Highlights
-
•
Ultrasonic-assisted thawing was applied to the thawing process of frozen white yak meat.
-
•
Ultrasound-assisted thawing can shorten the thawing time of frozen white yak meat.
-
•
Ultrasound-assisted thawing retains the qualities and nutritionals of white yak meat.
-
•
The 400 W is the appropriate ultrasonic power for thawing frozen white yak meat.
Keywords: White yak meat, Ultrasonic-assisted thawing, Thawing rate, Physicochemical quality, Microstructure
Abstract
The objective of this study was to assess the effects of ultrasound-assisted thawing (UAT) on the quality of longissimus dorsi muscles from white yak meat (WYM). Ultrasonic power levels of 0, 200, 400, and 600 W (frequency of 20 kHz) were used to assist thawing. The thawing rate, meat quality, nutrient substances, volatile compounds, and microstructure of the WYM were determined. The results showed that ultrasonic thawing treatment reduced thawing times by 30.95–64.28% compared to control. The meat quality results revealed that the thawing loss, cooking loss, L* and b* values, and pH values decreased significantly while the a* value and cutting force increased significantly (P < 0.05) at the lower 400 W power level compared with the control. In addition, the free amino acid (FAA), mineral, and vitamin (especially water-soluble vitamins) contents were significantly (P < 0.05) increased with the ultrasound treatment. UAT significantly (P < 0.05) increased the content of volatile compounds, an effect that was highest in the UAT-400 W group. Partial least squares discrimination analysis (PLS-DA) showed that 2,4-heptadienal was critical in distinguishing the UAT groups from the control. When the ultrasonic power was lower than 400 W, the muscle cell area was significantly (P < 0.05) increased but decreased when higher power was used. Therefore, UAT improves the thawing efficiency and quality of frozen WYM, particularly at a power level of 400 W, and these findings have potential applications in the meat industry.
1. Introduction
The white yak is a scarce and valuable livestock animal in the word which inhabits mainly Tianzhu County (Gansu, China; N36o58́, E103o08́; altitude:2393.69 m) [1]. With the improvement of artificial breeding technology, the number of white yak has increased to about 300 000 [2]. The white yak’s habitat is characterized by low temperatures and low levels of oxygen, as well as high solar radiation, which confer unique qualities on white yak meat (WYM), including a high protein content and unique flavor. WYM is considered a natural green food because the yak’s habitat is minimally affected by humans. In addition, WYM has numerous health benefits, including the enhancement of disease resistance, cell viability, and organ function [3].
White yak are generally slaughtered from the end of September to early November each year according to the local custom, and the WYM is usually frozen to prolong storage time and provide a year-round supply [4]. Because of this, thawing is necessary before using the meat. Many studies have reported that inappropriate thawing methods and conditions can lead to deterioration in the quality of the meat, including discoloration, poor flavor, and loss of nutrients due to potential physical and chemical changes [5], [6]. Hence, it is important to explore and optimize suitable thawing methods and conditions for the meat. Air- and water-thawing are conventionally used for thawing. However, not only are these methods inefficient, but the meat quality may undergo significant deterioration during the thawing process [6]. In recent years, novel thawing methods, such as ultra-high-pressure, microwave, and ultrasound have been adopted [7], [8], [9]. Ultra-high-pressure thawing has the advantages of reducing the thawing time and inhibiting microbial growth, but the method is relatively expensive [10]. Microwave thawing tends to cause uneven heating and hence difficulty in controlling the quality of the product. Moreover, microwave thawing cannot accommodate metal packaging [11]. These factors limit the general use of these methods.
Ultrasound is an environmentally friendly technique that has been widely used in the extraction of natural active substances and food analysis [12]. In recent years, ultrasound-assisted thawing (UAT) technology has achieved excellent results in shortening the thawing time and reducing the deterioration of meat quality [13]. In ultrasound-assisted thawing, part of the energy is converted into heat within the frozen meat, resulting in increased temperature at the meat surface while the temperature increase of the core remains lower [6]. Therefore, the thawed/frozen boundary can absorb more energy, and the high-speed jets caused by the ultrasonic cavitation effect will cause the asymmetric collapse of the bubble and micro-streaming, which can increase the efficiency of heat transfer [14]. Some studies have reported that UAT can significantly improve the thawing efficiency of many different types of food, compared to water-thawing. For example, the thawing times of frozen mango pulp [11], prok [6], and chicken breasts [10] were decreased by 73%, 80%, and 71%, respectively. While UAT can improve the efficiency of thawing, its influence on the quality of the thawed meat is two-sided [15]. The use of ultrasound with appropriate power results in the formation of micropores in the meat, which can increase the tenderness of the meat [16]. The gelling properties and water retention of the protein will also be improved [17] and the content of substances contributing to flavor will also increase due to lipid oxidation caused by free radicals [18]. However, high-power ultrasound may also have disadvantages, such as cell disruption, endogenous enzyme deactivation due to structural changes, and degradation of pigments [19], [20]. In addition, these impacts of ultrasound on the meat quality during thawing may vary due to the myofibril condition and composition of the meat [21]. As far as we know, the effects of UAT on the quality of WYM have not been reported. Therefore, the purpose of the study was to explore the effects of UAT on thawing time, meat quality (water loss, color, cutting force, pH), nutritional properties (free amino acids, vitamins, and minerals) and flavor ingredients of WYM.
2. Materials and methods
2.1. Materials
Ten white yaks (about 3 years of age) from the Tianrun White Yak Green Food Co., Ltd. (Tianzhu, China) were slaughtered by the standard procedure (GB/T19477-2018). To avoid microbial contamination during transport, the longissimus dorsi muscles were cut into pieces (10 × 10 × 5 cm, about 600 g) and vacuum-packed in polyethylene bags after cattle carcass aging for 72 h at 4 ℃. The samples were kept in cold-chain shipping boxes and transported to the laboratory within 3 h. Both the polyethylene bags and cold-chain shipping boxes were meticulously cleaned with 75% alcohol before use. A total of 60 chops were divided randomly into four groups. Meat pieces were packaged in polyethylene bags and frozen at −20 °C for 21 days [22].
Vitamin standards were obtained from Sigma-Aldrich Company (Shanghai, China). All other chemicals (analytical grade) were purchased from Jiancheng Technology Co., Ltd. (Nanjing, China).
2.2. Thawing process
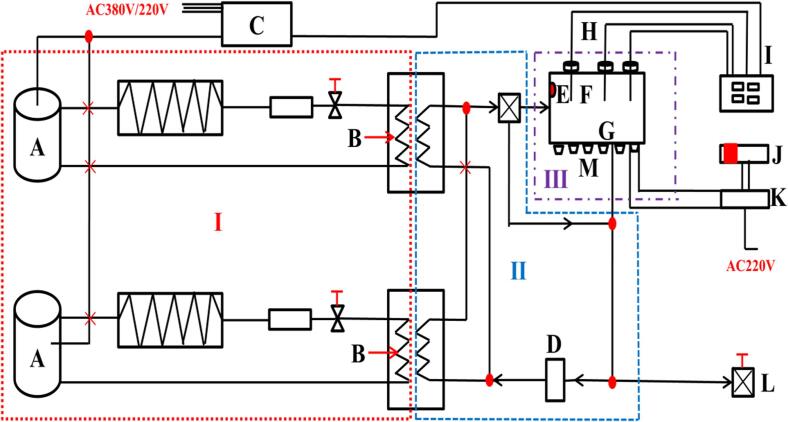
The frozen samples were thawed in an ultrasonic freezer (5-50PH, Ningbo Xinzhi Biotechnology Co., Ltd., Nanjing, China). Samples thawed in water at 25 ± 1 ℃ were used as controls. UAT-thawed samples were thawed at 200 W (UAT-200 W group), 400 W (UAT-400 W group), and 600 W (UAT-600 W group) in a water bath at 25 ± 1 °C with no pulse-off (frequency of 20 kHz). The schematic diagram of the ultrasound-assisted thawing device is shown in Fig. 1. The thawed samples were kept at 4 °C until analysis of indicators. All measurements of indicators were completed within three hours. Three independent tests were executed for each indicator determination.
Fig. 1.
Schematic diagram of the device of ultrasound-assisted thawing. A, compressor; B, plate heat exchanger; C, control panel; D, pump; E, coolant circulation inlet; F, ultrasonic thawing tank; G, coolant circulation outlet; H, thermocouple; I, temperature measuring system; J, ultrasonic controller; K, ultrasonic generator; L, air-vent; M, ultrasonic transducer.
2.3. Thawing curve
The probe of the thawing curve test system (Fig. 1 I) was inserted in the geometric center of the samples. The temperatures of the samples were recorded every 10 s.
2.4. Thawing loss and cooking loss
The thawing loss was measured according to the method of Sun et al.[23]. The samples were thawed until the temperature of the sample center reached 4 ℃ and were dried with filter paper to remove the surface water. The samples were weighed before (m0) and after (m1) thawing on a Secura electronic balance (M20S, Sartorius, Berlin, Germany). The formula for thawing loss is as follows:
The cooking loss was measured by the method of Zhang, Haili, Chen, Xia and Kong [16]. Thawed samples were heated in a plastic bag in an electro-thermostatic water bath until the central temperature reached 75 ℃. Samples were then immediately removed from the bag, cooled to below room temperature, and wiped dry with filter paper. The samples before (M0) and after (M1) cooking were accurately weighed. The formula for cooking loss is as follows:
2.5. Cutting force
Samples were cut into rectangular columns (1.5 × 1.5 × 5 cm). The cutting force was measured with a texture analyzer (TA.XT Plus, Isenso, Atlanta, USA). The compression distance was 60% and the load cell was 50 kg. Three samples were measured for each treatment.
2.6. pH and color
The pH of the samples was measured following the method of Gambuteanu and Alexe [6]. Briefly, 1 g of thawed white yak beef was mixed with 9 mL deionized water, homogenized with a homogenizer (B15, ATS, Toronto, Canada) and then filtered through filter paper (60 g/min, Double circle, Hangzhou, China). The handheld pH meter (PB-10, Sartorius, Berlin, Germany) was calibrated according to the supplier’s instructions. Standard solutions of pH7 and pH4 were used to calibrate the pH meter the first and second times after preheating for 30 min, respectively.
The color of the samples was determined by the method of Aminzare et al. [24]. The color parameters were measured using a chromameter (CR-410, Konica Minolta, Tokyo, Japan) at three different points of the sample surface using the Clelab system and color changes were described using L* (lightness), a* (redness), and b* (yellowness) values. The chromameter was calibrated with a white ceramic plate prior to use. Three samples were measured for each treatment.
2.7. Thiobarbital reaction materials (TBARS) and total volatile basic nitrogen (TVB-N)
The method used to determine the TBARS followed that described by Li et al. [22] with minor modifications. A total of 5 g meat sample was placed in a 100 mL test tube containing 20 mL of deionized distilled water and homogenized at 4000 rpm for 10 s using a homogenizer. A total of 1 mL of homogenate was transferred into a test tube containing butylated hydroxytoluene (BHT) (7.2%, 50 μL) and trichloroacetic acid (TCA) (15%, 2 mL) and boiled in water for 20 min. Afterward, the homogenate was cooled in water for 10 min. After centrifugation at 2000 g for 10 min, the absorbance of the supernatant at 532 nm was measured using a spectrophotometer (UV-756P, Shimadzu, Kyoto, Japan).
The TVB-N was measured as described by Aminzare et al. [24] Approximately 5 g meat was dispersed in 50 mL deionized water and stirred for 30 min before filtering. Boric acid (10 mL) and mixed indicator liquid (5 mL) were then added to the distillation condensing tube and distilled for 10 min. The mixed solution was titrated with HCl (0.01 mol/L) until turned to blue-purple. The formula of TVB-N is as follows:
where V1 is the titration volume of HCl in the sample, and V2 is the titration volume of HCl in the blank.
2.8. Free amino acids (FAA), minerals, and vitamins
The FAA of the samples were analyzed according to the method of Geng et al.[25]. In brief, 5 g of sample was homogenized in cold ion-exchanged water. The homogenate was centrifuged at 8000×g for 10 min before addition of 5 mL TCA (10%, w/w), followed by five washes with TCA (5%, w/w). The cleaning mixture was filtered through membrane (0.45 μm) and the filtrate was analyzed using an automatic amino acid analyzer (S433D, Sykam, Bavaria, Germany) at 39 °C equipped with a Shim-pack Amino-Li column (Shimadzu Corporation, Kyoto, Japan).
The mineral content of the samples was measured using the method of Hopcroft, Cowieson, Muir, & Groves [26]. Briefly, 1 g of sample was homogenized in a tissue grinding tube (YJQ, Solarbio, Beijing, China) and then freeze-dried. The powdered sample was digested in a digestion vessel containing 10 mL nitric acid and heated in a digestion furnace (KDN-04A, Xinrui, Shanghai, China). The heating process was divided into four phases: (1) the temperature was increased from room temperature to 90 ℃ at a rate of 2 ℃/min, (2) hydrogen peroxide was added to the digestion vessel after cooling to room temperature, (3) the temperature was raised to 120 ℃ at a rate of 5 ℃/min, (4) these conditions were maintained for 30 min. The sample was then transferred to a volumetric flask and diluted to 50 mL with deionized water. The samples were analyzed by a flame atomic absorption spectrophotometer (ICE-3000, Thermo Fisher Scientific, Massachusetts, USA).
The fat-soluble vitamin (Vitamin A and Vitamin E) content was measured using the method of GB 5009.154-2016. The samples were accurately weighed to 3 ± 0.01 g and added to a round flask containing 20 mL of warm water (50 ℃), following which the ascorbic acid (1 g), BHT (0.1 g), absolute ethanol (30 mL), and potassium hydroxide (15 mL, 0.2 mol/L) were added while shaking in a water bath constant temperature shaker for 30 min at 80 ℃. Vitamin B1, Vitamin B2, Vitamin B3, Vitamin B4, and Vitamin B5 contents were analyzed using the method of GB 5009.82–2016. Samples (5 ± 0.01 g) were added to a 150 mL conical flask containing 50 mL distilled water. The pH of the solution was adjusted to 1.7 ± 0.1 with hydrochloric acid (1 mol/L) then allowed to stand for 60 s. The conical flask was placed in an ultrasonic cleaner for 10 min at 10 W. All samples were then filtered with filter paper followed by secondary filtration with a microporous filtering film (0.45 μm, 565245, Dacheng, Shanghai, China). High-performance liquid chromatography (HPLC, E2695, Waters, Massachusetts, USA) equipped with a diode array detector was used to determine the vitamin content of the samples. A C30 column (250 × 4.6 mm, 3.0 µm particle size) was used at a temperature of 30 ℃ for HPLC measurement of the fat-soluble vitamins. Two solvents were used as mobile phases: water (solvent A) and carbinol (solvent B). A gradient-elution was applied as follows: 4% A, 0–20 min 96% B, 20–24 min 100% B, 24–30 min 96% B. The injection volume was 10 µL and the flow rate was 0.8 mL/min.
The C30 column was replaced by a C18 column (150 × 4.6 mm, 5.0 µm particle size) for measurement of the water-soluble vitamins. Fifty milliliters of carbinol, 2.0 g 1-octanesulfonic acid sodium, and 2.5 mL triethylamine were added to 1000 mL distilled water and the pH was adjusted to 3.0 ± 0.1 with glacial acetic acid. The mixture was filtered using microporous filtering film (0.45 μm) before use as the mobile phase. The injection volume was 10 µL and the flow rate was 1 mL/min.
A wavelength scanning range from 200 to 500 nm was used. The vitamins were identified by retention time and UV spectra in comparison with the vitamin standards under the same chromatographic conditions. The contents of vitamins were quantified using calibration curves at different wavelengths (Vitamin A: 325 nm; Vitamin B1: 365 nm; Vitamin B2: 462 nm; Vitamin B3: 261 nm; Vitamin B5: 294 nm; Vitamin B6: 395 nm; Vitamin B12: 365 nm; Vitamin E: 294 nm).
2.9. Volatile compounds
Ten grams of each sample, 2 g NaCl, and 5 μL 0.1% v/v (cyclohexanone/methanol) cyclohexanone solution were placed in a 30 mL headspace bottle. The bottle was sealed with parafilm (PM996, Parafilm, Detroit, USA) and heated in an electro-thermostatic water bath (HH-US, Spring Instrument Co., Ltb., Jintan, China) at 40 ℃ for 30 min. A solid-phase microextraction system (Supelco, Bellefonte, PA, USA) containing fused-silica fiber (10 mm length) coated with a 50/30 mm thickness of DVB/CAR/PDMS (divinylbenzene/carboxen/polydimethylsiloxane) was used to collect the volatile compounds of the samples. For quantification of the volatile compounds, an SPME device was inserted into an inlet of a Gas Chromatography-Mass Spectrometer (GC–MS) equipped with an HP-5MS column (30 m × 0.25 mm, film thickness, 0.25 μm). The GC oven temperature was increased from 45 °C to 250 °C at 15 °C/min and then held for 15 min. Mass spectra were obtained by electron ionization (EI) at 70 eV with a scan range of 10–550 mass units. The compounds were identified using the National Institute of Standards and Technology (NIST) and Kovats indices. The compounds were quantified from the ratio of peak area of unknown to the internal standard.
2.10. Microstructure
The organizational structure of the samples was measured with the method of Cardiff and Borowsky [27]. Three grams of sample were soaked in 100 mL of paraformaldehyde (4%, w/w) for 48 h to fix the muscle fibers. The fixed samples were placed in a tissue embedding box (YA0890, Beijing, Solarbio, China) and washed under running water for 30 min. Alcohol (75%, w/w) was used to remove the moisture of the sample. Samples were then bathed with xylene until transparent. The transparent tissue blocks were placed in the melted paraffin until the paraffin was completely immersed in the tissue block. The paraffin blocks were cut into thin slices with thicknesses of 5–8 μm. After removal of the paraffin with xylene, the muscle fibers were stained with hematoxylin solution (10%, w/w) for 1 min at 60 ℃, rinsed under running water for 1 h and dehydrated with absolute ethyl alcohol for 10 min. The muscle fibers were then stained with eosin solution (10%, w/w) for 5 min, dehydrated and decolorized with absolute ethyl alcohol and xylene, respectively. After the tissues were fixed on the slide, an Olympus IX optical microscope (7L, Nikon, Tokyo, Japan) was used at 100x magnification to photograph the changes in tissue structure. The photographs were analyzed with Image-Pro Plus version 6.0. to measure the area and spacing of the muscle fibers.
2.11. Statistical analysis
Results were expressed as means ± standard error. The statistical analysis of the results was performed using an analysis of variance (ANOVA) method and a Duncan multiple range test at a 5% significance level, using the Statistical Product and Service Solutions (SPSS) package, version 24.0. (SPSS Inc., Chicago, IL, USA). The GC–MS data variables were normalized using Metaboanalyst version 4.0 (www.metaboanalyst.ca) by mean-centering and divided by the standard deviation of each variable before developing heatmaps and partial least squares-discriminant analysis (PLS-DA) models. PLS-DA was used for a supervised analysis. All of the different metabolites were identified and characterized using the loading plots and VIP value (cut-off > 1) from the PLS-DA analysis.
3. Results and discussion
3.1. Effect of the UAT on the thawing rate of WYM
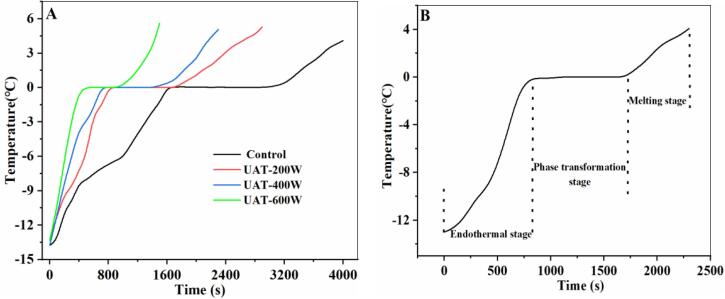
To grasp the characteristics of the thawing process, a temperature curve is necessary. The thawing curves of WYM are shown in Fig. 2. The thawing curves of WYM were divided into three stages: an endothermal stage (-13.5–0 ℃), a phase transformation stage (0–0.5 ℃), and a melting stage (0.5–4 ℃). The times of the three stages for the UAT groups were significantly (P < 0.05) lower than those of the control. The shortest thawing time was with UAT-600 W which was 64.28% lower than that of the control, followed by UAT-400 W and UAT-200 W. This means that UAT is helpful for shortening the WYM thawing time. This may be caused by cavitation where tiny holes appear in the structure of the frozen WYM when the ultrasound power reaches a certain level [28]. Thawed liquid can also generate microstreaming because of the ultrasound-induced cavitation, which assists with heat transmission and the dissolution of ice crystals [29]. Furthermore, turbulence is generated when ultrasonic waves disseminate through the thawed liquid, which can improve the heat transmission efficiency and reduce food damage [30]. Our results are supported by several previous studies. For example, the thawing time for frozen blocks of pacific cod decreased by 70% when the ultrasound power was 0.005 W/g, compared to thawing in water [17]. Similarly, when ultrasound (600 W) was used to thaw of pork meat, the thawing rate was improved by 0.84 °C/min [6].
Fig. 2.
Thawing curves of frozen WYM with different thawing treatments. UAT, ultrasound-assisted immersion thawing at different ultrasound powers (200, 400 and 600 W).
3.2. Effect of the UAT on thawing loss and cooking loss in WYM
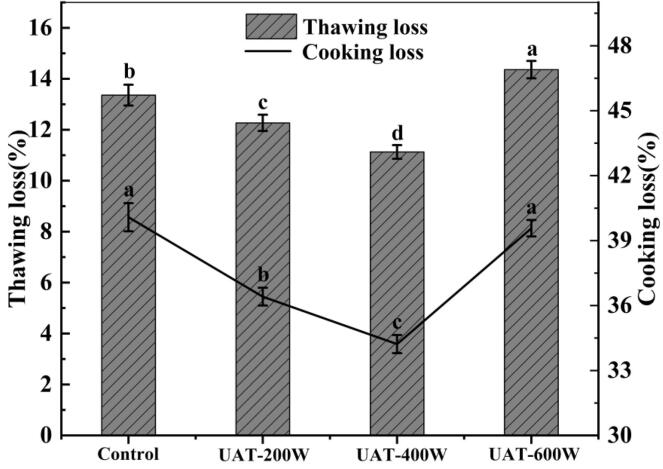
Thawing loss and cooking loss are critical factors influencing the quality of frozen meat [16]. The thawing loss and cooking loss of WYM are shown in Fig. 3. These values were lower in meat thawed at the 400 W ultrasound level but increased rapidly when the ultrasonic power was raised from 400 W to 600 W. The UAT-400 W sample had the least thawing loss and cooking loss, which were 29.02% and 33.22% lower than the control, respectively. The thawing time, ice crystal measurement and position, reabsorption of water, the integrity of muscle tissue, the condition of muscle protein, and the water retention capacity are the factors responsible for water loss during food thawing using ultrasound [23]. Myosin constitutes the largest proportion of WYM protein and contains a rich helical structure [3]. Ultrasound treatment has been shown to improve the structural characteristics of myosin, increasing the content of sulfhydryl groups and the transformation of α-helices to looser β-sheet structures, which may decrease dense aggregation and improve the water retention capacity of muscle proteins [8]. However, the myofibril structure is likely to be badly damaged at 600 W, causing a massive loss of water in myofibroblasts during thawing and cooking. A previous study found similar phenomena of thawing loss and cooking loss in the thawing of chicken breast meat [31].
Fig. 3.
Thawing loss and cooking loss of frozen WYM with different thawing treatments. UAT, ultrasound-assisted immersion thawing at different ultrasound powers (200, 400 and 600 W).
3.3. Effect of the UAT on the color, pH, and cutting force of WYM
Color is an important component of meat quality that also influences the consumers' willingness to buy meat [20]. The L*, a*, and b* values of WYM were determined to estimate changes in the color of these groups. The control and UAT-600 W samples had the highest L* and b* values, which were higher than those of the UAT-400 sample by about 17% and 27%, respectively. The sample treated with UAT-400 W had the highest a* value which was higher than the control by about 14.31%. The L* intensification in meat after thawing was detrimental because it was associated with free water spreading to the surface and reflecting more light [32]. There are two potential mechanisms for UAT thawing inducing a* value changes in meat: (1) the production of free radicals promotes oxidation leading to instability of heme pigments; (2) the chemical structures of the color pigments of myoglobin and hemoglobin may be changed due to the thermal and acoustic effects of the ultrasound [33]. Moreover, it is interesting that a recent research report shows that the formation of extracellular space may be the major reason for the increased L* and decreased a* value [34]. This finding is consistent with the results of color and microstructure observed in our experiments. Furthermore, lipid oxidation is the main reason for the increase in the b* value of meat [35] and the degree of lipid oxidation of different groups (Table 1) appears to be related to the b* value. Stadnik and Dolatowski [34] studied the effect of different ultrasound intensities on the color of beef and found a similar phenomenon.
Table 1.
Change in color, pH, cutting force, thiobarbital reaction materials (TBARS), and total volatile basic nitrogen (TVB-N) induced by ultrasonic powera, b.
| Samples | Colour |
pH | Cutting force (N) | TBARS (mg/kg) | TVB-N (mg/100 g) | ||
|---|---|---|---|---|---|---|---|
| L* | a* | b* | |||||
| Control | 29.51 ± 0.74a | 15.93 ± 0.48c | 3.77 ± 0.04a | 6.97 ± 0.08a | 35.47 ± 1.17d | 0.57 ± 0.05b | 10.42 ± 0.31b |
| UAT-200 W | 27.94 ± 0.12b | 17.36 ± 0.52b | 3.42 ± 0.09b | 6.35 ± 0.06b | 31.61 ± 0.90b | 0.46 ± 0.10c | 8.47 ± 0.33c |
| UAT-400 W | 25.27 ± 0.37c | 18.21 ± 0.28a | 3.01 ± 0.04c | 6.31 ± 0.07b | 33.83 ± 1.02c | 0.61 ± 0.08b | 6.69 ± 0.24d |
| UAT-600 W | 29.66 ± 0.24a | 15.97 ± 0.31c | 3.84 ± 0.06a | 6.84 ± 0.09a | 39.59 ± 1.06a | 0.79 ± 0.06a | 11.65 ± 0.15a |
UAT: ultrasonic-assisted thawing.
Values followed by different small letters within the same column denote significant differences (P < 0.05).
The pH value can influence the color, cooking loss, and the cutting force of the meat [24]. As shown in Table 1, the pH at UAT-400 W was significantly (P < 0.05) lower than that of UAT-600 W and the control. The TVB-N of the different groups are shown in Table 1, and the TVB-N results showed that the change of pH in different groups might relate to the degradation of nitrogenous substances during the thawing process of frozen WYM [36]. Moreover, the pH may also be the main factor causing the changes in thawing loss and cooking loss, which is closely related to the water retention capacity of the meat protein [37].
Tenderness is an important quality attribute of meat and cutting force is a commonly used index to evaluate meat tenderness. Table 1 shows that the UAT-400 W group presented a lower cutting force (31.83 ± 1.02 N), which indicated that UAT had a notable impact on the meat tenderness. The cutting force of meat is closely related to the moisture content and myofibrillar structure [38]. A suitable ultrasonic power might modify the structure of the meat and reduce the cutting force [32]. In addition, ultrasonic treatment increases the solubility of the meat protein due to the destruction of lysosomes which release tenderizing enzymes, which could increase the moisture content in the meat. It is notable that the higher ultrasonic power did not reduce the WYM cutting force. The enhanced water loss during thawing is the main reason for the increase in the WYM cutting force at higher ultrasonic power. This phenomenon was consistent with the viewpoint of a published study by Ma and Kim [39], in which slight damage to myofibrils helped to reduce the meat cutting force; however, when the damage was severe, the cutting force increased due to the decrease in the water retention capacity.
3.4. Effect of the UAT on the FAA, minerals, and vitamins of WYM
The FAA can produce volatile compounds through the Maillard reaction and Strecker degradation, which contribute to the taste and flavor of meat [40]. Changes in the FAA content of the WYM at various treatments are shown in Table 2. Ultrasound treatment could significantly (P < 0.05) increase the content of essential and non-essential FAA. The degradation of proteins and peptides causes an increase in FAA, and the Maillard reaction and loss with water cause a decrease in FAA [41]. Study by Kang, Gao, Ge, Zhou, and Zhang [42] showed that the cavitation caused by ultrasound in water can generate free radicals, which can increase the level of protein degradation during the thawing process of beef. However, the total FAA content of UAT-400 W and UAT-600 W were not significant (P > 0.05). It was reported that the high levels of ultrasound cause the water molecules to produce OH– leading to protein degradation while the H+ in the water might bind to negatively-charged free amino acids in the meat which may cause the negatively-charged polar FAA loss with water [43].
Table 2.
| FAA content (g/100 g) | Samples |
|||
|---|---|---|---|---|
| Control | UAT-200 W | UAT-400 W | UAT-600 W | |
| Essential FAA | ||||
| Threonine | 0.85 ± 0.02c | 0.96 ± 0.02b | 1.12 ± 0.05a | 1.14 ± 0.04a |
| Valine | 0.81 ± 0.04b | 0.91 ± 0.03a | 0.92 ± 0.07a | 0.95 ± 0.05a |
| Methionine | 0.47 ± 0.01c | 0.53 ± 0.04b | 0.60 ± 0.03a | 0.60 ± 0.02a |
| Isoleucine | 0.84 ± 0.01c | 0.96 ± 0.03b | 1.04 ± 0.01a | 1.05 ± 0.02a |
| Leucine | 1.58 ± 0.02c | 1.75 ± 0.01b | 1.96 ± 0.03a | 1.99 ± 0.02a |
| Phenylalanine | 0.97 ± 0.08b | 0.99 ± 0.04b | 1.05 ± 0.02a | 1.05 ± 0.07a |
| Lysine | 1.71 ± 0.13c | 1.92 ± 0.07b | 2.12 ± 0.01a | 2.11 ± 0.03a |
| Total | 7.24 ± 0.17c | 8.02 ± 0.16b | 8.81 ± 0.18a | 8.89 ± 0.14a |
| Non-essential FAA | ||||
| Aspartic acid | 1.65 ± 0.12c | 1.85 ± 0.17b | 2.18 ± 0.12a | 2.24 ± 0.11a |
| Serine | 0.70 ± 0.04c | 0.78 ± 0.02b | 0.88 ± 0.05a | 0.90 ± 0.03a |
| Glutamic acid | 2.63 ± 0.13c | 3.10 ± 0.14b | 3.51 ± 0.11a | 3.52 ± 0.27a |
| Proline | 1.45 ± 0.04c | 1.79 ± 0.07a | 1.66 ± 0.01b | 1.70 ± 0.04b |
| Glycine | 0.78 ± 0.01d | 0.85 ± 0.04c | 0.95 ± 0.01b | 1.02 ± 0.02a |
| Alanine | 1.12 ± 0.14b | 1.24 ± 0.10a | 1.24 ± 0.12a | 1.24 ± 0.11a |
| Cysteine | 0.09 ± 0.01b | 0.11 ± 0.01a | 0.12 ± 0.01a | 0.12 ± 0.04a |
| Tyrosine | 0.63 ± 0.02c | 0.70 ± 0.01b | 0.80 ± 0.08a | 0.84 ± 0.03a |
| Histidine | 0.90 ± 0.04b | 0.91 ± 0.03b | 1.01 ± 0.02a | 0.99 ± 0.02a |
| Arginine | 1.13 ± 0.04c | 1.32 ± 0.05b | 1.45 ± 0.05a | 1.46 ± 0.01a |
| Total | 11.08 ± 0.11d | 12.65 ± 0.12c | 13.80 ± 0.07b | 14.03 ± 0.09a |
| Total FAA | 18.32 ± 0.41c | 20.67 ± 0.37b | 22.61 ± 0.43a | 22.92 ± 0.27a |
| EFAA/NEFAA | 65.34 ± 0.34a | 63.40 ± 0.14b | 63.84 ± 0.61b | 63.36 ± 0.74b |
UAT: ultrasonic-assisted thawing.
Values followed by different small letters within the same row denote significant differences (P < 0.05).
Minerals and vitamins are very important for the human body's metabolism, growth, development, and health. The mineral and vitamin content of WYM at the different treatments are shown in Table 2. Ultrasonic treatment increases mineral and vitamin retention in meat. In addition, the mineral and vitamin content, except for Vitamin A and Vitamin E, significantly (P < 0.05) increased as the intensity of the ultrasound increased. The minerals and water-soluble vitamins in meat can be lost with water during thawing and cooking [44]. The ultrasonic treatment could reduce the water loss of meat, which may account for the observation that the minerals and water-soluble vitamins of the ultrasonic treatment groups were higher than those in the control. Vitamin A and Vitamin E are fat-soluble vitamins, which are less affected by water loss [45].
3.5. Effect of the UAT on the volatile compounds of WYM
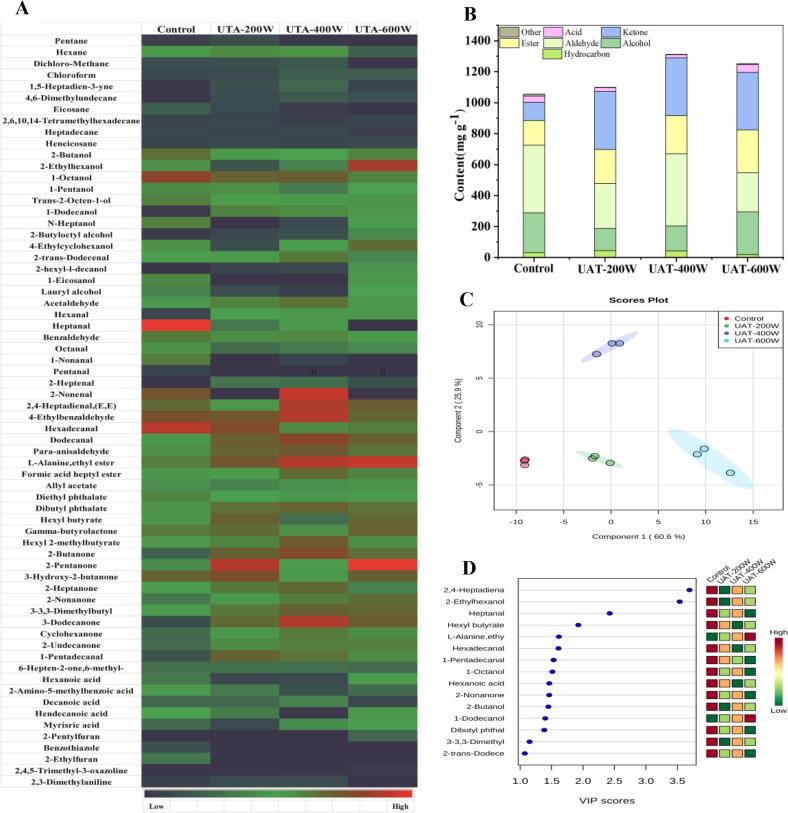
The heatmap (Fig. 4 A) shows all volatile compounds identified in the WYM. There were about 66 volatile compounds in WYM, which were divided into hydrocarbons, alcohols, aldehydes, esters, ketones, acids, and others. The total volatile compound content of UAT-400 W was significantly (P < 0.05) higher than those in the other groups (Table 3 and 4).
Fig. 4.
The heat map (A), volatile compounds contents (B), score-plot of PLS-DA analysis (C) and associated VIP score (D) of different treatment. PLS-DA, Partial least squares discrimination analysis; VIP, variables important in the projection.
Table 3.
| Minerals (mg/100 g) | Samples |
|||
|---|---|---|---|---|
| Control | UAT-200 W | UAT-400 W | UAT-600 W | |
| K | 263.55 ± 12.55c | 301.27 ± 4.31b | 307.21 ± 7.23b | 321.51 ± 5.66a |
| Ca | 29.76 ± 1.37c | 37.44 ± 1.14b | 38.21 ± 1.07b | 41.92 ± 1.26a |
| P | 206.03 ± 8.13b | 209.27 ± 9.24b | 237.26 ± 10.36a | 245.40 ± 11.46a |
| Fe | 34.27 ± 1.41a | 34.21 ± 2.11a | 36.95 ± 6.24a | 37.32 ± 5.81a |
| Zn | 7.31 ± 1.03c | 8.44 ± 0.33bc | 9.27 ± 1.06b | 11.45 ± 0.81a |
| Mg | 30.4 1 ± 1.61c | 35.27 ± 3.13b | 41.63 ± 2.44a | 42.33 ± 3.24a |
| Mn | 0.16 ± 0.03b | 0.18 ± 0.02b | 0.21 ± 0.01ab | 0.24 ± 0.02a |
| Cu | 0.19 ± 0.04b | 0.26 ± 0.03a | 0.24 ± 0.07a | 0.26 ± 0.01a |
UAT: ultrasonic-assisted thawing.
Values followed by different small letters within the same row denote significant differences (P < 0.05).
Table 4.
| Vitamins (ug/100 g) | Samples |
|||
|---|---|---|---|---|
| Control | UAT-200 W | UAT-400 W | UAT-600 W | |
| Vitamin A | 9.94 ± 0.62d | 11.80 ± 1.25c | 13.25 ± 1.08b | 16.30 ± 1.33a |
| Vitamin B1 | 271.54 ± 7.22b | 277.31 ± 2.25b | 292.69 ± 5.74a | 297.26 ± 3.72a |
| Vitamin B2 | 1788.97 ± 126.04c | 2427.26 ± 141.28b | 2990.74 ± 105.42a | 3009.26 ± 113.55a |
| Vitamin B3 | 6073.59 ± 130.72c | 6585.67 ± 393.04c | 7336.45 ± 232.52b | 8037.38 ± 351.54a |
| Vitamin B5 | 1047.89 ± 74.13b | 1191.78 ± 65.62b | 2073.29 ± 30.91a | 2061.34 ± 19.44a |
| Vitamin B6 | 259.67 ± 12.16b | 312.29 ± 49.21a | 310.41 ± 16.28a | 326.69 ± 24.88a |
| Vitamin B12 | 3394.87 ± 304.42a | 3369.23 ± 206.74a | 3322.12 ± 142.78a | 3422.18 ± 83.62a |
| Vitamin E | 9.45 ± 1.12b | 14.54 ± 2.04a | 15.37 ± 1.17a | 16.38 ± 1.06a |
UAT: ultrasonic-assisted thawing.
Values followed by different small letters within the same row denote significant differences (P < 0.05).
There were 9, 10, 10, and 7 types of hydrocarbons, including saturated and unsaturated hydrocarbons, detected in the control, UAT-200 W, UAT-400 W, and UAT-600 W groups, respectively. In addition, the hydrocarbon content the ultrasonic treatment groups was significantly higher than that in the control. Hydrocarbons usually have high odor thresholds, so the perception of their aroma is negligible [41]. However, some hydrocarbons are important intermediate materials for heterocyclic compounds [46]. It is possible that some hydrocarbons in the UAT-600 W group might transform into other substances, which might be responsible for the decrease in hydrocarbon content.
The degradation of lipids in meat produces alcohols, which have a higher odor detection threshold and contribute less to the aroma of meat [47]. With increasing carbon chain length, however, alcohols can produce unique aromas [30]. In our study, the alcohol content in the control group was significantly (P < 0.05) higher than that in the lower ultrasonic treatment group. The content of 1-octanol, which has a distinctive aroma, was the highest in the control, UAT-200 W, and UAT-400 W groups while the content of 2-ethylhexanol was highest in the UAT-600 W group. Some alcohols, such as 2-butanol, 2-ethylhexanol, trans-2-octen-1-ol, n-heptanol, 4-ethylcyclohexanol, and lauryl alcohol increased with the degree of lipid oxidation during thawing, while 1-dodecanol either decreased or, conversely, may have been oxidized to aldehydes or esters [43].
Aldehydes have a lower taste threshold and are the main compounds contributing to the odor of meat [41]. There are two sources of aldehydes: (1) oxidation of lipids, and (2) Strecker degradation of FAA [47]. The content of aldehydes in the UAT-400 W group was significantly (P < 0.05) higher than in the other groups, which might be due to lipid oxidation and Strecker degradation. Owing to the further oxidation of aldehydes to acids, the content of aldehydes in the UAT-600 W group was significantly (P < 0.05) lower than in the UAT-400 W group.
There were eight types of ester and five types of acid in WYM. Higher ester contents were detected in the ultrasound groups compared with the control group. In the ultrasound groups, the content of esters increased significantly (P < 0.05) with increasing ultrasonic power, which might be caused by the degradation of hydrocarbons [41]. The UAT-600 W group had the highest acid content, followed by the control, UAT-200 W, and UAT-400 W groups. This may be because some aldehydes were oxidized to acids. However, esters and acids are low in volatility and contribute only weakly to the flavor of meat [48].
Ketones have high detection thresholds and minor contributions to meat flavor; however, some ketones are intermediates in the formation of heterocyclic compounds [49]. The content of ketones in the ultrasonic treatment groups was significantly (P < 0.05) higher than that in the control group, with no significant (P > 0.05) differences among the ultrasound groups. There might be two reasons for this phenomenon: (1) the cavitation effect produced by ultrasound causes degradation of proteins and FAA; (2) the thermal effect of ultrasound causes lipid oxidation [50]. Zou et al. [43] found that the ketone content of beef increased with ultrasound power, which differed from our results. This might be due to some ketones being converted into intermediates of heterocyclic compounds under high-power ultrasonic conditions [51].
Other compounds with low levels and high thresholds were also detected, but these are unlikely to have any great effect on the aroma perception of WYM. The results of the volatile compounds showed that appropriate sonication was effective for better flavor production in WYM.
3.6. PLS-DA analysis of volatile compounds
The volatile compounds were analyzed by PLS-DA to acquire a greater depth of understanding of the different groups as this method is more effective in discriminating the groups based on the volatile compounds in food than principal component analysis [52]. Fig. 4C and D show the PLS-DA results of the four groups. The highest-ranking components accounted for 86.5% of the total variance, indicating that the changes in the volatile compounds among the different groups allow good discrimination and predictability in the model [53]. The first component of the cultivars accounted for 60.6% of their total variation. The control group and the ultrasonic treatment groups were successfully distinguished. The UAT-400 W group was separated from other groups when the second component accounting for 25.9% of their total variation was considered. Variables important in the projection (VIP) can help us understand the differences in the volatile compounds in the different groups. As shown in the VIP plots (Fig. 4D), the VIP values for 2,4-heptadienal, 2,4-ethylhexanol, heptanal, hexyl butyrate, L-alanine-ethy, hexadecanal, 1-pentadecanal, 1-octanol, hexanoic acid, 2-nonanone, 2-butanol, 1-dodecanol, dibutyl phthalate, 3–3,3-dimethyl and 2-trans-dodecenal were greater than 1, suggesting that these volatile compounds were the contributors in the UAT groups and the control group [54], and 2,4-heptadienal showed the highest VIP, which indicate that 2,4-heptadiena is the key biomarker of the different groups. The PLS-DA has been widely used in screening different sets of characteristic biomarkers. Zhou et al., (2020) screened acetic acid, 3-methyl-butanoic acid, and 3-methyl-butanal from 36 volatile compounds by PLS-DA as key biomarkers in normal ham and defective ham and the results were shown to be accurate by the Odor/Taste active values test [55]. Gong et al., (2019) found that the hexanoic acid and hexanal screened by PLS-DA can accurately determine whether ‘Fuji’ and ‘Delicious’ apples are infected by Penicillium expansum [52]. The above studies demonstrate the accuracy of PLS-DA in selecting key biomarkers among differently treated samples.
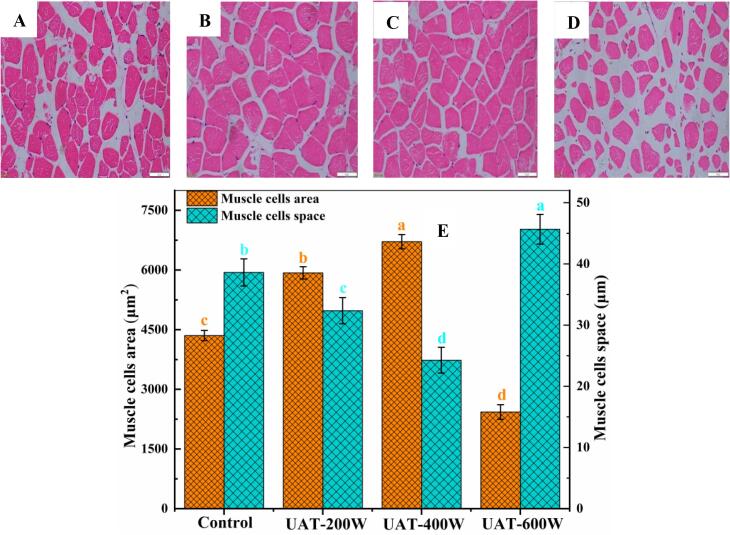
3.7. Microstructure of thawed WYM
The cavitation produced ultrasound often causes changes in the microstructure of meat. The effect of UAT on the microstructure of WYM during thawing was evaluated in this study. As shown in Fig. 5, the muscle fiber membrane of the control sample was damaged, and the boundaries between the adjacent muscle fibers were blurred. The muscle fiber membrane and boundaries were clear when the ultrasonic power was at 200 W and 400 W. Following the use of ultrasonic power at 600 W, the muscle fiber membranes were more severely ruptured than the control and the boundaries had disappeared. Fig. 5 E shows the muscle fiber area and muscle fiber space in the different treatment groups. The UAT-400 W group had the greatest muscle fiber area and the smallest muscle fiber space. This result is not consistent with the observation of Sir et al. [56] that ultrasonic treatment can increase the muscle fiber space of beef. However, a recent study showing that grass carp muscle treated by proper ultrasound power can reduce muscle fiber space supports our results [57].
Fig. 5.
Microstructure of frozen WYM with different thawing treatments. UAT, ultrasound-assisted immersion thawing at different ultrasound powers (200, 400 and 600 W).
4. Conclusions
The effects of different power levels of ultrasound at a 20 kHz frequency on the thawing rate and quality of white yak meat (WYM) were investigated. Ultrasound-assisted thawing (UAT) could significantly reduce of thawing time due to cavitation effects. UAT can also reduce the damage to WYM quality during thawing, although this did not hold when the ultrasonic power was 600 W. An appropriate ultrasonic power (UAT-400 W) can effectively reduce the thawing loss of WYM, helping to avoid mineral and water-soluble vitamin loss during thawing and leading to a decrease in the cutting force and L* value. Meanwhile, samples treated with UIT-400 W had the most complete microstructure, consistent with the findings that the UAT-400 W group had the lowest cooking losses and highest a* values. Furthermore, the degree of lipid oxidation in the UAT-400 W group was lower than in the other groups with consistent b* values. The overall content and type of volatile compounds in the UAT groups were higher than those of the control. The key biomarker of the different groups selected by PLS-DA was 2,4-heptadienal. Therefore, it is proposed that the application of UAT-400 W treatment increases the thawing efficiency and minimizes the unwanted changes induced by thawing in WYM.
CRediT authorship contribution statement
Zonglin Guo: Investigation, Methodology. Xiangzhen Ge: Data curation. Lihua Yang: Software, Validation. Guoyuan Ma: Resources, Writing - original draft. Jibing Ma: Formal analysis. Qun-li Yu: Conceptualization, Supervision, Validation, Project administration. Ling Han: Conceptualization, Supervision, Validation, Project administration.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This work was supported by the Funding Code of Fostering Foundation for the Excellent Ph.D. Dissertation of Gansu Agricultural University (YB2020005), the Industrialization of the Cascade Processing of Ecological Beef and Mutton (2020C-18), the National Natural Science Foundation of China (31771905), the China Agriculture Research System (No. CARS-37).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ultsonch.2020.105345.
Contributor Information
Qun-li Yu, Email: 1149569474@qq.com.
Ling Han, Email: hltgggyx@163.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Ruan C.M., Wang J., Yang Y.X., Hu J.J., Ma Y.J., Zhang Y., Zhao X.X. Proteomic analysis of Tianzhu White Yak (Bos grunniens) testis at different sexual developmental stages. Animal Sci. J. 2019;01:1–11. doi: 10.1111/asj.13157. [DOI] [PubMed] [Google Scholar]
- 2.Yang E.G., Basang B.G., Zhu W.D., An Y.B., Luo X.L. Screening for signatures of selection of Tianzhu white yak using genome-wide re-sequencing. Anim. Genet. 2019;50:534–538. doi: 10.1111/age.12817. [DOI] [PubMed] [Google Scholar]
- 3.Ma Y., Yuan Y., Bi X., Zhang L., Xing Y., Che Z. Tenderization of yak meat by the Combination of papain and high-pressure processing treatments. Food Bioprocess Technol. 2019;12:681–693. [Google Scholar]
- 4.Gaerrang K. Contested understandings of yaks on the eastern Tibetan Plateau: market logic, Tibetan Buddhism and indigenous knowledge. Area. 2017;49:526–532. [Google Scholar]
- 5.James S.J., James C., Purnell G. Microwave-assisted thawing and tempering. Microwave Process. Foods. 2017;7:252–272. [Google Scholar]
- 6.Gambuteanu C., Alexe P. Comparison of thawing assisted by low-intensity ultrasound on technological properties of pork Longissimus dorsi muscle. J. Food Sci. Technol. 2015;52:2130–2138. doi: 10.1007/s13197-013-1204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jia G., Nirasawa S., Ji X., Luo Y., Liu H. Physicochemical changes in myofibrillar proteins extracted from pork tenderloin thawed by a high-voltage electrostatic field. Food Chem. 2017;240:910–916. doi: 10.1016/j.foodchem.2017.07.138. [DOI] [PubMed] [Google Scholar]
- 8.Wang B., Du X., Kong B., Liu Q., Li F., Pan N., Xia X. Effect of ultrasound thawing, vacuum thawing, and microwave thawing on gelling properties of protein from porcine longissimus dorsi. Ultrason. Sonochem. 2019 doi: 10.1016/j.ultsonch.2019.104860. [DOI] [PubMed] [Google Scholar]
- 9.Li D., Zhao H., Muhammad A.I., Song L., Guo M., Liu D. The comparison of ultrasound-assisted thawing, air thawing and water immersion thawing on the quality of slow/fast freezing bighead carp (Aristichthys nobilis) fillets. Food Chem. 2020 doi: 10.1016/j.foodchem.2020.126614. [DOI] [PubMed] [Google Scholar]
- 10.Li W., Wang P., Xu X., Xing T., Zhou G. Use of low-field nuclear magnetic resonance to characterize water properties in frozen chicken breasts thawed under high pressure. Eur. Food Res. Technol. 2014;239:183–188. [Google Scholar]
- 11.Liu Y., Chen S., Pu Y., Muhammad A.I., Hang M., Liu D., Ye T. Ultrasound-assisted thawing of mango pulp: effect on thawing rate, sensory, and nutritional properties. Food Chem. 2019;286:576–583. doi: 10.1016/j.foodchem.2019.02.059. [DOI] [PubMed] [Google Scholar]
- 12.Qiu L., Zhang M., Chitrakar B., Bhandari B. Application of power ultrasound in freezing and thawing processes: Effect on process efficiency and product quality. Ultrason. Sonochem. 2020 doi: 10.1016/j.ultsonch.2020.105230. [DOI] [PubMed] [Google Scholar]
- 13.Miles C.A., Morley M.J., Rendell M. High power ultrasonic thawing of frozen foods. J. Food Eng. 1999;39:151–159. [Google Scholar]
- 14.Cheng X.F., Zhang M., Adhikari B. Effects of ultrasound-assisted thawing on the quality of edamames [Glycine max, (L.) Merrill] frozen using different freezing methods. Food Sci. Biotechnol. 2014;23:1095–1102. [Google Scholar]
- 15.Wu X.F., Zhang M., Adhikari B., Sun J. Recent developments in novel freezing and thawing technologies applied to foods. Crit. Rev. Food Sci. Nutr. 2017;57:3620–3631. doi: 10.1080/10408398.2015.1132670. [DOI] [PubMed] [Google Scholar]
- 16.Kissam A.D., Nelson R.W., Ngao J., Hunter P. Water-thawing of fish using low frequency acoustics. J. Food Sci. 1982;47:71–75. [Google Scholar]
- 17.Kang D.C., Zou Y.H., Cheng Y.P., Xing L.J., Zhou G.H., Zhang W.G. Effects of power ultrasound on oxidation and structure of beef proteins during curing processing. Ultrason. Sonochem. 2016;33:47–53. doi: 10.1016/j.ultsonch.2016.04.024. [DOI] [PubMed] [Google Scholar]
- 18.Tao Y., Sun D.W. Enhancement of food processes by ultrasound: A review. Crit. Rev. Food Sci. Nutr. 2015;55:570–594. doi: 10.1080/10408398.2012.667849. [DOI] [PubMed] [Google Scholar]
- 19.Nowacka M., Wedzik M. Effect of ultrasound treatment on microstructure, colour and carotenoid content in fresh and dried carrot tissue. Appl. Acoust. 2016;103:163–171. [Google Scholar]
- 20.Chemat F., Zille H., Khan M.K. Applications of ultrasound in food technology: processing, preservation and extraction. Ultrason. Sonochem. 2011;18:813–835. doi: 10.1016/j.ultsonch.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 21.O'Donnell C.P., Tiwari B.K., Bourke P., Cullen P.J. Effect of ultrasonic processing on food enzymes of industrial importance. Trends Food Sci. Technol. 2010;21:358–367. [Google Scholar]
- 22.Li F., Wang B., Liu Q., Chen Q., Zhang H., Xia X., Kong B. Changes in myofibrillar protein gel quality of porcine longissimus muscle induced by its stuctural modification under different thawing methods. Meat Sci. 2019;147:108–115. doi: 10.1016/j.meatsci.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 23.Sun Q., Chen Q., Xia X., Kong B., Diao X. Effects of ultrasound-assisted freezing at different power levels on the structure and thermal stability of common carp (Cyprinus carpio) proteins. Ultrasonics Sonochemistry. 2019;54:311–320. doi: 10.1016/j.ultsonch.2019.01.026. [DOI] [PubMed] [Google Scholar]
- 24.Aminzare M., Tajik H., Aliakbarlu J., Hashemi M., Raeisi M. Effect of cinnamon essential oil and grape seed extract as functional-natural additives in the production of cooked sausage-impact on microbiological, physicochemical, lipid oxidation and sensory aspects, and fate of inoculated Clostridium perfringens. J. Food Saf. 2018;38 [Google Scholar]
- 25.Geng J.T., Takahashi K., Kaido T., Kasukawa M., Okazaki E., Osako K. Relationship among pH, generation of free amino acids, and Maillard browning of dried Japanese common squid Todarodes pacificus meat. Food Chem. 2019;283:324–330. doi: 10.1016/j.foodchem.2019.01.056. [DOI] [PubMed] [Google Scholar]
- 26.Hopcroft R.L., Cowieson A.J.W.I., Groves P.J. Changes to mineral levels in the yolk of meat chicken embryos during incubation. Poult. Sci. 2018;7:1–6. doi: 10.3382/ps/pey423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cardiff R.D., Borowsky A.D. At last: classification of human mammary cells elucidates breast cancer origins. J. Clinical Investigation. 2014;124:78–80. doi: 10.1172/JCI73910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiu-xia L., Pan S., Yingying M., Luyun C., Jian-rong L. Effect of ultrasonic thawing on the water holding capacity, physico-chemical properties, and structure of frozen tuna fish (Thunnus tonggol) myofibrillar proteins. J. Sci. Food Agric. 2019;99:5081–5093. doi: 10.1002/jsfa.9752. [DOI] [PubMed] [Google Scholar]
- 29.Cheng L., Sun D.W., Zhu Z., Zhang Z. Emerging techniques for assisting and accelerating food freezing processes: A review of recent research progresses. Crit. Rev. Food Sci. Nutr. 2017;57:769–781. doi: 10.1080/10408398.2015.1004569. [DOI] [PubMed] [Google Scholar]
- 30.Cai L., Zhang W., Cao A., Cao M., Li J. Effects of ultrasonics combined with far infrared or microwave thawing on protein denaturation and moisture migration of Sciaenops ocellatus (red drum) Ultrason. Sonochem. 2019;55:96–104. doi: 10.1016/j.ultsonch.2019.03.017. [DOI] [PubMed] [Google Scholar]
- 31.Shi H., Zhang X., Chen X., Fang R., Zou Y., Wang D., Xu W. How ultrasound combined with potassium alginate marination tenderizes old chicken breast meat: Possible mechanisms from tissue to protein. Food Chem. 2020 doi: 10.1016/j.foodchem.2020.127144. [DOI] [PubMed] [Google Scholar]
- 32.Reyes-Villagrana R.A., Huerta-Jimenez M., Salas-Carrazco J.L., Carrillo-Lopez L.M., Alarcon-Rojo A.D., Sanchez-Vega R., Garcia-Galicia I.A. High-intensity ultrasonication of rabbit carcases: a first glance into a small-scale model to improve meat quality traits. Ital. J. Anim. Sci. 2020;19:544–550. [Google Scholar]
- 33.Hughes J.M., Clarke F.M., Purslow P.P., Warner R.D. Meat color is determined not only by chromatic heme pigments but also by the physical structure and achromatic light scattering properties of the muscle. Compr. Rev. Food Sci. Food Saf. 2020;19:1–20. doi: 10.1111/1541-4337.12509. [DOI] [PubMed] [Google Scholar]
- 34.Stadnik J., Dolatowski Z.J. Influence of sonication on Warner-Bratzler shear force, colour and myoglobin of beef (m. semimembranosus) European Food Res. Technol. 2011;233:553–559. [Google Scholar]
- 35.Yi G., Grabež V., Bjelanovic M., Slinde E., Olsen K., Langsrud O., Egelandsdal B. Lipid oxidation in minced beef meat with added Krebs cycle substrates to stabilise colour. Food Chem. 2015;187:563–571. doi: 10.1016/j.foodchem.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 36.Yang X., Wang H., Badoni M. Effects of meat pH and the initial numbers of spores of clostridium estertheticumon the development of blown pack spoilage of vacuum-packaged beef. Int. J. Food Sci. Technol. 2013;49:1619–1625. [Google Scholar]
- 37.Xu D., Wang Y., Jiao N., Qiu K., Zhang X., Wang L., Yin J. The coordination of dietary valine and isoleucine on water holding capacity, pH value and protein solubility of fresh meat in finishing pigs. Meat Sci. 2020;163 doi: 10.1016/j.meatsci.2020.108074. [DOI] [PubMed] [Google Scholar]
- 38.Zhang M., Haili N., Chen Q., Xia X., Kong B. Influence of ultrasound-assisted immersion freezing on the freezing rate and quality of porcine longissimus muscles. Meat Sci. 2018;136:1–8. doi: 10.1016/j.meatsci.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 39.Ma D., Kim Y.H.B. Proteolytic changes of myofibrillar and small heat shock proteins in different bovine muscles during aging: Their relevance to tenderness and water-holding capacity. Meat Sci. 2020;163 doi: 10.1016/j.meatsci.2020.108090. [DOI] [PubMed] [Google Scholar]
- 40.Spanier A.M., Flores M., Toldrá F., Aristoy M.C., Bett K.L., Bystricky P., Bland J.M. Meat flavor: contribution of proteins and peptides to the flavor of beef. Quality of Fresh Processed Foods. 2004;542:33–49. doi: 10.1007/978-1-4419-9090-7_3. [DOI] [PubMed] [Google Scholar]
- 41.Qi J., Li X., Zhang W., Wang H., Zhou G., Xu X. Influence of stewing time on the texture, ultrastructure and in vitro digestibility of meat from the yellow-feathered chicken breed. Animal Sci. J. 2017;89:474–482. doi: 10.1111/asj.12929. [DOI] [PubMed] [Google Scholar]
- 42.Kang D., Gao X., Ge Q., Zhou G., Zhang W. Effects of ultrasound on the beef structure and water distribution during curing through protein degradation and modification. Ultrason. Sonochem. 2017;38:317–325. doi: 10.1016/j.ultsonch.2017.03.026. [DOI] [PubMed] [Google Scholar]
- 43.Zou Y., Kang D., Liu R., Qi J., Zhou G., Zhang W. Effects of ultrasonic assisted cooking on the chemical profiles of taste and flavor of spiced beef. Ultrason. Sonochem. 2018;46:36–45. doi: 10.1016/j.ultsonch.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 44.Farfán N.B., Sammán N. Retention of nutrients in processed cuts of Creole cattle. J. Food Compos. Anal. 2003;16:459–468. [Google Scholar]
- 45.Piironen V., Varo P., Koivistoinen P. Stability of tocopherols and tocotrienols in food preparation procedures. J. Food Compos. Anal. 1987;1:53–58. [Google Scholar]
- 46.Ventanas S., Mustonen S., Puolanne E., Tuorila H. Odour and flavour perception in flavoured model systems: Influence of sodium chloride, umami compounds and serving temperature. Food Qual. Prefer. 2010;21:453–462. [Google Scholar]
- 47.Watanabe A., Kamada G., Imanari M., Shiba N., Yonai M., Muramoto T. Effect of aging on volatile compounds in cooked beef. Meat Sci. 2015;107:12–19. doi: 10.1016/j.meatsci.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 48.Lv T., Wang Y., Pan D., Cao J., Zhang X., Sun Y., Liu Y. Effect of trypsin treatments on the structure and binding capacity of volatile compounds of myosin. Food Chem. 2017;214:710–716. doi: 10.1016/j.foodchem.2016.07.115. [DOI] [PubMed] [Google Scholar]
- 49.Zhuang K., Wu N., Wang X., Wu X., Wang S., Long X., Wei X. Effects of 3 feeding modes on the volatile and nonvolatile compounds in the edible tissues of female chinese mitten crab (eriocheir sinensis) J. Food Sci. 2016;81:S968–S981. doi: 10.1111/1750-3841.13229. [DOI] [PubMed] [Google Scholar]
- 50.Khan M.I., Jo C., Tariq M.R. Meat flavor precursors and factors influencing flavor precursors-A systematic review. Meat Sci. 2015;110:278–284. doi: 10.1016/j.meatsci.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 51.Girard B., Durance T. Headspace volatiles of sockeye and pink salmon as affected by retort process. J. Food Sci. 2000;65:34–39. [Google Scholar]
- 52.Gong D., Bi Y., Jiang H., Xue S., Wang Z., Li Y., Prusky D. A comparison of postharvest physiology, quality and volatile compounds of “Fuji” and “Delicious” apples inoculated with Penicillium expansum. Postharvest Biol. Technol. 2019;150:95–104. [Google Scholar]
- 53.Centonze V., Lippolis V., Cervellieri S., Damascelli A., Casiello G., Pascale M., Longobardi F. Discrimination of geographical origin of oranges (Citrus sinensis L. Osbeck) by mass spectrometry-based electronic nose and characterization of volatile compounds. Food Chem. 2019;277:25–30. doi: 10.1016/j.foodchem.2018.10.105. [DOI] [PubMed] [Google Scholar]
- 54.Lei T., Lin X.H., Sun D.W. Rapid classification of commercial Cheddar cheeses from different brands using PLSDA, LDA and SPALDA models built by hyperspectral data. J. Food Meas. Charact. 2019:11694. [Google Scholar]
- 55.Zhou C.Y., Le Y., Zheng Y.Y., Wang J.J., Li G., Bai Y., Cao J.X. Characterizing the effect of free amino acids and volatile compounds on excessive bitterness and sourness in defective dry-cured ham. LWT. 2020 [Google Scholar]
- 56.Sir I.O., Vén C., Balla C., Jónás G., Zeke I. Application of an ultrasonic assisted curing technique for improving the diffusion of sodium chloride in porcine meat. J. Food Eng. 2009;91:353–362. [Google Scholar]
- 57.Shi Z., Zhong S., Yan W., Liu M., Yang Z., Qiao X. The effects of ultrasonic treatment on the freezing rate, physicochemical quality, and microstructure of the back muscle of grass carp (Ctenopharyngodon idella) LWT. 2019;111:301–308. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.