Abstract
Rationale: Long noncoding RNAs (lncRNAs) are emerging as important regulators of diverse biological functions. Their role in pulmonary arterial hypertension (PAH) remains to be explored.
Objectives: To elucidate the role of TYKRIL (tyrosine kinase receptor–inducing lncRNA) as a regulator of p53/ PDGFRβ (platelet-derived growth factor receptor β) signaling pathway and to investigate its role in PAH.
Methods: Pericytes and pulmonary arterial smooth muscle cells exposed to hypoxia and derived from patients with idiopathic PAH were analyzed with RNA sequencing. TYKRIL knockdown was performed in above-mentioned human primary cells and in precision-cut lung slices derived from patients with PAH.
Measurements and Main Results: Using RNA sequencing data, TYKRIL was identified to be consistently upregulated in pericytes and pulmonary arterial smooth muscles cells exposed to hypoxia and derived from patients with idiopathic PAH. TYKRIL knockdown reversed the proproliferative (n = 3) and antiapoptotic (n = 3) phenotype induced under hypoxic and idiopathic PAH conditions. Owing to the poor species conservation of TYKRIL, ex vivo studies were performed in precision-cut lung slices from patients with PAH. Knockdown of TYKRIL in precision-cut lung slices decreased the vascular remodeling (n = 5). The number of proliferating cell nuclear antigen–positive cells in the vessels was decreased and the number of terminal deoxynucleotide transferase–mediated dUTP nick end label–positive cells in the vessels was increased in the LNA (locked nucleic acid)-treated group compared with control. Expression of PDGFRβ, a key player in PAH, was found to strongly correlate with TYKRIL expression in the patient samples (n = 12), and TYKRIL knockdown decreased PDGFRβ expression (n = 3). From the transcription factor–screening array, it was observed that TYKRIL knockdown increased the p53 activity, a known repressor of PDGFRβ. RNA immunoprecipitation using various p53 mutants demonstrated that TYKRIL binds to the N-terminal of p53 (an important region for p300 interaction with p53). The proximity ligation assay revealed that TYKRIL interferes with the p53–p300 interaction (n = 3) and regulates p53 nuclear translocation.
Conclusions: TYKRIL plays an important role in PAH by regulating the p53/PDGFRβ axis.
Keywords: human precision-cut lung slices, long noncoding RNAs, platelet-derived growth factor receptor β, vascular remodeling, smooth muscle cells
At a Glance Commentary
Scientific Knowledge on the Subject
Long noncoding RNAs (lncRNAs) have been shown to have roles in various diseases and have been identified as potential therapeutic targets. However, the role of lncRNA in the pathogenesis of pulmonary arterial hypertension remains largely unknown.
What This Study Adds to the Field
This study identifies a novel lncRNA, TYKRIL (tyrosine kinase receptor–inducing lncRNA), which is a checkpoint molecule in p53/PDGFRβ (platelet-derived growth factor receptor β) signaling with functional relevance in both hyperproliferating pulmonary artery smooth muscle cells and pericytes, suggesting that it may serve as a novel therapeutic target in pulmonary arterial hypertension.
Pulmonary arterial hypertension (PAH, group 1 pulmonary hypertension) is a debilitating disease in which remodeled pulmonary vasculature increases pulmonary vascular resistance. The resulting increased afterload leads to right ventricle hypertrophy and can ultimately result in right heart failure (1). The maladaptive inward remodeling of the pulmonary artery in PAH is characterized mainly by the hyperproliferation of various resident cells such as pulmonary artery smooth muscle cells (PASMCs), although recent studies have shown that some nonresident cells, such as pericytes, also contribute (2). Various treatments for PAH are available, but as yet none achieve optimal outcomes (3, 4). The most important cell types that contribute to vascular remodeling are PASMCs, fibroblasts, endothelial cells, and pericytes. Therefore, there is a need to identify and to target common molecules in these cell types that drive the vascular remodeling and to explore their therapeutic potential.
Human genome analysis has shown that 80% of the human genome is transcribed into noncoding RNAs, of which the majority are long noncoding RNAs (lncRNAs), with transcripts >200 nucleotides in length. lncRNAs, which lack protein coding ability, are expressed in a wider diversity of species than other RNAs such as mRNA, microRNA, or small nucleolar RNA. Despite their poor species conservation and low abundance, lncRNAs play significant roles in various biological processes, including X chromosome inactivation, genomic imprinting, cell differentiation, and developmental patterning (5–8). They regulate various molecular pathways by, for example, acting as an RNA decoy, microRNA sponge, or ribonucleoprotein component, or by the recruitment of chromatin modifiers, inhibition of translation, or splicing (9–13). lncRNAs have been shown to have roles in various diseases and have been identified as potential therapeutic targets (14–16). However, the role of lncRNAs in the pathogenesis of PAH remains largely unknown.
The most common cell types known to be involved in vascular remodeling are PASMCs, fibroblasts, endothelial cells, and pericytes; thus, delineating and targeting a common molecule in these cell types is important. In this process, we have identified a novel lncRNA, TYKRIL (tyrosine kinase receptor–inducing lncRNA), which is commonly upregulated in human PASMCs (hPASMCs) and pericytes under hyperproliferative conditions. The aims of this study were to elucidate the role of TYKRIL as a regulator of the p53/PDGFRβ (platelet-derived growth factor receptor β) signaling pathway and to investigate its role in idiopathic PAH (IPAH) by using in vitro and ex vivo models. Some of the results of these studies have been previously reported in the form of an abstract (17).
Methods
Cell Isolation
PASMCs and human lung pericytes were isolated as described previously (18, 19). Lung pericytes were cultured in pericyte growth medium (ScienCell), and hPASMCs were cultured in smooth muscle cell growth medium 2 (PromoCell).
RNA Sequencing
RNA deep sequencing was performed by analyzing ribosomal-depleted total RNA from human pericytes and hPASMCs. The RNA was isolated using a RNeasy Mini Kit (Qiagen) according to the manufacturer’s instructions. A HiSeq 2000 flow cell (Illumina) was used for the sequencing.
RNA-guided Gene Activation
Human pericytes were transduced with a constitutive dCas9-VP64 lentiviral expression vector. Guide RNAs (gRNAs) directed against the putative TYKRIL promoter region were designed using the CRISPR (clustered regularly interspaced short palindromic repeats) design tool (Zhang Lab; http://crispr.mit.edu/). gRNA blocks expressing the respective gRNA sequences (IDT) were amplified by PCR according to the manufacturer’s instructions and expressed in human pericytes.
Proximity Ligation Assay
The proximity ligation assay was performed using a Duolink In Situ Red Starter Kit Mouse/Rabbit (DUO92101; Sigma), according to the manufacturer’s instructions.
Proliferation and Apoptosis Assays
The effects of TYKRIL knockdown on the proliferation and apoptosis of hPASMCs from donors and patients with IPAH were assessed, respectively, with a calorimetric BrdU (5-bromo-2’-deoxyuridine) incorporation assay kit and a Cell Death Detection ELISA kit (both from Roche), according to the manufacturer’s instructions.
Precision-Cut Lung Slices
Samples of lung tissue were obtained from patients with IPAH. Tissue sections 400-μm thick were sectioned using a vibratome (Leica Biosystems). GapmeRs (single-stranded oligonucleotides for silencing lncRNA, Exiqon) were transfected using Lipofectamine 3000 (Thermo Fisher Scientific). The medial wall thickness was measured for all the vessels 20–150 μm in size within the lung sections. Sections were stained for medial wall thickness, proliferating cell nuclear antigen (PCNA), and terminal deoxynucleotide transferase–mediated dUTP nick end label.
RNA Fluorescence In Situ Hybridization
The Stellaris probe design tool (LGC Biosearch Technologies) was used to design RNA fluorescence in situ hybridization (RNA-FISH) probes. Cells were incubated with Quasar 570 dye–labeled RNA-FISH oligos against TYKRIL (LGC Biosearch Technologies), according to the manufacturer’s instructions. The imaging was performed using a Zeiss epifluorescent microscope at 100× magnification with an oil objective.
RNA Immunoprecipitation of p53 Mutants
p53 was immunoprecipitated using a p53 C-term-Trap_A kit (ChromoTek), according to the manufacturer’s instructions. The agarose or nickel beads were further processed for either immunoblotting or qRT-PCR.
Results
Under Hyperproliferative Conditions, TYKRIL Was Upregulated and Functionally Relevant in hPASMCs and Pericytes
Human PASMCs and lung pericytes exposed to hypoxia and derived from patients with IPAH exhibited hyperproliferative and apoptosis-resistant phenotypes (Figures 1A–1H). RNA sequencing of both cell types was performed to identify lncRNAs with the greatest physiological importance under these hyperproliferative conditions. Among the top 50 upregulated lncRNAs, TYKRIL was found to be commonly upregulated across all conditions (Figure 1I and Tables E1–E4 in the online supplement). qRT-PCR analyses performed on nuclear and cytoplasmic fractions showed that TYKRIL was present in both fractions (Figure 1J), which was confirmed by RNA-FISH (Figure E1A). Apart from estimated secondary structure and genomic sequence coverage, Northern blotting has shown that TYKRIL has only one known transcript variant (Figures 1I and E1B and E1C). The upregulation of TYKRIL expression was validated in hypoxia and IPAH using qRT-PCR (Figures 1K–1N).
Figure 1.
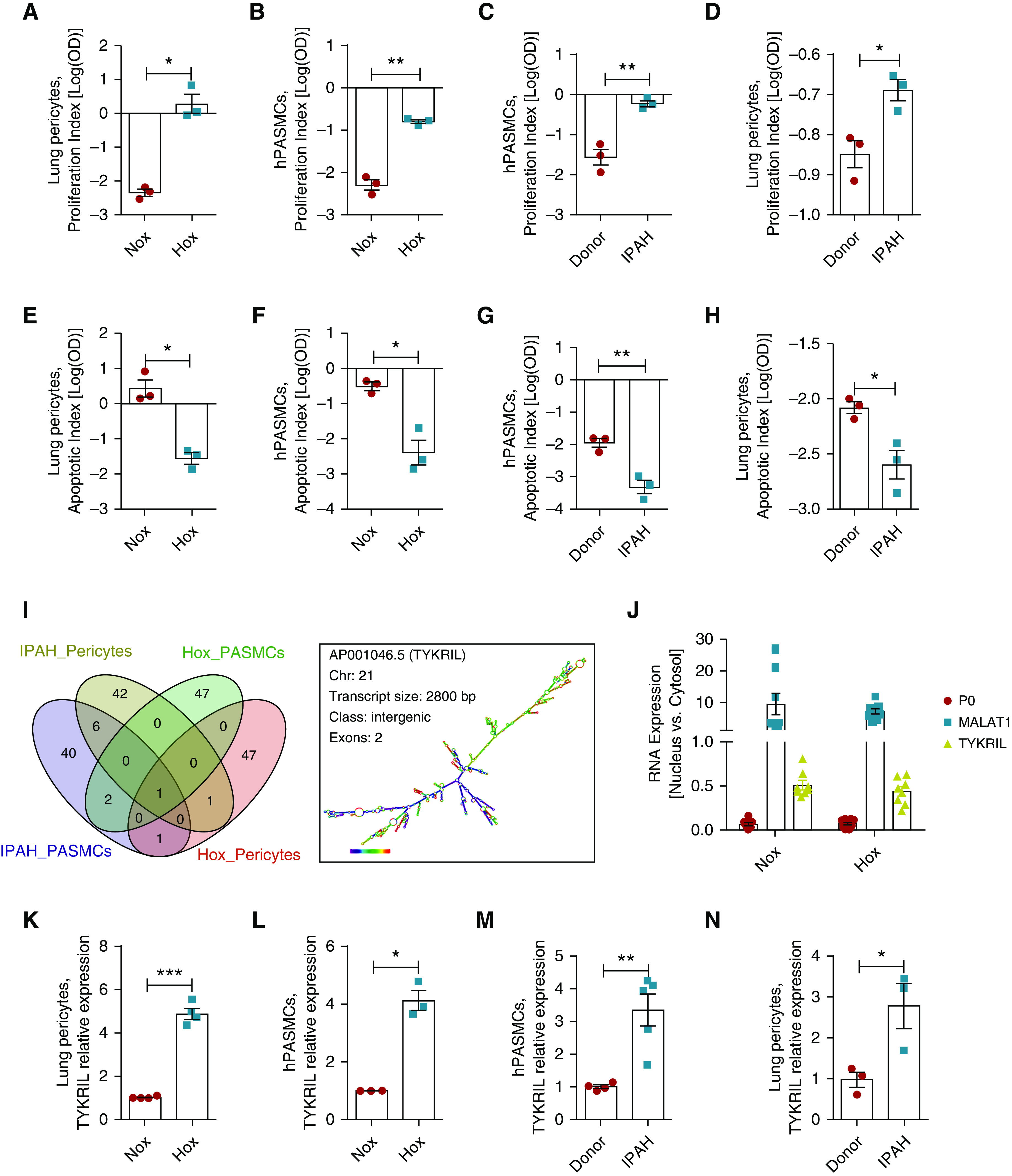
The novel long noncoding RNA (lncRNA) TYKRIL (tyrosine kinase receptor–inducing lncRNA) was a widely regulated lncRNA in the hyperproliferative phenotype. The hyperproliferative and apoptotic-resistant phenotype was observed in (A and E) lung pericytes and (B and F) human pulmonary arterial smooth muscle cells (hPASMCs) exposed to hypoxia compared with normoxia and in (C and G) hPASMCs and (D and H) lung pericytes from patients with IPAH compared with controls (n = 3). (I) Venn diagram generation of upregulated lncRNAs in various vascular cells with the hyperproliferative phenotype and identification of TYKRIL as a commonly upregulated lncRNA under hyperproliferative conditions and description of TYKRIL (n = 2–5). (J) Expression analyses of TYKRIL in cytosolic and nuclear fractions demonstrated that TYKRIL was present in both cellular compartments in pericytes (n = 8). (K–N) The upregulation of TYKRIL under hypoxia and idiopathic PAH conditions was confirmed using quantitative PCR analyses (n = 3–5). Paired t test, *P < 0.05, **P < 0.01, and ***P < 0.001 compared with control. Hox = hypoxia; IPAH = idiopathic pulmonary arterial hypertension; MALAT1 = metastasis-associated lung adenocarcinoma transcript 1; Nox = normoxia; P0 = ribosomal protein lateral stalk subunit P0.
To study the functional relevance of TYKRIL in hPASMCs and lung pericytes under these hyperproliferative conditions, two distinct LNA (locked nucleic acid) GapmeRs (single-stranded oligonucleotides for silencing lncRNA in cell cultures) were used to knock down TYKRIL (Figures 2A–2D). Silencing TYKRIL resulted in a reduction in proliferation (Figures 2E–2H) and the induction of a proapoptotic phenotype (Figures 2I–2L).
Figure 2.
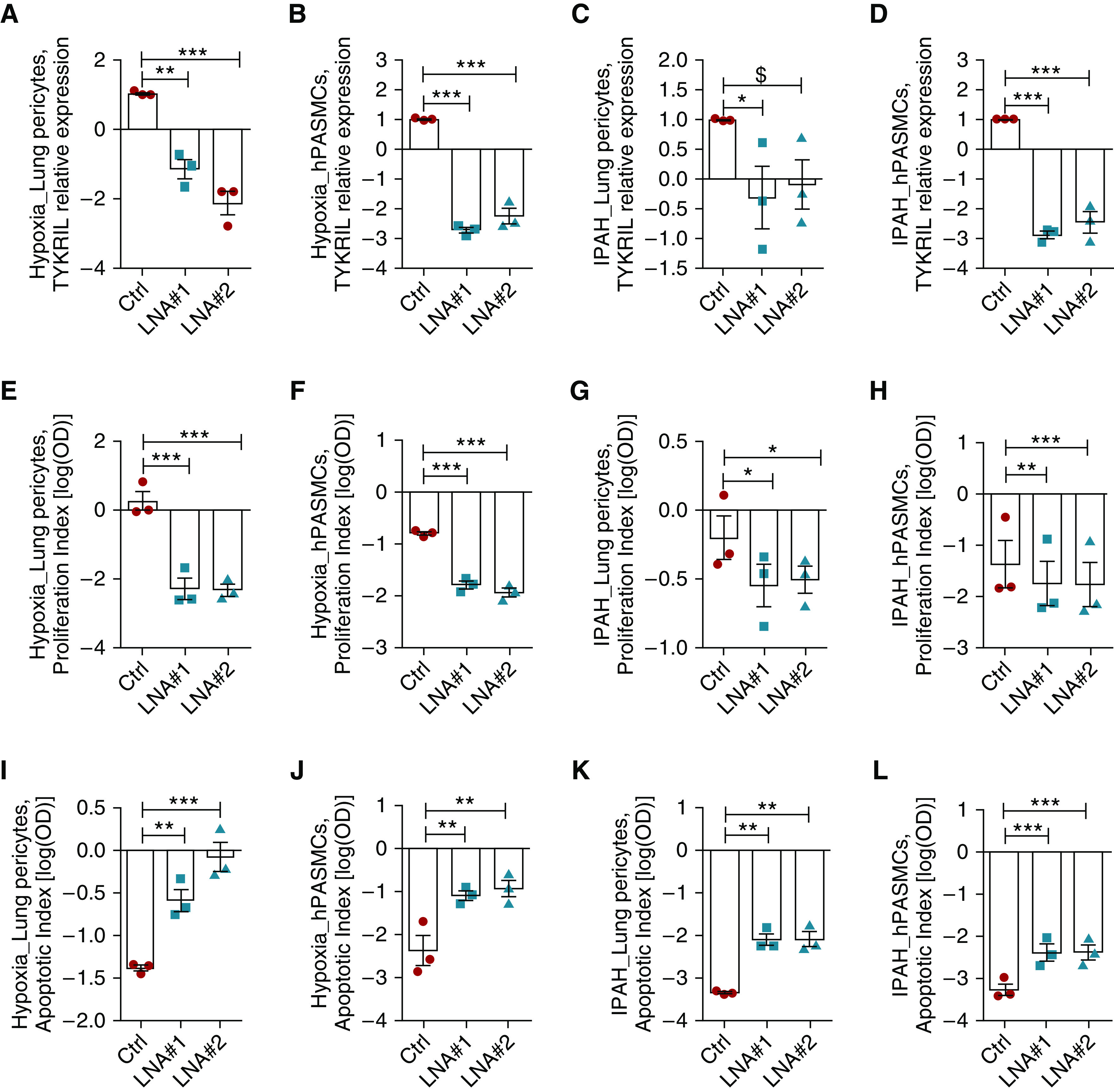
TYKRIL (tyrosine kinase receptor–inducing long noncoding RNA) induced the proproliferative and antiapoptotic phenotype in lung pericytes and human pulmonary arterial smooth muscle cells (hPASMCs) under idiopathic pulmonary arterial hypertension (IPAH) and hypoxic conditions. (A–D) Knockdown of TYKRIL using GapmeRs in both lung pericytes and hPASMCs. (E–H) The proproliferative phenotype induced under these conditions was reversed with TYKRIL knockdown in lung pericytes and hPASMCs (n = 3). (I–L) Increased apoptosis was observed with TYKRIL knockdown in lung pericytes and hPASMCs exposed to hypoxia and isolated from patients with IPAH (n = 3). One-way ANOVA followed by Dunnett’s multiple comparison test, $P = 0.08, *P < 0.05, **P < 0.01, and ***P < 0.001 compared with control. Ctrl = control; LNA = locked nucleic acid.
Furthermore, we studied the molecular regulation of TYKRIL that controls its upregulation in hypoxia and IPAH. Analyzing the TYKRIL promoter region using the eukaryotic promoter database has shown that −1,000 kb upstream of TSS of TYKRIL has six HRE-binding regions, suggesting the possibility of TYKRIL regulation by HIF1α (Figure E2A). Silencing of HIF1α under hypoxia downregulated the TYKRIL expression in hPASMCs and pericytes (Figures E2B and E2C). Interestingly, TYKRIL was induced upon treatment with several pro–pulmonary hypertension (PH) factors such as PDGF, IL18, and TGFβ in hPASMCs (Figure E2D).
TYKRIL Knockdown Reduces Vascular Remodeling in an Ex Vivo Model of the Lungs of Patients with IPAH
An expression analysis of microdissected vessels from the lungs of patients with IPAH showed significant upregulation of TYKRIL expression compared with that in vessels from healthy donors (Figure E3A). Importantly, effective silencing of TYKRIL (Figures 3A–3C) in the viable precision-cut lung slices (PCLS) from the lungs of patients with IPAH was performed using LNA GapmeRs; this resulted in decreased medial wall thickness (Figures 3D and 3E) similar to the paclitaxel-treated IPAH PCLS used as a positive control and sildenafil as a negative control (Figures E3B and E3C), reduced the number of PCNA-positive cells (Figures 3D and 3F), and increased the terminal deoxynucleotide transferase–mediated dUTP nick end label–positive cells (Figures 3D and 3G) in pulmonary vessels of 20–150 μm in size.
Figure 3.

Studying the role of TYKRIL (tyrosine kinase receptor–inducing long noncoding RNA) in pulmonary hypertension (PH) ex vivo model using precision-cut lung slices (PCLS). (A) Representative image of experimental setup for PCLS from patients with PH. (B) Representation of TYKRIL treatment timeline on idiopathic pulmonary arterial hypertension (IPAH)-derived PCLS. (C) GapmeR-mediated knockdown of TYKRIL in PCLS derived from patients with IPAH. (D) Representative images of medial wall thickness, in situ proliferation (proliferating cell nuclear antigen [PCNA]), and apoptosis (terminal deoxynucleotide transferase–mediated dUTP nick end label [TUNEL]-TMR) of small pulmonary vessels from PCLS of patients with PH. Arrows indicate PCNA (brown) and TUNEL+ (red) cells. Scale bar, 100 μm. (E) Medial wall thickness was significantly reduced upon TYKRIL knockdown in PCLS from patients with IPAH (n = 5). (F and G) Reduced number of PCNA+ and increased TUNEL+ cells per vessels were observed upon TYKRIL knockdown in PCLS from patients with IPAH (n = 3). One-way ANOVA followed by Dunnett’s multiple comparison test, *P < 0.05, **P < 0.01, and ***P < 0.001 compared with Ctrl. Ctrl = control; LNA = locked nucleic acid GapmeR; MWT = medial wall thickness; T1 = treatment 1; T2 = treatment 2; TMR = tetramethylrhodamine.
TYKRIL Plays Significant Roles in Pericyte Survival and Functioning by Regulating PDGFRβ Expression
Knocking down TYKRIL effectively reduced the expression of TYKRIL in primary human pericytes (Figure 4A). This resulted in a similar phenotypical shift to that observed in the lung pericytes and hPASMCs, with reduced proliferation (Figure 4B), impaired recruitment to the endothelial cells (Figure 4C), and reduced pericyte survival (Figure 4D). To identify the downstream targets of TYKRIL, RNA sequencing was performed on primary human pericytes after the knockdown of TYKRIL. This showed downregulation of the expression of various tyrosine kinase receptors (Figure 4E). The tyrosine kinase receptor PDGFRβ is known to play important roles in pericyte survival and functioning, as well as in PAH, so this was investigated further. TYKRIL showed a strong correlation with PDGFRβ (Figure 4F), and knocking down TYKRIL significantly reduced the expression of PDGFRβ (Figure 4G). PDGF stimulation experiments further demonstrated that the TYKRIL-dependent reduction of PDGFRβ expression impaired downstream signaling, as shown by the reduced phosphorylation of AKT and ERK1/2 (Figures E4A–E4C).
Figure 4.
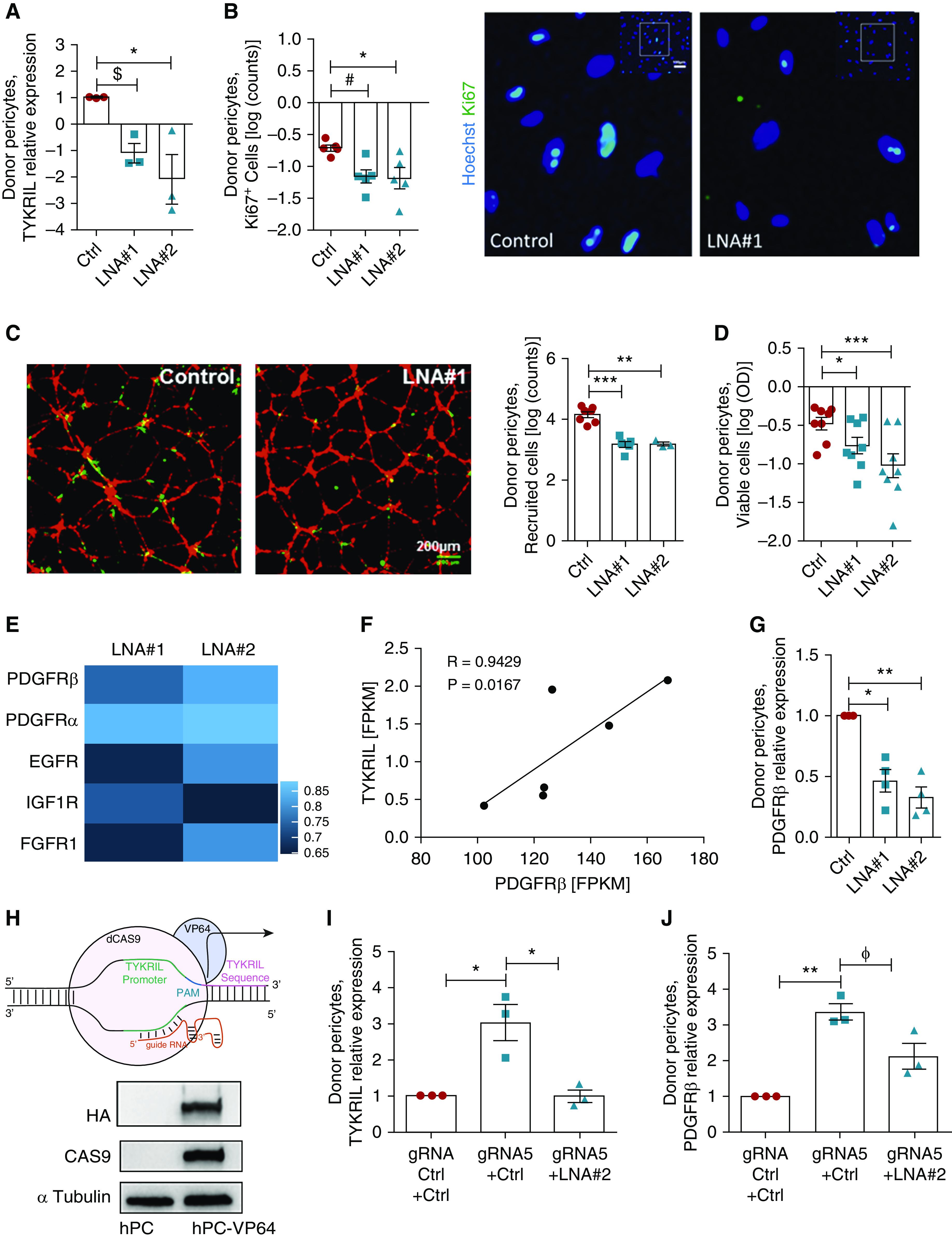
TYKRIL (tyrosine kinase receptor–inducing long noncoding RNA) plays a significant role in pericyte survival and function via the PDGFRβ (platelet-derived growth factor receptor β). (A) TYKRIL was silenced using the LNA GapmeRs LNA #1 and LNA #2. (B) Pericyte proliferation was reduced with the silencing of TYKRIL (n = 18–36 random fields of view [RFVs] from at least three experiments). (C) TYKRIL loss impaired the recruitment of pericytes (green) to human umbilical vein endothelial cells (red) in three-dimensional Matrigel coculture assays (n = 9–21 RFVs from three to seven assays per condition). Scale bar, 200 μm. (D) MTT assays (n = 8) and automated cell count analyses (n = 9–15 RFVs from three to five independent experiments). (E) RNA sequencing reveals that various tyrosine kinase receptors were downregulated after TYKRIL knockdown in human pericytes; scale represents log2FC. (F) TYKRIL significantly correlated with PDGFRβ expression under both normoxic (n = 3) and hypoxic (n = 3) conditions. (G) The knockdown of TYKRIL downregulated PDGFRβ in pericytes. (H) Representation of the RNA-guided gene activation CRISPR (clustered regularly interspaced short palindromic repeats) system. TYKRIL overexpression using CRISPR dCAS9-VP64 system significantly increased the expression of (I) TYKRIL and (J) PDGFRβ, which was blunted by knocking down TYKRIL. One-way ANOVA followed by Dunnett’s and Tukey’s multiple comparison test, $P = 0.06, #P = 0.053, ɸP = 0.07, *P < 0.05, **P < 0.01, and ***P < 0.001 compared with control. Ctrl = control; dCAS9 = dead Cas9; EGFR = epidermal growth factor receptor; FGFR1 = fibroblast growth factor receptor 1; FPKM = fragments per kilobase million; gRNA = guide RNA; HA = HATag; hPC = human pericytes; IGF1R = insulin-like growth factor 1 receptor; LNA = locked nucleic acid GapmeR; MTT = 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; PAM = protospacer adjacent motif; Ki67 = antibody against Ki67 antigen; VP64 = VP64 tag.
To confirm these observations, an overexpression study of TYKRIL was performed using the CRISPR-dCas9-VP64 system (Figure 4H), which allows to upregulate genes in their endogenous context. The RNA-guided gene activation of TYKRIL resulted in significant upregulation of both TYKRIL and PDGFRβ (Figures 4I and 4J). Importantly, cotransfection of LNA GapmeRs with guide RNA abrogated the upregulation of TYKRIL and blunted the increase in PDGFRβ expression (Figures 4I and 4J).
TYKRIL Acts as a Protein Decoy, Thereby Reducing p53–p300 Complex Formation
To obtain further insights into the molecular mechanism of TYKRIL, a transcription factor assay profiling analysis was performed to assess the impact of the loss of TYKRIL on transcription factor activity. The tumor suppressor p53 was observed to be most prominently activated after silencing TYKRIL (Figure 5A). This was confirmed by luciferase reporter constructs that contained p53 regulatory elements or promoter sequences of the known p53 targets p21 and BAX (an apoptosis regulator) (Figure E5A). Pathway analysis from the RNA sequencing have shown that p53 signaling pathway was regulated upon TYKRIL knockdown in both IPAH PASMCs and IPAH pericytes (Figures E5B–E5D). It is known that p53 regulates the expression of lncRNAs (20, 21), but there is little information about the lncRNA-dependent regulatory mechanism of its activity.
Figure 5.
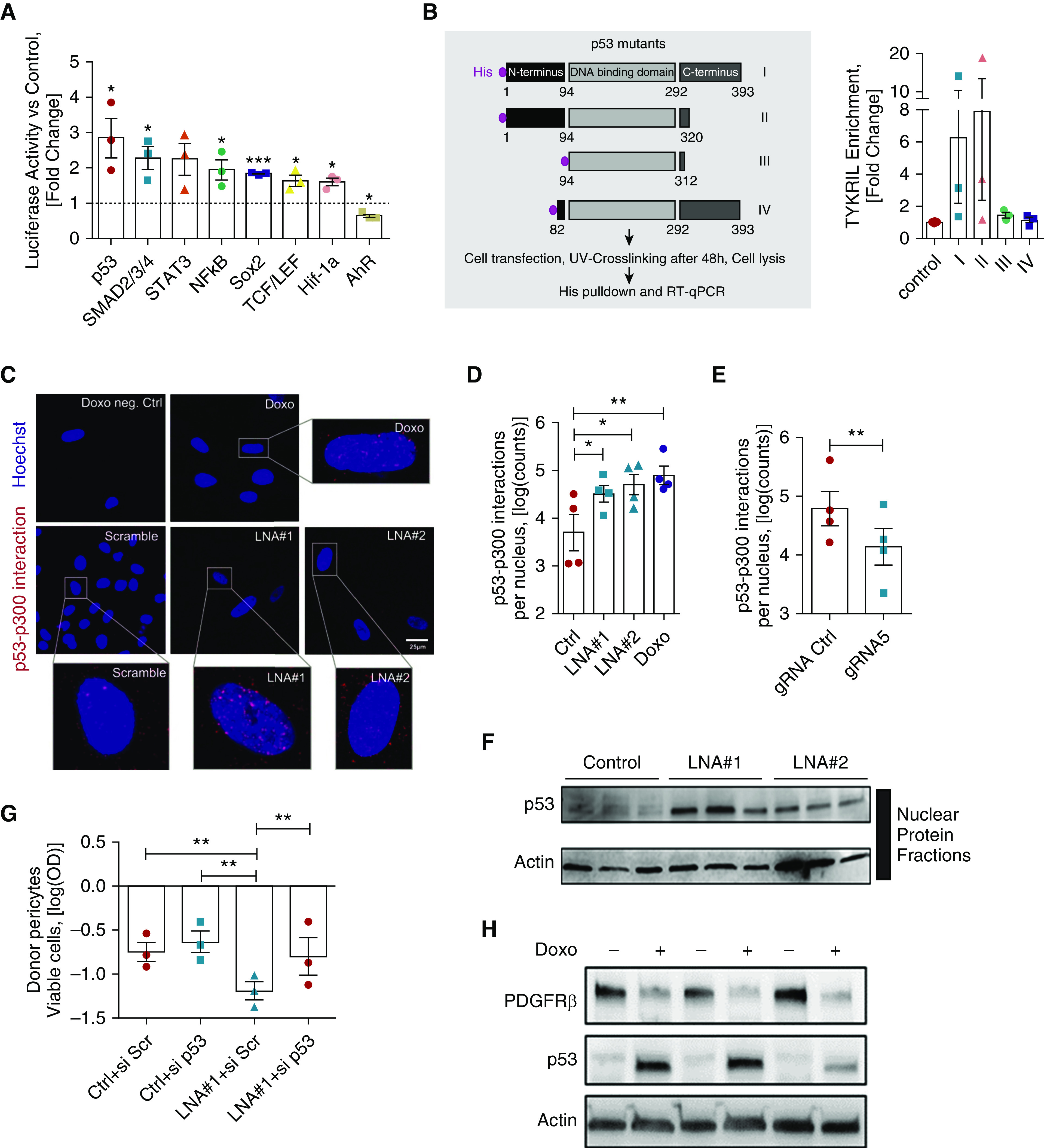
TYKRIL (tyrosine kinase receptor–inducing long noncoding RNA) acts a protein decoy, resulting in the decreased formation of p53–p300 complexes. (A) Luciferase reporter arrays showed p53 activation with TYKRIL silencing in primary human pericytes (n = 3). (B) Pulldown experiments with histidine-tagged p53 mutants revealed binding of TYKRIL on the N-terminus of p53 as no TYKRIL enrichment was detected in p53 mutants (III, IV) lacking the N-terminus (n = 3). (C and D) Specific proximity ligation assays demonstrated sparse p53–p300 interactions in scrambled controls compared with doxorubicin-treated human pericytes. Similarly, TYKRIL knockdown resulted in a significant increase in nuclear p53–p300 interactions (n = 3). (E) RNA-guided gene activation significantly repressed the formation of nuclear p53–p300 complexes, as indicated by quantitative proximity ligation assays (n = 4). (F) p53 immunoblotting confirmed nuclear p53 levels were increased in pericytes following TYKRIL knockdown (n = 3). (G) TYKRIL mediated the cell viability loss via p53 as determined via MTT. (H) PDGFRβ (platelet-derived growth factor receptor β) was downregulated following p53 stabilization. One-way ANOVA followed by Dunnett’s multiple comparison test, *P < 0.05, **P < 0.01, and ***P < 0.001 compared with control. Ctrl = control; Doxo = doxorubicin; gRNA = guide RNA; LNA = locked nucleic acid GapmeR; MTT = 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; Scr = scrambled; si = small interfering; UV = ultraviolet.
To identify the binding site, we performed pulldown experiments with histidine-tagged p53 mutants. These showed that TYKRIL binds to the full length p53 and p53 lacking the C-terminus (Mutants I, II; Figure 5B). However, TYKRIL was not enriched in p53 mutants that lack the N-terminus of p53 (Mutants III, IV; Figure 5B) that contains transcriptional activation domains. These results illustrate that TYKRIL binding requires the N-terminus of p53. RNA immunoprecipitation studies additionally showed that human TYKRIL does not interact with the mouse p53, possibly owing to its lack of conserved N-terminus with the human p53 (Figures E5E and E5F), which is required for the TYKRIL interaction. p53 is tightly regulated by posttranslational modifications, such as acetylation and phosphorylation (22). Coactivators, such as acetyltransferase p300, mediate p53 acetylation and bind to the p53 transcriptional activation domains, resulting in p53 stabilization and translocation of the p53–p300 complex into the nucleus (23, 24). We therefore used a proximity ligation assay (25) to analyze the p53–p300 interaction following TYKRIL knockdown. The inhibition of TYKRIL expression was associated with a significant increase in the formation of nuclear p53–p300 complexes (Figures 5C–5D), whereas RNA-guided TYKRIL overexpression resulted in a reduction of p53–p300 levels in the nucleus (Figure 5E). These results demonstrated that TYKRIL acts as a p53 decoy molecule that prevents p53 activation by blocking p53–p300 interaction. These findings were further corroborated by immunoblotting using nuclear extracts, which demonstrated enhanced nuclear p53 protein levels in TYKRIL knockdown conditions (Figure 5F).
To confirm the causal involvement of p53 as a mediator of the TYKRIL-dependent decrease in pericyte viability, we silenced p53 and showed that this prevented the TYKRIL-dependent loss of pericyte viability (Figure 5G). The doxorubicin-induced activation of endogenous p53 further confirmed the p53-dependent PDGFRβ repression (Figure 5H).
PDGFRβ Expression Was Regulated by TYKRIL and TYKRIL Expression Positively Correlated with PDGFRβ in Patients with IPAH
To further evaluate the TYKRIL-mediated regulation of PDGFRβ expression under the disease condition, the expression of TYKRIL and PDGFRβ in the human lung homogenates were measured. TYKRIL and PDGFRβ showed a significant positive correlation in lung tissues from healthy controls and patients with IPAH (Figure 6A). Knockdown studies were performed in hPASMCs and lung pericytes exposed to hypoxia and isolated from patients with IPAH. Silencing TYKRIL resulted in decreased PDGFRβ in hPASMCs and pericytes exposed to hypoxia (Figures 6B and 6C) and isolated from the patients with IPAH (Figures 6D and 6E). In line with the results from the brain-derived pericytes, the regulation of PDGFRβ expression by TYKRIL was also observed in both hPASMCs and lung-derived pericytes under hyperproliferative conditions.
Figure 6.
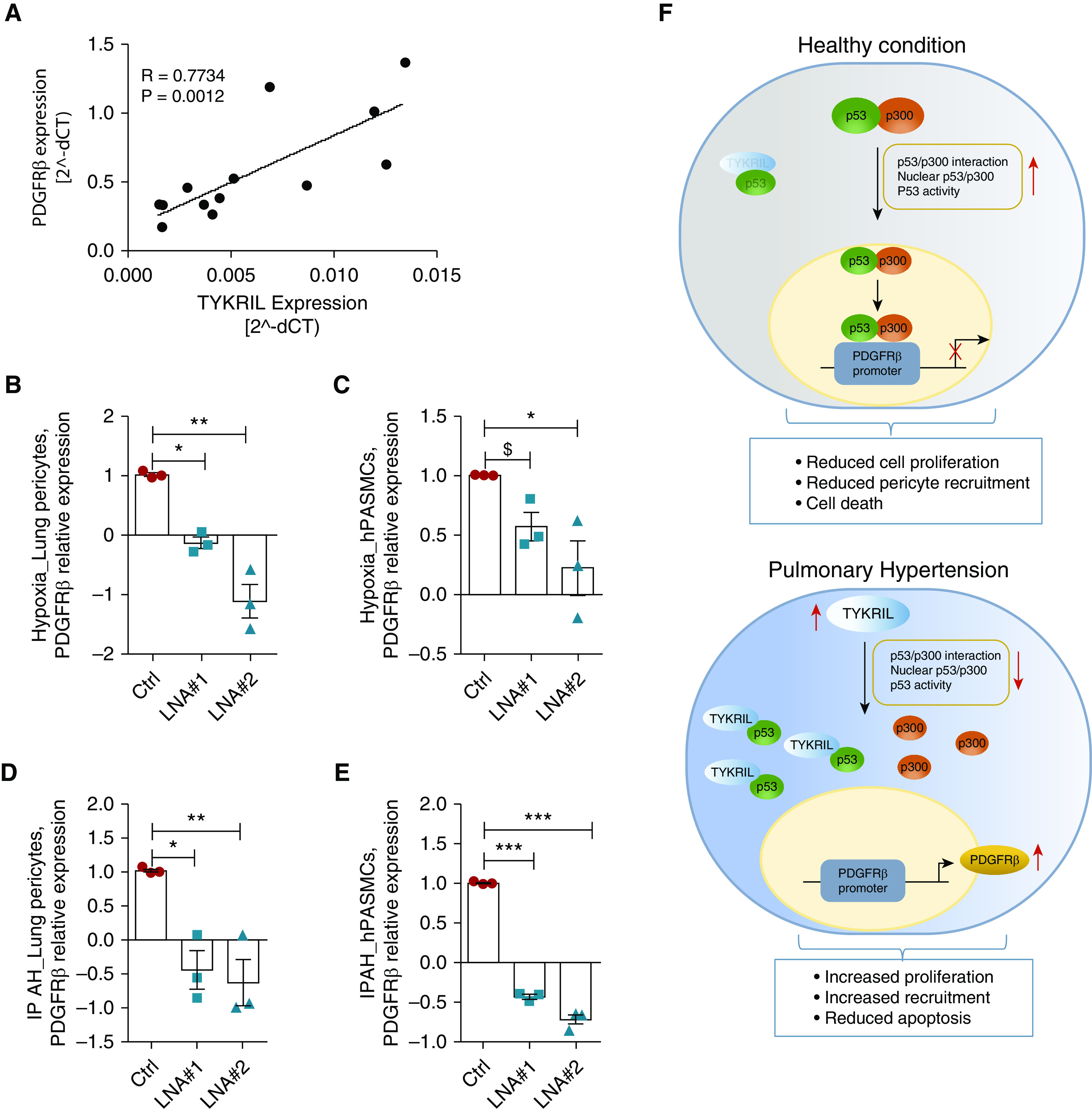
The TYKRIL (tyrosine kinase receptor–inducing long noncoding RNA)/p53/PDGFRβ (platelet-derived growth factor receptor β) signaling axis in lung pericytes and human pulmonary arterial smooth muscle cells (hPASMCs) exposed to hypoxia and from patients with idiopathic pulmonary arterial hypertension (IPAH). (A) TYKRIL and PDGFRβ showed a strong correlation in lung homogenates from patients with IPAH (n = 13). qRT-PCR analyses showed that TYKRIL knockdown downregulated PDGFRβ expression in (B) lung pericytes exposed to hypoxia and in (D) pericytes from patients with IPAH (n = 3). The expression of the PDGFRβ mRNA was downregulated with TYKRIL knockdown in (C) PASMCs exposed to hypoxia and (E) isolated from patients with IPAH (n = 3). (F) A representative image summarizing the TYKRIL molecular mechanism modulating p53/PDGFRβ signaling. One-way ANOVA followed by Dunnett’s multiple comparison test, $P = 0.17, *P < 0.05, **P < 0.01, and ***P < 0.001 compared with control. Ctrl = control; dCT = delta CT; LNA = locked nucleic acid GapmeR.
TYKRIL under PAH Conditions Regulates Non-p53/PDGFRβ Signaling Pathways
RNA sequencing data have shown that TYKRIL regulates 354 genes commonly upon TYKRIL knockdown in both IPAH pericytes and IPAH PASMCs. It was also observed that TYKRIL distinctly regulates 4,392 genes in IPAH PASMCs and 1,246 genes in IPAH pericytes (Figures E6A and E6B). Pathway analysis revealed that calcium signaling, estrogen signaling, tryptophan metabolism, and various other pathways were commonly targeted by TYKRIL in both PASMCs and pericytes under disease conditions (Figures E6C and E6D). Several distinct pathways such as ABC transporters, adrenergic signaling, and Rap1 signaling pathways were regulated by TYKRIL only in IPAH pericytes (Figures E7A and E7B). In IPAH PASMCs, TYKRIL specifically regulates various signaling pathways such as AMPK signaling, cancer-related pathways, and Wnt signaling (Figures E8A and E8B). In addition to proproliferative pathways, TYKRIL controlled various vasoconstriction pathways by regulating genes such as PDE5A and GUCY1A1 in PASMCs (Figures E9A and E9B). Functionally, TYKRIL knockdown in IPAH PASMCs reduced the gel contraction compared with control (Figure E9C), suggesting that TYKRIL mediated vasomodulation.
Discussion
This study demonstrated that TYKRIL, a previously unknown lncRNA, plays a vital role in maintaining the hyperproliferative phenotype of PASMCs and pericytes, thereby contributing to the pathological inward remodeling of vessels in PAH. It acts as a novel checkpoint molecule in the tyrosine kinase signaling pathway by acting as a p53 decoy, thereby modulating the expression of PDGFRβ. This interpretation is based on five results. First, RNA sequencing data from screening PASMCs and pericytes showed that TYKRIL was widely upregulated under all the hyperproliferative conditions. Second, silencing TYKRIL resulted in a significant reduction in the proproliferative and antiapoptotic phenotypes in both PASMCs and pericytes; together with the first result, this shows that TYKRIL plays a significant role in maintaining the hyperproliferating phenotype. Third, correlation analyses and expression studies showed that TYKRIL regulated PDGFRβ expression, with a strong correlation between TYKRIL and PDGFRβ in the patient samples, suggesting that TYKRIL has a significant role in regulating tyrosine kinase signaling. Fourth, transcription factor array and pulldown studies showed that TYKRIL bound strongly to p53 and regulated its activity by disrupting the formation of p53–p300 complexes, suggesting its role as a p53 decoy. Fifth, ex vivo studies using PCLS derived from the lungs of patients with IPAH demonstrated that silencing TYKRIL using LNA GapmeR technology reversed the pulmonary vascular remodeling. This suggested that TYKRIL may have therapeutic potential for PAH and hypoxia-associated PH.
Previous studies have shown that lncRNAs are involved in and functionally relevant to a spectrum of biological events. They regulate signaling pathways by acting as an RNA decoy, microRNA sponge, or ribonucleoprotein component, or by the recruitment of chromatin modifiers, translation inhibition, or splicing (26–29). Recent studies have shown the involvement of lncRNAs in various lung diseases, including H19 in idiopathic pulmonary fibrosis (30, 31), SCAL1 in chronic obstructive pulmonary disease (32), MALAT1 in lung cancer (33), and H19 and MANTIS in PH (34, 35). In addition, the present study identified and investigated the role of the novel lncRNA TYKRIL in IPAH.
PH is a multifactorial disease characterized mainly by elevated pulmonary pressure caused by excessive remodeling of the pulmonary vasculature (1) as a result of the hyperproliferating phenotype of resident and nonresident cells in the vasculature. Studies have shown that the dysregulated expression of lncRNAs can result in the hyperproliferative phenotype under various pathological conditions and that targeting the lncRNAs reverses this phenotype (36–41). The most common cell types known to be involved in vascular remodeling are PASMCs, fibroblasts, endothelial cells, and pericytes; thus, delineating and targeting a common molecule in these cell types is important (4). We identified TYKRIL to be strongly induced and functionally relevant under various hyperproliferating conditions in both PASMCs and pericytes. In addition, our studies indicate that pro-PH factors such as HIF, growth factors (PDGF and TGFβ), and proinflammatory cytokines (IL18) play an important role in the induction of TYKRIL. TYKRIL was also upregulated in endothelial cells exposed to hypoxia and in adventitial fibroblasts isolated from patients with IPAH (data not shown). In addition, we observed that TYKRIL exerted pathophysiological relevant functions in commercially available primary human pericytes, where it modulated differentiation, proliferation, and recruitment to endothelial cells. These findings suggest TYKRIL induction as one of the common downstream mechanism of PH pathogenesis.
Notably, the investigation of molecular mechanisms revealed that TYKRIL interacts with p53, thereby interfering with the p300 interaction that modulates PDGFRβ expression. To the best of our knowledge, TYKRIL is the first lncRNA to be observed to regulate the p53/PDGFRβ axis. It has previously been reported that p53 is a major regulator of PDGFRβ and that it downregulates PDGFRβ expression (42, 43); conversely, mutants of p53 have been found to drive PDGF signaling in various malignant diseases (44). Studies have shown that p53 also regulates the expression of various other lncRNAs (19, 45); however, few studies have addressed the regulation of p53 functionality by lncRNAs. Li and colleagues (46) recently showed that the lncRNA PURPL suppressed basal levels of p53 and promoted tumor growth. Specifically, they demonstrated that PURPL inhibited p53–MYBBP1A interactions by direct binding to MYBBP1A. Another lncRNA, PANDAR, has been shown to block CDKN1A gene transcription in gastric cancer by competing with the p53 binding site (45). These studies have demonstrated that lncRNAs are capable of modulating p53 activity by binding to its interaction partners or to the p53 target site. We found that TYKRIL interacts with p53; specifically, histidine-tagged mutant experiments identified the binding site as the N-terminus of p53. Similar to the findings of Li and colleagues (46), TYKRIL blocks the interaction with a p53 coactivator, namely, p300. The present study showed, using nuclear immunoblotting and proximity ligation assays, that the binding of TYKRIL to the N-terminus of p53 blocked p53–p300 interactions. We therefore propose that TYKRIL acts as a p53 decoy molecule, thereby regulating PDGFRβ, a known direct target of p53. We would like to clarify that our data demonstrate a binding of TYKRIL to p53; however, our data do not rule out the possibility that other molecules or proteins may also be involved in the TYKRIL–p53 complex formation.
Zhang and colleagues (43) reported that increased levels of p53 resulted in the downregulation of PDGFRβ on protein level. Consistent with that finding, we observed that the activation of endogenous p53 by doxorubicin treatment resulted in the upregulation of p53 and downregulation of PDGFRβ, whereas the knockdown of p53 rescued cell viability loss upon TYKRIL silencing; this suggested a similar regulatory p53–PDGFRβ pathway in pericytes. Our finding that TYKRIL had an ultimate impact on PDGFRβ expression was further supported by CRISPR Cas9-mediated overexpression and TYKRIL loss-of-function experiments; these showed that the loss of PDGFRβ in pericytes under TYKRIL knockdown conditions were accompanied by disrupted AKT and ERK downstream signaling. It is well established that p53 and PDGFRβ both play important roles in the pathogenesis of PAH (47, 48), even though data on their expression and transcriptional activity are scarce. Notably, our study revealed that TYKRIL regulated PDGFRβ expression in both PASMCs and pericytes isolated from patients with IPAH and exposed to hypoxia. Apart from p53/PDGFRβ signaling, TYKRIL also modulates several known PH pathways such as calcium signaling, estrogen signaling, inflammatory, metabolic, cancer, and vasomodulatory pathways in a cell type–specific context. In line, TYKRIL knockdown resulted in reduced contractility of IPAH-PASMCs. These findings suggest that TYKRIL, via regulation of P53 and other transcription factors (Smad2/3, Stat3, etc.), modulates various signaling pathways driving both vascular remodeling and vasomodulation, and inhibition of TYKRIL may therefore offer a therapeutic option for PH.
The findings of this study identified, for the first time, that TYKRIL is a checkpoint molecule in p53/PDGFRβ signaling with functional relevance in both hyperproliferating PASMCs and pericytes, suggesting that it may serve as a novel therapeutic target in PAH. An ex vivo model was used to explore the therapeutic option using GapmeR technology because of the poor conservation of TYKRIL across the model species. A study by Nickel and colleagues (49) reported that treating lung organ cultures from patients with PAH with elafin resulted in the regression of neointima and that treatment of SU/Hox rats with elafin resulted in a reduction in obliterative pulmonary vessels and improved right ventricular systolic pressure (49). Similarly, in the present study, an ex vivo model with PCLS from samples of patients with IPAH was used to target TYKRIL using GapmeRs. This showed that silencing TYKRIL resulted in a decrease in PCNA-positive cells and reduced apoptosis and subsequently medial wall thickness in distal pulmonary vessels, suggesting the potential of TYKRIL as a therapeutic target option for PH.
Studying the translational aspect of human-specific lncRNA is challenging, especially with constrains in using conventional rodent disease models. To address this challenge, we used human diseased PCLS to study human lncRNAs and have successfully identified the specific function of a nonconserved human lncRNA. Other alternative approaches to study the regulation and function of nonconserved human lncRNAs include generation of complex human organoid models (50), transplantation of human cells into xenograft or immunodeficient rodents, and the use of humanized mouse models (51). In a recent study Ruan and colleagues used a liver-specific humanized mouse model to study human lncRNAs and have successfully identified the specific function of a nonconserved human lncRNA (52). Thus, recent technical advances in tissue engineering may offer various humanized mouse models, organoid models mimicking the disease condition, and use of precision-cut organ slices from patients to predict the translational transferability of the nonconserved human lncRNAs.
Acknowledgments
Acknowledgment
The authors thank Ariane Fischer, Marion Muhly-Reinholz, Andrea Knau, and Tobias Hirnet for excellent technical assistance. They also thank Dr. Jochen Wilhelm for assisting in statistical analysis and Volker Doetsch for providing luciferase reporter constructs.
Footnotes
Supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)—Projektnummer 268555672—SFB 1213, project A01 and A05. The study was also supported by the European Research Council grant Angilnc (to S.D.), Excellence Cluster Cardio-Pulmonary System (ECCPS)/Cardio-Pulmonary Institute (Exc2026 to C.M.Z., S.D., and S.S.P.), a Goethe University Startup grant (to C.M.Z.), the SFB834 (to F.C.B., S.U., and S.D.), ECCPS (DFG), the LOEWE Center for Cell and Gene Therapy (State of Hessen to C.M.Z. and S.D.), German Center for Cardiovascular Research (DZHK to S.D. and A.M.Z.), LOEWE Center for Cell and Gene Therapy (State of Hessen to S.D. and S.U.), LOEWE program Medical RNomics (State of Hessen to O.R.), and by the SFB-1213 (Projektnummer 268555672) projects A01 and A05 (C.V. and S.S.P.).
Author Contributions: C.M.Z., C.V., J.-N.B., O.R., N.J., S.S.P., S. Dimmeler, and S.U. designed the research study. C.M.Z., A.W., F.C.B., S.F.G., C.V., R.M.W., K.Y., O.R., S. Demolli, and F.K. conducted the experiments. C.M.Z., A.W., C.V., D.J., K.M.M., and F.K. acquired the data. C.M.Z., C.V., A.W., J.-N.B., D.J., T.W., A.G., V.A.d.J.P., W.C., S.U., and F.K. analyzed the data. C.M.Z., S.S.P., S. Dimmeler, K.Y., V.A.d.J.P., A.G., and R.M.W. provided samples and reagents. C.M.Z., S.S.P., S. Dimmeler, A.M.Z., and W.S. took the lead in writing the manuscript. All authors provided critical feedback and helped shape the research, analysis, and manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201910-2041OC on July 7, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. D’Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, et al. Survival in patients with primary pulmonary hypertension: results from a national prospective registry. Ann Intern Med. 1991;115:343–349. doi: 10.7326/0003-4819-115-5-343. [DOI] [PubMed] [Google Scholar]
- 2. Ricard N, Tu L, Le Hiress M, Huertas A, Phan C, Thuillet R, et al. Increased pericyte coverage mediated by endothelial-derived fibroblast growth factor-2 and interleukin-6 is a source of smooth muscle-like cells in pulmonary hypertension. Circulation. 2014;129:1586–1597. doi: 10.1161/CIRCULATIONAHA.113.007469. [DOI] [PubMed] [Google Scholar]
- 3. Hill NS, Badesch D, Benza RL, D’Eletto TA, Farber HW, Gomberg-Maitland M, et al. Perspectives on oral pulmonary hypertension therapies recently approved by the U.S. Food and Drug Administration. Ann Am Thorac Soc. 2015;12:269–273. doi: 10.1513/AnnalsATS.201501-020AS. [DOI] [PubMed] [Google Scholar]
- 4. Pullamsetti SS, Schermuly R, Ghofrani A, Weissmann N, Grimminger F, Seeger W. Novel and emerging therapies for pulmonary hypertension. Am J Respir Crit Care Med. 2014;189:394–400. doi: 10.1164/rccm.201308-1543PP. [DOI] [PubMed] [Google Scholar]
- 5. Chow J, Heard E. X inactivation and the complexities of silencing a sex chromosome. Curr Opin Cell Biol. 2009;21:359–366. doi: 10.1016/j.ceb.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 6. Ginger MR, Shore AN, Contreras A, Rijnkels M, Miller J, Gonzalez-Rimbau MF, et al. A noncoding RNA is a potential marker of cell fate during mammary gland development. Proc Natl Acad Sci USA. 2006;103:5781–5786. doi: 10.1073/pnas.0600745103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Amaral PP, Neyt C, Wilkins SJ, Askarian-Amiri ME, Sunkin SM, Perkins AC, et al. Complex architecture and regulated expression of the Sox2ot locus during vertebrate development. RNA. 2009;15:2013–2027. doi: 10.1261/rna.1705309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang X, Lian Z, Padden C, Gerstein MB, Rozowsky J, Snyder M, et al. A myelopoiesis-associated regulatory intergenic noncoding RNA transcript within the human HOXA cluster. Blood. 2009;113:2526–2534. doi: 10.1182/blood-2008-06-162164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kino T, Hurt DE, Ichijo T, Nader N, Chrousos GP. Noncoding RNA gas5 is a growth arrest- and starvation-associated repressor of the glucocorticoid receptor. Sci Signal. 2010;3:ra8. doi: 10.1126/scisignal.2000568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cesana M, Cacchiarelli D, Legnini I, Santini T, Sthandier O, Chinappi M, et al. A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell. 2011;147:358–369. doi: 10.1016/j.cell.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tsai MC, Manor O, Wan Y, Mosammaparast N, Wang JK, Lan F, et al. Long noncoding RNA as modular scaffold of histone modification complexes. Science. 2010;329:689–693. doi: 10.1126/science.1192002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang KC, Yang YW, Liu B, Sanyal A, Corces-Zimmerman R, Chen Y, et al. A long noncoding RNA maintains active chromatin to coordinate homeotic gene expression. Nature. 2011;472:120–124. doi: 10.1038/nature09819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yoon JH, Abdelmohsen K, Srikantan S, Yang X, Martindale JL, De S, et al. LincRNA-p21 suppresses target mRNA translation. Mol Cell. 2012;47:648–655. doi: 10.1016/j.molcel.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ, et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071–1076. doi: 10.1038/nature08975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lin N, Chang KY, Li Z, Gates K, Rana ZA, Dang J, et al. An evolutionarily conserved long noncoding RNA TUNA controls pluripotency and neural lineage commitment. Mol Cell. 2014;53:1005–1019. doi: 10.1016/j.molcel.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boudewijn IM, Roffel MP, Vermeulen CJ, Nawijn MC, Kok K, Terpstra MM, et al. INDURAIN Investigators A novel role of bronchial microRNAs and long noncoding RNAs in asthma remission Am J Respir Crit Care Med 2020202614–618. [DOI] [PubMed] [Google Scholar]
- 17. Zehendner CM, Valasarajan C, Thal S, Werner A, Boeckel J-N, Bischoff FC, et al. Long noncoding RNA Tykril plays a role in pulmonary hypertension by controlling the p53 mediated regulation of PDGFRβ. Circulation. 2019;140(Suppl_1):A14368. doi: 10.1164/rccm.201910-2041OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 19. Perez-Pinera P, Kocak DD, Vockley CM, Adler AF, Kabadi AM, Polstein LR, et al. RNA-guided gene activation by CRISPR-Cas9-based transcription factors. Nat Methods. 2013;10:973–976. doi: 10.1038/nmeth.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sánchez Y, Segura V, Marín-Béjar O, Athie A, Marchese FP, González J, et al. Genome-wide analysis of the human p53 transcriptional network unveils a lncRNA tumour suppressor signature. Nat Commun. 2014;5:5812. doi: 10.1038/ncomms6812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang A, Xu M, Mo YY. Role of the lncRNA-p53 regulatory network in cancer. J Mol Cell Biol. 2014;6:181–191. doi: 10.1093/jmcb/mju013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–310. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- 23. Teufel DP, Freund SM, Bycroft M, Fersht AR. Four domains of p300 each bind tightly to a sequence spanning both transactivation subdomains of p53. Proc Natl Acad Sci USA. 2007;104:7009–7014. doi: 10.1073/pnas.0702010104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dyson HJ, Wright PE. Role of intrinsic protein disorder in the function and interactions of the transcriptional coactivators CREB-binding protein (CBP) and p300. J Biol Chem. 2016;291:6714–6722. doi: 10.1074/jbc.R115.692020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Söderberg O, Gullberg M, Jarvius M, Ridderstråle K, Leuchowius KJ, Jarvius J, et al. Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nat Methods. 2006;3:995–1000. doi: 10.1038/nmeth947. [DOI] [PubMed] [Google Scholar]
- 26. Shi X, Sun M, Liu H, Yao Y, Song Y. Long non-coding RNAs: a new frontier in the study of human diseases. Cancer Lett. 2013;339:159–166. doi: 10.1016/j.canlet.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 27. Zhang R, Guo Y, Ma Z, Ma G, Xue Q, Li F, et al. Long non-coding RNA PTENP1 functions as a ceRNA to modulate PTEN level by decoying miR-106b and miR-93 in gastric cancer. Oncotarget. 2017;8:26079–26089. doi: 10.18632/oncotarget.15317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Salamo O, Mortaz E, Mirsaeidi M. Noncoding RNAs: new players in pulmonary medicine and sarcoidosis. Am J Respir Cell Mol Biol. 2018;58:147–156. doi: 10.1165/rcmb.2017-0196TR. [DOI] [PubMed] [Google Scholar]
- 29. Jiang D, Liang J. A long noncoding RNA links TGF-β signaling in lung fibrosis. Am J Respir Crit Care Med. 2019;200:123–125. doi: 10.1164/rccm.201812-2313ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lu Q, Guo Z, Xie W, Jin W, Zhu D, Chen S, et al. The lncRNA H19 mediates pulmonary fibrosis by regulating the miR-196a/COL1A1 axis. Inflammation. 2018;41:896–903. doi: 10.1007/s10753-018-0744-4. [DOI] [PubMed] [Google Scholar]
- 31. Tang Y, He R, An J, Deng P, Huang L, Yang W. The effect of H19-miR-29b interaction on bleomycin-induced mouse model of idiopathic pulmonary fibrosis. Biochem Biophys Res Commun. 2016;479:417–423. doi: 10.1016/j.bbrc.2016.09.028. [DOI] [PubMed] [Google Scholar]
- 32. Thai P, Statt S, Chen CH, Liang E, Campbell C, Wu R. Characterization of a novel long noncoding RNA, SCAL1, induced by cigarette smoke and elevated in lung cancer cell lines. Am J Respir Cell Mol Biol. 2013;49:204–211. doi: 10.1165/rcmb.2013-0159RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ji P, Diederichs S, Wang W, Böing S, Metzger R, Schneider PM, et al. MALAT-1, a novel noncoding RNA, and thymosin beta4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene. 2003;22:8031–8041. doi: 10.1038/sj.onc.1206928. [DOI] [PubMed] [Google Scholar]
- 34. Su H, Xu X, Yan C, Shi Y, Hu Y, Dong L, et al. LncRNA H19 promotes the proliferation of pulmonary artery smooth muscle cells through AT1R via sponging let-7b in monocrotaline-induced pulmonary arterial hypertension. Respir Res. 2018;19:254. doi: 10.1186/s12931-018-0956-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Leisegang MS, Fork C, Josipovic I, Richter FM, Preussner J, Hu J, et al. Long noncoding RNA MANTIS facilitates endothelial angiogenic function. Circulation. 2017;136:65–79. doi: 10.1161/CIRCULATIONAHA.116.026991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12:861–874. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- 37. Saeinasab M, Bahrami AR, González J, Marchese FP, Martinez D, Mowla SJ, et al. SNHG15 is a bifunctional MYC-regulated noncoding locus encoding a lncRNA that promotes cell proliferation, invasion and drug resistance in colorectal cancer by interacting with AIF. J Exp Clin Cancer Res. 2019;38:172. doi: 10.1186/s13046-019-1169-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang N, Zhang C, Wang W, Liu J, Yu Y, Li Y, et al. Long noncoding RNA DANCR regulates proliferation and migration by epigenetically silencing FBP1 in tumorigenesis of cholangiocarcinoma. Cell Death Dis. 2019;10:585. doi: 10.1038/s41419-019-1810-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhang J, Yao T, Wang Y, Yu J, Liu Y, Lin Z. Long noncoding RNA MEG3 is downregulated in cervical cancer and affects cell proliferation and apoptosis by regulating miR-21. Cancer Biol Ther. 2016;17:104–113. doi: 10.1080/15384047.2015.1108496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nie FQ, Sun M, Yang JS, Xie M, Xu TP, Xia R, et al. Long noncoding RNA ANRIL promotes non-small cell lung cancer cell proliferation and inhibits apoptosis by silencing KLF2 and P21 expression. Mol Cancer Ther. 2015;14:268–277. doi: 10.1158/1535-7163.MCT-14-0492. [DOI] [PubMed] [Google Scholar]
- 41. Wang X, Li M, Wang Z, Han S, Tang X, Ge Y, et al. Silencing of long noncoding RNA MALAT1 by miR-101 and miR-217 inhibits proliferation, migration, and invasion of esophageal squamous cell carcinoma cells. J Biol Chem. 2015;290:3925–3935. doi: 10.1074/jbc.M114.596866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Weissmueller S, Manchado E, Saborowski M, Morris JP, IV, Wagenblast E, Davis CA, et al. Mutant p53 drives pancreatic cancer metastasis through cell-autonomous PDGF receptor β signaling. Cell. 2014;157:382–394. doi: 10.1016/j.cell.2014.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yang W, Wetterskog D, Matsumoto Y, Funa K. Kinetics of repression by modified p53 on the PDGF beta-receptor promoter. Int J Cancer. 2008;123:2020–2030. doi: 10.1002/ijc.23735. [DOI] [PubMed] [Google Scholar]
- 44. Cheng L, Huang Z, Zhou W, Wu Q, Donnola S, Liu JK, et al. Glioblastoma stem cells generate vascular pericytes to support vessel function and tumor growth. Cell. 2013;153:139–152. doi: 10.1016/j.cell.2013.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Liu J, Ben Q, Lu E, He X, Yang X, Ma J, et al. Long noncoding RNA PANDAR blocks CDKN1A gene transcription by competitive interaction with p53 protein in gastric cancer. Cell Death Dis. 2018;9:168. doi: 10.1038/s41419-017-0246-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li XL, Subramanian M, Jones MF, Chaudhary R, Singh DK, Zong X, et al. Long noncoding RNA PURPL suppresses basal p53 levels and promotes tumorigenicity in colorectal cancer. Cell Rep. 2017;20:2408–2423. doi: 10.1016/j.celrep.2017.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schermuly RT, Dony E, Ghofrani HA, Pullamsetti S, Savai R, Roth M, et al. Reversal of experimental pulmonary hypertension by PDGF inhibition. J Clin Invest. 2005;115:2811–2821. doi: 10.1172/JCI24838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ghofrani HA, Morrell NW, Hoeper MM, Olschewski H, Peacock AJ, Barst RJ, et al. Imatinib in pulmonary arterial hypertension patients with inadequate response to established therapy. Am J Respir Crit Care Med. 2010;182:1171–1177. doi: 10.1164/rccm.201001-0123OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nickel NP, Spiekerkoetter E, Gu M, Li CG, Li H, Kaschwich M, et al. Elafin reverses pulmonary hypertension via caveolin-1-dependent bone morphogenetic protein signaling. Am J Respir Crit Care Med. 2015;191:1273–1286. doi: 10.1164/rccm.201412-2291OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fatehullah A, Tan SH, Barker N. Organoids as an in vitro model of human development and disease. Nat Cell Biol. 2016;18:246–254. doi: 10.1038/ncb3312. [DOI] [PubMed] [Google Scholar]
- 51. Zhang Y, Pitchiaya S, Cieślik M, Niknafs YS, Tien JC, Hosono Y, et al. Analysis of the androgen receptor-regulated lncRNA landscape identifies a role for ARLNC1 in prostate cancer progression. Nat Genet. 2018;50:814–824. doi: 10.1038/s41588-018-0120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ruan X, Li P, Chen Y, Shi Y, Pirooznia M, Seifuddin F, et al. In vivo functional analysis of non-conserved human lncRNAs associated with cardiometabolic traits. Nat Commun. 2020;11:45. doi: 10.1038/s41467-019-13688-z. [DOI] [PMC free article] [PubMed] [Google Scholar]


