Highlights
-
•
First report on the current information on the effects of ultrasonic treatment on rice grain and rice starch.
-
•
Ultrasound imparts morphological, textural, and physicochemical changes in rice rain and rice starch.
-
•
Ultrasonication enhances the formation of metabolites, uptake of fortificants, and softening of rice texture.
-
•
Ultrasonic treatment can be used for applications towards the improvement of rice functional properties.
Keywords: Ultrasonication, Rice starch, Rice grain, Texture
Abstract
As a green, nonthermal, and innovative technology, ultrasonication generates acoustic cavitation in an aqueous medium, developing physical forces that affect the starch chemistry and rice grain characteristics. This review describes the current information on the effect of ultrasonication on the morphological, textural, and physicochemical properties of rice starch and grain. In a biphasic system, ultrasonication introduced fissures and cracks, which facilitated higher uptake of water and altered the rice starch characteristics impacting textural properties. In wholegrain rice, ultrasonic treatment stimulated the production of health-related metabolites, facilitated the higher uptake of micronutrient fortificants, and enhanced the palatability by softening the rice texture. This review provides insights into the future direction on the utilization of ultrasonication for the applications towards the improvement of rice functional properties.
1. Introduction
Rice (Oryza sativa L.) is one of the important cereal staple crops, which feeds almost 3.5 billion people worldwide. Starch is the most abundant biomolecule in rice grain containing 72–82% of the dry weight of brown rice grain and approximately 90% of milled rice grain [1]. Compared to other cereal starches (wheat, barley, or rye), the advantages of rice starch are its consumer acceptance, bland flavor, small granules, white color, increased freeze-thaw stability of pastes, greater acid resistance, and a wide range of amylose/amylopectin ratios [2]. Rice is considered to be hypoallergenic because rice does not contain proteins such as gliadins or parts of proteins that are normally associated with the celiac disease [3]. Moreover, rice is easily digested and absorbed compared to other gluten-free raw materials such as corn, sorghum, buckwheat, millet, and potato [4]. In line with this, rice is used in making gluten-free food products [5], [6]. With growing rice consumption demand in Asia and Africa, there is a need to look into effective strategies to increase the nutritional density by promoting whole grain consumption and enhancing milled rice nutrition through fortification. At present brown rice consumption accounts for only 1% of consumption due to poor texture and fast rancidity trigger preventing the optimum storage and reducing the shelf life. Sonication techniques offer wide applications to overcome these limitations.
Sonication is a technique that utilizes the ultrasonic wave, covering the frequency range of low-energy (high frequency) diagnostic ultrasound (10 to 1 MHz), extended range ultrasound (1 MHz – 100 kHz), and power ultrasound (100–16 kHz) [7]. Moreover, ultrasound applied in food materials can be classified into modifiers (high-power ultrasound) and sensors (low-power ultrasound) [8]. The low-power ultrasound having a high frequency (>1 MHz) are involved in the noninvasive analysis [9], which corresponds to smaller wavelength applications in making sonograms similar to ultrasonography used in medical diagnosis [10]. Moreover, low-intensity ultrasound applied in food materials includes pulse-echo, continuous wave (through transmission), and process tomography (ultrasonic imaging). Detailed reviews on low-power ultrasound in food applications are found elsewhere [8], [9], [11], [12]. The high-power ultrasound (>5 W/cm2) at a lower frequency (around 40 kHz) [13] have been utilized in modifying food physicochemical properties and structures affording beneficial effects on the preservation [14], [15], maintaining quality [16], [17], and ensuring the safety of food products [18]. Moreover, the latter type of ultrasound was utilized in the functionalizing properties of rice starch and rice grain.
Ultrasonication is a nonthermal technique widely used in food science and technology. It has been applied in various food materials for the processing, preservation, and quality of products. Extensive reviews have been done on the application of ultrasonication on various food processing technologies [13], [19], [20], [21], [22], [23], [24], food safety with antimicrobial efficacy [18], bioactive compound extraction [25], [26], food fermentation [27], and food degradation [28]. Likewise, reviews on the effects of ultrasonic treatment on starches from various sources have been extensively reported [29], [30], [31], but no systematic meta-analysis is available for ultrasonic-treated (UT) rice.
This current paper reviews the effects of ultrasonication specifically on rice grain and starch properties and provides insights on the future direction on the utilization of ultrasonication for the applications towards the improvement of rice functional properties. In this review paper, we have discussed the potential applications of UT rice (milled, brown, and germinated sprouts) in addressing the texture, digestibility, and nutritional factors.
2. Rice starch structure and properties
Rice starch is composed of the two major polysaccharides: amylose and amylopectin. Amylose is a long-chain α-1,4-linked glucose molecule (105 – 106 molecular weight) with a few α-1,6 branch points. In contrast, amylopectin is a highly branched glucan with the branch points at α-(1,6) bonds having a molecular weight of 107-109. Amylopectin constitutes 65%-85% in starch granules in indica, while in waxy rice its composition reaches 100% making it the major composition of starch. In terms of apparent amylose content, rice is classified into five categories: waxy (lower than 2% amylose), very low (3% − 9% amylose), low (10% − 19% amylose), intermediate (20% − 25% amylose), and high (25% − 33% amylose). A rice variety having apparent amylose content as high as 35% is a rice mutant and is classified as an amylose extender (ae) [32].
The complexity of rice starch can be divided into multiple levels. Tran et al. [33] categorized rice starch into six levels. The first two levels describe the molecular structure of starch. The first level explains the linear chains of starch polymer dealing with an α-1,4 linkage between glucose molecules, while the second level shows branching (1 → 6)-α glycosidic bonds, which differentiates amylose and amylopectin. These levels can be characterized using high-performance size exclusion chromatography (HPSEC) for amylose and whole starch molecular size distribution and fluorophore-assisted carbohydrate electrophoresis (FACE) or high-performance anion-exchange chromatography (HPAEC) for amylopectin chain-length distribution [34]. The molecular weight distribution of debranched starch is estimated using SEC. It is classified into four categories in terms of the degree of polymerization (DP): long-chain amylose (DP > 1000), intermediate chain amylose (DP = 1000–121), medium-chain amylopectin (DP 120–37), and short-chain amylopectin (36–6) [35]. On the other hand, the fine amylopectin structure is classified into four groups expressed in terms of the DP, namely A (DP = 6–12), B1 (DP = 13–24), B2 (DP = 25–36), and B3 chains (DP > 37) as determined using HPAEC [36]. The molecular weights of amylose and amylopectin are 3.85 × 105 and 1.48 × 109, respectively, as determined by HPSEC equipped with refractive index and multi-angle laser light scattering [37]. The third level describes the starch chain aggregation of starch molecules entwining into a helical structure and the helices aggregate to form crystallites. Level 3 can be measured through analytical techniques such as differential scanning calorimeter (DSC), 13C cross-polarization magic angle spinning (CP/MAS) NMR, X-ray diffractometry (XRD), and small-angle neutron (SANS) or Xray scattering (SAXS) [38]. Starch XRD patterns originate from various glycosidic coiling conformations. These distinctive lamellar arrangements have been termed as the starch polymorphs [39]. Native starch polymorphs have been categorized into A, B, and C (A + B) [40]. Moreover, the packing of starch double helices within the A-type polymorph is relatively compact with low water content. In contrast, the B-type polymorph has a more open structure containing a hydrated helical core [41]. Rice starch displays a typical A polymorph diffraction patterns and with strong reflections at 2θ = 15, 17, 18, and 23° [42], while the ae rice mutant exhibited Type-B [43] or type-C [44] polymorphism. The fourth level is the growth ring structure illustrating the alternating crystalline and amorphous lamellae, which can be characterized by scanning electron microscopy (SEM), transmission electron microscopy (TEM), and atomic force microscopy (AFM). The size of rice starch blocklets is an average of 100 nm as estimated by AFM. Moreover, rice blocklets are proposed to make up of 280 amylopectin side-chain clusters and alternating with amorphous regions estimated to be about 4 nm long [45]. The fifth level corresponds to granular structure such as shape and size characterized through light microscopy and SEM and its size can be measured using dynamic light scattering [46]. Rice starch granules have size ranges from 3 to 8 µm having a polyhedral, oval, irregular, angular, or smooth in shape [47]. Lastly, level 6 represents the whole grain structure showing the starch granules interacting between other biomolecular constituents (e.g. proteins, lipids, and non-starch polysaccharides). These complex multiple level structures of starch and the changes they undergo during processing are the major determinants of starch functionality and food quality. Thus, the physical and chemical changes that ultrasonication imparts on the starch granules would most likely be defined by the responses of these multi-scale starch structures in the presence of sound waves.
3. Ultrasonic treatment of rice grain and rice starch
Ultrasonic treatment of rice grain is generally done using ultrasonic bath system. Most bath systems use single frequency and some are designed to vary the acoustic energy density or power output amplitude. The rice grains can be placed in a beaker with distilled or deionized water in certain ratios and immersed in the ultrasonic bath with enough levels of water to stabilize the beaker. Other systems use the direct immersion of rice grain in the sonic bath with water. During sonication, the temperature increases, which can be controlled by adding ice in the bath system. A thermostat can be used to monitor the temperature. The treatment time is generally monitored.
In most reported procedures, the ultrasonic treatment of rice starch is done using the ultrasonic probe system, which is equipped with a booster horn (probe) in varying lengths and diameters. The probe tip diameter dictates the amount of sample that can be effectively processed. Smaller tip diameters (Microtip probes) deliver high intensity sonication but the energy is focused within a small, concentrated area. Larger tip diameters can process larger volumes, but offer lower intensity. The probe receives its energy from the transducer and resonates at a specified frequency, which transmits ultrasonic energy to the sample. Commercial probe sonicator systems are equipped with a generator that run within a narrow frequency range (e.g., +/−500 Hz at 20 kHz). Modern generators will automatically tune to the required resonance but some older generators require manual tuning. The generators are designed to run with compatible piezoelectric converters/transducers that converts high frequency electrical energy into high frequency mechanical vibration. Modern ultrasonic probe systems are equipped with an amplitude control that allows the ultrasonic vibrations at the probe tip to be set to any desired level. The distance the tip travels is dependent on the amplitude; as the amplitude setting increases, the sonication intensity will increase within the sample. Sonication of rice starch are conducted by dissolving the starch in distilled or de-ionized water in a beaker and the probe is dipped into the liquid solution at a distance above the bottom of the beaker and positioned at the center of the beaker. The beaker containing the sample is usually immersed in a cold-water bath to counter the heat given off by the sonication process and the temperature can be monitored. The same process can be done for the UT of rice grain using the ultrasonic probe system.
In general, the choice between bath and probe is dependent on the sample. Probes are preferred for dissolved samples where the volume and viscosity changes during the processing. The ultrasonic probe processor is designed to deliver a constant amplitude to the liquid sample. As the liquid sample is processed, the load on the probe will vary due to changes in the viscosity, concentration, and temperature of the liquid sample. As the load on the probe increases, the power supply will adjust due to the increased resistance to the movement of the probe to ensure that the excursion at the probe tip remains constant. For biphasic systems, such as the rice grain in water, the ultrasonic bath is preferred because it generally gives uniform sound waves within the bath. The ultrasonic systems as well as the parameters used in the reviewed articles are summarized in Table 1, Table 2.
Table 1.
Ultrasonic conditions applied in rice grain and its effects.
| Rice Samples | Equipment | Output Frequency (Hz) | Acoustic Energy Density/Power Output/ Amplitude (%) | Temperature (℃) | Sonication Time (mins) | Results | References | |
|---|---|---|---|---|---|---|---|---|
| Long grain | Tank | 25, 40 & 80 | 0.03 W/cm3 | 75 | up to 600 |
|
[114] | |
| Probe | 20 | 0.01 W/cm3 | 75 | 300 |
|
|||
| Medium grain cultivar M202 | Bath | 16 | 2000 W | 25, 40, & 55 | 30 |
|
[50] | |
| Brown Rice (Huaidao 5) with enzymatic treatment | Probe | - | - | 40 | 30 |
|
[51] | |
| Brown Japonica type (Ilpum) | Bath | 400 | 185 W | 162 J/cm3333 J/cm3 | 25 ± 250 ± 3 | 30 60 |
|
[71] |
| Brown Japonica Rice Sanqiuding QTXD | Bath | 28 | 400 W 17.83 W/cm2 |
– | 30 |
|
[96] | |
| Milled Rice (cultivar RD6) | Bath | 40 | 400 W 40, 70, 100% |
30 ± 1 | 15 30 |
|
[77] | |
| Milled Rice (Soa Hai) | Bath | 40 | 180 W | – | 1, 3, & 5 |
|
[89] | |
| Milled Rice (TH82) | Bath | 53 | – | – | 5, 15, 25 & 35 |
|
[52] | |
| Brown Rice (RD31) | Bath | 40 | – | 30 ± 2 | 30 |
|
[84], [73] | |
| Milled Rice | Bath | 40 | 150, 300, 450, 600 W | 4 | 10–120 |
|
[53] | |
| Brown and Red Rice | Tank | 25 | 2000 W 1.25 W/cm2 100% |
23–24 | 5 |
|
[97], [98] | |
| Two white rice cultivars (Khao Dawk Mali 105 and Chai-Nat1) | Bath | 60 | 665 W 40, 70, 100% |
30–46 | 15 30 |
|
[72] | |
| Brown Japonica rice (HeituXZ) | Bath | 28 | 400 W 17.83 W/cm2 |
– | 5–30 |
|
[99] | |
| Milled Rice (IR64) | Bath | 40 | 130 W | – | 5 |
|
[113] | |
| Milled Rice (Huanggan Shanlan) | Bath | – | 160, 240, 320, 400 W | – | 30 |
|
[88] | |
| Brown Rice (IR64 and IR65) | Bath | 40 | 130 W | – | 5–60 |
|
[54] | |
Table 2.
Studies on the effects of ultrasonic treatments on rice starch properties.
| Rice Starch Sample | Type |
Ultrasonic Conditions |
Effects of Ultrasonic Treatment | Reference | |||
|---|---|---|---|---|---|---|---|
| Frequency (kHz) | Power Output (W) | Sonication Time (mins) | Other Parameters | ||||
| Waxy Rice Starch | Probe | 20 | 600 | – | – |
|
[67] |
| Rice starch | Probe | 30 | 1, 3, & 5 | – |
|
[68] | |
| Long-grain rice flour (RL-100) | Probe | 20 | 750 | 10 & 20 | 40 ℃, 75% AS |
|
[62] |
| Long-grain rice flour (RL-100) | Probe | 15, 30, or 60 | 50 ℃, 100% AS |
|
[61] | ||
| Waxy rice starch | Probe | 211 | 2.5 & 4.1 | 15, 30, 45 & 60 | 25–70 ℃, intensities 0.11 & 0.18 W/cm2 |
|
[60] |
| Rice (18.96% AC) | Probe | 20 | 30 | 20 ℃ |
|
[56] | |
| Broken Rice (27.27% AC) | Bath | 40 | 300 | 60 | 70 °C |
|
[75] |
| Nonwaxy rice starch (Japonica rice) | Probe | 24 | 100, 500 & 1,000 | 0–120 | Φ6 or Φ10 A |
|
[82] |
| Rice (18.96% AC) | Probe | 20 | 170 | 30 | 20 °C |
|
[57] |
| Native rice starch (AC not given) | Probe | 16* | 10 | 20 ℃, 100% AS |
|
[86] | |
| Rice starch (21% AC) | Probe | 22 | 150, 300, 450 & 600 | 20 | 25 ℃ |
|
[58] |
| Rice (PR-123) | Probe | 24 | 100 | 15 & 30 | 100% AS |
|
[81] |
| Rice starch (29.5%, AC) | Bath | 20 | 170 | 60 | 25–35 ℃ |
|
[74] |
AC = Amylose content; A = amplitude; AS = amplitude setting.
*conducted with ozonolysis.
4. Changes in physicochemical properties of ultrasonicated rice grain
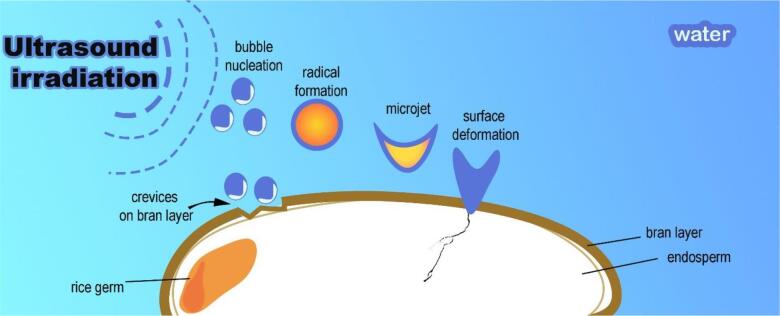
Ultrasonic treatment of rice grain affects the physicochemical properties of the kernel. Table 1 summarized the different ultrasonic parameters applied to rice grains. Ultrasound generates acoustic cavitation, which produced collapsing bubbles in the aqueous medium. Fig. 1 illustrates the mechanism of collapse of these microbubbles generating micro-jet, shearing force, and local heating [48]. In the rice-water biphasic systems, sonication induced microporous formation, cell wall exposure, and rice matrix loosening (Fig. 2). Rice grain, experienced surface erosion via a cavitational collapse in the surrounding liquid, inducing destruction of the rice surface “shell” and grain fragmentation [49]. The fissures and cracks in ultrasonicated rice grain structure are observed through scanning electron microscopy [50], [51], [52], [53] and synchrotron x-ray tomography [54]. The mechanical jet impact of sound waves caused the packing of starch granules in rice to loosen cell wall matrix. The ultrasound vibrations (40–53 kHz) for 5 mins induced cracks and fissures on the kernel maintaining the head rice grain without breaking the kernels. Extending the sonication time to 30 and 60 mins increased the degree of broken kernels. The formation of cracks and fissures manifests in the increased porosity of the UT rice [54]. The porosity in the food matrix produced after ultrasonication enhances the water mass transfer and the hydration process [55].
Fig. 1.
Mechanism of fissure on rice induced by ultrasonic irradiation.
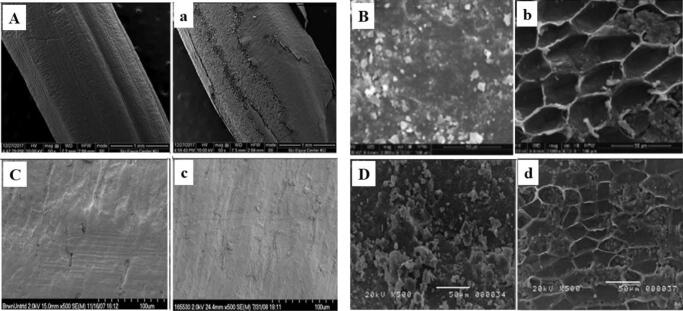
Fig. 2.
Scanning electron microscopy micrographs of rice grain before (A-D) and after (a-d) sonication. (A, C) Brown rice; (B, D) milled rice. Sonication conditions: (a) 40 kHz, 150 W, 30 mins, 30 °C [84]; (b) 40 kHz, 300 W, 10 mins, 4 °C [53]; (c) 16 kHz, 2 kW, 30 mins, 55 °C [50]; (d) 53 kHz, 5 mins, 55 °C [52]. Reprinted with permission.
5. Effects of ultrasonic treatment on rice grain and rice starch properties
The UT of rice starch or rice grain imparts physical and chemical changes on the starch granules affecting its properties and function. This section surveys the effect of ultrasonic treatment on rice starch properties (Table 2).
5.1. Size and shape of starch granules
The morphology of native starch has shown to be affected by UT (Fig. 3). Aqueous and ethanolic rice starch suspension exposed to 20 kHz (170 W) ultrasonication for 30 min showed small fissures and depressions on the granule surface as visualized by SEM [56]. Further investigation in the porosity of sonicated rice starch in terms of specific surface area (SBET), suspended in ethanol, showed a significant increase in the average diameter of mesopores and formed new pores with diameter ranges from 1.7 to 300 nm [57]. UT (24 kHz) with increasing ultrasonic power (150 to 600 W) caused the peeling off of the outer layer of the starch granules [58]. The effect of low-frequency ultrasound (20–24 kHz) on rice starch is attributed to mechanical action as the micro-bubbles collapse on the surface [57]. The observed formation of fissures and pores, as well as the degradation of the starch granule under ultrasonic conditions, were shown to be induced by the shear forces and micro-jets of the collapsing bubble during cavitation [59]. Starch granule pitting, however, was not observed when low power ultrasound (2.5 and 4.1 W) was applied [60], suggesting that the magnitude of ultrasonic power is an essential parameter of the mechanical effect in distorting the starch granules.
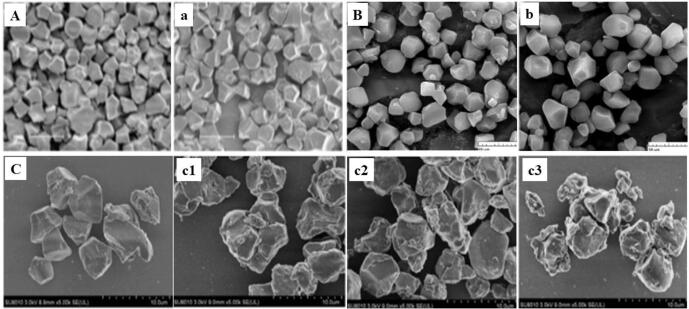
Fig. 3.
Scanning electron microscopy micrographs of rice starch before (A-C) and after (a-c) sonication. Amylose content (A) 3.25%, (B) 18.96%, (C) 21%. Sonication conditions: (a) 211 kHz, 4.1 W, 30 mins, 25 °C [60]; (b) 20 kHz, 170 W, 30 mins, 20 °C [56]; (c1-c3) 22 kHz, 20 mins, 25 °C at (c1) 150 W, (c2) 300 W, (c3) 600 W [58]. Reprinted with permission.
The granular starch shape is also affected by UT. Increasing the temperature of the aqueous system during ultrasonication changed the shape of the UT starch. At room temperature (25 ℃), starch exposed with low power ultrasound (2.5 and 4.1 W, 211 kHz) showed no morphological changes but when exposed at a higher temperature (70 ℃), UT starch becomes smaller [60]. Moreover, with increasing sonication time (15, 30, and 45 mins), starch diameter (average diameter D4,3) slightly shifted from 7.2 to 5.4 µm as shown in the dynamic light scattering experiment [60]. Contrary to the general trend, not all UT rice starch showed fissures and cracks. Wang and Wang [61], [62] observed no damage on UT starch even with the application of ultrasonic frequency (20 kHz, 750 W) arguing that only non-covalent bonding between the starch and protein was most likely affected by ultrasonication. Hence, these differences in UT rice starch morphology can be attributed to the differences in frequency, power, and temperature of applied ultrasonic parameters. It would be interesting to cover the range of ultrasonic power, frequency, and temperature to systematically observe the effect on rice starch morphology and relate it to its properties.
5.2. Starch structure distribution
Ultrasonication has been shown to cause degradation of water-soluble polymers through molecular scission [63], [64], [65]. In general, ultrasonication of starch either caused degradation via molecular scission or had little effect on the molecules as determined by diverse techniques [31]. The deterioration of a polymer can be attributed to the mechanical and chemical effects of cavitation. For the mechanical impact, a shear force can break the starch chain. High localized temperature (>5,000 K) and pressure (70–100 MPa) can cause chemical reactions within the cavitation bubbles and the surrounding liquid [66]. Mainly, the homolytic reaction of a water molecule produced radicals, hydrogen (H•), and hydroxide (OH•), and the recombination of these radicals can form molecular products. These radicals likely caused chemical changes in starch by molecular scission of the chains [63]. UT on rice starch has shown to cause degradation in the molecular composition of starch. Using gel permeation chromatography (GPC), UT on waxy rice starch showed deterioration as evidenced by the decrease in the number of average molecular weight when it was exposed to high power ultrasound (20 kHz, 600 W) [67]. This drop in the molecular size was attributed to radical-induced (OH•) scission of the starch glycosidic bond. As most of rice starch experiments implied low powered UT, application of SEC and SEC-MALLI-RI did not show significant changes in molecular weight distribution upon ultrasonication both in non-waxy and waxy rice [60], [61], [68]. With the use of HPAEC, the UT showed no significant effect on chain length distribution of rice starch with 21% amylose content. However, using relatively lower ultrasonic power (22 kHz, 150 W, and 300 W) the rice starch caused a slight decrease in the percentage of the B2 (DP = 25–36) and B3 (DP > 37) chains and an increase in A chains (DP = 6–12). A more intensive ultrasound setting (450 W and 600 W) resulted to a higher proportion of B chains and lower value of A chains [58]. These results suggest that ultrasonic disruption occurred preferentially in the amorphous regions of the granule structure rather than the crystalline region, while the application of stronger ultrasonic power leads to further disruption of crystalline regions, double helixes distortion, and A chain reduction. As studies have shown, applying high power UT can induce the molecular disruption and degradation of rice starch. There is still uncertainty on how ultrasonication affects the molecular structure of rice starch with different amylose/amylopectin ratio. To fully understand this gap, a wider range of rice starch with contrasting amylose contents may be utilized in future studies. Moreover, a benchmark study on using isolated amylose and amylopectin from rice starch can also give meaningful insights into the effect of UT.
5.3. Crystalline pattern
In general, ultrasound is insufficient in disrupting, or even in distorting the starch granule crystallites; however, this physical treatment can affect changes in the amorphous regions, as well as interrupt and destroy the double-helical order in both the amorphous and crystalline regions [29]. Similar to maize [69] and potato [70] starches, ultrasound did not induce a polymorphic change in rice starch (A-type diffraction pattern is still intact), but changes in percent crystallinity has been observed. W. Yang et al. [58] reported that UT does not affect crystalline pattern type, while relative crystallinity shows a decrease in increasing ultrasonic power (34.60% to 26.24%). Moreover, the Fourier transform infrared spectroscopy (FTIR), and Raman spectroscopy results indicated that ultrasound treatments decreased the starch crystallinity. Using the absorbance ratio of 1022/995 and 1047/1022 cm−1, it was shown that with the increasing ultrasonic power (150 to 600 W), the 1022/995 cm−1 ratio increased, while the 1047/1022 cm−1 ratio decreased indicative of decreasing starch crystallinity.
Cui et al. [50] reported higher ΔH and relative crystallinity of isolated starch from UT brown rice, which was due to the formation or rearrangement of new intermolecular interaction among starch molecules. In contrast to this, several works show that ultrasonication weakens the double helices and disrupts the crystalline region of extracted starch from UT rice, resulting in lower relative crystallinity value [53], [71], [72]. While Dang et al. [73] reported no significant changes in starch relative crystallinity after ultrasonication as measured by XRD, the DSC results showed a decrease in ΔH value by 7.2%. These results suggested that ultrasound irradiation might act on starch by weakening the short-range crystallinity. To fully understand changes in the thermal and pasting properties of UT rice, determining the molecular structure of isolated starch is highly recommended. It is also essential to elucidate the changes in the lamellae structure of UT rice starch using advanced x-ray techniques such as small-angle x-ray scattering and neutron scattering.
Using Raman spectroscopy, a remarkable decrease in the relative height of the band at 480 cm−1 (peak with a strong and positive correlation with the crystallinity of starch) was noticed as ultrasonic power increased. While using synchrotron wide-angle x-ray scattering (WAXS), the relative crystallinity of rice starch decreased after ultrasonication (20 kHz, 170 W) with a combination of ice recrystallization [74]. In contrast, a combination of glycerol addition during UT (40 kHz, 300 W) leads to the increase of starch relative crystallinity, which can be caused by the reduction of amylose content in UT starch [75]. Compared to other starches, very few studies were done in testing the effect of ultrasound on rice varieties that differ in amylose content. It is interesting to explore this gap because differences in rice amylose content affect the texture, digestibility, and nutritional aspects. Studying the impact of different ultrasonic parameters in different rice varieties may be explored in future studies.
5.4. Digestibility of ultrasonicated rice starch
During ultrasonication, strong shear forces can disrupt granule crystallinity and depolymerize starch chains. Moreover, ultrasonication induced the formation of pores in starch granules and disruption of the double-helix structure, contributing to enzyme accessibility at the sites susceptible to the digestive enzymes [76]. Starch digestibility is affected by the alteration of starch molecular architecture. Rice grain shows changes in its digestibility after UT. Using in vitro hydrolysis, UT rice shows a higher glycemic index compared to the control, which is attributed to the higher access of enzymatic activity in the deteriorated starch crystalline region [77], [72]. Faster digestion rate is also observed in UT rice assisted with the ice recrystallization process [74]. On the other hand, Dang et al. [73] reported that the glycemic index of UT brown rice has no significant difference compared to native brown rice. Because of these effects, several works have been done in determining the effect of ultrasonication on starch digestibility [76], [78], [79], [80]. Kaur and Gill [81] observed that increasing sonication (24 kHz, 100 W) durations from 15 to 30 mins caused an increase in the content of RDS and RS, subsequently decreasing the SDS content. Recently, Keeratiburana et al. [74] reported that UT rice starch (20 kHz, 170 W, 1hr also treated with ice recrystallization process) demonstrated higher susceptibility to digestive Porcine pancreatic α-amylase and amyloglucosidase-catalyzed hydrolysis. An increase in starch enzymatic degradation is aligned with a decrease in starch relative crystallinity, as shown in wide-angle x-ray scattering results.
These observations suggest that different ultrasonic parameters and rice varieties may give variation in crystallinity and digestibility of UT rice. For future studies, it is interesting to explore the starch hydrolysis of contrasting rice samples (varieties with difference in amylose content) treated with ultrasonication (different intensity and duration) and how this physical treatment affects the deterioration of resistant starch or formation of amylose–lipid complexes.
5.5. Thermal properties
The disruption of the amorphous and crystalline regions in rice starch upon UT can be indicated by changes in the thermal properties, enthalpy change (ΔH), and the gelatinization temperatures, including onset (To), peak (Tp), and conclusion (Tc) temperatures [31]. For isolated rice starch treated with high-intensity ultrasonication (20 kHz, 750 W), no effect in the thermal properties (To, Tp, and ΔH) were observed [61]. However, Yu et al. [82] reported the effect of ultrasound in To, Tp, and ΔH in two ultrasonic amplitudes (6 and 10) and three ultrasonic powers (100, 500, and 1000 W) in increasing sonication time. In<60 min, a decrease in all thermal parameters was observed, while an increase in onset and peak temperatures were noted at longer sonication time (120 min). Similarly, W. Yang et al. [58] observed a decrease in ΔH in low-power ultrasound; however, no significant changes occurred in To, Tp, and Tc in UT rice starch. In low-power UT (150 and 300 W), the decrease in enthalpy change indicates the loss of starch molecular order. Other UT starches exhibit the same thermal behavior [69], [83], which corresponds to the disruption of the starch region. In contrast, high ultrasonic power applied by Yu et al. [82] (1000 W) and W. Yang et al. [58] (450 and 600 W) induced a sharp increase in enthalpy. This increase is attributed to the starch structural damage caused by high-power ultrasound, making it easier for the penetration of water molecules into starch granules, causing the amylose to leach out from the interior starch region. Hence, on the starch surface, the leached amylose might partially gelatinize under local high temperatures preventing water penetration and causing the increase in enthalpy change. These results indicate that the application of different ultrasonic power on the same starch (e.g., rice starch) causes diversity in thermal properties.
5.6. Pasting and viscoelastic properties
Ultrasonication not only induced bulk structural changes on rice grain but also affected the properties of the UT rice. Table 3 summarizes the impact of ultrasonication on rice thermal, crystalline, and pasting properties. In terms of pasting properties, an increase in the peak and final viscosities are observed in isolated starch, even if different UT conditions are applied in the rice samples [50], [51], [71], [72], [77], [84]. The elevated peak viscosity is caused by the softening of the rice starch matrix resulting in an easier rupture of starch during gelatinization.
Table 3.
Percent (%) change in thermal properties, relative crystallinity, and pasting properties of sonicated rice grain with respect to raw rice.
| Rice Type | References | Amylose Content (%) |
Ultrasonic Conditions |
Thermal Properties |
Relative Crystallinity |
Pasting Properties |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Frequency (kHz) | Power (W) | Temperature(°C) | Time (mins) | To | Tp | ΔH | Peak Viscosity | Final Viscosity | ||||
| Brown Rice | [50] | – | 16 | 2000 | 25, 40, 55 | 30 | 5.9 ↓ | 4.6 ↓ | 31.0 ↑ | 10.0 ↑ | 9.9 ↑ | 6.8 ↑ |
| [51]* | – | 20 | 750 | 40 | 30 | – | – | – | – | 18.6 ↑ | 37.6 ↑ | |
| [71] | – | 400 | 158 | 25, 50 | 30, 60 | 4.8 ↑ | 1.7 ↑ | 4.5 ↓ | 39.8 ↓ | 25.1 ↑ | 34.2 ↑ | |
| [84] | 35.53 | 40 | 150 | 30 | 30 | – | – | – | – | 6.2 ↑ | 2.4 ↑ | |
| [73] | 35.53 | 40 | 180 | 30 | 30 | 0.3 ↑ | 1.7 ↑ | 7.2 ↓ | 3.6 ↑ | – | – | |
| Milled Rice | [77] | 7.04 | 400 | 40 | 30 | 15, 30 | 6.9 ↓ | 0.7 ↓ | 61.3 ↓ | – | 24.7 ↑ | 21.4 ↑ |
| [53] | – | 40 | 0 – 600 | 4 | 10–120 | – | – | – | 25.1 ↓ | – | – | |
| [72] | 16.9 & 29.35 | 60 | 665 | 30 | 15, 30 | 3.4 ↓ | 1.9 ↓ | 44.8 ↓ | 27.7 ↓ | 279.6 ↑ | 105.4 ↑ | |
↑ = increase; ↓ = decrease with respect to the raw non-sonicated rice..
*with enzymatic treatment.
Heating starch up to its gelatinization temperature, where heat and water transfer phenomena occur, results in disordered crystallinity due to the absorption of water in the granular structure. This event leads to an increase in the viscosity of aqueous rice suspension and thus UT affects the starch pasting properties [56], [85]. Specifically, in rice, UT starch exhibited contrasting pasting behaviors depending on the sonication parameters (frequency, intensity, amplitude, and sonication time). Wang and Wang [61], [62] reported that the peak and breakdown viscosities of rice starch increased after sonication. Recently, W. Yang et al. [58] also observed a similar trend in rice peak viscosity values, mainly when rice starch was treated in increasing ultrasonic power levels (150–600 W). These elevating values of pasting viscosity might be attributed to the degradation of the amylopectin region in the starch molecular architecture, which increases the capacity of the starch to undergo swelling. Contrastingly, lowering paste viscosity was reported after ultrasonication due to the differences in ultrasonic frequency and also due to the varieties used with differences in amylose content. Chung et al. [68] reported a decrease in a hot paste and alkaline viscosity of starch after UT which implicate the changes in its physicochemical properties due to disruption of swollen granules. While Zuo et al. [60] reported that UT waxy rice starch has a lower peak and final viscosities compared to non-sonicated rice suspension, suggesting the possibility that shorter starch molecules (amylopectin) come close to each other in forming an intermolecular network causing the increase in pasting and viscoelastic behavior of UT rice sample. Ultrasonication increased the viscosity and consistency index of native rice starch as well [86]. Sonication treatment time influences rice viscoelasticity. Elastic (G’) and viscous (G”) values of UT rice increased after 15 min exposure then drop after 30 min. This rheological pattern after ultrasonic exposure attributed to the induced starch damage under the shear forces, and thus, the leaching out of amylose molecules occurs. The shear action within the fluid layers gets reduced, contributing to the lowering of viscosity [81].
6. Application of ultrasonic treatment on rice grain to enhance nutrition and improve texture
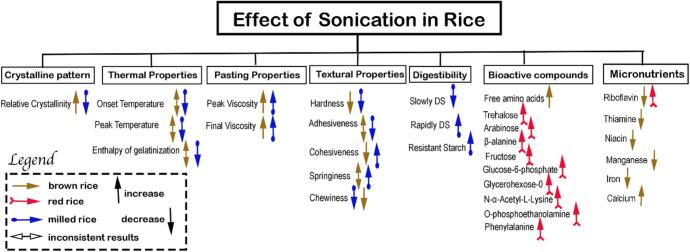
The UT of rice grain not only implied changes in the physical properties of rice to improve the texture of brown rice, these applications also offered wide-array of benefits to fortify nutrients, explore nutritional benefits in germinated sprouts and fermented products. Fig. 4 summarizes the changes observed as a result of treating rice under ultrasonic conditions.
Fig. 4.
Summary of the effects of ultrasonication in brown, red, and milled rice. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
6.1. Improving hydration process, cooking properties, and textural attributes of brown rice
The presence of bran in wholegrain or brown rice results in the restriction of water diffusion during cooking, which makes brown rice harder in texture and has lower palatability in comparison to milled rice. In line with this, brown rice is treated with different physical treatments such as high hydrostatic pressure, pulse-field, cold plasma, and ultrasound, which tends to improve the eating and cooking quality [87]. Among these physical methods, ultrasonication has shown success in improving rice quality by providing softer texture and shortening cooking time. The effect of ultrasonication resulted in loosened rice matrix allowing for faster absorption of water and quicker gelatinization. This enhanced hydration process in sonicated food improved the cooking quality. Cui et al. [50] reported the reduction in cooking time of UT brown rice from 39.6 to 33 mins, which was attributed to the improved water absorption of the processed rice (2.78 to 3.08, water uptake). Similar observations were reported by Zhang et al. [51]; the enhanced water absorption of UT brown rice effectively decreased from 28.7 mins to 24.3 mins with enzymatic treatment only and further reduced to 22.3 mins with ultrasonic and enzymatic treatments. Moreover, an increase in water uptake of UT Thai brown rice [84], [73] reduced the cooking time of Shanlan rice after UT [88]. Volume expansion is also affected by ultrasonication; Dang et al. [73] reported an increase in volume expansion ratio of brown rice (2.83 ± 0.24 to 3.33 ± 0.00) after ultrasonication. For milled rice, ultrasonication produced microporous formation and cell wall exposure [52], [53], [89]. Similar to brown rice, micropores on milled rice surface increased the contact area between rice and water, facilitating the improved water penetration into the rice during ultrasonication. This enhances water penetration as indicated by the increase in the activity and freedom of tightly bound protons as determined by low-field nuclear magnetic resonance [53].
The fissures and cracks in UT rice also impact rice textural attributes as shown in Table 4. These defects result from loosening the starch matrix, making the rice grain structurally weaker, and produced softer texture upon cooking. The texture is an essential attribute in determining the eating quality of cooked rice. Defined as the required force to remove the adhered food material to the mouth during normal eating [90], stickiness or adhesiveness is an essential physical and sensory property, which critically influence the preference of consumers [91]. The physical and chemical characteristics of the leached materials (amylose and amylopectin) during soaking and cooking, as well as due to UT is likely the primary determining factor in lowering hardness [92], [93]. UT of brown rice has lower adhesiveness compared to raw rice [84], [71]. The decrease of stickiness in UT rice may be attributed to the degradation of leached starch during ultrasonication. For non-waxy milled rice, our study on the stickiness of the cooked UT milled rice decreased but shows no significant difference to cooked raw milled rice [54]. A decrease in chewiness value was observed in UT brown rice [84], [71]. The springiness, defined as the rate at which a deformed material goes back to its undeformed condition after removing the deforming force, shows a decrease (from 0.590 ± 0.023 to 0.504 ± 0.0191) in UT brown rice [51] but increased significantly in UT milled rice (non-waxy) [54].
Table 4.
Percent (%) change in the instrumental textural properties of sonicated rice grain with respect to raw rice.
| Type of Rice | References | Amylose Content (%) |
Ultrasonic Conditions |
Hardness | Adhesiveness | Cohesiveness | Springiness | Chewiness | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Frequency (kHz) | Power (W) | Temperature(°C) | Time (mins) | ||||||||
| Brown Rice | [51]* | – | 20 | 750 | 40 | 30 | 18.8 ↓ | 4.1 ↑ | 20.3 ↓ | 14.6 ↓ | 34.9 ↓ |
| [71] | – | 400 | 158 | 25 & 50 | 30 & 60 | 50.5 ↓ | 22.5 ↓ | – | 2.1 ↑ | 46.8 ↓ | |
| [84] | 35.53 | 40 | 150 | 30 | 30 | 10.7 ↓ | 30.1 ↓ | 5.6 ↓ | – | 11.3 ↓ | |
| [54] | 21.9 | 40 | 130 | <10 | 15 | 8.9 ↓ | 47.9 ↓ | 0.7 ↓ | 7.8 ↑ | – | |
| Milled Rice | [54] | 21.9 | 40 | 130 | <10 | 15 | 14.8 ↓ | 16.6 ↓ | 1.9 ↓ | 111.8 ↑ | – |
↑ = increase; ↓ = decrease with respect to the raw non-sonicated rice.
*with enzymatic treatment.
As amylose being the primary determinant for rice hardness, it is crucial to explore the effect of ultrasonic treatment on rice varieties having different or contrasting amylose contents. Besides, molecular characterization of leached starch after ultrasonication or during cooking of UT rice is vital to determine further how leached starch affects rice stickiness.
Furthermore, as a sensory property, the hardness of cooked rice is commonly measured [94]. Generally, cooked UT brown rice shows lower instrumental hardness compared to cooked untreated rice as measured by a texture analyzer. With higher sonication time (60 mins) and temperature (50 ℃), UT significantly decreased the hardness of cooked brown rice compared to milder conditions (at 25 ℃ for 30 min) [71]. UT brown rice with an additional cellulase treatment reported a softer texture (from 1192.3 ± 70.9 to 946 ± 70.2 g) and its sensory analysis shows a higher acceptance value for this treated rice [51]. Dang et al. [84] reported similar textural attributes for UT Thai brown rice. Because of the absence of the bran layer, the hardness of cooked milled rice (non-waxy) is more dramatically affected by ultrasonication inferred in our recent results [54]. Likewise, Li et al. [53] showed a significant decrease in the hardness of cooked UT rice as determined by instrumental analysis. These outcomes are advantageous to favor consumer acceptability with preference toward eating rice with a softer texture, particularly for nutritious brown rice.
6.2. Enhancing intrinsic health-related metabolites of wholegrain rice through ultrasonic treatment
Besides UT being used as a texture-improving pretreatment, previous studies show that ultrasonication, acting as an abiotic elicitor, shows capabilities to initiate stress response such as accelerating enzymatic activities [95], which significantly alters the production of primary and secondary metabolites, and minerals during rice germination and storage [96], [97], [98], [99]. Similarly, enhanced nutritional attributes are also observed in ultrasonic-treated crops such as wheat [100], oat [101], soybean [102], broccoli [103], and tomato [104], [105]. Furthermore, UT bean and lotus seeds offer a possibility in increment germination productivity in a larger-scale farm [49].
In this subsection, changes in the profiles of primary metabolites (fatty acid, starch, and amino acid), secondary metabolites (γ-aminobutyric acid and other health-related compounds), and mineral of UT rice during germination and storage are discussed. Free fatty acid (FFA), lipase activity, and lipid oxidation of germinated rice are affected by ultrasonication [96]. Initially, brown rice treated with 30 min ultrasonication (28 kHz, 400 W, 17.83 W/cm2) shows significantly lower FFA content compared to raw rice. The decrease in FFA value in UT rice could be attributed to the enhancement of the mass transfer process and permeability of cytoplasmatic membranes and diffusion rates of intracellular compounds with rice matrix. In contrast to this, the opposite pattern happens during storage; UT brown rice shows an elevation of FFA value attributed to the enhanced lipase activity, which could be associated with higher lipase-substrate interaction. It is known that FFA produced from lipase-catalyzed hydrolysis is prone to oxidation, so an increase in lipid oxidation activity is observed as determined by increasing thiobarbituric acid reactive substance (TBARS) values during the storage process.
Enhanced starch hydrolysis is observed in germinated rice after ultrasonication as determined by lower starch content and higher reducing sugar level compared with untreated rice [97], [99]. For morphological attributes, rice grain fissures, changes in starch microstructure, and enhanced cell membrane permeability could improve the water intake during germination, providing more substrate for enzymatic hydrolysis and enhancing the release of the enzyme from the cell wall for amylase activity. In contrast, UT could affect the synthesis, transformation, and active conformation of the endogenous proteins during rice germination [95].
Like other significant macromolecules, the free amino acid of wholegrain rice is also affected by ultrasonication. In UT brown rice, free amino acids such as Glu, Gly, Ala, Tyr, Val, Ile, Leu, Phe, Pro, Trp, and His show improved accumulation [99]. The higher accumulation of proline is observed in UT brown rice serving as an important nitrogen source. Pro is a vital signaling indicator that accumulates in plants in response to environmental stress [106]. On the other hand, ultrasonication of red rice resulted in the increased accumulation of Ala and Phe during germination [98].
Furthermore, the enhancement of health-related rice metabolites was also observed. Among these metabolites, the nutritious γ-aminobutyric acid (GABA), considered as a neurotransmitter inhibitor, was found to improve significantly [98], [99]. These results suggest that ultrasonication induces stress to rice grain, affecting the physiological role of amino acid metabolism and promote the synthesis of byproducts of the metabolic pathways. An increase in value proposition is the enriched nutritious metabolites upon UT, which can benefit human health in combating cellular energy deprivation and oxidative damage. Moreover, UT red rice also shows a significant increase in riboflavin (Vitamin B2), arabinose, β-alanine, fructose, glucose-6-phosphate, glycerohexose-0, N-α-Acetyl-L-Lysine, O-phosphoethanolamine, phenylalanine, and trehalose as determined by gas chromatography-mass spectroscopy [98]. Also, loss of thiamin (Vitamin B1), riboflavin (B6), and niacin (B3) were observed in brown rice after 8 h soaking and UT for 60 mins at 50℃ [71].
Endogenous minerals present in wholegrain rice are also affected by ultrasonication. Some minerals remained unchanged (Mg, Cu, P), whereas Mn and Fe contents lessened, while Ca level increased after UT [96] as determined by ICP-OES quantitatively and micro-XRF for mineral spatial distribution. In addition, a decrease in Fe and P contents were also observed in brown non-waxy rice after 15 min of UT [54]. The elevated Ca value could be associated with Ca2+- activated lipase derived from rice bran. Moreover, a simulated gastrointestinal digestion model shows that Ca in UT rice shows increased bio accessibility [99].
Changes in metabolite profiles of wholegrain rice are stimulated by ultrasonication, which led to the changes in primary and secondary metabolites in germinated rice sprouts. Though the application of UT on brown and red rice sprouts have been shown, it is still important to explore the effect of ultrasonication on the production of health-associated metabolites (e.g., phenolic acids, anthocyanidins) on black and other colored rice. Also, by utilizing advanced mass spectroscopic techniques (e.g., LC-MS, MALDI-TOF), future studies can profile the metabolites induced by this physical treatment.
6.3. Ultrasonication as a postharvest technique for micronutrient fortification
Fortifying rice is an effective and economical way to elevate micronutrient intake in countries where rice is a staple food. Different non-destructive techniques have been extensively utilized for micronutrient fortification of rice, which includes the following: soaking, parboiling, coating, germination, foliar spray, fertilization, and dusting [107]. The potential of the sonication technique expands the arsenal for the choice of technology as a postharvest technique to increase vitamin and mineral uptake during rice fortification. The formation of fissures and cracks after UT in rice may have been seen as a drawback in rice quality, but it provides an advantage to allow micronutrients to be easily absorbed during soaking. Ultrasound produced microchannels and cavities, which enhance the hydration process and micronutrient uptake in the food matrix through capillarity and by sponge effect (the food matrix behaves like a sponge due to the alternative squeezing and relaxing of the structure) [108]. The formed cavities are the primary determining features for the higher micronutrient uptake in UT rice grain. Sonicated rice has 140% higher uptake and a 93.9% higher adsorption rate of pantothenic acid (Vit B5) than non-sonicated rice [52]. Riboflavin (Vit B2) and pyridoxine (Vit B6) also show enhanced uptake in sonicated milled rice [109]. We reported that sonicated rice has a 4,054-fold increase in folic acid content upon fortification compared to its endogenous content with a 93.53% retention after washing and cooking [110], [111], [112]. Recently, our results show that sonication followed by soaking in aqueous iron solution resulted in a 28-fold increase in iron uptake (321 ± 13.43 mg of iron per kg of rice) compared to the endogenous iron content of milled rice with retention of 82.9% upon washing and cooking. The UT brown rice also showed a dramatic increase in iron content after 30 mins soaking and further increased after soaking for 60 min [54]. Besides, the spatial distribution of iron fortificant into the uncooked grains showed inward diffusion at different rates reaching into the kernel core as determined by micro-x-ray fluorescence spectroscopy [113]. The premix grains with a specific nutrient added can be mixed with regular rice to afford a fortified rice mixture loaded with multiple nutrients that can be tailored to address specific needs. A fortified rice mixture can be designed to address the nutrient needs of pregnant women containing premix grains that are loaded individually with folic acid and Fe. Addressing stunting growth in school-aged children, fortified rice mixture loaded with premix grains containing Fe, Zn, and vitamin B complexes can be prepared. This tailored approach to address malnutrition is advantageous from the point-of-view of government implementors as it targets specific sectors of society.
Compared to other fortification methods, the relatively low premix-to-rice ratio using the sonication technique is compensated by the fact that the fortificant is embedded at high concentration in the core kernel [113] without introducing added chemicals such as binders, waxes, and gums. The absence of these chemicals ensures retention of cooking qualities of fortified kernels and promotes overall consistency in the cooked fortified rice mixture. Sonication technique showed improvement in the textural properties producing softer premix grains, which is highly favored by consumers, especially in South East Asia. Moreover, the observed leaching of water-soluble phosphorus during the sonication process suggests the potential removal of anti-nutrients such as phytic acid and phytates (IP6) [113]. The observed higher uptake rate of Fe into the core of the uncooked milled rice was reflected in the high retention after cooking. In the removal of phytic acid, these Fe incorporated and retained in the rice may be readily available for use in the body to counter iron-deficiency anemia. Iron introduced into the rice by other fortification techniques may not be readily available as it undergoes strong complexation with IP6. The approach of sonicating the rice offers the dual benefit of leaching the potentially anti-nutrient IP6 and higher Fe uptake upon soaking, which could result to increased Fe bioavailability. Further study though is needed to confirm the proposed higher bioavailability of Fe in UT fortified rice.
The success achieved in increasing micronutrients in rice grain through ultrasonic pretreatment provides further room for enriching vitamins and minerals in the staple food. Reports done so far are for single-nutrient fortification. Multi-nutrient incorporation in rice can be explored to study the competitive effects of different nutrients. It would also be beneficial if the stability and the complexation of these multi-nutrient fortificants in sonicated rice can be well-established. Evaluating the sensory qualities of UT fortified rice to fully understand its textural attributes is also encouraged. Detailed information on the costs of implementing a rice fortification process using sonication has not been carried out yet. Still, the fixed cost associated with the process utilizes minimal investment that includes sonicators and drying platforms.
6.4. Ultrasonication used for enhancing rice fermented products
Ultrasound can influence fermentation through the improvement of mass transfer and cell permeability leading to the enhancement in the process in terms of efficiency and production rates [27]. The cooking time is a vital determinant in Chinese rice wine production because minimum cooking time and gelatinization is a critical factor in brewing. Li et al. [53] reported that UT shortens the soaking and cooking time of rice, which contributes to the decrease in the fermentation time of Chinese rice wine. While, recently, Z. Yang et al. [88] observed a 16.6% increase in rice wine yield due to the increased water absorption (8.2%) during the cooking process resulting in the promotion of saccharification and production of alcohol. Moreover, high-quality rice wine is produced from UT rice because of the presence of a higher concentration of organic acids such as oxalic acid, tartaric acid, and malic acid. The demonstration of the utility of sonication in rice wine production can be fine-tuned by manipulating the sonic conditions and rice varieties to achieve higher concentrations of polyphenols towards improvement in quality, taste, and health benefits. The use of germinated brown rice in wine production can be facilitated by sonication as it has shown to increase the nutritional value of germinated brown rice. Unlike ordinary fermented rice wine, the germinated brown rice wine is richer in alcohol-soluble ingredients and aromas. The effect of sonicating the germinated brown rice prior to wine production is seen as a feasible process in affording wine with new composition of nutritional ingredients and healthy flavor compounds. The effects of various UT conditions on rice on the rate of the liquefaction/saccharification of starch and the fermentation of sugars is worth pursuing as the sonic waves affect the rice starch properties and cooking qualities. In traditional Chinese wine rice production, the glutinous rice variety is preferred. Since the waxy rice variety responds differently to sonication in terms of textural and rheological properties associated with interactions between amylopectin molecules, the effect of sonicated waxy rice in rice wine production is seen to afford a unique composition.
7. Summary and future insights
Ultrasonication is a useful technology in modifying the composition, structure, and properties of rice starch. In general, UT induced the formation of fissures in rice starch and decreased the granular size as observed in the isolated starch from UT rice grain. Optimizing sonication parameters to enhance the porosity but with no effect on rice starch molecular structure is critical to deploy this technique to improve the texture of brown rice and to fortify essential nutrients in the milled rice. Similarly, variations in the thermal, crystallinity, pasting, and viscoelastic properties of rice starch have to be considered in the applied ultrasonic parameters. Furthermore, the changes in rice starch structure generally affected its physicochemical structure, texture, and digestibility. The decrease in relative crystallinity and enthalpy of gelatinization and an increase in the peak viscosity of UT rice starch are attributed to the softening of the rice starch matrix and the quickening of the gelatinization process. This technique decreases the hardness of milled rice and brown rice, making it more palatable to consumers. Moreover, ultrasonication can be deployed for the induction of health-promoting metabolites and enhancing the nutritional benefits of whole grain rice and germinated sprouts.
While there is a vast advancement in the understanding of the effect of ultrasonication in the chemistry of rice starch and grain, further studies and improvements are still needed to address the practical utilization of ultrasonication in rice starch and grain for various applications. The following future directions can be explored.
-
(1)
The investigation of the impact of ultrasonic treatment in pure amylose and pure amylopectin isolated from rice starch is worth pursuing because it would give a benchmark data on the molecular level effects of ultrasonication in rice sample. The dimension of amylose helix and the helication of amylose can potentially be affected by the sonic waves and may impart unique interaction resulting to novel properties. Employing a larger number of rice genotypes with different amylose/amylopectin ratios will help test its broader applicability. Conducting sensory evaluation in ultrasonic-treated samples will provide insights as to its textural attributes and in assessing the product suitability from the consumer perspectives.
-
(2)
The cavitation, chemical, and mechanical effect thresholds in a wide range of frequency on rice grains is worth investigating to optimize the condition that promotes favorable eating qualities while maintaining the head rice quality. Varying the power and intensity of ultrasonication will give insights on how ultrasonic parameters induced changes in starch properties, which can be taken into consideration for the processing of rice-based products. Moreover, performing advanced x-ray experimentations on the effect of ultrasonication on the amorphous and crystalline lamellae of rice starch will give vital insights on how mechanical disruptions of the starch architecture affect thermal, pasting, rheological and digestibility properties. It is also important to observe the polymorph transition of A-type to V-type starch pattern as a product of rice starch ultrasonication.
-
(3)
To further understand the effect of ultrasonication on the production of rice grain metabolites, the systematic investigation of increasing ultrasonic power and intensity is recommended. It would be worth investigating the metabolite profile in ultrasonically treated pigmented rice as it could help promote or eliminate the endogenous antioxidants. The response of UT rice towards enzymatic activities needs to be explored further. Ultrasonication is an excellent method to increase the nutritional functionality of rice, particularly in germinated sprouts and it would be interesting to explore the resulting genetic profile of germinated sprouts.
-
(4)
The effect of sonication in the presence of oils or lipids can promote complexation in rice starch leading to the potential formation of starch-lipid complexes that would increase the composition of resistant starch. Since the formation and stabilization of these complexes involve a series of noncovalent interactions including hydrogen bonds, hydrophobic attractions, and van der Waals forces, its complexation chemistry would potentially be affected by the sound waves resulting to unique conformations and properties. It is also worth exploring the potential formation of ternary starch–lipid–protein complexes under ultrasonic condition. The formation of these complexes can be influenced by sonic waves affecting the functional, nutritional, and textural properties. The helical inclusion complexes of the linear amylose fraction in rice with different volatile aroma and flavor compounds can be explored in the presence of sonic wav While there is a vast advancement in the understanding of the effect of ultrasonication in the chemistry of rice starch and grain, further studies and improvements are still needed to address the practical utilization of ultrasonication in rice starch and grain for various applications. The following future directions can be explored.
CRediT authorship contribution statement
Aldrin P. Bonto: Conceptualization, Writing - original draft. Rhowell N. Tiozon: Visualization, Writing - original draft. Nese Sreenivasulu: Conceptualization, Supervision. Drexel H. Camacho: Conceptualization, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This work was supported by the Commission on Higher Education (CHED), Philippines, through its CHED-GIA grant and the De La Salle University, Philippines, through the University Research Coordination Office (DLSU-URCO). A. P. Bonto and R. N. Tiozon Jr., would like to acknowledge the Department of Science and Technology DOST, Philippines, through the Science Education Institute (SEI) - Accelerated Science and Technology Human Resource Development (ASTHRDP) program for the graduate scholarships.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ultsonch.2020.105383.
Contributor Information
Nese Sreenivasulu, Email: n.sreenivasulu@irri.org.
Drexel H. Camacho, Email: drexel.camacho@dlsu.edu.ph.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.J. Bao, Rice starch, in: J. Bao (Ed.), Rice Chem. Technol., 4th ed., Elsevier Inc. in cooperation with AACC International, 2019: pp. 55–108. https://doi.org/10.1016/B978-0-12-811508-4.00003-4.
- 2.C. Mitchell, Rice Starches: Production and Properties, in: J. Bemiller, R. Whistler (Eds.), Starch Chem. Technol., 3rd Editio, Elsevier Inc., 2009: pp. 569–578. https://doi.org/10.1016/B978-0-12-746275-2.00013-6.
- 3.Wu T., Wang L., Li Y., Qian H., Liu L., Tong L., Zhou X., Wang L., Zhou S. LWT - Food Science and Technology Effect of milling methods on the properties of rice flour and gluten-free rice bread. LWT - Food Sci. Technol. 2019;108:137–144. doi: 10.1016/j.lwt.2019.03.050. [DOI] [Google Scholar]
- 4.Gujral H.S., Rosell C.M. Functionality of rice flour modified with a microbial transglutaminase. J. Cereal Sci. 2004;39:225–230. doi: 10.1016/j.jcs.2003.10.004. [DOI] [Google Scholar]
- 5.Yano H. Recent practical researches in the development of gluten-free breads. Npj Sci. Food. 2019;3 doi: 10.1038/s41538-019-0040-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El Khoury D., Balfour-Ducharme S., Joye I.J. A review on the gluten-free diet: Technological and nutritional challenges. Nutrients. 2018;10:1410. doi: 10.3390/nu10101410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patist A., Bates D. Ultrasonic innovations in the food industry: From the laboratory to commercial production. Innov. Food Sci. Emerg. Technol. 2008;9:147–154. doi: 10.1016/j.ifset.2007.07.004. [DOI] [Google Scholar]
- 8.Awad T.S., Moharram H.A., Shaltout O.E., Asker D., Youssef M.M. Applications of ultrasound in analysis, processing and quality control of food: A review. Food Res. Int. 2012;48:410–427. doi: 10.1016/j.foodres.2012.05.004. [DOI] [Google Scholar]
- 9.Mulet A., Benedito J., Golás Y., Cárcel J.A. Noninvasive ultrasonic measurements in the food industry. Food Rev. Int. 2002;18:123–133. doi: 10.1081/FRI-120014354. [DOI] [Google Scholar]
- 10.Carovac A., Smajlovic F., Junuzovic D. Application of Ultrasound in Medicine. Acta Inform. Medica. 2011;19:168. doi: 10.5455/aim.2011.19.168-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.J. Welti-Chanes, M. Morales-de la Peña, D.A. Jacobo-Velázquez, O. Martín-Belloso, Opportunities and Challenges of Ultrasound for Food Processing: An Industry Point of View, in: D. Bermudez-Aguirre (Ed.), Ultrasound Adv. Food Process. Preserv., 2017: pp. 457–497. https://doi.org/10.1016/B978-0-12-804581-7.00019-1.
- 12.N.J. Watson, M.J.W. Povey, N.G. Parker, Acoustic Microscopy, in: M. Villamiel, J. V. García-Pérez, A. Montilla, J.A. Cárcel, J. Benedito (Eds.), Ultrasound Food Process. Recent Adv., First, John Wiley & Sons Ltd, 2017: pp. 230–251. https://doi.org/10.1201/9781420017373.ch29.
- 13.L. Paniwnyk, Application of Ultrasound, in: D.-W. Sun (Ed.), Emerg. Technol. Food Process., Elsevier, London, U.K., 2014: pp. 271–291. https://doi.org/10.1016/b978-0-12-411479-1.00015-2.
- 14.Alarcon-Rojo A.D., Carrillo-Lopez L.M., Reyes-Villagrana R., Huerta-Jiménez M., Garcia-Galicia I.A. Ultrasound and meat quality: A review. Ultrason. Sonochem. 2019;55:369–382. doi: 10.1016/j.ultsonch.2018.09.016. [DOI] [PubMed] [Google Scholar]
- 15.S. Ercan C. Soysal Use of ultrasound in food preservation Nat. Sci. 5 2013 5 13 https://doi.org/doi.org/10.4236/ns.2013.58A2002.
- 16.Nicolau-Lapeña I., Lafarga T., Viñas I., Abadias M., Bobo G., Aguiló-Aguayo I. Ultrasound Processing Alone or in Combination with Other Chemical or Physical Treatments as a Safety and Quality Preservation Strategy of Fresh and Processed Fruits and Vegetables: A Review. Food Bioprocess Technol. 2019;12:1452–1471. doi: 10.1007/s11947-019-02313-y. [DOI] [Google Scholar]
- 17.Soltani Firouz M., Farahmandi A., Hosseinpour S. Recent advances in ultrasound application as a novel technique in analysis, processing and quality control of fruits, juices and dairy products industries: A review. Ultrason. Sonochem. 2019;57:73–88. doi: 10.1016/j.ultsonch.2019.05.014. [DOI] [PubMed] [Google Scholar]
- 18.Sango D.M., Abela D., Mcelhatton A., Valdramidis V.P. Assisted ultrasound applications for the production of safe foods. J. Appl. Microbiol. 2014;116:1067–1083. doi: 10.1111/jam.12468. [DOI] [PubMed] [Google Scholar]
- 19.Ashokkumar M. Applications of ultrasound in food and bioprocessing. Ultrason. Sonochem. 2015;25:17–23. doi: 10.1016/j.ultsonch.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 20.Chemat F., Zill-E-Huma M.K. Khan, Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011;18:813–835. doi: 10.1016/j.ultsonch.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 21.Gallo M., Ferrara L., Naviglio D. Application of Ultrasound in Food Science and Technology : A Perspective. Foods. 2018;7:164. doi: 10.3390/foods7100164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kentish S., Feng H. Applications of Power Ultrasound in Food Processing. Annu. Rev. Food Sci. Technol. 2014;5:263–284. doi: 10.1146/annurev-food-030212-182537. [DOI] [PubMed] [Google Scholar]
- 23.Knorr D., Zenker M., Heinz V., Lee D.U. Applications and potential of ultrasonics in food processing. Trends Food Sci. Technol. 2004;15:261–266. doi: 10.1016/j.tifs.2003.12.001. [DOI] [Google Scholar]
- 24.Majid I., Nayik G.A., Nanda V. Ultrasonication and food technology: A review. Cogent Food Agric. 2015;1:1071022. doi: 10.1080/23311932.2015.1071022. [DOI] [Google Scholar]
- 25.Chemat F., Rombaut N., Sicaire A.G., Meullemiestre A., Fabiano-Tixier A.S., Abert-Vian M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review, Ultrason. - Sonochemistry. 2017;34:540–560. doi: 10.1016/j.ultsonch.2016.06.035. [DOI] [PubMed] [Google Scholar]
- 26.Hasan M.M., Bashir T., Bae H. Use of Ultrasonication Technology for the Increased Production of Plant Secondary Metabolites. Molecules. 2017;22:1046. doi: 10.3390/molecules22071046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ojha K.S., Mason T.J., O’Donnell C.P., Kerry J.P., Tiwari B.K. Ultrasound technology for food fermentation applications. Ultrason. Sonochem. 2017;34:410–417. doi: 10.1016/j.ultsonch.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 28.Pingret D., Fabiano-Tixier A.S., Chemat F. Degradation during application of ultrasound in food processing: A review. Food Control. 2013;31:593–606. doi: 10.1016/j.foodcont.2012.11.039. [DOI] [Google Scholar]
- 29.Bemiller J.N. Physical Modification of Starch. Elsevier Ltd. 2018 doi: 10.1016/B978-0-08-100868-3.00005-6. [DOI] [Google Scholar]
- 30.BeMiller J.N., Huber K.C. Physical Modification of Food Starch Functionalities. Annu. Rev. Food Sci. Technol. 2015;6:19–69. doi: 10.1146/annurev-food-022814-015552. [DOI] [PubMed] [Google Scholar]
- 31.Zhu F. Impact of ultrasound on structure, physicochemical properties, modifications, and applications of starch. Trends Food Sci. Technol. 2015;43:1–17. doi: 10.1016/j.tifs.2014.12.008. [DOI] [Google Scholar]
- 32.De Guzman M.K., Parween S., Butardo V.M., Alhambra C.M., Anacleto R., Seiler C., Bird A.R., Chow C.P., Sreenivasulu N. Investigating glycemic potential of rice by unraveling compositional variations in mature grain and starch mobilization patterns during seed germination. Sci. Rep. 2017;7:5854. doi: 10.1038/s41598-017-06026-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tran T.T.B., Shelat K.J., Tang D., Li E., Gilbert R.G., Hasjim J. Milling of rice grains. the degradation on three structural levels of starch in rice flour can be independently controlled during grinding. J. Agric. Food Chem. 2011;59:3964–3973. doi: 10.1021/jf105021r. [DOI] [PubMed] [Google Scholar]
- 34.Gilbert R.G., Witt T., Hasjim J. What is being learned about starch properties from multiple-level characterization. Cereal Chem. 2013;90:312–325. doi: 10.1094/CCHEM-11-12-0141-FI. [DOI] [Google Scholar]
- 35.Butardo V.M., Anacleto R., Parween S., Samson I., de Guzman K., Alhambra C.M., Misra G., Sreenivasulu N. Systems genetics identifies a novel regulatory domain of amylose synthesis. Plant Physiol. 2017;173:887–906. doi: 10.1104/pp.16.01248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hanashiro I., Abe J.I., Hizukuri S. A periodic distribution of the chain length of amylopectin as revealed by high-performance anion-exchange chromatography. Carbohydr. Res. 1996;283:151–159. doi: 10.1016/0008-6215(95)00408-4. [DOI] [Google Scholar]
- 37.Wani A.A., Singh P., Shah M.A., Schweiggert-Weisz U., Gul K., Wani I.A. Rice Starch Diversity: Effects on Structural, Morphological, Thermal, and Physicochemical Properties- A Review. Compr. Rev. Food Sci. Food Saf. 2012;11:417–436. doi: 10.1111/j.1541-4337.2012.00193.x. [DOI] [Google Scholar]
- 38.Wu A.C., Witt T., Gilbert R.G. Characterization methods for starch-based materials: State of the art and perspectives. Aust. J. Chem. 2013;66:1550–1563. doi: 10.1071/CH13397. [DOI] [Google Scholar]
- 39.Ong M.H., Blanshard J.M.V. The Significance of Starch Polymorphism in Commercially Produced Parboiled Rice. Starch - Stärke. 1995;47:7–13. doi: 10.1002/star.19950470104. [DOI] [Google Scholar]
- 40.Tester R.F., Karkalas J., Qi X. Starch - Composition, fine structure and architecture. J. Cereal Sci. 2004;39:151–165. doi: 10.1016/j.jcs.2003.12.001. [DOI] [Google Scholar]
- 41.Hsein-Chih H.W., Sarko A. The double-helical molecular structure of crystalline A-amylose. Carbohydr. Res. 1978;60:27–40. doi: 10.1016/s0008-6215(00)83568-5. [DOI] [Google Scholar]
- 42.Zhou X., Ying Y., Hu B., Pang Y., Bao J. Physicochemical properties and digestibility of endosperm starches in four indica rice mutants. Carbohydr. Polym. 2018;195:1–8. doi: 10.1016/j.carbpol.2018.04.070. [DOI] [PubMed] [Google Scholar]
- 43.Takahashi T., Fujita N. Thermal and rheological characteristics of mutant rice starches with widespread variation of amylose content and amylopectin structure. Food Hydrocoll. 2017;62:83–93. doi: 10.1016/j.foodhyd.2016.06.022. [DOI] [Google Scholar]
- 44.Wei C., Qin F., Zhou W., Yu H., Xu B., Chen C., Zhu L., Wang Y., Gu M., Liu Q. Granule structure and distribution of allomorphs in C-type high-amylose rice starch granule modified by antisense RNA inhibition of starch branching enzyme. J. Agric. Food Chem. 2010;58:11946–11954. doi: 10.1021/jf103412d. [DOI] [PubMed] [Google Scholar]
- 45.Dang J.M.C., Copeland L. Imaging rice grains using atomic force microscopy. J. Cereal Sci. 2003;37:165–170. doi: 10.1006/jcrs.2002.0490. [DOI] [Google Scholar]
- 46.Chiou H., Fellows C.M., Gilbert R.G., Fitzgerald M.A. Study of rice-starch structure by dynamic light scattering in aqueous solution. Carbohydr. Polym. 2005;61:61–71. doi: 10.1016/j.carbpol.2005.02.011. [DOI] [Google Scholar]
- 47.Kong X., Sun X., Xu F., Umemoto T., Chen H., Bao J. Morphological and physicochemical properties of two starch mutants induced from a high amylose indica rice by gamma irradiation. Starch/Staerke. 2014;66:157–165. doi: 10.1002/star.201300024. [DOI] [Google Scholar]
- 48.Li W., Gamlath C.J., Pathak R., Martin G.J.O., Ashokkumar M. Ultrasound – The Physical and Chemical Effects Integral to Food Processing. Ref. Modul. Food Sci. 2019 doi: 10.1016/b978-0-08-100596-5.22679-6. [DOI] [Google Scholar]
- 49.Mason T.J., Paniwnyk L., Lorimer J.P. The uses of ultrasound in food technology. Ultrason. Sonochem. 1996;3:S253–S260. [Google Scholar]
- 50.Cui L., Pan Z., Yue T., Atungulu G.G., Berrios J. Effect of ultrasonic treatment of brown rice at different temperatures on cooking properties and quality. Cereal Chem. 2010;87:403–408. doi: 10.1094/CCHEM-02-10-0034. [DOI] [Google Scholar]
- 51.Zhang X., Wang L., Cheng M., Wang R., Luo X., Li Y., Chen Z. Influence of ultrasonic enzyme treatment on the cooking and eating quality of brown rice. J. Cereal Sci. 2015;63:140–146. doi: 10.1016/j.jcs.2015.03.002. [DOI] [Google Scholar]
- 52.Bonto A.P., Camacho K.S.I., Camacho D.H. Increased vitamin B5 uptake capacity of ultrasonic treated milled rice: A new method for rice fortification. LWT - Food Sci. Technol. 2018;95:32–39. doi: 10.1016/j.lwt.2018.04.062. [DOI] [Google Scholar]
- 53.Li S., Luo Z., Guan X., Huang K., Li Q., Zhu F., Liu J. Effect of ultrasonic treatment on the hydration and physicochemical properties of brewing rice. J. Cereal Sci. 2019;87:78–84. doi: 10.1016/j.jcs.2019.03.002. [DOI] [Google Scholar]
- 54.Bonto A.P., Tiozon R.N., Jr, Rojviriya C., Sreenivasulu N., Camacho D.H. Sonication increases the porosity of uncooked rice kernels affording softer textural properties, loss of intrinsic nutrients and increased uptake capacity during fortification. Ultrason. Sonochem. 2020;68 doi: 10.1016/j.ultsonch.2020.105234. [DOI] [PubMed] [Google Scholar]
- 55.Miano A.C., Pereira C., Castanha N., Divino M., Esteves P., Augusto D. Enhancing mung bean hydration using the ultrasound technology: description of mechanisms and impact on its germination and main components. Sci. Rep. 2016;6:38996. doi: 10.1038/srep38996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sujka M., Jamroz J. Ultrasound-treated starch: SEM and TEM imaging, and functional behaviour. Food Hydrocoll. 2013;31:413–419. doi: 10.1016/j.foodhyd.2012.11.027. [DOI] [Google Scholar]
- 57.Sujka M. Ultrasonic modification of starch – Impact on granules porosity. Ultrason. Sonochem. 2017;37:424–429. doi: 10.1016/j.ultsonch.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 58.Yang W., Kong X., Zheng Y., Sun W., Chen S., Liu D., Zhang H., Fang H., Tian J., Ye X. Controlled ultrasound treatments modify the morphology and physical properties of rice starch rather than the fine structure. Ultrason. Sonochem. 2019;59 doi: 10.1016/j.ultsonch.2019.104709. [DOI] [PubMed] [Google Scholar]
- 59.Zuo Y.Y.J., Hébraud P., Hemar Y., Ashokkumar M. Quantification of high-power ultrasound induced damage on potato starch granules using light microscopy. Ultrason. Sonochem. 2012;19:421–426. doi: 10.1016/j.ultsonch.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 60.Zuo J.Y., Knoerzer K., Mawson R., Kentish S., Ashokkumar M. The pasting properties of sonicated waxy rice starch suspensions. Ultrason. Sonochem. 2009;16:462–468. doi: 10.1016/j.ultsonch.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 61.Wang L., Wang Y.J. Rice starch isolation by neutral protease and high-intensity ultrasound. J. Cereal Sci. 2004;39:291–296. doi: 10.1016/j.jcs.2003.11.002. [DOI] [Google Scholar]
- 62.Wang L., Wang Y.J. Application of High-Intensity Ultrasound and Surfactants in Rice Starch Isolation. Cereal Chem. 2004;81:140–144. doi: 10.1094/CCHEM.2004.81.1.140. [DOI] [Google Scholar]
- 63.Czechowska-Biskup R., Rokita B., Lotfy S., Ulanski P., Rosiak J.M. Degradation of chitosan and starch by 360-kHz ultrasound. Carbohydr. Polym. 2005;60:175–184. doi: 10.1016/j.carbpol.2004.12.001. [DOI] [Google Scholar]
- 64.Ogutu F.O. Ultrasonic Modification of Selected Polysaccharides-Review. J. Food Process. Technol. 2015;6:1000446. doi: 10.4172/2157-7110.1000446. [DOI] [Google Scholar]
- 65.Tayal A., Khan S.A. Degradation of a water-soluble polymer: molecular weight changes and chain scission characteristics. Macromolecules. 2000;33:9488–9493. doi: 10.1021/ma000736g. [DOI] [Google Scholar]
- 66.Kentish S., Ashokkumar M. The Physical and Chemical Effects of Ultrasound. Springer; New York, NY: 2010. [DOI] [Google Scholar]
- 67.Isono Y., Watanabe T., Kumagai T. Ultrasonic Degradation of Waxy Rice Starch. Biosci. Biotechnol. Biochem. 1994;58:1799–1802. doi: 10.1271/bbb.58.1799. [DOI] [Google Scholar]
- 68.Chung K.M., Moon T.W., Kim H., Chun J.K. Physicochemical properties of sonicated mung bean, potato, and rice starches. Cereal Chem. 2002;79:631–633. doi: 10.1094/CCHEM.2002.79.5.631. [DOI] [Google Scholar]
- 69.Amini A.M., Razavi S.M.A., Mortazavi S.A. Morphological, physicochemical, and viscoelastic properties of sonicated corn starch. Carbohydr. Polym. 2015;122:282–292. doi: 10.1016/j.carbpol.2015.01.020. [DOI] [PubMed] [Google Scholar]
- 70.Cao M., Gao Q. Effect of dual modification with ultrasonic and electric field on potato starch. Int. J. Biol. Macromol. 2020;150:637–643. doi: 10.1016/j.ijbiomac.2020.02.008. [DOI] [PubMed] [Google Scholar]
- 71.Park D.J., Han J.A. Quality controlling of brown rice by ultrasound treatment and its effect on isolated starch. Carbohydr. Polym. 2016;137:30–38. doi: 10.1016/j.carbpol.2015.10.045. [DOI] [PubMed] [Google Scholar]
- 72.Kunyanee K., Luangsakul N. The effects of ultrasound – assisted recrystallization followed by chilling to produce the lower glycemic index of rice with different amylose content. Food Chem. 2020;323 doi: 10.1016/j.foodchem.2020.126843. [DOI] [PubMed] [Google Scholar]
- 73.Dang L., Therdthai N., Ratphitagsanti W. Effects of ultrasonic and enzymatic treatment on physical and chemical properties of brown rice. J. Food Process Eng. 2019;42 doi: 10.1111/jfpe.13016. [DOI] [Google Scholar]
- 74.Keeratiburana T., Hansen A.R., Soontaranon S., Tongta S., Blennow A. Porous rice starch produced by combined ultrasound-assisted ice recrystallization and enzymatic hydrolysis. Int. J. Biol. Macromol. 2020;145:100–107. doi: 10.1016/j.ijbiomac.2019.12.144. [DOI] [PubMed] [Google Scholar]
- 75.Setyawati Y.D., Ahsan S.F., Ong L.K., Soetaredjo F.E., Ismadji S., Ju Y.H. Production of glutinous rice flour from broken rice via ultrasonic assisted extraction of amylose. Food Chem. 2016;203:158–164. doi: 10.1016/j.foodchem.2016.02.068. [DOI] [PubMed] [Google Scholar]
- 76.Flores-Silva P.C., Roldan-Cruz C.A., Chavez-Esquivel G., Vernon-Carter E.J., Bello-Perez L.A., Alvarez-Ramirez J. In vitro digestibility of ultrasound-treated corn starch. Starch/Staerke. 2017;69 doi: 10.1002/star.201700040. [DOI] [Google Scholar]
- 77.Kunyanee K., Luangsakul N. The utilization of ultrasound and chilling treatment to reduce GI in Thai glutinous rice (RD6) Int. J. Agric. Technol. 2018;14:1365–1378. [Google Scholar]
- 78.Ding Y., Liang Y., Luo F., Ouyang Q., Lin Q. Understanding the mechanism of ultrasonication regulated the digestibility properties of retrograded starch following vacuum freeze drying. Carbohydr. Polym. 2020;228 doi: 10.1016/j.carbpol.2019.115350. [DOI] [PubMed] [Google Scholar]
- 79.Flores-Silva P.C., Alvarez-Ramirez J., Bello-Perez L.A. Effect of dual modification order with ultrasound and hydrothermal treatments on starch digestibility. Starch/Staerke. 2018;70:1–19. doi: 10.1002/star.201700284. [DOI] [Google Scholar]
- 80.Ding Y., Luo F., Lin Q. Insights into the relations between the molecular structures and digestion properties of retrograded starch after ultrasonic treatment. Food Chem. 2019;294:248–259. doi: 10.1016/j.foodchem.2019.05.050. [DOI] [PubMed] [Google Scholar]
- 81.Kaur H., Gill B.S. Effect of high-intensity ultrasound treatment on nutritional, rheological and structural properties of starches obtained from different cereals. Int. J. Biol. Macromol. 2019;126:367–375. doi: 10.1016/j.ijbiomac.2018.12.149. [DOI] [PubMed] [Google Scholar]
- 82.Yu S., Zhang Y., Ge Y., Zhang Y., Sun T., Jiao Y., Zheng X.Q. Effects of ultrasound processing on the thermal and retrogradation properties of nonwaxy rice starch. J. Food Process Eng. 2013;36:793–802. doi: 10.1111/jfpe.12048. [DOI] [Google Scholar]
- 83.Yang Q.Y., Lu X.X., Chen Y.Z., Luo Z.G., Xiao Z.G. Fine structure, crystalline and physicochemical properties of waxy corn starch treated by ultrasound irradiation. Ultrason. Sonochem. 2019;51:350–358. doi: 10.1016/j.ultsonch.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 84.Dang L., Therdthai N., Ratphitagsanti W. Improvement of structure and cooking quality of brown rice using ultrasonic and enzymatic treatments. J. Food Process. Preserv. 2018;42:1–8. doi: 10.1111/jfpp.13814. [DOI] [Google Scholar]
- 85.Chan H.T., Bhat R., Karim A.A. Effects of sodium dodecyl sulphate and sonication treatment on physicochemical properties of starch. Food Chem. 2010;120:703–709. doi: 10.1016/j.foodchem.2009.10.066. [DOI] [Google Scholar]
- 86.Ibanoglu S., Özaslan Z.T., Ibanoglu E. Combined effect ozonation and ultrasonication on rheological and thermal properties of rice starch in aqueous phase. Qual. Assur. Saf. Crop. Foods. 2018;10:69–74. doi: 10.3920/QAS2017.1096. [DOI] [Google Scholar]
- 87.Xia Q., Green B.D., Zhu Z., Li Y., Taghi S.M. Innovative processing techniques for altering the physicochemical properties of wholegrain brown rice (Oryza sativa L.) – opportunities for enhancing food quality and health attributes. Crit. Rev. Food Sci. Nutr. 2019;59:3349–3370. doi: 10.1080/10408398.2018.1491829. [DOI] [PubMed] [Google Scholar]
- 88.Yang Z., Liu S., Lin X., Wang L., Li C. Effects of ultrasonic treatment on the cooking and fermentation properties of Shanlan rice. J. Cereal Sci. 2020;95 doi: 10.1016/j.jcs.2020.103003. [DOI] [Google Scholar]
- 89.Jadwong K., Therdthai N. Effects of Ultrasonic and Enzymatic Treatment on Cooking and Eating Quality of Sao Hai Rice. Food Appl. Biosci. J. 2018;6:153–165. [Google Scholar]
- 90.Szczesniak A.S. Classification of Textural Characteristics. J. Food Sci. 1963;28:385–389. doi: 10.1111/j.1365-2621.1963.tb00215.x. [DOI] [Google Scholar]
- 91.Chen J., Feng M., Gonzalez Y., Pugnaloni L.A. Application of probe tensile method for quantitative characterisation of the stickiness of fluid foods. J. Food Eng. 2008;87:281–290. doi: 10.1016/j.jfoodeng.2007.12.004. [DOI] [Google Scholar]
- 92.Li H., Fitzgerald M.A., Prakash S., Nicholson T.M., Gilbert R.G. The molecular structural features controlling stickiness in cooked rice, a major palatability determinant. Sci. Rep. 2017;7:43713. doi: 10.1038/srep43713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Leelayuthsoontorn P., Thipayarat A. Textural and morphological changes of Jasmine rice under various elevated cooking conditions. Food Chem. 2006;96:606–613. doi: 10.1016/j.foodchem.2005.03.016. [DOI] [Google Scholar]
- 94.Patindol J., Gu X., Wang Y. Chemometric analysis of cooked rice texture in relation to starch fine structure and leaching characteristics. Starch/Stärke. 2010;62:188–197. doi: 10.1002/star.200900181. [DOI] [Google Scholar]
- 95.Wang D., Yan L., Ma X., Wang W., Zou M., Zhong J., Ding T., Ye X., Liu D. Ultrasound promotes enzymatic reactions by acting on different targets: Enzymes, substrates and enzymatic reaction systems. Int. J. Biol. Macromol. 2018;119:453–461. doi: 10.1016/j.ijbiomac.2018.07.133. [DOI] [PubMed] [Google Scholar]
- 96.Xia Q., Wang L., Yu W., Li Y. Investigating the influence of selected texture-improved pretreatment techniques on storage stability of wholegrain brown rice: Involvement of processing-induced mineral changes with lipid degradation. Food Res. Int. 2017;99:510–521. doi: 10.1016/j.foodres.2017.06.020. [DOI] [PubMed] [Google Scholar]
- 97.Ding J., Hou G.G., Dong M., Xiong S., Zhao S., Feng H. Physicochemical properties of germinated dehulled rice flour and energy requirement in germination as affected by ultrasound treatment. Ultrason. Sonochem. 2018;41:484–491. doi: 10.1016/j.ultsonch.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 98.Ding J., Ulanov A.V., Dong M., Yang T., Nemzer B.V., Xiong S., Zhao S., Feng H. Enhancement of gama-aminobutyric acid (GABA) and other health-related metabolites in germinated red rice (Oryza sativa L.) by ultrasonication. Ultrason. Sonochem. 2018;40:791–797. doi: 10.1016/j.ultsonch.2017.08.029. [DOI] [PubMed] [Google Scholar]
- 99.Xia Q., Tao H., Li Y., Pan D., Cao J., Liu L., Zhou X., Barba F.J. Characterizing physicochemical, nutritional and quality attributes of wholegrain Oryza sativa L. subjected to high intensity ultrasound-stimulated pre-germination. Food Control. 2020;108 doi: 10.1016/j.foodcont.2019.106827. [DOI] [Google Scholar]
- 100.Ding J., Hou G.G., Nemzer B.V., Xiong S., Dubat A., Feng H. Effects of controlled germination on selected physicochemical and functional properties of whole-wheat flour and enhanced γ -aminobutyric acid accumulation by ultrasonication. Food Chem. 2018;243:214–221. doi: 10.1016/j.foodchem.2017.09.128. [DOI] [PubMed] [Google Scholar]
- 101.J. Ding J. Johnson Y. Fang H. Feng Enhancement of γ-aminobutyric acid, avenanthramides, and other health-promoting metabolites in germinating oats (Avena sativa L.) treated with and without power ultrasound Food Chem. 283 (2019) 239 247 10.1016/j.foodchem.2018.12.136. [DOI] [PubMed]
- 102.Yang H., Gao J., Yang A., Chen H. The ultrasound-treated soybean seeds improve edibility and nutritional quality of soybean sprouts. Food Res. Int. 2015;77:704–710. doi: 10.1016/j.foodres.2015.01.011. [DOI] [Google Scholar]
- 103.Aguilar-Camacho M., Welti-Chanes J., Jacobo-velázquez D.A. Combined effect of ultrasound treatment and exogenous phytohormones on the accumulation of bioactive compounds in broccoli florets. Ultrason. Sonochem. 2019;50:289–301. doi: 10.1016/j.ultsonch.2018.09.031. [DOI] [PubMed] [Google Scholar]
- 104.Pinheiro J., Alegria C., Abreu M., Gonçalves E.M., Silva C.L.M. Influence of postharvest ultrasounds treatments on tomato (Solanum lycopersicum, cv. Zinac) quality and microbial load during storage. Ultrason. Sonochem. 2015;27:552–559. doi: 10.1016/j.ultsonch.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 105.Lu C., Ding J., Kyung H., Feng H. High intensity ultrasound as a physical elicitor affects secondary metabolites and antioxidant capacity of tomato fruits. Food Control. 2020;113 doi: 10.1016/j.foodcont.2020.107176. [DOI] [Google Scholar]
- 106.Kavi Kishor P.B., Sreenivasulu N. Is proline accumulation per se correlated with stress tolerance or is proline homeostasis a more critical issue? Plant, Cell Environ. 2014;37:300–311. doi: 10.1111/pce.12157. [DOI] [PubMed] [Google Scholar]
- 107.Saha S., Roy A. Whole grain rice fortification as a solution to micronutrient deficiency: Technologies and need for more viable alternatives. Food Chem. 2020;326 doi: 10.1016/j.foodchem.2020.127049. [DOI] [PubMed] [Google Scholar]
- 108.Miano A.C., Augusto P.E.D. The ultrasound assisted hydration as an opportunity to incorporate nutrients into grains. Food Res. Int. 2018;106:928–935. doi: 10.1016/j.foodres.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 109.Camacho D.H. Vitamin Fortification of Milled Rice: A New Approach to Address Micronutrient Malnutrition, Policy Br. AKI Res. Grants Food Stud. 2018;8:1–4. [Google Scholar]
- 110.Tiozon R.N., Jr. Efficient Fortification of Folic acid in rice through ultrasonic treatment and absorption. MS Chemistry Thesis, De La Salle University. 2019 doi: 10.1016/j.foodchem.2020.127629. [DOI] [PubMed] [Google Scholar]
- 111.Tiozon R.N., Jr, Camacho D.H., Bonto A.P., Oyong G.G., Sreenivasulu N. Efficient fortification of folic acid in rice through ultrasonic treatment and absorption. Food Chem. 2021;335 doi: 10.1016/j.foodchem.2020.127629. [DOI] [PubMed] [Google Scholar]
- 112.Tiozon R.N., Jr, Camacho D.H., Bonto A.P., Oyong G.G., Sreenivasulu N. Dataset on the folic acid uptake and the effect of sonication-based fortification on the color, pasting and textural properties of brown and milled rice. Data in Brief. 2020;32 doi: 10.1016/j.dib.2020.106198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bonto A.P., Jearanaikoon N., Sreenivasulu N., Camacho D.H. High uptake and inward diffusion of iron fortificant in ultrasonicated milled rice. LWT - Food Sci. Technol. 2020;128 doi: 10.1016/j.lwt.2020.109459. [DOI] [Google Scholar]
- 114.Wambura P., Yang W., Wang Y. Power ultrasound enhanced one-step soaking and gelatinization for rough rice parboiling. Int. J. Food Eng. 2008;4:1–12. doi: 10.2202/1556-3758.139. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.