Highlights
-
•
Ultrasonic duration had the greater effect on properties of cellulose nanoparticles.
-
•
Ultrasound significantly improved hydrophobicity of cellulose nanoparticles.
-
•
Cellulose treated by intense ultrasound were easier to adsorb at O/W interface.
-
•
The adsorbed nanoparticles formed an interfacial film at the interface of droplets.
Keywords: Ultrasound, Cellulose nanoparticles, Interfacial properties, Emulsifying properties
Abstract
Cellulose microparticles from ginkgo seed shells were treated by ultrasonic treatments within the selected output powders (150–600 W) and durations (10–60 min) to produce cellulose nanoparticles. The main aim of this study was to investigate effects of ultrasonic conditions on the interfacial property and emulsifying property of those cellulose nanoparticles. Compared to ultrasonic output powers, ultrasonic durations showed the greater influence on morphology and physical properties of cellulose nanoparticles. Atomic force microscopy revealed that noodle-like cellulose particles with 1100 nm in length gradually became the short rod-like nanoparticles with 300 nm in length with increasing of ultrasonic duration from 10 min to 60 min. Moreover, results of contact angles indicated that ultrasound could significantly improve hydrophobicity of cellulose nanoparticles. The interfacial shear rheology showed that although all cellulose nanoparticles exhibited the similar interface adsorption behavior which showed the initial lag-phase of adsorption, followed by the interface saturation, the time of this initial lag-phase was affected by ultrasonic conditions. The increase of ultrasonic duration and ultrasonic power could shorten the time of this initial lag-phase, suggesting the resulting cellulose nanoparticles easier adsorption at the O/W interface. It was probably attributed to its small size and high hydrophobicity induced by intense ultrasonic treatments. Meanwhile, the cellulose nanoparticles with small size and higher hydrophobicity exhibited the better emulsifying ability to stabilize oil-in-water emulsions due to the formation of the viscoelastic interfacial film. This study improved understanding about changes in interfacial and emulsifying properties of cellulose nanoparticles caused by ultrasonic treatments.
1. Introduction
Cellulose nanoparticles have attracted considerable attention in food, pharma, and cosmetics fields due to its exceptional mechanical properties, large specific surface area, high aspect ratio, environmental benefits, and low cost [1], [2]. In contrast to conventional surfactants, Pickering emulsions stabilized by those nanocellulsoe exhibited the outstanding stabilization against coalescence and creaming [3], [4]. The excellent stabilization mechanism is attributed to the accumulation of particles at the oil–water interface to form a densely packed layer which is closely related to the long-term stability of emulsions [5]. So far, cellulose nanoparticles have been successfully isolated from many agricultural wastes, such as pistachio shells [6], corn cob [7], vine shoots [8]. Those agricultural wastes are regarded as attractive sources to extract nanocellulose due to containing plenty of cellulose fibers. Ginkgo seed shells are main by-products during the production of ginkgo seed fleshes. A large amount of seed shells directly burned or buried in the soil every year. Thus it is significant to isolate nanocellulose using ginkgo seed shells.
Among various methods for preparing cellulose nanocrystals (CNCs), the acid hydrolysis method is the most well-known and widely used based on the fact that disordered or amorphous regions of cellulose are preferentially hydrolyzed, while crystalline regions have a better ability to resist acid attack [2]. After acid hydrolysis, the targeted cellulose nanoparticle commonly exhibited specific morphology and properties depending on the raw materials and hydrolysis conditions. However, it should be noted that the morphology and physical properties of cellulose nanoparticles are closely related to properties of Pickering emulsions [9]. For example, the aspect ratios of cellulose particles affect coverage ratio of droplets in stabilized emulsions [10]. The crystalline allomorph of CNCs influences the stabilization of Pickering emulsions [11]. In contrast to acid hydrolysis, ultrasonication, as a widely accepted mechanical method, can effectively change morphology and properties during the preparation of cellulose nanoparticles [1], [12], [13]. Li, Yue [1] reported that large size of MCC with irregular shapes gradually became nano-sized NCC with rod shape by the increase of ultrasonic time ranging from 5 to 15 min under the 1500 W ultrasonic power. Wong, Kasapis [14] revealed that ultrasonication (150 W for 60 min) significantly reduced the molecular weight and increased crystallinity index of bacterial cellulose and plant cellulose. Moreover, thermal properties of cellulose nanoparticles were also affected by ultrasonic treatments [12], [15]. Those changes induced by ultrasonication are primarily attributed to the acoustic cavitation effect [1], [12].
Although changes in morphology and properties of cellulose nanoparticles caused by ultrasonication have been reported in many studies, the effect of ultrasonication on the interfacial adsorption behavior of cellulose nanoparticles at the O/W interface has not been reported until now. Interfacial performance of cellulose nanoparticles at the O/W interface is of profound importance for the stabilization of Pickering emulsions. The adsorbed cellulose particles at the O/W interface result in the formation of an interfacial film. The strong interfacial film contributes to the long-term stability of emulsions [16]. Nevertheless, this adsorption behavior of cellulose nanoparticles is closely related to its crystalline allomorph, morphology, amphipathicity, and surface charge density [17]. In this case, it is of significance to reveal the changes in morphology and properties of cellulose particles caused by ultrasonication and further study effects of those changes on the interfacial property and emulsifying property of cellulose nanoparticles.
Therefore, in order to study effects of different ultrasonic conditions on interfacial property and emulsifying property of cellulose nanoparticles, the present work applied ultrasound within the selected output powders (150–600 W) and duration (10–60 min) to treat cellulose microparticles obtained from acid hydrolysis. The resulting cellulose nanoparticles were characterized by particle size, micromorphology, crystallinity, wettability. The dynamic interfacial tension and interfacial shear rheology were used to study interfacial properties of cellulose nanoparticles. The oil-in-water emulsions were further prepared to study the emulsifying property of cellulose nanoparticles.
2. Materials and methods
2.1. Materials and reagents
Ginkgo seed shells were collected from Jiangsu, China. All reagents, including Sodium hydroxide (NaOH), sulfuric acid (H2SO4), sodium chlorite (NaCLO2), and acetic acid, were AR grade and purchased from Sinopharm Chemical Reagent Co., Ltd. Commercial corn oil was obtained from Auchan supermarket. Cellulose membrane dialysis tube was provided from Shanghai Macklin Biochemical Co., Ltd. The ginkgo shell mainly contains cellulose (44.34%, w/w), hemicellulose (7.0%, w/w), lignin (46.32%, w/w), and protein (1.4%, w/w) [18].
2.2. Production of cellulose particles
The production of cellulose particles from ginkgo seed shells was performed based on our previous method [18]. Briefly, alkali and bleaching treatments were applied to purify cellulose. Those purified cellulose were further hydrolyzed by H2SO4 (62%) at 45 °C for 30 min to remove amorphous regions of cellulose. After acid hydrolysis, the resulting cellulose sediments were dialyzed using dialysis membrane (12–14 kDa molecular weight cut off) for 4 days to attain a constant pH. The final suspensions were stored in a refrigerator at 4 °C.
2.3. Ultrasonic treatments
Cellulose suspensions (0.3%, w/v) were submitted to ultrasonic treatments using a 1200 W ultrasonic processor (TL-1200Y, Jiangsu Tenlin Instrument Co., Ltd, China.) with a 15 mm diameter titanium probe. Cellulose suspension (80 mL) was put into a 150 mL glass vessel which was immersed in an ice-water bath and the probe was dipped into cellulose suspensions with a depth of 15 mm. The suspensions were treated by at 20 kHz at different levels of output power (150, 300, 450, 600 W) for different time (10, 30, 60 min). The pulse duration of on-time 1 s and off-time 1 s was applied. During the process of ultrasonic treatments, ice was added into the ice-water bath to maintain the temperature of the suspension below 20 °C. The Energy density (J/mL) was calculated based on the previous method [19] by the following equation:
| (1) |
where power drawn was measured by a power meter (power bay equipment, Shenzhen Northmeter Co., Ltd., China) and time represents effective processing time with omitting the off-time and volume is 80 mL.
2.4. Characterization of the cellulose nanoparticles
2.4.1. Particle size and zeta potential
Particle size and zeta potential of 0.03% (w/v) cellulose nanoparticle suspensions were measured by the DLS instrument (Zetasizer Nano ZS, Malvern Instruments, Worcestershire, UK) in duplicates at the room temperature.
2.4.2. Micromorphology
Atomic force microscopy (AFM) was used to measure the micromorphology of cellulose nanoparticles based on the previous method [18]. After dilution of cellulose suspensions to 0.003% (w/v), diluted suspensions (10 μL) were dropped on a fresh mica surface and dried at room temperature. NanoScope Analysis software was applied to analyze the diameter and height of the cellulose nanoparticles
2.4.3. X-ray diffraction
D2 PHASER (AXS, Germany) was applied to measure X-ray diffraction of cellulose nanoparticles at 40 kV, 40 mA, and Cu-Ká radiation (wave length 0.154 nm). The powder samples were scanned from 2θ = 3° to 50° with a scan rate of 1° min−1. The crystallinity index (CrI) of cellulose was calculated by the following equation [20]:
| (2) |
where I002 represents the intensity of the peak corresponding to cellulose I and Iam is the intensity of the cellulose amorphous region.
2.4.4. Contact angle of cellulose nanoparticles
The sessile drop method was applied to determine the static water contact angles of cellulose nanoparticles. The self-standing film of cellulose nanoparticles was formed based on the previous study [11]. Deionized water (2 μL) was carefully deposited on the surface of films using the optical contact angle meter (OCA15, Dataphysics instruments, Germany) equipped with a high-precision injector. Before taking a picture of the droplet, let it stand for 5 min at the surface of films.
2.4.5. Interfacial tension
The dynamic interfacial tension between nanoparticles suspensions (0.3%, w/v) and corn oil was measured by the pendant drop method. The experiment details were seen from our previous study [18]. The optical contact angle meter recorded the change of the oil/water interface and the Young-Laplace equation was used to calculate the interfacial tension. Distilled water was used as a control.
2.4.6. Interfacial shear rheology
The DHR-3 rheometer (Waters, USA) equipped with Du Noüy ring (20 mm diameter) was used to measure the interfacial shear rheology between nanoparticles suspensions (0.3%, w/v) and corn oil based on the previous method with some modifications [21]. In brief, 20 mL of suspensions were added to a PTFE circular trough. Then, the ring was positioned just below the air/water surface. After that, 20 mL of the oil phase was carefully poured on top of the aqueous phase to ensure the ring was positioned at the water/oil interface. Time sweep tests were conducted for 30 min at a constant temperature of 25 °C.
2.5. Preparation of emulsions
The oil-in-water emulsions were prepared by corn oil and cellulose nanoparticles in 20 mM NaCl at the required concentrations to match an oil/water ratio of 50/50. The final cellulose concentration was 0.15% (w/v) referring to whole emulsions. In order to avoid other high energy treatments affected physical properties and morphology of cellulose nanoparticles, emulsions were prepared merely using an IKAUltra-Turrax T25 homogenizer at 12,000 rpm for 3 min.
2.6. Emulsion properties
2.6.1. Particle size and distribution
The laser diffraction particle analyzer (S3500, Microtrac Instruments Ltd., USA) was applied to determine the particle size and size distribution of emulsions. The area-weighted mean diameter (D[3,2]) were evaluated according to Eq. (1), where ni is the number of particles of diameter di.
| (3) |
2.6.2. Microstructure of emulsions
The optical microscopy (Nikon Instrument Inc., Japan) was applied to visualize emulsion droplets based on the previous study [18]. Confocal laser scanning microscopy (Leica TCS SP8, Mannheim, Germany) was applied to observe the adsorbed cellulose nanoparticles at the O/W interface of droplets. The oil phase in emulsions was stained by Nile Red dye (0.01%, w/v) and cellulose particles were stained with Calcofluorwhite (1.0%, w/v). The details of measurements could be seen from the previous method [22].
2.6.3. Surface coverage ratio
The surface coverage (C) depends on morphology and size of particles, the amount of particles, and the volume of oil [10]. Based on the following equation, the coverage ratio was determined:
| (4) |
where mp represents the mass of cellulose nanoparticles, D represents the diameter of the droplet (D3,2), h represents the height of particles measured by AFM, ρ represents the cellulose nanoparticles density (1.6 g/cm3), Voil represents the volume of oil trapped in the emulsion after centrifugation.
2.6.4. Appearance of stored emulsions
The fresh emulsions were stored at the ambient environment for 10 days. After that, a digital camera was used to picture the appearance of stored emulsions.
2.7. Data analysis
The results were expressed as the mean ± standard deviation. The significance analysis (p < 0.05) was conducted by SPSS 20.0 software (IBM, Chicago, U.S.A.). The experiments were performed three times.
3. Results and discussion
3.1. Particle size and zeta potential of cellulose nanoparticles
Fig. 1 shows the appearance of cellulose suspensions and morphology of cellulose nanoparticles after different ultrasonic treatments. Clearly, transparency of suspensions gradually increased with the increase of ultrasonic duration from 10 to 60 min. This phenomenon was attributed to the decrease in particle size induced by ultrasonic treatments, as evidenced by AFM photos (Fig. 1(ii)) and Table 2. The similar phenomenon was also reported by some previous studies [12], [15], [23]. Nevertheless, it is worth noting that, with respect to suspensions with the same ultrasonic duration, transparency of suspensions did not occur obvious changes although the ultrasonic output power increased from 150 W to 600 W. For example, as shown in Fig. 1(a), all suspensions were opaque after different power treatments for 10 min. However, when the ultrasonic duration prolonged to 60 min, the suspension treated by only 150 W exhibited the complete transparency. It indicated that the ultrasonic duration exhibited the greater influence on changing particle size in comparison to the ultrasonic output power. Moreover, the length reduction of cellulose particles also evidenced this result. As shown in Table 2, the length of cellulose after 10 min of ultrasonic treatment merely decreased from 1125 to 955 nm under different ultrasonic powers ranging from 150 to 600 W. However, the length of cellulose treated by 150 W decreased sharply from 1125 to 321 nm with the increase of ultrasonic duration from 10 to 60 min. The corresponding morphology of cellulose nanoparticles was shown in Fig. 1(ii). The increase of ultrasonic power did not cause obvious changes in particle morphology, while the increase of ultrasonic duration resulted in that the long noodle-like cellulose particles gradually became the short rod-like nanoparticles. This difference in the effect of power and time on particle size could be attributed be their energy density. As shown in Table 1, energy density derived from 10 min of ultrasonic duration increased 330 to 605 J/mL with the increase of powers from 150 to 600 W. However, with increasing duration from 10 to 60 min, energy density derived from 150 W increased sharply from 330 to 1980 J/mL. It indicated that the increased energy density by extending the ultrasonic duration was greatly higher than that by increasing the ultrasonic power. As reported in previous studies on preparation of nanocellulose, ultrasonic treatments can change particle size due to sound energy provided by the acoustic cavitation: the formation, growth, and collapse of bubbles in aqueous solution [1]. The violent collapse of the bubbles produces microjets and shock waves on the surfaces of suspended cellulose particles, resulting in the scission effect on micron-sized cellulose particles [12], [24]. This scission effect can break the relatively weak interfaces among the fibers, which are bonded to each other mainly by van der Waals forces and hydrogen bonds [13]. The higher energy density is more effective to break the relatively weak interfaces and disintegrate the micron sized cellulose particles into nanoparticles [25]. Therefore, ultrasonic time causing large changes in energy density had a greater influence on particle size compared to ultrasonic powers.
Fig. 1.
Cellulose suspensions (i) treated by different ultrasonic duration, (a) 10 min; (b) 30 min; (c) 60 min. The morphology (ii) of partial cellulose nanoparticles characterized by AFM method.
Table 2.
The length, diameter, zeta potential, and crystallinity of cellulose nanoparticles after different ultrasonic treatments.
| Samples | Diameter (nm) | Length (nm) | Zeta potential (mV) | Crystallinity (%) | |
|---|---|---|---|---|---|
| control | 14212 ± 1080a | \ | –22.3 ± 1.1b | 72.06 | |
| 150 W | 10 min | 65 ± 3.2b | 1125 ± 10.4a | −42.1 ± 1.6a | 70.78 |
| 30 min | 42 ± 2.5c | 843 ± 25.7d | −43.6 ± 2.1a | 66.67 | |
| 60 min | 30 ± 1.5e | 321 ± 17.8 g | −42.2 ± 2.9a | 63.23 | |
| 300 W | 10 min | 63 ± 2.0b | 1040 ± 16.4b | −42.6 ± 1.5a | 69.41 |
| 30 min | 38 ± 1.6d | 807 ± 13.4e | −42.3 ± 2.0a | 67.14 | |
| 60 min | 31 ± 2.1e | 312 ± 22.1 g | −43.7 ± 2.3a | 62.33 | |
| 450 W | 10 min | 60 ± 3.0b | 987 ± 21.6c | −42.6 ± 1.4a | 68.55 |
| 30 min | 38 ± 1.1d | 787 ± 16.9e | −43.1 ± 2.0a | 65.67 | |
| 60 min | 29 ± 0.8e | 308 ± 18.1 g | −44.3 ± 2.1a | 59.30 | |
| 600 W | 10 min | 62 ± 2.6b | 955 ± 20.6c | −42.8 ± 2.4a | 66.14 |
| 30 min | 38 ± 1.5d | 746 ± 16.2f | −43.0 ± 1.2a | 63.48 | |
| 60 min | 24 ± 1.7f | 310 ± 15.9 g | −42.5 ± 2.9a | 58.33 | |
Different letters in the same column indicate significant difference (P < 0.05).
Table 1.
The energy densities (J/mL) of the different ultrasonic treatments.
| Ultrasonic treatments | Treatment 1 | Treatment 2 | Treatment 3 | Treatment 4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Power (W) | 150 | 450 | 450 | 600 | ||||||||
| Effective time (min) | 5 | 15 | 30 | 5 | 15 | 30 | 5 | 15 | 30 | 5 | 15 | 30 |
| Energy density (J/mL) | 330 | 990 | 1980 | 420 | 1260 | 2520 | 510 | 1530 | 3060 | 605 | 1815 | 3630 |
Effective time is effective processing time with omitting the off-time.
The zeta potential of the control sample reached –22 mV, indicating that the surface of particles was grafted with a certain charged sulphate groups after acid hydrolysis [2]. After ultrasonic treatments, the zeta potential value of the resulting nanoparticle suspensions decreased sharply to approximately −42 mV. This may be attributed to that ultrasound provided more drastic agitation and greater contact between cellulose nanoparticles and oxygen, causing the generation of more negative charge due to partial oxidation of the particles [26]. However, the increase of ultrasonic intensity did not cause the significant change in zeta potential values (Table 2). The similar results were also reported by Costa, Gomes [26], who reported ultrasonic treatments caused an increase of zeta potential values from −24.3 to −55.5 mV, but the values kept unchanged with the increase of treatments intensity.
3.2. X-ray diffraction
The X-ray diffraction of ginkgo cellulose particles obtained from different ultrasonic powers was shown in Fig. 2. All the diffraction diagrams were composed of an amorphous broad hump and crystalline peaks. The obvious peaks at 2θ = 16.5° and 22.0° for ginkgo cellulose were regarded as cellulose I [18]. It is apparent that ultrasonic treatments did not change the cellulose type. Nevertheless, there was a decrease in the intensity of the peak at 22.0° with the increase of ultrasonic powers. This peak intensity is closely related to crystallinity index (CrI) of cellulose [12]. Table 2 exhibited changes in CrI values of cellulose particles after different ultrasonic treatments in detail. CrI values of all samples occurred the reduction after ultrasonic treatments. Moreover, higher energy density caused a larger reduction in CrI value. The drastic decrease of CrI value was observed from 72% (no ultrasound) to 58% (600 W for 60 min). This decreased crystallinity of cellulose could be attributed to that intense cavitation forces derived from ultrasonic treatments caused scission of the long cellulose particles into short cellulose nanoparticles and partial collapse of the crystal structure [26]. The decreased CrI values induced by ultrasonic treatments were also previously reported in bacterial cellulose [12] and NCC [1].
Fig. 2.
X-ray diffraction patterns of cellulose particles obtained after 60 min of ultrasonic duration under different powers.
3.3. The static water contact angles
The static water contact angles of cellulose nanoparticle films were determined to reveal the change in amphipathicity after ultrasonic treatments. As shown in Fig. 3, it is obvious that ultrasonic treatments significantly changed contact angle values of cellulose nanoparticles. The contact angle value of cellulose particles with 150 W for 10 min treatment was only 28.2°, suggesting particles showed stronger hydrophilicity. With the increase of ultrasonic power or duration, contact angle values of cellulose particles gradually increased. It indicated that ultrasonic treatments could improve the hydrophobicity of cellulose nanoparticles. The similar result was also reported by Costa, Gomes [26], who found cellulose nanofibers showed the higher hydrophobicity with the increase of ultrasonic power ranging from 225 to 675 W. In previous studies of CNCs, the amphiphilic character of CNCs is closely related to the hydrophobic (2 0 0)β crystalline edge [17], [27]. They revealed that the (2 0 0)β crystalline edge is responsible for the wettability of the CNCs at the O/W interface and directly interacts with the interface, resulting in CNCs adsorption at the O/W interface. However, (2 0 0) crystalline plane is of minor importance in terms of surface exposure since it is located at the corners of the cellulosic crystals [27]. Ultrasonic treatments disintegrated long cellulose nanoparticles into short rod-like nanoparticles while destroyed partial crystal structure. This process may expose more (2 0 0)β crystalline planes, causing higher hydrophobicity of cellulose nanoparticles.
Fig. 3.
The static water contact angles of different cellulose nanoparticles films.
3.4. Interfacial properties
3.4.1. Interfacial tension
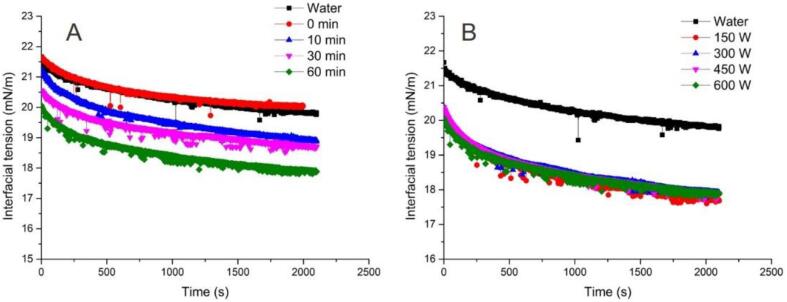
The physical properties of small particles can influence the interfacial activity [28]. The present work showed that ultrasonic treatments could effectively change the morphology, particle size and amphipathy of cellulose nanoparticles. Therefore, the dynamic interfacial tension of obtained cellulose nanoparticles was measured at the oil–water interface in a 0.3% w/v dispersion. Fig. 4A showed the interfacial tension of cellulose particles which were obtained after different ultrasonic duration under the same power (600 W). It could be found that the interfacial tension between deionized water and commercial corn oil slightly decreased from 21.6 mN/m to 20.0 mN/m after equilibrium. The decreased interfacial tension was attributed to contaminants in commercial oil [29]. As expected, the cellulose microparticles without the ultrasonic treatment (0 min) nearly showed no interfacial activity, as evidenced by the overlapping trends of interfacial tensions curves with that of water. This is because the large solid particles have a higher adsorption energy barrier [30]. However, after ultrasonic treatments, the resulting cellulose nanoparticles significantly lowered the interfacial tension. Moreover, with increasing of ultrasonic duration, cellulose nanoparticles caused the greater reduction of interfacial tension. It could be explained by that those cellulose nanoparticles could be easier to adsorb at the oil/water interface due to their small size and higher hydrophobicity induced by long ultrasonic duration. Fig. 4B exhibited the interfacial tension of cellulose particles which were obtained after different ultrasonic powers under the same duration (60 min). It is obvious that there was no obvious difference in interfacial tension between cellulose nanoparticles with different ultrasonic powers. It might be attributed to the similar particle size and amphipathy of those cellulose particles. It could be confirmed by results of the particle size (Table 2) and contact angles (Fig. 3).
Fig. 4.
Interfacial tension of cellulose nanoparticles obtained after 600 W for different time (A) and cellulose nanoparticles with 60 min of ultrasonic duration under different powers (B).
3.4.2. Interfacial shear rheology
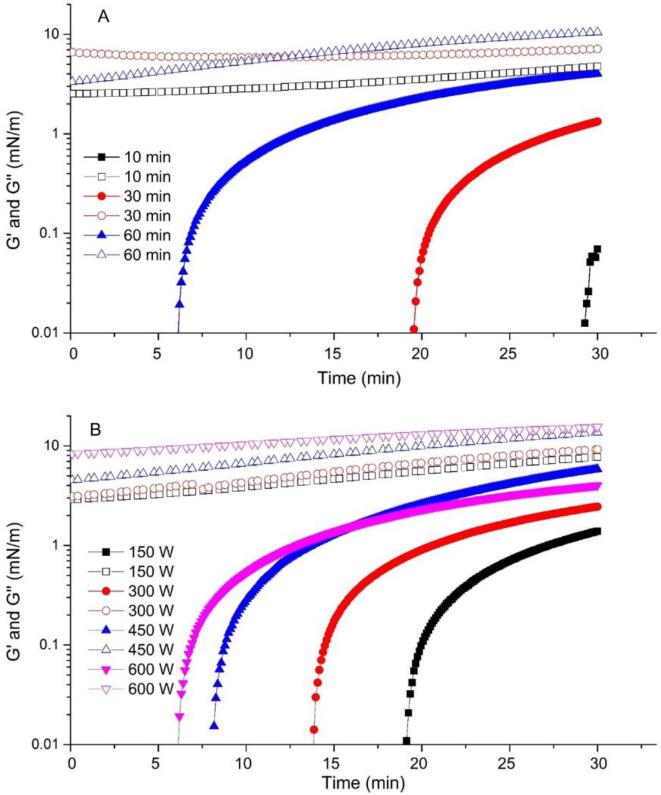
To gain the knowledge of the interfacial film formed by cellulose nanoparticles adsorbed at the oil/water interface, the interfacial shear rheology was determined. The time sweep experiments were shown in Fig. 5, which could provide the visual insight into the process of cellulose particles adsorption at the interface [21]. Clearly, the initial lag-phase (without the observation of the storage modulus G’) was observed during cellulose nanoparticles adsorption process. This was followed by the sharp increase of G’, indicating cellulose nanoparticles adsorption. The lag-phase is attributed to nanoparticle diffusion to the interface as described by the model of Ward and Tordai or a kinetic adsorption barrier [28], [31], [32]. However, in contrast to the time scale of hours for CNCs adsorption at the A/W interface [31], the time scale of present lag-phase was only minutes. This indicated that the lower kinetic adsorption barrier for cellulose nanoparticles adsorbed at the O/W interface compared to the A/W interface, probably due to enhanced wetting of cellulose nanoparticles by oil compared to air [33].
Fig. 5.
Interfacial rheology of cellulose nanoparticles obtained after 600 W for different time (A) and cellulose nanoparticles with 60 min of ultrasonic duration under different powers (B). Closed symbols and open symbols is storage modulus G’ and loss modulus G”, respectively.
Interestingly, the lag-phase time depended on ultrasonic treatment conditions. As shown in Fig. 5A, the emergence of G’ of cellulose particles obtained from 10 min of ultrasonic treatment was nearly at 30 min. As for nanoparticles obtained from 60 min of ultrasonic duration, the lag-phase time was decreased to 7 min. This decreased lag-phase time indicated earlier adsorption for cellulose nanoparticles at the O/W interface, due to its small particle size and higher hydrophobicity. Moreover, those nanoparticles lowered interfacial tension, resulting in the decrease of the particle adsorption energy [33]. However, the G” was always larger than G’ and no crossover of G’ and G” within the measured time sweep. It indicated that adsorbed cellulose nanoparticles did not completely form a viscoelastic interfacial film [31]. One reason may be due to that the sweep time was too short to observe the crossover of G’ and G”. Another reason could be closely related to electrostatic repulsion derived from charged cellulose particles [31], [33]. Electrostatic repulsion can limit particle adsorption at the O/W interface due to the repulsive force within particles in the bulk and adsorbed particles, causing an insufficient surface coverage [34]. Bertsch, Diener [31] found that no interfacial shear rheology signal was measured below 0.3% CNCs due to the insufficient surface coverage. The adsorption-limiting effect of electrostatic repulsion on CNCs adsorption was previously shown [31], [33], [35].
Additionally, as shown in Fig. 5B, the ultrasonic output power also influenced the adsorption behavior of cellulose nanoparticles. The lag-phase time gradually shortened with the increase of ultrasonic power. It indicated that cellulose nanoparticles obtained from higher output power treatment adsorbed easily at the O/W interface. Nevertheless, it is obvious to find that the ultrasonic duration had a greater impact than the power on interfacial shear rheology of cellulose nanoparticles by comparing Fig. 5A with 5B. It was attributed to that larger changes in physical properties were induced by the ultrasonic duration. It is consistent with results of particle size, morphology and amphipathy.
3.5. Emulsions stabilized by cellulose nanoparticles
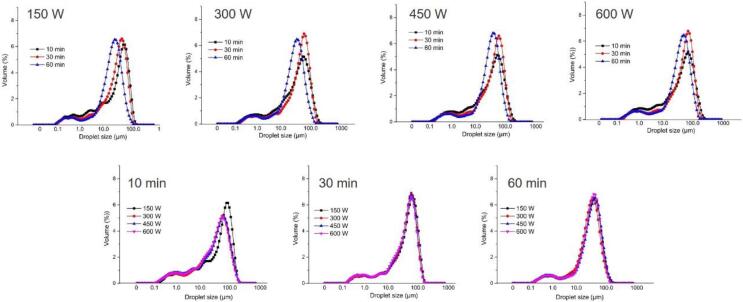
The emulsifying property and interfacial behavior of cellulose nanoparticles were further investigated by preparing oil in water emulsions. Fig. 6 shows droplet size distribution of emulsions stabilized by cellulose nanoparticles obtained from different ultrasonic treatments. All emulsions exhibited bimodal distributions, with similar first peaks located at around 1 μm and second high peaks. As for emulsions stabilized by cellulose nanoparticles which were obtained from different ultrasonic durations under the same ultrasonic power, the high peak gradually moved from large size to small size with increasing of durations. Nevertheless, under the same ultrasonic duration, emulsions stabilized by cellulose nanoparticles obtained from different powers showed the similar droplet size distribution. The results indicated that ultrasonic durations had a greater impact on droplet size distribution of emulsions compared to ultrasonic powers. This could be attributed to larger changes in physical properties of cellulose nanoparticles caused by ultrasonic durations in comparison to ultrasonic powers. The influence of physical properties, such as size, aspect ratio, on emulsions droplet changes were previously shown for cellulosic nanorods [10], bacterial cellulose nanofibrils [36], and cellulose nanoparticles [18]. Moreover, the corresponding droplet size of emulsions was tabulated in Table 3. As expected, emulsions stabilized by cellulose nanoparticles which were obtained after lower ultrasonic powers and short durations had the larger droplet size. This is because larger particles caused larger droplet size for particles to be properly located around the droplets [4]. With the increase of ultrasonic durations or powers, droplet size of emulsions stabilized by those resulting nanoparticles gradually decreased. The optical microscope (Fig. 8) showed changes in droplet size of emulsions stabilized by different cellulose nanoparticles, which large droplets of emulsions gradually decreased to small and uniform droplets with increasing of ultrasonic conditions.
Fig. 6.
Particle size distribution of emulsions stabilized by cellulose nanoparticles obtained from different ultrasonic treatments.
Table 3.
Particle size and coverage ratio of emulsions stabilized by different cellulose nanoparticles.
| Ultrasonic treatments | Particle size (μm) |
Coverage ratio (%) | ||
|---|---|---|---|---|
| Fresh emulsions | Stored emulsions | |||
| 150 W | 10 min | 40.82 ± 2.12aB | 58.21 ± 3.21aA | 38.98 |
| 30 min | 34.18 ± 1.43cB | 43.54 ± 1.32dA | 55.32 | |
| 60 min | 25.16 ± 1.55eB | 27.32 ± 0.53gA | 76.41 | |
| 300 W | 10 min | 37.55 ± 2.01bB | 55.29 ± 2.87bA | 40.21 |
| 30 min | 32.78 ± 2.37cB | 38.23 ± 1.99eA | 57.88 | |
| 60 min | 22.94 ± 1.11fB | 25.21 ± 2.43gA | 77.91 | |
| 450 W | 10 min | 36.44 ± 1.57bB | 51.27 ± 3.82cA | 39.92 |
| 30 min | 30.69 ± 2.42 dB | 34.32 ± 1.27fA | 61.29 | |
| 60 min | 20.31 ± 1.04gA | 23.24 ± 2.21hA | 80.21 | |
| 600 W | 10 min | 37.42 ± 1.33bB | 50.33 ± 2.59cA | 40.32 |
| 30 min | 27.32 ± 1.22 dB | 33.42 ± 2.34fA | 62.71 | |
| 60 min | 19.12 ± 0.91gA | 21.73 ± 1.20hA | 78.15 | |
Different lowercase letters in the same column indicate significant difference in droplet size between different emulsions (P < 0.05). Different capital letters in the same line represent significant differences in droplet size of emulsions after storage (P < 0.05).
Fig. 8.
Optical and CLSM images of emulsions stabilized by cellulose nanoparticles obtained by different ultrasonic treatments. The black colour represents the oil phase (stained by Nile Red); the green colour represents the cellulose nanoparticles (stained by Calcofluor-white). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
On the other hand, after 10 days storage, all emulsions occurred the separation of cream layer and water phase (Fig. 7). However, with the increase of ultrasonic durations, emulsions stabilized by the resulting cellulose nanoparticles exhibited obviously less separation of water phase. It indicated that the storage stability of emulsions was improved. The storage stability of Pickering emulsions is closely related to the interfacial film formed by adsorbed particles at the O/W interface [16]. For charged cellulose particles (regardless of crystalline origin), the electrostatic repulsion plays a very important role in influencing the formation of interfacial layer [17], [33]. Kalashnikova, Bizot [17] found that cellulose nanocrystals with a surface charge density above 0.03 e/nm2 were not able to efficiently stabilize at the oil/water interface. In order to avoid the adsorption-limit effect derived from the electrostatic repulsion, this work added 20 mM NaCl into cellulose nanoparticles suspensions to induce charge screening [33]. For emulsions stabilized by cellulose nanoparticles obtained from 10 min of ultrasonic treatments, the worse stability was observed. One reason could be due to these nanoparticles showing long size and low hydrophobicity. Another reason was attributed to the low surface coverage (38.98%) caused by long cellulose nanoparticles, as shown in Table3. It is in agreement with the previous study [10], in which long cellulose nanocrystals cause low surface coverage while short nanocrystals result in high surface coverage. The low surface coverage may be insufficient to form the viscoelastic interfacial film to protect emulsions against coalescence. It explained the obvious increase in droplet size of emulsions stabilized by long cellulose nanoparticles obtained from 10 min of treatments (Table 3) after storage. With the increase of ultrasonic duration, the stability of emulsions was significantly improved (Fig. 7). The enhanced stability could be probably attributed to higher surface coverage caused by cellulose nanoparticles with smaller size and high hydrophobicity. As the above described, the prolonged ultrasonic duration prepared short cellulose nanoparticles with high hydrophobicity. The nanoparticles showed relatively higher emulsifying capacity due to easier adsorption at the O/W interface. This interfacial behavior of short nanoparticles benefited the formation of formed the viscoelastic interfacial film at the interface of droplets. The formed viscoelastic interfacial film can effectively prevent droplets against coalescence [5]. Thus emulsions stabilized by cellulose nanoparticles obtained from 60 min ultrasonic treatment had an only slight increase in droplet size and exhibited the relatively good storage stability. Nevertheless, these emulsions still occurred the separation of water phase, which was attributed to the density contrast of cream layer and water. In previous studies of long-term stability of emulsions stabilized by cellulose, the benign storage stability of those emulsions is not only related to the adsorption behavior of cellulose (the interfacial film) at the interface but also related to the network formation [37], [38], [39].
Fig. 7.
Appearance of emulsions stabilized by cellulose nanoparticles obtained by different ultrasonic treatments after 10 days storage.
CLSM images (Fig. 8) further provided the evidence of the interfacial film. Black spheres represented the oil droplets and green particles were stained cellulose nanoparticles by calcofluor-white. It was obvious that emulsions stabilized by cellulose nanoparticles obtained from 600 W for 10 min treatment exhibited large droplets and the surface of droplets was merely covered by little green particles that resulted in the low surface coverage. Nevertheless, the clear and complete “green rings” were observed in emulsions stabilized by cellulose nanoparticles obtained from 600 W for 60 min ultrasonic treatment. This ‘green rings’ evidenced cellulose nanoparticles acting as Pickering stabilizers, suggesting the formation of an viscoelastic interfacial film [22]. Thus those emulsions showed better storage stability.
4. Conclusion
The present work applied ultrasonic treatments within the selected output powders (150–600 W) and duration (10–60 min) to produce cellulose nanoparticles from ginkgo seed shells. The resulting nanoparticles were characterized using Atomic force microscopy (AFM) and X-ray diffraction and further investigated effects of ultrasonic conditions on the interfacial property and emulsifying property. Compared to the increase of ultrasonic output powers, the increase of ultrasonic duration was more effective to produce nano-sized cellulose particles. Ultrasound resulted in the decrease of the crystallinity index. Moreover, ultrasound also significantly improved hydrophobicity of cellulose nanoparticles. This increased hydrophobicity promoted cellulose nanoparticles adsorb at the O/W interface more easily, resulting in shortening the time of initial lag-phase during the interface adsorption process. In addition, the resulting cellulose nanoparticles were further applied to prepare oil-in-water emulsions. The result showed that ultrasound could improve the emulsifying ability by decreasing particle size and increasing hydrophobicity of cellulose nanoparticles. The emulsions stabilized by cellulose nanoparticles with small size and high hydrophobicity exhibited the better storage stability due to the formation of the viscoelastic interfacial film.
CRediT authorship contribution statement
Yang Ni: Methodology, Data curation, Writing - original draft. Jinwei Li: Conceptualization, Validation, Writing - review & editing. Liuping Fan: Conceptualization, Supervision, Validation, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors acknowledge financial support of the National Key research & Development Plan (2018YFC1603705-03),Jiangsu Key R & D plan, China (BE2019309), China National Natural Science Foundation (31871840), Postgraduate Research & Practice Innovation Program of Jiangsu Province, China (KYCX20_1877), which has enabled us to carry out this study.
References
- 1.Li W., Yue J., Liu S. Preparation of nanocrystalline cellulose via ultrasound and its reinforcement capability for poly(vinyl alcohol) composites. Ultrason. Sonochem. 2012;19(3):479–485. doi: 10.1016/j.ultsonch.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 2.Youssef H., Lucia L.A., Rojas O.J. Cellulose nanocrystals: chemistry, self-assembly, and applications. Chem. Rev. 2010;110(6):3479–3500. doi: 10.1021/cr900339w. [DOI] [PubMed] [Google Scholar]
- 3.Xiao J., Li Y., Huang Q. Recent advances on food-grade particles stabilized Pickering emulsions: fabrication, characterization and research trends. Trends Food Sci. Technol. 2016;55:48–60. [Google Scholar]
- 4.Tavernier I. Food-grade particles for emulsion stabilization. Trends Food Sci. Technol. 2016;50:159–174. [Google Scholar]
- 5.Dickinson E. Use of nanoparticles and microparticles in the formation and stabilization of food emulsions. Trends Food Sci. Technol. 2012;24(1):4–12. [Google Scholar]
- 6.Kasiri N., Fathi M. Production of cellulose nanocrystals from pistachio shells and their application for stabilizing Pickering emulsions. Int. J. Biol. Macromol. 2018;106:1023–1031. doi: 10.1016/j.ijbiomac.2017.08.112. [DOI] [PubMed] [Google Scholar]
- 7.Silvério H.A. Extraction and characterization of cellulose nanocrystals from corncob for application as reinforcing agent in nanocomposites. Ind. Crops Prod. 2013;44:427–436. [Google Scholar]
- 8.El Achaby M. Production of cellulose nanocrystals from vine shoots and their use for the development of nanocomposite materials. Int. J. Biol. Macromol. 2018;117:592–600. doi: 10.1016/j.ijbiomac.2018.05.201. [DOI] [PubMed] [Google Scholar]
- 9.Kalashnikova I. New Pickering emulsions stabilized by bacterial cellulose nanocrystals. Langmuir. 2011;27(12):7471–7479. doi: 10.1021/la200971f. [DOI] [PubMed] [Google Scholar]
- 10.Kalashnikova I. Cellulosic nanorods of various aspect ratios for oil in water Pickering emulsions. Soft Matter. 2013;9(3):952–959. [Google Scholar]
- 11.Li X. Cellulose nanocrystals (CNCs) with different crystalline allomorph for oil in water Pickering emulsions. Carbohydr. Polym. 2018;183:303–310. doi: 10.1016/j.carbpol.2017.12.085. [DOI] [PubMed] [Google Scholar]
- 12.Abral H. Preparation of nano-sized particles from bacterial cellulose using ultrasonication and their characterization. Carbohydr. Polym. 2018;191:161–167. doi: 10.1016/j.carbpol.2018.03.026. [DOI] [PubMed] [Google Scholar]
- 13.Chen W. Individualization of cellulose nanofibers from wood using high-intensity ultrasonication combined with chemical pretreatments. Carbohydr. Polym. 2011;83(4):1804–1811. [Google Scholar]
- 14.Wong S.-S., Kasapis S., Huang D. Molecular weight and crystallinity alteration of cellulose via prolonged ultrasound fragmentation. Food Hydrocolloids. 2012;26(2):365–369. [Google Scholar]
- 15.Wong S.-S., Kasapis S., Tan Y.M. Bacterial and plant cellulose modification using ultrasound irradiation. Carbohydr. Polym. 2009;77(2):280–287. [Google Scholar]
- 16.Dickinson E. Biopolymer-based particles as stabilizing agents for emulsions and foams. Food Hydrocoll. 2017;68:219–231. [Google Scholar]
- 17.Kalashnikova I. Modulation of cellulose nanocrystals amphiphilic properties to stabilize oil/water interface. Biomacromolecules. 2012;13(1):267–275. doi: 10.1021/bm201599j. [DOI] [PubMed] [Google Scholar]
- 18.Ni Y., Li J., Fan L. Production of nanocellulose with different length from ginkgo seed shells and applications for oil in water Pickering emulsions. Int. J. Biol. Macromol. 2020 doi: 10.1016/j.ijbiomac.2020.01.263. [DOI] [PubMed] [Google Scholar]
- 19.Taha A. Effect of different oils and ultrasound emulsification conditions on the physicochemical properties of emulsions stabilized by soy protein isolate. Ultrason. Sonochem. 2018;49:283–293. doi: 10.1016/j.ultsonch.2018.08.020. [DOI] [PubMed] [Google Scholar]
- 20.Winuprasith T., Suphantharika M. Microfibrillated cellulose from mangosteen (Garcinia mangostana L.) rind: Preparation, characterization, and evaluation as an emulsion stabilizer. Food Hydrocoll. 2013;32(2):383–394. [Google Scholar]
- 21.Li W. Improvement of emulsifying properties of soy protein through selective hydrolysis: Interfacial shear rheology of adsorption layer. Food Hydrocoll. 2016;60:453–460. [Google Scholar]
- 22.Du Le H. Pickering emulsions stabilised by hydrophobically modified cellulose nanocrystals: Responsiveness to pH and ionic strength. Food Hydrocoll. 2020;99 doi: 10.1016/j.foodchem.2020.126650. [DOI] [PubMed] [Google Scholar]
- 23.Jiang L. Fabrication and characterisation of cellulose nanocrystals from microcrystalline cellulose by esterification and ultrasound treatment. Micro Nano Lett. 2018;13(11):1574–1579. [Google Scholar]
- 24.Tischer P.C.S.F., Sierakowski M.R., Westfahl H. Nanostructural reorganization of bacterial cellulose by ultrasonic treatment. Biomacromolecules. 2010;11(5):1217–1224. doi: 10.1021/bm901383a. [DOI] [PubMed] [Google Scholar]
- 25.Shanmugam A., Ashokkumar M. Ultrasonic preparation of stable flax seed oil emulsions in dairy systems – Physicochemical characterization. Food Hydrocoll. 2014;39:151–162. [Google Scholar]
- 26.Costa A.L.R. Cellulose nanofibers from banana peels as a Pickering emulsifier: High-energy emulsification processes. Carbohydr. Polym. 2018;194:122–131. doi: 10.1016/j.carbpol.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 27.Cherhal F., Cousin F., Capron I. Structural description of the interface of pickering emulsions stabilized by cellulose nanocrystals. Biomacromolecules. 2016;17(2):496–502. doi: 10.1021/acs.biomac.5b01413. [DOI] [PubMed] [Google Scholar]
- 28.Deshmukh O.S. Hard and soft colloids at fluid interfaces: Adsorption, interactions, assembly & rheology. Adv. Colloid Interface Sci. 2015;222:215–227. doi: 10.1016/j.cis.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 29.Seta L. Rheology and adsorption behaviour of β-casein and β-lactoglobulin mixed layers at the sunflower oil/water interface. Colloids Surf., A. 2014;441:669–677. [Google Scholar]
- 30.Tamayo Tenorio A. Interfacial properties of green leaf cellulosic particles. Food Hydrocoll. 2017;71:8–16. [Google Scholar]
- 31.Bertsch P. Adsorption and interfacial layer structure of unmodified nanocrystalline cellulose at air/water interfaces. Langmuir. 2018;34(50):15195–15202. doi: 10.1021/acs.langmuir.8b03056. [DOI] [PubMed] [Google Scholar]
- 32.Hu J.W., Han G.B., Ren B. Theoretical consideration on preparing silver particle films by adsorbing nanoparticles from bulk colloids to an air-water interface. Langmuir. 2004;20(20):8831–8838. doi: 10.1021/la049842s. [DOI] [PubMed] [Google Scholar]
- 33.Bergfreund J. Adsorption of charged anisotropic nanoparticles at oil–water interfaces. Nanoscale Adv. 2019;1(11):4308–4312. doi: 10.1039/c9na00506d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bertsch P. Designing cellulose nanofibrils for stabilization of fluid interfaces. Biomacromolecules. 2019;20(12):4574–4580. doi: 10.1021/acs.biomac.9b01384. [DOI] [PubMed] [Google Scholar]
- 35.Bertsch P., Fischer P. Interfacial rheology of charged anisotropic cellulose nanocrystals at the air-water interface. Langmuir. 2019;35(24):7937–7943. doi: 10.1021/acs.langmuir.9b00699. [DOI] [PubMed] [Google Scholar]
- 36.Li Q. Flexible cellulose nanofibrils as novel pickering stabilizers: The emulsifying property and packing behavior. Food Hydrocoll. 2019;88:180–189. [Google Scholar]
- 37.Jia X. Stabilizing oil-in-water emulsion with amorphous cellulose. Food Hydrocoll. 2015;43:275–282. [Google Scholar]
- 38.Winuprasith T., Suphantharika M. Properties and stability of oil-in-water emulsions stabilized by microfibrillated cellulose from mangosteen rind. Food Hydrocoll. 2015;43:690–699. [Google Scholar]
- 39.Zhao Y. Effect of regenerated cellulose fiber on the properties and microstructure of emulsion model system from meat batters. Food Hydrocoll. 2019;87:83–89. [Google Scholar]