Abstract
During primate arboreal locomotion, substrate orientation modifies body axis orientation and biomechanical contribution of fore- and hindlimbs. To characterize the role of cortical oscillations in integrating these locomotor demands, we recorded electrocorticographic activity from left dorsal premotor, primary motor, and supplementary motor cortices of three common marmosets moving across a branch-like small-diameter pole, fixed horizontally or vertically. Animals displayed behavioral adjustments to the task, namely, the horizontal condition mainly induced quadrupedal walk with pronated/neutral forelimb postures, whereas the vertical condition induced walk and bound gaits with supinated/neutral postures. Examination of cortical activity suggests that β (16–35 Hz) and γ (75–100 Hz) oscillations could reflect different processes in locomotor adjustments. During task, modulation of γ ERS by substrate orientation (horizontal/vertical) and epoch (preparation/execution) suggests close tuning to movement dynamics and biomechanical demands. β ERD was essentially modulated by gait (walk/bound), which could illustrate contribution to movement sequence and coordination. At rest, modulation of β power by substrate orientation underlines its role in sensorimotor processes for postural maintenance.
Keywords: body posture, β desynchronization, γ synchronization, gait, substrate orientation
Introduction
Most of our knowledge on the neurophysiological mechanisms of locomotion is derived from studies of stepping movements in cats, rats and primates (Drew and Marigold 2015; Kiehn 2016). These studies demonstrate that, although locomotor movements are mostly driven by pattern and rhythm generating networks in the spinal cord, several regions of the brain, brainstem, and cerebellum are also involved in several aspects of gait control (Kiehn 2016). Cortical activity is thought to reflect the supervision of downstream circuits, triggering gait motor programs in the brainstem and spinal cord (DiGiovanna et al. 2016) and mastering coordination between gait and other motor activities. In arboreal primates, locomotion is coupled with prehensile fore- and hindlimb movements (Schmitt 2003b). Prehensile movements are controlled by a frontoparietal cortical network that has been largely documented in primates (Raos et al. 2004; Shimazu et al. 2004; Davare et al. 2006; Tia et al. 2017), but was never examined during whole-body locomotion in ethologically relevant, naturalistic conditions. Here we address this question by examining electrocorticographic (ECoG) activity recorded from the frontal motor cortex of common marmosets (Callithrix jacchus) moving over horizontal/vertical substrates that resemble branches on which they normally travel in their arboreal environment.
In a locomotor task, varying substrate orientation drastically modifies body axis orientation as well as hand posture and biomechanics (kinematics, forces, and muscle activity). Forelimb grip posture can vary between horizontal/vertical substrates, in terms of ulnar/radial wrist deviation (mouse lemurs, Reghem et al. 2012) or extent of limb protraction/retraction at touch down and lift off (tamarins, Nyakatura et al. 2008). Locomotion on horizontal and vertical substrates is associated with distinct functional differentiation of fore- and hindlimbs (Hesse et al. 2015; Hanna et al. 2017). The horizontal condition is typically characterized by a net-braking role of forelimbs and a net-propulsive role of hindlimbs, whereas the vertical condition is characterized by a net-propulsive role of both, with greater contribution of hindlimbs. In the vertical condition, the hindlimbs tend to be used in compression (to push onto the substrate) while the forelimbs tend to be used in traction (to pull away from the substrate). Besides, in horizontal condition, small substrate diameter imposes strong balance requirements, inducing adjustments in posture (high forelimb protraction and elbow flexion; small shoulder height), kinematics (long contact duration and low velocity) and forces (low peak substrate reaction forces; Schmitt 1999, 2003b; Young et al. 2016).
Although previous work documented biomechanical adjustments to substrate orientation, few studies targeted the neurophysiological underpinnings at the cortical level. In this work, we were interested in spotting which features of cortical oscillations, if any, reflect locomotor adjustments to substrate orientation. We focused on respective contributions of spectral power in β (16–35 Hz) and γ (75–100 Hz) frequency bands because we had previously reported the involvement of β and γ oscillations for grasping movements in marmosets (Tia et al. 2017). γ activity was reported as a good indicator of neuronal dynamics, in terms of firing rate and synchrony, compared to lower frequencies (Ray et al. 2008). γ synchronization in the sensorimotor cortex closely reflects movement-related cortical processing, whereas β desynchronization generally displays spatial and temporal features that are less specific to the task (Crone et al. 2006; Miller et al. 2007). These characteristics suggest that β and γ power could be involved, albeit to different extents, in adjustments to locomotor task requirements. More specific to gait control, prior investigations showed that locomotor tasks requiring enhanced muscle activity correlate with stronger multiunit activity in rats (DiGiovanna et al. 2016) and γ power in humans (Gwin et al. 2011). Skilled paw placement in rats (DiGiovanna et al. 2016) and precise walking in humans (Bruijn et al. 2015) also modulate cortical involvement by shifting the time of peak firing rate and increasing β power at push-off, respectively. Based on these considerations, we forecast modulations of β and γ power, reflecting adjustments to substrate orientation and tuning to task epoch (preparation, execution).
Considering recorded areas, we focused this study on the frontal motor cortex including dorsal premotor (6Dr and 6Dc), supplementary motor (6M) and primary motor (4) areas of common marmosets. Previous work suggests that motor areas could underpin distinct roles during walking. Premotor and supplementary motor cortices are involved in gait planning and initiation, as well as the execution of movement sequences (Sahyoun et al. 2004; Takakusaki 2013; Koenraadt et al. 2014). A specific role of supplementary motor cortex was highlighted in postural adjustments (Takakusaki 2013). Finally, primary motor cortex is known to modulate the recruitment of synergistic muscle groups during gait execution/modification (Sahyoun et al. 2004; Drew and Marigold 2015).
Our hypothesis in which adaptation to substrate orientation rests on adjustments of cortical oscillatory activity stands on evidence that gait generation, as well as prehensile movement, are fundamentally driven by descending motor pathways, with specific involvement of premotor and primary motor cortices (Davare et al. 2011; Gwin et al. 2011; Foster et al. 2014). Thus, we designed the present experiment to investigate activity in the frontal motor cortex of three common marmosets moving on a small-diameter, motionless pole oriented either horizontally or vertically (Fig. 1). It is worth mentioning that the small size of common marmosets represented an optimal condition to study whole-body movements along different axes. Moreover, lissencephalic brains and thin dura mater of common marmosets allowed the use of epidural ECoG arrays to record over multiple areas with minimal damage to the brain tissues (Hashikawa et al. 2015; Tia et al. 2017). Finally, our protocol was designed to emphasize volitional engagement of the animals and task complexity, which enhances motor cortex activity during locomotor tasks (rats, DiGiovanna et al. 2016).
Figure 1.
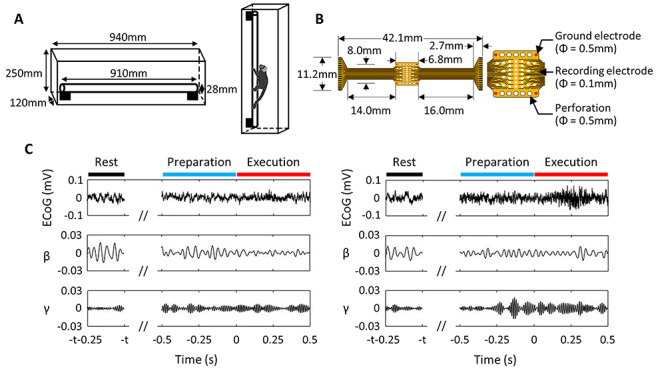
Experimental setup. (A) Schema of the cage used for horizontal and vertical locomotion tasks. The animals moved over a 28-mm diameter substrate made of an 18-mm diameter wooden pole covered with a 5-mm-thick urethane sheet. (B) 64-channel electrocorticographic (ECoG) array (left) and zoom-in of the recording area (right), adapted from Tia et al. (2017) Creative Commons permission © 2017 by John Wiley & Sons Ltd (C) Typical ECoG signal recorded from a channel in premotor area 6Dc of MK1. Left panels represent horizontal condition and right panels, vertical condition. Top panels represent the raw signal; second and third panels represent β (16–35 Hz) and γ (75–100 Hz) components obtained after band-pass filtering. Data is aligned with respect to movement onset (time = 0). Rest was recorded ≥1 s before movement onset. Duration separating rest from movement onset is designated as t.
Materials and Methods
Ethical Approval
The data presented here were recorded from three purpose-bred adult common marmosets (Callithrix jacchus; MK1, male, 4.3 years, 358 g; MK2, male, 2.8 years, 305 g; MK3, male, 3.3 years, 381 g), housed at RIKEN Brain Science Institute (Wako, Japan). The animals were not food deprived, but in order to maintain a high motivation for the task, daily food was provided at the end of each testing session. Water was always available ad libitum. All procedures were performed in accordance with the Laboratory Animal Welfare Act and The Guide for the Care and Use of Laboratory Animals (National Institutes of Health, Bethesda, MD) and were approved by the Institutional Animal Research Committee at RIKEN (IRB approval number H24-2-228). All adequate measures were taken to minimize pain and discomfort.
Task
Common marmosets were trained to walk freely on an elevated wooden pole (diameter, 18 mm; length, 910 mm) covered with a urethane sheet (5-mm thick), oriented either horizontally or vertically (Fig. 1A). On the basis of preliminary observations, pole diameter was selected as the smallest on which the animals would succeed to move. After each walk along the pole, the animal received a food reward from the experimenter. The number of trials performed per animal and condition is displayed in Table 1. Locomotor movements were monitored throughout experiments by a video camera at 30 frames/s (HDR-HC9, Sony Corporation, Japan). Gaits were classified as 1) walk, when a forelimb was temporally paired with a hindlimb, and the body was always supported by two, three, or four limbs at a time, and 2) bound, when the left and right forelimbs were temporally paired, as were the left and right hindlimbs, with both limbs contacting the substrate simultaneously (Supplementary Table 1; Hildebrand 1980; Hunt et al. 1996; Shapiro et al. 2016). Forelimb postures were classified as pronation, neutral and supination based on the direction in which the palm was pointing and on the degree of rotation of the forearm along its longitudinal axis (Supplementary Fig. 1; Soubeyrand et al. 2017). On the horizontal substrate, pronation referred to a posture in which the palm faced downward and touched the upper side of the pole. Neutral posture referred to the palm facing medially and touching the left/right side of the pole. On the vertical substrate, supination referred to a posture in which the palm faced toward the animal and touched the side of the pole opposite to the animal. Neutral posture referred to the palm facing medially and touching the left/right side of the pole.
Table 1.
Number of artifact-free ECoG epochs (left values in each cell) and trials that animals completed (right values in each cell) per epoch and condition
| MK1 | MK2 | MK3 | ||
|---|---|---|---|---|
| Horizontal | Rest | 335/335 | 98/98 | 237/249 |
| Preparation | 43/83 | 67/71 | 55/106 | |
| Execution | 54/83 | 71/71 | 54/106 | |
| Number of sessions | 5 | 2 | 3 | |
| Vertical | Rest | 212/219 | 16/17 | 192/266 |
| Preparation | 47/72 | 15/19 | 73/114 | |
| Execution | 53/72 | 16/19 | 73/114 | |
| Number of sessions | 6 | 2 | 9 |
Neural Recordings with ECoG Arrays
Local field potentials were recorded over the frontal cortex using 8 × 8 micro-ECoG arrays purposely built in our laboratory (Fig. 1B; Castagnola et al. 2013; Castagnola et al. 2014). Contact diameter was 100 μm and interelectrode distance was 900 μm in the mediolateral and 700 μm in the anteroposterior direction. Four electrodes (500-μm diameter) located at each corner of the ECoG array acted as a linked reference. The presence of multiple perforations through the device ensured effective contact with the brain surface and free flow of cerebrospinal fluid. The micro-ECoG arrays were connected to a wireless headstage (Triangle Biosystems International, Durham, NC). The signals were filtered (1–1000 Hz) and digitized at 2713 Hz using the Digital Lynx data acquisition system (Fig. 1C; Neuralynx, Bozeman, MT). Recordings were performed over a period of 10 weeks for MK1, 1 week for MK2 and 14 weeks for MK3.
Surgical Procedure
The surgical procedure of micro-ECoG implantation has been previously reported (Tia et al. 2017; Kosugi et al. 2018). Anesthesia was introduced with an intraperitoneal injection of medetomidine, midazolam and butorphanol (0.05, 0.5, and 0.5 mg/kg, respectively). Atropine (0.10 mg/kg) and prednisolone (0.15 mg/kg) were injected intramuscularly immediately after anesthesia. During the surgery, anesthesia was maintained by inhalation of 1.5–2.5% isoflurane and the oxygen saturation level was continuously monitored. When intensive postsurgical care was required (e.g., suture repair), the animals were anesthetized with isoflurane (0.5–3%) and administrated with lidocaine (subcutaneous injection) for analgesia.
For implantation of a micro-ECoG electrode array, a craniotomy of 9 × 5 mm (coordinates relative to bregma: 0–9 mm anterior and 2–7 mm lateral) was performed in the left hemisphere, leaving the dura intact. The ECoG sheet was laid onto the dura using a micromanipulator, and then a piece of artificial dura mater was placed between the array and the skull in order to maintain the electrodes in place and to ensure proper signal-to-noise ratio. A head chamber made of Ultem (height, 15 mm; width, 18 mm; length, 16 mm), a polyetherimide polymer with high dielectric strength, solvent resistance and mechanical properties, was attached to the skull with stainless screws and dental acrylics. The area inside the chamber was washed with sterilized saline every day for up to 7–10 days following the surgery for a reduction of reactive tissue around the electrode arrays. The chamber was then filled with a silicone polymer (Kwik-Cast, World Precision Instruments, Sarasota, FL, USA) in order to improve the stability of the micro-ECoG sheet.
Recording Locations
In order to identify the cortical regions covered by the array, histological mapping was performed at the end of the experiment, using the procedure described in our previous study (Fig. 2; Tia et al. 2017). The monkey was anesthetized with ketamine (15 mg/kg) and pentobarbital (75 mg/kg) and was perfused through the heart with phosphate-buffered saline and paraformaldehyde. The postmortem brain was measured and photographed. A block of cortex containing the recorded areas was cut parasagittally and 50-μm histological sections were mounted and Nissl stained. The recording location of the entire array over the cortex was then reconstructed. Histological borders were plotted as transition zones of various width, reflecting sources of uncertainty such as test–retest variability (assessed by repeated plotting by the same observer on different days) and interference of histology artifacts. Criteria described in previous studies (Krubitzer and Kaas 1990; Burman et al. 2006; Burman et al. 2008; Burman et al. 2015) were used to distinguish between prefrontal, premotor, supplementary motor, primary motor, and somatosensory areas. Details of the criteria were summarized in supplementary materials (Supplementary methods—Criteria for histological mapping).
Figure 2.
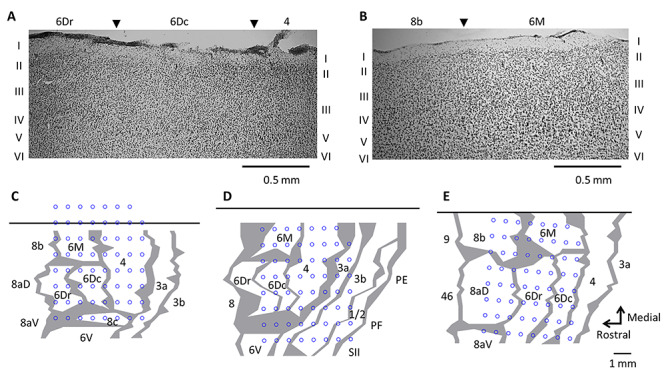
(A–B) Parasagittal sections illustrating cytoarchitectural characteristics of premotor (6Dr and 6Dc), supplementary motor (6M) and primary motor (4) cortex. Panels A and B respectively represent sections from MK2 and MK3. C-E. Reconstruction of array location over the frontal motor cortex by histological assessment for MK1 (C), MK2 (D), and MK3 (E). The top black horizontal line represents the midline. Gray lines indicate architectonic borders, reconstructed from sagittal sections. The thickness of the lines represents zones of uncertainty. Blue circles refer to the location of each of 63 recording electrodes.
For all monkeys, a subset of electrodes was located over the left dorsal premotor, supplementary motor and primary motor cortices. In MK1, recordings were mainly made from prefrontal (area 8c), dorsal premotor (areas 6Dr and 6Dc), supplementary motor (area 6M) and primary motor (area 4) cortices in the left hemisphere (Fig. 2C). In MK2, recordings were made from premotor (areas 6V, 6Dr, and 6Dc), supplementary motor (area 6M), primary motor (area 4) and somatosensory (areas 3a, 3b, and 1/2) cortices (Fig. 2D). In MK3, recordings were made from prefrontal (areas 8aD and 8b), dorsal premotor (areas 6Dr, 6Dc), supplementary motor (area 6M) and primary motor (area 4) cortices (Fig. 2E).
ECoG Signal Processing
Analyses were performed on ECoG signals measured during all locomotion sequences, namely when the monkeys moved across any section of the pole without interruption. Three epochs were identified from the recorded movies: “rest,” “preparation” (500-ms interval preceding movement onset), and “execution” (500-ms interval from the start of fore−/hindlimb movement initiating locomotion). The physiological reason for not evaluating the cortical activity of entire locomotion periods is that gait initiation involves cortical processes but locomotion is mostly driven by central pattern generators, where motor cortex has less contribution (Kiehn 2016). We also excluded from rest epochs the periods within 1 s before the movement onset to 1 s after the termination of locomotion, and segmented ECoG signals according to the time of epochs.
During preprocessing, we rejected channels that failed to record reliable physiological signal by assessing power spectrum of the segmented ECoG signals. This procedure excluded two channels (32 and 62) for MK1, three channels (21, 32, 62) for MK2, and 12 channels (1, 12, 18, 19, 20, 27, 32, 37, 42, 46, 52, and 62) for MK3. The periods including large amplitude artifacts, which are problematic for accurate independent component analysis (ICA) decomposition, were removed from each epoch (Rogasch et al. 2014), and we kept epochs longer than 100 ms for further analysis. This procedure rejected 120 epochs (13.9% of the all epochs) for MK1, 12 epochs for MK2 (4.1% of the all epochs), and 271 epochs for MK3 (28.4% of the all epochs) (Table 1). ICA was then applied in order to remove components reflecting residual artifacts. In particular, independent components of which the weighted matrix showed spatial discontinuity due to sudden amplitude fluctuations in one channel (Mognon et al. 2011) were subtracted from the original signals. Artifact-free signals were transformed into power spectra using a complex Morlet wavelet of three cycles, and averaged power spectra across β (16–35 Hz) and γ (75–100 Hz) frequencies were obtained.
Statistical Analysis
First, we aimed to investigate β and γ spectral power at rest. Power spectra were averaged across channels for each cortical area (6M, 6Dr, 6Dc, and 4, where ECoG signals were measured in all monkeys). Three-way analyses of variance with aligned rank transform (ART-ANOVAs) were applied to area-averaged data from all trials/monkeys, with TASK (horizontal, vertical) and MONKEY (MK1, MK2, and MK3) as between-subject factors and AREA (6M, 6Dr, 6Dc, and 4) as the within-subject factor. If the assumption of sphericity was violated, Greenhouse–Geisser correction was applied. Post hoc pairwise comparisons were performed using Wilcoxon rank-sum test with Bonferroni–Holm correction. We considered P < 0.05 as statistically significant.
Next, we studied β and γ event-related desynchronization and event-related synchronization (ERD/ERS) during locomotion. ERD/ERS were defined as changes in power spectrum relative to the resting period over the same substrate, expressed as percentages of the resting power. Statistical significance of single channel ERD/ERS was assessed by bootstrap tests. For each epoch (preparation, execution) and task (vertical, horizontal) during walk gaits, we randomly resampled the mean power spectrum for each trial and created 1000 bootstrap datasets, which were normalized relative to the actual mean of power spectra during the resting period. A histogram of these bootstrapped ERD/ERS values was then used to test statistical significance. If the 2.5th percentile (ERS) or the 97.5th percentile (ERD) of the distribution of bootstrapped ERD/ERS values was larger or smaller than 0%, respectively, we deemed the difference between task epoch and rest as significant. We conducted a similar analysis for each epoch (preparation, execution) and gait (walk, bound) during the vertical task.
Finally, we assessed modulations of ERD/ERS due to task (horizontal, vertical) and gait (walk, bound). Bound gait pattern almost always occurred in vertical condition and therefore could not be used for horizontal/vertical comparison. Thus, to evaluate the effect of task, four-way ART-ANOVAs were applied to ERD/ERS values that were averaged across channels for each cortical area and obtained during walk gaits only, with TASK (horizontal, vertical), EPOCH (preparation and execution) and MONKEY (MK1, MK2 and MK3) as between-subject factors and AREA (6M, 6Dr, 6Dc and 4) as the within-subject factor. To assess the effect of gait, four-way ART-ANOVAs were applied to area-averaged ERD/ERS obtained during vertical task only. These ANOVAs included GAIT (walk and bound), EPOCH (preparation and execution) and MONKEY (MK1, MK2, and MK3) as between-subject factors and AREA (6M, 6Dr, 6Dc, and 4) as the within-subject factor. If the assumption of sphericity was violated, Greenhouse–Geisser correction was applied. Analyses were performed using Matlab R2016a and the Fieldtrip toolbox (Oostenveld et al. 2011).
Results
Behavioral Adjustments to Substrate Orientation
Representative movies of the locomotion task are provided in the supplementary materials (Supplementary Movies 1–4). After selecting images with sufficient visibility for classification, we combined data from all animals in a pooled ensemble of 713 grips for horizontal condition and 636 grips for vertical condition. Video recordings showed that the animals used different forelimb postures, which could be distinguished by the degree of pronation/supination (Supplementary Fig. 1). The horizontal task mainly involved pronation and neutral grips (55.5% and 44.5%; Supplementary Figs 2 and 3), whereas the vertical task mainly involved supination and neutral grips (75.6% and 24.4%; Supplementary Figs 4 and 5). In contrast with the forelimb, only one posture was identified for the hindlimb in which the thumb was brought in opposition to the other four fingers to form a power grip.
Regarding gait, video recordings allowed identification of a subset of locomotor sequences defined as walks and bounds (Supplementary Table 1). More precise classification (e.g., diagonal/lateral sequence) was rendered difficult by the low visibility, namely, not all limb movements within a gait were clearly identifiable. In horizontal condition, marmosets preferred walks over bounds (96.3% vs. 3.7% of total gait duration; Supplementary Movies 1 and 2). In vertical condition, marmosets also preferred walks, although they performed a larger proportion of bounds (63.4% vs. 36.6%; Supplementary Movies 3 and 4). Altogether, these results highlight some degree of locomotor adjustments to substrate orientation, both in terms of forelimb posture and movement sequence.
β and γ Power Modulation during Locomotion
Figure 3 illustrates power spectrum for horizontal and vertical conditions obtained from a single channel in area 4 of MK1–3. As can be seen, power during task preparation/execution exceeded power at rest in the frequencies above 60 Hz. By contrast, in the frequencies below 35 Hz, power was generally lower during task than at rest. These results agree with largely documented task-related power variations (Crone et al. 2006; Miller et al. 2007). Noteworthily, β power at rest appears to be higher in the horizontal compared to vertical condition.
Figure 3.
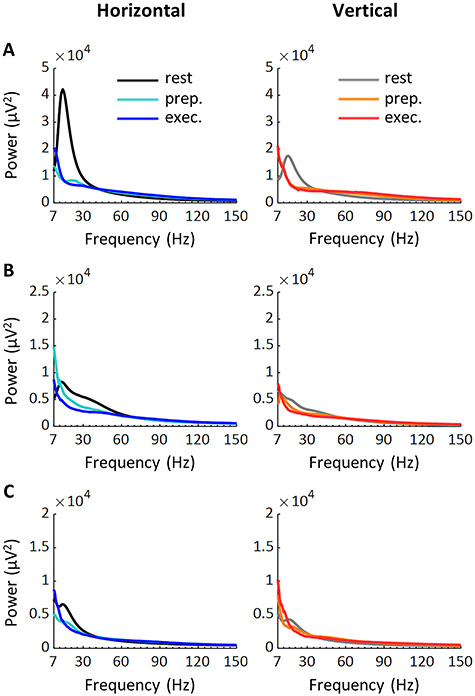
Power spectrum during locomotor tasks. Top, middle, and bottom panels represent a channel in area 4 of MK1 (A), MK2 (B), and MK3 (C), respectively. Prep., preparation; exec., execution.
To determine whether substrate orientation modulates resting activity, we conducted ART-ANOVAs with TASK and MONKEY as between-subject factors and AREA as the within-subject factor (Fig. 4; Supplementary Table 2). ANOVAs conducted on β and γ power both revealed significant TASK*MONKEY*AREA interactions (β power: F5.04, 2732 = 83.6, p < 0.001; γ power: F3.77, 2043 = 19.2, p < 0.001). Post hoc analyses confirmed higher β power on horizontal than vertical substrate in area 6Dc (MK1 and MK2), 6M (MK1 and MK2) and 4 (MK1, MK2, and MK3; P < 0.05). γ differences were more mitigated, with higher values on horizontal than vertical substrate in area 4 for MK2, but lower values on horizontal than vertical substrate in area 6Dr and 6Dc for MK3.
Figure 4.
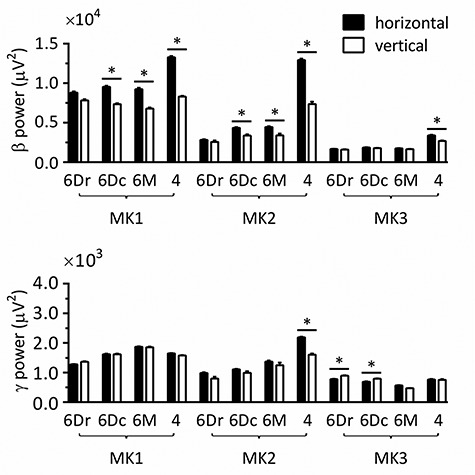
Spectral power in β and γ bands at rest. Pairwise comparisons were performed by Wilcoxon rank-sum test with Bonferroni–Holm correction, after three-way ANOVAs with aligned rank transform (factors: monkey, task, and cortical area). 6Dr, 6Dc, 6M, and 4 refer to the corresponding Brodmann areas. All measurements are expressed as mean ± standard error of the mean (SEM). *P < 0.05, by Wilcoxon pairwise comparison with Bonferroni–Holm correction.
Table 2.
Summary of results from behavioral and neural recordings
| Behavior | ECoG | ||
|---|---|---|---|
| Task | Gait | Forelimb posture | Spectral power |
| Horizontal | Walk (≈ 96%) Bound (≈ 4%) | Pronation (≈ 56%) Neutral (≈ 44%) | Higher βrest power than verticalLower γ ERSwalk than vertical |
| Both | γ ERSexecution > γ ERSpreparation | ||
| Vertical | Walk (≈ 63%) Bound (≈ 37%) | Supination (≈ 76%) Neutral (≈ 24%) | Lower βrest power than horizontalHigher γ ERSwalk than horizontalβ ERDwalk > β ERDbound |
Next, we examined ERD/ERS during locomotion. In order to dissociate task (horizontal and vertical) from gait (walk and bound) effects, we conducted two sets of analyses. In the first set, we evaluated task effects considering only walk gaits, so as to eliminate possible sources of variation due to gait. In the second set, we evaluated gait effects considering only vertical task, so as to eliminate possible sources of variation due to task.
We calculated ERD/ERS for each channel by normalizing power during task by power at rest over the same pole orientation. Figure 5 displays ERD/ERS over time for a channel in area 4 of MK1. Only trials in which video recordings allowed clear identification of walk gaits initiated by the right forelimb are included in this figure. In agreement with power variations described earlier (Fig. 3), both tasks and epochs (preparation and execution) induced ERD in the β frequency and ERS in the γ frequency ranges, with stronger ERS during execution than preparation.
Figure 5.
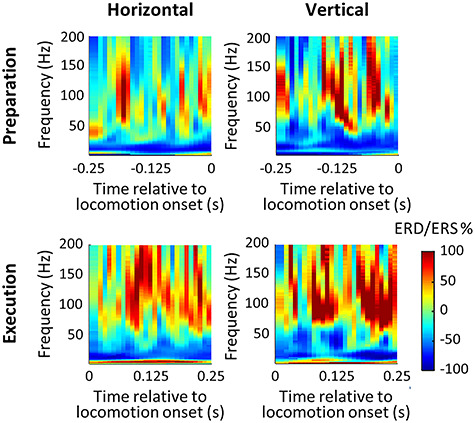
Time-frequency plot of a channel in area 4 of MK1 during preparation and execution of locomotion on horizontal and vertical substrates. Only trials in which video recordings allowed clear identification of walk gaits initiated by the right forelimb were included. Power spectrum for each condition was normalized by the averaged power spectrum at rest over the same substrate. Preparation was defined as the 250-ms interval preceding locomotion onset, and execution, as the 250-ms following locomotion onset.
Statistical significance of single channel ERD/ERS was determined by the bootstrap method. As shown in figure 6A, β ERD and γ ERS were distributed over the sensorimotor cortex of MK1 during preparation and execution of horizontal and vertical tasks during walk gait. Similarly, MK2 and MK3 displayed significant ERD/ERS over several recording sites (Supplementary Fig. 6).
Figure 6.
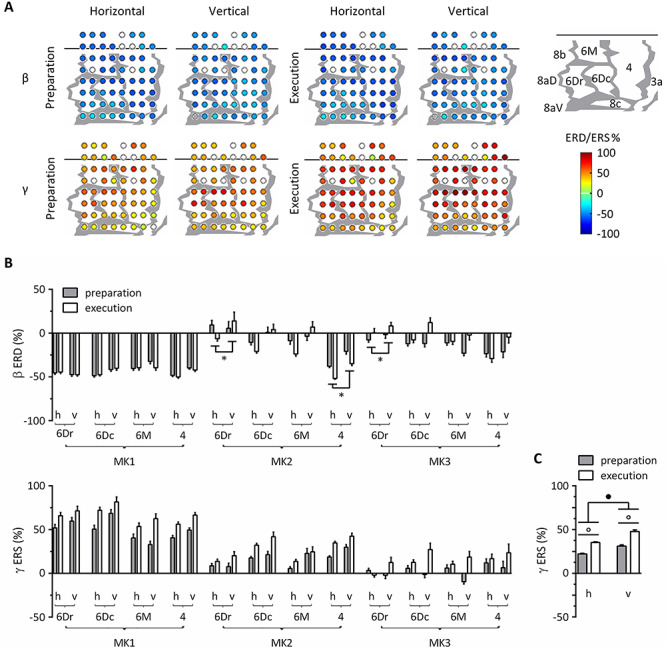
Task-related modulation of β and γ power, considering walk gaits only. (A) ERD/ERS in β and γ bands over the sensorimotor cortex of MK1. Differences from the baseline resting power were statistically assessed by the bootstrap method (P < 0.05). Electrodes with statistically significant ERD/ERS were represented as filled colored circles, whereas those with nonsignificant ERD/ERS were represented as open circles. Blue colors denote ERD and red colors denote ERS, ranging from −100% to 100%. Bad channels were excluded from analysis and were not represented. Gray lines indicate architectonic borders between cortical areas, reconstructed from histological assessment. The cortical map of MK1 is represented in the upper right corner. (B) ERD/ERS in β and γ bands across monkeys, areas, tasks and epochs. All measurements are expressed as mean ± SEM. *P < 0.05, difference between the task conditions; h, horizontal; v, vertical. 6Dr, 6Dc, 6M, and 4 refer to the corresponding Brodmann areas. (C) Task- and epoch-related differences in γ ERS averaged across monkeys and cortical areas. •P < 0.001, main effect of task; °P < 0.001, main effect of epoch.
To further evaluate task-related differences, we conducted ART-ANOVAs with TASK, EPOCH and MONKEY as between-subject factors and AREA as the within-subject factor (Fig. 6B-C; Supplementary Table 3). The ANOVA on β ERD showed significant TASK*MONKEY*AREA (F5.65, 1036 = 3.39, P = 0.005) and EPOCH*TASK (F1, 367 = 5.97, P = 0.030) interactions. Post hoc tests revealed inconsistent results across monkeys. β ERD was more prominent for horizontal than vertical task in area 6Dr (MK2 and MK3) and 4 (MK2). The ANOVA on γ ERS showed significant MONKEY*AREA interaction (F5.70, 1198 = 7.50, P < 0.001), as well as main effects for TASK (F1, 420 = 15.9, P < 0.001) and EPOCH (F1, 420 = 17.5, P < 0.001). These main effects suggested that γ ERS took higher values for vertical than horizontal task and increased from preparation to execution (Fig. 6C). Of note, the lack of interaction between MONKEY and TASK/EPOCH shows that all animals followed the same trends regarding task- and epoch-related γ power variations.
In the second set of analyses, we evaluated the effect of gait considering only the vertical task. As shown in Figure 7A, β ERD and γ ERS were distributed over the sensorimotor cortex of MK1 during preparation and execution of walk and bound gaits. Similarly, MK2 and MK3 displayed significant ERD/ERS over several recording sites (Supplementary Fig. 7). In order to assess gait-related differences, we conducted ART-ANOVAs with GAIT, EPOCH and MONKEY as between-subject factors and AREA as the within-subject factor (Fig. 7B; Supplementary Table 4). The ANOVA on β ERD showed significant MONKEY*AREA interaction (F5.57, 499 = 18.1, P < 0.001) and a main effect for GAIT (F1, 179 = 17.4, P < 0.001) which suggests that β ERD was stronger for walk compared to bound. The ANOVA on γ ERS showed significant GAIT*MONKEY*AREA interaction (F5.41, 716 = 2.96, P = 0.019), but post hoc tests failed to reveal consistent pairwise differences for all three marmosets.
Figure 7.
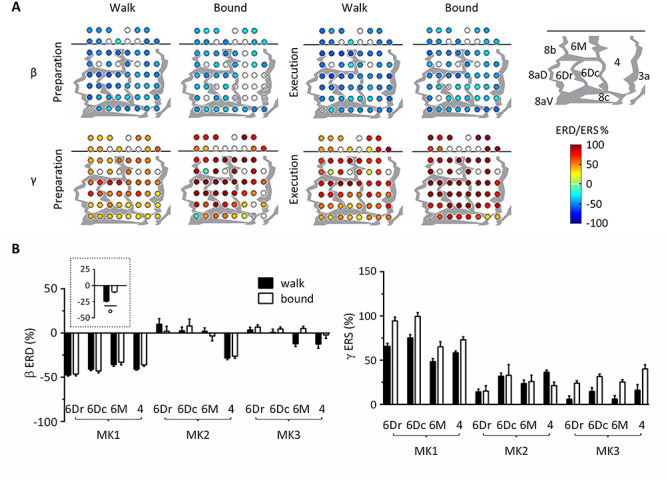
Gait-related modulation of β and γ power, considering vertical task only. (A) ERD/ERS in β and γ bands over the sensorimotor cortex of MK1. Differences from baseline resting power were statistically assessed by the bootstrap method (P < 0.05). Electrodes with statistically significant ERD/ERS were represented as filled colored circles, whereas those with nonsignificant ERD/ERS were represented as open circles. Blue colors denote ERD and red colors denote ERS, ranging from −100% to 100%. Bad channels were excluded from analysis and were not represented. Gray lines indicate architectonic borders between cortical areas, reconstructed from histological assessment. The cortical map of MK1 is represented in the upper right corner. (B) ERD/ERS in β and γ bands across monkeys, areas and gaits. Preparation and execution epochs were merged in the main plots since ANOVAs with aligned rank transform (factors: monkey, gait, epoch, and cortical area) found no significant main effect nor interaction with epoch. The plot surrounded by a dot line emphasizes gait-related differences in β ERD averaged across monkeys, epochs, and cortical areas. All measurements are expressed as mean ± SEM. °P < 0.001, main effect of gait. 6Dr, 6Dc, 6M, and 4 refer to the corresponding Brodmann areas.
Discussion
Using epicortical recordings, we investigated oscillatory dynamics in the frontal motor cortex of common marmosets during locomotion over horizontal and vertical substrates. We focused on ethologically relevant paradigms that emphasized task complexity and volitional engagement of the animals (see also, DiGiovanna et al. 2016), by using small-diameter, motionless poles to model branches on which marmosets normally travel in natural behavioral contexts. In addition to behavioral adjustments (forelimb posture and gait), our results showed that substrate orientation and gait modulate β and γ ERD/ERS in the frontal motor cortex (Table 2). We also detected significant effects of substrate orientation on β power at rest. In the next sections, we will discuss these results and propose some hypotheses regarding underlying mechanisms.
Behavioral Adjustments to Substrate Orientation
In contrast with widely documented stepping movements, our experimental task strongly relied on fore- and hindlimb prehensile movements (Schmitt and Lemelin 2002; Rossignol and Frigon 2011; Sustaita et al. 2013; Drew and Marigold 2015) and showed differences in forelimb posture depending on substrate orientation. Similar findings were described in tamarins (Saguinus oedipus), where the extent of relative fore- and hindlimb protraction/retraction at touch down/lift-off varies with substrate incline, possibly to reduce the retarding role of forelimbs during stance phase and to emphasize hindlimb propulsion on inclined substrates (Nyakatura et al. 2008). In mouse lemurs (Microcebus murinus), forelimb grips differ between horizontal and vertical conditions, with a trend to ulnar deviation on vertical substrates, which was suggested to generate more powerful forelimb action and allow more secure grip on relatively thick vertical substrates (Reghem et al. 2012).
Considering gait selection, marmosets displayed different proportions of walks and bounds on horizontal and vertical substrates. Horizontal/vertical orientations are associated with distinct functional differentiation of fore- and hindlimbs (Hesse et al. 2015; Hanna et al. 2017), which may influence gait preference. Greater balance requirements on horizontal substrates (Schmitt 1999, 2003b; Young et al. 2016) could also explain the large predominance of walk gaits. While bounds allow bursts of speed with good absorption of impact by fore- and hindlimbs in small agile mammals (Hildebrand 1980), walk gaits allow higher stability since the body is always supported by two, three or four limbs at a time (Hildebrand 1980; Shimada et al. 2017). Concerning walk gaits, contrary to the primate-typical diagonal sequence, marmosets preferentially use a lateral sequence gait, which has been related to their unusual habitat of broad tree trunks and branches (Schmitt 2003a; Shimada et al. 2017).
Body Posture and β Oscillations at Rest
Examination of spectral power showed that, at rest, our animals displayed higher β power in the frontal motor cortex (areas 6Dc, 6M, and 4) on horizontal than vertical pole. A first explanation could be that of different body posture and muscular activity required to oppose gravity (Gwin et al. 2011; DiGiovanna et al. 2016). Regarding forelimb posture, previous work described modulation of β local field potentials at stable hold in macaque premotor and primary motor cortex (Spinks et al. 2008). Further experiments in humans indicate that body axis orientation and whole-body posture, namely the arrangement of body segments in space, can modulate oscillatory brain activity. Compared to lying horizontally, sitting down and standing upright increase β and γ frequency oscillations (Thibault et al. 2014; Spironelli and Angrilli 2017). For humans, supine compared to seated posture induces a redistribution of gravitational loads, moving blood toward the head and mimicking increased blood pressure. Horizontal lying position stimulates arterial and cardiopulmonary baroreceptors, leading to a decrease in sympathetic nervous system activity, which would result in cortical inhibition (Spironelli et al. 2016; Thibault and Raz 2016).
Generalizing the findings in humans to common marmosets must be tempered by possible cross-species differences in cardiovascular and sympathetic nervous responses to changes in body posture. Moreover, in our experiment, the effects of substrate orientation were clear in the β, but not in the γ band activity, where contradictory results were obtained across areas and monkeys. The specificity to β oscillations seems hardly compatible with cortical inhibition hypothesis in humans. An alternative would be a context-dependent hypothesis, whereby interactions between the current posture and the surrounding environment influence brain activity. For instance, human activities are mostly carried out in seated/upright posture while resting occurs in supine posture; thus, cortical activity would be inhibited in the latter (Thibault and Raz 2016). Marmosets are quadrupedal animals, and their locomotor and food foraging activity is performed on branches of various orientations. It was reported that, in callitrichid monkeys, locomotion preferentially occurs on horizontal and oblique branches (Chamove and Goldsborough 2004; Pines et al. 2005), while feeding on gum exudates involves vertical tree trunks (Youlatos 2009). Thus, a variety of orientations would correspond to active postures.
Finally, evidence in favor of tuning of neuronal discharge by body posture comes from a recent study exploring posterior parietal and frontal motor cortices in freely behaving rats (Mimica et al. 2018). Albeit it is difficult to conclude on the physiological mechanism in our experiment, ECoG recordings are particularly advantageous to further address this question, since this method offers spatially and temporally high-quality signal while allowing naturalistic experimental tasks.
β and γ Power Modulation Reflect Different Aspects of Locomotor Adjustments
Examination of spectral power over several recording areas showed that both tasks (horizontal and vertical), gaits (walk and bound) and epochs (preparation and execution) induced significant β ERD and γ ERS, which represent typical neural signatures of movement (Crone et al. 2006; Miller et al. 2007; Babiloni et al. 2016). Our results further showed that substrate orientation modulates γ ERS with higher values for vertical than horizontal task. This may reflect differences in cortical processing of limb biomechanics during the locomotion task (Hesse et al. 2015; Hanna et al. 2017). During human locomotion, γ power enhancement in the sensorimotor cortex is most pronounced when maximum muscle activity is required, namely, at the end of contralateral stance phase (Gwin et al. 2011). Besides, previous findings globally concur with the role of cortical oscillations, especially γ frequencies, in adjusting the motor command during dynamic force production (Omlor et al. 2007). Higher γ ERS on vertical substrates could reflect increased propulsive force necessary to work against gravity, as compared to horizontal substrates (Hesse et al. 2015; Hanna et al. 2017). In addition to task effects, epoch-related differences in γ ERS indicate that cortical activity closely reflects animals’ motor state, from movement programming to actual execution (Combrisson et al. 2017; Tia et al. 2017).
Contrary to γ ERS, results showed very scarce task- and epoch-related modulation of β ERD. β and γ oscillations could reflect distinct aspects of motor-related cortical processing. In the literature, β waves were reported to less closely reflect movement-related features than γ (Crone et al. 2006; Miller et al. 2007). Interestingly, we found β—but not γ—modulation by gait patterns, which somehow contrasts with these observations. β activity in the motor system is known to have a role in processing and prediction of rhythmic patterns, and β oscillatory networks over frontal and parietal regions are involved in adapting to changes in gait tempo (Wagner et al. 2016). Previous work on corticomuscular coherence supports the view that β band is involved in the synergistic control of different muscle groups; thus, its properties could vary depending on coordination required by the task (Reyes et al. 2017). Taken together, we assume that β oscillations may underpin cortical processing of movement sequences and γ oscillations more reflect actual limb biomechanics. However, we cannot exclude the possibility that our analyses failed to detect gait-related γ modulation due to small dataset, since the bound gait occurred only in the vertical task condition. Future investigation with larger datasets, including various gaits on horizontal substrate and ground, will help to shed more light on gait-related differences in cortical activity.
Methodological Considerations and Limitations
One may wonder whether muscle activity that occurs during locomotor tasks could be mixed into the ECoG signals. In contrast to scalp electroencephalography (EEG), muscle activity contamination into ECoG signals should rarely happen. First, the signal-to-noise ratio is better for ECoG than EEG because the signals are recorded close to cortical sources (Darvas et al. 2010; McCrimmon et al. 2018). Second, for our ECoG recording, reference electrodes were located intracranially and close to the recording electrodes. This electrode montage helps to cancel out signals equally put into two channels, such as electromyographic activity. Finally, during signal preprocessing, we removed segments with large amplitude artifacts and applied ICA to remove components reflecting residual artifacts (Mognon et al. 2011; Rogasch et al. 2014).
The current study did not focus on cortical activation patterns of full gait cycles. Instead, we assessed β and γ oscillations during preparation (500-ms interval before the movement onset) and the first 500 ms of execution epochs. One technical reason is the recording setup that could not provide ECoG signals of entire trials due to motion noise from naturalistic locomotion. The wireless headstage provides robust signals when marmosets are at rest. However, if the headstage touches objects (e.g., the surrounding cage) or the animals move their head too quickly, it will cause saturation of the signals and take hundreds of milliseconds to return to baseline. The other physiological reason is that gait initiation involves cortical processes but locomotion is mostly driven by central pattern generators, where motor cortex has less contribution (Kiehn 2016).
The last drawback of this study is that we could not dissociate activities between fore- and hindlimb regions of the motor cortex, which will motivate future work. We would expect cortical activity to reflect distinct biomechanical contributions of fore- and hindlimbs depending on substrate orientation (Hesse et al. 2015; Hanna et al. 2017).
Concluding Remarks
In this work, we investigated features of frontal motor cortex oscillations associated with locomotor adjustments to substrate orientation in common marmosets. The novelty of our approach lies in the naturalistic conditions of the task which involves prehensile movements, whereas standard protocols generally involve stepping movements. Arboreal locomotion requires coordinated interlimb movement sequence as well as controlled limb kinematics, grasping posture and force production to provide an efficient hold onto the substrate (Reghem et al. 2012; Hesse et al. 2015). In our results, behavioral adjustments to substrate orientation were visible through changes in preferred forelimb posture and gait. Neurophysiological investigation supports the view that β and γ oscillations in frontal motor cortex fulfill partly different roles during locomotion (see also in human, Seeber et al. 2015). Modulation of γ ERS by substrate orientation and epoch potentially reflects close relation to the temporal dynamics of movement and highlights the role of γ waves in adjusting the motor command to sensory information (Crone et al. 2006; Omlor et al. 2007). By comparison, changes in β ERD with gait could depict a role in the synergistic control of different muscle groups (Reyes et al. 2017) to achieve interlimb coordination. We do not exclude a possible similar role of γ waves, which could be addressed by future experiments. During static posture, we essentially observed that substrate orientation modulated β power. Earlier studies described synchrony of β waves with motor unit activity, as well as close links between β oscillations and tonic muscle contraction (Kilavik et al. 2013). We proposed a second possible hypothesis which is that β modulation could be driven by interactions between the body posture and surrounding environment (see also Thibault and Raz 2016); yet, deeper investigation is needed to clarify this question.
By providing the first description of cortical mechanisms involved in marmoset locomotion, this work constitutes a basis for future studies on locomotor impairments resulting from neurological diseases, injuries or aging, and possible therapeutic/rehabilitative treatments (Bowes et al. 2013; Takemi et al. 2014). Our results draw attention to cortical processing of body posture, biomechanical features and movement sequences during locomotion, which are crucial aspects when evaluating injuries/treatments and generalizing to other species. Further insights into neurophysiological mechanisms of locomotion could be gained by studying coordination between cortical areas and subcortical structures, as well as examining brain activity at single-cell level. It is also important to consider differences in muscle architectural properties between human and animal models, since they can affect biomechanics and neuronal control of movement (Ogihara et al. 2017).
Marmosets hold a specific niche as a simian primate relying mostly on indirect pathways from the motor cortex to spinal motoneurons (Lemon and Griffiths 2005; Kondo et al. 2015; Walker et al. 2016). The evolution of prehensile hands has been related to arboreal locomotion, visually guided predation and fruit/flower foraging (Almécija and Sherwood 2017). However, arboreal locomotion does not necessarily require high levels of digital dexterity and, to our knowledge, no link has been established between the degree of arboreality and corticospinal anatomy (Iwaniuk et al. 1999). That being said, we can presume that corticospinal anatomy should impact the coordination of cortical and spinal processes for locomotor control. It is still to be determined to what extent locomotor functions, such as arboreality and preferences for branch sizes, were associated with certain neurophysiological mechanisms for gait control.
Supplementary Material
Notes
We thank Dr Miki Taoka for helpful advice on the histological study and Dr Alberto Ansaldo, Dr Yumiko Yamazaki, Mr Masakado Saiki, Mr Masayuki Inada, Mr Taku Koike, and Mr Takafumi Nakamura for their technical assistance. Conflicts of Interest: All authors declare no competing financial interests.
Funding
The Japan Agency for Medical Research and Development (AMED, to A.I., Brain/MINDS project); JST PRESTO (to M.T. #JPMJPR18J6); and the Italian Ministry of Education, Universities and Research (to L.F., PRIN 2015, in collaboration with the Robotics Brain and Cognitive Sciences Department of the Italian Institute of Technology).
References
- Almécija S, Sherwood C. 2017. Hands, brains, and precision grips: Origins of tool use behaviors In: Kaas J, editor. Evolution of Nervous Systems. 2nd ed. Oxford: Academic Press, pp. 299–315. [Google Scholar]
- Babiloni C, Del Percio C, Vecchio F, Sebastiano F, Di Gennaro G, Quarato PP, Morace R, Pavone L, Soricelli A, Noce G et al.. 2016. Alpha, beta and gamma electrocorticographic rhythms in somatosensory, motor, premotor and prefrontal cortical areas differ in movement execution and observation in humans. Clin Neurophysiol. 127:641–654. [DOI] [PubMed] [Google Scholar]
- Bowes C, Burish M, Cerkevich C, Kaas J. 2013. Patterns of cortical reorganization in the adult marmoset after a cervical spinal cord injury. J Comp Neurol. 521:3451–3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijn SM, Van Dieën JH, Daffertshofer A. 2015. Beta activity in the premotor cortex is increased during stabilized as compared to normal walking. Front Hum Neurosci. 9:593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burman KJ, Bakola S, Richardson KE, Yu HH, Reser DH, Rosa MG. 2015. Cortical and thalamic projections to cytoarchitectural areas 6Va and 8C of the marmoset monkey: connectionally distinct subdivisions of the lateral premotor cortex. J Comp Neurol. 523:1222–1247. [DOI] [PubMed] [Google Scholar]
- Burman KJ, Palmer SM, Gamberini M, Rosa MG. 2006. Cytoarchitectonic subdivisions of the dorsolateral frontal cortex of the marmoset monkey (Callithrix jacchus), and their projections to dorsal visual areas. J Comp Neurol. 495:149–172. [DOI] [PubMed] [Google Scholar]
- Burman KJ, Palmer SM, Gamberini M, Spitzer MW, Rosa MG. 2008. Anatomical and physiological definition of the motor cortex of the marmoset monkey. J Comp Neurol. 506:860–876. [DOI] [PubMed] [Google Scholar]
- Castagnola E, Ansaldo A, Maggiolini E, Angotzi GN, Skrap M, Ricci D, Fadiga L. 2013. Biologically compatible neural interface to safely couple nanocoated electrodes to the surface of the brain. ACS Nano. 7:3887–3895. [DOI] [PubMed] [Google Scholar]
- Castagnola E, Ansaldo A, Maggiolini E, Ius T, Skrap M, Ricci D, Fadiga L. 2014. Smaller, softer, lower-impedance electrodes for human neuroprosthesis: a pragmatic approach. Front Neuroeng. 7:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamove AS, Goldsborough S. 2004. Callitrichid monkey branch preference. Lab Primate News. 43:1–6. [Google Scholar]
- Combrisson E, Perrone-Bertolotti M, Soto JL, Alamian G, Kahane P, Lachaux JP, Guillot A, Jerbi K. 2017. From intentions to actions: neural oscillations encode motor processes through phase, amplitude and phase-amplitude coupling. Neuroimage. 147:473–487. [DOI] [PubMed] [Google Scholar]
- Crone NE, Sinai A, Korzeniewska A. 2006. High-frequency gamma oscillations and human brain mapping with electrocorticography. Prog Brain Res. 159:275–295. [DOI] [PubMed] [Google Scholar]
- Darvas F, Scherer R, Ojemann JG, Rao R, Miller KJ, Sorensen LB. 2010. High gamma mapping using EEG. Neuroimage. 49:930–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davare M, Andres M, Cosnard G, Thonnard JL, Olivier E. 2006. Dissociating the role of ventral and dorsal premotor cortex in precision grasping. J Neurosci. 26:2260–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davare M, Kraskov A, Rothwell JC, Lemon RN. 2011. Interactions between areas of the cortical grasping network. Curr Opin Neurobiol. 21:565–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiGiovanna J, Dominici N, Friedli L, Rigosa J, Duis S, Kreider J, Beauparlant J, Brand R, Schieppati M, Micera S, et al.. 2016. Engagement of the rat hindlimb motor cortex across natural locomotor behaviors. J Neurosci. 36:10440–10455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew T, Marigold DS. 2015. Taking the next step: cortical contributions to the control of locomotion. Curr Opin Neurobiol. 33:25–33. [DOI] [PubMed] [Google Scholar]
- Foster JD, Nuyujukian P, Freifeld O, Gao H, Walker R, S IR, T HM, Murmann B, M JB, Shenoy KV. 2014. A freely-moving monkey treadmill model. J Neural Eng. 11:046020. [DOI] [PubMed] [Google Scholar]
- Gwin JT, Gramann K, Makeig S, Ferris DP. 2011. Electrocortical activity is coupled to gait cycle phase during treadmill walking. Neuroimage. 54:1289–1296. [DOI] [PubMed] [Google Scholar]
- Hanna JB, Granatosky MC, Rana P, Schmitt D. 2017. The evolution of vertical climbing in primates: evidence from reaction forces. J Exp Biol. 220:3039–3052. [DOI] [PubMed] [Google Scholar]
- Hashikawa T, Nakatomi R, Iriki A. 2015. Current models of the marmoset brain. Neurosci Res. 93:116–127. [DOI] [PubMed] [Google Scholar]
- Hesse B, Nyakatura JA, Fischer MS, Schmidt M. 2015. Adjustments of limb mechanics in cotton-top tamarins to moderate and steep support orientations: significance for the understanding of early primate evolution. J Mamm Evol. 22:435–450. [Google Scholar]
- Hildebrand M. 1980. The adaptive significance of tetrapod gait selection. Am Zool. 20:255–267. [Google Scholar]
- Hunt KD, Cant JG, Gebo DL, Rose MD, Walker SE, Youlatos D. 1996. Standardized descriptions of primate locomotor and postural modes. Primates. 37:363–387. [Google Scholar]
- Iwaniuk AN, Pellis SM, Whishaw IQ. 1999. Is digital dexterity really related to corticospinal projections?: a re-analysis of the Heffner and Masterton data set using modern comparative statistics. Behav Brain Res. 101:173–187. [DOI] [PubMed] [Google Scholar]
- Kiehn O. 2016. Decoding the organization of spinal circuits that control locomotion. Nat Rev Neurosci. 17:224–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilavik BE, Zaepffel M, Brovelli A, MacKay WA, Riehle A. 2013. The ups and downs of beta oscillations in sensorimotor cortex. Exp Neurol. 245:15–26. [DOI] [PubMed] [Google Scholar]
- Koenraadt KL, Roelofsen EG, Duysens J, Keijsers NL. 2014. Cortical control of normal gait and precision stepping: an fNIRS study. Neuroimage. 85(Pt 1):415–422. [DOI] [PubMed] [Google Scholar]
- Kondo T, Yoshihara Y, Yoshino-Saito K, Sekiguchi T, Kosugi A, Miyazaki Y, Nishimura Y, Okano HJ, Nakamura M, Okano H. 2015. Histological and electrophysiological analysis of the corticospinal pathway to forelimb motoneurons in common marmosets. Neurosci Res. 98:35–44. [DOI] [PubMed] [Google Scholar]
- Kosugi A, Takemi M, Tia B, Castagnola E, Ansaldo A, Sato K, Awiszus F, Seki K, Ricci D, Fadiga L, et al.. 2018. Accurate motor mapping in awake common marmosets using micro-electrocorticographical stimulation and stochastic threshold estimation. J Neural Eng. 15:036019. [DOI] [PubMed] [Google Scholar]
- Krubitzer LA, Kaas JH. 1990. The organization and connections of somatosensory cortex in marmosets. J Neurosci. 10:952–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon RN, Griffiths J. 2005. Comparing the function of the corticospinal system in different species: organizational differences for motor specialization? Muscle Nerve. 32:261–279. [DOI] [PubMed] [Google Scholar]
- McCrimmon CM, Wang PT, Heydari P, Nguyen A, Shaw SJ, Gong H, Chui LA, Liu CY, Nenadic Z, Do AH. 2018. Electrocorticographic encoding of human gait in the leg primary motor cortex. Cereb Cortex. 28:2752–2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KJ, Leuthardt EC, Schalk G, Rao RP, Anderson NR, Moran DW, Miller JW, Ojemann JG. 2007. Spectral changes in cortical surface potentials during motor movement. J Neurosci. 27:2424–2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimica B, Dunn BA, Tombaz T, Bojja VS, Whitlock JR. 2018. Efficient cortical coding of 3D posture in freely behaving rats. Science. 362:584–589. [DOI] [PubMed] [Google Scholar]
- Mognon A, Jovicich J, Bruzzone L, Buiatti M. 2011. ADJUST: an automatic EEG artifact detector based on the joint use of spatial and temporal features. Psychophysiology. 48:229–240. [DOI] [PubMed] [Google Scholar]
- Nyakatura JA, Fischer MS, Schmidt M. 2008. Gait parameter adjustments of cotton-top tamarins (Saguinus oedipus, Callitrichidae) to locomotion on inclined arboreal substrates. Am J Phys Anthropol. 135:13–26. [DOI] [PubMed] [Google Scholar]
- Ogihara N, Oishi M, Kanai R, Shimada H, Kondo T, Yoshino-Saito K, Ushiba J, Okano H. 2017. Muscle architectural properties in the common marmoset (Callithrix jacchus). Primates. 58:461–472. [DOI] [PubMed] [Google Scholar]
- Omlor W, Patino L, Hepp-Reymond MC, Kristeva R. 2007. Gamma-range corticomuscular coherence during dynamic force output. Neuroimage. 34:1191–1198. [DOI] [PubMed] [Google Scholar]
- Oostenveld R, Fries P, Maris E, Schoffelen J-M. 2011. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pines M, Kaplan G, Rogers L. 2005. Use of horizontal and vertical climbing structures by captive common marmosets (Callithrix jacchus). Appl Anim Behav Sci. 91:311–319. [Google Scholar]
- Raos V, Umilta MA, Gallese V, Fogassi L. 2004. Functional properties of grasping-related neurons in the dorsal premotor area F2 of the macaque monkey. J Neurophysiol. 92:1990–2002. [DOI] [PubMed] [Google Scholar]
- Ray S, Crone NE, Niebur E, Franaszczuk PJ, Hsiao SS. 2008. Neural correlates of high-gamma oscillations (60–200 Hz) in macaque local field potentials and their potential implications in electrocorticography. J Neurosci. 28:11526–11536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reghem E, Byron C, Poudebat E. 2012. Hand posture of Microcebus murinus during arboreal locomotion. J Zool. 288:76–81. [Google Scholar]
- Reyes A, Laine CM, Kutch JJ, Valero-Cuevas FJ. 2017. Beta band corticomuscular drive reflects muscle coordination strategies. Front Comput Neurosci. 11:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogasch NC, Thomson RH, Farzan F, Fitzgibbon BM, Bailey NW, Hernandez-Pavon JC, Daskalakis ZJ, Fitzgerald PB. 2014. Removing artefacts from TMS-EEG recordings using independent component analysis: importance for assessing prefrontal and motor cortex network properties. Neuroimage. 101:425–439. [DOI] [PubMed] [Google Scholar]
- Rossignol S, Frigon A. 2011. Recovery of locomotion after spinal cord injury: some facts and mechanisms. Annu Rev Neurosci. 34:413–440. [DOI] [PubMed] [Google Scholar]
- Sahyoun C, Floyer-Lea A, Johansen-Berg H, Matthews PM. 2004. Towards an understanding of gait control: brain activation during the anticipation, preparation and execution of foot movements. Neuroimage. 21:568–575. [DOI] [PubMed] [Google Scholar]
- Schmitt D. 1999. Compliant walking in primates. J Zool. 248:149–160. [Google Scholar]
- Schmitt D. 2003a. Evolutionary implications of the unusual walking mechanics of the common marmoset (C. jacchus). Am J Phys Anthropol. 122:28–37. [DOI] [PubMed] [Google Scholar]
- Schmitt D. 2003b. Substrate size and primate forelimb mechanics: implications for understanding the evolution of primate locomotion. Int J Primatol. 24:1023–1036. [Google Scholar]
- Schmitt D, Lemelin P. 2002. Origins of primate locomotion: gait mechanics of the woolly opossum. Am J Phys Anthropol. 118:231–238. [DOI] [PubMed] [Google Scholar]
- Seeber M, Scherer R, Wagner J, Solis-Escalante T, Müller-Putz GR. 2015. High and low gamma EEG oscillations in central sensorimotor areas are conversely modulated during the human gait cycle. Neuroimage. 112:318–326. [DOI] [PubMed] [Google Scholar]
- Shapiro LJ, Kemp AD, Young JW. 2016. Effects of substrate size and orientation on Quadrupedal gait kinematics in mouse lemurs (Microcebus murinus). J Exp Zool A Ecol Genet Physiol. 325:329–343. [DOI] [PubMed] [Google Scholar]
- Shimada H, Kanai R, Kondo T, Yoshino-Saito K, Uchida A, Nakamura M, Ushiba J, Okano H, Ogihara N. 2017. Three-dimensional kinematic and kinetic analysis of quadrupedal walking in the common marmoset (Callithrix jacchus). Neurosci Res. 125:11–20. [DOI] [PubMed] [Google Scholar]
- Shimazu H, Maier MA, Cerri G, Kirkwood PA, Lemon RN. 2004. Macaque ventral premotor cortex exerts powerful facilitation of motor cortex outputs to upper limb motoneurons. J Neurosci. 24:1200–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soubeyrand M, Assabah B, Bégin M, Laemmel E, Dos Santos A, Crézé M. 2017. Pronation and supination of the hand: anatomy and biomechanics. Hand Surg Rehabil. 36:2–11. [DOI] [PubMed] [Google Scholar]
- Spinks RL, Kraskov A, Brochier T, Umilta MA, Lemon RN. 2008. Selectivity for grasp in local field potential and single neuron activity recorded simultaneously from M1 and F5 in the awake macaque monkey. J Neurosci. 28:10961–10971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spironelli C, Angrilli A. 2017. Posture used in fMRI-PET elicits reduced cortical activity and altered hemispheric asymmetry with respect to sitting position: an EEG resting state study. Front Hum Neurosci. 11:621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spironelli C, Busenello J, Angrilli A. 2016. Supine posture inhibits cortical activity: Evidence from Delta and alpha EEG bands. Neuropsychologia. 89:125–131. [DOI] [PubMed] [Google Scholar]
- Sustaita D, Pouydebat E, Manzano A, Abdala V, Hertel F, Herrel A. 2013. Getting a grip on tetrapod grasping: form, function, and evolution. Biol Rev Camb Philos Soc. 88:380–405. [DOI] [PubMed] [Google Scholar]
- Takakusaki K. 2013. Neurophysiology of gait: from the spinal cord to the frontal lobe. Mov Disord. 28:1483–1491. [DOI] [PubMed] [Google Scholar]
- Takemi M, Kondo T, Yoshino-Saito K, Sekiguchi T, Kosugi A, Kasuga S, Okano HJ, Okano H, Ushiba J. 2014. Three-dimensional motion analysis of arm-reaching movements in healthy and hemispinalized common marmosets. Behav Brain Res. 275:259–268. [DOI] [PubMed] [Google Scholar]
- Thibault RT, Lifshitz M, Jones JM, Raz A. 2014. Posture alters human resting-state. Cortex. 58:199–205. [DOI] [PubMed] [Google Scholar]
- Thibault RT, Raz A. 2016. Imaging posture veils neural signals. Front Hum Neurosci. 10:520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tia B, Takemi M, Kosugi A, Castagnola E, Ansaldo A, Nakamura T, Ricci D, Ushiba J, Fadiga L, Iriki A. 2017. Cortical control of object-specific grasp relies on adjustments of both activity and effective connectivity: a common marmoset study. J Physiol (Lond). 595:7203–7221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner J, Makeig S, Gola M, Neuper C, Müller-Putz G. 2016. Distinct β band oscillatory networks subserving motor and cognitive control during gait adaptation. J Neurosci. 36:2212–2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J, MacLean J, Hatsopoulos NG. 2016. The marmoset as a model system for studying voluntary motor control. Dev Neurobiol. 1–13. [DOI] [PubMed] [Google Scholar]
- Youlatos D. 2009. Locomotion, postures, and habitat use by pygmy marmosets Cebuella pygmaea In: Ford S, Porter L, Davis L, editors. The smallest anthropoids. Boston, MA: Springer, pp. 279–297. [Google Scholar]
- Young JW, Stricklen BM, Chadwell BA. 2016. Effects of support diameter and compliance on common marmoset (Callithrix jacchus) gait kinematics. J Exp Biol. 219:2659–2672. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


