Abstract
Several subsets of regulatory CD4+ T cells (CD4+ Tregs) have been described in peripheral blood and tumor microenvironment of breast cancer (BC) patients and may play a role in the progression of BC. High-risk human papilloma virus (HR-HPV) has a causal role in cervical, head, and neck tumors but the role of HR-HPV in evoking neoplasia in BC is still unclear. In this study we assessed the prevalence of CD4+CD25+ FOXP3+ regulatory T cells (CD4+Tregs) and CD3+ CD8+ T cells by flow cytometry in peripheral blood from a total of 55 Egyptian women, including 20 treatment-naïve BC, 15 with breast benign lesions (BBL), and 20 healthy volunteers (HV). HR-HPV genotypes type 16, 18, and 31 were investigated in breast tissue from all BC and BBL patients using Real-Time PCR. HR-HPV was detected in 4/20 (20%) and 0/15 (0%) BC and BBL patients respectively. The frequency of CD4+ Tregs was significantly higher in BC compared to BBL and HV, (P < 0.001). In addition, we observed a significantly higher frequency of CD3+ CD8+ T cells in peripheral blood of patients with late stage III BC compared to early stage I and II BC (P = 0.011). However, there was no significant association between the ratio of CD8+ T cell to CD4+ Tregs frequencies and the expression of Estrogen Receptor (ER), Progesterone Receptor (PR), and Human Epidermal Growth Factor Receptor 2 (HER2). These results lead us to postulate that the association between the frequency of CD4+ Tregs and CD8+ T cells in the peripheral blood may be a prognostic or predictive parameter in Egyptian women with BC. In addition, HR-HPV infection may be implicated in the development of some types of BC in Egyptian women.
Keywords: breast cancer, HPV, Treg
Introduction
Breast cancer (BC) is the second-leading cause of death from cancer in Egyptian women. The overall incidence of cancer is 157.0 per 100,000 Egyptian females with the highest incidence being BC (32%) and a three-fold increase in incidence of cancer is predicted by 2050.1 While the etiology of BC is still unknown, numerous risk factors have been identified. Increased public awareness and regular screening can play a vital role in the prevention, early detection, and treatment of BC.2 Virus infections including human parvovirus (B19), Epstein Barr Virus (EBV), and high-risk human papilloma virus (HR-HPV) have a causal role in some thyroid and cervical tumors.3–6 In addition, HR-HPV infections are associated with many oropharyngeal cancers.7 However, the role of HR-HPV in evoking neoplasia in BC is still unclear.8,9
Human papilloma virus is a small, circular, double-stranded DNA virus that includes more than 120 known subtypes.10 HR-HPV genotypes 16, 18, and 31 encode a series of early (E1 − E7) and late (L1 and L2) proteins. HR-HPV E6 and E7 early proteins are oncoproteins that stimulate cell cycle progression by inhibiting tumor suppressor genes p53 and p110 RB resulting in cellular transformation and cancer.11,12 In addition, HR-HPV E5 and E6 early proteins are known to disrupt cytokeratin causing perinuclear cytoplasmic clearing and nuclear enlargement.13
The expression of hormone receptors such as estrogen receptor (ER) and progesterone receptor (PR) in BC predicts the response to growth factors, patient prognosis, and response to targeted therapy.14 Patients positive for these receptors tend to have improved prognosis and better response to hormonal therapy.15 In contrast, BC patients expressing Human Epidermal Growth Factor Receptor 2 (HER2) have increased disease recurrence and poor prognosis.16 Triple negative breast cancer is a unique subtype constituting about 20% of breast cancer cases is characterized by the lack of ER, PR, and HER2 expression and poor clinical outcome.17,18 Metaplastic carcinoma is an aggressive type of BC characterized by low hormone receptor expression and poor prognosis.19
Several subsets of CD4+CD25+ FOXP3+ regulatory T cells (CD4+Tregs) have been described in peripheral blood and tumor microenvironment from BC patients with invasive high-grade breast carcinomas, and may play a role in the progression of breast cancer.20–24 CD4+ Tregs express high levels of FOX P3, CD25, Glucocorticoid-induced TNFR family related protein (GITR), Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), and CD103.25–27 In addition, decreased expression of CD127 on human CD4+ Tregs is inversely correlated with FoxP3 expression.28,29 “Naturally” occurring CD4+ Tregs are derived in the thymus, while “Adaptive” CD4+ Tregs are believed to come from mature T cells in the periphery under specific conditions of persistent antigenic stimulation.30 CD4+Tregs exert their non-antigen specific inhibitory effects on T cell proliferation and cytokine production through a cell-cell contact-dependent mechanism.31,32 These cells have also been shown to inhibit the cytotoxic function and decrease the expression of major histocompatibility class I molecules on target cells. CD4+ Tregs were shown to secrete immunosuppressive cytokines interleukin-10 and Transforming Growth Factor-β (TGF-β) that suppress the induction of antitumor immunity.33,34
The relationship between the severity of BC and the frequency of CD4+ Tregs in the peripheral blood is not well defined. Quantification of CD4+ Tregs may be valuable for assessing BC progression and as an important therapeutic target. It has been reported that the number of CD4+ Tregs was significantly higher in patients with invasive high-grade BC and may correlate with both shorter relapse-free and overall survival time.22,35,36 These findings suggest that increased frequency of CD4+ Tregs in BC patients may play a key role in the prognosis of BC.37,38 However, the frequency of CD4+ Tregs did not directly correlate with the clinical stage of BC in other studies.39 The aim of this study was to assess the frequency of CD4+ regulatory T cells, CD8+ T cells, and human papilloma virus infection in Egyptian Women with breast cancer compared to women with benign breast lesions (BBL) and healthy volunteers (HV).
Material and methods
Population and source of samples
All women over the age of 18 visiting the oncology clinic at Al-Azhar university hospital between February 2018 and March 2019 were invited to volunteer in the study. Inclusion criteria included all new treatment naïve cases of histologically confirmed BC at any stage. Women that were diagnosed with benign breast lesion, as well as women residing in the same areas and admitted to the hospital, for a wide spectrum of acute, non-neoplastic conditions unrelated to known or likely risk factors for BC were invited to volunteer as controls in the study. Exclusion criteria included Patients with systemic therapy prior to surgery, bilateral BC, metastatic or recurrent disease, and cancer of other origin. Peripheral blood and frozen breast tissue samples were collected according to the ethical regulations at Al-Azhar university hospital. Breast tissue samples were not collected from healthy volunteers. Demographic and clinical information was obtained from medical records. The study received approval from the Institutional Review Board at Al-Azhar University and each volunteer gave informed written consent for participation in the study. Specimens were collected from 20 treatment-naïve women with primary BC, 15 benign breast lesions (BBL), and 20 healthy volunteers (HV) after providing informed written consent. To avoid a potential influence of major surgical procedures, as well as of neoadjuvant treatment, blood was drawn after core biopsy but before definitive surgery.
Clinicopathological risk factors were assessed for age, lymph node metastasis, tumor size, histologic grade, tumor stage, and hormonal receptor status. BC is regarded to be hormonal receptor positive if at least 1% stained positive for ER or PR assays as previously described.40 Expression of ER, PR, and HER2 receptors was assessed by immunohistochemistry staining performed in Al-Azhar University hospital clinical laboratory as previously described.41 Test results were obtained from the patient medical records. Tumor stages were classified according to the American Joint Committee on Cancer (AJCC)-TNM (tumor, node, metastases) classification.
Detection and genotyping of HR-HPV by RT-PCR
DNA was extracted using the genomic DNA extraction kit for tissues (Invitrogen), following the manufacturer’s instructions. The quality of the isolated DNA was checked by polymerase chain reaction (PCR) of control genes with primers generating fragments of 200, 400, and 600 bp as previously described.42 Specimens were frozen at −70°C for molecular analysis. Three HR-HPV types 16, 18, and 31 were assessed by RT-PCR using the genesis® Advanced Kit specific for each type (Primedesign Ltd, UK) following manufacture protocol. Briefly, genomic DNA is amplified using primers specific to the early E6 gene for types 16, 18, and 31. The β-Actin gene was used as an internal control. The PCR products were analyzed by the “One step Plus™ Real Time PCR System (Applied Biosystem). Several measures were undertaken to avoid DNA contamination, including the use of distinct areas for sample preparation, PCR setup, post-PCR analysis, and equipment. In addition, we used designated sets of pipettes and pipette tips with aerosol filters, lab coats, glove boxes, and waste baskets for the pre-PCR and post-PCR areas.
Immunophenotyping of CD4+ Tregs and CD8+ T cells
Blood samples from patients with primary BC, BBL, and HV were procured from treatment-naïve patients prior to any surgery to avoid a potential influence of major surgical procedures and neoadjuvant treatment. Flow Cytometry testing was performed in a single laboratory using fresh blood samples on the same day of collection as previously described.43,44. Two ml peripheral blood sample was collected in EDTA tubes. About 100 µl of whole blood was incubated for 15 min at room temperature with combinations of monoclonal antibodies against CD3 PerCP, CD4 FITC, CD8 PE, CD25 APC, and intracellular transcription factor FOXP3 PE, using immobilization buffer (BD, Biosciences, San Jose, USA). Red cell lysis was performed with FACS Lysing Solution (BD Biosciences). Isotype controls, IgG1 FITC, IgG2 PE, IgG1 PerCP, and IgG1 APC were used for detection of non-specific binding background (BD Biosciences). Compensation settings were established before sample acquisition using color calibrate beads. A minimum of 30,000 CD3+ T cells per sample was acquired using a 4 color FACSCalibur (Beckton Dickenson (BD), USA) and analysis was performed by Cell-Quest Pro software (BD, USA). Gating was performed using the fluorescence-minus-one (FMO) control for each marker. Results were expressed as percent of CD25+ FOXP3+ CD4+ Tregs from the CD4+ lymphocyte gate or CD3+ CD8+ T cells from the lymphocyte gate after subtraction of the isotype control background values (<0.1%).
Statistical analysis
Statistical analysis was done using IBM SPSS® Statistics version 23 (IBM® Corp., Armonk, NY, USA). Numerical data were expressed as mean and standard deviation or median and range as appropriate. Qualitative data were expressed as frequency and percentage. For not normally distributed quantitative data, comparison between two groups was done using Mann-Whitney test (non-parametric t-test). All tests were two-tailed. When more than two groups of data sets were compared, one-way analysis of variance (ANOVA) was performed as described. A P-value <0.05 was considered significant.
Results
Patient demographics and disease characteristics
The mean age of patients with BC, BBL, and HV was 50.6 ±11.7, 55.0 ±13.7, 64.3 ±9.9 respectively (Table 1). There was no significant difference in age between patients with BC compared to BBL (P = 0.188). HR-HPV types 16, 18, and 31 were assessed because they are very common and may play a causal role in evoking neoplasia.3,4,6,45 HR-HPV was detected in breast tissue from 4/20 BC patients compared to 0/15 of BBL patients. The prevalence of HR-HPV genotypes was 3/4 (75%), 1/4 (25%), and 0/4 (0%) for genotypes 16, 18, and 31, respectively. None of the patients were infected with more than 1 HR-HPV genotype.
Table 1.
Patient demographics and disease characteristics.
| BC (n = 20) | BBL (n = 15) | HV (n = 20) | P | |
|---|---|---|---|---|
| Age in years (mean ± SD) | 50.6 ± 11.7 | 55.0 ± 13.7 | 64.3 ± 9.9 | 0.18 |
| HPV | ||||
| Positive | 4 | 0 | 0 | ND |
| Negative | 16 | 15 | 20 | |
| Percent lymphocyte subsets | ||||
| CD4+ Tregs (mean ± SD) | 4.39 ± 1.31 | 1.73 ±.74 | 1.73 ± .63 | 0.001 |
| CD8+ (mean ± SD) | 22.51 ± 5.51 | 23.93 ± 6.30 | 23.95 ± 8.08 | 0.8 |
| CD4+ (mean ± SD) | 43.16 ± 8.05 | 43.16 ± 7.39 | 40.17 ± 7.68 | 0.35 |
| Histology type, n (%) | ||||
| Carcinoma in situ | 1 (5.3%) | |||
| Invasive duct carcinoma | 14 (73.7%) | |||
| Invasive mammary carcinoma | 1 (5.3%) | |||
| Metaplastic carcinoma | 3 (15.8%) | |||
| Benign mammary lesion | 4 (33.3%) | |||
| Fibroadenoma | 3 (25.0%) | |||
| Fibrocystic changes | 1 (8.3%) | |||
| Benign ductal epithelial cells | 1 (8.3%) | |||
| Benign mastitis | 1 (8.3%) | |||
| Benign unspecified | 2 (16.7%) | |||
| Unknown | 1 | 3 | ||
| Tumor size | ||||
| <2 cm | 2 (10.5%) | 1 (11.1%) | ||
| 2−5 cm | 12 (63.2%) | 3 (33.3%) | 0.31 | |
| >5 cm | 5 (26.3%) | 5 (55.6%) | ||
| Unknown | 1 | 3 | ||
ND: not done because the group is <5; BBL: breast benign lesions; HV: healthy volunteers; SD: standard deviation.
P < 0.05 is significant.
Metaplastic carcinoma and invasive duct carcinoma were found in 3/4 (75%) and 1/4 HR-HPV infected patients respectively compared to 0/15 and 13/15 HPV negative BBL patients respectively. These results suggest that HR-HPV infection may be associated with more aggressive BC in Egyptian women. Ten of 20 patients with BC (50%) were diagnosed with early stage disease (stage I and II) while 6/20 (30%) were diagnosed with advanced disease (stage III).
The number of BC patients expressing ER, PR, or HER2 was 13/20 (65%), 10/20 (50%), and 4/20 (20%) respectively (Table 2). While 3/20 (15%) BC patients lacked expression of all three hormonal receptors (triple negative). All four HR-HPV positive BC patients expressed ER and PR receptors while only 1/4 HR-HPV positive BC patient expressed all three hormonal receptors (Table 3).
Table 2.
Frequency of CD4+ Tregs and CD8+ T cells in BC patients according to age, HPV, and tumor characteristics.
| Item | n (%) | CD4+ T regs (mean ± SD) | P | CD8+ T cells (mean ± SD) | P | |
|---|---|---|---|---|---|---|
| 1. | Age in years | 0.12 | 0.77 | |||
| <50 | 7 (35%) | 3.77 ± 1.08 | 21.95 ± 6.42 | |||
| >50 | 8 (40%) | 4.76 ± 1.62 | 20.40 ± 4.13 | |||
| Unknown | 5 (25%) | |||||
| 2. | HPV | ND | ND | |||
| Positive | 4 (20%) | 4.23 ± 1.10 | 22.57 ± 8.88 | |||
| Negative | 16 (80%) | 4.43 ± 1.39 | 22.49 ± 4.77 | |||
| 3. | Grade | ND | ND | |||
| G1 & G2 | 16 (80%) | 4.17 ± 1.35 | 22.08 ± 6.05 | |||
| G3 | 3 (15%) | 5.04 ± .71 | 23.74 ± 2.33 | |||
| Unknown | 1 (5%) | |||||
| 4. | Pathology | 0.68 | 0.5 | |||
| IDC | 14 (70%) | 4.37 ± 1.40 | 22.60 ± 4.91 | |||
| Others | 5 (25%) | 4.14±1.10 | 21.63 ± 7.91 | |||
| Unknown | 1 (5%) | |||||
| 5. | LN metastasis | 0.19 | 0.1 | |||
| Positive | 8 (40%) | 4.12 ± 1.57 | 19.74 ± 3.98 | |||
| Negative | 8 (40%) | 4.77 ± .76 | 24.87 ± 7.06 | |||
| Unknown | 4 (20%) | |||||
| 6. | Stage | 0.79 | 0.01 | |||
| Early (I & II) | 10 (50%) | 4.52 ± 1.41 | 19.22 ± 4.33 | |||
| Advanced (III) | 6 (30%) | 4.32 ± .97 | 27.44 ± 5.35 | |||
| Unknown | 4 (20%) | |||||
| 7. | ER | ND | ND | |||
| Positive | 13 (65%) | 4.46 ± 1.40 | 22.85 ± 6.17 | |||
| Negative | 4 (20%) | 3.68 ± 1.24 | 20.57 ± 5.54 | |||
| Unknown | 3 (15%) | |||||
| 8. | PR | 0.16 | 0.19 | |||
| Positive | 10 (50%) | 4.74 ± 1.28 | 24.42 ± 5.76 | |||
| Negative | 7 (35%) | 3.61 ± 1.29 | 19.30 ± 5.14 | |||
| Unknown | 3 (15%) | |||||
| 9. | HER2 | ND | ND | |||
| Positive | 4 (20%) | 4.04 ± 1.24 | 22.85 ± 4.94 | |||
| Negative | 13 (65%) | 5.06 ± 1.69 | 20.56 ± 9.23 | |||
| Unknown | 3 (15%) | |||||
| 10. | Triple negative | ND | ND | |||
| Yes | 3 (15%) | 3.17 ± .87 | 23.09 ± 2.78 | |||
| No | 14 (70%) | 4.51 ± 1.36 | 22.14 ± 6.49 | |||
| Unknown | 3 (15%) |
ND: not done because the group is <5; BBL: breast benign lesions; HV: healthy volunteers; SD: standard deviation.
P < 0.05 is significant.
Table 3.
Demographics and characteristics of HR-HPV positive BC patients.
| Patients | HR-HPV genotype | Age (years) | %CD4+ Tregs | %CD8+ T cells | Sizea | Grade | Stage | Pathology | ER | PR | HER2 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 16 | 45 | 5.53 | 22 | 2−5 | II | II | IDC | + | + | − |
| 2 | 16 | 37 | 3.15 | 22 | >5 | II | III | MC | + | + | − |
| 3 | 16 | 43 | 4.74 | 12.3 | 2−5 | II | II | MC | + | + | − |
| 4 | 18 | 50 | 3.5 | 33.9 | <2 | II | I | MC | + | + | + |
IDC: invasive duct carcinoma; MC: metaloplastic carcinoma; ER: estrogen receptor; PR: progesterone receptor; HER2: human epidermal growth factor receptor 2; (+): positive; (−): negative.
Size in centimeters.
Prevalence of CD4+ Tregs and CD8+ T cells
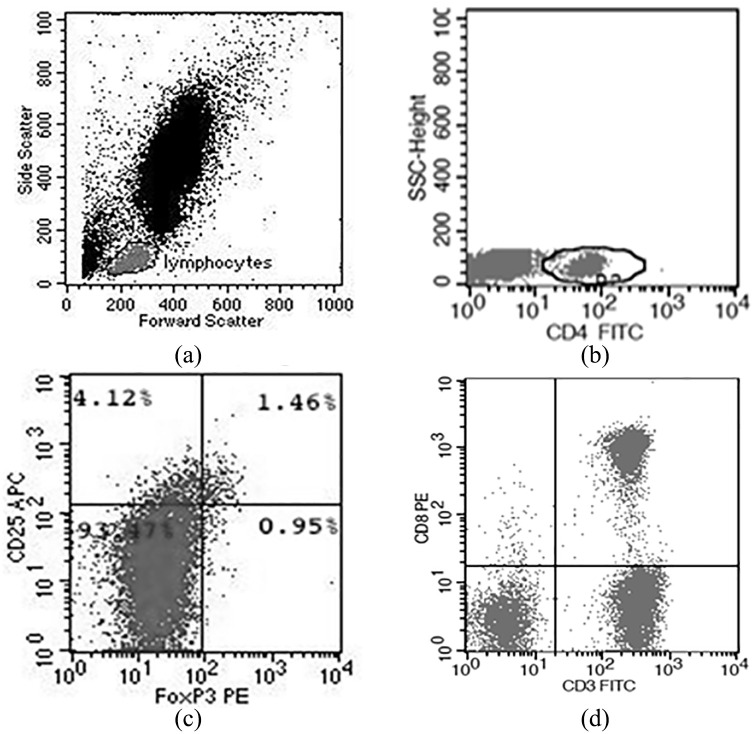
We characterized CD4+ Tregs by the expression of CD4, CD25, and FOXP3.25 Representative plots of the gating and frequency of frequency of CD4+ Tregs are shown in Figure 1. We observed a significantly higher frequencey of CD4+ Tregs in patients with BC compared to BBL and HV (P < 0.001; Table 1). In contrast, there was no significant difference in the total percentage of CD8+ and CD4+ T cells between the three groups (Table 1).
Figure 1.
Flow cytometric analysis. (a) Using typical forward and side scatter characteristics, a gate was first set on lymphocytes. (b) From the lymphocyte gate, percent CD4+ T cells were determined. (c) From the CD4+ gate, the percent CD25+ FoxP3+ CD4+ Tregs are shown. (d) From the lymphocyte gate, percent CD3+ CD8+ T cells are shown.
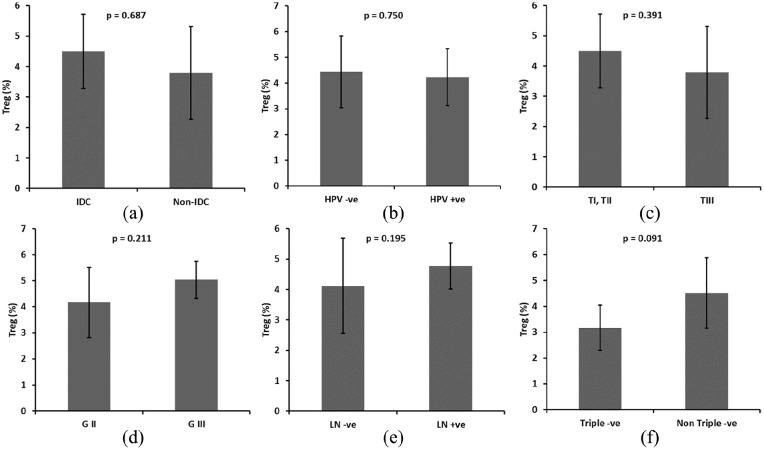
We assessed the histologic grade and disease stage as variables to gain further insight into the association between the frequency of CD4+ Tregs and tumor biology. In addition, we analyzed the frequency of CD4+ Tregs among clinically defined surrogates of BC molecular subtypes for ER, PR, and Her2 expression in order to explore whether any particular clinical disease correlates were associated with increased number of CD4+ Tregs. While invasive duct carcinoma (IDC) was the most prevalent BC subtype, there was no significant difference in frequency of CD4+ Tregs between patients with IDC and other pathological subtypes [P = 0.687; Figure 2(a)]. In addition, we observed no significant difference in frequency of CD4+ Tregs between BC patients with and without HR-HPV infection [P = 0.750; Figure 2(b)], lymph node metastases [P = 0.195; Figure 2(c)], poorly differentiated grade III tumor [P = 0.211; Figure 2(d)], late stage III tumor [P = 0.391; Figure 2(e)], and triple negative tumor [P = 0.091; Figure 2(f)].
Figure 2.
Relation between the frequency of CD25+ FOXP3+ CD4+ Tregs in (a) HPV positive (+ve) versus HPV negative (−ve). (b) IDC vs. non-IDC. (c) Small tumor size (TI, TII) versus large tumors (TIII). (d) Well differentiated (G2) versus poorly differentiated (G3). (e) Node negative patients versus node positive patients. (f) Triple −ve versus non-triple −ve patients. Groups were compared using t tests (a, b, c, e, f) and ANOVA (d). Bars represent standard deviation.
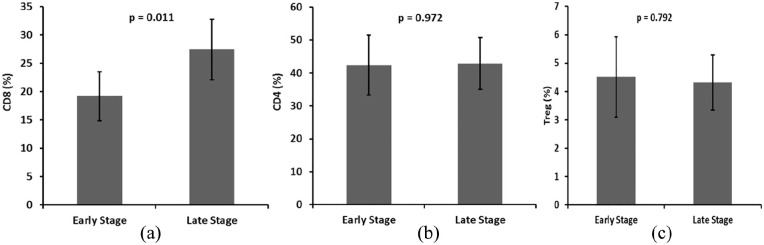
Tumor-infiltrating CD8+ T lymphocytes in BC patients were reported to have antitumor activity resulting in a favorable effect on patients survival.46 In this study we observed a significantly higher frequencey of CD8+ T cells in peripheral blood of patients with late stage III compared to early stage I and II BC [P = 0.011; Figure 3(a)]. In contrast, there was no significant difference in the frequency of CD4+ T cells (Figure 3(b); P = 0.792) or CD4+ Tregs (Figure 3(c); P = 0.972) between early compared to late stage BC patients. In addition, there was no significant association between the ratio of CD8+ T cell to CD4+ Tregs frequencies and the expression of ER, PR, or HER2 (data not shown).
Figure 3.
Frequency of (a) CD3+ CD8+ T cells. (b) CD4+ T cells. (c) CD25+ FOXP3+ CD4+ Tregs in relation to early (I, II) versus late (III) stage of BC. Mann-Whitney U test was performed to access statistical significance. Bars represent standard deviation. A significant difference in the frequency of CD3+ CD8+ T cells was found between early and late tumor stages (P = 0.011).
Discussion
While most HPV infections are cleared by the host immune response, a persistent HPV infection is established in some individuals by evading immune-surveillance and generating an anti-inflammatory microenvironment resulting in suppression of Natural Killer cell activity and antigen presentation.47–49 In addition, HPV infection promotes T-helper cell type 2 and CD4+ Tregs phenotypes and reduces T-helper cell type 1 phenotype, leading to suppression of cellular and humoral immunity and contributes to HPV persistence. In this study, we demonstrated the presence of HR-HPV in the tissues of 20% of BC specimens which is consistent with previously published worldwide data.8,50–52 The variability in HR-HPV detection rate between previously published studies may be a result of differences in study population, sample source, detection method, and viral load. Our results lead us to hypothesize that HR-HPV may be implicated in the development of some types of BC in Egyptian women and the potential prevention of some breast cancers by HPV vaccination.8
It has long been hypothesized that hormone dependent oncogenic viruses may have causal roles in some cancers.53 Expression of HR-HPV DNA in BC9 and HR-HPV proteins in cervical cancer54 has been reported. Meta-analysis of case-control studies suggests that HPV infection is a potential risk factor in BC.55,56 The degree of risk may be influenced by patient nationality, HPV subtype, and type of tissue infected. Recent studies have demonstrated that exosomes containing microRNAs play a pathogenic role in HR-HPV mediated inflammation and development of cervical cancer.5,6 In addition, Human Parvovirus infection (B19) and Epstein Barr Virus (EBV) may play an important role in thyroid cancer development and progression via increased inflammation in thyroid tissue.3,4 The presence of the HR-HPV was shown to be associated with increased inflammatory cytokines and BC progression.9
CD4+ Tregs have an immunosuppressive effect that may promote tumor growth and progression of BC. In addition, heterogeneous expression of hormone receptors and growth factors in BC affects patient-specific adaptive immune responses.57 In this study we assessed the prevalence of CD4+ Tregs in the peripheral blood of Egyptian females with BC. We observed a significantly higher frequency of CD4+ Tregs in patients with BC compared to BBL and HV. These results lead us to postulate that the composition of T cell subsets in peripheral blood of Egyptian patients with BC may favor an immunoregulatory phenotype that is distinct from BBL and HV. Our finding is consistent with previously reported analysis of freshly isolated lymphocytes from normal and malignant breast tissue samples.58
A number of studies have demonstrated that increased CD4+ Tregs frequency was associated with poor prognostic characteristics such as higher histological grade, lymph node metastasis.20,25,59,60 In addition, the mRNA expression of FOXP3, CTLA-4, and GITR in CD4+ Tregs from peripheral blood in BC patients was significantly increased in comparison with healthy individuals.25 In contrast, a higher level of CD4+ Tregs and a lower ratio of CD4+ Tregs and CD8+ T cells was shown to be associated with better overall survival independent of HPV status and age in patients with oral and oropharyngeal squamous cell carcinomas.61 In this study, we observed no significant increase in the frequency of CD4+ Tregs in BC patients with poor prognostic characteristics such as higher histological grade, lymph node metastasis, and presence of high-risk HPV which may be due to the relatively small number of study participants. The lack of sample size power calculation is the limitation of this study and may not have permitted discernable differences in the frequency of CD4+ Tregs in BC patients with poor prognostic characteristics. We postulate that circulating CD4+ Tregs may be used as a prognostic or predictive parameter in Egyptian women with BC and should be assessed in a larger longitudinal study with sufficient follow-up time.
A positive correlation was observed in multiple studies between the number of tumor infiltrating cytotoxic CD8+ T cells and clinical outcome in BC that may improve patient survival.24,37,46,60,62 Decreased number of tumor infiltrating CD8+ T cells in BC was significantly associated with lymph node metastasis and a higher disease stage. These results suggest that cytotoxic CD8+ T cells are heavily involved in antitumor immune responses. However, CD8+ T cell infiltrates were also shown to be associated with higher histological grade and basal phenotype, and inversely associated with ER and PR expression. Estrogen has been shown to inhibit CD8+ T cell infiltration of tumors in ER+ BC and correlating with decreased overall survival.63 In addition, CD8+ T cells may play a role in the dissemination of circulating breast cancer cells.64
In our study, we performed multivariate least square regression analysis after controlling for age to determine the association between clinical disease stage and the frequency of CD4+ Tregs and CD8+ T cells. We observed a significantly higher frequency of CD8+ T cells, but not CD4+ Tregs, in the peripheral blood from late compared to early stage BC. These results suggest that the total CD8+ T cell counts are not constant during the course of BC and may serve as an important biomarker of disease. In addition, the ratio of CD4+ Tregs and CD8+ T cells was different between early and late stage of BC suggesting that different mechanisms control CD4+ Tregs and CD8+ T cells homeostasis. Our results are consistent with previously reported analysis of freshly isolated lymphocytes from a small set of normal and malignant breast tissue samples.65
The association between levels of lymphocyte subsets in peripheral blood and different BC phenotypes has been reported and may be used as a marker for aggressive phenotypes.66 In addition, assessing the changes in peripheral blood cellular subsets may be a useful tool to evaluate the response of BC patients to therapy.67 The role of CD8+ T cell frequency in the peripheral blood and the ratio of CD4+ Tregs and CD8+ T cells as a prognostic marker in Egyptian women with BC are not well defined and should be assessed in a large prospective study.
In vitro data often do not adequately address the complexity of in vivo immune processes. However, our study provides important information on the role of various risk factors including HR-HPV in the pathogenesis of BC in Egypt. The cross-sectional design does not permit definitive analysis of the predictive value of CD4+ Tregs and CD8+ T cell frequencies in BC. Nevertheless, we believe that our study provided an important evaluation of CD4+ Tregs and CD8+ T cell frequencies in Egyptian women with BC. Our results support the design of subsequent longitudinal studies to directly examine the association between the frequency of CD4+ Tregs and CD8+ T cells in the peripheral blood and the response to BC treatment.
Conclusion
Our results lead us to postulate that the association between the frequency of CD4+ Tregs and CD8+ T cells in the peripheral blood may be a prognostic or predictive parameter in Egyptian women with BC. In addition, HR-HPV infection may be implicated in the development of some types of BC in Egyptian women.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by the Science and Technology Development Fund (STDF), Egypt, Grant # 22914.
Ethical approval: Ethical approval for this study was obtained from the Institutional Review Board at Al-Azhar University.
Informed consent: Written informed consent was obtained from all the subjects before the study.
ORCID iD: Mohamed Elrefaei  https://orcid.org/0000-0002-2030-4779
https://orcid.org/0000-0002-2030-4779
References
- 1. Ibrahim AS, Khaled HM, Mikhail NN, et al. (2014) Cancer incidence in Egypt: Results of the national population-based cancer registry program. Journal of Cancer Epidemiology 2014: 437971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. El Saghir NS. (2008) Responding to the challenges of breast cancer in Egypt and other Arab countries. Journal of the Egyptian National Cancer Institute 20: 309–312. [PubMed] [Google Scholar]
- 3. Etemadi A, Mostafaei S, Yari K, et al. (2017) Detection and a possible link between parvovirus B19 and thyroid cancer. Tumour Biology 39: 1010428317703634. [DOI] [PubMed] [Google Scholar]
- 4. Moghoofei M, Mostafaei S, Nesaei A, et al. (2019) Epstein-Barr virus and thyroid cancer: The role of viral expressed proteins. Journal of Cellular Physiology 234: 3790–3799. [DOI] [PubMed] [Google Scholar]
- 5. Nahand JS, Mahjoubin-Tehran M, Moghoofei M, et al. (2020) Exosomal miRNAs: Novel players in viral infection. Epigenomics 12: 353–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sadri Nahand J, Moghoofei M, Salmaninejad A, et al. (2020) Pathogenic role of exosomes and microRNAs in HPV-mediated inflammation and cervical cancer: A review. International Journal of Cancer 146: 305–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. You EL, Henry M, Zeitouni AG. (2019) Human papillomavirus-associated oropharyngeal cancer: Review of current evidence and management. Current Oncology 26: 119–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Heng B, Glenn WK, Ye Y, et al. (2009) Human papilloma virus is associated with breast cancer. British Journal of Cancer 101: 1345–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Khodabandehlou N, Mostafaei S, Etemadi A, et al. (2019) Human papilloma virus and breast cancer: The role of inflammation and viral expressed proteins. BMC Cancer 19: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baseman JG, Koutsky LA. (2005) The epidemiology of human papillomavirus infections. Journal of Clinical Virology 32(Suppl 1): S16–S24. [DOI] [PubMed] [Google Scholar]
- 11. Pang CL, Thierry F. (2013) Human papillomavirus proteins as prospective therapeutic targets. Microbial Pathogenesis 58: 55–65. [DOI] [PubMed] [Google Scholar]
- 12. McLaughlin-Drubin ME, Munger K. (2009) Oncogenic activities of human papillomaviruses. Virus Research 143: 195–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fernandes A, Bianchi G, Feltri AP, et al. (2015) Presence of human papillomavirus in breast cancer and its association with prognostic factors. Ecancermedicalscience 9: 548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Early Breast Cancer Trialists’ Collaborative Group (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet 365: 1687–1717. [DOI] [PubMed] [Google Scholar]
- 15. Arpino G, Weiss H, Lee AV, et al. (2005) Estrogen receptor-positive, progesterone receptor-negative breast cancer: Association with growth factor receptor expression and tamoxifen resistance. Journal of the National Cancer Institute 97: 1254–1261. [DOI] [PubMed] [Google Scholar]
- 16. De Laurentiis M, Arpino G, Massarelli E, et al. (2005) A meta-analysis on the interaction between HER-2 expression and response to endocrine treatment in advanced breast cancer. Clinical Cancer Research 11: 4741–4748. [DOI] [PubMed] [Google Scholar]
- 17. Bauer KR, Brown M, Cress RD, et al. (2007) Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: A population-based study from the California cancer registry. Cancer 109: 1721–1728. [DOI] [PubMed] [Google Scholar]
- 18. Dent R, Trudeau M, Pritchard KI, et al. (2007) Triple-negative breast cancer: Clinical features and patterns of recurrence. Clinical Cancer Research 13: 4429–4434. [DOI] [PubMed] [Google Scholar]
- 19. Zhang Y, Lv F, Yang Y, et al. (2015) Clinicopathological features and prognosis of metaplastic breast carcinoma: Experience of a major Chinese cancer center. PLoS One 10: e0131409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Decker T, Fischer G, Bucke W, et al. (2012) Increased number of regulatory T cells (T-regs) in the peripheral blood of patients with HER-2/neu-positive early breast cancer. Journal of Cancer Research and Clinical Oncology 138: 1945–1950. [DOI] [PubMed] [Google Scholar]
- 21. Bates GJ, Fox SB, Han C, et al. (2006) Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. Journal of Clinical Oncology 24: 5373–5380. [DOI] [PubMed] [Google Scholar]
- 22. Liyanage UK, Moore TT, Joo HG, et al. (2002) Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. Journal of Immunology 169: 2756–2761. [DOI] [PubMed] [Google Scholar]
- 23. Wang Y, Sun J, Zheng R, et al. (2016) Regulatory T cells are an important prognostic factor in breast cancer: A systematic review and meta-analysis. Neoplasma 63: 789–798. [DOI] [PubMed] [Google Scholar]
- 24. Bailur JK, Gueckel B, Derhovanessian E, et al. (2015) Presence of circulating HER2-reactive CD8 + T-cells is associated with lower frequencies of myeloid-derived suppressor cells and regulatory T cells, and better survival in older breast cancer patients. Breast Cancer Research 17: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Khalife E, Khodadadi A, Talaeizadeh A, et al. (2018) Overexpression of regulatory T cell-related markers (FOXP3, CTLA-4 and GITR) by peripheral blood mononuclear cells from patients with breast cancer. Asian Pacific Journal of Cancer Preventive 19: 3019–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang J, Yang J. (2016) Identification of CD4(+)CD25(+)CD127(-) regulatory T cells and CD14(+)HLA(-)DR(-)/low myeloid-derived suppressor cells and their roles in the prognosis of breast cancer. Biomedical Reports 5: 208–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fontenot JD, Gavin MA, Rudensky AY. (2003) FOXP3 programs the development and function of CD4+CD25+ regulatory T cells. Nature Immunology 4: 330–336. [DOI] [PubMed] [Google Scholar]
- 28. Liu W, Putnam AL, Xu-Yu Z, et al. (2006) CD127 expression inversely correlates with FOXP3 and suppressive function of human CD4+ T reg cells. Journal of Experimental Medicine 203: 1701–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Seddiki N, Santner-Nanan B, Martinson J, et al. (2006) Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. Journal of Experimental Medicine 203: 1693–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Workman CJ, Szymczak-Workman AL, Collison LW, et al. (2009) The development and function of regulatory T cells. Cellular and Molecular Life Sciences 66: 2603–2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Baecher-Allan C, Brown JA, Freeman GJ, et al. (2001) CD4+CD25 high regulatory cells in human peripheral blood. Journal of Immunology 167: 1245–1253. [DOI] [PubMed] [Google Scholar]
- 32. Scotto L, Naiyer AJ, Galluzzo S, et al. (2004) Overlap between molecular markers expressed by naturally occurring CD4+CD25+ regulatory T cells and antigen specific CD4+CD25+ and CD8+CD28- T suppressor cells. Human Immunology 65: 1297–1306. [DOI] [PubMed] [Google Scholar]
- 33. Strauss L, Bergmann C, Szczepanski M, et al. (2007) A unique subset of CD4+CD25 high FOXP3+ T cells secreting interleukin-10 and transforming growth factor-beta1 mediates suppression in the tumor microenvironment. Clinical Cancer Research 13: 4345–4354. [DOI] [PubMed] [Google Scholar]
- 34. Levings MK, Sangregorio R, Sartirana C, et al. (2002) Human CD25+CD4+ T suppressor cell clones produce transforming growth factor beta, but not interleukin 10, and are distinct from type 1 T regulatory cells. Journal of Experimental Medicine 196: 1335–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu S, Foulkes WD, Leung S, et al. (2014) Prognostic significance of FOXP3+ tumor-infiltrating lymphocytes in breast cancer depends on estrogen receptor and human epidermal growth factor receptor-2 expression status and concurrent cytotoxic T-cell infiltration. Breast Cancer Research 16: 432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Banin-Hirata BK, de Oliveira CEC, Losi-Guembarovski R, et al. (2018) The prognostic value of regulatory T cells infiltration in HER2-enriched breast cancer microenvironment. International Reviews of Immunology 37: 144–150. [DOI] [PubMed] [Google Scholar]
- 37. Matsumoto H, Thike AA, Li H, et al. (2016) Increased CD4 and CD8-positive T cell infiltrate signifies good prognosis in a subset of triple-negative breast cancer. Breast Cancer Research and Treatment 156: 237–247. [DOI] [PubMed] [Google Scholar]
- 38. Xu L, Zhou Y, Xiao DM, et al. (2010) The change of CD4+ CD25 high CCR6+ regulatory T cells in breast cancer patients. Sichuan Da Xue Xue Bao Yi Xue Ban 41: 415–419. [PubMed] [Google Scholar]
- 39. Perez SA, Karamouzis MV, Skarlos DV, et al. (2007) CD4+CD25+ regulatory T-cell frequency in HER-2/neu (HER)-positive and HER-negative advanced-stage breast cancer patients. Clinical Cancer Research 13: 2714–2721. [DOI] [PubMed] [Google Scholar]
- 40. Hammond ME, Hayes DF, Dowsett M, et al. (2010) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. Archives of Pathology & Laboratory Medicine 134: 907–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Park C, Park K, Kim J, et al. (2016) Prognostic values of negative estrogen or progesterone receptor expression in patients with luminal B HER2-negative breast cancer. World Journal of Surgical Oncology 14: 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. van Dongen JJ, Langerak AW, Bruggemann M, et al. (2003) Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: Report of the BIOMED-2 concerted action BMH4-CT98-3936. Leukemia 17: 2257–2317. [DOI] [PubMed] [Google Scholar]
- 43. Elrefaei M, Burke CM, Baker CA, et al. (2010) HIV-specific TGF-beta-positive CD4+ T cells do not express regulatory surface markers and are regulated by CTLA-4. AIDS Research and Human Retroviruses 26: 329–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Boldt A, Borte S, Fricke S, et al. (2014) Eight-color immunophenotyping of T-, B-, and NK-cell subpopulations for characterization of chronic immunodeficiencies. Cytometry Part B: Clinical Cytometry 86: 191–206. [DOI] [PubMed] [Google Scholar]
- 45. Kobayashi K, Hisamatsu K, Suzui N, et al. (2018) A Review of HPV-Related Head and Neck Cancer. Journal of Clinical Medicine 7: 241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mahmoud SM, Paish EC, Powe DG, et al. (2011) Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. Journal of Clinical Oncology 29: 1949–1955. [DOI] [PubMed] [Google Scholar]
- 47. Deligeoroglou E, Giannouli A, Athanasopoulos N, et al. (2013) HPV infection: Immunological aspects and their utility in future therapy. Infectious Diseases in Obstetrics and Gynecology 2013: 540850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Song D, Li H, Li H, et al. (2015) Effect of human papillomavirus infection on the immune system and its role in the course of cervical cancer. Oncology Letters 10: 600–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nunes RAL, Morale MG, Silva GAF, et al. (2018) Innate immunity and HPV: Friends or foes. Clinics (Sao Paulo) 73: e549s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pereira Suarez AL, Lorenzetti MA, Gonzalez Lucano R, et al. (2013) Presence of human papilloma virus in a series of breast carcinoma from Argentina. PLoS One 8: e61613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. de Leon DC, Montiel DP, Nemcova J, et al. (2009) Human papillomavirus (HPV) in breast tumors: Prevalence in a group of Mexican patients. BMC Cancer 9: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Antonsson A, Spurr TP, Chen AC, et al. (2011) High prevalence of human papillomaviruses in fresh frozen breast cancer samples. Journal of Medical Virology 83: 2157–2163. [DOI] [PubMed] [Google Scholar]
- 53. Lawson JS, Glenn WK, Heng B, et al. (2009) Koilocytes indicate a role for human papilloma virus in breast cancer. British Journal of Cancer 101: 1351–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Miao H, Cao B, Ge W, et al. (2019) Expression of p16 and p27 protein in cervical exfoliated cells and its relationship with high risk human papilloma virus in cervical lesions. Journal of Biological Regulators and Homeostatic Agents 33: 197–203. [PubMed] [Google Scholar]
- 55. Bae JM, Kim EH. (2016) Human papillomavirus infection and risk of breast cancer: A meta-analysis of case-control studies. Infectious Agents and Cancer 11: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ren C, Zeng K, Wu C, et al. (2019) Human papillomavirus infection increases the risk of breast carcinoma: A large-scale systemic review and meta-analysis of case-control studies. Gland Surgery 8: 486–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Varn FS, Mullins DW, Arias-Pulido H, et al. (2017) Adaptive immunity programmes in breast cancer. Immunology 150: 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ruffell B, Au A, Rugo HS, et al. (2012) Leukocyte composition of human breast cancer. Proceedings of the National Academy of Sciences USA 109: 2796–2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhou Y, Shao N, Aierken N, et al. (2017) Prognostic value of tumor-infiltrating FOXP3+ regulatory T cells in patients with breast cancer: A meta-analysis. Journal of Cancer 8: 4098–4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Meng S, Li L, Zhou M, et al. (2018) Distribution and prognostic value of tumor infiltrating T cells in breast cancer. Molecular Medicine Reports 18: 4247–4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lukesova E, Boucek J, Rotnaglova E, et al. (2014) High level of Tregs is a positive prognostic marker in patients with HPV-positive oral and oropharyngeal squamous cell carcinomas. Biomed Research International 2014: 303929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kuznetsova M, Lopatnikova J, Shevchenko J, et al. (2019) Cytotoxic activity and memory T cell subset distribution of in vitro-stimulated CD8(+) T cells specific for HER2/neu epitopes. Frontiers in Immunology 10: 1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ali HR, Provenzano E, Dawson SJ, et al. (2014) Association between CD8+ T-cell infiltration and breast cancer survival in 12,439 patients. Annals of Oncology 25: 1536–1543. [DOI] [PubMed] [Google Scholar]
- 64. Xue D, Xia T, Wang J, et al. (2018) Role of regulatory T cells and CD8(+) T lymphocytes in the dissemination of circulating tumor cells in primary invasive breast cancer. Oncology Letters 16: 3045–3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rech AJ, Mick R, Kaplan DE, et al. Homeostasis of peripheral FOXP3(+) CD4 (+) regulatory T cells in patients with early and late stage breast cancer. Cancer Immunology, Immunotherapy 2010; 59: 599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Jia Y, Xu L, Lin Q, et al. Levels of lymphocyte subsets in peripheral blood prior treatment are associated with aggressive breast cancer phenotypes or subtypes. Medical Oncology 2014; 31: 981. [DOI] [PubMed] [Google Scholar]
- 67. Fernandes A, Pesci-Feltri A, Garcia-Fleury I, et al. Lymphocyte subsets predictive value and possible involvement of human papilloma virus infection on breast cancer molecular subtypes. World Journal of Clinical Oncology 2018; 9: 123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]