We compare the effectiveness of an early childhood development intervention delivered through individual home visits or mother–child group sessions in a cluster randomized control trial.
Abstract
Video Abstract
OBJECTIVES:
Poor early childhood development in low- and middle-income countries is a major public health problem. Efficacy trials have shown the potential of early childhood development interventions but scaling up is costly and challenging. Guidance on effective interventions’ delivery is needed. In an open-label cluster-randomized control trial, we compared the effectiveness of weekly home visits and weekly mother-child group sessions. Both included nutritional education, whose effectiveness was tested separately.
METHODS:
In Odisha, India, 192 villages were randomly assigned to control, nutritional education, nutritional education and home visiting, or nutritional education and group sessions. Mothers with children aged 7 to 16 months were enrolled (n = 1449). Trained local women ran the two-year interventions, which comprised demonstrations and interactions and targeted improved play and nutrition. Primary outcomes, measured at baseline, midline (12 months), and endline (24 months), were child cognition, language, motor development, growth and morbidity.
RESULTS:
Home visiting and group sessions had similar positive average (intention-to-treat) impacts on cognition (home visiting: 0.324 SD, 95% confidence interval [CI]: 0.152 to 0.496, P = .001; group sessions: 0.281 SD, 95% CI: 0.100 to 0.463, P = .007) and language (home visiting: 0.239 SD, 95% CI: 0.072 to 0.407, P = .009; group sessions: 0.302 SD, 95% CI: 0.136 to 0.468, P = .001). Most benefits occurred in the first year. Nutrition-education had no benefit. There were no consistent effects on any other primary outcomes.
CONCLUSIONS:
Group sessions cost $38 per child per year and were as effective on average as home visiting, which cost $135, implying an increase by a factor of 3.5 in the returns to investment with group sessions, offering a more scalable model. Impacts materialize in the first year, having important design implications.
What’s Known on This Subject:
In low- and middle-income countries millions of young children have poor development. Efficacy trials show that stimulation and nutritional interventions benefit children’s development but evidence on cost-effective methods of going to scale is urgently needed.
What This Study Adds:
Mother-child group sessions were as effective on average as home visits in improving child cognition and language in Odisha, India. Groups required 28% of the cost of home visits, substantially improving scalability of child psychosocial interventions.
Poverty, malnutrition, and poor stimulation are preventing millions of young children in low- and middle-income countries from reaching their developmental potential.1 These disadvantages affect brain architecture and function with lifelong consequences.2,3
Efficacy trials of psychosocial stimulation in early childhood improve disadvantaged children’s development,4,5 with limited evidence of sustained benefits into adulthood6,7 International agencies and professionals have called for these interventions to be scaled up.4,8,9 However, existing evidence says little about the costs and challenges of scaling up these interventions. Furthermore, even the most effective programs can fade out or not replicate.10,11 Trials of home visits,12,13 mother-child groups,14,15 clinic visits,16,17 and mixtures thereof18 all show some success in improving development but have used different outcomes, curricula, duration, and intervention intensities, making it difficult to evaluate the relative cost-effectiveness of alternative deliveries.
Previously,19 we adapted the Reach-Up and Learn13 home visiting intervention to the Odisha, India context. This had moderate impacts on children’s development but was labor-intensive and costly. Groups may reduce the cost because several mothers and children can participate in a session simultaneously. It is unclear, however, whether they can deliver the same impacts. Implementation is more challenging because facilitators must engage with children of different developmental levels and relate to several mothers at the same time, and mothers may have difficulty attending. However, mothers and children may learn more because of interactions and synergies within the peer group. We compare the effectiveness of the same early childhood development (ECD) intervention delivered to mothers and children in groups or through individual home visits. Given the poor nutritional status of children in this context, we included a nutritional education component. We collected midline data to evaluate progress over time. The combination of using local women with group delivery offers a potentially low-cost and genuinely scalable model. Hence, testing its effectiveness is critical.
Methods
We conducted an open-label, cluster randomized control trial in 3 districts: Cuttack, Salepur, and Bolangir. A total of 192 villages (clusters) were stratified by district and randomized into 4 groups: control, nutritional education, and combined nutritional education with ECD interventions delivered either through individual home visits or through mother–child group sessions.
The study was implemented by Pratham Education Foundation (Pratham). The Reach-Up and Learn13 curriculum was adapted to the local context by the Ambedkar University Centre for Early Childhood Education and Development (CECED). Data were collected by 2 independent organizations, Abdul Latif Jameel Poverty Action Laboratory and Morsel Research and Development, with training and support by study investigators. Measurements new to India were piloted and adapted to the context. Data were collected at baseline and 12 and 24 months later.
The study protocol was approved by the Institute for Financial Management and Research, India (IRB00007107, FWA00014616, IORG0005894), Yale University Human Subjects Committee (1112009492), University College London Ethics Committee (2168/002), Indian Council of Medical Research (5/7/822/2012/RCH), University of Pennsylvania Office of Regulatory Affairs (815027 IRB no. 8), and the Pratham Education Foundation FWA for the Protection of Human Subjects (FWA00019832). Trial registration numbers are ISRTN:18811205 and AEA RCT Registry: 0000958.
Participants
The sampling frame consisted of 300 villages in which Pratham had recently worked. On the basis of official sources and primary data, villages with <6 expected children of target ages, where facilities were shared, or scheduled for relocation were dropped, leaving 192 study villages. Children were identified through prebaseline household censuses and were deemed eligible if they were singletons, aged 7 to 16 months by the beginning of the intervention, and had no obvious disability. This age range was chosen because pregnant women often go away for their deliveries and most return home by 7 months.
In villages with ≤8 eligible children, all were approached to participate. In villages with >8 eligible children, clusters of children who lived within 0.7 km of each other were identified, and 1 child per cluster was randomly selected by using a random number generator in Stata-13 (Stata Corp, College Station, TX). This child and the 7 nearest neighbors were selected; remaining children were placed on reserve lists. Whenever possible, selected children who were unavailable for baseline were replaced by reserve-list children.
Participants were recruited between August 31 and December 19, 2015, and written or oral consent (depending on literacy) was obtained from both household heads and primary caregivers by survey staff. Households were asked again for consent before each survey round. Informed consent to participate in the intervention was collected from all households approached at baseline.
Interventions
All interventions were conducted weekly for 24 months, beginning December 2015. Home visits lasted ∼60 minutes, group meetings lasted 90 minutes, and nutritional education visits 40 minutes. The nutritional education arm was delivered individually and was focused on improving the quality of children’s diets and basic hygienic practices in households through games, stories, and cooking demonstrations. Home visiting and group sessions were both focused on psychosocial stimulation and included some nutritional education content. Stimulation was based on the Reach-Up and Learn home visiting program,13 which has a structured home visiting curriculum, previously adapted to the local context.19Facilitators showed mothers how to play and interact with and respond to their children in ways likely to promote development. They demonstrated play activities and encouraged mothers to participate by using toys made from locally available materials and purpose-designed books. Mothers were given the play materials to use at home and then exchanged weekly. The same toys were used ∼3 times but not repeated within <5 weeks, so they remained reasonably novel. Exchanging toys limits the materials required, reducing costs.
The home visiting curriculum was adapted for groups of 7 to 8 children. All mothers and children performed the same activities at the start of sessions, such as free play, singing, review of previous week’s activities, and child-rearing discussions. The groups were then divided into 2 by children’s ages for specific age-related play activities.
Pratham recruited 141 female facilitators from local communities. Average age was 25 years, 40% had bachelor’s degrees, 55% had completed secondary schooling, and 5% had not completed secondary school. Initial training lasted 3 weeks, followed by 3 short refresher trainings spread over the intervention. Facilitators were trained and supervised weekly by 28 mentors with social science degrees and experience working with children (see Supplemental Information for intervention details).
For ethical considerations, all study participants were given a Health and Nutrition Service Link (HNSL) service (Supplemental Information), informing them of the Integrated Child Development Services relating to health and nutrition.
Outcomes
The primary outcomes were children’s development, growth, and morbidity.
At baseline, because of time constraints, children were assessed by using an adapted version of the Ages and Stages Questionnaire, third edition (ASQ-3).20 At 12 and 24 months after baseline, children’s cognitive, language, and motor development were measured with the Bayley Scales of Infant and Toddler Development, third edition (Bayley-III)21 at centers accompanied by their caregivers. Testers had tertiary education or equivalent experience working with children and were trained for 8 weeks. Interobserver reliabilities were assessed before and during the study, and intraclass correlations ranged from 0.82 to 0.99 (n = 205).
Children were measured at home in all rounds using World Health Organization (WHO) guidelines22: lengths or heights were measured with an infantometer or a stadiometer, respectively, and weights were measured with the Seca 876. Morbidity was assessed at midline and endline through mothers’ reports on occurrences of diarrhea, fever, and cough in the previous 2 weeks by using WHO definitions.23
Children’s socioemotional development was assessed at endline by maternal reports by using the Strengths and Difficulties Questionnaire.24 Test items were combined into externalizing, internalizing, and prosocial scores.
Secondary outcomes were measured at all rounds in homes. Home-environment quality was assessed by using the UNICEF Family Care Indicators (FCI),25 including a play-materials scale, recording presence of certain types of toys and books, and a play-activities scale, including caregiver involvement with children in various play activities in the last 3 days. Selected items from the Responsivity and Involvement subscales of the Infant-Toddler Home Observation for the Measurement of the Environment (IT-HOME)26 were included at endline. The Knowledge of Infant Development27 was used to assess knowledge of child-rearing practices. All instruments measuring outcomes were translated into Odiya and extensively piloted.
Sample Size
Assuming 10% attrition and intracluster correlations up to 0.3, a target sample size of 1440 children was chosen (7.5 per village or cluster). This yielded power of 80%, with minimum detectable effects of 0.19 to 0.32 SDs on primary outcomes, and a minimum detectable difference in effects of 0.32 SD between treatment arms.
A total of 1449 children were initially selected from all 192 villages, out of which 1243 were successfully interviewed at baseline. Another 158 were successfully replaced (from reserve lists), leaving a final baseline sample of 1401 children.
Randomization and Blinding
Randomization of clusters to each arm was completed before baseline by a researcher not otherwise involved with the study using a random number generator in Stata-13.
Participants and intervention staff could not be masked to treatment status. Testers and enumerators were blind to treatment status, although enumerators could have potentially inferred treatment status from toys in intervened households.
Statistical Analysis
We calculated composite scores for the Bayley-III scales. We present intention-to-treat (ITT) differences between each intervention group and the control, measured in control group SD. SEs were clustered at the village level, and two-sided P values were calculated by using t tests adjusting for multiple hypotheses testing by the Romano–Wolf stepdown procedure.28 Outcomes in the same panel of each table were tested jointly as a group. For all analyses, we controlled for covariates (baseline ASQ-3 scores, maternal education, first-born, child’s age and sex) to improve precision. Missing covariates were replaced by sample means of the same variables. For robustness, see Supplemental Table 4.
Results
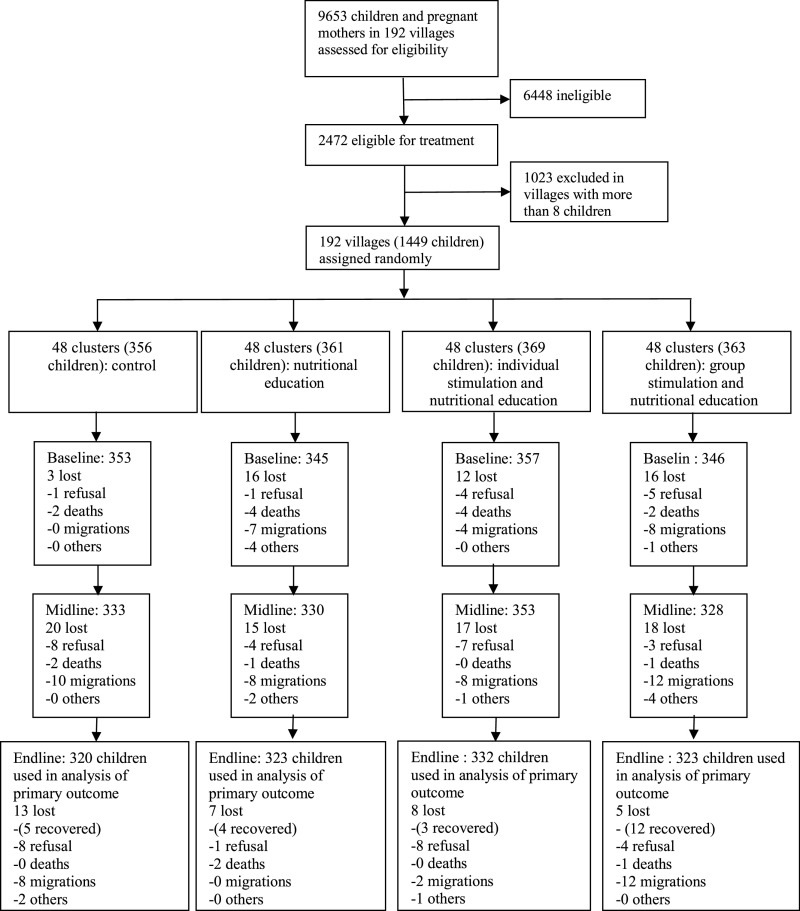
No clusters were lost during the study. A total of 1331 (92%) children were reinterviewed at midline and 1298 (90%) at endline. Analyses were conducted on children with completed endline testing with a total of 1298 for the Bayley-III and 1275 for anthropometric outcomes (Fig 1). Losses were balanced across groups.
FIGURE 1 d.
Participant flow diagram showing clusters and children from initial screening to endline analysis. Boxes display number of children included in the analysis in each wave, along with the reasons for attrition between waves. Baseline loss numbers refer to those children not replaced.
Table 1 shows baseline characteristics across all 4 groups: between 12% and 14% of children were stunted, and 58% to 64% of mothers had attained at least primary education. Access to clean water and good hygiene was limited, with 44% to 46% of households owning a toilet and 62% to 68% reporting purification of drinking water. Only the ASQ-3 communication score was significantly different across groups; we control for this in all analyses.
TABLE 1.
Sample Characteristics at Baseline
| Control | Nutritional Education | Home Visiting | Group Sessions | |
|---|---|---|---|---|
| Child characteristics | ||||
| Male, n (%) | 167 (52) | 171 (54) | 166 (52) | 145 (44) |
| Age, mo, mean (SD) | 12 (2.70) | 12 (2.68) | 12 (2.61) | 12 (2.69) |
| HAZ, mean (SD) | −0.69 (1.27) | −0.67 (1.20) | −0.71 (1.25) | −0.59 (1.19) |
| Stunted at baseline (HAZ <−2), n (%) | 39 (12) | 36 (12) | 45 (14) | 48 (14) |
| No. siblings, mean (SD) | 0.70 (0.82) | 0.73 (0.84) | 0.72 (0.85) | 0.69 (1.04) |
| Mother is primary caregiver, n (%) | 295 (92) | 289 (90) | 297 (92) | 301 (90) |
| Primary caregiver has at least primary education, n (%) | 204 (64) | 207 (64) | 202 (62) | 195 (58) |
| ASQ-3 scores, mean (SD) | ||||
| Communication | 82.33 (21.07) | 86.23 (19.29) | 84.78 (18.19) | 88.34 (19.32) |
| Gross motor | 85.84 (26.77) | 88.33 (25.12) | 88.34 (24.32) | 88.28 (26.44) |
| Fine motor | 93.14 (18.95) | 95.09 (17.80) | 95.29 (16.46) | 95.50 (16.90) |
| Problem solving | 92.88 (21.16) | 96.16 (21.58) | 96.33 (19.72) | 95.21 (18.94) |
| Personal social | 77.42 (21.67) | 80.54 (20.23) | 80.54 (20.39) | 80.78 (19.58) |
| Household characteristics | ||||
| Primary caregiver Raven’s raw score, mean (SD) | 11.88 (5.28) | 12.48 (5.63) | 12.12 (4.88) | 11.71 (5.17) |
| Has access to electricity, n (%) | 293 (92) | 289 (90) | 284 (88) | 304 (92) |
| Owns a toilet, n (%) | 146 (46) | 146 (46) | 145 (44) | 154 (46) |
| Household purifies drinking water, n (%) | 201 (64) | 219 (68) | 201 (62) | 227 (68) |
| Home environment and knowledge of child development scores, mean (SD) | ||||
| FCI play materials (0–7) | 2.70 (1.61) | 2.84 (1.64) | 2.9 (1.56) | 2.58 (1.51) |
| FCI play activities (0–7) | 2.53 (1.38) | 2.78 (1.35) | 2.74 (1.52) | 2.56 (1.37) |
| Knowledge index (18–54) | 38.38 (4.84) | 38.70 (5.20) | 38.67 (4.39) | 39.05 (4.72) |
HAZ, height-for-age z score.
During the trial, of caregivers interviewed at baseline, 11.2% in the nutritional education arm, 14.1% in the home visiting arm, and 25.5% in the group sessions arm (group sessions versus home visiting P < .05) stopped participating. However, data collection and measurement continued to include them. The average number of sessions attended varied by arm. Of children in the endline analysis sample, attendance rates were 84% in nutritional education, 75% in home visiting, and 51% in the group sessions arm (group sessions versus home visiting P < .05). The most common reason for missing sessions was temporary migration for the home visiting and nutritional education arms and mothers being too busy for the group sessions arm.
The Bayley-III scores were generally lower than the test reference population, with smaller SDs (Supplemental Table 4). Cronbach alphas were ≥0.8 for all subscales, and discriminant validity and stability over time were adequate (Supplemental Information).
Over the first year, the home visiting group improved significantly in cognition but not language, whereas the group sessions improved significantly in both cognition and language (Table 2). Over 2 years, home visiting had 0.324 SD impact on cognition (95% confidence interval [CI]: 0.152 to 0.496; stepdown P = .001) and a 0.239 SD on language (95% CI: 0.072 to 0.407; stepdown P = .009). Group sessions had a 0.281 SD impact on cognition (95% CI: 0.100 to 0.463; stepdown P = .007) and a 0.302 SD on language (95% CI: 0.136 to 0.468; stepdown P = .001). Differences in average impacts between home visiting and group sessions over 2 years were not significant. For both cognition and language, there was no significant difference between the impact at midline and at endline for either home visiting or group sessions. Neither treatment arm affected motor skills.
TABLE 2.
Treatment Effect on Bayley-III Composite Score and the Strengths and Difficulties Questionnaire
| Point Estimate | CI: Lower Bound | CI: Upper Bound | P (Unadjusted) | P (Adjusted) | n | |
|---|---|---|---|---|---|---|
| Panel 1: Bayley-III composite scores baseline to midline | ||||||
| Nutritional education | ||||||
| Cognitive | −0.144 | −0.320 | 0.033 | .082 | .359 | 1270 |
| Language | 0.037 | −0.144 | 0.217 | .613 | .956 | 1273 |
| Motor | −0.036 | −0.223 | 0.150 | .683 | .956 | 1269 |
| Group sessions and nutritional education | ||||||
| Cognitive | 0.298 | 0.104 | 0.493 | .004 | .018 | 1270 |
| Language | 0.313 | 0.115 | 0.510 | .004 | .006 | 1273 |
| Motor | 0.011 | −0.210 | 0.232 | .900 | .956 | 1269 |
| Home visiting and nutritional education | ||||||
| Cognitive | 0.313 | 0.132 | 0.494 | .002 | .006 | 1270 |
| Language | 0.156 | −0.053 | 0.365 | .090 | .359 | 1273 |
| Motor | −0.058 | −0.262 | 0.146 | .611 | .956 | 1269 |
| Panel 2: Bayley-III composite scores baseline to endline | ||||||
| Nutritional education | ||||||
| Cognitive | 0.037 | −0.145 | 0.220 | .673 | .709 | 1298 |
| Language | 0.127 | −0.038 | 0.292 | .103 | .342 | 1298 |
| Motor | 0.075 | −0.087 | 0.238 | .354 | .657 | 1298 |
| Group sessions and nutritional education | ||||||
| Cognitive | 0.281 | 0.100 | 0.463 | .002 | .007 | 1298 |
| Language | 0.302 | 0.136 | 0.468 | <.001 | .001 | 1298 |
| Motor | 0.144 | −0.036 | 0.324 | .110 | .342 | 1298 |
| Home visiting and nutritional education | ||||||
| Cognitive | 0.324 | 0.152 | 0.496 | <.001 | .001 | 1298 |
| Language | 0.239 | 0.072 | 0.407 | .001 | .009 | 1298 |
| Motor | 0.055 | −0.108 | 0.218 | .475 | .709 | 1298 |
| Panel 3: Strengths and Difficulties Questionnaire | ||||||
| Nutritional education | ||||||
| Externalizing | 0.010 | −0.157 | 0.177 | .900 | .991 | 1297 |
| Internalizing | −0.058 | −0.209 | 0.094 | .438 | .949 | 1297 |
| Prosocial | 0.073 | −0.082 | 0.229 | .328 | .903 | 1297 |
| Group sessions and nutritional education | ||||||
| Externalizing | −0.039 | −0.211 | 0.134 | .651 | .981 | 1297 |
| Internalizing | −0.008 | −0.151 | 0.134 | .908 | .991 | 1297 |
| Prosocial | 0.179 | 0.021 | 0.336 | .018 | .126 | 1297 |
| Home visiting and nutritional education | ||||||
| Externalizing | 0.043 | −0.117 | 0.204 | .583 | .981 | 1297 |
| Internalizing | −0.025 | −0.172 | 0.122 | .750 | .981 | 1297 |
| Prosocial | 0.215 | 0.057 | 0.373 | .008 | .054 | 1297 |
Estimated coefficients are expressed in SDs of the control group. Sample size includes all target children who completed the relevant subscales of the Bayley-III at endline and midline for panel 1, and at endline for panel 2, and all mothers who completed the Strengths and Difficulties Questionnaire (at endline) in panel 3. Estimates in all panels controlled for baseline ASQ-3 problem solving, communication, fine motor, gross motor, and personal-social scales, as well as child sex, child age in days, maternal education, and parity with a dummy for first child. Estimated coefficients are expressed in SDs of the control group. The P values are two-tailed P values calculated using the T-bootstrap method (5000 replications) to account for the finite sample and for maximum comparability with the adjusted P values. Adjusted P values are two-tailed P values corrected for multiple hypotheses testing by using the Romano–Wolf stepdown procedure (5000 replications). CIs were constructed by using conventional critical values for individual hypotheses. The multiple hypotheses testing was applied within panels. All replications were done within district used in the randomization protocol and corrected for cluster effects at the village level.
The nutritional education arm had no significant impact on any of the Bayley-III outcomes. There were no significant differences among study arms in weight-for-age or height-for-age z scores (Supplemental Table 6). For morbidity (Supplemental Table 7), the nutritional education arm had lower fever prevalence at 12 months (95% CI: −0.190 to −0.045; stepdown P = .008), and group sessions had a significant reduction in fever at 24 months (95% CI: −0.168 to −0.027; stepdown P = .031). There were no other significant treatment effects. These results are relative to mean reported occurrences of 2.3% diarrhea, 46.7% coughing, and 31.4% fever over 2 weeks in the control group at endline.
In Table 2 panel 3, we report no impacts on Strengths and Difficulties Questionnaire internalizing and externalizing problems. The home visiting arm had a marginally significant 0.215 SD impact on prosocial skills (95% CI: 0.057 to 0.373; stepdown P = .054).
There was no significant effect on knowledge of child development in any of the treatments once adjustment for multiple testing was done (Table 3). The FCI play activities significantly increased in both home visiting and group sessions (home visiting: 0.383 SD; 95% CI: 0.242 to 0.524; stepdown P < .001, group sessions: 0.331 SD; 95% CI: 0.195 to 0.468; stepdown P < .001). Impacts were not significant on FCI play materials once intervention materials were excluded; the group sessions arm had a 0.221 SD effect on the IT-HOME Involvement scale (95% CI: 0.071 to 0.371; stepdown P = .034). Neither home visiting nor group sessions had impacts on the IT-HOME Responsivity scale once P values were adjusted for multiple testing.
TABLE 3.
Treatment Effects on FCI, IT-HOME, and Maternal Knowledge on Child Development
| Point Estimate | CI: Lower Bound | CI: Upper Bound | P (Unadjusted) | P (Adjusted) | n | |
|---|---|---|---|---|---|---|
| Nutritional education | ||||||
| FCI variety materials | 0.130 | −0.009 | 0.270 | .045 | .249 | 1298 |
| FCI variety activities | 0.301 | 0.162 | 0.440 | <.001 | <.001 | 1298 |
| IT-HOME responsivity | 0.129 | 0.002 | 0.256 | .037 | .228 | 1298 |
| IT-HOME involvement | 0.132 | −0.029 | 0.293 | .100 | .437 | 1298 |
| Maternal knowledge on child development | −0.005 | −0.082 | 0.072 | .862 | .862 | 1298 |
| Group sessions and nutritional education | ||||||
| FCI variety materials | 0.066 | −0.078 | 0.210 | .324 | .668 | 1298 |
| FCI variety activities | 0.331 | 0.195 | 0.468 | <.001 | <.001 | 1298 |
| IT-HOME responsivity | 0.144 | 0.028 | 0.260 | .014 | .109 | 1298 |
| IT-HOME involvement | 0.221 | 0.071 | 0.371 | .003 | .034 | 1298 |
| Maternal knowledge on child development | 0.035 | −0.044 | 0.114 | .254 | .648 | 1298 |
| Home visiting and nutritional education | ||||||
| FCI variety materials | 0.055 | −0.088 | 0.198 | .427 | .668 | 1298 |
| FCI variety activities | 0.383 | 0.242 | 0.524 | <.001 | <.001 | 1298 |
| IT-HOME responsivity | 0.145 | 0.037 | 0.254 | .006 | .064 | 1298 |
| IT-HOME involvement | 0.099 | −0.058 | 0.257 | .199 | .620 | 1298 |
| Maternal knowledge on child development | 0.075 | −0.003 | 0.152 | .013 | .109 | 1298 |
Estimated coefficients are expressed in SDs of the control group. Estimates in all panels controlled for baseline ASQ-3 problem solving, communication, fine motor, gross motor, and personal-social scales, as well as child sex, age in days, maternal education, and parity with a dummy for first child. Estimated coefficients are expressed in SDs of the control group. The P values are two-tailed P values calculated by using the T-bootstrap method (5000 replications) to account for the finite sample and for maximum comparability with the adjusted P values. Adjusted P values are two-tailed P values corrected for multiple hypotheses testing by using the Romano–Wolf stepdown procedure (5000 replications) for all outcomes together. CIs were constructed by using conventional critical values for individual hypotheses. All replications are done within strata defined by the preprogram variables used in the randomization protocol and corrected for cluster effects at the village level.
Allowing for all overhead, training, materials, and personnel salaries, home visiting cost $135 per child per year assuming each home visitor performs 15 visits weekly. Group sessions cost $38 per child per year assuming each facilitator runs 8 groups per week with 8 children per group (see Supplemental Information for details).
Discussion
Delivering the same ECD intervention via home visiting or group sessions had similar ITT impacts on children’s cognitive and language development. This is the first study to our knowledge comparing different ECD intervention delivery systems with a randomized design. Group sessions cost only 28% of home visiting in this context, with equivalent average effectiveness. At $38 per child per year, the group sessions model is genuinely scalable. Pratham is established nationally with capacity to select local women, train them, monitor fidelity, and run the program.
When designing Reach-Up and Learn, it was assumed that home visiting would facilitate teaching by targeting individual children’s proximal zone of development, help integrate play into everyday activities, and assist home visitors’ relationship with mothers.13 It is striking that, despite lower compliance for group sessions (attendance was 51% compared with 75% for home visiting), we observed similar ITT. We hypothesize that mothers and children in groups learned skills through observing others and isolated mothers received support by interacting with them. We also hypothesize that group sessions aided cultural acceptance of the promoted child stimulation and rearing practices. It remains possible that the type of intervention delivery may affect the sustainability of benefits, and longer-term follow-up is essential to answer this question.
The group model should be replicable in other poor rural Indian contexts and probably in other Asian countries with similar cultural contexts, where groups are acceptable, making it relevant for large populations. For example, in a recent Bangladeshi study, a similar ECD intervention16 was more effective when delivered to pairs of mothers and children than found in previous Bangladeshi studies when delivered individually; however, no direct comparison within the same experimental setup was attempted, in contrast to this study.
A disadvantage of group sessions compared with home visiting was that more mothers refused to enroll, dropped out, or attended poorly. There was concern that children who attended less were more disadvantaged, but their initial height-for-age, ASQ-3 scores, and home backgrounds were not worse. Implementation research10 is necessary at both community and program levels to determine how to increase attendance. The greatest dropout of group children (11.8%) occurred in the first 4 weeks; we need to improve outreach and understand the causes of lower attendance and adapt the program delivery appropriately.
Two years is longer than many interventions, and nearly all developmental improvements took place in the first year. In the second year, advantages were maintained, although group participation declined. The limited available data13,18,29 indicate that smaller improvements usually occur in the second year. This suggests a model with less intensive intervention in the second year or limiting it to one year. However, the effects of duration on sustainability of benefits need to be examined first.
The nutritional education intervention, worryingly, had no impact on growth. Stunting often increases over this age range in deprived contexts,30 and interventions may need to begin earlier to prevent it. It is estimated that if all known effective nutritional interventions were implemented together, only 20% of stunting would be reduced.31 Successful programs reducing stunting in early childhood have usually taken multisector approaches.32 Household food security was low in this population (14.6% of households reported at least 1 household member skipping meals in the past week), and nutritional supplementation may have been required. A further problem was poor sanitation, with less than half of households owning toilets, adding to risks of diarrhea or enteropathy.
The study strengths are its rigorous design, use of well-established developmental tests with midpoint evaluations, and use of a feasible delivery strategy, conducted by a national nongovernmental organization with readily available staff.
Study limitations are that morbidity occurrence was measured only on 2 occasions, so small effects may have been missed. The Bayley-III was not standardized for India; however, it appears valid in this population with acceptable levels of internal reliability, discriminant validity, and stability over time. Our minimum detectable effect between treatment arms (0.32 SD) was too large to detect differences between treatments; however, differences in impacts were minimal and all <0.06 SD. Despite the large size of our study, involving 1400 children, challenges not testable here may arise at scale.
In conclusion, nutritional education alone is unlikely to solve the problem of malnutrition in the context of food insecurity and poor hygiene, and more comprehensive programs may be required. The finding that a much cheaper ECD intervention delivered to groups of mother-child dyads had similar average impacts as individual home visiting has crucially important implications and should facilitate increasing coverage with limited resources in rural India and, if replicated elsewhere, in other countries.
Glossary
- ASQ-3
Ages and Stages Questionnaire, third edition
- Bayley-III
Bayley Scales of Infant and Toddler Development, third edition
- CECED
Centre for Early Childhood Education and Development
- CI
confidence interval
- ECD
Early Child Development
- FCI
Family Care Indicators
- HNSL
Health and Nutrition Service Link
- IT-HOME
Infant-Toddler Home Observation for the Measurement of the Environment
- ITT
intention-to-treat
- WHO
World Health Organization
Footnotes
Dr Grantham-McGregor conceptualized and designed the study, designed the initial curriculum and adapted it to this study’s setting, contributed to the design of the data collection instruments and to the interpretation of the data analysis, and drafted the initial manuscript; Ms Adya, Ms Day, Ms Kochar, Ms Makkar supported the adaptation of the curriculum and intervention to the setting, trained and supervised staff for implementation and data collection, and critically reviewed the manuscript for important intellectual content; Mr Attanasio, Ms Augsburg, Mr Behrman, Mr Meghir conceptualized and designed the study, contributed to the design and adaptation of the curriculum and the design of the data collection instruments, conducted analysis and interpretation of the data, and contributed to the drafting of the manuscript; Ms Caeyers contributed to the design of the data collection instruments and interpretation of the data analysis and critically reviewed the manuscript; Ms Jervis supported the adaptation of the curriculum and intervention to the setting, trained and mentored implementation staff, contributed to the design of the data collection instruments, conducted analysis and interpretation of data, and drafted the initial manuscript; Mr Phimister contributed to the design of the data collection instruments, supported training of data collection staff, conducted analysis and interpretation of data, and drafted the initial manuscript; Ms Rubio-Codina conceptualized and designed the study, supported the adaptation of the curriculum and intervention to the setting, trained and mentored implementation staff, contributed to the design of the data collection instruments, conducted analysis and interpretation of data, and critically reviewed the manuscript for important intellectual content; Ms Vats supported the adaptation of the intervention, trained and managed implementation staff, and critically reviewed the manuscript; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
This trial has been registered with the ISRTN Register (http://isrtn.org) (identifier 18811205) and the AER RCT Registry (0000958).
Deidentified individual participant data (including data dictionaries) will be made available, in addition to study protocols and the informed consent form. The data will be made available through the World Bank Open Data Depository within a year after publication.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Funded and supported by the National Institutes of Health under grant R01 HD 72120; the Strategic Intervention Evaluation Fund, World Bank; the Economic and Social Research Council; the Cowles Foundation at Yale University; the Population Studies Center at the University of Pennsylvania; and the Center for Research in Inclusive Education, Chile under grant PIA ANID 160009. The study funders reviewed and approved the preliminary study design, but had no further role in study design, data collection, data analysis, data interpretation or the writing of this article. Any opinions, findings, and recommendations are the authors and do not necessarily reflect the views of any of the institutions they represent, including those of the IDB, its Board of Directors or the countries they represent. All authors declare no competing interests. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Lu C, Black MM, Richter LM. Risk of poor development in young children in low-income and middle-income countries: an estimation and analysis at the global, regional, and country level. Lancet Glob Health. 2016;4(12):e916–e922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson SB, Riis JL, Noble KG. State of the art review: poverty and the developing brain. Pediatrics. 2016;137(4):e20153075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Black MM, Walker SP, Fernald LCH, et al.; Lancet Early Childhood Development Series Steering Committee . Early childhood development coming of age: science through the life course. Lancet. 2017;389(10064):77–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Britto PR, Lye SJ, Proulx K, et al.; Early Childhood Development Interventions Review Group, for the Lancet Early Childhood Development Series Steering Committee . Nurturing care: promoting early childhood development. Lancet. 2017;389(10064):91–102 [DOI] [PubMed] [Google Scholar]
- 5.Aboud FE, Yousafzai AK. Global health and development in early childhood. Annu Rev Psychol. 2015;66:433–457 [DOI] [PubMed] [Google Scholar]
- 6.Walker SP, Chang SM, Vera-Hernández M, Grantham-McGregor S. Early childhood stimulation benefits adult competence and reduces violent behavior. Pediatrics. 2011;127(5):849–857 [DOI] [PubMed] [Google Scholar]
- 7.Tanner JC, Candland T, Odden WS. Later Impacts of Early Childhood Interventions: A Systematic Review. Washington, DC: Independent Evaluation Group, World Bank Group; 2015. Available at: https://ieg.worldbankgroup.org/sites/default/files/Data/reports/chapters/ecd-later-outcomes_overview-introduction.pdf. Accessed June 10, 2020 [Google Scholar]
- 8.World Health Organization Nurturing care for early childhood development: a framework for helping children survive and thrive to transform health and human potential. Available at: https://apps.who.int/iris/bitstream/handle/10665/272603/9789241514064-eng.pdf?ua=1. Accessed May 1, 2020
- 9.Richter LM, Daelmans B, Lombardi J, et al.; Paper 3 Working Group and the Lancet Early Childhood Development Series Steering Committee . Investing in the foundation of sustainable development: pathways to scale up for early childhood development. Lancet. 2017;389(10064):103–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tomlinson M, Hunt X, Rotheram-Borus MJ. Diffusing and scaling evidence-based interventions: eight lessons for early child development from the implementation of perinatal home visiting in South Africa. Ann N Y Acad Sci. 2018;1419(1):218–229 [DOI] [PubMed] [Google Scholar]
- 11.Duncan GJ, Magnuson K. Investing in preschool programs. J Econ Perspect. 2013;27(2):109–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vazir S, Engle P, Balakrishna N, et al. . Cluster-randomized trial on complementary and responsive feeding education to caregivers found improved dietary intake, growth and development among rural Indian toddlers. Matern Child Nutr. 2013;9(1):99–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grantham-McGregor S, Smith JA. Extending the Jamaican early childhood development intervention. J Appl Res Child. 2016;7(2):4 [Google Scholar]
- 14.Fernald LC, Kagawa RM, Knauer HA, Schnaas L, Guerra AG, Neufeld LM. Promoting child development through group-based parent support within a cash transfer program: experimental effects on children’s outcomes. Dev Psychol. 2017;53(2):222–236 [DOI] [PubMed] [Google Scholar]
- 15.Singla DR, Kumbakumba E, Aboud FE. Effects of a parenting intervention to address maternal psychological wellbeing and child development and growth in rural Uganda: a community-based, cluster randomised trial. Lancet Glob Health. 2015;3(8):e458–e469 [DOI] [PubMed] [Google Scholar]
- 16.Hamadani JD, Mehrin SF, Tofail F, et al. . Integrating an early childhood development programme into Bangladeshi primary health-care services: an open-label, cluster-randomised controlled trial. Lancet Glob Health. 2019;7(3):e366–e375 [DOI] [PubMed] [Google Scholar]
- 17.Chang SM, Grantham-McGregor SM, Powell CA, et al. . Integrating a parenting intervention with routine primary health care: a cluster randomized trial. Pediatrics. 2015;136(2):272–280 [DOI] [PubMed] [Google Scholar]
- 18.Yousafzai AK, Rasheed MA, Rizvi A, Armstrong R, Bhutta ZA. Effect of integrated responsive stimulation and nutrition interventions in the Lady Health Worker programme in Pakistan on child development, growth, and health outcomes: a cluster-randomised factorial effectiveness trial. Lancet. 2014;384(9950):1282–1293 [DOI] [PubMed] [Google Scholar]
- 19.Andrew A, Attanasio O, Augsburg B, et al. . Effects of a scalable home-visiting intervention on child development in slums of urban India: evidence from a randomised controlled trial. J Child Psychol Psychiatry. 2020;61(6):644–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bricker D, Squires J, Mounts L, et al. . Ages and Stages Questionnaire. Baltimore: Paul H. Brookes; 1999 [Google Scholar]
- 21.Bayley N. Bayley Scales of Infant and Toddler Development, 3rd ed. San Antonio: Psych Corp, Pearson; 2006 [Google Scholar]
- 22.World Health Organization WHO child growth standards. 2006. Available at: https://www.who.int/childgrowth/standards/en/. Accessed October 13, 2019
- 23.Levine GA, Walson JL, Atlas HE, Lamberti LM, Pavlinac PB. Defining pediatric diarrhea in low-resource settings. J Pediatric Infect Dis Soc. 2017;6(3):289–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goodman R. The Strengths and Difficulties Questionnaire: a research note. J Child Psychol Psychiatry. 1997;38(5):581–586 [DOI] [PubMed] [Google Scholar]
- 25.Kariger P, Frongillo EA, Engle P, Britto PM, Sywulka SM, Menon P. Indicators of family care for development for use in multicountry surveys. J Health Popul Nutr. 2012;30(4):472–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caldwell BM, Bradley RH. Home Observation for Measurement of the Environment: Administration Manual. Tempe, AZ: Family & Human Dynamics Research Institute, Arizona State University; 2016 [Google Scholar]
- 27.MacPhee D. Knowledge of Infant Development Inventory. Princeton, NJ: Educational Testing Service; 1981 [Google Scholar]
- 28.Romano JP, Wolf M. Exact and approximate stepdown methods for multiple hypothesis testing. J Am Stat Assoc. 2005;100:94–108 [Google Scholar]
- 29.McKay H, Sinisterra L, McKay A, Gomez H, Lloreda P. Improving cognitive ability in chronically deprived children. Science. 1978;200(4339):270–278 [DOI] [PubMed] [Google Scholar]
- 30.Victora CG, de Onis M, Hallal PC, Blössner M, Shrimpton R. Worldwide timing of growth faltering: revisiting implications for interventions. Pediatrics. 2010;125(3). Available at: www.pediatrics.org/cgi/content/full/125/3/e473 [DOI] [PubMed] [Google Scholar]
- 31.Bhutta ZA, Das JK, Rizvi A, et al.; Lancet Nutrition Interventions Review Group, the Maternal and Child Nutrition Study Group . Evidence-based interventions for improvement of maternal and child nutrition: what can be done and at what cost? Lancet. 2013;382(9890):452–477 [DOI] [PubMed] [Google Scholar]
- 32.Hossain M, Choudhury N, Adib Binte Abdullah K, et al. . Evidence-based approaches to childhood stunting in low and middle income countries: a systematic review. Arch Dis Child. 2017;102(10):903–909 [DOI] [PMC free article] [PubMed] [Google Scholar]