Newborns and infants of women with disabilities may be at greater risk for adverse health outcomes than those of women without disabilities.
Abstract
Video Abstract
CONTEXT:
Women with disabilities are at elevated risk for pregnancy, delivery, and postpartum complications. However, there has not been a synthesis of literature on the neonatal and infant health outcomes of their offspring.
OBJECTIVE:
We examined the association between maternal disability and risk for adverse neonatal and infant health outcomes.
DATA SOURCES:
Cumulative Index to Nursing and Allied Health Literature, Embase, Medline, and PsycINFO were searched from database inception to January 2020.
STUDY SELECTION:
Studies were included if they reported original data on the association between maternal physical, sensory, or intellectual and/or developmental disabilities and neonatal or infant health outcomes; had a referent group of women with no disabilities; were peer-reviewed journal articles or theses; and were written in English.
DATA EXTRACTION:
We used standardized instruments to extract data and assess study quality. DerSimonian and Laird random effects models were used for pooled analyses.
RESULTS:
Thirty-one studies, representing 20 distinct cohorts, met our inclusion criteria. Meta-analyses revealed that newborns of women with physical, sensory, and intellectual and/or developmental disabilities were at elevated risk for low birth weight and preterm birth, with smaller numbers of studies revealing elevated risk for other adverse neonatal and infant outcomes.
LIMITATIONS:
Most studies had moderate (n = 9) or weak quality (n = 17), with lack of control for confounding a common limitation.
CONCLUSIONS:
In future work, researchers should explore the roles of tailored preconception and perinatal care, along with family-centered pediatric care particularly in the newborn period, in mitigating adverse outcomes among offspring of women with disabilities.
The World Health Organization defines disability as an umbrella term for impairments, activity limitations, and participation restrictions reflecting the interaction between features of an individual’s body and the society or environment in which the individual lives.1 Disabilities can be categorized broadly as physical, affecting mobility, flexibility, or dexterity; sensory, affecting hearing or vision; and intellectual or developmental, affecting cognitive functioning or adaptive behavior such as conceptual, social, and practical skills.2 Because of stigma associated with disability and sexuality and concerns about medication use in pregnancy, childbearing among women with disabilities was relatively uncommon historically.3,4 However, in the last 20 years, pregnancy rates have increased in this population in the United States and elsewhere,5,6 with pregnancy rates among women with physical and sensory disabilities now similar to those without disabilities.4–7 In the United States, it is estimated that there are between 4 and 9 million parents with disabilities.8
Women of reproductive age with disabilities experience significant socioeconomic, health, and health care disparities; they are more likely than those without disabilities to live in poverty, experience violence, have chronic health conditions and mental illness,9–13 and encounter barriers accessing and navigating preventive and prenatal care.10,14–16 There is a growing body of literature indicating they are at increased risk for pregnancy, delivery, and postpartum complications, including gestational hypertension, cesarean delivery, postpartum hospitalization,17 and postpartum depression.18 Given the link between poor preconception and perinatal health and offspring complications,19,20 it is possible then that newborns and infants of women with disabilities may be at elevated risk for adverse health outcomes. However, to our knowledge, there has not been a synthesis of the literature on this topic. The neonatal period and infancy are critical periods for determining long-term health and developmental trajectories.21,22 A better understanding of the health outcomes of newborns and infants of women with disabilities would inform the development of tailored services to support their needs.
Our objective of this systematic review and meta-analysis was to examine the health outcomes of newborns and infants born to women with physical, sensory, and intellectual and/or developmental disabilities compared with those born to women without these disabilities.
Methods
Search Strategy
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines23 to identify studies published from database inception to January 2, 2020, in the Cumulative Index to Nursing and Allied Health Literature, Embase, Medline, and PsycINFO databases. We used an adapted version of a validated search strategy for disability,24 which includes keywords and subject headings for disability generally (eg, “activity limitation”) and physical, sensory, and intellectual and/or developmental disabilities specifically. In consultation with a research librarian, we added keywords and subject headings for indicators of neonatal health (eg, “preterm birth”) and health care use (eg, “neonatal intensive care unit”) and infant health (eg, “infant mortality”) and health care use (eg, “emergency department visit”) (Supplemental Table 1). To identify studies that may have been missed in database searches, we hand searched reference lists of original articles selected for full-text review and review articles.
Selection Criteria
To be included in the review, studies were required to (1) report original data on the association between maternal physical (eg, arthritis, spinal cord injury), sensory (eg, hearing loss), or intellectual and/or developmental disabilities (eg, autism spectrum disorder, Down syndrome) and neonatal or infant health; (2) use an observational design with a referent group of women with no disabilities (eg, cohort, case-control, or cross-sectional studies); (3) be peer-reviewed journal articles or theses; and (4) be written in English. Studies in which authors (1) examined conditions that were not clearly disabilities (eg, diabetes without evidence of functional limitations) or (2) reported on the health of newborns or infants of women with only psychiatric disorders were excluded. Although women with psychiatric disorders experience many of the same risk factors for adverse outcomes as women with other disabilities (eg, poverty), we did not include this group because there is a sizeable body of literature on the health outcomes of their newborns and infants,25–27 and the focus on physical, sensory, and intellectual and/or developmental disabilities in our review aligns with the National Institutes of Health’s call for research in this area.28 (Studies in which authors included psychiatric disorders within their definition of “any” disability were included, however, and the impact of this decision on the results was tested in additional analyses.) All eligible studies were included in the qualitative synthesis.
Data Extraction
Three authors (L.A.T., F.M., and D.S.) independently screened titles and abstracts. After agreement, the same authors then independently retrieved and reviewed relevant full-text articles in detail. They independently extracted data from studies that met the inclusion criteria using a standardized data extraction form created a priori on the basis of the Strengthening the Reporting of Observational Studies in Epidemiology statement.29 The following data were extracted: study period and location, study design and data sources, sample size, exclusion criteria, disability definition and measurement, outcome definition(s) and measurement, and confounders. To calculate pooled effect estimates, frequencies and percentages as well as odds ratios (ORs) and 95% confidence intervals (CIs) were extracted. Discrepancies in data extraction were resolved through discussion with the senior author (H.K.B.).
Quality Assessment
Three authors (L.A.T., F.M., and D.S.) independently assessed the quality of each study using an adapted version of the Effective Public Health Practice Project Quality Assessment tool, a validated30,31 and widely used tool in health research.17,22 Studies were rated as weak, moderate, or strong overall on the basis of the following criteria: (1) study design, (2) selection bias (sample representativeness, response rate), (3) confounding (percentage of confounders controlled for), (4) detection bias (reliability and validity of the data sources), and (5) attrition bias (loss to follow-up, missing data) (Supplemental Table 2). Informed by existing literature regarding risks for adverse neonatal and infant outcomes,32–35 we identified the following confounders a priori: demographics (eg, maternal age, race and ethnicity), socioeconomic status (eg, education, employment), comorbidities (eg, chronic health conditions, mental illness), lifestyle behaviors (eg, smoking), and social support (eg, marital status). Discrepancies in ratings were resolved through discussion with the senior author (H.K.B.).
Data Synthesis
DerSimonian and Laird36 random effects models were used to calculate pooled ORs and 95% CIs for outcomes examined by at least 3 studies. When there were multiple studies published by using the same data sources with overlapping samples and study periods, we included the study with the largest cohort in the meta-analysis. Analyses were conducted for any disability (as defined by the study authors) and by disability type, as numbers allowed. The source of variance was examined across studies by calculating the Q statistic (ie, the weighted sum of squared differences between the effects of individual studies and the pooled effect across studies) and I2 statistic (ie, the percent of variation across studies that results from real heterogeneity, not chance).37 Although heterogeneity is to be expected,38 to address potential bias introduced by significant heterogeneity, we (1) conservatively excluded meta-analyses with I2 values ≥75% (corresponding to the Cochrane Handbook for Systematic Reviews of Interventions classification of “considerable” heterogeneity)39 and (2) calculated 95% prediction intervals for the remaining meta-analyses to show the range of true effects in similar studies (for analyses with at least 5 studies).40,41 In sensitivity analyses, fixed effects models were used to re-estimate pooled ORs for studies with a nonsignificant Q statistic and small I2 value, assuming the true effect was the same for all studies.37 The effect of removing individual studies from the meta-analysis was also tested to identify influential studies. Although we planned to generate a funnel plot to test for publication bias, we had an insufficient number of studies for any given analysis to do so.42 Two additional analyses were undertaken to test the effect of analysis decisions on results. First, we tested the impact of substituting alternative studies into the meta-analysis from among those not included, having used the same data sources. Second, we tested the impact of excluding studies that included psychiatric disorders in their definition of any disability. For our analyses, we used Comprehensive Meta-Analysis version 3.0 (Biostats Inc, Englewood, NJ), and we generated figures using R version 3.6.0.
Results
Study Selection
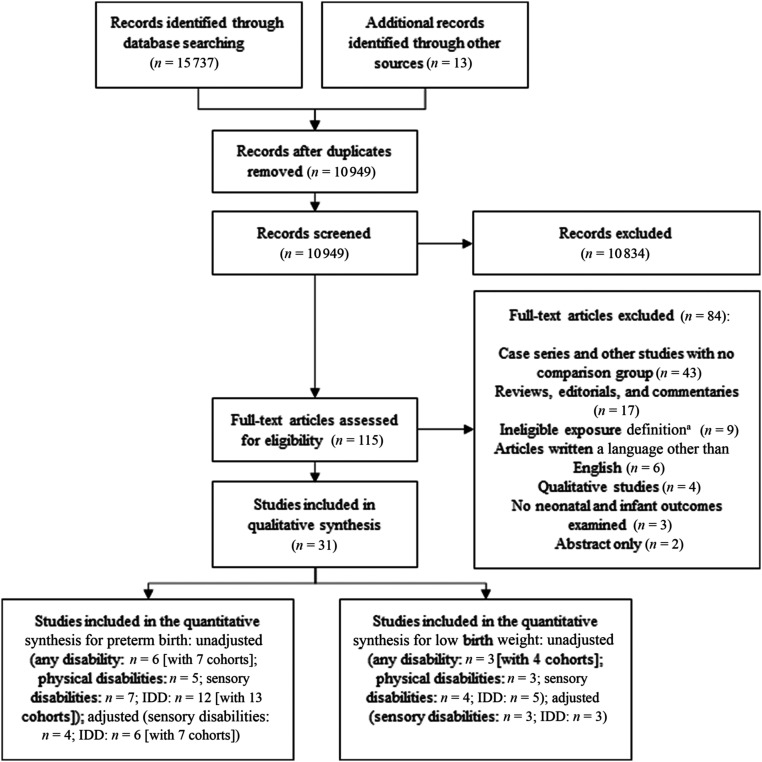
The article selection process is outlined in Fig 1. Database searches returned 15 737 articles, and 13 articles were identified from other sources. After removal of duplicates and title and abstract screening, we identified 115 articles to review in detail. After full-text review, 84 studies were removed because they had no comparison group (n = 43); were reviews, editorials, or commentaries (n = 17); had ineligible exposure definitions (n = 9); were not written in English (n = 6); were qualitative studies (n = 4); did not examine neonatal or infant outcomes (n = 3); or were abstracts (n = 2). Thirty-one studies,6,43–72 representing >13 million unique women, including 30 139 with any disability, 10 198 with physical disabilities, 4436 with sensory disabilities, and 9535 with intellectual and/or developmental disabilities in 20 distinct cohorts (including 1 US study in which authors examined 2 states separately50 and 1 Australian study in which authors examined Aboriginal and non-Aboriginal women separately49), met our criteria. In 20 studies, authors used the same data sources and study periods (representing 7 distinct cohorts): 5 used the US (MA) Pregnancy to Early Life Longitudinal database,46,47,59,61,62 3 used the US Nationwide Inpatient Sample of the Healthcare Cost and Utilization Project,43,60,67 3 used California administrative data,6,48,55 2 used the Pregnancy Risk Assessment Monitoring System for Rhode Island,65,68 2 used Washington State administrative data,64,70 3 used Swedish administrative data,53,54,72 and 2 used the UK Millennium Cohort Study.52,71
FIGURE 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses diagram for study selection. a “Ineligible exposure definition” included studies examining chronic conditions without evidence of functional limitations, studies that examined individual disabilities (eg, lupus), studies in which disability was not identified before pregnancy, and studies in which disability was not defined. IDD, intellectual and/or developmental disability.
Study Characteristics
Study characteristics are summarized in Supplemental Table 3. Nineteen studies were conducted in the United States,* 5 in the United Kingdom,51,52,56,69,71 3 in Sweden,53,54,72 2 in Australia,49,57 and 1 each in Canada45 and Israel.67 Twenty studies were retrospective cohort studies,† 2 were prospective cohort studies,44,57 and 9 were cross-sectional studies.‡ Studies had between 6244 and 6 453 012 participants,55 with all but 444,57,63,64 having a total sample size >1500. In 11 studies, authors examined the impact of maternal disability overall,§ 14 examined intellectual and/or developmental disabilities only,‖ 4 examined sensory disabilities only,60,62,67,70 and only 2 examined physical disabilities only.63,66 Of the studies in which authors examined the impact of maternal disability overall, 10 compared an “any disability” group with a “no disability” referent group, and 7 also reported outcomes by disability type.6,45–48,55,56,69 In 7 studies, authors included psychiatric disorders within their definition of any disability.46,47,56,58,65,69,71 In 23 studies, authors used health administrative data (eg, hospital discharge data, insurance claims) to identify disability status,¶ 7 used self-report measures of disability,52,56–58,65,69,71 and 1 used a clinical measure.44 Authors of most studies examined outcomes related to maternal and neonatal health (see Tarasoff et al17 for a review of studies on maternal outcomes). Among neonatal and infant outcomes, the most commonly reported were preterm birth (primarily defined as <37 weeks’ gestation; n = 29)6,43,45–60,62–72 and low birth weight (n = 14).43,50,52,57–64,66,69,70 Other neonatal outcomes were very preterm birth (primarily defined as <32 weeks’ gestation; n = 8), postterm birth (n = 6), very low birth weight (n = 6), high birth weight (n = 4), small for gestational age (n = 11), large for gestational age (n = 7), low Apgar scores (n = 9), NICU admission (n = 6), neonatal mortality (n = 5), and neonatal morbidity (n = 1). In few studies did authors examined infant outcomes (eg, infant mortality, n = 2; hospital admission, n = 1). Outcomes were primarily ascertained from health administrative data; in 4 studies, authors used maternal self-report data.52,56,69,71 Studies varied with respect to their control for confounding factors.
Quality of Included Studies
Study quality is described in Supplemental Table 4. Overall, studies were rated as strong (n = 5),45–47,49,62 moderate (n = 9),# or weak (n = 17).** Retrospective and prospective cohort studies (n = 22) were rated as having moderate risk of bias for study design, and cross-sectional studies (n = 9) were rated as having high risk. Concerning selection bias, many studies were population based and had good generalizability. Studies with moderate or high selection bias ratings were those using Medicaid data,50 with urban clinic samples,44,57,63 and/or with response rates that were <80% or unreported.44,52,56,58,65,69,71 Authors of 14 studies did not control for confounders.†† Just more than half (n = 17) controlled for demographics,‡‡ 12 for socioeconomic status,§§ 10 for comorbidities,‖‖ 9 for lifestyle behaviors, ¶¶ and 6 for social support.46,50,51,53,54,62 Only 5 studies were rated as having low risk of detection bias6,44,57,63,67; most were rated as having moderate or high risk of bias because the authors provided limited or no data on the validity and/or reliability of their data sources. Finally, 15 studies44,50–52,55–59,63,65–69 were rated as having high risk of attrition bias, primarily because the authors did not report on the frequency of missing data.
Synthesis of Results
In the studies, authors primarily examined indicators of fetal growth and birth timing; fewer studies examined indicators of neonatal or infant morbidity and mortality. The results for outcomes that could be pooled, as well as those reported descriptively, on the basis of available data, are as follows.
Fetal Growth and Birth Timing
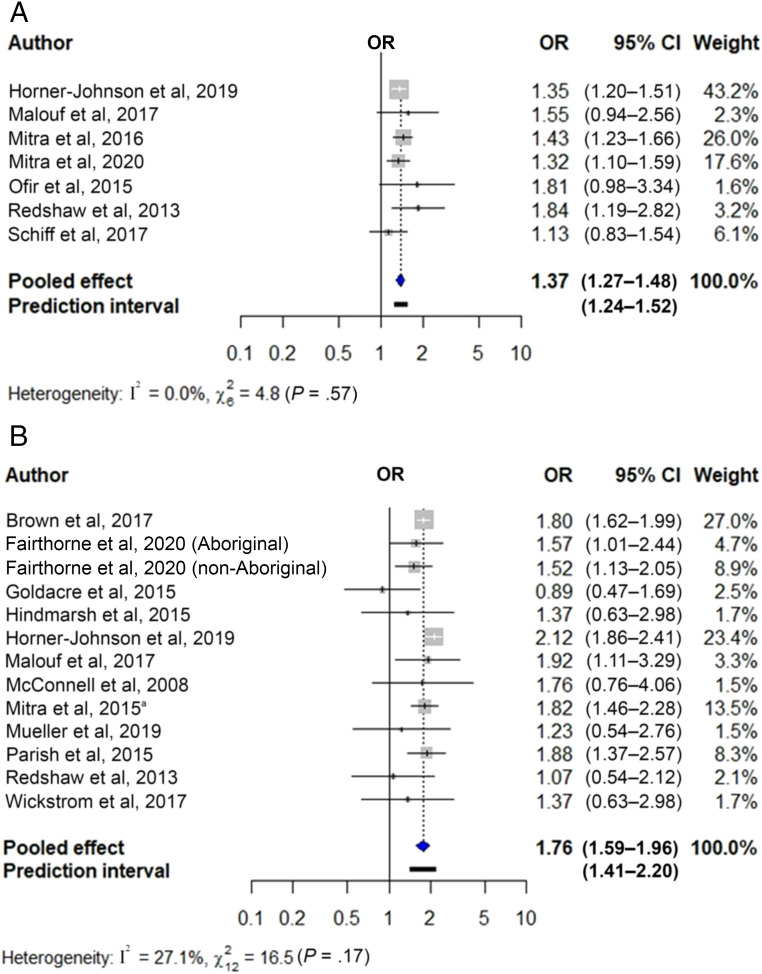
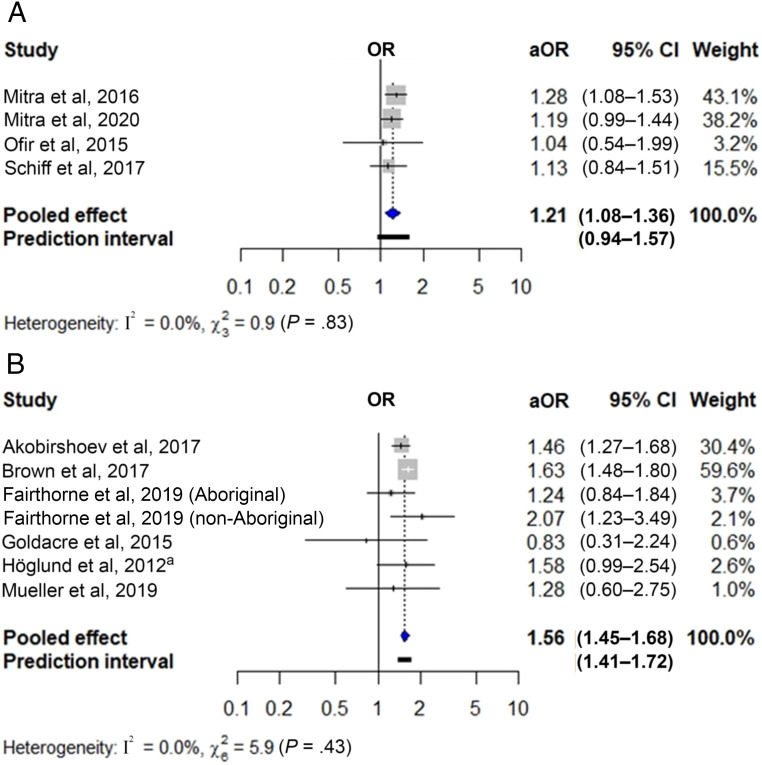
Figure 2 shows the pooled unadjusted ORs for preterm birth in newborns of women with sensory (Fig 2A) and intellectual and/or developmental disabilities (Fig 2B). These were 1.37 for sensory (95% CI 1.27–1.48; 7 studies, n = 11 191 691) and 1.76 for intellectual and/or developmental disabilities (95% CI 1.59–1.96; 12 studies with 13 cohorts; n = 8 783 563). Heterogeneity was nonsignificant. Figure 3 shows the pooled adjusted ORs of preterm birth in newborns of women with sensory (Fig 3A) and intellectual and/or developmental disabilities (Fig 3B), which were 1.21 (95% CI 1.08–1.36; 4 studies; n = 4 728 092) and 1.56 (95% CI 1.45–1.68; 6 studies with 7 cohorts; n = 5 178 838), respectively. Heterogeneity was nonsignificant, but the 95% prediction interval crossed the null value for the first analysis. Findings were robust to use of fixed effects models (Supplemental Table 5) and removal of individual studies from the meta-analysis (Supplemental Table 6).
FIGURE 2.
Unadjusted association between maternal disability status and preterm birth. The figure displays the unadjusted ORs and 95% CIs for the association between (A) sensory disabilities and (B) intellectual and/or developmental disabilities and preterm birth, along with the weight of each study. Measures of heterogeneity (Q and I2 statistics) and prediction intervals for analyses with at least 5 studies are also presented. a Ref 59.
FIGURE 3.
Adjusted association between maternal disability status and preterm birth. The figure displays the adjusted odds ratios (aORs) and 95% CIs for the association between (A) sensory disabilities and (B) intellectual and/or developmental disabilities and preterm birth, along with the weight of each study. Measures of heterogeneity (Q and I2 statistics) and prediction intervals for analyses with at least 5 studies are also presented. a Ref 54.
Notably, meta-analyses of 7 studies (n = 6 863 019) in which authors examined any disability and preterm birth and 5 studies (n = 6 639 560) in which authors examined physical disability and preterm birth were not presented because of considerable heterogeneity (I2 values ≥75%); however, these studies indicated increased risk for preterm birth in these groups (Supplemental Table 7). Unadjusted pooled analyses for very preterm birth in newborns of women with intellectual and/or developmental disabilities (Supplemental Fig 6) and findings from studies on birth timing that could not be pooled (Supplemental Table 7) generally supported increased risk for very preterm birth but not postterm birth in newborns of women with disabilities compared with those without disabilities.
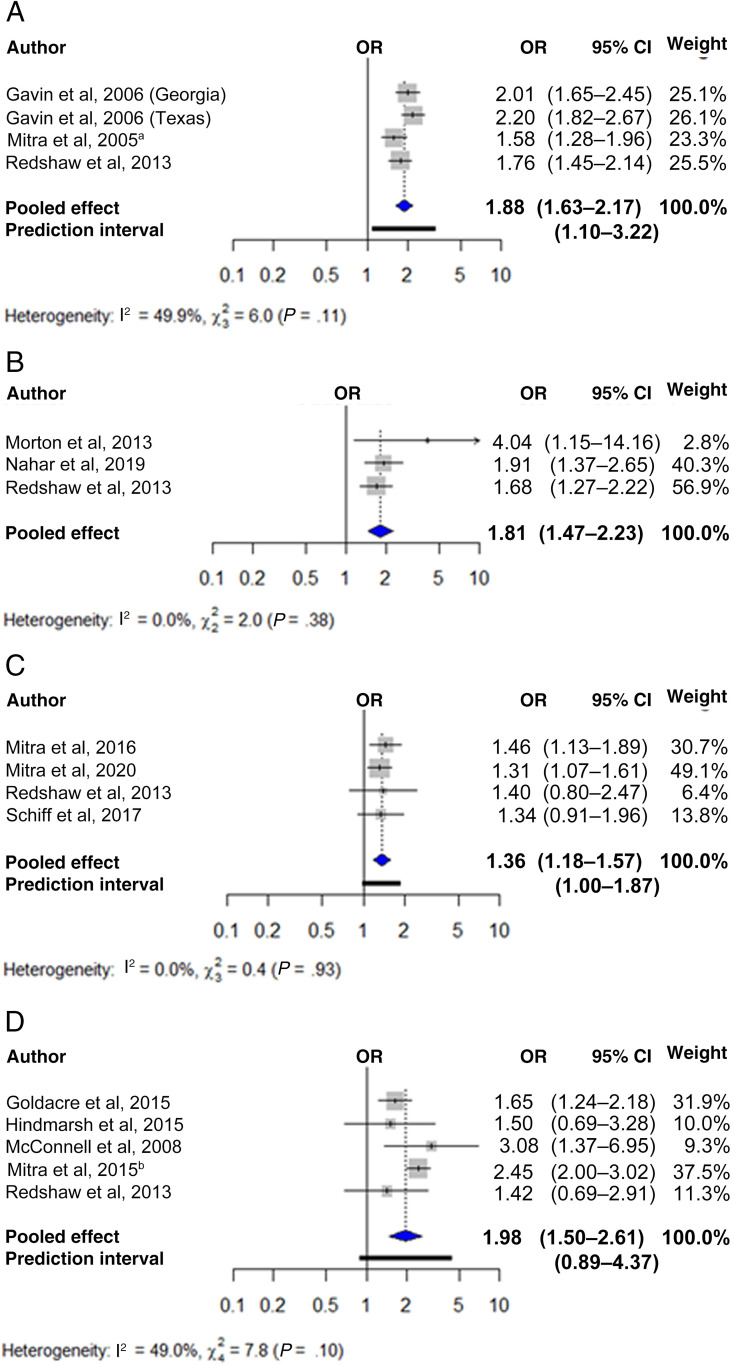
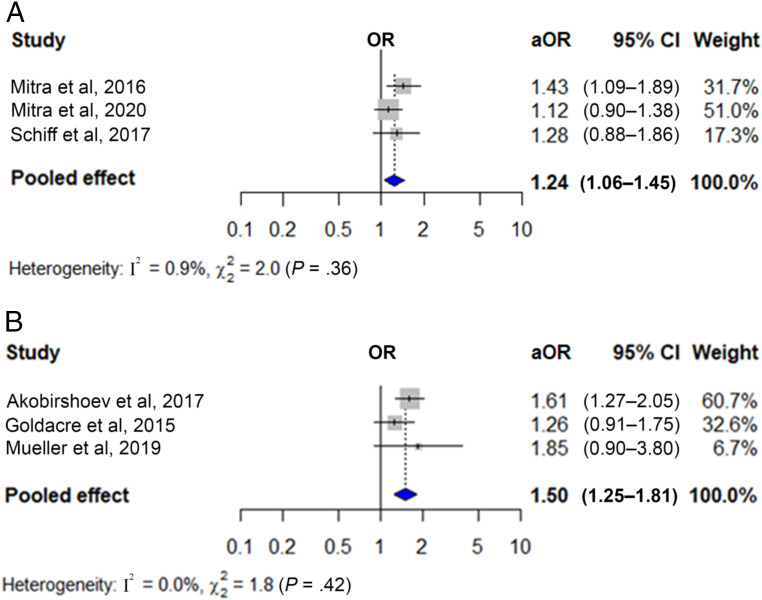
Figure 4 shows the pooled unadjusted ORs for low birth weight in newborns of women with any disability (Fig 4A) and physical (Fig 4B), sensory (Fig 4C), and intellectual and/or developmental disabilities (Fig 4D). These were 1.88 for any (95% CI 1.63–2.17; 3 studies with 4 cohorts; n = 167 684), 1.81 for physical (95% CI 1.47–2.23; 3 studies; n = 173 031), 1.36 for sensory (95% CI 1.18–1.57; 4 studies; n = 4 485 221), and 1.98 for intellectual and/or developmental disabilities (95% CI 1.50–2.61; 5 studies; n = 1 151 920). Heterogeneity was nonsignificant, but the 95% prediction interval crossed the null value for the last analysis. Figure 5 shows the pooled adjusted ORs for low birth weight in newborns of women with sensory (Fig 5A) and intellectual and/or developmental disabilities (Fig 5B), which were 1.24 (95% CI 1.06–1.45; 3 studies; n = 4 462 351) and 1.50 (95% CI 1.25–1.81; 3 studies; n = 4 442 979), respectively. Heterogeneity was nonsignificant. Findings were generally robust to the use of fixed effects models (Supplemental Table 5) and removal of individual studies from the meta-analysis (Supplemental Table 6).
FIGURE 4.
Unadjusted association between maternal disability status and low birth weight. The figure displays the unadjusted ORs and 95% CIs for the association between (A) any disability, (B) physical disabilities, (C) sensory disabilities, and (D) intellectual and/or developmental disabilities and low birth weight, along with the weight of each study. Measures of heterogeneity (Q and I2 statistics) and prediction intervals for analyses with at least 5 studies are also presented. a Ref 58. b Ref 59.
FIGURE 5.
Adjusted association between maternal disability status and low birth weight. The figure displays the adjusted odds ratios (aORs) and 95% CIs for the association between (A) sensory disabilities and (B) intellectual and/or developmental disabilities and low birth weight, along with the weight of each study. Measures of heterogeneity (Q and I2 statistics) and prediction intervals for analyses with at least 5 studies are also presented.
Pooled analyses for very low birth weight in newborns of women with sensory disabilities (Supplemental Fig 7), small for gestational age (Supplemental Fig 8) in newborns of women with intellectual and/or developmental disabilities, as well as findings from studies on fetal growth that could not be pooled (Supplemental Table 7) generally supported elevated risk of restricted, but not excessive, fetal growth among newborns of women with disabilities compared with those without disabilities.
Neonatal and Infant Morbidity and Mortality
In examining other neonatal outcomes, we found that newborns of women with sensory (Supplemental Fig 9A) but not intellectual and/or developmental disabilities (Supplemental Fig 9 B and C) were at increased risk for 5-minute Apgar scores <7. However, the latter group was at increased risk of 1-minute Apgar scores <7 (Supplemental Fig 10), NICU admission (Supplemental Fig 11), and neonatal mortality (Supplemental Fig 12). Findings from studies that could not be pooled (Supplemental Table 7) generally supported increased risk for low Apgar scores, NICU admission, longer hospital stay, and neonatal morbidity and mortality but not congenital anomalies among newborns of women with disabilities. In examining infant outcomes, we found that infants of women with intellectual and/or developmental disabilities were not at increased risk of hospitalization (Supplemental Fig 13). Findings from studies that could not be pooled (Supplemental Table 7) were nonsignificant for hospitalizations and problems requiring a doctor and were significant for infant mortality. However, authors of few studies contributed data to these analyses.
Sensitivity Analyses
Results were mostly unchanged when we substituted different studies into our analyses from among those in which authors used the same data sources (Supplemental Table 8). When we removed studies in which authors included psychiatric disorders in their overall disability definition, the association with low birth weight could no longer be meta-analyzed, but results of the remaining study50 (which included 2 cohorts) suggested increased risk.
Discussion
Summary of Findings
In our systematic review of 31 studies, representing >13 million unique women, we found that newborns of women with physical, sensory, and intellectual and/or developmental disabilities may be at elevated risk for adverse health outcomes compared with those of women without these disabilities, with particularly strong evidence of elevated risk for low birth weight across disability groups. Studies in which authors examined preterm birth as well as other neonatal outcomes were also consistent with elevated risk. However, findings from the small number of studies in which authors examined infant outcomes were more inconsistent, with some evidence of increased risk of infant mortality but no other adverse infant health outcomes. Findings were generally robust in sensitivity analyses, although some heterogeneity across studies should be acknowledged.
Comparison With Previous Research
This is the first systematic review and meta-analysis to examine risk for adverse health outcomes among newborns and infants of women with disabilities compared with those of women without disabilities. Our findings are generally consistent with a 2011 summary of the literature, in which authors found elevated rates of preterm birth and other adverse neonatal outcomes among women with physical disabilities,73 and with other studies focused on specific disabilities such as spinal cord injury,74 muscular dystrophy,75 lupus,76 and autism spectrum disorder.77 Other studies on specific disabilities, such as multiple sclerosis,78,79 have yielded conflicting results, suggesting a need for further research to understand how social, health, and health care factors associated with different disabilities may influence risk.
Explanation for Findings
Several factors may explain the adverse health outcomes observed in offspring of women with disabilities. Reproductive-aged women with disabilities experience significant socioeconomic and health disparities, including high rates of poverty, chronic health conditions, and mental illness,11,12 with many disabilities or secondary conditions requiring use of potentially teratogenic medications.11,80 Women with disabilities are also less likely than their peers to be married or partnered10,12 and more likely to experience violence and isolation.81–83 Numerous studies indicate that women with disabilities encounter barriers accessing and navigating perinatal care16,55,56 and are at greater risk for pregnancy, delivery, and postpartum complications.17 In our review, women with intellectual and/or developmental disabilities were an especially vulnerable group. Along with the disparities described above, these women experience a lack of adequate sexual and reproductive health education, high rates of unintended pregnancy,84 short interpregnancy intervals,85 and difficulty understanding and following medical advice.83,86,87 Finally, women with disabilities may be hesitant to ask for help or avoid accessing services because of fears of stigma, their parenting being scrutinized, and child welfare involvement.88,89 Together, these disparities may contribute to adverse outcomes for their offspring. This hypothesis is consistent with research revealing that much of the variance in adverse health and developmental outcomes among older children of women with disabilities is driven by the mother’s circumstances (eg, low socioeconomic status, lack of adequate supports), not her disability per se.88,90,91 However, few studies have isolated the effects of disability from pathologizing assumptions and disability-based discrimination or marginalization.88In our review, few studies comprehensively accounted for social, health, and health care factors that could explain disparities. Further research is needed to understand the contribution of these factors to adverse neonatal health outcomes because many are modifiable and could be valuable targets for supportive interventions for women with disabilities and their offspring.
Limitations
Our methods and the included studies are not without limitations. Our protocol was not registered a priori. We excluded studies not written in English and identified no studies from low- and middle-income countries. Given the socioeconomic, health, and health care disparities experienced by women with disabilities in low- and middle-income countries,92,93 it is possible that their offspring fare even worse. Thus, the generalizability of our findings may be limited. Not all studies defined disability in the same way. For example, several studies included psychiatric disorders within their definition of any disability; however, this heterogeneity did not appear to affect results. Authors of several studies based their definition of disability on diagnostic codes, which may not accurately reflect functional limitations or lived experiences of disability and may miss less obvious disabilities.55,94 Moreover, most of the included studies examined disability overall (n = 11) or intellectual and/or developmental disabilities only (n = 14). As well, most studies examined neonatal outcomes, with few studies examining infant outcomes. Results of these smaller analyses should be interpreted with caution, particularly those with significant heterogeneity. Finally, just more than half (n = 17) of the studies were rated as weak in quality, with lack of control for confounding being a common limitation. There is a need for more high-quality research in this area.
Clinical Implications
Although women with disabilities are becoming pregnant at increasing rates,5 research and clinical practice have not kept pace. To our knowledge, there are no preconception health interventions tailored to women with disabilities, few clinical practice guidelines specific to this population in pregnancy,95 and limited supports for disabled parents with infants and older children.8 This lack of practical support is concerning88,96: From their summary of the literature on parents with disabilities, McConnell and Hahn found that, “antipathy to parents and parenting with disabilities affects women and mothers and, by extension, their children, in profound ways.”88(p3) To address the disparities found in our review, in future work, researchers should explore how tailored preconception and prenatal care could benefit women with disabilities.11
In addition, future research could be used to explore the role of family-centered pediatric care in better supporting neonatal and infant health.47 As noted in the American Academy of Pediatrics 2003 Report of the Task Force on the Family, “The health and well-being of children are inextricably linked to their parents’ physical, emotional and social health, social circumstances, and child-rearing practices.”97(p1541) Because pediatricians are often the main point of contact for women in the immediate postpartum period, in addition to providing breastfeeding support and screening for postpartum depression, they could potentially support women with disabilities and in turn their infants by connecting them with peer support, community-based parenting programs, and other services they may need (eg, nutrition, housing). To facilitate pediatricians in caring for families of women with disabilities, there would be a need for provider education and training on the social circumstances, medical risks, and stigma faced by mothers with disabilities, as well as their health care–related needs, such as longer and more frequent appointments, accessibility and communication requirements, and other accommodations such as home or virtual visits. Such efforts could make a positive impact on the health of offspring of women with disabilities.
Conclusions
In our systematic review and meta-analysis, we found that offspring of women with physical, sensory, and intellectual and/or developmental disabilities are at elevated risk for low birth weight, with studies of preterm birth and other adverse neonatal outcomes also suggesting elevated risk. Notably, there were fewer studies in which authors examined infant health outcomes, with mixed results, highlighting the need for more research in this area. In future studies, researchers should explore the social, health, and health care factors explaining the disparities experienced by women with disabilities and their offspring. Interventions to address these disparities should also be explored, with disability-competent preconception and perinatal care and family-centered approaches to pediatric care potentially having a positive impact on health outcomes. Future research and interventions regarding the health of women with disabilities and their offspring should be attentive to the multiple social and health challenges women with disabilities face.
Acknowledgment
We thank Mary-Rose Faulkner for her assistance with data collection.
Glossary
- CI
confidence interval
- OR
odds ratio
Footnotes
Dr Tarasoff collected and interpreted the data, drafted the initial manuscript, and reviewed and revised the manuscript; Ms Murtaza, Ms Carty, and Ms Salaeva collected and interpreted the data and critically reviewed the manuscript for important intellectual content; Ms Hamilton conceptualized and designed the study and critically revised the manuscript for important intellectual content; Dr Brown conceptualized and designed the study, conducted the data analyses, and critically revised the manuscript for important intellectual content; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health (award 5R01HD092326). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This research was undertaken, in part, thanks to funding from the Canada Research Chairs Program and the University of Toronto Scarborough Research Competitiveness Fund to Dr Brown. Dr Tarasoff is supported by a Canadian Institutes of Health Research postdoctoral fellowship. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.World Health Organization International Classification of Functioning, Disability and Health (ICF). Geneva, Switzerland: World Health Organization; 2001 [Google Scholar]
- 2.The Eunice Kennedy Shriver National Institute of Child Health and Human Development. Intellectual and developmental disabilities (IDDs): condition information. 2016. Available at: https://www.nichd.nih.gov/health/topics/idds/conditioninfo/default. Accessed March 10, 2020
- 3.Silver MG. Eugenics and compulsory sterilization laws: providing redress for victims of a shameful era in United States history. George Washington Law Rev. 2004;72(4):862–892 [PubMed] [Google Scholar]
- 4.Horner-Johnson W, Darney BG, Kulkarni-Rajasekhara S, Quigley B, Caughey AB. Pregnancy among US women: differences by presence, type, and complexity of disability. Am J Obstet Gynecol. 2016;214(4):529.e1-529.e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown HK, Chen S, Guttmann A, et al. Rates of recognized pregnancy in women with disabilities in Ontario, Canada. Am J Obstet Gynecol. 2020;222(2):189–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horner-Johnson W, Biel FM, Darney BG, Caughey AB. Time trends in births and cesarean deliveries among women with disabilities. Disabil Health J. 2017;10(3):376–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iezzoni LI, Yu J, Wint AJ, Smeltzer SC, Ecker JL. Prevalence of current pregnancy among US women with and without chronic physical disabilities. Med Care. 2013;51(6):555–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Council on Disability. Rocking the Cradle: Ensuring the Rights of Parents with Disabilities and Their Children. Washington, DC: National Council on Disability; 2012. Available at: https://www.ncd.gov/sites/default/files/Documents/NCD_Parenting_508_0.pdf. Accessed April 1, 2018 [Google Scholar]
- 9.Parish SL, Rose RA, Andrews ME. Income poverty and material hardship among US women with disabilities. Soc Serv Rev. 2009;83(1):33–52 [Google Scholar]
- 10.Wisdom JP, McGee MG, Horner-Johnson W, Michael YL, Adams E, Berlin M. Health disparities between women with and without disabilities: a review of the research. Soc Work Public Health. 2010;25(3):368–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tarasoff LA, Lunsky Y, Chen S, et al. Preconception health characteristics of women with disabilities in Ontario: a population-based, cross-sectional study [published online ahead of print July 14, 2020]. J Womens Health (Larchmt). doi: 10.1089/jwh.2019.8273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitra M, Clements KM, Zhang J, Smith LD. Disparities in adverse preconception risk factors between women with and without disabilities. Matern Child Health J. 2016;20(3):507–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iezzoni LI, Yu J, Wint AJ, Smeltzer SC, Ecker JL. General health, health conditions, and current pregnancy among U.S. women with and without chronic physical disabilities. Disabil Health J. 2014;7(2):181–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mosher W, Bloom T, Hughes R, Horton L, Mojtabai R, Alhusen JL. Disparities in receipt of family planning services by disability status: new estimates from the National Survey of Family Growth. Disabil Health J. 2017;10(3):394–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Hearn A. Deaf women’s experiences and satisfaction with prenatal care: a comparative study. Fam Med. 2006;38(10):712–716 [PubMed] [Google Scholar]
- 16.Mitra M, Akobirshoev I, Moring NS, et al. Access to and satisfaction with prenatal care among pregnant women with physical disabilities: findings from a national survey. J Womens Health (Larchmt). 2017;26(12):1356–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tarasoff LA, Ravindran S, Malik H, Salaeva D, Brown HK. Maternal disability and risk for pregnancy, delivery, and postpartum complications: a systematic review and meta-analysis. Am J Obstet Gynecol. 2020;222(1):27.e1-27.e32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitra M, Iezzoni LI, Zhang J, Long-Bellil LM, Smeltzer SC, Barton BA. Prevalence and risk factors for postpartum depression symptoms among women with disabilities. Matern Child Health J. 2015;19(2):362–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhutta ZA, Lassi ZS, Blanc A, Donnay F. Linkages among reproductive health, maternal health, and perinatal outcomes. Semin Perinatol. 2010;34(6):434–445 [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization. Meeting to Develop a Global Consensus on Preconception Care to Reduce Maternal and Childhood Mortality and Morbidity: World Health Organization Headquarters, Geneva, 6–February 7, 2012: Meeting Report. Geneva, Switzerland: World Health Organization; 2013. Available at: https://apps.who.int/iris/handle/10665/78067. Accessed January 5, 2020 [Google Scholar]
- 21.Raju TNK, Pemberton VL, Saigal S, Blaisdell CJ, Moxey-Mims M, Buist S; Adults Born Preterm Conference Speakers and Discussants . Long-term healthcare outcomes of preterm birth: an executive summary of a conference sponsored by the national Institutes of health. J Pediatr. 2017;181:309–318.e1 [DOI] [PubMed] [Google Scholar]
- 22.Bell K, Corbacho B, Ronaldson S, Richardson G, Torgerson D, Robling M; Building Blocks trial group . The impact of pre and perinatal lifestyle factors on child long term health and social outcomes: a systematic review. Health Econ Rev. 2018;8(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P; PRISMA Group . Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walsh ES, Peterson JJ, Judkins DZ; Expert Panel on Health Care Disparities Among Individuals With Disabilities . Searching for disability in electronic databases of published literature. Disabil Health J. 2014;7(1):114–118 [DOI] [PubMed] [Google Scholar]
- 25.Vigod SN, Kurdyak PA, Dennis CL, et al. Maternal and newborn outcomes among women with schizophrenia: a retrospective population-based cohort study. BJOG. 2014;121(5):566–574 [DOI] [PubMed] [Google Scholar]
- 26.Frayne J, Nguyen T, Allen S, Hauck Y, Liira H, Vickery A. Obstetric outcomes for women with severe mental illness: 10 years of experience in a tertiary multidisciplinary antenatal clinic. Arch Gynecol Obstet. 2019;300(4):889–896 [DOI] [PubMed] [Google Scholar]
- 27.Grigoriadis S, Graves L, Peer M, et al. Maternal anxiety during pregnancy and the association with adverse perinatal outcomes: systematic review and meta-analysis. J Clin Psychiatry. 2018;79(5):17r12011. [DOI] [PubMed] [Google Scholar]
- 28.National Institutes of Health Pregnancy in women with disabilities (R01). 2011. Available at: https://grants.nih.gov/grants/guide/pa-files/PAR-11-258.html. Accessed January 2, 2020
- 29.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–349 [DOI] [PubMed] [Google Scholar]
- 30.Armijo-Olivo S, Stiles CR, Hagen NA, Biondo PD, Cummings GG. Assessment of study quality for systematic reviews: a comparison of the Cochrane Collaboration Risk of Bias Tool and the Effective Public Health Practice Project Quality Assessment Tool: methodological research. J Eval Clin Pract. 2012;18(1):12–18 [DOI] [PubMed] [Google Scholar]
- 31.Thomas BH, Ciliska D, Dobbins M, Micucci S. A process for systematically reviewing the literature: providing the research evidence for public health nursing interventions. Worldviews Evid Based Nurs. 2004;1(3):176–184 [DOI] [PubMed] [Google Scholar]
- 32.Nair M, Knight M, Kurinczuk JJ. Risk factors and newborn outcomes associated with maternal deaths in the UK from 2009 to 2013: a national case-control study. BJOG. 2016;123(10):1654–1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Misra DP, Guyer B, Allston A. Integrated perinatal health framework. A multiple determinants model with a life span approach. Am J Prev Med. 2003;25(1):65–75 [DOI] [PubMed] [Google Scholar]
- 34.Heaman M, Kingston D, Chalmers B, Sauve R, Lee L, Young D. Risk factors for preterm birth and small-for-gestational-age births among Canadian women. Paediatr Perinat Epidemiol. 2013;27(1):54–61 [DOI] [PubMed] [Google Scholar]
- 35.Amjad S, MacDonald I, Chambers T, et al. Social determinants of health and adverse maternal and birth outcomes in adolescent pregnancies: a systematic review and meta-analysis. Paediatr Perinat Epidemiol. 2019;33(1):88–99 [DOI] [PubMed] [Google Scholar]
- 36.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188 [DOI] [PubMed] [Google Scholar]
- 37.Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods. 2006;11(2):193–206 [DOI] [PubMed] [Google Scholar]
- 38.Higgins JPT. Commentary: heterogeneity in meta-analysis should be expected and appropriately quantified. Int J Epidemiol. 2008;37(5):1158–1160 [DOI] [PubMed] [Google Scholar]
- 39.Higgins JPT, Thomas J, Chandler J, eds., et al. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed Chichester, United Kingdom: John Wiley & Sons; 2019 [Google Scholar]
- 40.Partlett C, Riley RD. Random effects meta-analysis: coverage performance of 95% confidence and prediction intervals following REML estimation. Stat Med. 2017;36(2):301–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guddat C, Grouven U, Bender R, Skipka G. A note on the graphical presentation of prediction intervals in random-effects meta-analyses. Syst Rev. 2012;1(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lau J, Ioannidis JPA, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. BMJ. 2006;333(7568):597–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Akobirshoev I, Parish SL, Mitra M, Rosenthal E. Birth outcomes among US women with intellectual and developmental disabilities. Disabil Health J. 2017;10(3):406–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bowling M, Keltner BR. Primary health care for children of mothers with intellectual limitations. Pediatr Nurs. 1996;22(4):312–315, 319 [PubMed] [Google Scholar]
- 45.Brown HK, Cobigo V, Lunsky Y, Vigod SN. Maternal and offspring outcomes in women with intellectual and developmental disabilities: a population-based cohort study. BJOG. 2017;124(5):757–765 [DOI] [PubMed] [Google Scholar]
- 46.Clements KM, Mitra M, Zhang J, Iezzoni LI. Pregnancy characteristics and outcomes among women at risk for disability from health conditions identified in medical claims. Womens Health Issues. 2016;26(5):504–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clements KM, Zhang J, Long-Bellil LM, Mitra M. Emergency department utilization during the first year of life among infants born to women at risk of disability. Disabil Health J. 2020;13(1):100831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Darney BG, Biel FM, Quigley BP, Caughey AB, Horner-Johnson W. Primary cesarean delivery patterns among women with physical, sensory, or intellectual disabilities. Womens Health Issues. 2017;27(3):336–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fairthorne J, Bourke J, O’Donnell M, et al. Pregnancy and birth outcomes of mothers with intellectual disability and their infants: advocacy needed to improve well-being. Disabil Health J. 2020;13(2):100871. [DOI] [PubMed] [Google Scholar]
- 50.Gavin NI, Benedict MB, Adams EK. Health service use and outcomes among disabled Medicaid pregnant women. Womens Health Issues. 2006;16(6):313–322 [DOI] [PubMed] [Google Scholar]
- 51.Goldacre AD, Gray R, Goldacre MJ. Childbirth in women with intellectual disability: characteristics of their pregnancies and outcomes in an archived epidemiological dataset. J Intellect Disabil Res. 2015;59(7):653–663 [DOI] [PubMed] [Google Scholar]
- 52.Hindmarsh G, Llewellyn G, Emerson E. Mothers with intellectual impairment and their 9-month-old infants. J Intellect Disabil Res. 2015;59(6):541–550 [DOI] [PubMed] [Google Scholar]
- 53.Höglund B, Lindgren P, Larsson M. Pregnancy and birth outcomes of women with intellectual disability in Sweden: a national register study. Acta Obstet Gynecol Scand. 2012;91(12):1381–1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Höglund B, Lindgren P, Larsson M. Newborns of mothers with intellectual disability have a higher risk of perinatal death and being small for gestational age. Acta Obstet Gynecol Scand. 2012;91(12):1409–1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Horner-Johnson W, Biel FM, Caughey AB, Darney BG. Differences in prenatal care by presence and type of maternal disability. Am J Prev Med. 2019;56(3):376–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Malouf R, Henderson J, Redshaw M. Access and quality of maternity care for disabled women during pregnancy, birth and the postnatal period in England: data from a national survey. BMJ Open. 2017;7(7):e016757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McConnell D, Mayes R, Llewellyn G. Women with intellectual disability at risk of adverse pregnancy and birth outcomes. J Intellect Disabil Res. 2008;52(pt 6):529–535 [DOI] [PubMed] [Google Scholar]
- 58.Mitra M, Clements KM, Zhang J, Iezzoni LI, Smeltzer SC, Long-Bellil LM. Maternal characteristics, pregnancy complications, and adverse birth outcomes among women with disabilities. Med Care. 2015;53(12):1027–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mitra M, Parish SL, Clements KM, Cui X, Diop H. Pregnancy outcomes among women with intellectual and developmental disabilities. Am J Prev Med. 2015;48(3):300–308 [DOI] [PubMed] [Google Scholar]
- 60.Mitra M, Akobirshoev I, McKee MM, Iezzoni LI. Birth outcomes among U.S. women with hearing loss. Am J Prev Med. 2016;51(6):865–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mitra M, Parish SL, Akobirshoev I, Rosenthal E, Moore Simas TA. Postpartum hospital utilization among Massachusetts women with intellectual and developmental disabilities: a retrospective cohort study. Matern Child Health J. 2018;22(10):1492–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mitra M, McKee MM, Akobirshoev I, et al. Pregnancy, birth, and infant outcomes among women who are deaf or hard of hearing. Am J Prev Med. 2020;58(3):418–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morton C, Le JT, Shahbandar L, Hammond C, Murphy EA, Kirschner KL. Pregnancy outcomes of women with physical disabilities: a matched cohort study. PM R. 2013;5(2):90–98 [DOI] [PubMed] [Google Scholar]
- 64.Mueller BA, Crane D, Doody DR, Stuart SN, Schiff MA. Pregnancy course, infant outcomes, rehospitalization, and mortality among women with intellectual disability. Disabil Health J. 2019;12(3):452–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mwachofi AK. A comparative analysis of pregnancy outcomes for women with and without disabilities. J Health Dispar Res Pract. 2017;10(1):28–50 [Google Scholar]
- 66.Nahar I. Adverse Birth Outcomes Among Women with Physical Disabilities: A Retrospective Cohort Study in South Carolina [master’s thesis]. Columbia, SC: University of South Carolina; 2019. Available at: https://scholarcommons.sc.edu/etd/5163. Accessed August 13, 2019 [Google Scholar]
- 67.Ofir D, Kessous R, Belfer N, Lifshitz T, Sheiner E. The influence of visual impairment on pregnancy outcomes. Arch Gynecol Obstet. 2015;291(3):519–523 [DOI] [PubMed] [Google Scholar]
- 68.Parish SL, Mitra M, Son E, Bonardi A, Swoboda PT, Igdalsky L. Pregnancy outcomes among U.S. women with intellectual and developmental disabilities. Am J Intellect Dev Disabil. 2015;120(5):433–443 [DOI] [PubMed] [Google Scholar]
- 69.Redshaw M, Malouf R, Gao H, Gray R. Women with disability: the experience of maternity care during pregnancy, labour and birth and the postnatal period. BMC Pregnancy Childbirth. 2013;13(1):174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schiff MA, Doody DR, Crane DA, Mueller BA. Pregnancy outcomes among deaf women in Washington State, 1987-2012. Obstet Gynecol. 2017;130(5):953–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Šumilo D, Kurinczuk JJ, Redshaw ME, Gray R. Prevalence and impact of disability in women who had recently given birth in the UK. BMC Pregnancy Childbirth. 2012;12(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wickström M, Höglund B, Larsson M, Lundgren M. Increased risk for mental illness, injuries, and violence in children born to mothers with intellectual disability: a register study in Sweden during 1999-2012. Child Abuse Negl. 2017;65:124–131 [DOI] [PubMed] [Google Scholar]
- 73.Signore C, Spong CY, Krotoski D, Shinowara NL, Blackwell SC. Pregnancy in women with physical disabilities. Obstet Gynecol. 2011;117(4):935–947 [DOI] [PubMed] [Google Scholar]
- 74.Crane DA, Doody DR, Schiff MA, Mueller BA. Pregnancy outcomes in women with spinal cord injuries: a population-based study. PM R. 2019;11(8):795–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Petrangelo A, Alshehri E, Czuzoj-Shulman N, Abenhaim HA. Obstetrical, maternal and neonatal outcomes in pregnancies affected by muscular dystrophy. J Perinat Med. 2018;46(7):791–796 [DOI] [PubMed] [Google Scholar]
- 76.Abdwani R, Al Shaqsi L, Al-Zakwani I. Neonatal and obstetrical outcomes of pregnancies in systemic lupus erythematosus. Oman Med J. 2018;33(1):15–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sundelin HE, Stephansson O, Hultman CM, Ludvigsson JF. Pregnancy outcomes in women with autism: a nationwide population-based cohort study. Clin Epidemiol. 2018;10:1817–1826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Finkelsztejn A, Brooks JB, Paschoal FM Jr., Fragoso YD. What can we really tell women with multiple sclerosis regarding pregnancy? A systematic review and meta-analysis of the literature. BJOG. 2011;118(7):790–797 [DOI] [PubMed] [Google Scholar]
- 79.Goldacre A, Pakpoor J, Goldacre M. Perinatal characteristics and obstetric complications in mothers with multiple sclerosis: record-linkage study. Mult Scler Relat Disord. 2017;12:4–8 [DOI] [PubMed] [Google Scholar]
- 80.Byrnes L, Hickey M. Perinatal care for women with disabilities: clinical considerations. J Nurse Pract. 2016;12(8):503–509 [Google Scholar]
- 81.Mitra M, Manning SE, Lu E. Physical abuse around the time of pregnancy among women with disabilities. Matern Child Health J. 2012;16(4):802–806 [DOI] [PubMed] [Google Scholar]
- 82.Barrett KA, O’Day B, Roche A, Carlson BL. Intimate partner violence, health status, and health care access among women with disabilities. Womens Health Issues. 2009;19(2):94–100 [DOI] [PubMed] [Google Scholar]
- 83.Llewellyn G, McConnell D. Mothers with learning difficulties and their support networks. J Intellect Disabil Res. 2002;46(pt 1):17–34 [DOI] [PubMed] [Google Scholar]
- 84.Horner-Johnson W, Dissanayake M, Wu JP, Caughey AB, Darney BG. Pregnancy intendedness by maternal disability status and type in the United States. Perspect Sex Reprod Health. 2020;52(1):31–38 [DOI] [PubMed] [Google Scholar]
- 85.Brown HK, Ray JG, Liu N, Lunsky Y, Vigod SN. Rapid repeat pregnancy among women with intellectual and developmental disabilities: a population-based cohort study. CMAJ. 2018;190(32):E949–E956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lunsky Y, Straiko A, Armstrong S. Women be healthy: evaluation of a women’s health curriculum for women with intellectual disabilities. J Appl Res Intellect Disabil. 2003;16(4):247–253 [Google Scholar]
- 87.Abells D, Kirkham YA, Ornstein MP. Review of gynecologic and reproductive care for women with developmental disabilities. Curr Opin Obstet Gynecol. 2016;28(5):350–358 [DOI] [PubMed] [Google Scholar]
- 88.McConnell D, Hahn L. Growing up with Parents with Disabilities In: Hupp S, Jewell JD, eds. The Encyclopedia of Child and Adolescent Development. John Wiley & Sons, Inc.; 2020 [Google Scholar]
- 89.Xie E, Gemmill M. Exploring the prenatal experience of women with intellectual and developmental disabilities: in a southeastern Ontario family health team. Can Fam Physician. 2018;64(suppl 2):S70–S75 [PMC free article] [PubMed] [Google Scholar]
- 90.Emerson E, Brigham P. The developmental health of children of parents with intellectual disabilities: cross sectional study. Res Dev Disabil. 2014;35(4):917–921 [DOI] [PubMed] [Google Scholar]
- 91.Andrews EE, Ayers K. Parenting with Disability: Experiences of Disabled Women In: Miles-Cohen SE, Signore C, eds. Eliminating Inequities for Women with Disabilities: An Agenda for Health and Wellness. Washington, DC: American Psychological Association; 2016:209–225 [Google Scholar]
- 92.Casebolt MT. Barriers to reproductive health services for women with disability in low- and middle-income countries: a review of the literature. Sex Reprod Healthc. 2020;24:100485. [DOI] [PubMed] [Google Scholar]
- 93.Nguyen TV, King J, Edwards N, Pham CT, Dunne M. Maternal healthcare experiences of and challenges for women with physical disabilities in low and middle-income countries: a review of qualitative evidence. Sex Disabil. 2019;37:175–201 [Google Scholar]
- 94.Brown HK, Carty A, Havercamp SM, Parish S, Lunsky Y. Identifying reproductive-aged women with physical and sensory disabilities in administrative health data: a systematic review. Disabil Health J. 2020;13(3):100909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.American College of Obstetricians and Gynecologists ACOG Committee Opinion; number 275, Obstetric management of patients with spinal cord injuries. Obstet Gynecol. 2002;100(3):625–627 [DOI] [PubMed] [Google Scholar]
- 96.Kirshbaum M, Olkin R. Parents with physical, systemic, or visual disabilities. Sex Disabil. 2002;20(1):65–80 [Google Scholar]
- 97.Schor EL; American Academy of Pediatrics Task Force on the Family . Family pediatrics: report of the Task Force on the Family. Pediatrics. 2003;111(6 pt 2):1541–1571 [PubMed] [Google Scholar]