Abstract
COVID-19 is a viral pandemic that primarily manifests with respiratory distress but may also lead to symptoms and signs associated with the gastrointestinal tract. It is characteristically associated with a hyper-immune response, also referred to as a ‘cytokine storm’. Probiotics are living microorganisms that have been shown to have positive effects on immune response in man with some bacteria; some strains of Bifidobacteria, for example, possess especially potent immune modulating effects. These bacteria have the potential to ameliorate the ‘cytokine storm’ through a differential effect on pro- and anti-inflammatory cytokines. In the management of COVID-19 and other coronovirus-mediated illnesses, probiotic bacteria also have the potential to enhance vaccine efficacy.
Keywords: bifidobacterium, covid-19, probiotic
Introduction
COVID-19 is a new disease caused by a novel coronavirus, SARS-CoV-2, that primarily affects the lungs and airways. While the complete pathophysiology of the effect of this virus in man remains to be defined, it is evident that its pathological impacts include both direct effects of viral invasion and a complex immunological response.1 Indeed, elevated serum levels of the pro-inflammatory cytokines have been reported to be predictive of a poor prognosis in patients with COVID-19.2 Some strains of the probiotic species bifidobacteria have been shown to exert immune modulating and anti-inflammatory effects on human immune system.3
Autophagy and immune responses in COVID-19
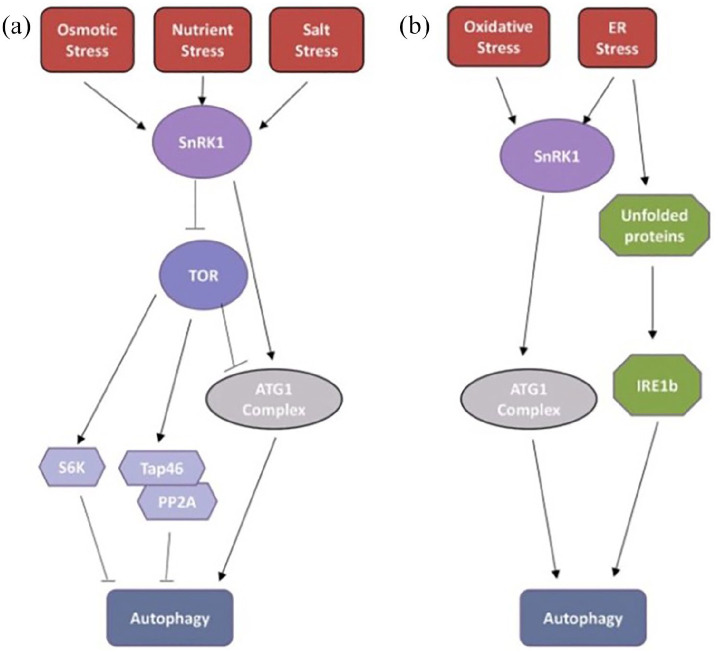
Autophagy is the cell’s self-destruction process. It is a process that begins with the endoplasmic reticulum (ER) under stress and ends with destructive metabolites in the cell and nucleus, which are collected in autophagosomes. While osmotic stresses cause autophagy via the target of rapamycin (TOR) kinase pathway, oxidative and ER stress conditions lead to autophagy via the inositol-requiring enzyme 1 (IRE1) pathway (Figure 1).4
Figure 1.
Over the TOR and IRE1b pathways of stress factors leading to autophagy mechanisms of action (Copyright from Plant Physiology).4
Certain foods that cause ER stress (such as trehalose saccharides) and infections (such as influenza and coronavirus) initiate the inflammation cascade and promote viral replication by accelerating the process of autophagy. Coronavirus induces the formation of membrane vesicles which viral replication and transcription complexes are associated. These membrane vesicles lead to ER stress mediated autophagy.5 Although there is no evidence that SARS-Cov2 causes ER stress-mediated autophagy, Fung et al.5 showed that viral replication could be decreased by blocking the IRE1 pathway in coronavirus infected patients with bronchitis. Likewise, in relation to autophagy, Kong et al.6 reported that interleukin-6 may increase autophagy via the IRE1 pathway.
Interleukin-17 (originating mainly from IL-17-producing T cells) is a pro-inflammatory and procarcinogenic cytokine that plays an important role in the adaptive immune system.7 IL-17 is also a potent inducer of ER stress and autophagy via IRE1.8 Accordingly, IL-17 blockade has been shown to prevent ER stress-related autophagy and inflammation.8 IL-17 also plays an important role in inflammatory bowel disease.7 Since effects of this novel coronavirus on the gastrointestinal tract have been well documented,9 it is reasonable to propose that these effects might be mediated through IL-17. Also, Hou et al.10 showed that the presence of excessive IL-6 promotes the generation of Th17 cells and that IL-6 and IL-17 synergistically promote viral replication and may be important targets for anti-coronovirus therapies. Corneth et al.11 demonstrated that an absence of the IL-17 receptor leads to a decrease in IL-6 production. However, Colaneri et al.12 reported that anti-IL-6 therapy alone failed to reduce intensive care admission rates and mortality in covid-19 patients.
Therapeutic implications
These findings suggest that a strategy that incorporates a more comprehensive approach to immune modulation rather than the inhibition of individual cytokines may be more effective against the cytokine storm in virus-infected patients. IL-17 and IL-6, promote viral persistence by immune interactions through autophagy. Schett et al.13 reported that blocking IL-17 might reduce viral replication in COVID-19 patients. The pathogenesis of the immune response to coronavirus bears a striking resemblance to TH17-Th1driven autoimmune diseases and these TH17-Th1 immune interactions appear to play an important role in virus replication. The immune modulating effects of bifidobacteria species include an anti-IL-17 effect which could prove important in both treatment and vaccine development. Furthermore, it seems clear that immune interactions, through autophagy, will affect coronaviral replication. In this context, certain strains of bifidobacteria might play an important role in the management COVID-19.
Bifidobacteria in the management of COVID-19
Although there is no clear evidence that bifidobacteria could be used to treat viruses, it has been showed that probiotic bifidobacteria decreased, not only the duration of respiratory, symptoms caused by the common cold coronavirus but also days with fever.14 Also, a report revealed that some patients with COVID-19 demonstrated changes in the gut microbiome which feature decreased numbers of bifidobacteria and lactobacilli.15 It also needs to be stressed that probiotic strains of lactobacilli have also been shown to exert beneficial effect in some viral infections.16
Some probiotic strains of bifidobacteria possess the aforementioned IL-17 inhibitory effect.7 Bifidobacterium animalis subsp. Lactis-BB12 is a Gram-positive, anaerobic commensal-derived probiotic. Wang et al.17 showed that Bifidobacterium animalis subsp. Lactis-BB12 significantly suppressed both TNF-α and NF-b in inflammatory diseases. Also, Jungersen et al.18 demonstrated that immune modulation by BB-12 response is dose-dependent. In our previous reports, we demonstrated that a intracolonic single dose of Bifidobacterium animalis sp. Lactis-BB12 led to rapid mucosal healing in an ulcerative colitis patient.7,19 This effect was related to the IL-17 inhibitory effect of the BB-12 strain. Zuo et al.20 showed that patients with COVID-19 had a significantly disrupted gut microbiome, characterised by enrichment of opportunistic pathogens and depletion of beneficial commensal bacteria. Interestingly, the bloom of opportunistic pathogens correlated positively with the numbers of Th17 cells.21 Groeger et al.22 reported that the oral administration of B. infantis 35624 could reduce systemic inflammatory biomarkers in both gastrointestinal and extra-intestinal inflammatory disorders; an effect associated with a lowering of levels of TNF alpha, IL-6 and C-reactive protein. Also, Han et al.23 showed that probiotic bifidobacteria can protect against intestinal damage induced by autophagy. Some species of bifidobacteria are likely to prevent the replication of coronaviruses by reducing ER stress-related autophagy through an effect on IL-17.6
One striking difference between influenza-related lung injury and COVID-19-related lung injury relates to the prominence of angiogenesis in the pathology of COVID-19.24 Interestingly, IL-17 has been shown to be a proangionesis cytokine, especially in the context of cancer metastases.7
For all of these reasons the administration of a single high dose of an appropriate strain of bifidobacterium (such as BB-12, or infantis) and especially in patients with gastrointestinal symptoms (diarrhoea, abdominal pain, vomiting), may be postulated to have a role in the management of coronavirus infected patients. In support of this proposal it should be noted that Jayawardena25 reported that bifidobacterium probiotic strains either reduced the severity or shortenes the duration of viral infections; in another report, Bifidobacterium infantis reduced the duration of acute respiratory infections illness in children and adults.26
There are some findings that suggest that intestinal dysbiosis might play a role in the failure to respond to vaccines.27 In this regard, gut microbiota could affect intestinal immune responses by acting as immune modulators as well as natural vaccine adjuvants.27 Also, Rizzardini et al.28 reported that the consumption of the probiotic strain BB-12 significantly increased antigen-specific immune responses in healthy individuals receiving influenza vaccination. Similarly, probiotic bifidobacteria might play an important role in the efficacy of vaccines against SARS-CoV-2. Certain bifidobacteria have a prominent cell surface exopolysaccharide (EPS). Schiavi et al.29 showed that EPS produced by B. longum 35624 (infantis) played an essential role in the anti-inflammatory effects of this bacterium and removal of exopolysaccharide resulted, not only in loss of these anti-inflammatory effects, but to a transformation to become an inducer of local TH17 responses. In other experiments, Lee et al.30 showed that EPS- protein conjugate vaccines could enhance immunogenity. Our studies indicated that the maintenance of the unique electrophysiological properties of BB-12 in an aerobic environment for up to 6 months could be attributed to the integrity of its EPS.31 Also, Salazar et al.32 reported that EPS derived from Bifidobacterium animalis sp.lactis exhibited metabolic activity in in vitro conditions. There are two basic types of vaccines; peptide –based vaccines and lipopeptides derived from microbes. Unlike peptide-based vaccines, the immune response to lipopeptides derived from microbes can be generated without the use of adjuvant due to their electrogenic activity and hygrophilic features.33 Lipopeptides derived from microbial origin provide intrinsic adjuvant-like activity by signalling via Toll-like receptor 2.33 Given the electrogenic activity and hygrophilic properties attributed to EPS, bifidobacteria such as B. İnfantis and BB-12 might play an important role in the development of vaccines against the coronavirus. Hence, the probiotic bacterial polysaccharide structure can be considered as a lipopeptide based vaccines.
Conclusion
Given the close relationship that seems to exist between viral replication and gastrointestinal immunity, a probiotic strategy that targets and modulates the immune response might be effective in reducing viral replication. The known anti-inflammatory activity of certain bifidobacteria suggest that these bacteria could play an important role in abrogating the inflammatory response that seems so characteristic of this virus. New approaches using specific single strain probiotic preparations hold promise both in terms of abrogating the immune response to the virus and enhancing vaccine efficacy.
Footnotes
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: HSB declares no conflict of interest. EMMQ is a consultant to 4D Pharma, Alimentary Health, Allergan, Biocodex, Ironwood, Precision Biotics, Salix and Vibrant, has research support from 4D Phrama, Biomerica, Vibrant and Zealand and holds equity in Alimentary Health.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Hüseyin S Bozkurt  https://orcid.org/0000-0003-2097-2950
https://orcid.org/0000-0003-2097-2950
References
- 1. Wong SH, Lui RN, Sung JJ. (2020) Covid-19 and the digestive system. Journal of Gastroenterology and Hepatology 35(5): 744–748. [DOI] [PubMed] [Google Scholar]
- 2. Zhao M. (2020) Cytokine storm and immunomodulatory therapy in COVID-19: Role of chloroquine and anti-IL-6 monoclonal antibodies. International Journal of Antimicrobial Agents 55(6): 105982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bozkurt HS, Quigley EMM, Kara B. (2019) Bifidobacterium animalis subspecies lactis engineered to produce mycosporin-like amino acids in colorectal cancer prevention. SAGE Open Medicine 7: 2050312119825784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Soto-Burgos J, Zhuang X, Jiang L, et al. (2018) Dynamics of autophagosome formation. Plant Physiology 176(1): 219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fung S, Liu DX. (2019) The ER stress sensor IRE1 and MAP kinase ERK modulate autophagy induction in cells infected with coronavirus infectious bronchitis virus. Virology 533: 34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kong EY, Cheng SH, Yu KN. (2018) Induction of autophagy and interleukin 6 secretion in bystander cells: Metabolic cooperation for radiation-induced rescue effect? Journal of Radiation Research 59(2): 129–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bozkurt HS, Kara B. (2020) A new treatment for ulcerative colitis: Intracolonic bifidobacterium and xyloglucan application. European Journal of Inflammation 18: 205873922094262. [Google Scholar]
- 8. Kim SR, Kim HJ, Kim DI, et al. (2015) Blockade of interplay between IL-17A and endoplasmic reticulum stress attenuates LPS-induced lung injury. Theranostics 5(12): 1343–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gu J, Han B, Wang J. (2020) COVID-19: Gastrointestinal manifestations and potential fecal-oral transmission. Gastroenterology 158(6): 1518–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hou W, Jin Y-H, Kang HS, et al. (2014) Interleukin-6 (IL-6) and IL-17 synergistically promote viral persistence by inhibiting cellular apoptosis and cytotoxic T cell function. Journal of Virology 88(15): 8479–8489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Corneth OBJ, Mus AMC, Asmawidjaja PS, et al. (2014) Absence of interleukin-17 receptor a signaling prevents autoimmune inflammation of the joint and leads to a Th2-like phenotype in collagen-induced arthritis. Arthritis & Rheumatology 66(2): 340–349. [DOI] [PubMed] [Google Scholar]
- 12. Colaneri M, Bogliolo L, Valsecchi P, et al. (2020) Tocilizumab for treatment of severe COVID-19 patients: Preliminary results from SMAtteo COvid19 REgistry. Microorganisms 8(5): 695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schett G, Sticherling M, Neurath MF. (2020) COVID-19: Risk for cytokine targeting in chronic inflammatory diseases? Nature Reviews Immunology 20(5): 271–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. de Vrese M, Winkler P, Rautenberg P, et al. (2005) Effect of lactobacillus gasseri PA 16/8, Bifidobacterium longum SP 07/3, B. bifidum MF 20/5 on common cold episodes: A double blind, randomized, controlled trial. Clinical Nutrition 24(4): 481–491. [DOI] [PubMed] [Google Scholar]
- 15. Xu K, Cai H, Shen Y, et al. (2020) Management of coronavirus disease-19 (COVID-19): The Zhejiang experience. Zhejiang da xue xue bao. Yi xue ban = Journal of Zhejiang University. Medical Sciences 49(2): 147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Doron S, Snydman DR. (2015) Risk and safety of probiotics. Clinical Infectious Diseases 60(Suppl. 2): S129–S134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang Z, Wang J, Cheng Y, et al. (2011) Secreted factors from Bifidobacterium animalis subsp. lactis inhibitNF-kB-mediated interleukin-8 gene expression in Caco-2 Cells. Applied and Environmental Microbiology 77(22): 8171–8174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jungersen M, Wind A, Johansen E, et al. (2014) The Science behind the Probiotic Strain Bifidobacterium animalis subsp. lactis BB-12®. Microorganisms 2(2): 92–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bozkurt HS, Quigley EMM. (2019) Bifidobacteria and Mucosal-Associated Invariant T (MAIT) cells: A new approach to colorectal cancer prevention? Gastrointestinal Disorders 1(2): 266–272. [Google Scholar]
- 20. Zuo T, Zhang F, Lui GCY, et al. (2020) Alterations in gut microbiota of patients with COVID-19 during time of hospitalization. Gastroenterology 159(3): 944–955.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Atarashi K, Tanoue T, Ando M, et al. (2015) Th17 cell induction by adhesion of microbes to intestinal epithelial cells. Cell 163: 367–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Groeger D, O’Mahony L, Murphy EF, et al. (2013) Bifidobacterium infantis 35624 modulates host inflammatory processes beyond the gut. Gut Microbes 4(4): 325–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Han C, Ding Z, Shi H, et al. (2016) The role of probiotics in lipopolysaccharide-induced autophagy in intestinal epithelial cells. Cellular Physiology and Biochemistry 38(6): 2464–2478. [DOI] [PubMed] [Google Scholar]
- 24. Ackermann M, Verleden SE, Kuehnel M, et al. (2020) Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. New England Journal of Medicine 383(2): 120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jayawardena R, Sooriyaarachchi P, Chourdakis M, et al. (2020) Enhancing immunity in viral infections, with special emphasis on COVID-19: A review. Diabetes & Metabolic Syndrome 14(4): 367–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. King S, Glanville J, Sanders ME, et al. (2014) Effectiveness of probiotics on the duration of illness in healthy children and adults who develop common acute respiratory infectious conditions: A systematic review and meta-analysis. British Journal of Nutrition 112(1): 41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ciabattini A, Olivieri R, Lazzeri E, et al. (2019) Role of the microbiota in the modulation of vaccine immune responses. Frontiers in Microbiology 10: 1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rizzardini G, Eskesen D, Calder PC, et al. (2012) Evaluation of the immune benefits of two probiotic strains Bifidobacterium animalis ssp. lactis, BB-12 and Lactobacillus paracasei ssp. paracasei,L. casei 431w in an influenza vaccination model: A randomised, double-blind,placebo-controlled study. British Journal of Nutrition 107(6): 876–884. [DOI] [PubMed] [Google Scholar]
- 29. Schiavi E, Gleinser M, Molloy E, et al. (2016) The surface-associated exopolysaccharide of Bifidobacterium longum 35624 plays an essential role in dampening host proinflammatory responses and repressing local TH17 responses. Applied and Environmental Microbiology 82(24): 7185–7196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee CJ, Lee LH, Lu CS, et al. (2001) Bacterial polysaccharides as vaccines—immunity and chemical characterization. Advances in Experimental Medicine and Biology 491: 453–471. [DOI] [PubMed] [Google Scholar]
- 31. Bozkurt K, Denktas C, Ozdemir O, et al. (2019) Charge Transport in Bifidobacterium animalis subsp.lactis BB-12 under various Atmospheres. Open Journal of Applied Sciences 9(6): 506–514. [Google Scholar]
- 32. Salazar N, Ruas-Madiedo P, Kolida S, et al. (2009) Bifidobacterium longum IPLA E44 and Bifidobacterium animalis subsp. lactis IPLA R1 modify the composition and metabolic activity of human faecal microbiota in pH-controlled batch cultures. International Journal of Food Microbiology 135(3): 260–267. [DOI] [PubMed] [Google Scholar]
- 33. Zaman M, Toth I. (2013) Immunostimulation by synthetic lipopeptide-based vaccine candidates: Structure-activity relationships. Frontiers in Immunology 4: 318. [DOI] [PMC free article] [PubMed] [Google Scholar]