Highlights
-
•
Ultrasound freeze-thawing pretreatments improved the vacuum freeze-drying.
-
•
Pretreatment reduced the drying time and energy consumption.
-
•
Pretreatment changed the microstructure to various extent.
-
•
Ultrasound pretreatment retained most of the quality characteristics.
-
•
Pretreatment had no effect on the function groups and structure of pectin.
Keywords: Okra, Ultrasound, Freeze-thawing, Vacuum-freeze drying efficiency, Quality characteristics
Abstract
Vacuum freeze-drying is a new and high technology on agricultural product dehydrating dry, but it faces the high cost problem caused by high energy consumption. This study investigated the effect of ultrasound (US), freeze-thawing (including the freeze-air thawing (AT), freeze-water thawing (WT), freeze-ultrasound thawing (UST), and freeze-air ultrasound thawing (AT + US)) pretreatments on the vacuum freeze-drying efficiency and the quality of dried okra. The results indicated that the application of ultrasound and different freeze-thawing pretreatments reduced the drying time by 25.0%–62.50% and the total energy consumption was 24.28%–62.35% less. The AT pretreatment reduced the time by of okra slices by 62.50% and the total energy consumption was 62.35% less. The significant decrease in drying time was due to a change in the microstructure caused by pretreatment. Besides, the okra pretreated with the US retained most of the quality characteristics (flavor, color, hardness, and frangibility) among all methods, while, AT + US had the most changeable characteristics in quality, which is deprecated in our study. The okra pretreated with the US and AT, separately, had the best dry matter content loss (9.008%, 5.602%), lower chlorophyll degradation (5.05%, 5.44% less), and higher contents of total phenolics, total flavonoids, and pectin, with strong antioxidant capacity, compared to other methods. The pretreatments did not have a large effect on the functional groups and the structure of pectin, but slightly affected the viscosity. It can be concluded that AT and US pretreatment methods are better than others.
1. Introduction
Okra is an annual herb used in catering, health care, medical treatment and other industries [1]. It is rich in protein, carotene, vitamins, trace elements, flavonoids, viscous substances, etc. [2]. However, because the okra has a wide surface area, a high moisture content and a high respiration, it is susceptible to external environmental influences. Thus, its economic value can be negatively affected by the fast degradation of nutritional and organic qualities, such as freshness, the richness of nutrients, during transportation, processing and storage. Notably, the harvesting time usually couples with the high-temperature environment, which makes the storage procedure of okra facing challenges [3].
Drying is widely used to obtain food products with long shelf life with lower storage cost [4]. With the continuous improvement in life quality, consumers required more dried products with a better organic quality (texture, color and luster) and no loss in the nutritional characteristics. Thereby, this life amelioration provided a broad prospect for development of the dried products [5]. In recent years, the common methods of drying okra slices were, e.g., open-sun, hot air drying (HD), vacuum freeze‐drying (VFD), microwave drying (MWD), natural convective drying (NCD), and vacuum drying (VD), Microwave assisted hot air drying [6], [7], [8], [9], [10]. Each drying method has its advantages and drawbacks, such as microwave drying has a high heating efficiency; however, the disparity in the absorption of microwave energy caused by the unequal internal moisture of the material at the late drying stage, is likely to lead to local scorching and poor quality of the dried material [11]. Instead, the VFD method retains the color, aroma, taste, shape and nutritional components of the product to the maximum extent. This method also maintained its inherent form, without hardening the surface of the material, as well as extending the period of storage [12]. However, VFD requires a long-time processing and it is expensive, to remediate this issue, the material can be pretreated before drying to make the drying process more effective and save energy.
There are many ways of pretreatment for fruits and vegetables [14]. The chemical pretreatment method can improve the quality of the drying process, such as using sodium metabisulfite pretreatment on okra [13]. Problems associating with this pretreatment method are food safety and loss of water-soluble substances, such as pectin. Hot water blanching pretreatment is a standard physical pretreatment for okra [13]. It can destroy enzymes and reduce microorganisms, which is suitable for okra drying. But it also causes degradation of its heat-sensitive substances, such as flavonoids and phenolic compounds. Some studies use the pulsed electric field to pretreat okra, since okra mucus is easily soluble in water and affected during the pretreatment process [14]. In depth, pectin is the main component of okra mucus and is a biologically active ingredient. It may cause the electrochemical reactions to corrode the electrode, and the hydrostatic pressure may also damage the shape of the material, making it unsuitable for industrial production. Ultrasound is a non-thermophysical pretreatment methods, which produces a cavitation effect when propagating in an elastic medium, thereby forming an inertial flow sponge effect, promoting the mass transfer and protecting heat-sensitive substances [6]. Some researchers have shown that pre-drying okra by ultrasound can change the microstructure of materials, promote drying efficiency and rehydration capability [15], [16]. It can also increase the shrinking density, porosity and facilitate the extraction of compounds as well as bioactive ingredients [17], [18].
The freeze-thaw processing technology is also one of the non-thermophysical pretreatment methods [14]. Its principle is based on ice crystallization which is formed by free water pierce into the cell wall during the expansion process of frozen water. This allows the free water overflows the cell to promote mass transfer. Freeze-thaw processing is mostly used for meat products [19], however, in recent years, experts have also used it for fruit and vegetable pretreatment [12], [20]. Currently, relevant researches mainly focus on freezing and freeze-thawing cycle process [21], [22]. Some experts have studied that the freeze-thaw pretreatment of carrot roots may reduce the drying time, nevertheless, some limitations have been considered, such as lack of ability to inactivate browning reaction enzymes, severe nutritional loss and high cost [23]. Ultrasound-assisted thawing pretreatment is then suggested creatively, and a standard ultrasound-freeze-thaw pattern is established to enhance the vacuum freeze-drying process.
Previously, some experts have studied ultrasound-assisted thawing on mango pulp and demonstrated that ultrasound processing under optimized conditions could serve as a possible alternative to conventional thawing processing of mango pulp [24]. Other experts also have indicated that ultrasound and freezing/thawing treatment on blueberries can increase the porosity of blueberries, becoming softer, chewy and sticky berries [21]. Okra itself has a loose and porous structure, with more pectin content between cells. The outer epidermis has a protective layer, which is not conducive to the diffusion of water. In fact, okra produces polyphenol oxidase and peroxidase enzymes, which are difficult to inactivate during the freezing process; thus, the shelf life of the product is affected [11]. To our knowledge, the effects of ultrasound/freeze-thaw pretreatment on the vacuum freeze-drying of okra have not been studied yet. Furthermore, only little is known about the influence of ultrasound/freeze-thaw pretreatment on the physicochemical properties of the dried products.
Based on the above-mentioned emerging non-thermal physical pretreatment methods, this study explores single and combined pretreatment methods, that is, ultrasound (US), freeze-thaw (water thawing (WT), ultrasound thawing (UST), and air thawing (AT)), and ultrasound-freeze-thaw (AT + US) pretreatments. This study aims at investigating the drying characteristics of the pretreated okra in terms of the cell viability, the microstructure, drying kinetics and energy consumption, to propose an action mechanism of the pretreatment process. It also explores the effects of VFD on the product quality characteristics (hardness, color, flavor, and contents of chlorophyll, flavonoids and phenolic compounds and antioxidant properties), and the influence of the pretreatment on the pectin content, microstructure and rheological properties of okra pectin.
2. Materials and methods
2.1. Raw materials
Fresh okra pods were obtained from a green ginseng food specialty store in Fujian, the initial water content is 87.17 ± 0.68 g/100 g. Okra pods with uniform length and size were sorted out and stored at 4 ℃ for drying experiments.
2.2. Okra pretreatments
2.2.1. Ultrasound (US)
The okra was processed by using an ultrasound generator (developed by Jiangsu University). The sonication was undertaken under the following conditions: an ultrasound frequency of 40 kHz working on a sweeping mode, the power density is 25 W/L, and the ultrasound time is 30 min [11].
2.2.2. Freeze-thawing
Fresh okra samples were frozen at temperature −20 °C for 24 h [12], then thawed by using four different thawing modes [22], [24]. A portion of the frozen okra was thawed in the water at 25 ℃ (WT), which was the frequently used temperature for thawing [25], [26]. And a second portion was thawed at 25 °C under the ultrasound conditions (UST). The third portion was thawed under the air condition and the same temperature as the second (AT). The fourth portion of the frozen okra did as the third, followed by sonication under the conditions mentioned previously, this is mentioned as AT + US.
2.3. Vacuum freeze-drying of okra samples
The unpretreated sample, ultrasound pretreated sample and frozen samples thawed by different methods (WT, UST, AT, and AT + US) were de-stemmed and cut into slices of 1 cm thickness. The slices were put in the VF-dryer to undergo the drying stages of pre-freezing, main drying, and final drying under a vacuum pressure of 0.518 Mbar (2-6DLSC+, Martin Ltd., Germany). The samples were prone to pre-freezing at −20 °C for 3 h, then dried at −18 °C, which is the main drying, and the final drying at 20 °C [12]. Throughout the entire freeze-drying time, moisture measurements were undertaken at regular intervals by weighing the samples until the okra moisture content (MC) was lower 0.1 g/g. Then the process was stopped, thus the total drying time can be determined. For comparison, okra slices were dehydrated using the vacuum pulsation drying method at 60 ℃ [11].
2.4. Analysis of drying characteristics
2.4.1. Moisture ratio (MR)
The differences in the drying process were examined by determining the change in the moisture ratio with time. The MR was calculated as follows [27]:
| (1) |
where Mo, Mt is the initial and at time t (g/g) dry basis MC of okra (g/g).
2.4.2. Drying rate (DR)
From the drying curves (plots of the MC (g water/g sample, dry basis) versus time), DR (g /g.min−1, dry basis), was calculated using Eq. (2):
| (2) |
where are the dry basis MC at the drying time t1, t2 [28].
2.4.3. Microstructure analysis
2.4.3.1. Cell viability
The pretreated sample was first stained with fluorescein diacetate dye solution (0.01 mg/mL in acetone-water) and was then observed under a fluorescence microscope [29].
2.4.3.2. Scanning electron microscopy (SEM)
For a SEM microscope (Hitachi S-3400N, Hitachi Ltd., Japan) analysis, unpretreated and pretreated samples were cut into 1.1 ± 0.25 mm thicknesses, then lyophilized in a vacuum freeze drier [4].
2.5. Total energy consumption
The total energy consumption (TEC) during processing (including the pretreatment and drying steps) of fresh okra was calculated by using Eq. (3).
| (3) |
where m is the weight of fresh okra samples to be dried (kg). Ep and Ed represent the energy consumption during the pretreatments and drying processes, respectively, and were measured by an electricity meter connected with the vacuum freeze-drier [12].
2.6. Quality characteristics of okra samples
2.6.1. Chlorophyll content
Using 20 mL acetone(80 ± 5%), the chlorophyll was extracted from in 1.0 ± 0.05 g of the okra powder. After that, the mixture was filtered, and the absorbance of the filtrate was subsequently measured at and , during 20 min after completing of the extraction process. The 80% acetone solution was used as a blank.
| (4) |
where C is the concentration of chlorophyll (L/g), V is acetone’s volume (L), M is sample’s weight (g) [11].
2.6.2. Measurement of total flavonoids (TFC), total phenolic acid (TPC) and antioxidant capacity of okra
A weight of 1.0 g of dried okra powder (fresh and pretreated) was dispersed into 40 mL of a 90% methanol solution. Then, ultrasound assisted extraction was carried out at 40 °C for 30 min. After standing for 0.5 h, the reaction mixture was centrifuged at 10000 rpm. The supernatant was put in a beaker. The residue was re-extracted in 90% methanol solution, following the same procedure as before. The two supernatants were combined, and methanol was removed in the temperature of 50 ± 5 °C by a rotary evaporator. The obtained concentrated extract was diluted to 10 mL with water [30]. This extract was used for TFC, TPC, DPPH and ABTS radical-scavenging ability [31].
2.6.3. Color
In this experiment, the colorimeter (Konica Minolta, Inc., Japan) was used to measure color [32].
2.6.4. Hardness and frangibility
A texture analyzer (TAXT2i Ltd., Vienna 153Court, UK) was utilized to test the hardness and frangibility of the dried okra. The parameters were as follows: probe P/2; test force using compression mode; RETURN TO START test; pre-experimental, experimental, after experiment speed 1.0, 2.0, 10.0 mm/s; compression 10%; trigger value/induction: Auto-5.0 g [11].
2.6.5. Electronic nose analysis
The changes in the flavor of the dried okra samples were analyzed using an electronic nose system (FOX 3000, Alpha MOS, Toulouse, France) [4].
2.7. Pectin analysis
2.7.1. Determination of pectin content
In a 50 mL tube which kept in a shaking water bath, 2.5 g okra were mixed with 35 mL absolute ethanol (85 °C). After 10 min, then cooling, the total volume of the mixture was made ~50 mL with absolute ethanol. The mixture was then centrifuged at 10,000 rpm for 5 min. Then do again, until no sugar was detected in the washing solution. The precipitate was then put in a conical flask containing a sulfuric acid solution (pH 0.5) (Adjusting the pH of pure water with concentrated sulfuric acid), and heated at 85 ± 5 °C for 1 h, with intermittent shaking, and then filtered. After cooling, water was used to ensure volume of filtrate to 100 mL. Then, 1.0 mL of the filtrate mixed with 0.25 mL of a 1.0% carbazole-9-ethanol solution, with continuous shaking. After that, that solution quickly added 5.0 mL of sulfuric acid, with vigorous shaking. The mixture was immediately heated at 85 °C for 20 min and shook. Then reaction mixture was quickly cooled under running water. Within 1.5 h, the absorbance of the reaction solution was measured at 525 nm. The pectin content was determined based on the galacturonic acid content [34].
2.7.2. Pectin extraction
Dried okra powder (5 g) was mixed with 100 mL of 75% ethanol, then the mixture was stirred at a speed of 300 rpm in the whole process. The mixture was then filtered under suction; the insoluble residue was then air-dried at 25 ℃. Soon after, the dried residue was dissolved in a phosphate buffer solution (pH 3.9) at a solid-to-liquid ratio of 1:42. The mixture was heated at 60 ℃ for 1 h, then separated at 10,000 rpm for 10 min. The mixture was prepared twice. Afterward, the two supernatants were combined, then deproteinized with 40% of chloroform and 10% of butanol reagent overnight; the operation was repeated until there was no white foam in the water layer. The obtained extract was dialyzed for 48 h at room temperature. During dialysis, the water was changed three times a day. The pectin extract was then freeze-dried [33], [34].
2.7.3. FT-IR spectroscopy of pectin
The sample was then scanned at a range 500–4000 cm−1 by using a FT-IR spectrometer (Nexus 670, Nicolet Co., USA). The scanning was done at a resolution of 4 cm−1, and an average scan of 32 times [33], [59].
2.7.4. Rheological properties
The pectin extracted by the dried okra samples was dissolved in water at a concentration of 8 mg/mL, then warmed at 40 °C in a water bath for 24 h. The viscosity of the sample was tested at 25 °C with a rheometer. Measurements were taken at a shear rate ranging from 0.1 to 100.0 (s−1), a test duration of 300 s, and 60 measurement points [33], [34].
2.8. Statistical analysis
Using SPSS 22.0 statistical software (SPSS Inc., USA) analyzed data. One-way ANOVA (analysis of variance) was used for comparison between groups, and the differences between the Mean ± SD were considered significant at p < 0.05.
3. Result and discussion
3.1. Effect of pretreatments on the drying process of okra
3.1.1. Microstructure
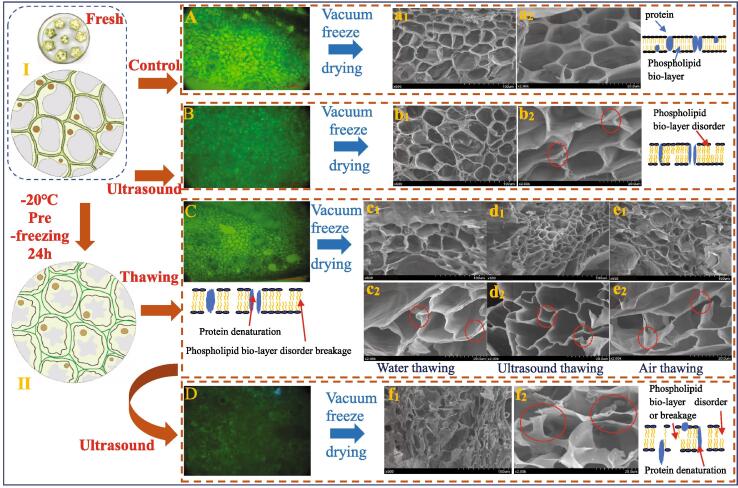
As shown in Fig. 1, the microstructure analysis is used to show the flow chart of the entire experiment. The unpretreated okra has a rectangular or cube-shaped complete honeycomb structure [11]. Pectin and hemicellulose molecules in the cell wall are tightly connected together, and cells has no gap. The cell wall is composed of cellulose, hemicellulose, and pectin, which maintained the cell shape while the membrane layer maintained the turgidity and vitality of the cell [35]. Okra retains the original features and textural characteristics of plant tissues through tissue structure or cell adhesion. The density of the tissue and the pectin cover made the intrinsic water of okra transportable only through the wooden ducts, thus limiting the dehydration of okra [11], [23]. For plant tissues with high water content, there is 50%–95% free water inside the cell, 5–40% fixed water and 1–3% bound water [22]. From Fig. 1, compared to the unpretreated sample, the US pretreatment showed no effect on cell viability, and the cell structure did not change significantly. However, from Fig. 2, the total drying time was saved by 25%. It can be inferred that the inertial flow and sponge effect caused by the US acoustic cavitation trigger the formation of micropores between cells that alleviated the gap between cells [3]. This situation resulted in the displacement of pectin and hemi-fiber molecules, which boosted the diffusion of free water between the cell walls [11].
Fig. 1.
Scanning electron microscopy images of cell viability and structure of okra after pretreatment and vacuum freeze-drying. Ⅰ & Ⅱ: Cell morphology change and water transfer caused by the alteration of osmotic pressure during freezing; A: Cell viability of fresh samples; B, C & D: Cell viability of the sample after ultrasound, freeze-thawing and freeze-thawing/ultrasound pretreatment; a1-a2: The SEM images of control sample after vacuum freeze drying; b1-b2: The SEM images of sample after ultrasound pretreatment and vacuum freeze drying; c1-e2: The SEM images of sample after freeze-thawing pretreatment and vacuum freeze drying; f1-f2: The SEM images of sample after freeze-thawing/ultrasound pretreatment and vacuum freeze drying.
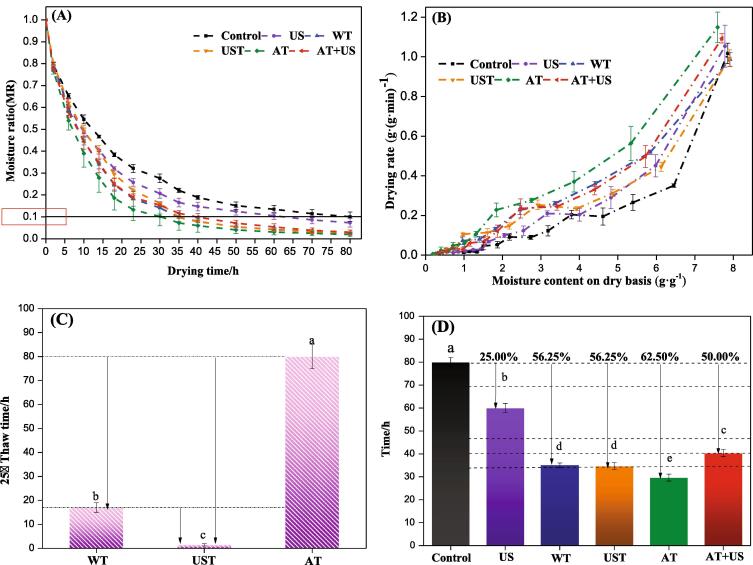
Fig. 2.
Effect of pretreatment methods on drying kinetics during vacuum freeze-drying of okra. Control: un-pretreatment; US: ultrasound pretreatment; WT: water thawing pretreatment; UST: ultrasound thawing pretreatment; AT: air thawing pretreatment; AT + US: air thawing + ultrasound pretreatment. (A): Effect of pretreatment on the change of moisture ratio with time during the vacuum freeze drying process of okra; (B): Effect of pretreatments on the drying rate of okra during the vacuum freeze drying; (C): Effect of pretreatments on the thawing time of okra; (D): Effect of pretreatments on the total vacuum freeze-drying time of okra.
Freezing the vegetable or fruit, followed by thawing, destroyed the cell's internal structure. As shown in Fig. 1, the SEM images showed that the integrity of the cell changes due to the growth of ice crystals, and gaps between cells increased significantly due to shrinkage. During freezing, large ice crystals were formed inside the cells. Due to internal and external osmotic pressure, the water inside the cells diffused outward during the melting process, and as a result, the increased cell gap promoted water transfer [23]. Moreover, the cell membrane was affected because of the growth of ice crystals, causing the deposition of some phospholipid molecules and the appearance of the micropores (Fig. 1), thereby promoting the diffusion of water [22]. Cell wall degradation, cell membrane rupture, and changes in osmotic pressure, all have an impact on cell viability [22]. According to the cell viability diagram (Fig. 1), it can be found that the ultrasound has no effect on cell viability, but after freezing and thawing, cell viability disappears. Other studies also found that the highest drying rate shown by frozen-thawed samples was presumably attributable to the formation of spaces in the tissue due to ice crystals that then facilitated water transfer during drying [23].
3.1.2. Drying characteristics
The effects of ultrasound freeze–thaw pretreatment on the moisture ratio (MR) and drying rate (DR) of okra during vacuum freeze-drying (VFD) are shown in Fig. 2a and b. As the VFD is progressing, the water froze into ice crystals and sublimates. The curves in the Fig. 2a and b represent the rate of ice crystal sublimation during vacuum freeze-drying after different pretreatments. After five hours from the beginning of the drying process, the water content of the US pretreated okra is lower compared with the unpretreated sample. Overall, the moisture content curve of the pretreated samples is steadily lower than the control (Fig. 2a). The drying rate curves showed that in the initial drying period, the drying rate was high. When the critical moisture content was reached, the drying rate attained a plateau, and then decreased. This was because the okra needed more energy to remove the binding water after losing free water during the drying process [11]. The drying rate of the sample after ultrasound pretreatment is significantly greater than that of the unpretreated one. These US improvements have led to significant changes in the microstructure of the okra. US pretreatment facilitated the formation of micropores within the cells, destroyed the structure of the fiber ducts, and made the tissues of the okra loose and porous. As a result, the diffusion of water was increased from the inside of the okra tissue to the outside [3]. At the same time, the effect of ultrasound might damage the fluff on the outer surface of the okra, therefore, reducing the time required for the water evaporation process [11]. Compared to other thermal drying methods, the microchannels formed by pretreatments maintained their pore effects during the vacuum freeze-drying process, thereby maximizing the impact of ultrasound pretreatment [36].
Similarly, the moisture ratio of okra subjected to different ultrasound freeze–thaw pretreatments (WT, UST, AT, AT + US) was also lower than the control. Among them, the dried okra pretreated with the AT shows the lowest moisture ratio and the largest drying rate as well, that is, the water removal rate of this dried sample was the fastest. As shown in Fig. 2c, the UST sample melted significantly faster than WT and AT during the thawing process. It only took 1–2 min to melt, which was 85%–90% faster than WT and AT. The AT sample showed the fastest VFD speed (- 20 °C), and the slowest thawing speed (25 °C) which compared to other pretreatments. Before the drying time of 35 h, the WT is dehydrated faster than the UST pretreated sample, after that, the drying speeds of the two samples were similar (Fig. 2a and b). As can be noticed, the WT pretreated sample is significantly dehydrated faster in the early and later drying stage than the UST sample. Moreover, the internal structure of the WT sample subjected to the freeze–thaw pretreatment has changed significantly (Fig. 1). The ice crystals formed gaps inside the plant tissue, allowing free water to diffuse inside okra tissues and promote mass transfer [12]. The drying characteristic of the AT + US pretreatment was better than the unpretreated and UST pretreated samples, but, still inferior to the US pretreatment- drying characteristics. Pectin is a component of the cell wall, which together with other polysaccharides, binds the surrounding cells together and firmly glues them in the middle layer [54]. Some studies have found that after heating the plant tissues, the main structure of pectin is composed of homogalacturonan acid chains [55]. The structure is split by β-elimination, and the pectin in the middle layer is filtered out [56]. In our study, after freezing and thawing, the structure of the pectin chain between the samples also changed, which weakened the adhesion of the cell wall and significantly softened the tissue. For this cause, the ultrasound pretreatment has made the internal structure heterogeneous, so that its drying properties are intricate. Compared to the unpretreated sample, when the moisture ratio reached 0.1, US, WT, UST, AT and AT + US pretreatments shortened significantly the total drying time of okra by 25%, 56.25, and 56.25%, 62.5% and 50%, respectively (Fig. 2d). The total energy consumption of US, WT, UST, AT and AT + US pretreatment and drying process are reduced by 24.28%, 56.12%, 56.12%, 62.35%, 49.22% (Table 1). The results suggest that the total energy consumption (TEC) during processing (including the pretreatment and drying steps) and the effect on the vacuum freeze-drying time are due to changes in the microstructure caused by the different pretreatment processes [12].
Table 1.
Effect of pretreatment on chlorophyll content, TFC, TPC, pectin content, dry matter loss of okra after vacuum freeze-drying and total energy consumption during the process.
| Chlorophyll content (g/L) | TFC (%) | TPC (%) | Pectin content (%) | Solid loss (%) | Total energy consumption (kWh/kg) | |
|---|---|---|---|---|---|---|
| Control | 1.305 ± 0.003a | 1.012 ± 0.015b | 49.472 ± 0.359a | 15.827 ± 0.093a | – | 300.70 ± 0.25a |
| US | 1.239 ± 0.002b | 0.994 ± 0.002d | 47.908 ± 0.281b | 13.099 ± 0.154c | 9.008 ± 5.455a | 227.70 ± 0.20b |
| WT | 1.138 ± 0.004c | 0.889 ± 0.007f | 34.090 ± 0.194c | 12.531 ± 0.150e | 18.846 ± 10.280ab | 131.95 ± 0.22d |
| UST | 1.143 ± 0.002c | 0.923 ± 0.019e | 33.883 ± 0.082c | 12.802 ± 0.130d | 22.874 ± 8.654ab | 131.95 ± 0.35d |
| AT | 1.234 ± 0.005b | 1.138 ± 0.004a | 33.867 ± 0.112c | 13.556 ± 0.134b | 5.602 ± 9.960a | 113.20 ± 0.30e |
| AT + US | 1.077 ± 0.003d | 8.310 ± 0.068g | 33.867 ± 0.112c | 11.605 ± 0.021f | 34.145 ± 12.999b | 152.70 ± 0.33c |
| Hot | 0.742 ± 0.007e | 9.870 ± 0.060c | 20.67 ± 0.09d | – | – | – |
3.2. Effect of pretreatments on physical quality of dried okra
3.2.1. Electronic nose
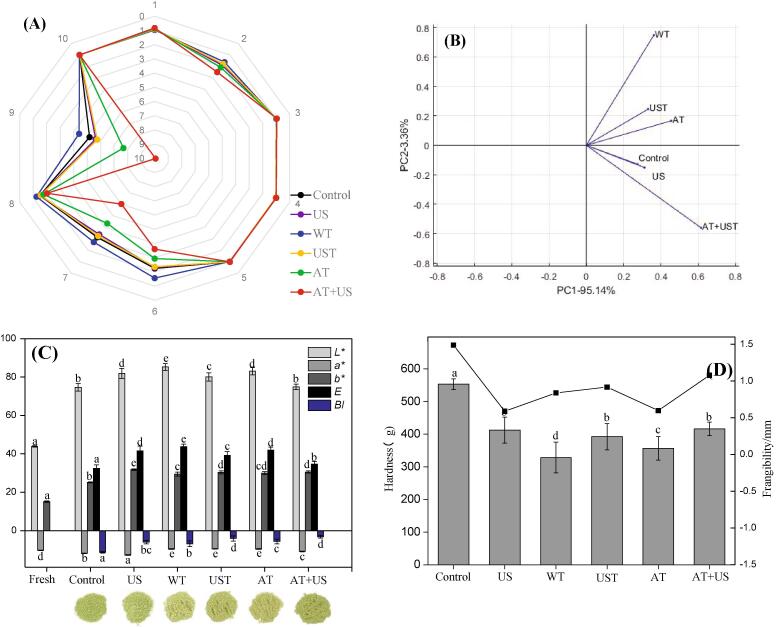
The electronic nose established a radar fingerprint from the response value of the sensor (Fig. 3A), identified the components in the gas, and analyzes them. Each curve represented the flavor intensity of each sample. As shown in Fig. 3A, the serial numbers 1, 2, 3, 4, 5, 6, 7, 8, 9, and 10 represented the sensors W-1C, W-5S, W-3C, W-6S, W-5C, W-1S, W-1W, W-2S, W-2W, and W-3S, respectively. Among them, the sensors W-1C, W-3C, W-6S, W-5C, and W-3S have no difference in response value, which means that there is no difference in the aromatic components, benzenes, ammonias, hydrides, and the short-chain and long-chain alkanes, between the unpretreated and the processed samples. The response values of the sensor W-5S were slightly different from those of W-2S. The response values of the dried okra samples pretreated with US and UST samples overlap with the dried control sample, AT and AT + US were slightly higher than the dried control sample, while WT are slightly lower. All the pretreated dried samples showed no significant impact on nitrogen oxides, alcohols, aldehydes and ketones. The most significant differences in the response value are found between the sensors W-1S, W-1 W, and W-2 W. Results also show that US, UST, and WT pretreatment dried samples have significantly indifferent response values, which are significantly lower than control. These results suggest that the US and UST pretreated dried samples have no effect on methyl and organic sulfur compounds besides WT. The dried samples pretreated with AT and AT + US showed response values perfectly higher than the control sample, that is, the AT and AT + US pretreatments promoted loss of methyl and organic sulfide compounds [12]. The PCA analysis of the electronic nose can quickly distinguish volatile compounds in the different samples. It can be seen from Fig. 3B that the contribution ratio of the first, second principal component are 95.14%, 3.36%. The total contribution ratio is 98.50%, which exceeds 85%, therefore it can reflect the principal component data. Among them, there is no difference between the US pretreated dried sample and the unpretreated sample. The WT and UST pretreated dried samples have no difference in the first principal component, but they have a significant difference in the second principal component. The AT and AT + US pretreated dried samples have a significant difference at the first and second principal components, which is consistent with the trend of a radar chart reported previously [5]. In this study, combined with the micrographs, we can find that the US pretreatment method has little effect on cell viability and cell wall. The UST, WT, AT, and AT + US pretreatment methods require a freezing and thawing process, which affects cell viability and wrinkles. Shrinkage of cell, rupture of cell membrane and volatile components overflow, contributed to the loss of methyl and organic sulfides [12]. However, UST pretreatment is the best method for thawing because it reduced the thawing time. Some studies showed the role of ultrasound in protecting certain chemical components from damage [58], such as the work on pumpkins and found that ultrasound pretreatment can reduce the damage of natural compounds [57]. The results showed that freeze–thaw pretreatment will cause the degradation of volatile components, US pretreatment will not affect volatile components, UST pretreatment can reduce the loss of volatile components by freeze–thaw.
Fig. 3.
Effect of pretreatments on the quality of okra subjected to the vacuum freeze-drying. Control: un-pretreatment; US: ultrasound pretreatment; WT: water thawing pretreatment; UST: ultrasound thawing pretreatment; AT: air thawing pretreatment; AT + US: air thawing + ultrasound pretreatment. (A): Radar chart of the effect of pretreatments on flavor substances of okra after vacuum freeze drying; (B): Effect of pretreatments on main components of flavor substances of okra after the vacuum freeze-drying; (C): Effect of pretreatments on the color of okra after the vacuum freeze-drying; (D): Effect of pretreatments on hardness (bar graph) and frangibility (line graph) of okra after the vacuum freeze-drying.
3.2.2. Color
Color is one of the most critical quality evaluation indicators of dried okra and is a key quality factor affecting consumer acceptance and product market value. The color is mainly measured through the color parameters L*, a*, b*, ΔE, and BI. For freeze-dried samples, as shown in Fig. 3C, the L* value represents the whiteness of the sample, the whiteness values of VFD samples is higher than the fresh sample. However, the AT + US pretreated dried sample’s whiteness values and the unpretreated sample are significantly indifferent. The L* values of all other pretreated dried samples were significantly higher than that of the unpretreated dried sample, which were presented following the order: WT > US and AT > UST. In this context, higher whiteness values to indicated less browning. The a* value represents the red-green spectrum. As known, okra is a green plant, so the color values of all dried samples are negative, the smaller the value, the greener the sample. It can be seen from the figure that among the dried products, the greenness value of the US pretreated dried sample was the smallest, and the green values of the WT, UST and AT pretreatment dried samples were significantly indifferent, but were greater than that of the unpretreated dried sample. These results suggested that the US pretreatment has an ability to protect the green color from damage caused by the freeze-thawing process. The b* value represents the blue-yellow spectrum; the positive value indicates that the sample color is yellowish. All the dried products had b* values significantly higher than the fresh, which means that some browning occurs during the drying process. The yellow value of the pretreated dried samples is higher than that of the non-pretreated dried sample. This may be due to the air presented in the sample environment during the pretreatment process which caused some oxidation reactions to occur. The total color difference (ΔE) is a value used to evaluate the difference between the dry product and the fresh sample. A value greater than 3 implies that there is a very large difference between the dry sample and the fresh sample [5]. Among them, the dried samples pretreated with WT have the largest color difference, followed by the dried samples pretreated with US and AT, then UST, and lastly AT + US. As can be seen, the browning index (BI) of all pretreated dried products is low, indicating that the pretreatments lessen the effect of browning reactions happens during the drying process, that is, better color retention. The BI values of the pretreated are higher than the control, but the values are not much different between the pretreated dried samples. Such higher BI values may occur in part from enzymatic and non-enzymatic browning of food reactions during the pre-treatment process [11], [12].
3.2.3. Texture
For fruit and vegetable crisps, the texture is one of the indicators for evaluating quality. The evaluation of okra is usually based on two indicators, hardness, and frangibility. Hardness is an index for evaluating the softness and hardness of the material, and the test can only be performed when the water activity of the sample is below 0.4. From Fig. 3D, compared to the unpretreated dried okra, the hardness/frangibility value of the pretreated okra sample are significantly lower. This decrease is probably ascribed to the changes in the microstructure after the pretreatment. The cell damage, the formation of larger gaps, and the deformation of the duct structure weakened the cellular structure (Section 3.1.1). The results showed that the hardness of samples obtained by US, UST, AT pretreatment was greater than that of WT and AT; the frangibility of samples obtained by US and AT pretreatment were better than WT, UST, AT + US. As can be seen, dried samples after pretreatments become crisper and there was no systematic trend of the hardness and frangibility among the samples pretreated with the different methods, which may be attributable to the complex organizational structure of okra interacting with each other [11].
3.3. Effect of pretreatments on chemical composition quality of dried okra
Compared to heat dried samples (such as vacuum pulse drying), the chemical quality of vacuum freeze-dried samples was better. The problems of long drying time and large energy consumption were improved by some pretreatments before vacuum freeze-drying, but it is also very important to discuss the influence of pretreatments on the loss of chemical composition. It can be seen that dried sample after pretreatments have a certain loss in dry matter compared to the unpretreated samples (Table 1). Compared to other pretreatments, the dry matter loss of AT + US, UST and WT pretreatment were more (18.846%–34.145%) and there was no significant difference. In contrast to that, the least dry matter loss was shown in the dried samples pretreated with US and AT (9.000% and 5.602%, respectively). Among all samples, a significant difference in the loss of dry matter was only found between the dried samples pretreated with AT + US and the ones pretreated with the US and AT. The dry matter loss is mainly related to the loss of nutrients during the pretreatment.
Chlorophyll is a type of green pigment that can be used for photosynthesis in higher plants. Chlorophyll is insoluble in water, but soluble in solvents such as ethanol, ether and acetone. As can be depicted in Table 1, compared with the heat drying, the freeze-drying has a higher retention of chlorophyll. Similar finding was reported previously [26]. The main factors that degrades chlorophyll are light, oxygen and enzymes. In this study, the freeze-dryer was placed away from light to exclude the effect of light while the oxygen was isolated by the vacuum freeze-drying environment. The main drying process was undertaken in a low temperature environment, as consequence, the enzyme activity was inhibited. In the end of the vacuum freeze-drying, the terminal drying temperature was increased, and the enzyme activity was recovered which might be due to the loss of chlorophyll in the dried products. The influential order for the different pretreatments on the chlorophyll retention of the dried samples as follows: US and AT ˃ WT and UST ˃ AT + US. The results of chlorophyll retention of the pretreated dried sample were consistent with their color measurement results indicating that the US pretreatment had the best green retention (Section 3.2.2).
The total flavonoid content of the dried pretreated okra is shown in Table 1. Compared to the unpretreated sample, the dried sample pretreated with AT showed the highest total flavonoid content, followed by the US, UST, WT, and AT + US. This indicates that the freeze-drying effectively retains flavonoids. Freezing and thawing had a favorable effect on the total flavonoids, and cell damage promoted the extraction of total flavonoids, which explained that the dry sample pretreated by AT had the highest content of total flavonoids. The US pretreatment reduced the loss of flavonoids during drying. In addition, phenolic compounds are the most important chemical constituents of okra. It can be noticed that the phenolic compounds of the vacuum freeze-dried products are significantly better than that of the heat dried product. This is possibly attributed to the less degradation of the heat-sensitive phenolic compounds resulting from the low temperature environment of the freeze dry process. Among the five pretreatment methods, the US pretreated dried sample had the least degradation of the phenolic compounds, which was due to the less damage to the cell structure caused by the ultrasound pretreatment. On the contrary, the freeze-thawing pretreatments (WT, UST, AT, and AT + US) retain less phenolic compounds after drying compared with the US pretreatment. Due to the greater damage to the cell structure caused by the freeze-thawing pretreatments. Despite that, the ultrasound and freeze-thawing pretreatments combined with the vacuum freeze-drying improved the drying efficiency. Although the okra quality was deteriorated by the vacuum freeze-drying, it was still better than the heat dried sample. A study on orange drying methods showed that oranges have the highest TPC content after freeze drying [37]. A study of concentrated broccoli showed that the freezing process can maximize the retention of total phenol content [38].
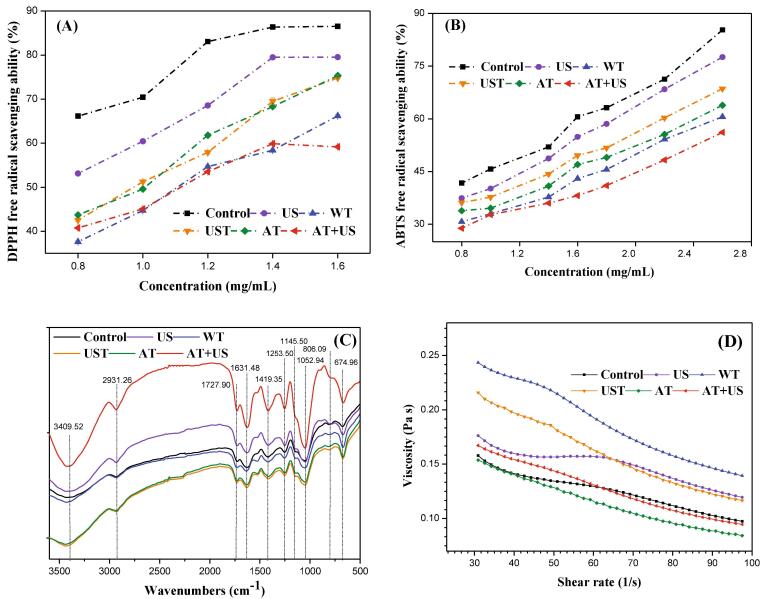
Human rehabilitation needs large amounts of antioxidant substances for protecting human tissue cells against oxidative damage. High content of antioxidants can enhance therapeutic effect and body immunity [60]. Chlorophyll and flavonoid are antioxidant substances which are widely available in rehabilitation cure plant [43], [44], [45]. The antioxidant properties of the dried okra extract are also important indexes to evaluate the quality of okra. This study explored the scavenging ability of the dried okra extracts on DPPH and ABTS free radicals. In Fig. 4Ⅰ and II, the scavenging potential of the un-pretreated dried sample on the free radicals of DPPH and ABTS, at various concentration gradients, was substantially greater. The US dried sample reveals significantly higher DPPH free radical scavenging rate than the dried samples pretreated with the different freeze-thawing processes (Fig. 4I). Moreover, the samples pretreated with UST and AT have significantly higher free radical scavenging ability than the samples pretreated with WT and AT + US. Similarly, the scavenging ability of all okra dried samples on the ABTS free radical follows the order: US ˃ UST ˃ AT ˃ WT ˃ AT + US. These results indicate that the antioxidant activity of the dried sample pretreated with the ultrasound is stronger than that of the dried samples subjected to the different freeze–thaw pretreatments. The good performance was displayed by the dried samples pretreated with US and UST in terms of the antioxidant activity was probably resulted from the enhancement of the chemical reactions induced by the ultrasound waves. Studies have shown that ultrasound could improve the antioxidant properties and the dried product has better antioxidant activity than the fresh sample [40], [31]. The DPPH free radical scavenging ability of the sample is related to the total flavonoid content [31]. Ultrasound cavitation generates hydroxyl groups that can be involved in chemical reactions against components that are easily oxidized, such as flavonoids, producing hydroxylated derivatives, which enhances the antioxidant activity of the component, in addition, the position of the hydroxylation against the effects of the antioxidant activity are also important [41]. Thereby the enhanced hydroxylated compounds improve antioxidant activity, and the impact of this activity on the hydroxylation position is also significant [43]. Other studies have shown that phenolic acid is also one of the main components of okra extract with antioxidant activity [42]. In addition, chlorophyll content also affects the antioxidant activity of okra extract. A study has showed that porphyrin seems to be an essential chemical structure for the antioxidant activity of chlorophyll. Chlorophyll did not decompose the hydroperoxides but reduced free radicals such as 1,1‐diphenyl‐2‐picrylhydrazyl [43]. In this study, From Table 1, we can find that the chlorophyll content in the dried product extract after US, UST and AT pretreatment is higher, which may be the main reason for the higher antioxidant activity (Fig. 4).
Fig. 4.
Effect of pretreatments on antioxidant properties, pectin structure and rheological properties of okra. Control: un-pretreatment; US: ultrasound pretreatment; WT: water thawing pretreatment; UST: ultrasound thawing pretreatment; AT: air thawing pretreatment; AT + US: air thawing + ultrasound pretreatment. (A): Effect of pretreatments on the DPPH free radical scavenging ability of the freeze-dried okra extract; (B): Effect of pretreatments on the ABTS free radical scavenging ability of the freeze-dried okra extract; (C): Effect of pretreatments on the structure of pectin obtained from the freeze-dried okra extract; (D): Effect of pretreatment on rheological properties of pectin from the freeze-dried okra extract.
3.4. Effect of pretreatments on rheological properties of okra pectin
3.4.1. Pectin content
Okra pectin is an acidic heteropolysaccharide with polygalacturonic acid as the main chain. The galacturonic acid content is usually used to indicate the concentration of pectin [46]. The pectin content of the dried control sample (15.827%) was significantly reduced after pretreatments. The pectin content of the dried samples pretreated with US, WT, UST, AT, and AT + US is 13.099%, 12.531%, 12.802%, 13.556%, and 11.605%, respectively. As seen, the loss of the pectin of the dried sample pretreated with AT is lower compared to that of the dried sample pretreated with the US and freeze–thaw process. The ultrasound and freeze–thaw pretreatments damage the cell structure of okra, thus lead to more solubility of the mucus pectin in water, that is, more loss of pectin in these samples versus least loss in the AT sample.
3.4.2. Fourier transform infrared spectrum (FT-IR)
The Fourier transform infrared spectrum analysis of the pectin sample was depicted in Fig. 4III. The results showed that pectin presented similar structural characterization, indicating that the pretreatment had no significant effect on the functional groups of okra pectin. The broad strong absorption peak located between 3300 and 3500 cm−1 refers to the intermolecular and intramolecular hydrogen bonds, which are absorbed due to O–H stretching vibration [47]. In the case of a pectin sample, the O–H absorption region is due to intermolecular and intramolecular hydrogen bonding of the galacturonic acid polymer [47]. After AT + US pretreatment, the pectin O–H extracted from the sample stretches and vibrates, the peak becomes narrow. This suggests that the pectin hydroxyl group is partially oxidized [33]. The absorption peak at 2931 cm−1 (3000–2800 cm−1) refers to the absorption of the C–H bond [48]. The carboxylate group has two bands, an asymmetric stretching vibration at 1631 cm−1, and a weaker symmetrical stretching vibration at 1419 cm−1 [49]. The weaker symmetrical –COO stretch corresponds to a characteristic wavelength of the polygalacturonic acid in the pectin [47]. The effect of the displacement of the wavelengths observed is the result of several factors associated with the areas where the polymer COO– groups of galacturonic acid were inserted and the physical state of a given sample. The two strong absorption peaks locate at 1075 and 1040 cm−1 are characteristics of the type of glycosides bond between sugar units [47]. The absorption peaks found between 1300 and 800 cm−1 are collectively referred to as the “fingerprint” region and are unique to each compound. These absorption peaks are usually difficult to explain [49]. The two strong absorption peaks observed at 1056 cm−1 and 1041 cm−1 may suggest a pyran-type glycosides bonds linking sugar units in the okra pectin sample [49]. The absorption peaks located at 820–950 cm−1 are characteristic absorption peaks of various carbohydrates of α, and β configurations [50]. The existence of the weak absorption peaks at 906 cm−1 and 856 cm−1 are the result of β-glycosidic bonds of the okra pectin sample [50], [53].
3.4.3. Rheological properties
Pectin are complex polysaccharides found in higher plants and are composed mainly of α-1,4-linked d-galacturonic acid (GalA) chains (called homogalacturonan or smooth regions). Pectin also contain hairy regions (called rhamnogalacturonan I) with GalA rhamnose regions. Okra pectin extracted with hot buffer consisted of an unusual pectic rhamnogalacturonan I structure in which acetyl groups and alpha galactose residues are substituted on rhamnose residues within the backbone [33].
A stress scan of the pectin sample was performed. The shear frequency changes when the stress reaches 10%, which cause no damage to the gel structure. Based on Fig. 4IV, the viscosity of the pectin from the control dried sample and the pretreated dried samples showed a decreasing trend with increasing shear rates, from 30 s−1 to 100 s−1. Compared with the control sample, the viscosity of the US pretreated dried sample first showed a downward trend with increasing the shear rates from 30 s−1 to 45 s−1. After that, the viscosity is relatively stable thereafter at the shear rates of 45–65 s−1, then it decreases gradually to lower levels with increasing the shear rates, from 65 s−1 to 100 s−1. At the shear rates 65–100 s−1, both the control sample and the US pretreated dried sample showed a gentle viscosity decrease. The WT, UST, AT, and AT + US pretreated dried samples showed a similar decreasing tendency of the pectin viscosity with increasing the shear rate values from 30 s−1 to 100 s−1. Except for UST sample, the viscosity of the pectin of these samples decreased steadily with increasing the shear rates, showing the phenomenon of shear-thinning, which is one of non-Newtonian fluids. Hydrocolloids such as xanthan gum, galactomannans, and pectin exhibit high viscosity at low concentration and strong shear thinning [33]. This character in the viscosities could be attributed to the different macromolecular compositions of the samples. The high shear-thinning behavior of polysaccharides allows liquid foods to be pumped easily and imparts a thinner consistency during swallowing [52]. As can be seen, the viscosity of the pectin from the dried samples pretreated with the US and UST decreases slowly under the shear rates ranging between 45 s−1 and 65 s−1, and 48 s−1 and 50 s−1, respectively. This gentle interval decrease in the pectin viscosity from the US and UST samples indicates that these pretreatments affect the interaction of the pectin molecules under force, and therefore show characteristics of Newtonian fluids. Except for the AT + US and AT pretreated dried samples, all other pretreated dried samples show higher pectin viscosity under the shear rates ranging between 30 s−1 and 100 s−1 compared with that of the control. In addition, the viscosity levels of the pectin from the AT pretreated dried sample determined in the shear rate range of 30–100 s−1 are lower than those of the control sample. However, the viscosity of pectin, which is very complicated folded shape molecule, is directly proportional to the chain length [51]. A study’s results suggested that the structural variation within the okra pectin greatly affect their rheological behavior and it is suggested that acetylation of the pectin plays an important role through hydrophobic associations [34]. A study also have shown that specific interactions take place among the polymeric chains that modify the rheological behavior of okra pectin [33]. The pectin from the AT pretreated dried sample differs from that of samples pretreated by other methods in that it does not contact with water, so it is speculated that the increase in viscosity may be related to water molecules. A large number of hydrophilic groups can be fully hydrated in water, which may be the reason for the contact with water to increase the viscosity [30].
4. Conclusions
In this work, the results indicated that the use of the ultrasound pretreatment, the freeze–thaw pretreatments (water thawing, ultrasound thawing, air thawing), and the ultrasound-freeze-thawing pretreatment significantly affected the vacuum freeze-drying process and the quality of dried okra slices. All pretreatment methods significantly shortened the drying time and reduced the total energy consumption due to the changes in microstructure and cell viability. The dried okra pretreated with the US protected the green color, retained flavor, hardness and fragility, increased total phenolic content and increased antioxidant capacity compared to unpretreated samples. The dried okra pretreated with US and AT has the lower chlorophyll degradation, higher total flavonoids and pectin, with strong antioxidant capacity. The biggest change in the quality characteristics (dry matter, chlorophyll content, TFC, TPC and pectin content) was found in the dried sample pretreated with AT + US. Additionally, all pretreatments have no remarkable effect on the functional groups and the structure of pectin, but slightly affected the viscosity. Therefore, these pretreatment methods could be helpful in improving the vacuum freeze-drying process, with retaining the original quality of okra.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors are grateful for the support provided by the National Key Research and Development Program of China (NO. 2017YFD0400903-01, 2016YFD0400705-04), the National Natural Science Foundation of China (31801599), Science and Technology Major Project of Anhui (NO. 18030701152), the Key Research and Development Program of Jiangsu (NO. BE2019366) and 333 high level talents of Jiangsu (NO. 2018-26).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ultsonch.2020.105300.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Adelakun O.E., Oyelade O.J., Ade-Omowaye B.I.O., Adeyemi I.A., Van de Venter M. Chemical composition and the antioxidative properties of Nigerian okra seed (Abelmoschus esculentus Moench) flour. Food Chem. Toxicol. 2009;47:1123–1126. doi: 10.1016/j.fct.2009.01.036. [DOI] [PubMed] [Google Scholar]
- 2.Arapitsas P. Identification and quantification of polyphenolic compounds from okra seeds and skins. Food Chem. 2008;110:1041–1045. doi: 10.1016/j.foodchem.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 3.Feng Y., Yu X., Yagoub A.A., Xu B., Wu B., Zhang L., Zhou C. Vacuum pretreatment coupled to ultrasound assisted osmotic dehydration as a novel method for garlic slices dehydration. Ultrason. Sonochem. 2019;50:363–372. doi: 10.1016/j.ultsonch.2018.09.038. [DOI] [PubMed] [Google Scholar]
- 4.Wang L., Xu B., Wei B., Zeng R. Low frequency ultrasound pretreatment of carrot slices: effect on the moisture migration and quality attributes by intermediate-wave infrared radiation drying. Ultrason. Sonochem. 2018;40:619–628. doi: 10.1016/j.ultsonch.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Wang X., Feng Y., Zhou C., Sun Y., Wu B., Yagoub A.A., Aboagarib E.A.A. Effect of vacuum and ethanol pretreatment on infrared-hot air drying of scallion (Allium fistulosum) Food Chem. 2019;295:432–440. doi: 10.1016/j.foodchem.2019.05.145. [DOI] [PubMed] [Google Scholar]
- 6.Gökçe Kocabay Ö., İsmail O. Investigation of rehydration kinetics of open-sun dried okra samples. Heat Mass Transf. 2017;53:2155–2163. [Google Scholar]
- 7.Gong X., Huang X., Yang T., Wen J., Zhou W., Li J. Effect of drying methods on physicochemical properties and antioxidant activities of okra pods. J. Food Process. Preserv. 2019;43 [Google Scholar]
- 8.Aamir M., Boonsupthip W. Effect of microwave drying on quality kinetics of okra. J. Food Sci. Technol. 2017;54:1239–1247. doi: 10.1007/s13197-017-2546-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar D., Prasad S., Murthy G.S. Optimization of microwave-assisted hot air-drying conditions of okra using response surface methodology. J. Food Sci. Technol. 2014;51:221–232. doi: 10.1007/s13197-011-0487-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma H., Huang L., Peng L., Wang Z., Jiang N., Liu C., Li D., Zhang Z., Liu C., Wang D., Niu L., Zhang M. Evaluation of freeze drying combined with microwave vacuum drying for functional okra snacks: antioxidant properties, sensory quality, and energy consumption. LWT – Food Sci. Technol. 2017;82:216–226. [Google Scholar]
- 11.Xu X., Zhang L., Feng Y., Yagoub A.A., Sun Y., Ma H., Zhou C. Vacuum pulsation drying of okra (Abelmoschus esculentus L. Moench): better retention of the quality characteristics by flat sweep frequency and pulsed ultrasound pretreatment. Food Chem. 2020;326 doi: 10.1016/j.foodchem.2020.127026. [DOI] [PubMed] [Google Scholar]
- 12.Feng Y., Tan C., Zhou C., Yagoub A.A., Xu B., Sun Y., Ma H., Xu X., Yu X. Effect of freeze-thaw cycles pretreatment on the vacuum freeze-drying process and physicochemical properties of the dried garlic slices. Food Chem. 2020;324 doi: 10.1016/j.foodchem.2020.126883. [DOI] [PubMed] [Google Scholar]
- 13.Sobukola O. Effect of pre-treatment on the drying characteristics and kinetics of okra (Abelmoschus esculetus (L.) Moench) Slices. Int. J. Food Eng. 2009;5 doi: 10.2202/1556-3758.1191. [DOI] [Google Scholar]
- 14.Deng L.Z., Mujumdar A.S., Zhang Q., Yang X.H., Wang J., Zheng Z.A., Gao Z.J., Xiao H.W. Chemical and physical pretreatments of fruits and vegetables: Effects on drying characteristics and quality attributes – a comprehensive review. Crit. Rev. Food Sci. Nutr. 2019;59:1408–1432. doi: 10.1080/10408398.2017.1409192. [DOI] [PubMed] [Google Scholar]
- 15.Tüfekçi S., Özkal S.G. Enhancement of drying and rehydration characteristics of okra by ultrasound pre-treatment application. Heat Mass Transf. 2017;53:2279–2286. [Google Scholar]
- 16.Nowacka M., Wiktor A., Śledź M., Jurek N., Witrowa-Rajchert D. Drying of ultrasound pretreated apple and its selected physical properties. J. Food Eng. 2012;113:427–433. [Google Scholar]
- 17.Nowacka M., Wedzik M. Effect of ultrasound treatment on microstructure, color and carotenoid content in fresh and dried carrot tissue. Appl. Acoust. 2016;103:163–171. [Google Scholar]
- 18.Wang H., Zhao Q.S., Wang X.D., Hong Z.D., Zhao B. Pretreatment of ultrasound combined vacuum enhances the convective drying efficiency and physicochemical properties of okra (Abelmoschus esculentus) LWT – Food Sci. Technol. 2019;112:108–201. [Google Scholar]
- 19.Xia X., Kong B., Liu Q., Liu J. Physicochemical change and protein oxidation in porcine longissimus dorsi as influenced by different freeze–thaw cycles. Meat Sci. 2009;83:239–245. doi: 10.1016/j.meatsci.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Ando Y., Maeda Y., Mizutani K., Wakatsuki N., Hagiwara S., Nabetani H. Impact of blanching and freeze-thaw pretreatment on drying rate of carrot roots in relation to changes in cell membrane function and cell wall structure. LWT – Food Sci. Technol. 2016;71:40–46. [Google Scholar]
- 21.Nowak K.W., Zielinska M., Waszkielis K.M. The effect of ultrasound and freezing/thawing treatment on the physical properties of blueberries. Food Sci. Biotechnol. 2019;28:741–749. doi: 10.1007/s10068-018-0528-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li D., Zhu Z., Sun D.W. Effects of freezing on cell structure of fresh cellular food materials: a review. Trends Food Sci. Tech. 2018;75:46–55. [Google Scholar]
- 23.Ando Y., Maeda Y., Mizutani K., Wakatsuki N., Hagiwara a S., Nabetani H. Impact of blanching and freeze-thaw pretreatment on drying rate of carrot roots in relation to changes in cell membrane function and cell wall structure. LWT - Food Sci. Technol. 2016;71:40–46. [Google Scholar]
- 24.Liu Y., Chen S., Pu Y., Muhammad A.I., Hang M., Liu D., Ye T. Ultrasound-assisted thawing of mango pulp: effect on thawing rate, sensory, and nutritional properties. Food Chem. 2019;286:576–583. doi: 10.1016/j.foodchem.2019.02.059. [DOI] [PubMed] [Google Scholar]
- 25.Tan M., Lin Z., Zu Y., Zhu B., Cheng S. Effect of multiple freeze-thaw cycles on the quality of instant sea cucumber: Emphatically on water status of by LF-NMR and MRI. Food Res. Int. 2018;109:65–71. doi: 10.1016/j.foodres.2018.04.029. [DOI] [PubMed] [Google Scholar]
- 26.Ando Y., Hagiwara S., Nabetani H., Sotome I., Okunishi T., Okadome H., Orikasa T., Tagawa A. Effects of prefreezing on the drying characteristics, structural formation and mechanical properties of microwave-vacuum dried apple. J. Food Process Eng. 2019;244:170–177. [Google Scholar]
- 27.Xie Y., Gao Z., Liu Y., Xiao H. Pulsed vacuum drying of rhizoma dioscoreae slices. LWT – Food Sci. Technol. 2017;80:237–249. [Google Scholar]
- 28.Xie L., Zhang C., Wang J. Unified and explicit expressions of three-dimensional Green's functions and their first derivatives for piezoelectric solids with general anisotropy. Int. J. Solids Struct. 2018;155:1–14. [Google Scholar]
- 29.Velickova E., Tylewicz U., Rosa M.D., Winkelhausen E., Kuzmanova S., Romani S. Effect of pulsed electric field coupled with vacuum infusion on quality parameters of frozen/thawed strawberries. J. Food Eng. 2018;233:57–64. [Google Scholar]
- 30.Jiang N., Liu C., Li D., Zhang Z., Liu C., Wang D., Niu L., Zhang M. Evaluation of freeze drying combined with microwave vacuum drying for functional okra snacks: antioxidant properties, sensory quality, and energy consumption. LWT - Food Sci. Technol. 2017;82:216–226. [Google Scholar]
- 31.An K., Zhao D., Wang Z., Wu J., Xu Y., Xiao G. Comparison of different drying methods on Chinese ginger (Zingiber officinale Roscoe): changes in volatiles, chemical profile, antioxidant properties, and microstructure. Food Chem. 2016;197:1292–1300. doi: 10.1016/j.foodchem.2015.11.033. [DOI] [PubMed] [Google Scholar]
- 32.Wang J., Law C.L., Nema P.K., Zhao J.H., Liu Z.L., Deng L.Z., Gao Z.J., Xiao H.W. Pulsed vacuum drying enhances drying kinetics and quality of lemon slices. J. Food Eng. 2018;224:129–138. [Google Scholar]
- 33.Chen Y., Zhang J., Sun H., Wei Z. Pectin from Abelmoschus esculentus: optimization of extraction and rheological properties, nt. J. Biol. Macromol. 2014;70:498–505. doi: 10.1016/j.ijbiomac.2014.07.024. [DOI] [PubMed] [Google Scholar]
- 34.Sengkhamparn N., Sagis L.M.C., Vries R., Schols H.A., Sajjaanantakul T., Voragen A.G.J. Physicochemical properties of pectins from okra (Abelmoschus esculentus (L.) Moench) Food Hydrocoll. 2010;24:35–41. [Google Scholar]
- 35.Tan L., Eberhard S., Pattathil S., Warder C., Glushka J., Yuan C., Hao Z., Zhu X., Avci U., Miller J.S., Baldwin D., Pham C., Orlando R., Darvill A., Hahn M.G., Kieliszewski M.J., Mohnen D. An arabidopsis cell wall proteoglycan consists of pectin and arabinoxylan covalently linked to an arabinogalactan protein. Plant Cell. 2013;25:270–287. doi: 10.1105/tpc.112.107334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reyes A., Vega R., Bustos R., Araneda C. Effect of processing conditions on drying kinetics and particle microstructure of carrot. Dry. Technol. 2008;26:1272–1285. [Google Scholar]
- 37.Farahmandfar R., Tirgarian B., Dehghan B., Nemati A. Changes in chemical composition and biological activity of essential oil from Thomson navel orange (Citrus sinensis L. Osbeck) peel under freezing, convective, vacuum, and microwave drying methods. Food Sci. Nutr. 2020;8:124–138. doi: 10.1002/fsn3.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Azhar A.N.H., Panirselvam M., Amran N.A., Ruslan M.S.H., Samsuri S. Retention of total phenolic content and antioxidant activity in the concentration of broccoli extract by progressive freeze concentration. Int. J. Food Eng. 2020 doi: 10.1515/ijfe-2019-0237. [DOI] [Google Scholar]
- 40.Siewea F., Kudrea T., Bettadaiahb B.K., Narayana B. Effects of ultrasound-assisted heating on aroma profile, peptide structure, peptide molecular weight, antioxidant activities and sensory characteristics of natural fish flavouring. Ultrason. Sonochem. 2020;65 doi: 10.1016/j.ultsonch.2020.105055. [DOI] [PubMed] [Google Scholar]
- 41.Ashokkumar M., Sunartio D., Kentish S., Mawson R., Simons L., Vilkhu K., Versteeg C.K. Modification of food ingredients by ultrasound to improve functionality: a preliminary study on a model system. Innov. Food Sci. Emerg. Technol. 2008;9:155–160. [Google Scholar]
- 42.Sobukola O. Effect of pre-treatment on the drying characteristics and kinetics of okra (Abelmoschus esculetus (L.) Moench) slices. Int. J. Food Eng. 2009;5:234–247. [Google Scholar]
- 43.Endo Y., Usuki R., Kaneda T. Antioxidant effects of chlorophyll and pheophytin on the autoxidation of oils in the dark. II. The mechanism of antioxidative action of chlorophyll. J. Am. Oil Chem. Soc. 1985;62:1387–1390. [Google Scholar]
- 44.Cao X., Zhang M., Mujumdar A.S., Zhong Q., Wang Z. Effects of ultrasound pretreatments on quality, energy consumption and sterilization of barley grass in freeze drying. Ultrason. Sonochem. 2018;40:333–340. doi: 10.1016/j.ultsonch.2017.06.014. [DOI] [PubMed] [Google Scholar]
- 45.Shyu Y., Lin J., Chang Y., Chiang C., Yang D. Evaluation of antioxidant ability of ethanolic extract from dill (Anethum graveolens L.) flower. Food Chem. 2009;115:515–521. [Google Scholar]
- 46.Hosseini S., Khodaiyan F., Yarmand M. Optimization of microwave assisted extraction of pectin from sour orange peel and its physicochemical properties. Carbohydr. Polym. 2016;140:59–65. doi: 10.1016/j.carbpol.2015.12.051. [DOI] [PubMed] [Google Scholar]
- 47.Sengkhamparn N., Verhoef R., Bakx E.J., Schols H.A., Sajjaanantakul T., Voragen A.G.J. Okra pectin contains an unusual substitution of its rhamnosyl residues with acetyl and alpha-linked galactosyl groups. Carbohyd. Res. 2009;344:1842–1851. doi: 10.1016/j.carres.2008.11.022. [DOI] [PubMed] [Google Scholar]
- 48.Kong L., Yu L., Feng T., Yin X., Liu T., Dong L. Physicochemical characterization of the polysaccharide from Bletilla Striata: Effect of drying method. Carbohydr. Polym. 2015;125:1–8. doi: 10.1016/j.carbpol.2015.02.042. [DOI] [PubMed] [Google Scholar]
- 49.Yan J.K., Wu L.X., Qiao Z.R., Cai W.D., Ma H.L. Effect of different drying methods on the product quality and bioactive polysaccharides of bitter gourd (Momordica charantia, L.) slices. Food Chem. 2019;277:588–596. doi: 10.1016/j.foodchem.2018.08.012. [DOI] [PubMed] [Google Scholar]
- 50.Ying Z., Han X., Li J. Ultrasound-assisted extraction of polysaccharides from mulberry leaves. Food Chem. 2011;127:1273–1279. doi: 10.1016/j.foodchem.2011.01.083. [DOI] [PubMed] [Google Scholar]
- 51.Kjoniksen A.L., Hiorth M., Nystrom B. Association under shear flow in aqueous solutions of pectin. Eur. Polm. J. 2005;41:761–770. [Google Scholar]
- 52.Kontogiorgos V., Margelou I., Georgiadis N., Ritzoulis C. Rheological characterization of okra pectins. Food Hydrocoll. 2012;29:356–362. [Google Scholar]
- 53.Xu B., Yuan J., Wang L., Lu F., Wei B., Azam R.S.M., Ren X., Zhou C., Ma H., Bhandari B. Effect of multi-frequency power ultrasound (MFPU) treatment on enzyme hydrolysis of casein. Ultrason. Sonochem. 2020;63 doi: 10.1016/j.ultsonch.2019.104930. [DOI] [PubMed] [Google Scholar]
- 54.Bolwell G.P. Dynamic aspects of the plant extracellular matrix. Int. Rev. Cytol. 1993;146:261–324. [Google Scholar]
- 55.Sila D.N., Van Buggenhout S., Duvetter T., Fraeye I., De Roeck A., Van Loey A. Pectins in processed fruit and vegetables: part II – structure-function relationships. Compr. Rev. Food Sci. Food Saf. 2009;8:86–104. [Google Scholar]
- 56.Van Buggenhout S., Sila D.N., Duvetter T., Van Loey A., Hendrickx M. Pectins in processed fruits and vegetables: part III – texture engineering. Compr. Rev. Food Sci. Food Saf. 2009;8:105–117. [Google Scholar]
- 57.Rojas M., Silveira I., Augusto P.E.D. Ultrasound and ethanol pre-treatments to improve convective drying: Drying, rehydration and carotenoid content of pumpkin. Food Bioproc. Tech. 2020;119:20–30. [Google Scholar]
- 58.Rahaman A., Zeng X.A., Kumaric A., Rafiqd M., Siddeege A., Manzoor M.F., Balochf Z., Ahmed Z. Influence of ultrasound-assisted osmotic dehydration on texture, bioactive compounds and metabolites analysis of plum. Ultrason. Sonochem. 2019;58 doi: 10.1016/j.ultsonch.2019.104643. [DOI] [PubMed] [Google Scholar]
- 59.Li M., Jiang H., Zhang L., Yu X., Liu H., Yagoub A.A., Zhoua C. Synthesis of 5-HMF from an ultrasound-ionic liquid pretreated sugarcane bagasse by using a microwave-solid acid/ionic liquid system. Ind. Crops Prod. 2020;149 [Google Scholar]
- 60.Zhao Q., Yua X., Zhou C., Yagou A.A., Ma H. Effects of collagen and casein with phenolic compounds interactions on protein in vitro digestion and antioxidation. LWT-Food Sci. Technol. 2020;124 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.