Highlights
-
•
Novel fluidized bed drying methods saved seaweed drying time significantly.
-
•
Airborne ultrasound assisted drying method retained highest total phenolic content.
-
•
Page model was the best fitting ultrasound assisted fluidized bed drying kinetics.
-
•
Seaweed drying after pretreatments saved more energy.
Keywords: Seaweeds, Airborne acoustics, Pre-treatment, Ultrasound, Fluidized bed drying, Drying kinetics
Abstract
In this study, ultrasound either as a pretreatment technique or as an integrated technique was employed to enhance fluidized bed drying of Ascophyllum nodosum, and drying kinetics and dried product quality were assessed. In order to compare technology efficiency and dried product qualities, oven drying and fluidized bed drying (FBD) were employed. The novel drying methods included airborne ultrasound-assisted fluidized bed drying (AUA), ultrasound pre-treatment followed by FBD (USP), and hot water blanching pre-treatment followed byFBD (HWB). Six drying kinetics models were used to describe the drying curves, among which the Page model was the best in fitting USP and AUA. Model by Millidi et al. was employed to describe HWB. Airborne ultrasound in AUA did not reduce energy consumption or drying time, but retained total phenolic content (TPC) as well as colour, and exhibited the highest yield among the novel drying methods. USP and HWB showed lower energy consumption and drying time considerably, but the TPC was the lowest among the studied methods. At the same time, USP dried product exhibited the lowest aw, followed by HWB and then AUA. This studyalso demonstrated that FBD could be a very practical drying method on Irish brown seaweed, and ultrasound-assisted drying methods may have potential developments in Irish brown seaweed drying process.
1. Introduction
Nowadays, the seaweed market scale all over the world is over 6 billion US Dollars per year, among which, 85% consists of food products and other 15% is contributed by seaweed extractions such as carrageenan, agar and alginates [1]. Seaweed is widely consumed and farmed as a low-calorie, nutritious food in many Asian countries especially in Korea, Indonesia, China and Japan [2]. Some of the edible seaweed referred to as ‘Kombu’ is sold in the dried form [3]. In the western countries, seaweed is normally regarded as a good resource of phycocolloids in the food processing industry such as agars, carrageenan and alginates [4]. In addition, seaweeds have been used as a fertilizer for centuries due to its high content of organic compounds such as amino acids, vitamins, proteins and manyother polysaccharides [5]. Many studies have proved that it can increase nutrient absorption and the growth rate of crops [6], [7]. Seaweed is a good source of biologically active phytochemicals and metabolites including fatty acids, polysaccharides, polyphenols, vitamins, minerals, meroterpenoids, etc. [8], [9]. These biologically active phytochemicals and metabolites may have potential treatment properties in a variety of diseases such as thyroid-related diseases, thrombosis, upper respiratory infection, tumour, obesity, diabetes etc. [8], [10], [11]. Seaweed can also beessential raw material for the biofuel, pharmaceutical and cosmetic industrie [12], [13], [14]. Due to the potential beneficial effects of various seaweed origin polyphenols on different cardiovascular-associated disorders and cancers such as hypertension, diabetes mellitus type 2, metabolic syndrome and breast cancer, total polyphenol content (TPC) is one of the key parameters to evaluate drying methods in present study [15], [16], [17]. North Atlantic rockweed (Ascophyllum nodosum) studied in the present paper, a typical brown seaweed is commonly harvested in northwest Europe, including Ireland, used for animal feed, fertiliser and alginate production [18], [19]. In order to optimise the use of all biological substances in fresh and perishable seaweedand extend shelf life of seaweed related products, an efficient preservation process is required prior to industrial or domestic seaweed use.
Drying is the most common method for the food industry [20], [21], [22], [23], which can also be used to further stabilize the biomass of fresh, harvested seaweed [24]. However, the quality of seaweed can be significantly affected by the drying process, especially when processed using high temperatures [25]. Low-cost methods of drying such as solar drying require a large space and a stable climate condition [26]. In Ireland, rotary dryers are commonly used for producing seaweed powder [27]. Some researchers have studied the effect on seaweed quality by different drying processes. Wong and Cheung [28] declared that oven drying is better than freeze-drying in terms of improving extractability and digestibility of protein in three seaweed species. Different drying methods were studied by Ling et al. [29],in which, oven drying and shade drying have shown better ability in retaining the biologically active phytochemicals in samples. Cruces et al. [30] claimed that freeze-drying is the best method of retaining the antioxidant activity of seaweed samples. Another factor to consider in this process is the energy consumption of drying technology. Among various drying methods, fluidized bed drying (FBD) offers many significant advantages such as high heat and mass transfer, high drying rate and even moisture reduction with less drying time. It can mix the entire solid product efficiently with drying air and provide with uniform drying temperature and longer constant drying rate period [31]. However hotspot formation in FBD dryers can result in a high moisture variation which may damage the product with loss of quality [31].
In order to develop a low-cost, efficient, drying technique with minimal impact to phytochemicals in seaweed, pre-treatments using ultrasound, microwave and osmosis have also been investigated [32], [33], [34]. Ultrasound, as a promising technique in the food industry has already shown the potential in accelerating freezeing, drying process, inactivating microbes, etc [35], [36], [37], [38], [39], [40], [41]. Pre-treatment operations such as hot water blanching, ultrasound, microwave etc, can modify the tissue structure and result in a shorter drying time [42]. In comparison with widely applied treatments such as hot water blanching, the non-thermal attribute of ultrasound can improve the final product quality and reduce the drying time [43]. The use of an ultrasound technique in the drying process canbeapplied in two ways: ultrasound pre-treatment before the drying process or airborne ultrasound irradiation during drying. Many studies have proven this technique to be efficient in relation to both drying time and product quality [44], [45], [46]. In terms of ultrasound pre-treatment in foods such as fruits and vegetables, drying time were shortened significantly in previous studies [47], [48], [49]. Ultrasound can also be employed directly during the drying process. Airborne ultrasound-assisted drying technologies also increases the water effective diffusivity while reducing the processing time. It was reported that drying time of zucchini, apple and strawberry was reduced by 13 to 44% [50], [51], [52]. Kroehnke et al. [53] even claimed an efficient hybrid convective drying method assisted by both ultrasound and microwave for carrot drying.
In order to investigate the effectiveness of fluidized bed drying combined with other novel technologies, hot water blanching, power ultrasound pre-treatment and airborne ultrasound combined with fluidized bed drying of Ascophyllum nodosum were explored in this study. Conventional oven drying, fluidized bed drying alone andcombinations offluidized bed drying techniques were conducted in order to compare various parameters of novel methods.
2. Materials and methods
2.1. Seaweed sampleand chemicals
Fresh Ascophyllum nodosum (moisture content of 73.08 ± 0.29%,w.b.) was harvested from the west coast of Ireland in November 2019. Fresh seaweed samples were washed thoroughly with tapwater to remove salt and surface impurities. Samples were then wiped with tissue to remove surface water and were then ground until approximately 1 to 2 cm in length. All samples were stored at −20 °C prior to further processing.
All reagents (ethanol, methanol, sodium carbonate, Folin-Ciocaltreau, gallic acid) were purchased from Sigma-Aldrich, AUA. Maximum recovery diluent CM0733 (MRD) and plate count agar CM0325 (PCA) were purchased from Oxoid, UK.
2.2. Drying methods
After defrosting the seaweedat 4 °C, 200 g of seaweed was used for each drying method.
2.2.1. Oven drying
Conventional oven drying technique was carried out in using an oven (Gallenkamp Plus II, Gemini, Netherland), at 50 °C. The seaweeds were evenly placed in a tray (36 × 26 cm) in the oven.
2.2.2. Fluidized bed drying (FBD)
Afluidized bed dryer (Sherwood Tornado M501, Sherwood Scientific, U.K.) was used to dry the seaweed samples. The drying temperature was set at 50 °C. The superficial velocity of hot air was 6.7 m/s. Initial loading height was about 9 ± 0.2 cm. The environmental relative humidity is 47.63 ± 2.51. Pulsed air flow was produced by the pulser inside fluidized bed dryer in order to homogenize the samples inside the dryer. The pulsing of the valve occurred in cycles of approximately 2.5 s closed and 2.5 s open. Samples were weighed every 10 min for the first 30 min of drying and every 15 min for the second 30 min, and every 30 min thereafter.
2.2.3. Airborne ultrasound-assisted (AUA) fluidized bed drying
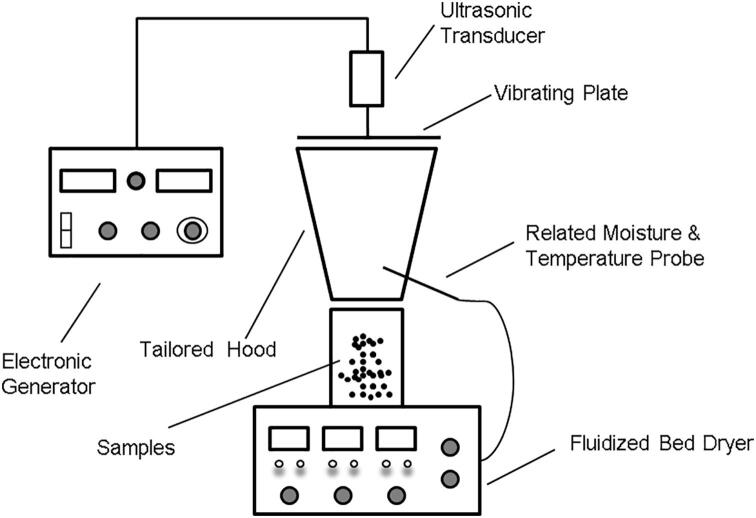
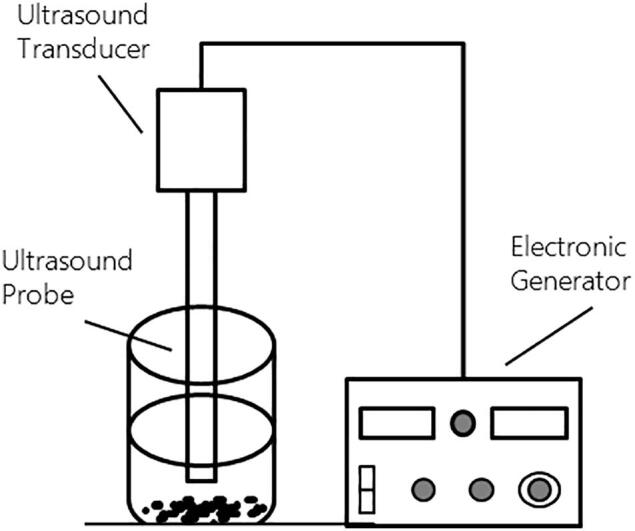
The AUA drying was conducted in an experimental setup combining the fluidized bed dryer aforementioned and an airborne ultrasound instrument (Pusonics S.L., Madrid, Spain), as illustrated in Fig. 1. A 26 kHz electronic wave was generated, amplified and transferred to the transducer, which connected to a horizontal vibration plate. Airborne ultrasound from the plate was transmitted downwards to the samples being dried by the fluidized bed dryer in the cylinder and conical hood. The temperature and velocity setting were the same as in FDB drying, while the power of the air-borne ultrasound was 170 W.
Fig. 1.
Experiment setup for airborne ultrasound-assisted (AUA) fluidized bed drying.
2.2.4. Ultrasound pre-treatment (USP)followed by FBD drying
The samples were mixed with distilled water in a beakerat a ratio of 1:4 solid/water as recommended by Fernandes and Rodrigues [54] and Kadam et al. [55]. An ultrasound processer (500 W, UIP500hdT, Hislscher, Germany) at 20 kHz with a 13 mm diameter probe was employed in the ultrasound pre-treatment. The probe was submerged 30 mm under the water surface. The equipment configuration is shown in Fig. 2. After 10 min ultrasound pre-treatment at an amplitude of 100%, surface water was removed by vacuum filtering and blotting using a tissue. Thereafter, the samples were transferred to the fluidized bed for drying under the same drying conditions as for FBD drying.
Fig. 2.
Experiment setup for ultrasound water bath pretreatment.
2.2.5. Hot water blanching (HWB) followed by FBD drying
The samples were blanched at 70 °C for 8 min in 800 ml distilled water in a beaker placed in a hot water bath (T100, Grant Instruments, U.K). Thereafter, the samples were filtered, then blotted to remove surface water, and finally dried as in FBD drying.
2.3. Moisture content measurement and electric power consumption measurement
Moisture content was evaluated by drying at 105 °C overnight in anoven (Model 28, Binder, Germany). Energy consumption of the various processes was determined, in terms of electric power consumption, using a power meter (PM 231E, Brennenstuhl, Germany). Total energy consumption measurement includes energy consumed at various stages of the drying process required to achieve 10% moisture content.
2.4. Colourimetric characterization
The colour was measured using a colourimeter (CR-400, Konica Minolta, Japan). It was calibrated with a white reference tile. Colour parameters of defrostednon-treated seaweed, as the control, were collected after washing and grounding into small pieces. After drying samples were wrapped with a film evenly and tested directly. Colour was expressed by means of CIE Lab coordinates (L*, a* and b*). Total colour difference (ΔE*) caused by the different drying methods was evaluated using Eq. (1). Defrosted sample before processing was taken as the reference.
| (1) |
where L* is lightness, a* is redness and b* is yellowness, subscript r indicates reference value from the defrost sample as a control. Eight replicates were conducted foreach sample.
2.5. Water activity (aw)
After calibration of standard solutions, a water activity meter (Series 3, AquaLab, USA) was used to measure water activity of samples. Measurements were performed at 23 ± 0.36 °C in three replicates.
2.6. Rehydration capacity
Ten grams of dried seaweed were immersed in 500 ml of distilled water kept at room temperature for 1 h. After rehydration, the seaweeds were drained using a wire mesh sieve and then blotted with tissue paper to remove surface water. The rehydration capacity of dried seaweed is estimated by the moisture content of the rehydrated sample.
2.7. Total phenolic content (TPC) measurement
Extractions of phenolic compounds were performed a method modified from Rajauria et al. [56]. Methanol (60%) was used as the extractant. Two grams of powdered seaweed samples, ground by a ball miler (MM 400, Retsch, Germany), were mixed with 20 ml methanol in dark conditions, shaking at 170 rpm at room temperature overnight. After filtration with a muslin cloth, the extract was concentrated using a nitrogen dryer at room temperature for 8 h, followed by freeze-drying. The freeze-dried samples were stored at −80 °C prior to further test.
Each freeze-dried extract was dissolved in water to prepare a solution at 0.8 mg/ml. The Folin-Coitreaunmethod, used by Ainsworth and Gillespie [57] and Ganesan and Bhaskar [58] with minor modification was employed in TPC evaluation in this study. Gallic acid standard solutions were used for calibration. After incubation with Folin-Coiltreau solution and sodium carbonate solution at dark for 30 min, the absorbance of the sample was recorded at 720 nm using a spectrophotometer (Epoch 2, Biotek, U.S.A.). Results are expressed as mg GA equivalents per gram dried seaweed sample.
2.8. Total viable count (TVC)
TVC evaluation was conducted based on ISO 4833-1:2013 [59]. Ten grams of samples were blended and diluted with MRD into different concentrations. One ml of each dilution was pipetted into a Petri dish. The pour plate method was applied using PCA as a growth medium. After 48 h incubation at 30 °C, colonies were counted and expressed as log CFU/gram. Three replicates were employed.
2.9. Scanning electron microscope (SEM) analysis
Dried seaweed samples were rehydrated as in Section 2.6 and immediately frozen in liquid nitrogen, followed by freeze-drying. The dried samples were mounted on stubs and then coated with a 5 nm layer of Gold by Emitech K575X Peltier Cooled Sputter Coating Unit (Quorum Technologies). Sample surfaces were photographed with a scanning electron microscope (Regulus 8230, Hitachi, Japan).
2.10. Mathematical modelling
In the drying process, the sample was weighed at fixed time points until a constant weight was achieved. Moisture content (w.b.) at any specifictime point (Mtw) can be evaluatedby Eq. (2):
| (2) |
where M0, Mtw and MLt are the initial moisture content (w.b.), moisture content and moisture loss at a given time. W0 and Wt are the initial sample weight (w.b.) and sample weight at a given time.
Moisture ratio (MR) was used for drying kinetics study, calculated as in Eq. (3)
| (3) |
where M0d and Mtd are initial moisture content (d.b.) and moisture content at a given time, Me is the equilibrium moisture content (d.b.). Six kinetic models shown in Table 1 were used forthe drying kinetics study.
Table 1.
Six mathematical models applied describing drying kinetics.
| Model | Equation | Reference |
|---|---|---|
| Newton | Kumari and Khatkar (2018) | |
| Henderson and Pabis | Touré (2019) | |
| Page | Yang et al. (2018) | |
| Weibull | Ju et al. (2018) | |
| Midilli et al. | Midillietal. (2002) | |
| Wang and Singh | Zhao et al. (2017) |
2.11. Statistical analysis
Triplicates were performed for all drying and quality measurements unless otherwise stated. Pearson correlation analysis and principal component analysis (PCA) were performed to explore the correlation and correspondence between aw and dried sample colour using XLSTAT (version 2020.3, Redmond, Washington, USA). Effects of drying methods were evaluated using one-way analysis of variance (ANOVA) with Post HocTukey test, using SPSS (v20.0.0, IBM, U.S.A.). The significance level was defined as P < 0.05. Parameters of the six models were estimated using SPSS (v20, IBM, U.S.A.). The fitness of models was evaluated based on the sum square error (SSE; Eq. (4)), regression coefficient (R2), root mean square error (RMSE; Eq. (5)), chi-square (χ2; Eq. (6)), Akaike information criterion (AIC; Eq. (7)) and Bayesian information criterion (BIC, Eq. (8)).
| (4) |
| (5) |
| (6) |
| (7) |
| (8) |
Where MRexp and MRpred are experimental and predicted moisture ratio, N represents the number of observations and c represents the number of constants in models.
3. Results and discussion
3.1. Drying kinetics, energy consumption and drying yield
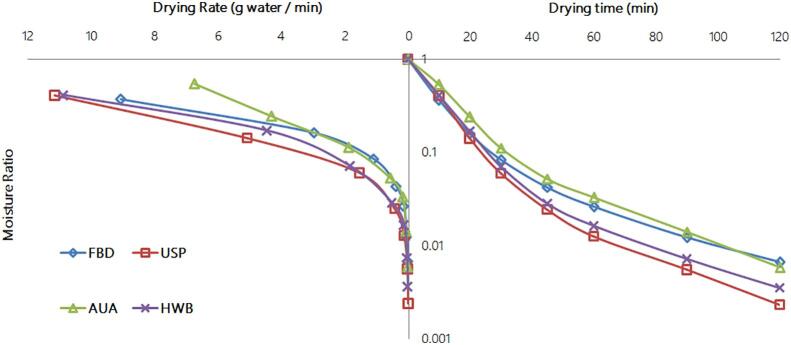
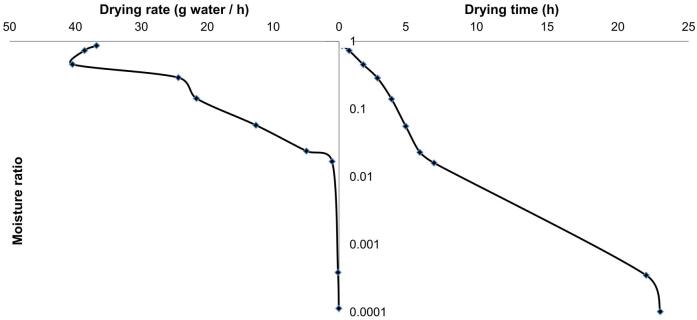
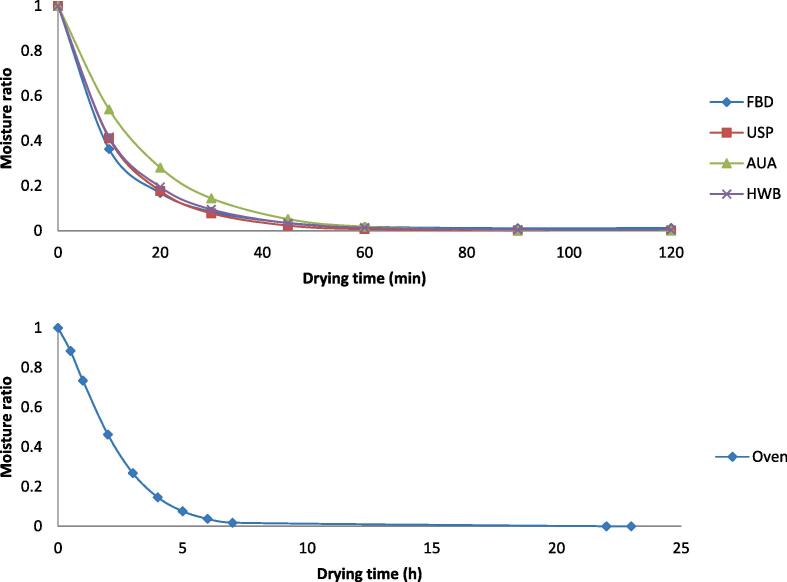
Drying rates and drying curves of the studied methods are shown in Fig. 3, Fig. 4. In a typical drying process, the drying rate goes through three stages: increasing, constant and decreasing stages [60]. The drying rate curve for the oven drying exhibited an increasing stage when the moisture content was still high at the beginning of the drying process and a subsequent fallingstagewhich was the predominant stage of the drying. However, in Fig. 3, all the other drying methods, which employed FBD drying, showed onlythe falling stage from the beginning. The falling stage as predominant drying stage for all the methods studied indicated that diffusion was the dominant mechanism for drying seaweed using FDB method. Similar results were also statedby Horuz et al. [61]. The occurrence of the increasing stage in oven drying was due to heating-up of the seaweed samples slowly in the oven. The other drying methods, where FBD drying was employed, could sharply heat up all seaweed sample particles in the bed. As a result, no increasing drying rate stage existed and their drying rates were much higher than the oven drying. Their drying rates rapidly reduced with reduction of MR until MR dropped down to about 0.1 for FBD, AUA and HWB and well below 0.1 for USP. As shown in Fig. 3, in general, among the drying methods employing FBD, USP had the highest drying rate, followed by HWB, and then FBD. It was interesting that AUA had the lowest drying rate among them, even though many researches pointing out that airborne ultrasound can enhance drying and reducing drying time [62], [63], [64]. In these previous studies, drying conditionsvaried a lot compared to this experiment, in terms of air velocity, drying methods and airborne ultrasound plate positions, etc. From this perspective, optimization of airborne ultrasound setups can be studied in further research. The higher drying rate for USP and HWB could result from the disruption of the cell structure of the seaweed samples bypower ultrasound [55] and blanching [65], which improved moisture diffusion within the seaweed matrix during drying.
Fig. 3.
Drying curves and drying rate evolution for the fluidized bed drying (FBD), ultrasound pre-treatment assisted FBD (USP), airborne ultrasound-assisted FBD (AUA) and hot water blanching assisted FBD (HWB) Note: all data are the means from 3 replicates.
Fig. 4.
Drying curve and drying rate evolution for theoven drying Note: all data are the means from 3 replicates.
Five drying kinetic models, as listed in Table 1, were used to investigate the drying methods. Parameters of the five drying kinetic models along with regression coefficients, RMSE, χ2, AIC and BIC are shown in Table 2.Wang and Singh model didnot show a good fit for the five drying methods in this experiment due to low R2 value. As for the other models they had similar R2 around 0.99, low RMSE from 0.0056 to 0.0189, AIC (<−26.92) and BIC (<−58.88). Page model fitted oven drying better due to its lowest χ2 (0.0001), AIC (−89.76) and BIC (−97.11), as similar results reported by Djaeni and Sari [66] studied on seaweed. The Page model was also employed in the present study to describe USP and AUA due to its smaller deviation at low MR points, which could predict the drying timemore accurately. The Page model was employed in previous literature for Ascophyllum nodosum [34], [67]. Midilliet al. model showed the best fit to FBD and HWB based on lowest RMSE. Mirzaee et al. [68] claimed that Midilli et al. model could satisfactorily fit apricot drying process. Kinetics models picked for all drying methods are shown in Fig. 5. The drying times to 10% moisture content predicted based on chosen models were 80 min for USP, HWB and FBD, 100 min and 6.5 h for AUA and oven drying respectively. Oven drying was much slower than other drying methods. Similarly, Moreira et al. claimed that it needed 5.5 h to dry Ascophyllum nodosum to 10% moisture content at 50℃by convective air drying (2 m/s) [69].
Table 2.
Parameters and regression coefficient of the different models applied to drying kinetics of five drying methods.
| Model | Parameter | FBD | USP | AUA | HWB | Oven |
|---|---|---|---|---|---|---|
| Newton | k | 0.0938 | 0.0919 | 0.0673 | 0.0881 | 0.4214 |
| R2 | 0.998 | 0.999 | 0.998 | 0.998 | 0.991 | |
| RMSE | 0.0184 | 0.0134 | 0.0157 | 0.0186 | 0.0434 | |
| X2 | 0.0004 | 0.0002 | 0.0003 | 0.0004 | 0.0021 | |
| AIC | −57.556 | −62.659 | −60.1 | −57.311 | −60.006 | |
| BIC | −63.956 | −69.059 | −66.5 | −63.711 | −69.006 | |
| Henderson and Pabis | a | 0.9941 | 1.0023 | 1.0071 | 0.9992 | 1.0557 |
| k | 0.0933 | 0.0921 | 0.0677 | 0.088 | 0.4459 | |
| R2 | 0.9981 | 0.999 | 0.998 | 0.998 | 0.989 | |
| RMSE | 0.0182 | 0.0132 | 0.0154 | 0.0187 | 0.0392 | |
| X2 | 0.0004 | 0.0002 | 0.0003 | 0.0005 | 0.0019 | |
| AIC | −52.061 | −57.228 | −54.73 | −51.678 | −61.814 | |
| BIC | −61.981 | −67.149 | −64.651 | −61.598 | −69.163 | |
| Page | k | 0.0419 | 0.0116 | 0.0129 | 0.0125 | 0.0638 |
| n | 1.0176 | 1.3941 | 1.2897 | 1.3436 | 1.9933 | |
| R2 | 0.999 | 0.999 | 0.997 | 0.999 | 0.999 | |
| RMSE | 0.0116 | 0.0087 | 0.0189 | 0.0128 | 0.011 | |
| X2 | 0.0002 | 0.0001 | 0.0005 | 0.0002 | 0.0001 | |
| AIC | −59.339 | −63.945 | −51.497 | −57.747 | −89.761 | |
| BIC | −69.26 | −73.865 | −61.417 | −67.668 | −97.111 | |
| Weibull | a | 1.0054 | 1.0092 | 1.0127 | 1.0062 | 0.97672 |
| k | 0.0429 | 0.0124 | 0.0141 | 0.0131 | 0.05271 | |
| n | 1.0119 | 1.3775 | 1.268 | 1.3323 | 2.10705 | |
| R2 | 0.906 | 0.999 | 0.999 | 0.998 | 0.999 | |
| RMSE | 0.0079 | 0.0154 | 0.013 | 0.0178 | 0.01066 | |
| X2 | 0.0001 | 0.0004 | 0.0003 | 0.0005 | 0.00016 | |
| AIC | −56.158 | −45.443 | −48.117 | −43.154 | −85.234 | |
| BIC | −73.097 | −62.382 | −65.056 | −60.093 | −95.506 | |
| Midilli et al. | a | 1.0003 | 1.0004 | 1.0012 | 0.9996 | 0.9916 |
| k | 0.1526 | 0.0753 | 0.0478 | 0.1091 | 0.2999 | |
| n | 0.8218 | 1.0768 | 1.1247 | 0.9048 | 1.337 | |
| b | 0.0001 | 0.0001 | 0.0001 | 0 | −0.0001 | |
| R2 | 0.999 | 0.998 | 0.999 | 0.999 | 0.999 | |
| RMSE | 0.0056 | 0.0153 | 0.0105 | 0.0127 | 0.0106 | |
| X2 | 0.0001 | 0.0005 | 0.0002 | 0.0003 | 0.0002 | |
| AIC | −43.037 | −26.924 | −32.93 | −29.913 | −78.002 | |
| BIC | −74.99 | −58.877 | −64.883 | −61.866 | −91.955 | |
| Wang and Singh | a | −0.0262 | −0.0259 | −0.0235 | −0.0251 | −0.1779 |
| b | 0.0002 | 0.0002 | 0.0001 | 0.0001 | 0.0059 | |
| R2 | 0.773 | 0.815 | 0.846 | 0.824 | 0.939 | |
| RMSE | 0.1997 | 0.1843 | 0.1637 | 0.1738 | 0.1039 | |
| X2 | 0.0532 | 0.0453 | 0.0357 | 0.0403 | 0.0132 | |
| AIC | −13.774 | −15.063 | −16.957 | −15.998 | −40.384 | |
| BIC | −23.694 | −24.984 | −26.878 | −25.919 | −47.733 | |
Note: FBD, fluidized bed drying; USP, ultrasound pre-treatment; HWB, hot water blanching; Cl, confidence interval; RMSE, root mean square error; AIC, Akaike’s information criterion; BIC, Bayesian information criterion; all data are the means from 3 replicates.
Fig. 5.
Drying kinetics of all drying methods in fitted modelsNote: all data are the means from 3 replicates.
Table 3 shows the energy consumption for each method studied. It can be seen that HWB and USP required 1.63 ± 0.02 and 1.58 ± 0.02 kWh respectively to complete the drying process. Energy consumption in HWB for its pre-treatment did not cover that for heating-up of the water bath prior to blanching, meaning less energy was considered in the present study. Among the studied drying methods, oven drying and AUA consumed energy the most. Long processing time of the oven method leadstohigh energy consumption. As for AUA, it was caused by the consumption by the airborne ultrasound generator, which could not reduce the drying time in the present experimental setup in addition to the energy consumption for drying. In general, FBD was a better choice in this study in terms of both energy consumption and process complexity.
Table 3.
Energy consumption, drying time and product yield of dried seaweeds dried to 10% moisture content (w.b.) by the five drying methods.
| Energy (kWh) | Predicted Drying time (min) | Yield (g / 200 g fresh seaweed) | |
|---|---|---|---|
| FBD | 1.59 ± 0.13a | 80 | 60.51 ± 1.00a |
| USP | 1.63 ± 0.02a,b | 80 | 49.70 ± 3.63b |
| HWB | 1.58 ± 0.02b | 80 | 50.89 ± 0.28b |
| AUA | 2.47 ± 0.16c | 100 | 58.17 ± 1.42a |
| Oven | 2.51 ± 0.11c | 390 | 55.16 ± 4.59a,b |
Note: Data in the same column with the same letter are not significantly different (P > 0.05); all data are the means from 3 replicates.
Although HWB and USP demanded lower energy, their product yields were about 50 g dried seaweeds per 200 g fresh seaweeds, lower than the other methods by 5–10 g, as exhibited in Table 3. USP yield was lower significantly (P < 0.05) than FBD, AUA and oven. It is quite reasonable since solid loss occurred inthepre-treatments, especially for USP as the high power ultrasound cavitation in the pre-treatment would disrupt texture of the sample, resulting in insoluble and even some insoluble solids segment leachedout into the water. Solid lost for the pre-treatments were measured, they were7.65 ± 0.09 g and 6.32 ± 0.19 g for USP and HWB, respectively. FBD had the highest yield, followed by AUA, and then oven drying.
3.2. Water activity (aw) and colour
Water activities of fresh seaweedof ~0.99 were considerably decreased to between 0.19 and 0.37 after drying to 10% moisture content, as listed in Table 4. It is interesting to find that HWB and USP caused significantly (P < 0.05) lower water activity values than the other methods, by over 0.1. Water activities for the other methods varied from 0.321 to 0.373. The lower water activities in HWB and USP would attribute to the solid loss in their pre-treatments. Normally, food products with aw at 0.3 have the most stable status with regards to lipid oxidation, non-enzymatic browning, enzyme activity and the microbial parameters. However, froma preservation perspective, the water activity of dehydrated food only requires no more than 0.62 [70]. Hurdle technologies could be taken into consideration to combat the deleterious effects of seaweed [71]. And a higher value ofawof Ascophyllum nodosum could be allowed for long term preservation, and at the same time, the drying time, as well as the energy consumption, could be reduced.
Table 4.
Water activities and colour values of seaweed after drying and after rehydration.
| Samples | After Drying |
After rehydration |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| aw | L* | a* | b* | ΔE* | L* | a* | b* | ΔE* | |
| Oven | 0.373a | 26.58 ± 1.63a | −0.03 ± 0.01a | 4.14 ± 0.28a | 7.76 ± 0.69a | 29.78 ± 0.01a,c | −3.18 ± 0.27a | 14.97 ± 0.23a | 4.66 ± 0.34a |
| FBD | 0.351b | 31.48 ± 0.98a | −1.00 ± 0.12b | 5.99 ± 0.41b | 6.52 ± 0.12a | 28.79 ± 0.04b | −3.54 ± 0.27a,b | 14.79 ± 0.58a | 4.57 ± 0.59a |
| USP | 0.193c | 26.95 ± 2.03a | −0.53 ± 0.07c | 4.49 ± 0.31b | 7.40 ± 0.81a | 31.40 ± 0.62b,c | −3.70 ± 0.02a,b | 16.05 ± 1.12a | 6.40 ± 0.97a |
| HWB | 0.238d | 27.93 ± 1.83a | −0.70 ± 0.03b,c | 5.42 ± 0.30b | 6.36 ± 0.54a | 31.97 ± 0.19c | −4.23 ± 0.20b | 16.83 ± 0.47a | 7.49 ± 0.31a |
| AUA | 0.321e | 29.70 ± 4.40b | −0.87 ± 0.25b,c | 5.33 ± 1.09b | 7.39 ± 0.87a | 28.49 ± 0.59c | −3.75 ± 0.04a,b | 14.35 ± 1.00a | 4.46 ± 0.57a |
Note: Data in the same column with the same letter are not significantly different (P > 0.05); all data are the means from 3 replicates.
Colour of dried and rehydrated seaweed samples are listed in Table 4. Colour values L* (28.33 ± 2.23), a* (−0.31 ± 0.24) and b* (11.6 ± 1.33) of the defrost sample were also measured, based on which total colour changes (ΔE*) were calculated. As showed in Table 4, in general, after drying, L* values of the seaweed samples varied gently, a* decreased slightly, but b* considerably reduced. The huge reduction of b* caused large ΔE* (>6) for all the samples, among which samples dried by USP and AUA had higher values of ΔE* (>7.0) and the oven-dried sample had the highest (>7.7). ΔE* can be classified respectively as small difference when ΔE* ≤ 1.5, the distinct difference when 1.5 < ΔE* ≤ 3, and very distinct difference when ΔE* > 3 [72].This means that the dried sample’s colours differed distinctly from the defrost one. The highest ΔE* value for the oven-dried sample could belinked to the degradation of some pigments (i.e. carotenoids) during the long processing time [73]. Tekin et al., [74] pointed out that increase of a*, a decrease of b* of oven drying was related to browning reactions and degradation of the heat-stable green and yellowish pigments. The higher ΔE* values of AUA- and USP-dried samples indicated that ultrasound application could stimulate pigment degradation. It can be found also in the research by Tekin et al. [74]. After rehydration, compared to the defrosted sample, L* became higher, especially for samples with pre-treatment; a* values were much lower, indicating much stronger green colour, and their b* values were all higher, even though the values for dried samples were much lower than the defrosted one. Their ΔE* values were smaller than those for dried samples but still were over 3, indicating distinct different from the defrosted sample. Colour for the two pre-treatments methods after rehydration differed the most from the defrosted seaweed. They have more distinct variance in comparison with other methods.
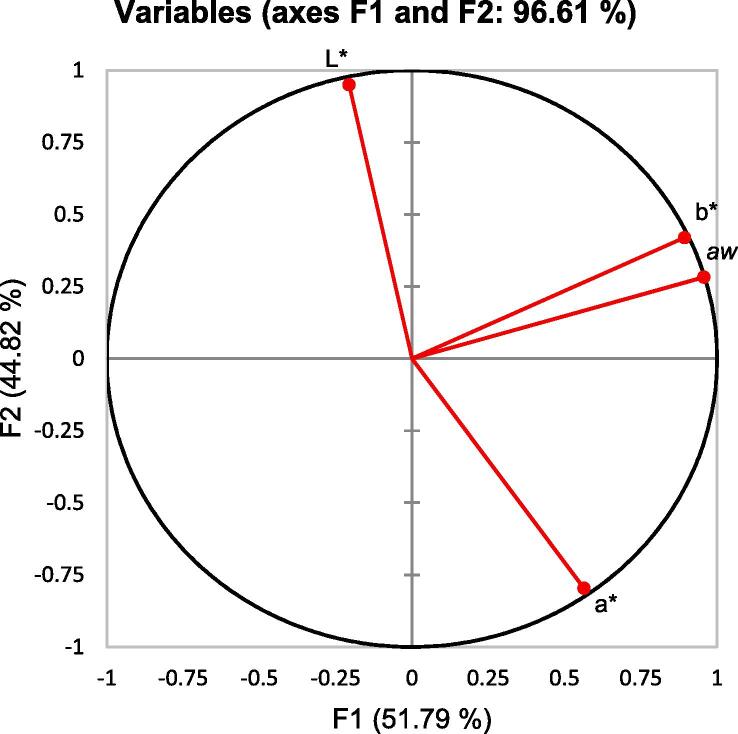
To verify the correlation between water activity and colour of dried samples, the Pearson correlation analysis among aw and L*, a*, b* was applied and is reported in Table 5. Overall, b* value showed a high correlation coefficient with aw (r2 = 0.960). PCA was introduced to analyze the correspondence between aw and dried sample colour. Analyzed data were illustrated in the circle of correlations Fig. 6. The first two principal components (expressing 96.61% of initial variances of aw and L*, a*, b* were selected to evaluate the correlation. The first component (F1, 51.79%) was expressed dominantly by aw and b* value which showed that the yellowness of dried seaweed was strongly correlated with aw.
Table 5.
Pearson’s correlation coefficients of aw, L*, a* and b*
| Variables | aw | L* | a* | b* |
|---|---|---|---|---|
| aw | 1.000 | 0.087 | 0.329 | 0.960 |
| L* | 1 | −0.822 | 0.177 | |
| a* | 1 | 0.134 | ||
| b* | 1 |
Note: all data are the means from 3 replicates.
Fig. 6.
Correlation circle of aw, L*, a* and b* Note: all data are the means from 3 replicates.
3.3. Rehydration capacity
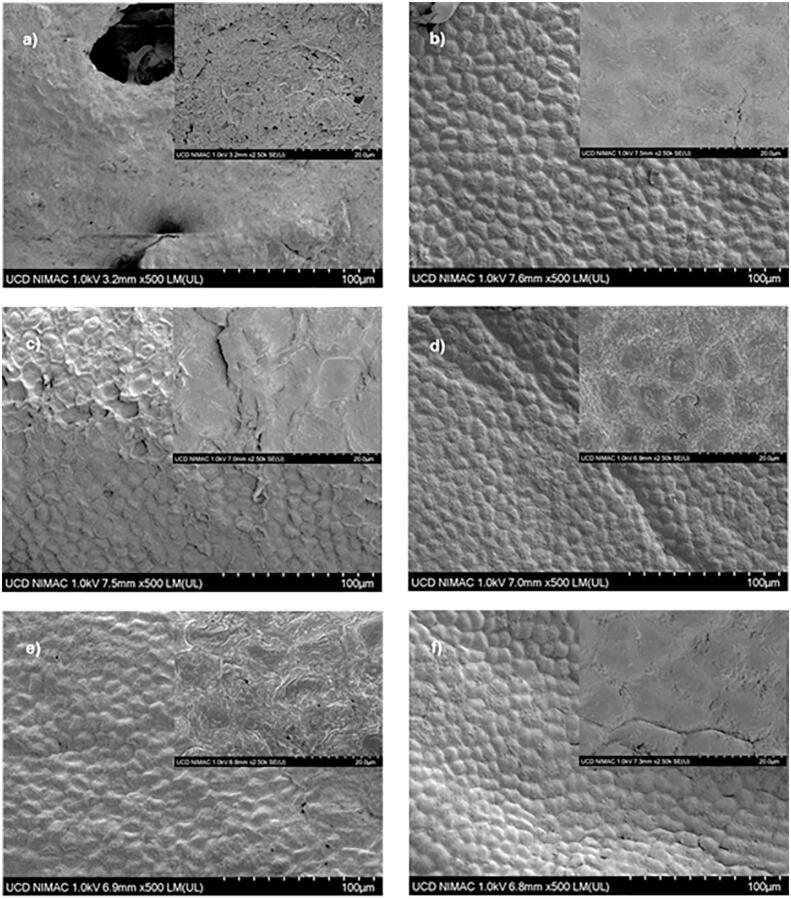
Seaweed is oftendried to increase shelf life. Dried seaweeds are commonly rehydrated before consumption in order to recover some of the properties, and thatrehydration capacity is one of the quality parameters toward different drying technologies. Rehydration is composed of three simultaneous steps: water absorption, swelling and soluble compounds diffusion [75]. It is generally accepted that the degree of rehydration is dependent on the degree of cellular and structural disruption. After drying, there can be cellular rupture and dislocation, resulting in a dense structure of collapsed as shown in Fig. 8. The moisture contents of dried samples in this study, listed in Table 6, exceeded defrost sample’s by 3–6% after one-hour rehydration. This fact could be explained by the loss of solids of the seaweed during the rehydration process, which could lead to more porous seaweed structure to be replaced by water, resulting better hydrophilic properties [34], [67]. USP and HWB samples showed higher moisture content than other drying methods. During the pre-treatment processes, the solid matrix of the samples was disrupted, which could cause water easier and faster to diffuse into the seaweed samples during the rehydration process. Oven, FBD and AUA have similar rehydration ability.
Fig. 8.
Microscopic structure of the Ascophyllum nodosum samples: a) after defrosting, and after rehydration of samples dried byb) Fluidized bed drying, c) oven drying,d) ultrasound pre-treated fluidized bed drying, e) airborne ultrasound-assisted fluidized bed drying and f) hot water blanching pre-treated fluidized bed drying.
Table 6.
Moisture content of defrost sample and rehydrated samples.
| Samples | Moisture content (%) |
|---|---|
| defrost | 73.08 ± 0.68 |
| Oven | 76.44 ± 1.39a |
| FBD | 76.33 ± 0.33a |
| USP | 79.00 ± 1.18a,b |
| HWB | 79.27 ± 0.87b |
| AUA | 77.38 ± 0.90a,b |
Note: Data in the same column with the same letter are not significantly different (P > 0.05); all data are the means from 3 replicates.
3.4. Total phenolic content (TPC)
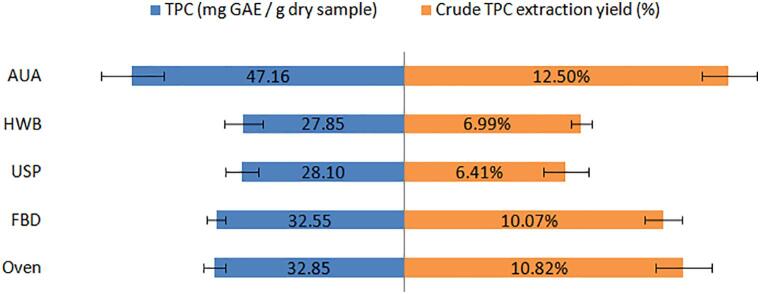
Crude extraction yields from 2 g of dried samples areshown in Fig. 7 for the five drying methods. The extraction yields for oven and FBD were similar, indicating fluidized bed drying would not affect the extraction yield. Due to the solid loss during the pre-treatments, HWB (6.99 ± 0.39%) and USP (6.41 ± 0.86%) only had 50% of yield in comparison with AUA. On the other hand, AUA dried samples hadthe highest yield (12.50 ± 1.03%). Yuan et al. [76] employed microwave-assisted extraction to extract antioxidant compounds from Ascophyllum nodosum. The extraction yield was enhanced, from 10.41% by conventional methanol extraction, to 12.46% by the novel extraction technology. In the present study, AUA achieved similar enhancement effect, increasing the extraction from 10.82% (for oven drying) to 12.50%, indicating AUA could improvethe extractability of phenolic compounds from seaweeds, although the objective of the irradiation of airborne ultrasoundwas not initially intended for extractionpurposes. The higher extraction yield for the samples dried by using AUA couldbe caused by ultrasonication generated cavitation and/or micro-channels, which increases the extractability of phenolic compounds by increasing the disruption of the seaweed cells structure.
Fig. 7.
TPC and crude extractionyieldforthefive drying methods Note: all data are the means from 3 replicates.
In the present study, TPC values varied from 27.85 to 47.16 GAE/g dried samples. This result was similar to those reported by Moreira et al. [69]. According to their study, 50 °C convective air-dried A. nodosum contained 31.8 ± 1.31 mg phloroglucinol equivalent /g of dry sample. However, TPC in this study was much higher than that reported by Sabrina et al. [77], where TPC was 12.1 ± 0.2 mg GAE/g oven-dried Irish brown seaweed at 40 °C for 24 h. The variation may be caused by the harvest season and geographical conditions [78].
As demonstrated in Fig. 7 oven and FBD dried samples had similar TPC (32.85 ± 2.10 and 32.55 ± 1.81 mg GAE/g dried sample, respectively). HWB (27.85 ± 3.2) and USP (28.10 ± 2.81) samples had the lowest TPC, as expected, mainly due to the solid loss as discussed above. TPC in AUA dried samples (47.16 ± 5.73 mg GAE/g dried sample) was significantly higher than other methods (P < 0.05). This may predominantly result from the much higher extraction yield as aforementioned. Other researchers also pointed out that airborne ultrasound could cause the change of microstructure and increase extractability of many bioactives such as total phenolic compounds and vitamins [63], [79]. Rodríguez et al. [80] reported airborne ultrasound could reduce TPC loss during convective drying of apple at low drying temperature (30 °C), in comparison with convective drying without ultrasound. It is to say, convective drying with airborne ultrasound resulted in higher TPC levels in the dried apple than convective drying without ultrasound. However, in their study, the effect of ultrasound depended on the drying temperature. A higher drying temperature (50 or 70 °C) caused alower TPC in apple dried with ultrasound.
3.5. Total viable count (TVC)
The total viable count was carried out for all samples to assess the microbial load. In fresh samples (before any washing or treatment) the highest microbial count of 2.89 log10 was observed. After washing and grinding, the microbial load showed a reduction of 0.35 log10. After the final processing steps (freezing, respective drying treatments) microbial load was reduced to below the detection limit (moulds were not tested in present study). Although air drying at low temperature (50 to 60 °C) alone is not an effective method to inactivite microbes, dehydration process may affect cellular components, induce DNA and RNA breakdown, protein denaturation and cell wall damage [81].
3.6. SEM analysis
SEM analysis was carried out to explore the effect of different drying methods from seaweed cell structure perspective. SEM observations of the seaweed samplesat different magnifications (500× and 2500×) are presented in Fig. 8 Ascophyllum nodosum often has epidermal shedding, which is devoid ofcell contents, removable from the thallus surface [82], [83]. The shedding covered the defrosted seaweed sample, as shown in Fig. 8a, while Fig. 8b-f exhibits clear cellular structure. In the oven-dried sample, as illustrated in Fig. 8c, there was still some detritus of the shedding left on the thallus surface, while in other samples dried by methods involving FBD, few detritus could be seen. This indicates that the shedding at the surface of the thallus would be removed under the drying conditions of FBD, which would be the high speed of blowing air in the dryer. In Fig. 8f, a few tiny fissures can be seen and shallow pits at the cellular structure in the HWB dried sample, which would be caused by solid lost during blanching. As in Fig. 8d-e, there existed a similar pattern in the larger magnifications, which was distinguished from the others. The samples for these two pictures were subjected to ultrasound, in which Fig. 8d is for power ultrasound pre-treatment in water, and Fig. 8e for irradiation by airborne ultrasound during drying. These patterns would be related to the effect of ultrasound, such as cavitation or sponge effect. Drying methods can affect samples cell structure and porosity significantly [84]. Cárcel et al. [85] and Ozuna et al. [86] reported airborne ultrasound-assisted convective drying could generate micro-channels in their apple samples, caused by “sponge effect”. However, in the present study, only surface SEM was performed, and the matrix structure could not be seen. In order to identify the effect of ultrasound, a further SEM should be conducted in future to observe the cross-section of the samples.
4. Conclusions
In this study, a quantitative analysis of the drying kinetics and the product quality indicators of dried Ascophyllum nodosum were performed for five novel drying methods. All methods involving FBD performed better than oven drying in drying time, quality control and energy consumption. Moreover, FBD had a better production yield than other methods with comparable drying time. AUA showed better retention of total phenolics in Ascophyllum nodosum. On the other hand, both pre-treatment technologies were superior in relation to energy and drying time, exhibiting more efficiency comparing with other methods. The mechanism of the drying methods should be investigated further by examining the microstructure of cross-sections oftreated samples. In order to explore and optimize ultrasound-assisted drying for practical applications, further research and development of this technology is required.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
The authors would like to acknowledge UCD-CSC Scholarship Scheme supported by University College Dublin (UCD) and China Scholarship Council (CSC) for this study.
References
- 1.Ferdouse F. Food and Agriculture Organization of the United Nations; 2018. The Global Status of Seaweed Production, Trade and Utilization. [Google Scholar]
- 2.Taboada C., Millán R., Míguez I. Composition, nutritional aspects and effect on serum parameters of marine algae Ulva rigida. J. Sci. Food Agric. 2010;90(3):445–449. doi: 10.1002/jsfa.3836. [DOI] [PubMed] [Google Scholar]
- 3.Ronan J.M. High proportions of inorganic arsenic in Laminaria digitata but not in Ascophyllum nodosum samples from Ireland. Chemosphere. 2017;186:17–23. doi: 10.1016/j.chemosphere.2017.07.076. [DOI] [PubMed] [Google Scholar]
- 4.Torres M., Kraan S., Domínguez H. Seaweed biorefinery. Rev. Environ. Sci. Bio/Technol. 2019;18(2):335–388. [Google Scholar]
- 5.Khan W. Seaweed extracts as biostimulants of plant growth and development. J. Plant Growth Regul. 2009;28(4):386–399. [Google Scholar]
- 6.Wang M. Responses of soil microbial communities to a short-term application of seaweed fertilizer revealed by deep amplicon sequencing. Appl. Soil Ecol. 2018;125:288–296. [Google Scholar]
- 7.Tujula N.A. Variability and abundance of the epiphytic bacterial community associated with a green marine Ulvacean alga. ISME J. 2010;4(2):301–311. doi: 10.1038/ismej.2009.107. [DOI] [PubMed] [Google Scholar]
- 8.Liu L. Towards a better understanding of medicinal uses of the brown seaweed Sargassum in Traditional Chinese Medicine: a phytochemical and pharmacological review. J. Ethnopharmacol. 2012;142(3):591–619. doi: 10.1016/j.jep.2012.05.046. [DOI] [PubMed] [Google Scholar]
- 9.Pal A., Kamthania M.C., Kumar A. Bioactive compounds and properties of seaweeds—a review. Open Access Library J. 2014;1(4):1–17. [Google Scholar]
- 10.Mohy El-Din S.M., El-Ahwany A.M. Bioactivity and phytochemical constituents of marine red seaweeds (Jania rubens, Corallina mediterranea and Pterocladia capillacea) J. Taibah Univ. Sci. 2016;10(4):471–484. [Google Scholar]
- 11.Barzkar N. Metabolites from marine microorganisms, micro, and macroalgae: immense scope for pharmacology. Mar. Drugs. 2019;17(8):464. doi: 10.3390/md17080464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pereira L., Gheda S.F., Ribeiro-Claro P.J. Analysis by vibrational spectroscopy of seaweed polysaccharides with potential use in food, pharmaceutical, and cosmetic industries. Int. J. Carbohydr. Chem. 2013;2013 [Google Scholar]
- 13.Herrmann C. Ensiling of seaweed for a seaweed biofuel industry. Bioresour. Technol. 2015;196:301–313. doi: 10.1016/j.biortech.2015.07.098. [DOI] [PubMed] [Google Scholar]
- 14.Helou A.M. Analysis of illicit drugs and pharmaceuticals in edible seaweeds by liquid chromatography-tandem mass spectrometry. Anal. Methods. 2018;10(38):4702–4710. [Google Scholar]
- 15.Gómez-Guzmán M. Potential role of seaweed polyphenols in cardiovascular-associated disorders. Mar. Drugs. 2018;16(8):250. doi: 10.3390/md16080250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu Y. Anti-oxidant and anti-inflammatory activities of ultrasonic-assistant extracted polyphenol-rich compounds from Sargassum muticum. J. Oceanol. Limnol. 2019;37(3):836–847. [Google Scholar]
- 17.Zhang J. Antidiabetic properties of polysaccharide-and polyphenolic-enriched fractions from the brown seaweed Ascophyllum nodosum. Can. J. Physiol. Pharmacol. 2007;85(11):1116–1123. doi: 10.1139/Y07-105. [DOI] [PubMed] [Google Scholar]
- 18.D.J. McHugh. Production and utilization of products from commercial seaweeds. 1987.
- 19.Bertness M.D. Sinauer Associates, Incorporated; 2014. Marine Community Ecology and Conservation. [Google Scholar]
- 20.Qu J.-H., Sun D.-W., Cheng J.-H., Pu H. Mapping moisture contents in grass carp (Ctenopharyngodon idella) slices under different freeze drying periods by Vis-NIR hyperspectral imaging. LWT-Food Sci. Technol. 2017;75:529–536. [Google Scholar]
- 21.Ma J., Sun D.-W., Qu J.-H., Pu H. Prediction of textural changes in grass carp fillets as affected by vacuum freeze drying using hyperspectral imaging based on integrated group wavelengths. LWT-Food Sci. Technol. 2017;82:377–385. [Google Scholar]
- 22.Ma J., Qu J.-H., Sun D.-W. Developing hyperspectral prediction model for investigating dehydrating and rehydrating mass changes of vacuum freeze dried grass carp fillets. Food Bioprod. Process. 2017;104:66–76. [Google Scholar]
- 23.Pu Y.-Y., Sun D.-W. Vis-NIR hyperspectral imaging in visualizing moisture distribution of mango slices during microwave-vacuum drying. Food Chem. 2015;188:271–278. doi: 10.1016/j.foodchem.2015.04.120. [DOI] [PubMed] [Google Scholar]
- 24.Mujumdar A.S. Handbook of Industrial Drying. CRC Press; 2006. Principles, classification, and selection of dryers; pp. 28–57. [Google Scholar]
- 25.Chan J.-C.-C., Cheung P.-C.-K., Ang P.O. Comparative studies on the effect of three drying methods on the nutritional composition of seaweed Sargassum hemiphyllum (Turn.) C. Ag. J. Agric. Food. Chem. 1997;45(8):3056–3059. [Google Scholar]
- 26.Milledge J.J., Harvey P.J. Ensilage and anaerobic digestion of Sargassum muticum. J. Appl. Phycol. 2016;28(5):3021–3030. [Google Scholar]
- 27.Walsh M., Watson L. Irish Sea Fisheries Board; 2011. A Market Analysis Towards the Further Development of Seaweed Aquaculture in Ireland; Part 1. [Google Scholar]
- 28.Wong K., Cheung P.C. Influence of drying treatment on three Sargassum species. J. Appl. Phycol. 2001;13(1):43–50. [Google Scholar]
- 29.Ling A.L.M. Effect of different drying techniques on the phytochemical content and antioxidant activity of Kappaphycus alvarezii. J. Appl. Phycol. 2015;27(4):1717–1723. [Google Scholar]
- 30.Cruces E. Comparison of different techniques for the preservation and extraction of phlorotannins in the kelp Lessonia spicata (Phaeophyceae): assays of DPPH, ORAC-PGR, and ORAC-FL as testing methods. J. Appl. Phycol. 2016;28(1):573–580. [Google Scholar]
- 31.Sivakumar R. Fluidized bed drying of some agro products–a review. Renew. Sustain. Energy Rev. 2016;61:280–301. [Google Scholar]
- 32.Mothibe K.J. Use of ultrasound pretreatment in drying of fruits: drying rates, quality attributes, and shelf life extension. Drying Technol. 2011;29(14):1611–1621. [Google Scholar]
- 33.Lee J.-S., Tham H.J., Wong C.S. Osmotic dehydration of Kappaphycus alvarezii. J. Appl. Phycol. 2014;26(2):1063–1070. [Google Scholar]
- 34.López-Hortas L. Alternative environmental friendly process for dehydration of edible Undaria pinnatifida brown seaweed by microwave hydrodiffusion and gravity. J. Food Eng. 2019;261:15–25. [Google Scholar]
- 35.Tian Y., Chen Z., Zhu Z., Sun D.-W. Effects of tissue pre-degassing followed by ultrasound-assisted freezing on freezing efficiency and quality attributes of radishes. Ultrason. Sonochem. 2020;67:105162. doi: 10.1016/j.ultsonch.2020.105162. [DOI] [PubMed] [Google Scholar]
- 36.Tian Y., Zhang P., Zhu Z., Sun D.-W. Development of a single/dual-frequency orthogonal ultrasound-assisted rapid freezing technique and its effects on quality attributes of frozen potatoes. J Food Eng. 2020;286:110112. [Google Scholar]
- 37.Pan Y., Zhang Y., Cheng J.H., Sun D.-W. Inactivation of listeria monocytogenes at various growth temperatures by ultrasound pretreatment and cold plasma. LWT-Food Sci. Technol. 2020;118:108635. [Google Scholar]
- 38.Zhang P., Zhu Z., Sun D.-W. Using power ultrasound to accelerate food freezing processes: effects on freezing efficiency and food microstructure. Crit. Rev. Food Sci. Nutr. 2018;58(16):2842–2853. doi: 10.1080/10408398.2018.1482528. [DOI] [PubMed] [Google Scholar]
- 39.Zheng L.Y., Sun D.-W. Innovative applications of power ultrasound during food freezing processes - A review. Trends Food Sci. Tech. 2006;17(1):16–23. [Google Scholar]
- 40.Sun D.-W., Li B. Microstructural change of potato tissues frozen by ultrasound-assisted immersion freezing. J. Food Eng. 2003;57(4):337–345. [Google Scholar]
- 41.Li B., Sun D.-W. Effect of power ultrasound on freezing rate during immersion freezing. J. Food Eng. 2002;55(3):277–282. [Google Scholar]
- 42.Fernandes F.A., Rodrigues S. Ultrasound as pre-treatment for drying of fruits: dehydration of banana. J. Food Eng. 2007;82(2):261–267. [Google Scholar]
- 43.Nowacka M. Drying of ultrasound pretreated apple and its selected physical properties. J. Food Eng. 2012;113(3):427–433. [Google Scholar]
- 44.Dehghannya J., Naghavi E.A., Ghanbarzadeh B. Frying of potato strips pretreated by ultrasound-assisted air-drying. J. Food Process. Preserv. 2016;40(4):583–592. [Google Scholar]
- 45.Ricce C. Ultrasound pre-treatment enhances the carrot drying and rehydration. Food Res. Int. 2016;89:701–708. doi: 10.1016/j.foodres.2016.09.030. [DOI] [PubMed] [Google Scholar]
- 46.Szadzińska J. Microwave-and ultrasound-assisted convective drying of raspberries: drying kinetics and microstructural changes. Drying Technol. 2019;37(1):1–12. [Google Scholar]
- 47.Oliveira F.I. Dehydration of Malay apple (Syzygium malaccense L.) using ultrasound as pre-treatment. Food Bioprocess Technol. 2011;4(4):610–615. [Google Scholar]
- 48.Rodríguez Ó. Application of power ultrasound on the convective drying of fruits and vegetables: effects on quality. J. Sci. Food Agric. 2018;98(5):1660–1673. doi: 10.1002/jsfa.8673. [DOI] [PubMed] [Google Scholar]
- 49.Žlabur J.Š. Effect of ultrasound pre-treatment and drying method on specialized metabolites of honeyberry fruits (Lonicera caerulea var. kamtschatica) Ultrason. Sonochem. 2019;56:372–377. doi: 10.1016/j.ultsonch.2019.04.034. [DOI] [PubMed] [Google Scholar]
- 50.Bagheri N., Dinani S.T. Investigation of ultrasound-assisted convective drying process on quality characteristics and drying kinetics of zucchini slices. Heat Mass Transf. 2019;55(8):2153–2163. [Google Scholar]
- 51.Fijalkowska A. Ultrasound as a pretreatment method to improve drying kinetics and sensory properties of dried apple. J. Food Process Eng. 2016;39(3):256–265. [Google Scholar]
- 52.Gamboa-Santos J. Air-borne ultrasound application in the convective drying of strawberry. J. Food Eng. 2014;128:132–139. [Google Scholar]
- 53.Kroehnke J. Ultrasound-and microwave-assisted convective drying of carrots–process kinetics and product’s quality analysis. Ultrason. Sonochem. 2018;48:249–258. doi: 10.1016/j.ultsonch.2018.05.040. [DOI] [PubMed] [Google Scholar]
- 54.Fernandes F.A., Rodrigues S. Application of ultrasound and ultrasound-assisted osmotic dehydration in drying of fruits. Drying Technol. 2008;26(12):1509–1516. [Google Scholar]
- 55.Kadam S.U., Tiwari B.K., O’Donnell C.P. Effect of ultrasound pre-treatment on the drying kinetics of brown seaweed Ascophyllum nodosum. Ultrason. Sonochem. 2015;23:302–307. doi: 10.1016/j.ultsonch.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 56.Rajauria G. Effect of hydrothermal processing on colour, antioxidant and free radical scavenging capacities of edible Irish brown seaweeds. Int. J. Food Sci. Technol. 2010;45(12):2485–2493. [Google Scholar]
- 57.Ainsworth E.A., Gillespie K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat. Protoc. 2007;2(4):875–877. doi: 10.1038/nprot.2007.102. [DOI] [PubMed] [Google Scholar]
- 58.Ganesan P., Kumar C.S., Bhaskar N. Antioxidant properties of methanol extract and its solvent fractions obtained from selected Indian red seaweeds. Bioresour. Technol. 2008;99(8):2717–2723. doi: 10.1016/j.biortech.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 59.ISO, E., 4833-1 (2013): Microbiology of the food chain-Horizontal method for the enumeration of microorganisms-Part 1: Colony count at 30 degrees C by the pour plate technique. International Organization for Standardization, Geneva, Switzerland, 2013.
- 60.Schmitz-Schug I., Kulozik U., Foerst P. Modeling spray drying of dairy products–impact of drying kinetics, reaction kinetics and spray drying conditions on lysine loss. Chem. Eng. Sci. 2016;141:315–329. [Google Scholar]
- 61.Horuz E. Drying kinetics of apricot halves in a microwave-hot air hybrid oven. Heat Mass Transf. 2017;53(6):2117–2127. [Google Scholar]
- 62.Beck S.M. Enhancement of convective drying by application of airborne ultrasound–a response surface approach. Ultrason. Sonochem. 2014;21(6):2144–2150. doi: 10.1016/j.ultsonch.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 63.Fan K., Zhang M., Mujumdar A.S. Application of airborne ultrasound in the convective drying of fruits and vegetables: a review. Ultrason. Sonochem. 2017;39:47–57. doi: 10.1016/j.ultsonch.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 64.Dibagar N. Deep bed rough rice air-drying assisted with airborne ultrasound set at 21 kHz frequency: a physicochemical investigation and optimization. Ultrason. Sonochem. 2019;53:25–43. doi: 10.1016/j.ultsonch.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 65.Del Rosario E.Z., Mateo W. Hot water blanching pre-treatments: enhancing drying of seaweeds (Kappaphycus alvarezii S.) Open Sci. J. 2019;4(1) [Google Scholar]
- 66.Djaeni M., Sari D.A. Low temperature seaweed drying using dehumidified air. Procedia Environ. Sci. 2015;23(2) [Google Scholar]
- 67.Chenlo F. Air-drying and rehydration characteristics of the brown seaweeds, Ascophylum nodosum and Undaria pinnatifida. J. Appl. Phycol. 2018;30(2):1259–1270. [Google Scholar]
- 68.Mirzaee E., Rafiee S., Keyhani A. Evaluation and selection of thin-layer models for drying kinetics of apricot (cv NASIRY) Agric. Eng. Int.: CIGR J. 2010;12(2):111–116. [Google Scholar]
- 69.Moreira R. Aqueous extracts of Ascophyllum nodosum obtained by ultrasound-assisted extraction: effects of drying temperature of seaweed on the properties of extracts. J. Appl. Phycol. 2017;29(6):3191–3200. [Google Scholar]
- 70.Barbosa-Cánovas G.V. Food & Agriculture Org; 2003. Handling and Preservation of Fruits and Vegetables by Combined Methods for Rural Areas: Technical Manual. [Google Scholar]
- 71.Barbosa-Cánovas G.V. John Wiley & Sons; 2008. Water Activity in Foods: Fundamentals and Applications. [Google Scholar]
- 72.Adekunte A. Effect of sonication on colour, ascorbic acid and yeast inactivation in tomato juice. Food Chem. 2010;122(3):500–507. [Google Scholar]
- 73.Kumar V., Fotedar R. Agar extraction process for Gracilaria cliftonii (.) Carbohydr. Polym. 2009;78(4):813–819. [Google Scholar]
- 74.Tekin Z.H. Dehydration of green beans using ultrasound-assisted vacuum drying as a novel technique: drying kinetics and quality parameters. J. Food Process. Preserv. 2017;41(6) [Google Scholar]
- 75.Lee K.T., Farid M., Nguang S.K. The mathematical modelling of the rehydration characteristics of fruits. J. Food Eng. 2006;72(1):16–23. [Google Scholar]
- 76.Yuan Y. Microwave assisted extraction of phenolic compounds from four economic brown macroalgae species and evaluation of their antioxidant activities and inhibitory effects on α-amylase, α-glucosidase, pancreatic lipase and tyrosinase. Food Res. Int. 2018;113:288–297. doi: 10.1016/j.foodres.2018.07.021. [DOI] [PubMed] [Google Scholar]
- 77.S. Cox, S. Gupta, N. Abu-Ghannam. Effect Of Different Rehydration Temperatures On The Moisture And Phytochemical Constituents Of Dried Edible Irish Brown Seaweed. 2012.
- 78.Stengel D.B., Connan S., Popper Z.A. Algal chemodiversity and bioactivity: sources of natural variability and implications for commercial application. Biotechnol. Adv. 2011;29(5):483–501. doi: 10.1016/j.biotechadv.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 79.Szadzińska J. The effect of high power airborne ultrasound and microwaves on convective drying effectiveness and quality of green pepper. Ultrason. Sonochem. 2017;34:531–539. doi: 10.1016/j.ultsonch.2016.06.030. [DOI] [PubMed] [Google Scholar]
- 80.Rodríguez Ó. Influence of power ultrasound application on drying kinetics of apple and its antioxidant and microstructural properties. J. Food Eng. 2014;129:21–29. [Google Scholar]
- 81.Bourdoux S. Performance of drying technologies to ensure microbial safety of dried fruits and vegetables. Compr. Rev. Food Sci. Food Saf. 2016;15(6):1056–1066. doi: 10.1111/1541-4337.12224. [DOI] [PubMed] [Google Scholar]
- 82.Halat L. Epidermal shedding in Ascophyllum nodosum (Phaeophyceae): seasonality, productivity and relationship to harvesting. Phycologia. 2015;54(6):599–608. [Google Scholar]
- 83.Garbary D.J. Cell division in the absence of mitosis: the unusual case of the fucoid Ascophyllum nodosum (L.) Le Jolis (Phaeophyceae) Algae. 2009;24(4):239–248. [Google Scholar]
- 84.Krokida M.K., Maroulis Z.B. Structural properties of dehydrated products during rehydration. Int. J. Food Sci. Technol. 2001;36(5):529–538. [Google Scholar]
- 85.Cárcel J. Food process innovation through new technologies: use of ultrasound. J. Food Eng. 2012;110(2):200–207. [Google Scholar]
- 86.Ozuna C. Influence of material structure on air-borne ultrasonic application in drying. Ultrason. Sonochem. 2014;21(3):1235–1243. doi: 10.1016/j.ultsonch.2013.12.015. [DOI] [PubMed] [Google Scholar]