Abstract
Objectives:
Nowadays, it is believed that more than 240 million people worldwide are infected with the hepatitis B virus (HBV), associated with irreversible health-related consequences, represented by hepatic failure, cirrhosis, and hepatocellular carcinoma, and already are ranked as 15th cause of human death around the world.
Methods:
A systematic review and a meta-analysis were performed to evaluate the effectiveness of vaccination and immunization on health professionals against HBV. The review was registered at the PROSPERO database (CRD42017075643). A search for cross-sectional studies was performed in PubMed, Scopus, Web of Science, LILACS, BBO, Cochrane Library, and in the gray literature. The Effective Public Health Practice Project Modified Scale was used to evaluate the internal quality of the studies included.
Results:
A total of 1865 articles were identified after the removal of duplicates. Of these, 790 studies remained after screening the titles and abstracts. Finally, ten studies remained after full-text reading for qualitative analysis, all of which were used in the meta-analysis. A significant difference was found in the vaccine protocol for health professionals immunized against hepatitis B, compared to those not immunized. The risk ratio was 7.37 (95% confidence interval = 3.92–13.83; P < 0.00001).
Conclusion:
This study showed that the vaccine protocol is effective in immunizing health professionals against hepatitis B.
Keywords: Health personnel, hepatitis b vaccines, meta-analysis, systematic review
Introduction
Hepatitis B remains one of the most relevant concerns on global public health.[1-3] Nowadays, it is believed that more than 240 million people worldwide are infected with the hepatitis B virus (HBV), and according to the Global Burden of Disease Study 2010 (GBD 2010), hepatitis associated to its irreversible health-related consequences, represented by hepatic failure, cirrhosis, and hepatocellular carcinoma, already is ranked as 15th cause of human death around of the world.
The HBV is transmitted by percutaneous or mucous membrane exposure to infectious blood and body fluids that contain blood.[4] Percutaneous exposures that have resulted in HBV transmission also include transfusion of blood or blood products manipulation, contaminated equipment used for therapeutic injections or needle pricks or for endogenous illegal drug application, as well as other healthcare-related procedures such as injuries from sharp instruments sustained by hospital personnel. In addition, occasional outbreaks of hepatitis B have been associated with tattooing and acupuncture.[5]
This incident places healthcare workers, especially medical, nurse, and dental professionals at higher occupational risk when compared to other common communicable diseases, including human immunodeficiency virus (HIV) and hepatitis C virus.[6-8] Thus, measures of individual protection and the vaccination of workers who may come into contact with blood or other body fluids, or even with sharp instruments or contaminated surfaces, should be adopted to prevent transmission of hepatitis.[9] Vaccination is the most persuasive intervention for infectious disease prevention,[10] and the HBV vaccine is the most currently used measure of disease prevention.[11] The administration of this vaccine is considered safe, and its efficacy is considered high; its score conversion can be attested by 90–95% of immunocompetent adults.[12]
However, it should highlight that some individuals who fail to elicit a protective antibody response after hepatitis B vaccination remains at risk for HBV infection,[7] but the vast majority of those who do not achieve anti-HBs seroprotective levels respond to a booster vaccine and may be considered protected against HBV.[13]
After primary immunization received with the hepatitis B vaccine, the titer of antibody to hepatitis B surface antigen (anti-HBs) considered seroprotective is ≥10 milli-international units per milliliter (mIU/ml).[14] However, anti-HBs levels decline as soon as the time period progress, and many previously vaccinated individuals may have anti-HBs below the threshold of protection considered as accepted, attested 10–15 years after the first series of doses.[15,16] It is noteworthy that a health professional under these circumstances invariably inserted his or her peers and patients at hepatitis risk.
According to these premises, as well as in an endeavor to analyze whether health professionals are or are not immunized against HBV, we performed a systematic review sought to answer the following question: Are healthcare workers immunized after receiving the hepatitis B vaccination according to the recommended guidelines?
Methods
Herein we described the sequence from previously published systematic reviews.[17-19]
Protocol and registration
This study was conducted from July to December 2017 at University Positivo, Curitiba, Paraná, Brazil. We previously have registered the study at the protocol at the PROSPERO database (CRD42017075643) and followed the recommendations of the MOOSE statement to report this systematic review.[20]
Information sources and search strategy
The search strategy included indexed terms, such as MeSH terms and loose words in the article appearing in the title and abstract. The strategy was defined based on the study question formed by the acronym PECOS, which stands for:
Population (P): Healthcare workers
Intervention (E): Effective vaccination
Comparison (C): Not effective vaccination
Outcome (O): Immunization after being given the hepatitis B vaccination according to the recommended guidelines
Study design (S): Observational studies.
This review included trials from the following electronic databases: MEDLINE through PubMed, Scopus, Web of Science, Latin American and Caribbean Health Sciences Literature database (LILACS), Brazilian Library in Dentistry (BBO), and Cochrane Library. No restrictions were made regarding publication date or language.
We also used other sources to identify a greater number of articles published in literature. We searched the abstracts of annual conferences of the International Association for Dental Research and contacted authors of relevant abstracts for further information. We explored the gray literature using the System for Information on Gray Literature in Europe database, as well as dissertations and theses, using the full texts of the ProQuest Dissertations and Theses database and the Capes Theses database.
We searched the following clinical trial registries to locate unpublished and ongoing trials related to the review question: Current Controlled Trials (www.controlled-trials.com), International Clinical Trials Registry Platform (http://apps.who.int/trialsearch/), ClinicalTrials.gov (www.clinicaltrials.gov), Rebec (www.rebec.gov.br), and EU Clinical Trials Register (https://www.clinicaltrialsregister.eu).
Eligibility criteria
We included all studies that identified the number of healthcare workers who were not seropositive after receiving the hepatitis B vaccine. Non-controlled clinical trials, editorial letters, pilot studies, historical reviews, in vitro studies, and cohort and descriptive studies, such as case reports and case series, were excluded from the study.
Study selection and data collection process
Primarily, the articles were selected by title and abstract according to a previously described search strategy. Articles that appeared in more than one database were considered only once. Full-text articles were obtained even when the title and abstract had insufficient information to make a clear decision; subsequently, four reviewers classified those which met the inclusion criteria. The data were extracted using customized extraction forms, and the following data were recorded for each study included:
Details of study methods, including study design and setting.
Details of the participants, comprising only healthcare workers
Only studies that included results of anti-HBs blood tests
Data from publications of unrestricted date – article publication dates were not defined.
If there were multiple reports of the same study (i.e., reports with different follow-ups), the data from all these reports were extracted and included on a single data collection form to avoid data overlap.
Risk of bias of individual studies
The internal quality of the included studies was assessed by two independent reviewers using the Effective Public Health Practice Project (EPHPP) Modified Scale.[16] This quality assessment tool evaluates the design and quality of observational studies and facilitates the incorporation of quality assessments into the interpretation of the meta-analysis results, although it is not used as criteria for the inclusion or exclusion of articles.
The quality assessment instrument used herein contains the following components: (1) Selection bias, (2) study design, (3) identification and treatment of confounders, (4) blinding of outcome assessors and of participants, (5) reliability and validity of data collection methods, and (6) withdrawals of consent and dropouts. The components are rated as strong, moderate, or weak, according to a standardized dictionary (http://www.ephpp.ca/ PDF/QADictionary_dec 2009.pdf).[21]
The overall rating given to the study is determined by assessing the previous six rating components. In the original instrument,[21] studies having no weak ratings and at least four strong ratings should be considered strong. Those with less than four strong ratings and one weak rating are to be considered moderate. Finally, those with two or more weak ratings should be considered weak.
Strong and moderate studies were included in the review.[21] We listed the important confounders that should have been taken into consideration in the study: Whether the research used health professionals, the number of relevant participants, the participants’ report on whether they followed all or part of the vaccination protocol, and whether the studies reported the results of anti-HBs blood tests. If the article covered two or three of these confounding factors, the study was considered strong; if the article covered only one of the factors, the study was considered moderate, and if the article did not cover any of the factors, the study was considered weak.
The inclusion criteria defined for evaluating the quality of this study consisted of articles that considered only professionals in the health area, that reported completion of the vaccination protocol, with 3 doses as having been administered on 0, 1st, and 6th month, respectively, and that included the anti-HBs test to determine if, in fact, the immunization should be performed earlier than 1 year of age.
Summary measures and synthesis of the results
The RevMan 5.3 program (Review Manager, The Cochrane Collaboration, Copenhagen, Denmark) was used to conduct all the analyses that could be extracted from the study. The data from eligible studies were dichotomous (presence or absence of immunization). Risk ratio effects models were applied to the dichotomous data.
Assessment of the quality of evidence using grading of recommendations: Assessment, development, and evaluation (GRADE)
We graded the quality of the evidence for each pair of comparison across studies and for the ranking of treatments (body of evidence) using the GRADE (http://www.gradeworkinggroup.org/) and following the recommendations for assessment of the quality of evidence for network meta-analysis.
From the initial classification, it is possible to identify and judge the aspects that can reduce or increase the level of evidence. The factors responsible for the reduction in the level of evidence are risk of bias, imprecision, inconsistency, indirectness of evidence, and publication bias) to possibly downgrade the quality of the evidence (1 or 2 levels). In addition, if the level has not been lowered due to the factors presented above, the evidence from observational studies can be high considering three factors: Great magnitude of effect; dose-response gradient; and residual confounding factors, which increase confidence in the estimate. Each one of these topics was assessed as “no limitations,” “serious limitations,” or “very serious limitations” to allow for categorization of the quality of the evidence for each outcome into high, moderate, low, and very low.
Results
Study selection
The EndNote program was used to refine the search after database screening and removal of duplicates; it identified 1835 studies [Figure 1]. A total of 790 studies remained after title and abstract screening. This number was further reduced to 10 after careful examination of the abstracts.
Figure 1.
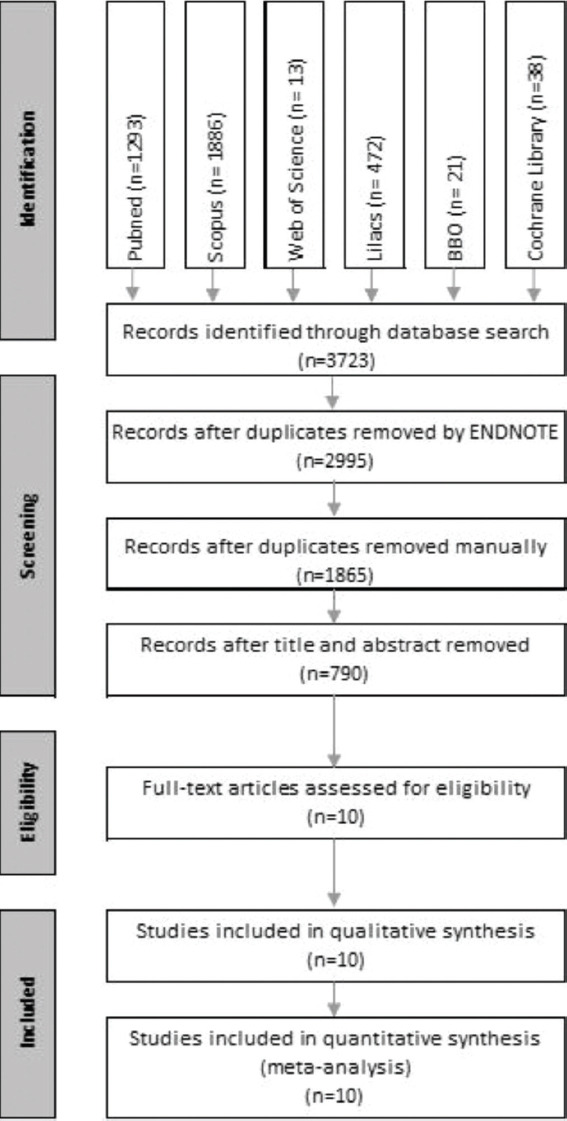
Flow diagram of the studies
The characteristics of the included studies[22-31] and their locations of origin of the studies were: Catalonia, Chile, Costa Rica, United States, India, Israel, Pakistan, Peru, Valencia, and Taiwan. The average age of the participants in 6 articles[12,13,26,28,30,31] was 37.8 years, the youngest participant being 18 years old, and the oldest is 70 years old. In only one article, the age of the participants was not reported.[27] In three articles, the authors separated the participants into groups of different age ranges but did not go into details.[24,25,29]
In eight articles, the percentage of male gender participants was stated: 34.2%[24-31]. In the other two articles, these data were not mentioned.[22,23] In relation to the number of participants, a disparity was observed among the studies, ranging from 34 to 2058 participants. The number of participants in the respective studies by Herrer[22] and Villena[25] was the same: 211 health professionals. The average number of participants in all the articles was 740.
All the participants were health professionals, but four articles did not report what specific fields these professionals belonged to Racela et al.,[23] Sabido et al.,[24] Yen et al.,[26] Zeeshan et al.[29] In the other six articles, the professional nurses were part of the research. Doctors appeared in four articles;[22,24,27,31] technicians (no particular area mentioned) in three articles;[22,25,31] laboratorians (no specific field mentioned), in two articles;[27,30] and dentists, in only one article.[22]
Three types of vaccines against HBV were applied to the participants: Engerix-B;[24,26,28-31] Cuban anti-HBV;[25] and Heptavax-B.[22,23,27] The interval between the first and second dose was 1 month, while the interval between the second and third dose was 6 months in six articles;[26-31] in two articles,[24,25] the interval was 1 month between the first and second dose and 2 months between the second and third dose. In one article,[22] the interval between the doses was 2 months between the first and the second dose and a variation of 4–6 months between the second and the third doses. Racela et al.[23] did not report this data.
The anti-HBs test was requested in all the articles, but the time after the third dose and the collection was reported in seven articles.[23,24,26-30] The period between the third dose and the anti-HBs test was between 6 and 8 weeks,[29] 6 months,[22,23] 7 months,[31] 21-120 days, and 1 month.[30] The laboratory method for evaluating the anti-HBs test was: Enzyme immunoassay method in six articles,[25,26,27,28-30] enzyme immunoassay and radioimmunoassay method in one article,[23] radioimmunoassay method in one article,[24] immunoradiometric assay in one article,[22] and quantitative immunoassay in another article.[31]
In relation to the location of the health service where the surveys were carried out, six articles reported that they were carried out at a hospital;[22-25,29,31] two at a medical center;[26,27] two at a laboratory;[23,30] and only one article did not report the location[28].
Other data evaluated in some articles were seroconversion, seroprotection, and hyperresponsiveness. These data were computed in five articles[23-25,28,29].
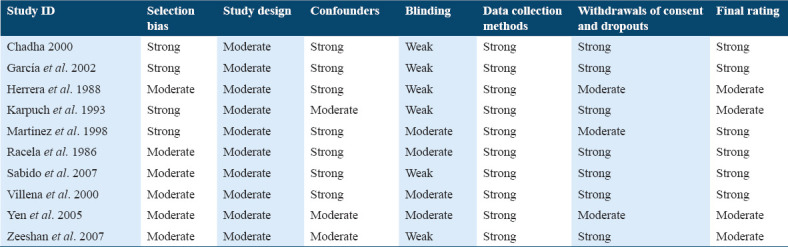
Assessment of the risk of bias
The studies were evaluated for quality using the EPHPP tools [Table 1]. Ten studies were selected being that six were considered strong,[23-25,28,30,31] and four were considered moderate,[22,26,27,29] according to the quality assessment components. For this reason, all the studies were included in the meta-analysis.
Table 1.
Quality assessment components and final rating of the studies
Meta-analysis
The meta-analysis was performed on studies classified as strong and moderate, based on the final rating of quality assessment components.
Frequency of individuals immunized against hepatitis B
This analysis was based on 10 studies.[22-31] The risk ratio of those immunized and those not immunized for HBV was 7.37, with a 95% confidence interval [CI] of 3.92–13.83 (P < 0.001). Based on these studies, a significant statistical difference could be identified among the groups [Figure 2]. The data were heterogeneous (Chi-square test, P < 0.00001; I2 = 98%; Figure 2), which means that none of the studies included in the analysis shared a common effect size.
Figure 2.
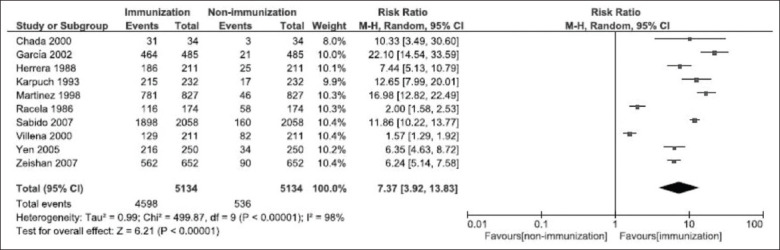
Forest plot of the frequency of immunized and non-immunized individuals
Sensitivity analysis
We sought to identify the factors warranting the heterogeneity observed by considering the local characteristics of where the research was carried out, such as the primary health care system and the systemic and socioeconomic conditions of the researched populations.
Assessment of the quality of evidence
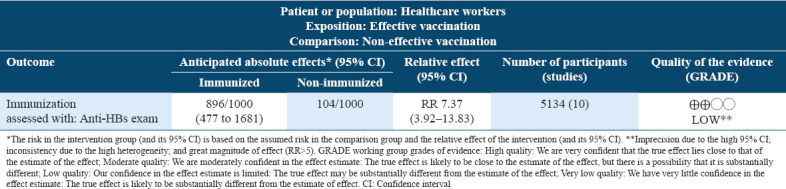
In the summary of findings in Table 2, the meta-analysis was graded as low in the quality of evidence. The reasons for downgrading the evidence were that all studies are observational, had imprecision with a high 95% CI and statistical heterogeneity (for the meta-analysis). The reason for the increase the evidence was the great magnitude of effect (RR>5).
Table 2.
Summary of findings table
Discussion
Viral hepatitis resulted in 1.34 million of deaths in 2015, a number similar to the deaths caused by tuberculosis and HIV in the same year. However, the mortality rates for tuberculosis and HIV are declining, whereas hepatitis death rates seem to be increasing.[27]
All the articles evaluated in the present systematic review have demonstrated that health professionals are mostly immunized against HBV after receiving 3 doses of the vaccine protocol. This result agrees with the vast literature on the subject, in which positive rates of antibodies against HBsAg (anti-HBs) is ≥10 mIU/mL have been reported to range from 85% to 95% of the vaccines.[32]
The practice of a health-related activity represents a risk factor for acquiring HBV infection due to the conglomerate of patients with various infectious diseases and the risk of accidents occurring in many procedures.[33] The risk of HBV transmission to healthcare professionals is 3–5 times higher than to people in general.[34] Therefore, it is extremely important that health professionals be vaccinated and prove their immunization.[33] Healthcare workers should be trained to update their knowledge of prevention and control measures.[35]
The protocol for unvaccinated individuals, or those for which no prior vaccination information is available, consists of a series of 3 doses of hepatitis B vaccine (0, 1, and 6 months), followed by testing for anti-HBs, performed 1–2 months after the last dose to confirm immunity.[2,36] Positive results for anti-HBs equal to or >10 mIU/mL confirm immunization.[37] Vaccinated professionals who’s anti-HBs level remains below 10 mIU/mL, even after the booster dose, are considered “non-responders.”[38] They are also regarded as susceptible to infection; therefore, if exposed to HBV, they should be administered anti-hepatitis B immunoglobulin.[39]
Even though the HBV vaccine is highly effective, some healthy individuals do not respond satisfactorily to vaccination against hepatitis B, ranging from 5% to 10%.[40] The reasons for this lack of response are not defined, but the authors believe that the leading causes are advanced age (<40 years), male sex, obesity, alcoholism, smoking, genetic predisposition, chronic diseases, and immunosuppressant drug use.[41]
Another critical factor that should be addressed is the durability of this immunization. Studies show that 77% of health professionals have adequate levels of antibodies even after 18 years of the vaccine protocol.[42] The maintaining of a long-term antibody response is critical for protective immunity against HBV infection, particularly among healthcare professionals. The age factor may interfere with the durability of antibody maintenance. A study found that a group of participants between 5 and 19 years of age had a better level of anti-HBs (10 mIU/mL) after 22 years of primary immunization compared to other groups.[43]
Regarding the immune response of health professionals who had inadequate levels of anti-HBs after years of vaccination, it was found that only one booster dose of the vaccine could elicit a rapid response.[9] Protective levels were developed at 53% on day 7% and 94% on day 21, with an average increase of 100 times the level of antibodies observed in this period. Other studies with a shorter follow-up time also documented a rapid immune response to a booster dose of the vaccine in health workers and other populations.[15,37,41]
The fast response to one booster administered in health professionals with no anti-HBs or with inadequate levels of it suggests that they will be protected after the re-exposure to HBV, a response called anamnestic response.[44] These data support current guidelines that contraindicate revaccination for healthy health professionals.[45]
Regarding the meta-analysis, high heterogeneity was observed among the ten studies included (98%). A sensitivity analysis was performed to verify the possible heterogeneity between the studies. When the studies by Racela[23] and Villena[25] were removed because they were deemed as less adequate in observing heterogeneity, the remaining eight studies were heterogeneous at 94%, proving that this heterogeneity occurs at random. One possible explanation may be that the seroconversion evaluations in the Villena[25] study were performed 30 days after the second dose and 15 days after the third dose. In other articles, the evaluations were mostly performed only after the third dose. In the study of Racela,[23] the sample was subdivided and evaluated at different times: 0 and 6 months after the third dose, with 41 subjects, 7 and 24 months after the third dose, with 75 subjects. Moreover, two tests were used to evaluate this seroconversion, thus presenting a variation which may have influenced the observance of heterogeneity in the meta-analysis.
Conclusion
This systematic review demonstrated that there was immunization against HBV in health professionals. This positive result indicates that there is awareness, concern, and care on the part of health professionals. However, these results should be interpreted with caution due to the high heterogeneity found and the quality of evidence being considered low.
The large number of articles presented in the literature and researched in this meta-analysis showed the effectiveness of the vaccine protocol in the immunization against hepatitis B for health professionals. New studies that evaluate the effectiveness of hepatitis B vaccination should be conducted to confirm these positive results.
Authors’ Declaration Statements
Ethics approval and patients’ consent
Not applicable, as no human subjects were involved in this study.
Availability of data and material
Data related to this paper will be made available by the corresponding author upon receiving legitimate requests.
Competing interests
On behalf of both the authors, the corresponding author states that there are no conflicts of interest.
Funding statement
No funding was available for this study in any form.
Authors’ Contributions
JLG and LMW conceptualized and designed this study, performed database search, study selection, data abstraction, risk of bias assessment, analysis, and first and final draft of this manuscript. CK, LFAK, PPS, SPB, AFG, and EP contributed to the study selection, data abstraction, risk of bias assessment, and hard editing of the first draft.
Acknowledgment
This work was done by the authors independently, and it is not related to their affiliated institutes.
ORCID link of the submitting author: https://orcid.org/0000-0001-9633-0474.
References
- 1.Li X, Xu Y, Dong Y, Yang X, Ye B, Wang Y, et al. Monitoring the efficacy of infant hepatitis B vaccination and revaccination in 0- to 8-year-old children: Protective anti-HBs levels and cellular immune responses. Vaccine. 2018;36:2442–9. doi: 10.1016/j.vaccine.2018.03.044. [DOI] [PubMed] [Google Scholar]
- 2.Garbin CA, Wakayama B, Saliba TA, Junior OA, Garbin AJ. A cross-sectional study on dental surgeons'immune status against hepatitis B virus in the public health system. Rev Inst Med Trop Sao Paulo. 2020;62:e18. doi: 10.1590/S1678-9946202062018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Almutairi R, Almutairi M, Alsugair A, Alseraikh M, Almutairi H. Senior health sciences students'perception of occupational risk of viral hepatitis and attitudes toward patients diagnosed with viral hepatitis B and C. Int J Health Sci. 2017;11:1–7. [PMC free article] [PubMed] [Google Scholar]
- 4.Alter MJ. Epidemiology of hepatitis B in Europe and worldwide. J Hepatol. 2003;39:S64–9. doi: 10.1016/s0168-8278(03)00141-7. [DOI] [PubMed] [Google Scholar]
- 5.Alter MJ. Epidemiology of viral hepatitis and HIV co-infection. J Hepatol. 2006;44:S6–9. doi: 10.1016/j.jhep.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Batista SM, Andreasi MS, Borges AM, Lindenberg AS, Silva AL, Fernandes TD, et al. Author information seropositivity for hepatitis B virus, vaccination coverage, and vaccine response in dentists from Campo Grande, Mato Grosso do Sul, Brazil. Mem Inst Oswaldo Cruz. 2006;101:263–7. doi: 10.1590/s0074-02762006000300006. [DOI] [PubMed] [Google Scholar]
- 7.Qiu S, He P, Fang X, Tong H, Lv J, Liu J, et al. Significant transcriptome and cytokine changes in hepatitis B vaccine non-responders revealed by genome-wide comparative analysis. Hum Vaccin Immunother. 2018;14:1763–72. doi: 10.1080/21645515.2018.1450122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alawad M, Alturki A, Aldoghayyim A, Alrobaee A, Alsoghair M. Knowledge, attitudes, and beliefs about HIV/AIDS and people living with HIV among medical students at Qassim university in Saudi Arabia. Int J Health Sci. 2019;13:22–30. [PMC free article] [PubMed] [Google Scholar]
- 9.Ferreira RC, Guimarães AL, Pereira RD, Andrade RM, Xavier RP, Martins AM. Vacinação contra hepatite B e fatores associados entre cirurgiões-dentistas. Rev Bras Epidemiol. 2012;15:315–23. doi: 10.1590/s1415-790x2012000200009. [DOI] [PubMed] [Google Scholar]
- 10.Anania C, Olivero F, Spagnolo A, Chiesa C, Pacifico L. Immune response to vaccines in children with celiac disease. World J Gastroenterol. 2017;23:3205–13. doi: 10.3748/wjg.v23.i18.3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oliveira AC, Paiva MH, Paula AO, Gama CS. Cobertura vacinal e situação sorológica para hepatite B entre alunos de um curso de medicina expostos a material biológico: Uma análise quantitativa. Online Braz J Nurs. 2010;9:1–12. [Google Scholar]
- 12.Hess L, Riesenberg K, Rolston KV, Nesher L. Administering an additional hepatitis B vaccination dose after 18 years maintains adequate long-term protection levels in healthcare workers. Infect Dis. 2020;52:330–5. doi: 10.1080/23744235.2020.1718201. [DOI] [PubMed] [Google Scholar]
- 13.Bruce MG, Bruden D, Hurlburt D, Zanis C, Thompson G, Rea L, et al. Antibody levels and protection after hepatitis B vaccine: Results of a 30-year follow-up study and response to a booster dose. J Infect Dis. 2016;214:16–22. doi: 10.1093/infdis/jiv748. [DOI] [PubMed] [Google Scholar]
- 14.Chaves SS, Fischer G, Groeger J, Patel PR, Thompson ND, Teshale EH, et al. Persistence of long-term immunity to hepatitis B among adolescents immunized at birth. Vaccine. 2012;30:1644–9. doi: 10.1016/j.vaccine.2011.12.106. [DOI] [PubMed] [Google Scholar]
- 15.Honorati MC, Palareti A, Dolzani P, Busachi CA, Rizzoli R, Facchini A. A mathematical model predicting anti-hepatitis B virus surface antigen (HBs) decay after vaccination against hepatitis B. Clin Exp Immunol. 1999;116:121–6. doi: 10.1046/j.1365-2249.1999.00866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leuridan E, Van Damme P. Hepatitis B and the need for a booster dose. Clin Infect Dis. 2011;53:68–75. doi: 10.1093/cid/cir270. [DOI] [PubMed] [Google Scholar]
- 17.de Geus JL, Wambier LM, Bortoluzzi MC, Loguercio AD, Kossatz S, Reis A. Does smoking habit increase the micronuclei frequency in the oral mucosa of adults compared to non-smokers? A systematic review and meta-analysis. Clin Oral Investig. 2018;22:81–91. doi: 10.1007/s00784-017-2246-4. [DOI] [PubMed] [Google Scholar]
- 18.Al-Hazzaa HM. Physical inactivity in Saudi Arabia revisited: A systematic review of inactivity prevalence and perceived barriers to active living. Int J Health Sci. 2018;12:44–52. [PMC free article] [PubMed] [Google Scholar]
- 19.Al Dalbhi S, Alshahrani HA, Almadi A, Busaleh H, Alotaibi M, Almutairi W, et al. Prevalence and mortality due to acute kidney injuries in patients with influenza A (H1N1) viral infection: A systemic narrative review. Int J Health Sci. 2019;13:56–62. [PMC free article] [PubMed] [Google Scholar]
- 20.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 21.Thomas BH, Ciliska D, Dobbins M, Micucci S. A process for systematically reviewing the literature: Providing the research evidence for public health nursing interventions. Worldviews Evid Based Nurs. 2004;1:176–84. doi: 10.1111/j.1524-475X.2004.04006.x. [DOI] [PubMed] [Google Scholar]
- 22.Herrera MG, Taylor L, Visoná KA. Respuesta inmunehumoral contra la vacuna del virus de hepatitis B H-B-VAX en el personal hospitalario. Rev Costarric Cienc Med. 1988;9:41–7. [Google Scholar]
- 23.Racela LS, Tegtmeier GE, Hodges GR, Reed JS. Comparison of two testing methods to determine hepatitis B surface antibody response to hepatitis B vaccine among health-care workers. Am J Clin Pathol. 1986;86:527–9. doi: 10.1093/ajcp/86.4.527. [DOI] [PubMed] [Google Scholar]
- 24.Sabido M, Gavalda L, Olona N, Ramon JM. Timing of hepatitis B vaccination:Its effect on vaccine response in health care workers. Vaccine. 2007;25:7568–72. doi: 10.1016/j.vaccine.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 25.Villena EZ, Griego AG, Albajés VR, Barrios RF. Inmunogenicidad de la vacuna recombinante cubana contra la hepatitis B en trabajadores de la salud peruanos. Rev Cuba Invest Bioméd. 2000;19:45–50. [Google Scholar]
- 26.Yen YH, Chen CH, Wang JH, Lee CM, Changchien CS, Lu SN. Study of hepatitis B (HB) vaccine non-responsiveness among health care workers from an endemic area (Taiwan) Liver Int. 2005;25:1162–8. doi: 10.1111/j.1478-3231.2005.01157.x. [DOI] [PubMed] [Google Scholar]
- 27.Karpuch J, Scapa E, Eshchar J, Waron M, Bar-Shany S, Shwartz T. Vaccination against hepatitis B in a general hospital in Israel:Antibody level before vaccination and immunogenicity of vaccine. Isr J Med Sci. 1993;29:449–52. [PubMed] [Google Scholar]
- 28.Martinez NT, Burillo JM, Bermudez BP, Alvarez JB. Factors associated with inadequate response to hepatitis B vaccination in health care personnel. Rev Esp Salud Publica. 1998;72:509–15. [PubMed] [Google Scholar]
- 29.Zeeshan M, Jabeen K, Ali AN, Ali AW, Farooqui SZ, Mehraj V, et al. Evaluation of immune response to hepatitis B vaccine in health care workers at a tertiary care hospital in Pakistan: An observational prospective study. BMC Infect Dis. 2007;7:120. doi: 10.1186/1471-2334-7-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chadha MS, Arankalle VA. Ten-year serological follow up of hepatitis B vaccine recipients. Indian J Gastroenterol. 2000;19:168–71. [PubMed] [Google Scholar]
- 31.García PC, De la Cerda SG, Arellano MC, Godoy GR, Covarrubias FC, Potin SM, et al. Inmunogenicidad de una vacuna recombinante anti hepatitis B en personal de salud. Rev Chilena Infectol. 2002;19:133–9. [Google Scholar]
- 32.Barash C, Conn MI, DiMarino AJ, Marzano J, Allen ML. Serologic hepatitis B immunity in vaccinated health care workers. Arch Intern Med. 1999;159:1481–3. doi: 10.1001/archinte.159.13.1481. [DOI] [PubMed] [Google Scholar]
- 33.Silva FA., Jr O risco dos profissionais de saúde àinfecção por hepatites B e C. NBC. 2014;4:1–6. [Google Scholar]
- 34.Ministério da S, Fundaçä o. Brasil: Ministério da S, Fundaçäo Nacional de Saúde; 2014. Nacional, de Saúde, Manual de Procedimentos Para Vacinaçäo; pp. 73–7. [Google Scholar]
- 35.Hosny G, Samir S, El-Sharkawy R. An intervention significantly improve medical waste handling and management:A consequence of raising knowledge and practical skills of health care workers. Int J Health Sci. 2018;12:56–66. [PMC free article] [PubMed] [Google Scholar]
- 36.Souza FO, Freitas PS, Araújo TM, Gomes MR. Vacinação contra hepatite B e anti-HBS entre trabalhadores da saúde. Cad Saúde Colet. 2015;23:172–9. [Google Scholar]
- 37.Jack AD, Hall AJ, Maine N, Mendy M, Whittle HC. What level of hepatitis B antibody is protective? J Infect Dis. 1999;179:489–92. doi: 10.1086/314578. [DOI] [PubMed] [Google Scholar]
- 38.Chiaramonte M, Ngatchu T, Majori S, Baldo V, Moschen ME, Renzulli G, et al. Response to an extra dose of hepatitis B vaccine and specific antibody persistence in non-responders to primary immunization. Scand J Gastroenterol. 1995;30:601–3. doi: 10.3109/00365529509089796. [DOI] [PubMed] [Google Scholar]
- 39.Zuckerman JN. Review: Hepatitis B immune globulin for prevention of hepatitis B infection. J Med Virol. 2007;79:919–21. doi: 10.1002/jmv.20816. [DOI] [PubMed] [Google Scholar]
- 40.Davis JP. Experience with hepatitis A and B vaccines. Am J Med. 2005;118:7S–15. doi: 10.1016/j.amjmed.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 41.Hennig BJ, Fielding K, Broxholme J, Diatta M, Mendy M, Moore C, et al. Host genetic factors and vaccine-induced immunity to hepatitis B virus infection. PLoS One. 2008;3:e1898. doi: 10.1371/journal.pone.0001898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gara N, Abdalla A, Rivera E, Zhao X, Werner JM, Liang TJ, et al. Durability of antibody response against hepatitis B virus in healthcare workers vaccinated as adults. Clin Infect Dis. 2015;60:505–13. doi: 10.1093/cid/ciu867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McMahon BJ, Dentinger CM, Bruden D, Zanis C, Peters H, Hurlburt D, et al. Antibody levels and protection after hepatitis B vaccine:Results of a 22-year follow-up study and response to a booster dose. J Infect Dis. 2009;200:1390–6. doi: 10.1086/606119. [DOI] [PubMed] [Google Scholar]
- 44.Bagheri-Jamebozorgi M, Keshavarz J, Nemati M, Mohammadi-Hossainabad S, Rezayati MT, Nejad-Ghaderi M, et al. The persistence of anti-HBs antibody and anamnestic response 20 years after primary vaccination with recombinant hepatitis B vaccine at infancy. Hum Vaccin Immunother. 2014;10:3731–6. doi: 10.4161/hv.34393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kolawole OM, Wahab AA, Adekanle DA, Sibanda T, Okoh AI. Seroprevalence of hepatitis B surface antigenemia and its effects on hematological parameters in pregnant women in Osogbo, Nigeria. Virol J. 2012;9:317. doi: 10.1186/1743-422X-9-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data related to this paper will be made available by the corresponding author upon receiving legitimate requests.


