Abstract
Aim:
The present review aimed to consolidate and analyze the recent information about the use of zebrafish in studies concerning cisplatin-induced ototoxicity and otoprotection.
Material and methods:
The PubMed, Web of Science, and Scopus databanks were searched using the following MESH terms: zebrafish, cisplatin, ototoxicity. The identified publications were screened according to inclusion and exclusion criteria and the 26 qualifying manuscripts were included in the full-text analysis. The experimental protocols, including cisplatin concentrations, the exposure duration and the outcome measurements used in zebrafish larvae studies, were evaluated and the reported knowledge was summarized.
Results:
Twenty-six substances protecting from cisplatin-induced toxicity were identified with the use of zebrafish larvae. These substances include quinine, salvianolic acid B, berbamine 6, benzamil, quercetin, dexmedetomidine, dexamethsanone, quinoxaline, edaravone, apocynin, dimethyl sulfoxide, KR-22335, SRT1720, ORC-13661, 3-MA, D-methionine, mdivi-1, FUT-175, rapamycin, Z-LLF-CHO, ATX, NAC, CYM-5478, CHCP1, CHCP2 and leupeptin. The otoprotective effects of compounds were attributed to their anti-ROS, anti-apoptotic and cisplatin uptake-blocking properties. The broadest range of protection was achieved when the experimental flow used preconditioning with an otoprotective compound and later a co-incubation with cisplatin. Protection against a high concentration of cisplatin was observed only in protocols using short exposure times (4 and 6 h).
Conclusions:
The data extracted from the selected papers confirm that despite the differences between the human and the zebra fish hearing thresholds (as affected by cisplatin), the sensory cells of zebrafish and larval zebrafish are a valuable tool which could be used: (i) for the discovery of novel otoprotective substances and compounds; (ii) to screen their side effects and (iii) to extend the knowledge on the mechanisms of cisplatin-induced inner ear damage. For future studies, the development of a consensus experimental protocol is highly recommended.
Keywords: animal models, cisplatin, otoprotection, ototoxicity, zebrafish
Introduction
Hearing loss is a widespread human sensory disability adversely affecting the communication, social isolation, depression and the quality of life of the affected persons. One of the common causes of hearing loss is the exposure to ototoxic substances such as heavy metals or ototoxic drugs, aminoglycoside antibiotics (neomycin, gentamycin), loop diuretics, and platinum-based cytostatic drugs (cisplatin, oxaliplatin, and carboplatin). The platinum-containing medications are used for the treatment of solid cancers (e.g., head and neck carcinomas, lung carcinomas, cervical carcinomas, or melanomas).1,2 Unfortunately, the incidence of cisplatin-induced ototoxicity is high and the cisplatin-induced hearing loss is not only bilateral, progressive and irreversible but also often associated with vertigo and tinnitus.3,4 According to the literature, children are more prone to develop hearing loss following cisplatin treatment than adults. Loss of hearing at an early age has negative psychosocial consequences, negatively affecting the development of the affected child.5,6
Recent research has concentrated on understanding the mechanisms of cisplatin-induced inner ear damage and on identifying anti-ototoxic substances. Since auditory cell-lines cannot substitute the mature hearing organ, animal models are used in ototoxicity research, including rats, guinea pigs, mice and zebrafish (Danio rerio).
Despite many differences, the zebrafish and human models share considerable similarities and zebrafish can be used to screen conditions observed in human pathologies.7 The easy accessibility to the hearing organ, the small size and the structural and functional similarities between zebrafish and mammalian hair cells, make zebrafish a valuable animal model for studying cisplatin-induced hearing loss. The zebrafish possesses hair cells on the outside of its body in a sensory system called the lateral line. In the lateral line, mechanosensory hair cells are organized into small groups called neuromasts. Each neuromast contains 10–20 hair cells and associated supporting cells.8–14 In zebrafish larvae, neuromasts are mature with functional hair cells by 3 days post-fertilization (dpf).15 The physiological similarities between lateral line and hair cells of the inner ear, easy visualization of the lateral line, and full maturity by 3 days post-fertilization make the lateral line of larvae zebrafish an ideal model for screening large numbers of individual drugs and drugs combinations. The adult zebrafish are used less frequently in the ototoxicity-related studies than the larval zebrafish. There are several advantages of using zebrafish larvae in this type of research, including their permeability to small molecules, their small size and transparency, their rapid generation time and the lateral hair cell similarity to the mature hair cells in the inner ear of adult zebrafish.
Although the zebrafish model offers many advantages over the other animal models, it is essential to remember that in humans, cisplatin induces a hearing loss in the high frequencies, while in zebrafish, only the low frequencies are affected. Also, the sensory hair cells of the fish can regenerate, which a feature not observed in mammals. Therefore, only the acute ototoxicity can be studied using the zebrafish model, not matching the chronic ototoxicity that induces a permanent hearing loss in humans.16
The present review focused on the lateral line of zebrafish larvae and adult animals in studies of cisplatin-induced sensory hair cell loss and otoprotection. The main goal was to analyze the usefulness of the zebrafish model to identify new substances protecting against cisplatin and to explore new knowledge about the mechanisms mediating ototoxicity. The experimental protocols used in the included publications, the protective mechanisms and the adverse effects of the identified substances were evaluated.
In this scoping review, articles published between 2009 and 2020 were considered.
Methods
The present study searched for papers published between January 2009 and May 2020, using the following databanks:
US National Library of Medicine - National Institutes of Health (PubMed);
Scopus;
Web of Science.
The search was restricted to publications in the English language. The keywords included the following combination of mesh terms: zebrafish AND cisplatin AND ototoxicity. Full-text articles were downloaded when the title, abstract, or keywords suggested that the study may be eligible for this review. The selection procedure followed the inclusion and exclusion criteria summarized below (see also Figure 1).
Figure 1.
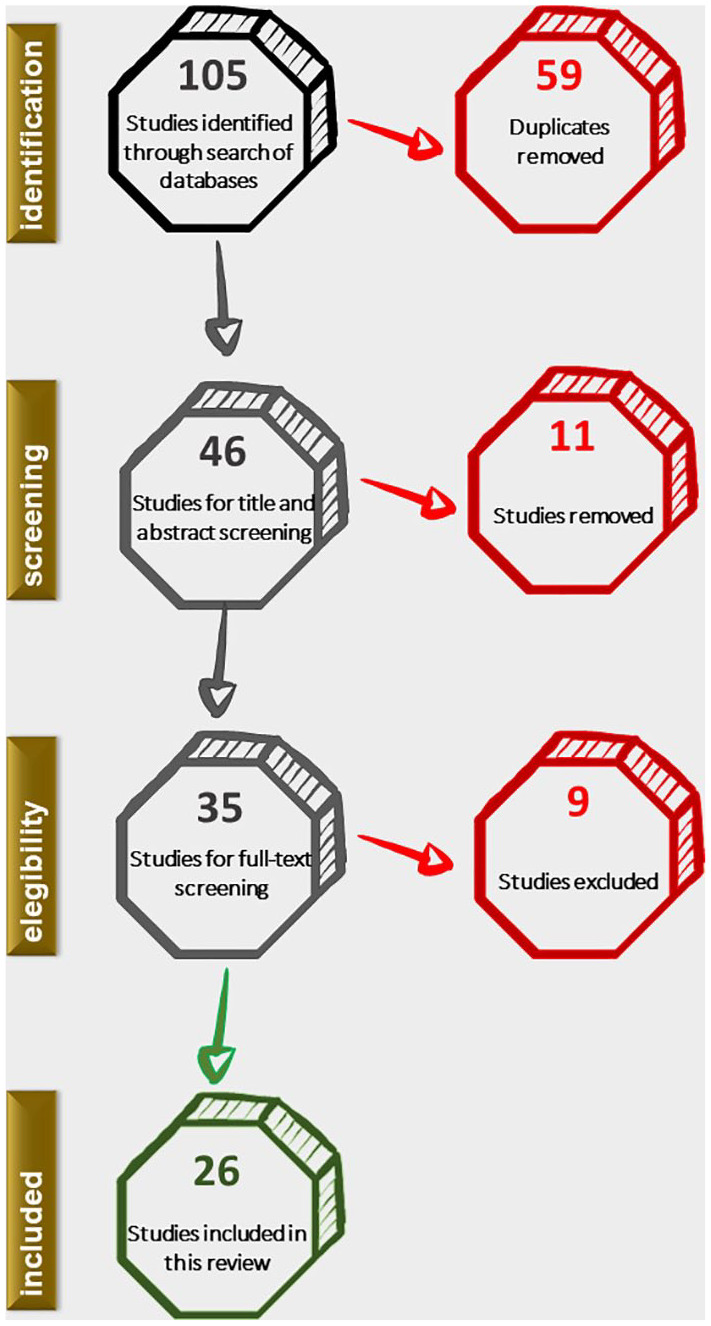
Flowchart of the study.
Inclusion criteria
articles published in the last 12 years (2009–2020)
original research
articles dedicated to studying molecules or compounds protecting from cisplatin-induced toxicity to sensory hair cells
using zebrafish1
Exclusion criteria
full text not available
literature review
lack of information about the experimental groups
After applying the selection criteria, 26 papers were selected for analysis. The following information was extracted from each publication (for the complete dataset see Table A1 in the Appendix section):
the objective of the study
the experimental flow (length of exposure to cisplatin, used concentration of cisplatin, conditions of treatment with compound/molecule, the sample size2)
mechanism of action of compound/molecule used
the adverse effects of the compound/molecule
the outcome measurements
The extracted information was analyzed and the information was summarized and presented in the Results section.
Results
The focus of selected literature
The present review has identified research articles, in which a lateral line of zebrafish was used to study cisplatin-induced hearing loss and otoprotection. The general goals of all the papers were: (i) to identify molecules and compounds protecting the sensory hair cells from cisplatin toxicity (fourteen papers); (i) to develop new knowledge about the mechanisms mediating cisplatin-induced hair cell damage (five papers); (ii) to screen the ototoxicity of various drugs (two papers); (iii) to investigate their synergistically ototoxic effect on hair cells (three articles) and to develop behavioral methods dedicated to zebrafish (two papers).
Zebrafish culture conditions
In all studies, zebrafish embryos were kept at 28.5°C on a 14 h light/10 h dark cycle. The embryos were maintained in Petri dishes in embryo media (EM). Two types of EM were used. The first EM was composed of 15.0 mM NaCl, 0.5 mM KCl, 1.0 mM MgSO4, 0.12 mM KH2PO4, 0.074 mM Na2HPO4, 1.0 mM CaCl2, 0.5 mM NaHCO3 in distilled water.17–28 The second EM was compsed of 14.9 mM NaCl, 0.503 mM KCl, 0.994 mM MgSO4, 0.150 mM KH2PO4, 42 mM Na2HPO4, 0.986 mM CaCl2, 0.714 mM NaHCO3 in distilled water.12,29–31,32,33 In addition, the embryos were also kept in E3 embryo media consisting of 5 mM NaCl, 0.17 mM KCl, 0.33 mM CaCl2, 0.33 MgSO4 or in a standard bath solution containing: 120 mM NaCl, 2 mM KCl, 10 mM HEPES (2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid), 2 mM CaCl2 and 0.7 mM NaH2PO4 adjusted to pH 7.2.21,34 For the anesthesia, MS-222 (3-aminobenzoic acid ethyl ester, methanesulfonate salt) was used. Various sample size was used in the studies, ranging from 3 to 30 zebrafish per experimental group.
Experimental protocols
Based on the literature, cisplatin at low concentrations (50–100 μm) causes the death of lateral line hair cells in zebrafish larvae after 24 h, whereas cisplatin at higher concentrations (250–500 μm) is toxic after 6 h.11,12 In the selected articles, the zebrafish were exposed to cisplatin for either 24 h (six studies), 6 h (six studies), 4 h (seven studies), and 16 h (one study). In one article, two exposure times were used (12 and 24 h). In the research dedicated to adult zebrafish, the incubation time used was 45 min and 24 h. The results were examined after one cisplatin injection (four articles). In one article, there was no information on that topic.
Various cisplatin concentrations and different exposure times were applied in the studies. Thirteen studies used cisplatin in a concentration lower than 200 µM. Three studies applied cisplatin in a concentration ranging from 200 to 800 µM, whereas seven studies used cisplatin at concentrations ranging between 0.8 and 1 mM (Table 1). In studies focusing at the inner ear of adult zebrafish, 25 mg/kg cisplatin was injected. Two articles provided no information about the concentrations used. The data are summarized in Table 1.
Table 1.
Concentration and duration of exposure to cisplatin (CIS) based on the extracted data.
| Article | Cisplatin concentration | Duration of exposure to cisplatin | Experimental flow |
|---|---|---|---|
| Vlasits et al. 12 | 0–100 µM | 24 h | Pre-treatment for 1 h, then co-exposure for 24 h |
| Todd et al. 5 | 0–1000 µM | 4 h | CIS exposure for 4 h |
| Monroe et al. 35 | 100 µM | 45 min | Co-exposure for 45 min |
| Hong et al. 24 | 1000 µM | 4 h | Co-exposure for 4 h |
| Lee et al. 23 | 1000 µM | 4 h | Co-exposure for 4 h |
| Choi et al.42 | 1000 µM | 6 h | Co-exposure for 6 h |
| Min et al. 26 | 1000 µM | 6 h | Pre-treatment for 150 min, then CIS exposure for 6 h |
| Niihori et al. 6 | 1000 μM | 4 h | Pre-treatment for 12 h, then co-exposure for 4 h |
| Monroe et al. 33 | 100–500 µM | 45 min | CIS exposure for 45 min,regeneration for 3 h, then co-exposure for 15 h (cell culture) |
| Coffin et al. 31 | 250–1000 µM | 6 h | Pre-treatment for 1 h, then co-exposure for 6 h |
| Uribe et al. 18 | 250–1500 μM | 4 h | Co-exposure for 4 h |
| Kitcher et al. 30 | 25–200 µM | 24 h | Co-exposure for 24 h |
| Monroe et al.36 | 25 mg/kg | Single microinjection | CIS microinjection, 24 after drug microinjection |
| Mackenzie and Raible 29 | 50 µM | 24 h | Co-exposure for 24 h |
| Shin et al. 37 | 50 µM | 24 h | Pre-treatment for 1 h, then CIS exposure for 24 h |
| Thomas et al. 27 | 50 µM | 24 h | Pre-treatment for 1 h, then CIS exposure for 24 h |
| Zheng et al. 38 | 50 µM | 24 h | Pre-treatment for 2 h, then co-exposure for 24 h |
| Hirose et al. 22 | 50 µM | 6 h | Co-exposure for 6 h |
| Kruger et al. 25 | 500 µM | 6 h | Co-exposure for 6 h |
| Vargo et al. 21 | 50–200 µM | 16 h | Co-exposure for 16 h |
| Thomas et al. 19 | 50–500 µM | 6 and 24 h | Pre-treatment for 1 h, then co-exposure for 6 h and 24 h |
| Rocha-Sanchez et al. 34 | 50–800 µM | 6 h | Pre-treatment for 2 h, then co-exposure for 6 h |
| Gu et al. 39 | 60 µM | 24 h | Pre-treatment for 4 h, then CIS exposure for 24 h |
| Pang et al. 28 | 600 µM | 12 and 24 h | Pre-treatment for 1 h, then co-exposure for 12 h and 24 h |
| Wang et al. 40 | Data not available | 24 h | Co-exposure for 24 h |
Based on the protocols used, three main experimental designs were identified. In the first design, simultaneous incubation with tested compounds (apocynin, berbamine, edaravone, quercetin, ORC-13661, mdivi-1, NAC, CYM-5478) and cisplatin was used.20,21,23–25,30 The second experimental strategy used preconditioning with an otoprotective substance (quinoxaline, Z-LLF-CHO, benzamil, quinine, leupeptin, 3-MA, D-methionine, rapamycin, Sal B, SRT1720 and FUT-175) followed by simultaneous incubation with cisplatin.12,19,31,34,38 The last experimental strategy consisted of preconditioning with otoprotective medication (dexmedetomidine, KR-22335, CHCP1, CHCP2, ATX) and later a sole exposure to cisplatin.17,26,27,40 In one experimental protocol using adult zebrafish, a single microinjection of cisplatin and drug was applied, whereas when cell cultures were used, the experimental procedure included incubation with cisplatin and then co-incubation with the otoprotective substances.33,35,36
In the studies dedicated to apocynin, L-Serine, CHCP1, Rapamycin, Sal B, KR-22335, ORC-13661, ATX, NAC, CYM-5478, and curcuminoids zebrafish served as a second model in addition to mice, guinea pig, cancer cell lines and HEI-OC1 mouse cell line derived from the organ of Corti.17,20,27,28,30,35,38–40
The details regarding the experimental design for otoprotective screening are summarized in Table 2.
Table 2.
The experimental conditions of otoprotective experiments.
| Article | Name of substance | Functional target | The optimal concentration of otoptotective substance | Cisplatin concentration | Experimental flow |
|---|---|---|---|---|---|
| Monroe et al. 36 | CLEFMA and EF24 | Oxidative stress | 5 mg/kg | 25 mg/kg | CIS microinjection, 24 h after the compound microinjection |
| Monroe et al. 35 | L-Serine | Oxidative stress | 100 µM | 100 µM | Co-exposure for 45 min |
| Hong et al. 24 | Edaravone | Oxidative stress | 750 µM | 1000 µM | Co-exposure for 4 h |
| Lee et al. 23 | Quercetin | Oxidative stress | 100 µM | 1000 µM | Co-exposure for 4 h |
| Choi et al. 42 | Apocynin | Oxidative stress | 125–250 µM | 1000 µM | Co-exposure for 6 h |
| Kruger et al.25 | Berbamine | MET-channel | 1 and 10 µM | 500 µM | Co-exposure for 6 h |
| Vargo et al. 21 | Mdivi-1 | MET-channel | 3–7 µM | 50-100 µM | Co-exposure for 16 h |
| Kitcher et al. 30 | ORC-13661 | MET-channel | 2.2 µM | 200 µM | Co-exposure for 24 h |
| Wang et al. 39 | NAC | Oxidative stress | Data not available | 1000 µM | Co-exposure for 24 h |
| Wang et al. 39 | CYM-5478 | Oxidative stress | Data not available | 20 µM | Co-exposure for 24 h |
| Shin et al. 37 | KR-22335 | Oxidative stress | 1, 10, 100 µg/mL | 50 µM | Pre-treatment for 60 min, then CIS exposure for 24 h |
| Thomas et al. 27 | CHCP2 | Oxidative stress | 100 µM | 50 µM | Pre-treatment for 60 min, then CIS exposure for 24 h |
| Pang et al. 28 | Rapamycin | Autophagy | 10 µM | 600 µM | Pre-treatment for 60 min, then co-exposure for 12 h |
| Pang et al. 28 | SRT1720 | Autophagy | 5 µM | 600 µM | Pre-treatment for 60 min, then co-exposure for 12 h |
| Vlasits etal. 12 | Benzamil | MET-channel | 50 µM | 0–100 µM | Pre-treatment for 60 min, then co-exposure for 24 h |
| Coffin et al. 31 | Leupeptin | Calpains | 500 µM | 250 µM | Pre-treatment for 60 min, then co-exposure for 6 h |
| Coffin et al. 31 | Z-LLF-CHO | Protease | 25 µM | 750 µM | Pre-treatment for 60 min, then co-exposure for 6 h |
| Coffin et al. 31 | FUT-175 | Proteasome | 10 µM | 500–1000 µM | Pre-treatment for 60 min, then co-exposure for 6 h |
| Coffin et al. 31 | D-methionine | Oxidative stress | 5 mM | 250–1000 µM | Pre-treatment for 60 min, then co-exposure for 6 h |
| Coffin et al. 31 | 3-MA | Autophagy | 5 mM | 250, 750 µM | Pre-treatment for 60 min, then co-exposure for 6 h |
| Thomas et al. 19 | Quinine | MET-channel | 100 µM | 250–500 µM | Pre-treatment for 60 min, then co-exposure for 6 h |
| Zheng et al. 38 | Sal B | Oxidative stress | 40 µM | 0–50 µM | Pre-treatment for 120 min, then co-exposure for 24 h |
| Rocha-Sanchez et al. 34 | Quinoxaline | Oxidative stress | 300 µM | 50–400 µM | Pre-treatment for 120 min, then co-exposure for 6 h |
| Min et al. 26 | Dexmedetomidine | Oxidative stress | 0.1, 1, 10 µM | 1000 µM | Pre-treatment for 150 min, then CIS exposure for 6 h |
| Gu et al. 40 | ATX-LPN | Oxidative stress | 50 μg/mL | 60 µM | Pre-treatment for 240 min, then CIS exposure for 24 h |
Berbamine, quinine, and leupeptin offered protection against high concentrations of cisplatin (250–500 µM), whereas ORC-13661, benzamil, L-Serine and mdivi-1 protected against cisplatin used in lower concentration (0–200 µM).12,19,21,25,30,31,35 Dexmedetomidine, quercetin, and edaravone protected against 1000 µM cisplatin.23,24,26 FUT-175 (500–1000 µM), quinoxaline (50–400 µM), D-methionine (250–1000 µM), and KR-22335 offered relatively broad protection whereas CHCP2, Sal B, and ATX-LPN exhibited a narrow protection profile (<50 and <60 µM), respectively. Rapamycin and SRT1720 protected the lateral line of larval zebrafish against 12 h exposure to 600 µM cisplatin.17,28,31,34,38,40 The proteasome inhibitor (Z-LLF-CHO) protected from the exposure to 750 μM cisplatin but had no effect when the lower concentration of cisplatin was used.31 The curcuminoids offered protection against a single cisplatin injection (25 mg/kg).36
Five anti-cancer drugs (sunitinib, raloxifene, dactinomycin, carmustine, and exemestane) were identified as ototoxic substances by using zebrafish larvae.22 Moreover, drugs such as doxorubicin, vincristine and vinorelbine were shown to have synergistic ototoxic effects.22 Interestingly, carboplatin caused no ototoxicity in the zebrafish, possibly due to differences in the genome and proteome between mammals and fish, which affects the targeting ability of carboplatin in fish.22
Mechanism of otoprotective action of studied compounds and molecules
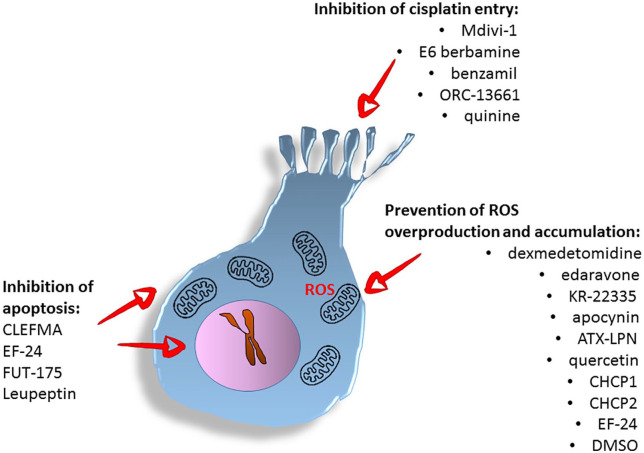
This review has identified three general strategies used to protect the sensory hair cells from cisplatin-induced damage (see Figure 2).
Figure 2.
Summary of anti-ototoxic strategies and respective compounds.
The physiological production of reactive oxygen species is essential for cellular metabolism; however, overproduction or accumulation of ROS can lead to apoptosis. The first type of otoprotective strategy aims at the reduction of overproduction and/or accumulation of ROS to restore cellular homeostasis leading to cell survival. In agreement with the above notion, the otoprotective action of dexmedetomidine, edaravone, KR-22335, apocynin, ATX-LPN, quercetin, and the CHCP1 and CHCP2 molecules were related to their ability to decrease ROS production.17,20,23,26 Similarly, the EF-24 was suggested to prevent intracellular ROS formation via inhibition of NF-kB-induced signaling and suppressed expression of oncogenic miRNAs, including miR-21.33 Also dimethyl sulfoxide (DMSO) used at low concentrations is a known scavenger of the hydroxyl radicals.41
Apoptosis is a programmed cell death initiated via the intrinsic or extrinsic pathway. The intrinsic pathway can be started by intracellular processes, such as damage to DNA or overproduction of ROS, both known to be induced by cisplatin. In contrast, the extrinsic pathway can be activated by extracellular ligands binding the transmembrane death receptors. At the point of initiation and execution of apoptosis, several proteolytic proteins from the family of caspases and sometimes from the family of calpains may be activated. Targeting the apoptotic or the autophagy pathways by otoprotective substances and compounds may lead to cell survival and is the second type of otoprotective strategy.42,43 Studies of the inner ear of adult zebrafish have suggested that synthetic curcumin analogs (CLEFMA or EF-24) protect the auditory system against cisplatin-induced damage by inhibition of the apoptotic pathway. Following 48 h exposure to cisplatin, curcuminoids administration induced significant recovery of ABR thresholds (0.1–3 kHz), when compared to cisplatin only.36 Also, the serin protease inhibitor FUT-175 might function as an apoptosis blocker and protect hair cells from death via interaction with the intrinsic and extrinsic apoptotic pathways.31 Leupeptin, inhibits serine and cysteine proteases—plasmin, trypsin, papain, calpain, and cathepsin B, of which calpain and cathepsin were implicated to be involved in apoptotic processes.44
The therapeutic impact of quinoxaline (Qx) against cisplatin was attributed to the prevention of apoptosis of the sensory hair cells in a still indeterminate way.34 The cellular process of autophagy, in which the damaged mitochondria are being eliminated from the cells, thus, promoting cell survival, can counteract apoptosis.45 In agreement with that, the experiments demonstrated that the exposure of larval zebrafish to autophagy modulators (3-MA and rapamycin) prevented the cisplatin-induced damage to the lateral line.28,31
The third otoprotective strategy involves blocking the entry of toxic substances into the inner ear and, in particular, into hair cells.31,46 Lateral line hair cells share mechanisms of mechanotransduction (MET) with hair cells of the inner ear.19 Data in the literature suggest that blocking MET channel prevents the intracellular accumulation of cisplatin.19 Currently, it is unclear if cisplatin enters the lateral line of zebrafish directly through the MET channels. However, studies using blockers of MET channels (Mdivi-1, E6 berbamine, benzamil and ORC-13661, quinine) in larval zebrafish have confirmed that blocking of MET channels protects from the cisplatin-induced hair cell death.12,19,21,25 Lastly, blockade of mechanotransduction using quinine protected from the cisplatin-induced hair cell loss. However, quinine is a well-known ototoxin; therefore, its medical usefulness as an otoprotector might be negligible.19,47
The adverse effects of the tested compounds/molecule
The adverse effects of the compounds and molecules used as otoprotectors were identified during data extraction. The adverse effect was found to be time and dose-dependent. Mdivi-1 in doses higher than 10 μM was toxic to zebrafish. CHCP1 started to be lethal, for the zebrafish larvae, in concentrations above 100 μM.21 On the one hand, Z-LLF-CHO presented otoprotective properties; however, continuous exposure to Z-LLF-CHO was toxic to the hair cells.31 A similar dependence was ecountered with DMSO at a concentration of 0.01%, which was toxic to zebrafish, as opposed to lower concentration which could induce otoprotection.48 The DMSO concentration of 0.5% or higher were shown to be toxic to the auditory hair cells in the rat cochlear explant cultures.49 In combination with cisplatin, DMSO has induced more extensive hair cell death than cisplatin alone.18 The protective effect of quinoxaline depends on the incubation protocol. The otoprotection D-methionine was limited to incomplete hair cell survival seen in all cases.31 Incubation with flubendazole during recovery after cisplatin exposition blocked producing the new hair cells in zebrafish.50 The adverse effects of other substances were not reported.
Outcome measurements
In the majority of the reviewed studies, the assessment of the otoprotective and ototoxic effects involved sensory hair cell counting (eighteen articles).12,17,18,20–22,24,25,27,28,30,31,32–34,39,40 An additional method, evaluating the hair cell function, was the uptake of FM1-43 dye (four articles) and the recording of microphonic potentials (two articles).19,21,25,34,43 In six articles, the TUNEL assay was applied, detecting single-stranded brakes in the chromosomal DNA (a feature of apoptosis).20,23,24,34,37,38 The proliferation assay was used in two articles, whereas the fluorescent platinum analog (Rho-Pt) uptake assay was used in one article.27,29,34 In two studies, anatomic changes were correlated with behavioral modification observed in the zebrafish with the help of rheotaxis.6,32 In another two studies, in which the inner ear cell of zebrafish was cultured, spectrophotometry was used.33,35 Lastly, one publication assessed the hearing abilities of adult zebrafish by measuring auditory brainstem responses (ABR).36
Discussion
This review aimed to assess the usefulness of the lateral line in zebrafish for studying cisplatin-induced ototoxicity and otoprotection. Twenty-two studies published between January 2009 and May 2020 dedicated to the zebrafish larvae and four dedicated to the studies of the inner ear in adult zebrafish met the inclusion criteria. All articles have confirmed the usefulness of the lateral line in the high-throughput screening of otoprotective substances. These studies employed anatomical and behavioral assays to measure the experimental outcome.
In the inner ear, cisplatin accumulates predominantly in the stria vascularis. However, the most significant damage induced by cisplatin is seen in the sensory hair cells, the supporting cells in the mammalian vestibular system (utricle), and the regenerative potential of the utricle.14,51,52 Cisplatin induces cytotoxicity by binding to the nuclear DNA, leading to apoptosis, particularly in the proliferating cells.53 In addition to that, cisplatin can mediate the activation of NADPH oxidase 3 (NOX3), which catalyzes the production of superoxide, representing reactive oxygen species (ROS). Overproduction of ROS can activate the signal transducer and activator of transcription 1 (STAT1), inducing the inflammation.46 Previous studies have shown that ROS might induce autophagy, which, depending on the stimulation context, can promote either cell survival or lead to cell death.54,55
The critical step responsible for the ototoxic properties of cisplatin is its transport inside the sensory cell. Several studies have reported that mechanotransducer (MET) channels mediate the entry of cisplatin into cochlear hair cells, but it remains unclear whether MET channels are blocked during that process.30,46,53 A study using larval zebrafish confirmed that cisplatin-induced damage to the hair cells relies on functional MET channels.19 Furthermore, the effect of platinum (II) complexes on adult zebrafish auditory system suggested that cisplatin, and to a limited extent phenanthriplatin, can induce hair cells loss in particular regions of the saccule but not the utricle,56 suggesting either various regional susceptibility to cisplatin or its distinct diffusion or transport pattern.
Studies using larvae zebrafish included in the present review confirmed the otoprotective properties of astaxanthin (ASX), N-acetylcysteine (NAC), rapamycin, aalvianolic acid B, SRT1720, E6 berbamine, quercetin, dexamethasome, dexmedetomidine, edaravone, quinine, dimethyl sulfoxide (DMSO), FUT-175, benzamil, apocynin, and flubendazole. What is more, the clinical adverse effects of the above molecules are already known, as ASX, NAC, rapamycin, dexamethasone, quinine, dexmedetomidine, DMSO, edaravone, flubendazole are used in clinical trials or practice (see Table 3). Interestingly, the screening of new molecules by the zebrafish model, identified new otoprotective compounds such as CHCP1, CHCP2, apocynin, quinoxaline, ORC-13661, SRT1720, CYM-5478, mdivi-1, KR-22335, leupeptin, and 3-MA. Although no information is yet available on the impact of these compounds on cisplatin-efficiency, their small size makes a local delivery possible, which could be a solution to avoid the risk of antitumor interference and other adverse effects.
Table 3.
Candidate molecules and compounds for the protection against cisplatin induced-hearing loss.
| Molecule or compound | IUPAC name | Natural occurrence | Direct effect on cancer cells | Modulation of cisplatin anti-cancer cytotoxicity | Known adverse effects in humans | Use in human trials |
|---|---|---|---|---|---|---|
| 3-MA | 3-Methylpentane | A structural isomer of hexane | No data available | No data available | Irritation, headache, drowsiness, dizziness, loss of coordination, convulsions, and coma57 | Technically possible |
| Apocynin | 1-(4-hydroxy-3-methoxyphenyl)ethanone | Apocynin is a natural polyphenolic compound isolated from various plants such as Apocynum cannabinum, Picrorhiza kurroa | Apocynin inhibits the NF-kB activation in androgen-independent rat prostate cancer cell lines58 | No data available | Irritating to eyes, respiratory system and skin59 | No data available |
| Astaxanthin (ATX) | 3,3'-Dihydroxy-beta,beta-carotene-4,4'-dione | Astaxanthin is a keto-carotenoid in the terpenes class of chemical compounds. It is classified as a xanthophyll but it is a carotenoid with no vitamin A activity. It is found in the majority of aquatic organisms with red pigment. | Enhances CTL activity and IFN-gamma production against Meth-A tumor cells in parallel to suppression of tumor growth60 | No data available | Doses of up to 50 mg have been tolerated. An upper toxicity limit is not known61 | Technically possible |
| Benzamil | 3,5-diamino-N-(N'-benzylcarbamimidoyl)-6-chloropyrazine-2-carboxamide | Synthetic molecule | No data available | Amiloride (10, 30, and 100 μmol/L) concentration-dependently potentiated erlotinib-induced inhibition of cell proliferation and colony formation in the four pancreatic cancer cell lines62 | Irritating to eyes, respiratory system and skin63 | Technically possible |
| CHCP1 | Not available | Not available | Not available | Not available | Not available | No data available |
| CHCP2 | Not available | Not available | Not available | Not available | Not available | No data available |
| CLEFMA | (Z)-4-[(3E,5E)-3,5-bis[(2-chlorophenyl)methylidene]-4-oxopiperidin-1-yl]-4-oxobut-2-enoic acid | Synthetic molecule | IC50 for the human lung carcinoma A549 cell line measured by MTT test - 13.82 µM (24 h ) and 16.05 (48 h) | Decreases the cisplatin-induced ROS production, decreases the motility of cancer cells in the presence of cisplatin36 | No data available | No data available |
| CYM-5478 | 2-[[4-[5-(3,4-diethoxyphenyl)-1,2,4-oxadiazol-3-yl]-2,3-dihydro-1H-inden-1-yl]amino]ethanol | No data available | No data available | No data available | No data available | No data available |
| Dexamethasone | (8S,9R,10S,11S,13S,14S,16R,17R)-9-fluoro-11,17-dihydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-3-one | Dexamethasone is a synthetic adrenal corticosteroid | Induction of apoptosis and enhancement chemosensitivity of GRα-rich colon cancer cell lines64 | Dexamethasone reduces cisplatin efficiency in different human NSCLC cell lines in a p53-dependent manner65 | Vertigo, acne, insomnia, increased appetite, irritability, muscle weakness, impaired wound healing, amnesia increased blood sugar levels66 | Technically possible |
| Dexmedetomidine | 5-[(1S)-1-(2,3-dimethylphenyl)ethyl]-1H-imidazole | Dexmedetomidine is an imidazole derivate and active d-isomer of medetomidine | Dexmedetomidine promots cell proliferation, migration and upregulats antiapop- totic protein in human lung carcinoma and neuroglioma cell lines67 | no data available | low or high blood pressure (hypotension or hypertension), slow heart rate (bradycardia), nausea, dry mouth, irregular heartbeat, fever, vomiting, low blood plasma68 | technically possible |
| Dimethyl sulfoxide (DMSO) | Methylsulfinylmethane | Synthetic molecule | DMSO inhibits the tumor volume growth in breast cancer bearing mice (except for 0.25 mg/g) in a time and dose-dependent way.37 | DMSO did not have any effect on cisplatin's cytoxicity on human cervical (KB-3-1) or colorectal (DLD-1) carcinoma cells69 | Garlic-like taste (a few minutes after instillation), odor on breath 70 | Technically possible |
| D-methionine | (2R)-2-amino-4-methylsulfanylbutanoic acid | D-methionine is an optically active form of methionine having D-configuration. It is a methionine and a D-alpha-amino acid | No data available | No data available | No data available | Technically possible |
| E6 Berbamine | (20,21,25-trimethoxy-15,30-dimethyl-7,23-dioxa-15,30-diazaheptacyclo[22.6.2.23,6.18,12.114,18.027,31.022,33]hexatriaconta-3(36),4,6(35),8,10,12(34),18,20,22(33),24,26,31-dodecaen-9-yl) 4-nitrobenzoate | Berbamine is a natural compound derived from the Berberis amurensis plant, E6 Berbamine is a synthetic molecule | Induction of apoptosis in breast cancer and lung cancer cell lines71 | No data available | Jaundice, stomach upset, lethargy, nose bleed, skin and eye irritation, kidney irritation72 | Technically possible |
| Edaravone | 5-methyl-2-phenyl-4H-pyrazol-3-one | Not available | Lack of cytostatic effect in colon carcinoma cells73 | No data available | Bruising, gait disturbance, headache,skin inflammation or rash, eczema, respiratory disorder,oxygen deficiency74 | Technically possible |
| EF24 | (3E,5E)-3,5-bis[(2-fluorophenyl)methylidene]piperidin-4-one | Synthetic molecule | Cytostatic effect75 | Decreases the cisplatin-induced ROS production, decreases the motility of cancer cells in the presence of cisplatin36 | No data available | No data available |
| Flubendazole (Flu, microtubule assembly blocker) | Methyl N-[6-(4-fluorobenzoyl)-1H-benzimidazol-2-yl]carbamate | Flubendazole is a member of the class of mebendazole. The benzoyl group is replaced by a p-fluorobenzoyl group | Flubendazole inhibited breast cancer cells proliferation in dose- and time-dependent manner50 | No data available | Abdominal pain, headache, dizziness, diarrhoea76 | Technically possible |
| FUT-175 | (6-carbamimidoylnaphthalen-2-yl) 4-(diaminomethylideneamino)benzoate;methanesulfonic acid | Nafamostat Mesylate (MN) is the mesylate salt form of nafamostat | NM significantly inhibits proliferation, migration, and invasion in MDA-MB231 triple-negative breast cancer (TNBC) cells77 | No data available | Nausea, vomiting, itching and eruption78 | Technically possible |
| KR-22335 | 3-Amino-3-(4-fluoro-phenyl)-1H-quinoline-2,4-dione | Synthetic molecule | No data available | KR-22332 does not appear to interfere with the antitumor effect of chemotherapeutic37 | No data available | No data available |
| Leupeptin | N-Acetyl-L-leucyl-L-leucyl-L-argininal hemisulfate salt | Organic compound produced by actinomycetes | No data vailable | No data availabe | No data available | Technically possible |
| L-serine | (S)-2-Amino-3-hydroxypropanoic acid H-Ser-OH; | Serine is a nonessential amino acid derived from glycine. L-serine is the L-enantiomer of serine. | No data available |
L-serine inhibits the antitumor effect of cisplatin in human gastric cancer cell lines SGC7901, BGC823, and MGC803 in a dose-dependent manner79 | Tiredness, anxiety, chronic fatigue, neurotoxicity, depression80 | Technically possible |
| Mdivi-1 | 3-(2,4-dichloro-5-methoxyphenyl)-2-sulfanylidene-1H-quinazolin-4-one | Synthetic molecule | No data available | Synergistic action with cisplatin81 | No data available | No data available |
| NAC | N-Acetyl-L-cysteine ((2R)-2-acetamido-3-sulfanylpropanoic acid) | NAC is an essentially prodrug that is converted to cysteine (in the intestine by the enzyme aminoacylase 1) and absorbed in the intestine into the blood stream. Cysteine is a key constituent to glutathione and hence administration of acetylcysteine replenishes glutathione stores. | Growth inhibition of several cancers82 | No data available | Unlikely cause of clinically apparent liver injury83 | Technically possible |
| ORC-13661 | (1R,8S)-4-[(4-chlorophenyl)carbamoylamino]-11-methyl-5-thia-11-azatricyclo[6.2.1.02,6]undeca-2(6),3-diene-3-carboxamide | No data available | No data available | No data available | No data available | No data available |
| Quercetin | 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxychromen-4-one | Quercetin is a flavonoid widely distributed in many plants, vegetables and fruits | Growth inhibition in the human breast carcinoma cell line58 | Synergistic action with cisplatin against human oral squamous cell carcinoma (OSCC) (cell lines Tca-8113 and SCC-15)84 | Headache (oral use), numbness and tingling (oral use), shortness of breath (intravenous use), nausea and vomiting (intravenous use), kidney damage (intravenous use greater than 945 mg/m2)85 | Technically possible |
| Quinine | (R)-[(2S,4S,5R)-5-ethenyl-1-azabicyclo[2.2.2]octan-2-yl]-(6-methoxyquinolin-4-yl)methanol | Quinine is a quinidine alkaloid isolated from the bark of the cinchona tree | Quinine possess cytotoxic effect on laryngeal cancer cells (147.58 μM/mL for 24 h and 123.74 μM/mL for 48 h)86 | No data available | Headache, ringing in the ears, trouble seeing, and sweating, deafness, low blood platelets, an irregular heartbeat, during pregnancy is harm to the baby87 | Technically possible |
| Quinoxaline | Quinoxaline | Synthetic molecule | Cytostatic effect88 | No data available | No data available | No data available |
| Rapamycin | (1R,9S,12S,15R,16E,18R,19R,21R,23S,24Z,26E,28E,30S,32S,35R)-1,18-dihydroxy-12-[(2R)-1-[(1S,3R,4R)-4-hydroxy-3-methoxycyclohexyl]propan-2-yl]-19,30-dimethoxy-15,17,21,23,29,35-hexamethyl-11,36-dioxa-4-azatricyclo[30.3.1.04,9]hexatriaconta-16,24,26,28-tetraene-2,3,10,14,20-pentone | Isolated from Streptomyces hygroscopicus found in an Easter Island soil sample | Used for therapy of carcinoma89 leukemia (B-CLL)90 melanoma91 | Synergistic action with cisplatin against various types of cancer92,93 | Stomatitis, behavioral disturbance, rash, pyrexia, pneumonia, gastroenteritis, aggression, agitation, amenorrhea, hypercholesterolemia, elevated partial thromboplastin time, neutropenia, infectiorapa94 | Technically possible |
| Salvianolic acid B (Sal B) | (2R)-2-[(E)-3-[(2R,3R)-3-[(1R)-1-carboxy-2-(3,4-dihydroxyphenyl)ethoxy]carbonyl-2-(3,4-dihydroxyphenyl)-7-hydroxy-2,3-dihydro-1-benzofuran-4-yl]prop-2-enoyl]oxy-3-(3,4-dihydroxyphenyl)propanoic acid | Sal B is a compound extracted from Salvia miltiorrhiza (Danshen) | Slows the growth of breast cancer cells95 and and lung cancer cells96 | No data available | Allergy, dizziness, headache, mild GI symptoms, and reversible thrombocytopenia97 | Technically possible |
| SRT1720 (small-molecule activator of the sirtuin subtype SIRT1) | N-[2-[3-(piperazin-1-ylmethyl)imidazo[2,1-b][1,3]thiazol-6-yl]phenyl]quinoxaline-2-carboxamide;2,2,2-trifluoroacetic acid | No data available | Cytostatic effects on various cancer88 | No data available | Stomatitis, behavioral disturbance, rash, pyrexia, pneumonia, gastroenteritis, aggression, agitation, amenorrhea, hypercholesterolemia, elevated partial thromboplastin time, neutropenia, infection | Technically possible |
| Z-LLF-CHO | Benzyl N-[(2S)-4-methyl-1-[[(2S)-4-methyl-2-[(1-oxo-3-phenylpropan-2-yl)amino]pentanoyl]amino]-1-oxopentan-2-yl]carbamate | No data available | Z-LLF-CHO induces early tumor regression and a delay in tumor progression in a murine model of Burkitt's lymphoma98 | No data available | No data available | No data available |
The otoprotective effect of various substances observed in zebrafish was also demonstrated in other animal models and humans. The administration of dexamethasone delivered by intratympanic injection in cancer patients provided narrow protection against hearing-loss (at 6 kHz).3 Lack of otoprotection was observed in patients who have received cisplatin chemotherapy and an injection of poloxamer hydrogel containing dexamethasone (OTO-104).99 Currently, the OTO-104 is tested in patients with unilateral Meniere’s Disease (administrated by a single intratympanic injection).100 Animal studies with guinea pigs demonstrated an otoprotective effect of dexamethasone only in co-administration with curcumin, whereas when both substances were administrated alone provided no protection in the zebrafish.26,36,101 To consider that data in the literature show that dexamethasone reduces the cisplatin efficiency.65
N-Acetylcysteine (NAC) which was found protective in zebrafish, presented conflicting results in humans. One study found that a local delivery of NAC provided an otoprotection at 8 kHz (using the patient’s opposite ear as a control);70 another study by Yoo et al, reported no differences between cancer patients treated with NAC and an untreated group.102 There was no protective effect of an oral low-dose NAC in patients with head and neck cancer from cisplatin-induced toxicities and oxidative stress.103 Currently, there is an IV phase clinical trial of the effectiveness of intratympanic administration of N-acetylcysteine in patients treated with cisplatin.104
Studies in zebrafish confirmed the otoprotective effect of D-methionine observed in guinea pigs. Guinea pigs, after a local application of D-methionine in the round window, presented improved otoacoustic emissions.105 Multiple studies have demonstrated the D-methionine protection against cisplatin, amikacin and on permanent noise-induced hearing loss.106 Nevertheless, there is no information about the effect of D-methionine on cancer cells. Currently, the efficacy of L-serine, astaxanthin (ATX) and rapamycin are assessed in clinical trials for treating Alzheimer's disease, glucose intolerance and amyotrophic lateral sclerosis (ALS), which will help us understand their adverse effects.107–109
Similarities observed in the results obtained from different animal models and humans, support the idea of using of zebrafish for preclinical drug-screening. Nevertheless, not all substances identified by the zebrafish are acceptable for use in humans. The differences between results in otoprotective studies in humans and animals are caused by differences in the drug administration protocol, cohort size and cancer types.110
The majority of studies used anatomical or histological/immunohistological assays considered a gold standard in the ototoxicity research to measure the experimental outcome. Few studies employed specific, zebrafish-related behavioral methods such as rheotaxis. The rheotaxis uses the physiological principle of fish facing the oncoming current of water, which can be already observed in zebrafish larvae because the lateral line is entirely sensitive to the environment from 5 dpf.111 There is a dose-dependent relationship between cisplatin exposure, progressive hair cell damage, and reduced fish swimming behavior. Moreover, in response to otoprotective substances such as dexamethasone (5 μM+ cisplatin 1000 μM), the rheotaxis of zebrafish improved significantly.6 These results indicate that detecting changes in the swimming behavior could serve as a biomarker for the functionality of hair cells of fish. The automated swimming apparatus provides quick testing of large numbers of zebrafish and opens a new field in the ototoxic studies.6,32 Nevertheless, a few model limitations must be considered. Firstly, not only the lateral line but also the visual system are essential for rheotaxis.112 Secondly, higher concentrations of cisplatin may affect other systems of zebrafish (e.g., neurotransmitters or motor neurons), changing their swimming behavior.6
While collecting data for the present review, we have identified four articles dedicated to adult zebrafish inner ear (⩾6 mpf) and cisplatin-induced ototoxicity that were published between 2009 and 2020. Two of the manuscripts were studying the curcuminoids-dependent otoprotection against cisplatin.33,36 The third article focused on the effect of the platinum (II) complex on ABR hearing thresholds and found that the exposure to platinum (II) complex resulted in decreased hearing thresholds similarly as in response to cisplatin.56 The aim of the fourth article was to study whether L-serine might impact on the reduction of cisplatin-mediated ROS generation in vestibular tissue.35
The zebrafish larvae model offers two significant advantages: (i) it can be used during a screening of novel substances and compounds against cisplatin-induced hair cell damage; (ii) it can promote a better understanding of the mechanisms related to cisplatin-induced hearing loss.21 However, studies using zebrafish are not free of limitations. The first limitation is the ability of zebrafish hair cells to regenerate through the proliferation of supporting cells, which is not the case for mammalian hair cells. Subsequently, diverse damage protocols appear to induce different pathways leading to hair cell loss.111 This implies the importance of testing zebrafish over a longer time. The second limitation is the fact that the zebrafish hearing range is low and does not reflect this of humans, whereas cisplatin primarily affects the hair cells in high frequencies.113 In addition, larvae are sensitive to frequencies up to 1200 Hz, while the inner ear hair cells of the adult zebrafish can detect sound frequencies up to 4000 Hz. The inner ear of zebrafish is sensitive to a broader spectrum of frequencies, whereas the zebrafish lateral line is restricted to detecting low-frequency sounds.113,114 The third limitation is the lack of a stria vascularis in contrast to the human inner ear; thus, damage through strial mechanism could not be evaluated.24 The fourth limitation is that the zebrafish shares only 70% homology with the human genome, and therefore, some proteins that in humans will be targeted by the ototoxic drugs may no longer be a good target, or simply are absent in fish. It suggests that zebrafish could be used as a model only for studying acute ototoxicity at low frequencies; however, some findings may still be applicable to humans.19 The fifth pitfall of the presented studies is the varying sample size. Because of the character of our review, the sample size was not used as an exclusion criterion. In large part, the ototoxic studies focused on the zebrafish lateral line. In contrast, it is supposed that the zebrafish's inner ear may display different sensitivity to drugs or different times of response and regeneration.14 Nonetheless, the zebrafish lateral line is a relatively easy, quick, and inexpensive alternative to the ototoxicity studies in rodents.
The studies using zebrafish larvae demonstrated that cisplatin-induced hair cell loss could be reduced by lowering the levels of ROS, by the apoptosis and by inhibiting the MET channel. However, the effectiveness of substances and compounds tested still has to be proven under other experimental conditions. Importantly, the present review identified significant discrepancies between the protocols used, suggesting a need for the establishment of a consensus method to test the anti-ototoxic properties of compounds in zebrafish. These protocols should implement optimized concentrations of cisplatin and standardized incubation times. A practical suggestion to improve data presentation is using RDI (relative dose intensity), reflecting the ratio of “delivered” to the “planned” dose intensity and can be expressed as a percentage.
Conclusion
Despite a relatively low number of studies in the past 12 years, zebrafish prove to be a useful model for studying ototoxicity, especially during high throughput screening of new ototoxic compounds. However, the present study identified a need for developing a consensus protocol that should be used during future ototoxic studies. It is recommended to develop a standardized range of cisplatin concentration, duration of exposure, and the sequence of exposure concerning tested compounds. The generation of an agreed-upon experimental protocol should be on the priority list of researchers using the zebrafish model to study ototoxicity. This field of science would also benefit from standardization of outcome measures and units used, as well as from precise specification of observed effects and molecules (e.g., instead of using ROS, referring to a precise molecule that is produced, such as peroxide, superoxide, hydroxyl radical, singlet oxygen, or alpha-oxygen). Finally, even though zebrafish does not offer an ideal model for cisplatin-induced hearing loss, the mechanism of the damage to zebrafish hair cells is likely similar to that in humans, which is encouraging for further use of this model in ototoxicity and otoprotection-related studies.
Supplemental Material
Supplemental material, Supplementary for Use of zebrafish larvae lateral line to study protection against cisplatin-induced ototoxicity: A scoping review by Ewa Domarecka, Magda Skarzynska, Agnieszka J Szczepek and Stavros Hatzopoulos in International Journal of Immunopathology and Pharmacology
When in addition to zebrafish, other animals were studied, only the data related to zebrafish were acquired.
The sample size was not considered as an exclusion criteria.
Footnotes
List of abbreviations: 3-MA 3-methyladenine
ATX Astaxanthin
CHCP1 Cisplatin Hair Cell Protectant 1
CHCP2 Cisplatin Hair Cell Protectant 2
CYM-5478 an S1P2 selective agonist
Danio rerio zebrafish
dpf days post-fertilization
EM embryo media
FUT-175 Nafamostat Mesilate
KR-22335 3-Amino-3-(4-fluoro-phenyl)-1H-quinoline-2,4-dione
mdivi-1 Mitochondrial Division Inhibitor 1
MET mechanotransducer
NAC N-acetylcysteine
ORC-13661 Oricula Therapeutics' first product
RDI relative dose intensity
ROS Reactive oxygen species
SRT1720 a specific SIRT1 activator/ a cell-permeable inhibitor of the mitochondrial SIRT3
Z-LLF-CHO benzyl N-[(2S)-4-methyl-1-[[(2S)-4-methyl-2-[(1-oxo-3-phenylpropan-2-yl)amino]pentanoyl]amino]-1-oxopentan-2-yl]carbamate
Authors’ note: Agnieszka J Szczepek is also affiliated with Faculty of Medicine and Health Sciences, University of Zielona Góra, Zielona Góra, Poland.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Agnieszka J Szczepek  https://orcid.org/0000-0002-9292-6606
https://orcid.org/0000-0002-9292-6606
Stavros Hatzopoulos  https://orcid.org/0000-0002-9509-9722
https://orcid.org/0000-0002-9509-9722
Supplemental material: Supplemental material for this article is available online.
References
- 1. Szczepek AJ. (2017) Ototoxicity: Old and new foes. In: Hatzopoulos S. (ed) Advances in Clinical Audiology. IntechOpen; https://www.intechopen.com/books/advances-in-clinical-audiology/ototoxicity-old-and-new-foes (accessed 1 January 2020). [Google Scholar]
- 2. Arslan E, Orzan E, Santarelli R. Global problem of drug-induced hearing loss. Annals of the New York Academy Sciences 1999; 884(1): 1–14. [DOI] [PubMed] [Google Scholar]
- 3. Marshak T, Steiner M, Kaminer M, et al. Prevention of cisplatin-induced hearing loss by intratympanic dexamethasone: A randomized controlled study. Otolaryngology Head and Neck Surgery 2014; 150(6): 983–990. [DOI] [PubMed] [Google Scholar]
- 4. Rybak LP. Mechanisms of cisplatin ototoxicity and progress in otoprotection. Current Opinion in Otolaryngology & Head and Neck Surgery 2007; 15(5): 364–369. [DOI] [PubMed] [Google Scholar]
- 5. Wiedenhoft H, Hayashi L, Coffin AB. PI3K and Inhibitor of apoptosis proteins modulate gentamicin- induced hair cell death in the zebrafish lateral line. Frontiers in Cellular Neuroscience 2017; 11: 326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Niihori M, Platto T, Igarashi S, et al. Zebrafish swimming behavior as a biomarker for ototoxicity-induced hair cell damage: A high-throughput drug development platform targeting hearing loss. Translational Research 2015; 166(5): 440–450. [DOI] [PubMed] [Google Scholar]
- 7. Ou HC, Santos F, Raible DW, et al. Drug screening for hearing loss: Using the zebrafish lateral line to screen for drugs that prevent and cause hearing loss. Drug Discovery Today 2010; 15(7–8): 265–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Buck LM, Winter MJ, Redfern WS, et al. Ototoxin-induced cellular damage in neuromasts disrupts lateral line function in larval zebrafish. Hearing Research 2012; 284(1–2): 67–81. [DOI] [PubMed] [Google Scholar]
- 9. Ou HC, Raible DW, Rubel EW. Cisplatin-induced hair cell loss in zebrafish (Danio rerio) lateral line. Hearing Research 2007; 233(1–2): 46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vlasits AL, Simon JA, Raible DW, et al. Screen of FDA-approved drug library reveals compounds that protect hair cells from aminoglycosides and cisplatin. Hearing Research 2012; 294(1–2): 153–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shin YS, Song SJ, Kang S, et al. Novel synthetic protective compound, KR-22335, against cisplatin-induced auditory cell death. Journal of Applied Toxicology 2014; 34(2): 191–204. [DOI] [PubMed] [Google Scholar]
- 12. Hirose Y, Simon JA, Ou HC. Hair cell toxicity in anti-cancer drugs: Evaluating an anti-cancer drug library for independent and synergistic toxic effects on hair cells using the zebrafish lateral line. Journal of the Association for Research in Otolaryngology 2011; 12(6): 719–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee SK, Oh KH, Chung AY, et al. Protective role of quercetin against cisplatin-induced hair cell damage in zebrafish embryos. Human & Experimental Toxicology 2015; 34(11): 1043–1052. [DOI] [PubMed] [Google Scholar]
- 14. Hong SJ, Im GJ, Chang J, et al. Protective effects of edaravone against cisplatin-induced hair cell damage in zebrafish. International journal of pediatric otorhinolaryngology 2013; 77(6): 1025–1031. [DOI] [PubMed] [Google Scholar]
- 15. Mackenzie SM, Raible DW. Proliferative regeneration of zebrafish lateral line hair cells after different ototoxic insults. PLoS One 2012; 7(10): e47257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kruger M, Boney R, Ordoobadi AJ, et al. Natural bizbenzoquinoline derivatives protect zebrafish lateral line sensory hair cells from aminoglycoside toxicity. Frontiers in Cellular Neuroscience 2016; 10: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Min TJ, Kim WY, Ha YR, et al. Dexmedetomidine preconditioning attenuates Cisplatin-induced ototoxicity in zebrafish. Clinical and Experimental Otorhinolaryngology 2014; 7(4): 275–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rocha-Sanchez SM, Fuson O, Tarang S, et al. Quinoxaline protects zebrafish lateral line hair cells from cisplatin and aminoglycosides damage. Scientific Reports 2018; 8(1): 15119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Thomas AJ, Wu P, Raible DW, et al. Identification of small molecule inhibitors of cisplatin-induced hair cell death: Results of a 10,000 compound screen in the zebrafish lateral line. Otology & Neurotology 2015; 36(3): 519–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pang J, Xiong H, Zhan T, et al. Sirtuin 1 and autophagy attenuate cisplatin-induced hair cell death in the mouse cochlea and zebrafish lateral line. Frontiers in Cellular Neuroscience 2018; 12: 515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goldsmith JR, Jobin C. Think small: Zebrafish as a model system of human pathology. BioMed Research International 2012; 2012: 817341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Owens KN, Coffin AB, Hong LS, et al. Response of mechanosensory hair cells of the zebrafish lateral line to aminoglycosides reveals distinct cell death pathways. Hearing Research 2009; 253(1–2): 32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vernon PJ, Tang D. Eat-me: Autophagy, phagocytosis, and reactive oxygen species signaling. Antioxidants & Redox Signaling 2013; 18(6): 677–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang J, Song Q, Yu D, et al. Ontogenetic development of the auditory sensory organ in zebrafish (Danio rerio): Changes in hearing sensitivity and related morphology. Scientific Reports 2015; 5: 15943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Coffin AB, Ou H, Owens KN, et al. Chemical screening for hair cell loss and protection in the zebrafish lateral line. Zebrafish 2010; 7(1): 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.https://www.mathesongas.com/pdfs/msds/MAT29460.pdf (2008, accessed 24 July 2020).
- 27. Tunçer S, Gurbanov R, Sheraj I, et al. Low dose dimethyl sulfoxide driven gross molecular changes have the potential to interfere with various cellular processes. Scientific Reports 2018; 8(1): 14828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Coffin AB, Williamson KL, Mamiya A, et al. Profiling drug-induced cell death pathways in the zebrafish lateral line. Apoptosis 2013; 18(4): 393–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kitcher SR, Kirkwood NK, Camci ED, et al. ORC-13661 protects sensory hair cells from aminoglycoside and cisplatin ototoxicity. JCI Insight 2019; 4(15). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Harris JA, Cheng AG, Cunningham LL, et al. Neomycin-induced hair cell death and rapid regeneration in the lateral line of zebrafish (Danio rerio). Journal of the Association for Research in Otolaryngology 2003; 4(2): 219–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Uribe PM, Mueller MA, Gleichman JS, et al. Dimethyl sulfoxide (DMSO) exacerbates cisplatin-induced sensory hair cell death in zebrafish (Danio rerio). PLoS One 2013; 8(2): e55359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Thomas AJ, Hailey DW, Stawicki TM, et al. Functional mechanotransduction is required for cisplatin-induced hair cell death in the zebrafish lateral line. Journal of Neuroscience 2013; 33(10): 4405–4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Monroe JD, Moolani SA, Irihamye EN, et al. Effects of L-serine against cisplatin-mediated reactive oxygen species generation in zebrafish vestibular tissue culture and HEI-OC1 auditory hybridoma cells. Neurotoxicity Research. Epub ahead of print 27 March 2020. DOI: 10.1007/s12640-020-00188-y. [DOI] [PubMed] [Google Scholar]
- 34. Katary MA, Abdelsayed R, Alhashim A, et al. Salvianolic acid b slows the progression of breast cancer cell growth via enhancement of apoptosis and reduction of oxidative stress, inflammation, and angiogenesis. International Journal of Molecular Sciences 2019; 20(22): 5653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhao X, Fu J, Tang W, et al. Inhibition of serine metabolism promotes resistance to cisplatin in gastric cancer. OncoTargets and Therapy 2020; 13: 4833–4842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Borse V, Al Aameri RFH, Sheehan K, et al. Epigallocatechin-3-gallate, a prototypic chemopreventative agent for protection against cisplatin-based ototoxicity. Cell Death & Disease 2017; 8(7): e2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Breglio AM, Rusheen AE, Shide ED, et al. Cisplatin is retained in the cochlea indefinitely following chemotherapy. Nature Communications 2017; 8(1): 1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chaitanya GV, Babu PP. Activation of calpain, cathepsin-b and caspase-3 during transient focal cerebral ischemia in rat model. Neurochemical Research 2008; 33(11): 2178–2186. [DOI] [PubMed] [Google Scholar]
- 39. Campbell KCM, Fox DJ. Cisplatin-induced hearing loss. In: Le Prell C, Lobarinas E, Popper A, Fay R. (eds) Translational Research In Audiology, Neurotology, and the Hearing Sciences; Springer Handbook of Auditory Research. Schwitzerland: Springer, 2016, pp. 141–164. [Google Scholar]
- 40. Todd DW, Philip RC, Niihori M, et al. A fully automated high-throughput zebrafish behavioral ototoxicity assay. Zebrafish 2017; 14(4): 331–342. [DOI] [PubMed] [Google Scholar]
- 41. Jyonouchi H, Sun S, Iijima K, et al. Antitumor activity of astaxanthin and its mode of action. Nutrition and Cancer 2000; 36(1): 59–65. [DOI] [PubMed] [Google Scholar]
- 42. Choi J, Im GJ, Chang J, et al. Protective effects of apocynin on cisplatin-induced ototoxicity in an auditory cell line and in zebrafish. Journal of Applied Toxicology 2013; 33(2): 125–33. [DOI] [PubMed] [Google Scholar]
- 43. Vargo JW, Walker SN, Gopal SR, et al. Inhibition of mitochondrial division attenuates cisplatin-induced toxicity in the neuromast hair cells. Frontiers in Cellular Neuroscience 2017; 11: 393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang W, Shanmugam MK, Xiang P, et al. Sphingosine 1-phosphate receptor 2 induces otoprotective responses to cisplatin treatment. Cancers (Basel) 2020; 12(1): 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kros CJ, Steyger PS. Aminoglycoside- and cisplatin-induced ototoxicity: Mechanisms and otoprotective strategies. Cold Spring Harbor Perspectives in Medicine 2019; 9(11): a033548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gu J, Chen Y, Tong L, et al. Astaxanthin-loaded polymer-lipid hybrid nanoparticles (ATX-LPN): Assessment of potential otoprotective effects. Journal of Nanobiotechnology 2020; 18(1): 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Repine JE, Pfenninger OW, Talmage DW, et al. Dimethyl sulfoxide prevents DNA nicking mediated by ionizing radiation or iron/hydrogen peroxide-generated hydroxyl radical. Proceedings of the National Academy of Sciences 1981; 78(2): 1001–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Qi W, Ding D, Salvi RJ. Cytotoxic effects of dimethyl sulphoxide (DMSO) on cochlear organotypic cultures. Hearing Research 2008; 236(1–2): 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zou L, Xue Y, Jones M, et al. The effects of quinine on neurophysiological properties of dopaminergic neurons. Neurotoxicity Research 2018; 34(1): 62–73. [DOI] [PubMed] [Google Scholar]
- 50. Suli A, Watson GM, Rubel EW, et al. Rheotaxis in larval zebrafish is mediated by lateral line mechanosensory hair cells. PLoS One 2012; 7(2): e29727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Monroe JD, Rajadinakaran G, Smith ME. Sensory hair cell death and regeneration in fishes. Frontiers in Cellular Neuroscience 2015; 9: 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yuan H, Wang X, Hill K, et al. Autophagy attenuates noise-induced hearing loss by reducing oxidative stress. Antioxid Redox Signal 2015; 22(15): 1308–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Monroe JD, Hodzic D, Millay MH, et al. Anti-cancer and ototoxicity characteristics of the curcuminoids, CLEFMA and EF24, in combination with cisplatin. Molecules 2019; 24(21): 3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.https://www.caymanchem.com/msdss/11976m.pdf (accessed 22 July 2020).
- 55. Li X, Guo S, Xiong XK, et al. Combination of quercetin and cisplatin enhances apoptosis in OSCC cells by downregulating xIAP through the NF-κB pathway. Journal of Cancer 2019; 10(19): 4509–4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Costa-Machado LF, Fernandez-Marcos PJ. The sirtuin family in cancer. Cell Cycle 2019; 18(18): 2164–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ge H, Ni S, Wang X, et al. Dexamethasone reduces sensitivity to cisplatin by blunting p53-dependent cellular senescence in non-small cell lung cancer. PLoS One 2012; 7(12): e51821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mosley CA, Liotta DC, Snyder JP. Highly active anti-cancer curcumin analogues. Advances in Experimental Medicine and Biology 2007; 595: 77–103. [DOI] [PubMed] [Google Scholar]
- 59.https://www.rxlist.com/amiloride-hydrochloride-side-effects-drug-center.htm (2017, accessed 22 July 2020).
- 60.https://www.drugs.com/cdi/dimethyl-sulfoxide.html (2020, accessed 22 July 2020).
- 61. Mander S, You DJ, Park S, et al. Nafamostat mesilate negatively regulates the metastasis of triple-negative breast cancer cells. Archives of Pharmacal Research 2018; 41(2): 229–242. [DOI] [PubMed] [Google Scholar]
- 62. He J, Zhou J, Yang W, et al. Dexamethasone affects cell growth/apoptosis/chemosensitivity of colon cancer via glucocorticoid receptor α/NF-κB. Oncotarget 2017; 8(40): 67670–67683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bae-Jump VL, Zhou C, Boggess JF, et al. Synergistic effect of rapamycin and cisplatin in endometrial cancer cells. Cancer 2009; 115(17): 3887–3896. [DOI] [PubMed] [Google Scholar]
- 64. Wang C, Datoo T, Zhao H, et al. Midazolam and dexmedetomidine affect neuroglioma and lung carcinoma cell biology in vitro and in vivo. Anesthesiology 2018; 129(5): 1000–1014. [DOI] [PubMed] [Google Scholar]
- 65. Jeong CH, Joo SH. Downregulation of reactive oxygen species in apoptosis. Journal of Cancer Prevention 2016; 21(1): 13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Yang R, Miki K, He X, et al. Prolonged treatment with N-acetylcystine delays liver recovery from acetaminophen hepatotoxicity. Critical Care 2009; 13(2): R55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.https://www.drugs.com/mtm/dexmedetomidine.html (2018, accessed 22 July 2020).
- 68.https://www.drugs.com/sfx/edaravone-side-effects.html (2019, accessed 22 July 2020).
- 69.https://www.drugs.com/sfx/quinine-side-effects.html (2019, accessed 22 July 2020).
- 70. Kim HS, Lee KE, Oh JH, et al. Cardiac arrest caused by nafamostat mesilate. Kidney Research and Clinical Practice 2016; 35(3): 187–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.https://www.drugoffice.gov.hk/eps/do/en/consumer/news_informations/dm_19.html (accessed 22 July 2020).
- 72. Hall MD, Telma KA, Chang KE, et al. Say no to DMSO: Dimethylsulfoxide inactivates cisplatin, carboplatin, and other platinum complexes. Cancer Research 2014; 74(14): 3913–3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Dankort D, Curley DP, Cartlidge RA, et al. Braf(V600E) cooperates with Pten loss to induce metastatic melanoma. Nature Genetics 2009; 41(5): 544–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Monroe JD, Millay MH, Patty BG, et al. The curcuminoid, EF-24, reduces cisplatin-mediated reactive oxygen species in zebrafish inner ear auditory and vestibular tissues. Journal of Clinical Neuroscience 2018. b; 57: 152–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zheng YT, Yang HY, Li T, et al. Amiloride sensitizes human pancreatic cancer cells to erlotinib in vitro through inhibition of the PI3K/AKT signaling pathway. Acta Pharmacologica Sinica 2015; 36(5): 614–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zheng Z, Wang Y, Yu H, et al. Salvianolic acid B inhibits ototoxic drug-induced ototoxicity by suppression of the mitochondrial apoptosis pathway. Journal of Cellular and Molecular Medicine 2020; 24(12): 6883–6897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Tusskorn O, Khunluck T, Prawan A, et al. Mitochondrial division inhibitor-1 potentiates cisplatin-induced apoptosis via the mitochondrial death pathway in cholangiocarcinoma cells. Biomedicine & Pharmacotherapy 2019; 111: 109–18. [DOI] [PubMed] [Google Scholar]
- 78.https://www.drugs.com/drp/astaxanthin-capsules-and-oral-powder.html (accessed 22 July 2020)
- 79.https://www.drugs.com/npp/danshen.html (2019, accessed 22 July 2020).
- 80. Orlowski RZ, Eswara JR, Lafond-Walker A, et al. Tumor growth inhibition induced in a murine model of human Burkitt’s lymphoma by a proteasome inhibitor. Cancer Res 1998; 58(19): 4342–8. [PubMed] [Google Scholar]
- 81. Kontoghiorghes GJ, Kontoghiorghe CN. Prospects for the introduction of targeted antioxidant drugs for the prevention and treatment of diseases related to free radical pathology. Expert Opinion on Investigational Drugs 2019; 28(7): 593–603. [DOI] [PubMed] [Google Scholar]
- 82. Law BK, Chytil A, Dumont N, et al. Rapamycin potentiates transforming growth factor beta-induced growth arrest in nontransformed, oncogene-transformed, and human cancer cells. Molecular and Cellular Biology 2002; 22(23): 8184–8198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.https://www.rxlist.com/consumer_quercetin/drugs-condition.htm (accessed 22 July 2020).
- 84. Liu Z, Ji J, Zheng D, et al. Protective role of endothelial calpain knoc out in lipopolysaccharide-induced acute kidney injury via attenuation of the p38-iNOS pathway and NO/ROS production. Experimental & Molecular Medicine 2020; 52(4): 702–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kokura S, Yoshida N, Sakamoto N, et al. The radical scavenger edaravone enhances the antitumor effects of CPT-11 in murine colon cancer by increasing apoptosis via inhibition of NF-kappaB. Cancer Letters 2005; 229(2): 223–233. [DOI] [PubMed] [Google Scholar]
- 86. Hu Z, Li S, Yang L. Preparation of berbamine loaded chitosan-agarose microspheres and in vitro release study. Polímeros 2012; 22(5): 422–426. [Google Scholar]
- 87. Tang XL, Yan L, Zhu L, et al. Salvianolic acid A reverses cisplatin resistance in lung cancer A549 cells by targeting c-met and attenuating Akt/mTOR pathway. Journal of Pharmacological Sciences 2017; 135(1): 1–7. [DOI] [PubMed] [Google Scholar]
- 88. Guo S, Lin CM, Xu Z, et al. Co-delivery of cisplatin and rapamycin for enhanced anti-cancer therapy through synergistic effects and microenvironment modulation. ACS Nano 2014; 8(5): 4996–5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Decker T, Hipp S, Ringshausen I, et al. Rapamycin-induced G1 arrest in cycling B-CLL cells is associated with reduced expression of cyclin D3, cyclin E, cyclin A, and survivin. Blood 2003; 101(1): 278–285. [DOI] [PubMed] [Google Scholar]
- 90. Shin YS, Song SJ, Kang SU, et al. A novel synthetic compound, 3-amino-3-(4-fluoro-phenyl)-1H-quinoline-2,4-dione, inhibits cisplatin-induced hearing loss by the suppression of reactive oxygen species: In vitro and in vivo study. Neuroscience 2013; 232: 1–12. [DOI] [PubMed] [Google Scholar]
- 91.https://www.drugs.com/dexamethasone.html (2019, accessed 22 July 2020).
- 92. Choi JA, Kim JY, Lee JY, et al. Induction of cell cycle arrest and apoptosis in human breast cancer cells by quercetin. International Journal of Oncology 2001; 19(4): 837–44. [DOI] [PubMed] [Google Scholar]
- 93. Hou ZJ, Luo X, Zhang W, et al. Flubendazole, FDA-approved anthelmintic, targets breast cancer stem-like cells. Oncotarget 2015; 6(8): 6326–6340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Tuorkey MJ. Cancer therapy with phytochemicals: Present and future perspectives. Biomedical and Environmental Sciences 2015; 28(11): 808–19. [DOI] [PubMed] [Google Scholar]
- 95.https://www.accessdata.fda.gov/drugsatfda_docs/label/2004/21083s017,21110s020lbl.pdf (2004, accessed 22 July 2020)
- 96. Krishnaveni M, Suresh K. Induction of apoptosis by quinine in human laryngeal carcinoma cell line. International Journal of Current Research and Academic Review 2015; 3(3): 169–178. [Google Scholar]
- 97. Ghysen A, Dambly-Chaudière C. The lateral line microcosmos. Genes & Development 2007; 21(17): 2118–30. [DOI] [PubMed] [Google Scholar]
- 98. Coffin AB, Ramcharitar J. Chemical ototoxicity of the fish inner ear and lateral line. Advances in Experimental Medicine and Biology 2016; 877: 419–37. [DOI] [PubMed] [Google Scholar]
- 99. Sheth S, Mukherjea D, Rybak LP, et al. Mechanisms of cisplatin-induced ototoxicity and otoprotection. Frontiers in Cellular Neuroscience 2017; 11: 338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.https://clinicaltrials.gov/ct2/show/NCT03664674 (2018, accessed 24 July 2020).
- 101. Salehi P, Akinpelu OV, Waissbluth S, et al. Attenuation of cisplatin ototoxicity by otoprotective effects of nanoencapsulated curcumin and dexamethasone in a guinea pig model. Otology & Neurotology 2014; 35(7): 1131–1139. [DOI] [PubMed] [Google Scholar]
- 102. Campbell KC, Meech RP, Klemens JJ, et al. Prevention of noise- and drug-induced hearing loss with D-methionine. Hearing Research 2007; 226(1–2): 92–103. [DOI] [PubMed] [Google Scholar]
- 103. Yoo J, Hamilton SJ, Angel D, et al. Cisplatin otoprotection using transtympanic L-N-acetylcysteine: A pilot randomized study in head and neck cancer patients. Laryngoscope 2014; 124(3): E87–E94. [DOI] [PubMed] [Google Scholar]
- 104.https://www.clinicaltrials.gov/ct2/show/NCT04226456 (2020, accessed 24 July 2020).
- 105.https://clinicaltrials.gov/ct2/show/NCT02997189 (2017, accessed 24 July 2020).
- 106. Visacri MB, Quintanilha JCF, de Sousa VM, et al. Can acetylcysteine ameliorate cisplatin-induced toxicities and oxidative stress without decreasing antitumor efficacy? A randomized, double-blind, placebo-controlled trial involving patients with head and neck cancer. Cancer Medicine 2019; 8(5): 2020–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Riga MG, Chelis L, Kakolyris S, et al. Transtympanic injections of N-acetylcysteine for the prevention of cisplatin-induced ototoxicity: A feasible method with promising efficacy. American Journal of Clinical Oncology 2013; 36(1): 1–6. [DOI] [PubMed] [Google Scholar]
- 108. Wimmer C, Mees K, Stumpf P, et al. Round window application of D-methionine, sodium thiosulfate, brain-derived neurotrophic factor, and fibroblast growth factor-2 in cisplatin-induced ototoxicity. Otology & Neurotology 2004; 25(1): 33–40. [DOI] [PubMed] [Google Scholar]
- 109.https://clinicaltrials.gov/ct2/show/NCT03062449 (2019, accessed 24 July 2020).
- 110.https://clinicaltrials.gov/ct2/show/NCT03310359 (2019, accessed 24 July 2020).
- 111.https://www.foodsweeteners.com/l-serine-side-effects (2015, accessed 22 July 2020).
- 112. Slattery EL, Oshima K, Heller S, et al. Cisplatin exposure damages resident stem cells of the mammalian inner ear. Developmental Dynamics 2014; 243(10): 1328–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Monroe JD, Hruska HL, Ruggles HK, et al. Anti-cancer characteristics and ototoxicity of platinum(II) amine complexes with only one leaving ligand. PLoS One 2018; 13(3): e0192505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.https://clinicaltrials.gov/ct2/show/NCT03359538 (2017, accessed 24 July 2020).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary for Use of zebrafish larvae lateral line to study protection against cisplatin-induced ototoxicity: A scoping review by Ewa Domarecka, Magda Skarzynska, Agnieszka J Szczepek and Stavros Hatzopoulos in International Journal of Immunopathology and Pharmacology