Graphical abstract
Keywords: Wastewater treatment, Disinfection, Hydrodynamic Cavitation, Plasma discharge, Radicals
Highlights
-
•
Water disinfection by simultaneous treatment with hydrodynamic cavitation and plasma discharge.
-
•
Decomposition of organic pollutants in water under hydrodynamic cavitation and plasma.
-
•
Easily scalable hybrid technology combining cavitation and plasma.
-
•
Intense generation of radicals, UV light, shock waves and charged particles.
-
•
Prolonged oxidation takes place in the solution/suspension after the end of the treatment.
Abstract
Over the last two decades, the scientific community and industry have made huge efforts to develop environmental protection technologies. In particular, the scarcity of drinking water has prompted the investigation of several physico-chemical treatments, and synergistic effects have been observed in hyphenated techniques. Herein, we report the first example of water treatment under simultaneous hydrodynamic cavitation and plasma discharge with the intense generation of radicals, UV light, shock waves and charged particles. This highly reactive environment is well suited to the bulk treatment of polluted water (i.e. E. coli disinfection and organic pollutant degradation). We have developed a new prototype and have efficiently applied this hybrid technology to water disinfection and the complete degradation of methanol in water with the aim of demonstrating its scalability. We have analyzed the mechanisms of water disinfection under the abovementioned conditions and verified them by measuring cavitation noise spectra and plasma emission spectra. We have also used the degradation of textile dyes and methanol solutions as an indicator for the formation of radicals.
1. Introduction
The results of demographic studies allow us to assume that the world population will reach 10 billion people by 2050, and, even now, more than one billion people are suffering from water scarcity. The risk of epidemics and environmental pollution associated with industrial activity may also increase sharply. Organic contaminants, aromatic compounds and pharmaceuticals in drinking water can cause many diseases, including hormone disruption and cancer. These challenges make finding new water-treatment technologies increasingly urgent.
Conventional wastewater methods often do not remove complex organic contaminants [1]. Moreover, techniques for water disinfection either have severe limitations, are associated with the use of toxic substances (like chlorine) or are quite expensive. The use of chlorine, ozone and UV light are well known disinfection techniques.
It is commonly known that chlorination is harmful. In addition, some microbes are resistant to chlorine [2], [3], [4], making the method of limited applicability.
Ozonation is an expensive method for water disinfection. While ozone itself is a powerful oxidant that can directly oxidize unsaturated organic compounds, it is also spontaneously converted into a more reactive unselective species, •OH, in water. However, ozone is difficult to disperse or dissolve into water, leading to lower gas–liquid mass transfer. For this reason, various mixing technologies have to be used to enhance ozonation and increase efficiency, although this leads to the drawback of high costs [5], [6], [7], [8], [9], [10].
Some types of another advanced oxidation processes based on mixing technologies, such as the use of hydrogen peroxide, persulfate and derivative compounds have the same limits [11], [12].
UV-treatment methods are very effective for water disinfection, although careful filtration is needed before irradiation. This limits the economic efficiency of the technique [13].
Recent technological advances in physicochemical transformations can appreciably contribute to the development of more sustainable industrial processes. Powerful acoustic cavitation and hot spots that are generated by ultrasound in solutions and suspensions may be able to dramatically promote the process [14], [15]. Shock waves and microjets from collapsing cavitation in liquid–solid slurries produce high-velocity interparticle collisions, the impacts of which are sufficient to melt most metals
[16]. Ultrasound has also found important applications in the initiation and enhancement of degradation and catalytic reactions in both homogeneous and heterogeneous systems [17], [18], [19], [20], [21], [22], [23]. High-power ultrasound and cavitation are used in wastewater treatment for the activation of reagents (for example, one of promising methods of water treatment based on the use of persulfate and peroxymonosulfate involves ultrasound-assisted activation [24], [25], [26], [27], [28], [29], [30]) for wastewater treatment, or to enhance ozonation or another types of advanced oxidation processes [31], [32].
Hydrodynamic cavitation has been widely used in environmental remediation as it offers the advantages of process acceleration and higher energy efficiency [33]. It has also been combined with ozonation on a pilot scale [34]. Hydrodynamic cavitation releases large amounts of energy (shock waves, microjets, shear forces, turbulences, etc.) in a flowing liquid during the extreme implosion of cavitation bubbles, which is caused by a drop and successive rise in local pressure [35]. The mechanisms of the effects caused by hydrodynamic cavitation differ from those of ultrasound. Besides the cavitation number, other hydrodynamic factors, such as inlet pressure, flow rate, velocities in the constrictions, the ratio between total hole perimeter and the total area of the openings, and the ratio between the total hole area and the cross-sectional area of the pipe, complicate the rationalization [36], [37].
We have recently shown that an ultrasonic field affects electrical discharge in water [38], and, in continuous flow, very large volumes of water can be treated. This is due to the fact, that the discharge becomes volumetric in the cavitation zone, thus liquid can be treated in a continuous flow. Some physical aspects of plasma formation are reported in [38].
In the present work, we investigate the disinfection potential of a new hybrid technique that simultaneously combines hydrodynamic cavitation and plasma discharge. Our preliminary findings lead us to believe that this may be an innovative approach that can overcome the limitations of previous methods. We have developed a prototype to treat water in continuous flow with plasma discharge in a cavitation zone that is generated by the hydrodynamic unit. The prototype is an original tool that is well suited to both scale-up and numbering-up. The main goals of our technology are: the disinfection of water and the removal of persistent organic pollutants (POPs) from wastewater. This work deals with the design of a new process for water disinfection.
We have developed the following model in order to prove the disinfection power of the “hydrodynamic cavitation – plasma discharge” hybrid technique.
During the growth stage, the radius of cavitation bubbles increases significantly, and the gas pressure inside the bubble may be very low. According to Pashen's rule, an electric discharge occurs at low gas pressures. Consequently, the presence of an electric field can lead to cavitation bubbles becoming lined up in strings [39]. In this case, the discharge develops inside the bubbles and also jumps from bubble to bubble [40], [41], [42]. A so-called microchannel is formed between the electrodes, a dynamic effect that continuously forms and disappears in the ultrasonic and electric fields. If the abovementioned hypothesis is correct, we should observe an average glow pattern over the entire volume of the treated liquid. In fact, this is exactly what was observed at the start of plasma discharge in the cavitation zone, together with the generation of hydroxyl radicals, which are produced by water cleavage during cavitation bubble collapse [43].
| (1) |
If there are organic molecules present in the treated water, there are numerous specific reactions that are capable of producing •OH radicals. Hydroxyl radicals have very high reduction potential (2.7 eV), and thus radicals are formed both at the liquid/gas interface and within bubbles [44], [45].
Ozone, which is formed in the presence of ultraviolet radiation and discharges inside the bubbles, is another potential source of radicals. As mentioned above, ozone can react with dissolved substances at the gas/liquid interface, as well as initiate the further production of •OH radicals.
| (2) |
The reaction products are rapidly spread in the liquid flow, and their distribution is facilitated by cavitation. The free radicals formed can lead to microorganism inactivation and therefore to water disinfection. •OH radicals can penetrate bacteria cell walls and membranes, and cause severe damage. This process is based on chemical oxidation, meaning that microorganisms and viruses cannot develop resistance.
Of course, the physical effects of cavitation on microorganisms is an important stage of disinfection. The shock waves and high temperatures that occur when cavitation bubbles collapse lead to the weakening of microorganism structure, facilitating the penetration of radicals and UV. It is well known that UV light suppresses the activity of microorganisms. In addition, UV radiation activates oxidation reactions as it provides additional energy for chemical-bond breakage [46].
Simultaneous hydrodynamic-cavitation and plasma-discharge treatment cause shock-waves via the action of collapsing bubbles, ultraviolet radiation, hydroxyl radicals and ozone formation. These intense effects in the treated liquid cause:
-
-
Homogenization
-
-
Disinfection by cavitation
-
-
Disinfection by radicals and ozone
-
-
Disinfection by UV light
-
-
Prolonged oxidation after treatment
In summary, the proposed hybrid technology for water disinfection causes a synergistic effect to occur between the two energy sources. Although there is a need for further investigation, we can speculate that this technology has impressive potential uses in a wide number of industrial and urban applications.
2. Materials and methods
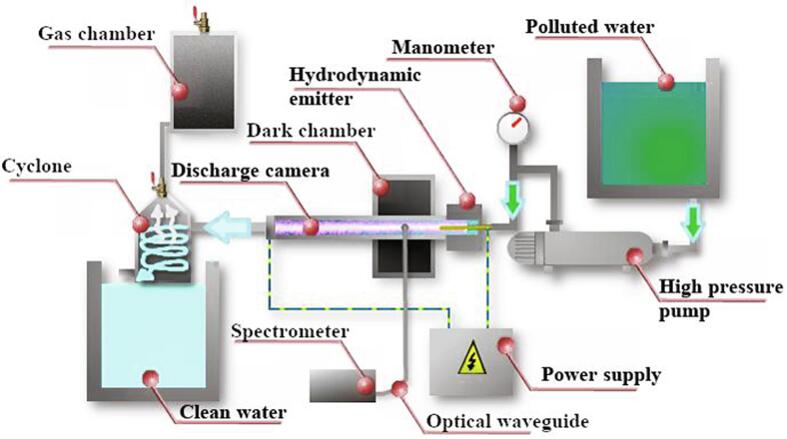
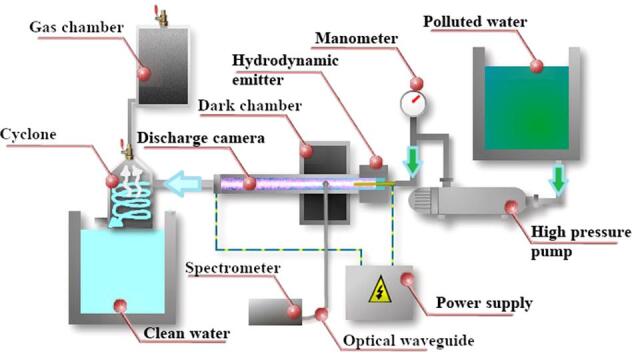
The hybrid reactor designed for the experimental work is depicted in Fig. 1.
Fig. 1.
Scheme of the laboratory setup for water treatment under hydrodynamic cavitation and plasma discharge.
The combined reactor is equipped with two tanks (contaminated and treated water), a high-pressure pump, a discharge chamber with two electrodes, a hydrodynamic cavitation unit and a closed safety vessel to collect gases and vapor. The flow-rate of the laboratory setup was 1 m3/h. A photograph of the discharge chamber is shown in Fig. 2.
Fig. 2.
Intense UV emission in the plasma discharge chamber.
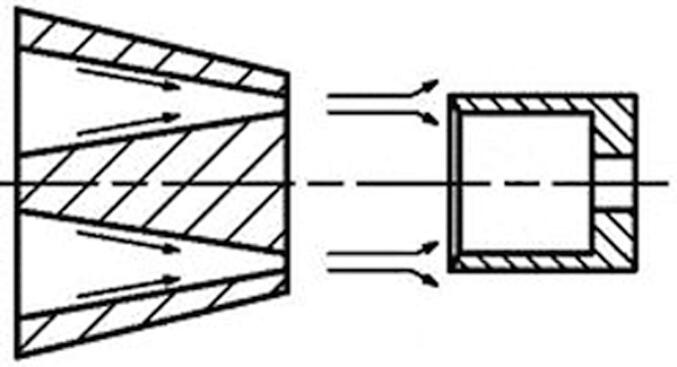
The voltage from the power supply was applied to two replaceable electrodes that are located at each end of the discharge chamber. Electrodes made of graphite, silver and brass were used. Hydrodynamic cavitation in the liquid flow was generated by an efficient unit (emitter), which has been described in a previous paper [47]. A scheme of the emitter is shown in Fig. 3. The operation of the emitter is based on the generation of oscillations in a liquid media, when the jet from the nozzle interacts with a barrier of a certain shape and size. The perturbations caused by the obstacle affect the jet base, causing autooscillations. In the experimental setup we used an annular slotted nozzle, which was formed by two conical surfaces. The barrier had the shape of a hollow cylinder, dissected along the elements. Thus, the barrier consisted of cantilever plates, arranged circumferentially. The hydrodynamic emitter was fixed at the inlet of the discharge chamber, and the contaminated water was circulated by a high-pressure pump. Ideally, the electric potential should be applied directly to the hydrodynamic emitter. However, for safety reasons, the emitter could not be used as an electrode. One electrode (ring shape that matches the internal diameter of the discharge chamber – 8 mm and the outer diameter of the discharge chamber – 10 mm) was fixed close to the hydrodynamic emitter. The thickness of the electrode was 4 mm. The second electrode was fixed at the endo of the discharge chamber. The chamber was a 200 mm long quartz cylinder, thus the distance between the electrodes was 200 mm. Linear velocity of water inside the chamber was about 5–6 m/s. All experiments were carried out at the same discharge voltage of 15 kV. The pressure at the inlet of the hydrodynamic emitter was 6 MPa (this is the optimal value for the emitter used, since the rarefication in the discharge chamber reached at this inlet pressure is 0,8 bar, further increase of the inlet pressure leads to a slow grows of the rarefication to a maximum value of 0,93 bar at 120 bar at the inlet). The pressure at the outlet of the hydrodynamic emitter was atmospheric.
Fig. 3.
Schematic of the hydrodynamic emitter.
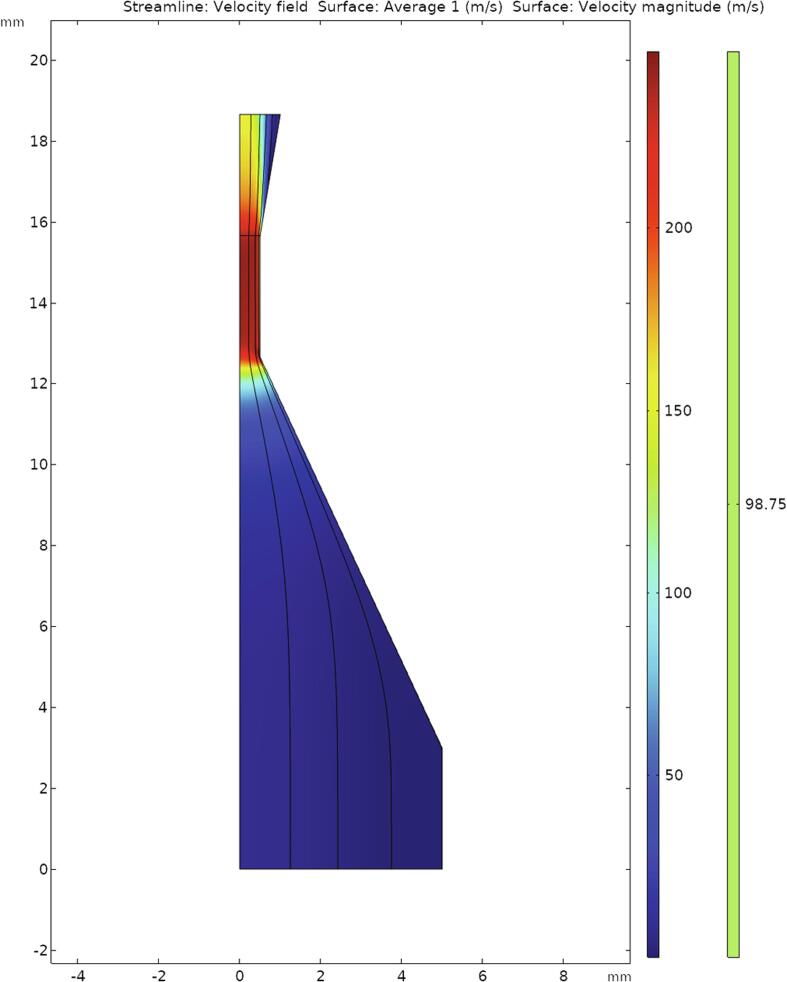
The original design of the hydrodynamic emitter enables a strong cavitation inside the whole discharge chamber. This is achieved by hydrodynamic flows, which cause pressure reduction inside the chamber. The cavitation number of the system is 0.0027, which confirms the hypothesis. The number was calculated from the following values: water density at 25˚C – 997.07 kg/m3, average flow velocity at the outlet of the hydrodynamic emitter – 99 m/s (the value is taken from the model of the emitter, using the software Comsol Multyphysics, the modelling results are shown in Fig. 4), vapor pressure at 25˚C – 3160 Pa, pressure at the outlet of the emitter – 16212 Pa (measured value).
Fig. 4.
Modelling result of the hydrodynamic emitter.
We used a cyclone to separate the gas produced during the treatment. The composition of the released gas was analyzed using gas chromatography “CrystalLux-4000 m” provided by “RPC Meta-chrom” Ltd. was used (more information about the device and methods can be found in https://www.meta-chrom.ru/catalog/chromatographs/crystallux-4000m/). During water treatment approximately 500–1000 cm3 of gas was produced per minute.
Emission spectra were recorded to study the discharge parameters in relation to the material used for the electrodes. A spectrometer QE65000, with high spectral response and high optical resolution in a small footprint, was used. For complete details of this detector, visit www.hamamatsu.com. Spectra were recorded in a wavelength range of 200–1000 nm. The sensor was mounted directly to the setup in a chamber closed from light.
The cavitation noise spectra were measured using a dynamic pressure sensor PS 01–03 with a single crystal element, operating in a wide dynamic range with a sensitivity of 400 nC/bar and a nonlinearity < 2% at a signal frequency of over 20 kHz. The sensor was placed at the entrance to the discharge chamber.
The concentration of silver ions in the treated water was measured using the potentiometric method on the «Expert 001» device with an Ag2S electrode. The device was provided by “Econics-Expert Ltd.”. It ia a universal ion-meter programmed for different tasks. A standard silver nitrate solution was used to calibrate the instrument.
The disinfection power of the combined treatment was determined using a suspension of E. coli M−17−A in pre-sterilized water samples. The daily culture was bred according to the optical bacterial standard. The water sample was inoculated with 1000 cells/ml. Water samples were taken before and after treatment. After that they were placed into Petri cups and bred at 37 °C in a nutritious culture medium. The number of colonies was counted in accordance with Russian standard 2116–50. For this purpose, the method of accelerated determination of the coli index using nitrocellulose membrane filters was used. In accordance with the standard 2116–50 each sample was divided into 5 samples in order to ensure accurate results.
Unlike sterilization, disinfection is not sporicidal. In fact, it eliminates many or all pathogenic microorganisms, but does not necessarily kill bacterial spores. In this paper, we are prudently only claiming powerful disinfection.
3. Results and discussion
Herein, we have investigated the effect of simultaneous treatment by hydrodynamic cavitation and plasma discharge on bacteria present in water, namely a suspension of E. coli. Water samples were collected after bacteriologically contaminated water underwent one, two or three passages through the hybrid reactor. Experiments were conducted using electrodes made of three different materials: silver, graphite and brass. The microbiological analysis of the samples after incubation counted the E. coli colony number. The results are summarized in Table 1. The initial concentration of bacteria in water was 25.0 × 107 per mL.
Table 1.
Influence of electrode material on the efficiency of water disinfection during simultaneous treatment by hydrodynamic cavitation and plasma discharge.
| Electrode material | Round of treatment | Concentration of bacteria × 107, bacteria/mL | E. coli lethality, % |
|---|---|---|---|
| Silver | 1 | 0.5 | 98.0 |
| 2 | <0.001 | >99.99 | |
| 3 | <0.001 | >99.99 | |
| Graphite | 1 | 11.0 | 56.0 |
| 2 | <0.001 | >99.99 | |
| 3 | <0.001 | >99.99 | |
| Brass | 1 | 9.0 | 44.0 |
| 2 | 1.0 | 96.0 | |
| 3 | <0.001 | >99.99 |
In Table 1 shows the disinfection efficiency’s dependence on the electrode material used; the most effective decontamination was with silver electrodes and the worst with brass electrodes. As mentioned above, we have hypothesized that combined treatment with hydrodynamic cavitation and plasma discharge will lead to disinfection via shock-waves, the action of free-radicals, ozone formation and via UV light, followed by post-treatment oxidative effects. We carried out a number of experiments, which aimed to prove the abovementioned effects and to understand what causes the difference in antibacterial activity when different electrodes are used.
We assumed that the high effectiveness of silver electrodes may be caused by the catalytic leaching of silver ions in the treated water. After treatment with silver electrodes, we measured the concentration of silver ions and found up to 0.8 mg/L in the treated water. The maximum permissible concentration of silver ions in water differs from country to country. For example, in the sanitary norms for drinking water that are in force in Russia, the concentration of ions should not exceed 0.05 mg/L. The US Environment Protection Agency recommends limiting the concentrations of silver ions to 0.1 mg/L. In some EU countries, the maximum concentration of silver is not controlled, while other countries have determined a concentration of no >0.01 mg/L can be present. Silver electrodes can therefore not be used in our setup for drinking water treatment. Having taken this into account, it was important to understand how other factors could affect disinfection.
In order to understand, if the antibacterial effect of the setup with silver electrodes was caused only by the silver ions, we have carried out an experiment, in which a silver wire was put into the water. After that, the water was ionized with silver ions by applying electrical current to the wire. The concentration of ions was increased to 0.8 mg/L. We have found, that after such treatment the lethality of E. coli was 46%, while after treatment by the hydrodynamic cavitation and the plasma discharge the lethality was 98% (for silver electrodes).
Thus, the antibacterial effect must be caused not only by the silver ions, but also by the abovementioned phenomena: acoustical cavitation, free radicals, ozone and UV-light. Further experiments were carried out in order to understand, how the material of the electrode affect the different phenomena and confirm the presence of the abovementioned effects.
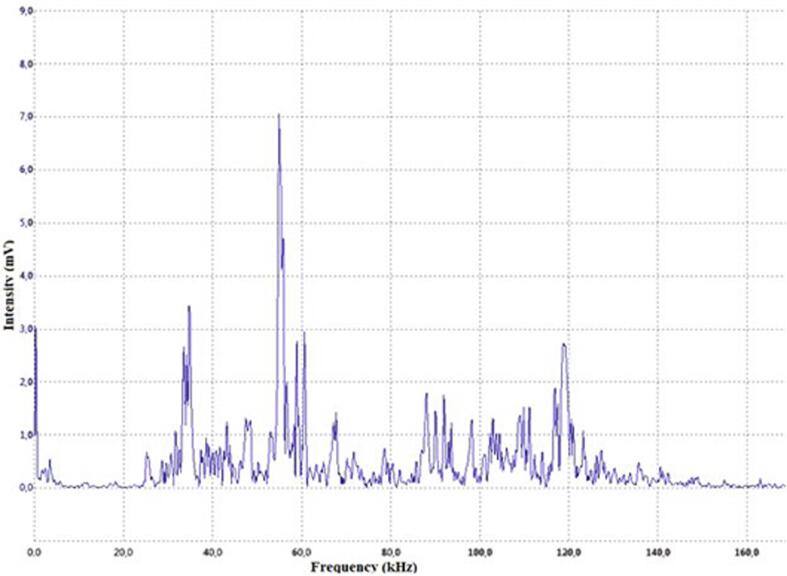
In order to confirm the intense hydrodynamic cavitation, we measured the acoustical spectrum of the noise created during the experimental run. The results of that measurement are summarized in Fig. 5.
Fig. 5.
The spectrum of cavitation noise recorded during the operation of the experimental setup.
The formation of a cavitation zone is accompanied by the appearance of cavitation noise, whose intensity rises with the acoustic pressure in the liquid. The spectrum of cavitation noise measured in the liquid flow during sonoplasma discharge is shown above. The spectrum does not depend on the electrode material. The spectrum shows peaks in a wide range of frequencies, which indicates the presence of intense cavitation in the fluid stream – an avalanche-like reproduction of cavities initiated by a chain mechanism. The effect of cavitation alone on microorganisms is well studied and has been reported, for example, in [48]. In our experimental setup, microorganism suppression of up to 95% was reached, and similar values cannot be achieved using hydrodynamic cavitation alone. Experiments with E. coli show, that maximal suppression of microorganisms with hydrodynamic cavitation only in our setup is about 10%. At the same time, the use of the discharge without cavitation has almost no effect in a water stream. Simultaneous use of both: the discharge and the cavitation cause a synergetic effect. Cavitation facilitates the formation of a discharge throughout the volume due to the fact, that inside the cavitation bubble there is rarified gas, in which it is much easier to create a discharge compared to a liquid medium. Thus, the need of a hybrid technology is obvious.
In order to confirm the formation of •OH radicals in the treated liquid, we used methanol as a model organic contaminant. The experiments in the laboratory setup show that simultaneous treatment with hydrodynamic cavitation and plasma discharge is effective for the decomposition of this pollutant. The treatment of a 7% methanol solution at a flow rate of 1000 L/hour using graphite electrodes caused a 5-fold decrease in the concentration of methanol, the solution was completely mineralized when it was treated 3 times in a row. We also compared the methanol concentration after treatment using different electrodes in the experimental setup, and found that it was 1.5% after treatment using graphite electrodes, 2% using brass electrodes and 1.8% using silver electrodes.
The plasma decomposition of organic compounds is a developing technique in the field of advanced oxidation processes. According to [47], the multistep oxidation of methanol can be considered a typical example of the mineralization of organic molecules, as depicted in the following equation:
| (3) |
The process can be considered an indicator for •OH radical formation. As we can see from the equation, the products of the reaction are water and carbon dioxide. This was confirmed experimentally during the treatment by an analyses of the gas composition. This experiment was carried out using graphite electrodes. The results are reported in Table 2.
Table 2.
Composition of the gas formed during the simultaneous treatment of a water and methanol solution under hydrodynamic cavitation and plasma discharge.
| Test | Mass fraction % |
||
|---|---|---|---|
| Hydrogen | Carbon dioxide | Methane | |
| water | 55.5 | <1 | – |
| 7% methanol in H2O | 38.8 | 9.5 | 8.4 |
It should be noted that this reaction only describes the process of methanol oxidation in the system due to hydroxide, and is an idealized model of the decomposition of the substance in a plasma discharge. Under real conditions, other products can be formed in the intermediate stages and as resulting by-products. This is evidenced by the fact that a significant amount of methane (up to 8%) is present in the mixture of gases formed during the treatment of the methanol solution. In this case, the percentage of hydrogen in the mixture is 1.5-times lower than in the processing of clean water.
A possible reason for this is the formation of methane molecules from atomic hydrogen formed in the process:
| (4) |
The results of the experiment indicate that •OH radicals are formed during the simultaneous treatment of contaminated water with hydrodynamic cavitation and plasma discharge. The electrode material significantly affects radical formation and, consequently, disinfection power in water. The effect achieved when graphite electrodes are used is higher than when brass electrodes are used, while the reduction of methanol concentration in water is also more effective when graphite materials are used. The effect of •OH radicals when silver electrodes are used is supported by the inhibiting effect of silver ions in solution on microorganisms.
Another indication of the presence of free radicals in the treated water is the prolonged oxidation after the treatment. We assume, that the prolonged oxidation is caused by the secondary products of the free radicals (hydrogen peroxide, oxides of metals etc.), thus the prolonged oxidation can be seen as an indication of the presence of free radicals during the treatment. The lifetime of the OH radicals is 10−9 s, thus it is challenging to register them directly and we can build a hypothesis about their presence based on secondary effects caused by them.
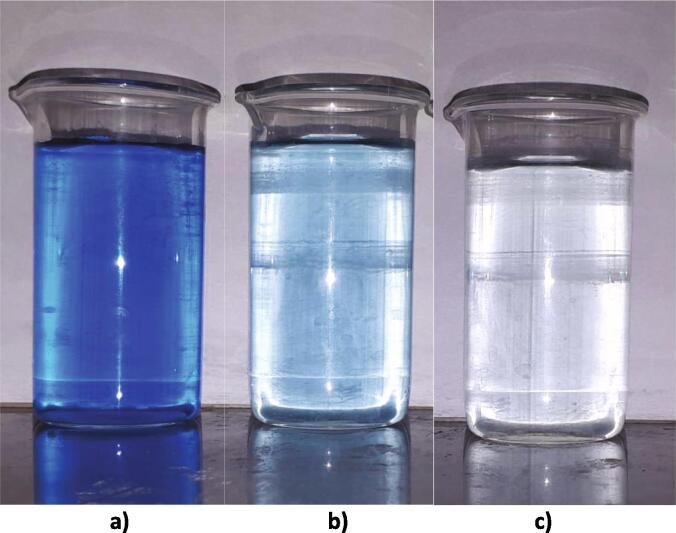
Prolonged oxidation was investigated using a model solution with a high concentration of E132 dye (~1000 mg/L). Immediately after processing, the adsorption of light at a certain wavelength fell >2-fold compared to the original solution. After that, the solution was kept for 60 h in the dark. During this exposure, the solution continued to bleach without reprocessing. Photos of the solution before and after treatment and 60 h after treatment are shown in Fig. 6.
Fig. 6.
Photos of the initial solution E132 (a), the solution after treatment (b) and 60 h after treatment (c).
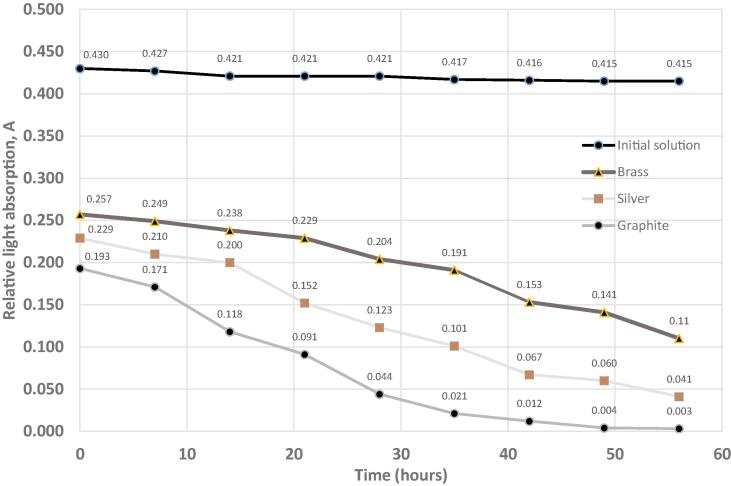
We measured the absorption of white light by the E132 dye solution before and after treatment over time. The measurements were carried out after treatment in the setup with various electrode materials. The results of the measurements are reported in Fig. 7.
Fig. 7.
Change in the absorption of white light by the E132 dye solution over time after dilution and treatment with hydrodynamic cavitation and plasma.
As shown in Fig. 5, the change of light absorption immediately after treatment was the highest when graphite electrodes were used, while the drop in light absorption over time was also higher when the graphite electrodes were used. The results are in line with the results obtained after the decomposition of the methanol solution, and indicate that the amount of radicals formed depend on the material of the electrodes used.
After 60 h, the solution completely lost its color and the light transmission coefficient became that of a pure water sample. At the same time, the light transmission coefficient through the untreated solution showed no significant changes over time. We have thus observed that oxidation continued after treatment. It is also likely that chemical changes occur in the structure of the dye during processing, making it less stable.
As mentioned above, apart from disinfection via cavitation and radical formation, we also expected to observe disinfection by UV light. We carried out spectroscopic studies in order to understand whether the effect of UV light depends on the electrode material.
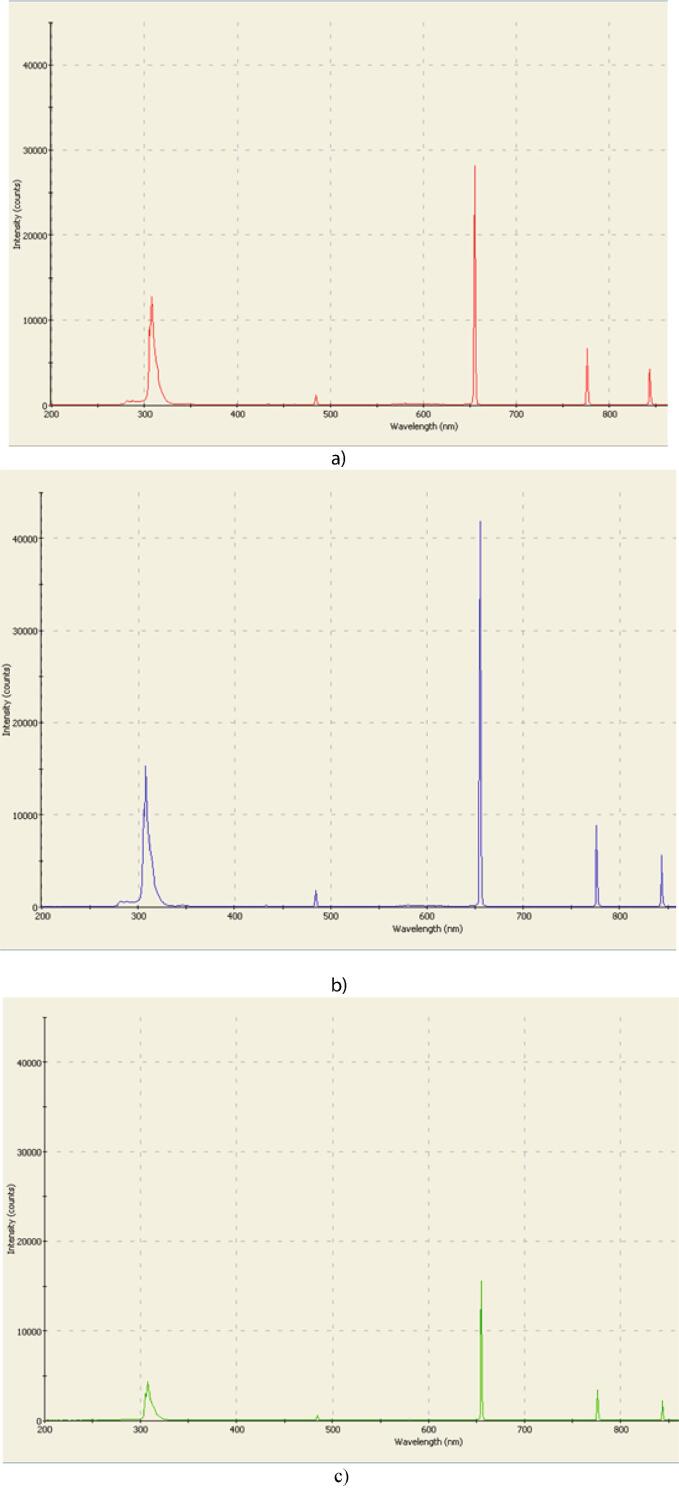
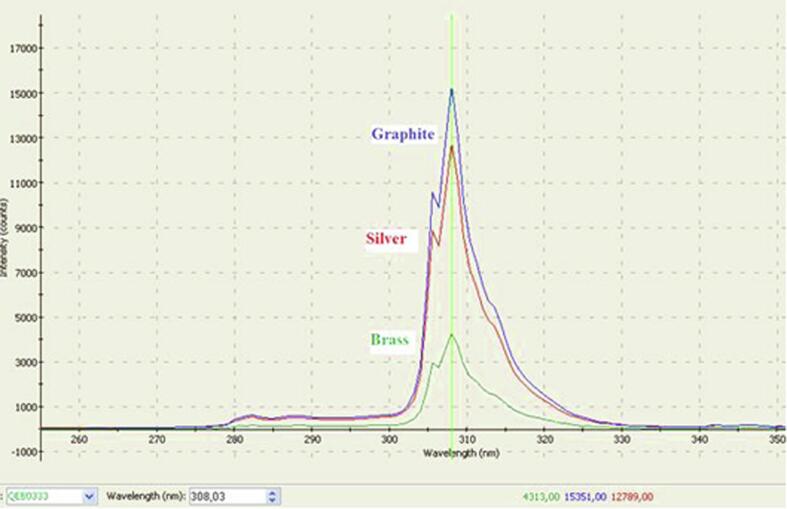
We observed that the intensity of the glow changed greatly when using electrodes made of different materials. The obtained spectra are shown in Fig. 8 and the UV peak is shown in Fig. 9. The brightest plasma discharge-glow was observed when the graphite electrodes were used. The lowest discharge-glow intensity was observed in all spectral regions when the brass electrodes were used. These results are in line with the measured disinfection efficiencies. This clearly indicates that the effect of UV radiation on the treated liquid plays important role in the disinfection process.
Fig. 8.
Full spectrum of the plasma glow using silver (a), graphite (b) and brass (c) electrodes (wavelength range 198–1002 nm).
Fig. 9.
UV peak (280–330 nm) of the plasma-glow spectrum using different electrode materials.
4. Conclusions
We have shown that the simultaneous, continuous-flow treatment of polluted water with hydrodynamic cavitation and plasma discharge causes microorganism suppression (E. coli) and the decomposition of organic pollutants (methanol 7%). The proposed hybrid technology combines cavitational [49] and plasma effects [50] and is easily scalable, which makes it suitable for industrial applications. We have shown that the treated liquid is affected by cavitation, radical generation and UV light. Moreover, prolonged oxidation takes place in the treated liquid after the treatment ends. The disinfection efficiency is significantly affected by the electrode material, and is enhanced when silver electrodes are used, due to the leaching of silver ions into the water. As the Ag + concentration is over the admitted limits, silver electrodes cannot be used for the treatment of drinking water. The use of graphite electrodes for this purpose is therefore recommended.
Also it is important to understand, that while the method is universal the final result may vary in each case. For example, in case of very high concentrations of oil products in the water treatment with the suggested method may lead to the appearance of unwanted by-products including some of oxygenated organic compounds [47], [51]. In this case preliminary water treatment may be required.
The excellent results achieved in the degradation of methanol in water have prompted us to further investigate the effect of simultaneous treatment by hydrodynamic cavitation and plasma discharge on various POPs in water.
The water treatment under the present conditions can be easily integrated into any systems and does not requires any chemical reagents. The low power needed of 1–4 kW is related to the POPs characteristics and concentration. In Russian Federation 1 kW*h costs approximately 0.05 euro. The total cost of all equipment depends on the concrete characteristics of the technological process and the flow rate. The internal estimated cost of discharge chambers, electrodes and power blocks is in the range of 1000–5500 euro (since the blocks should be integrated into water treatment lines the pumps of such lines would be used). Maintenance costs are in the range from 50 euro to 300 euro per year. Very recently Marsalek et al. applied in lab scale hydrodynamic cavitation with cold
plasma discharge to inactivate cyanobacteria in water [52]. To the best of our knowledge this is the only case reported so far, while few other investigations were dealing with treatments under acoustic cavitation and plasma [53]. Thus, there is huge potential in the design of industrial applications of the proposed technique for water decontamination and purification from specific organic pollutants.
CRediT authorship contribution statement
Vladimir O. Abramov: Conceptualization, Methodology. Anna V. Abramova: Writing - original draft, Supervision. Giancarlo Cravotto: Supervision, Writing - review & editing. Roman V. Nikonov: Visualization, Methodology. Igor S. Fedulov: Investigation, Software, Validation. Vladimir K. Ivanov: Investigation, Software, Data curation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Anna V. Abramova, Email: anna_v_abramova@mail.ru.
Giancarlo Cravotto, Email: giancarlo.cravotto@unito.it.
References
- 1.Suares S., Carballa M., Omil F., Lema J.M. How are pharmaceutical an personal care products (PPCPs) removed from urban waste water. Rev. Environ. Sci. Biotechnol. 2008;7:125–128. [Google Scholar]
- 2.MacKenzie W.R., Hoxie N.J., Proctor M.E., Gradus M.S., Blair K.E., Peterson D.E., Kazmierchak J.J., Addiss D.G., Fox K.R., Rose J.B., Davis J.P. A massive outbreak in Milwaukee of cryptosporidium infection transmitted through the public water supply. New England J. Med. 1994;331(3):161–167. doi: 10.1056/NEJM199407213310304. [DOI] [PubMed] [Google Scholar]
- 3.Eisenberg J.N.S., Seto E.Y.W., Colford J.M., Jr., Olivieri A., Spear R.C. An analysis of the Milwaukee cryptosporidiosis infection transmitted through the public water supply. Epidemiology. 1998;9(3):255–263. [PubMed] [Google Scholar]
- 4.Driedger A.M., Rennecker J.L., Marines B. Sequential inactivation of cryptosporidium parvum oocysts with ozone and free chlorine. Water Res. 2000;34(14):3591–3597. doi: 10.1016/s0043-1354(02)00092-1. [DOI] [PubMed] [Google Scholar]
- 5.Burleson G.R., Murray T.M., Pollard M. Inactivation of viruses and bacteria by ozone, with and without sonication. J. Appl. Microbiol. 1975;29:340–344. doi: 10.1128/am.29.3.340-344.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dahl E. Physicochemical aspects of disinfection of water by means of ultrasound and ozone. Water Res. 1976;10:677–684. [Google Scholar]
- 7.Wu Z., Franke M., Ondruschka B., Zhang Y., Ren Y., Braeutigam P., Wang W. Enhanced effect of suction-cavitation on the ozonation of phenol. J. Hazard. Mater. 2011;190:375–380. doi: 10.1016/j.jhazmat.2011.03.054. [DOI] [PubMed] [Google Scholar]
- 8.Wu Z., Ondruschka B., Zhang Y., Bremner D.H., Shen H., Franke M. Chemistry driven by suction. Green Chem. 2009;11:1026. [Google Scholar]
- 9.Wu Z., Cravotto G., Ondruschka B., Stolle A., Li W. Decomposition of chloroform and succinic acid by ozonation in a suction-cavitation system: Effects of gas flow. Sep. Purif. Technol. 2016;161:25–31. [Google Scholar]
- 10.Wu Z., Shen H., Ondruschka B., Zhang Y., Wang W., Bremner D.H. Removal of blue-green algae using the hybrid method of hydrodynamic cavitation and ozonation. J. Hazard. Mater. 2012;235–236:152–158. doi: 10.1016/j.jhazmat.2012.07.034. [DOI] [PubMed] [Google Scholar]
- 11.Boczkaj G., Fernandes A. Wastewater treatment by means of Advanced Oxidation Processes at basic pH conditions: A review. Chem. Eng. J. 2017;320:608–633. [Google Scholar]
- 12.Gagol M., Przyjazny A., Boczkaj G. Wastewater treatment by means of advanced oxidation processes based on cavitation - A Review. Chem. Eng. J. 2018;338:599–627. [Google Scholar]
- 13.Turtoi M. Ultraviolet light potential for wastewater disinfection. Annals. Food Sci. Technol. 2013;14(1):153–164. [Google Scholar]
- 14.Gogate P.R., Shirgaonkar I.Z., Sivakumar M., Senthilkumar P., Vichare N.P., Pandit A.B. Cavitation reactors: Efficiency assessment using a model reaction. AIChEJ. 2001;47:2526–2538. [Google Scholar]
- 15.Cintas P., Luche J.-L. Green chemistry: The sonochemical approach. Green Chem. 1999;1:115–125. [Google Scholar]
- 16.Suslick K.S. Sonochemistry. Science. 1990;247:1439–1445. doi: 10.1126/science.247.4949.1439. [DOI] [PubMed] [Google Scholar]
- 17.Wu Z., Lifka J., Ondruschka B. Comparison of energy efficiency of various ultrasonic devices in aquasonochemical reactions. Chem. Eng. Technol. 2006;29:610–615. [Google Scholar]
- 18.Wu Z., Ondruschka B. Ultrasound-assisted oxidative desulfurization of liquid fuels and its industrial application. Ultrason. Sonochem. 2010;17:1027–1032. doi: 10.1016/j.ultsonch.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 19.Wu Z., Ondruschka B., Cravotto G. Degradation of phenol under combined irradiation of microwaves and ultrasound. Environ. Sci. Technol. 2008;42:8083–8087. doi: 10.1021/es8013375. [DOI] [PubMed] [Google Scholar]
- 20.Wu Z., Cravotto G., Adrians M., Ondruschka B., Li W. Critical factors in sonochemical degradation of fumaric acid. Ultrason. Sonochem. 2015;27:148–152. doi: 10.1016/j.ultsonch.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 21.Wu Z., Ondruschka B., Cravotto G., Garella D., Asgari J. Oxidation of primary aromatic amines under irradiation with ultrasound and/or microwaves. Synth. Commun. 2008;38:2619–2624. [Google Scholar]
- 22.Wu Z., Cravotto G., Gaudino E.C., Giacomino A., Medlock J., Bonrath W. Ultrasonically improved semi-hydrogenation of alkynes to (Z-)alkenes over novel lead-free Pd/Boehmite catalysts. Ultrason. Sonochem. 2017;35:664–672. doi: 10.1016/j.ultsonch.2016.05.019. [DOI] [PubMed] [Google Scholar]
- 23.Cravotto G., Binello A., Di Carlo S., Orio L., Wu Z.-L., Ondruschka B. Oxidative degradation of chlorophenol derivatives promoted by microwaves or power ultrasound: A mechanism investigation. Environ. Sci. Pollut. R. 2010;17:674–687. doi: 10.1007/s11356-009-0253-y. [DOI] [PubMed] [Google Scholar]
- 24.Fedorov K., Plata-Gryl M., Ali Khan J., Boczkaj G. Ultrasound-assisted heterogeneous activation of persulfate and peroxymonosulfate by asphaltenes for the degradation of BTEX in water. J. Hazard. Mater. 2020;397 doi: 10.1016/j.jhazmat.2020.122804. [DOI] [PubMed] [Google Scholar]
- 25.Yuan R., Jiang Z., Wang Z., Gao S., Liu Z., Li M., Boczkaj G. Hierarchical MnO2 nanoflowers blooming on 3D nickel foam: A novel micro-macro catalyst for peroxymonosulfate activation. J. Colloid Interface Sci. 2020;571 doi: 10.1016/j.jcis.2020.03.041. [DOI] [PubMed] [Google Scholar]
- 26.Yuan R., Jiang Z., Wang Z., Gao S., Liu Z., Boczkaj G., Li M., Ma J. 3D mesoporous α-Co(OH)2 nanosheets electrodeposited on nickel foam: A new generation of macroscopic cobalt-based hybrid for peroxymonosulfate activation. Chem. Eng. J. 2019;380 [Google Scholar]
- 27.Fernandes A., Makos P., Boczkaj G. Treatment of bitumen post oxidative effluents by sulfate radicals based advanced oxidation processes (S-AOPs) under alkaline pH conditions. J. Clean. Prod. 2018;195:374–384. [Google Scholar]
- 28.Fernandes A., Makos P., Ali Khan J., Boczkaj G. Pilot scale degradation study of 16 selected volatile organic compounds by hydroxyl and sulfate radical based advanced oxidation processes. J. Clean. Prod. 2019;208:54–64. [Google Scholar]
- 29.Abramov V.O., Abramova A., Keremetin P., Mullakaev M., Vexler G., Mason T. Ultrasonically improved galvanochemical technology for the remediation of industrial wastewater. Ultrason. Sonochem. 2014;21:812–818. doi: 10.1016/j.ultsonch.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 30.Abramov V.O., Abramova A., Bayazitov V., Kruchinina N.E., Gutul T.D., Sidorenko A.S. Use of ultrasound for stabilisation of nanodispersed structure of alumosilicic reagents for wastewater treatment. J. Env. Prot. Ecol. 2018;19(2):638–645. [Google Scholar]
- 31.Gagol M., Cako E., Fedorov K., Darvishi Cheshmeh Soltani R., Przyjazny A., Boczkaj G. Hydrodynamic cavitation based advanced oxidation processes: Studies on specific effects of inorganic acids on the degradation effectiveness of organic pollutants. J. Mol. Liquids. 2020;307 [Google Scholar]
- 32.Gagol M., Przyjazny A., Boczkaj G. Effective method of treatment of industrial effluents under basic pH conditions using acoustic cavitation – a comprehensive comparison with hydrodynamic cavitation processes. Chem. Eng. Process. 2020;128:103–113. [Google Scholar]
- 33.Gogate P.R. Hydrodynamic cavitation for food and water processing. Food Bioprocess Technol. 2011;4:996–1011. [Google Scholar]
- 34.Li W.X., Tang C.D., Wu Z., Wang W.M., Zhang Y.F., Zhao Y., Cravotto G. Eutrophic water purification efficiency using a combination of hydrodynamic cavitation and ozonation on a pilot scale. Environ. Sci. Pollut. Res. 2015;22(8):6298–6307. doi: 10.1007/s11356-014-3889-1. [DOI] [PubMed] [Google Scholar]
- 35.Sivakumar M., Pandit A.B. Wastewater treatment: a novel energy efficient hydrodynamic cavitational technique. Ultrason. Sonochem. 2002;9:123–131. doi: 10.1016/s1350-4177(01)00122-5. [DOI] [PubMed] [Google Scholar]
- 36.Wu Z., Ferreira D.F., Crudo D., Bosco V., Stevanato L., Costale A., Cravotto G. Plant and biomass extraction and valorisation under hydrodynamic cavitation. Processes. 2019;7(12):965. [Google Scholar]
- 37.Zupanc M., Pandur Ž., Stepišnik Perdih T., Stopar D., Petkovšek M., Dular M. Effects of cavitation on different microorganisms: The current understanding of the mechanisms taking place behind the phenomenon. A review and proposals for further research. Ultrason. Sonochem. 2019;57:147–165. doi: 10.1016/j.ultsonch.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 38.Abramov V.O., Andriyanov V., Kisterev E.V., Klassen N.V., Shekhtman A.V., Bulychev N.A. Physicochemical processes in a sonoplasma discharge. Inorg. Mater. Appl. Res. 2011;2(1):76–80. [Google Scholar]
- 39.Foster J., Sommers B.S., Gucker S.N., Bankson I.M. Perspectives on the interaction of plasmas with liquid water for water purification. IEE Trans. Plasma Science. 2012;40(5):1311–1323. [Google Scholar]
- 40.Babaeva N.Y., Kushner M.J. Effect of inhomogeneities on streamer propagation: II. Steamer dynamics in high pressure humid air with bubbles, Plasmas Sources. Sci. Technol. 2009;18(3) [Google Scholar]
- 41.Yamabe C., Takeshita F., Miichi T., Hayashi N., Ihara S. Water treatment using discharge on the surface of a bubble in water. Plasma Process Polym. 2005;2(3):246–251. [Google Scholar]
- 42.Foster J.E., Weatherford B., Gillman E., Yee B. Underwater operation of DBD plasma jet. Plasma Sources Sci. Technol. 2010;19(2) [Google Scholar]
- 43.Cravotto G., Cintas P. Power ultrasound in organic synthesis: moving cavitational chemistry from academia to innovative and large-scale applications. Chem. Soc. Rev. 2006;35(2):180–196. doi: 10.1039/b503848k. [DOI] [PubMed] [Google Scholar]
- 44.M.A. Malik, Water purification by plasmas: Which reactor are most with energy efficient? Plasma Chem. Plasma Process, 30(1) 21-31.
- 45.Kurahashi M., Katsura S., Mizuno A. Radical formation due to discharge inside bubble in liquid. J. Electrost. 1997;42(1/2):93–105. [Google Scholar]
- 46.Glaze W., Kang J.-W., Chaplin D.H. The chemistry of water treatment processes involving ozone, hydrogen peroxide and ultraviolet radiation. Ozone Sci. Eng. 1987;9(4):335–352. [Google Scholar]
- 47.Abramov V.O., Abramova A.V., Bayazitov V.M., Mullakaev M.S., Marnosov A.V., Ildyakov A.V. Acoustic and sonochemical methods for altering the viscosity of oil during recovery and pipeline transportation. Ultrason. Sonochem. 2017;35:389–396. doi: 10.1016/j.ultsonch.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 48.Mezule L., Tsyfansky S., Yakushevich V., Juhna T. A simple technique for water disinfection with hydrodynamic cavitation: Effect on survival of Escherichia coli. Desalination. 2009;248(1–3):152–159. [Google Scholar]
- 49.Wu Z., Tagliapietra S., Giraudo A., Martina K., Cravotto G. Harnessing cavitational effects for green process intensification. Ultrason. Sonochem. 2019;52:530–546. doi: 10.1016/j.ultsonch.2018.12.032. [DOI] [PubMed] [Google Scholar]
- 50.Locke B.R., Sato M., Sunka P., Hoffmann M.R., Chang J.-S. Electrohydraulic discharge and nonthermal plasma for water treatment. Ind. Eng. Chem. Res. 2006;45:882–905. [Google Scholar]
- 51.Makos P., Przyjazny A., Boczkaj G. Methods of assaying volatile oxygenated organic compounds in effluent samples by gas chromatography - a review. J. Chromatogr. A. 2019;1592:143–160. doi: 10.1016/j.chroma.2019.01.045. [DOI] [PubMed] [Google Scholar]
- 52.Marsalek B., Marsalkova E., Odehnalova K., Pochyly F., Rudolf P., Stahel P., Rahel J., Cech J., Fialova S., Zezulka T. Removal of Microcystis aeruginosa through the combined effect of plasma discharge and hydrodynamic cavitation. Water. 2020;12(1):8. [Google Scholar]
- 53.Fang Y., Hariu D., Yamamoto T., Komarov S. Acoustic cavitation assisted plasma for wastewater treatment: Degradation of Rhodamine B in aqueous solution. Ultrason. Sonochem. 2019;52:318–325. doi: 10.1016/j.ultsonch.2018.12.003. [DOI] [PubMed] [Google Scholar]