Abstract
The increase of cerebral blood flow evoked by neuronal activity is essential to ensure enough energy supply to the brain. In the neurovascular unit, endothelial cells are ideally placed to regulate key neurovascular functions of the brain. Nevertheless, some outstanding questions remain about their exact role neurovascular coupling (NVC). Here, we postulated that the tissue-type plasminogen activator (tPA) present in the circulation might contribute to NVC by a mechanism dependent of its interaction with endothelial N-Methyl-D-Aspartate Receptor (NMDAR). To address this question, we used pharmacological and genetic approaches to interfere with vascular tPA-dependent NMDAR signaling, combined with laser speckle flowmetry, intravital microscopy and ultrafast functional ultrasound in vivo imaging. We found that the tPA present in the blood circulation is capable of potentiating the cerebral blood flow increase induced by the activation of the mouse somatosensorial cortex, and that this effect is mediated by a tPA-dependent activation of NMDAR expressed at the luminal part of endothelial cells of arteries. Although blood molecules, such as acetylcholine, bradykinin or ATP are known to regulate vascular tone and induce vessel dilation, our present data provide the first evidence that circulating tPA is capable of influencing neurovascular coupling (NVC).
Keywords: Endothelial cells, NDMA receptors, neurovascular coupling, tissue-type plasminogen activator, vascular biology
Introduction
Regulation of the cerebral blood flow (CBF) plays a critical role in brain functions with its alteration as a cause or a consequence of several brain disorders, including stroke and Alzheimer’s Disease.1 Therefore, understanding the cellular and molecular mechanisms underlying physiological and/or pathophysiological hemodynamic signals elicited by neuronal activation should lead to a better understanding of brain health and diseases. The control of the functional hyperemia, also known as neurovascular coupling (NVC),1 has been reported to involve almost all cells of the neurovascular unit (neurons, astrocytes, vascular smooth muscle cells, pericytes and endothelial cells),1,2 with mechanisms that may differ between arteries/arterioles and capillaries.3,4
Tissue-type plasminogen activator (tPA) is a serine protease initially characterized for its role in fibrinolysis by its ability to convert plasminogen into plasmin,5 and is therefore used in the treatment of stroke.6 tPA was also described as a neuromodulator7,8 and a gliotransmitter9 implicated in various brain functions.10–12 A number of its cerebral parenchymal effects are related to its ability to influence N-methyl-D-Aspartate receptor (NMDAR) signaling.8,13,14 Therefore, tPA was previously described as an actor of NVC via a mechanism involving neuronal NMDAR signaling.15 However, the complete mechanism of action of tPA on NVC remains to be clarified since tPA may originate either from neurons16 or endothelial cells17 and NMDAR also being expressed on neurons and on endothelial cells.18–20 When expressed on brain endothelial cells, NMDAR is involved in the maintenance of the integrity of the blood–brain barrier (BBB),18,19 and their tPA-dependent modulation results in the passage of immune cells across the BBB in neuroinflammatory conditions.21
Whereas important studies have described signaling pathways in astrocytes, vascular smooth muscle cells, pericytes and endothelial cells involved in the control of hemodynamic signal induced by neuronal activity, some outstanding questions remain. Endothelial cells are key component in the regulation of vascular tone in the brain, and many circulating molecules are capable to have an influence on it, such as acetylcholine, bradykinin or ATP.22,23 As endothelial cells are localized at the interface between the circulation and the brain parenchyma, the question whether blood components are capable of specifically influencing NVC is still debated. Here, we hypothesized that circulating tPA may participate in hemodynamic responses induced by neuronal activation. To address this question, we took advantages of laser speckle contrast imaging, functional UltraSound (fUS) imaging24 and intravital imaging along with pharmacological and genetic approaches. We found that plasmatic tPA can potentiate the CBF increase evoked by activation of the mouse barrel cortex. Moreover, our study shows that this effect is mediated by a tPA-dependent activation of NMDAR expressed on endothelial cells of arteries and arterioles. Thus, while mechanisms reported so far to control NVC mainly arise from the parenchyma towards the vessels, we provide here the evidences of a secondary mechanism coming from the blood stream towards endothelial cells in the control of NVC.
Materials and methods
Animals
All experiments were performed on male tPA wt, tPA−/− (Centre Universitaire de Ressources Biologiques, Caen, France) or C57BL/6 (Janvier labs, Le Genest-Saint-Isle, France) aged from 8 to 12 weeks. Mice were housed in plastic cages on a 12-h light cycle with ad libitum access to water and food. During experiments, body temperature was maintained with electric heating pads with thermal feedback, and hearth rate and blood O2 saturation were monitored using the MouseOx+ device (Starr Life Sciences Corporation). All experiments and analysis were randomized and performed blind. Experiments were performed in accordance with the European directive (2010/63/UE) and French ethical laws (act no. R214; 87–137 du code rural). The project was approved by the ethical committee CENOMEXA (under the identification number #11104) and the experiments were performed following the ARRIVE guidelines (Animal Research: Reporting of in Vivo Experiments; http://www.nc3rs.org.uk).
Animals preparation
Anesthesia was initially induced using 5% isoflurane (Forene®, AbbVie) in 70% N2O/30% O2 and then maintained using 2% isoflurane in 70% N2O/30% O2. Mice were intubated, placed under mechanical ventilation, and fixed in a stereotaxic frame. The skull was exposed, and lidocaine (Xylocaine 5% spray®, AstraZeneca) was applied. Whiskers on the left side of the mouse were cut to let only about 1 cm. A catheter was placed in the tail vein, to allow further IV injection. Anesthesia was then switch to medetomidine (Domitor®, Pfizer, 0.1 mg/kg). Isoflurane, N2O and O2 were stopped 10 min after the bolus injection. A waiting time of a least 20 min was then respected to discard N2O and isoflurane from the mouse body and to allow the stabilization of the CBF. Preliminary experiments were performed in order to evaluate physiological parameters, especially blood pCO2 and pH, in mice placed in experimental conditions. Blood pressure was measured using the tail-cuff method. These experiments revealed normal values for these parameters in our conditions. These controls were repeated regularly.
Pharmacology
During experiments, different treatments were injected through the tail vein of the mice. The total volume injected was 300 µL as follows: a bolus of 150 µL, 10 min before the beginning of the CBF response measurement, followed by an infusion (10 µL/min). Dosages were as follows: rtPA = 10 mg/kg; AP5 (2-amino-5-phosphonopentanoic acid, noncompetitive antagonist of NMDARs) = 0.3 mg/kg; MK-801 (competitive antagonist of NMDARs) = 0.3 mg/kg; Glunomab® and control IgG = 5 mg/kg. As a control, HEPES (0.3 M) and saline (0.9%) were injected.
rtPA preparation
rtPA was prepared from Actilyse® (Boehringer Ingelheim). In order to eliminate the arginine buffer contained in the commercial solution, rtPA was dialyzed in HEPES buffer (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 0.3 M, pH 7.4, Sigma-Aldrich) using dialysis cassettes (Slide-A-Lyzer® 10 K; ThermoScientific).
Laser speckle flowmetry
CBF responses to whiskers stimulations in medetomidine-anesthetized mice were determined using laser speckle flowmetry directly through the skull. Images were acquired using a Laser Speckle Contrast Imager (moorFLPI-2, Moor Instruments; Sample interval: 1 Hz, exposure time: 20 ms, FOV = 6 mm × 11.3 mm, images resolution 752 × 580). Stimulation were performed by mechanically shaking (4–5 Hz) mice whiskers on the left side over a period of 30 s, followed by a 90-s rest periods, three times (total time = 390 s). Images were analyzed using the moorFLPIReviewV40 software (moorFLPI-2, Moor Instruments). The average of the images obtained during rest was subtracted to the average of the images obtained during stimulation, revealing the area where the CBF had changed during the stimulation. The average CBF signal of this area was then extracted, and the percent of increase of the CBF was calculated based on the baseline signal during rest. Values of the 3 stimulations were then averaged (See Suppl. Figure 1).
Intravital microscopy
The day before the experiment, animals were anesthetized with isoflurane and placed in a stereotaxic device. The skull was exposed, and the area above the S1bf cortex was thinned with a drill. On the day of the experiment, anesthetized mice were placed in a stereotaxic device and aqueous medium was deposed between the thin-skull window and the ×25 immersive objective. Just before the imaging, an IV injection of FITC Dextran (100 µL; 70,000 kDa; Sigma Aldrich) was performed to visualize the lumen of blood vessels. Acquisitions were performed using a Leica TCS SP5 MP microscope on confocal mode (1392 × 1040 pixels; 0702 µm/pixel, 145 ms/frame).
Mechanical whiskers stimulation was performed during a single 10-s period. Images were analyzed using ImageJ software (NIH) as follows: images obtained during rest and during stimulation were averaged separately and a line ROI was drawn perpendicularly to the vessel. An intensity curve of fluorescence along the line was obtained from this ROI, and vessel diameter was measured by measuring the distance between the two borders of the curve at 50% of the peak of intensity. Vessels were then separated in three groups depending on their resting diameter size (>25 µm; 25 µm>× >15 µm; <15 µm).
Hydrodynamic transfection
Hydrodynamic transfection was conducted as previously described.25 Mice were injected with 100 µg of pLIVE plasmid alone, as a control, pLIVE plasmid encoding wild-type rat tPA (pLIVE-tPA) or tPA mutants (pLIVE-tPA-K2*). Briefly, a large volume (10% of body weight) of plasmid-containing saline buffer (0.9% NaCl) was injected in the tail vein of the mice in less than 5 s. This approach with the pLIVE vector allows the constitutive hepatic expression of the insert during few days. Laser speckle flowmetry was realized before the transfection (see Laser speckle flowmetry section). At the end of this first measurement, mice received an intraperitoneal injection of atipamezole (0.5 mg/kg, Antisedan®, Pfizer) to facilitate their wake up; 48 h after the transfection, a second CBF measurement was realized (see Laser speckle flowmetry section). Right after, blood sampling was performed by retro-orbital puncture. Blood was anticoagulated using citrate. Blood samples were then subject to a 1500g centrifugation for 15 min, to separate cells from plasma. Supernatant was separated and subjected to a 10,000g centrifugation, to separate plasma from remaining platelets.
Zymography
The presence of free plasmatic tPA after hydrodynamic transfection was detected by direct fibrin autography following sodium dodecylsulphate polyacrylamide gel electrophoresis (SDS-PAGE) performed as previously described.26 Plasma samples and reference tPA (0.25 nM, 10 µL) were subjected to SDS electrophoresis (8% polyacrylamide gels, under non-reducing conditions). SDS was then exchanged with 2.5% Triton X-100. After washing off excess Triton X-100 with distilled water, the gel was carefully overlaid on a 1% agarose gel containing 1 mg/mL bovine fibrinogen, 100 nM plasminogen and 0.2 NIH U/mL of bovine thrombin. Zymograms were allowed to develop at 37°C for 12 h and photographed at regular intervals using dark-ground illumination. Active proteins in plasma samples were identified by reference to the migration of known tPA.
Functional ultrasound imaging
Introduced in Macé et al.,24 functional ultrasound (fUS) enables a fast tracking of hemodynamic changes in depth in animal brains summited to an external stimulation. fUS sequences and parameters in the present study reproduce the methology described in Tiran et al.,27 where fUS was applied to different models of rodents, especially in mice’s and describes the response to whiskers stimulation.
The day before the experiment, animals were anesthetized with isoflurane and placed in a stereotaxic frame. The skin was cut to expose the skull and the area above the S1bf cortex was thinned with a drill.
The animal preparation was then the same as for the Laser speckle flowmetry experiments (see Laser speckle flowmetry section), except that ultrasound gel was applied between the ultrasound probe and the mouse skull to ensure good acoustic coupling. The probe was positioned over the coronal plane corresponding to the somatosensory barrel field cortex (S1bf; bregma −1.5 mm). Stimulations were performed the same way as for the Laser speckle flowmetry experiments (see Laser speckle flowmetry section).
Ultrafast acquisition was performed using an ultrasonic sequence based on compounded plane wave transmission (11 angles from −10° to 10° by steps of 2°) and a 15 MHz ultrasonic probe (Vermon, France, 100 µm × 100 µm in plane pixels, 300 µm slice thickness, elevation focus 8 mm) with a frame rate of 500 Hz; 200 images were acquired every second for 390 s. For each block of 200 images, blood signal was extracted from tissue signal using singular value decomposition filters28 and excluding the 60 most energetic singular values.
For each pixel, the correlation coefficient was calculated between the normalized Power Doppler (PD) intensity along time and a step function following the stimulation pattern. An activation map was reconstructed by keeping only pixels with a correlation coefficient higher than 0.2 and superimposing the corresponding pixels on the mean Doppler image. An artefact caused by the mechanical respirator was masked on the bottom left of the image, without significant impact on the activation in the cortex.
The activated area was determined as the pixels with a correlation coefficient higher than 0.2 corresponding to two times the spatial standard deviation of CBV baseline estimated in a non-activated area. The quantification of the relative PD increase is performed of the mean PD signal in the activated area.
Immunohistochemistry on isolated brain vessels
Brain vessels of C57BL/6 mice were isolated as previously described.29 Deeply anesthetized mice were transcardially perfused with cold heparinized saline (15 mL/min). Brain were then dissociated in a solution of HEPES and HBSS and then centrifuged at 2000g at 4°C during 10 min. The pellet was then resuspended in a solution of HEPES/HBSS and Dextran (Dextran from Leuconostoc spp. Mr ∼70,000, Sigma-Aldrich) and centrifuged at 4400g at 4°C during 15 min. Vessels were then suspended in a solution of HEPES/HBSS and BSA 1% and filtrated on a 20-µm mesh filter. Vessels were then detached from the filter in a PBS solution. Vessels were then put on a poly-lysine coated slides, cryoprotected overnight in a 20% sucrose PBS solution and fixated during 15 min with PBS 0.1 M. pH 7.4 containing 2% paraformaldehyde and 0.2% picric acid. Slide was stored at −80°C before processing. Vessels were then co-incubated overnight with rabbit anti-PDGF-Rβ (1:1000, ab32570, Abcam) primary antibodies and FITC-conjugated mouse anti-αSMA (1:500, ab8211, Abcam) primary antibodies, or with goat anti-GluN1 (1:200, sc-1467, Santa-Cruz) primary antibodies, FITC-conjugated mouse anti-αSMA (1:500, ab8211, Abcam) primary antibodies and rabbit anti-laminin (1:2000, ab11575, Abcam) primary antibodies. Primary antibodies were revealed using Fabʹ2 fragment anti rabbit and goat IgG linked to CY3 and Alexa Fluor 647 (1:600, Jackson ImmunoResearch) co-incubated 90 min at room temperature. Vessels were then coverslipped using mounting medium containing DAPI. Images were digitally captured using an epifluorescence microscope (Leica DM6000). Fluorescent intensity of the GluN1 staining in vessels was assessed using ImageJ software (NIH).
Statistical analysis
Results are expressed as the mean ± the standard deviation (SD). For curves displayed, curves in transparency represent the SEM in order to preserve the scale. Statistical analysis was performed using Mann–Whitney test, Wilcoxon test or t-test with the Statistica software (Statsoft). For the GluN1 fluorescence intensity and the pool meta-analysis, normality was assessed using D'Agostino and Pearson normality test. Values were considered statistically different if probability value, p < 0.05.
Results
Circulating tPA contributes to the increase of CBF during NVC
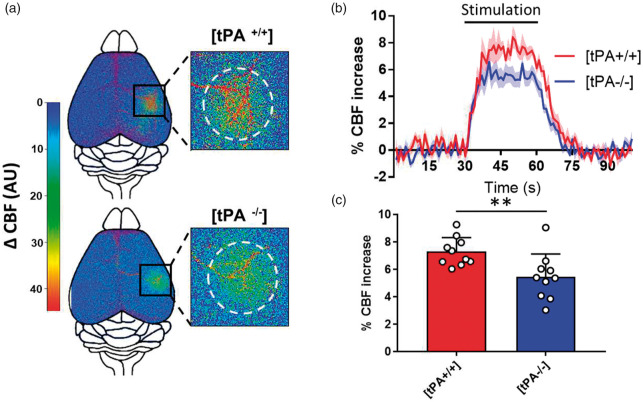
We examined the CBF increase produced by the activation of the whisker barrel cortex in wild type (WT) versus tPA-deficient mice (tPA−/−) by using time-lapse laser speckle flowmetry (Suppl. Figure 1). Using this procedure, we showed that the CBF increase evoked by whiskers stimulation is significantly impaired in tPA-deficient mice (tPA−/−) when compared to WT littermates (Figure 1(a) to (c); +7.29% of CBF increase for WT mice, n = 10, vs. +5.45% for tPA−/− mice, n = 10, i.e. −25.24% for tPA−/− mice compared to tPAWT mice, p-value = 0.0042). There is no modification of the resting CBF either before or after whiskers stimulation, neither heterogeneity of CBF increase in between the three stimulations performed prior and after treatments (Suppl. Figure 2, n = 33, p = 0.1577). Physiological parameters, including heart rate and arterial O2 saturation were not affected by whiskers stimulation (Suppl. Figure 3(a) and (b), n = 5). Furthermore, there is no difference in blood pressure (mean, systolic or diastolic) between tPA WT and tPA−/− mice (Suppl. Figure 3(c), n = 12 per group), as well as no difference in coagulation factors such as fibrinogen or plasminogen, or cleaved kininogen between tPA WT and tPA−/− mice (Suppl. Figure 4, n = 5 per group).
Figure 1.
Functional hyperemia is reduced in tPA−/− mice. (a) Pseudo colored representative subtraction maps of the CBF highlighting CBF change during whiskers stimulation of tPA WT and tPA−/− mice. Maps were obtained by subtracting Laser Doppler speckle images recorded during stimulation to images recorded during rest. Color intensity goes from blue (no change during stimulation) to red (strong CBF increase during stimulation). (b) Mean CBF signal traces from the S1bf during whiskers stimulation of tPA WT ( ) and tPA−/− (
) and tPA−/− ( ) mice extracted from Laser Doppler speckle images (curves in transparency define the SEM, n = 10 per group). (c) Quantification of the CBF increase during whiskers stimulation in tPA WT and tPA−/− mice. Circles represent values for each mouse (Mean ± SD, Mann–Whitney test, n = 10 per group).
) mice extracted from Laser Doppler speckle images (curves in transparency define the SEM, n = 10 per group). (c) Quantification of the CBF increase during whiskers stimulation in tPA WT and tPA−/− mice. Circles represent values for each mouse (Mean ± SD, Mann–Whitney test, n = 10 per group).
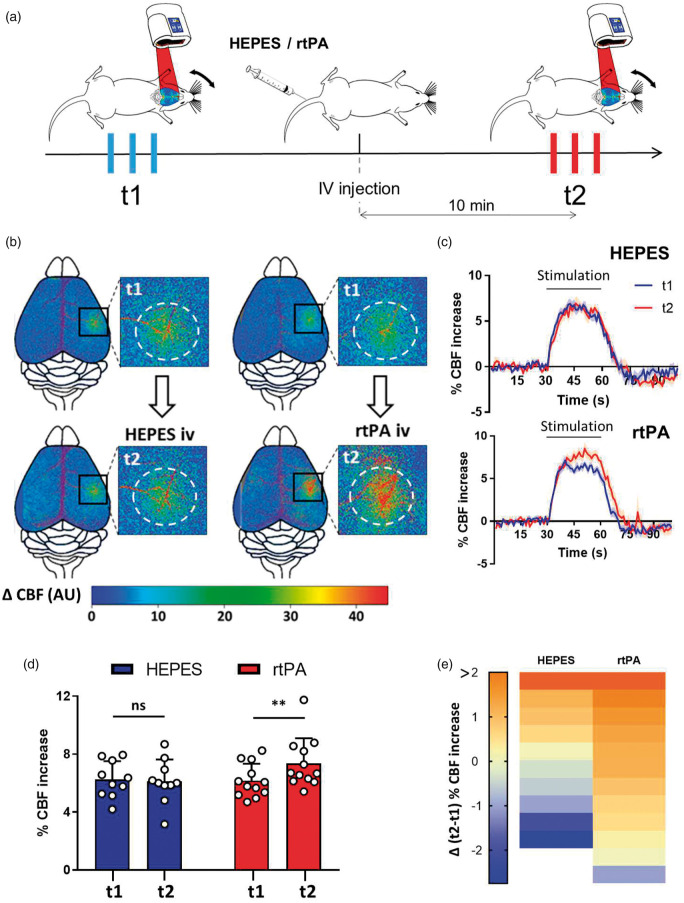
To specifically study the role of circulating tPA, we investigated whether intravenously injected recombinant tPA could rescue the phenotype observed in tPA−/− mice. Arginine-free recombinant tPA (rtPA, Altilyse®, Boehringer Ingelheim) was injected intravenously (10 mg/kg, 200 µL injected 10% in bolus followed by 90% in infusion for 15 min (300 µL/h)). PAI-1 level is higher in tPA−/− mice (Suppl. Figure 4), but the dose of rtPA injected is believed to be sufficient to saturate all available PAI-1 in the blood. The increase of the CBF in response to whiskers stimulation was measured prior to (t1) and 10 min after (t2) the initiation of rtPA infusion, each animal being thus its own control (Figure 2(a)). Control group was injected with HEPES. Neither injection of rtPA or HEPES influenced resting CBF at t2 (Suppl. Figure 5(a) and (b)). Control experiments were performed by injecting rtPAAlexa555 in the circulation to determine whether vascular rtPA may reach the brain parenchyma approximatively 10 min after injection (Suppl. Figure 5(c) to (f)). In these conditions, our data show that the vascular rtPA did not reach the brain parenchyma (somatosensorial cortex) when evaluated 10 min after intravenous injection. A positive control shows the extravasation of vascular rtPAAlexa555 in the median eminence, an area with fenestrated vessels.
Figure 2.
Vascular rtPA increases the functional hyperemia in tPA−/− mice. (a) Schematic representation of the experimental timeline, including a first train of stimulations as a control followed by an IV injection and a second train of stimulations 10 min after the injection. (b) Pseudo colored representative subtraction maps of the CBF highlighting CBF change during whiskers stimulation of tPA−/− mice before and after the injection of HEPES (200 µL, 0.3 M) or rtPA (200 µL, 10 mg/kg). Color intensity goes from blue (no change during stimulations) to red (strong CBF increase during stimulation). (c) Mean CBF signal trace from the S1bf during whiskers stimulation of tPA−/− mice before and after the injection of HEPES (200 µL, 0.3 M;  ) or rtPA (200 µL, 10 mg/kg;
) or rtPA (200 µL, 10 mg/kg;  ) extracted from Laser Doppler speckle images (curves in transparency define the SEM, n = 10 for the HEPES group, n = 12 for the rtPA group). (d) Quantification of the CBF increase during whiskers stimulation in tPA−/− mice before (t1) and after (t2) IV injection of HEPES (200 µL, 0.3 M) or rtPA (200 µL, 10 mg/kg). Circles represent values for each mouse (Mean ± SD, Wilcoxon test, n = 10 for the HEPES group, n = 12 for the rtPA group). (e) Diagram showing the evolution (delta) of the hemodynamic response of each mouse between the control condition and after the IV injection of HEPES (200 µL, 0.3 M) or rtPA (200 µL, 10 mg/kg).
) extracted from Laser Doppler speckle images (curves in transparency define the SEM, n = 10 for the HEPES group, n = 12 for the rtPA group). (d) Quantification of the CBF increase during whiskers stimulation in tPA−/− mice before (t1) and after (t2) IV injection of HEPES (200 µL, 0.3 M) or rtPA (200 µL, 10 mg/kg). Circles represent values for each mouse (Mean ± SD, Wilcoxon test, n = 10 for the HEPES group, n = 12 for the rtPA group). (e) Diagram showing the evolution (delta) of the hemodynamic response of each mouse between the control condition and after the IV injection of HEPES (200 µL, 0.3 M) or rtPA (200 µL, 10 mg/kg).
We found that intravenous rtPA infusion led to a significant increase of the hyperemia induced by whiskers stimulation (Figure 2(b) to (e); HEPES injected tPA−/− mice, 6.26% at t1 vs. 6.12% at t2, i.e. −2.19% for t2/t1, p-value = 0.9219; rtPA injected tPA−/− mice, 6.17% at t1 vs. 7.36% at t2, i.e. +19.39% for t2/t1, p-value = 0.0068; n = 10/12). These data demonstrate that the rtPA present in the bloodstream contributes to the increase of CBF during NVC.
A similar set of experiments was performed using wild-type animals instead of tPA−/− mice, in order to determine whether increasing the levels of circulating tPA may promote NVC. Our data demonstrate that even in the presence of endogenous tPA, increasing rtPA levels in the bloodstream leads to an increase of the hyperemia induced by whiskers stimulation (Suppl. Figure 6(a) to (d); HEPES injected tPA WT mice, 6.31% at t1 vs. 6.17% at t2, i.e. −2.21% for t2/t1, p-value = 0.8438; rtPA injected tPA WT mice, 6.17% at t1 vs. 7.12% at t2, i.e. +15.39% for t2/t1, p-value = 0.0117; n = 8–9 per group). A pooled analysis comparing all tPA−/− mice (n = 38), treated or not with rtPA during our experiments confirms that plasmatic tPA promotes CBF increase induced by whiskers stimulation (Suppl. Figure 7(a) to (c); HEPES injected tPA−/− mice, 6.27% at t1 vs. 6.31% at t2, i.e. +0.64% for t2/t1, p-value = 0.8982; rtPA injected tPA−/− mice, 6.05% at t1 vs. 6.98% at t2, i.e. +15.18% for t2/t1, p-value = 0.001; n = 38 in each group).
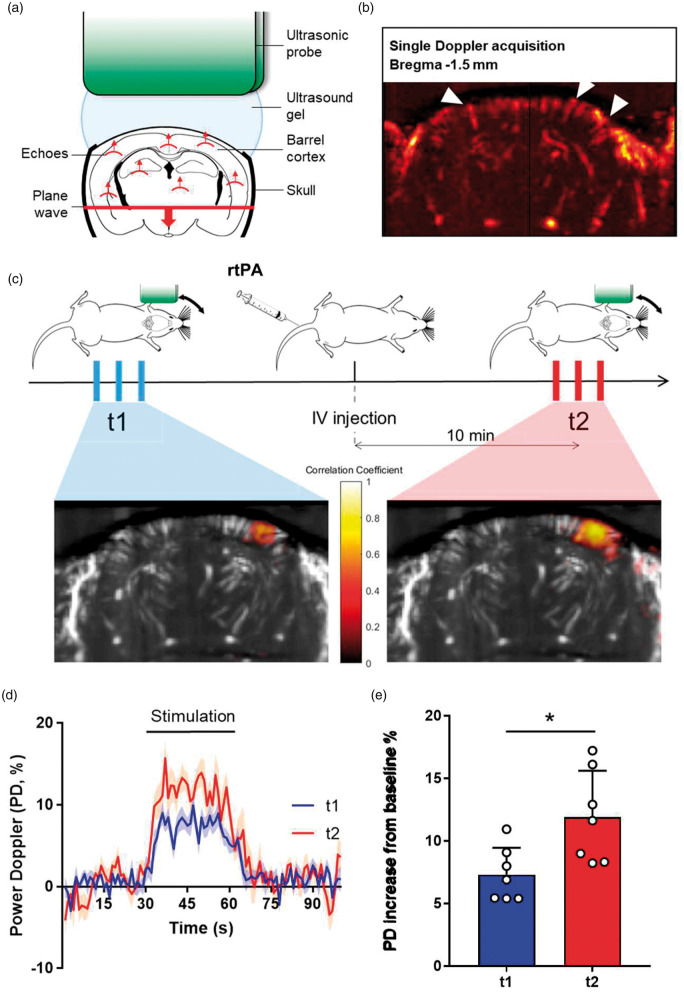
We then used in vivo functional ultrasound (fUS) imaging to confirm the function of tPA on NVC (Figure 3(a) to (e)). As reported above, when injected intravenously, rtPA led to an increase of the hyperemia induced by whiskers stimulation in the corresponding barrel cortex (+7.31% at t1 vs. +11.91% at t2 for rtPA-treated tPA−/− mice (n = 7), i.e. +62.93% for t2/t1, p-value = 0.0042, n = 7). Altogether, these multimodal data demonstrate that intravenous rtPA is implicated in the modulation of the CBF increase during NVC.
Figure 3.
The promotion of the functional hyperemia by rtPA is not restricted to the surface of the brain but involved the whole barrel cortex. (a) Schematic representation of functional ultrasound acquisition. (b) Ultrasensitive Doppler image of the brain of a C57BL/6 mouse through a thinned skull window, revealing blood vessels, and particularly penetrating arterioles (white arrows). (c) Schematic representation of the experimental timeline, including a first train of stimulations as control followed by an IV injection of rtPA and a second train of stimulations 10 min after the injection. Bottom images represent an example of the activation maps when stimulating the left whiskers before and after the injection of rtPA. Colormap corresponds to the correlation coefficient between the normalized PD intensity along time and a step function representing the stimulation pattern. (d) Relative augmentation of the Power Doppler in the activated area (seen in c) during whiskers stimulation of tPA−/− mice before ( ) or after (
) or after ( ) intravenous injection of rtPA (curves in transparency define the SEM, n = 7). (e) Quantification of the relative PD intensity increase in the activated area (seen in c) during whiskers stimulation in tPA−/− mice. Circles represent values for each mouse (Mean ± SD, Wilcoxon test, n = 7).
) intravenous injection of rtPA (curves in transparency define the SEM, n = 7). (e) Quantification of the relative PD intensity increase in the activated area (seen in c) during whiskers stimulation in tPA−/− mice. Circles represent values for each mouse (Mean ± SD, Wilcoxon test, n = 7).
Circulating tPA enhances Whiskers stimulation-induced vasodilation, a phenomenon restricted to larger vessels
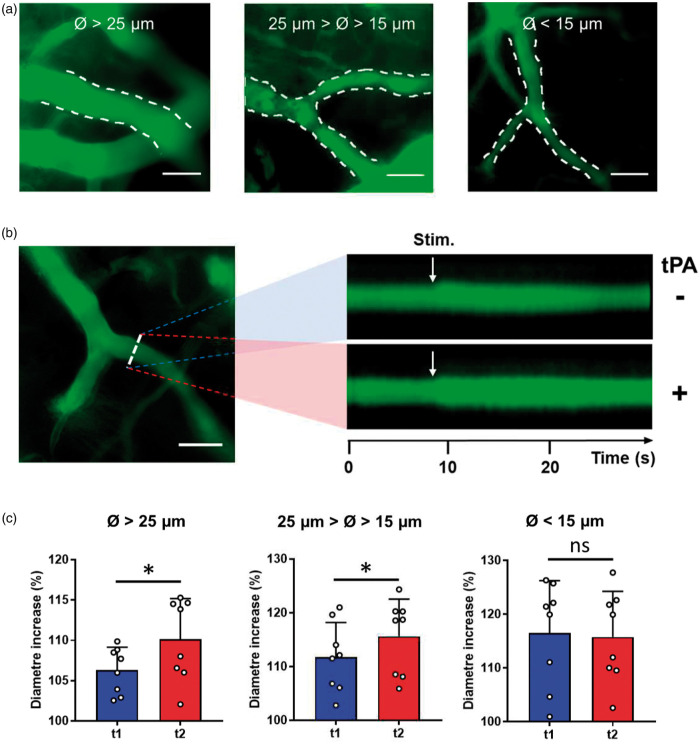
We used intravital microscopy in tPA−/− mice with or without intravenous infusions of rtPA to examine responses of individual vessels. We observed that rtPA treatment leads to a more pronounced dilation of the vessels in the barrel cortex during whiskers stimulation (Figure 4(a) and (b)). We then separated vessels according to their diameter (d) as follows: d >25 µm, 15 < d < 25 and d < 15 µm (Figure 4(c)). All types of vessels showed a dilation in response to whiskers stimulation: +6.3% for vessels of d >25 µm; +11.8% for 15<d < 25 µm; +16.5% for d < 15 µm). However, a differential effect of rtPA was observed according to the diameter of vessels. Indeed, although rtPA treatment led to an increase of the dilation of the larger vessels, rtPA did not affect the dilation of small vessels (Figure 4(b) and (c); d >25 µm, +3.8%, p = 0.0391; 15<d < 25 µm, +3.8%, p = 0.0391; d < 15 µm, −0.7%, p = 0.6406; n = 8 vessels in each group).
Figure 4.
Vascular rtPA promotes whiskers stimulation induced dilation of large vessels. (a) Intravital microscopy images of vessels of different diameters revealed by in IV injection of FITC dextran. Images obtained from the S1bf cortex of tPA−/− mice (scale bar = 50 µm). (b) Left: Intravital microscopy images of a vessel from the S1bf cortex of a tPA−/− mouse (scale bar = 50 µm). Vessels were labelled using an IV injection of FITC dextran. Right: line-scans of the same vessel during whiskers stimulation before (top) and after (bottom) rtPA (10 mg/kg) injection. (c) Quantification from intravital microscopy images of the vessels dilation during whiskers stimulation according to their diameters before ( ) and after (
) and after ( ) rtPA (10 mg/kg) injection. Circles represent values for each vessel (Mean ± SD, Wilcoxon test, n = 8–9 per group).
) rtPA (10 mg/kg) injection. Circles represent values for each vessel (Mean ± SD, Wilcoxon test, n = 8–9 per group).
NMDAR expressed on endothelial cells mediates the tPA-dependent modulation of NVC
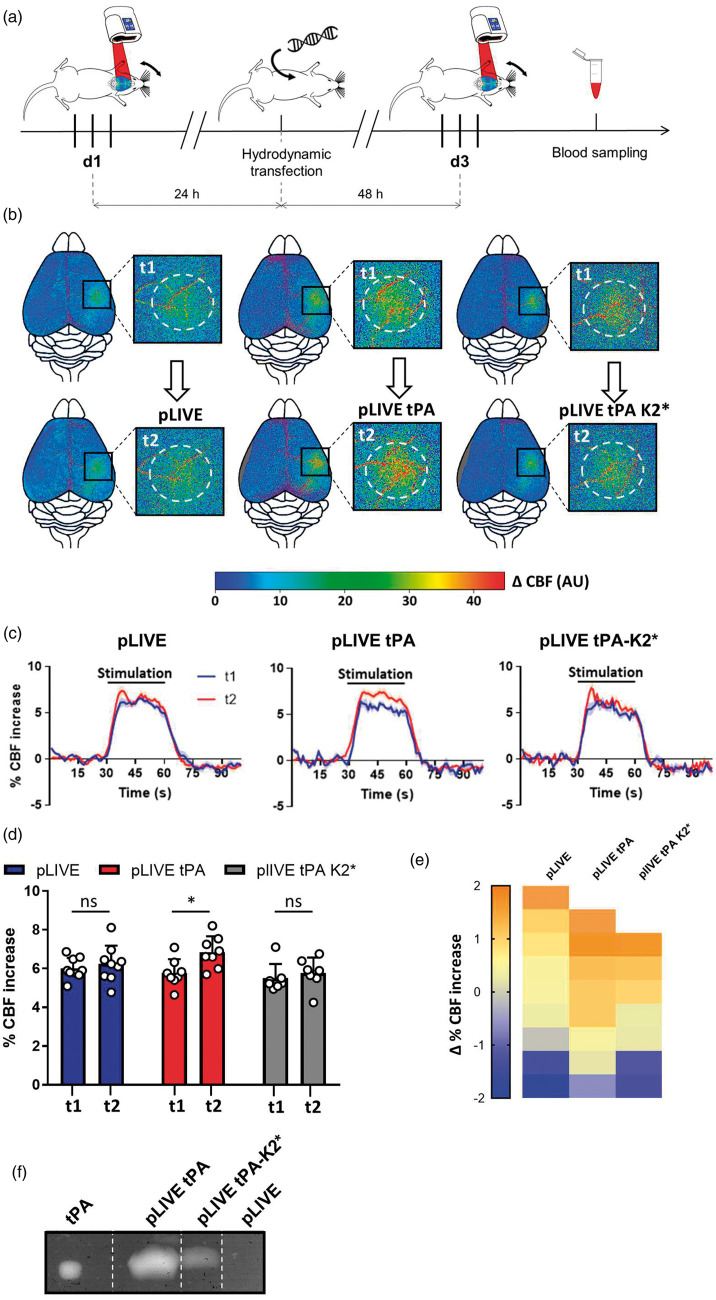
We then postulated that the tPA present in the bloodstream could influence NVC by its ability to modulate NMDAR signaling on endothelial cells. In order to address this question, we used a molecular tool, tPA-K2*, a tPA mutated on the amino-acid in position 254 of the Lysine Binding Site (LBS) containing kringle 2 domain which is not capable to bind and activate NMDAR.30 Then, we proposed that a chronic overexpression of tPA by the liver cells and its subsequent release in the circulation may promote the increased CBF induced by whiskers stimulation in tPA−/− mice. We thus used a set of expression vectors driven by a liver-specific promoter (pLIVE plasmids) that promotes sustained tPA secretion in the circulation, as we previously characterized25 encoding for either a WT tPA (tPAWT) or a tPA-K2* (pLIVE-tPAWT, pLIVE-tPA-K2*; Figure 5). The liver was transfected in vivo by a hydrodynamic transfection of pLIVE constructs. Each animal was tested before (d1) and after (d3) hydrodynamic transfection and was thus its own control (Figure 5(a)). Our data showed that although tPAWT expression promoted the CBF increase induced by whiskers stimulation, the expression of tPA-K2* did not (Figure 5(b) to (e); pLIVE transfected tPA−/− mice, 6.01% at d1 vs. 6.24% at d3, i.e. +3.82% for d3/d1, p-value = 0.4961; pLIVE-tPAWT transfected tPA−/− mice, 5.77% at d3 vs. 6.86% at d1, i.e. +18.89% for d3/d1, p-value = 0.0391; pLIVE-tPA-K2* transfected tPA−/− mice, 5.50% at d1 vs. 5.77% at d3, i.e. +4.90% for d3/d1, p-value = 0.6875; n = 7–9). To control the efficiency of the transfection, we performed zymography assays from blood samples collected at d3 to measure tPA plasmatic concentration and activity.25 As expected, we detected tPA proteolytic activity in the plasma of tPA−/− mice transfected with pLIVE-tPAWT or pLIVE-tPA-K2*, but not with the pLIVE empty plasmid (Figure 5(f)). These data demonstrate that tPA can promote the CBF increase induced by whiskers stimulation through a mechanism involving its K2 domain, suggesting a mechanism dependent on NMDAR.
Figure 5.
Chronic expression of tPA restrained to the blood circulation promotes functional hyperemia in tPA−/− mice. (a) Schematic representation of the experimental timeline, including a first train of stimulations as control followed 24 h later by the hydrodynamic transfection, and by a second train of stimulations 48 h after the transfection; Three different plasmids were transfected: pLIVE (empty), pLIVE-tPA and pLIVE- tPA-K2*. (b) Pseudo colored representative subtraction maps of the CBF highlighting CBF change during whiskers stimulation of tPA−/− mice before and after the transfection. Color intensity goes from blue (no change during stimulations) to red (strong CBF increase during stimulation). (c) Mean CBF signal trace from the S1bf during whiskers stimulation of tPA−/− mice before ( ) and after (
) and after ( ) the transfection extracted from Laser Doppler speckle images (curves in transparency define the SEM, n = 7–9 per group). (d) Quantification of the CBF increase during whiskers stimulation in tPA−/− mice before (t1) and after (t2) the transfection. Circles represent values for each mouse (Mean ± SD, Wilcoxon test, n = 7–9 per group). (e) Diagram showing the evolution (delta) of the hemodynamic response of each mouse between the control condition and after the hydrodynamic transfection. (f) Representative fibrin-agar zymography assays performed from plasma of pLIVE, pLive-tPA and pLIVE-tPA-K2* transfected mice, which demonstrate the presence of free plasmatic tPA and tPA-K2* after the transfection. rtPA was used as standard in the zymography assays.
) the transfection extracted from Laser Doppler speckle images (curves in transparency define the SEM, n = 7–9 per group). (d) Quantification of the CBF increase during whiskers stimulation in tPA−/− mice before (t1) and after (t2) the transfection. Circles represent values for each mouse (Mean ± SD, Wilcoxon test, n = 7–9 per group). (e) Diagram showing the evolution (delta) of the hemodynamic response of each mouse between the control condition and after the hydrodynamic transfection. (f) Representative fibrin-agar zymography assays performed from plasma of pLIVE, pLive-tPA and pLIVE-tPA-K2* transfected mice, which demonstrate the presence of free plasmatic tPA and tPA-K2* after the transfection. rtPA was used as standard in the zymography assays.
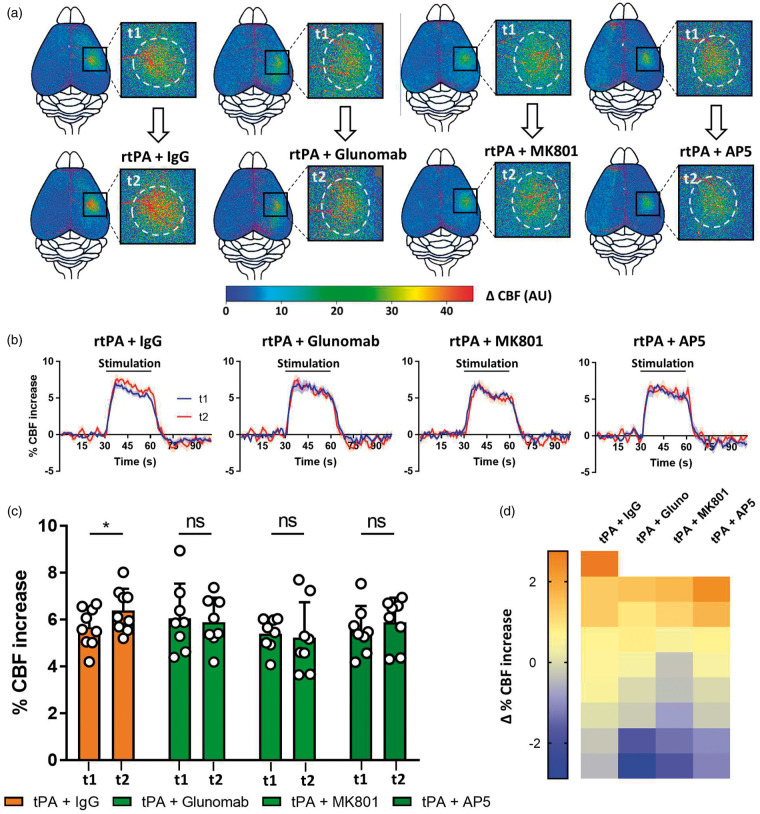
Since the mutation of the LBS within the kringle 2 domain of tPA may also interfere with NMDAR-independent functions of tPA, we performed complementary experiments using Glunomab®. Glunomab® is a monoclonal antibody targeting the binding site of tPA on the GluN1 subunit of NMDAR, used as a competitive antagonist of tPA on NMDAR.21,31 The aim of this experiment was not to target tPA itself, but directly the binding site of tPA on NMDAR and thus to block subsequent NMDAR signaling.21,31 In agreement with the above data and our hypothesis that circulating tPA may contribute to NVC by acting on NMDAR expressed on endothelial cells, Glunomab® blocked the rtPA-induced increase of hyperemia during whiskers stimulation (Figure 6(a) to (d); rtPA + control IgG injected tPA−/− mice, 5.66% at t1 vs. 6.39% at t2, i.e. +12.89% for t2/t1, p-value = 0.0391; rtPA + Glunomab® injected tPA−/− mice, 6.05% at t1 vs. 5.89% at t2, i.e. −2.64% for t2/t1, p-value > 0.9999; n = 8–9).
Figure 6.
Inhibition of the interaction between vascular rtPA and NMDARs prevents the rtPA-induced potentiation of functional hyperemia. (a) Pseudo colored representative subtraction maps of the CBF highlighting CBF change during whiskers stimulation of tPA−/− mice before and after the injection of rtPA (10 mg/kg) and a control IgG (5 mg/kg), rtPA (10 mg/kg) and Glunomab® (5 mg/kg), rtPA (10 mg/kg) and MK-801 (0.4 mg/kg), or rtPA (10 mg/kg) and AP5 (0.4 mg/kg). Color intensity goes from blue (no change during stimulations) to red (strong CBF increase during stimulation). (b) Mean CBF signal trace from the S1bf during whiskers stimulation of tPA−/− mice before ( ) and after (
) and after ( ) the injection of rtPA (10 mg/kg) and a control IgG (5 mg/kg), rtPA (10 mg/kg) and Glunomab® (5 mg/kg), rtPA (10 mg/kg) and MK-801 (0.4 mg/kg), or rtPA (10 mg/kg) and AP5 (0.4 mg/kg) extracted from Laser Doppler speckle images (curves in transparency define the SEM, n = 8–10 per group). (c) Quantification of the CBF increase during whiskers stimulation in tPA−/− mice before (t1) and after (t2) IV injection of rtPA (10 mg/kg) and a control IgG (5 mg/kg), rtPA (10 mg/kg) and Glunomab® (5 mg/kg), rtPA (10 mg/kg) and MK-801 (0.4 mg/kg), or rtPA (10 mg/kg) and AP5 (0.4 mg/kg). Circles represent values for each mouse (Mean ± SD, Wilcoxon test, n = 8–10 per group). (d) Diagram showing the evolution (delta) of the hemodynamic response of each mouse between the control condition and after the IV injection of rtPA (10 mg/kg) and a control IgG (5 mg/kg), rtPA (10 mg/kg) and Glunomab® (5 mg/kg), rtPA (10 mg/kg) and MK-801 (0.4 mg/kg), or rtPA (10 mg/kg) and AP5 (0.4 mg/kg).
) the injection of rtPA (10 mg/kg) and a control IgG (5 mg/kg), rtPA (10 mg/kg) and Glunomab® (5 mg/kg), rtPA (10 mg/kg) and MK-801 (0.4 mg/kg), or rtPA (10 mg/kg) and AP5 (0.4 mg/kg) extracted from Laser Doppler speckle images (curves in transparency define the SEM, n = 8–10 per group). (c) Quantification of the CBF increase during whiskers stimulation in tPA−/− mice before (t1) and after (t2) IV injection of rtPA (10 mg/kg) and a control IgG (5 mg/kg), rtPA (10 mg/kg) and Glunomab® (5 mg/kg), rtPA (10 mg/kg) and MK-801 (0.4 mg/kg), or rtPA (10 mg/kg) and AP5 (0.4 mg/kg). Circles represent values for each mouse (Mean ± SD, Wilcoxon test, n = 8–10 per group). (d) Diagram showing the evolution (delta) of the hemodynamic response of each mouse between the control condition and after the IV injection of rtPA (10 mg/kg) and a control IgG (5 mg/kg), rtPA (10 mg/kg) and Glunomab® (5 mg/kg), rtPA (10 mg/kg) and MK-801 (0.4 mg/kg), or rtPA (10 mg/kg) and AP5 (0.4 mg/kg).
Furthermore, these data were confirmed by using common NMDAR antagonists: MK-801 (Dizocilpine), a non-competitive NMDAR antagonist, and D-2-amino-5-phosphonopentanoate (AP5), a competitive antagonist of NMDAR (Figure 6(a) to (d)). Both MK-801 and AP5 blocked the rtPA effect on the CBF increase induced by whiskers stimulation (rtPA + MK801 injected tPA−/− mice, 5.39% at t1 vs. 5.23%, at t2 i.e. −2.96% for t2/t1, p-value = 0.7422; rtPA + AP5 injected tPA−/− mice, 5.54% at t1 vs. 5.89% at t2, i.e. +6.31% for t2/t1, p-value = 0.6406; n = 9 per groups). Control experiments with injection of MK801 or AP5 alone showed no effect of these molecules on NVC (Suppl. Figure 8(a) to (d)). Altogether, these data demonstrate that the plasmatic tPA triggers NVC through a mechanism dependent of its ability to activate the NMDAR present at the surface of endothelial cells.
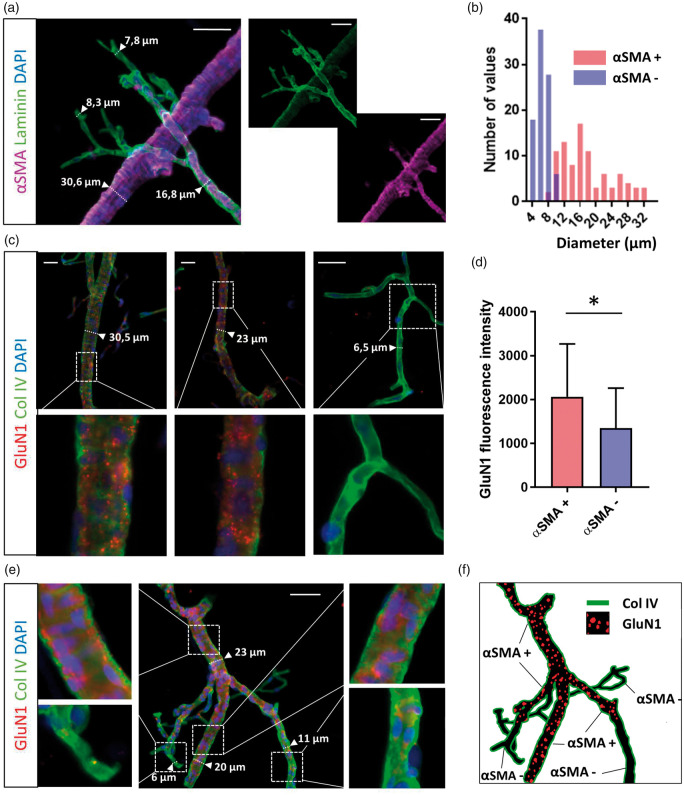
To understand why the dilation of only larger vessels is affected by rtPA, we sought to investigate the distribution of NMDAR in brain vessels. To do so, we performed immunostaining for GluN1-containing NMDAR from isolated brain vessels. Vessels were discriminated by positive or negative immunostaining for alpha-SMA (smooth muscle actin; Figure 7(a)). As expected, αSMA staining was positive for vessels of more than 8–9 µm in diameter (Figure 7(b)). In agreement with the above data, immunostainings revealed the presence of NMDAR preferentially on alpha-SMA positive vessels, i.e. arteries and arterioles (Figure 7(c) to (f); n = 34 for alpha-SMA positive vessels, n = 29 for alpha-SMA negative vessels, p-value = 0.011).
Figure 7.
Expression of NMDARs is restricted to arteries and arterioles. (a) Epifluorescence images of isolated vessels from C57BL/6 mice brain revealing (scale bar = 50 µm): α-SMA (purple), laminin (green) and cells nuclei (blue). (b) Quantification of the number of vessels positive (red) or negative (blue) for α-SMA staining depending on their diameter. Only vessels over 8–9 µm in diameter express α-SMA. (c) Epifluorescence images of isolated vessels from C57BL/6 mice brain revealing GluN1 subunit of the NMDAR (red), collagen IV (green) and cells nuclei (blue), depending on vessel diameter. (d) Quantification of the GluN1 staining fluorescence intensity in α-SMA positive vessels and in α-SMA negative vessels (ANOVA test, n = 34 for the α-SMA + group and n = 29 for the α-SMA – group, from four different mice). (e–f) Epifluorescence images of isolated cerebrovascular tree from C57BL/6 mice brain revealing GluN1 staining (red), Col IV (green) and cells nuclei (blue), and (f) corresponding schematic representation.
Overall, we demonstrate here for the first time that circulating tPA is necessary for the full increase of CBF during NVC by promoting the signaling of NMDAR expressed at the luminal side of endothelial cells, a phenomenon restricted to larger vessels (arteries and arterioles).
Discussion
CBF rapidly increases in response to neural activation, a phenomenon termed functional hyperemia or neurovascular coupling (NVC), providing a local supply of substrates and energy to neurons.1 NVC is a complex mechanism involving several different cell types and signaling pathways among the neurovascular unit, especially in neurons and astrocytes. Many different molecules are involved, and their mechanisms of action are not yet completely understood. At the synaptic level, NMDAR activation contributes to this process.1 Indeed, an important part of the vascular response is mediated by the activation of post-synaptic NMDAR. NMDAR are associated to the neuronal NO synthase (nNOS),32 whose activation leads to the production and release of the vasodilator nitric oxide (NO).33 Accordingly, NMDAR can regulate nNOS activity by increasing intra-cellular Ca2+ concentration and regulating the phosphorylation of the nNOS.34
The serine protease tPA (69 kDa), in addition to its role in fibrinolysis,6 has more recently emerged as a pleiotropic neuromodulator implicated in various aspects of brain functions, including learning and memory processes and anxiety behavior.7,14 In 2008, using a mouse model of whiskers stimulation,15 Park et al., proposed tPA as an actor of NVC15 for its ability to influence NMDAR signaling.8 When compared with wild-type mice, CBF increase in the barrel cortex of tPA deficient mice showed a sustained attenuation during whiskers stimulation. In this pioneer publication, superfusion of rtPA on the surface of the brain parenchyma of tPA-deficient mice restored NVC, with NMDAR and activation of NOS implicated as mediators of this response.15 Our present data confirm the role of tPA in NVC, providing the demonstration that in addition to its role at the synapse level, circulating tPA plays also a significant role. Indeed, although tPA deficient mice showed an impaired CBF increase induced by whiskers stimulation, this lack of response can be rescued after intravenous injection of rtPA. Besides similarities in our model and the one used by Park et al., we can note a difference in the increase produced by whiskers stimulation of mice. In their study, whiskers stimulation induced a 20% increase of the CBF, whereas our model provides an 8% increase of CBF. This can be explained by our use of a laser speckle imaging system instead of a Doppler laser fiber, allowing us to measure the blood flow of the entire brain surface through the intact skull, and giving us the advantage to measure the mean CBF increase of the whole S1bf. We also choose to anesthetize our mice with medetomidine. Medetomidine is an alpha-2-adrenergic receptor antagonist having little effect on brain vessels, and widely used for rodent functional magnetic resonance imaging (fMRI) studies. Medetomidine can be used for longitudinal studies such as hydrodynamical transfection model (Figure 5). On the other hand, medetomidine causes a bradycardia that can diminish the CBF increase. Overall, when looking at the difference in the CBF increase between tPA WT and tPA KO mice (Figure 1(b)), the effect size is comparable between studies.
Emerging evidence suggest an important role of endothelial cells in NVC, but the nature of how it contributes to the dialogue between neurons, smooth muscle cells and astrocytes in NVC remains unknown. Endothelial cells would indeed be the support of the propagation of a retrograde activity-induced signal that would triggers the dilation of larger vessels upstream of the activated area.1,2 Furthermore, the presence of NMDAR on endothelial cells was reported by several groups18–20 with roles in the control of the homeostasis of the BBB on healthy and injured brains.35 NMDAR was also reported to act on the cerebral blood vessel tone.36,37 Particularly, several studies have showed that NMDAR expressed on endothelial cells is involved in the dilation mechanism of middle cerebral arteries isolated from mice.38 The activation of endothelial NMDAR elicited by astrocytes thus leads to the production of NO by the eNOS and to the dilation of brain vessels.39,40 A recent study demonstrated that loss of function of endothelial NMDAR leads to a 50% reduction of CBF increased during whiskers stimulation.41 This study used grin1fl/fl· Cre+/− mice in which the Cre recombinase is driven by Tie2 promoter elements, leading to a complete loss of function of all endothelial NMDAR, while we have performed intravenous injections of NMDA receptors inhibitors in tPA−/− mice with an effect only on the luminal NMDA receptors. Additional investigations are needed to investigate whether the specific contribution of endothelial NMDAR to NVC is dependent on their localization side and signalization input (luminal or parenchymal). Interestingly, and consistent with our data, this loss of function has no effect on resting CBF.41
Accordingly, plasmatic tPA was also reported to have vasodilation properties on peripherical vessels and to reduce brain vessels reactivity,42–44 cerebral vascular resistance and systemic blood pressure,45 suggesting that tPA may modulate cerebrovascular tone during functional hyperemia. It is also interesting to note that an inactive tPA variant (tPA-S481A) was capable of preventing the impairment of autoregulation when administered 30 min after fluid percussion injury through the inhibition of the NMDAR over-activation.46 Similarly, a tPA variant tPA-(A296-299), characterized to prevent the binding of endogenous tPA to NMDAR, was reported to prevent impairment of cerebral autoregulation and necrosis of hippocampal neurons after stroke.47 Here we demonstrate that the circulating tPA can act on endothelial NMDAR to promote brain vessels dilation during functional hyperemia. Thus, deletion and rescue experiments were performed using a mutated form of tPA, tPA-K2* previously reported to not bind with NMDAR and thus to not promote their signaling,30 and a monoclonal antibody (Glunomab®) characterized as an antagonist of tPA on NMDAR signaling.21 Our data were also confirmed by using different modalities of in vivo imaging including the largely used laser speckle imaging and intravital microscopy imaging, in addition to the more recent promising methodology of fUS.24,27,48 Our data show that the intravenous injection of rtPA has no effect on the resting CBF (Suppl. Figure 1), which can be surprising given the previous discussed studies reporting an effect of both tPA and NMDAR on vascular tone.38,42 However, those studies were not performed in vivo, but mostly on isolated arteries, thus may be lacking other regulatory mechanisms compensating this phenomenon in vivo, such as autoregulation. tPA is also mostly inhibited in the blood circulation in physiological conditions, and a large part of the rtPA injected in our model is dedicated to the saturation of the inhibitors present in the blood stream. This could explain the lack of direct effect of rtPA on vessel tone just after its injection.
In addition, it is interesting to note that tPA was previously reported to influence NO and reactive oxygen species (ROS) production on endothelial cells by a mechanism dependent of NMDAR.19 This is to be compared with the study of LeMaistre et al., which has showed that the dilation of the middle cerebral artery mediated by the activation of NMDAR required an intact endothelium and the presence of eNOS, supporting the hypothesis of an action of tPA directly on the vessels.
Traditionally, regulation of CBF is thought to occur at the level of arterioles.3 However, capillaries in the brain are also wrapped by contractile cells called pericytes,49 for which the exact role in NVC is debated. However, we found that rtPA only promotes vasodilation of large vessels of at least 15 µm of diameter, corresponding to arterioles and arteries (Figure 4). Consistent with this, we show that the expression of NMDAR is restricted to the vessels of these diameters. These data are correlated with the fact that during NVC, NMDAR and the subsequent signaling pathways are associated with arteriolar dilation rather than capillaries, suggesting different signalization pathways between arteries and capillaries.50 This leads us to think that similar organization could occur in endothelial cells, and that endothelial NMDAR activation is specific of arterial dilation. In agreement with that, previous findings have shown that tPA is primarily associated with precapillary arterioles in the CNS.51
It is known for a long time that blood molecules, such as acetylcholine, bradykinin or ATP can regulate vascular tone and induce vessel dilation. Other events such as shear stress can also induce vessel dilation in the brain. However, the effects of these molecules are independent of the NVC by itself. Our study reports for the first time that a molecule present in the circulation (here tPA) contributes to the CBF increase observed during NVC. We found that circulating tPA, potentially released in the blood circulation by endothelial cell during neuronal activity, can interact with endothelial NMDAR and promote the dilation of large vessels surrounded by smooth muscle cells. Circulating tPA is thus necessary for the full increase of CBF during NVC.
Although NVC is the basis of blood oxygen level dependent (BOLD) fMRI, our understanding of the underlying signaling mechanisms is still incomplete. Our study provides important information to understand the complexity of fMRI, with the demonstration that a vascular molecule such as tPA may contribute to NVC by interacting with NMDAR expressed on the luminal side of endothelial cells. The finding that tPA modulates functional hyperemia raises the possibility that levels of plasmatic tPA may contribute to the alterations in NVC that occur in aging1,52 or brain pathologies such as Alzheimer’s disease or ischemic stroke.1 Indeed, the levels of the principal inhibitor of tPA in the blood stream, PAI-1, that are increased during Alzheimer Disease,53 raise the possibility that this increased tPA inhibition in the circulation could participate in the impairment of NVC. Moreover, stroke leads to a profound alteration in NVC in the acute phase after the ischemic event, worsening cerebral perfusion and promoting brain damages,1 an effect which could be explained by alterations of the vascular levels of active tPA.
Altogether, our present study demonstrates that the vascular tPA plays a role in neurovascular coupling by influencing endothelial NMDA receptors. Further investigations are needed to understand how this new mechanism of action of tPA contributes to brain functions and dysfunctions.
Supplemental Material
Supplemental material, JCB883599 Supplemetal Material for Circulating tPA contributes to neurovascular coupling by a mechanism involving the endothelial NMDA receptors by Antoine Anfray, Antoine Drieu, Vincent Hingot, Yannick Hommet, Mervé Yetim, Marina Rubio, Thomas Deffieux, Mickael Tanter, Cyrille Orset and Denis Vivien in Journal of Cerebral Blood Flow & Metabolism
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by the Institut National de la Santé et de la Recherche Médicale (INSERM), Caen Normandie University, the Regional Council of Lower Normandy. This project is also part of the following European research programs: 1/European Marie Curie International Training Network “NeuroInflammation, FP7; 2/NeuroAtlantic, An Atlantic innovation platform on diagnosis and treatment of neurological diseases and aging, EAPA_791/2018-NEUROATLANTIC; 3/H2020, International Marie Curie Training Network, ENTRAIN, Neuroinflammation, H2020-MSCA-ITN-2018 number 813294.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
Study design: DV, AA and CO Conducting experiments and acquiring data: AA, AD, VH, CO, TD, MR, YH and MY Analyzing data: AA, VH and YH. Writing the article: DV, AA, and MT.
Supplemental material
Supplemental material for this article is available online.
References
- 1.Iadecola C.The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron 2017; 96: 17–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen BR, Kozberg MG, Bouchard MB, et al. A critical role for the vascular endothelium in functional neurovascular coupling in the brain. J Am Heart Assoc 2014; 3: e000787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hill RA, Tong L, Yuan P, et al. Regional blood flow in the normal and ischemic brain is controlled by arteriolar smooth muscle cell contractility and not by capillary pericytes. Neuron 2015; 87: 95–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hall CN, Reynell C, Gesslein B, et al. Capillary pericytes regulate cerebral blood flow in health and disease. Nature 2014; 508: 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collen D, Lijnen HR.Basic and clinical aspects of fibrinolysis and thrombolysis. Blood 1991; 78: 3114–3124. [PubMed] [Google Scholar]
- 6.Vivien D, Gauberti M, Montagne A, et al. Impact of tissue plasminogen activator on the neurovascular unit: from clinical data to experimental evidence. J Cereb Blood Flow Metab 2011; 31: 2119–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Samson AL, Medcalf RL.Tissue-type plasminogen activator: a multifaceted modulator of neurotransmission and synaptic plasticity. Neuron 2006; 50: 673–678. [DOI] [PubMed] [Google Scholar]
- 8.Nicole O, Docagne F, Ali C, et al. The proteolytic activity of tissue-plasminogen activator enhances NMDA receptor-mediated signaling. Nat Med 2001; 7: 59–64. [DOI] [PubMed] [Google Scholar]
- 9.Cassé F, Bardou I, Danglot L, et al. Glutamate controls tPA recycling by astrocytes, which in turn influences glutamatergic signals. J Neurosci 2012; 32: 5186–5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pawlak R, Nagai N, Urano T, et al. Rapid, specific and active site-catalyzed effect of tissue-plasminogen activator on hippocampus-dependent learning in mice. Neuroscience 2002; 113: 995–1001. [DOI] [PubMed] [Google Scholar]
- 11.Pawlak R, Magarinos AM, Melchor J, McEwen B, Strickland S.Tissue plasminogen activator in the amygdala is critical for stress-induced anxiety-like behavior. Nat Neurosci 2003; 6: 168–174. [DOI] [PubMed] [Google Scholar]
- 12.Chevilley A, Lesept F, Lenoir S, Ali C, Parcq J, Vivien D.Impacts of tissue-type plasminogen activator (tPA) on neuronal survival. Front Cell Neurosci 2015; 9: 415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parcq J, Bertrand T, Montagne A, et al. Unveiling an exceptional zymogen: the single-chain form of tPA is a selective activator of NMDA receptor-dependent signaling and neurotoxicity. Cell Death Differ 2012; 19: 1983–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hébert M, Anfray A, Chevilley A, et al. Distant space processing is controlled by tPA-dependent NMDA receptor signaling in the entorhinal cortex. Cereb Cortex 2017; 27: 4783–4796. [DOI] [PubMed] [Google Scholar]
- 15.Park L, Gallo EF, Anrather J, et al. Key role of tissue plasminogen activator in neurovascular coupling. Proc Natl Acad Sci U S A 2008; 105: 1073–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lochner JE, Honigman LS, Grant WF, et al. Activity-dependent release of tissue plasminogen activator from the dendritic spines of hippocampal neurons revealed by live-cell imaging. J Neurobiol 2006; 66: 564–577. [DOI] [PubMed] [Google Scholar]
- 17.Angles-Cano E, Balaton A, Bonniec BL, et al. Production of monoclonal antibodies to the high fibrin-affinity, tissue- type plasminogen activator of human plasma. Demonstration of its endothelial origin by immunolocalization. Blood 1985; 66: 913–920. [PubMed] [Google Scholar]
- 18.András IE, Deli MA, Veszelka S, et al. The NMDA and AMPA/KA receptors are involved in glutamate-induced alterations of occludin expression and phosphorylation in brain endothelial cells. J Cereb Blood Flow Metab 2007; 27: 1431–1443. [DOI] [PubMed] [Google Scholar]
- 19.Reijerkerk A, Kooij G, Van Der Pol SMA, et al. The NR1 subunit of NMDA receptor regulates monocyte transmigration through the brain endothelial cell barrier. J Neurochem 2010; 113: 447–453. [DOI] [PubMed] [Google Scholar]
- 20.Scott GS, Bowman SR, Smith T, et al. Glutamate-stimulated peroxynitrite production in a brain-derived endothelial cell line is dependent on N-methyl-D-aspartate (NMDA) receptor activation. Biochem Pharmacol 2007; 73: 228–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Macrez R, Ortega MC, Bardou I, et al. Neuroendothelial NMDA receptors as therapeutic targets in experimental autoimmune encephalomyelitis. Brain 2016; 139: 2406–2419. [DOI] [PubMed] [Google Scholar]
- 22.Kisler K, Nelson AR, Montagne A, et al. Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat Rev Neurosci 2017; 18: 419–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hillman EMC.Coupling mechanism and significance of the BOLD signal: a status report. Annu Rev Neurosci 2014; 37: 161–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Macé E, Montaldo G, Cohen I, et al. Functional ultrasound imaging of the brain. Nat Methods 2011; 8: 662–664. [DOI] [PubMed] [Google Scholar]
- 25.Marcos-Contreras OA, Martinez de Lizarrondo S, Bardou I, et al. Hyperfibrinolysis increases blood-brain barrier permeability by a plasmin- and bradykinin-dependent mechanism. Blood 2016; 128: 2423–2434. [DOI] [PubMed] [Google Scholar]
- 26.Gaussem P, Grailhe P, Anglés-Cano E.Sodium dodecyl sulfate-induced dissociation of complexes between human tissue plasminogen activator and its specific inhibitor. J Biol Chem 1993; 268: 12150–12155. [PubMed] [Google Scholar]
- 27.Tiran E, Ferrier J, Deffieux T, et al. Transcranial functional ultrasound imaging in freely moving awake mice and anesthetized young rats without contrast agent. Ultrasound Med Biol 2017; 43: 1679–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Demené C, Deffieux T, Pernot M, et al. Spatiotemporal clutter filtering of ultrafast ultrasound data highly increases Doppler and fUltrasound sensitivity. IEEE Trans Med Imaging 2015; 34: 2271–2285. [DOI] [PubMed] [Google Scholar]
- 29.Boulay A-C, Saubaméa B, Declèves X, et al. Purification of mouse brain vessels. J Vis Exp JoVE 2015; 10: e53208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parcq J, Bertrand T, Baron AF, et al. Molecular requirements for safer generation of thrombolytics by bioengineering the tissue-type plasminogen activator A chain. J Thromb Haemost 2013; 11: 539–546. [DOI] [PubMed] [Google Scholar]
- 31.Macrez R, Obiang P, Gauberti M, et al. Antibodies preventing the interaction of tissue-type plasminogen activator with N-Methyl-d-Aspartate receptors reduce stroke damages and extend the therapeutic window of thrombolysis. Stroke 2011; 42: 2315–2322. [DOI] [PubMed] [Google Scholar]
- 32.Christopherson KS, Hillier BJ, Lim WA, et al. PSD-95 Assembles a ternary complex with the N-Methyl-d-aspartic acid receptor and a bivalent neuronal NO synthase PDZ domain. J Biol Chem 1999; 274: 27467–27473. [DOI] [PubMed] [Google Scholar]
- 33.Busija DW, Bari F, Domoki F, Louis T.Mechanisms involved in the cerebrovascular dilator effects of N-methyl-D-aspartate in cerebral cortex. Brain Res Rev 2007; 56: 89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rameau GA, Chiu L-Y, Ziff EB.NMDA receptor regulation of nNOS phosphorylation and induction of neuron death. Neurobiol Aging 2003; 24: 1123–1133. [DOI] [PubMed] [Google Scholar]
- 35.Mehra A, Ali C, Parcq J, et al. The plasminogen activation system in neuroinflammation. Biochim Biophys Acta 2016; 1862: 395–402. [DOI] [PubMed] [Google Scholar]
- 36.Fiumana E, Parfenova H, Jaggar JH, et al. Carbon monoxide mediates vasodilator effects of glutamate in isolated pressurized cerebral arterioles of newborn pigs. Am J Physiol 2003; 284: H1073–H1079. [DOI] [PubMed] [Google Scholar]
- 37.Parfenova H, Fedinec A, Leffler CW.Ionotropic glutamate receptors in cerebral microvascular endothelium are functionally linked to heme oxygenase. J Cereb Blood Flow Metab 2003; 23: 190–197. [DOI] [PubMed] [Google Scholar]
- 38.LeMaistre JL, Sanders SA, Stobart MJ, et al. Coactivation of NMDA receptors by glutamate and d-serine induces dilation of isolated middle cerebral arteries. J Cereb Blood Flow Metab 2012; 32: 537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu L, Hogan-Cann AD, Globa AK, et al. Astrocytes drive cortical vasodilatory signaling by activating endothelial NMDA receptors. J Cereb Blood Flow Metab 2019; 39: 481–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stobart JLL, Lu L, Anderson HDI, et al. Astrocyte-induced cortical vasodilation is mediated by D-serine and endothelial nitric oxide synthase. Proc Natl Acad Sci U S A 2013; 110: 3149–3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hogan-Cann AD, Lu P, Anderson CM.Endothelial NMDA receptors mediate activity-dependent brain hemodynamic responses in mice. Proc Natl Acad Sci USA 2019; 116: 10229–10231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nassar T, Akkawi S, Shina A, et al. In vitro and in vivo effects of tPA and PAI-1 on blood vessel tone. Blood 2004; 103: 897–902. [DOI] [PubMed] [Google Scholar]
- 43.Nassar T, Yarovoi S, Fanne RA, et al. Regulation of airway contractility by plasminogen activators through N-Methyl-D-aspartate receptor–1. Am J Respir Cell Mol Biol 2010; 43: 703–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heyman SN, Hanna Z, Nassar T, et al. The fibrinolytic system attenuates vascular tone: effects of tissue plasminogen activator (tPA) and aminocaproic acid on renal microcirculation. Br J Pharmacol 2004; 141: 971–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cipolla MJ, Lessov N, Clark WM.Postischemic attenuation of cerebral artery reactivity is increased in the presence of tissue plasminogen activator. Stroke 2000; 31: 940–945. [DOI] [PubMed] [Google Scholar]
- 46.Armstead WM, Bohman L-E, Riley J, et al. tPA-S481A prevents impairment of cerebrovascular autoregulation by endogenous tPA after traumatic brain injury by upregulating p38 MAPK and inhibiting ET-1. J Neurotrauma 2013; 30: 1898–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Armstead WM, Hekierski H, Yarovoi S, et al. tPA variant tPA-A296-299Prevents impairment of cerebral autoregulation and necrosis of hippocampal neurons after stroke by inhibiting upregulation of ET-1. J Neurosci Res 2018; 96: 128–137. [DOI] [PubMed] [Google Scholar]
- 48.Errico C, Pierre J, Pezet S, et al. Ultrafast ultrasound localization microscopy for deep super-resolution vascular imaging. Nature 2015; 527: 499–502. [DOI] [PubMed] [Google Scholar]
- 49.Kisler K, Nelson AR, Rege SV, et al. Pericyte degeneration leads to neurovascular uncoupling and limits oxygen supply to brain. Nat Neurosci 2017; 20: 406–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mishra A, Reynolds JP, Chen Y, et al. Astrocytes mediate neurovascular signaling to capillary pericytes but not to arterioles., Astrocytes mediate neurovascular signaling to capillary pericytes but not to arterioles. Nat Neurosci Nat Neurosci 2016; 19: 1619–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Levin EG, del Zoppo GJ.Localization of tissue plasminogen activator in the endothelium of a limited number of vessels. Am J Pathol 1994; 144: 855–861. [PMC free article] [PubMed] [Google Scholar]
- 52.Toth P, Tarantini S, Tucsek Z, et al. Resveratrol treatment rescues neurovascular coupling in aged mice: role of improved cerebromicrovascular endothelial function and downregulation of NADPH oxidase. Am J Physiol 2014; 306: H299–H308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wilkerson WR, Sane DC.Aging and thrombosis. Semin Thromb Hemost 2002; 28: 555–568. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, JCB883599 Supplemetal Material for Circulating tPA contributes to neurovascular coupling by a mechanism involving the endothelial NMDA receptors by Antoine Anfray, Antoine Drieu, Vincent Hingot, Yannick Hommet, Mervé Yetim, Marina Rubio, Thomas Deffieux, Mickael Tanter, Cyrille Orset and Denis Vivien in Journal of Cerebral Blood Flow & Metabolism