Highlights
-
•
Conventional pasteurization drastically decreases Lactobacillus sp.
-
•
Thermosonication (TS) for less 9 min is sufficient for processing pulque.
-
•
Physicochemical properties of pulque were not modified by the use of TS.
-
•
Thermosonication extending shelf life of pulque up to 24 days.
-
•
TS is a viable technology for processing pulque.
Keywords: Pulque, Thermosonication, Shelf life, Physicochemical properties, Sensory quality
Abstract
The aim of this study was to evaluate thermosonication as an alternative method for the pasteurization of pulque in order to improve its shelf life and retain its quality parameters.
Thermosonication was carried out at 50 °C using amplitudes of 75% (for 6 and for 9 min), 85% (for 4 and for 6 min), and 95% (for 3 and for 5 min). These were the optimal conditions found for processing pulque by thermosonication. Physicochemical (acidity, color, alcohol content, and sensory analysis) and microbiological (lactic acid bacteria and yeasts) parameters were determined during 30 days for storage at 4 ± 1 °C. Conventional pasteurization (63 °C, 30 min) and raw pulque were used as controls. According to the results, the shelf life of pulque was extended up to 24 days storage at 4 °C. After this time, the quality of beverage decreased, due that the microbial load increases. Thermosonication treatments at 75% and 85% showed a higher content of LAB (6.58–6.77 log CFU/mL) and yeasts (7.08–7.27 log CFU/mL) than conventional pasteurization (3.64 log CFU/mL of LAB and 3.97 log CFU/mL of yeasts) at 24 days of storage. Raw pulque demonstrated up to 7.77 log CFU/mL of yeasts and 7.51 log CFU/mL of LAB. Pulque processed by thermosonication exhibited greater lightness, sensory acceptance, a maximal acidity of 0.83 g/lactic acid, and an alcohol content of 4.48–4.95% v/v. The thermosonication process preserves sensory and physicochemical properties better than conventional pasteurization. Lactic acid bacteria such as Lactobacillus kefiri, Lactobacillus acidophilus, and Lactobacillus hilgardii and yeasts such as Saccharomyces cereviasiae were identified in thermosonicated pulque.
1. Introduction
The Conventional pasteurization (CP) method has long been used for microbial inactivation to ensure the safety and extended shelf life of a variety of foods and beverages [1], [2]. Many investigations have demonstrated that CP adversely affects the sensory quality (flavor, texture, aroma, and color) and nutritional values of processed foods [3], [4]. Due to consumer demand for high-quality foods, new safe and effective techniques have been developed and tested for food processing and preservation [5]. An alternative method for processing beverages and foods is Ultrasound high intensity (UHI).
Ultrasound waves result from the conversion of electrical into mechanical energy by means of piezoelectric materials [6], [7]. During processing, ultrasonic energy propagates in the liquid food, generating pressure variations that form bubbles. These collapse violently in succeeding cycles of compression, resulting in regions of high temperature and pressure, this phenomenon known as cavitation [8]. When UHI is combined with heat (temperatures ≥ 50 °C), it is known as Thermosonication (TS) [9]. This process has been considered a potential method for the inactivation of microorganisms in aqueous solutions and liquid food, due that there is a synergistic effect of both the heat and the sound waves [10], [11]. These act simultaneously and consequently, high sensitivity in the cell wall occurs, causing the death of the bacteria [12], [13]. Therefore, to ensure the effectiveness of the thermosonication treatment it is necessary to consider variables such as the pH of the product, and the frequency, amplitude, temperature, and time of the treatment [14], [15]. Normally, frequencies ranging from 20 to 100 kHz are used for processing [7]. The thermosonication method has been utilized in order to extend shelf life, the pasteurization and the homogenization of many products and, the same time the method retains its nutritive and sensory properties [7], [16]. Particularly in fermented beverages, these products generally have a short shelf life due to their high microbial content. Interest in non-dairy fermented beverages has increased in recent times, because these products are free of fats, milk allergens, and cholesterol [17], [18]. A non-dairy, fermented, and alcoholic beverage possessing interesting properties has been produced and consumed in Mexico since Olmec times [19], [20]. This beverage is known as pulque.
Pulque is produced by the fermentation of a fresh sap known as aguamiel, which is extracted from different species of Agave (maguey), such as Agave americana, A. atrovirens, A. mapisaga, and A. salmiana [21], [22]. For the production of pulque, it is necessary to collect of fresh aguamiel in open barrels and to add a portion of previously elaborated pulque [21]. Then, the fermentation process takes place at room temperature during 3–8 h; after this time, pulque is obtained. Lactic acid bacteria (LAB), Acetic acid bacteria (AAB), and yeasts are the main microorganisms that participate in the biochemical changes during the fermentation process. As a result, pulque is slightly acidic, white, viscous, and with an alcohol content ranging from 4 to 9% v/v [21]. The metabolites produced by microbial activity include organic acids, such as lactic and acetic, produced by LAB and AAB (Gluconobacter and Acetobacter), respectively. Ethanol is produced by Saccharomyces cerevisiae and Zymomonas mobilis [20].
Microorganisms such as lactic acid bacteria and yeasts are very important in pulque, due that they contribute to its physicochemical, sensory, and microbiological properties [19], [20], [21], [22], [23]. According to [23], parameters that determine quality in pulque are its acidity, alcohol content, flavor, color, and aroma.
Pulque is an important source of protein (6%), vitamins (riboflavin, 24%; niacin, 23%; thiamine, 10%, and vitamin C, 48%), and minerals such as calcium (8%) and iron (51% not associated with hemoglobin) [21]. In addition, some species of LAB isolated from pulque have demonstrated probiotic activity, such as Leuconostoc mesenteroides, L. citreum, L. kimchi, and Lactobacillus acidophilus [19], [20], [21], [22]. These confer protection against gastrointestinal infections caused by pathogenic microorganisms [22]. However, pulque entails the technological problem of having a short shelf life of at least 3 days under refrigeration. This is caused by its higher microbial load that generates rapid decomposition [23]. As a result, it minimizes its distribution and commercialization. Some investigations have reported the use of different methods for preserving the beverage, such as conventional pasteurization; if preservatives and filtration are added to the process, its sensory and microbiological properties are reduced [19]. Pulque is a beverage with relevant properties and there is a need to test emerging technologies to preserve its quality. Therefore, the objective of this study was to evaluate thermosonication as an alternative method for processing pulque in order to extend its shelf life, conserve its sensory, physicochemical, and microbiological properties during storage, and compare these effects with those of samples prepared with conventional pasteurization and samples that are unprocessed.
2. Materials and methods
2.1. Samples and treatments
A fresh batch of pulque was obtained from a rural producer at “Hacienda La Palmita” located in San Miguel de Allende, Guanajuato, Mexico. Samples were transported at 4 °C and collected in a sterile container.
The experimental strategy was followed as proposed by [23]. Treatments of thermosonication were performed at 50 ± 2 °C in 500 W high-intensity ultrasound equipment (Hielscher, Inc., NJ., USA). An ice bath was employed to control the temperature and thermocoupling to measure the latter. At first, optimal pasteurization conditions by thermosonication were determined in 150 mL of pulque by applying the following amplitudes and times: at 65% for 5, 7, 10, 13, and 17 min; at 75% for 3, 6, 9, 12, and 15 min; at 85% for 2, 4, 6, 8, and 10 min, and at 95% for 1, 3, 5, 7, and 9 min. The conditions at 75% (for 6 and 9 min), at 85% (for 4 and 6 min), and at 95% (3 and 5 min) were selected as optimal for the pasteurization of pulque. These conditions were utilized to conduct studies of the shelf life of pulque, processing 1L samples for each treatment.
On the other hand, under sterile conditions, the thermal Conventional pasteurization (CP) treatment was applied at 63 °C for 30 min in 1 L of pulque. Then, cooling was carried out to cause thermal shock. Samples processed both the Conventional pasteurization and Thermosonication methods, including raw pulque, were stored in a sterile container at 4 ± 1 °C for 30 days. Microbiological and physicochemical parameters were monitored every 72 h for each treatment. All conditions were evaluated by triplicate.
2.2. Microbial analysis
The spread-plate method was employed to determine LAB and yeasts. Samples were diluted several times (10−1 to 10−6) in sterile buffered peptone water 0.1% w/v (Difco, Co., Tacuba, Mexico City, Mexico). Then, a 1 mL volume of several diluted samples were mixed with 15 mL Man, Rogosa, and Sharp agar (Sigma Aldrich Co., St. Louis, MO, USA) for determining LAB. Previously, this agar was supplemented with 100 mg/L of cycloheximide (Sigma Aldrich Co., St. Louis, MO, USA) and the plates were incubated at 37 °C for 48 h [25]. Potato dextrose agar (Dibico Co., Cuauhtitlán Izcalli, Edo. de México, Mexico) was used for the enumeration of yeasts, and incubation was carried out at 27 °C for 5 days. All analyses were performed in triplicate.
2.3. Identification of microorganisms
Isolation and identification of LAB and yeasts strains from raw and thermosonicated pulque was conducted following the method reported by [23]. Subsequently, the colonies that tested positive for biochemical tests were subjected to molecular identification. In order to identify the yeasts, a PCR was performed to amplify the D1/D2 dominion of the gene that codes for subunit 23S of the rRNA using the following primers: NL1 (5′-GCATATCAATAAGCGGAGGAAAAG-3′) and NL4 (5′-GGTCCGTGTTTCAAGACGG-3′). For lactic acid bacteria, the subunit 16S of rRNA was amplified. Then, the primers used were pA (5′-AGAGTTTGATCCTGGCTCAG-3′) and pH (5′-AAGGAGGTGATCCAGCCGCA-3′). Total genomic DNA extraction became a pre-treatment to remove lipids, salts, and proteins, obtaining the cell pellet. Cells were subjected to lysis by heat, and DNA was purified with phenol-chloroform and ethanol precipitation [24]. The products obtained were sent to the Macrogen Laboratory (Geumcheon-gu, Seoul, South Korea) for sequencing. Microorganisms were identified using the BLAST database (Basic Local Alignment Search Tool) (http://www.ncbi.nlm.nih.gov/BLAST/).
2.4. Scanning Electron Microscope (SEM) analysis
SEM was carried out to observe the damage of the thermosonication on the morphology of the lactic acid bacteria and yeasts. The culture and incubation of these microorganisms were performed as mentioned in Section 2.2; these were isolated previously from pulque. Each microorganism sample was coated with gold and observed with the high resolution microscope, coupled with Electron Backscatter Diffraction (JEOL, Ltd., Tokyo, Japan). The LAB and yeasts of raw pulque were employed as a control.
2.5. Physicochemical analysis
2.5.1. Titratable acidity
Titratable acidity (TA) was determined in a 20 mL sample and titrated with a 0.1 N NaOH standard [25]. Phenolphthalein was utilized as an indicator. The volume of NaOH was converted into g of lactic acid per 100 mL of sample applying the following formula:
| (1) |
2.5.2. Lightness determination
Color in samples of pulque was determined with a Hunter-Lab model 45/0-L colorimeter (Hunter Associates Laboratory, Inc., Reston, VA, USA). Color value was expressed as Lightness (L*) on a scale from 0 (darkness) to 100 (lightness) [23].
2.5.3. Alcoholic content
Pulque samples of 200 mL were distilled and alcohol content was determined in a pycnometer at 15 °C following methods 942.08, 957.03, and 26.1.08 described in [26]. The alcoholic content was expressed as a percentage (% v/v) of ethanol.
2.6. Sensory analysis
Sensory analysis was evaluated as described by [23] with some modifications. Samples of raw and thermosonicated pulque were evaluated at 1, 24, and 30 storage days at 4 ± 1 °C by a Panel of 10 judges. The analysis consisted of an evaluation of three sensory attributes, including color, flavor, and odor, following the hedonic scale: 1 = Dislike extremely; 2 = Dislike very much; 3 = Dislike moderately; 4 = Dislike slightly; 5 = Neither like or dislike; 6 = Like slightly; 7 = Like moderately; 8 = Like very much, and 9 = Like extremely, according to [27]. In addition, the intensity of the acidity and alcohol perception was measured using the following scale: 1 = very weak; 2 = weak; 3 = intermediate; 4 = strong, and 5 = very strong.
2.7. Statistical analyses
ANOVA and TUKEY tests were applied to determine statistically significant differences among the treatments, considering a p < 0.05 significance level. All statistical analyses were performed with JMP® ver. 8.0 statistical software.
3. Results and discussion
3.1. Determination of optimal pasteurization conditions for pulque processed by thermosonication
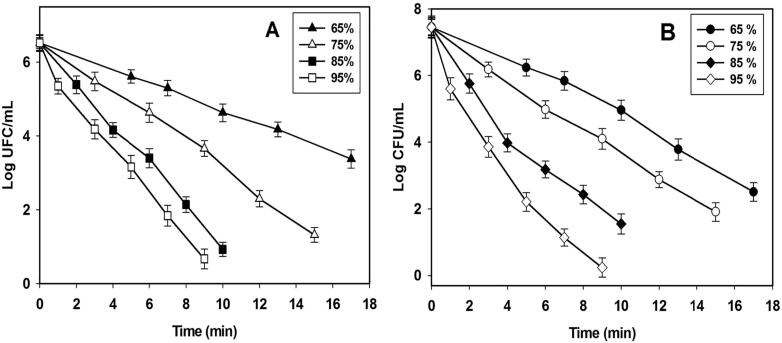
Lactic acid bacteria and yeasts were selected for determining optimal conditions because they represent two of more important species of pulque. These microorganisms confer physicochemical and microbiological properties that determine beverage quality. Samples of raw pulque were processed by TS at different conditions in order to obtain survival curves of the microbial reduction of LAB (Fig. 1A) and yeasts (Fig. 1B). In the case of LAB, survival curves conducted at 65% barely showed a 0.91 log CFU/mL reduction during 5 min, while increases in the processing time ranging from 7 to 17 min reductions from 1.22 to 3.14 log CFU/mL were achieved (Fig. 1A). This means that conditions at 65% allowed higher LAB survival. A higher content of LAB in pulque could generate faster decomposition and reduce shelf life [23]. Contrariwise, the results obtained when an amplitude of 75% was employed exhibited major reductions from 1.89, 2.86, 4.22, and 5.20 log CFU/mL for 6, 9, 12, and 15 min, respectively (Fig. 1A); in other words, the number of surviving cells was lower than 65%. Treatments tested with higher amplitudes, such as 85% and 95%, exhibited higher microbial reduction than treatments at 65% and 75%. For example, at 85% during 6, 8, and 10 min, maximal microbial reduction ranged from 3.12 to 5.59 log CFU/mL, while at 95% for 3 up to 9 min, reductions were achieved that ranged from 2.34 to 5.85 log CFU/mL. As expected, the microbial reduction of LAB was found to that depend on the amplitude and processing times and the higher amplitude the used, the more cells are inactivated. Therefore, the survival curves decrease linearly with time. Similar behavior has been reported by [15] in Enterobacter aerogenes. When yeasts were thermosonicated at 65% for 10, 13, and 17 min, reductions of 2.49, 3.67, and 4.94 log CFU/mL were achieved (Fig. 1B). As for LAB, these conditions allowed high yeast survival and poor inactivation, and the shelf life of pulque could be reduced, due that a yeast content of 5–7 log CFU/mL could cause deterioration of the pulque and a high production of ethanol [23]. Treatments at 85% during 4–10 min demonstrated reductions of 3.47 up to 5.90 Log CFU/mL. Similar reductions were reported by [4] in Saccaromyces cerevisiae when TS treatments were applied 50 °C for 10 min. They suggest that the action of the temperature and of the ultrasound waves increased the sensitivity of the yeasts. Treatment at 95% during 3 and 5 min exhibited reductions of 3.59 and 7.21 log CFU/mL of the yeasts. These conditions revealed a higher reduction of LAB. However, this suggests that pulque could present alterations in flavor and aroma. Therefore, these treatments are considered lethal; they reduce significant amounts of yeasts and LAB. Treatments at 75% and 85% exhibited reductions that could be considered sub-lethal. As a result, the sensory and physicochemical properties could be being retained. According to the results of the survival curves for LAB and yeasts, it is considered that optimal treatments to process the pulque comprised amplitudes at 75% (for 6 and 9 min) and at 85% (for 4 and 6 min).
Fig. 1.
Survival curves of Lactic Acid Bacteria (A) and yeasts (B) in pulque processed by thermosonication at 50 °C and different amplitudes of 65%, 75%, 85%, and 95%. Each value is expressed as mean ± standard deviation.
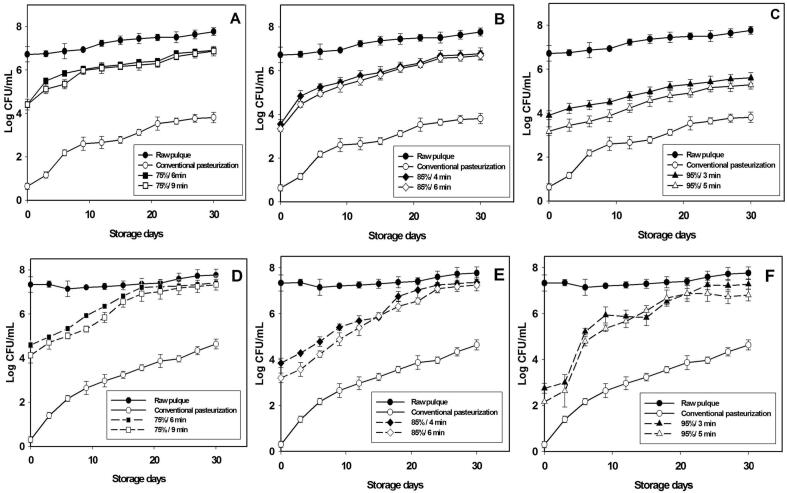
3.2. LAB and microbial yeast growth during storage in processed pulque
Optimal conditions for pulque processing by TS were evaluated for 30 days, finding which treatments at 75% for 6 and 9 min exhibited a LAB load ranging from 6.30 to 6.91 log CFU/mL on days 21 and 30 of storage at 4 °C (Fig. 2A). The growing total microbial load under these conditions was 2.47 and 2.49 log CFU/mL, respectively. Pulque processed at 85% for 4 and 6 min showed a maximal growth from day 24 up to day 30 with counts of 6.62–6.78 log CFU/mL for both conditions (Fig. 2B). The growth of LAB obtained by treatments at 75% and 85% was greater than that reported by [15] in samples of ayran (<1 log CFU/mL) processed by TS for 30 days of storage.
Fig. 2.
Growing of Lactic Acid Bacteria (A-C), and yeasts (D-F) in raw pulque (●), conventionally pasteurized pulque (○), and pulque processed by thermosonication at 75%, 6 min (■); 75%, 9 min (□); 85%, 4 min (♦); 85%, 6 min (◊), 95%, 3 min (▲); 95%, 5 min (▲) stored for 30 days at 4 ± 1 °C. Each value is expressed as mean ± standard deviation.
Conventional pasteurization demonstrated a LAB growth of 3.64–3.81 log CFU/mL from day 24 to day 30 of storage (Fig. 2). In contrast, the treatment with the highest amplitude was applied at 95% for 3 and for 5 min (Fig. 2C). These conditions exhibited a higher content (5.16–5.61 log CFU/mL) of LAB than conventional pasteurization. It could be suggested that conventional pasteurization did not conserve the LAB and, because the latter was more lethal, the physicochemical quality could be affected as a result. Raw pulque showed the highest LAB content (7.77 log CFU/mL) of all treatments tested, at day 30 of storage. [23] It was observed that, at a range of 6–7 log CFU/mL the quality parameters of LAB (acidity, sensory, and microbiological) could be conserved in pulque during storage. These counts were obtained in the treatments at 75% and 85%. Regarding the development of yeasts, these play an important role in the production of ethanol in pulque and contribute to its flavor, odor, and sensory properties [28]. A similar development of yeasts occurred during day 30 of storage in thermosonication treatments at 75%, 85%, and 95%. On day 24, amounts ranging from 6.87 to 7.27 log CFU/mL were obtained under these conditions at 50 °C (Fig. 2D, 2E, and 2F). The pulque processed by conventional pasteurization (Fig. 2) demonstrated the lowest development (3.97–4.64 log CFU/mL) of yeasts, while the raw pulque had the highest content of all of the evaluated treatments (Fig. 2). A high content of yeast generates very fast fermentation, causing a high production of ethanol and shortening the shelf life of the pulque [19]. In general, conventional pasteurization showed microbial stability during storage period, with lower counts of yeasts and LAB. Microbial control is necessary in fermented beverages in order to maintain the stability of the product during storage, due that physicochemical parameters are directly related to the microbial content [15]. Of the three Thermosonication amplitudes evaluated, the best were at 75% and 85%; counts were lower at 95%. Pulque processed by TS revealed adequate microbial stability at day 24 of storage. After this time, the pulque could undergo altered sensory qualities. Therefore, thermosonication proves to be a suitable technology to process pulque in less than 9 min at 50 °C. This is more efficient than conventional pasteurization, which used (30 min, 60 °C).
3.3. Microorganisms identified in the processed pulque
The identified microorganisms in raw and thermosonicated pulque were Lactobacillus kefiri, L. acidophilus, and L. hilgardii. The yeast identified was Saccaromyces cerevisiae, which is recognized as the main ethanol production on pulque and provides sensory properties [19], [23]. Lactocabillus in pulque processed by TS could provide benefits for the health of the consumer. L. kefiri possesses a strong ability to modulate gut microbiotic composition, leading to a reduction of several bacterial genera directly involved in the onset of the proinflammatory response and gastrointestinal diseases [30], while L. acidophilus enhances the immune response, improves lactose digestion, minimizes the effect of antibiotics on normal bacterial flora, inflammatory syndrome bowel is reduced [31], and L. hilgardii is used for technological purposes [32].
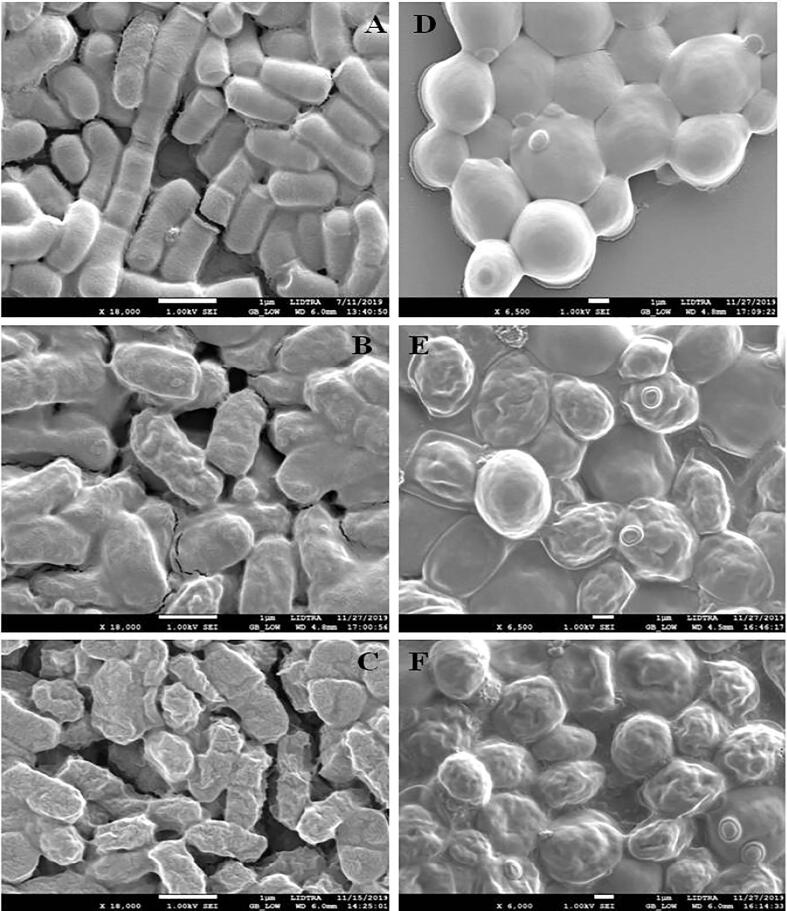
3.4. Morphological analysis of SEM
SEM is a technique utilized to visualize the morphology of single bacteria cell. SEM images of lactic acid bacteria and yeasts of raw and processed pulque by thermosonication are shown in Fig. 3. The SEM image of microorganism on raw pulque (control) confirms that LAB are short, plump rods that can present in chains or palisades (Fig. 3A). Yeasts are elongated or spherical (Fig. 3D). It can also be clearly observed that LAB and yeasts processed by thermosonication exerted a considerable effect on their morphology. At 85% for 6 min, LAB and yeasts demonstrated roughness, slight elongation, and pore formation (Fig. 3B and E). Mainly in the treatment at 95% during 5 min, it exhibited greater damage, such as greater elongation of the cell body, larger pores, and greater roughness (Fig. 3C and F). Similar changes in the morphology of microorganisms processed by TS were observed by [33]. These authors reported modification, erosion, and the perforation of cell membranes; in consequence, this causes inactivation of the microbial load. When the conditions are lethal, microorganisms do not possess the ability to multiply and regenerate; in contrast, when the damage is sub-lethal, bacteria could multiply again. Therefore, the thermosonication treatment at 85% during 6 min gave rise to less morphological damage than the treatment at 95% for 5 min. Therefore, this is relevant in terms of conserving the LAB and yeasts, because are charged with conferring the physicochemical and sensory properties on the beverage.
Fig. 3.
SEM micrographs of Lactic Acid Bacteria and yeasts in raw (A and D) and processed pulque by thermosonication at 85% for 6 min (B and E) and at 95% for 5 min (C and F).
3.5. Physicochemical parameters of pulque during storage
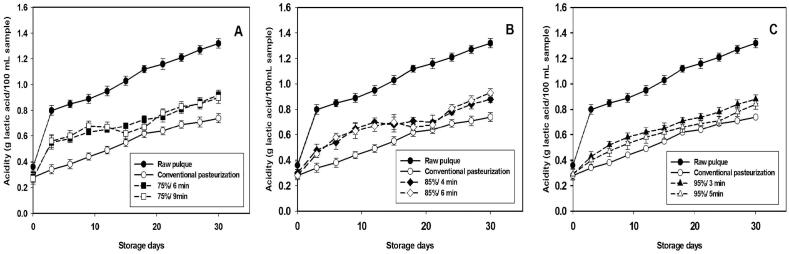
3.5.1. Titratable acidity
Acidity affects the storage stability and quality of pulque. The Titratable acidity (TA) values in pulque samples during storage are presented in Fig. 4. The control sample (raw pulque) had the highest titratable acidity on day 30 of storage, which might have resulted from the high microbial counts. There were significant differences (p < 0.05) in titratable acidity among raw pulque, thermosonication treatments, and conventional pasteurization. As expected, because thermosonication and conventional pasteurization samples had a lower microbial load of LAB during storage. These bacteria are responsible for the production of lactic acid. A similar behavior was reported by [23] in pulque processed by ohmic heating. The values of titratable acidity of raw pulque ranged from 0.36 up to 1.32 g of lactic acid/100 mL during 30 storage days. At the same time, the values of titratable acidity in TS samples at 75%, 85%, and 95% were 0.28–0.93 g lactic acid/100 mL (Fig. 4A, B, and C). According to [34], Mexican standard NMX-V-037-1972, titratable acidity in pulque must be 0.75 g lactic acid/100 mL sample. This parameter was close to being achieved on days 21 (0.68–0.78 g lactic acid/100 mL) and 24 (0.71–0.83 g lactic acid/100 mL) of storage. However, what determines the acceptance of the beverage will be the sensory analysis. Titratable acidity values reported by [35], [36] on yogurt were ranged from 0.6 up to 1.10 g lactic acid/100 mL; these are higher than those found in thermosonicated pulque. The titratable acidity values during the storage period increased in thermosonication treatments, similarly to in the samples processed by conventional pasteurization, which exhibited titratable acidity values ranging from 0.28 to 0.74 g lactic acid/100 mL. On days 21 and 24, these values were 0.64 and 0.69 g lactic acid/100 mL, respectively. Conventional pasteurization exhibited lower values of titratable acidity than those of thermosonication treatments. This could be due to that a result of lower microbial growth during the storage period is due to that conventional pasteurization causes a lethal effect on bacteria, while thermosonication treatments have a sub-lethal effect (Fig. 2). The acidity levels of fermented beverages are directly associated with microbial load and activity conditions [36]. Parameters such as acidity and the microbial load of LAB determine the quality of pulque; therefore, the conditions tested for thermosonication have demonstrated to conserve both of these parameters.
Fig. 4.
Acidity changes in raw (●) and processed pulque by thermosonication at 75%, 6 min (■); 75%, 9 min (□); 85%, 4 min (♦); 85%, 6 min (◊), 95%, 3 min (▲); 95%, 5 min (▲), and conventional pasteurization (○) stored 30 days at 4 ± 1 °C. Each value is expressed as mean ± standard deviation.
3.5.2. Alcoholic content
The ethanol content in pulque is important because the latter is considered an alcoholic beverage. Table 1 shows the alcohol content during storage in raw and pulque processed by thermosonication and conventional pasteurization. On day 1 values of the alcohol content (ethanol % v/v) did not show significant changes (p < 0.05) between the raw pulque and thermosonication treatments. All treatments exhibited an increase in alcohol content during the storage period, but lowest ethanol values were found in samples processed by conventional pasteurization (4.34 ± 0.03% v/v on day 24 and 4.38 ± 0.03% v/v on day 30 of storage). Instead, thermosonication treatments with the highest alcohol content were at 75% (for 6 and 9 min) and 85% (for 4 and 6 min), demonstrating values ranging from 4.75 to 4.95% v/v at day 24 of storage. At day 30, these values increased from 4.89 to 5.14% v/v of ethanol. A similar alcohol content ranging from 4 to 5% v/v was reported by [28] in the burukutu alcoholic beverage. Samples processed at 95% during 3 and 5 min exhibited an alcohol content ranging from 4.61 ± 0.03% v/v–4.48 ± 0.02% v/v at day 24 of storage, while at day 30 of storage, these increased up to 4.75 ± 0.04% v/v–4.60 ± 0.02% v/v. The alcohol content in fermented beverages is directly related to the multiplication of yeasts during the storage period [37]. The treatment that showed greater development of yeasts was thermosonication compared to conventional pasteurization (Fig. 2D-F). However, both treatments, that is, conventional pasteurization and thermosonication, complied with Mexican standard NMX-V-037-1972 for commercial pulque, which establishes an alcohol content ranging from 4.0 to 6.0% v/v. The experimental results demonstrated that thermosonication treatments did not affect the alcohol content of the beverage, which was conserved up to 24 storage days.
Table 1.
Alcoholic content in raw and processed pulque by thermosonication and conventional pasteurization stored for 30 days at 4 ± 1 °C.
| Alcoholic content (% V/V) | |||||
|---|---|---|---|---|---|
| Storage days | 1 | 8 | 15 | 24 | 30 |
| Treatment | |||||
| Raw pulque | 3.37 ± 0.02Ad | 6.18 ± 0.01Ac | 6.93 ± 0.02Ab | 7.16 ± 0.04Aa | 6.86 ± 0.03Ab |
| 1CP | 3.33 ± 0.03Ad | 3.93 ± 0.01Fc | 4.26 ± 0.01Eb | 4.34 ± 0.03Fab | 4.38 ± 0.02Fa |
| 75%, 6 min | 3.37 ± 0.02Ae | 4.59 ± 0.007Bd | 4.80 ± 0.01Bc | 4.95 ± 0.001Bb | 5.14 ± 0.04Ba |
| 75%, 9 min | 3.34 ± 0.02Ad | 4.52 ± 0.007Cc | 4.67 ± 0.02Cb | 4.75 ± 0.02Cb | 4.93 ± 0.07Ca |
| 85%, 4 min | 3.36 ± 0.04Ad | 4.42 ± 0.007Dc | 4.74 ± 0.02BCb | 4.88 ± 0.03Ba | 4.91 ± 0.04Ca |
| 85%, 6 min | 3.33 ± 0.03Ad | 4.39 ± 0.00Dc | 4.68 ± 0.01Cb | 4.75 ± 0.04Cb | 4.89 ± 0.03CDa |
| 95%, 3 min | 3.36 ± 0.007Ad | 4.31 ± 0.02Ec | 4.53 ± 0.03Db | 4.61 ± 0.03Db | 4.75 ± 0.04DEa |
| 95%, 5 min | 3.34 ± 0.02Ad | 4.29 ± 0.007Ec | 4.33 ± 0.020Ec | 4.48 ± 0.02Eb | 4.60 ± 0.02Ea |
The results are expressed as the mean (n = 2) ± SD of two independent experiments. Different uppercase by columns (treatments) and lowercase letters by rows (storage days) indicate significant difference (p < 0.05) using the Tukey test.
: Conventional pasteurized (63 °C/30 min).
3.5.3. Lightness (L*)
The lightness value or the whiteness is an important quality parameter of pulque, and a high value is desired for conservation during storage. Untreated pulque revealed an initial L* value of 38.73 ± 0.01; as storage elapses, lightness decreases to 23.76 ± 0.06 and 18.20 ± 0.01 on days 24 and 30 of storage, respectively (Table 2). It is clear that, during storage, there was a loss of lightness in all treatments, but this loss was greater in conventional pasteurization. Thermosonication treatments exhibited values of lightness in ranging from 31.93 to 33.69 at 24 storage days, while conventional pasteurization had a value of 27.18. This suggests that microorganisms and enzymes could cause degradation of carbohydrates and changes in color. Similar behavior was obtained by [23] in pulque processed by ohmic heating. However, [29] reported that the phenomenon of cavitation may induce changes in color due to the acceleration of chemical reactions, the rise of the diffusion rate, dispersion, the formation of aggregates, and particle breakdown. Thermosonication treatments up to 24 storage days conserved the lightness; after this time, the values decreased significantly (p < 0.05). On the other hand, conventional pasteurization did not conserve the lightness after 15 storage days. Therefore, thermosonication demonstrated that it could comprise an appropriate technology for processing pulque and for conserving its color and physicochemical parameters.
Table 2.
Color in raw and processed pulque by thermosonication and conventional pasteurization stored for 30 days at 4 ± 1 °C.
| L* |
||||
|---|---|---|---|---|
| Storage days | 1 | 15 | 24 | 30 |
| Treatment | ||||
| Raw pulque | 38.73 ± 0.01a | 34.17 ± 0.06d | 23.76 ± 0.06 g | 18.20 ± 0.01f |
| 1CP | 37.85 ± 0.05d | 34.10 ± 0.03d | 27.18 ± 0.02f | 24.17 ± 0.06e |
| 75%, 6 min | 38.63 ± 0.03ab | 35.83 ± 0.09a | 33.69 ± 0.05a | 26.88 ± 0.05d |
| 75%, 9 min | 38.66 ± 0.01ab | 35.34 ± 0.15ab | 33.49 ± 0.05ab | 27.74 ± 0.04c |
| 85%, 4 min | 38.58 ± 0.02bc | 34.90 ± 0.03bc | 32.68 ± 0.04c | 29.00 ± 0.02b |
| 85%, 6 min | 37.96 ± 0.04d | 34.50 ± 0.21 cd | 33.31 ± 0.14b | 28.30 ± 0.04b |
| 95%, 3 min | 38.48 ± 0.03c | 35.93 ± 0.07a | 31.98 ± 0.04d | 28.19 ± 0.07b |
| 95%, 5 min | 38.61 ± 0.01b | 34.99 ± 0.17bc | 31.93 ± 0.07e | 29.16 ± 0.01a |
L*: lightness value.
Each value is expressed as mean ± standard deviation (n = 2). Means in the same column followed by different lowercase letters represent significant differences (p < 0.05).
: Conventional pasteurized.
3.6. Sensory analysis
Changes in sensory properties in raw and processed pulque are shown in Table 3. On day 1 of storage, there were no significant differences (p < 0.05) between the thermosonication and conventional pasteurization treatments of raw pulque in the parameters of aroma and color. After conventional pasteurization, flavors revealed a significant difference (p < 0.05) with respect to the raw pulque. On day 24 of storage, raw pulque presented the lowest acceptability with a score of 1.0 (Dislike extremely) in the parameters of flavor, aroma, and color. According to [21], [23], after day 3 of storage at 4 °C, the sensory quality of raw pulque is seriously affected. Regarding the intensity of acidity and the perception of alcohol at day 24 of storage, these were very strong for raw pulque, resulting in its not being suitable for consumption. Samples processed by thermosonication up to day 24 of storage at 4 °C were evaluated as “Like moderately” in flavor, aroma, and color. The highest score of acceptance was obtained in these parameters in the samples thermosonicated at 85% during 4 and 6 min. Thermosonication treatments had a better acceptance on a scale of –intermediate- intensity of acidity and alcohol perception. Similar results were reported by [23] on pulque processed by ohmic heating. These authors reported that the sensory properties were conserved up to day 22.
Table 3.
Sensory analysis in raw and processed pulque by thermosonication and conventional pasteurization stored for 1, 24, and 30 days at 4 ± 1 °C.
| Storage days |
Day 1 |
Day 24 |
Day 30 |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter/ Treatments | Flavor | Aroma | Color | 2Acidity | 2Alcohol perception | Flavor | Aroma | Color | 2Acidity | 2Alcohol perception | Flavor | Aroma | Color | 2Acidity | 2Alcohol perception |
| Raw pulque | 8.05bc | 8.05a | 8.10a | 2.30a | 2.90a | 1.00e | 1.00d | 1.00e | 5.00a | 5.00a | 1.00c | 1.00d | 1.00e | 5.00a | 5.00a |
| 1CP | 6.80e | 7.95a | 7.90a | 1.45c | 2.05b | 6.55d | 6.30c | 5.50d | 2.50c | 2.25c | 1.30c | 4.20b | 2.90bc | 3.75d | 2.35d |
| 75%, 6 min | 7.35de | 8.15a | 8.10a | 2.00ab | 2.85a | 7.10 cd | 7.35ab | 7.15bc | 3.35b | 3.10b | 4.30a | 2.70c | 2.90bc | 4.10 cd | 4.25b |
| 75%, 9 min | 8.35abc | 8.05a | 8.15a | 2.00ab | 2.95a | 7.50abc | 7.45ab | 7.40abc | 3.20b | 3.35b | 2.65b | 2.75c | 2.75bcd | 4.60b | 4.20bc |
| 85%, 4 min | 8.60ab | 8.10a | 8.05a | 2.05ab | 2.90a | 8.20a | 7.65ab | 7.85ab | 3.15b | 3.40b | 4.25a | 3.95b | 2.95b | 4.15c | 4.0bc |
| 85%, 6 min | 8.75a | 8.20a | 8.20a | 2.00ab | 2.95a | 8.05ab | 7.85a | 7.90a | 3.50b | 3.30b | 3.05b | 4.85a | 3.70a | 4.20c | 4.20bc |
| 95%, 3 min | 7.90 cd | 8.10a | 8.15a | 1.90abc | 2.85a | 7.35bc | 6.90bc | 7.65ab | 3.15b | 3.20b | 2.70b | 3.75b | 2.40 cd | 3.85 cd | 4.15bc |
| 95%, 5 min | 8.00c | 8.05a | 8.05a | 1.80f | 2.90a | 6.80 cd | 7.10ab | 6.80c | 3.45b | 3.10b | 2.75b | 3.75b | 2.30d | 4.0 cd | 3.85c |
Means in the same column followed by different lowercase letters represent significant differences (p < 0.05).
Sensory code: 1 = Dislike extremely; 2 = Dislike very much; 3 = Dislike moderately; 4 = Dislike slightly; 5 = Neither like or dislike; 6 = Like slightly; 7 = Like moderately; 8 = Like very much; 9 = Like extremely
CP: Conventional pasteurization (63 °C, 30 min).
Intensity scale: 1 = very weak; 2 = weak; 3 = Intermediate; 4 = strong; 5 = very strong.
On the other hand, at day 30 of storage raw and processed pulque by conventional pasteurization and thermosonication exhibited a loss of sensory parameters and a higher intensity of acidity and alcohol perception. Similar results were reported by [37] in thermosonicated beer that, at day 30 days of storage, showed alterations in sensory properties. The maximal duration of the sensory aroma of pulque was 24 storage days at 4 °C. After this time, no treatment preserved the sensory properties. Sensory analysis of parameters of flavor and aroma were carried out to detect metallic notes during 1, 8, 15, 24, and 30 storage days (data not shown). In the obtained results, the metallic note was not detected in any of the pulque samples processed by thermosonication. Even the samples of raw and processed pulque by CP demonstrated the absence of metallic notes at the same storage time. Similar results were obtained by [38] in fruit juices processed by ultrasound high intensity; these authors did not find a metallic taste. In general, the sensory attributes of thermosonicated pulque exhibited greater acceptance than those of pulque processed by conventional pasteurization. This could suggest that thermosonication is a viable technology for processing pulque and preserving its sensory properties up to day 24 at 4 °C.
4. Conclusions
The optimal conditions found for processing pulque by thermosonication were at 75% for 6 and 9 min and 85% for 4 and 6 min. These conditions allowed the survival of lactic acid bacteria and yeasts that can develop during storage and that can preserve the quality of pulque. Thermosonication achieves the extension of the shelf life of pulque up to day 24 of storage at 4 °C. At the same time, sensory and physicochemical properties such as color, alcohol content, and acidity were conserved. Conventional pasteurization revealed a lower growth rate of LAB and yeasts during the storage period as a result; it retains some parameters, such as acidity and alcohol content, but not color and sensory quality. Therefore, conventional pasteurization could extend the shelf life in pulque up to day 24 of storage, but does no retain all of the quality parameters, which are important for acceptance of the beverage by the consumer. The LAB identified were L. kefiri, L. acidophilus, and L. hilgardii. These strains could have some applications on the food industry. The process times applied by thermosonication were less than 9 min, which are more efficient than those of the conventional pasteurization process (63 °C for 30 min), further saving energy. In other words, thermosonication can be utilized as an alternative method for processing pulque and preserving its quality during storage.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
Alejandra Elizabeth Alcántara-Zavala thanks CONACYT-México for her PhD fellowship, Adair Jiménez for his support in SEM analyses, and Luz María Reyna Aviléz-Arellano, José Juan Velés-Medina, and Verónica Flores-Casamayor for their technical support.
Author contributions
Alejandra. E. Alcántara-Z., preparation, creation, and presentation of the published work, specifically writing the initial draft, analysis and organization of the data, Juan de Dios Figueroa-C., oversight and leadership responsibility for research activity planning and execution, J. Francisco Pérez-R collaborated with the ultrasound equipment, Gerónimo Arámbula-V contributed with ideas, the formulation of overarching research goals, and aims, and Dalia E. Miranda-C provided the laboratory work to identify microorganisms by PCR.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ultsonch.2020.105290.
Contributor Information
Alejandra Elizabeth Alcántara-Zavala, Email: alejandra.alcantara@cinvestav.mx.
Juan de Dios Figueroa-Cárdenas, Email: jfigueroa@cinvestav.mx.
Juan Francisco Pérez-Robles, Email: jfperez@cinvestav.mx.
Gerónimo Arámbula-Villa, Email: garambula@cinvestav.mx.
Dalia E. Miranda-Castilleja, Email: emc_as10@hotmail.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Lee H., Zhou B., Liang W., Feng H., Martin S.E. Inactivation of Escherichia coli cells with sonication, manosonication, thermosonication, and manothermosonication: microbial responses and kinetics modeling. J. Food Eng. 2009;93:354–364. doi: 10.1016/j.jfoodeng.2009.01.037. [DOI] [PubMed] [Google Scholar]
- 2.Xu J., Zhang M., Cao P., Adhikari B., Yang C. Microorganisms control and quality improvement of stewed pork with carrots using ZnO nanoparticles combined with radio frequency pasteurization. Food Biosci. 2019;32 doi: 10.1016/j.fbio.2019.100487. [DOI] [Google Scholar]
- 3.R. Kadkhodaee, R., and M. J. P., Ultrasonic inactivation of Bacillus alpha-amylase. I. Effect of gas content and emiting face of probe. Ultrason. Sonoch., 15 (2007) 133–142. DOI:10.1016/j.ultsonch.2007.02.005. [DOI] [PubMed]
- 4.Gao S., Hemar Y., Ashokkumar M., Paturel S., Lewis G.D. Inactivation of bacteria and yeast using high-frequency ultrasound treatment. Sci. Water Res. 2014;60:93–104. doi: 10.1016/j.ultsonch.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 5.Villamiel M., De Jong P. Influence of high-intensity ultrasound and heat treatment in continuous flow on fat, proteins, and native enzymes of milk. J. Agric. Food Chem. 2000;48:472–478. doi: 10.1021/jf990181s. [DOI] [PubMed] [Google Scholar]
- 6.A. Esparza, L. M., Velázquez, E. R. M., Roig, X. A., García, G. H.S., Sayago, A. G. S., Montalvo, G. E., Technology thermosonication: an alternative processing for fruit and vegetable juices, Trends Food Sci. Technol., 61 (2017) 26–37. DOI:10.1016/j.tifs.2016.11.020.
- 7.Wang S., Kang J., Zhang X., Guo Z. Dendrites fragmentation induced by oscillating cavitation bubbles in ultrasound field. Ultrasonics. 2018;83:26–32. doi: 10.1016/j.ultras.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Ashokkumar M. The characterization of acoustic cavitation bubbles – an overview. Ultrason. Sonochem. 2011;18:864–872. doi: 10.1016/j.ultsonch.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 9.M. García, M. Costa, T. V. Fonteles, and S. Tibérito D. J., Alcántara, A. L., M., M. R. Narciso, F. A. Rodrigues, High-intensity ultrasound processing of pineapple juice, Food Bioprocess, Technol., 6 (2013) 997–1006. DOI:10.1007/s11947-011-0746-9.
- 10.Pingret D., Chemat F., Fabiano-Tixier A.S. Degradation during application of ultrasound in food processing: a review. Food Control. 2013;31:593–606. doi: 10.1016/j.foodcont.2012.11.039. [DOI] [Google Scholar]
- 11.Piyasena P., Mohareb E., Mckellar R.C. Inactivation of microbes using ultrasound: a review. Int. J. Food Microbiol. 2003;87:207–216. doi: 10.1016/S0168-1605(03)00075-8. [DOI] [PubMed] [Google Scholar]
- 12.Jambrak A.R., Šimunek M., Evačić S., Markov K. Influence of high power ultrasound on selected moulds, yeasts and Alicyclobacillus acidoterrestris in apple, cranberry and blueberry juice and nectar. Ultrasonics. 2018;83:3–17. doi: 10.1016/j.ultras.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 13.Kiang W.S., Bhat R., Rosma A., Cheng L.H. Effects of thermosonication on the fate of Escherichia coli O157:H7 and Salmonella enteritidis in mango juice. Appl. Microbiol. 2012;56:251–257. doi: 10.1111/lam.12042. [DOI] [PubMed] [Google Scholar]
- 14.Paniwnyk L. Applications of ultrasound in processing of liquid foods: a review. Ultrason. Sonochem. 2017;38:794–806. doi: 10.1016/j.ultsonch.2016.12.025. [DOI] [PubMed] [Google Scholar]
- 15.Erkaya E.M., Bas T., Mustafa M., Fatih S. Effect of thermosonication on physicochemical, microbiological and sensorial characteristics of ayran during storage. Ultrason. Sonochem. 2015;23:406–412. doi: 10.1016/j.ultsonch.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 16.Morales J.A.T., Santo B.H., Miranda J.R. Effect of ultrasound on the techno-functional properties of food components/ingredients: a review. Ultrason. Sonochem. 2019;61 doi: 10.1016/j.ultsonch.2019.104787. [DOI] [PubMed] [Google Scholar]
- 17.Kandylis P., Pissaridi K., Bekatorou A., Kanellaki M., Koutinas A.A. Dairy and non-dairy probiotic beverages. Curr. Opin. Food Sci. 2016;7:58–63. doi: 10.1016/j.cofs.2015.11.012. [DOI] [Google Scholar]
- 18.Granato D., Branco G.F., Nazzaro F., Cruz A.G., Faria A.F. Functional foods and non-dairy probiotic food development: trends, concepts, and products. Compr. Rev. Food Sci. Food Saf. 2010;9:292–302. doi: 10.1111/j.1541-4337.2010.00110.x. [DOI] [PubMed] [Google Scholar]
- 19.Giles G.M., Sandoval G.J.G., Matus V., Campos Q.I., Bolívar F., Escalante A. In vitro and in vivo probiotic assessment of Leuconostoc mesenteroides P45 isolated from pulque, a Mexican traditional alcoholic beverage. Springerplus. 2016;5:1–10. doi: 10.1186/s40064-016-2370-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Escalante A., Giles G.M., Hernández G., Córdova A.M.S., López M.A., Gosset G., Bolívar Analysis of bacterial community during the fermentation of pulque, a traditional Mexican alcoholic beverage, using a polyphasic approach. Int. J. Food Microbiol. 2008;124:126–134. doi: 10.1016/j.ijfoodmicro.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 21.A. Escalante, A. López, S. D. R., Velázquez, G. J. E., Gílez, G. M., Bolívar, F., López, Pulque, a traditional Mexican alcoholic fermented beverage: historical, microbiological, and technical aspects, Front. Microbiol., 7 (2016) 1–18. DOI:10.3389/fmicb.2016.01026. [DOI] [PMC free article] [PubMed]
- 22.Maravilla E.T., Lenoir M., Reyes L.M., Allain P., Sokol T., Langella P., Pardo M.E.S., Humarán L.G.B. Identification of novel anti-inflammatory probiotic strains isolated from pulque. Appl. Microbiol. Biotechnol. 2016;100:385–396. doi: 10.1007/s00253-015-7049-4. [DOI] [PubMed] [Google Scholar]
- 23.Zavala A.E.A., Cárdenas J.D.D.F., Sánchez E.M., Flores H.E.M., Tapia J.A.A., Medrano S.M.A. Application of ohmic heating to extend shelf life and retain the physicochemical, microbiological, and sensory properties of pulque. Food Bioprod. Process. 2019;118:139–148. doi: 10.1016/j.fbp.2019.09.007. [DOI] [Google Scholar]
- 24.Tapia A.A., Ramírez M.C.E., Tamplin M.L., Iturriaga M.H. High-throughput sequencing of microbial communities in poro cheese, an artisanal Mexican cheese. Food Microbiol. 2014;44:136–141. doi: 10.1016/j.fm.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 25.AOAC, Official Method of Analysis, Method 942.15, Quantitative Chemistry, 2005.
- 26.AOAC, Official Method of Analysis, Methods 948.08, 957.03, 26.1.08, Determination of Alcohol, 1998.
- 27.M. Everitt, Chapter 8: Consumer-target Sensory Quality., A. Pullman University, WA, USA: Editorial Washington State University, Global Issues in Food Science and Technology, 2009, pp. 117–128.
- 28.Adesulu A.D.A., Dahunsi A.T., Olayanju S.O. Synergistic microbial interactions between lactic acid bacteria and yeasts during production of Nigerian indigenous fermented foods and beverages. Food Control. 2019;110:106963. doi: 10.1016/j.foodcont.2019.106963. [DOI] [Google Scholar]
- 29.Cansino S.C., Hernández I.R., Olivares L.D., Bustos D.P.J., Ortega J.A.A., Moreno E.R. Effect of ultrasound on survival and growth of Escherichia coli in cactus pear juice during storage. Brazilian J. Microbiol. 2016;47:431–437. doi: 10.1016/j.bjm.2016.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Toscano M., De Grandi R., Leonardo V., Mattina R., Drago L. Ability of Lactobacillus kefiri LKF01 (DSM32079) to colonize the intestinal environment and modify the gut microbiota composition of healthy individuals. Dig. Liver Dis. 2017;49:261–267. doi: 10.1016/j.dld.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 31.Panghal A., Janghu S., Virkar K., Gat Y., Kumar V., Chhikara N. Potential non-dairy probiotic products– a healthy approach. Food Biosci. 2018;21:80–89. doi: 10.1016/j.fbio.2017.12.003. [DOI] [Google Scholar]
- 32.Bossi A., Rinalducci S., Zolla L., Antonioli P., Righetti P.G., Zapparoli G. Effect of tannic acid on Lactobacillus hilgardii analysed by a proteomic approach. Appl. Microbiol. 2007;102:787–795. doi: 10.1111/j.1365-2672.2006.03118.x. [DOI] [PubMed] [Google Scholar]
- 33.Cameron M., McMaster L.D., Britz T.J. Electron microscopic analysis of dairy microbes inactivated by ultrasound. Ultrason. Sonochem. 2008;15:960–964. doi: 10.1016/j.ultsonch.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 34.NMX-V-037-1972, Mexican Standard. Pulque manejado a granel. https//www.economia-nmx.gob.mx/normasmx/detallenorma [Accessed 21 Marzo 2020].
- 35.Gursoy O., Yilmaz Y., Gokce O., Ertan K. Effect of ultrasound power on physicochemical and rheological properties of yoghurt drink produced with thermosonicated milk. J. Food Agric. 2016;28:235–241. doi: 10.9755/ejfa.2015-09-719. [DOI] [Google Scholar]
- 36.Senadeera S.S., Prasanna P.H.P., Jayawardana N.W.I.A., Gunasekara D.C.S., Senadeera P., Chandrasekara A. Antioxidant, physicochemical, microbiological, and sensory properties of probiotic yoghurt incorporated with various Annona species pulp. Heliyon. 2018;4 doi: 10.1016/j.heliyon.2018.e00955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deng Y., Bi H., Yin H., Yu J., Dong J., Yang M., Ma Y. Influence of ultrasound assisted thermal processing on the physicochemical and sensorial properties of beer. Ultrason. Sonochem. 2018;40:166–173. doi: 10.1016/j.ultsonch.2017.07.017. [DOI] [PubMed] [Google Scholar]
- 38.Simunek M., Jambrak R.A., Petrovic M., Hrvoje J., Major N., Herceg Z., Mirjana H., Tomislava V. Aroma profile and sensory properties of ultrasound treated apple juice and nectar. Food Technol. Biotechnol. 2013;51:101–111. ISSN 1330-9862. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.