Using a simulation model of HPV, we find 3 interventions to increase HPV vaccination are a high-value use of state funding over 50 years.
Abstract
Video Abstract
OBJECTIVES:
We sought to prioritize interventions for increasing human papillomavirus (HPV) vaccination coverage based on cost-effectiveness from a US state perspective to inform decisions by policy makers.
METHODS:
We developed a dynamic simulation model of HPV transmission and progression scaled to a medium-sized US state (5 million individuals). We modeled outcomes over 50 years comparing no intervention to a one-year implementation of centralized reminder and recall for HPV vaccination, school-located HPV vaccination, or quality improvement (QI) visits to primary care clinics. We used probabilistic sensitivity analysis to assess a range of plausible outcomes associated with each intervention. Cost-effectiveness was evaluated relative to a conservative willingness-to-pay threshold; $50 000 per quality-adjusted life-year (QALY) .
RESULTS:
All interventions were cost-effective, relative to no intervention. QI visits had the lowest cost and cost per QALY gained ($1538 versus no intervention). Statewide implementation of centralized reminder and recall cost $28 289 per QALY gained versus QI visits. School-located vaccination had the highest cost but was cost-effective at $18 337 per QALY gained versus QI visits. Scaling to the US population, interventions could avert 3000 to 14 000 future HPV cancers. When varying intervention cost and impact over feasible ranges, interventions were typically preferred to no intervention, but cost-effectiveness varied between intervention strategies.
CONCLUSIONS:
Three interventions for increasing HPV vaccine coverage were cost-effective and offered substantial health benefits. Policy makers seeking to increase HPV vaccination should, at minimum, dedicate additional funding for QI visits, which are consistently effective at low cost and may additionally consider more resource-intensive interventions (reminder and recall or school-located vaccination).
What’s Known on This Subject:
Human papillomavirus (HPV) vaccination offers substantial benefits for cancer prevention, yet uptake remains well below national targets. Although interventions to address low HPV vaccination coverage have been tested, the cost-effectiveness of these interventions over the long-term is unexplored.
What This Study Adds:
We find 3 interventions for increasing HPV vaccination are cost-effective at common thresholds compared with status quo. HPV vaccination interventions are a high-value use of state funding and could prevent from 3000 to 14 000 incident HPV cancers over the next 50 years.
Human papillomavirus (HPV) infections cause 34 800 cancers annually in the United States, 92% of which could be prevented through HPV vaccination.1 Yet, coverage remains low, with only 68% of US adolescents ages 13 to 17 having received at least 1 dose of HPV vaccine and only 51% up to date with all recommended doses.2 With limited and shrinking budgets for state-based government programs in preventive care3 and increasing centralization of primary care in cost-conscious health care systems,4 it is important to identify not just effective but high-value strategies for improving HPV vaccine uptake.
Programmatic interventions focused on primary care are more successful at increasing vaccination than interventions focused on risk perception or vaccine confidence.5,6 Successful HPV vaccination interventions, often led by state health departments or large health care systems, include facilitating action through parent reminders,7 prompting providers through clinical quality improvement (QI) efforts,8 and increasing access through school-located vaccination.9 Yet, direct comparison of the costs and benefits of vaccination interventions is rare, and connections to outcomes beyond vaccination are needed. In addition, state-based comprehensive cancer control plans reveal that, although nearly all states prioritize HPV cancer prevention, few are engaged in evidence-based activities to increase HPV vaccination.10,11 Therefore, we sought to provide state health departments and policy makers with empirical guidance for prioritizing HPV vaccine interventions based on value, defined as increased healthy life-years per dollar spent.
Methods
To estimate the value of HPV vaccine coverage interventions, we developed a compartmental, dynamic model of HPV infection, transmission, and progression to cancer in a heterosexual US population. We compared cost, individuals vaccinated, cancer incidence, and quality-adjusted life-years (QALYs) associated with a statewide implementation of each of 3 evidence-based interventions for increasing HPV vaccination coverage versus no intervention (vaccination as normal) over a 50-year time horizon. We considered costs and benefits from the state perspective. The study was deemed exempt by the University of North Carolina’s Institutional Review Board. We followed best practices according to the Consolidated Health Economic Evaluation Reporting Standards.12
HPV Transmission Model Overview
The model started with 5 million adults and children (the median population size of a US state) stratified by sex and age, corresponding to the US population.13 New individuals entered the population at birth according to estimated US fertility rates. All individuals progressed through 11 nonoverlapping age groups in which they could die of competing disease at age- and sex-specific mortality rates.14 Sexual transmission of HPV was based on a susceptible-infected-recovered-susceptible framework.15 We separately considered 7 vaccine-preventable types of HPV (16, 18, 31, 33, 45, 52, and 58) and a single combined estimate of high-risk HPV types without vaccine protection. Prevalent HPV infection could either be cleared or could progress at type-, sex-, and age-specific rates to 1 of 6 HPV cancers (cervical, vaginal, vulvar, penile, anal, and oropharyngeal). The full details of the model structure appear in the Supplemental Information.
The HPV vaccine has high efficacy against covered HPV types when the full series is completed,16,17 and partial series completion also confers substantial benefit.18–20 Therefore, we separately model vaccine initiation (receiving at least 1 HPV vaccine dose) and vaccine follow-through (completion of the full series among those with at least 1 dose), with the assumption that those who initiate but fail to complete the vaccine series receive only partial protection (one-half the efficacy of the full series).
Model Input Data
Model inputs came directly from large national data sources when available, with additional parameters drawn from other published estimates. Data on population age structure, births, and deaths came from 2010 census data and the Centers for Disease Control and Prevention (CDC) WONDER database.13,14 Data on sexual behavior, type-specific infectivity rates, and vaccine efficacy came from HPV modeling literature.21–24 Age- and type-specific HPV prevalence were generated by using the NHANES.25 Finally, data on HPV vaccine initiation and follow-through by age, sex, and year were derived from provider-verified estimates by using the 2006 to 2017 National Immunization Survey–Teen.26 Transitions through disease states for which direct estimates are not available (HPV progression, clearance, and natural immunity) were calibrated within a plausible range to match prevaccine HPV prevalence by type and age and HPV cancer incidence by sex, site, and age by using estimates from the Surveillance, Epidemiology and End Results Program.25,27
Comparison Interventions
We evaluated 3 evidence-based interventions, assuming a one-time, year-long implementation. The population-level impact of each intervention is determined by a combination of 2 factors. Reach is the total number of individuals potentially exposed to the intervention (both vaccinated and unvaccinated), whereas effectiveness describes the impact of the intervention on HPV vaccination coverage for routine (11–12 years old) or catch-up (13–17 years old). We separately model initiation (increase in receipt of 1 vaccine among unvaccinated individuals) and follow-through (receipt of additional doses among those with at least 1 dose of the HPV vaccine). Because reach is generally proportional to the total cost of an intervention, we used trials with cost data to estimate both a feasible reach for each intervention and the expected per-person cost when implemented statewide (Table 1, Supplemental Tables 12 and 13). Base case data on the effectiveness of each intervention by age and sex were collected from randomized controlled trials, with additional data on best- and worst-case scenarios from randomized controlled trials or single-arm studies (Table 1, Supplemental Table 14).
TABLE 1.
Intervention Characteristics
| Target Population | Centralized Reminder and Recall, 11- to 17-y-Olds Included in Immunization Information System | School-Located Vaccination, 11- to 13-y-Olds in Public Middle Schools | QI Visits, 11- to 17-y-Olds With Participating Providers |
|---|---|---|---|
| Expected reach | |||
| 11- to 13-y-olds | 249 400 | 247 254 | 246 500 |
| 14- to 17-y-olds | 219 300 | 0 | 216 750 |
| Expected improvement in HPV vaccine initiation rate,a % (range) | |||
| Girls 11–13 | 2.0 (1.0–6.0) | 13.0 (2.0–17.7) | 2.6 (0.9–4.2) |
| Boys 11–13 | 5.0 (1.0–6.0) | 13.0 (2.0–17.7) | 2.6 (0.9–3.1) |
| Girls 14–17 | 3.0 (1.9–6.0) | — | 0.0 (0.0–3.3) |
| Boys 14–17 | 2.0 (0.3–6.0) | — | 0.0 (0.0–8.0) |
| Expected improvement in HPV vaccine follow-through rate,b % (range) | |||
| Girls 11–13 | 8.0 (0.0–16.6) | 4.0 (0.0–18) | 0.0 (0.0–1.3) |
| Boys 11–13 | 8.0 (0.0–16.6) | 4.0 (0.0–18) | 0.0 (0.0–1.3) |
| Girls 14–17 | 8.0 (0.0–16.6) | — | 0.0 (0.0–1.3) |
| Boys 14–17 | 8.0 (0.0–16.6) | — | 0.0 (0.0–1.3) |
| Cost per individual reached,c $ (range) | 2.10 (1.33–2.70) | 12.29 (9.22–15.36) | 0.14 (0.09–0.60) |
—, not applicable.
Estimated percentage point increase in vaccination in unvaccinated individuals.
Estimated percentage point increase in vaccine follow-through rates in vaccine initiators.
Costs are updated to 2018 US dollars by using the Consumer Price Index.28
Centralized Reminder and Recall
Centralized reminder and recall uses a regional or statewide immunization information system that stores vaccination histories from participating providers and health departments.7 Eligible individuals (or their parents and/or guardians) receive a call, e-mail, text, or mailing about being due or past due for recommended vaccines. To estimate the potential intervention reach, we assume that 86% of adolescents have at least 1 record in the immunization information system.29–31 Estimates of effect account for missing or incorrect contact information. Resources involved in reminder and recall include staff time and intervention materials (eg, postage and printing for reminder letters).29,32 Studies suggest reminders increase HPV vaccine initiation in boys (5.0% [range: 1.0%–6.0%]) and girls (2.0% [range: 1.0%–6.0%]),33–35 and recall likely increases HPV vaccine follow-through for both groups (8.0% [range: 0.0%–16.0%]).33,36,37
School-Located Vaccination
In US school-located vaccination models, parents provide consent for their children to be vaccinated by trained community vaccinators at school.38,39 Because school-located health clinics are uncommon in the United States, we consider the model of holding mass vaccination days, which would potentially reach those ages 11 to 13 who attend a public school (87%).40 The costs for school-located vaccination include time and resources to create information materials, obtain permission from parents, schedule the event, administer the vaccination, and process insurance claims for privately insured adolescents.41 Given existing coverage of the HPV vaccine through the federally funded Vaccine for Children program and its designation as an essential health benefit through the Patient Protection and Affordable Care Act, we assume vaccine purchase costs are negligible.42 School-located vaccination is resource intensive and generates substantial improvement in HPV vaccine initiation for 11- to 13-year-old girls and boys (13.0% [range: 2.0%–17.7%]) as well as likely increasing follow-through (4.0% [range: 0.0%–18%]).41,43,44
QI Visits
CDC funds state health departments to conduct QI visits with primary care clinics participating in the Vaccines for Children program. The CDC QI curriculum was originally called the Assessment, Feedback, Incentives eXchange program but was remodeled in 2019 as the Immunization Quality Improvement for Providers. These in person or webinar sessions provide clinic vaccination coverage estimates, facilitate goal setting, and support implementation of best practices.45 Effects potentially reach adolescents whose regular source of care is a participating clinic (85%). Costs for these QI visits are those associated with travel and staff time for conducting training either in person or online.46,47 QI visits increase HPV vaccine initiation among adolescents from ages 11 to 12 (girls: 2.6% [range: 0.9%–4.2%]; boys: 2.6% [range: 0.9%–3.1%]); however, the impact on follow-through is small and not statistically significant (both: 0% [range: 0%–1.3%]).48–50
Outcome Measures
We considered total intervention costs from a state government perspective in 2018 US dollars. Because all intervention costs were assumed to occur in the first year, they were not discounted. We did not include savings resulting from increased vaccination (eg, lower cancer treatment costs) because the state government would not directly bear most of these costs in the future. We estimated the number of additional individuals initiating HPV vaccination, total cases of HPV cancers averted, and QALYs gained over a 50-year time horizon. By design, interventions affect primarily HPV vaccine initiation; however, we also report increases in HPV vaccine completion. QALYs associated with each intervention were calculated by using previously reported sex- and age-specific decrements associated with incidence of HPV cancer51 and were discounted at a rate of 3% per year. Outcomes are provided as incremental cost-effectiveness ratios (ICERs), which are used to report the additional cost per improved outcome (QALYs gained, additional individuals initiating HPV vaccine, or cancers averted).
Analyses
Using identical starting conditions, we simulated HPV vaccination, transmission, and cancer progression for 50 years after each intervention. We first report each outcome relative to no intervention then compare each sequentially to the next most effective alternative. Interventions were dropped from comparison when either dominated (the next best intervention was both more effective and less expensive) or dominated by extension (the next best intervention had a lower ICER).52
We performed both deterministic and probabilistic sensitivity analyses to assess the robustness of our findings. For each alternative, we compared a best-case (highest effectiveness and lowest cost estimate) and worst-case (lowest effectiveness and highest cost) scenario. Then, to create a plausible range of intervention outcomes, we varied all cost and effectiveness parameters by sampling 500 sets of values from a uniform distribution for each parameter across the range of these estimates.
For cost per QALY gained, we compared against a conservative willingness-to-pay threshold, with interventions that cost <$50 000 per QALY gained considered cost-effective.52 No willingness-to-pay thresholds have been established for cost per additional individual initiating and completing HPV vaccination or cost per cancer case averted, yet these outcomes may be important for decision-makers. Therefore, we present cost-effectiveness acceptability curves for each outcome, indicating the percentage of considered probabilistic scenarios in which each intervention would be preferred across a range of possible willingness-to-pay thresholds.
Results
Interventions Compared With No Intervention
Each of the interventions increased the number of adolescents initiating HPV vaccine relative to no intervention: an additional 3733 HPV vaccine initiators from QI visits, 6993 from reminder and recall, and 18 721 from school-located vaccinations (Table 2). Comparable gains were seen in HPV vaccine completion (Supplemental Table 15). The interventions reduced total cancer incidence over 50 years; 44 cases were averted for QI visits, 76 for reminder and recall, and 212 for school-located vaccination, which represent 3000 to 14 000 cancers averted, if scaled to the full US population (330 million). The interventions also increased QALYs gained (an additional 42 QALYs for QI visits, 75 for reminder and recall, and 204 for school-located vaccination). The total intervention costs for the state were an estimated $64 855 for QI visits, $984 270 for reminder and recall, and $3 038 752 for school-located vaccination. Each intervention was well below the $50 000 per QALY threshold, when compared with no intervention (QI visits: $1538 per QALY gained; reminder and recall: $13 183 per QALY gained; school-located vaccination: $14 871 per QALY gained).
TABLE 2.
Base Case Cost-effectiveness of Strategies for Increasing HPV Vaccination
| Approach | Cost, $ | Additional Individuals Vaccinated, n | Incremental Vaccination, n | Cost per Additional Person Vaccinated, $ | Cancers Averted, n | Incremental Cancers Averted, n | Cost per HPV Cancer Averted, $ | QALYs Gained, n | Incremental QALYs Gained, n | Cost per QALY Gained, $ | Cost per QALY Gained Versus No Intervention, $ |
|---|---|---|---|---|---|---|---|---|---|---|---|
| QI visits | 64 855 | 3733 | 3733 | 17 | 44 | 44 | 1475 | 42 | 42 | 1538 | 1538 |
| Reminder and recall | 984 270 | 6993 | 3260 | 282 | 76 | 32 | 28 333 | 75 | 33 | 28 290a | 13 183 |
| School-located vaccination | 3 038 752 | 18 721 | 14 988a | 198a | 212 | 168a | 17 691a | 204 | 162a | 18 337a | 14 871 |
QALYs are discounted at 3% per year.
Indicates that school-located vaccination has extended dominance compared with reminder and recall intervention; comparisons were made to QI visits instead.
Interventions Compared With Each Other
Beyond the gains achievable by QI visits, the use of reminder and recall would result in an additional 33 QALYs for an additional $919 415 ($28 290 per QALY gained; Table 2). School-located vaccination dominates reminder and recall by extension (improved outcomes at a lower ICER); therefore, we compared school-located vaccination directly to QI visits. School-located vaccination results in an additional 254 QALYs gained, at just under 3 million in incremental cost, resulting in an ICER of $18 337 per QALY gained, making it cost-effective under conservative willingness-to-pay thresholds.
Comparing best- and worst-case scenarios from literature-based estimates revealed changes in per-person intervention costs had minimal impact on the relative cost-effectiveness of alternatives (Table 3). Increasing intervention effectiveness similarly changed the absolute, but not relative, ICERs. However, at the lowest effectiveness, school-located vaccination resulted in only 33 QALYs gained, lower than the base case QALYs for either QI visits or reminder and recall (42 and 75, respectively) and only slightly higher than each of these interventions at their lowest effectiveness (15 and 19, respectively). When compared with QI visits, school-located vaccination was no longer cost-effective ($166 232 per QALY gained), making QI visits the preferred alternative across the worst-case effectiveness scenarios.
TABLE 3.
Best- and Worst-Case Cost-effectiveness of Interventions for Increasing HPV Vaccination
| Interventions | Total Cost, $ | Incremental QALYs Gained Versus Next Best, n | Incremental Cost per QALY Gained Versus Next Best, $ | QALYs Gained Versus No Intervention, n | Cost per QALY Gained Versus No Intervention, $ |
|---|---|---|---|---|---|
| Worst-case costs | |||||
| QI visits | 97 283 | 42 | 2307 | 42 | 2307 |
| Reminder and recall | 1 734 190 | 33 | 50 366 | 75 | 23 228 |
| School-located vaccination | 3 797 821 | 162a | 22 817a | 204 | 18 586 |
| Best-case costs | |||||
| QI visits | 41 693 | 42 | 989 | 42 | 989 |
| Reminder and recall | 623 371 | 33 | 17 898 | 75 | 8349 |
| School-located vaccination | 2 279 682 | 162a | 10 213a | 204 | 11 156 |
| Worst-case effectiveness | |||||
| QI visits | 64 855 | 15 | 4388 | 15 | 4388 |
| Reminder and recall | 984 270 | 4 | 221 546 | 19 | 51 995 |
| School-located vaccination | 3 038 752 | 18a | 166 232a | 33 | 93 014 |
| Best-case effectiveness | |||||
| QI visits | 64 855 | 72 | 900 | 72 | 900 |
| Reminder and recall | 984 270 | 56 | 16 421 | 128 | 7688 |
| School-located vaccination | 3 038 752 | 219a | 13 610a | 291 | 10 459 |
Costs and QALYs discounted at 3% per year.
Indicates that school-located vaccination has dominance and extended dominance compared with reminder and recall intervention; comparisons were made to QI visits.
Probabilistic Sensitivity Analysis
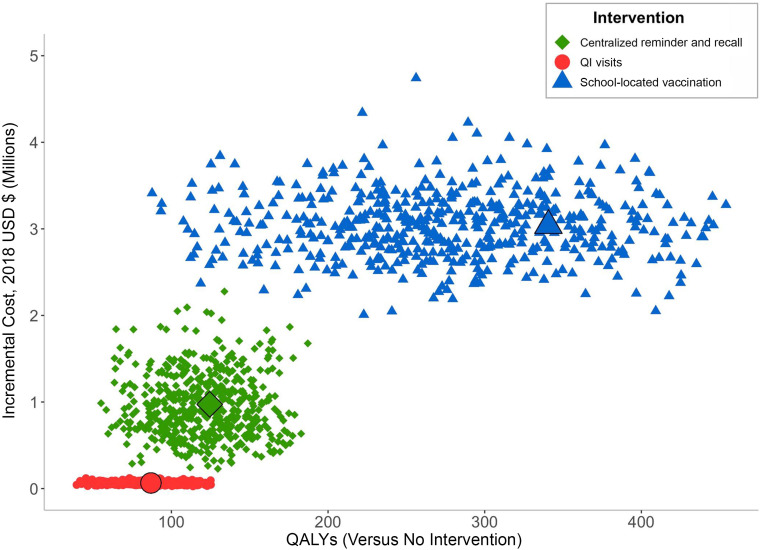
Although the expected cost of the 3 interventions did not overlap, the total QALYs gained from each intervention revealed moderate overlap, with a particularly wide uncertainty range for the effectiveness of school-located vaccination (Fig 1). Relative to no intervention, all 3 interventions were cost-effective in 100% of the scenarios. When comparing interventions with each other at a willingness to pay of $50 000 per QALY gained, school-located vaccination was the preferred option in 83% of scenarios, reminder and recall was preferred in 12%, and QI visits were preferred in the remaining 5% of scenarios.
FIGURE 1.
Incremental cost and QALYs gained. In the figure, we compare 500 simulations of cost and QALYs gained for each intervention compared with no intervention scenario. The largest points represent base case estimates.
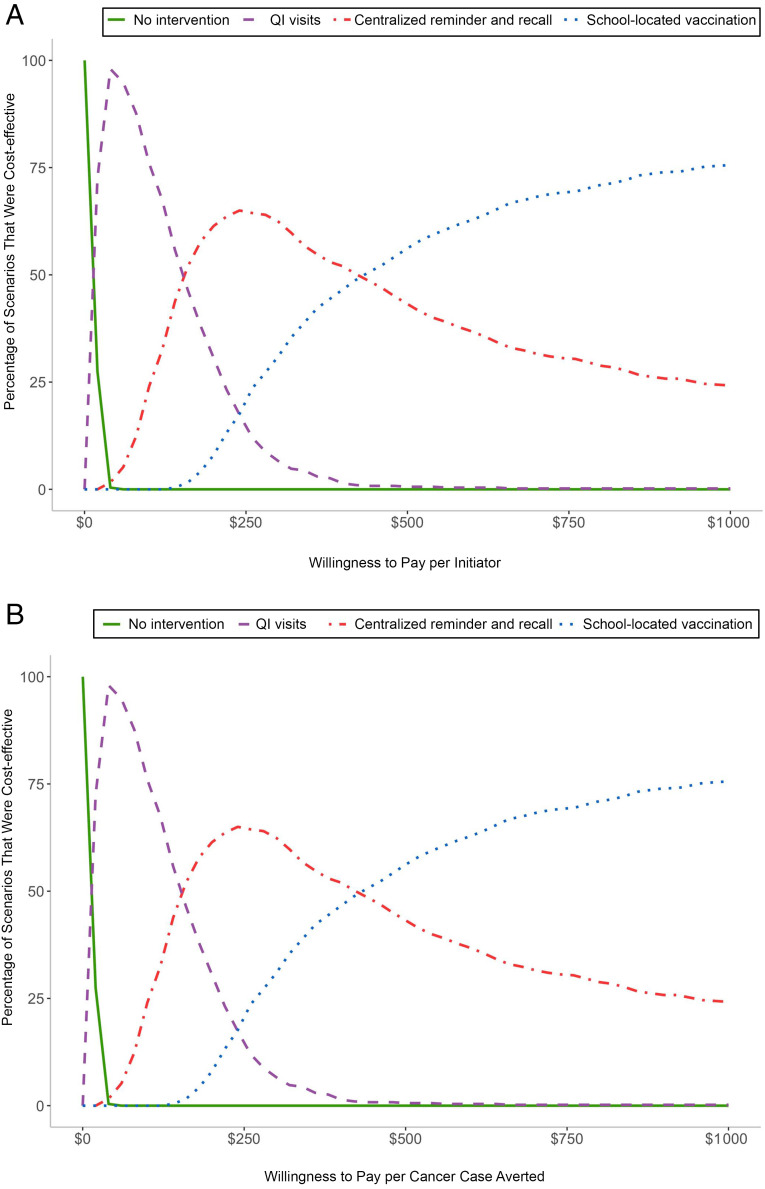
When considering short- and medium-term benefits, recommendations vary on the basis of a state’s willingness to pay for improved outcomes (Fig 2). When willing to pay at least $13 per additional initiator, $18 per completer, or $1500 per cancer case averted, QI visits were preferable to no intervention in most scenarios. Additional gains from school-located vaccination were not worth the additional costs until a state was willing to pay at least $350 per additional initiator, $420 per additional completer, or $26 000 per cancer case averted (Fig 2, Supplemental Fig 11). Even at higher thresholds, centralized reminder and recall remains preferable in a small percent of scenarios for all outcomes (10%–25%) because it would achieve similar or better outcomes to school-located vaccination at a similar or lower cost (dominant).
FIGURE 2.
Cost-effectiveness acceptability by willingness to pay. A, Cost per HPV vaccine initiator. B, Cost per cancer case averted. In the figure, we compare 500 simulations of each intervention using net monetary benefit across a range of willingness-to-pay thresholds for each outcome considered.
Discussion
Statewide implementation of QI visits, centralized reminder and recall, and school-located vaccination were each cost-effective at increasing HPV vaccine coverage, relative to no intervention. The increases in HPV vaccination rates after a one-time implementation of these interventions led to a meaningful reduction in HPV cancers over 50 years. When compared with the status quo, all 3 interventions were well below standard cost-effectiveness thresholds in the United States, suggesting these interventions are a high-value use of state prevention resources.
If choosing among the 3 interventions, school-located vaccination is generally preferred because it has the largest impact and is cost-effective relative to alternatives. Policy makers, however, should interpret these findings with some caution because school-located vaccination may fail to be cost-effective or, in some cases, may be dominated, when gains are on the lower end of observed effectiveness. Finally, when considering shorter-term outcomes, QI visits are preferred to no intervention, even at low willingness-to-pay thresholds ($13 per initiator or $1500 per cancer averted), whereas school-located vaccination was only likely to be preferable when states were willing to pay at least $350 per individual initiating HPV vaccine or $26 000 per cancer case averted.
The effectiveness and cost-effectiveness of vaccine interventions have been studied extensively,5,6 but evidence suggests HPV vaccination rates may be harder to influence via policy or programmatic intervention53,54 compared with other vaccines, perhaps because of patient and provider hesitency.55,56 Previous studies have revealed, for example, that reminder and recall interventions have an estimated median effect of 7 percentage points across other adolescent vaccines7 but an increase of only a 2 to 6 percentage points for HPV vaccination.33,34 Consistent with this, we find the cost per vaccine initiator is generally higher than what has been reported for other adolescent vaccines.7,57 However, when linking these vaccination gains to long-term outcomes, we find cost per QALY gained is even lower for HPV vaccination than for other vaccine-based interventions,58,59 likely because of the relatively high population burden of HPV.
In our study, we examine HPV interventions from the perspective of a hypothetical US state, assuming demographics, disease burden, and vaccine uptake reflective of the United States as a whole. There is considerable variation across US states in HPV vaccine uptake, with lower initiation in states with a higher HPV cancer burden.60 Although our analysis presents cost-effectiveness across a range of intervention outcomes, the underlying characteristics of states could also influence the cost-effectiveness of these interventions, with states at a higher burden of HPV disease or lower HPV vaccination rates potentially seeing an even greater benefit from these interventions.
Our findings should be interpreted in light of the limitations of existing intervention evidence, model simplifications, and project scope. First, we used the costs and reach associated with previous implementation of each intervention, which may vary by setting and may change at scale in ways we cannot capture here. States weighing intervention options would additionally need to consider contextual details of program implementation: for example, buy-in of school districts or providers as well as quality and completeness of immunization information systems, which could have an impact on program effectiveness. Secondly, we made several simplifications when modeling HPV infection. Notably, we only consider heterosexual transmission of HPV, a simplification made in nearly all models of HPV infection22 but one that may nonetheless obscure nuances of transmission patterns61 and populations with high rates of HPV cancers.62,63 We also note our model structure did not allow for the consideration of co-occurring infections. Although we retained the highest risk infections when multiple exposures occurred, we could not model an additive or multiplicative effect of concurrent infections. Our model estimated the intervention benefits specific to HPV vaccination and HPV cancers from the perspective of a state agency. We did not include vaccine cost, nor did we include cost savings associated with future cancers because states are unlikely to cover these costs directly. We compared data on 3 interventions with sufficient data on both intervention cost and effectiveness. Additional evidence on other interventions, including multilevel interventions, is needed.
Our analysis provides a rare comparison of cancer control interventions from a state perspective and presents decision-makers with evidence on cost-effectiveness for both short- and long-term outcomes. In future work, researchers may also consider that each intervention has the potential to improve receipt of other adolescent vaccines in addition to the HPV vaccine and that the HPV vaccine also prevents genital warts, benefits that would improve cost-effectiveness estimations.
Conclusions
Scaling up interventions to increase HPV vaccination could prevent thousands of cancers nationally over the next 50 years, providing a high-value use of state prevention funding. Although most states support efforts to increase HPV vaccine uptake, they are of varying quality and intensity. State health departments should strengthen and scale-up existing QI visit programs because they have a high benefit relative to their cost. In addition, policy makers and advocates should consider devoting resources to more intensive interventions, including reminder and recall or school-located vaccination, which could prevent thousands of HPV cancers in the United States in the coming decades.
Glossary
- CDC
Centers for Disease Control and Prevention
- HPV
human papillomavirus
- ICER
incremental cost-effectiveness ratio
- QALY
quality-adjusted life-year
- QI
quality improvement
Footnotes
Dr Spencer conceptualized and designed the study, conducted analysis, drafted the initial manuscript, and reviewed and revised the manuscript; Drs Wheeler, Trogdon, Weinberger, Coyne-Beasley, and Brewer contributed meaningfully to study design and interpretation of the data and critically reviewed and revised the manuscript; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
FINANCIAL DISCLOSURE: Dr Brewer has received research grants from Merck and Pfizer and served as a paid advisory board member for Merck; Dr Trogdon has received research grants from Merck; Dr Wheeler has received grant funding from Pfizer’s Independent Grants for Learning & Change; Dr Coyne-Beasley has served as an advisory board member for Sanofi Pasteur; and Drs Spencer and Weinberger have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Dr Spencer received support for this work from the University of North Carolina Cancer Care Quality Training Program (National Cancer Institute: T32CA11633) and the Dana-Farber Training in Oncology Population Sciences program (National Cancer Institute: T32CA092203). Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Senkomago V, Henley SJ, Thomas CC, Mix JM, Markowitz LE, Saraiya M. Human papillomavirus-attributable cancers - United States, 2012–2016. MMWR Morb Mortal Wkly Rep. 2019;68(33):724–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walker TY, Elam-Evans LD, Yankey D, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years - United States, 2018. MMWR Morb Mortal Wkly Rep. 2019;68(33):718–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Himmelstein DU, Woolhandler S. Public health’s falling share of US health spending. Am J Public Health. 2016;106(1):56–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The Physicians Foundation Health reform and the decline of physician private practice. Available at: https://physiciansfoundation.org/wp-content/uploads/2018/02/Health_Reform_and_the_Decline_of_Physician_Private_Practice.pdf. Accessed October 27, 2020
- 5.Brewer NT, Chapman GB, Rothman AJ, Leask J, Kempe A. Increasing vaccination: putting psychological science into action. Psychol Sci Public Interest. 2017;18(3):149–207 [DOI] [PubMed] [Google Scholar]
- 6.Briss PA, Rodewald LE, Hinman AR, et al.; The Task Force on Community Preventive Services . Reviews of evidence regarding interventions to improve vaccination coverage in children, adolescents, and adults. Am J Prev Med. 2000;18(suppl 1):97–140 [DOI] [PubMed] [Google Scholar]
- 7.Jacobson Vann JC, Jacobson RM, Coyne-Beasley T, Asafu-Adjei JK, Szilagyi PG. Patient reminder and recall interventions to improve immunization rates. Cochrane Database Syst Rev. 2018;(1):CD003941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Community Preventive Services Task Force Increasing Appropriate Vaccination: Provider Assessment and Feedback. Atlanta, GA: Community Preventive Services Task Force; 2018 [Google Scholar]
- 9.Community Preventive Services Task Force Increasing Appropriate Vaccination: Vaccination Programs in Schools and Organized Child Care Centers. Atlanta, GA: Community Preventive Services Task Force; 2010 [Google Scholar]
- 10.Meyerson BE, Zimet GD, Multani GS, Levell C, Lawrence CA, Smith JS. Increasing efforts to reduce cervical cancer through state-level comprehensive cancer control planning. Cancer Prev Res (Phila). 2015;8(7):636–641 [DOI] [PubMed] [Google Scholar]
- 11.Townsend JS, Steele CB, Hayes N, Bhatt A, Moore AR. Human papillomavirus vaccine as an anticancer vaccine: collaborative efforts to promote human papillomavirus vaccine in the national comprehensive cancer control program. J Womens Health (Larchmt). 2017;26(3):200–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Husereau D, Drummond M, Petrou S, et al. Consolidated health economic evaluation reporting standards (CHEERS) statement. Eur J Health Econ. 2013;14(3):367–372 [DOI] [PubMed] [Google Scholar]
- 13.Social Explorer. Results. Available at: www.socialexplorer.com/pub/reportdata/HtmlResults.aspx?reportid=R12026676. Accessed October 16, 2018
- 14.Centers for Disease Control and Prevention About compressed mortality, 1999–2016. Available at: http://wonder.cdc.gov/cmf-icd10.html. Accessed October 16, 2018
- 15.Rock K, Brand S, Moir J, Keeling MJ. Dynamics of infectious diseases. Rep Prog Phys. 2014;77(2):026602. [DOI] [PubMed] [Google Scholar]
- 16.Vesikari T, Brodszki N, van Damme P, et al. A randomized, double-blind, phase III study of the immunogenicity and safety of a 9-valent human papillomavirus L1 virus-like particle vaccine (V503) versus Gardasil® in 9–15-year-old girls. Pediatr Infect Dis J. 2015;34(9):992–998 [DOI] [PubMed] [Google Scholar]
- 17.Apter D, Wheeler CM, Paavonen J, et al.; HPV PATRICIA Study Group . Efficacy of human papillomavirus 16 and 18 (HPV-16/18) AS04-adjuvanted vaccine against cervical infection and precancer in young women: final event-driven analysis of the randomized, double-blind PATRICIA trial. Clin Vaccine Immunol. 2015;22(4):361–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perkins RB, Lin M, Wallington SF, Hanchate A. Impact of number of human papillomavirus vaccine doses on genital warts diagnoses among a national cohort of US adolescents. Sex Transm Dis. 2017;44(6):365–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hariri S, Schuler MS, Naleway AL, et al. Human papillomavirus vaccine effectiveness against incident genital warts among female health-plan enrollees, United States. Am J Epidemiol. 2018;187(2):298–305 [DOI] [PubMed] [Google Scholar]
- 20.Basu P, Bhatla N, Ngoma T, Sankaranarayanan R. Less than 3 doses of the HPV vaccine - review of efficacy against virological and disease end points. Hum Vaccin Immunother. 2016;12(6):1394–1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim JJ, Goldie SJ. Health and economic implications of HPV vaccination in the United States. N Engl J Med. 2008;359(8):821–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brisson M, Laprise J-F, Drolet M, et al. Comparative cost-effectiveness of the quadrivalent and bivalent human papillomavirus vaccines: a transmission-dynamic modeling study. Vaccine. 2013;31(37):3863–3871 [DOI] [PubMed] [Google Scholar]
- 23.Chesson HW, Flagg EW, Koutsky L, et al. Modeling the impact of quadrivalent HPV vaccination on the incidence of Pap test abnormalities in the United States. Vaccine. 2013;31(29):3019–3024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Durham DP, Ndeffo-Mbah ML, Skrip LA, Jones FK, Bauch CT, Galvani AP. National- and state-level impact and cost-effectiveness of nonavalent HPV vaccination in the United States. Proc Natl Acad Sci USA. 2016;113(18):5107–5112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Center for Health Statistics; Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey. Available at: https://www.cdc.gov/nchs/nhanes/index.htm. Accessed February 13, 2019
- 26.Centers for Disease Control and Prevention Datasets and related documentation for the National Immunization Survey - Teen, 2008–2014. Available at: https://www.cdc.gov/nchs/nis/data_files_teen.htm. Accessed February 13, 2019
- 27.Surveillance, Epidemiology, and End Results Program; National Cancer Institute. SEER*Explorer. Available at: https://seer.cancer.gov/explorer/application.php?site=47&data_type=1&graph_type=2&compareBy=race&chk_sex_1=1&chk_race_5=5&chk_race_4=4&chk_race_3=3&chk_race_6=6&chk_race_2=2&chk_age_range_1=1&chk_data_type_1=1&advopt_precision=1&advopt_display=1&showDataF. Accessed February 13, 2019
- 28.US Bureau of Labor Statistics. Consumer Price Index. Available at: https://www.bls.gov/cpi/. Accessed April 15, 2018
- 29.Kempe A, Saville AW, Beaty B, et al. Centralized reminder/recall to increase immunization rates in young children: how much bang for the buck? Acad Pediatr. 2017;17(3):330–338 [DOI] [PubMed] [Google Scholar]
- 30.Koepke R, Petit AB, Ayele RA, et al. Completeness and accuracy of the Wisconsin immunization registry: an evaluation coinciding with the beginning of meaningful use. J Public Health Manag Pract. 2015;21(3):273–281 [DOI] [PubMed] [Google Scholar]
- 31.Hurley LP, Beaty B, Lockhart S, et al. Randomized controlled trial of centralized vaccine reminder/recall to improve adult vaccination rates in an accountable care organization setting. Prev Med Rep. 2019;15:100893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rask KJ, LeBaron CW, Starnes DM. The costs of registry-based immunization interventions. Am J Prev Med. 2001;21(4):267–271 [DOI] [PubMed] [Google Scholar]
- 33.Szilagyi PG, Albertin C, Humiston SG, et al. A randomized trial of the effect of centralized reminder/recall on immunizations and preventive care visits for adolescents. Acad Pediatr. 2013;13(3):204–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rand CM, Brill H, Albertin C, et al. Effectiveness of centralized text message reminders on human papillomavirus immunization coverage for publicly insured adolescents. J Adolesc Health. 2015;56(suppl 5):S17–S20 [DOI] [PubMed] [Google Scholar]
- 35.Staras SAS, Vadaparampil ST, Livingston MD, Thompson LA, Sanders AH, Shenkman EA. Increasing human papillomavirus vaccine initiation among publicly insured Florida adolescents. J Adolesc Health. 2015;56(suppl 5):S40–S46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kharbanda EO, Stockwell MS, Fox HW, Andres R, Lara M, Rickert VI. Text message reminders to promote human papillomavirus vaccination. Vaccine. 2011;29(14):2537–2541 [DOI] [PubMed] [Google Scholar]
- 37.Rand CM, Vincelli P, Goldstein NPN, Blumkin A, Szilagyi PG. Effects of phone and text message reminders on completion of the human papillomavirus vaccine series. J Adolesc Health. 2017;60(1):113–119 [DOI] [PubMed] [Google Scholar]
- 38.Middleman A. School-located vaccination for adolescents: past, present, and future and implications for HPV vaccine delivery. Hum Vaccin Immunother. 2016;12(6):1599–1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kempe A, Allison MA, Daley MF. Can school-located vaccination have a major impact on human papillomavirus vaccination rates in the United States? Acad Pediatr. 2018;18(2S):S101–S105 [DOI] [PubMed] [Google Scholar]
- 40.National Center for Education Statistics. Back to school statistics. Available at: https://nces.ed.gov/fastfacts/display.asp?id=372. Accessed January 21, 2020
- 41.Daley MF, Kempe A, Pyrzanowski J, et al. School-located vaccination of adolescents with insurance billing: cost, reimbursement, and vaccination outcomes. J Adolesc Health. 2014;54(3):282–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schuchat A. HPV “coverage”. N Engl J Med. 2015;372(8):775–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hayes KA, Entzel P, Berger W, et al. Early lessons learned from extramural school programs that offer HPV vaccine. J Sch Health. 2013;83(2):119–126 [DOI] [PubMed] [Google Scholar]
- 44.Middleman AB, Won T, Auslander B, Misra S, Short M. HPV vaccine uptake in a school-located vaccination program. Hum Vaccin Immunother. 2016;12(11):2872–2874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Centers for Disease Control and Prevention. At-a-glance. Available at: https://www.cdc.gov/vaccines/programs/afix/index.html. Accessed February 8, 2019
- 46.Gilkey MB, Moss JL, Roberts AJ, Dayton AM, Grimshaw AH, Brewer NT. Comparing in-person and webinar delivery of an immunization quality improvement program: a process evaluation of the adolescent AFIX trial. Implement Sci. 2014;9(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fontanesi J, De Guire M, Kopald D, Holcomb K. The price of prevention. cost of recommended activities to improve immunizations. Am J Prev Med. 2004;26(1):41–45 [DOI] [PubMed] [Google Scholar]
- 48.Gilkey MB, Dayton AM, Moss JL, et al. Increasing provision of adolescent vaccines in primary care: a randomized controlled trial. Pediatrics. 2014;134(2):e346–e353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moss JL, Reiter PL, Dayton A, Brewer NT. Increasing adolescent immunization by webinar: a brief provider intervention at federally qualified health centers. Vaccine. 2012;30(33):4960–4963 [DOI] [PubMed] [Google Scholar]
- 50.Perkins RB, Zisblatt L, Legler A, Trucks E, Hanchate A, Gorin SS. Effectiveness of a provider-focused intervention to improve HPV vaccination rates in boys and girls. Vaccine. 2015;33(9):1223–1229 [DOI] [PubMed] [Google Scholar]
- 51.Chesson HW, Ekwueme DU, Saraiya M, Dunne EF, Markowitz LE. The cost-effectiveness of male HPV vaccination in the United States. Vaccine. 2011;29(46):8443–8450 [DOI] [PubMed] [Google Scholar]
- 52.Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. [published correction appears in JAMA. 2016;316(18):1924]. JAMA. 2016;316(10):1093–1103 [DOI] [PubMed] [Google Scholar]
- 53.Walling EB, Benzoni N, Dornfeld J, et al. Interventions to improve HPV vaccine uptake: a systematic review. Pediatrics. 2016;138(1):e20153863. [DOI] [PubMed] [Google Scholar]
- 54.Moss JL, Reiter PL, Truong YK, Rimer BK, Brewer NT. School entry requirements and coverage of nontargeted adolescent vaccines. Pediatrics. 2016;138(6):e20161414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McRee A-L, Gilkey MB, Dempsey AF. HPV vaccine hesitancy: findings from a statewide survey of health care providers. J Pediatr Health Care. 2014;28(6):541–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gilkey MB, Calo WA, Marciniak MW, Brewer NT. Parents who refuse or delay HPV vaccine: differences in vaccination behavior, beliefs, and clinical communication preferences. Hum Vaccin Immunother. 2017;13(3):680–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jacob V, Chattopadhyay SK, Hopkins DP, Murphy Morgan J, Pitan AA, Clymer JM; Community Preventive Services Task Force . Increasing coverage of appropriate vaccinations A community guide systematic economic review. Am J Prev Med. 2016;50(6):797–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Michaelidis CI, Zimmerman RK, Nowalk MP, Smith KJ. Cost-effectiveness of programs to eliminate disparities in elderly vaccination rates in the United States. BMC Public Health. 2014;14:718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wateska AR, Nowalk MP, Zimmerman RK, Smith KJ, Lin CJ. Cost-effectiveness of increasing vaccination in high-risk adults aged 18-64 years: a model-based decision analysis. BMC Infect Dis. 2018;18(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moss JL, Reiter PL, Brewer NT. Correlates of human papillomavirus vaccine coverage: a state-level analysis. Sex Transm Dis. 2015;42(2):71–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brisson M, Bénard É, Drolet M, et al. Population-level impact, herd immunity, and elimination after human papillomavirus vaccination: a systematic review and meta-analysis of predictions from transmission-dynamic models. Lancet Public Health. 2016;1(1):e8–e17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Clifford GM, Tully S, Franceschi S. Carcinogenicity of human papillomavirus (HPV) types in HIV-positive women: a meta-analysis from HPV infection to cervical cancer. Clin Infect Dis. 2017;64(9):1228–1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marra E, Lin C, Clifford GM. Type-specific anal human papillomavirus prevalence among men, according to sexual preference and HIV status: a systematic literature review and meta-analysis. J Infect Dis. 2019;219(4):590–598 [DOI] [PubMed] [Google Scholar]