Highlights
-
•
US pre-treatment has optimally improved functional properties of Lac-cross-linked α-LA.
-
•
α-LA was used as a natural emulsifier for CLA emulsion stabilization.
-
•
No sign of phase separation was noted throughout the storage studies.
-
•
Lac-cross-linked US α-LA emulsion had the highest stability and smallest particle size.
Abbreviations: CLA, Conjugated linoleic acid; US, Ultrasound; α-lactalbumin treated with US pre-treatment and cross-linking with laccase, Laccase cross-linking US-α-lactalbumin; α-lactalbumin treated with US pre-treatment, US-α-lactalbumin; PUFAs, Polyunsaturated fatty acids; Z-Ave, Average particle size; PDI, Polydispersity index; TGase, Transglutaminase; WPI, Whey protein isolate; EAI, Emulsifying activity index; ESI, Emulsifying stability index; CI, Creaming index; AP, Adsorbed protein; CDs, Conjugated Dienes; CLSM, Confocal laser scanning microscopy; PV, Peroxide value; EC, Electrical conductivity; FBPI, Fava bean protein isolate
Keywords: Ultrasound, Laccase, Cross-linking reaction, Conjugated linoleic acid (CLA), Oxidative stability
Abstract
α-lactalbumin was modified by ultrasound (US, 20 kHz, 43 ± 3.4 W/cm−2) pre-treatments (0, 15, 30 and 60 min) and laccase cross-linking of sonicated α-lactalbumin was used to evaluate the physical and oxidative stability of conjugated linoleic acid (CLA) emulsions. The emulsions prepared with laccase cross-linking US-α-lactalbumin (α-lactalbumin treated with US pre-treatment) and US-α-lactalbumin were scrutinized for oxidative and physical stability at room temperature for two weeks of storage. Laccase cross-linking US-α-lactalbumin (Lac-US-α-lactalbumin) revealed improved physical stability in comparison with US-α-lactalbumin, specified by droplet size, structural morphology, adsorbed protein, emulsifying properties and creaming index. SDS-PAGE analysis showed that there was formation of polymers in Lac-US-α-lactalbumin emulsion. Surface hydrophobicity of Lac-US-α-lactalbumin was higher than that of US-α-lactalbumin, and gradually enhanced with the increase of ultrasound time. More importantly, the measurements of peroxide values and conjugated dienes were used to study the oxidative stability of the CLA emulsions. The Lac-US-α-lactalbumin emulsion proved to be reducing the synthesis of fatty acid hydroperoxides and less conjugated dienes compared to the native and US-α-lactalbumin emulsions. This study revealed that the combination of US pre-treatment and laccase cross-linking might be an effective technique for the modification of CLA emulsions.
1. Introduction
Polyunsaturated fatty acids (PUFAs), e.g., conjugated linoleic acid (CLA; C18:2), has been proved to be useful due to various health advantages, like cancer inhibition, beneficial for infant development and improving cardiac health [1]. Oxidation of the PUFAs in food matrices was speed up by the availability of oxygen, enzymes, metals, light, heat and metalloproteins, etc., which might result in the development of variation in colour, off-flavours and loss of the other nutrients and production of other potentially deleterious substances [2].
However, it is essential to create an effective protective method during the application of food to control the oxidation of the PUFAs. In the past, different kinds of approaches and various studies were carried out to identify how to increase the functional attributes of emulsion. Conditions such as pressure, temperature, pH and protein concentration have been identified to perform an ideal process for emulsification [3], [4], [5], [6]. Recently, enzymatic tailoring of proteins like cross-linking or hydrolysis of proteins has also been attempted. Additionally, cross-linking with the usage of different enzymes like peroxidase, tyrosinase, transglutaminase [7], [8], [9], is the alternate method to increase the emulsifying properties of proteins. Laccase (EC 1.10.3.2) is a blue copper oxidase that can catalyze the oxidation of various aromatic substrates. A byproduct H2O formed as a result of an oxidation reaction, where oxygen is used as electron acceptor [10]. It has been reported that laccase can polymerize different kinds of components like lignin and other aromatic compounds [11], [12]. It has also shown that tyrosine and tyrosine containing peptides can be oxidized through laccase [13]. Therefore phenolic groups of protein are not considered to be unique for the laccase-induced reaction [14].
Functional attributes of proteins can be enhanced by using different techniques including heating, ultrasound, microwave and high-pressure treatment [15]. Recently ultrasound technology is getting more attention and has been widely exploited in food processing for both liquid and solid media [16]. Ultrasound can easily process high reproducible foods within short period of time, resulting in least processing cost, producing optimal products and lesser operational time as compared to conventional process [17]. Similarly, ultrasound technology can be applied to ensure food safety and quality [18].
α-lactalbumin is a crucial component of whey protein and is mainly used in food processing [19]. Previously Jiang et al. [20] reported that laccase-treated α-lactalbumin in presence of ferulic acid enhanced its antioxidant and gel properties. Furthermore, Sato et al. [21] revealed that emulsion stabilized with sodium caseinate treated with laccase and ferulic acid was kinetically stable throughout seven days of storage. It has been demonstrated that physical features of WPI-beet pectin complex coacervations were significantly altered if ferulic acid was used for laccase-catalyzed cross-linking [22]. However, the purpose of our study is to apply laccase cross-linking of sonicated α-lactalbumin to enhance physical and oxidative stability of CLA emulsion without using linking/bridging agents such as ferulic acid, as these linking/bridging agents probably have some undesirable effects in food and other applications. Another objective of our study is to find the possible changes in oxidative stability of CLA emulsion upon application of combined treatment of US and laccase cross-linking of α-lactalbumin.
2. Materials and methods
α-lactalbumin was gifted from Davisco Foods International, Eden Prairie, MN, USA. According to the manufacturers, the composition of α-lactalbumin powders contained 91.6 g per 100 g protein. Laccase (20 U/g) was obtained from Yuanye Co, (Shanghai, China). Nile blue, Nile red and SDS (sodium dodecyl sulfate was procured from Sigma-Aldrich Co. (St. Louis, MO, USA).
2.1. α-lactalbumin stock solution and ultrasound treatments
A complete dissolution of α-lactalbumin (100 mg/mL) was achieved by mixing for 3–4 h in deionized water with a magnetic stirrer at 25 °C. The pH was adjusted to 7.0 by adding 2.0 mol/L NaOH and kept overnight at 4 °C for complete dissolution. Subsequently, the α-lactalbumin solution was sonicated by using an ultrasound processor (DY-1200Y, Deyang yibang, instruments Co. Ltd, Shanghai, China) with a 2-cm diameter titanium probe. Samples were treated at 20 kHz at 600 W for 0, 15, 30 and 60 min (pulse duration of working-time 2 s and rest-time 4 s to avoid the probe damage). By using calorimetric method [23], the ultrasound intensity (43 ± 3.4 W/cm2) was calculated. The sonication probe was kept at 2 cm inside the solution, and ice cubes were put in order to maintain the sample temperature<20 °C.
2.2. Laccase cross-linking of US-treated α-lactalbumin
Laccase was added into US-treated α-lactalbumin suspension (100 mg/mL) with an amount of 85 U/g protein to speed up the cross-linking reaction and then kept at 40 °C for 10 h. subsequently, the mixture was heated for 15 min at 75 °C to stop the laccase activity. Laccase-treated sonicated α-lactalbumin samples were cooled down and lyophilized for further analysis. α-lactalbumin without laccase treatment used as a control.
2.3. Preparation of oil in water emulsions
The emulsion was formed by mixing CLA oil with Lac-US-α-lactalbumin or US-α-lactalbumin dispersions with a ratio of 1:4 by using a blender (IKA, T18 digital Ultra-Turrax, Germany) operating at 1200 rpm for 5 min. The coarse emulsion was then homogenized for four cycles at 80 MPa using a high pressure homogenizer (HPH/AH-100D, ATS Engineering Ltd, Jiangsu, China) to form a CLA emulsion. Moreover, the α-lactalbumin solution was sonicated for 0, 15, 30, and 60 min, afterwards treated with laccase and finally prepared for CLA emulsion. The corresponding emulsion samples were marked as Lac-α-lactalbumin-0 min, Lac-US-α-lactalbumin-15 min, Lac-US-α-lactalbumin-30 min and Lac-US-α-lactalbumin-60 min. The α-LA solution was sonicated for 0, 15, 30 and 60 min and then prepared for CLA emulsion, marked as α-lactalbumin-0 min, US-α-lactalbumin-15 min, US-α-lactalbumin-30 min and US-α-lactalbumin-60 min. The measured temperature of the Lac-US-α-lactalbumin and US-α-lactalbumin emulsion was below 40 °C. The ultrasound and laccase cross-linking conditions were selected according to the related recent research work [24], [25], [26].
2.4. Surface hydrophobicity measurement
The surface hydrophobicity of samples was measured by using 1-Anilino-8-naphthalenesulfonate acid (ANS) as a fluorescent probe, based on a method of Kato et al. with slight modifications [27]. All samples were diluted with 10 mol/L PBS (pH 7.0) and the protein concentration ranged from 1 to 5 mg/mL. A 20 μL of 8.0 mol/L ANS was added to 2 mL samples and put in the dark at 25 °C for 15 min before measurement. The fluorescence intensity scanning was performed using a F-7100 fluorescence spectrophotometer at the excitation wavelength of 390 nm and emission wavelength of 470 nm. Both slits of excitation and emission were set at 2.5 nm with a scanning speed of 240 nm/min. The initial slope of fluorescence intensity versus corresponding sample concentrations was presented as surface hydrophobicity. Distilled water was used as blank.
2.5. Characterization of emulsion
2.5.1. pH determination, temperature variation and determination of electrical conductivity
Electrical conductivity, change in temperature and pH determination of emulsion samples were measured, being based on the previous method by Jambrak et al. [28]. By using a thermometer, the temperature of the cross-linking and non-cross-linking samples was measured and then the averages were taken after every treatment. The pH of emulsion was measured with a pH meter (Sartorius-PB-10, China) at room temperature. The rate of deviations in conductivities was analyzed at room temperature using electrical conductivity meter (DD-307A, Leici, Shanghai).
2.5.2. Particle size
Particle size distribution of emulsion samples was evaluate by a Zetasizer machine (Nano-ZS90, Malvern Ltd, UK). The concentration of emulsion samples was diluted to 1 mg/mL with 0.01 mol/L PBS (pH 7.0). Particle size distribution results obtained from three independent experiments were shown by the intensity.
2.5.3. Zeta potential
For the measurement of z-potential of oil droplets, a Zetasizer Nano-ZS90 (Malvern Instruments Ltd., Worcestershire, UK) was used. The emulsions were diluted to 2 mg/mL and then injected into the instrument for measurements.
2.5.4. Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE)
The molecular weight distribution of emulsion samples was evaluated by SDS-PAGE as described by Tong et al. [29] with minor changes. Our used gels were composed of 12% acrylamide separating gel and 5% acrylamide staking gel. Emulsion samples were diluted in deionized water (2.5 mg protein/mL) and the resulting sample was dissolved in buffer (1:1) to attain dissociating conditions. The samples were heated at 100 °C for 5 min and then the samples were cooled immediately. Electrophoresis was run for 90 min at 100 V. Coomassie Brilliant Blue R-250 solution was prepared to dye protein samples and the gels were decolorized with a mixture of methanol and glacial acetic acid.
2.5.5. Emulsifying properties
Emulsifying activity index (EAI) and emulsifying stability index (ESI) of emulsion were determined according to the earlier report [30]. The emulsion was taken (50 µL) from the bottom at 0 and 30 min after Lac-US-α-lactalbumin and α-lactalbumin treatment and then mixed with 5 mL of SDS (1.0 mg/mL) and vortex for 6 s. The absorbance was measured at wavelength of 500 nm with UV-spectrophotometer (UV −6100 Shanghai, China). Following equations were used to determine the EAI and ESI.
| (1) |
c × ∅x (1-θ) × 10000
| (2) |
where T shows turbidity with value (2.303), DF represents dilution factor, c denotes the concentration of the samples (g/mL), θ shows oil fraction, ∅ marks optical path. A0 and A30 are absorbance at 0 and 30 min.
2.5.6. Creaming stability
A recently described method [31] was used to describe the creaming index (CI) in the Lac-US- α-lactalbumin and US-α-lactalbumin emulsion. Glass tubes containing fresh Lac-US-α-lactalbumin and US-α-lactalbumin emulsion were kept at room temperature. Following equation was used to measure CI% throughout the storage period of two weeks.
| (3) |
where Ht shows the total height of the emulsion and Hc is the height of supernatant layer
2.5.7. 2.5.7. Adsorbed protein
The evaluation of adsorbed protein (AP) was measured through the interface of the fresh Lac-US-α-lactalbumin and US-α-lactalbumin emulsion with minor modification, as described previously by Liu and Tang [32]. In brief, 1 mL of Lac-US-α-lactalbumin or US-α-lactalbumin was centrifuged at 15,000 g for 45 min at ambient temperature. The supernatant was then filtered by a 0.22 µm filter. Loery method [33] was applied to find the concentration of filtrate (Cf) protein (mg/mL). The following equation (Eq. (4)) was used to calculate the AP (%).
| (4) |
Where the preliminary concentration of proteins (mg/mL) of Lac- US-α-lactalbumin or US-α-lactalbumin is Co solutions, while Cf is the protein concentration of filtrate protein (mg/mL).
2.5.8. Confocal laser scanning microscopy (CLSM)
A CLSM (Leica TCS SP2, Heidelberg, Germany) technique has been used to determine the structural changes in Lac-US-α-lactalbumin and α-lactalbumin emulsion. Nile red and Nile blue were used as fluorescence dyes for oil phase and protein respectively, with excitation wavelength of 488 and 633 nm. A 5 µL stained emulsion was positioned on a microscope slide and then slightly enclosed with a coverslip. Lastly, the CLSM images were obtained with a 40X magnification lens. Nile blue and Nile red were used to obtain all fluorescence images. The overlay could allow two dyes relating each other. Particle in red are usually dyed with Nile red while green particles of protein are dyed with Nile blue. On overlay image the appearance of protein coated oil droplets was predominantly yellow color (green + red - yellow).
2.5.9. Lipid oxidation
In detail, 50 µL of emulsion was dissolved in 750 µL of acetone/ isopropanol (2:1, v/v) and vortex for 60 s, and mixed the emulsion samples and centrifuged at 550 × g for 10 min at 4 °C. After centrifugation, the upper organic phase 100 µL was collected, diluted and vortexed with 2.4 mL isooctane. After that, the absorbance was recorded at 234 nm through UV-spectrophotometer (UV-6100, Metash and Shanghai, China). Finally, concentration calculation of the conjugated dienes (CDs) was done by using 25,200 M1cm1 as the molar extinction coefficient [34].
2.5.10. Peroxide values (PV)
The concentration of Lac- US-α-lactalbumin and US-α-lactalbumin emulsion was scrutinized with a reported literature to evaluate the primary product of lipid oxidation [35]. The CLA emulsions (0.3 mL) was mingled with 1.5 mL of acetone/2-propanol (iso-propanol) at 3:1, (v:v). After centrifugation, the upper portion of supernatant was separated to determine the lipid hydroperoxides. The mentioned sample was added to a newly autoclaved tube (200 μL) and then mixed it in 2.8 mL of methanol/1-butanol (2:1, v:v) solution. Moreover, the thiocyanate (15 μL, 3.97 M ammonium thiocyanate) and ferrous (15 μL) were added and mixed in the solution. After fully mixed with the help of the stir bar, the samples were kept at room temperature for 20 min. Subsequently, the absorbance was recorded with the help of a spectrophotometer UV-spectrophotometer (UV-6100, Metash and Shanghai, China) at 510 nm. The value of PV has been expressed as a millimole of hydroperoxide kg−1 of Lac- US-α-lactalbumin and US-α-lactalbumin emulsion with the aid of standard curve that was formed through a series of BSA hydroperoxide standard solutions. For the identification of peroxidase values, emulsions were stored at 23 °C and samples were periodically taken for the identification of peroxidize values.
2.6. Statistical analysis
SPSS 22.0 was used to analyze data for one-way ANOVA and multiple comparisons of Duncan. P < 0.05 represented statistically significant.
3. Results and discussion
3.1. Particle size distribution
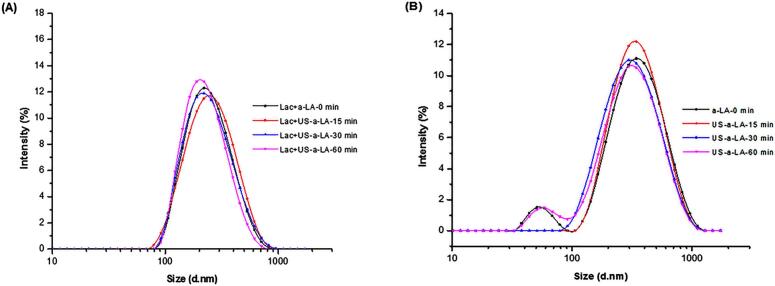
The main character which altered the functional and physical properties is particle size [36]. The particle size distribution in Lac-US-α-lactalbumin and US-α-lactalbumin emulsion is shown in Fig. 1A and 1B, respectively. The distribution of Lac-US-α-lactalbumin and US-α-lactalbumin emulsion varied from 100 to 1000 nm and was lying within the range of normal distribution. As shown in Fig. 1A, the Lac-US-α-lactalbumin emulsion exhibited a unimodal and narrowed distribution, compared with US-α-lactalbumin emulsion. The unimodal distribution showed a better emulsion stability. Lac-US-α-lactalbumin-30 min sample depicted the smallest particle size and narrowest peak among others, showing its better stability. The particle size results were further confirmed with the CLSM.
Fig. 1.
Effect of US pre-treatment (0, 15, 30 and 60 min) and cross-linking on the distribution of particle size of laccase cross-linking US-α-LA (A) and US-α-LA (B).
The particle size was further evaluated as average particle size (Z-Ave) of Lac-US-α-lactalbumin and US-α-lactalbumin emulsion, shown in Fig. 2A. It is depicted that an increase of ultrasound duration in the range of 0–60 min, led to a steady reduction of Z-Ave (278–205 nm) of Lac-US-α-lactalbumin and US-α-lactalbumin emulsion. Regarding bimodal droplet size distribution, the sample, US-α-lactalbumin-60 min emulsion showed a secondary peak (Fig. 1B). It resembled the peaks noticed in control. The association of droplet coalescence might be due to the secondary peak in control while the treated emulsion showed the formation of aggregates. However, Lac-US-α-lactalbumin-30 min showed the lowest Z-Ave and polydispersity index (205 nm, 0.2), which showed the maximum stability in comparison with native and treated samples. The production of acoustic bubbles which increased the oil droplet disruptions might be the main cause of obtained results [37]. Moreover, the accumulation of oil droplets in the non-cross link emulsion was caused by an increased particle size [38]. Furthermore, the decline in particle size might be due to the share energy waves and cavitation effects produced by the ultrasonic treatment [39]. Previously, Sato et al. [21] reported emulsion cross-linked with laccase and ferulic acid also presented the secondary peaks and increased in Z-Ave in treated samples in comparison with native proteins.
Fig. 2.
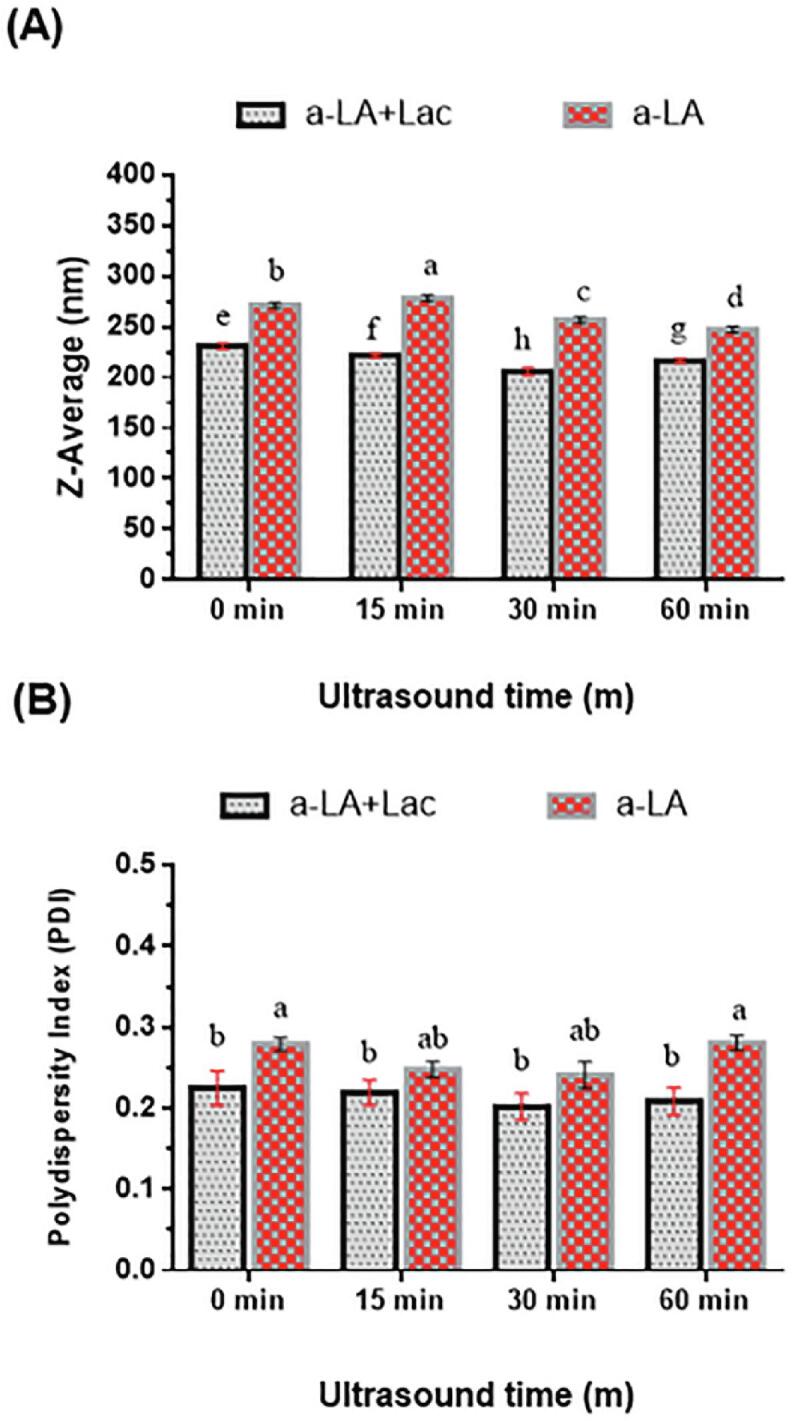
Effect of US pre-treatment (0, 15, 30 and 60 min) and cross-linking on the Z-Average (A) and PDI (B) of laccase cross-linking US-α-LA and US-α-LA. Data were represented as mean and SD (n = 3), significant differences (p < 0.05) in between treatments were differentiated with lower case letters.
The polydispersity index (PDI) is an indicator to evaluate the dispersion of emulsion. Thus, emulsion with bigger PDI values suggested a broad size distribution, showing that emulsion was more susceptible toward Ostwald ripening [40]. The PDI of Lac-US-α-lactalbumin and US-α-lactalbumin was presented in Fig. 2B. The outcomes showed that the values of PDI of Lac-US-α-lactalbumin and US-α-lactalbumin emulsion varied from 0.28 to 0.20, respectively. The PDI values below 0.3 in all the samples exhibited better dispersibility and stability. The Lac-US-α-lactalbumin emulsion showed the least PDI (0.25–0.2) compared with US-α-lactalbumin emulsion (0.28–0.24). These results meant that combined treatment of US pre-treatment and enzymatic cross-linking was in a better dispersion state. Power ultrasound pre-treatments were found to be increased in PDI when applied to reconstituted milk protein concentrate [41].
3.2. Zeta potential
The stability of emulsion can be improved by enhancing the repulsive forces among oil droplets [42], which can be arbitrated on z-potential values of emulsion [43]. The results are depicted in Fig. 3. All treatments had negative values of absolute zeta potential because of the negative charge of α-lactalbumin at pH 7.0 greater than its isoelectric point [36]. The value of absolute zeta-potential of US-α-lactalbumin was determined to be 37–48 mV (highly stable), which increased significantly (p < 0.05) after Lac-US-α-lactalbumin treatments with the increase in ultrasound duration from 0 to 60 min. A gradual increase in Lac-US-α-lactalbumin was noticed from 44 to 56 mV (highly stable). This might be linked to the fact that ultrasonication might disrupt protein aggregates, resulting in more exposure of negative amino acid residues to the surface of α-lactalbumin molecules, which could be the cause of increasing the repulsive forces among droplets. Recently, it was reported that absolute zeta potential values of ultrasound-treated myofibril protein increased with the ultrasonic capacity ranging from 0 to 1000 W [44]. Moreover, these results were in agreement with a previous study that WPI pretreated by TGase showed comparatively greater (p < 0.05) values of zeta-potential than a counterpart dispersion [45].
Fig. 3.
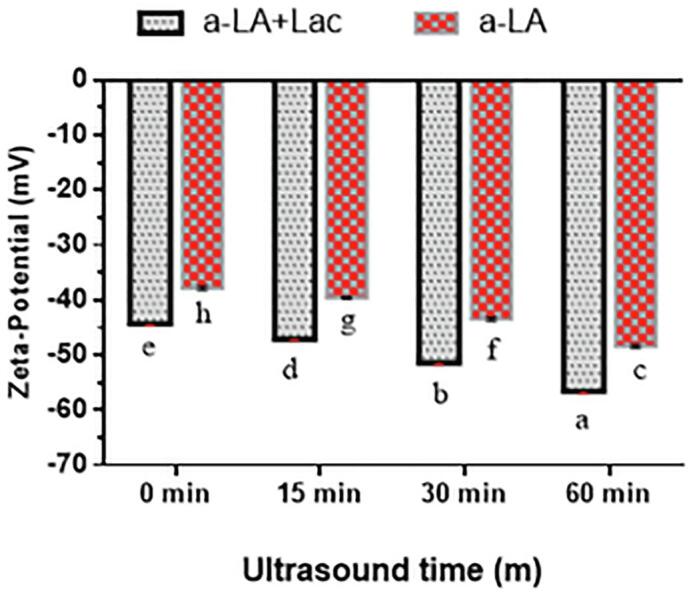
Effect of US pre-treatment (0, 15, 30 and 60 min) and cross-linking on the zeta-potential of laccase cross-linking US-α-LA and US-α-LA. Data were represented as mean and SD (n = 3), significant differences (p < 0.05) in between treatments were differentiated with lower case letters.
3.3. Emulsifying properties
The emulsifying activity index (EAI) presented the ability of proteins absorbed at the interface between oil droplets and water during the development of the emulsion to avoid gravitational separation, flocculation and coalescence [46]. As depicted in Fig. 4A. The EAI of Lac-US-α-lactalbumin and US-α-lactalbumin significantly increased (p < 0.05) from 76.54 to 142.93 m2/g, (approximate increase 86.7%) and from 67.41 to 137.08 m2/g, with the duration of ultrasound from 0 to 60 min, with only an alteration noted from 139.58 to 142.93 m2/g and 120.83 to 137.08 m2/g at > 30 min. These results indicated that the combined US pre-treatment and laccase cross-linking led to the improvement in emulsion stability. Moreover, ultrasound stimulated the interface between proteins and oils or protein and protein. Another possible explanation for improved EAI was because of reduced particle size. It was formerly stated that TGase-treated protein had higher EAI than native protein with pH increased from 4 to 6 [47]. Interestingly, EAI of myofibrillar protein was significantly increased by 95.07% after ultrasound treatment from 0 to 6 min [48].
Fig. 4.
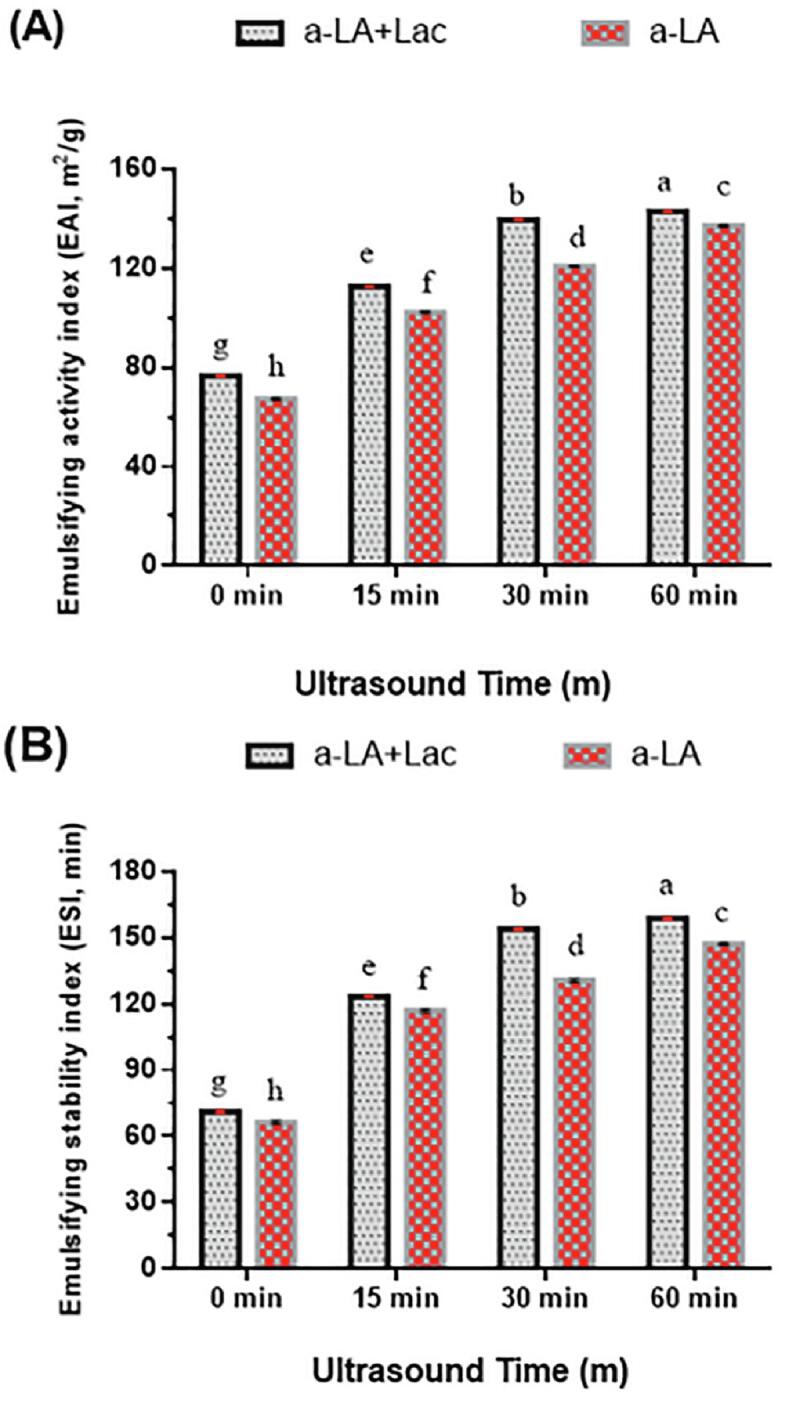
Effect of US pre-treatment (0, 15, 30 and 60 min) and cross-linking on the emulsifying properties (EAI) (A) and emulsifying stability (ESI) (B) of laccase cross-linking US-α-LA and US-α-LA. Data were represented as mean and SD (n = 3), significant differences (p < 0.05) in between treatments were differentiated with lower case letters.
Moreover, the emulsifying stability index (ESI) stated to the capacity of a protein to sustain emulsion constancy for a predetermined period [46]. The ESI of Lac-US-α-lactalbumin and US-α-lactalbumin is presented in Fig. 4B. The ESI of Lac-US-α-lactalbumin and US-α-lactalbumin significantly increased (p < 0.05) from 71.23 to 158.70 min, (an approximate increase 122.7%) and from 66.42 to 147.26 min, with the duration of ultrasound from 0 to 60 min, with only a very minor variation was observed at > 30 min. The higher ESI could be due to the development of large WPI polymers formed by laccase and US pre-treatment. Recently Wang et al. [49] reported that the increase in emulsion stability was inferred to the formation of large WPI polymers produced by TGase and superfine crushing treatment. Moreover, an increase in ESI of cross-linked casein was observed compared with casein at the same protein concentration [50].
3.4. Creaming stability
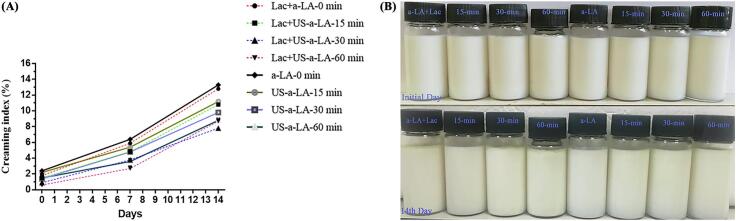
By observation of creaming index, stability of emulsion can be predicted. The density differences causes creaming and oil layer occurred at the surface of the emulsion as large oil droplets moved up [30]. In general, poor stability of emulsion has a direct relationship with higher index values of the creaming. The creaming layer and visual appearance of the Lac-US-α-lactalbumin and US-α-lactalbumin were considered as indicators to evaluate the stability of creaming between 1 and 14 days of storage. CI percentage and images of Lac-US-α-lactalbumin and US-α-lactalbumin emulsion were presented in Fig. 5A and B, respectively. Results indicated that the CI of all the Lac-US-α-lactalbumin emulsions slightly increased from the initial day to 14 days of storage. Lac-US-α-lactalbumin (15 to 60 min) emulsions had lower creaming index than control sample (0 min). As observed from current studies, CI reduced with the increase of ultrasound duration. The Lac-US-α-lactalbumin emulsion at 60 min showed the minimum CI value. Moreover, Lac-US-α-lactalbumin emulsion depicted no apparent emulsion stratifications during 14 days of storage at room temperature. However, the US-α-lactalbumin emulsion also indicated the CI of all the US-α-lactalbumin gradually increased from 0 to 14 days. The untreated (control) α-lactalbumin emulsion showed the worst stability with the maximum CI value (13.3%) upon visual appearance. This might be linked to the fact that the inhomogeneous distribution of oil droplets. Furthermore, the US-α-lactalbumin showed good stability without any phase separation throughout the storage of 14 days at room temperature. The lower values of CI in the Lac-US-α-lactalbumin and US-α-lactalbumin emulsion might be ascribed to the greater ratio of adsorbed protein fraction (Fig. 6) and the small average particle of oil droplets (Fig. 2A). Similarly, it has been previously described for stabilized soy protein isolate emulsion that the decrease in CI was associated with the increase in US intensity [51]. Sato et al. [21] revealed that all the emulsions treated with laccase and ferulic acid were kinetically stable for seven days of storage at pH 7.0.
Fig. 5.
Effect of US pre-treatment (0, 15, 30 and 60 min) and cross-linking on the creaming index (CI %) (A) and typical visual image (B) of laccase cross-linking US-α-LA and US-α-LA stored at room temperature for 14 days.
Fig. 6.
Effect of US pre-treatment (0, 15, 30 and 60 min) and cross-linking on adsorbed protein (AP %) of laccase cross-linking US-α-LA and US-α-LA. Data were represented as mean and SD (n = 3), significant differences (p < 0.05) in between treatments were differentiated with lower case letters.
3.5. Adsorbed protein
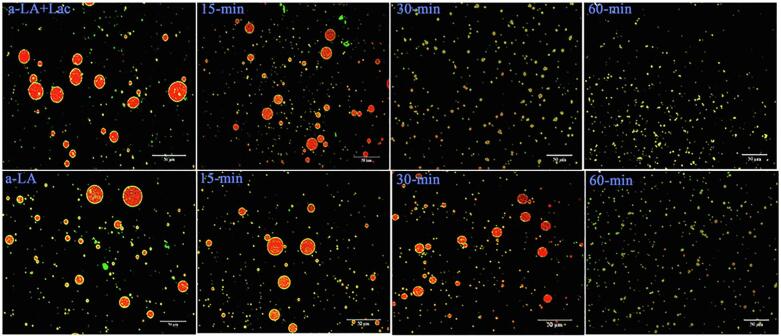
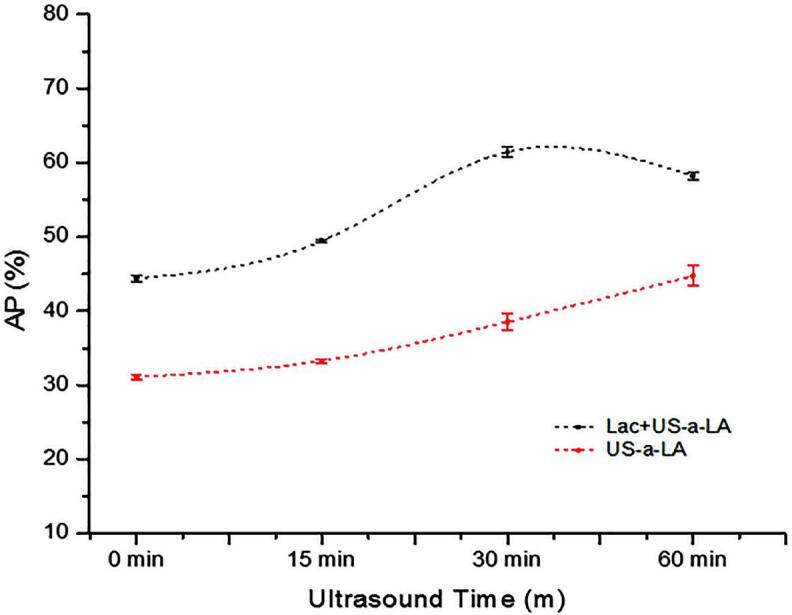
The emulsion stability is influenced by the formation of protein-membrane, which is formed as a result of the absorption of protein molecules on the oil surface [52]. The adsorbed protein of Lac-US-α-lactalbumin and US-α-lactalbumin is depicted in Fig. 6. The value of adsorbed protein of Lac-US-α-lactalbumin and α-lactalbumin emulsion was 44.2% and 31.1%, respectively. With the increase of US duration (0–60 min), the protein absorption of both Lac-US-α-lactalbumin and US-α-lactalbumin remarkably increased (p < 0.05). Lac-US-α-lactalbumin showed maximum value of 61.4% at moderate US treatment (600 W, 30 min). In contrast, US-α-lactalbumin showed maximum protein adsorption of 44.7% after US treatment of 60 min. This finding was consistent with the CLSM microscopy (Fig. 7). A comparison of Fig. 6 with Fig. 2A also showed a close relationship between droplet size and protein adsorption for Lac-US-α-lactalbumin and US-α-lactalbumin. In most cases, the smaller droplet size had the higher protein absorption. Similarly, a previous study reported an inverse relationship between droplet size and protein absorption for emulsion modified by combined US-papain hydrolysis and papain hydrolysis [50].
Fig. 7.
The structure morphology of ultrasound pretreatment (0, 15, 30 and 60 min) and cross-linking on the CLSM micrograph of laccase cross-linking US-α-LA and US-α-LA. Red color denoted oil phase and green represented protein phase. Magnification 40X, and scale bar represent 50 µm. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.6. pH, temperature variation and electrical conductivity determination
The stability of an emulsion can also be evaluated through electrical conductivity [53]. pH, temperature and electrical conductivity (EC) of Lac-US-α-lactalbumin and US-α-lactalbumin emulsion are shown in Table 1. As presented in Table 1, sonication was not induced any noticeable changes in the pH of Lac-US-α-lactalbumin and US-α-lactalbumin emulsion. On the contrary, its value of EC was significantly (p < 0.05) altered, particularly for the Lac-US-α-lactalbumin emulsion after US treatment (15 to 60 min). The increase in EC might be due to the impact of sonotrode and metal erosion during treatments and the release of metal particles. Also, the EC was related to temperature and the fraction of oil. Moreover, a significant (p < 0.05) reduction of EC was observed in US-α-lactalbumin emulsion in comparison to Lac-US-α-lactalbumin one. This was due to the presence of ion aggregates which didn’t take part in the conduction process, the other possible reason could be due to decrease in viscosity which can apparently reduce EC [54]. Previously, the researcher also noted that the formation of hydroxyl radicals and additional species resulted in better conductivity during sonication [55]. Bernardi et al. [56] noticed the increase in EC of oil in water nanoemulsion stabilized by rice bran oil, due to the small particle size and pH value.
Table 1.
Variation in pH, electrical conductivity (mS/cm−1), temperature (oC), and an average increase in temperature of laccase cross-linking α-LA and US-α-LA emulsion. α-LA, control (0 min); Laccase cross-linking US-α-LA (15 min); Laccase cross-linking US-α-LA (30 min); Laccase cross-linking US-α-LA (60 min) and without laccase cross-linking, ultrasound pretreated α-LA, Control (0 min); US-α-LA (15 min); US-α-LA (30 min) and US-α-LA (60 min).
| Treatments | pH | Electrical Conductivity (mS/cm−1) | Temperature (oC) | ‾xc (oC) |
|---|---|---|---|---|
| α-LA + Lac 0 min | 7.0 ± 0.1b | 3.16 ± 0.02a | 23.5 ± 0.1e | – |
| α-LA + Lac 15 min | 7.1 ± 0.1a | 3.27 ± 0.01a | 28.4 ± 0.1d | 4.9 |
| α-LA + Lac 30 min | 7.1 ± 0.1a | 3.32 ± 0.01a | 31.2 ± 0.1a | 7.7 |
| α-LA + Lac 60 min | 7.2 ± 0.1a | 3.22 ± 0.01a | 31 ± 0.1a | 7.5 |
| α-LA-0 min | 7.0 ± 0.1b | 1.16 ± 0.1b | 24 ± 0.2e | – |
| US-α-LA 15 min | 7.1 ± 0.1a | 1.11 ± o.1c | 31.1 ± 0.15a | 7.1 |
| US-α-LA 30 min | 7.2 ± 0.1a | 1.16 ± 0.1b | 29.5 ± 0.1c | 5.5 |
| US-α-LA 60 min | 7.1 ± 0.1a | 1.04 ± 0.1d | 30.1 ± 0.2b | 6.1 |
Each value represents the mean of three independent experiments. Different superscript small letters indicate statistical significance at a value of (p < 0.05).
Indicates the average increase in temperature after each different treatment.
Effect of temprature on Lac-US-α-lactalbumin and US-α-lactalbumin emulsion is shown in Table 1. A rise in the temperature (up to 31 °C) was observed in Lac-US-α-lactalbumin and US-α-lactalbumin emulsion, being lower than protein denaturation temperature [57]. This might be in virtue of high energy input up to 20 kHz probe. Our recent result was in line with Jambrak et al. [24], who proved that a remarkable increase in temperature was noted after US-treated α-lactalbumin due to ultrasound vibration and micro heating of the systems.
3.7. Confocal laser scanning microscopy (CLSM)
The microstructure of Lac-US-α-lactalbumin and US-α-lactalbumin emulsion was measured by using confocal laser scanning microscopy to see a direct image of droplet conduct. In Fig. 7, oil and protein emitted reddish and greenish-yellowish fluorescent light respectively. For fresh emulsions of Lac-US-α-lactalbumin and US-α-lactalbumin, the distribution of oil droplets was homogeneous, there were some bigger droplets but the diameter of most droplets were lesser than the range of 1 µm with a small amount of flocculation discernible (panel Lac-US-α-lactalbumin 30 min, 60 min and panel US-α-lactalbumin 60 min, Fig. 7). Coalescence was found to occur during the emulsion of native Lac-US-α-lactalbumin and US-α-lactalbumin as distribution of large oil droplets was depicted (panels Lac-α-lactalbumin and α-lactalbumin, Fig. 7). It could be due to the strong attractive interaction. Another possible explanation for these large oil droplets might be due to inadequate emulsification, resulting in uneven distribution. For the Lac-US-α-lactalbumin emulsion, the droplets stayed almost stable, showing good storage stability against coalescence. Moreover, the droplets of emulsion were homogeneously dispersed and no concentration-effect was showing small oil droplets as observed in comparison within the absence of Lac-US-α-lactalbumin (panel Lac-US-α-lactalbumin 15 to 30 min, Fig. 7). There was an increase in droplet dispersion in the US-α-lactalbumin emulsions significantly enhanced the creaming of droplets. Because of this clarification occurred on the top with large oil droplets, which ultimately fused into a yellowish coloured separate layer (Fig. 5B). Ma et al. [38] also stated that the laccase-treated vanillic acid-WPI emulsion showed good storage stability after 72 h in comparison without cross-linking. Moreover, Sato et al. [21] also reported that flocculation and inhomogeneous dispersions were observed in sodium caseinate emulsion treated with laccase and ferulic acid at different acidic pH in comparison with neutral pH.
3.8. Lipid oxidation
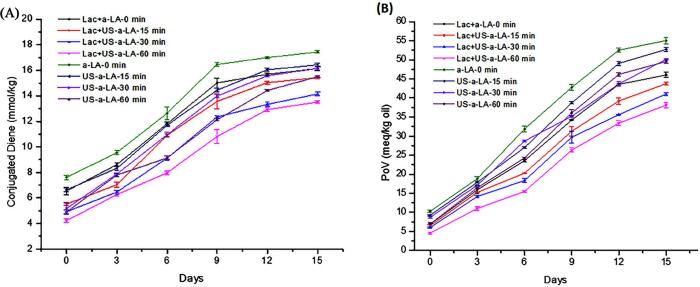
Conjugated dienes (CDs) are often used to determine primary oxidation products or to characterize antioxidative properties [58]. During storage, we studied the link between physical and oxidative stability of emulsion treated with Lac-US-α-lactalbumin and US-α-lactalbumin. In all emulsions, we observed the progression of lipid oxidation, an increased formation of CDs above baseline was observed after the 3rd day of storage. On the 9th day the levels of CDs were maximum in all Lac-US-α-lactalbumin and US-α-lactalbumin emulsions of 4–16 mmol/kg (Fig. 8A). Lac-US-α-lactalbumin-60 min emulsion displayed significantly lower CDs on days 3rd to 15th compared with other Lac-US-α-lactalbumin emulsions. In contrast, the US-α-lactalbumin-15 min and US-α-lactalbumin-30 min emulsions possessed a same level of CDs compared to US-α-lactalbumin-60 min and native (α-lactalbumin-0 min) emulsions. These results suggested that laccase cross-linking with US pre-treatments enhanced the capability of α-lactalbumin to sustain stability to lipid oxidation in CLA oil in water emulsions. It has been proposed that laccase cross-linking combined with ultrasound pre-treatments probably resulted in enhancing antioxidant properties of the native protein, its scavenging ability and chelate metal ions [59]. A recent study by Liu et al. [60] found that the enzymatic hydrolysis of (FBPI) fava bean protein isolate modified by alcalase could improve its oxidative and physical stability of the emulsion. The author ascribed that the antioxidant properties of native proteins were enhanced by moderate hydrolysis and transition metals. In addition, free radicals could be scavenged by hydrolysates, which resulted in reducing emulsion lipids oxidation.
Fig. 8.
Effect of US pre-treatment (0, 15, 30 and 60 min) and cross-linking on the conjugated dienes (CDs) (A) and peroxide value (PV) (B) of laccase cross-linking US-α-LA and US-α-LA stored at room temperature for 15 days. Data were represented as mean and SD (n = 3), significant differences (p < 0.05) in between treatments were differentiated with lower case letters.
3.9. Peroxide values (PV)
The main cause of oil deterioration was oxidation and the primary product during this reaction was hydroperoxide produced as a result of the reaction between unsaturated fattyacid and oxygen. The oxidation of Lac-US-α-lactalbumin and US-α-lactalbumin emulsions were observed and showed as peroxide values (PV) during two weeks of storage. The development of main oxidation products in Lac-US-α-lactalbumin and US-α-lactalbumin emulsions were measured as PV(Fig. 8B), progressively improved throughout storage time with significant (P < 0.05) statistical increase. The PV of Lac-US-α-lactalbumin emulsion increased with the storage time prolonged. PV values were 46.03, 43.74, 41.02 and 38.10 meq/kg oil for emulsions treated with native (control), Lac-US-α-lactalbumin-15 min, 30 min and 60 min, respectively, after 15 days of storage at ambient temperature. The Lac-US-α-lactalbumin-15 min and 30 min, had a similar level of PV than Lac-US-α-lactalbumin-60 min emulsions. Besides, the emulsions treated without laccase were also increased with time from initial to 15th day of storage. The PV values of the control emulsion reached the maximum value (55.04 meq/kg oil) on day 15. However, the PV values of the US-α-lactalbumin-30 min and US-α-lactalbumin-60 min emulsion reached the maximum levels of 50.10 and 49.54 meq/kg oil, respectively, on day 15, which was much lower than that of the US-α-lactalbumin-30 and native emulsions. US-α-lactalbumin emulsion showed low oxidative stability due to its large particle sizes, thus led to creaming and accruing direct exposure of oxygen in the headspace. On the contrary, there was an increase in the interfacial area due to the smaller particle size of Lac-US-α-lactalbumin emulsion, which might enhance the vulnerability of emulsion to react with oxygen in a continuous phase when there was a large availability of oxygen. The above findings were in line with Lethuat et al. [61], who investigated conjugated diene in emulsion made and stabilized from sunflower oil and bovine serum albumin. Moreover, in another study conducted by Let et al. [62] showed less oxidation with smaller droplets in milk stabilized fish oil emulsions. Likewise, higher stability with smaller droplet sizes was observed in caseinate-stabilized emulsion [63].
3.10. Surface hydrophobicity measurement
To estimate the variations in protein conformation surface hydrophobicity is used as structural characterization [64]. Surface hydrophobicity of Lac-US-α-lactalbumin and US-α-lactalbumin is shown in Fig. 9. Lac-US-α-lactalbumin and US-α-lactalbumin produced a substantial rise (p < 0.05) in surface hydrophobicity with US time from 0 to 60 min. This outcome was in agreement with Yuan et al. [25] stated that surface hydrophobicity of laccase-catalyzed α-lactalbumin in the presence of ferulic acid showed a remarkable increase in comparison with α-lactalbumin and α-lactalbumin incubated with laccase. Furthermore, it was also reported that surface hydrophobicity of WPI increased with an increase of ultrasound power (0–600 W) and pH value (3–11) [65]. This showed that cavitation produced during ultrasound could induce the generation of laccase-catalyzed α-lactalbumin conjugate. Similarly, it was also known that ultrasound pretreatment could result in increasing surface hydrophobicity of the transglutaminase cross-linked wheat gluten gels and soy protein isolates [66].Fig.
Fig. 9.
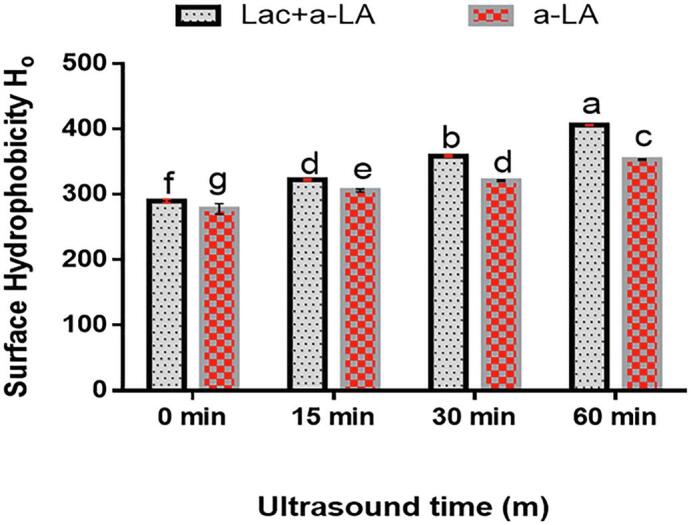
Effect of US pre-treatment (0, 15, 30 and 60 min) and cross-linking on surface hydrophobicity of laccase cross-linking US-α-LA and US-α-LA. Data were represented as mean and SD (n = 3), significant differences (p < 0.05) in between treatments were differentiated with lower case letters.
3.11. Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE)
The effect of combined ultrasound pretreatment (0, 15, 30 and 60 min) and laccase cross-linking is evaluated by using SDS-PAGE under reducing conditions (Fig. 10). Compared with US-α-lactalbumin emulsion, Lac-US-α-lactalbumin emulsion had the formation of polymers around at 120 kDa. Furthermore, Yuan et al. [25] stated that there was production of polymers, trimers and dimers of 40–120 kDa in α-lactalbumin treated with laccase and FA. However, with US time prolonged, molecular weight distribution of Lac-US-α-lactalbumin and US-α-lactalbumin emulsion was not changed.
Fig. 10.
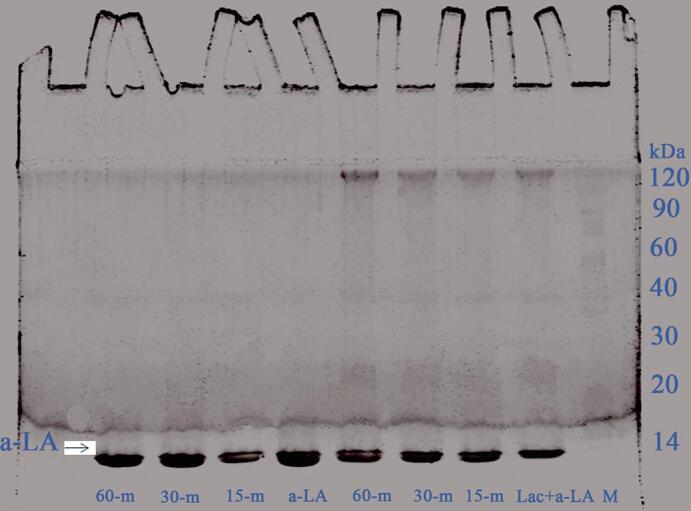
Effect of US pre-treatment (0, 15, 30 and 60 min) and cross-linking on the SDS-PAGE of laccase cross-linking US-α-LA and US-α-LA emulsion. M represents the molecular marker.
4. Conclusion
This study revealed the promising use of combined treatment of US pre-treatment and laccase cross-linking to improve the oxidative stability of α-lactalbumin-stabilized CLA emulsions. We found a significant increase in emulsifying properties and oxidative stability of CLA emulsions after combined treatment of US pre-treatment and laccase cross-linking. Lac-US-α-lactalbumin emulsion showed no sign of phase separation, lower creaming index, smaller particle size and a higher ratio of adsorbed protein at the oil–water interface than US-α-lactalbumin emulsion. Our derived results have shown the possibility for the formation of stable CLA emulsions in the food and beverage industries.
CRediT authorship contribution statement
Abdul Qayum: Data curation, Writing - original draft. Meng Li: Investigation. Ruijie Shi: Resources, Software. Akhunzada Bilawal: Methodology, Validation. Munkh-Amgalan Gantumur: . Muhammad Hussain: Data curation. Muhammad Ishfaq: . Syed Waqas Ali Shah: Formal analysis. Zhanmei Jiang: Supervision, Funding acquisition, Conceptualization. Juncai Hou: Supervision, Project administration.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was supported by project for the National Key Research and Development Program of China (No.2016YFD0400605) and Key Program of Heilongjiang Province of China (No. 2019ZX07B02-04). We thank Dvisco food international (Eden prairie, MN, USA) for providing α-LA powders.
Contributor Information
Zhanmei Jiang, Email: zhanmeijiang@neau.edu.cn.
Juncai Hou, Email: jchou@neau.edu.cn.
References
- 1.Chen B., McClements D.J., Decker E.A. Design of Foods with Bioactive Lipids for Improved Health. Annu. Rev. Food Sci. Technol. 2013;4(1):35–56. doi: 10.1146/annurev-food-032112-135808. [DOI] [PubMed] [Google Scholar]
- 2.Shahidi F., Zhong Y. Lipid oxidation and improving the oxidative stability. Chem. Soc. Rev. 2010;39(11):4067. doi: 10.1039/b922183m. [DOI] [PubMed] [Google Scholar]
- 3.Schultz S., Wagner G., Urban K., Ulrich J. High-Pressure Homogenization as a Process for Emulsion Formation. Chem. Eng. Technol. 2004;27(4):361–368. doi: 10.1002/ceat.200406111. [DOI] [Google Scholar]
- 4.Neirynck N., Van der Meeren P., Lukaszewicz-Lausecker M., Cocquyt J., Verbeken D., Dewettinck K. Influence of pH and biopolymer ratio on whey protein–pectin interactions in aqueous solutions and in O/W emulsions. Colloids Surf., A. 2007;298(1-2):99–107. doi: 10.1016/j.colsurfa.2006.12.001. [DOI] [Google Scholar]
- 5.Neirynck N., Dewettinck K., Meeren P.-V.-D. Influence of protein concentration and homogenization pressure on O/W emulsifying and emulsion-stabilizing properties of sodium caseinate and whey protein isolate. Milchwissenschaft. 2009;64:36–40. http://hdl.handle.net/1854/LU-623706. [Google Scholar]
- 6.Khalloufi S., Corredig M., Goff H.D., Alexander M. Flaxseed gums and their adsorption on whey protein-stabilized oil-in-water emulsions. Food Hydrocolloids. 2009;23(3):611–618. doi: 10.1016/j.foodhyd.2008.04.004. [DOI] [Google Scholar]
- 7.Junwen L., Tiejing L., Xinhuai Z. Hydrogen peroxide and ferulic acid-mediated oxidative cross-linking of casein catalyzed by horseradish peroxidase and the impacts on emulsifying property and microstructure of acidified gel. Afr. J. Biotechnol. 2009;8:6993–6999. doi: 10.4314/ajb.v8i24.68786. [DOI] [Google Scholar]
- 8.Onwulata C.I., Tomasula P.M. Gelling Properties of Tyrosinase-Treated Dairy Proteins. Food Bioprocess Technol. 2010;3(4):554–560. doi: 10.1007/s11947-008-0124-4. [DOI] [Google Scholar]
- 9.Færgemand M., Murray B.S., Dickinson E. Cross-Linking of Milk Proteins with Transglutaminase at the Oil−Water Interface. J. Agric. Food Chem. 1997;45(7):2514–2519. doi: 10.1021/jf9609789. [DOI] [Google Scholar]
- 10.Solomon E.I., Sundaram U.M., Machonkin T.E. Multicopper Oxidases and Oxygenases. Chem. Rev. 1996;96(7):2563–2606. doi: 10.1021/cr950046o. [DOI] [PubMed] [Google Scholar]
- 11.Littoz F., McClements D.J. Bio-mimetic approach to improving emulsion stability: Cross-linking adsorbed beet pectin layers using laccase. Food Hydrocolloids. 2008;22(7):1203–1211. doi: 10.1016/j.foodhyd.2007.06.009. [DOI] [Google Scholar]
- 12.Mattinen M., Suortti T., Gosselink R., Argyropoulos D.S., Evtuguin D., Suurnakki A., De Jong E., Tamminen T. Polymerization of different lignins by laccase. Bio Resources. 2008;3:549–565. doi: 10.15376/biores.3.2.549-565. [DOI] [Google Scholar]
- 13.Mattinen M.-L., Kruus K., Buchert J., Nielsen J.H., Andersen H.J., Steffensen C.L. Laccase-catalyzed polymerization of tyrosine-containing peptides. FEBS J. 2005;272(14):3640–3650. doi: 10.1111/j.1742-4658.2005.04786.x. [DOI] [PubMed] [Google Scholar]
- 14.Mattinen M.-L., Hellman M., Permi P., Autio K., Kalkkinen N., Buchert J. Effect of Protein Structure on Laccase-Catalyzed Protein Oligomerization. J. Agric. Food Chem. 2006;54(23):8883–8890. doi: 10.1021/jf062397h. [DOI] [PubMed] [Google Scholar]
- 15.Uluko H., Zhang S., Liu L.u., Tsakama M., Lu J., Lv J. Effects of thermal, microwave, and ultrasound pretreatments on antioxidative capacity of enzymatic milk protein concentrate hydrolysates. J. Funct. Foods. 2015;18:1138–1146. doi: 10.1016/j.jff.2014.11.024. [DOI] [Google Scholar]
- 16.Chandrapala J., Oliver C., Kentish S., Ashokkumar M. Ultrasonics in food processing. Ultrason. Sonochem. 2012;19(5):975–983. doi: 10.1016/j.ultsonch.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 17.Chandrapala J., Oliver C., Kentish S., Ashokkumar M. Ultrasonics in food processing – Food quality assurance and food safety. Trends Food Sci. Technol. 2012;26(2):88–98. doi: 10.1016/j.tifs.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 18.Chemat F., Zill-e-Huma, Khan M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011;18(4):813–835. doi: 10.1016/j.ultsonch.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 19.Thalmann C., Lötzbeyer T. Enzymatic cross-linking of proteins with tyrosinase. Eur. Food Res. Technol. 2002;214(4):276–281. doi: 10.1007/s00217-001-0455-0. [DOI] [Google Scholar]
- 20.Jiang Z., Yuan X., Yao K., Li X., Zhang X., Mu Z., Jiang L., Hou J. Laccase-aided modification: Effects on structure, gel properties and antioxidant activities of α-lactalbumin. LWT. 2017;80:355–363. doi: 10.1016/j.lwt.2017.02.043. [DOI] [Google Scholar]
- 21.Sato A.C.K., Perrechil F.A., Costa A.A.S., Santana R.C., Cunha R.L. Cross-linking proteins by laccase: Effects on the droplet size and rheology of emulsions stabilized by sodium caseinate. Food Res. Int. 2015;75:244–251. doi: 10.1016/j.foodres.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 22.Chen B., Li H., Ding Y., Suo H. Formation and microstructural characterization of whey protein isolate/beet pectin coacervations by laccase catalyzed cross-linking. LWT - Food Sci. Technol. 2012;47(1):31–38. doi: 10.1016/j.lwt.2012.01.006. [DOI] [Google Scholar]
- 23.Margulis M.A., Margulis I.M. Calorimetricmethod for measurement of acoustic power absorbed in a volume of a liquid. Ultrason. Sonochem. 2003;10:343–345. doi: 10.1016/S1350-4177(03)00100-7. [DOI] [PubMed] [Google Scholar]
- 24.Jambrak A.R., Mason T.J., Lelas V., Krešić G. Ultrasonic effect on physicochemical and functional properties of α-lactalbumin. LWT - Food Sci. Technol. 2010;43(2):254–262. doi: 10.1016/j.lwt.2009.09.001. [DOI] [Google Scholar]
- 25.Yuan X., Li X., Zhang X., Mu Z., Gao Z., Jiang L., Jiang Z. Effect of ultrasound on structure and functional properties of laccase-catalyzed α-lactalbumin. J. Food Eng. 2018;223:116–123. doi: 10.1016/j.jfoodeng.2017.12.008. [DOI] [Google Scholar]
- 26.Ma L., Li A., Li T., Li M., Wang X., Hussain M.A., Qayum A., Jiang Z., Hou J. Structure and characterization of laccase-crosslinked α-lactalbumin: Impacts of high pressure homogenization pretreatment. LWT. 2020;118:108843. doi: 10.1016/j.lwt.2019.108843. [DOI] [Google Scholar]
- 27.Kato, A Nakai, S.1980. Hydrophobicity determined by a fluorescence probe method and its correlation with surface properties of proteins, BBA - Protein Struct. 624 13– 20.doi: /10.1016/0005-2795(80)90220-2. [DOI] [PubMed]
- 28.Jambrak Anet Režek, Lelas Vesna, Mason Timothy J., Krešić Greta, Badanjak Marija. Physical properties of ultrasound treated soy proteins. J. Food Eng. 2009;93(4):386–393. doi: 10.1016/j.jfoodeng.2009.02.001. [DOI] [Google Scholar]
- 29.Li Tong, Hu Jialun, Tian Ran, Wang Kaili, Li Jinpeng, Qayum Abdul, Bilawal Akhunzada, Gantumur Munkh-Amgalan, Jiang Zhanmei, Hou Juncai. Citric acid promotes disulfide bond formation of whey protein isolate in non-acidic aqueous system. Food Chem. 2021;338:127819. doi: 10.1016/j.foodchem.2020.127819. [DOI] [PubMed] [Google Scholar]
- 30.Klompong Vilailak, Benjakul Soottawat, Kantachote Duangporn, Shahidi Fereidoon. Antioxidative activity and functional properties of protein hydrolysate of yellow stripe trevally (Selaroides leptolepis) as influenced by the degree of hydrolysis and enzyme type. Food Chem. 2007;102(4):1317–1327. doi: 10.1016/j.foodchem.2006.07.016. [DOI] [Google Scholar]
- 31.Arancibia Carla, Riquelme Natalia, Zúñiga Rommy, Matiacevich Silvia. Comparing the effectiveness of natural and synthetic emulsifiers on oxidative and physical stability of avocado oil-based nanoemulsions. Innovative Food Sci. Emerg. Technol. 2017;44:159–166. doi: 10.1016/j.ifset.2017.06.009. [DOI] [Google Scholar]
- 32.Liu Fu, Tang Chuan-He. Emulsifying Properties of Soy Protein Nanoparticles: Influence of the Protein Concentration and/or Emulsification Process. J. Agric. Food Chem. 2014;62(12):2644–2654. doi: 10.1021/jf405348k. [DOI] [PubMed] [Google Scholar]
- 33.Lowry O.H., Rosebrough N.J., Farr L.A., Randall R.J. Protein measurement whit the folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 34.Estevez M., Kylli P., Puolanne E., Kivikari R., Heinonen M. Fluorescence spectroscopy as a novel approach for the assessment of myofibrillar protein oxidation in oil-in-water emulsions. Meat Sci. 2008;80:1290–1296. doi: 10.1016/j.lwt.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 35.Shantha N.C., Decker E. A, Rapid, sensitive, iron-based spectrophotometric methods for determination of peroxide values of food lipids. J. AOAC. Int. 1994;77:421–424. https://www.ncbi.nlm.nih.gov/pubmed/8199478 [PubMed] [Google Scholar]
- 36.McClements David Julian. Protein-stabilized emulsions. Curr. Opin. Colloid Interface Sci. 2004;9(5):305–313. doi: 10.1016/j.cocis.2004.09.003. [DOI] [Google Scholar]
- 37.Hu Hao, Li-Chan Eunice C.Y., Wan Li, Tian Ming, Pan Siyi. The effect of high intensity ultrasonic pre-treatment on the properties of soybean protein isolate gel induced by calcium sulfate. Food Hydrocolloids. 2013;32(2):303–311. doi: 10.1016/j.foodhyd.2013.01.016. [DOI] [Google Scholar]
- 38.Ma Hairan, Forssell Pirkko, Partanen Riitta, Buchert Johanna, Boer Harry. Improving Laccase Catalyzed Cross-Linking of Whey Protein Isolate and Their Application as Emulsifiers. J. Agric. Food Chem. 2011;59(4):1406–1414. doi: 10.1021/jf103591p. [DOI] [PubMed] [Google Scholar]
- 39.Gülseren İbrahim, Güzey Demet, Bruce Barry D., Weiss Jochen. Structural and functional changes in ultrasonicated bovine serum albumin solutions. Ultrason. Sonochem. 2007;14(2):173–183. doi: 10.1016/j.ultsonch.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 40.Qayum A., Hussain M., Meng L., Jiaqi L., Ruijie S., Tianqi L., Anwar A., Ahmed Z., Hou J., Jiang Z. Gelling, microstructure and water-holding properties of alpha- lactalbumin emulsion gel: Impact of combined ultrasound pretreatment and laccase cross- linking. J. Food. Hydrocolloids. 2021;110 doi: 10.1016/j.jfoodhyd.2020.106122. [DOI] [Google Scholar]
- 41.Yanjun Sun, Jianhang Chen, Shuwen Zhang, Hongjuan Li, Jing Lu, Lu Liu, Uluko H., Yanling Su, Wenming Cui, Wupeng Ge, Jiaping Lv. Effect of power ultrasound pre-treatment on the physical and functional properties of reconstituted milk protein concentrate. J. Food Eng. 2014;124:11–18. doi: 10.1016/j.jfoodeng.2013.09.013. [DOI] [Google Scholar]
- 42.McClements David Julian, Rao Jiajia. Food-Grade Nanoemulsions: Formulation, Fabrication, Properties, Performance, Biological Fate, and Potential Toxicity. Crit. Rev. Food Sci. Nutr. 2011;51(4):285–330. doi: 10.1080/10408398.2011.559558. [DOI] [PubMed] [Google Scholar]
- 43.R. J. Hunter, Zeta Potential in Colloidal Sciences: Principles and Applications, ACADEMIC PRESS INC. USA, 1981. https://www.elsevier.com/books/zeta- potential-in-colloidscience/hunter/978-0-12-361961-7.
- 44.Zhang Ziye, Regenstein Joe M., Zhou Peng, Yang Yuling. Effects of high intensity ultrasound modification on physicochemical property and water in myofibrillar protein gel. Ultrason. Sonochem. 2017;34:960–967. doi: 10.1016/j.ultsonch.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 45.Wang Wan, Zhong Qixin, Hu Zhixiong. Nanoscale Understanding of Thermal Aggregation of Whey Protein Pretreated by Transglutaminase. J. Agric. Food Chem. 2013;61(2):435–446. doi: 10.1021/jf304506n. [DOI] [PubMed] [Google Scholar]
- 46.Shen Xue, Fang Tianqi, Gao Feng, Guo Mingruo. Effects of ultrasound treatment on physicochemical and emulsifying properties of whey proteins pre- and post-thermal aggregation. Food Hydrocolloids. 2017;63:668–676. doi: 10.1016/j.foodhyd.2016.10.003. [DOI] [Google Scholar]
- 47.Motoki M., Nio N. Crosslinking between Different Food Proteins by Transglutaminase. J. Food Sci. 1983;48:561–566. [Google Scholar]
- 48.Li Ke, Fu Lei, Zhao Ying-Ying, Xue Si-Wen, Wang Peng, Xu Xing-Lian, Bai Yan-Hong. Use of high-intensity ultrasound to improve emulsifying properties of chicken myofibrillar protein and enhance the rheological properties and stability of the emulsion. Food Hydrocolloids. 2020;98:105275. doi: 10.1016/j.foodhyd.2019.105275. [DOI] [Google Scholar]
- 49.Wang Chunyan, Li Tianqi, Ma Ling, Li Tong, Yu Haiying, Hou Juncai, Jiang Zhanmei. Consequences of superfine grinding treatment on structure, physicochemical and rheological properties of transglutaminase-crosslinked whey protein isolate. Food Chem. 2020;309:125757. doi: 10.1016/j.foodchem.2019.125757. [DOI] [PubMed] [Google Scholar]
- 50.Chen Lin, Chen Jianshe, Ren Jiaoyan, Zhao Mouming. Effects of Ultrasound Pretreatment on the Enzymatic Hydrolysis of Soy Protein Isolates and on the Emulsifying Properties of Hydrolysates. J. Agric. Food Chem. 2011;59(6):2600–2609. doi: 10.1021/jf103771x. [DOI] [PubMed] [Google Scholar]
- 51.Taha Ahmed, Hu Tan, Zhang Zhuo, Bakry Amr M., Khalifa Ibrahim, Pan Siyi, Hu Hao. Effect of different oils and ultrasound emulsification conditions on the physicochemical properties of emulsions stabilized by soy protein isolate. Ultrason. Sonochem. 2018;49:283–293. doi: 10.1016/j.ultsonch.2018.08.020. [DOI] [PubMed] [Google Scholar]
- 52.Dickinson Eric. Biopolymer-based particles as stabilizing agents for emulsions and foams. Food Hydrocolloids. 2017;68:219–231. doi: 10.1016/j.foodhyd.2016.06.024. [DOI] [Google Scholar]
- 53.Wei Z., Ming-Yuan L., Mei-qin L.N., Tao L., Chun Y. electrical conductivity and stability of o/w emulsions. China. Academic. J. Electric. Pub. H. 2008:11–15. http://www.syxbsyjg.com/EN/Y2008/V24/I5/592. [Google Scholar]
- 54.Southall J.P., StA Hubbard H.V., Johnston S.F., Rogers V., Davies G.R., McIntyre J.E., Ward I.M. Ionic conductivity and viscosity correlations in liquid electrolytes for incorporation into PVDF gel electrolytes. Solid State Ionics. 1996;85:51–60. doi: 10.1016/0167-2738(96)00040-9. [DOI] [Google Scholar]
- 55.Petrier Christian, Jeunet Andre, Luche Jean Louis, Reverdy Gilbert. Unexpected frequency effects on the rate of oxidative processes induced by ultrasound. J. Am. Chem. Soc. 1992;114(8):3148–3150. doi: 10.1021/ja00034a077. [DOI] [Google Scholar]
- 56.Bernardi Daniela S, Pereira Tatiana A, Maciel Naira R, Bortoloto Josiane, Viera Gisely S, Oliveira Gustavo C, Rocha-Filho Pedro A. Formation and stability of oil-in-water nanoemulsions containing rice bran oil: in vitro and in vivo assessments. J Nanobiotechnol. 2011;9(1):44. doi: 10.1186/1477-3155-9-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Giroux Hélène J, Britten Michel. Heat treatment of whey proteins in the presence of anionic surfactants. Food Hydrocolloids. 2004;18(4):685–692. doi: 10.1016/j.foodhyd.2003.11.012. [DOI] [Google Scholar]
- 58.R. Abuzaytoun and F. Shahidi, F. Oxidative stability of flax and hemp oils. J. Amer. Oil Chem. Soc., 83 (2006) 855–861. doi:10.1007/s11746-006-5037-7.
- 59.Sarmadi Bahareh H., Ismail Amin. Antioxidative peptides from food proteins: A review. Peptides. 2010;31(10):1949–1956. doi: 10.1016/j.peptides.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 60.Liu Chang, Bhattarai Mamata, Mikkonen Kirsi S., Heinonen Marina. Effects of Enzymatic Hydrolysis of Fava Bean Protein Isolate by Alcalase on the Physical and Oxidative Stability of Oil-in-Water Emulsions. J. Agric. Food Chem. 2019;67(23):6625–6632. doi: 10.1021/acs.jafc.9b00914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lethuaut Laurent, Métro François, Genot Claude. Effect of droplet size on lipid oxidation rates of oil-in-water emulsions stabilized by protein. J Amer Oil Chem Soc. 2002;79(5):425. doi: 10.1007/s11746-002-0500-z. [DOI] [Google Scholar]
- 62.L. B. Mette, C. Jacobsen, M. Ann-Dorit, Sørensen and A. S. Meyer. Homogenization conditions affect the oxidative stability of fish oil enriched milk emulsions: lipid oxidation. J. Agric. Food Chem. 55 (2007) 1773−1780. doi:10.1021/jf062391s. [DOI] [PubMed]
- 63.Ries D., Ye A., Haisman D., Singh H. Antioxidant properties of caseins and whey proteins in model oil-in-water emulsions. Int. Dairy J. 2010;20(2):72–78. doi: 10.1016/j.idairyj.2009.09.001. [DOI] [Google Scholar]
- 64.Jakopovic K.L., Barukcic I., Bozanic R. Physiological significance, structure and isolation of α- lactalbumin. Mljekarstvo. 2016;66(1):3–11. doi: 10.15567/mljekarstvo.2016.0101. [DOI] [Google Scholar]
- 65.Gao Hao, Ma Ling, Li Tianqi, Sun Dongxue, Hou Joucai, Li Aili, Jiang Zhanmei. Impact of ultrasonic power on the structure and emulsifying properties of whey protein isolate under various pH conditions. Process Biochem. 2019;81:113–122. doi: 10.1016/j.procbio.2019.03.012. [DOI] [Google Scholar]
- 66.Qin Xin-Sheng, Luo Shui-Zhong, Cai Jing, Zhong Xi-Yang, Jiang Shao-Tong, Zhao Yan-Yan, Zheng Zhi. Transglutaminase-induced gelation properties of soy protein isolate and wheat gluten mixtures with high intensity ultrasonic pretreatment. Ultrason. Sonochem. 2016;31:590–597. doi: 10.1016/j.ultsonch.2016.02.010. [DOI] [PubMed] [Google Scholar]