Abstract
Objective:
Foodborne diseases caused by non-typhoid Salmonella and the emergence of antimicrobial resistance remain as a public health challenge, especially in developing countries. The current study aimed to estimate the pooled prevalence and the antimicrobial resistance patterns of non-typhoid Salmonella in Ethiopia.
Methods:
Literature search was conducted from major electronic databases and indexing services. Both published and unpublished studies addressing the prevalence and antimicrobial resistance profiles of Salmonella in Ethiopia from 2010 to 2020 and those studies reported sample size and the numbers of isolates/number of positive samples were included in the study. Data were extracted using format prepared in Microsoft Excel. The identified data were exported to EndNote to remove duplicated studies, then after the remained articles were screened using title, abstract, and full text to identify studies that meet the inclusion criteria and finally appraised for methodological validity using JBI guideline. The pooled prevalence of Salmonella and its drug resistance pattern was computed by a random-effects model. I2 test statistic was used to test heterogeneity across studies. The presence of publication bias was evaluated using the Begg’s and Egger’s tests.
Results:
A total of 49 eligible articles, 33 of them on human stools, 15 of them on animal origin foods, and one both on human stools and animal origin foods, were included in the study. The pooled prevalence of Salmonella among human stools and animal origin foods in Ethiopia was 4.8% (95% CI: 3.9, 5.9) and 7.7% (95% CI: 5.6, 10.4), respectively. The subgroup analysis detected high pooled prevalence, 7.6% (95% CI: 5.3, 10.7) among outpatients and low, 3.7% (95% CI: 2.6, 5.1) in food handlers. The pooled resistant level of Salmonella was 80.6% (95% CI 72.6, 86.7) for ampicillin and 63.5% (95% CI 53.7, 72.4) for tetracycline. Low pooled resistance pattern was reported in ciprofloxacin, 8.7% (95% CI 5.6, 13.3) and ceftriaxone 12.2% (95% CI 7.9, 18.3). There was some sort of publication bias.
Conclusion:
High pooled prevalence of Salmonella among human stools and animal origin foods which were 4.8% and 7.7% respectively, and high Salmonella resistance, >72% to ampicillin and tetracycline were detected in Ethiopia. Antimicrobial stewardship efforts and infection control strategies are required to mitigate this major public health concern.
Keywords: Animal origin food, antimicrobial resistance, Ethiopia, human stool, Salmonella
Background
Nontyphoidal Salmonella are a leading cause of foodborne illness responsible for more than 93 million cases of gastroenteritis and about 155,000 deaths each year.[1] Foodborne diseases caused by non-typhoid Salmonella still remains as a public health challenge. All countries around the globe are suffering from foodborne disease outbreaks. However, the relative contributions of animal products to human salmonellosis differ across countries. For instance, beef is reported to be the vector of 7% of the 1.7 million cases of foodborne disease in England and Wales[2] and out of more than one million people sickened by Salmonella each year in the United States, poultry was the pathogenic vehicle for 20% of these cases.[3] The problem is more serious in developing countries, where more than one billion cases of gastroenteritis are reported and five million individuals die annually.[4]
Nontyphoidal Salmonella enters the food chain at any point in livestock feed and in food processing. Apart from sporadic infections, outbreaks associated with the consumption of contaminated animal products have been recorded in several countries. Poultry and other food animals are considered the common reservoirs of Salmonella where undercooked food products mainly consumption of contaminated food of animal origin such as poultry products, beef, and pork as well as contact with infected animals are the major sources of human infection with nontyphoidal Salmonella.[5,6]
The emergences of antimicrobial-resistant Salmonella spp. are other global challenges, especially in developing countries where there is an increased misuse of antimicrobial agents in humans as well as in animals and is associated with a variety of biological, pharmacological, and societal variables with the worst combinations in developing countries.[7] Antimicrobial-resistant Salmonella is one of the global problems in present-day clinical practices, and recently, a strain on the verge of pan-resistance was reported.[8] The prevalence of multidrug resistance (MDR) Salmonella has increased and outbreaks due to MDR strains were reported in sub-Saharan Africa.[9,10] Infections with MDR pathogens are associated with excess morbidity and mortality, probably because of the co-selection of traits of drug resistance and virulence.[11]
In developing countries including Ethiopia, where a high frequency of resistant Salmonella spp. against different antimicrobial agents have been reported,[12] the therapeutic management of the disease is difficult because isolation of Salmonella spp. in most laboratories remains a challenge due to inadequate laboratory facilities required for the accurate detection of bacteria and to perform antimicrobial susceptibility testing.[7] Hence, drug sensitivity tests are not routinely carried out and treatment alternatives are not available in most health care facilities.[13,14]
Moreover, surveillance and monitoring systems are not in place and the pharmacoepidemiology of the bacteria among the different population and their food is not well described despite the importance of the disease caused by the bacteria. However, pooling previous prevalence and antimicrobials susceptibility patterns among human stools and animal origin foods could provide insight about the overall pooled prevalence of the isolates and the proportions of drug-resistant isolates reported by different cross-sectional studies. Therefore, the main aim of this study was to estimate the pooled prevalence and antimicrobial susceptibility of Salmonella in human stool and animal origin food specimens using systematic review and meta-analysis. The findings of this systematic review and meta-analysis are expected to provide evidence for policy formulation, prevention, and control of Salmonellosis and Antimicrobial resistance in human and animals.
Methods
Study protocol
The Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) reporting items were followed for reporting of identification of records, screening of titles and abstracts as well as evaluation of eligibility of full texts for final inclusion to ensure inclusion of relevant information/studies into this systematic review and meta-analysis.[15] PRISMA checklist was also strictly followed while conducting this systematic review and meta-analysis. The protocol of this systematic review and meta-analysis has been submitted for registration in the Prospero database. Two authors (DA and Na) independently screened the titles and abstracts of studies with predefined inclusion criteria. Two authors (DA and NA) also independently collected full-texts of studies and evaluated the eligibility of them for final inclusion.
Inclusion criteria
All Prospective cross-sectional studies aimed to assess prevalence and antimicrobial susceptibility patterns of Salmonella by collecting human stools from food handlers working in towns and universities student cafeteria or restaurants, patients including under-five children that sought medical service for reasons of gastroenteritis/diarrhea, and foods of animal origin(restricted to raw egg, milk, and meat produced for human consumption) samples in Ethiopia that were conducted/published from 2010 to 2020 were included in the study. Studies reported both sample size of the study and the numbers of isolates/number of positive samples, and published in English language, the articles reported detection by culture and confirmed by biochemical test of Salmonella, determine antimicrobial resistance patterns for ceftriaxone (CRO, 30 mg), chloramphenicol (C, 30 mg), ciprofloxacin (CIP, 5 mg), gentamicin (GM, 10 mg), ampicillin (AMP, 10 mg), and tetracycline (T, 30 mg) using disk diffusion method and defined antimicrobial resistance range according to according to the Clinical Laboratory Standards Institute were included in the study.[16]
Exclusion criteria
Studies that were conducted on target population with a pre-existing medical condition such as HIV, diabetic, and other chronic conditions, used microscopic, molecular, and serological methods to detect and isolate Salmonella, not reported the numerator (i.e., number positive) and denominator (i.e., number tested) information by sample type (human stool and food of animal origin), and all review articles and original articles conducted outside of Ethiopia were excluded from the study. And also, articles with irretrievable full texts, records with unrelated outcome measures, and articles with missing or insufficient outcomes, and also studies which did not adhere to standard operating procedures were excluded from the study.
Search strategy and identification of studies
The search strategy used aims to find both published and unpublished articles. The data were collected by searching articles published in English from electronic databases and indexing services such as PubMed/Medline, Google Scholar, Embase, Direct Science, World cat, and University repositories which were used as the main source of data. The reference list of identified articles was also searched for additional studies. Subject headings relevant to each database were used, like MeSH term for PubMed/Medline. In addition, Google and hand searching were employed to retrieve grey literature. Salmonella* and Ethiopia were the main MeSH terms in electronic searches. The additional search terms used in this review were “Foodborne pathogen,” “diarrhea/diarrhea,” “antimicrobial resistance,” “antibiotic resistance,” “humans,” “animals, “and “a specific region name” that appeared in the title, abstract, or keywords. The Boolean logical connectors (AND and OR) and truncation were applied for appropriate search and identification of records for the research question. Articles published from 2010 to 2020 were included in the study. The last search was conducted on March 24, 2020.
Study selection
Articles identified from various electronic databases, indexing services, and directories were exported to EndNote reference software version 8[17] which was used to remove duplicated studies. After duplicates were removed using EndNote, some duplicates were addressed manually due to variation in reference styles across sources. The titles and abstracts were screened to identify studies that potentially meet the inclusion criteria. Full-text articles were reviewed to determine if each article met predetermined inclusion and exclusion criteria. Studies that are deemed to meet inclusion by title/abstract screening undergone full-text appraisal for methodological validity using standardized critical appraisal instruments from the Joanna Briggs Institute (JBI) Instrument.[18] Full texts of articles that were selected to be included were extracted systematically using the Excel sheet.
Data extraction
From each included article, data such as author, year of study/publication, location/region, mean age with standard deviation, sample size, isolation/identification methods, number of Salmonella isolated or detected, and number of antimicrobial-resistant isolates against the selected antimicrobial agents, number of MDR isolates, study design, sampling technique, and sample types were extracted using standardized data abstraction format prepared in Microsoft Excel. During data extraction conditions and time of transport to laboratory, amount of sample tested, media used, and temperature and gas conditions of incubation were considered.
Quality assessment
The articles were assessed prior to inclusion to maintain methodological validity using the JBI critical appraisal checklist for studies reporting prevalence data by two authors. The assessment tool consisted of nine questions about the quality of the study for which articles receive values representing the extent to which they met criteria using Yes, No, Unclear, and Not applicable reply to each nine questions. This critical appraisal was conducted to assess the internal (systematic error) and external (generalizability) validity of studies and to reduce the risk of biases. The mean score of two authors was taken for final decision and studies with a score yes, greater than or equal to five were included in the study.[18]
Outcome measurements
The primary outcome measure is the prevalence of Salmonella among human stools and animal origin foods in Ethiopia, which was calculated by dividing the numbers of Salmonella isolates by the total number of examined samples (study participants). The secondary outcome is antimicrobial resistance patterns of Salmonella isolated from human stools and animal origin foods in Ethiopia which was also calculated by dividing the numbers of resistant Salmonella isolates against the selected antimicrobial agents (CRO, 30 mg, C, 30 mg, CIP, 5 mg, GM, 10 mg, AMP, 10 mg, and T, 30 mg) by the total number of isolated Salmonella. This systematic review and meta-analysis are aimed to assess the pooled estimates of prevalence and antimicrobial resistance profile of Salmonella among human stools and animal origin of foods in Ethiopia. Subgroup analysis was also conducted among different study participants and types of food of animal origin
Data processing and analysis
After extraction of relevant data from studies that were included into the study using format prepared in Microsoft Excel, data were entered into comprehensive meta-analysis software version 3 to analysis pooled estimate of outcome measures and subgroup analyses. Subgroup analysis of the primary outcome (prevalence of Salmonella) was done based on study participants/type of food, locations/directions of the country, and year of studies categories. The methodological validity of individual studies included in this study was assessed using the JBI critical appraisal checklist to assess the validity of studies and to reduce the risk of biases, considering variation in true effect sizes across study population, locations/directions of country, and year of studies, random-effects model was used for the analysis at 95% confidence level. The significance of heterogeneity of the studies was assessed using I2 statistics. P < 0.05 was used to define a significant degree of heterogeneity within studies. Publication bias was assessed using comprehensive meta-analysis software and presented using with funnel plots of the standard error of Logit event rate along with testing its asymmetry using Begg’s and Egger’s test (Begg and Mazumdar,1994, Sterne and Egger, 2001).
Results
Search results
A total of 339 potentially relevant studies published from 2010 to 2020 were identified by online searching electronic database such as PubMed/MEDLINE, Google Scholar, Embase, Science Direct, and Worldcat. From where 162 duplicated articles were removed with the help of EndNote and manual tracing. The remaining 177 articles after removing duplicates were screened using their titles, by which 75 articles were excluded from the study. The 102 articles selected by title screening were further screened using their abstracts. By abstract screening, 32 articles were removed. The remaining 70 articles were evaluated by reviewing full text against eligibility criteria and PRISMA statement.[15] From these, 21 articles were excluded due to the outcome of interest missed/different, sample size was not mentioned, used Salmonella isolates from stoke/storage, different/absence of study design, and Poor-quality assessment results.
Finally, a total of 49 articles those fulfilled the eligibility criteria and quality assessment were included for systematic review and meta-analysis [Figure 1]. The included articles were divided into two groups, those articles which gave a report about Salmonella prevalence or antimicrobial resistance patterns among human stool or animal food origin. Some studies reported both prevalence and antimicrobial resistance of Salmonella.
Figure 1.
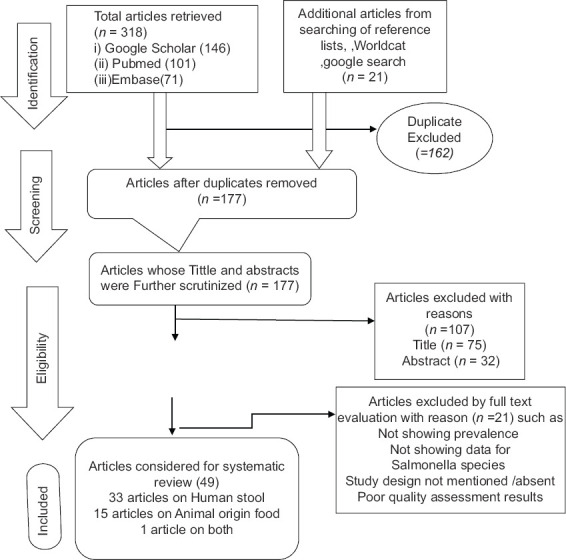
PRISMA flow chart describing the selection process of articles used for systematic review and meta-analysis
Study characteristics
A total of 49 eligible articles, 33 of them on human stools, 15 of them on animal origin foods, and one of them on both human stools and animal origin foods, were included in this systematic review and meta-analysis. All the included articles were cross-sectional study design in study type. The included articles were carried out on stools of humans with different age groups and animal food types included raw meat, egg, and milk.
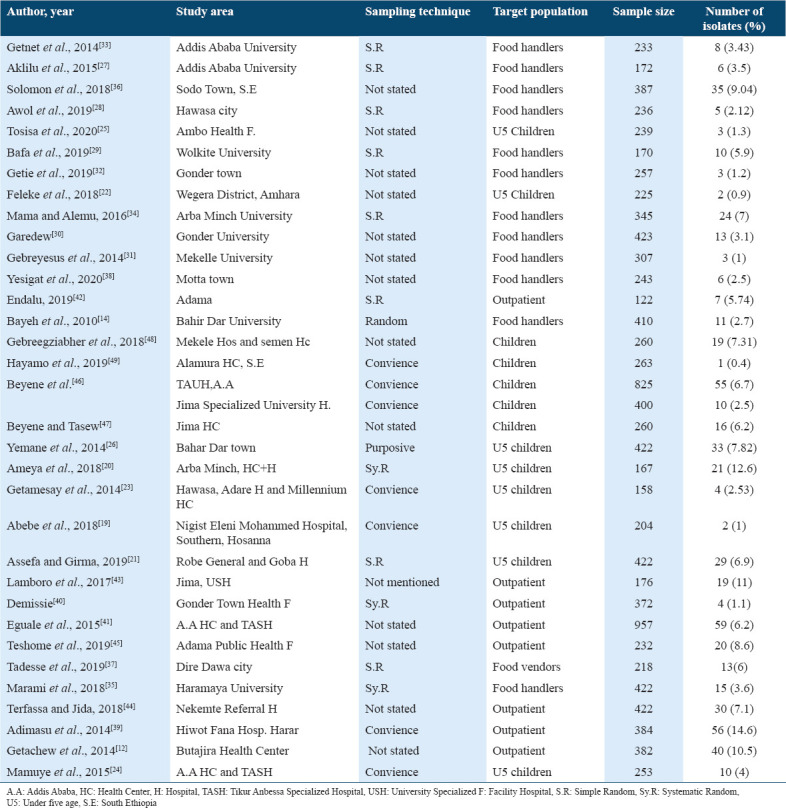
Among 34 articles that were conducted on human stools, eight articles were done on under-five children with diarrhea,[19-26] 13 articles were on food handlers who were working in different town’s and universities’ student cafeterias and restaurants,[14,27-38] eight articles were done on general outpatients (all age group) with diarrhea,[12,39-45] and five articles were on diarrheic children with age up to 14 years.[46-49] From 34 articles describing prevalence of Salmonella on human stool that were included in this review and meta-analysis, most of them were conducted in southern (13 articles), northern (9 articles), and central (8 articles) Ethiopia. Three and only one articles were conducted in eastern and western Ethiopia, respectively [Table 1].
Table 1.
Characteristics of the studies describing the prevalence of Salmonella isolates in Human stools in Ethiopia published/done from 2010 to 2020
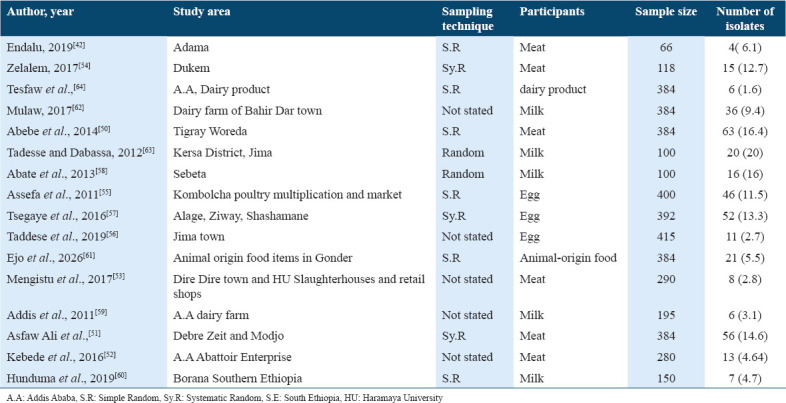
Out of 16 articles on animal origin foods: 6, 3, and 7 of them were on meat,[42,50-54] egg,[55-57] and milk,[58-64] respectively. From 16 articles describing prevalence of Salmonella in animal origin foods that were used in this review and meta-analysis, most of them were conducted in central (7/16), northern (4/16), southern (4/16), and only one in eastern Ethiopia. No study on Salmonella prevalence in animal origin food was obtained from western Ethiopia [Table 2].
Table 2.
Characteristics of the studies describing the prevalence of Salmonella isolates in Animal Origin Foods in Ethiopia published/done from 2010 to 2020
Quality assessment result
The quality score of included articles ranges from 56% to 89% as per JBI critical appraisal checklist for studies reporting prevalence data. Two studies were excluded from systematic review and meta-analysis since they scored <56%.
Primary outcome
Prevalence of Salmonella in human
A total of 34 articles, with 10,968 study participants from where 592 Salmonella isolates were isolated, were used in this meta-analysis. The 34 included studies revealed that the pooled prevalence of Salmonella among human stools in Ethiopia was 4.8% (95% CI: 3.9, 5.9) [Figure 2]. High heterogeneity was observed across the included studies (I2 = 82.47, P < 0.001). Therefore, a random effect meta-analysis model was computed to estimate the pooled prevalence of Salmonella among Human Stools in Ethiopia.
Figure 2.
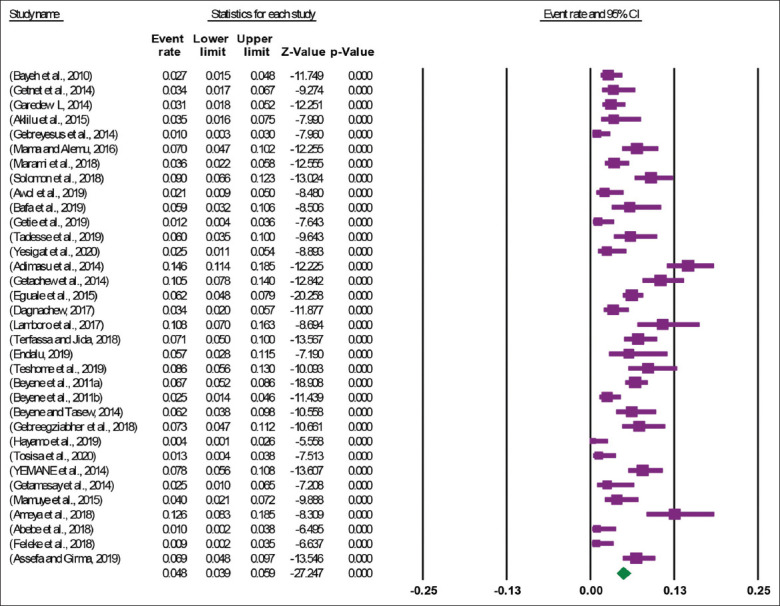
Forest plot of the pooled prevalence of Salmonella among Human stools in Ethiopia
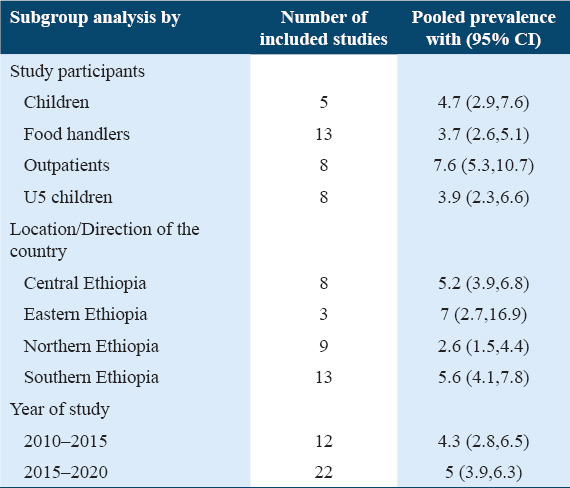
Subgroup analysis prevalence of Salmonella among human stools
In this meta-analysis, the subgroup analysis was performed based on study populations, the location/direction of the country where studies were conducted as well as the year of study. Accordingly, the subgroup analysis detected that the highest pooled prevalence was detected among outpatients which were 7.6% (95% CI: 5.3, 10.7), followed by children, 4.7% (95% CI: 2.9, 7.6), under-five children, 3.9% (95% CI: 2.3, 6.6), and food handlers, 3.7% (95% CI: 2.6, 5.1) with I2 values of the Logit event estimates in children, food handlers, outpatients, and under-five children were 77.02%, 75.59%, 85.32%, and 83.25%, respectively (P = 0.000). The subgroup analysis based on location indicated that pooled prevalence was 7% (95% CI: 2.7, 16.9) in eastern Ethiopia, 5.6% (95% CI: 4.1, 7.8) in southern Ethiopia, 5.2% (95% CI: 3.9, 6.8) in central Ethiopia, and 2.6% (95% CI:1.5, 4.4) in northern Ethiopia. Pooled estimates for Western Ethiopia were not calculated due to insufficient data (only one article). The I2 values of the Logit event estimates in Central, eastern, northern, and southern Ethiopia were 60.08%, 93.38%, 83.70%, and 80 .36%, respectively (P = 0.000). With regard to the year of study, the prevalence of Salmonella was 4.3% (95%CI: 2.8, 6.5) and 5% (95% CI: 3.9, 6.3) from studies that were published from 2010 to 2015 and 2015 to 2020, respectively [Table 3]. The I2 values of the Logit event estimates in the 2010-2015 and 2015-2020 were 89.74% and 75.96%, respectively (P = 0.000).
Table 3.
Subgroup pooled prevalence of Salmonella among Human stools in Ethiopia, (n=34)
Prevalence of Salmonella in animal origin foods
A total of 16 articles with 4426 study participants from where 380 Salmonella isolates were isolated were included in this meta-analysis that was conducted to determine the pooled prevalence of Salmonella in animal origin foods. The analysis of 16 studies showed that the pooled prevalence of Salmonella in animal origin foods in Ethiopia was 7.7% (95% CI: 5.6, 10.4) [Figure 3]. High heterogeneity was observed across the included studies (I2 = 88.95, P = 0.000). Therefore, a random effect meta-analysis model was computed to determine the pooled prevalence of Salmonella in animal origin foods in Ethiopia.
Figure 3.
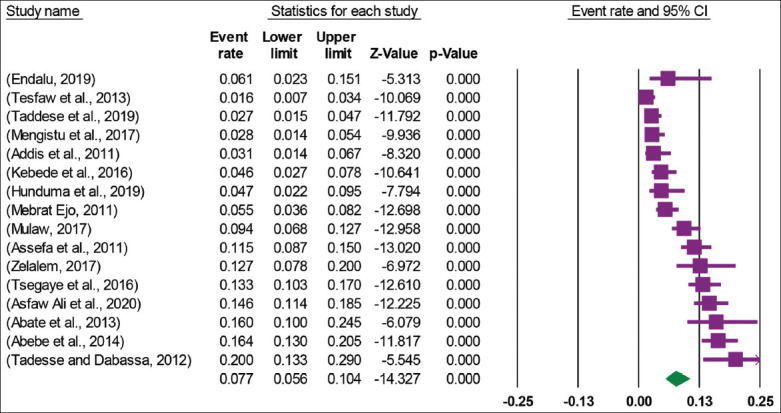
Forest plot of the pooled prevalence of Salmonella among animal origin foods in Ethiopia
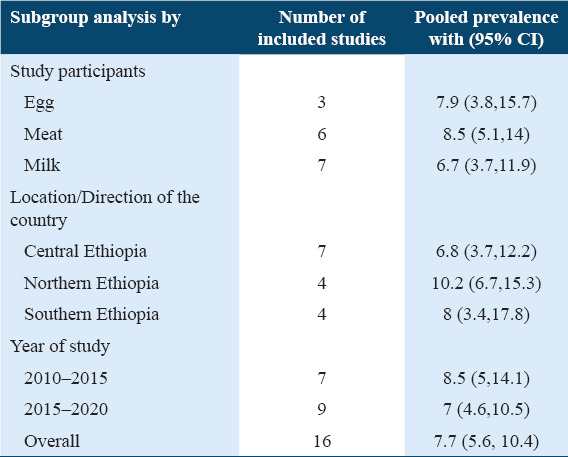
Subgroup analysis prevalence of Salmonella among animal origin foods
The subgroup analysis was conducted based on animal food types (meat, egg, and milk), the location/direction of the country where studies were conducted as well as the year of study. Accordingly, the subgroup analysis detected that the highest pooled prevalence was detected in northern Ethiopia which was 10.2% (95% CI: 6.7, 15.3) followed by southern Ethiopia, 8% (95% CI: 3.4, 17.8) and central Ethiopia, 6.8% (95% CI: 3.7, 12.2). Pooled estimates for western and eastern Ethiopia were not calculated due to the absence/inadequate articles (no study in western and one study in eastern Ethiopia. The I2 values of the Logit event estimates in Central, northern, and southern Ethiopia were 89.03%, 87.30%, and 92 .53%, respectively (P = 0.000). The subgroup analysis based on animal origin foods indicated that pooled prevalence was 8.5% (95% CI: 5.1, 14) in meat, 7.9% (95% CI: 3.8, 15.7) in egg, and 6.7% (95% CI: 3.7, 11.9) in milk with I2 values of the Logit event estimates in egg, meat, and milk were 92.43%, 88.58%, and 69.15%, respectively (P = 0.000). With regard to the year of study, the prevalence of Salmonella in animal origin foods was 8.5% (95% CI: 5, 14.1) and 7% (95% CI: 4.6, 10.5) in studies that were published from 2010 to 2015 and 2015 to 2020, respectively. The I2 values of the Logit event estimates in the 2010-2015 and 2015-2020 were 91.23% and 87.49%, respectively (P = 0.000) [Table 4].
Table 4.
Subgroup pooled prevalence of Salmonella in animal origin foods in Ethiopia, (n=16)
Secondary outcome measures
Antimicrobial resistance patterns of Salmonella isolated from both human stools and animal origin foods
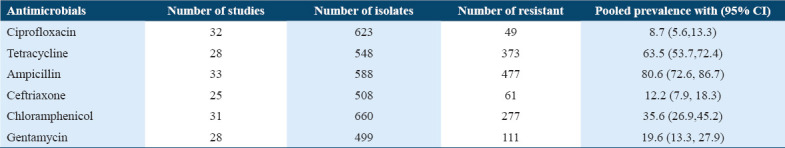
The Salmonella isolates showed different antimicrobial resistance profiles against selected agents. The meta-analysis revealed a high-pooled resistant level of Salmonella that was 80.6% (95% CI 72.6, 86.7) resistance for ampicillin and 63.5% (95% CI 53.7, 72.4) for tetracycline with I2 values of the Logit event estimates 63.97% and 67.38%, respectively. However, low pooled resistance pattern was reported in ciprofloxacin, 8.7% (95% CI 5.6, 13.3), ceftriaxone 12.2% (95% CI 7.9, 18.3), gentamycin 19.6% (95% CI 13.3,27.9), and chloramphenicol 35.6% (95% CI 26.9, 45.2) [Table 5] with I2 values of the Logit event estimates of 47.72%, 46.02%, 65.43%, and 74.87%, respectively.
Table 5.
Antimicrobial resistance patterns of Salmonella isolated from both human stools and animal origin foods
Publication bias
Funnel plots of standard error with Logit event rate (prevalence Salmonella in human stools and animal origin foods) supported with statistical tests confirmed that there is evidence of publication bias on studies reporting the prevalence Salmonella in human stools and animal origin foods in Ethiopia (Begg’s test, P = 0.000 and Egger’s test, P = 0.000) [Figure 4].
Figure 4.
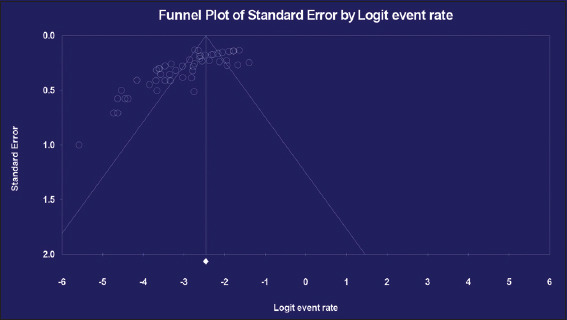
Funnel plot for the prevalence of Salmonella among human stools and animal origin foods in Ethiopia
Discussion
We used a systematic method to identify articles reporting the prevalence and antimicrobial resistance profile of Salmonella in human stools and animal origin foods in Ethiopia. A total of 49 original articles, 33 of them on human stools, 15 of them on animal origin foods, and 1 on both, were included in this systematic review and meta-analysis to determine the pooled prevalence of Salmonella and its antimicrobial resistance status. All the included articles were cross-sectional study design in study type.
In this meta-analysis, the pooled prevalence of Salmonella among human stools was 4.8% (95% CI: 3.9, 5.9). A similar study in the Middle Eastern and Northern Africa reported higher pooled Salmonella prevalence which was 6.6% (95% CI: 5.4–7.9%). Moreover, higher pooled prevalence reports were reported in Morocco, 17.9%, (95% CI: 5.7–34.8%), Tunisia 10.2% (95% CI: 4.3–18.0%), and Sudan 9.2% (95% CI: 6.5–12.2%). However, lower than current pooled prevalence reports were reported from Jordan 1.1% (95% CI: 0.1–3.0%), Oman 1.2% (95% CI: 1.2–1.3%), and Palestine, 1.2% (95% CI: 0.4–2.1%).[65]
The subgroup analysis based on study populations reported the highest pooled prevalence among outpatients which was 7.6% (95% CI: 5.3, 10.7) followed by children, 4.7% (95% CI: 2.9, 7.6), under-five children, 3.9% (95% CI: 2.3, 6.6), and food handlers, 3.7% (95% CI: 2.6, 5.1). This indicates a higher prevalence of Salmonella in general outpatients than children in contrast to another study which stated higher occurrence of Salmonellosis in diarrheic children, 9.97% (95% CI −0.41, 20.36) compared to diarrheic adults, 8.22% (95% CI 5.75, 10.69),[66] and other reports that stated children as a higher risk for Salmonella than adults.[67] This might be due to more raw animal origin food consumption by adults than children in the country.
The pooled prevalence of Salmonella in outpatients attending different health facilities in Ethiopia was 7.6% (95% CI: 5.3, 10.7). In line with this, other studies in Ethiopia which were conducted from 1974 to 2010 reported a similar pooled prevalence was 8.22% (95%, CI: 5.75, 10.69).[66] The present study reported pooled prevalence of Salmonella in children less than fifteen was 4.7% (95% CI: 2.9, 7.6) and in under five age children was 3.9% (95% CI: 2.3, 6.6) which is less than pooled prevalence reported by Tadesse which was 9.97% (95% CI: −0.41, 20.36).[66] This less pooled prevalence in the current study might be due to increased awareness about hygiene and health information dissemination by health extension works mainly by focusing on children to decrease children morbidity and mortality due to diarrhea.
In the current study, subgroup analysis showed pooled prevalence of 3.7% (95% CI: 2.6, 5.1) among food handlers. In support of this report, a study in the Middle Eastern and northern Africa reported similar pooled Salmonella prevalence which was 3.8% (95% CI: 1.0–8.0%) among food handlers.[65] However, other studies in Ethiopia reported lower pooled Salmonella prevalence among food handlers was 1.09% (95% CI: 0.73, 1.44).[66] This might be due to the current high workload for food handlers due to high population growth result in high customers which in turn affect their hygiene by affecting the environmental sanitation including toilet hygiene which increase the exposure of food handlers to pathogen including Salmonella.
The analysis of 16 studies showed that the pooled prevalence of Salmonella in animal origin foods in Ethiopia was 7.7% (95% CI: 5.6, 10.4). The subgroup analysis based on animal origin foods indicated that pooled prevalence was 8.5% (95% CI: 5.1, 14) in meat, 7.9% (95% CI: 3.8, 15.7) in egg, and 6.7% (95% CI: 3.7, 11.9) in milk. Similar pooled prevalence was reported by Zelalem’s study on meat and meat products which was 9% (95% CI: 6.0, 12.0).[68] This result is also in line with a study in Portugal where the prevalence of Salmonella spp. in meats was 6% (95% CI: 4, 9%).[69] However, lower prevalence was reported by the study conducted on ground beef in the United States was 1.9%.[70] This difference might be due to poor food handling practice, lack of slaughtering facility and poor animal health management at primary production, and substandard transport of animal meat contribute to the high prevalence Salmonella pathogen in animal origin foods in Ethiopia.
The subgroup analysis indicated highest pooled prevalence in northern Ethiopia which was 10.2% (95% CI: 6.7, 15.3) followed by southern Ethiopia, 8% (95% CI: 3.4, 17.8), central Ethiopia, 6.8% (95% CI: 3.7, 12.2), and 7% (95% CI: 2.7, 16.9) in eastern Ethiopia among animal origin foods, and 5.6% (95% CI: 4.1, 7.8) in southern Ethiopia, 5.2% (95% CI: 3.9, 6.8) in central Ethiopia, and 2.6% (95% CI: 1.5, 4.4) among human stools. This variation might be due to the difference in basic environmental conditions, behavioral characteristics of animal caregivers, the sociodemographic, environmental, and behavioral characteristics of households.
The pooled prevalence of Salmonella was 4.3% (95% CI: 2.8, 6.5) in human stools and 8.5% (95% CI: 5, 14.1) in animal origin foods from studies published from 2010 to 2015 and 5% (95% CI: 3.9, 6.3) in human stools and 7% (95% CI: 4.6, 10.5) in animal origin foods in studies that were published from 2015 to 2020. This slight difference indicates that no improvements in hygienic and sanitary measures practiced since 2010 might have not reduced the occurrence of Salmonella infections. Moreover, the slight increases in infections in humans reflect the absence of substantial improvements in the hygienic and sanitary practices of the general population in proportion to population growth.
This meta-analysis also revealed the high-pooled resistant level of Salmonella that was 80.6% (95% CI 72.6, 86.7) for ampicillin and 63.5% (95% CI 53.7, 72.4) for tetracycline. However, low pooled resistance pattern was reported in ciprofloxacin, 8.7% (95% CI 5.6, 13.3), ceftriaxone 12.2% (95% CI 7.9, 18.3), gentamycin 19.6% (95% CI 13.3, 27.9), and chloramphenicol 35.6% (95% CI 26.9, 45.2). In contrast to this study, other studies detected 12–63% Salmonella resistance to ciprofloxacin and 16.2–49.1% Salmonella resistance to ampicillin. In our study, higher and lower prevalence of Salmonella resistance to ampicillin and ciprofloxacin, respectively, than other systematic reviews done elsewhere from 2000 to 2017 was reported.[71] In line with the present study, another study reported high resistance, which is 100% resistance to ampicillin, but unlike the present study, high (100%) to ceftriaxone was reported.[56]
However, systematic review by Zelalem et al. reported <10% antimicrobial resistance profile of bacteria by the majority of estimates[68] which is less than the report of the current study which reported 80.6% (95% CI 72.6, 86.7) Salmonella resistance against ampicillin. Other systematic reviews also reported less (0–52%) Salmonella resistance against ampicillin than the current systematic review and meta-analysis. This study also reported 0–9.25% resistance of Salmonella isolates against chloramphenicol,[72] which is less than a report from our systematic review and meta-analysis which reported, 35.6% (95% CI 26.9, 45.2).
The increase in the resistance to the antimicrobial agents in this review and meta-analysis in comparison to other studies could be associated with extensive use of antimicrobials not only as therapeutic agents for human infections but also for prophylaxis and growth promotion in animal husbandry. The driving force behind the increasing rates of resistance of Salmonella to antimicrobial agents both in human and animals can ultimately be found in the abuse and misuse of antibacterial agents in patients/livestock and releasing into the environment and also inappropriate use of antibiotics, inadequate infection prevention and control programs, limited laboratory capacity, poor surveillance, population growth, and migration, as well as inadequate sanitation. Moreover, the high resistance to ampicillin and tetracycline in this review may be due to easily availability of these drugs and widely usage of it in both human and veterinary medicine.
Strength and limitation
This study has the following strengths; it is the first systematic review and meta-analysis conducted in Ethiopia to show the national pooled prevalence and antimicrobial resistance profiles of Salmonella isolates in both human stools and animal origin foods. The reviewed articles have the same study design and the same laboratory method used to isolate the pathogen and assess the antimicrobial resistance. It has analyzed articles from most parts of the country where eligible studies have been retrieved from by comprehensive research strategy. It has included many articles conducted for more than 10 years that will enable the reader to understand the trend changing with time. The subgroup analysis was conducted. Nevertheless, this study has limitations such as studies included for analysis were heterogeneous as some were done in food handlers, outpatients, different age group children, and different types of animal foods (milk, meat, and egg) and also there was some sort of publication bias. No study from western Ethiopia was found to include in subgroup analysis.
Conclusion and Recommendation
The pooled prevalence of Salmonella among human stools and animal origin foods in Ethiopia was 4.8% (95% CI: 3.9, 5.9) and 7.7% (95% CI: 5.6, 10.4), respectively. The subgroup analysis detected higher pooled prevalence among outpatients, 7.6% (95% CI: 5.3, 10.7) than pooled prevalence among children, 4.7% (95% CI: 2.9, 7.6), and under-five children, 3.9% (95% CI: 2.3, 6.6), whereas lower prevalence among food handlers, 3.7% (95% CI: 2.6, 5.1). Higher and lower pooled prevalence also was reported eastern Ethiopia 7% (95% CI: 2.7, 16.9) and central Ethiopia 2.6% (95% CI: 1.5, 4.4), respectively, in human stools, but in northern Ethiopia which was 10.2% and Central Ethiopia, 6.8% (95% CI: 3.7, 12.2) in case of animal origin foods. The subgroup analysis based on animal origin foods indicated that pooled prevalence was 8.5% (95% CI: 5.1, 14) in meat, 7.9% (95% CI: 3.8, 15.7) in egg, and 6.7% (95% CI: 3.7, 11.9) in milk. The prevalence of Salmonella in animal origin foods was higher in studies published from 2010 to 2015, 8.5% (95% CI: 5, 14.1) compared to those published from 2015 to 2020, 7% (95% CI: 4.6, 10.5).
The pooled resistant level of Salmonella was 80.6% (95% CI 72.6, 86.7) resistance for ampicillin and 63.5% (95% CI 53.7, 72.4) for tetracycline, respectively. However, low pooled resistance pattern was reported in ciprofloxacin, 8.7% (95% CI 5.6, 13.3), ceftriaxone 12.2% (95% CI 7.9, 18.3), gentamycin 19.6% (95% CI 13.3, 27.9), and chloramphenicol 35.6% (95% CI 26.9, 45.2). This systematic review and meta-analysis showed ciprofloxacin, ceftriaxone gentamycin, and slightly chloramphenicol is effective, but ampicillin and tetracycline are ineffective. It is clear that the Salmonella disease burden is of public and economic importance. Therefore, routine diagnosis of Salmonella, appropriate use of antimicrobials based on laboratory result, educating communities on hygienic practices and animal/food handle and use, and antimicrobial resistance surveillance system is necessary to reduce both the Salmonella prevalence and antimicrobial resistance in Ethiopia.
Authors’ Declaration Statements
Ethical approval and patients consent
Not applicable since this study is a systematic review and meta-analysis where possible to get study participants to consent, moreover no individual’s information to be exposed.
Availability of data and material
The data used in this study are available and will be provided by the corresponding author on a reasonable request.
Competing interest
The authors declare that they have no competing interests.
Funding statement
None.
Authors’ Contributions
DA and NA conceived and designed the study. Both authors collected scientific literature, critically appraised individual articles for inclusion, analyzed, and interpreted the findings. DA drafted the manuscript, critically reviewed it, and prepared the final version for publication. Both authors read and approved the final version.
Acknowledgment
Authors thank Shifera Leta, Mekonnen Sisay, and Jemal Yusuf for guiding/helping me in preparation of this document and helping us in searching articles from the Embase database and also authors of articles that were used in this review and meta-analysis.
References
- 1.Andrews AH, Blowey R, Eddy RG, Boyd H. United States: John Wiley &Sons; 2008. Bovine Medicine: Diseases and Husbandry of Cattle. [Google Scholar]
- 2.Heredia NL, Wesley IV, Garcia JS. United States: John Wiley &Sons; 2009. Microbiologically Safe Foods. [Google Scholar]
- 3.Hoffman S, Maculloch B, Batz M. Economic Burden of Major Foodborne Illnesses Acquired in the United States. United States Department of Agriculture, Economic Information Bulletin. 2015 [Google Scholar]
- 4.Gould G, Russell N. Berlin, Germany: Springer; 2003. Major, New, and Emerging Food-poisoning and Food-spoilage Microorganisms, in Food Preservatives; pp. 1–13. [Google Scholar]
- 5.Braden CR. Salmonella enterica serotype enteritidis and eggs:A national epidemic in the United States. Clin Infect Dis. 2006;43:512–7. doi: 10.1086/505973. [DOI] [PubMed] [Google Scholar]
- 6.Foley SL, Nayak R, Hanning IB, Johnson TJ, Han J, Ricke SC. Population dynamics of Salmonella enterica serotypes in commercial egg and poultry production. Appl Environ Microbiol. 2011;77:4273–9. doi: 10.1128/AEM.00598-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sosa AD, Okeke I, Amábile-Cuevas CF, Kariuki S, Hsueh PR, Byarugaba DK. Berlin, Germany: Springer; 2010. Antimicrobial Resistance in Developing Countries. [Google Scholar]
- 8.Le Hello S, Harrois D, Bouchrif B, Sontag L, Elhani D, Guibert V, et al. Highly drug-resistant Salmonella enterica serotype kentucky st198-x1: A microbiological study. Lancet Infect Dis. 2013;13:672–9. doi: 10.1016/S1473-3099(13)70124-5. [DOI] [PubMed] [Google Scholar]
- 9.Kariuki S, Revathi G, Kariuki N, Muyodi J, Mwituria J, Munyalo A, et al. Increasing prevalence of multidrug-resistant non-typhoidal salmonellae, Kenya, 1994-2003. Int J Antimicrob Agents. 2005;25:38–43. doi: 10.1016/j.ijantimicag.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 10.Niehaus AJ, Apalata T, Coovadia YM, Smith AM, Moodley P. An outbreak of foodborne salmonellosis in rural Kwazulu-Natal, South Africa. Foodborne Pathog Dis. 2011;8:693–7. doi: 10.1089/fpd.2010.0749. [DOI] [PubMed] [Google Scholar]
- 11.Angulo FJ, Mølbak K. Human health consequences of antimicrobial drug-resistant Salmonella and other foodborne pathogens. Clin Infect Dis. 2005;41:1613–20. doi: 10.1086/497599. [DOI] [PubMed] [Google Scholar]
- 12.Getachew M, Mulugeta G, Lema T, Aseffa A. Prevalence and antimicrobial susceptibility patterns of Salmonellaserovars and Shigellaspecies in Butajira, Central Ethiopia. J Microb Biochem Technol. 2014;6:S2–006. [Google Scholar]
- 13.World Health Organization. Foodborne Disease Outbreaks: Guidelines for Investigation and Control. Geneva: World Health Organization; 2008. [Google Scholar]
- 14.Bayeh A, Fantahun B, Belaye B. Prevalence of Salmonella typhi and intestinal parasites among food handlers in Bahir Dar town, Northwest Ethiopia. EJHD. 2010;24:46–50. [Google Scholar]
- 15.Hutton B, Catala-Lopez F, Moher D. The prisma statement extension for systematic reviews incorporating network meta-analysis: Prismanma. Med Clin. 2016;147:262–6. doi: 10.1016/j.medcli.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 16.Patel J, Cockerill F, Bradford P. London, United Kingdom: Clinical and Laboratory Standards Institute; 2014. M100-s24 Performance Standards for Antimicrobial Susceptibility Testing, Twenty-fourth Informational Supplement. [Google Scholar]
- 17.London S, Gurdal O, Gall C. Automatic export of pubmed®citations to endnote®. Med Ref Serv Quart. 2010;29:146–53. doi: 10.1080/02763861003723317. [DOI] [PubMed] [Google Scholar]
- 18.Schultz T, Florence Z. Adelaide: Joanna Briggs Institute; 2007. Joanna Briggs Institute Meta-analysis of Statistics Assessment and Review Instrument. [Google Scholar]
- 19.Abebe W, Earsido A, Taye S, Assefa M, Eyasu A, Godebo G. Prevalence and antibiotic susceptibility patterns of Shigellaand Salmonellaamong children aged below five years with diarrhoea attending nigist eleni mohammed memorial hospital, South Ethiopia. BMC Pediatr. 2018;18:241–1. doi: 10.1186/s12887-018-1221-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ameya G, Tsalla T, Getu F, Getu E. Antimicrobial susceptibility pattern, and associated factors of Salmonellaand Shigellainfections among under five children in Arba Minch, South Ethiopia. Ann Clin Microbiol Antimicrob. 2018;17:1. doi: 10.1186/s12941-018-0253-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Assefa A, Girma M. Prevalence and antimicrobial susceptibility patterns of Salmonellaand Shigellaisolates among children aged below five years with diarrhea attending robe general hospital and goba referral hospital, South East Ethiopia. Trop Dis Travel Med Vaccines. 2019;5:19. doi: 10.1186/s40794-019-0096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feleke H, Medhin G, Abebe A, Beyene B, Kloos H, Asrat D. Enteric pathogens and associated risk factors among under-five children with and without diarrhea in Wegera district, Northwestern Ethiopia. Pan Afr Med J. 2018;29:72. doi: 10.11604/pamj.2018.29.72.13973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Getamesay M, Getenet B, Ahmed Z. Prevalence of Shigella, Salmonellaand Campylobacterspecies and their susceptibility patters among under five children with diarrhea in Hawassa town, South Ethiopia. Ethiop J Health Sci. 2014;24:101–8. doi: 10.4314/ejhs.v24i2.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mamuye Y, Amaru GM, Mekonnen AB, Desta K, Fantaw S. Isolation and antibiotic susceptibility patterns of Shigella and Salmonellaamong under 5 children with acute diarrhoea: A cross-sectional study at selected public health facilities in Addis Ababa, Ethiopia. J Clin Microbiol. 2015;2015:1000186. [Google Scholar]
- 25.Tosisa W, Mihret A, Ararsa A, Eguale T, Abebe T. Prevalence and antimicrobial susceptibility of Salmonella and Shigella species isolated from diarrheic children in ambo town. BMC Pediatr. 2020;20:91. doi: 10.1186/s12887-020-1970-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yemane G, Mulam G, Gaim T. Prevalence and antimicrobial susceptibility of Salmonellaspecies in diarrheal children under five-years of age in Bahir Dar town, Ethiopia. Int J Int Sci Inn Tech Sec A. 2014;3:12–7. [Google Scholar]
- 27.Aklilu A, Kahase D, Dessalegn M, Tarekegn N, Gebremichael S, Zenebe S, et al. Prevalence of intestinal parasites, Salmonellaand Shigellaamong apparently health food handlers of Addis Ababa university student's Cafeteria, Addis Ababa, Ethiopia. BMC Res Notes. 2015;8:17. doi: 10.1186/s13104-014-0967-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Awol N, Nigusse D, Ali M. Prevalence and antimicrobial susceptibility profile of Salmonella and Shigella among food handlers working in food establishment at Hawassa citym, Southern Ethiopia. BMC Res Notes. 2019;12:1–7. doi: 10.1186/s13104-019-4725-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bafa TA, Sherif EM, Hantalo AH, Woldeamanuel GG. Magnitude of enteropathogens and associated factors among apparently healthy food handlers at wolkite university student's Cafeteria, Southern Ethiopia. BMC Res Notes. 2019;12:567. doi: 10.1186/s13104-019-4599-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garedew L. Identification of drug-resistant Salmonella from food handlers at the university of Gondar, Ethiopia. BMC Res Notes. 2014;7:545. doi: 10.1186/1756-0500-7-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gebreyesus A, Adane K, Welekidan LN, Dejene TA, Belay S, Alemu M, et al. Prevalence of Salmonella typhi and intestinal parasites among food handlers in mekelle university student Cafeteria, Mekelle, Ethiopia. Food Control. 2014;44:45–8. [Google Scholar]
- 32.Getie M, Abebe W, Tessema B. Prevalence of enteric Bacteria and their antimicrobial susceptibility patterns among food handlers in Gondar town, Northwest Ethiopia. Antimicrob Resist Infect Control. 2019;8:111. doi: 10.1186/s13756-019-0566-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Getnet F, Awulachew E, Ashuro Z. Prevalence and antimicrobial resistance of Salmonella isolated from food handlers in addis ababa university students'cafeteria, Ethiopia. Afr J Basic Appl Sci. 2014;6:210–6. [Google Scholar]
- 34.Mama M, Alemu G. Prevalence, antimicrobial susceptibility patterns and associated risk factors of Shigella and Salmonella among food handlers in Arba Minch university, South Ethiopia. BMC Infect Dis. 2016;16:686. doi: 10.1186/s12879-016-2035-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marami D, Hailu K, Tolera M. Prevalence and antimicrobial susceptibility pattern of Salmonella and Shigellaspecies among asymptomatic food handlers working in Haramaya university cafeterias, Eastern Ethiopia. BMC Res Notes. 2018;11:74. doi: 10.1186/s13104-018-3189-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Solomon FB, Wada FW, Anjulo AA, Koyra HC, Tufa EG. Burden of intestinal pathogens and associated factors among asymptomatic food handlers in South Ethiopia:Emphasis on salmonellosis. BMC Res Notes. 2018;11:502. doi: 10.1186/s13104-018-3610-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tadesse G, Mitiku H, Teklemariam Z, Marami D. Salmonellaand Shigellaamong asymptomatic street food vendors in the Dire Dawa city, Eastern Ethiopia: Prevalence, antimicrobial susceptibility pattern, and associated factors. Environ Health Insights. 2019;13:1–8. doi: 10.1177/1178630219853581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yesigat T, Jemal M, Birhan W. Prevalence and associated risk factors of Salmonella, Shigella, and intestinal parasites among food handlers in Motta town, North West Ethiopia. CJID. 2020;2020:6425946. doi: 10.1155/2020/6425946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adimasu DA, Kebede A, Menkir S, Abubeker R, Mihre A, Fantaw S, et al. Prevalence of antibiotic resistant Salmonella species and selected intestinal protozoan parasites in Harar Hiwot Fana hospital, Ethiopia. Am J Biochem Mol Bio. 2014;4:99–111. [Google Scholar]
- 40.Demissie A. Prevalence and antimicrobial susceptibility patterns of Shigella and Salmonella species among patients with diarrhea attending Gondar town health institutions, Northwest Ethiopia. Sci J Public Health. 2014;2:469–75. [Google Scholar]
- 41.Eguale T, Gebreyes WA, Asrat D, Alemayehu H, Gunn JS, Engidawork E. Non-typhoidal salmonellosis, antimicrobial resistance and co-infection with parasites among patients with diarrhea and gastroenteritis in Addis Ababa, Ethiopia. Trop Med Int Health. 2015;20:S1–263. doi: 10.1186/s12879-015-1235-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Endalu M. Assam: AAU Institutional Repository; 2019. Occurrence of Salmonella in Selected Beef Chain Locations and Stool of Diarrheic Patients in Adama, Ethiopia. [Google Scholar]
- 43.Lamboro T, Ketema T, Bacha K. Prevalence and antimicrobial resistance in Salmonella and Shigella species isolated from out patients, Jimma university specialized hospital, Southwest Ethiopia. AJBAS. 2017;9:118–25. doi: 10.1155/2016/4210760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Terfassa A, Jida M. Prevalence and antibiotics susceptibility pattern of Salmonella and Shigella species among diarrheal patients attending nekemte referral hospital, Oromia, Ethiopia. Int J Microbiol. 2018;2018:6. doi: 10.1155/2018/9214689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Teshome B, Teklemariam Z, Ayana DA, Marami D, Asaminew N. Salmonella and Shigellaamong patients with diarrhea at public health facilities in Adama, Ethiopia: Prevalence, antimicrobial susceptibility pattern, and associated factors. SSAGE Open Med. 2019;7:1–8. doi: 10.1177/2050312119846041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beyene G, Nair S, Asrat D, Mengistu Y, Engers H, Wain J. Multidrug resistant Salmonella concord is a major cause of salmonellosis in children in Ethiopia. J Infect Dev. 2011;5:23–33. doi: 10.3855/jidc.906. [DOI] [PubMed] [Google Scholar]
- 47.Beyene G, Tasew H. Prevalence of intestinal parasite, Shigella and Salmonella species among diarrheal children in Jimma health center, Jimma Southwest Ethiopia:A cross sectional study. Ann Clin Microbiol Antimicrob. 2014;13:10. doi: 10.1186/1476-0711-13-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gebreegziabher G, Asrat D, Amanuel YW, Hagos T. Isolation and antimicrobial susceptibility profile of Shigella and Salmonella species from children with acute diarrhoea in mekelle hospital and semen health center, Ethiopia. Ethiop J Health Sci. 2018;28:197–206. doi: 10.4314/ejhs.v28i2.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hayamo M, Alemayehu T, Tadesse B, Mitiku E, Bedawi Z. Washington, DC: Research Square; 2019. Shigella and Salmonella, Antibiotics Susceptibility Pattern and Associated Risk Factors Among Diarrheic Children in Southern Ethiopia: A Cross Sectional Study. [Google Scholar]
- 50.Abebe M, Tafese B, Adane H. Antimicrobial resistance of Salmonella serovars isolated from food of bovine origin in selected woredas of Tigray, Ethiopia. World J Med Sci. 2014;11:342–7. [Google Scholar]
- 51.Asfaw Ali D, Tadesse B, Ebabu A. Prevalence and antibiotic resistance pattern of salmonella isolated from caecal contents of exotic chicken in Debre Zeit and Modjo Ethiopia. Int J Microbiol. 2020;2020:1910630. doi: 10.1155/2020/1910630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kebede A, Kemal J, Alemayehu H, Mariam SH. Isolation, identification, and antibiotic susceptibility testing of Salmonella from slaughtered bovines and ovines in Addis Ababa abattoir enterprise, Ethiopia: A cross-sectional study. Int J Bacteriol. 2016;2016:3714785. doi: 10.1155/2016/3714785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mengistu S, Abayneh E, Shiferaw D, Coli E. o157: H7 and Salmonella species:Public health importance and microbial safety in beef at selected slaughter houses and retail shops in Eastern Ethiopia. J Vet Sci Technol. 2017;8:2. [Google Scholar]
- 54.Zelalem S. Barbheta: AAU Institutional Repository; 2017. Assessment of the Contamination of Beef with Salmonella and Knowledge, Attitudes and Beef Handling Practices Along Beef Supply Chain in Dukem Town, Ethiopia. [Google Scholar]
- 55.Assefa M, Teklu A, Negussie H. The prevalence and public health importance of salmonella from chicken table eggs, Ethiopia. Am Eur J Agric Environ Sci. 2011;11:512–8. [Google Scholar]
- 56.Taddese D, Tolosa T, Gelalcha BD, lakow M, Olani A, Shumi E. Antibiograms and risk factors of Salmonellaisolates from laying hens and eggs in Jimma town, South Western Ethiopia. BMC Res Notes. 2019;12:472. doi: 10.1186/s13104-019-4516-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tsegaye S, Beyene W, Birhanu BT, Tessema TS, Feleke A, et al. Prevalence and antimicrobial susceptibility pattern of Salmonella species from exotic chicken eggs in alage, Ziway and Shashemene, Ethiopia. Afr J Basic Appl Sci. 2016;8:180–4. [Google Scholar]
- 58.Abate AA, Rakshit SK, Anal AK. Genotypic and phenotypic characterization of antimicrobial resistance patterns of Salmonella strains isolated from raw milk in Sebeta, Ethiopia. Int J Adv Life Sci. 2013;6:192–9. [Google Scholar]
- 59.Addis Z, Kebede N, Worku Z, Gezahegn H, Yirsaw A, Kassa T. Prevalence and antimicrobial resistance of Salmonella isolated from lactating cows and in contact humans in dairy farms of Addis Ababa: A cross sectional study. BMC Infect Dis. 2011;11:222. doi: 10.1186/1471-2334-11-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hunduma D, Alonso S, Agga G, Kerro O, Wieland B, Desta H, et al. United States: IAFP; 2019. Occurrence and Antimicrobial Resistance Patterns of Escherichia coli o157:H7 and Non-typhoid Salmonella in Milk and Feces of Lactating Dairy Cows and Camels in Borana, Southern Ethiopia; pp. 2–249. [Google Scholar]
- 61.Ejo M, Garedew L, Alebachew Z, Worku A. Prevalence and antimicrobial resistance of Salmonella isolated from animal-origin food items in Gondar, Ethiopia. Biomed Res Int. 2016;2016:4290506. doi: 10.1155/2016/4290506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mulaw G. Prevalence and antimicrobial susceptibility of Salmonellaspecies from lactating cows in dairy farm of bahir dar town, Ethiopia. J Microbiol Res. 2017;11:1578–85. [Google Scholar]
- 63.Tadesse T, Dabassa A. Prevalence and antimicrobial resistance of Salmonella isolated from raw milk samples collected from Kersa district, Jimma zone, Southwest Ethiopia. J Med Sci. 2012;12:224–8. [Google Scholar]
- 64.Tesfaw L, Taye B, Alemu S, Alemayehu A, Sisay Z, Negussie H. Prevalence and antimicrobial resistance profile of Salmonella isolates from dairy products in Addis Ababa, Ethiopia. Afr J Microbiol Res. 2013;7:5046–50. [Google Scholar]
- 65.Al-Rifai RH, Chaabna K, Denagamage T, Alali WQ. Prevalence of enteric non-typhoidal Salmonella in humans in the Middle East and North Africa:A systematic review and meta-analysis. Zoonoses Public Health. 2019;66:701–28. doi: 10.1111/zph.12631. [DOI] [PubMed] [Google Scholar]
- 66.Tadesse G. Prevalence of human salmonellosis in Ethiopia:A systematic review and meta-analysis. BMC Infect Dis. 2014;14:88. doi: 10.1186/1471-2334-14-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Crump JA, Sjölund-Karlsson M, Gordon MA, Parry CM. Epidemiology, clinical presentation, laboratory diagnosis antimicrobial, resistance and antimicrobial management of invasive Salmonella infections. Clin Microbiol Rev. 2015;28:901–37. doi: 10.1128/CMR.00002-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zelalem A, Sisay M, Vipham JL, Abegaz K, Kebede A, Terefe Y. The prevalence and antimicrobial resistance profiles of bacterial isolates from meat and meat products in Ethiopia:A systematic review and meta-analysis. Int J Food Contam. 2019;6:1. [Google Scholar]
- 69.Xavier C, Gonzales-Barron U, Paula V, Estevinho LM. Meta-analysis of the incidence of foodborne pathogens in portuguese meats and their products. Food Res Int. 2014;55:311–23. [Google Scholar]
- 70.US Department of Agriculture, Forest Service and Interior Service. Progress Report on Salmonella and Campylobacter Testing of Raw Meat and Poultry Products, 1998-2013. United States: US Department of Agriculture, Forest Service and Interior Service; 2014. [Google Scholar]
- 71.Britto CD, Wong VK, Dougan G, Pollard AJ. A systematic review of antimicrobial resistance in Salmonella enterica serovar typhi, the etiological agent of typhoid. PLoS Negl Trop Dis. 2018;12:e0006779. doi: 10.1371/journal.pntd.0006779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tadesse G. A meta-analysis of the proportion of animal Salmonella isolates resistant to drugs used against human salmonellosis in Ethiopia. BMC Infect Dis. 2015;15:84. doi: 10.1186/s12879-015-0835-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used in this study are available and will be provided by the corresponding author on a reasonable request.


