Highlights
-
•
Bioactive compounds such as total phenols maintained better by ultrasonication.
-
•
Weight loss of ultrasonicated arils was lower during storage.
-
•
Microbial load of arils decreased by ultrasonication within storage time.
Keywords: Pomegranate arils, Ultrasound treatment, Microbial loads, Bioactive compounds
Abstract
Difficulty of Pomegranate fruit peeling and arils separation are the main motivations of progressive ready-to-eat pomegranate fresh arils industry. Also, extracted pomegranate arils are highly perishable due to water loss and microbial contamination expose. The aim of the current work was then to evaluate the effect of ultrasound for 15 and 30 min on maintenance of bioactive compounds and microbial load reduction of pomegranate arils cv. Rabbab. Treated arils were kept at 5 °C and analyzed during 15 days of storage. The most and least weight loss of arils obtained in control and 30 min treated samples, respectively. After 12 days of storage, all samples were decayed except those treated for 15 and 30 min. The ultrasound treatment significantly prevented degradation of anthocyanin and ascorbic acid compounds. Total phenol and antioxidant activity decreased during storage. At the end of storage, the most total phenol content (3898.6 mg GAE L−1) was found in arils treated for 30 min whereas the most anthocyanin (91.93 mg L−1), total antioxidant activity (82.65%), and ascorbic acid (2.53 mg L−1) were found in arils treated for 15 min. Ultrasound treated arils had lower microbial load (total mesophilic bacteria in control and 30-min treated samples) in each stage during storage. At the end of storage, the microbial load in treated and control arils was 0.7 and 0.2 Log CFU g−1, respectively). Overall, ultrasound treatment effectively reduced weight loss and preserved bioactive compounds during storage.
1. Introduction
Ultrasound waves are non-toxic, safe, and ecofriendly treatments. Ultrasound technology has been used widely in almost all fields of food industry including microbial inactivation [1], filtration [2], [3], [4] and extraction [5], [6], [7]. Ultrasonic has been applied as a technique to control disease, and to preserve fresh products such as litchi [8], apple [9] and vegetables such as lettuce [10], spinach leaves [11] and cucumbers [12]. The inhibitory effect of ultrasound at 40 KHz, for 10 min on microbial pollution of peach fruit has been reported [13]. Using of ultrasonic treatments has been shown to be promising as a technology for maintaining quality and to prolong the storability and shelf life of plums [14] and tomatoes [15]. Ultrasound power and treatment time are the main factors which can affect ultrasound efficiency [16]. Using 250 W of ultrasound for 9.8 min has been considered as a potential method of diminishing fungal contamination and preserving biochemical compounds in strawberry fruit [17].
Pomegranate (Punica granatum L.) is a main resource of polyphenol compounds with high antioxidant capacity [18]. Currently, the use of minimally processed arils is expanding due to consumer demands. However extracted arils are highly perishable which shorten its shelf life [18], [19], [20]. Decay is a critical challenge for maintenance of pomegranate arils. Botrytis cinerea, Aspergillus niger, Penicillium spp. and Alternaria spp are the main pathogens cause contamination of arils [21] Hence, new techniques are needed to preserve arils quality considered as human-safe. It has been reported the effects of storage temperature [22], and coating treatments [23], [18] on contamination reduction and prolongation of the pomegranate arils shelf life. To date, rare data has been reported on the effects of ultrasonic waves on pomegranate arils after harvest. So, the aim of this experiment was to determine the effect of ultrasonic time on maintenance of biochemicals and microbial decontamination of pomegranate arils were kept in cold storage time.
2. Materials and methods
2.1. Fruit sample preparation
The pomegranates (Punica granatum L. cv. Rabab) were harvested, at full maturity stage, from a commercial orchard in Neyriz, Fars province, Iran and were transported to the Postharvest Lab of Shiraz University immediately before processing. After washing with tap water arils were extracted and those with same color placed in a basket, washed under water, rinsed and finally dried at room temperature.
2.2. Ultrasonic treatment
Arils were immersed in a water bath ultrasonic chamber (China) containing sterile water and subjected to frequency of 40 KHz for 15 and 30 min. Then, arils placed in a basket till air-dried. One part of prepared arils were immediately analyzed in order to demonstrate day zero quality and thereafter, about 300 g of arils were randomly divided into three lots of 100 g, were put on the polystyrene boxes, then were kept at 5 °C and 90% RH [21] to be analyzed at 3-day intervals for 15 days.
2.3. Fruit quality studies
2.3.1. Weight loss (WL)
For measuring WL, boxes containing arils were weighted at day zero (W1), and also at other sampling times (W2). The weight loss was determined by the following Eq. (1) [24].
| (1) |
2.3.2. Titratable acidity and water total soluble solids
Approximately 30 g of arils was juiced using the mortar and pestle. This juice was used to measure Total soluble solids (TSS), titratable acidity (TA), and ascorbic acid content. TSS was determined by means of hand-held refractometer (ATAGO, Japan). TA was measured by taking 5 mL of juice and this was titrated with 0.1 mol L−1 NaOH to pH 8.2 and expressed as a percentage of anhydrous citric acid. pH was determined by pH meter (JENWAY 351, England).
2.3.3. Ascorbic acid assay
Ascorbic acid was measured by method reported [25]. Briefly, 10 mL of 1% meta phosphoric acid was blended with 100 µL of fruit extract, then 1 mL of mixture was added to 9 mL of 2,6-dichlorophenol indophenol, and the absorbance was recorded using the spectrophotometer (Spectronic 20D, USA) at 515 nm.
2.3.4. Total anthocyanins
Anthocyanin concentration was determined based on pH differential procedure [26]. Absorbance was recorded at 510 and 700 nm by microplate spectrophotometer (Epoch Biotech, Germany). Results were expressed as cyanidin-3-glucoside equivalent per L of fruit extract (mg L−1).
2.3.5. Total phenolic content
To prepare arils juice, arils of each replicate was extracted and centrifuged for 15 min at 10,000g. Total phenolic content (TPC) was measured by the Folin-Ciocalteu reagent [27]. Briefly, 700 µL of extracted juice was mixed with 900 µL of 2% sodium carbonate. After 3 min incubation at room temperature, 180 µL of 50% folin was added, and the mixture was incubated for 30 min at same condition. The absorbance was recorded at 750 nm using microplate spectrophotometer (Epoch Biotech, Germany). The concentration of TPC was expressed as mg gallic acid equivalent per L (mg GAE L-1).
2.3.6. Total Antioxidant Activity (TAA)
To prepare arils juice, arils of each replicate was extracted and centrifuged for 15 min at 10,000g. TAA of each extract was measured using DPPH radical procedure [28]. Afterwards, 100 µL of extract was incorporated with 1 mL of DPPH (0.1 mM) and 1 mL of Tris-HCl (pH 7.5) buffer and was incubated at the room temperature for 30 min. Then, the absorbance was recorded using microplate spectrophotometer (Epoch Biotech, Germany) at 517 nm. Antioxidant activity was evaluated by the following Eq. (2):
| (2) |
2.3.7. Color measurement
The external color was assessed by measuring L* (lightness), a* (redness) and b* (yellowness(chromaticity index with Minolta Chroma-meter (CR-400, Japan). chroma (C*) and hue angle (h°), were then reported according to the following Eqs. (3) and (4) [29].
| (3) |
| (4) |
2.4. Microbiological evaluations
From each treatment, 10 g of arils were homogenized and serially diluted with saline solution (NaCl 0.9%) in triplicate. Microbial colonies were obtained by means of the pour plate method on the Plate Count Agar (PCA, Sigma Aldrich, USA) for mesophilic aerobic bacteria incubated at 30 °C for 48 h and also applied the Yeast extract Glucose Chloramphenicol Agar (YGC, Sigma Aldrich. USA) for molds and yeast incubated at 25 °C for 72 h. Microbial counts were expressed as log CFU g−1 [30].
2.5. Statistical analysis
A complete randomized design (CRD) was applied to analysis of the treatments. Quality parameters were analyzed considering two-way analysis of variance with SAS (v. 9.2). LSD (least significant difference) test at P = 0.01 was used for determining different between the means. Principal component analysis (PCA) was carried out with GenStat software (v. 12.1) software. Pearson correlation coefficients were measured with SPSS software (v. 16.0) for all traits to explore the relationship between them.
3. Results and discussion
3.1. Visual observation
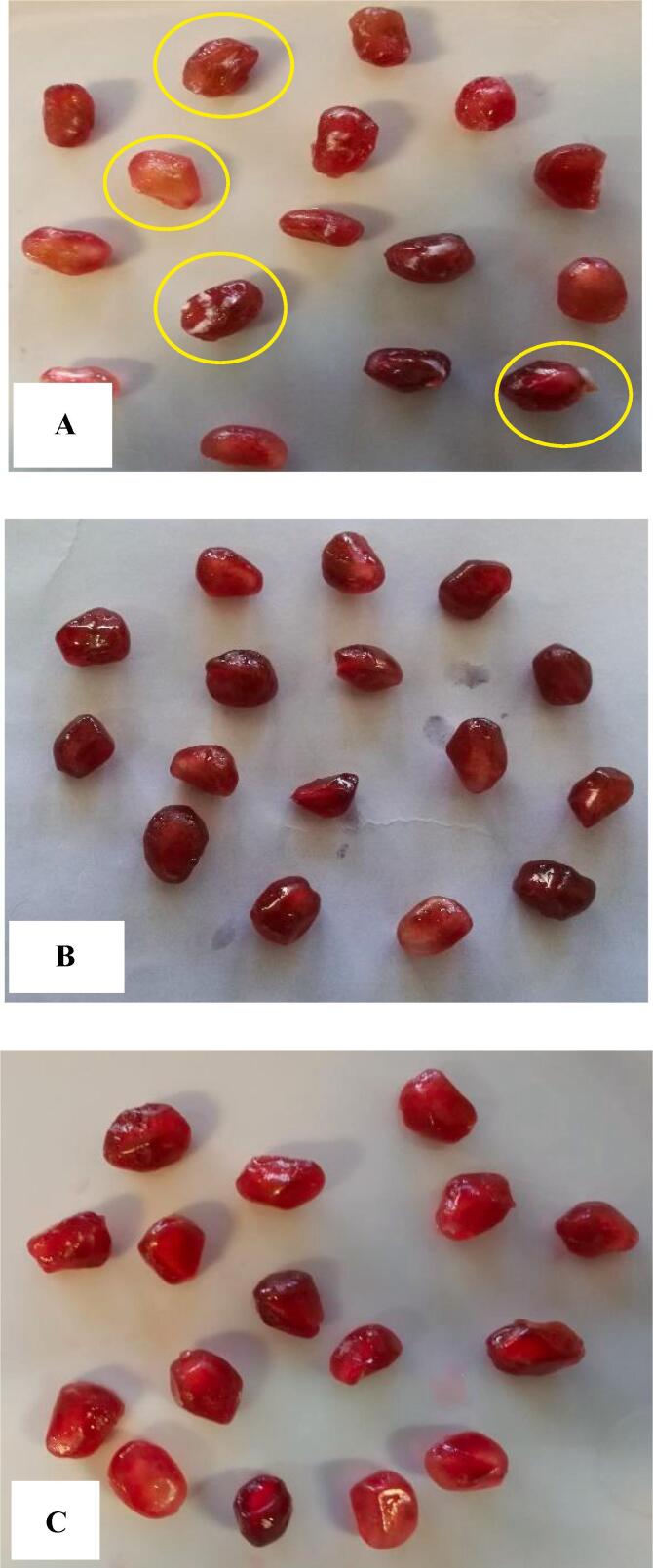
During storage, apparent signs of fungal growth were showed. After nine days of cold storage the first signs of fungal contamination were recorded in control samples. Among ultrasonic treated samples, those that exposed for 15 min, demonstrated the decay symptoms after 15 days and 30-min treated samples kept their healthy appearance until the end of storage (Fig. 1).
Fig. 1.
Photographs of control arils (a), arils subjecetd to ultrasound treatment for 15 min (b), and 30 min (c) after 12 days of storage at 5 °C.
3.2. Fruit quality (WL, TSS, TA and pH)
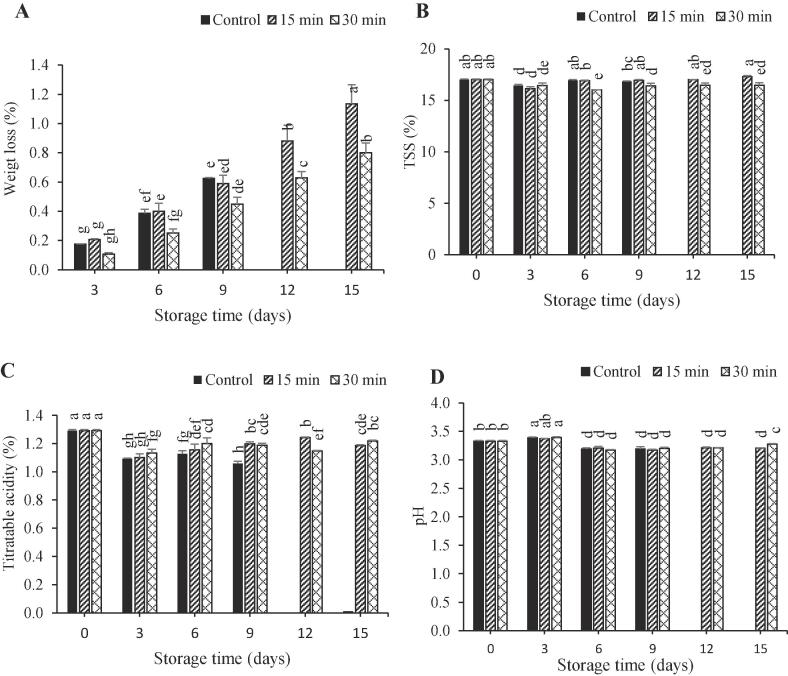
Overall arils WL increased in all treatments during storage (Fig. 2. A) however, it was more in the control samples than the treated ones. The least WL was recorded in arils which were exposed to ultrasound for 30 min. Control samples had the most WL after 9 days of storage (Fig. 2A). In addition, at final storage time, WL in arils treated with ultrasound for 30 min was 0.34% lower than those treated for 15 min. These results are in alignment with those reported for nectarine [31] and cucumbers [12]. The reduced WL may be due to the boosting effect of ultrasound waves on hydrogen bonds between water molecules and macromolecules [32].
Fig. 2.
Effect of ultrasonic treatments on weight loss (a), total soluble solids (TSS) (b), titratable acidity (c) and pH (d) in pomegranate arils cv. Rabbab during storage at 5 °C and 90 ± 5% RH.
Total soluble solids (TSS) decreased during the first three days (Fig. 2. B). Soluble solids content increased after three days of storage which may be due to break down of polysaccharide into monosaccharide [19]. Similar results have been reported in cucumbers [12] and white nectarines [31]. Titratable acidity (TA) decreased during the first three days of storage (Fig. 2. C) and then increased. A significant difference was found between the TA of control and ultrasound-treated arils after 9 days of storage. By the end of the storage time, TA in arils treated with ultrasound for 30 min was higher than in those treated by ultrasound for 15 min but not significant. The obtained results were in agreement with those reported for strawberries [17] and plum [14]. It should be interpreted that the ultrasonication may decreases the ripening rate through declining the ethylene production and respiratory rate [32], [33]. Arils pH changes were not significant during storage (Fig. 2. D).
3.3. Ascorbic acid (AA)
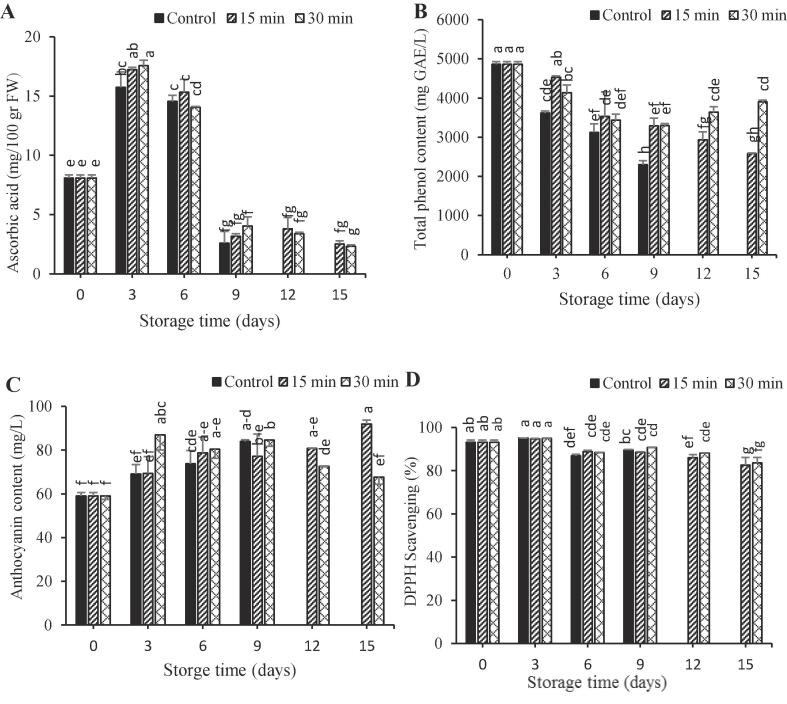
AA increased in the first three days and then decreased gradually. The possible reason for the loss of ascorbic acid during storage is its oxidation [34]. The ascorbic acid content of arils treated with ultrasound for 30 min was more than other samples after 9 days of storage (Fig. 3. A). At final storage time, the effectiveness did not depend on the ultrasonic time. These findings were in agreement with those reported in previous works [17] which underlined the fact that the ultrasonic method is efficient in maintaining AA in strawberries, plums and cherry tomatoes, respectively. The positive effect of ultrasonic treatment might be related to the elimination of dissolved oxygen which is essential for ascorbic acid degradation during cavitation and suppression of the activity of ascorbic acid oxidase [35].
Fig. 3.
Effect of ultrasonic treatments on ascorbic acid (a), total phenol (b), anthocyanin (c), and antioxidant activity (d) of pomegranate aril cv. Rabbab during storage at 5 °C and 90 ± 5% RH.
3.4. Total phenolic content (TPC), total anthocyanins content (TAC) and colour
Overall, TPC declined during storage. The amount of decrease was more in control than treated ones. This may be due to cell membrane destruction as a result of the fruit senescence [36]. Ultrasound treatment significantly reduced the degradation rate of phenolic compounds. The TPC in arils treated with ultrasound for 30 min was higher than control arils after 9 days of storage (Fig. 3. B). The effect of ultrasound treatment was not related to the ultrasonic time and there was no any significant difference among various ultrasound times. The highest TPC was achieved at the last day of storage in arils treated with ultrasound for 30 min which was significantly different from the ones treated with ultrasound for 15 min. The inhibitory effect of ultrasound on the loss of TPC during storage was similarly reported on Prunus salicina L. [14]. Also, tomato treated with 40%–100% power level for 4 min showed intensified TPC [37].
Total anthocyanins content (TAC) increased during storage. The increase in TAC during storage has been reported in raspberry [38] and cherries [39]. This may be due to the activity of anthocyanins biosynthesis enzyme [40]. TAC raised in both control and ultrasound-treated arils; however, the rate of increase was greater in the latter (Fig. 3. C). This findings were in alignment with other research in strawberry [41], and litchi [8]. Explanation for this observation is that using of ultrasonic decreased the activities of polyphenol oxidase and peroxidase enzyme, and maintained anthocyanins and TPC throughout the storage period. Therefore, it can be inferred that the enzymatic degradation of pigments is inhibited to some extent by ultrasound irradiation [8].
In 9th day of storage, ultrasound-treated arils had significantly higher lightness compared with control. There was no significant difference in chroma value between treated arils for 30 min and control after nine days of cold storage, however the remarkable decrease in (C*) was observed in arils treated for 15 min (Table 1). As it is illustrated in Fig. 1, hue angle of the arils reduced during the storage. Nevertheless, the arils treated with ultrasound had higher hue angle than the control. The increase in ultrasound treatment time increased hue angle significantly. Our results are in alignment with other studies on strawberry [42], [43]. Colour of the arils are due to the presence of various anthocyanins such as cyanidin-3 glucoside, delphinidin-3 glucoside and pelargonidin-3 glucoside [19]. There was a remarkable correlation between total anthocyanins with TPC (R2 = 0.58, Table 2). Higher redness (a*) was found in ultrasound-treated arils for 30 min and control samples after 9 days of storage. This can be confirmed with total anthocyanin samples presented in Fig. 3C. The yellowness (b*) showed stability in all samples during 9 days of storage, however significantly it was reduced in those treated for 15 min.
Table 1.
Effect of ultrasonic treatments on colour indices of pomegranate arils cv. Rabbab during storage at 5 °C and 90 ± 5% RH.
| Treatment | Storage time (day) | L* | a* | b* | H° | C° |
|---|---|---|---|---|---|---|
| control | 1 | 12.81b | 13.57bc | 10.05a | 36.52 a | 16.89 bc |
| 9 | 12.66b | 19.67a | 10.36a | 27.88 bc | 22.24 a | |
| 15 min | 1 | 12.81b | 13.57bc | 10.05a | 36.52 a | 16.89 bc |
| 9 | 17.61a | 12.25c | 6.20b | 26.73c | 13.73c | |
| 30 min | 1 | 12.81b | 13.57bc | 10.05a | 36.52 a | 16.89 bc |
| 9 | 16.97a | 17.21ab | 10.83a | 32.70 ab | 20.41ab |
Different letters within the same column in each treatment indicates a significant difference (p < 0.05).
Table 2.
Correlation coefficients between measured factors of pomegranate arils cv. Rabbab during storage at 5 °C and 90 ± 5% RH.
| WL | TPC | AA | TAA | TAC | TSS | TA | pH | |
|---|---|---|---|---|---|---|---|---|
| WL | 1 | |||||||
| TPC | 0.03 | 1 | ||||||
| AA | −0.21 | 0.53** | 1 | |||||
| TAA | 0.25* | 0.86** | 0.50** | 1 | ||||
| TAC | 0.44** | 0.58** | 0.36** | 0.87** | 1 | |||
| TSS | 0.34** | 0.82** | 0.42** | 0.98** | 0.89** | 1 | ||
| TA | 0.27* | 0.86** | 0.38** | 0.97** | 0.85** | 0.98** | 1 | |
| pH | 0.31* | 0.85** | 0.47** | 0.99** | 0.88** | 0.99** | 0.98** | 1 |
* Significant at P = 0.05, ** Significant at P = 0.01
weight loss (WL), total phenolic content (TPC), ascorbic acid (AA), total antioxidant activity (TAA), total anthocyanin (TAC), total soluble solids (TSS), titratable acidity (TA).
3.5. Total antioxidant activity (TAA)
TAA increased in first three days and then decreased. The amount of decrease in the TAA of control samples was higher than that of ultrasound-treated arils (Fig. 4. D). The reduction in TAA was related to senescence due to increased degradative processes which results in decreasing the bioactive compounds [30]. The effectiveness of ultrasound treatment was not affected by ultrasonic time and there was no difference between different sonication times after 15 days. Similarly, another research indicated that the ultrasonic wave could improve the TAA of cherry tomatoes. Ultrasonication, as an abiotic stress, could promote the synthesis of bioactive compounds through the simulation of their physiological activities [43]. In present study, the correlation coefficient of TAA with TPC, and ascorbic acid content was 0.86 and 0.50, respectively (Table 2). The ultrasound treatment stimulates OH− and hydrogen peroxide production in which they have biocidal effect on food materials. They trigger phenolic compounds accumulation which can increase its TAA [44]. A direct correlation was distinguished between arils TAA and total anthocyanins (R2 = 0.87) (Table 2). In pomegranate arils, increased antioxidant capacity was related to total anthocyanins and ascorbic acid content [18]. In this research, ultrasound treatments were effective on maintaining antioxidants during storage.
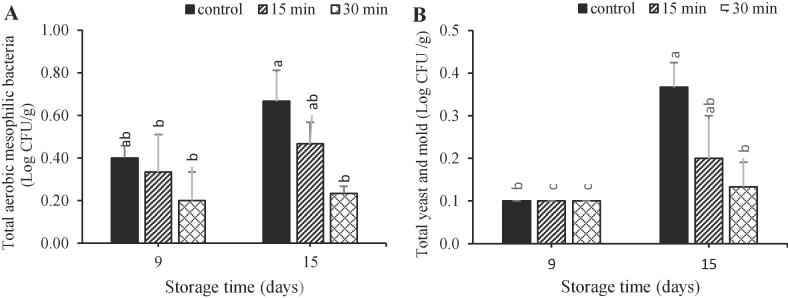
Fig. 4.
Effect of ultrasonic treatments on total yeast/mold counts (a) and total aerobic mesophilic bacteria (b) (Log CFU g−1) of pomegranate aril cv. Rabbab during storage at 5 °C and 90 ± 5% RH.
3.6. Microbial analysis
The effects of ultrasound treatment on microbial load are presented in Fig. 4. The microbial load raised during storage in control and ultrasound-treated arils, but with a significant higher rate in control samples. This results were similarity those reported on strawberry [41] and pineapple fruit [45]. Ultrasonication for 30 min was more effective than 15 min in decreasing the populations of bacteria, and molds. Similarly, ultrasound treatment for 20 min reduced food born pathogens on lettuce more than 5 min treatment [10]. Ultrasonic inhibition of pathogens occurs by free radical and the physical break down of cell membranes [46].
Natural antioxidants are also able to reduce contamination of pathogens and enhance the shelf life. In literature, there are multiple investigations indicate the antioxidant, antimicrobial and bioactivities of olive polyphenols [47], [48]. Also, the result of other study [49] showed that polyphenols and other antioxidant compounds could have antimicrobial properties, which underlined the fact that ultrasonic treatment has been able to increase resistance to pathogens by preserving phenolic compounds and maintaining antioxidant capacity.
3.7. Principal component analysis (PCA)
In the present study, PCA was performed through a physicochemical evaluation in different treatments and storage times (Fig. 5). The variables taken into consideration were TSS, TA, WL, TPC, ascorbic acid (AA), TAA, and total anthocyanin. According to PCA, a range of parameters were responsible for enhancing shelf life of arils. As shown in Fig. 5A, a close relationship existed among TPC, AA, total anthocyanin as well as TAA. The TPC, AA, total anthocyanin and TAA were placed on the upper right side plot. These attributes were apparently associated with 15 and 30 min treatments. Therefore, increase of the TAA was related to arils shelf life enhancement caused by ultrasound treatment. As it is seen in Fig. 5.B, a close relationship existed among TSS, total anthocyanin and TAA with 6 and 9 day of storage.
Fig. 5.
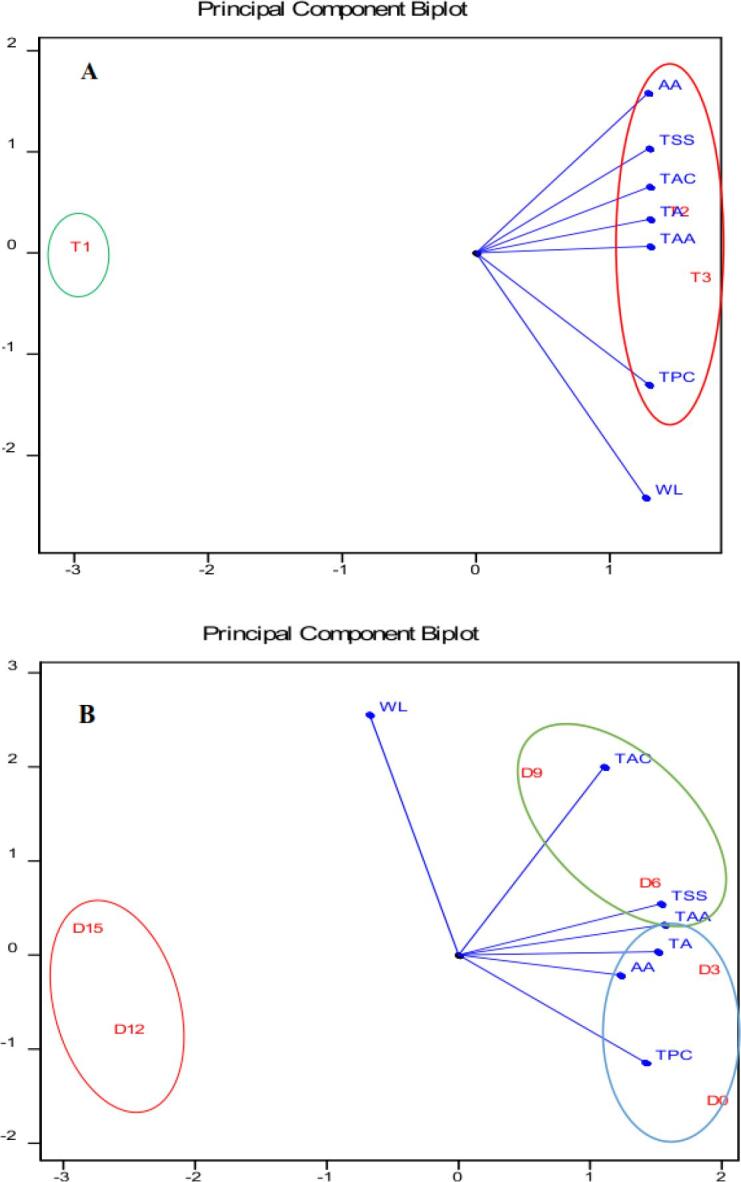
PCA of measured parameters of pomegranate arils in different treatments (control: T1, 15 min: T2, 30 min: T3). (A) and different storage times (B). total soluble solids (TSS), titratable acidity (TA), weight loss (WI), total phenolic content (TPC), ascorbic acid (AA), total antioxidant activity (TAA), total anthocyanin (TAC).
4. Conclusion
The results obtained from current study showed that the ultrasonic treatment at appropriate time is an effective technique for prolonging the storage life of arils. In more detail, applying 40 kHz ultrasound for 30 min was suitable for decreasing microbial load and preserving the qualitative characteristics (ascorbic acid and other bioactive compounds) of arils. Eventually, it was revealed that ultrasonication for 30 min extends the pomegranate arils storage time up to 15 days at cold storage.
CRediT authorship contribution statement
Azam Amiri: Conceptualization, Project administration, Funding acquisition, Resources. Asghar Ramezanian: Formal analysis, Visualization, Writing - original draft. Seyed Mohammad Hassan Mortazavi: Methodology, Software, Writing - review & editing. Seyed Mohammad Hashem Hosseini: Writing - review & editing.
Declaration of Competing Interest
There are no conflicts of interest. (i) no support, financial or otherwise, has been received from any organization that may have an interest in the submitted work; and (ii) there are no other relationships or activities that could appear to have influenced the submitted work; (iii) to the best of our knowledge, the named authors have no conflict of interest, financial or otherwise.
References
- 1.Zinoviadou K.G., Galanakis C.M., Brnčić M., Grimi N., Boussetta N., Mota M.J., Barba F.J. Fruit juice sonication: implications on food safety and physicochemical and nutritional properties. Food Res. Int. 2015;77:743–752. doi: 10.1016/j.foodres.2015.05.032. [DOI] [Google Scholar]
- 2.Galanakis C.M. Separation of functional macromolecules and micromolecules: from ultrafiltration to the border of nanofiltration. Trends Food Sci. Technol. 2015;42:44–63. doi: 10.1016/j.tifs.2014.11.005. [DOI] [Google Scholar]
- 3.Galanakis C.M., Markouli E., Gekas V. Recovery and fractionation of different phenolic classes from winery sludge using ultrafiltration. Sep. Purif. Technol. 2013;107:245–251. doi: 10.1016/j.seppur.2013.01.034. [DOI] [Google Scholar]
- 4.Galanakis C.M., Kotanidis A., Dianellou M., Gekas V. Phenolic content andantioxidant capacity of Cypriot Wines. Czech J. Food Sci. 2015;33:126–136. doi: 10.17221/335/2014-CJFS. [DOI] [Google Scholar]
- 5.Galanakis C.M. Recovery of high added-value components from food wastes: conventional, emerging technologies and commercialized applications. Trends Food Sci. Technol. 2012;26:68–87. [Google Scholar]
- 6.Deng Q., Zinoviadou K.G., Galanakis C.M., Orlien V., Grimi N., Vorobiev E. The effects of conventional and non-conventional processing on glucosinolates andits derived forms, isothiocyanates: extraction, degradation, and applications. Food Eng. Rev. 2015;7:357–381. doi: 10.1007/s12393-014-9104-9. [DOI] [Google Scholar]
- 7.Galanakis C.M. Emerging technologies for the production of nutraceuticals from agricultural by-products: a viewpoint of opportunities and challenges. Food Bioprod. Process. 2013;91:575–579. doi: 10.1016/j.fbp.2013.01.004. [DOI] [Google Scholar]
- 8.Chen Y., Jiang Y., Yang S., Yang E., Yang B., Prasad K.N. Effects of ultrasonic treatment on pericarp browning of postharvest litchi fruit. J. Food Biochem. 2012;36:613–620. [Google Scholar]
- 9.Jang J.H., Moon K.D. Inhibition of polyphenol oxidase and peroxidase activities on fresh-cut apple by simultaneous treatment of ultrasound and ascorbic acid. Food Chem. 2011;124:444–449. [Google Scholar]
- 10.Sagong H.G., Lee S.Y., Chang P.S., Heu S., Ryu S., Choi Y.J., Kang D.H. Combined effect of ultrasound and organic acids to reduce Escherichia coli O157: H7, Salmonella Typhimurium, and Listeria monocytogenes on organic fresh lettuce, International journal of. Food Microbiol. 2011;145:287–292. doi: 10.1016/j.ijfoodmicro.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 11.Zhou B., Feng H., Luo Y. Ultrasound enhanced sanitizer efficacy in reduction of Escherichia coli O157: H7 population on spinach leaves. J. Food Sci. 2009;74:M308–M313. doi: 10.1111/j.1750-3841.2009.01247.x. [DOI] [PubMed] [Google Scholar]
- 12.Feng L., Zhang M., Adhikari B., Guo Z. Effect of ultrasound combined with controlled atmosphere on postharvest storage quality of cucumbers (Cucumis sativus L.) Food Bioproc. Tech. 2018;11:1328–1338. doi: 10.1007/s11947-018-2102-9. [DOI] [Google Scholar]
- 13.Yang Z., Cao S., Cai Y., Zheng Y. Combination of salicylic acid and ultrasound to control postharvest blue mold caused by Penicillium expansum in peach fruit. Innov. Food Sci. Emerg. Technol. 2011;12:310–314. [Google Scholar]
- 14.Chen Z., Zhu C. Combined effects of aqueous chlorine dioxide and ultrasonic treatments on postharvest storage quality of plum fruit (Prunus salicina L.) Postharvest Biol. Technol. 2011;61:117–123. [Google Scholar]
- 15.Brilhante São José J.F., Dantas Vanetti M.C. Effect of ultrasound and commercial sanitizers in removing natural contaminants and Salmonella enterica Typhimurium on cherry tomatoes. Food Control. 2012;24:95–99. [Google Scholar]
- 16.Valero M., Recrosio N., Saura D., Muñoz N., Martí N., Lizama V. Effects of ultrasonic treatments in orange juice processing. J. Food Eng. 2007;80:509–516. [Google Scholar]
- 17.Cao S., Hu Z., Pang B. Optimization of postharvest ultrasonic treatment of strawberry fruit. Postharvest Biol. Technol. 2010;55:150–153. doi: 10.1016/j.postharvbio.2009.11.002. [DOI] [Google Scholar]
- 18.Saba M.K., Amini R. Nano-ZnO/carboxymethyl cellulose-based active coating impact on ready-to-use pomegranate during cold storage. Food Chem. 2017;232:721–726. doi: 10.1016/j.foodchem.2017.04.076. [DOI] [PubMed] [Google Scholar]
- 19.Özdemir K.S., Gökmen V. Extending the shelf-life of pomegranate arils with chitosan-ascorbic acid coating. LWT - Food Sci. Technol. 2017;76:172–180. doi: 10.1016/j.lwt.2016.10.057. [DOI] [Google Scholar]
- 20.Martínez-Romero D., Castillo S., Guillén F., Díaz-Mula H.M., Zapata P.J., Valero D., Serrano M. Aloe vera gel coating maintains quality and safety of ready-to-eat pomegranate arils. Postharvest Biol. Technol. 2013;86:107–112. doi: 10.1016/j.postharvbio.2013.06.022. [DOI] [Google Scholar]
- 21.Soloklui A.A.G., Gharaghani A., Oraguzie N., Ramezanian A. Shelf life and biochemical changes of ready-to-eat arils among nineteen Iranian pomegranate cultivars (Punica granatum L.) during storage. J. Food Sci. Technol. 2019;56:1416–1426. doi: 10.1007/s13197-019-03620-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Grady L., Sigge G., Caleb O., Opara U.L. Effects of storage temperature and duration on chemical properties, proximate composition and selected bioactive components of pomegranate (Punica granatum L.) arils. LWT - Food Sci. Technol. 2014;57:508–515. [Google Scholar]
- 23.Ghasemnezhad M., Zareh S., Rassa M., Sajedi R.H. Effect of chitosan coating on maintenance of aril quality, microbial population and PPO activity of pomegranate (Punica granatum L. cv. Tarom) at cold storage temperature. J. Agric. Food Chem. 2013;93:368–374. doi: 10.1002/jsfa.5770. [DOI] [PubMed] [Google Scholar]
- 24.Razzaq K., Khan A.S., Malik A.U., Shahid M., Ullah S. Role of putrescine in regulating fruit softening and antioxidative enzyme systems in ‘Samar Bahisht Chaunsa’mango. Postharvest Biol. Technol. 2014;96:23–32. doi: 10.1016/j.postharvbio.2014.05.003. [DOI] [Google Scholar]
- 25.Klein B., Perry A. Ascorbic acid and vitamin A activity in selected vegetables from different geographical areas of the United States. J. Food Sci. 1982;47:941–945. doi: 10.1111/j.1365-2621.1982.tb12750.x. [DOI] [Google Scholar]
- 26.Rapisarda P., Fanella F., Maccarone E. Reliability of analytical methods for determining anthocyanins in blood orange juices. J. Agric. Food Chem. 2000;48:2249–2252. doi: 10.1021/jf991157h. [DOI] [PubMed] [Google Scholar]
- 27.Meyers K.J., Watkins C.B., Pritts M.P., Liu R.H. Antioxidant and antiproliferative activities of strawberries. J. Agric. Food Chem. 2003;51:6887–6892. doi: 10.1021/jf034506n. [DOI] [PubMed] [Google Scholar]
- 28.Çam M., Hışıl Y., Durmaz G. Classification of eight pomegranate juices based on antioxidant capacity measured by four methods. Food Chem. 2009;112:721–726. doi: 10.1016/j.foodchem.2008.06.009. [DOI] [Google Scholar]
- 29.Pathare P.B., Opara U.L., Al-Said F.A.-J. Colour measurement and analysis in fresh and processed foods: a review. Food Bioproc. Tecch. 2013;6:36–60. doi: 10.1007/s11947-012-0867-9. [DOI] [Google Scholar]
- 30.Sogvar O.B., Saba M.K., Emamifar A., Hallaj R. Influence of nano-ZnO on microbial growth, bioactive content and postharvest quality of strawberries during storage. Innov. Food Sci. Emerg. Technol. 2016;35:168–176. doi: 10.1016/j.foodchem.2017.04.076. [DOI] [Google Scholar]
- 31.Temizkan R., Atan M., Büyükcan M.B., Caner C. Efficacy evaluation of ultrasound treatment on the postharvest storability of white nectarine by both physicochemical and image processing analyses. Postharvest Biol. Technol. 2019;154:41–51. doi: 10.1016/j.postharvbio.2019.04.014. [DOI] [Google Scholar]
- 32.Li N., Chen F., Cui F., Sun W., Zhang J., Qian L., Yang Y., Wu D., Dong Y., Jiang J. Improved postharvest quality and respiratory activity of straw mushroom (Volvariella volvacea) with ultrasound treatment and controlled relative humidity. Sci. Hortic. 2017;225:56–64. doi: 10.1016/j.scienta.2017.06.057. [DOI] [Google Scholar]
- 33.Wang W., Ma X., Zou M., Jiang P., Hu W., Li J., Zhi Z., Chen J., Li S., Ding T. Effects of ultrasound on spoilage microorganisms, quality, and antioxidant capacity of postharvest cherry tomatoes. J. Food Sci. 2015;80:C2117–C2126. doi: 10.1111/1750-3841.12955. [DOI] [PubMed] [Google Scholar]
- 34.Owusu-Yaw J., Marshall M., Koburger J., Wei C. Low pH inactivation of pectinesterase in single strength orange juice. J. Food Sci. 1988;53:504–507. doi: 10.1111/j.1365-2621.1988.tb07742.x. [DOI] [Google Scholar]
- 35.Ali G., Russly A., Jamilah B., Azizah O., Mandana B. Effect of heat and thermosonication on kinetics of peroxidase inactivation and vitamin C degradation in seedless guava (Psidium guajava L.) Int Food Res J. 2011;18:1289. doi: 10.1016/j.eaef.2017.10.002. [DOI] [Google Scholar]
- 36.Macheix J., Fleuriet A., Billot J. The Main Phenolics of Fruits, Fruit Phenolics. CRC Press; 1990. p. 392. [Google Scholar]
- 37.Pinheiro J., Alegria C., Abreu M., Gonçalves E.M., Silva C.L. Influence of postharvest ultrasounds treatments on tomato (Solanum lycopersicum, cv. Zinac) quality and microbial load during storage. Ultrason. Sonochem. 2015;27:552–559. doi: 10.1016/j.ultsonch.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 38.Hassanpour H. Effect of Aloe vera gel coating on antioxidant capacity, antioxidant enzyme activities and decay in raspberry fruit. LWT-Food Sci. Technol. 2015;60:495–501. doi: 10.1016/j.lwt.2014.07.049. [DOI] [Google Scholar]
- 39.Gonçalves B., Silva A.P., Moutinho-Pereira J., Bacelar E., Rosa E., Meyer A.S. Effect of ripeness and postharvest storage on the evolution of colour and anthocyanins in cherries (Prunus avium L.) Food Chem. 2007;103:976–984. doi: 10.1016/j.foodchem.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 40.Miguel G., Fontes C., Antunes D., Neves A., Martins D. Anthocyanin concentration of “Assaria” pomegranate fruits during different cold storage conditions. J. Biomed. Biotec. 2004;2004:338–342. doi: 10.1155/S1110724304403076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alexandre E.M., Brandão T.R., Silva C.L. Efficacy of non-thermal technologies and sanitizer solutions on microbial load reduction and quality retention of strawberries. J. Food Eng. 2012;108:417–426. [Google Scholar]
- 42.Aday M.S., Temizkan R., Büyükcan M.B., Caner C. An innovative technique for extending shelf life of strawberry: ultrasound. LWT - Food Sci. Technol. 2013;52:93–101. doi: 10.1016/j.lwt.2014.01.006. [DOI] [Google Scholar]
- 43.Wang J., Han T., Li L., Wang K., Sun S. Effect of ultrasonic treatment on the peaches quality during storage. J. Shihezi Univ. (Natural Sci.) 2006;24:732–735. [Google Scholar]
- 44.Gani A., Baba W.N., Ahmad M., Shah U., Khan A.A., Wani I.A., Masoodi F., Gani A. Effect of ultrasound treatment on physico-chemical, nutraceutical and microbial quality of strawberry. LWT - Food Sci. Technol. 2016;66:496–502. [Google Scholar]
- 45.Khayankarn S., Uthaibutra J., Setha S., Whangchai K. Using electrolyzed oxidizing water combined with an ultrasonic wave on the postharvest diseases control of pineapple fruit cv. ‘Phu Lae’. Crop Prot. 2013;54:43–47. doi: 10.1016/j.cropro.2013.07.004. [DOI] [Google Scholar]
- 46.Phull S., Newman A., Lorimer J., Pollet B., Mason T. The development and evaluation of ultrasound in the biocidal treatment of water. Ultrason. Sonochem. 1997;4:157–164. doi: 10.1016/S1350-4177(97)00029-1. [DOI] [PubMed] [Google Scholar]
- 47.Galanakis C.M., Tsatalas P., Charalambous Z., Galanakis I.M. Control of microbial growth in bakery products fortified with polyphenols recovered from olive mill wastewater. Environ. Technol. Innov. 2018;10:1–15. doi: 10.1016/j.eti.2018.01.006. [DOI] [Google Scholar]
- 48.Galanakis C.M. Phenols recovered from olive mill wastewater as additives in meat products. Trends Food Sci. Technol. 2018;79:98–105. doi: 10.1016/j.tifs.2018.07.010. [DOI] [Google Scholar]
- 49.Sun J., Janisiewicz W.J., Nichols B., Jurick W.M., Chen P. Composition of phenolic compounds in wild apple with multiple resistance mechanisms against postharvest blue mold decay. Postharvest Biol. Technol. 2017;127:68–75. doi: 10.1016/j.postharvbio.2017.01.006. [DOI] [Google Scholar]