Graphical abstract
Keywords: Convective drying, Rehydration, Ethanol, Ultrasound
Highlights
-
•
Microscopic and macroscopic structural changes were detailed.
-
•
Ethanol and ultrasound reduced drying time in ~50%
-
•
Energy consumption was reduced from 42 to 62%
-
•
Ethanol and ultrasound improved rehydration rate and final moisture.
-
•
Mechanisms were discussed.
Abstract
Ultrasound was combined with ethanol to improve different aspects of carrot convective drying, evaluating both processing and product quality. The ultrasound in water treatment resulted in cellular swelling and small impact on texture. Differently, the ultrasound in ethanol and ethanol treatments modified both carrot microstructure (cell wall modifications of parenchymatic tissue) and macrostructure (shrinkage and resistance to perforation). Pre-treatments with ultrasound in ethanol and ethanol improved the drying kinetics, reducing the processing time (~50%) and the energy consumption (42–62%). These pre-treatments also enhanced rehydration, whose initial rate and water retention were higher than the control. In addition, the carotenoid content was preserved after drying, for all the treatments. Any impact on shrinkage was observed. A mechanistic discussion, based on structural modification (microstructure and macrostructure) and physical properties of water and ethanol, was provided. As conclusion, this work not only described positive aspects of combining the technologies of ultrasound and ethanol as pre-treatments to convective drying, but also proposed mechanisms to explain the phenomena.
1. Introduction
Drying is an important operation in the food industry, producing safe and stable food products, and also reducing post-harvest losses. This operation allows for obtaining various products such as snacks, soups and dried fruits [1], which can be consumed directly or after rehydration.
Drying has numerous advantages in food preservation. However, conventional convective drying is a long process, which also presents a high energy consumption [2]. Moreover, the long processing time, associated with high temperatures, can result in undesirable changes, such as nutrient degradation or poor rehydration capacity.
Therefore, different strategies are being studied to enhance food drying, including the application of pre-treatments [3]. In this context, both ethanol and ultrasound can be used as a promising alternative in food processing.
The pre-treatment with ultrasound has been studied in different products, while the studies using pre-treatments with ethanol are now increasing. However, the combination of both approaches (conducting ultrasound processing with ethanol) was only recently proposed. Rojas et al. [4] conducted a work combining ethanol and ultrasound (Ethanol + US) pre-treatments to convective drying of pumpkin. The combined treatment reduced the drying time and the energy consumption during processing, improved the rehydration and avoid carotenoid degradation. The same combination (Ethanol + US) was also proposed prior to apple convective drying [5], with smaller processing time (3 min). They obtained significative reduction of drying time, although the combination did not minimize the degradation the phenolic compounds. The combination Ethanol + US was also evaluated on melon, with two ethanol concentrations (50 and 100%) and convective drying at 60° C. The treatment with a higher concentration of ethanol obtained shorting drying time, but there was degradation of phenolic compounds, ascorbic acid and carotenoids, when compared to dry samples without treatment and fresh melons [6]. However, in all woks, the authors did not evaluate the product structure nor performed the pre-treatment using water in the ultrasonic bath. Moreover, the three works evaluated homogeneous vegetables, while it would be interesting to study a matrix with different structures.
Three other works employed the combination Ethanol + US, but as pre-treatment to different drying techniques, whose mechanisms are different from convective drying. These pre-treatments were evaluated prior to the infrared drying of potatoes [7] and garlic [8], reducing the drying time. However, the rehydration properties of potatoes were impaired due to the structure modification associated with its composition, while allicin losses were registered in the garlic samples. The Ethanol + US combination was also investigated in pulsed vacuum drying of apple, reducing the drying time and liberating free amino acids [9]. These different results reinforce the need to evaluate other structures and quality parameters by using these pre-treatments.
Therefore, although being promising, the combination of ethanol and ultrasound (Ethanol + US) technologies as pre-treatments to convective drying still needs to be better understood. Particularly, its effect on product structure, the subsequent impact on processing, and how different tissues are affected by those pre-treatments still must be better described. Based on this context, the present work was proposed.
Carrot was selected as a food matrix for drying, not only due to its commercial importance, but also as this vegetable can be consumed both directly dried (as a health-claim snack or composing other products, such as cereal bars, breakfast cereals, granola, cookies, etc) or after rehydration (as in soups, puree, creams or cakes, among other possibilities). In addition, carrots present an appreciated nutritional composition, due to its high carotenoid content, which is interesting in this study as a quality parameter. Moreover, carrots exhibit two distinct structural regions (cortex and core) with parenchymatic tissue and two vascular tissues (xylem and phloem), representing a typical anisotropy of food matrix. Their complex structure can provide an important opportunity to describe the mechanisms of mass transfer during drying under a more realistic perspective.
Consequently, the present work studied the effect of ethanol pre-treatment along with ultrasound on carrot structure and convective drying, also evaluating the energy consumption, and quality parameters (evaluated through the texture, shrinkage, rehydration kinetics and carotenoid retention).
2. Material and methods
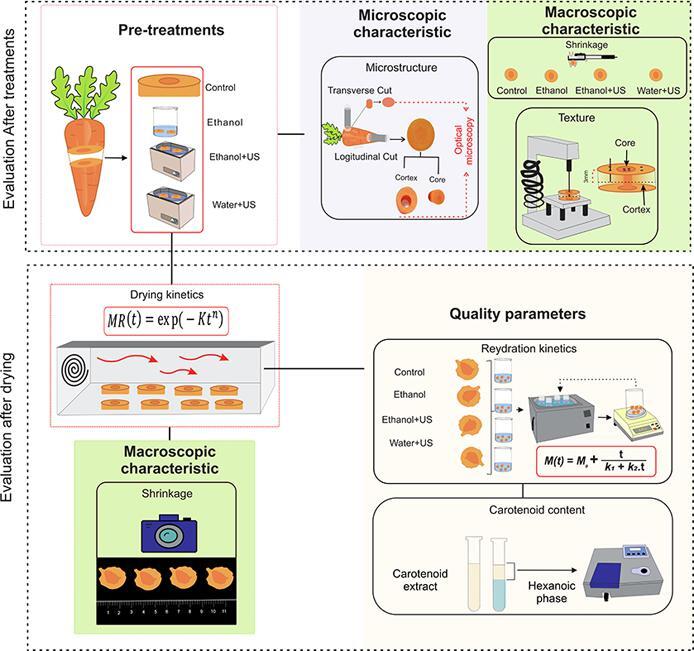
The experimental flowchart is described in Fig. 1. This work described ethanol and/or ultrasound pre-treatments to carrot convective drying and their influence on drying and rehydration kinetics, microstructure, texture, shrinkage and carotenoid content. Carrot was chosen by being a widely consumed vegetable, with good nutritional value (in special related with its carotenoid content), and the product can be consumed directly dried or rehydrated (such as in soups). Moreover, it is constituted by two different fibrous parts (demonstrated on Fig. 1, Fig. 2), which make its study interesting from a structural point of view: the core, which is the internal and softer pulp; and the cortex, which is the external and harder pulp.
Fig. 1.
Illustrative diagram of sample preparation and analyses.
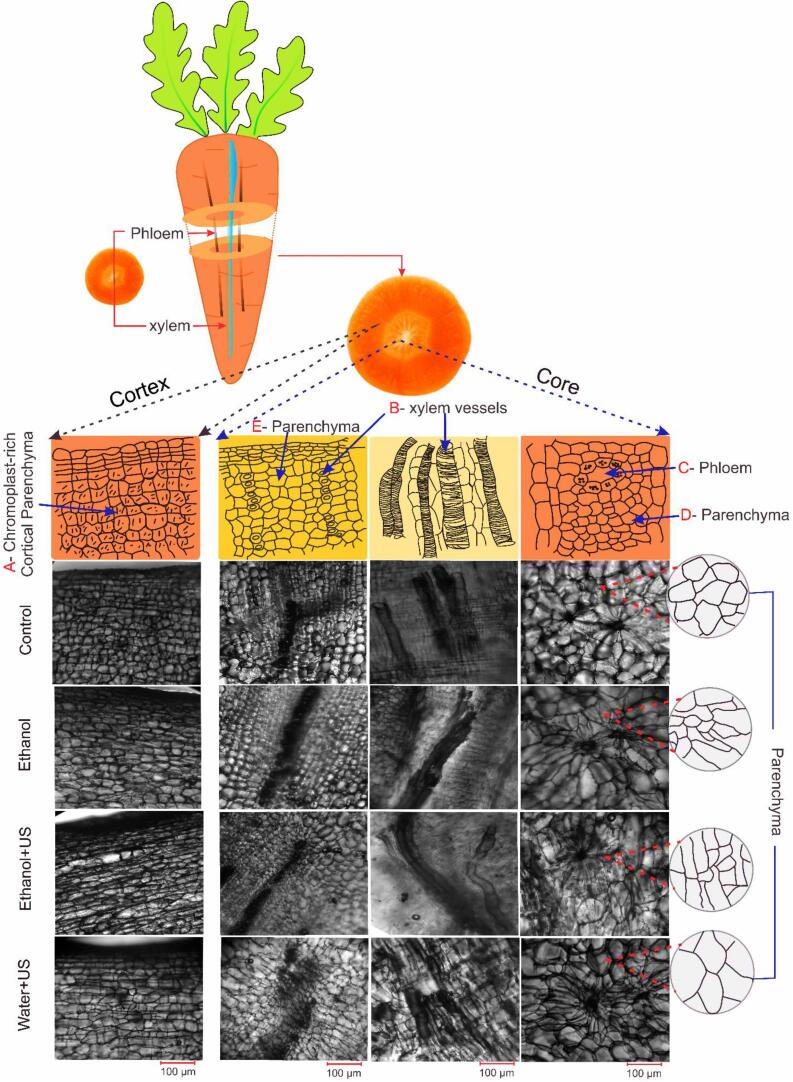
Fig. 2.
Carrot microscopic tissues of the core and cortex from the Control, Ethanol, Ethanol + US and Water + US pre-treated samples.
2.1. Sample preparation
Carrots (Daucus carota) free of injuries and homogeneous in colour were obtained in a local market of Piracicaba-SP, being stored at ~ 6 °C before processing. The carrots were cut in 5 mm slices (cutter Britânia, Brazil), totalling 32 slices per treatment. The slices were then submitted to different treatments.
2.2. Pre-treatments
In order to better understand the effect of ethanol and ultrasound on carrots, the following pre-treatments were applied: Ethanol (E); Ethanol + Ultrasound (Ethanol + US); Water + Ultrasound (Water + US); and Control. Ethanol pre-treatment evaluates only the effect of ethanol. It is impossible to isolate the pure ultrasound effect since a fluid must transmit the waves through a liquid until the solid product in the ultrasonic bath. Therefore, water was used in the treatment Water + US to closely evaluate the ultrasound effect. The effect of the two technologies combination was observed in the Ethanol + US pre-treatment. Control is a sample that did not undergo any pre-treatment before drying, being only cut to standard size and dried under the same conditions as the other treatments.
The carrot slices were submerged in ethanol (99.8% v/v) for 30 min (Ethanol). The ethanol and ultrasound action time were chosen based on the work Rojas et al. [4] that obtained the highest drying time reduction with combination Ethanol + US for 30 min. After the immersion time, the samples were removed from ethanol, which was drained, and the samples were superficially dried with absorbent paper.
Ultrasound was applied in an ultrasonic bath (Q13/25, Ultronique, Brazil; frequency of 25 kHz) at 20 °C (±1 °C), using ethanol (99.8% v/v) or water, for 30 min. To certify the maintenance of the temperature during the ultrasonic pre-treatment (20 °C), an auxiliary thermostatic water bath (ColdLab CL 16–40 - Brazil) and a heat exchanger recirculating a cold solution of ethanol/water were used. Moreover, the good practices described by Vinatoru [10] were applied. The actual delivered volumetric power was 23.9 and 25.7 W/L (calculated through the calorimetric method according to [11]) for water and ethanol, respectively. After ultrasound processing, the samples were removed from ethanol or water, and their surfaces were drained and superficially dried with absorbent paper.
2.3. Structural analysis
Microstructural evaluation was carried out using the optical L1000 microscopy model (Bioval, Curitiba, Brazil) with 20 W halogen lamp and a portable camera of 5 megapixels. The carrots slices were cut into 20 μm dishes using a handheld microtome (Ancap, São Paulo, Brazil) and observed with 10-fold magnification lens. For better observing, a blue dye of 0.1% toluidine was used. Then, the microstructure was verified on the in natura sample and pre-treated samples. The images were captured after securing a representative field. The images were captured in core and cortex of carrot.
2.4. Texture analysis
Ethanol and ultrasound may act differently on the cortex and core regions of carrot due to different composition and structure of tissues and cells, which are distributed along carrot length. Therefore, this work analysed the effects of pre-treatments at the macrostructural level through texture analysis. For this, it was considered both the core and cortex in order to understand and describe the mechanism in materials with different structures such as carrots.
Texture analysis was performed in the in natura carrot slices and those after the pre-treatments. Texture was assessed by a penetration test using a texture analyser (TA.XT Plus, Stable Micro Systems Ltd., Surrey, UK) with a 50 kgf load cell (490.3 N). A cylindrical probe of 2 mm diameter was used to penetrate the thickness of the samples at a constant rate of 1 mm/s until the distance of 3 mm. The curve force (N) versus penetration (mm), was used to describe the texture. The analysis was performed with five carrot slices for each treatment, considering both the core and cortex. Each slice was perforated 4 times in the core and 4 times in the cortex. Illustrative scheme of the analysis is described in Fig. 1.
2.5. Drying
Convective drying was conducted in a tray dryer (UOP8MKII, Armfield®, England) at 40 °C with air velocity of 1 m/s. This temperature was selected in order to minimize carotenoid degradation, once a decrease of 52.5 and 57.8% were observed in the carotenoid content of dried carrots at 50 and 70 °C respectively [12]. The carrot slices were placed on perforated metal trays, allowing the hot air flows through all their surfaces. Samples remained in the dryer until constant weight. The sample mass was recorded continuously through the UOP8-MKII-306 software (Armfield®), without the need to withdrawal of samples.
The moisture at each time was obtained by the mass balance, considering the initial (of in natura samples) and final (after drying) moistures, which were obtained after completely drying crushed carrots at 105 °C with the aid of moisture analyser (MX-50, A & D Company, Tokyo, Japan). It is important to highlight the sample “moisture” is a lumped parameter that includes both volatile liquids: the remaining water and the absorbed ethanol [13].
The drying curves were elaborated according to the dimensionless moisture (MR) during the processing time, calculated according to Eq. (1) where Mt is the moisture (% d.b.) content during the drying process time (t), Me (% d.b.) is the equilibrium moisture and Mp (% d.b.) is the carrot moisture after pre-treatment. In the case of the Control sample, Mp (% d.b.) is equal to the in natura carrots moisture.
| (1) |
Drying kinetics was evaluated using the Page Model (Eq. (2)), where k (h-n) is the drying rate parameter and n is the dimensionless shape parameter. According to Simpson et al., [14] interpretation of Page Model, the parameter k is associated with the “diffusion coefficient” and sample geometry, while the parameter n describes the “diffusion type”: n > 1 is related with super diffusion, while n < 1 is related with sub diffusion. When n ≠ 1, mechanisms other than diffusion are important; for example, the “super diffusion process” (n > 1) may indicate the importance of capillarity [13].
| (2) |
2.6. Estimative of the total energy consumption
An estimative of the total energy consumption (TEC) (Eq. (3)) during processing (including pre-treatment and drying) was calculated according to Motevali et al.[2]; Onwude et al. [15] and Rojas et al. [4]. It is important to mention this approach does not consider the strict energy consumption during processing, being an estimative of the energy consumption by considering the performed pre-treatments. Even so, it is useful for comparison purposes. The total energy consumption (TEC, Eq. (3)) was calculated considering 1 kg of in natura sample () and the two terms: EPT represents the energy consumption during pre-treatments (Eq. (4)), and ED represents the energy consumption during convective drying (Eq. (5)).
| (3) |
| (4) |
where W is the US actual volumetric power (W/L - determined by the calorimetric method depending on whether water or ethanol was used), V is the volume of water or ethanol (L) used in the US bath, tP is the time of pre-treatment.
| (5) |
Where is the cross-sectional area of drying (m2), is the ambient air density (25 °C), is the specific heat capacity (J/kg‧K) of ambient air, is the temperature difference between the ambient air and drying air, is the air velocity (m/s), and is the drying time needed to the samples reach a moisture 20%w.b. For the calculations, the initial sample mass, the histories of temperature and air velocity were considered for each treatment and drying process replicate.
2.7. Shrinkage
The shrinkage of carrot slices was measured after pre-treatments and also after drying. Carrot slices radial area was used as the shrinkage evaluation parameter. For this, carrot slices with standard dimensions (5 mm thick and 3.5 cm diameter) were used.
The in natura samples and those after pre-treatments were circular; so, the area formula of a circle was used to calculate their area. The diameter was verified with the aid of a digital calliper.
After drying, the samples lose their circular shape, so it is not possible anymore to calculate the area by considering a circular shape. Therefore, the projected areas were measured by image analysis. The dried samples were placed on a dark (black) surface near to a scale reference. Their images were captured at the same distance and then processed in ImageJ version 1.52a (National Institutes of Health, USA) software. The accurate photos have been converted to grayscale (8 bits). With the help of the command “set scale” and a ruler close to the samples, the scale was defined. Then the images were converted to binary scale using the “threshold” command. For the results, we used the command “Analyse”, which provides a response window with the area of the calculated samples. For visualization and better understanding of the results, the deformation was expressed as shrinkage ratio (%).
Shrinkage assessment was divided into three ratios, in order to evaluate the deformation during pre-treatment, during drying and also the whole process treatments: (1) Ratio between the area after pre-treatments (before drying) and the initial area (of in natura sample); (2) Ratio between final area (after drying) and the area after pre-treatments (before drying); and (3) Ratio between final area (after drying) and the initial area (of in natura sample), considering each treatment.
2.8. Rehydration kinetics
The rehydration process was conducted at 25 ± 1 °C (water bath MA 095 / CFRE, Marconi). The dried carrot slices were submerged in distilled water (4 g of dried product with 20%w.b moisture was used with 1 L of distilled water). The sample moisture over the rehydration time was calculated by mass balance, considering the carrot initial moisture obtained with the moisture analyser, as described before.
For this, the samples were taken from the water, drained and dried superficially, weighed, and then returned to the water again. This step was performed every 5 min for the first 25 min, then every 10 min until 130 min, and then every 30 min until reaching constant mass.
The rehydration data were fitted using the Peleg Model (Eq. (6)) (Peleg, 1979), where M(t) is moisture content in dry basis (d.b., g water/g dry matter) at time t (min), M0 is initial moisture content (d.b.), and k1 (min·d.b−1) and k2 (d.b−1) are parameters related with the water absorption rate and quantity: the reciprocal of k1 represents the maximum water absorption rate (at the beginning of rehydration), and the reciprocal of k2 is associated with the water retention capacity.
| (6) |
2.9. Total carotenoid content
Prolonged exposure to hot air may cause nutrient degradation during drying. Therefore, the present work evaluated the carotenoid content over the proposed treatments in comparison with the in natura carrot in order to evaluate the nutritional quality. To avoid errors due to lack of homogeneity between samples and also to avoid over-drying effects, all treatments were dried to the final moisture of 20% (in wet basis). This value has been selected as a reference as the maximum moisture value recommended for dehydrated fruits and vegetables [17].
After drying with the different pre-treatments, the samples were rehydrated for 8 h at 25 °C and the total carotenoid content was measured according to the methodology described by Potosí-Calvache et al. [18] with modifications.
About 0.25 g of sample was weighed and transferred to aluminium-coated glass tubes in order to protect the samples from light and oxygen. Then, 21 mL of ethanol:hexane (4:3) solution was added (ethanol 99.8% from Scientific Exodus, São Paulo, Brazil and hexane 98.5% from Labsynth, São Paulo, Brazil). Samples were triturated with the solution for 1 min using a rotor–stator homogenizer (Superohm, São Paulo, Brazil) and the probe was washed with an additional 21 mL of ethanol:hexane solution, which was reserved. The tube with sample and solvent was then stirred in a thermal bath at 250 rpm and 25 °C for 30 min (DUBNOFF MA 095 / CFRE, Marconi, Brazil). Then, the solvent was separate from the sample and transferred to other vessel protected from light and oxygen. After this, the decanted residue was mixed with the solution used for washing the homogenizer probe in a tube that was stirred for 30 min at the same conditions. Subsequently, the solvent was removed from the residue and added to the vessel containing the solvent from the first extraction. 5 mL of distilled water was added to this vessel, manually stirred and allowed to stand for 5 min to separate the phases (aqueous phase and hexanoic phase). The volume of the hexanoic phase was noted for subsequent calculation of carotenoid content.
After obtaining the phase of interest (the hexanoic phase), its absorbance was read at 450 nm (FEMTO 600S, São Paulo, Brazil) using hexane for calibration. The carotenoid content of the extracts was calculated by Eq. (7) and expressed as β-carotene equivalents (µg)/ g of sample. Where 536.85 g/mol is the molecular weight of β-carotene, V is the volume (mL) of the hexanoic phase, m (g) is the mass of the used sample, and 137.4 mM−1 is the extinction coefficient for β-carotene in hexane.
| (7) |
2.10. Experimental design, regressions and statistics
The experiments were performed with three replicates. Results were analysed by analysis of variance (ANOVA) and differences among treatments were determined using the Tukey test at a 5% significance level using Statistica 7® software (Statsoft, USA).
The parameters of each model were iteratively adjusted to the experimental data by minimizing the sum of squared errors (SSE in Eq. (8)) between the experimental and the predicted values. A generalized reduced gradient algorithm was used, implemented in the ‘Solver’ tool of software Excel 2016 (Microsoft, USA). The different initial guesses of the parameters were evaluated to detect possible convergence to local optima.
| (8) |
3. Results and discussion
3.1. Effect of treatments on carrot microscopic and macroscopic structure
Fig. 2 shows the carrot structure before and after the different pre-treatments. The carrot has three main structures: Parenchyma, Phloem and Xylem. These tissues are distributed in both the core and cortex regions of the vegetable (Fig. 1, Fig. 3 shows the description for “core” and “cortex”).
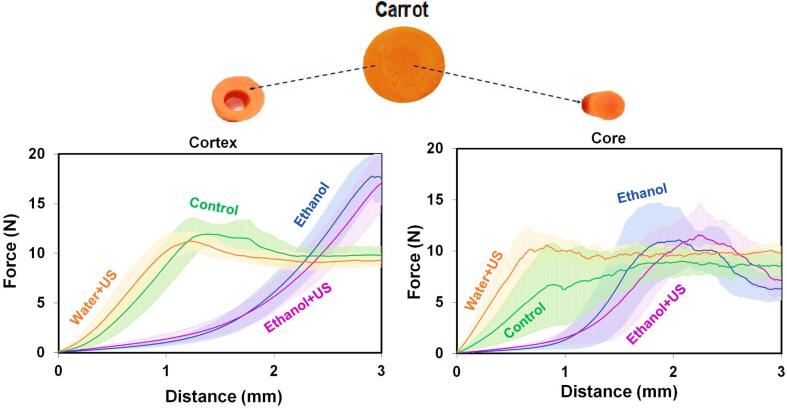
Fig. 3.
Resistance to puncture (Force (N) × Distance (mm)) of each carrot region (cortex and core) after pre-treatments. The shaded area in each treatment indicates the standard deviation.
The parenchyma is the predominant cellular tissue in the roots. It is composed by cells of polyhedral shape, being the fundamental filling tissue present throughout the root in which other tissues are found, such as the vascular (xylem, Fig. 2, B, and phloem, Fig. 2, C) tissue [19]. However, the structure of the parenchyma tissue can vary even in the same vegetable. In carrots, the parenchyma presented walls rich in elongated chromoplast at the cortex (Fig. 2, A), and larger cells in the core close to the phloem (Fig. 2, D) and xylem (Fig. 2, E).
According to Evert, Ray and Franklin [19] the xylem is responsible for nutrient transport and storage, and the phloem is the main nutrient conducting tissue. In carrots, Smith and Ho [20] reported the core region in carrots is mainly composed by vascular tissue, while the cortex region is predominantly parenchymal tissue, which in fact can be seen in Fig. 2. This confirms the difference between these two regions. Therefore, the objective of the following discussions is to describe and explain the structural changes that occurred with the use of pre-treatments in the tissues presented in both the core and on the cortex of the carrot.
The pre-treatments induced structural changes evidenced in some carrot tissues.
No structural changes were evidenced in the xylem (Fig. 2, B) and the phloem vessels (Fig. 2, C), in any of the treatments, which can be associated to its more rigid and compact structure. Smith and Ho [20] described that carrot xylems have a second lignified cell wall, which makes the xylem tracheal elements stronger than the parenchyma. A similar result was reported by Rojas; Augusto [21] in pumpkin.
After the treatments with Ethanol and Ethanol + US, the cortical parenchyma showed a slight shrinking of the cell wall when compared to the fresh sample (Control). Although cellular organization was maintained, this change on cell wall can be important to change its permeability – as discussed further. On the other hand, the parenchyma tissue next to the phloem (Fig. 2, D) and xylem (Fig. 2, E) at the core, obtained higher wrinkle levels of the cell wall, which became thinner and disorganized, losing their polyhedral shape. The changes in parenchyma cells can be associated with the loss of water, air and other compounds during the treatments with ethanol, altering the cells structure. This effect was also observed by [13] in pumpkin parenchyma
Canteri et al. [22] studied the composition of the cell wall and membrane of different vegetables, including carrots. They demonstrated that ethanol could extract polyphenols, some proteins and lipids from the cell wall and/or membrane. However, ethanol was not able to extract cellulose, lignin, pectin or hemicellulose from the cell wall. Consequently, this can help to explain the results here obtained. Once the main structural components of the cell wall are not extracted, but some components of the cell membrane and cell wall can be, the general structure of the cells is maintained but reducing the thickness. It may have contributed to the improvement of mass transfer (which can be seen in Fig. 6). However, although ethanol probably changes the cell wall and membrane composition, the measurable loss of solids in carrots were negligible.
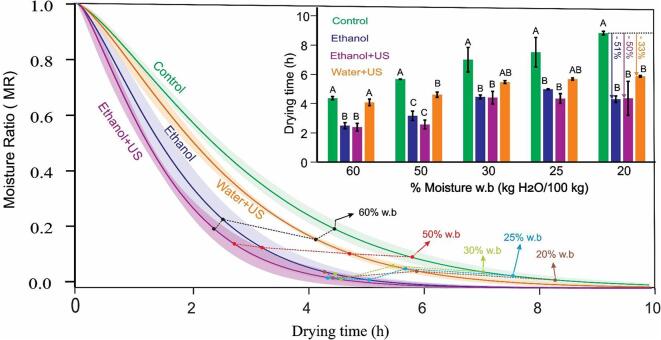
Fig. 6.
Convective drying kinetics of carrot slices with different pre-treatments (Control, Ethanol, Ethanol + US, Water + US; where MR is calculated in d.b according to Equation (2)). The curves are the data adjusted to the Page Model (Eq.2) and the shaded area indicates the standard deviation. Different moisture levels (in w.b.) are highlighted. Insert: the bar graph corresponds to the drying time to obtain different moistures (in % w.b). Vertical bars indicate standard deviation; different letters indicate statistically significant differences (p < 0.05) among treatments.
Unlike the observed in the Ethanol and Ethanol + US treatments, the Water + US treatment resulted in parenchyma cells swelling, at both the core and cortex, due to water inlet (Fig. 2, A). Xylem and phloem vessels can assist the transport and migration of water into the cells. These structures are responsible for transporting water and nutrients in the living carrot. However, Rojas; Augusto [21] demonstrated that water can be transported by capillarity in pumpkin xylem vessels during both drying and rehydration (https://youtu.be/o5vbxs1G81s). According to the authors, the water passes through the xylem vessels, from which begins to be distributed firstly through intercellular spaces, then diffuses through the walls and membranes into the cells.
Another factor associated with water migration into cells is the use of ultrasound, which improves mass transfer [23] due to direct and indirect effects [24].
The “sponge effect” is produced by mechanical waves passing through the product, and it helps to keep the microchannels unobstructed and favours the migration of water into the solid [13], [23], [25], [26], [27].
Moreover, it is widely described (but rarely demonstrated) in the literature that ultrasound treatments form new micro-channels in the product due to acoustic cavitation. However, this phenomenon could not be evidenced in carrots during pre-treatments with Ethanol + US and Water + US (Fig. 2), which can also explain the low influence of ultrasound treatments on drying time reduction (as it will be discussed on Section 3.2). The carrot has a stiffer or compact structure in comparison with other vegetables (such as potato [28] and melon, [29] for example), which may have contributed to little or no visible microchannel formation in the evaluated conditions. Moreover, the pronounced formation of microchannels may be associated with the level of power employed. Wang et al. [23] evaluated the use of ultrasound in a probe system as a pre-treatment of carrots and found that increasing the nominal power from 1800 to 3600 W/L resulted in a greater effect on the carrot structure, with the formation of microchannels. However, it is difficult to compare our results once only the nominal power was reported by Wang et al. [23] (and the difference between the nominal and actual delivered power can be in the order of 90–95% - [30]. Moreover, although interesting and valuable approach, the ultrasonic probe system has important drawbacks from an industrial point of view, such as high cost and wearing, and it is not suitable for scale production. Therefore, further studies are still needed to understand the effect of ultrasound conditions on the structure of different vegetables.
Structural differences and modifications can be manifested on macroscopic changes. Consequently, the texture was evaluated to complement the discussion of structural changes. Fig. 3 shows the texture, through a puncture assay, of carrot core and cortex after the different pre-treatments. Despite the high standard deviation range in all conditions, a qualitative discussion can be carried out.
In general, the graphics demonstrate the differences between the core and cortex regions of carrot. When compared to the cortex, the core region is less resistant, evidenced by the lowest time and force necessary to penetration for all conditions, except for – the Water + US treatment. The core region contains larger parenchymal cells (see Fig. 2, D) while the outer part of the cortex has smaller parenchymal cells (see Fig. 2, A) with a large amount of plastids containing carotenoids, which can explain the observed differences. It confirms the description of Zdunek e Umeda [31] which demonstrated that less energy is needed to fracture a tissue made up of larger cells.
In the texture curves, there was an increase in force as the probe exerts pressure on the cortex. At this stage, the cortex is deformed according to the applied force, but there is no perforation. This phase ends when the probe penetrates the first layers of tissue causing an irreversible rupture. The rupture occurs after ~1.25 mm for the Control and Water + US treatments, and ~2.8 mm for the treatments with ethanol (Ethanol and Ethanol + US). After rupture, the force exerted to maintain penetration in the cortex is approximately constant, which demonstrates the same profile of the layers of tissue along with its thickness. On the other hand, the behaviour in the pre-treatments with Ethanol and Ethanol + US indicates the first layers of tissue are more resistant than the adjacent layers, that is why the applied force increases until the rupture. The greater resistance of the first layers of tissue is caused by the superficial dehydration of the samples when using ethanol, forming a more resistant tissue external layer. This can be confirmed in the work of [13] where they demonstrated that ethanol only reachs the initial layers of tissue.
Therefore, in the cortex, the use of ultrasound had not changed the texture profile (since the treatment Water + US is similar to the Control, and the Ethanol + US is similar to the Ethanol), and the effect of ethanol on the carrot structure was higher than those of ultrasound.
Similar trend and behaviour were observed in the core. However, unlike the cortex, the Water + US treatment presented a more rigid structure than the Control. This may be associated with the migration of water to the cell during this pre-treatment, as demonstrated in Fig. 2D, the consequent increase in cell turgor contribute to a greater resistance of the tissue to perforation. The predominant absorption of water by the core can be associated with the presence of phloem and xylem vessels, which help the transport and migration of water into the cell. After the initial deformation, the core tissue broke at ~0.7 mm for Water + US treatment, ~0.9 mm for the Control and ~2 mm for Ethanol and Ethanol + US. After the rupture, the force exerted to maintain penetration into the core is approximately constant for Water + US similar to the Control, being slightly decreased in Ethanol and Ethanol + US – indicating dehydration and compactness in the surface layer of the samples that include ethanol during the pre-treatment.
Shrinkage was also investigated since it is another macroscopic characteristic which is correlated with microscopic structural modifications. Fig. 4 shows the visual appearance of carrot slices under different conditions: fresh and pre-treated (with ethanol and/or ultrasound) carrots before and after drying.
Fig. 4.
Shrinkage of pre-treated and Control carrot slices, after pre-treatment and drying.
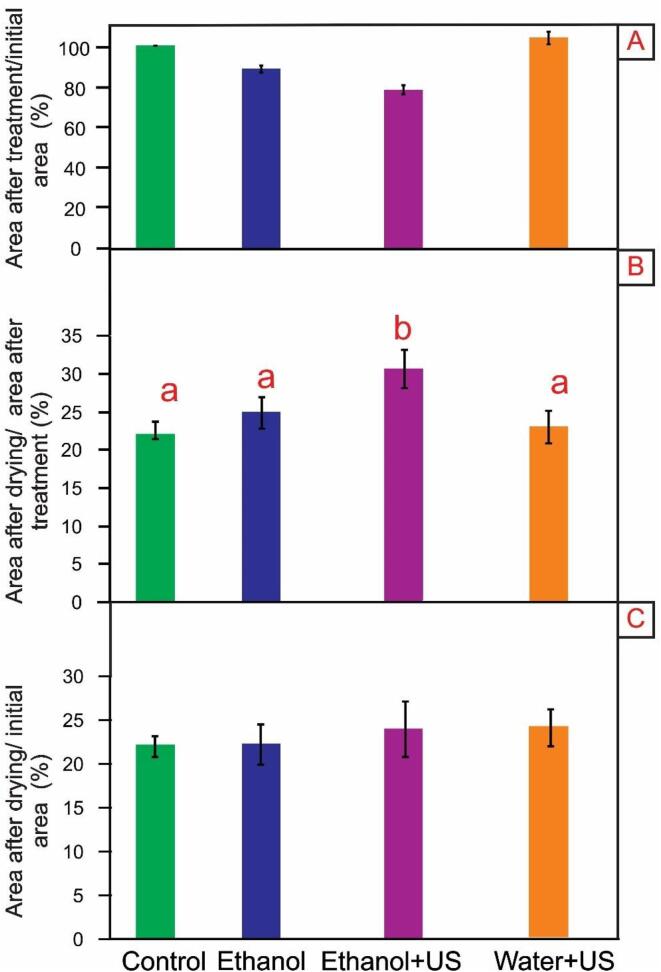
The shrinkage percentage of carrot slices are shown in Fig. 5, considering the changes due to only pre-treatment (Fig. 5A), only drying (Fig. 5B), and the whole process (Fig. 5C).
Fig. 5.
Shrinkage Ratio: A – Ratio of the final pre-treatment area to the initial Control area. All treatments were different from each other at 5% significance (Tukey). B – Ratio of final area of drying to initial area of treatments. Equal letters indicate similarity at the 5% significance level (Tukey). C – Ratio of final drying area to initial Control area. All treatments were equal at 5% significance level (Tukey).
Fig. 5A corresponds to the ratio between the area after pre-treatments (before drying) and the initial area (from the in natura sample). This allows us to visualize the radial shrinkage behaviour of carrot slices during pre-treatments. All treatments showed different shrinkage rates when compared to each other (p < 0.05). At this stage, the Ethanol treatment had about 12% shrinkage, already the Ethanol + US stands out with the greatest shrinkage, about 22%, this shrinkage levels could be explained because water and air has already been removed during the pre-treatment. On the other hand, the Water + US had a small (4%) increase in its area due to water absorption (which was also evidenced in microscopy and texture analyses).
Fig. 5B corresponds to the ratio between the area after drying and the area after pre-treatments, which indicates the behaviour of the carrot slices during drying. It is possible to verify that the treatment with Ethanol + US presents a lower shrinkage (70%) during drying, when compared to the Control (78%) and other pre-treatments (75 and 77% were obtained for the treatment Ethanol and Water + US, respectively). This is consistent because the Ethanol + US samples presented high retraction of cells due to the outflow of water, starting drying already with a higher shrinkage level than the Control (described in Fig. 5A). In the case of Water + US treatment, despite the water gain during treatment (Fig. 2 and Fig. 5A), it did not promote different shrinkage level compared to Control during drying.
Fig. 5C corresponds to the ratio between the final drying area and the initial area (fresh sample), thus describing the shrinkage of the final product. Although the treatments have different drying rates (Fig. 7, which will be explained later), this did not influence the shrinkage of the slices after drying. All presented equal shrinkage ratio (p > 0.05). This may have occurred due to the low temperature applied (40 °C), once at low temperatures, moisture is transported in a flat pattern with minimal stresses within the food.
Fig. 7.
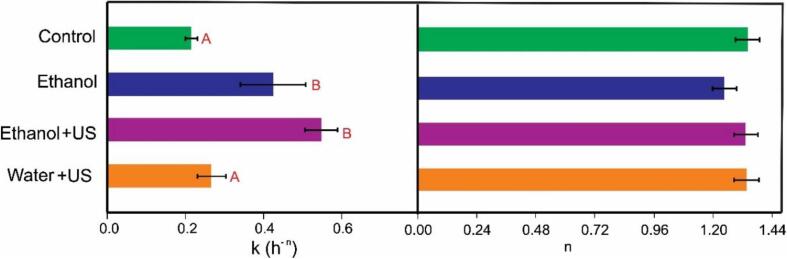
Parameters k and n of Page Model (Equation (2)). Horizontal bars indicate the standard deviation. Different letters indicate statistically significant differences (p < 0.05) among treatments. For the parameter n all treatments were equal (p > 0.05).
After drying, the carrot's cortex has shrunk towards the core, for all treatments, with pronounced deformation in the form of undulations (similar to a flower – Fig. 4). This behaviour was also observed in potato (Aprajeeta et al. [32]). They observed that during drying, moisture leaves the potato pores filled with water on the surface of the material, and this disturbs the mechanical balance of the cell walls (this occurs linearly over time). According to the authors, this effect causes shrinkage towards the core of the sample due to the contraction effect, which is the stress caused by the presence of spaces on the outer wall of the cell, while the inner wall remains the same. Possibly this mechanism also occurs in carrots. Another option is that the major quantity of vascular tissue in the core region provided more resistance to deformation.
3.2. Effect of ethanol and ultrasound pre-treatments on drying kinetics
The results of drying kinetics are presented in Fig. 6, with the data adjusted to the Page Model (Equation (2)). The inserted graphic in Fig. 6 shows the “drying time”, which was considered the time required to obtain different moisture levels (from 60 to 20%w.b.): therefore, the graphic allows to visualize the behaviour of the carrot slices during different drying phases.
In general, the Ethanol and Ethanol + US pre-treatments resulted in faster drying when compared to the Water + US treatment and the Control. Note the similarity in drying behaviour between Ethanol and Ethanol + US treatments, and between Water + US and Control: therefore, ethanol had a higher contribution in decreasing the drying time than ultrasound.
The Water + US pre-treatment resulted in drying time statistically equal to the Control at 60% moisture (p > 0.05). However, in the moisture range of 50 to 20%, this treatment dried faster than the Control. In fact, the time needed to finish drying (which we consider the final product moisture of 20% in wet basis - [17]) in the Water + US treatment was 33% smaller than the Control. On the other hand, the treatment of Ethanol and Ethanol + US required less drying time than Control and Water + US treatments. Considering the final moisture of 20% as the target, the drying time required was 51 and 50% less for the Ethanol and Ethanol + US treatments, respectively, than the required by Control. This result is similar to the reported for pumpkin cylinders [4].
Although the Water + US treatment has a positive contribution to drying process (reduces 33% of the drying time and does not differ from the Ethanol and Ethanol + US treatments (p > 0.05)), it was not possible to highlight drastically improving the process with the use of ultrasound technology, due to the small contribution of ultrasound in decreasing the drying time in the Ethanol + US treatment (the greatest effect was the use of ethanol).
However, this does not definitively exclude the participation of ultrasound on the drying rate. The slope of the drying curve of the Water + US denotes shorter drying time for this treatment compared to the control. Another fact that does not exclude the influence of ultrasound is related to water gain during pre-treatment.
In fact, during the immersion of the samples in ethanol (with or without ultrasound), part of the water outlets and ethanol inlets – which occur simultaneously. Consequently, the “moisture” in these treatments includes both water and ethanol (as described on section 2.5). Therefore, after pre-treatments, the carrot moisture was reduced 6% in the Ethanol pre-treatment, and 21% in the Ethanol + US; in relation to the Control. Although it is still a challenge to know the exact proportion of water and ethanol after pre-treatment, microscopic evidence shows that ethanol enters only the superficial part of the samples [13].
On the other hand, during pre-treatment using ultrasound and water, the sample absorbs water – the carrot moisture after pre-treatment Water + US was 38% higher than the control. In fact, ultrasound facilitates the entry of water into cells and intercellular spaces [26], [33]. This mechanism can help to unclog the natural pores of the samples, which can facilitate drying [26]. Consequently, it is interesting to observe although the pre-treatment Water + US increased the carrot moisture, there was no increase in drying time (Fig. 6).
Therefore, depending on the pre-treatment, the sample’s moisture in the beginning of drying is different. However, this was not the only factor affecting the process, once different slopes can be seen in the drying curves and the behaviour is consistent considering the time needed to reach different moisture levels (Fig. 6).
This reinforces the need to investigate parameters to maximize the effect of ultrasound for each material.
The adjusted data allowed obtaining the parameters k and n of the Page Model (Fig. 7).
The k parameter, which is associated with the drying rate, was lower for the Control (0.2139 ± 0.0162 h-n) and Water + US (0.2658 ± 0.0373 h-n) treatment and they did not differ between them (p = 0.62). On the other hand, the pre-treatments with ethanol resulted in higher k values. Although no statistical differences were observed between them at p < 0.05, this value was higher for Ethanol + US (0.5467 ± 0.0426 h-n) than Ethanol (0.4236 ± 0.0841 h-n) when considering p < 0.1. This result reinforces the idea of ethanol efficacy and its bigger influence than the pre-treatment with ultrasound during the following drying. Even so, this does not exclude the participation of ultrasound in improving drying (Fig. 6).
The n parameter can be interpreted as a behaviour index, once it indicates the mass transfer mechanism during processing. According to Simpson et al. [14] n < 1 indicates a sub diffusion process, and n > 1 a super diffusion process. Therefore, when n ≠ 1, mechanisms other than diffusion are important. For example, Rojas; Augusto [13] associated the “super diffusion process” (n > 1) with capillarity, while Rojas et al. [4] associated a reduction on n value due to ultrasound processing of pumpkin as the formation of isolated cavities and channels larger enough to do not promote capillarity.
All the treatments presented a super diffusive behaviour (n > 1). However, the structural changes mentioned in section 3.1 did not affect the n value once any significant difference was observed among treatments (p > 0.05). Therefore, the described structural changes were able to change the rate of water outflux the samples, but they did not alter the mechanisms of mass transfer.
Studies with different vegetable products reported drying acceleration by using pre-treatments with ultrasound in water bath [34]. Some examples with convective drying are those with potato [28] garlic [35] unripe banana [36] pineapple [37] mushrooms [38] and apple [39], [40]. The improvement of mass transfer by ultrasound can be achieved through two types of mechanisms: direct and indirect.
The direct mechanisms are mass transfer mechanisms, such as the so called inertial flow and sponge effect [24]. In the present work, these direct mechanisms could happen during the pre-treatment, inducing the water influx into the sample (treatment Water + US) and the ethanol influx and water outflux to the sample (treatment Ethanol + US). Consequently, the ultrasound direct mechanisms induced structural and compositional changes in carrots, which impacted further drying processing.
The indirect mechanisms are the structural changes induced by ultrasound, which are associated with the rupture of tissues and cells due to acoustic cavitation, resulting in the formation of microchannels [41], [42]. The opening of microchannels can improve mass transfer [28] such as the following drying after the pre-treatments with ultrasound. However, acoustic cavitation can also result in the formation of isolated channels without connection with each other and with an external medium, as well as channels with different tortuosity and permeability, which can affect the improvement in mass transfer.
In the present study, the effect of ultrasound was little evident to drying when compared with ethanol, as well as no open channels were noticeable and only slightly structural modifications (Fig. 2). It is worth mention the influence of ultrasound pre-treatment on drying rate will be influenced by different aspects associated with both the material and the ultrasonic processing [25], [28], [43], [44], [45]. For instance, the energy and time of sonification applied in this work were insufficient for the formation of many channels in carrot, probably due to its stiffer structure. Similar results were found by Siucińska et al.[46] who reported the ineffectiveness of ultrasound treatment in the mass transfer in cherries can be attributed to the specific morphological characteristics of the material since that fruits are covered by a hard and waxy peel. Moreover, Ricce et al. [26] studied pre-treatments of carrots with ultrasound in water (41 W/L, 25 kHz, up to 60 min). Although the authors did not evaluate structural changes, they reported small influence on drying rate, with a higher influence on the total amount of absorbed water. The authors suggested the ultrasonic direct effects (sponge effects) with water helps to extract the solids removed from the cells, avoiding the pores to clog during the following drying process. Nowacka; Wedzik [47] applied pre-treatments of vacuum-packed carrots with ultrasound in water (3–4 W/cm2; 21 and 35 kHz, up to 30 min). Although structural changes were observed, ultrasound did not change drying time.
The greatest effect in reducing drying time and increasing drying rate in carrots was due to the application of ethanol (Fig. 6). In fact, the use of ethanol is a rising trend in drying research, once this pre-treatment is reported to greatly reduce processing time [3]. Considering convective drying, ethanol pre-treatment was proposed for pumpkin [13] balls of mixed rice [48] bananas [49] pineapple [50] and apple [51]. Moreover, the application of ethanol as pre-treatment to infrared drying was recently studied for potatoes, in combination with mechanical perforation [52] and scallion, with the combination with vacuum [53]. All works showed improved drying processes.
Different mechanisms are related to ethanol improvement.
Ethanol is an organic solvent that promotes early evaporation during the process [54]. Moreover, Silva et al. [55] proposed possible improvements due to Marangoni Effect: this effect promotes mass transfer in an interface between two fluids with different surface tensions. This effect could occur during drying, where ethanol vaporizes firstly, leaving water on the sample surface and resulting in a gradient of water/ethanol concentration. Therefore, during drying, the Marangoni Effect is also observed due to the existing gradient of surface tension across the sample: this process is repeated during processing, generating a constant flow until it finds a surface tension equilibrium. Moreover, the xylems vessels can improve the Marangoni effect, as demonstrated by Rojas; Augusto [13]. Finally, the structural changes observed in parenchyma tissue with the use of ethanol (Fig. 2) can improve drying by removing air and water from the tissues, as well as promoting dissolution and disorganization of the cell wall compounds by ethanol [22]. All these structural changes may affect the cell wall and tissues permeability.
In addition, the effect of osmotic dehydration induced by ethanol may have contributed to the structural changes observed. In osmotic dehydration, water is removed due exposing the food to ethanol. This result in input of ethanol in the sample, while differences on osmotic pressure cause a displacement of water from the sample surface to the surrounding ethanol. In fact, Wang et al. [53] described the pre-treatment using ethanol and ethanol using vacuum showed a good osmotic dehydration effect on scallion. In the present study, the combination of structural changes, combined with the osmotic process and the Marangoni Effect, can explain the success of ethanolic pre-treatment in reducing drying time.
Recently ethanol and ultrasound started to be combined. The ethanol and ultrasound were studied as pre-treatments for convective drying of pumpkin [4] in infrared drying of garlic [8] and potato [7] slices and pulsed vacuum drying of apples [9]. In addition, the combination of ethanol pre-treatment and ultrasound assisted drying was studied for apple slices [4]. Therefore, the only three works in the literature combining ethanol and ultrasound as pre-treatments to convective drying were carried out with pumpkin [4]; apple [5] and melon [6].
Pumpkin cylinders were pre-treated using Ethanol + US (68 W/L, 25 kHz), up to 30 min [4]. A similar reduction in drying time was obtained between treatments with Ethanol and Ethanol + US – results similar to those reported in the present work. Even so, treatment with Ethanol + US showed a higher drying rate and improved rehydration, also preserving the carotenoid content. Apples were pre-treated with Ethanol + US combination (300 W, 21 kHz), up to 3 min [5]. The drying time was reduced from 9.7% to 18.3% compared to the control. However, the use of this pre-treatment was not able to minimize the degradation of polyphenols in apple tissue during drying. Melons were immersed in ethanol with two different concentrations (50 and 100%) with and without ultrasound (154 W, 25 kHz) and vacuum up to 10 min [6]. This treatment achieved a 56% reduction in drying time compared to the control. However, there was degradation of phenolic compounds, ascorbic acid and carotenoids. In all works the authors did not perform the treatment Water + US nor evaluated structure.
Considering infrared drying [7] the combination of ethanol and ultrasound using a probe reactor (48 W/L, 20 kHz, up to 3 min) provided a significant reduction in drying time, which was attributed to the greatest structural changes in potato tissue. However, the high structural modifications had a negative impact on rehydration. In infrared drying of garlic slices [8] ethanol and ultrasound pre-treatments (50 W/L, three frequency, 20, 40 and 60 kHz up to 30 min) shortened the drying time for Ethanol + US treatment compared to treatments with ethanol and Water + US only. However, the allicin content has decreased, the main bioactive substance in garlic.
In pulsed vacuum drying, the combination of ethanol with ultrasound by using the ultrasonic bath (300 W/L, 20 kHz, up to 30 min in temperatures of 60, 70 and 80 °C) reduced drying time by 27% (60 °C), 31% (70 °C), and 22% at 80 °C. Moreover, the total free amino acid was significantly increased with Ethanol + US for 30 min and 60 °C. This treatment also preserved the carbohydrates, phenolics, free total amino acids, as well as carboxylic acid [9].
In the present study, treatment with Ethanol + US ultrasound (25.7 W/L, 25 kHz, up to 30 min) contributed significantly to the improvement of drying in carrots, but the main effect was attributed to ethanol. Therefore, it is clear that the effect of ultrasound is different in each food matrix. For this reason, analyses combining reactor properties (dimensions, power, frequency) and process conditions (power, time, quantities, temperature), must be studied in order to determine which is the best condition to favours the improvement of drying without compromise quality parameters.
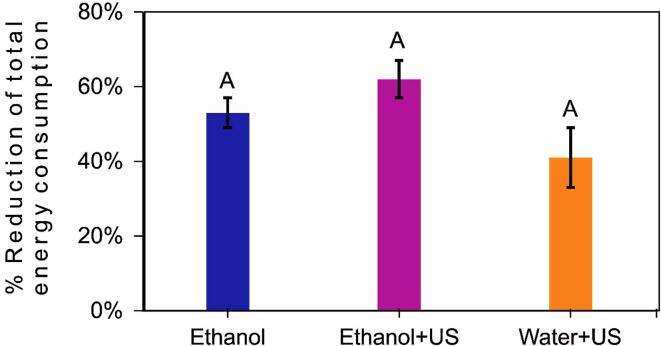
Moreover, it is important to highlight although the processing time reduction by itself is a very interesting result, this can also imply in reducing the energy consumption – which is relevant considering drying is a high-cost unit operation for the food industry, as this process consumes a lot of time and energy [56]. Therefore, the total energy consumed during pre-treatments and drying (until the final moisture of 20%w.b.) was estimated (Equation (4)). Fig. 8 shows the reduction (%) on the total energy consumption when pre-treatments were applied. Compared to Control, the energy was significantly reduced up to 53% ± 4% (Ethanol), 62% ± 5% (Ethanol + US) and 41% ± 8% (Water + US) for the applied pre-treatments. Non-significant differences were observed among pre-treatments (p > 0.05), once there were non-significant differences in the drying time reductions (Fig. 6). Even so, the values represent a relevant reduction. In addition, the energy consumption during pre-treatments that include US application, represent only 0.3% ± 0.1% of the energy consumed during drying, reflecting that indeed the drying process itself is a process of intensive energy consumption. Even so, considering that there were no differences between pre-treatments, only the use of Ethanol pre-treatment would already provide a significant energy improvement compared to the Control process. Therefore, considering all the limitations and simplifications of this approach of calculi, it contributes to demonstrating that the energy consumption with the proposed pre-treatments application could be compensated during drying, in a greater proportion.
Fig. 8.
Reduction (%) of the total energy consumed until reach final moisture of 20% w.b. for all pre-treatments regarding the Control treatment. Equal letters indicate non-significant differences (p > 0.05) by Tukey test.
However, we emphasize that the reduction in total energy consumption does not necessarily imply in a reduction of the total cost of production. The estimated costs depend on the socioeconomic and geographical contexts of each region. For example, energy, raw material, equipment, and ethanol costs vary widely, depending on that context. Therefore, each specific micro-context must be evaluated. However, the reduction in energy consumption is a desired result by itself, as it demonstrated scientific, social and environmental contributions.
Summarizing, the pre-treatments with Ethanol and Ethanol + US was more efficient in drying carrot slices, which was associated with structural changes in the parenchyma, the ethanol vapour pressure and the flux promoted by the Marangoni Effect. In fact, partial dehydration of the parenchyma with ethanol (Ethanol and Ethanol + US) was seen in Fig. 2, and greater shrinkage of the carrot slices was seen during these pre-treatments (Ethanol and Ethanol + US, Fig. 5A). Considering a target final moisture of 20% (wet basis, [17]), the evaluated pre-treatments reduced the drying time in 51 and 50% (Ethanol and Ethanol + US, respectively) while Water + US reduces 33%. In terms of energy consumption, all pre-treatments reduced the energy by 53% and 62% (Ethanol and Ethanol + US, respectively) while Water + US reduces 41%. Moreover, both pre-treatments and drying can affect quality parameters such as rehydration capacity and nutrient content. Therefore, it must also be evaluated, as described as follows.
3.3. Influence of pre-treatments on carrot quality: rehydration kinetics and carotenoid content
Drying is an operation that promotes several changes in food, including structural modifications and possible undesirable changes in nutritional value. Consequently, this section will discuss the obtained product quality, through both its rehydration behaviour and carotenoid content, always comparing the Control and pre-treated dried carrots.
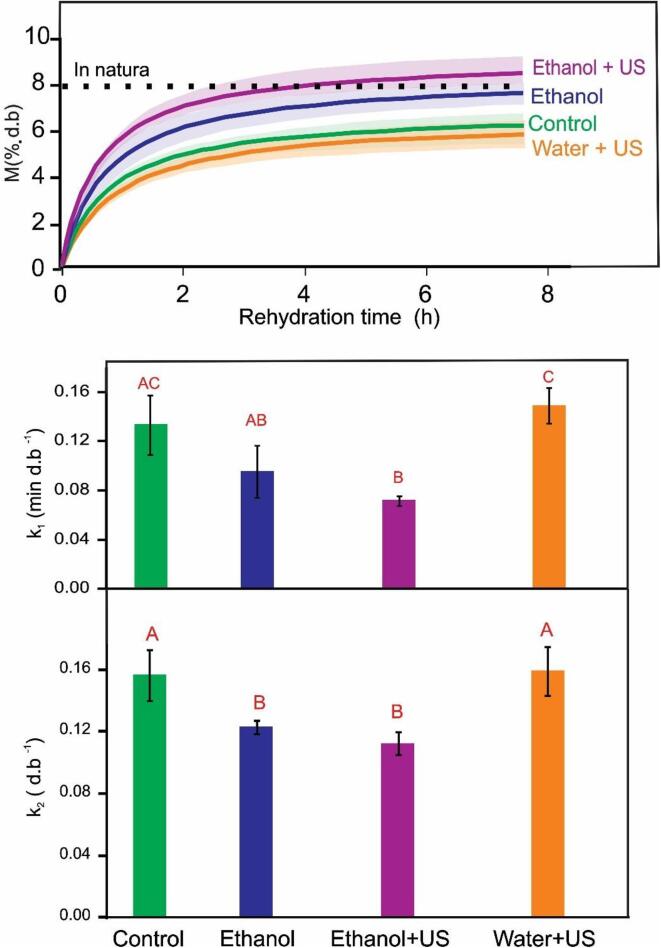
The results of rehydration kinetics are presented in Fig. 9, with the data adjusted to the Peleg Model (Equation (3)). The adjusted model allowed obtaining the parameters k1 and k2, which helps to describe the rehydration behaviour: the lower the value of k1, the higher the initial rate of rehydration, and the lower the value of k2, the higher is the equilibrium moisture content.
Fig. 9.
Rehydration kinetics of carrot slices with different pre-treatments (Control, Ethanol, Ethanol + US, Water + US). The curves are the data adjusted to the Peleg Model (Eq.3) and the shaded area indicates the confidence interval. Parameters k1 and k2 from Peleg Model. Vertical bars indicate the standard deviation. Different letters indicate statistically significant differences (p < 0.05) among treatments.
For parameter k1, no significant differences (p > 0.05) were found between Ethanol + US (0.0702 ± 0.2390 min d.b-1) and Ethanol (0.9540 ± 0.0209 min d.b-1) pre-treatments, while the Control (0.1327 ± 0.0239 min d.b-1) behaved similarly to the treatment with Ethanol and Water + US (0.1476 ± 0.0141 min d.b-1) (p > 0.05). For parameter k2, there were no significant differences (p > 0.05) between Ethanol (0.1229 ± 0.0042 d.b-1) and Ethanol + US (0.1124 ± 0.0074 d.b-1) treated carrots, but difference (p < 0.05) was achieved from Water + US treatment and Control. These results evidence the improvement of rehydration with Ethanol and Ethanol + US treatment with higher rehydration rate and equilibrium moisture. In fact, the Ethanol + US treated sample had water retention that exceeded the carrot original (in natura) moisture. The treatment with ethanol also presented a good capacity of incorporation of water reaching the same moisture content of the in natura carrot.
The improvement of rehydration with ethanol and/or ultrasound has been reported in other studies, such as Ethanol in pumpkin [13] and apple [51] ultrasound in carrot [23] and okra [57] and Ethanol + US in pumpkin [4]. In these works, the improvement was attributed to the structural changes in tissues and cells induced by pre-treatments, which facilitates the water transfer and/or retention. However, drastic structural changes can affect the quality of rehydration, such as after infrared drying of potatoes [52]: the Ethanol + US pre-treatment caused severe structural changes in the tissues and negatively impacted rehydration, which was slower than the Control treatment, and reaching a final moisture of only 74.6%w.b of the in natura vegetable. In that case, the main negative impact on rehydration was attributed to the application of high temperatures (80 °C) during infrared drying, that gelatinized the starch present in potato, causing a water migration resistance provided by the surface resistance of the crust (starch gelatinized). This reflects that the effectiveness of a pre-treatment improving rehydration also depends on the composition and structure of the raw material, in addition to the effects of the drying process.
On the other hand, as observed in Fig. 9, Control and Water + US have lost their rehydration capabilities. It is probably explained by the structural changes due to longer drying time in the case of the Control. In the case of Water + US samples, since during pre-treatment the water filled inside the cells causing their swelling, during drying that water had to leave the inside of the cells, then damaging the cell structure to a greater extent.
Summarizing, Control and Water + US treatments presented slower and smaller water absorption, which reinforces the negative structural modification due to drying. On the other hand, the pre-treatments with ethanol, with or without ultrasound, altered the carrot structure in a way that not only drying was improved, but they also avoid the negative aspects of drying in relation to rehydration. These results are important from both a perspective of application where the rehydration is necessary (such as in the formulation of soups, cakes and similar) or the description of structural modifications during pre-treatments and drying.
Carrots are rich in carotenoids, in special β-carotene [58] the natural pigment which give its intense orange colour. The carotenoid content varies according to the carrot cultivar, and its origin. In this work, in the in natura sample, a carotenoid content of 15.7 ± 1 mg/100 g of in natura sample was obtained, which was similar to the reported by Haque et al., [59] (10 mg/100 g of in natura sample), and Matějková; Petříková [60] (8.4 – 14.1 mg/100 g of in natura samples). Carotenoids are important components associated with human nutrition [61] and health benefits [62]. However, carotenoids are compounds whose stability to oxidation is low and they can be degraded due to moisture loss over the drying time in contact with oxygen [63]. Therefore, the drying process must maintain carotenoid concentration as high as possible.
In fact, different studies reported carotenoid degradation during drying of vegetables. Carotenoids degradation from 40% to 98.7% due to convective drying at 50–70 °C was reported in pumpkins [64] apricots [65] jackfruit bulb [66]. Considering carrot drying, [67] reported 17% degradation at 60 °C and 36% at 90 °C, while [12] reported degradation of 52.5% at 50 °C and 57.8% at 70 °C. However, convective drying conducted at 40 °C retained 92% of carrot carotenoids [68]. Based on this context, we selected 40 °C as processing temperature to avoid carotenoid degradation and maximizing the product quality.
Only one article reports the retention of carotenoid content with the application of pre-treatment with Ethanol and Ethanol + US: in the work of [4] pumpkin was convectively dried at 50 °C, with and without pre-treatments with Ethanol and Ethanol + US. The authors reported a 27% reduction in the carotenoid content in the Control treatment, while the pre-treatments could maintain the original carotenoid content.
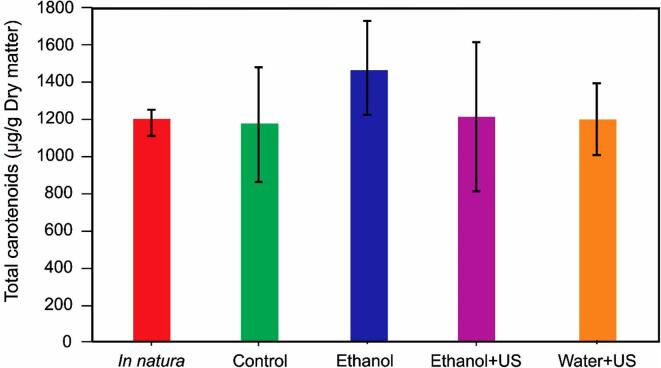
In the present study, there was no degradation of the total carotenoid content during drying for all treatments (Fig. 10, p > 0.05), which can be related with the drying temperature (40 °C). Therefore, under the conditions evaluated in this study, the treatments with Ethanol and Ethanol + US can help improve drying and rehydration without compromising the nutritional quality of the final product. It is worth mention this is an interesting result from both academic and industrial perspectives.
Fig. 10.
Total carotenoid content of the in-natura, and rehydrated samples (Control, Ethanol, Ethanol + US and Water + US). Vertical bars indicate the standard deviation. The carotenoids content of all treatments did not differ at p = 0.05.
3.4. Final consideration
This work shows a possibility of obtaining dried carrots quickly, with the same carotenoid content of fresh carrots and spent less energy by using ethanol and ultrasound as pre-treatment to drying. However, it is worth mentioning that, although the objective of our work has been accomplished, further aspects must be evaluated in future studies.
Therefore, we highlight this work opens the opportunity for future evaluations, considering different food products and processing conditions, and including:
-
•
Quantify the exact amount of ethanol and water after each pre-treatment and during the drying processing;
-
•
Quantify the residue of ethanol in different pre-treatments and drying conditions (we already started to develop this, demonstrating it is possible to achieve a negligible ethanol level in the dried product –[69]);
-
•
Evaluate the possibility of reusing ethanol, as well as different ethanolic solutions and also other compounds whose mechanisms and results can be similar or even better – what we call “drying accelerators”;
-
•
Expand and improve the energy consumption evaluation, considering further environmental analysis and Life Cycle Assessment (for example, as developed by Merone et al. [70]);
-
•
Applying ethanol at an industrial scale can be a challenge. Therefore, this theoretical basis serves as a support for future studies to evaluate the technical, operational, and industrial feasibility of applying ethanol, considering different aspects from costs, availability in each region and safety issues.
4. Conclusions
The use of ethanol and/or ultrasound was studied as pre-treatments to the convective drying of carrot slices. Both ethanol and ultrasound affected the parenchymatic cells, but the effect of ethanol was higher and mainly associated with the cell walls and membranes. The structural changes influenced the product texture (both cortex and core regions), shrinkage and improved mass transfer during both drying and rehydration. Ethanol and Ethanol + US pre-treatments reduced the drying time in ~50% when compared to the Control treatment, also increasing the water absorption and retention during rehydration. The energy consumption was reduced from 41–62% with pre-treatments application when compared to Control. Moreover, all the treatments could maintain the original carotenoid content. Therefore, treatments with Ethanol and Ethanol + US can be used to improve the drying process by convection of carrot slices without compromising the product's quality properties and also assist in reducing energy consumption.
CRediT authorship contribution statement
Karoline Costa Santos: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Data curation, Writing - original draft, Visualization. Jaqueline Souza Guedes: Methodology, Formal analysis, Investigation. Meliza Lindsay Rojas: Methodology, Formal analysis, Data curation, Writing - review & editing, Visualization. Gisandro Reis Carvalho: Methodology, Formal analysis, Visualization. Pedro Esteves Duarte Augusto: Conceptualization, Methodology, Formal analysis, Resources, Writing - review & editing, Supervision, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors are grateful to the São Paulo Research Foundation (FAPESP, Brazil) for funding the project n° 2019/05043-6 and the GR Carvalho post-doctoral fellowship (2018/17844-0); this study was financed in part by the “Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brazil (CAPES)” – Finance Code 001, through the KC Santos M.Sc. scholarship; to the National Council for Scientific and Technological Development (CNPq, Brazil) for the JS Guedes M.Sc. scholarship (131235/2020-6) and the productivity grant of PED Augusto (306557/2017-7); and to the Fondo Nacional de Desarrollo Científico, Tecnológico y de Innovación Tecnológica (FONDECYT, Peru) from the ‘‘Consejo Nacional de Ciencia, Tecnología e Innovación Tecnológica” (CONCYTEC, Peru) for funding the project n° 409-2019-FONDECYT.
References
- 1.M. Maleki, F. Shahidi, M.J. Varidi, E. Azarpazhooh, Hot air drying kinetics of novel functional carrot snack: Impregnated using polyphenolic rich osmotic solution with ultrasound pretreatment, J. Food Process Eng. (2019). doi: 10.1111/jfpe.13331.
- 2.Motevali A., Minaei S., Khoshtagaza M.H. Evaluation of energy consumption in different drying methods. Energy Convers. Manage. 2011;52(2):1192–1199. doi: 10.1016/J.ENCONMAN.2010.09.014. [DOI] [Google Scholar]
- 3.Llavata B., García-Pérez J.V., Simal S., Cárcel J.A. Innovative pre-treatments to enhance food drying: a current review. Curr. Opin. Food Sci. 2020;35:20–26. doi: 10.1016/j.cofs.2019.12.001. [DOI] [Google Scholar]
- 4.Rojas M.L., Silveira I., Augusto P.E.D. Ultrasound and ethanol pre-treatments to improve convective drying: drying, rehydration and carotenoid content of pumpkin. Food Bioprod. Process. 2020;119:20–30. doi: 10.1016/j.fbp.2019.10.008. [DOI] [Google Scholar]
- 5.J. Zubernik, M. Dadan, J. Cichowska, D. Witrowa-Rajchert, The impact of the pre-treatment in ethanol solution on the drying kinetics and selected properties of convective dried apples, Int. J. Food Eng. 20 (2019). doi: 10.1515/ijfe-2018-0338.
- 6.da Cunha R.M.C., Brandão S.C.R., de Medeiros R.A.B., da Silva Júnior E.V., Fernandes da Silva J.H., Azoubel P.M. Effect of ethanol pretreatment on melon convective drying. Food Chem. 2020;333:127502. doi: 10.1016/j.foodchem.2020.127502. [DOI] [PubMed] [Google Scholar]
- 7.Rojas M.L., Augusto P.E.D. Ethanol and ultrasound pre-treatments to improve infrared drying of potato slices. Innovative Food Sci. Emerg. Technol. 2018;49:65–75. doi: 10.1016/j.ifset.2018.08.005. [DOI] [Google Scholar]
- 8.Feng Y., Zhou C., ElGasim A., Yagoub A., Sun Y., Owusu-Ansah P., Yu X., Wang X., Xu X., Zhang J., Ren Z. Improvement of the catalytic infrared drying process and quality characteristics of the dried garlic slices by ultrasound-assisted alcohol pretreatment. LWT. 2019;116 doi: 10.1016/j.lwt.2019.108577. [DOI] [Google Scholar]
- 9.R. Amanor‐Atiemoh, C. Zhou, M. Abdullaleef Taiye, F. Sarpong, H. Wahia, A. Amoa‐Owusu, H. Ma, L. Chen, Effect of ultrasound‐ethanol pretreatment on drying kinetics, quality parameters, functional group, and amino acid profile of apple slices using pulsed vacuum drying, J. Food Process Eng. 43 (2020). doi: 10.1111/jfpe.13347.
- 10.Vinatoru M. Ultrasonically assisted extraction (UAE) of natural products some guidelines for good practice and reporting. Ultrason. Sonochem. 2015;25:94–95. doi: 10.1016/j.ultsonch.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Raso J., Mañas P., Pagán R., Sala F.J. Influence of different factors on the output power transferred into medium by ultrasound. Ultrason. Sonochem. 1999;5(4):157–162. doi: 10.1016/S1350-4177(98)00042-X. [DOI] [PubMed] [Google Scholar]
- 12.Md Saleh R., Kulig B., Hensel O., Sturm B. Investigation of dynamic quality changes and optimization of drying parameters of carrots (<scp> Daucus carota </scp> var. laguna) J. Food Process Eng. 2020;43 doi: 10.1111/jfpe.13314. [DOI] [Google Scholar]
- 13.Rojas M.L., Augusto P.E.D. Ethanol pre-treatment improves vegetable drying and rehydration: kinetics, mechanisms and impact on viscoelastic properties. J. Food Eng. 2018;233:17–27. doi: 10.1016/j.jfoodeng.2018.03.028. [DOI] [Google Scholar]
- 14.R. Simpson, C. Ramírez, H. Nuñez, A. Jaques, S. Almonacid, Understanding the success of Page’s model and related empirical equations in fitting experimental data of diffusion phenomena in food matrices, Elsevier, 2017. https://www.sciencedirect.com/science/article/pii/S0924224416304307 (accessed July 24, 2019).
- 15.Onwude D.I., Hashim N., Abdan K., Janius R., Chen G. The effectiveness of combined infrared and hot-air drying strategies for sweet potato. J. Food Eng. 2019;241:75–87. doi: 10.1016/j.jfoodeng.2018.08.008. [DOI] [Google Scholar]
- 17.Xiao Dong Chen, Kamlesh C. Patel, Biological Changes During food drying process, in: Dry. Tecnol. Food Process., Wiley Blackwell, 2008: pp. 90–109.
- 18.Potosí-Calvache D.C., Vanegas-Mahecha P., Martínez-Correa H.A. Secado convectivo de zapallo (Cucúrbita moschata): Influencia de la temperatura y velocidad de aire sobre la difusividad efectiva de humedad, contenido de carotenoides y fenoles totales. Dyna. 2017;84:112–119. doi: 10.15446/dyna.v84n202.63904. [DOI] [Google Scholar]
- 19.Evert Ray Franklin, Anatomia das Plantas de ESAU, 3rd ed., São Paulo, 2013.
- 20.Smith B.G., Ho C.A.L. Article 7 moisture. Int. J. Food Eng. 2007;3 doi: 10.2202/1556-3758.1242. [DOI] [Google Scholar]
- 21.Rojas M.L., Augusto P.E.D. Microstructure elements affect the mass transfer in foods: the case of convective drying and rehydration of pumpkin. LWT. 2018;93:102–108. doi: 10.1016/J.LWT.2018.03.031. [DOI] [Google Scholar]
- 22.Canteri M.H.G., Renard C.M.G.C., Le Bourvellec C., Bureau S. ATR-FTIR spectroscopy to determine cell wall composition: Application on a large diversity of fruits and vegetables. Carbohydr. Polym. 2019;212:186–196. doi: 10.1016/j.carbpol.2019.02.021. [DOI] [PubMed] [Google Scholar]
- 23.Wang L., Xu B., Wei B., Zeng R. Low frequency ultrasound pretreatment of carrot slices: effect on the moisture migration and quality attributes by intermediate-wave infrared radiation drying. Ultrason. Sonochem. 2018;40:619–628. doi: 10.1016/J.ULTSONCH.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 24.Miano A.C., Ibarz A., Augusto P.E.D. Mechanisms for improving mass transfer in food with ultrasound technology: Describing the phenomena in two model cases. Ultrason. Sonochem. 2016;29:413–419. doi: 10.1016/j.ultsonch.2015.10.020. [DOI] [PubMed] [Google Scholar]
- 25.Gamboa-Santos J., Montilla A., Cárcel J.A., Villamiel M., Garcia-Perez J.V. Air-borne ultrasound application in the convective drying of strawberry. J. Food Eng. 2014;128:132–139. doi: 10.1016/j.jfoodeng.2013.12.021. [DOI] [Google Scholar]
- 26.Ricce C., Rojas M.L., Miano A.C., Siche R., Augusto P.E.D. Ultrasound pre-treatment enhances the carrot drying and rehydration. Food Res. Int. 2016;89:701–708. doi: 10.1016/J.FOODRES.2016.09.030. [DOI] [PubMed] [Google Scholar]
- 27.Tao Y., Han M., Gao X., Han Y., Show P.-L., Liu C., Ye X., Xie G. Applications of water blanching, surface contacting ultrasound-assisted air drying, and their combination for dehydration of white cabbage: drying mechanism, bioactive profile, color and rehydration property. Ultrason. Sonochem. 2019;53:192–201. doi: 10.1016/j.ultsonch.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Miano A.C., Rojas M.L., Augusto P.E.D. Structural changes caused by ultrasound pretreatment: direct and indirect demonstration in potato cylinders. Ultrason. Sonochem. 2019;52:176–183. doi: 10.1016/j.ultsonch.2018.11.015. [DOI] [PubMed] [Google Scholar]
- 29.Fernandes F.A.N., Gallão M.I., Rodrigues S. Effect of osmotic dehydration and ultrasound pre-treatment on cell structure: melon dehydration. LWT – Food Sci. Technol. 2008;41(4):604–610. doi: 10.1016/j.lwt.2007.05.007. [DOI] [Google Scholar]
- 30.Carvalho G.R.d., Polachini T.C., Darros-Barbosa R., Bon J., Telis-Romero J. Effect of intermittent high-intensity sonication and temperature on barley steeping for malt production. J. Cereal Sci. 2018;82:138–145. doi: 10.1016/j.jcs.2018.06.005. [DOI] [Google Scholar]
- 31.Zdunek Artur, Umeda Mikio. Influence of cell size and cell wall volume fraction on failure properties of potato and carrot tissue. J. Texture Studies. 2005;36(1):25–43. doi: 10.1111/j.1745-4603.2005.00002.x. [DOI] [Google Scholar]
- 32.Aprajeeta J., Gopirajah R., Anandharamakrishnan C. Shrinkage and porosity effects on heat and mass transfer during potato drying. J. Food Eng. 2015;144:119–128. doi: 10.1016/j.jfoodeng.2014.08.004. [DOI] [Google Scholar]
- 33.Kadam S.U., Tiwari B.K., O’Donnell C.P., O’Donnell C.P. Effect of ultrasound pre-treatment on the drying kinetics of brown seaweed Ascophyllum nodosum. Ultrason. Sonochem. 2015;23:302–307. doi: 10.1016/j.ultsonch.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 34.Rodríguez Ó., Eim V., Rosselló C., Femenia A., Cárcel J.A., Simal S. Application of power ultrasound on the convective drying of fruits and vegetables: effects on quality: application of power ultrasound on convective drying. J. Sci. Food Agric. 2018;98(5):1660–1673. doi: 10.1002/jsfa.8673. [DOI] [PubMed] [Google Scholar]
- 35.Bozkir H., Rayman Ergün A., Tekgül Y., Baysal T. Ultrasound as pretreatment for drying garlic slices in microwave and convective dryer. Food Sci. Biotechnol. 2019;28(2):347–354. doi: 10.1007/s10068-018-0483-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.La Fuente C.I.A., Tadini C.C. Ultrasound pre-treatment prior to unripe banana air-drying: effect of the ultrasonic volumetric power on the kinetic parameters. J. Food Sci. Technol. 2018;55(12):5098–5105. doi: 10.1007/s13197-018-3450-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodríguez Ó., Gomes W., Rodrigues S., Fernandes F.A.N. Effect of acoustically assisted treatments on vitamins, antioxidant activity, organic acids and drying kinetics of pineapple. Ultrason. Sonochem. 2017;35:92–102. doi: 10.1016/j.ultsonch.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 38.Çakmak R.Ş., Tekeoğlu O., Bozkır H., Ergün A.R., Baysal T. Effects of electrical and sonication pretreatments on the drying rate and quality of mushrooms. LWT – Food Science and Technology. 2016;69:197–202. doi: 10.1016/j.lwt.2016.01.032. [DOI] [Google Scholar]
- 39.Nowacka M., Wiktor A., Śledź M., Jurek N., Witrowa-Rajchert D. Drying of ultrasound pretreated apple and its selected physical properties. J. Food Eng. 2012;113(3):427–433. doi: 10.1016/j.jfoodeng.2012.06.013. [DOI] [Google Scholar]
- 40.Fijalkowska A., Nowacka M., Wiktor A., Sledz M., Witrowa-Rajchert D. Ultrasound as a pretreatment method to improve drying kinetics and sensory properties of dried apple: ultrasound treatment before drying. J. Food Process. Eng. 2016;39(3):256–265. doi: 10.1111/jfpe.12217. [DOI] [Google Scholar]
- 41.Magalhães M.L., Cartaxo S.J.M., Gallão M.I., García-Pérez J.V., Cárcel J.A., Rodrigues S., Fernandes F.A.N. Drying intensification combining ultrasound pre-treatment and ultrasound-assisted air drying. J. Food Eng. 2017;215:72–77. doi: 10.1016/J.JFOODENG.2017.07.027. [DOI] [Google Scholar]
- 42.Huang C., Feng W., Xiong J., Wang T., Wang W., Wang C., Yang F. Impact of drying method on the nutritional value of the edible insect protein from black soldier fly (Hermetia illucens L.) larvae: amino acid composition, nutritional value evaluation, in vitro digestibility, and thermal properties. Eur. Food Res. Technol. 2019;245(1):11–21. doi: 10.1007/s00217-018-3136-y. [DOI] [Google Scholar]
- 43.J.A. Cárcel, J.V. García-Pérez, E. Riera, C. Rosselló, A. Mulet, Drying Assisted by Power Ultrasound, in: Mod. Dry. Technol., Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2014: pp. 237–278. doi: 10.1002/9783527631704.ch08.
- 44.Ozuna C., Gómez Álvarez-Arenas T., Riera E., Cárcel J.A., Garcia-Perez J.V. Influence of material structure on air-borne ultrasonic application in drying. Ultrason. Sonochem. 2014;21(3):1235–1243. doi: 10.1016/j.ultsonch.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 45.de la Fuente-Blanco S., Riera-Franco de Sarabia E., Acosta-Aparicio V.M., Blanco-Blanco A., Gallego-Juárez J.A. Food drying process by power ultrasound. Ultrasonics. 2006;44:e523–e527. doi: 10.1016/J.ULTRAS.2006.05.181. [DOI] [PubMed] [Google Scholar]
- 46.Siucińska K., Mieszczakowska-Frąc M., Połubok A., Konopacka D. Effects of ultrasound assistance on dehydration processes and bioactive component retention of osmo-dried sour cherries. J. Food Sci. 2016;81(7):C1654–C1661. doi: 10.1111/1750-3841.13368. [DOI] [PubMed] [Google Scholar]
- 47.Nowacka M., Wedzik M. Effect of ultrasound treatment on microstructure, colour and carotenoid content in fresh and dried carrot tissue. Appl. Acoust. 2016;103:163–171. doi: 10.1016/J.APACOUST.2015.06.011. [DOI] [Google Scholar]
- 48.Tatemoto Y., Mizukoshi R., Ehara W., Ishikawa E. Drying characteristics of food materials injected with organic solvents in a fluidized bed of inert particles under reduced pressure. J. Food Eng. 2015;158:80–85. doi: 10.1016/J.JFOODENG.2015.03.006. [DOI] [Google Scholar]
- 49.Corrêa J.L.G., Braga A.M.P., Hochheim M., Silva M.A. The influence of ethanol on the convective drying of unripe, ripe, and overripe bananas. Drying Technol. 2012;30(8):817–826. [Google Scholar]
- 50.Braga A.M.P., Pedroso M.P., Augusto F., Silva M.A. Volatiles identification in pineapple submitted to drying in an ethanolic atmosphere. Drying Technol. 2009;27(2):248–257. [Google Scholar]
- 51.Funebo T., Ahrne L., Prothon F., Kidman S., Langton M., Skjoldebrand C. Microwave and convective dehydration of ethanol treated and frozen apple - physical properties and drying kinetics. Int. J. Food Sci. Tech. 2002;37(6):603–614. [Google Scholar]
- 52.M.L. Rojas, I. Silveira, P.E.D. Augusto, Improving the infrared drying and rehydration of potato slices using simple approaches: Perforations and ethanol, J. Food Process Eng. (2019) e13089. doi: 10.1111/jfpe.13089.
- 53.Wang X., Feng Y., Zhou C., Sun Y., Wu B., Yagoub A.E.A., Aboagarib E.A.A. Effect of vacuum and ethanol pretreatment on infrared-hot air drying of scallion (Allium fistulosum) Food Chem. 2019;295:432–440. doi: 10.1016/j.foodchem.2019.05.145. [DOI] [PubMed] [Google Scholar]
- 54.A. Streitwiser, C.H. Heathcock, Introduction to organic chemistry, New York, 1976.
- 55.M.A. Silva, A.M.P. Braga, P.H.S. Santos, Enhancement of fruit drying: the ethanol effect, Proc. 18th Int. Dry. Symp. (IDS 2012). (2012) 11–15.
- 56.Nieto Calvache J.E., Fissore E.N., Latorre M.E., Soria M., De Escalada Pla M.F., Gerschenson L.N. Obtention of dietary fibre enriched fractions from peach bagasse using ethanol pre-treatment and microwave drying. LWT – Food Sci. Technol. 2015;62(2):1169–1176. doi: 10.1016/J.LWT.2015.01.045. [DOI] [Google Scholar]
- 57.Tüfekçi S., Özkal S.G. Enhancement of drying and rehydration characteristics of okra by ultrasound pre-treatment application. Heat Mass Transfer. 2017;53(7):2279–2286. doi: 10.1007/s00231-017-1983-x. [DOI] [Google Scholar]
- 58.Yara-Varón E., Fabiano-Tixier A.S., Balcells M., Canela-Garayoa R., Bily A., Chemat F. Is it possible to substitute hexane with green solvents for extraction of carotenoids? A theoretical versus experimental solubility study. RSC Adv. 2016;6(33):27750–27759. doi: 10.1039/c6ra03016e. [DOI] [Google Scholar]
- 59.M. Haque, M. Jilhaz, M. Maruf Hasan, M. Eshak Enan, S. Nazrul Islam, Estimation and comparison of total carotenoid contents in ethnic vegetables, fruits and some common non leafy vegetables of Bangladesh, ~ 5 ~ Int. J. Pharmacogn. Life Sci. 1 (2020). doi: 10.33545/27072827.2020.v1.i1a.2.
- 60.Matějková J., Petříková K. Variation in content of carotenoids and vitamin C in carrots. Not. Sci. Biol. 2010;2:88–91. doi: 10.15835/nsb245108. [DOI] [Google Scholar]
- 61.Rodriguez-Amaya D.B. Update on natural food pigments – a mini-review on carotenoids, anthocyanins, and betalains. Food Res. Int. 2019;124:200–205. doi: 10.1016/j.foodres.2018.05.028. [DOI] [PubMed] [Google Scholar]
- 62.Eggersdorfer M., Wyss A. Carotenoids in human nutrition and health. Arch. Biochem. Biophys. 2018;652:18–26. doi: 10.1016/j.abb.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 63.Catherine Nicolle, Gerad Simon, Emond Rock, Pierre Amouroux, Cristian Rémésy, Genetic Variability influences carotenoid, vitamin, phenolic, and mineral content in whitem yellow, purple, orage, and dark-orange carrot cultivars, Am. Soc. Hortic. Sci. 129 (2004) 523–529. doi: 10.21273/JASHS.129.4.0523.
- 64.Song J., Wei Q., Wang X., Li D., Liu C., Zhang M., Meng L. Degradation of carotenoids in dehydrated pumpkins as affected by different storage conditions. Food Res. Int. 2018;107:130–136. doi: 10.1016/j.foodres.2018.02.024. [DOI] [PubMed] [Google Scholar]
- 65.Fratianni A., Niro S., Messia M.C., Cinquanta L., Panfili G., Albanese D., Di Matteo M. Kinetics of carotenoids degradation and furosine formation in dried apricots (Prunus armeniaca L.) Food Res. Int. 2017;99:862–867. doi: 10.1016/j.foodres.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 66.Saxena A., Maity T., Raju P.S., Bawa A.S. Degradation kinetics of colour and total carotenoids in jackfruit (Artocarpus heterophyllus) bulb slices during hot air drying. Food Bioprocess Technol. 2012;5(2):672–679. doi: 10.1007/s11947-010-0409-2. [DOI] [Google Scholar]
- 67.Zielinska M., Markowski M. Color characteristics of carrots: effect of drying and rehydration. Int. J. Food Prop. 2012;15(2):450–466. [Google Scholar]
- 68.J. Frias, E. Peñas, P. Peñas, M. Mó, M. Ullate, C. Concepció, C. Vidal-Valverde, Influence of Drying by Convective Air Dryer or Power Ultrasound on the Vitamin C and β-Carotene Content of Carrots, J. Agric. Food Chem. 58 (2010) 10539–10544. doi: 10.1021/jf102797y. [DOI] [PubMed]
- 69.G.R. Carvalho, K.C. Santos, A.P. Massarioli, P.E.D. Augusto, Residue of ethanol in pineapple dried using ethanol and ultrasound as pre-treatments., in: Galoá (Ed.), 13o Lat. Am. Symp. Food Sci., Capinas, 2019. https://proceedings.science/slaca/slaca-2019/papers/residue-of-ethanol-in-pineapple-dried-using-ethanol-and-ultrasound-as-pre-treatments.
- 70.Merone D., Colucci D., Fissore D., Sanjuan N., Carcel J.A. Energy and environmental analysis of ultrasound-assisted atmospheric freeze-drying of food. J. Food Eng. 2020;283:110031. doi: 10.1016/j.jfoodeng.2020.110031. [DOI] [Google Scholar]