Abstract
Emulsion gels with low oil contents have been continuously developed in recent decades. In this study, the use of high-intensity ultrasound for the preparation of low oil emulsion gel (oil fraction of 0.25) was investigated. Specifically, defatted Antarctic krill protein (dAKP) was used to stabilize the interface of soybean oil and water. Then, the microstructure and the stabilization mechanism of the formed emulsion gel were evaluated by cryo-SEM, CLSM, zeta potential, rheological measurements, and FTIR. Besides, the particle diameter was measured to be around 5 μm. The results of CLSM indicated that the emulsion gel was the oil-in-water type. The emulsion gel exhibited gel-like viscoelastic behavior even at a low concentration of dAKP due to the formation of a rigid particle network while the rheological behavior of the emulsion gel was significantly affected by the concentration of dAKP. The stabilization of the emulsion gel can be maintained by space steric hindrance and hydrophobic interactions between particles in the emulsion gel system.
Keywords: Defatted antarctic krill protein, Ultrasound, Emulsion gel, Rheological properties
1. Introduction
Protein emulsions are prevalently applied as the food-grade delivery system of flavor and nutrition compounds for the development of foods, nourishments, pharmaceutics, and cosmetics [1], [2], [3], [4]. However, conventional emulsions stabilized by proteins exhibit weak stabilities due to protein aggregation, coalescence, and thermal denaturation [5], [6]. By comparison, the emulsion gels rely on their specific gel-like network structure and solid-like mechanical characteristics to highlight their outstanding stability [7], [8]. Emulsion gels are mainly formed by a protein-stabilized oil-in-water emulsion [9] with wide applications such as developing healthy meat products [10], [11], reducing trans-fat [12], improving food structuring [13], and amending heat-sensitive ingredients [14]. Consequently, a series of emulsions gels have fascinated considerable attention in recent decades.
Pickering high internal phase emulsions (HIPEs) that have porous structures with at least 74% of internal phase volume are one type of the extensively studied emulsion gels [15]. HIPEs made by droplets with rigid particle layers have a higher level of stability than conventional emulsions [16]. However, HIPEs with high oil contents for food products are not suitable for a direct diet and can cause diverse health problems with the intake of high oil to some extent. In the last decades, emulsion gels with low oil phase, such as emulsion filled gels, emulsion fluid gels, and emulsion particulate gels, were continuously developed to reduce the oil content and broaden the range of emulsion gels. However, these emulsion gels are complicated in preparation [17]. Two steps are indispensable in the conventional method of preparing an emulsion gel; the first is to obtain a protein-stabled emulsion and the second is to convert the liquid-like emulsion into emulsion gel [10]. For example, a low-in-fat emulsion gel (o/w: 15 g/100 g) stabilized with bovine gelatin was primarily pre-emulsified by homogenizer for 4 mins at 11,500 rpm; then, the final emulsion was acquired by a high-pressure valve homogenizer at 40 MPa and 4 MPa for two valves severally [18]. Hence, exploring one-step emulsion gel preparation methods is following up to simplify the process of emulsion gels preparation.
Several novel techniques have been exploited in producing emulsion gels. A miniature twin-screw extruder was first investigated in producing emulsion gel [19]. Alexandre et al. reported that the pendant drop method has high potentiality in producing emulsion gels [20]. Ultrasound techniques are proverbially used in characterizing emulsions, exhibiting the operating factors of ultrasonic emulsification, such as input power, irradiation time, and the ratio of oil to water [21]. Moreover, ultrasound enables emulsification through cavitation [21]. Ultrasonication was reported as an assistant to prepare emulsion gels in synergy together with other methods such as homogenization [22] while few studies considered a single application of ultrasound in the preparation of emulsion gels. It has a broad prospect of preparing emulsion gels using ultrasound, especially high-intensity ultrasound alone due to the expected features of ease of operation, high energy efficiency, cost-saving, and eco-friendly [23].
As mentioned earlier, protein plays a crucial role in the formation of stable emulsion gel. Plentiful proteins have been reported to be ingredients of the stabilized emulsion gels. Zein [11], potato protein [11], soy protein [9], myofibrillar protein [24], and whey protein [25] are frequently used in emulsion gel production. However, using Antarctic krill protein as a stabilizer has not been reported in emulsion gel production. Antarctic krill (Euphausia superba) is a public marine resource with enormous reserves and is rich in nutrients such as protein and lipid [26]. Besides, it was demonstrated that Antarctic krill contains around 77.9–83.1% of moisture, 11.9–15.4% of crude protein, and 0.5–3.6% of lipids [27]. Therefore, it is promising to use defatted Antarctic krill protein (dAKP) for the emulsion gel production.
This study illustrated that it is feasible to prepare emulsion gel stabilized by defatted Antarctic krill protein using high-intensity ultrasound. In this work, the microstructure, stability, and rheology of emulsion gel produced were evaluated, and the stability mechanism of the emulsion gel was briefly investigated.
2. Materials and methods
2.1. Materials
Antarctic krill (Euphausia superba) was purchased from Dalian Liaoyu Group Co., Ltd. (Dalian, China). Soybean oil was obtained from a local supermarket (Dalian, China). Nile Red and Nile Blue were acquired from Shanghai Aladdin Bio-Chem Technology Co., Ltd. (Shanghai, China). KBr used in the study was spectral grade, and other chemicals used were analytical grade.
2.2. Preparation of defatted Antarctic krill protein
Defatted Antarctic krill protein (dAKP) was obtained using the method developed by Wang et al. Frozen Antarctic krill was thawed at room temperature and then crushed into minced krill by a homogenizer. Anhydrous ethanol was added to minced krill (1:80, w/v); then, they were mixed at room temperature for 4 h; next, ethanol was removed to acquire defatted Antarctic krill powder. Alkaline solubilization and acidic precipitation processes were used to gain dAKP. Defatted Antarctic krill powder was stirred with 0.1 M NaOH at a concentration of 10% (w/v) for 100 min at 40 °C. The solution was centrifuged at 5000g for 20 min at 4 °C, and the supernatant was collected to be adjusted with 2 M HCl to pH 4.5. After the solution was placed at 4 °C for 30 min, it was centrifuged under the same condition, and the precipitate, dAKP, was collected. Afterward, dAKP was resuspended into distilled water and adjusted to pH 7.0 with 2 M NaOH. The obtained dAKP solution was stored at 4 °C, and the dried dAKP was obtained through vacuum freeze-drying.
The molecular weight of dAKP was analyzed by SDS-PAGE. The microstructure of dAKP was evaluated by Atomic Force Microscopy (AFM).
2.3. Nano-LC ESI-QTOF MS/MS analysis
Dried dAKP (1 mg) was rehydrated in 1 mL of 8 M urea and 100 mM ammonium bicarbonate (pH 8.2). The sample was reduced by 10 μL of 1 M DTT and incubated at 37 °C for 2 h. Until the temperature reduced to room temperature, 20 μL of 1 M IAA was added, and the sample was maintained in a dark place for 40 min. Then, the urea concentration was diluted to 1 M by 100 mM ammonium bicarbonate; the sample was digested by the added 40 μg trypsin at 37 °C for 16 h (overnight). The digests were purified by reversed-phase extraction using a C18 cartridge. Besides, eluted peptides were dried using a nitrogen blowing instrument. The hydrolysate peptides were resuspended in 80% methanol and separated and identified using nano-LC ESI-QTOF MS/MS (Bruker Daltonik GmbH, Germany).
2.4. Preparation of low oil emulsion gel
Emulsion gels were produced using dAKP as a emulsifier. The resuspended dAKP was boiled at 100 °C for 1 h and then cooled to room temperature. Afterward, 5 mL of soybean oil was added into 15 mL of dAKP solution (0.5%, 1.0%, 1.5%, and 2.0% w/v) at pH 4.5. Low oil emulsion gel was prepared by ultrasonic treatment in an ultrasonic cell disintegrator (SCIENTZ-IID, China) for 2 min at 6690 W/cm2. Besides, ice water was circulated continuously during sonication.
2.5. Cryo-scanning electron microscopy (cryo-SEM)
The microstructure of formed emulsion gel was elucidated by taking cryo-SEM photographs using SU8010/PP3010T (Hitachi, Japan). The frozen sample was sublimated for 20 min at −90 °C and then sputter-coated with thin (<20 nm) powdered gold. The photographs were taken at 1.0 k, 10.0 k, and 15.0 k magnification and 5.0 kV voltage.
2.6. Confocal laser scanning microscopy (CLSM)
The microstructure of emulsion gel droplets was observed using CLSM (Leica SP8, Germany). Specifically, 1 mL of emulsion gel was stained with 20 μL fluorescent colorant solution Nile Blue (1 mg/mL) and Nile Red (0.5 mg/mL) and coated with a glass coverslip before observation. He/Ne laser with an excitation wavelength of 633 nm for Nile Blue and argon laser with an excitation wavelength of 488 nm for Nile Red were employed to excite the fluorescent dyes.
2.7. Particle size and zeta-potential
The particle size and zeta-potential were determined using an ultrasonic particle size analyzer (DTI DT-1200, USA). Emulsion gel was diluted to the appreciate concentration before detection. All measurements were conducted in triplicate at 25 °C.
2.8. Rheological measurement
All rheological measurements were analyzed with a rheometer (TA Discovery HR-1, USA) equipped with parallel plate geometry (diameter of 60 mm, plate gap of 1 mm). Strain sweep was conducted at a frequency of 1 Hz which determined the linear viscoelastic region. Torage modulus (Gʹ) and loss modulus were measured by performing frequency sweep at a strain of 0.5%. Regarding apparent viscosity measurements, shear rate, as a function of shear stress, was increased from 0.1 to 400 s−1.
2.9. Fourier transform infrared spectroscopy (FTIR)
Infrared spectra were measured with a Fourier transform infrared instrument (PikerElmer Frontier, USA). First, 10 g of emulsion gel was frozen at −30 °C and thawed at room temperature. Then, it was centrifuged at 100,000g for 10 min using L550 centrifuge (Cence, China) to remove the separated oil phase. The above steps were repeated three times, and the remaining oil phase was separated three times by methanol/chloroform (1:3, v/v) extraction. After the freeze-drying processing, the interface protein was stored until it was analyzed for the structure.
The sample (2 mg) was finely grounded with KBr (200 mg) in mortar pressed into a disk under a pressure of 15 ton/m2 for 10 min. The spectra were scanned from 400 to 4000 cm−1 at a resolution of 4 cm−1. A KBr disk smeared with soybean oil and a disk using defatted Antarctic krill protein as samples were measured as controls.
3. Results and discussion
3.1. Molecular weight analysis and microstructure of dAKP
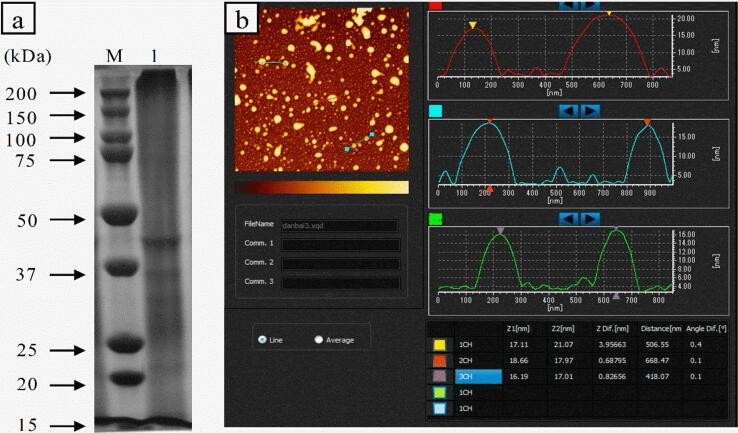
24 peptides with significant scores were identified using Nano-LC ESI-QTOF MS/MS (Table 1). Besides, the molecular weight of dAKP was defined by SDS-PAGE, and the pattern is illustrated in Fig. 1a. The traces of the protein band can be discerned in the range of 20–220 kDa. The bands representing myosin heavy chain (220 kDa), actin (42 kDa), beta-actin (Fragment, 22 kDa), and myosin heavy chain (Fragment, 25–37 kDa) can be visible in Fig. 1a, as demonstrated in Table 1. This result was previously testified by Shi et al. and Zheng et al. [27], [28].
Table 1.
Proteins from Antarctic krill protein identified by Nano-LC ESI-QTOF MS/MS.
| No. | Protein | Score | Monoisotopic mass | Sequence | emPAI |
|---|---|---|---|---|---|
| 1 | Tropomyosin | 581 | 32,643 | MDALESQLK | 3.79 |
| 2 | Tropomyosin | 532 | 32,679 | IVELEEELR | 3.37 |
| 3 | ATP synthase subunit beta | 352 | 55,881 | TIAMDGTEGLIR | 0.46 |
| 4 | Slow-type skeletal muscle actin 5 | 231 | 41,594 | IIAPPER | 0.79 |
| 5 | Myosin heavy chain type 1 | 226 | 219,576 | AEELEAAR | 0.12 |
| 6 | Fast-type skeletal muscle actin 10 | 224 | 41,814 | AVFPSIVGR | 0.92 |
| 7 | Actin 1 | 210 | 41,801 | AGFAGDDAPR | 0.66 |
| 8 | Fast-type skeletal muscle actin 12 | 196 | 41,824 | HQGVMVGMGQK | 0.92 |
| 9 | Actin | 189 | 41,825 | QEYDESGPSIVHR | 0.54 |
| 10 | Tubulin beta chain | 161 | 49,872 | YLTVAAIFR | 0.28 |
| 11 | Histone H2B (Fragments) | 156 | 12,767 | AMSIMNSFVNDIFER | 0.26 |
| 12 | Myosin heavy chain type 2 | 141 | 219,056 | TLLEQSDR | 0.09 |
| 13 | Fast-type skeletal muscle actin 8 | 130 | 41,867 | SYELPDGQVITIGNER | 1.54 |
| 14 | Tubulin alpha chain | 100 | 50,102 | DVNAAIATIK | 0.27 |
| 15 | Beta-actin (Fragment) | 94 | 21,479 | YPIEHGIITNWDDMEK | 0.99 |
| 16 | Arginine kinase 3 | 92 | 39,481 | LGVENLMK | 0.17 |
| 17 | Eukaryotic initiation factor 4A | 84 | 48,413 | MFVLDEADEMLSR | 0.06 |
| 18 | Arginine kinase 1 | 69 | 39,520 | ASVHVDLPGWTK | 0.17 |
| 19 | Elongation factor 1-alpha (Fragment) | 64 | 40,210 | RTGKELESTPK | 0.16 |
| 20 | ATP synthase subunit alpha | 62 | 59,188 | VLSIGDGIAR | 0.11 |
| 21 | Elongation factor 2 | 60 | 94,056 | LFDAIMNFK | 0.1 |
| 22 | Myosin heavy chain type 5 | 104 | 33,366 | MQDLVDKLQQK | |
| 23 | Myosin heavy chain (Fragment) | 104 | 29,269 | TLLEQSDR | |
| 24 | Myosin heavy chain (Fragment) | 86 | 28,352 | QIEEAEEIAALNLAK |
Fig. 1.
a: SDS-PAGE pattern of dAKP. Lane M: marker, Lane 1: dAKP. b: AFM images of dAKP (pH = 4.5). a: 3D image, b: the plane and data analysis diagrams.
The microstructure and morphology of dAKP were evaluated by AFM. As presented in Fig. 1b, protein particles performed needle-like and peak shapes, suggesting that protein particles were spherical or elliptical at pH 4.5 with heights ranging from about 16 nm to 24 nm. Furthermore, the aggregation behavior between dAKPs at the isoelectric point can be observed in Fig. 1b. Moreover, dAKP was expected to form stable emulsion gels by attaching to the oil–water surface to exert steric hindrance against coalescence.
3.2. Characterization and stability of dAKP stabilized low oil emulsion gel
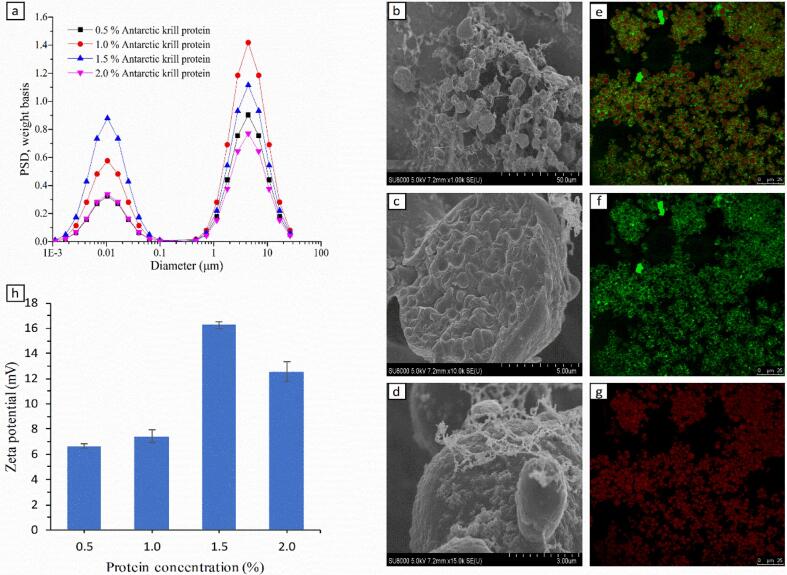
A low oil emulsion gel was prepared using high-intensity ultrasound with dAKP as the stabilizer. The particle size of the emulsion gels is presented in Fig. 2a. Two prominent peaks of the profile at 10 nm and 5 μm can be observed, attributed to dAKP and low oil emulsion gels, respectively. Different from previous reports [29], [30], the particle size did not decrease as the protein concentration increased while the quantity is heavily and irregularly influenced by protein concentration.
Fig. 2.
a: Particle size distribution of low oil emulsion gel stabilized by different concentrations of dAKP. b-d: The cryo-SEM (b: ×1.00 k, c: ×10.0 k, d: ×15.0 k) images of the low oil emulsion gel stabilized by 1.5% of dAKP. e-g: CLSM images of the low oil emulsion gel stabilized by 1.5% of dAKP. h: Zeta potential of low oil emulsion gel stabilized by different concentration dAKP (0.5%, 1.0%, 1.5%, and 2.0%).
The microstructure of low oil emulsion gel was further analyzed by cryo-SEM and CLSM. Cryo-SEM micrographs (Fig. 2b-d) illustrate that emulsion droplets were spherical and ellipsoidal; this was also confirmed by CLSM micrographs (Fig. 2e-g). The porosity of gel (Fig. 2b) is caused by random aggregation and cavitation during the high-intensity ultrasonic step. Fine filaments observed in Fig. 2d were attributed to the precipitation of protein crosslink in the sublimation step of gel; similar filaments between the protein network were observed in the previous report [31]. Such observation strengthened the occurrence of phase separation among protein networks [31]. Moreover, soybean oil and dAKP were stained by Nile Red and Nile Blue, respectively, as exhibited in Fig. 2e. Moreover, the oil phase and spherical droplets in green fluorescence were tightly surrounded by protein with red fluorescence, further demonstrating that the formed low oil emulsion gel is an oil-in-water emulsion gel. CLSM pictures (Fig. 2e) also indicate that the adjacent droplets were closely packed with each other to form a network structure, which was significant for the stability against coalescence of the emulsion gel. Additionally, oil droplets were evenly distributed in the CLSM image, reflecting that ultrasound is an effective method in forming an ordered emulsion system.
The extent of electrostatic repulsion between the droplet surfaces was checked by measuring zeta potential [32]. The zeta potential of emulsion gels stabilized by different concentrations of dAKP is illustrated in Fig. 2h. According to the report [33], the isoelectric point (pI) of Antarctic krill protein is around pH 4.5. The zeta potential values of the emulsion gels stabilized by different concentration proteins at pH 4.5 in this study were between +6 mV and +17 mV. This can be associated with the rearrangement of protein after ultrasonic treatment. Zeta potential value is a considerable parameter to evaluate the electrostatic stability of emulsions [34]. Particularly, emulsions with the high-value zeta potential should be stable due to the presence of strong repulsive forces between emulsion droplets while those droplets with little repulsion tend to coalesce rapidly ascribe to interparticle interaction [35]. Therefore, it can be speculated that the emulsion gel stabilized by 0.5% of dAKP, which was detected to exhibit the lowest zeta potential, should be the least stable one. This inference was verified by observing the storage stability of emulsion gels produced in this study (Fig. 3).
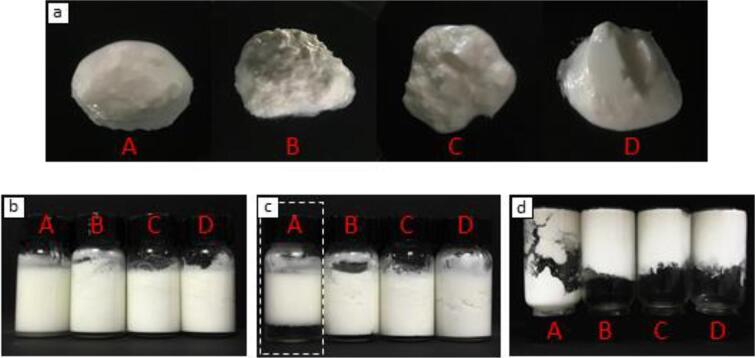
Fig. 3.
Appearance of emulsion gels stabilized by dAKP at different concentrations (A 0.5%, B 1.0%, C 1.5%, and D 2.0%). a, b, and d were freshly prepared emulsion gel. c was photographed after 7 days storage at 2 °C.
The apparent morphology of prepared emulsion gels was displayed in Fig. 3a. It can be revealed that the morphology of formed emulsions exhibited gel-like states. Moreover, the structure of the emulsion gel became more compact as the dAKP concentration increased (Fig. 3c). It was reported that the firmer and compacted emulsion gel disintegrated slowly [35], and this was consestent with our finding. As presented in Fig. 3c, low oil emulsion gel stabilized by 0.5% of dAKP performed creaming and phase separation after stored for 7 days. Meanwhile, emulsion stabilized by other concentrations of dAKP exhibited long-term storage stability, and were capable of building self-supporting gels (Fig. 3d). Combined with the results of zeta protein (Fig. 2h), it can be inferred that 1.5% of dAKP as a stabilizer could promote the formation of low oil emulsion gel.
3.3. Rheological measurements
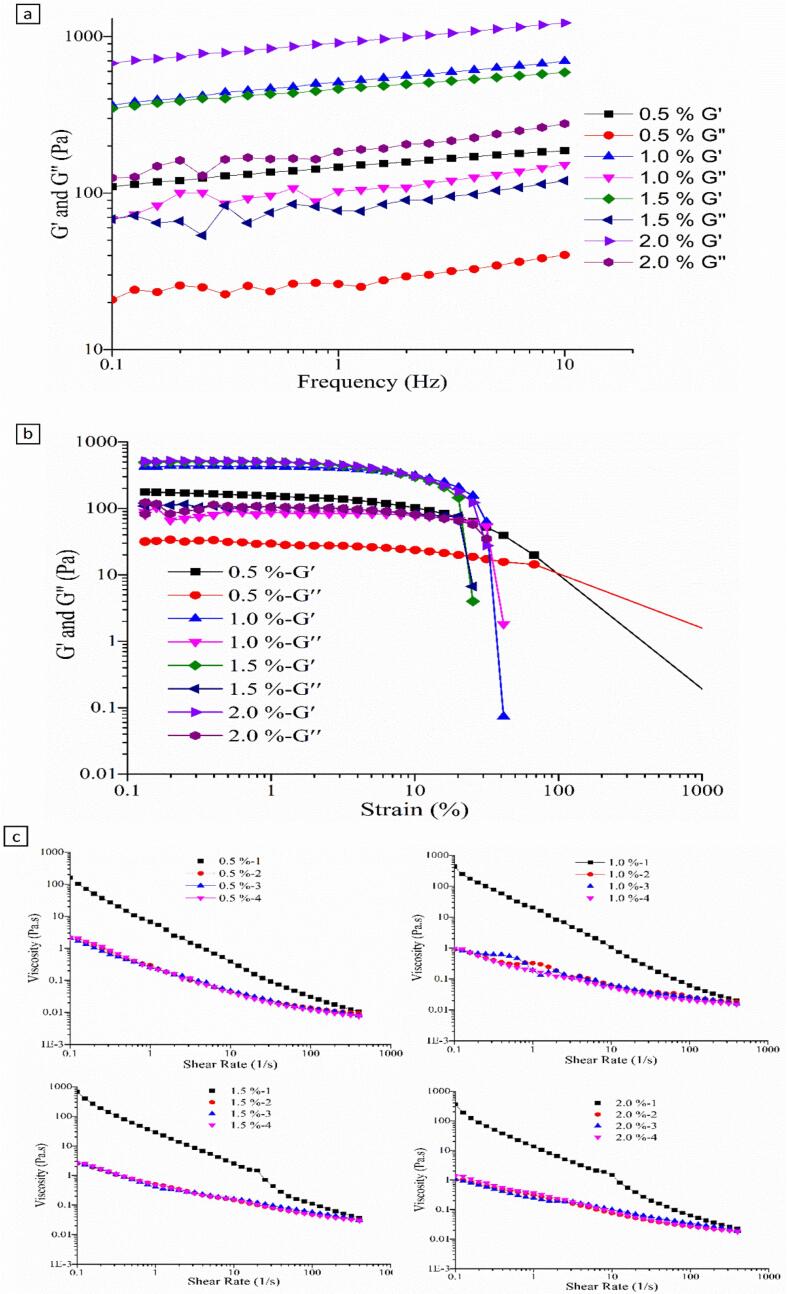
Rheology measurements were performed to obtain more information on the texture properties and the effects of dAKP concentration on the network structure of low oil emulsion gel. The storage modulus (G′) and loss modulus (G″) were provided to respond to the elastic and viscous of low oil emulsion gel stabilized by dAKP; G′ indicated the amount of stored energy in the viscoelastic system, the solid elastic behavior of formed network [36].
The frequency dependency of G′ and G″ of emulsion gels stabilized by different dAKP concentrations (0.5%, 1.0%, 1.5%, and 2.0%) was illustrated in Fig. 4a. For all cases, tested G′ was always higher than G″. Generally, a highly structured solid or gel performed an elastic rheological behavior (G′≫ G″) [37]. Thus, all emulsions in this study contained a well-developed gel-like structure, and the structure was formed even for a low concentration of dAKP (0.5%). As demonstrated in Fig. 4a, the higher the concentration of the dAKP, the higher their viscoelastic moduli (G′ and G″). Regarding the emulsion gels produced in this study, the amounts of G′ and G″ gradually increased as the dAKP concentration increased from 0.5% to 2.0%, indicating the formation of stronger gel-like structures. This was attributed to the gel properties being affected by the particle network involving dAKP in the continuous phase. Furthermore, the slight dependence of G′ and G″ upon the angular frequency was observed that both G′ and G″ for all emulsion gels increased faintly as the frequency increased, suggesting that the network formed in emulsion gels mainly constituted by non-covalent bonds in nature [38].
Fig. 4.
G′ and G″ versus (a) frequency and (b) strain (at 1 Hz) of emulsion gels stabilized by different dAKP concentration (0.5%, 1.0%, 1.5%, and 2.0%). c: Apparent viscosity versus shear rate of emulsion gels stabilized by 0.5%, 1.0%,1.5%, and 2.0% dAKP.
The previous measurements were performed for small deformation (linear viscoelastic regime), providing direct and indirect information on emulsion gel strength and microstructure, respectively. However, large and rapid deformations occurred frequently during actual food processing [36]. Therefore, amplitude sweeps of the emulsion gels stabilized by different concentrations of dAKPs were implemented at a fixed frequency of 1 Hz to obtain information on mechanical properties and the change in structure caused by large deformations. The changes of G′ and G″ as a function of strain from 0.1% to 1000% are displayed in Fig. 4b. In all investigated cases, G′ was much higher than G″ at low strain amplitude (γ < 10%), and both G′ and G″ were almost independent of the strain for small deformations. Furthermore, the values of G′ and G″ increased as the dAKP concentration increased in the individual linear viscoelastic regions, though less pronounced when the concentration was above 5%. Therefore, elastic-dominant behavior of all emulsion gels and an increase in the stiffness of emulsion gels at higher dAKP concentration were indicated. With further increase in the strain amplitude, both G′ and G″ decreased, and G′ decreased more rapidly than G″, leading to the observed crossover point (G″ > G′). The crossover point can be considered to be an indicator of the structural breakdown of the particle network [39], suggesting that emulsions started to show viscosity-dominant behavior after this degree of deformation.
Viscosities as a function of the shear rate of emulsion gels stabilized by 0.5%, 1.0%, 1.5%, and 2.0% of dAKP are exhibited in Fig. 4c. As demonstrated, the viscosities of emulsion gels decreased with the increasing of shear rate which indicated typical shear-thinning behavior [40]. This can be explained by the disintegration of oil droplet clusters in the emulsion gels during the shearing process, and the shear-thinning behavior also can be related to the non-Newtonian behavior of the continuous phase [29], [40]. It was observed that higher concentrations of dAKP (from 0.5% to 1.5%) resulted in increased viscosity along the entire shear-thinning curve, demonstrating that a more rigid particle network was created with the increased dAKP concentration. Interestingly, four characteristic regions were observed evidently to divide the shear-thinning curves of emulsion gels stabilized by 1.5% and 2.0% of dAKP. In the first region, a gradual decrease in viscosity can be observed under the low shear force. The viscosity stopped decreasing, and a plateau was then noticeable in the second region. The reasons for this phenomenon were that the reduction of viscosity was prohibited by a newly formed entangled network constituted by fine filaments presented in Fig. 2d during shearing. In the next region, the formed entangled network was disrupted by high shear force as the shear rate continued increasing, contributing to a sharp drop in the viscosity. In the last region, a steady downward trend of viscosity appeared, indicating that a well-oriented structure was formed. Similar shear-thinning behaviors were noticed in previous work [37]. Moreover, the thixotropy of emulsion gels increased, as indicated by increasing shear rate vs viscosity plot in the first measurement. Thixotropy is significant to flow assurance [41]. However, the thixotropic phenomena abated in all cases in three subsequent measurements, verifying that the structure of emulsion gels was destroyed by shear force.
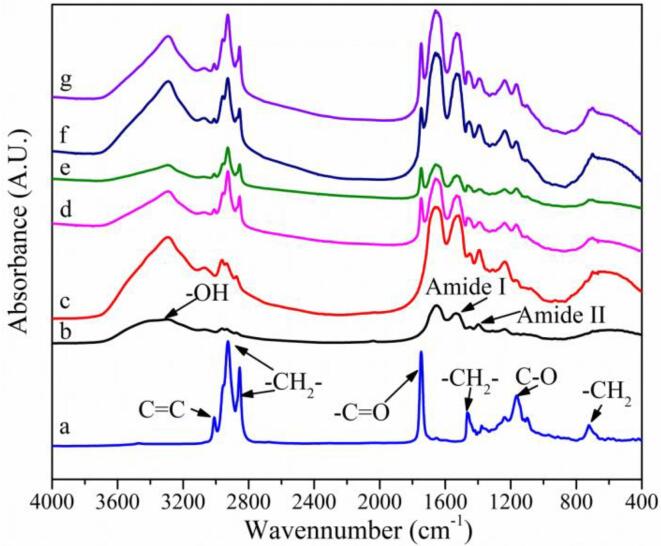
3.4. FTIR analysis of interface protein of emulsion gel
FTIR spectra for soybean oil (a), dAKP (b), the control of dAKP deoiling treatment (c) and interface protein of low oil emulsion gels stabilized by 0.5%, 1.0%, 1.5%, and 2.0% of dAKP (d-g, respectively) were carry out to verify the presence of connection between dAKPs (Fig. 5). As seen from the spectra, –OH peak around 3400 cm−1 in all case FTIR spectra for soybean oil (a), dAKP (b), the control of dAKP deoiling treatment (c), and interface protein of low oil emulsion gels stabilized by 0.5%, 1.0%, 1.5%, and 2.0% of dAKP (d-g, respectively) were conducted to verify the presence of a connection between dAKPs (Fig. 5). As observed from the spectra, –OH peak around 3400 cm−1 in all cases except soybean oil was an indicator of water. The peaks between 3000 cm−1 and 2800 cm−1 were associated with –CH2- asymmetric stretching vibration. Furthermore, the absorption peak of soybean oil at 3000 cm−1 for C C symmetric stretching vibration and 1750 cm−1 for C O stretching vibration, which were not found in b and c, were also exhibited in the infrared spectrum of spectra d-g. This was due to the residue of soybean oil. The characteristic bands of soybean oil at 1580 cm−1, 1290 cm−1, and 780 cm−1 corresponded to stretching vibration of C-O bonds and –CH2- bonds. Besides, the existence of C O bonds and N–H bonds was confirmed by the presence of peaks between 1600 cm−1 and 1200 cm−1 of dAKP. Thus, there were no crosslinks between interface proteins, and no chemical structure changes in the protein. The stable particle structures in emulsion gels were supposed to the interaction between soybean oil and dAKP. It can be inferred that the stabilization of emulsion gel was maintained by space steric hindrance and hydrophobic interactions between particles in the emulsion gel system.
Fig. 5.
The FTIR spectra of low oil emulsion gel, a: soybean oil, b: dAKP, c: the control of dAKP deoiling treatment, d-f: emulsion gels stabilized by 0.5%, 1.0%, 1.5%, and 2.0% dAKP, respectively.
4. Conclusions
The formation and stabilization mechanisms for the low oil emulsion gel stabilized by dAKP using high-intensity ultrasound formation were revealed in this work. Specifically, the microstructure of formed emulsion gel illustrated that dAKP encapsulated soybean oil by cavitation caused by high-intensity ultrasound, and dAKP tightly surrounded oil droplets in case of aggregation. In addition, the elastic gel network was formed without chemical modification or crosslink between proteins on emulsion droplets. To sum up, a green, economical method was proposed in this study to prepare low oil emulsion gel stabilized by dAKP, and the preparation of low oil emulsion gel has more application value in a wide range of fields such as food, nourishment, pharmaceutic, and cosmetics.
CRediT authorship contribution statement
Sijie Hu: Investigation, Data curation, Writing - original draft. Jianhai Wu: Investigation, Writing - original draft. Beiwei Zhu: Supervision. Ming Du: Supervision, Visualization. Chao Wu: Software, Investigation. Cuiping Yu: Resources, Investigation. Liang Song: Resources, Investigation. Xianbing Xu: Conceptualization, Methodology, Project administration.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This work was supported by the 13th Five Years Key Programs for Science and Technology Development of China (Grant No. 2017YFD0400103).
References
- 1.Linke C., Drusch S. Pickering emulsions in foods - opportunities and limitations. Crit. Rev. Food Sci. Nutr. 2018;58:1971–1985. doi: 10.1080/10408398.2017.1290578. [DOI] [PubMed] [Google Scholar]
- 2.Albert C., Beladjine M., Tsapis N., Fattal E., Agnely F., Huang N. Pickering emulsions: Preparation processes, key parameters governing their properties and potential for pharmaceutical applications. J. Controll. Release. 2019;309:302–332. doi: 10.1016/j.jconrel.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Yorgancioglu A., Bayramoglu E.E. Production of cosmetic purpose collagen containing antimicrobial emulsion with certain essential oils. Ind. Crops Prod. 2013;44:378–382. [Google Scholar]
- 4.Chen B., Meinertzhagen I.A., Shaw S.R. Circadian rhythms in light-evoked responses of the fly's compound eye, and the effects of neuromodulators 5-HT and the peptide PDF, Journal of comparative physiology. Sensory Neural Behav. Physiol. 1999;185:393–404. doi: 10.1007/s003590050400. [DOI] [PubMed] [Google Scholar]
- 5.Tang C.-H., Liu F. Cold, gel-like soy protein emulsions by microfluidization: Emulsion characteristics, rheological and microstructural properties, and gelling mechanism. Food Hydrocolloids. 2013;30:61–72. [Google Scholar]
- 6.McClements D.J. Protein-stabilized emulsions. Curr. Opin. Colloid Interface Sci. 2004;9:305–313. [Google Scholar]
- 7.Dickinson E. Emulsion gels: The structuring of soft solids with protein-stabilized oil droplets. Food Hydrocolloids. 2012;28:224–241. [Google Scholar]
- 8.Jiang Y., Liu L., Wang B., Yang X., Chen Z., Zhong Y., Zhang L., Mao Z., Xu H., Sui X. Polysaccharide-based edible emulsion gel stabilized by regenerated cellulose. Food Hydrocolloids. 2019;91:232–237. [Google Scholar]
- 9.Paglarini C.S., Martini S., Pollonio M.A.R. Physical properties of emulsion gels formulated with sonicated soy protein isolate. Int. J. Food Sci. Technol. 2019;54:451–459. [Google Scholar]
- 10.Paglarini C.D.S., Furtado G.D.F., Biachi J.P., Vidal V.A.S., Martini S., Forte M.B.S., Cunha R.L., Pollonio M.A.R. Functional emulsion gels with potential application in meat products. J. Food Eng. 2018;222:29–37. [Google Scholar]
- 11.Glusac J., Davidesko-Vardi I., Isaschar-Ovdat S., Kukavica B., Fishman A. Gel-like emulsions stabilized by tyrosinase-crosslinked potato and zein proteins. Food Hydrocolloids. 2018;82:53–63. [Google Scholar]
- 12.Montes de Oca-Ávalos J., Huck-Iriart C., Candal R., Herrera M. Sodium caseinate/sunflower oil emulsion-based gels for structuring food. Food Bioprocess Technol. 2016;9:981–992. [Google Scholar]
- 13.Patel A.R., Dumlu P., Vermeir L., Lewille B., Lesaffer A., Dewettinck K. Rheological characterization of gel-in-oil-in-gel type structured emulsions. Food Hydrocolloids. 2015;46:84–92. [Google Scholar]
- 14.Hu Z., Patten T., Pelton R., Cranston E.D. Synergistic stabilization of emulsions and emulsion gels with water-soluble polymers and cellulose nanocrystals. ACS Sustainable Chem. Eng. 2015;5:1023–1031. [Google Scholar]
- 15.Zhao Q., Jiang L., Lian Z., Khoshdel E., Schumm S., Huang J., Zhang Q. High internal phase water-in-oil emulsions stabilized by food-grade starch. J. Colloid Interface Sci. 2019;534:542–548. doi: 10.1016/j.jcis.2018.09.058. [DOI] [PubMed] [Google Scholar]
- 16.Zamani S., Malchione N., Selig M.J., Abbaspourrad A. Formation of shelf stable Pickering high internal phase emulsions (HIPE) through the inclusion of whey protein microgels. Food Funct. 2018;9:982–990. doi: 10.1039/c7fo01800b. [DOI] [PubMed] [Google Scholar]
- 17.Farjami T., Madadlou A. An overview on preparation of emulsion-filled gels and emulsion particulate gels. Trends Food Sci. Technol. 2019;86:85–94. [Google Scholar]
- 18.Lorenzo G., Checmarev G., Zaritzky N., Califano A. Linear viscoelastic assessment of cold gel-like emulsions stabilized with bovine gelatin. LWT - Food Sci. Technol. 2011;44:457–464. [Google Scholar]
- 19.Zhou C., Qiao M., Zhang X., Zhu Y., Zhang S., Chen J. Production of high internal phase emulsion with a miniature twin screw extruder. ACS Omega. 2019;4:9957–9963. doi: 10.1021/acsomega.9b01156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmit A., Courbin L., Marquis M., Renard D., Panizza P. A pendant drop method for the production of calibrated double emulsions and emulsion gels. RSC Adv. 2014;4:28504–28510. [Google Scholar]
- 21.Mantelet M., Panouillé M., Boué F., Bosc V., Restagno F., Souchon I., Mathieu V. Impact of sol-gel transition on the ultrasonic properties of complex model foods: Application to agar/gelatin gels and emulsion filled gels. Food Hydrocolloids. 2019;87:506–518. [Google Scholar]
- 22.Nourbehesht N., Shekarchizadeh H., Soltanizadeh N. Investigation of stability, consistency, and oil oxidation of emulsion filled gel prepared by inulin and rice bran oil using ultrasonic radiation. Ultrason. Sonochem. 2018;42:585–593. doi: 10.1016/j.ultsonch.2017.12.029. [DOI] [PubMed] [Google Scholar]
- 23.Nejatian M., Abbasi S., Kadkhodaee R. Ultrasonic-assisted fabrication of concentrated triglyceride nanoemulsions and nanogels. Langmuir. 2018;34:11433–11441. doi: 10.1021/acs.langmuir.8b01596. [DOI] [PubMed] [Google Scholar]
- 24.Zhuang X., Jiang X., Zhou H., Han M., Liu Y., Bai Y., Xu X.-L., Zhou G.-H. The effect of insoluble dietary fiber on myofibrillar protein emulsion gels: Oil particle size and protein network microstructure. LWT. 2019;101:534–542. doi: 10.1016/j.foodchem.2018.09.141. [DOI] [PubMed] [Google Scholar]
- 25.Fu W., Chen E., McClements D.J., Cao Y., Liu S., Li B., Li Y. Controllable viscoelastic properties of whey protein-based emulsion gels by combined cross-linking with calcium ions and cinnamaldehyde. ACS Appl. Bio Mater. 2018;2:311–320. doi: 10.1021/acsabm.8b00604. [DOI] [PubMed] [Google Scholar]
- 26.Bao J., Chen L., Liu T. Dandelion polysaccharide suppresses lipid oxidation in Antarctic krill (Euphausia superba) Int. J. Biol. Macromol. 2019;133:1164–1167. doi: 10.1016/j.ijbiomac.2019.04.205. [DOI] [PubMed] [Google Scholar]
- 27.Zheng H., Beamer S.K., Matak K.E., Jaczynski J. Effect of kappa-carrageenan on gelation and gel characteristics of Antarctic krill (Euphausia superba) protein isolated with isoelectric solubilization/precipitation. Food Chem. 2019;278:644–652. doi: 10.1016/j.foodchem.2018.11.080. [DOI] [PubMed] [Google Scholar]
- 28.Shi L., Beamer S.K., Yin T., Matak K.E., Yang H., Jaczynski J. Mass balance for isoelectric solubilization/precipitation of carp, chicken, menhaden, and krill. LWT – Food Sci. Technol. 2017;81:26–34. [Google Scholar]
- 29.Li S., Zhang B., Li C., Fu X., Huang Q. Pickering emulsion gel stabilized by octenylsuccinate quinoa starch granule as lutein carrier: Role of the gel network. Food Chem. 2019;305 doi: 10.1016/j.foodchem.2019.125476. [DOI] [PubMed] [Google Scholar]
- 30.Liu W., Gao H., McClements D.J., Zhou L., Wu J., Zou L. Stability, rheology, and β-carotene bioaccessibility of high internal phase emulsion gels. Food Hydrocolloids. 2019;88:210–217. [Google Scholar]
- 31.Geremias-Andrade I.M., Souki N.P.D.B.G., Moraes I.C.F., Pinho S.C. Rheological and mechanical characterization of curcumin-loaded emulsion-filled gels produced with whey protein isolate and xanthan gum. Lwt. 2017;86:166–173. [Google Scholar]
- 32.Maurya N.K., Mandal A. Investigation of synergistic effect of nanoparticle and surfactant in macro emulsion based EOR application in oil reservoirs. Chem. Eng. Res. Des. 2018;132:370–384. [Google Scholar]
- 33.Qi X.-M., Liao E., Wang L., Lin H., Xue C.-H. Extracting protein from antarctic krill (Euphausia superba) J. Aquat. Food Prod. Technol. 2015;25:597–606. [Google Scholar]
- 34.Aslan D., Dogan M. The influence of ultrasound on the stability of dairy-based, emulsifier-free emulsions: rheological and morphological aspect. Eur. Food Res. Technol. 2017;244:409–421. [Google Scholar]
- 35.Guo Q., Ye A., Lad M., Dalgleish D., Singh H. Effect of gel structure on the gastric digestion of whey protein emulsion gels. Soft Matter. 2014;10:1214–1223. doi: 10.1039/c3sm52758a. [DOI] [PubMed] [Google Scholar]
- 36.Zou Y., Yang X., Scholten E. Rheological behavior of emulsion gels stabilized by zein/tannic acid complex particles. Food Hydrocolloids. 2018;77:363–371. [Google Scholar]
- 37.Li M.-C., Wu Q., Song K., Lee S., Qing Y., Wu Y. Cellulose nanoparticles: structure–morphology–rheology relationships. ACS Sustainable Chem. Eng. 2015;3:821–832. [Google Scholar]
- 38.Wang X., Luo K., Liu S., Zeng M., Adhikari B., He Z., Chen J. Textural and rheological properties of soy protein isolate tofu-type emulsion gels: influence of soybean variety and coagulant type. Food Biophys. 2018;13:324–332. [Google Scholar]
- 39.Foudazi R., Qavi S., Masalova I., Malkin A.Y. Physical chemistry of highly concentrated emulsions. Adv. Colloid Interface Sci. 2015;220:78–91. doi: 10.1016/j.cis.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 40.Niknam R., Ghanbarzadeh B., Ayaseh A., Rezagholi F. The effects of Plantago major seed gum on steady and dynamic oscillatory shear rheology of sunflower oil-in-water emulsions. J. Texture Stud. 2018;49:536–547. doi: 10.1111/jtxs.12352. [DOI] [PubMed] [Google Scholar]
- 41.Guo L., Zhang J., Sun G., Bao Y. Thixotropy and its estimation of water-in-waxy crude emulsion gels. J. Petrol. Sci. Eng. 2015;131:86–95. [Google Scholar]