Highlights
-
•
Novel ultrasound-assisted basic electrolyzed water (BEW) extraction was established.
-
•
Ultrasound-assisted BEW successfully extracted marine Antarctic krill proteins.
-
•
Ultrasound-assisted BEW protected the active groups of proteins from oxidation.
-
•
Ultrasound-assisted BEW improved structural properties of Antarctic krill proteins.
-
•
Krill proteins-based emulsions were fabricated with superior long-term stability.
Keywords: Ultrasound, Basic electrolyzed water, Antarctic krill protein, Structural properties, Emulsions
Abstract
A novel protein extraction method of ultrasound-assisted basic electrolyzed water (BEW) was proposed, and its effects on the structural and functional properties of Antarctic krill proteins were investigated. Results showed that BEW reduced 30.9% (w/w) NaOH consumption for the extraction of krill proteins, and its negative redox potential (−800 ~ −900 mV) protected the active groups (carbonyl, free sulfhydryl, etc.) of the proteins from oxidation compared to deionized water (DW). Moreover, the ultrasound-assisted BEW increased the extraction yield (9.4%), improved the solubility (8.5%), reduced the particle size (57 nm), favored the transition of α-helix and β-turn to β-sheet, promoted the surface hydrophobicity and disulfide bonds formation of krill proteins when compared to BEW without ultrasound. These changes contributed to the enhanced foam capacity, foam stability and emulsifying capacity of the krill proteins. Notably, all the physicochemical, structural and functional properties of the krill proteins were comparable to those extracted by the traditional ultrasound-assisted DW. This study suggests that the ultrasound-assisted BEW can be a potential candidate to extract proteins, especially offering an alternative way to produce marine proteins with high nutritional quality.
1. Introduction
Antarctic krill is one of the most abundant biomass species in Southern Ocean, which is estimated at about 500 million metric tons [1]. Whole Antarctic krill contains 11.9–15.4% protein, 3% ash, 2% carbohydrates, 0.5–3.6% lipid and 77.9–83.1% water [2]. Thereinto, krill proteins have high nutritional values and even their biological values are higher than those of other meat and milk proteins, because it contains adequate levels of all essential amino acids [3], [4]. However, a great amount of krill is now used to produce low-value commodities such as animal and fish feed [5]. Recently, protein-based emulsions have received enormous attention, due to that they are promising in pharmaceutical, cosmetics and food fields. Therefore, a high value-added utilization of krill proteins for developing emulsions will provide sustainable marine proteins resource with high nutritional values to human diet.
At present, the frequently-used method for extracting plant or animal proteins is alkaline extraction [6], [7]. During the whole process, a large amount of alkaline solution (NaOH, KOH, etc.) will be consumed to adjust the pH of mixture reaching the target pH value, leading to a high economic investment. On this basis, basic electrolyzed water (BEW) is a promising reagent to be a candidate for traditional alkaline solution owing to its aqueous alkali nature. At present, BEW is the by-product of acidic electrolyzed water (AEW) which has a wide range of applications in food, agriculture, aquaculture, livestock industries [8], [9]. Additionally, BEW also possesses excellent functional characteristics, including typically higher pH > 12.0 and redox potential (ORP, −800 to −900 mV) [10], [11]. More importantly, BEW has a broad-spectrum ability of anti-bacteria and enzyme inactivation, which can effectively prevent the products from degradation [11], [12]. Furthermore, its negative redox potential can protect the active groups of proteins from oxidation and improve the quality of end products [10], [13]. Therefore, there is an urgent need for the development of BEW in food industry.
Ultrasound assisted extraction (UAE) is an effective extraction method of using ultrasonic waves as a pretreatment to extract proteins including rapeseed protein [12], rice bran [14], chicken egg shell membrane [15] and porcine placenta [16]. UAE treatment greatly improved the processing quality of food proteins. For example, the ultrasound treatment partially unfolded three-dimensional structure of proteins leading to the exposure of sulfhydryl group and hydrophobic groups [17], [18], which finally enhances the emulsifying properties of these proteins. Furthermore, such structural changes increase the protein flexibility, reduce the interfacial tension and strengthen the formation of protein membrane at the oil/water interface. However, the ultrasound treatment often induces the active substances or functional groups to generate highly reactive free radicals under extreme conditions of cavity collapse, which causes the oxidation of protein or lipid [19]. Taking above advantages of BEW into consideration, its negative redox potential can certainly reduce the oxidation or damage of protein or lipid caused by ultrasound.
Up to now, there are no reports of using BEW to extract marine animal proteins in combination with ultrasound. On this basis, this study aimed to extract the Antarctic krill proteins by the UAE and BEW treatment, and then evaluate their structural and functional properties. Notably, the effects of the ultrasound treatment (0, 10, 20, 30 min) on the structural changes of krill proteins were investigated from the viewpoint of the morphology, particle size, secondary structure, tertiary structure, surface hydrophobicity, etc. The functional properties of the extracted proteins were explored by determining their foaming capacity, foam stability, water/oil absorption capacity, emulsifying capability, etc. The generated knowledge will provide a new and effective extraction method of ultrasound-assisted BEW to produce various proteins, which facilitates the exploitation of natural protein resources with high quality.
2. Materials and methods
2.1. Materials
Antarctic krill (Euphausia superba) were purchased from Dalian Ocean Fishery Group Ltd. (Dalian, China). N-hexane and sodium azide were bought from Sigma-Aldrich, Inc. (St. Louis, MO, USA). All reagents were analytical grade and bought from Sangon Biotech Co., Ltd (Shanghai, China).
2.2. Preparation of basic electrolytic water
Basic electrolytic water (BEW) was prepared by our previous method [20]. BEW were produced by electrolyzing sodium chloride (0.5%, 1%, 1.5% NaCl, w/v) solution using electrolyzed water generator (FW-200, AMANO, Japan). A pH/ORP meter (Mettler-Toledo, Zurich, Switzerland) was used to measure pH (pH = 12.3 ± 0.12) and oxidation reduction potential (ORP = −850 ± 13.6 mV).
2.3. Extraction and analysis of Antarctic krill proteins
2.3.1. Extraction of proteins
Antarctic krill proteins were extracted by ultrasound-assisted BEW (produced by electrolyzing 0.5% sodium chloride) solution and ultrasound-assisted deionized water (DW). The procedure of ultrasound-assisted BEW was as follows: 20 g Antarctic krill flesh were mixed with BEW (1:10, w/v), and then the mixture was homogenized for 1 min at 800 rpm. The pH of the resulting slurry was adjusted to pH 12 by 1 M NaOH. For the ultrasound-assisted DW, the extraction procedure was the same to above one except replacing BEW with DW. Afterwards, 200 mL of resulting slurry was left in the beaker (diameter: 65 mm) with ice-water bath, and then treated by an ultrasound processor (NingBo Scientz Biotechnology Co. Ltd., Ningbo, China) with a 15 mm diameter titanium probe at 20 kHz (350W, 70% amplitude) for 0, 10, 20 and 30 min (pulse duration of on-time 6 s and off-time 3 s). And the ultrasound probe was submerged in a depth of 10 mm in the samples.
Subsequently, the mixture was stirred for 1 h at 25 °C. The mixture was centrifuged at 8000g for 10 min, and then the pH of supernatant was adjusted to 4.5 by 3 M HCl [6]. The precipitated proteins were washed by 3-stage countercurrent method [7], and then quick-frozen in liquid nitrogen. Afterwards, the protein samples were freeze-dried in a vacuum dryer (−76 °C, 1.3 × 10−2 mbar) to remove water [21]. The protein content was determined by combustion with a nitrogen analyzer (FP-428, Leco Corporation, USA).
2.3.2. Protein extraction yield and purity
The purity of krill proteins was determined by Kjeldahl method, and 6.25 was used as Kjeldahl nitrogen-to-protein conversion factor [7]. Protein yield of ultrasound-assisted BEW or DW were calculated by Eq. (1):
| (1) |
where W, P, V and C are the weight of dried protein, the protein purity, the solution amount (mL) for extraction and the protein content of extract, respectively [22].
2.3.3. Protein solubility
Protein solubility was determined according to the method of Anon et al. [21] with slight modification. Briefly, 0.1 g krill proteins were dissolved in sodium phosphate buffer (pH 7.0), and then centrifuged at 8,000g for 15 min (4 °C). The concentration of supernatant protein was measured by Coomassie brilliant blue method using BSA as a standard. Protein solubility was expressed as the mass percentage of supernatant proteins and total proteins before centrifugation. All determinations were conducted in triplicate.
2.3.4. Protein carbonyl content
The protein carbonyl content was determined using the 2,4-dinitrophenyl hydrazine (DNPH) as described by Wang et al. [23]. Briefly, 1 mL protein solution (2 mg/mL) was mixed with 3 mL of 10 mM 2,4-dinitrophenylhydrazine (DNPH) and incubated at 25 °C for 2 h. DNPH-reacted samples after 20% trichloroacetic acid (TCA) precipitation were collected by centrifugation at 10,000g for 15 min (4 °C), and then the unreacted DNPH was removed using ethanol/ethyl acetate solution (1:1, v/v). The resulting pellets were dissolved in 3 mL of 6 M guanidine hydrochloride. The absorbance (370 nm) of samples was determined using an UV–vis spectrophotometer (U-3900, Hitachi Corportion, Tokyo, Japan) and carbonyl content was expressed as nmol/mg protein using an absorption coefficient of 22,000 M−1 cm−1 [24].
2.3.5. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE)
SDS-PAGE was performed based on the method of Wang et al. [25] and Chen et al. [26]. SDS-PAGE was performed at 25 °C with 12% separating gel and 5% stacking gel. 20 μL protein solutions (2 mg/mL) were mixed with 5 μL loading buffer (0.0625 M Tris-HCl, 12% glycerin, 2% sodium dodecyl sulfate (SDS), 5% 2-mercaptoethanol, and 0.0025% bromophenol blue), and then heated at 100 °C for 10 min. Aliquots (10 μL) of the samples were loaded into the gels. The gels were stained with Coomassie Brilliant Blue-R250.
2.3.6. Atomic force microscopy (AFM) and particle size determination
Proteins (50 µg/mL) were dissolved in sodium phosphate buffer (pH 12.0), and then aliquots (10 µL) of the samples were dropped on a fresh mica surface, air-dried in a fume hood. Samples were scanned in tapping mode with the scan rate and step of 1.0 Hz and 2 µm, respectively. AFM images were analyzed by using NanoScope Analysis 1.8 (Bruker Co., Karlsruhe, Germany) [27].
The particle sizes of protein were measured by Zetasizer Nano ZS90 (Malvern Instruments, Worcestershire, UK). The determination was performed in a quartz cuvette with a 1.1 mL aliquot solution (0.3 cm/ml) with a light path of 1 cm [25].
2.3.7. Scanning electron microscopy (SEM)
The microstructure of dried Antarctic krill proteins was observed with a SEM (Carl Zesiss Evo18, Zesiss, Germany) at an accelerating voltage of 30 kV. Before measurement, the dried samples were coated with gold using an ion sputter coater.
2.4. Structural characterization
2.4.1. Secondary structure analysis
FT-IR analysis of krill proteins was performed by the method of Wang et al. [28]. The protein powder was mixed with potassium bromide at a ratio of 1:100. Then, a total of 64 scans from 500 to 4000 cm−1 against the background were taken at a resolution of 4 cm−1. Before further analysis, the 7-point Savitsky-Golay function was used to smooth the spectrum to reduce signal noise. Afterwards, PeakFit software 4.12 (SPSS Inc., Chicago, IL, USA) was used to analyze secondary structure components from the amide I band (1600–1700 cm−1), and peak area to different secondary structures was calculated.
2.4.2. Fluorescence measurement
The fluorescence spectra of protein samples were obtained using a fluorescence spectrophotometer (Hitachi F7100 Co., Japan) by the method of Jiang et al. [29] with some modifications. Protein solutions (0.2 mg/mL) were prepared, and then the excitation wavelength was 280 nm, and the emission spectra were recorded from 290 nm to 450 nm using a 4 nm slit. Each sample was repeated for five times.
2.4.3. Surface hydrophobicity (H0-ANS) measurements
H0-ANS was determined using 1-anilino-8-naphthalene-sulfonate (ANS) as a fluorescence probe according to the method of Kato et al. [30] and Haskard et al. [31]. 20 mL of ANS solution (8.0 mM in 0.01 M phosphate buffer, pH 12.0) was added to serial dilutions of samples containing 25–800 μg/mL target proteins. The fluorescence intensity at 364 nm (excitation) and 475 nm (emission) was measured (Hitachi F7100 Co., Japan). The initial slope of fluorescence intensity versus protein concentration was calculated as surface hydrophobicity (H0-ANS). All determinations were performed in triplicate.
2.4.4. Sulfhydryl contents
The free sulfhydryl (SH) of the proteins were determined using Ellman’s reagent DTNB. Protein samples (2 mg/ml) were prepared in buffer (0.086 M Trise, 0.09 M glycine, 4 mM Na2EDTA, pH = 8) to determine free SH. The mixtures were incubated at 25 °C for 24 h in a shaking water bath, and then centrifuged at 12,000g at 4 °C for 15 min. 30 µL of Ellman’s reagent solution (4 mg/mL of DTNB) was added to 3 mL mixtures. The solution was rapidly mixed and allowed to stand at 25 °C for 15 min, and then the absorbance was read at 412 nm, and the buffer was used as a reagent blank. The absorbance measurements were converted to free SH values using a calibration curve with reduced glutathione. All determinations were conducted in triplicate.
2.5. Functional properties
2.5.1. Determination of foaming capacity and foam stability
Foaming capacity (FC) and foam stability (FS) were measured according to the method of Timilsena et al. [32]. 1 g protein powder was dispersed in 20 mL of Milli-Q water. Foam was formed using shear emulsifying machine (AD500S-H, Suotn, Inc., Shanghai, China) at 10,000 rpm for 1 min. Total volumes were recorded before and after whipping and FC was calculated using Eq. (2). Total volume of the foam after 30 min formation were recorded and (FS) was evaluated using Eq. (3).
| (2) |
| (3) |
where V1 (mL) is the initial volume of protein solution, V2 (mL) is the volume of solution plus the foam and V3 is the volume after 30 min of foam formation.
2.5.2. Water absorption capacity (WAC) and oil absorption capacity (OAC)
The WAC was measured according to the method of Resendiz-Vazquez et al. [33]. The samples (0.5 g) were rehydrated with 10 mL distilled water in centrifuge tubes (15 mL) and dispersed for 30 s. The dispersion was allowed to stand at 25 °C for 15 min and then centrifuged at 2000g for 10 min. The supernatant was decanted, and the residue was weighed together with the centrifuge tube. The WAC was expressed as absorbed water (g)/protein (g). The OAC was determined by the same procedure for WAC by replacing water with Sunflower seed oil.
2.6. Emulsion preparation
Oil in water emulsions (O/W) were prepared according to Zhao et al. [34] with slight modifications. Briefly, protein solutions (2% w/v) were mixed with sunflower seed oil at oil–water ratio of 3:7 (Total volume = 50 mL), and sodium azide (0.02%, wt %) was added to suppress microbial growth. The shear emulsifying machine was used to homogenize the mixtures at 16,000 rpm for 3 min. All experiments were performed in ice-bath condition.
2.7. Creaming index (CI)
Freshly prepared emulsions were transferred into 1 mL glass tubes and stored at 4 °C for 30 d. The creaming index (CI) was calculated by the Eq. (4) [35]
| (4) |
where the Hc is the height (mm) of the cream layer (Hc) after 30 d storage; Ht is the total height (mm) of the emulsion at 0 d.
2.8. Droplet size
The size of emulsion droplet was measured by Mastersizer 3000 (Malvern Instruments, UK) according to the study [36]. The samples were diluted with deionized water. The refractive indices of water and sunflower seed oil were taken as 1.33 and 1.47, respectively. The droplet size d4,3 of the emulsions was calculated using Eq. (5)
(5)where ni is the number of droplets with diameter di, and d4,3 represents the volume-average droplet sizes of the emulsions in water dispersion.
2.9. Rheology of emulsions
The dynamic oscillatory measurements of emulsions were determined with the frequency ranging from 1 to 10 rad/s by a controlled stress rheometer (DSR200, Rheometric Scientific, USA). A parallel plate geometry (40 mm diameter) was employed, and the gap of two parallel plates was 1.0 mm. To determine the linear viscoelastic region, oscillation strain sweep was carried out ranging from 0.01 to 10% (frequency, 1 Hz) at 25 °C. Frequency sweeps (from 0.1 to 10 Hz) test was performed at a fixed strain amplitude of 0.1%. The elastic moduli (G′), loss moduli (G″) and loss tangent (tan δ) as a function of the frequency were recorded [37]. All the experiments were conducted in three replicates.
2.10. Statistical analysis
The experimental data were expressed as the mean ± standard deviation. One-way analysis of variance (ANOVA) was used to compare the value differences (p < 0.05) using SPSS 19.0 (SPSS Inc., Chicago, IL).
3. Results and discussion
3.1. Extraction of Antarctic krill proteins using ultrasound-assisted BEW and DW
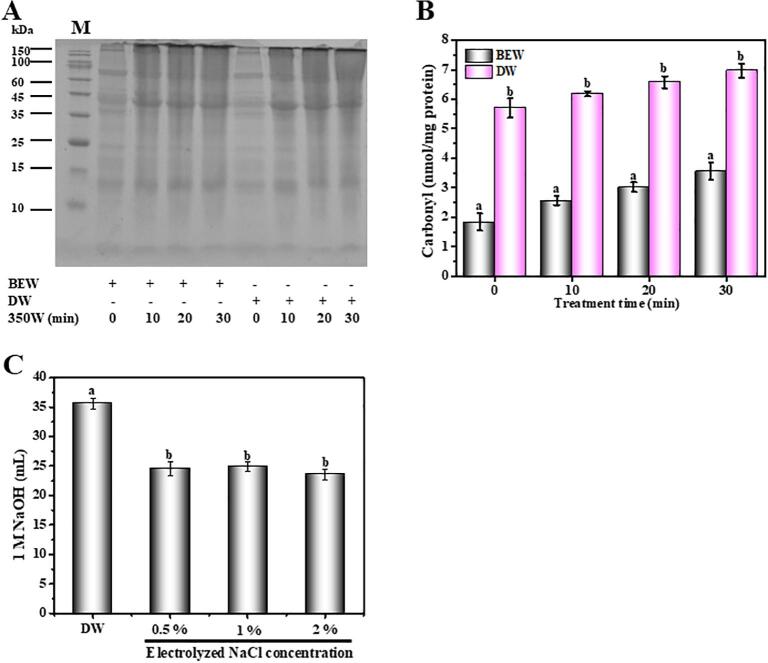
As shown in SDS-PAGE profiles (Fig. 1A), no significant difference was obverted in the composition of krill proteins extracted by the ultrasound-assisted BEW or DW, preliminarily suggesting that the ultrasound-assisted BEW was an effective method for the extraction of krill proteins. It was reported that the carbonyl group of protein was widely considered as an indicator for protein oxidation [38]. In this study, the carbonyl content of krill proteins obviously increased regardless of ultrasound-assisted BEW or DW with the ultrasound from 0 min to 30 min, which was attributed to the oxidation of active groups of amino acids driven by ultrasound treatment [39]. However, the ultrasound-assisted BEW treatment presented a significantly lower carbonyl content compared to the ultrasound-assisted DW (Fig. 1B), which was predominantly attributed to the antioxidant capacity of higher redox potential (−800 to −900 mV). The result was also supported by Athayde et al. [13], reporting that BEW had the antioxidant capacity due to its redox potential. Therefore, the ultrasound-assisted BEW could certainly maintain the nutritional values of natural proteins. In addition, the ultrasound-assisted BEW (electrolyzing 0.5% NaCl solution) reduced 30.9% NaOH consumption compared to DW (Fig. 1C), and no significant changes in NaOH consumption was further observed with the increase of electrolyzed NaCl concentration (1%, 1.5%, w/v). Therefore, 0.5% NaCl solution was used to produce BEW for the subsequent experiments.
Fig. 1.
Effects of ultrasound-assisted BEW or DW on the krill proteins composition (A), the carbonyl content (B) and the consumption volume of 1 M NaOH (C). Different letters (a, b, c, etc) indicate significant differences at p < 0.05.
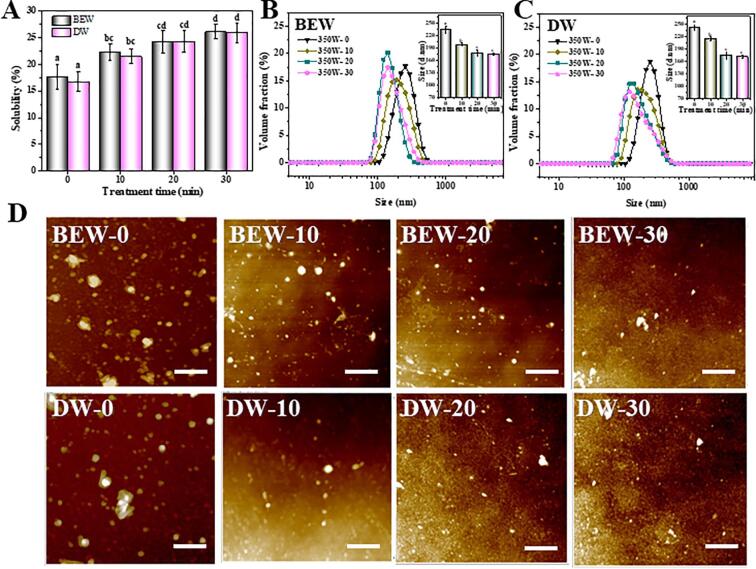
The yield and purity of krill proteins extracted by the ultrasound-assisted BEW or DW are showed in Table 1. Overall speaking, the ultrasound-assisted BEW increased the extraction yield and purity of krill proteins compared to individual BEW treatment, with their values reaching maximum 9.38% and 1.66%. Moreover, no significant difference was determined in these values between ultrasound-assisted BEW and ultrasound-assisted DW (Table 1). Additionally, the solubility of krill proteins after the ultrasound-assisted BEW and DW treatment also presented similar change trends (Fig. 2A). In detail, the ultrasound-assisted BEW significantly improved the solubility of krill proteins by 8.5%, and no significant difference was observed compared to the ultrasound-assisted DW. The protein solubility was increased because of the ultrasound cavitation-induced disruption of intermolecular hydrogen bonds of proteins, causing the disassociation of larger protein aggregates into soluble smaller aggregates [40]. In addition, the ultrasonic energy could also partially hydrolyze high molecular biopolymers [15].
Table 1.
Extraction yield and purity of krill protein. Different letters (a, b, c, etc) indicate significant differences at p < 0.05.
| Treatment (min) | Extraction yield (%) | Purity (%) |
|---|---|---|
| BEW BEW-10 | 68.03 ± 3.14a 72.88 ± 1.54b | 78.37 ± 2.56 80.03 ± 2.26 |
| BEW-20 | 77.44 ± 1.78c | 79.23 ± 2.55 |
| BEW-30 | 77.41 ± 2.39c | 79.47 ± 3.38 |
| DW | 68.79 ± 1.67a | 78.90 ± 2.42 |
| DW-10 | 73.20 ± 1.66b | 78.81 ± 2.34 |
| DW-20 | 77.87 ± 1.48c | 79.77 ± 3.17 |
| DW-30 | 77.66 ± 1.65c | 79.23 ± 2.51 |
Fig. 2.
Effects of ultrasound-assisted BEW or DW on the solubility (A), particle size (B and C) and microstructures (D) of krill proteins. 350W-0, 350W-10, 350W-20 and 350W-30 represent 350W ultrasound for 0, 10, 20 and 30 min; BEW-0, BEW-10, BEW-20 and BEW-30 represent 0.5% NaCl electrolyzed BEW treatment for 0, 10, 20 and 30 min; DW-0, DW-10, DW-20 and DW-30 represent DW treatment for 0, 10, 20 and 30 min. Scale bar = 600 nm. Different letters (a, b, c, etc) indicate significant differences at p < 0.05.
The effects of ultrasound-assisted BEW or DW on the particle size of krill proteins were examined by DLS analysis. In Fig. 2B, the particle sizes of proteins were significantly (p < 0.05) reduced from 234.4 nm to 178.3 nm with the ultrasound treatment from 0 min to 20 min, but no significant decrease (175.4 nm) was determined with the prolonging of ultrasound to 30 min. Such phenomenon caused by ultrasound has been reported in previous studies [41], [42]. The particle size of krill proteins extracted by the ultrasound-assisted DW showed similar results with the ultrasound-assisted BEW, and no significant difference was monitored (Fig. 2C). Meanwhile, the morphological details of krill proteins were also investigated by AFM. In Fig. 2D, massive aggregates were observed in the samples without ultrasound treatment. However, these aggregates disassociated after the ultrasound treatment, and the disassociation degree was enhanced with the increase of ultrasound time. All these facts visually showed that the ultrasound treatment indeed disassociated the protein aggregates, which supported the results in Fig. 2A. Furthermore, the smaller particle size also contributed to the increased protein solubility, because of its larger surface area and greater charge strengthening the protein-water interactions [43].
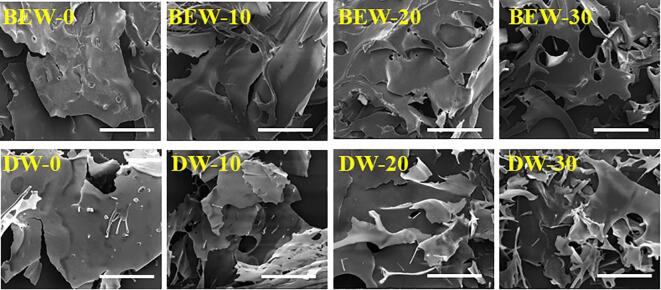
The microstructures of krill proteins treated by the ultrasound-assisted BEW or DW was observed using SEM (Fig. 3). Compared to the samples without ultrasound treatment, the ultrasound-treated samples exhibited more disordered structure and irregular fragments, suggesting that the ultrasound treatment greatly disrupted the natural distribution and arrangement of krill proteins. These were mainly generated by the cavitation forces exerted by the probe and microstreaming as well as turbulent forces during the ultrasonic treatment [29]. Based on these results, it could be concluded that the components and critical physicochemical properties of krill proteins extracted by the ultrasound-assisted BEW were comparable to those extracted by the ultrasound-assisted DW.
Fig. 3.
Effects of ultrasound-assisted BEW or DW on the morphology of krill proteins. BEW-0, BEW-10, BEW-20 and BEW-30 represent 0.5% NaCl electrolyzed BEW treatment for 0, 10, 20 and 30 min; DW-0, DW-10, DW-20 and DW-30 represent DW treatment for 0, 10, 20 and 30 min. Scale bar = 200 μm.
3.2. Structural properties of the extracted Antarctic krill proteins
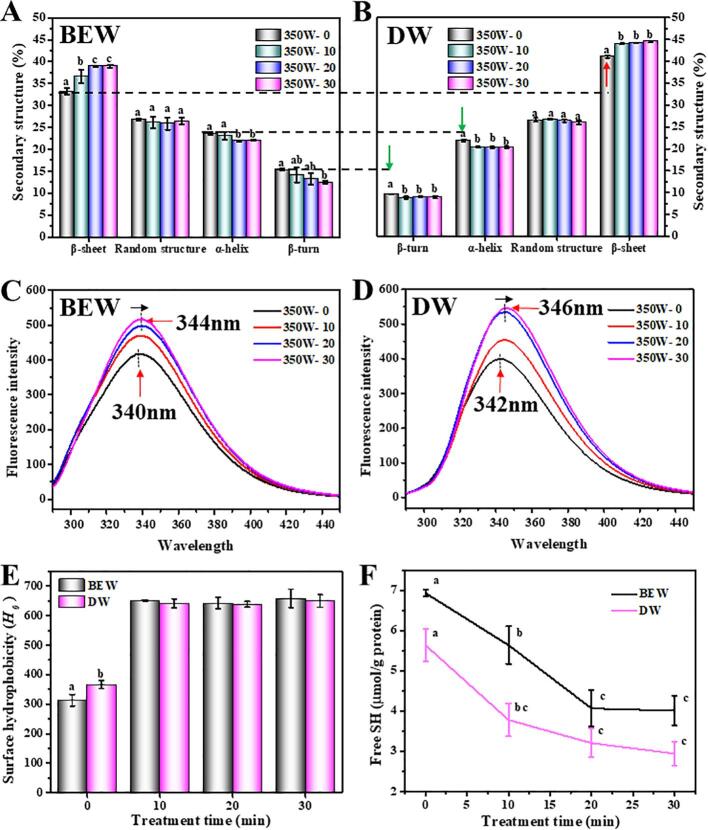
The structural characterization of Antarctic krill proteins extracted by the ultrasound-assisted BEW or DW were studied by FT-IR and fluorescence spectra. Fig. 4A shows the proportion of secondary structures of krill proteins extracted by the ultrasound-assisted BEW. In particular, the β-sheet conformation was found to dominate in the proteins followed by random coil, α-helix and β-turn. It was reported that the secondary structures of a protein were highly related to its emulsifying properties [44]. In this study, increasing the duration of ultrasound significantly elevated the β-sheet from 33.2% to 38.98%, but reduced the α-helix (from 23.6% to 22.1%) and β-turn (from 15.4% to 12.5%), suggesting that the ultrasound treatment favored the transition of α-helix and β-turn to β-sheet. The conformation of α-helix was mainly stabilized by intramolecular hydrogen bonds, and the disruption of hydrogen bonds would increase the flexibility of protein structures [45]. Overall speaking, the ultrasound-assisted DW (Fig. 4B) also presented the similar results in the secondary structures with the ultrasound-assisted BEW. However, the β-sheet of krill proteins treated by the ultrasound-assisted DW was obviously higher than that treated by the ultrasound-assisted BEW, conversely, the α-helix and β-turn were significantly lower. Such structural difference was mainly attributed to the protective function of BEW against the oxidation of functional groups (Fig. 1B), because Sun et al. [46] and Zhao et al. [38] proved that the oxidation treatment significantly increased the β-sheet and decreased the α-helix and β-turn of myofibrillar proteins and soybean proteins.
Fig. 4.
Effects of ultrasound-assisted BEW or DW on the secondary structures (A and B), tertiary structure (C and D), surface hydrophobicity (E), free sulfhydryl groups (F) of krill proteins. 350W-0, 350W-10, 350W-20 and 350W-30 represent 350W ultrasound for 0, 10, 20 and 30 min. Different letters (a, b, c, etc) indicate significant differences at p < 0.05.
Fluorescence spectroscopy can probe the microenvironment of the tryptophan (Trp) residues in proteins and can be used for studying protein folding/unfolding [25]. In Fig. 4C, the wavelength emission maxima (λmax) of proteins extracted by the ultrasound-assisted BEW had a red shift ranging from 340 nm to 344 nm with the increase of ultrasound time. Likewise, the λmax of proteins extracted by the ultrasound-assisted DW also displayed a red shift from 342 nm to 346 nm (Fig. 4D). A red shift in the λmax indicated that the tertiary conformation of protein molecules was opened, and more aromatic amino acids (Trp residues) were exposed to the polar environment, which was an indicator of protein unfolding [47]. In addition, ANS binds to the exposed hydrophobic patches in partially unfolded protein, and the increase in surface hydrophobicity (H0) is accompanied by an increase in the relative fluorescence intensity. In Fig. 4E, the ultrasound treatment obviously increased the surface hydrophobicity of krill proteins through the mechanical and cavitation effects generated by ultrasound. The ultrasonic cavitation induced a high degree of protein unfolding, causing the exposure of hydrophobic groups and regions to a more polar surrounding environment [48]. Additionally, Estévez [49] reported that the oxidation of proteins also led to partial unfolding of the structures, exposing the certain groups which were originally located inside proteins. All these factors collectively contributed to the red shift of λmax and the increased H0 of krill proteins. In addition, the exposure of functional groups (e.g. hydrophobic groups) in side chains caused the reduction of intramolecular hydrogen bonds[46], which might be the reason that the α-helix and β-turn decreased while β-sheet increased (Fig. 4A and B).
In Fig. 4F, the free sulfhydryl groups of proteins extracted by the ultrasound-assisted BEW decreased from 6.9 to 4.0 μmol/g protein with the increase of ultrasound time. Likewise, the free sulfhydryl groups of proteins treated by the ultrasound-assisted DW also exhibited a decreased trend, ranging from 5.6 to 2.9 μmol/g protein. Similar results have been reported in the ultrasound-treated soybean proteins [50] and squid mantle proteins [51]. Notably, the high-intensity ultrasound treatment markedly decreased the sulfhydryl groups of soybean proteins, and the cavitation-generated hydrogen peroxide was considered as the dominant factor to oxidize the free sulfhydryl groups [52].
However, it could be seen that the free sulfhydryl groups of proteins treated by the ultrasound-assisted BEW were obviously higher that those treated by the ultrasound-assisted DW. This phenomenon was certainly caused by the negative redox potential (−800 ~ −900 mV) of BEW, because it protected the free sulfhydryl groups from being oxidized by reactive free radicals. However, Xiong et al. [53] and Hu et al. [17] pointed out that the ultrasound treatment increased the content of sulfhydryl groups. The discrepancy in these results might depend on the difference in the ultrasound intensity, protein species, etc [53].
3.3. Functional properties of the extracted Antarctic krill proteins
The effects of ultrasound treatment on the foam capacity (FC) and foam stability (FS) of Antarctic krill proteins are shown in Fig. 5A. The FC of proteins extracted by the ultrasound-assisted BEW was significantly increased (p < 0.05) from 142.4% to 163.7% with the ultrasound from 0 to 30 min. The highest FC value was determined under 30 min ultrasound treatment. Meanwhile, the FS was also significantly (p < 0.05) increased from 67.2% to 72.7% under the same treatment (Fig. 5A). For the ultrasound-assisted DW, the FC and FS of krill proteins showed the similar trend with the ultrasound-assisted BEW treatment. Amir et al. [54] reported that the good formation capability of foam was greatly contributed by the reduced particle size, increased surface hydrophobicity, unfolded protein chains, exposed hydrophobic groups at the interface, etc. In this study, the ultrasound treatment unfolded and expanded the structures (Fig. 4C and D), reduced the particle size (Fig. 2C and D) and increased the hydrophobicity (Fig. 4E) of krill proteins, which would facilitate the diffusion of proteins at the interface and finally improve the FC. Meanwhile, the homogenization effect of ultrasound made the protein molecules more uniformly dispersed in solution, resulting in the increase of FS [55]. Similar results were previously reported that the FC and FS of pea protein isolates and jackfruit seed protein isolates were significantly increased by ultrasound treatment [33], [56].
Fig. 5.
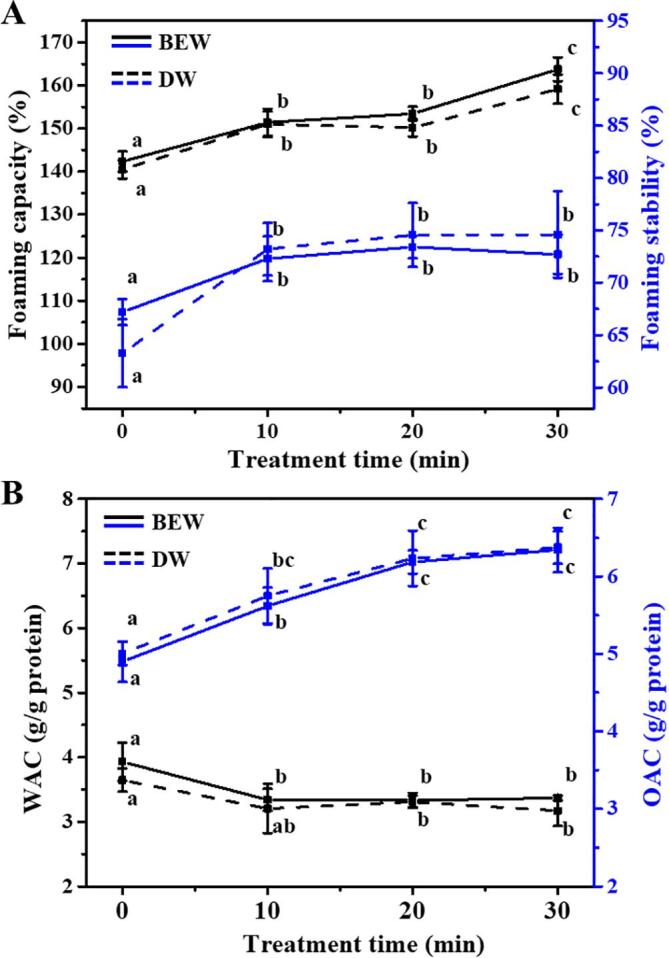
Effects of ultrasound-assisted BEW or DW on the foaming capacity (FC), foaming stability (FS), water absorption capacity (WAC) and oil absorption capacity (OAC) of krill proteins treated by different ultrasound time (0, 10, 20, 30 min). Different letters (a, b, c, etc) indicate significant differences at p < 0.05.
3.4. Water absorption capacity (WAC) and oil absorption capacity (OAC)
The interactions between protein and water as well as oil are important in food systems, because they affect the flavor and texture of food products [57]. In Fig. 5B, the WAC of proteins extracted by the ultrasound-assisted BEW was decreased from 3.93 to 3.36 g/g protein with the ultrasound from 0 to 30 min. However, the OAC values were increased from 4.90 to 6.34 g/g protein. No significant difference was observed between BEW and DW treatment. The decrease of WAC and the increase of OAC were certainly caused by the exposure of nonpolar residue side chains interacting with hydrocarbon chains in fat (oil) molecules [58]. Another probable reason was that the exposure of hydrophobic groups allowed the physical entrapment of oil [59].
3.5. Emulsifying performance of the extracted Antarctic krill proteins
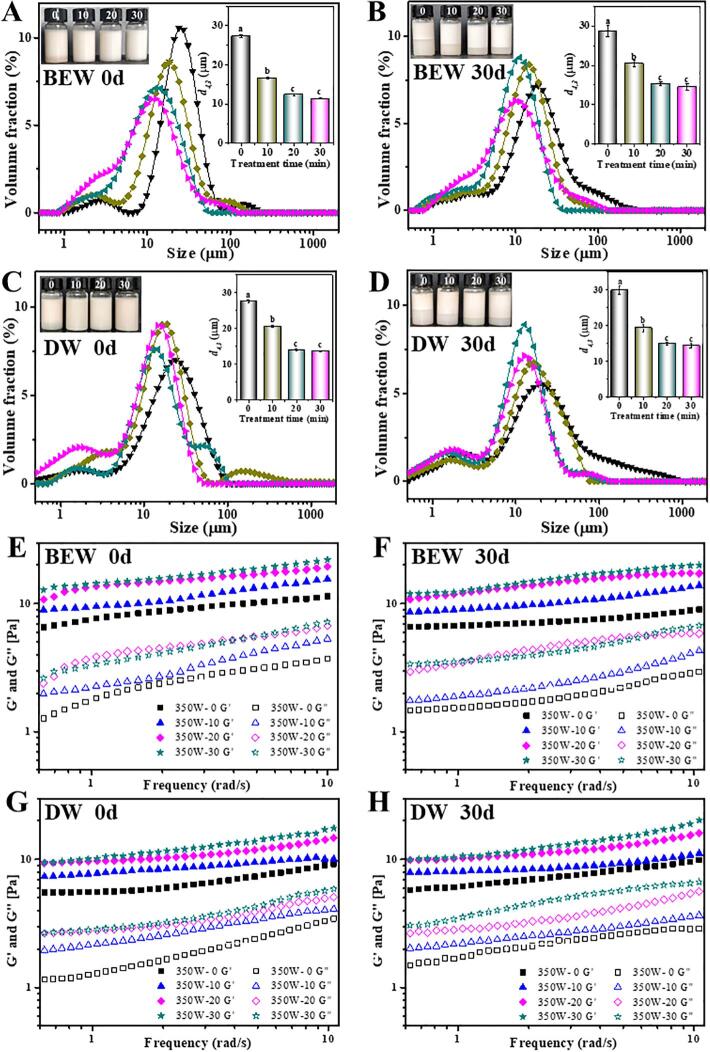
The extracted Antarctic krill proteins were used to evaluate their potential ability in fabricating emulsions. Fig. 6 shows the visual photographs and droplet sizes of the krill proteins-stabilized emulsions. The emulsions stabilized by the krill proteins (2%, w/v) exhibited an obvious creaming after storage at 4 °C for 30 d, regardless of BEW or DW extraction without ultrasound (Fig. 6A–D). However, the creaming degree of emulsions stabilized by the ultrasound-treated proteins was significantly decreased, especially after 20 min and 30 min treatment. Meanwhile, the CI values were calculated to quantitatively evaluate the emulsifying stability, and generally, the lower the CI value, the more stable the emulsion will be [35]. After 30 d storage, the CI values of emulsions stabilized by the proteins extracted by the ultrasound-assisted BEW were obviously decreased from 0.49 to 0.20 with the ultrasound time from 0 to 30 min (Fig. 6A and B). Similar results were also monitored for the samples extracted by the ultrasound-assisted DW, and the values were reduced from 0.51 to 0.22 (Fig. 6C and D). These results indicating that the ultrasound-assisted extraction facilitated the fabrication of more stable emulsions.
Fig. 6.
Effects of ultrasound-assisted BEW or DW on the visual morphology (A–D), particles size (A–D) and rheological properties (E–H) of krill proteins-stabilized emulsions after 0 d and 30 d storage. 350W-0, 350W-10, 350W-20 and 350W-30 represent 350W ultrasound for 0, 10, 20 and 30 min. Different letters (a, b, c, etc) indicate significant differences at p < 0.05.
It was reported that the decrease of protein particle size increased the adsorption rate of proteins at the oil-water interface, and the high absorption and migration rate effectively prevented gravitational separation, flocculation and coalescence [45], [60], which ultimately maintained the stability of emulsions. Meanwhile, the ultrasound-induced disordered and irregular structures also contributed to the stability of emulsions. Generally, disordered, small and flexible proteins more efficiently reduced the surface tension than ordered, larger and rigid proteins [61], because such proteins had balanced size and flexibility to facilitate their adsorption at the interface [45]. In addition, the fresh emulsions stabilized by the proteins extracted by the ultrasound-assisted BEW presented a significant decrease of droplet size from 27.3 μm to 12.4 μm with the ultrasound ranging from 0 min to 20 min. No obvious decrease was detected in the droplet size (11.8 μm) when the ultrasound was extended to 30 min. Likewise, almost the same change trend was observed for the proteins extracted by the ultrasound-assisted DW (Fig. 6C and D), with the droplet size decreasing from 27.6 μm to 13.4 μm. After 30 d storage, there was no great changes in the droplet sizes of all the proteins-stabilized emulsions, regardless of the ultrasound-assisted BEW or DW (Fig. 6B and D).
The rheological properties of krill proteins-stabilized emulsions are shown in Fig. 6E–H. Compared to samples without ultrasound treatment, the G′ and G″ value presented an increase regardless of ultrasound-assisted BEW (Fig. 6E) or DW (Fig. 6G) with the increase of ultrasound time, and G′ was predominantly larger than G″ throughout the whole measurement, implying a predominantly elastic gel-like structure. In addition, the loss tangent (tan δ, G″/G′) was used to determine whether elastic or viscous properties predominate in emulsions. Overall, the tan δ values of these emulsions were almost kept constant ~ 0.3, indicating the characteristics of predominantly weak and viscous gels [62]. Nevertheless, no significant changes in the G′, G″ and tan δ values were determined for all samples after 30 d storage at 4 °C (Fig. 6F, H), implying that the gel structures became much stable. It has reported that the formation of gel-like networks mainly originated from the interactions of protein molecules [63]. In this study, the ultrasonic treatment increased the hydrophobicity (Fig. 4E) and disulfide bonds (Fig. 4F) of the proteins. In addition, the increased β-sheet structure indicated the increased formation of intermolecular hydrogen bonds (Fig. 4A). Overall, the intermolecular interactions of proteins were increased after ultrasound-assisted treatment, which finally contributed to the enhanced emulsifying ability (Fig. 6). Based on above facts, it was firmly believed that the functional properties of krill proteins extracted by the ultrasound-assisted BEW could compete with those extracted by the ultrasound-assisted DW.
4. Conclusion
This study proved that the ultrasound-assisted basic electrolyzed water (BEW) was an effective and feasible method to extract marine krill proteins. Compared to ultrasound-assisted deionized water (DW), the ultrasound-assisted BEW reduced the consumption of 30.9% (w/w) NaOH. Moreover, the antioxidant capacity of negative redox potential of BEW could protect the active groups of proteins from oxidation. In addition, the ultrasound-assisted BEW increased the extraction yield and the solubility, reduced the particle size, favored the transition of α-helix and β-turn to β-sheet, elevated the surface hydrophobicity and promoted the formation of disulfide bonds of the krill proteins, in comparison with individual BEW treatment. All these properties contributed to the improved foam capacity (FC), foam stability (FS) and emulsifying capacity of the krill proteins. More importantly, the functional properties (foam capacity, foam stability, water absorption capacity, oil absorption capacity and emulsifying capacity) of the krill proteins extracted by ultrasound-assisted BEW were comparable to those extracted by ultrasound-assisted DW. In conclusion, this study indicates that the ultrasound-assisted BEW will become a novel candidate method with tremendous potential to extract various proteins, and does not produce negative effects on the functional properties of the extracted proteins.
CRediT authorship contribution statement
Yufeng Li: Investigation, Methodology, Data curation, Formal analysis, Writing - original draft. Qiao-Hui Zeng: Resources, Software. Guang Liu: Software, Revised the manuscript. Zhiyun Peng: Methodology, Writing - review & editing. Yixiang Wang: Review & editing. Yongheng Zhu: Writing - review & editing. Haiquan Liu: Software, Visualization. Yong Zhao: Funding acquisition, Investigation, Methodology. Jing Jing Wang: Investigation, Methodology, Project administration, Writing - original draft.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This research was supported by the National Key R&D Program of China (2018YFC1602205), the National Natural Science Foundation of China (31571917), Key Project of Shanghai Agriculture Prosperity through Science and Technology (2019-02-08-00-15-F01147), Shanghai Agriculture Applied Technology Development Program (T20170404).
Contributor Information
Yong Zhao, Email: yzhao@shou.edu.cn.
Jing Jing Wang, Email: w_j2010@126.com.
References
- 1.Liu S., Hu W., Fang Y., Cai Y., Zhang J., Liu J., Ding Y. Extraction of oil from wet Antarctic krill (Euphausia superba) using a subcritical dimethyl ether method. RSC Adv. 2019;9(59):34274–34282. doi: 10.1039/c9ra06238f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zheng H., Beamer S.K., Matak K.E., Jaczynski J. Effect of kappa-carrageenan on gelation and gel characteristics of Antarctic krill (Euphausia superba) protein isolated with isoelectric solubilization/precipitation. Food Chem. 2019;278:644–652. doi: 10.1016/j.foodchem.2018.11.080. [DOI] [PubMed] [Google Scholar]
- 3.Tou J.C., Jaczynski J., Chen Y.C. Krill for human consumption: nutritional value and potential health benefits. Nutr. Rev. 2007;65:63–77. doi: 10.1301/nr.2007.feb.63-77. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki T., Shibata N. The utilization of Antarctic krill for human food. Food Rev. Int. 1990;6(1):119–147. [Google Scholar]
- 5.Yoshitomi B., Aoki M., Oshima S.-I., Hata K. Evaluation of krill (Euphausia superba) meal as a partial replacement for fish meal in rainbow trout (Oncorhynchus mykiss) diets. Aquaculture. 2006;261(1):440–446. doi: 10.1016/j.aquaculture.2006.06.036. [DOI] [Google Scholar]
- 6.Qi X.-M., Liao E., Wang L., Lin H., Xue C.-H. Extracting protein from antarctic krill (Euphausia superba) J. Aquat. Food Prod. Technol. 2016;25(4):597–606. [Google Scholar]
- 7.Qi X., Liao E., Zhao K., Regenstein J.M., Mao X. Multi-stage countercurrent process for extracting protein from Antarctic Krill (Euphausia superba) J. Food Sci. Technol. 2018;55(11):4450–4457. doi: 10.1007/s13197-018-3368-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hricova D., Stephan R., Zweifel C. Electrolyzed water and its application in the food industry. J. Food Protect. 2008;71:1934–1947. doi: 10.4315/0362-028X-71.9.1934. [DOI] [PubMed] [Google Scholar]
- 9.Li Y., Tan L., Guo L., Zhang P., Malakar P.K., Ahmed F., Liu H., Wang J.J., Zhao Y. Acidic electrolyzed water more effectively breaks down mature Vibrio parahaemolyticus biofilm than DNase I. Food Control. 2020;117:107312. doi: 10.1016/j.foodcont.2020.107312. [DOI] [Google Scholar]
- 10.Brasil C.C.B., de Menezes C.R., Jacob‐Lopes E., Barin J.S., Zepka L.Q., Campagnol P.C.B., Wagner R., Cichoski A.J. Combined application of electrolysed water and ultrasound to improve the sanitation of knives in the meat industry. Int. J. Food Sci. Technol. 2020;55(3):1136–1144. doi: 10.1111/ijfs.14289. [DOI] [Google Scholar]
- 11.Xie J., Sun X., Pan Y., Zhao Y. Combining basic electrolyzed water pretreatment and mild heat greatly enhanced the efficacy of acidic electrolyzed water against Vibrio parahaemolyticus on shrimp. Food Control. 2012;23(2):320–324. doi: 10.1016/j.foodcont.2011.07.019. [DOI] [Google Scholar]
- 12.Dong X.-Y., Guo L.-L., Wei F., Li J.-F., Jiang M.-L., Li G.-M., Zhao Y.-D., Chen H. Some characteristics and functional properties of rapeseed protein prepared by ultrasonication, ultrafiltration and isoelectric precipitation: characteristics and functional properties of rapeseed protein. J. Sci. Food Agric. 2011;91(8):1488–1498. doi: 10.1002/jsfa.4339. [DOI] [PubMed] [Google Scholar]
- 13.Athayde D.R., Flores D.R.M., da Silva J.S., Genro A.L.G., Silva M.S., Klein B., Mello R., Campagnol P.C.B., Wagner R., de Menezes C.R., Barin J.S., Cichoski A.J. Application of electrolyzed water for improving pork meat quality. Food Res. Int. 2017;100:757–763. doi: 10.1016/j.foodres.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 14.Chittapalo T., Noomhorm A. Ultrasonic assisted alkali extraction of protein from defatted rice bran and properties of the protein concentrates. Int. J. Food Sci. Technol. 2009;44:1843–1849. doi: 10.1111/j.1365-2621.2009.02009.x. [DOI] [Google Scholar]
- 15.Jain S., Anal A.K. Optimization of extraction of functional protein hydrolysates from chicken egg shell membrane (ESM) by ultrasonic assisted extraction (UAE) and enzymatic hydrolysis. LWT - Food Sci. Technol. 2016;69:295–302. doi: 10.1016/j.lwt.2016.01.057. [DOI] [Google Scholar]
- 16.Tang W.-L., Zhang M., Fang Z. Optimization of ultrasound-assisted-extraction of porcine placenta water-soluble proteins and evaluation of the antioxidant activity. J. Food Sci. Technol. 2015;52(7):4042–4053. doi: 10.1007/s13197-014-1444-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu H., Wu J., Li-Chan E.C.Y., Zhu L.e., Zhang F., Xu X., Fan G., Wang L., Huang X., Pan S. Effects of ultrasound on structural and physical properties of soy protein isolate (SPI) dispersions. Food Hydrocolloids. 2013;30(2):647–655. doi: 10.1016/j.foodhyd.2012.08.001. [DOI] [Google Scholar]
- 18.Shanmugam A., Ashokkumar M. Characterization of ultrasonically prepared flaxseed oil enriched beverage/carrot juice emulsions and process-induced changes to the functional properties of carrot juice. Food Bioprocess Technol. 2015;8(6):1258–1266. doi: 10.1007/s11947-015-1492-1. [DOI] [Google Scholar]
- 19.Carpenter J., Saharan V.K. Ultrasonic assisted formation and stability of mustard oil in water nanoemulsion: effect of process parameters and their optimization. Ultrason. Sonochem. 2017;35:422–430. doi: 10.1016/j.ultsonch.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 20.Wang J.J., Zhang Z.H., Li J.B., Lin T., Pan Y.J., Zhao Y. Modeling Vibrio parahaemolyticus inactivation by acidic electrolyzed water on cooked shrimp using response surface methodology. Food Control. 2014;36(1):273–279. doi: 10.1016/j.foodcont.2013.08.031. [DOI] [Google Scholar]
- 21.Añón M.C., de Lamballerie M., Speroni F. Effect of high pressure on solubility and aggregability of calcium-added soybean proteins. Innov. Food Sci. Emerg. Technol. 2012;16:155–162. doi: 10.1016/j.ifset.2012.05.006. [DOI] [Google Scholar]
- 22.Yang X., Li Y., Li S., Oladejo A.O., Wang Y., Huang S., Zhou C., Ye X., Ma H., Duan Y. Effects of ultrasound-assisted alpha-amylase degradation treatment with multiple modes on the extraction of rice protein. Ultrason. Sonochem. 2018;40:890–899. doi: 10.1016/j.ultsonch.2017.08.028. [DOI] [PubMed] [Google Scholar]
- 23.Wang J., Zhao M., Qiu C., Sun W. Effect of malondialdehyde modification on the binding of aroma compounds to soy protein isolates. Food Res. Int. 2018;105:150–158. doi: 10.1016/j.foodres.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Boatright W.L., Hettiarachchy N.S. Effect of lipids on soy protein isolate solubility. J. Am. Oil Chem. Soc. 1995;72(12):1439–1444. [Google Scholar]
- 25.Wang J.J., Liu G.-Y., Liu G., Zeng Q.-H., Shen X., Hou Y.i., Li L., Hu S.-Q. The soluble recombinant N-terminal domain of HMW 1Dx5 and its aggregation behavior. Food Res. Int. 2015;78:201–208. doi: 10.1016/j.foodres.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 26.Chen B., Huang J., Li H., Zeng Q.-H., Wang J.J., Liu H., Pan Y., Zhao Y. Eradication of planktonic Vibrio parahaemolyticus and its sessile biofilm by curcumin-mediated photodynamic inactivation. Food Control. 2020;113:107181. doi: 10.1016/j.foodcont.2020.107181. [DOI] [Google Scholar]
- 27.Wang J.J., Liu G., Huang Y.-B., Zeng Q.-H., Hou Y.i., Li L., Ou S., Zhang M., Hu S.-Q. Dissecting the disulfide linkage of the N-terminal domain of HMW 1Dx5 and its contributions to dough functionality. J. Agric. Food Chem. 2017;65(30):6264–6273. doi: 10.1021/acs.jafc.7b02449. [DOI] [PubMed] [Google Scholar]
- 28.Wang J.J., Liu G., Huang Y.B., Zeng Q.H., Song G.S., Hou Y., Li L., Hu S.Q. Role of N-terminal domain of HMW 1Dx5 in the functional and structural properties of wheat dough. Food Chem. 2016;213:682–690. doi: 10.1016/j.foodchem.2016.07.026. [DOI] [PubMed] [Google Scholar]
- 29.Jiang L., Wang J., Li Y., Wang Z., Liang J., Wang R., Chen Y., Ma W., Qi B., Zhang M. Effects of ultrasound on the structure and physical properties of black bean protein isolates. Food Res. Int. 2014;62:595–601. doi: 10.1016/j.foodres.2014.04.022. [DOI] [Google Scholar]
- 30.Kato A., Nakai S. Hydrophobicity determined by a fluorescence probe method and its correlation with surface properties of proteins. Biochim. Biophys. Acta. 1980;624(1):13–20. doi: 10.1016/0005-2795(80)90220-2. [DOI] [PubMed] [Google Scholar]
- 31.Haskard C.A., Li-Chan E.C.Y. Hydrophobicity of bovine serum albumin and ovalbumin determined using uncharged (PRODAN) and anionic (ANS -) fluorescent probes. J. Agric. Food Chem. 1998;46(7):2671–2677. [Google Scholar]
- 32.Timilsena Y.P., Adhikari R., Barrow C.J., Adhikari B. Physicochemical and functional properties of protein isolate produced from Australian chia seeds. Food Chem. 2016;212:648–656. doi: 10.1016/j.foodchem.2016.06.017. [DOI] [PubMed] [Google Scholar]
- 33.Resendiz-Vazquez J.A., Ulloa J.A., Urías-Silvas J.E., Bautista-Rosales P.U., Ramírez-Ramírez J.C., Rosas-Ulloa P., González-Torres L. Effect of high-intensity ultrasound on the technofunctional properties and structure of jackfruit (Artocarpus heterophyllus) seed protein isolate. Ultrason. Sonochem. 2017;37:436–444. doi: 10.1016/j.ultsonch.2017.01.042. [DOI] [PubMed] [Google Scholar]
- 34.Zhao X.-H., Tang C.-H. Spray-drying microencapsulation of CoQ 10 in olive oil for enhanced water dispersion, stability and bioaccessibility: influence of type of emulsifiers and/or wall materials. Food Hydrocolloids. 2016;61:20–30. doi: 10.1016/j.foodhyd.2016.04.045. [DOI] [Google Scholar]
- 35.Lv W., Hu T., Taha A., Wang Z., Xu X., Pan S., Hu H. Lipo-dipeptide as an emulsifier: performance and possible mechanism. J. Agric. Food Chem. 2019;67(22):6377–6386. doi: 10.1021/acs.jafc.9b01721. [DOI] [PubMed] [Google Scholar]
- 36.Zou Y., Guo J., Yin S.-W., Wang J.-M., Yang X.-Q. Pickering emulsion gels prepared by hydrogen-bonded zein/tannic acid complex colloidal particles. J. Agric. Food Chem. 2015;63(33):7405–7414. doi: 10.1021/acs.jafc.5b03113. [DOI] [PubMed] [Google Scholar]
- 37.Li Y., Zeng Q.-H., Liu G., Chen X., Zhu Y., Liu H., Zhao Y., Wang J.J. Food-grade emulsions stabilized by marine Antarctic krill (Euphausia superba) proteins with long-term physico-chemical stability. LWT - Food Sci. Technol. 2020;128:109492. doi: 10.1016/j.lwt.2020.109492. [DOI] [Google Scholar]
- 38.Zhao J., Su G., Zhao M., Sun W. Physicochemical changes and in vitro gastric digestion of modified soybean protein induced by lipoxygenase catalyzed linoleic acid oxidation. J. Agric. Food Chem. 2019;67(50):13978–13985. doi: 10.1021/acs.jafc.9b05843. [DOI] [PubMed] [Google Scholar]
- 39.Sun Q., Chen Q., Xia X., Kong B., Diao X. Effects of ultrasound-assisted freezing at different power levels on the structure and thermal stability of common carp (Cyprinus carpio) proteins. Ultrason. Sonochem. 2019;54:311–320. doi: 10.1016/j.ultsonch.2019.01.026. [DOI] [PubMed] [Google Scholar]
- 40.Maity I., Rasale D.B., Das A.K. Sonication induced peptide-appended bolaamphiphile hydrogels for in situ generation and catalytic activity of Pt nanoparticles. Soft Matter. 2012;8(19):5301. doi: 10.1039/c2sm25126d. [DOI] [Google Scholar]
- 41.Jia J., Ma H., Zhao W., Wang Z., Tian W., Luo L., He R. The use of ultrasound for enzymatic preparation of ACE-inhibitory peptides from wheat germ protein. Food Chem. 2010;119(1):336–342. doi: 10.1016/j.foodchem.2009.06.036. [DOI] [Google Scholar]
- 42.Jambrak A.R., Mason T.J., Lelas V., Paniwnyk L., Herceg Z. Effect of ultrasound treatment on particle size and molecular weight of whey proteins. J. Food Eng. 2014;121:15–23. doi: 10.1016/j.jfoodeng.2013.08.012. [DOI] [Google Scholar]
- 43.Tang C.-H., Wang X.-Y., Yang X.-Q., Li L. Formation of soluble aggregates from insoluble commercial soy protein isolate by means of ultrasonic treatment and their gelling properties. J. Food Eng. 2009;92(4):432–437. doi: 10.1016/j.jfoodeng.2008.12.017. [DOI] [Google Scholar]
- 44.Xue S., Yu X., Li X., Zhao X., Han M., Xu X., Zhou G. Structural changes and emulsion properties of goose liver proteins obtained by isoelectric solubilisation/precipitation processes. LWT - Food Sci. Technol. 2019;102:190–196. doi: 10.1016/j.lwt.2018.12.019. [DOI] [Google Scholar]
- 45.Li K., Fu L., Zhao Y.Y., Xue S.W., Wang P., Xu X.L., Bai Y.H. Use of high-intensity ultrasound to improve emulsifying properties of chicken myofibrillar protein and enhance the rheological properties and stability of the emulsion. Food Hydrocolloids. 2020;98 [Google Scholar]
- 46.Sun W., Zhou F., Sun D.-W., Zhao M. Effect of oxidation on the emulsifying properties of myofibrillar proteins. Food Bioprocess. Technol. 2013;6(7):1703–1712. doi: 10.1007/s11947-012-0823-8. [DOI] [Google Scholar]
- 47.Keerati-u-rai M., Miriani M., Iametti S., Bonomi F., Corredig M. Structural changes of soy proteins at the oil–water interface studied by fluorescence spectroscopy. Colloids Surf. B. 2012;93:41–48. doi: 10.1016/j.colsurfb.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 48.Hu H., Li-Chan E.C.Y., Wan L.i., Tian M., Pan S. The effect of high intensity ultrasonic pre-treatment on the properties of soybean protein isolate gel induced by calcium sulfate. Food Hydrocolloids. 2013;32(2):303–311. doi: 10.1016/j.foodhyd.2013.01.016. [DOI] [Google Scholar]
- 49.Estévez M. Protein carbonyls in meat systems: a review. Meat Sci. 2011;89(3):259–279. doi: 10.1016/j.meatsci.2011.04.025. [DOI] [PubMed] [Google Scholar]
- 50.Hu H., Fan X., Zhou Z., Xu X., Fan G., Wang L., Huang X., Pan S., Zhu L. Acid-induced gelation behavior of soybean protein isolate with high intensity ultrasonic pre-treatments. Ultrason. Sonochem. 2013;20(1):187–195. doi: 10.1016/j.ultsonch.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 51.Higuera-Barraza O.A., Torres-Arreola W., Ezquerra-Brauer J.M., Cinco-Moroyoqui F.J., Rodríguez Figueroa J.C., Marquez-Ríos E. Effect of pulsed ultrasound on the physicochemical characteristics and emulsifying properties of squid (Dosidicus gigas) mantle proteins. Ultrason. Sonochem. 2017;38:829–834. doi: 10.1016/j.ultsonch.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 52.Gülseren İ., Güzey D., Bruce B.D., Weiss J. Structural and functional changes in ultrasonicated bovine serum albumin solutions. Ultrason. Sonochem. 2007;14(2):173–183. doi: 10.1016/j.ultsonch.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 53.Xiong W., Wang Y., Zhang C., Wan J., Shah B.R., Pei Y., Zhou B., Li J., Li B. High intensity ultrasound modified ovalbumin: structure, interface and gelation properties. Ultrason. Sonochem. 2016;31:302–309. doi: 10.1016/j.ultsonch.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 54.Amiri A., Sharifian P., Soltanizadeh N. Application of ultrasound treatment for improving the physicochemical, functional and rheological properties of myofibrillar proteins. Int. J. Biol. Macromol. 2018;111:139–147. doi: 10.1016/j.ijbiomac.2017.12.167. [DOI] [PubMed] [Google Scholar]
- 55.Mir N.A., Riar C.S., Singh S. Physicochemical, molecular and thermal properties of high-intensity ultrasound (HIUS) treated protein isolates from album (Chenopodium album) seed. Food Hydrocolloids. 2019;96:433–441. doi: 10.1016/j.foodhyd.2019.05.052. [DOI] [Google Scholar]
- 56.Xiong T., Xiong W., Ge M., Xia J., Li B., Chen Y. Effect of high intensity ultrasound on structure and foaming properties of pea protein isolate. Food Res. Int. 2018;109:260–267. doi: 10.1016/j.foodres.2018.04.044. [DOI] [PubMed] [Google Scholar]
- 57.Elsohaimy S.A., Refaay T.M., Zaytoun M.A.M. Physicochemical and functional properties of quinoa protein isolate. Ann. Agric. Sci. 2015;60(2):297–305. doi: 10.1016/j.aoas.2015.10.007. [DOI] [Google Scholar]
- 58.Higuera-Barraza O.A., Del Toro-Sanchez C.L., Ruiz-Cruz S., Márquez-Ríos E. Effects of high-energy ultrasound on the functional properties of proteins. Ultrason. Sonochem. 2016;31:558–562. doi: 10.1016/j.ultsonch.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 59.Meinlschmidt P., Sussmann D., Schweiggert‐Weisz U., Eisner P. Enzymatic treatment of soy protein isolates: effects on the potential allergenicity, technofunctionality, and sensory properties. Food Sci. Nutr. 2016;4(1):11–23. doi: 10.1002/fsn3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sun J., Liu W.-Y., Feng M.-Q., Xu X.-L., Zhou G.-H. Characterization of olive oil emulsions stabilized by flaxseed gum. J. Food Eng. 2019;247:74–79. doi: 10.1016/j.jfoodeng.2018.11.023. [DOI] [Google Scholar]
- 61.Bos M.A., van Vliet T. Interfacial rheological properties of adsorbed protein layers and surfactants: a review. Adv. Colloid Interface Sci. 2001;91(3):437–471. doi: 10.1016/s0001-8686(00)00077-4. [DOI] [PubMed] [Google Scholar]
- 62.Liu F., Tang C.-H. Cold, gel-like whey protein emulsions by microfluidisation emulsification: rheological properties and microstructures. Food Chem. 2011;127(4):1641–1647. doi: 10.1016/j.foodchem.2011.02.031. [DOI] [Google Scholar]
- 63.Zhang Y., Cui L., Xu H., Feng X., Wang B., Pukánszky B., Mao Z., Sui X. Poly(lactic acid)/cellulose nanocrystal composites via the Pickering emulsion approach: rheological, thermal and mechanical properties. Int. J. Biol. Macromol. 2019;137:197–204. doi: 10.1016/j.ijbiomac.2019.06.204. [DOI] [PubMed] [Google Scholar]