −5 to −3); melphalan (i.v.,140 mg/m2/day on day −2), bortezomib i.v. 1.3mg/m2 on days −6, −3, and +1
(Bashir et al) BuMel: Busulfan test dose followed by pharmacokinetically adjusted doses of Busulfan on days −7, −6, −5, and −4 to achieve a target daily area under the curve (AUC) of 5000 mmol-minute and melphalan 70 mg/m2 per day on days −2 and −1; melphalan 70mg/m2 on days −2 and −1
(Jung et al) BuMel: intravenous busulfan of 3.2 mg/kg was administered over 3 hours once daily from day −6 to days −4; melphalan of 70 mg/m2/day was administered on day −3 and day −2
(Byun et al) BuMel: Dosing not reported
(Rosinol et al) BuMel: intravenous busulfan at 9.6mg/kg; melphalan 140mg/m2
(Barta et al) BuMelVel: intravenous PK directed Busulfan to achieve target total AUC of 20,000 from days −6 to −3; melphalan 150mg/m2 onf day −2; intravenous Bortezomib given at 1mg/m2 on days −6,−3,+1,+4
M: months
TRM: Transplant-related mortality
PFS: Progression free survival
HDMel: High dose melphalan, melphalan at 200mg/m2 or less
sCR: stringent complete response
VGPR: very good partial remission
SOS: sinusoidal obstructive syndrome
Our results however seem better than other recent reports given the lack of maintenance therapy received by patients on our study, perhaps owing to the use of bortezomib in the combination and the PK dosing of the busulfan. To our knowledge, only one other group has prospectively evaluated BuMelVel as a high dose regimen for MM17 with a much shorter reported follow up of only 31.4 months. In their phase I/II study following a bortezomib based induction, they reported a higher response rate than ours with 83% of patients achieving a very good partial response (VGPR) or better but an inferior median PFS of only 26.8 months and two year PFS of just 56%. Interestingly, they reported less grade III mucositis (only 14.6%) even in the absence of palifermin and no SOS. These complication rates are similar to the report of Jung and colleagues utilizing BuMel high dose therapy18. Finally, the Spanish group compared data from their phase II BuMel study to a contemporaneous matched cohort treated on the GEM2000 protocol19 who received Mel200 high dose therapy. Induction therapy was with intensive chemotherapy comprising six alternating cycles of VBMCP (vincristine, carmustine, melphalan, cyclophosphamide, prednisone) and VBAD (vincristine, BCNU, adriamycin, dexamethasone) chemotherapy followed by ASCT. They found an improved PFS in the BuMel arm of 33 months compared to 24 months in the Mel200 arm. Response rates were similar, no SOS was seen, and they reported no grade III mucositis. Importantly, all three of these studies utilized non-PK adjusted busulfan dosing, had lower rates of mucositis, and seemingly inferior progression free survival as compared to our data potentially pointing to an under-dosing of the busulfan.
These data indicate that the combination of busulfan and melphalan appears to consistently lengthen duration of remission without necessarily deepening response rates. It has been postulated that the synergism between busulfan and melphalan could be due to complex genomic lesions that are more difficult for the myeloma stem cell to repair10. Potentially this synergism could more effectively target the myeloma stem cell population carrying driver mutations20 prolonging time to relapse without a further reduction in overall disease burden. Or possibly the common target sites on DNA are saturated prior to achieving dose limiting toxicity when a single alkylating agent is used. This remains to be clarified but could be a point of investigation in future studies utilizing this preparatory regimen.
Importantly, we report no cases of SOS and a low transplant related mortality of only 2% (1 death due to viral pneumonia at day 146 post-transplant) reinforcing that the preparatory regimen in myeloma can be intensified safely with improved duration of even an unmaintained PFS. Our data showed an impressive OS of 64% and based on the recent meta-analysis21 could perhaps have been longer had our patients received planned maintenance as did apparently the majority of the MEL200 comparator group. Although outcomes are improved by intensifying the preparatory regimen, the randomized data from the MD-Anderson group suggests that this is at the expense of increased non-hematological toxicity in particular mucositis10. We are unable to make a direct comparison in our study to the CIBMTR control group as mucositis rates are not reported to the CIBMTR; however, we do note that hospital stay, time to engraftment, and transplant related mortality were not different between the groups.
There appears to be increasing enthusiasm for intensifying the preparatory regimen in MM using other approaches, including 2 randomized phase II studies comparing bendamustine and melphalan22 as well as BEAM (carmustine, etoposide, cytarabine, Melphalan) (NCT03570983) to Mel200 in addition to the above mentioned PETHEMA-GEM study comparing BuMel to Mel20016. Similar to our work with busulfan, pharmacokinetic based melphalan dosing which was once thought not feasible is being explored utilizing propylene glycol free melphalan (Evomela)23,24. Finally, the incorporation of proteasome inhibitors into the preparatory regimen is particularly attractive as they have been shown to enhance the sensitivity of multiple myeloma tumor cells to chemotherapeutic agents without affecting normal hematopoietic cells25. Along these lines we have recently opened a phase 1/2 study evaluating the combination of carfilzomib, busulfan, and melphalan (NCT03795597) and have begun enrolling.
Conclusion:
In conclusion, we show that intensifying the preparatory regimen with PK-adjusted busulfan and combining it with both melphalan and bortezemib leads to an improved progression free survival with around one third of our patients being progression free 7 years out from transplant despite a lack of maintenance therapy. Our data along with the MD-Anderson data depicting a 20 month progression free survival advantage for busulfan and melphalan signal that a change be considered in the standard of care for high dose therapy for multiple myeloma patients. This benefit is on par with the PFS advantages seen with up front versus delayed ASCT and post-transplant maintenance.
Acknowledgement:
We would like to acknowledge the patients and family members who enrolled on this clinical trial as well as the CIBMTR for the support. Additionally, Otsuka Pharmaceutical Co for their financial support.
Financial disclosure and competing interest statement: This research was supported by Otsuka Pharmaceutical Co, Ltd. The authors have no other competing or conflicting interest to report.
CIBMTR SUPPORT LIST
The CIBMTR is supported primarily by Public Health Service U24CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); U24HL138660 from NHLBI and NCI; OT3HL147741, R21HL140314 and U01HL128568 from the NHLBI; HHSH250201700006C, SC1MC31881-01-00 and HHSH250201700007C from the Health Resources and Services Administration (HRSA); and N00014-18-1-2850, N00014-18-1-2888, and N00014-20-1-2705 from the Office of Naval Research; Additional federal support is provided by P01CA111412, R01CA152108, R01CA215134, R01CA218285, R01CA231141, R01HL126589, R01AI128775, R01HL129472, R01HL130388, R01HL131731, U01AI069197, U01AI126612, and BARDA. Support is also provided by Be the Match Foundation, Boston Children’s Hospital, Dana Farber, Japan Hematopoietic Cell Transplantation Data Center, St. Baldrick’s Foundation, the National Marrow Donor Program, the Medical College of Wisconsin and from the following commercial entities: AbbVie; Actinium Pharmaceuticals, Inc.; Adaptive Biotechnologies; Adienne SA; Allovir, Inc.; Amgen, Inc.; Anthem, Inc.; Astellas Pharma US; AstraZeneca; Atara Biotherapeutics, Inc.; bluebird bio, Inc.; Bristol Myers Squibb Co.; Celgene Corp.; Chimerix, Inc.; CSL Behring; CytoSen Therapeutics, Inc.; Daiichi Sankyo Co., Ltd.; Gamida-Cell, Ltd.; Genzyme; GlaxoSmithKline (GSK); HistoGenetics, Inc.; Incyte Corporation; Janssen Biotech, Inc.; Janssen Pharmaceuticals, Inc.; Janssen/Johnson & Johnson; Jazz Pharmaceuticals, Inc.; Kiadis Pharma; Kite Pharma; Kyowa Kirin; Legend Biotech; Magenta Therapeutics; Mallinckrodt LLC; Medac GmbH; Merck & Company, Inc.; Merck Sharp & Dohme Corp.; Mesoblast; Millennium, the Takeda Oncology Co.; Miltenyi Biotec, Inc.; Novartis Oncology; Novartis Pharmaceuticals Corporation; Omeros Corporation; Oncoimmune, Inc.; Orca Biosystems, Inc.; Pfizer, Inc.; Phamacyclics, LLC; Regeneron Pharmaceuticals, Inc.; REGiMMUNE Corp.; Sanofi Genzyme; Seattle Genetics; Sobi, Inc.; Takeda Oncology; Takeda Pharma; Terumo BCT; Viracor Eurofins and Xenikos BV. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government.
References:
- 1.Atttal M, et al. Lenalidomide, Bortezomib, Dexamethasone with Transplantation for Myeloma. NEJM. 2017; 376(14): 1311–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Costa L, et al. Recent trends in multiple myeloma incidence and survival by age, race, and ethnicity in the United States. Bld Adv. 2017; 1(4): 282–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hagen P, Stiff P. The Role of Salvage Second Autologous Hematopoietic Cell Transplantation in Relapsed Multiple Myeloma. BBMT. 2019; 25(3): e98–e107. [DOI] [PubMed] [Google Scholar]
- 4.Gore ME, et al. Intensive treatment of multiple myeloma-and criteria for complete remission. Lancet. 1989; 2:879. [DOI] [PubMed] [Google Scholar]
- 5.Bayraktar UD, Bashir Q, Qazilbash M, Champlin RE, Ciurea SO. Fifty years of melphalan use in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2013; 19: 344–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valdez BC, et al. Mechanistic studies on the synergistic cytotoxicity of the nucleoside analogs gemcitabine and clofarabine in multiple myeloma: relevance of p53 and its clinical implications. Experimental hematology. 2013; 41(8):719–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lonial S, et al. A phase I/II trial combining high dose melphalan and autologous transplant with bortezomib for multiple myeloma: dose and schedule finding study. Clin Cancer Res. 2010; 16:5079–5086. [DOI] [PubMed] [Google Scholar]
- 8.de Lima M, Couriel D, Shahjahan M. IV busulfan (Bu) with fludarabine (Flu) or cyclophosphamide (Cy)—comparing ablative preparative regimens for allogeneic transplantation in AML/MDS. Blood. 2004; 104:11.14976060 [Google Scholar]
- 9.Rodriguez T, et al. Busulfan, Melphalan, and Bortezomib versus High-Dose Melphalan as a Conditioning Regimen for Autologous Hematopoietic Stem Cell Transplantation in Multiple Myeloma. BBMT. 2017; 22(8): 1391–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bashir Q, et al. Conditioning with busulfan plus melphalan versus melphalan alone before autologous haemopoietic cell transplantation for multiple myeloma: an open-label, randomised, phase 3 trial. Lancet Hematology. 2019; 6(5): e266–e275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rajkumar SV, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncology. 2014; 15(12): e538–48. [DOI] [PubMed] [Google Scholar]
- 12.Durie BGM, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006; 20:1467–1473. [DOI] [PubMed] [Google Scholar]
- 13.Kumar SK, et al. Management of newly diagnosed symptomatic multiple myeloma: updated Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) consensus guidelines. Mayo Clin Proc. 2009; 84:1095–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greipp P, et al. International Staging System for Multiple Myeloma. JCO. 2005; 23(15): 3412–3420. [DOI] [PubMed] [Google Scholar]
- 15.Jones RJ, et al. Venoocclusive disease of the liver following bone marrow transplantation. Transplantation. 1987; 44:778–783. [DOI] [PubMed] [Google Scholar]
- 16.Rosinol L, et al. Bortezomib, Lenalidomide and Dexamethasone (VRD-GEM) As Induction Therapy Prior Autologous Stem Cell Transplantation (ASCT) in Multiple Myeloma (MM): Results of a Prospective Phase III Pethema/GEM Trial. Blood. 2017; 130(Suppl 1), 2017. [Google Scholar]
- 17.Park S, et al. A Phase I/II, Open-Label, Prospective, Multicenter Study to Evaluate theEfficacy and Safety of Lower Doses of Bortezomib Plus Busulfan and Melphalan as a Conditioning Regimen in Patients with MultipleMyeloma Undergoing Autologous Peripheral Blood Stem CellTransplantation: The KMM103 Study. BBMT. 2019; 25(7): 1312–1319. [DOI] [PubMed] [Google Scholar]
- 18.Jung SH, et al. Phase 2 Study of an Intravenous Busulfan and Melphalan Conditioning Regimen for Autologous Stem Cell Transplantation in Patients with Multiple Myeloma (KMM150). BBMT. 2018; 24(5): 923–929. [DOI] [PubMed] [Google Scholar]
- 19.Lahuerta J, et al. Busulfan 12 mg/kg plus melphalan 140 mg/m2 versus melphalan 200 mg/m2 as conditioning regimens for autologous transplantation in newly diagnosed multiple myeloma patients included in the PETHEMA/GEM2000 study. Haematologica. 2010; 95(11): 1913–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corre J, et al. Multiple myeloma clonal evolution in homogeneously treated patients. Leukemia. 2018; 32: 2636–2647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCarthy P, et al. Lenalidomide Maintenance After Autologous Stem-CellTransplantation in Newly Diagnosed Multiple Myeloma:A Meta-Analysis. JCO. 2017; 35(29): 3279–3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gomez-Arteaga A, et al. High-dose bendamustine and melphalan conditioning for autologous stem cell transplantation for patients with multiple myeloma. BMT. 2019; epub ahead of print (31190006) [DOI] [PubMed] [Google Scholar]
- 23.Shah G, et al. Feasibility, Tolerability, and Patient-Reported Outcomes with Pharmacokinetic (PK)-Directed Dosing of Evomela® (propylene glycol free melphalan) for Multiple Myeloma and AL Amyloidosis Patients Undergoing Autologous Hematopoietic Stem Cell Transplant (AHCT). BLOOD. 2017; 130: 2023. [Google Scholar]
- 24.Dhakal B, et al. Pharmacokinetics of High-Dose Propylene Glycol-Free Melphalan in Multiple Myeloma Patients Undergoing Autologous Hematopoietic Cell Transplantation. BBMT. 2018; 24(8): 1610–1614. [DOI] [PubMed] [Google Scholar]
- 25.Ma M, et al. The Proteasome Inhibitor PS-341 Markedly Enhances Sensitivity of Multiple Myeloma Tumor Cells to Chemotherapeutic Agents. Clinical Cancer Research. 2003; 9: 1136–1144. [PubMed] [Google Scholar]
- 26.Blanes M, et al. Intravenous busulfan plus melphalan versus melphalan aloneas conditioning regimen for patients with multiple myeloma. Ann of Hem. 2019; 98: 2013–2015. [DOI] [PubMed] [Google Scholar]
- 27.Byun J, et al. Busulfan plus melphalan versus high-dose melphalan as conditioning regimens in autologous stem cell transplantation for newly diagnosed multiple myeloma. Blood Research. 2018; 53(2): 105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barta S, et al. Pharmacokinetics-directed Intravenous Busulfan Combined With High-dose Melphalan and Bortezomib as a Conditioning Regimen for Patients With Multiple Myeloma. CLML. 2017; 17(10): 650–657. [DOI] [PubMed] [Google Scholar]


