Abstract
Pain in cancer is often underdiagnosed and undertreated. Breakthrough pain, in particular, severely impacts the quality of life of patients. In this study, we evaluated management and care of pain in Spain from the patient perspective by assessing the experience of 275 patients who had suffered breakthrough pain. Although most patients had suffered moderate-to-severe pain in the last 24 hours, pain relief was achieved in the majority of cases. The body areas with a higher pain intensity was felt varied based on primary cancer. Adherence to treatment was subpar, and patients were moderately concerned about addiction to treatment and adverse events. Doctors did not assess pain in every visit and there is room for improvement in its classification. Education strategies directed toward patients and health care personnel are needed to improve pain assessment, follow-up, and compliance. These could guide shared decision-making and improve communication about cancer pain to improve its care.
Keywords: breakthrough pain, cancer pain, chronic pain, patient experience, Spain
Introduction
Pain is a major source of distress associated with cancer and it significantly impacts patients’ quality of life, diagnosis and treatment (1). Although its prevalence varies between studies, it has been estimated that 55% of cancer patients undergoing treatment feel pain (2). Pain can be classified in multiple ways by evaluating domains such as location, intensity, temporal pattern, pathophysiology, and etiology (3,4). However, not all domains are evaluated in all cases, nor are other relevant aspects, such as characteristics of the disease (primary diagnosis, extent of the disease, metastasis), demographics, and psychological status (3).
In order to provide an adequate and personalized treatment of pain, it must be properly assessed and understood. One major type is breakthrough cancer pain (BTP), which is a “transitory exacerbation of pain that occurs, either spontaneously or associated with predictable factors or not, even though the baseline pain is relatively stable and well controlled” (5). The definition of BTP is still a source of debate, and there is variability on what constitute its minimum intensity, duration, and number of flares (6 –8). Additionally, not all clinicians consider opioids should be used to control this pain (6). The pathophysiology of BTP is heterogeneous, but the 2 main subtypes are incident pain (either voluntary, involuntary or intervention-related) and spontaneous pain (which is unpredictable) (9). The prevalence of BTP varies between studies (10), but its presence is associated with higher pain intensity, functional impairment, and a significant impact on quality of life (11,12). High prevalence rates of BTP in cancer patients were found in 2 recent studies in Spain, one of which notably showed that these high rates were not expected by doctors (13,14).
Although management of cancer pain has improved in recent years, it is still an ongoing challenge, with clinician and patient barriers—among others—that need to be overcome (15). A third of patients are found to be undertreated for pain (16). On this note, a study of 12 countries, not including Spain, found that 12% of patients reported health care professionals did not regard pain as a problem, and 50% perceived clinicians did not consider their quality of life to be important (17).
The Spanish Foundation for Excellence and Quality in Oncology (Fundación ECO) is committed to improving care of cancer patients. To this end, Fundación ECO directed this patient-centered study to understand their experience and unmet needs regarding pain. The aim of this article is to present the findings of the surveys carried out on pain (including BTP), the care received, the degree to which pain management was achieved, and the impact on quality of life as well as to discuss strategies to address the identified needs.
Methods
Study Design
This was a cross-sectional, multicenter, study. To calculate the sample size needed, we considered a margin of error of 6%, a 95% level of confidence, and a response distribution of 50%. This resulted in a required sample size of 275 patients. To guarantee this number was achieved in the event of possible withdrawals, 47 oncologists were invited to participate, their number being proportional to the population of their respective regions. Each oncologist recruited 4 to 6 patients until the required sample size of 275 was reached. From the study starting date, for 4 to 6 consecutive days, the first eligible patient of each day was asked to participate in this study. All patients provided written informed consent. Inclusion criteria were patients who were: over 18 years old; suffering from pain associated with cancer, including BTP; and, if taking analgesics, having initiated treatment at least 1 month prior to inclusion in this study. The only exclusion criterion was the patient’s lack of consent to participate.
An ad hoc questionnaire was provided to oncologic patients to assess pain associated with cancer, including BTP. Each oncologist collected the anonymized questionnaires and, once the last one was received, patient data were aggregated and recorded in a restricted-access online repository platform designed for this study. There was no patient follow-up after the questionnaire was submitted.
Due to the nature of the study, submission to the Spanish Agency of Medicines and Medical Products was not required. Nevertheless, this study was performed in accordance with the Declaration of Helsinki and submitted to the clinical research ethics committee, who issued a favorable opinion.
Variables Studied
Descriptive patient variables were recorded: demographics (age, sex, level of education, autonomous region of Spain) and clinical variables (type of cancer, pain associated with cancer [type, intensity, location, and progression], and impact on quality of life). Descriptive patient opinion variables were also recorded: attitude toward pain on the part of the oncologist and other health care professionals, quality of information received regarding pain and possible treatment, pain follow-up, adherence to recommended treatments, degree of personalization received, and general satisfaction with care compared with the patient’s expectations.
Data Analysis
Continuous variables were described using means ± SD, using medians and ranges in cases of large dispersion of data. Categorical variables were described using frequencies and CIs. Continuous variables were compared using independent t tests or Mann–Whitney U tests, as appropriate. All statistical analyses were carried out with SPSS version 21.0 (IBM Corp)
Results
Oncologist Profile
A total of 47 oncologists participated in this study (27 women, 20 men), with an average age of 38.9 (95% CI: 36.8-41.0) years and a median age of 38 (range, 30-55). No significant difference in age was found between women (39.0 years) and men (38.7 years; P = .094). Professional experience ranged from 2 to 28 years, with an average of 11.9 (95% CI: 10.1-13.7) and a median of 10 years. There was no significant difference in work experience between women (12.1 years) and men (11.7 years; P = .650). Most oncologists (46, 97.9%) worked in the public health care system, 3 of them in combination with private practice; only 1 oncologist worked exclusively in a private hospital. Oncologists saw an average of 20.4 (95% CI: 12-25) patients per day, with 30.5% of them reporting BTP.
Patient Demographics
A total of 275 patients representing 11 of the 17 autonomous regions of Spain were included in this study (Table 1). The average age was 62.4 (95% CI: 61.0-63.7) years and ranged from 19 to 86. Of these, 46.5% were women. Regarding education level, 14.2% had no studies, 36.0% had primary level studies, and 16.0% had a university degree. The most common primary tumor was lung (77, 28.0%), followed by breast (42, 15.3%) and colorectal (36, 13.1%).
Table 1.
Patient Characteristics.
| N (%) N = 275 |
|
|---|---|
| Age, average years (95% CI) | 62.4 (61.0-63.7) |
| Gender, female | 128 (46.5) |
| Education level | |
| No schooling | 39 (14.2) |
| Primary | 99 (36.0) |
| Junior high school | 40 (14.5) |
| Senior high school/vocational | 51 (18.5) |
| University | 44 (16.0) |
| Doctorate | 2 (0.7) |
| Primary tumor location | |
| Lung | 77 (28.0) |
| Breast | 42 (15.3) |
| Colorectal | 36 (13.1) |
| Head and neck | 29 (10.5) |
| Pancreas | 24 (8.7) |
| Prostate | 22 (8.0) |
| Gynecologic | 15 (5.5) |
| Melanoma | 6 (2.2) |
| Other | 24 (8.7) |
| Pain | |
| Noa | 25 (9.1) |
| Yes | 250 (90.9) |
| Chronic | 47 (17.1) |
| Breakthrough | 38 (13.8) |
| Chronic and breakthrough | 190 (69.1) |
a No pain at the time the patient was included in the study, other than common headaches, toothaches, contusions, and so on.
At the time of inclusion in the study, a considerable number of patients (250, 90.9%) felt pain. Most patients (190, 69%) reported feeling both chronic pain and BTP throughout the disease, while 38 (13.8%) and 47 (17.1%) felt only either BTP or chronic pain, respectively.
Pain Description
Patients reported the specific body area where they felt the most severe pain. Among all cancer types, the most common areas for pain were the abdomen (22.5%), torso (9.8%), neck (6.6%), spine (6.2%), and hips (5.8%) (Table 2). The most affected areas differed based on the primary cancer, with lung cancer patients feeling most pain in the spine (13%) and the lumbar area (11.7%), breast cancer patients feeling most pain in the lumbar area (23.8%) and the hips (19.1%), and colorectal cancer patients feeling most pain in the abdomen (66.7%).
Table 2.
Pain Location by Cancer Type.
| Body area with worst pain | Primary tumor | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Head and neck | Gynecologic | Colorectal | Breast | Melanoma | Pancreas | Prostate | Lung | Others | ||
| Head, n (%) | 2 (6.90%) | 0 (0.00%) | 0 (0.00%) | 1 (2.38%) | 0 (0.00%) | 1 (4.17%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 4 (1.45%) |
| Face, n (%) | 2 (6.90%) | 0 (0.00%) | 0 (0.00%) | 1 (2.38%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 3 (1.09%) |
| Neck, n (%) | 16 (55.17%) | 0 (0.00%) | 0 (0.00%) | 1 (2.38%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 1 (1.30%) | 0 (0.00%) | 18 (6.55%) |
| Right shoulder, n (%) | 0 (0.00%) | 0 (0.00%) | 1 (2.78%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 1 (4.55%) | 3 (3.90%) | 1 (4.17%) | 6 (2.18%) |
| Left shoulder, n (%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 2 (33.33%) | 0 (0.00%) | 0 (0.00%) | 7 (9.09%) | 1 (4.17%) | 10 (3.64%) |
| Shoulders, n (%) | 1 (3.45%) | 0 (0.00%) | 0 (0.00%) | 1 (2.38%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 1 (1.30%) | 0 (0.00%) | 3 (1.09%) |
| Right torso, n (%) | 0 (0.00%) | 1 (6.67%) | 1 (2.78%) | 1 (2.38%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 7 (9.09%) | 0 (0.00%) | 10 (3.64%) |
| Left torso, n (%) | 0 (0.00%) | 0 (0.00%) | 1 (2.78%) | 6 (14.29%) | 0 (0.00%) | 1 (4.17%) | 0 (0.00%) | 7 (9.09%) | 2 (8.33%) | 17 (6.18%) |
| Right arm, n (%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 2 (2.60%) | 0 (0.00%) | 2 (0.73%) |
| Left arm, n (%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 1 (4.17%) | 1 (0.36%) |
| Right abdomen, n (%) | 2 (6.90%) | 3 (20.00%) | 13 (36.11%) | 0 (0.00%) | 0 (0.00%) | 11 (45.83%) | 1 (4.55%) | 3 (3.90%) | 4 (16.67%) | 37 (13.45%) |
| Left abdomen, n (%) | 1 (3.45%) | 3 (20.00%) | 11 (30.56%) | 0 (0.00%) | 0 (0.00%) | 6 (25.00%) | 0 (0.00%) | 4 (5.19%) | 0 (0.00%) | 25 (9.09%) |
| Hips, n (%) | 0 (0.00%) | 0 (0.00%) | 2 (5.56%) | 8 (19.05%) | 0 (0.00%) | 0 (0.00%) | 1 (4.55%) | 4 (5.19%) | 1 (4.17%) | 16 (5.82%) |
| Right wrist, n (%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 1 (4.55%) | 0 (0.00%) | 0 (0.00%) | 1 (0.36%) |
| Left wrist, n (%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 1 (4.55%) | 0 (0.00%) | 0 (0.00%) | 1 (0.36%) |
| Genitals, n (%) | 0 (0.00%) | 3 (20.00%) | 1 (2.78%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 2 (9.09%) | 1 (1.30%) | 4 (16.67%) | 11 (4.00%) |
| Right leg, n (%) | 0 (0.00%) | 1 (6.67%) | 0 (0.00%) | 0 (0.00%) | 2 (33.33%) | 0 (0.00%) | 2 (9.09%) | 2 (2.60%) | 0 (0.00%) | 7 (2.55%) |
| Left leg, n (%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 1 (4.17%) | 0 (0.00%) | 1 (1.30%) | 1 (4.17%) | 3 (1.09%) |
| Legs, n (%) | 1 (3.45%) | 1 (6.67%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 1 (4.17%) | 3 (1.09%) |
| Right knee, n (%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 1 (4.17%) | 1 (0.36%) |
| Left knee, n (%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) |
| Knees, n (%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 1 (2.38%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 1 (0.36%) |
| Right foot, n (%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 1 (1.30%) | 0 (0.00%) | 1 (0.36%) |
| Head, n (%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 1 (2.38%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 1 (1.30%) | 0 (0.00%) | 2 (0.73%) |
| Back of neck, n (%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 1 (2.38%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 2 (2.60%) | 0 (0.00%) | 3 (1.09%) |
| Left dorsal, n (%) | 2 (6.90%) | 0 (0.00%) | 0 (0.00%) | 3 (7.14%) | 0 (0.00%) | 1 (4.17%) | 1 (4.55%) | 3 (3.90%) | 0 (0.00%) | 10 (3.64%) |
| Right dorsal, n (%) | 1 (3.45%) | 0 (0.00%) | 2 (5.56%) | 1 (2.38%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 2 (2.60%) | 0 (0.00%) | 6 (2.18%) |
| Left lumbar, n (%) | 0 (0.00%) | 1 (6.67%) | 0 (0.00%) | 1 (2.38%) | 0 (0.00%) | 0 (0.00%) | 1 (4.55%) | 2 (2.60%) | 0 (0.00%) | 5 (1.82%) |
| Right lumbar, n (%) | 1 (3.45%) | 0 (0.00%) | 0 (0.00%) | 2 (4.76%) | 1 (16.67%) | 0 (0.00%) | 3 (13.64%) | 2 (2.60%) | 0 (0.00%) | 9 (3.27%) |
| Lumbar area, n (%) | 0 (0.00%) | 2 (13.33%) | 0 (0.00%) | 10 (23.81%) | 0 (0.00%) | 2 (8.33%) | 4 (18.18%) | 9 (11.69%) | 2 (8.33%) | 29 (10.55%) |
| Left buttock, n (%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 1 (4.55%) | 0 (0.00%) | 1 (4.17%) | 2 (0.73%) |
| Right buttock, n (%) | 0 (0.00%) | 0 (0.00%) | 3 (8.33%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 1 (1.30%) | 0 (0.00%) | 4 (1.45%) |
| Buttocks, n (%) | 0 (0.00%) | 0 (0.00%) | 1 (2.78%) | 0 (0.00%) | 1 (16.67%) | 1 (4.17%) | 1 (4.55%) | 1 (1.30%) | 1 (4.17%) | 6 (2.18%) |
| Right calf, n (%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 1 (4.17%) | 1 (0.36%) |
| Left calf, n (%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) |
| Spine, n (%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 3 (7.14%) | 0 (0.00%) | 0 (0.00%) | 2 (9.09%) | 10 (12.99%) | 2 (8.33%) | 17 (6.18%) |
| Total, n (%) | 29 (100.00%) | 15 (100.00%) | 36 (100.00%) | 42 (100.00%) | 6 (100.00%) | 24 (100.00%) | 22 (100.00%) | 77 (100.00%) | 24 (100.00%) | 275 (100.00%) |
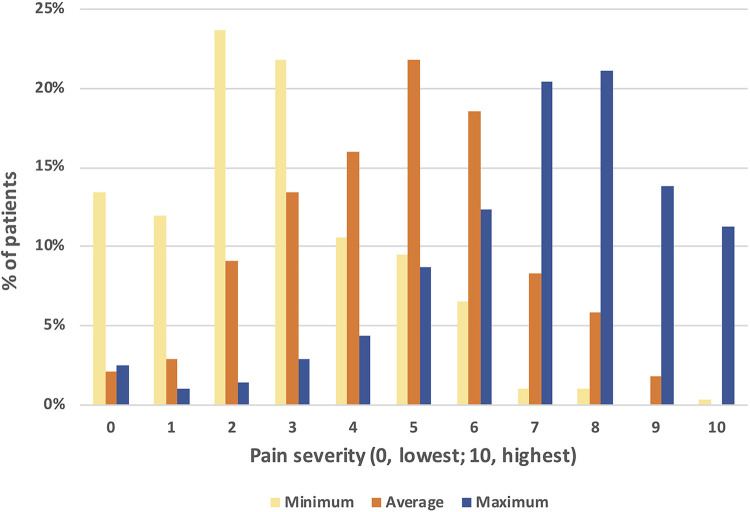
Patients reported the minimum, average, and maximum pain they had felt in the previous 24 hours on a scale from 0 (lowest) to 10 (highest; Figure 1). Almost half (127, 46.2%) of patients reported a maximum pain of ≥8. Almost 70% of patients (192, 69.8%) felt an average pain between 3 and 6, and also a minimum pain under 3 (195, 70.1%).
Figure 1.
Pain severity in the last 24 hours.
Patients reported feeling pain for an average of 10.3 (95% CI: 8.8-11.8) months and a median of 6 (range, 0.5-84) months. The average time patients had experienced BTP was 6.5 (95% CI: 5.1-7.6) months, with a median of 3 months (range, 0.3-6). Only 3 patients reported feeling no BTP.
When questioned about the cause of pain, 179 (65.1%) patients believed their pain was related only to the cancer, and 63 (22.9%) attributed it to both the cancer and the cancer treatment. Only 22 (8%) patients believed their pain was caused by the cancer treatment alone, and the remaining 11 (4%) patients attributed their pain to other causes or did not respond.
Impact of Pain on Patient Quality of Life
Patients reported the degree to which pain had affected their life, on a scale from 0 (not affected) to 10 (severely affected), in the following: general activity, mood, walking, work, relationships, sleep, and life enjoyment. The domains most affected were life enjoyment (average score, 6.34), general activity (6.31), and mood (6.2). Lower average scores were given to impact on work (6.03), relationships (5.03), and sleep (5.47), with the lowest score being for walking (4.98).
Care received for Pain
Most (213, 77.5%) patients reported being informed at diagnosis of the possibility of feeling pain associated with cancer, 31 (11.3%) patients reported not receiving this information, and another 31 (11.3%) did not remember. Most (227, 82.5%) patients also reported their oncologist informed them that pain can be controlled, while 24 (8.7%) patients said they were not informed of this, and another 24 (8.7%) did not remember.
Regarding monetarization of pain, 197 (72.9%) patients reported their doctor asked about it on every visit, 52 (19.3%) said it happened in most visits, 7 (2.6%) reported they were asked about it in less than half the visits, and 14 (5.2%) reported only occasionally being asked about pain. No patient reported not having been asked. Pain discussion was most commonly initiated by doctors (41.7%) than by patients (25.4%). Only in 16.9% of cases did the doctor ask about pain intensity; in 10.8% of total cases, the doctor examined the patient.
Oncologists were the main health care professionals (65%) involved in treating pain associated with cancer, followed by primary care doctors (11.0%), palliative care doctors (5.1%), anesthetists or pain unit doctors (4.9%), and radiation oncologists (4.6%).
Perspectives on Pain Relief Medication
Satisfaction with pain relief treatment was high, with 113 (43.1%) patients reporting being very satisfied and 133 (50.8%) being quite satisfied. Addiction to treatment was a cause for concern in almost half of patients, who were either very worried (38, 13.8%) or quite worried (80, 29.1%) about it. Additionally, over half of patients were either very worried (36, 13.1%) or quite worried (110, 40.0%) about the adverse events of pain relief treatment.
Questions about adherence to pain relief treatment showed that 46 (16.7%) patients sometimes forgot to take the medication, 48 (17.5%) did not take it at the indicated time, 87 (31.6%) stopped taking it once they felt better, and 142 (51.6%) stopped taking it if it did not agree with them. However, regarding treatment for BTP, almost all (264, 96%) patients followed treatment guidelines.
Regarding chronic pain management in the 24 hours prior to the patients’ visit, 191 (69.5%) patients reported relief over 70%. Eight (2.9%) patients experienced no or minimal relief, under 20%. In the case of BTP, 204 (74.2%) patients reported relief over 70%, and 7 (2.5%) patients experienced no or minimal relief, under 20%.
When asked about their degree of satisfaction with pain relief treatment, only a small percentage of patients reported being quite or very unsatisfied with the effectiveness of treatment (36, 13.1%), tolerance of adverse effects (43, 15.6%), and overall treatment (34, 12.4%).
Discussion
In this study, we evaluated the pain care that cancer patients receive in Spain from a patient-centered perspective. We found that most patients had suffered moderate-to-severe pain in the last 24 hours and had suffered from pain for an average of 10.3 months. We assessed the impact of pain on quality of life, adherence to treatment, and doctor attitudes to understand the different aspects involved in pain management.
A systematic review found the average prevalence of BTP was 61.0%, although there was high variability between studies, ranging 33.3% to 95.0%, with higher rates corresponding to patients in hospice (10). A recent study in Spain found that 91.3% of cancer patients with pain had suffered from BTP, although almost half of them had been undetected by doctors (13.) Our study did not address prevalence of BTP, as one of our inclusion criteria was that patients had suffered BTP at some point, although 3 of them later reported in the questionnaire that they had not experienced it.
The body areas where patients felt most pain varied depending on the primary cancer, in agreement with reports by others (18). Overall pain prevalence has also been found to vary based on primary cancer (14,17); however, we did not evaluate this due to the data being aggregated.
In contrast to the finding that pain is undertreated in approximately a third of cancer patients (16), most patients (93.9%) in our study were satisfied with the pain relief achieved with medication. This finding is also higher than a previous study in Spain, reporting that almost half of patients with BTP did not achieve pain control (14). While we observed that 96% of patients adhered to treatment for BTP, adherence to chronic pain treatment was subpar, with 1 in 6 patients forgetting to take the medication. The main patient barrier to adherence was negative side effects, with over half of patients not taking the medication for this reason. Patient education has proven to improve quality of life and adherence to treatment for cancer pain (19 –21) and to decrease pain intensity (19 –23). Future work in Spain could focus on carrying out patient education strategies using several methods (21): face-to-face coaching sessions, follow-up phone calls, and informational videos, among others. These initiatives could target patient compliance and educate on opioid treatment, its associated adverse events, and addiction, as these were a cause for concern for approximately half of patients in our study, which is in agreement with reports from other countries (24 –27).
Education of all health care personnel should also be considered, given that, in 35% of cases, patients reported that pain was not followed by an oncologist. Patients also reported the doctors examined them in only 10.8% of cases and assessed pain intensity in 16.9% of cases. On this note, oncologists in this study saw an average of 20.4 patients per day, a third of them reporting BTP, and a recent Delphi survey found that Spanish oncologists considered limited time to evaluate patients to be a barrier to managing BTP (28). Classifying pain in all of its domains can provide more information and improve understanding of patient needs to, ultimately, deliver a higher quality of pain care. Studies have shown that physicians consider there are deficiencies in pain management training (29 –31). On this note, a study in Spain found that almost half of oncologists had not read the guidelines on diagnosis and treatment of BTP (13). This further highlights the need to not only educate patients but also health care professionals. Additionally, significant differences were found on nurses’ training on pain management across 12 European countries (32). Initiatives to improve pain management by health care professionals could include mandatory palliative care rotations for trainees, developing multidisciplinary teams, creating educational courses, and encouraging interdisciplinary work (33). Most patients desire shared decision-making in pain management (34), which leads to more open communication and higher patient satisfaction (35).
This study provides, for the first time, the patient perspective on management of pain associated with cancer in Spain. We believe our findings can be useful for health care professionals and decision makers to better understand and address patient needs. They can also serve as a basis for implementing policies that address the issues found, such as the need for evaluating pain in several ways, most notably examining the patient and grading pain intensity. We suggest placing a stronger focus on education of patients and health care professionals, and implementing strategies that encourage open communication about pain.
Limitations
The main limitation of this study is the use of aggregated data of patient reports, which precludes establishing associations between the parameters evaluated. For example, studying the association between type of primary cancer and pain intensity, or that between information provided by the doctor and adherence to treatment could help develop guidelines and strategies toward personalization of pain care. Also, our sample size is relatively small, with a predominance of 3 cancer types (lung, breast, and colorectal). Future studies that address these limitations could further establish links between the parameters evaluated here and improve pain management. Finally, this study reports the experience of patients and their opinions; however, we cannot confirm the information they report, and we cannot rule out a possible bias in their expectations of care.
Conclusion
We evaluated the perspective of cancer patients in Spain regarding care of pain and found that, although almost half of them had felt a very high maximum pain in the previous 24 hours, the vast majority were satisfied with the degree of pain management achieved with analgesics. The body areas where patients felt most pain were the abdomen, torso, neck, spine, and hips, predominance varying with primary cancer type. The dimension of quality of life that patients considered were more affected by pain was life enjoyment and general activity. Our findings highlight some areas of improvement in pain management and could guide strategies that address them.
Acknowledgments
The authors thank all doctors and patients that participated in this study. The authors also thank Angela Rynne Vidal, PhD, for providing writing and editing support, funded by Fundación ECO.
Author Biographies
Jesús García-Foncillas, MD, PhD, is the head of the Cancer institute at the University Hospital “Fundación Jiménez Diaz”, Madrid, Spain and is also a professor of Oncology at the Autonomous University of Madrid. He obtained his degree in Medicine from University of Zaragoza (Zaragoza, Spain) and his PhD from University of Navarra, Pamplona, Spain. Prof García-Foncillas has authored more than 260 publications.
Antonio Antón-Torres, MD, is the head of the Medical Oncology Unit at Hospital Universitario Miguel Servet, Zaragoza, Spain. He is also an associate professor of Medicine at Zaragoza University. Prof Antón-Torres is a member of the European Society for Medical Oncology, the Spanish Society of Medical Oncology, and the American Society for Clinical Oncology, among others.
Fernando Caballero-Martínez, MD, PhD, is the dean of Medicine at Universidad Francisco de Vitoria, Madrid, Spain. He is also a professor of Medicine and scientific director at Universidad Francisco de Vitoria. Dr Caballero-Martínez has received several awards for his research and teaching experience, and has authored more than 70 publications.
Francisco J Campos, BS, is the head of the Consulting and Research Unit at Universidad Francisco de Vitoria, Madrid, Spain, where he develops scientific programs and secures funding to support them. He graduated in Chemistry at Autonomous University of Madrid and has published over 10 articles.
Margarita Feyjoo, MD, is the head of the Medical Oncology Unit at Hospital Universitario Sanitas La Moraleja, Madrid, Spain, and also teaches at Universidad Francisco de Vitoria, Madrid, Spain. Dr Feyjoo worked for 8 years in the pharmaceutical industry and is a member of Spanish Society of Medical Oncology.
Alfonso Gómez de Liaño, MD, is the head of the Genitourinary Oncology Unit at Complejo Hospitalario Universitario Insular Materno-Infantil, Las Palmas, Spain. He studied Medicine at Salamanca University and trained in Medical Oncology at Hospital Santa Creu i Sant Pau. He then completed a fellowship in clinical and immunotherapy research at St Bartholomew's Hospital, London, under the supervision of Prof Thomas Powles. His career to date has been devoted to clinical and translational research in the field of genitourinary tumours. He is also a member of the European Society for Medical Oncology, the Spanish Society of Medical Oncology, and the Spanish Oncology Genitourinary Group.
Diana Monge, MD, MPH, PhD, is a professor at Universidad Francisco de Vitoria, Madrid, Spain. She graduated in Medicine from Autonomous University of Madrid and obtained a PhD in Prevention and Public Health from King Juan Carlos University, Madrid, Spain. Dr Monge has authored 30 articles.
Carlos Camps, MD, PhD, is the head of the Medical Oncology Unit at General Valencia Hospital, Valencia, Spain, and a professor of Medicine at Valencia University. He is a member of the scientific committee of several institutions and a member of scientific societies, such as the European Society for Medical Oncology, the Spanish Society of Medical Oncology, and the American Society for Clinical Oncology. Prof Camps has supervised 12 doctoral thesis and is the author of more than 250 publications.
Authors' Note: This study was performed in accordance with the Declaration of Helsinki and submitted to the Clinical Research Ethics Committee at the San Carlos Clinical Hospital in Madrid (Spain), who issued a favorable opinion.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Kyowa Kirin.
ORCID iDs: Jesús García-Foncillas, MD, PhD  https://orcid.org/0000-0002-7591-8006
https://orcid.org/0000-0002-7591-8006
Alfonso Gómez de Liaño, MD  https://orcid.org/0000-0002-1844-8474
https://orcid.org/0000-0002-1844-8474
Diana Monge, MD, MPH, PhD https://orcid.org/0000-0002-3593-1820
References
- 1. Mantyh PW. Cancer pain and its impact on diagnosis, survival and quality of life. Nat Rev Neurosci. 2006;7:797–809. [DOI] [PubMed] [Google Scholar]
- 2. van den Beuken-Van Everdingen MHJ, Hochstenbach LMJ, Joosten EAJ, Tjan-Heijnen VCG, Janssen DJA. Update on prevalence of pain in patients with cancer: systematic review and meta-analysis. J Pain Symptom Manage. 2016;51:1070–1090. [DOI] [PubMed] [Google Scholar]
- 3. Knudsen AK, Aass N, Fainsinger R, Caraceni A, Klepstad P, Jordhøy M, et al. Classification of pain in cancer patients—a systematic literature review. Palliat Med. 2009;23:295–308. [DOI] [PubMed] [Google Scholar]
- 4. Caraceni A, Shkodra M. Cancer pain assessment and classification. Cancers. 2019;11:510 Epub ahead of print 2019 doi:10.3390/cancers11040510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Davies AN, Dickman A, Reid C, Stevens AM, Zeppetella G, Science Committee of the Association for Palliative Medicine of Great Britain and Ireland. The management of cancer-related breakthrough pain: Recommendations of a task group of the Science Committee of the Association for Palliative Medicine of Great Britain and Ireland. European J Pain. 2009;13:331–338. [DOI] [PubMed] [Google Scholar]
- 6. Boceta J, de la Torre A, Samper D, Farto M. Sánchez-de la Rosa R. Consensus and controversies in the definition, assessment, treatment and monitoring of BTcP: results of a Delphi study. Clin Transl Oncol. 2016;18:1088–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mercadante S, Lazzari M, Reale C, Cuomo A, Fusco F, Marchetti P, et al. Italian oncological pain survey (IOPS): a multicentre Italian study of breakthrough pain performed in different settings. Clin J Pain. 2015;31:214–221. [DOI] [PubMed] [Google Scholar]
- 8. Davies A, Buchanan A, Zeppetella G, Porta-Sales J, Likar R, Weismayr W, et al. Breakthrough cancer pain: an observational study of 1000 European oncology patients. J Pain Symptom Manage. 2013;46:619–628. [DOI] [PubMed] [Google Scholar]
- 9. Vellucci R, Mediati RD, Gasperoni S, Mammucari M, Marinangeli F, Romualdi P, et al. Assessment and treatment of breakthrough cancer pain: from theory to clinical practice. J Pain Res. 2017;10:2147–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Deandrea S, Corli O, Consonni D, Villani W, Teresa Greco M, Apolone G. Prevalence of breakthrough cancer pain: a systematic review and a pooled analysis of published literature. J Pain Symptom Manage. 2014;47:57–76. [DOI] [PubMed] [Google Scholar]
- 11. Caraceni A, Martini C, Zecca E, Portenoy RK, Ashby MA, Hawson G, et al. Breakthrough pain characteristics and syndromes in patients with cancer pain. An international survey. Palliat Med. 2004;18:177–183. [DOI] [PubMed] [Google Scholar]
- 12. Hjermstad MJ, Kaasa S, Caraceni A, Loge JH, Pedersen T, Haugen DF, et al. Characteristics of breakthrough cancer pain and its influence on quality of life in an international cohort of patients with cancer. BMJ Support Palliat Care. 2016;6:344–352. [DOI] [PubMed] [Google Scholar]
- 13. Camps Herrero C, Reina Zoilo JJ, Monge Martin D, Caballero Martínez F, Guillem Porta F, Aranda Aguilar E, et al. Active study: undetected prevalence and clinical inertia in the treatment of breakthrough cancer pain (BTcP). Clin Transl Oncol. 2019;21:380–390. [DOI] [PubMed] [Google Scholar]
- 14. Perez-Hernandez C, Blasco A, Gandara A, Mañas A, Jesús Rodríguez-López M, Martínez V, et al. Prevalence and characterization of breakthrough pain in patients with cancer in Spain: the CARPE-DIO study. Sci Rep. 2019;9:17701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Scarborough BM, Smith CB. Optimal pain management for patients with cancer in the modern era. CA: A Cancer J Clin. 2018;68:182–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Greco MT, Roberto A, Corli O, Deandrea S, Bandieri E, Cavuto S, et al. Quality of cancer pain management: an update of a systematic review of undertreatment of patients with cancer. J Clin Oncol. 2014;32:4149–4154. [DOI] [PubMed] [Google Scholar]
- 17. Breivik H, Cherny N, Collett B, de Conno F, Filbet M, Foubert AJ, et al. Cancer-related pain: a pan-European survey of prevalence, treatment, and patient attitudes. Ann Oncol. 2009;20:1420–1433. [DOI] [PubMed] [Google Scholar]
- 18. Grond S, Zech D, Diefenbach C, Radbruch L, Lehmann KA. Assessment of cancer pain: a prospective evaluation in 2266 cancer patients referred to a pain service. Pain. 1996;64:107–114. [DOI] [PubMed] [Google Scholar]
- 19. Jahn P, Kuss O, Schmidt H, Bauer A, Kitzmantel M, Jordan K, et al. Improvement of pain-related self-management for cancer patients through a modular transitional nursing intervention: a cluster-randomized multicenter trial. Pain. 2014;155:746–754. [DOI] [PubMed] [Google Scholar]
- 20. Lin CC, Chou PL, Wu SL, Chang YC, Lai YL. Long-term effectiveness of a patient and family pain education program on overcoming barriers to management of cancer pain. Pain. 2006;122:271–281. [DOI] [PubMed] [Google Scholar]
- 21. Oldenmenger WH, Geerling JI, Mostovaya I, Vissers KCP, de Graeff A, Reyners AKL, et al. A systematic review of the effectiveness of patient-based educational interventions to improve cancer-related pain. Cancer Treat Rev. 2018;63:96–103. [DOI] [PubMed] [Google Scholar]
- 22. Miaskowski C, Dodd M, West C, Schumacher K, Paul SM, Tripathy D, et al. Randomized clinical trial of the effectiveness of a self-care intervention to improve cancer pain management. J Clin Oncol. 2004;22:1713–1720. [DOI] [PubMed] [Google Scholar]
- 23. Oliver JW, Kravitz RL, Kaplan SH, Meyers FJ. Individualized patient education and coaching to improve pain control among cancer outpatients. J Clin Oncol. 2001;19:2206–2212. [DOI] [PubMed] [Google Scholar]
- 24. Potter VT, Wiseman CE, Dunn SM, Boyle FM. Patient barriers to optimal cancer pain control. Psychooncology. 2003;12:153–160. [DOI] [PubMed] [Google Scholar]
- 25. Ward SE, Goldberg N, Miller-McCauley V, Mueller C, Nolan A, Plank DP, et al. Patient-related barriers to management of cancer pain. Pain. 1993;52:319–324. [DOI] [PubMed] [Google Scholar]
- 26. Makhlouf SM, Pini S, Ahmed S, Bennett ML. Managing pain in people with cancer—a systematic review of the attitudes and knowledge of professionals, patients, caregivers and public. J Cancer Educ. 2020;35:214–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Savas M, Bayraktar-Ekincioglu A, Celebi N. An evaluation of cancer patients’ opinions about use of opioid analgesics and the role of clinical pharmacist in patient education in Turkey. Int J Clin Pharm. Epub ahead of print 2020 doi:10.1007/s11096-020-01098-x [DOI] [PubMed] [Google Scholar]
- 28. Herrero CC, Torres AA, Cruz-Hernández JJ, Cruz-Hernández JJ, Carrato A, Constenla M, et al. Working towards a consensus on the oncological approach of breakthrough pain: a Delphi survey of Spanish experts. J Pain Res. 2019;12:2349–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Odonkor CA, Osei-Bonsu E, Tetteh O, Tetteh O, Haig A, Samuel Mayer R, et al. Minding the gaps in cancer pain management education: a multicenter study of clinical residents and fellows in a low- versus high-resource setting. J Global Oncol. 2016;2:387–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Breuer B, Chang VT, von Roenn JH, von Gunten C, Neugut AJ, Kaplan R, et al. How well do medical oncologists manage chronic cancer pain? A national survey. Oncologist. 2015;20:202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Villegas Estévez F, López Alarcón MD, Alonso Babarro A, Gayoso LO, De Castro Carpeño J, Lería-Gelabert M, et al. Breakthrough cancer pain treatment in Spain: physicians’ perception of current opioids utilization and prescription. Curr Med Res Opin. 2020;36:1383–1391. [DOI] [PubMed] [Google Scholar]
- 32. Wengström Y, Rundström C, Geerling J, Pappa T, Weisse J, Williams SC, et al. The management of breakthrough cancer pain—educational needs a European nursing survey. Eur J Cancer Care. 2014;23:121 Epub ahead of print 2014 doi:10.1111/ecc.12118 [DOI] [PubMed] [Google Scholar]
- 33. Robbins JR, Kilari D, Johnston F. Palliative care education for oncologists: how are we doing? Ann Palliat Med. 2019;8:356–359. [DOI] [PubMed] [Google Scholar]
- 34. Brant JM, Stricker CT, Petok A, Sih-Meynier R, Wujcik D. Shared decision-making preferences and pain characterization in patients with cancer. J Clin Oncol. 2018;36:30. [Google Scholar]
- 35. Kehl KL, Landrum MB, Arora NK, Ganz PA, van Ryn M, Mack JW, et al. Association of actual and preferred decision roles with patient-reported quality of care. JAMA Oncol. 2015;1:50–58. doi:10.1001/jamaoncol.2014.112 [DOI] [PMC free article] [PubMed] [Google Scholar]