Abstract
Objective:
To understand the neurotoxic effects of manganese (Mn) exposure on monoaminergic function, utilizing [11C]dihydrotetrabenazine (DTBZ) positron emission tomography (PET) to measure vesicular monoamine transporter 2 (VMAT2).
Methods:
Basal ganglia and thalamic DTBZ binding potentials (BPND) were calculated on 56 PETs from 41 Mn-exposed workers. Associations between cumulative Mn exposure, regional BPND, and parkinsonism were examined by mixed linear regression.
Results:
Thalamic DTBZ BPND was inversely associated with exposure in workers with less than 3 mg Mn/m3-yrs, but subsequently remained stable. Pallidal DTBZ binding increased in workers with less than 2 mg Mn/m3-yrs of exposure, but decreased thereafter. Thalamic DTBZ binding was inversely associated with parkinsonism (P = 0.003).
Conclusion:
Mn-dose-dependent associations with thalamic and pallidal DTBZ binding indicate direct effects on monoaminergic VMAT2. Thalamic DTBZ binding was also associated with parkinsonism, suggesting potential as an early biomarker of Mn neurotoxicity.
Keywords: manganese, positron emission tomography, parkinsonism, vesicular monoamine transporter 2
In excess, manganese (Mn) is a neurotoxicant that targets basal ganglia,1 which makes Mn a candidate neurotoxin risk factor for parkinsonism and idiopathic Parkinson disease (PD). While the classic Mn-associated phenotype is quite distinct from PD,2 exposures in these historic cases were orders of magnitude higher than modern exposures. Expert phenotyping of welders with lower level, contemporary Mn exposures demonstrates a clinical phenotype and associated impairments much more closely resembling those seen in PD.3 Investigating the relationship between parkinsonism and in vivo neuronal integrity informs our understanding of the effects of Mn exposure on brain pathways relevant to human neurodegenerative disease.
PET-based approaches, utilizing radiotracers to assess dopaminergic neurons, have been used to investigate the role of Mn as a nigrostriatal toxin. The three presynaptic targets of PET radioligands include dopamine synthesis, dopamine reuptake, and the vesicular transport of monoamines. However, human studies of Mn toxicity using radioligands for the dopamine transporter (DAT), which is responsible for the reuptake of dopamine into dopaminergic nerve terminals, have been limited and provided inconsistent results.4–6 We previously used the presynaptic dopaminergic radio-ligand 6-[18F]fluoro-L-DOPA (FDOPA), which reflects neuronal aromatic L-amino acid decarboxylase activity,7 to investigate Mn-exposed in occupationally exposed welders and found lower caudate FDOPA uptake in comparison to non-exposed subjects.8 Although FDOPA uptake did not relate to Mn-dose, we hypothesized that this may be due to upregulation of dopa-decarboxylase in response to early nigral neurotoxicity,9 which could underestimate nigrostriatal neuronal loss and obscure the relationship with Mn-dose.
[11C]dihydrotetrabenazine (DTBZ) PET measurement of vesicular monoamine transporter 2 (VMAT2) is an alternative assessment of monoaminergic neuronal integrity that may not undergo as much compensatory upregulation as dopa-decarboxylase in the setting of dompaminergic dysfunction.10 DTBZ PET has been used to measure VMAT2 levels in striatal and extrastriatal regions including the thalamus, globus pallidus (GP), and substantia nigra (SN), all of which represent regions of interest in Mn toxicity.11–13 DTBZ PET in human Mn exposure is limited to a single study with three smelter workers demonstrating normal striatal uptake and one worker with a comorbid stroke demonstrating reduced uptake in the putamen.14 In the present study, we evaluated the relationship between Mn exposure, striatal, and extrastriatal VMAT2 as measured by DTBZ PET, and clinical parkinsonism in Mn-exposed welders and non-welders. We hypothesized Mn exposure would be associated with dose-dependent changes in striatal and extrastriatal DTBZ binding and that changes in DTBZ binding would be associated with a greater parkinsonism.
METHODS
Participants
All participants (n = 41) were workers age more than or equal to 18 years from the United States (US) Midwest recruited from May 2013 to September 2016. Nearly all workers were International Brotherhood of Boilermakers (IBB) union members, most of whom were employed at one of three welding worksites (n = 36) recruited from a previous cohort study.3 The remainder (n = 5) were carpenters who worked around welding in the same Midwestern region of the US. Exclusion criteria were history of stroke, brain tumor, any condition that could compromise the Unified Parkinson Disease Rating Scale motor subsection 3 (UPDRS3),15 or use of anti-parkinsonian medications, neuroleptics, or amphetamines. Of 43 potential participants, we excluded two workers: one due to a medical condition and one who was not examined by this study’s specialists. Of the 41 remaining workers, 26 conducted welding (welders or welder helpers, hereafter “Mn-exposed welders”), and 15 worked around welding fume (hereafter “Mn-exposed non-welders”). The latter included the five carpenters, two fabricators, one pipefitter, one outfitter, one tank tester, one painter, two quality assurance inspectors, one tool and die worker, and one steelworker. From this we created two categorical job groups based on job titles, Mn-exposed welders and Mn-exposed non-welders. Of the 41 workers, 15 participants completed repeated DTBZ imaging and examinations occurring from December 2014 to April 2017. Complete data were available for all baseline and repeat visits, providing a total of 56 scans. The Washington University in St. Louis Human Research Protection Office and the Radioactive Drug Research Committee approved this study, and all participants provided written informed consent. Data from participants who authorized sharing can be made available in accordance with US regulations.
Clinical Assessment
Blinded movement disorders specialists examined all participants using the UPDRS3 at the time of imaging. The two examiners were validated using standardized video ratings, a timed motor task, and against a third examiner’s video-based ratings. We also adjusted raw UPDRS3 scores for examiner and allowed for examiner by time differences as previously described.3
Magnetic Resonance Imaging (MRI) Acquisition
We acquired high resolution three-dimensional magnetization-prepared rapid gradient echo images on each participant using a Siemens 3T Magnetom Trio or Prisma scanner (Erlangen, Germany) (repetition time = 2400 ms, inversion time = 1000 ms, echo time = 3.14 ms, flip angle = 8°, 0.9 × 0.9 × 0.9 mm voxels).
PET Acquisition
DTBZ was synthesized as previously described.16 A Siemens/CTI EXACT+ scanner, which has 32 rings of BGO detector elements and acquires 63 simultaneous slices with 2.4 mm spacing and an axial field of view of 15.5 cm, was used to acquire scans in three-dimensional mode. Three retractable germanium 68 rod sources were used for transmission scans to measure individual attenuation factors. Transaxial and axial spatial resolution at slice center were 4.3 and 4.1 mm full width half maximum in three-dimensional mode. DTBZ (7.2 to 21.99 mCi) was intravenously injected over 20 seconds. Dynamic PET images were obtained for 60 minutes beginning with radioligand injection, with three 1-minute frames, four 2-minute frames, three 3-minute frames, and eight 5-minute frames. PET scans were reconstructed with filtered back projection with ramp filter cut off at the Nyquist frequency and included attenuation, scatter, and random correction.
Image Analysis
For each participant, we coregistered dynamically acquired PET image frames to each other and to the individuals’ MRI image using methods previously outlined.17 A priori MRI-defined volumes of interest (VOIs) included caudate, putamen, GP, thalamus, SN, and an occipital reference volume. We used FreeSurfer18 for segmentation of the subcortical deep nuclei on individual MRIs. Caudate, putamen, GP, and thalamus, were identified utilizing FreeSurfer, and a single investigator manually edited these volumes. The SN and the occipital volumes were manually traced following previously published methods.8,17 An independent second investigator then reviewed all VOIs. Finally, we eroded the VOIs to minimize partial volume effects on regional PET measurements. For the caudate, putamen, GP, and thalamus VOIs, we combined a Gaussian smoothing filter at 3 mm FWHM with thresholding set to 0.75 to erode the surface of the original region. The SN VOI was not large enough to erode.
For each participant, the FreeSurfer-defined VOIs and reference region were resampled in Talairach atlas space to 3 mm, and decay-corrected tissue activity curves were extracted from the dynamic PET data.19 We calculated VOI DTBZ BPND using the Logan graphical method20 with the occipital reference. Slopes were obtained from Logan plot points acquired 15 to 60 minutes after injection. We averaged left and right BPNDs for each VOI. All image analyses were conducted blinded to exposure status.
Exposure Assessment
At the time of imaging, all participants completed a validated, structured questionnaire.21 We also calculated cumulative Mn exposure in mg Mn/m3-yrs.3 The duration and intensity components of the mg Mn/m3-yrs metric require knowledge of start date, stop date, and job title, which demonstrate high (83.3% to 100%) agreement against employer records.21 Start and stop dates translate to duration in years, while job title determines intensity of Mn exposure. We were unable to conduct air monitoring at the study worksites, but we previously estimated that intensity of Mn exposure for the welders is eight times greater than that for Mn-exposed non-welders, who are working around welding fume at these worksites.22 More recently, we estimated that the mean air concentration among the welders in the full cohort is 0.14 mg Mn/m3.3 We used this value as a scalar to translate the product of duration and intensity of Mn exposure to a metric with greater comparability to prior studies and exposure limits. While this translation cannot affect P-values, we note that it implies that the mean estimated air concentration for a Mn-exposed welder in this cohort is 0.14 and 0.0175 mg Mn/m3 for Mn-exposed non-welders around welding fume at these sites. However, even at a single worksite, air concentration of Mn varies according to welding process and location. Flux core arc welding in confined spaces typically results in the highest air Mn concentrations.23 Hence, Mn air concentrations potentially as high as or higher than 1 mg Mn/m3 might have been experienced by some welders in the present study.
Statistical Analysis
All analyses were conducted in Stata. We used multivariable linear regression to examine associations within each a priori selected VOI (and the occipital reference) between Mn exposure and DTBZ BPND and between DTBZ BPND and examiner-adjusted UPDRS3. To maximize statistical power, we included all available scans. To account for dependence of data, that is, more than one scan in some participants, we used a linear mixed model and allowed for a random effect for each person using an independent variance-covariance structure.
In our primary analysis we did not transform our outcome variables so that the resulting beta estimates had the most straightforward interpretation. This approach was justified statistically, since our sample size was large enough that the outcome need not be distributed normally. However, we conducted a secondary analysis to directly compare our results to prior literature, which investigated the effect of age on DTBZ uptake.24 For this analysis, we took the natural logarithm of the outcome variables and repeated our primary analysis. In all models, we retained the independent variables as continuous measures and included the independent variable as a single variable. Then, because this approach constrains the association between the independent and outcome variables to be linear, we examined whether there was any evidence of a non-linear association. Specifically, we used local polynomial smoother graphs to visualize the shape of the association between the independent and outcome variables to examine the appropriateness of linear modeling, and to suggest alternative approaches such as spline models. We adjusted a priori for age in the above models unless noted, since age is associated with both striatal DTBZ BPND24,25 and the mg Mn/m3-yr exposure metric. We did not adjust for VOI volume because in no instance was volume associated with VOI DTBZ BPND.
For the 15 participants with two DTBZ scans, we calculated annual VOI rate of change in DTBZ BPND. We used this annual rate of change in VOI DTBZ BPND as an outcome in simple linear regression models to explore whether baseline age, cumulative Mn exposure, or other factors were associated with DTBZ BPND annual rate of change.
We report β estimates and 95% confidence intervals (CIs) from regression models as the measure of association. The linear regression β estimate represents the difference in the respective outcome in relation to one unit of change of the independent variable, that is, 1 mg Mn/m3-yr. In a worker, 1 mg Mn/m3-yr translates to 1 year of exposure at 1 mg Mn/m3. The Mn air concentration value of 1 mg Mn/m3 for a worker corresponds to 20% of the federal Occupational Safety and Health Administration (OSHA) Permissible Exposure Limit (PEL) ceiling.26 In models with UPDRS3 as the outcome and DTBZ BPND as the independent variable, we scaled the latter so that a “one unit change” was equivalent to a 0.1 decrease in the unitless DTBZ BPND for ease of interpretation. We used a two-sided α = 0.05 to determine significance.
RESULTS
Participants
Most participants were non-Hispanic white men (Table 1). The two job groups were similar in age and sex distribution. Mn-exposed welders had greater cumulative Mn exposure [mean 2.7 mg Mn/m3-yrs (SD 1.9)] than Mn-exposed non-welders [mean 0.3 mg Mn/m3-yrs (SD 0.2)] (Table 1). Average UPDRS3 scores were also higher in Mn-exposed welders [mean 9.2 (SD 6.4)], compared with Mn-exposed non-welders [mean 5.8 (SD 4.6)]. There was a significant positive association between cumulative Mn exposure and UPDRS3 score (P ≤ 0.01).
TABLE 1.
Characteristics of Participants (N = 41)
| Mn-Exposed Welders N = 26 n (%) | Mn-Exposed Non-welders N = 15 n (%) | Mn-Exposed Welders/Non-welders with Repeat Scans N = 15* n (%) | ||
|---|---|---|---|---|
| Male | 24 (92) | 14 (93) | 13 (87) | |
| Non-Hispanic white | 24 (92) | 15 (100) | 15 (100) | |
| Age, yrs† | Mean (SD) | 48.5 (12.0) | 49.1 (12.4) | 49.7 (11.9) |
| Median | 51.5 | 52 | 54 | |
| Range | 23–64 | 23–69 | 23–63 | |
| Welding fume exposure, yrs† | Mean (SD) | 24.7 (13.4) | 18.0 (13.8) | 26.7 (13.5) |
| Median | 26.0 | 15.9 | 33.1 | |
| Range | 0.6–45.3 | 0.1–47.1 | 0.6–45.3 | |
| Cumulative Mn exposure, Mg Mn/m3-yrs†,‡ | Mean (SD) | 2.7 (1.9) | 0.3 (0.2) | 2.9 (2.1) |
| Median | 2.0 | 0.3 | 2.3 | |
| Range | 0.1–6.3 | 0.001–0.8 | 0.1–6.3 | |
| UPDRS3† | Mean (SD) | 9.2 (6.4) | 5.8 (4.6) | 9.6 (7.1) |
| Median | 8.25 | 4.5 | 9.0 | |
| Range | 0.5–21.5 | 0.5–15 | 0.5–21.5 | |
| Signs of Parkinsonism (mean, SD)§ | Limb bradykinesia | 4.9 (4.1) | 3.1 (2.7) | 6.8 (4.3) |
| Limb rigidity | 2.4 (2.5) | 1.0 (1.7) | 3.5 (2.5) | |
| Action tremor | 0.3 (0.5) | 0.6 (0.9) | 0.2 (0.6) | |
| Rest tremor | 0.0 (0.0) | 0.2 (0.5) | 0.0 (0.0) | |
| Axial signs¶ | 1.7 (1.5) | 0.9 (1.1) | 2.6 (1.6) | |
| Time between baseline and follow up scans, yrs | Mean (SD) | – | – | 3.7 (1.0) |
| Median | – | – | 3.5 | |
| Range | – | – | 2.1–5.4 |
DTBZ, [11C]dihydrotetrabenazine; Mn, manganese; SD, standard deviation; UPDRS3, Unified Parkinson Disease Rating Scale motor subsection 3.
Includes 14 Mn-exposed welders and one Mn-exposed worker.
As of baseline DTBZ scan.
Weighted welding years multiplied by 0.14 mg Mn/m3.3 Among the welders the estimated time-weighted mean Mn air concentration during the years of exposure to welding fume ranged from 0.04 to 0.14 (mean 0.11, SD 0.04).
Derived from the UPDRS3.
Axial signs include gait, posture, postural stability, arising from a chair, global bradykinesia, neck rigidity, expression, and speech.
Age and DTBZ PET
Age was inversely associated with caudate, putamen, and SN DTBZ BPND, in a largely linear manner, with linear regression β estimates and intercepts in between those from previous studies.24,25 Age was not associated with GP or thalamic DTBZ BPND (see Table, Supplemental Digital Content 1, http://links.lww.com/JOM/A774, which demonstrates regional DTBZ BPND in relation to age).
Mn Exposure and DTBZ PET
After adjusting for age at scan, Mn-exposed welders had substantially lower thalamic DTBZ BPND than Mn-exposed non-welders, specifically 0.0267 (95% CI 0.0002, 0.0532) lower, or equivalently 10.6% (95% CI 0.55%, 20.7%) lower, for the log-transformed outcome (not in tables). Similarly, cumulative Mn exposure was associated with thalamic DTBZ BPND (Table 2); however, the relationship was non-linear. Specifically, there was an inverse association between Mn exposure and thalamic DTBZ BPND with less than 3 mg Mn/m3-yrs of exposure, and no association thereafter (Fig. 1A). The spline model was better than the linear model for Mn exposure (difference in Akaike information criterion [ΔAIC] = 5.5). The association between cumulative Mn exposure and GP DTBZ BPND was also non-linear. Pallidal DTBZ BPND appeared to increase with Mn exposures in welders and non-welders with less than 2 mg Mn/m3-yrs of exposure but decrease for those with exposures more than or equal to 2 mg Mn/m3-yrs (Fig. 1B). The spline model was also better than the linear exposure model (ΔAIC = 6.7). Given the non-linear relationships between exposure and DTBZ BPND in these two regions (thalamus, GP) we observed that the β estimates (and hence P-values) for these two regions were different depending on whether we did or did not include Mn non-welders, that is, less intensely exposed workers. There was no association between exposure status, mg Mn/m3-yrs, and DTBZ uptake in the other VOIs or the reference volume.
TABLE 2.
DTBZ BPND and its Relation with Cumulative Mn Exposure, * by Brain Region
| All Mn-Exposed Welders and Mn-Exposed Non-welders (N = 56)† | Mn-Exposed Welders Only (N = 40) | |||
|---|---|---|---|---|
| DTBZ BPND | Mean | SD | Mean | SD |
| Caudate | 1.23 | 0.19 | 1.23 | 0.20 |
| Globus pallidus | 1.35 | 0.20 | 1.36 | 0.21 |
| Putamen | 1.64 | 0.20 | 1.64 | 0.22 |
| Substantia nigra | 0.31 | 0.07 | 0.31 | 0.07 |
| Thalamus | 0.26 | 0.05 | 0.26 | 0.05 |
| Absolute Difference in DTBZ BPND per mg Mn/m3-yr‡ | ||||
| β Estimate (95% CI) | P-Value | β Estimate (95% CI) | P-Value | |
| Caudate | −0.0089 (-0.0408, 0.0231) | 0.59 | −0.0239 (-0.0790, 0.0313) | 0.40 |
| Globus pallidus | −0.0240 (-0.0590, 0.0110)§ | 0.18 | −0.0577 (-0.1107, −0.0047)§ | 0.03 |
| Putamen | −0.0122 (−0.0423, 0.0179) | 0.43 | −0.0383 (−0.0925, 0.0158) | 0.17 |
| Substantia nigra | −0.0029 (−0.0127, 0.0069) | 0.56 | −0.0093 (−0.0255, 0.0070) | 0.26 |
| Thalamus | −0.0088 (−0.0163, −0.0014)§ | 0.02 | −0.0048 (-0.0167, 0.0071)§ | 0.43 |
| Percent Difference in DTBZ BPND per mg Mn/m3-yr, %‡,¶ | ||||
| β Estimate (95% CI) | P-Value | β Estimate (95% CI) | P-Value | |
| Caudate | −0.74 (−3.42, 1.94) | 0.59 | −1.62 (−6.30, 3.06) | 0.50 |
| Globus pallidus | −1.72 (−4.27, 0.83) | 0.19 | −4.14 (−8.07, −0.22) | 0.04 |
| Putamen | −0.85 (−2.66, 0.96) | 0.36 | −2.35 (−5.59, 0.90) | 0.16 |
| Substantia nigra | −1.46 (−4.93, 2.01) | 0.41 | −3.66 (−9.49, 2.16) | 0.22 |
| Thalamus | −3.33 (−6.17, −0.49) | 0.02 | −1.51 (−6.20, 3.19) | 0.53 |
BPND, nondisplaceable binding potential; CI, confidence interval; DTBZ, [11C]dihydrotetrabenazine; Mn, manganese; SD, standard deviation.
Mg Mn/m3-yrs at the time of the respective (baseline or follow up) DTBZ scan.
Cross-sectional association, based on 56 scans (41 baseline scans in 41 participants, plus 15 follow up examinations among 15 of the 41 participants), including 40 scans (26 baseline scans and 14 follow up examinations) among Mn-exposed welders.
Adjusted for age (continuous) at the time of the scan. A one unit change in mg Mn/m3-yr represents, for example, one year of exposure at one mg Mn/m3, which is 20% of the federal Occupational Safety and Health Administration (OSHA) Permissible Exposure Limit ceiling for Mn.24
Local polynomial smoother indicated that linear modeling may not be appropriate for this brain region. See Fig. 1A for thalamus or Fig. 1B for globus pallidus.
Calculated by natural logarithm transforming DTBZ BPND.
FIGURE 1.
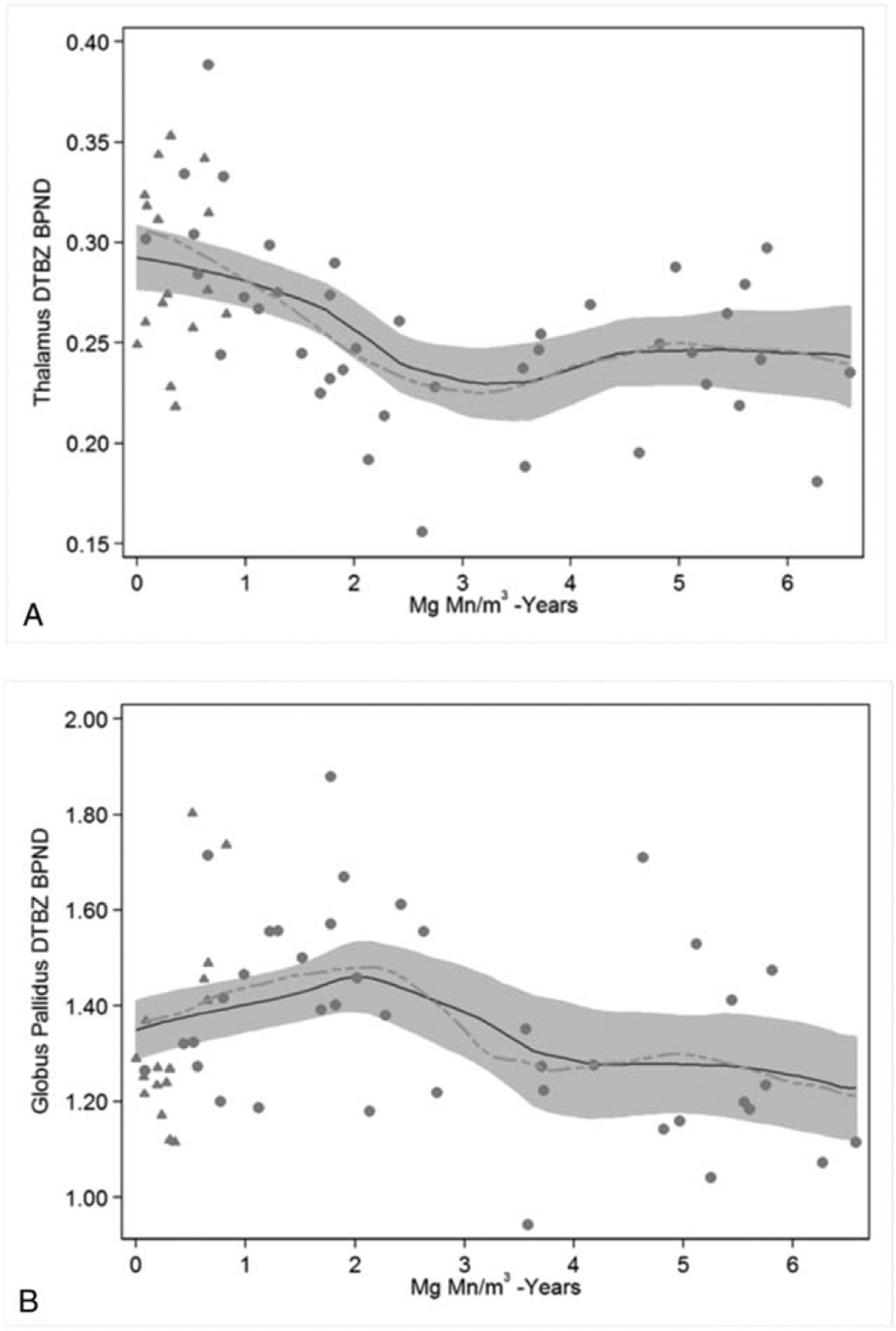
Thalamic and Pallidal DTBZ Binding in Relation to Cumulative Mn Exposure. (A) The cross-sectional association between cumulative Mn exposure (mg Mn/m3-yrs) and thalamus DTBZ BPND is inverse among those with less than 3 mg Mn/m3-yrs of exposure but relatively stable thereafter. Using two linear splines (not shown) with a knot at 3 mg Mn/m3-yrs, the respective DTBZ BPND estimates per mg Mn/m3-yr of exposure are −0.0268 (95% CI −0.0406, −0.0129) and 0.0121 (95% CI −0.0036, 0.0279). (B) The cross-sectional association between cumulative Mn exposure (mg Mn/m3-yrs) and globus pallidus DTBZ BPND is positive among those with less than 2mg Mn/m3-yrs of exposure and inverse among those with more than or equal to 2mg Mn/m3-yrs of exposure. Using two linear splines (not shown) with a knot at 2mg Mn/m3-yrs, the respective DTBZ BPND estimates per mg Mn/m3-yr of exposure are 0.1054 (95% CI 0.0169, 0.1938) and – 0.0901 (95% CI −0.1430, −0.0373). The lines represent the local polynomial smoother for all participants (solid line) or when restricted to Mn-exposed welders (dashed line). The grey band represents the respective 95% CI for all participants. (Grey triangle = Mn-exposed non-welders; grey dot = Mn-exposed welders).
DTBZ PET and UPDRS3
Thalamic DTBZ binding was inversely associated with UPDRS3. For each 0.1 unit decrease in thalamic DTBZ BPND, the UPDRS3 was an estimated 4.81 (95% CI 1.66, 7.96) points higher, even after adjustment for age (Table 3). In post hoc analyses, we found that 34.9% of the association between Mn exposure and UPDRS3 was mediated by thalamic DTBZ BPND, (95% CI 19.5%, 139%). There was a suggestion that SN DTBZ BPND was also inversely associated with UPDRS3 with a 2.13 (95% CI −0.07, 4.32) greater UPDRS3 score per 0.1 unit decrease in DTBZ BPND (Table 3). Mediation analysis indicated approximately 6.5% (95% CI 3.6%, 21.3%) of the association between cumulative Mn exposure and UPDRS3 was mediated by SN DTBZ BPND.
TABLE 3.
DTBZ BPND and UPDRS3, by Brain Region (N = 56)
| β Estimate for the Absolute Difference in UPDRS3 (95% CI)* | |||
|---|---|---|---|
| Per 0.1 Decrease in DTBZ BPND | Per One SD Decrease in DTBZ BPND | P-Value* | |
| Caudate | 0.52 (−0.34, 1.39) | 1.01 (−0.66, 2.69) | 0.24 |
| Globus pallidus | 0.33 (−0.42, 1.07) | 0.66 (−0.84, 2.17) | 0.39 |
| Putamen | 0.60 (−0.23, 1.42) | 1.17 (−0.45, 2.79) | 0.16 |
| Substantia nigra | 2.13 (−0.07, 4.32) | 1.47 (−0.05, 2.98) | 0.06 |
| Thalamus | 4.81 (1.66, 7.96) | 2.22 (0.77, 3.67) | 0.003 |
BPND, nondisplaceable binding potential; CI, confidence interval; DTBZ, [11C]dihydrotetrabenazine; SD, standard deviation; UPDRS3, Unified Parkinson Disease Rating Scale motor subsection 3.
Cross-sectional association between DTBZ BPND and UPDRS3 at the respective scan (41 baseline plus 15 follow up DTBZ scans). Adjusted for age (continuous) at the time of the scan and examiner. As presented in the table, positive β estimate values indicate greater severity of parkinsonism (higher UPDRS3) in relation to lower DTBZ BPND.
Longitudinal Change in DTBZ PET
In participants with repeat DTBZ PET scans, mean and median annual change in DTBZ BPND decreased over time in all brain volumes examined, after excluding one influential data point for SN (the only participant who had a marked increase in DTBZ BPND and the highest baseline UPDRS3). Excluding this participant, the median percent decrease relative to baseline DTBZ BPND was 0.90% per year for caudate, 1.00% for GP, 1.85% in putamen, 1.77% for SN, and 1.11% for thalamus. Neither age nor Mn exposure at baseline scan were associated with regional DTBZ BPND annual rate of change. The baseline UPDRS3 was associated with rate of change in SN DTBZ BPND after excluding the influential data point (Fig. 2), even after adjustment for age at baseline (P = 0.06). Moreover, only participants with a baseline PDRS3 more than 10 had an annual absolute rate of decline in SN DTBZ binding more than or equal to 0.02.
FIGURE 2.
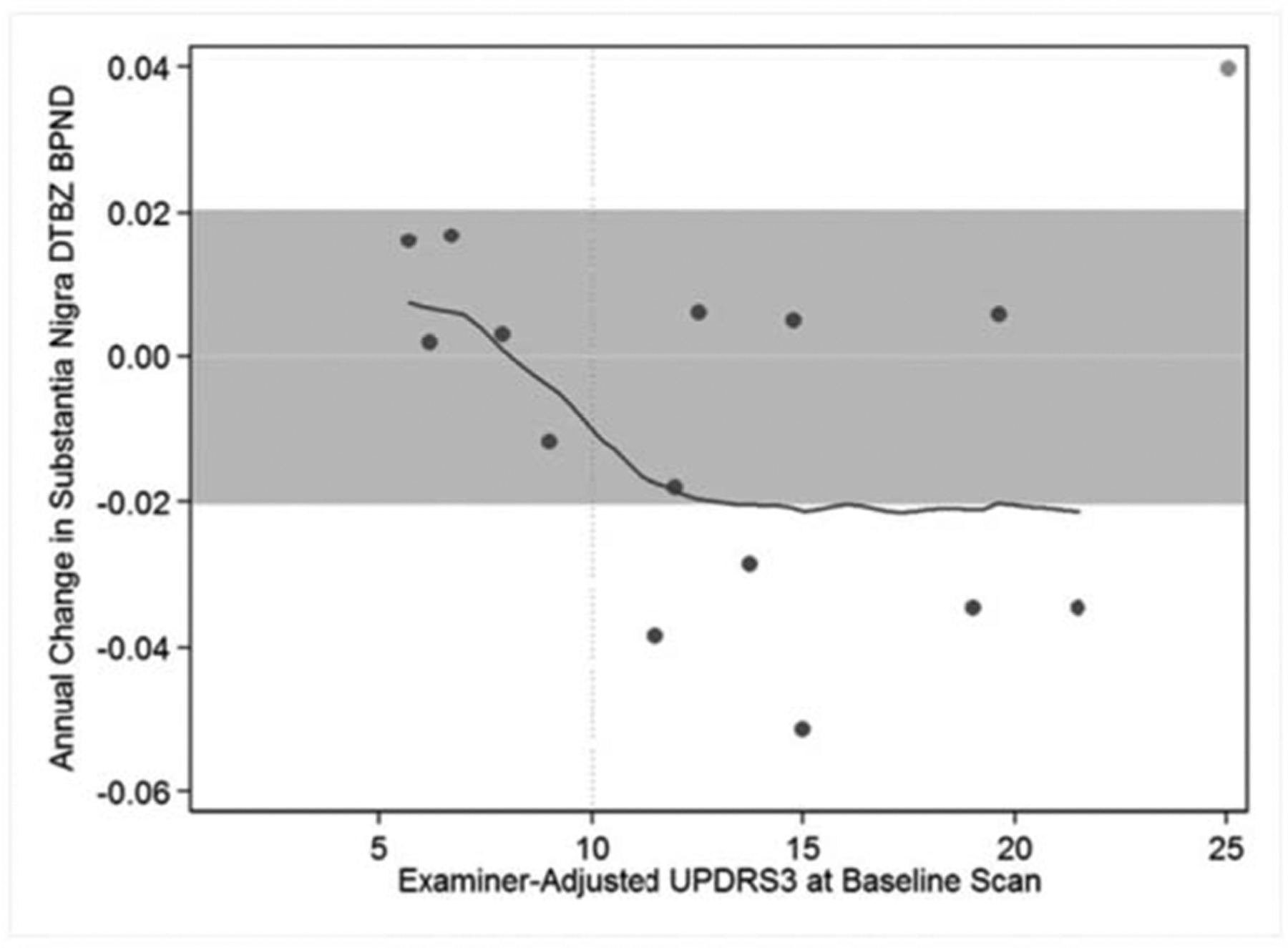
Baseline UPDRS3 in Relation to Annual Rate of Change in Substantia Nigra DTBZ BPND. Participants who had higher UPDRS3 scores at the baseline examination had a greater reduction in substantia nigra DTBZ BPND over time than participants with lower baseline UPDRS3 scores. The grey band represents annual “changes” in substantia nigra DTBZ BPND of only ±0.02 surrounding a change of zero (horizontal line). Only one participant (an influential point marked as a lighter grey dot) had a greater increase in DTBZ BPND, whereas five participants had greater decrease in DTBZ BPND. All five had a baseline UPDRS3 more than 10 (marked by the vertical line). A local polynomial smoother for all participants is shown (solid line).
DISCUSSION
In this molecular imaging study of VMAT2 in Mn-exposed welders and non-welders, we found Mn-dose-dependent DTBZ binding in the thalamus and GP. Thalamic DTBZ BPND was also inversely associated with parkinsonism. In contrast, we found no relationship between Mn exposure and DTBZ binding in the striatum, after adjusting for age. This parallels our previous cross-sectional analysis using FDOPA PET, in which we identified group differences in the caudate between Mn-exposed and non-exposed, but no Mn dose–response relationship.8 The lack of an association between striatal DTBZ uptake and Mn exposure may suggest that Mn preferentially affects striatal decarboxylase as measured by FDOPA more than striatal VMAT2. Alternatively, a significant component of early Mn toxicity may occur in extrastriatal regions. Nevertheless, our study adds to the literature by suggesting that Mn has diffuse, but complex effects in striatal and extrastriatal brain regions.
The GP has been a focus of Mn research due to the pathognomonic T1 MRI abnormalities.27 Our study suggests that Mn may induce compensatory upregulation of the GP monoaminergic neurons at low level Mn exposure, but, as exposure continues, these projections decline. While there are no other longitudinal DTBZ studies in Mn-exposed humans, a similar pattern of an early increase followed by progressive decline has been observed in pallidal FDOPA uptake in longitudinal studies with early PD patients.28
Thalamic VMAT2 binding demonstrated an association with both Mn exposure and parkinsonism, although this relationship was complex and not strictly linear. Most of the association between Mn exposure and thalamic DTBZ BPND occurred at less than or equal to 3 mg Mn/m3-yrs. The thalamus is a major output nucleus to the cortex from the basal ganglia direct and indirect pathways and has lower VMAT2 density than the striatum, as well as a greater density of dopamine type 3 receptors.29 Early neuronal loss in the thalamic centre-median/parafascicular complex is seen in early stage PD.30 Similar to early PD, a reduction of the thalamo-cortical excitatory projections may explain the inverse association between thalamic DTBZ binding and the UPDRS3 in Mn exposure.31 Although it is unclear why striatal DTBZ binding was not associated with severity of parkinsonism, the low density of VMAT2 and corresponding monoaminergic neurons may make the thalamus more sensitive to neurotoxic insult and could represent a biomarker for monitoring the early effects of Mn exposure.
We also observed an inverse trend between SN DTBZ BPND and UPDRS3, which is interesting in the context of our recent PET [11C](N-methyl)benperidol study in which we found Mn-dose-dependent upregulation of SN dopamine type 2 receptors (D2R).17 In that study, there was a positive linear association between SN D2R binding and UPDRS3. In sum, greater parkinsonism appears to be associated with lower nigral VMAT2 and higher D2R levels. Longitudinal studies using both ligands will be required to understand further the relative timing of these changes.
Although our longitudinal data based on 15 follow-up scans were underpowered to detect a dose–response association between change in DTBZ binding and Mn exposure, we did observe a decline in all brain regions DTBZ binding over time, consistent with the known association with age.24,25 Interestingly, in exploratory analyses we observed that the baseline UPDRS3 was potentially associated with the annual rate of change in substantia nigra DTBZ BPND. These longitudinal data suggest that workers with a greater parkinsonism may have a more aggressive decline in nigral VMAT2. Given that Mn exposure appears to contribute to progression of parkinsonism within the full cohort,3 we speculate that Mn exposure may contribute to decreased nigral DTBZ binding; however, this will require further study.
This study, in addition to our previous work in these workers demonstrating Mn-dose-dependent parkinsonism3 and D2R upregulation,17 provides compelling evidence that estimated average Mn exposures ranging from 0.0175 to 0.14 mg Mn/m3 are associated with biologically and clinically relevant health effects. Similarly, another study with welders with a mean Mn air concentration of 0.23 ± 0.18 mg Mn/m3 demonstrated higher thalamic GABA levels on magnetic resonance spectroscopy, as well a higher mean UPDRS3 score.32 We recently reported Mn-dose-dependent parkinsonism, using similar methods, in South African Mn miners with a mean estimated time-weighted concentration of 0.6 mg Mn/m3 even though there was evidence of healthy worker survivor effect in that study population.33 In addition, multiple studies have found a variety of cognitive and motor disturbances in workers exposed to mean inhalable Mn concentrations from 0.05 to 1.59 mg/m3 34; though not all of these effects were clinically relevant. In total, these and other studies suggest that there are adverse neurologic health effects from Mn exposures at or below the American Conference of Governmental Industrial Hygienists (ACGIH) threshold limit value for Mn of 0.1 mg Mn/m3.35
This study has several potential limitations. Our exposure metric relies on worker-reported histories, so some exposure misclassification of Mn is likely. However, our study is consistent with our previous studies using clinical3 and imaging17 methodologies that also demonstrated Mn-dose-dependent basal ganglia dysfunction. In addition, VMAT2 is present in all monoamine neurons,36 so non-dopaminergic neurons containing serotonin and noradrenaline may be alternative targets for Mn neurotoxicity. This is unlikely to affect interpretation of results in heavily dopaminergic areas like putamen,37 but we may be identifying non-dopaminergic neurotoxic effects in more neurochemically heterogeneous regions like thalamus. Further, while extrastriatal DTBZ binding is certainly lower in comparison to the striatum, we limited our analysis to brain regions that have been previously reported in DTBZ PET studies, and the associations with both exposure and the clinical outcome of interest are unlikely to be secondary to chance. While DTBZ BPND appeared to decline over time in all brain regions, we were underpowered to examine longitudinal associations with Mn exposure. Ultimately, demonstrating longitudinal decline in DTBZ binding in relation to Mn exposure would provide additional compelling evidence of the potential role of Mn as neurotoxin relevant to the pathogenesis of parkinsonism and specifically PD.
In conclusion, Mn-exposed welders and non-welders demonstrate Mn-dose-dependent associations with extrastriatal DTBZ (VMAT2) binding in the GP and thalamus indicating a complex relation between Mn exposure and monoaminergic VMAT2. Thalamic DTBZ binding was also inversely associated with parkinsonism, suggesting it may represent an early biomarker of Mn neurotoxicity. Estimated mean time-weighted Mn exposures for these workers were lower than the OSHA PEL for Mn of 5 mg Mn/m3.25 Given that we observe an association between Mn exposure and DTBZ binding in multiple extrastriatal regions below the OSHA exposure limits, our study provides additional evidence to support a reduction in the Mn OSHA PEL.
Supplementary Material
Clinical significance:
We found Mn-dose-dependent associations with thalamic and pallidal DTBZ binding in Mn-exposed welders and non-welders. Thalamic DTBZ binding was also associated with parkinsonism, suggesting that the thalamic monoaminergic system may be an early biomarker of Mn neurotoxicity and that the extrastriatal dopaminergic system may be critical to clinical Mn neurotoxicity.
Learning Objectives.
Discuss previous knowledge of manganese (Mn) as a neurotoxin associated with parkinsonism, including the limitations of radioligands used in previous studies of Mn as a nigrostriatal toxin.
Summarize the findings of the new study using [11C]dihydrotetrabenazine (DTBZ) positron emission tomography (PET) in Mn-exposed workers.
Identify exposure-related associations with DTBZ binding in selected brain areas and their implications for clinical Mn neurotoxicity.
Funding Sources:
This work was supported by NIH Grants [R01ES021488, K23ES021444, K24ES017765, R01ES013743, R01ES021488-02S1, P42ES004696, R01ES029524]; and the American Parkinson’s Disease Association.
Financial Disclosures of all Authors: Dr Criswell reports grant funding from the NIH including K23ES021444, R01ES029524, and R01ES013743.
Dr Searles Nielsen reports grant funding from the NIH including K24ES017765 and R01ES021488 and grants from Michael J. Fox Foundation and American Parkinson Disease Association.
Dr Perlmutter reports grant funding from NIH including R01ES021488, R01ES029524, and R01ES013743.
Dr Moerlein reports grant funding from the NIH including R01ES021488, R01ES029524.
Dr. Sheppard reports grant funding from NIH including R01ES029524, R01ES026187, and R01ES021488.
Mr Lenox-Krug reports funding from R01ES029524.
Dr Checkoway reports grant funding from the NIH including R01ES021488, R21ES026084, R01ES025991, and R01ES025792.
Dr Racette receives research support from NIH including K24ES017765, R01ES026891, R01ES026891-S1, R01ES021488, R01ES025991, R01ES025991-S1, and R01ES029524, Grant #10289-01 from the Michael J. Fox Foundation, and the American Parkinson Disease Association. Dr Racette has received honoraria (personal compensation) for lectures from Harvard University and the American Academy of Neurology. He has received personal compensation for peer review from the Parkinson Study Group and for service on the National Advisory Environmental Health Sciences Council for NIEHS.
Mr Warden has nothing to report.
Footnotes
Criswell, Nielsen, Warden, Perlmutter, Moerlein, Sheppard, Lenox-Krug, Checkoway, and Racette have no relationships/conditions/circumstances that present potential conflict of interest.
The JOEM editorial board and planners have no financial interest related to this research.
Supplemental digital contents are available for this article. Direct URL citation appears in the printed text and is provided in the HTML and PDF versions of this article on the journal’s Web site (www.joem.org).
REFERENCES
- 1.Parmalee NL, Aschner M. Manganese and aging. Neurotoxicology. 2016;56:262–268. [DOI] [PubMed] [Google Scholar]
- 2.Rodier J Manganese poisoning in Moroccan miners. Br J Ind Med. 1955;12:21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Racette BA, Searles Nielsen S, Criswell SR, et al. Dose-dependent progression of parkinsonism in manganese-exposed welders. Neurology. 2017;88: 344–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim Y, Kim JM, Kim JW, et al. Dopamine transporter density is decreased in parkinsonian patients with a history of manganese exposure: what does it mean? Mov Disord. 2002;17:568–575. [DOI] [PubMed] [Google Scholar]
- 5.Sikk K, Taba P, Haldre S, et al. Clinical, neuroimaging and neurophysiological features in addicts with manganese-ephedrone exposure. Acta Neurol Scand. 2010;121:237–243. [DOI] [PubMed] [Google Scholar]
- 6.Kim J, Kim JM, Kim YK, et al. Dopamine transporter SPECT of a liver cirrhotic with atypical parkinsonism. Ind Health. 2007;45:497–500. [DOI] [PubMed] [Google Scholar]
- 7.Yee RE, Irwin I, Milonas C, et al. Novel observations with FDOPA-PET imaging after early nigrostriatal damage. Mov Disord. 2001;16:838–848. [DOI] [PubMed] [Google Scholar]
- 8.Criswell SR, Searles Nielsen S, Warden M, et al. [18F]FDOPA positron emission tomography in manganese-exposed workers. Neurotoxicology. 2018;64:43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sossi V, de La Fuente-Fernandez R, Holden JE, et al. Increase in dopamine turnover occurs early in Parkinson’s disease: evidence from a new modeling approach to PET 18 F-fluorodopa data. J Cereb Blood Flow Metab. 2002;22:232–239. [DOI] [PubMed] [Google Scholar]
- 10.Adams JR, van Netten H, Schulzer M, et al. PET in LRRK2 mutations: comparison to sporadic Parkinson’s disease and evidence for presymptomatic compensation. Brain. 2005;128:2777–2785. [DOI] [PubMed] [Google Scholar]
- 11.Chang CC, Hsiao IT, Huang SH, et al. (1)(8)F-FP-(+)-DTBZ positron emission tomography detection of monoaminergic deficient network in patients with carbon monoxide related parkinsonism. Eur J Neurol. 2015;22:845–852. e859–860. [DOI] [PubMed] [Google Scholar]
- 12.Hsiao IT, Weng YH, Hsieh CJ, et al. Correlation of Parkinson disease severity and 18F-DTBZ positron emission tomography. JAMA Neurol. 2014;71:758–766. [DOI] [PubMed] [Google Scholar]
- 13.Cho SS, Christopher L, Koshimori Y, et al. Decreased pallidal vesicular monoamine transporter type 2 availability in Parkinson’s disease: the contribution of the nigropallidal pathway. Neurobiol Dis. 2019;124:176–182. [DOI] [PubMed] [Google Scholar]
- 14.Huang CY, Liu CH, Tsao E, et al. Chronic manganism: a long-term follow-up study with a new dopamine terminal biomarker of 18F-FP-(+)-DTBZ (18F-AV-133) brain PET scan. J Neurol Sci. 2015;353:102–106. [DOI] [PubMed] [Google Scholar]
- 15.Fahn S, Elton RL. Members of the UPDRS Development Committee. Unified Parkinson’s disease rating scale In: Fahn S, Marsden CD, Goldstein M, Calne DB, editors. Recent Developments in Parkinson’s Disease. New York: Macmillan; 1987. p. 153–163. [Google Scholar]
- 16.DaSilva JN, Kilbourn MR, Mangner TJ. Synthesis of a [11C]methoxy derivative of alpha-dihydrotetrabenazine: a radioligand for studying the vesicular monoamine transporter. Appl Radiat Isot. 1993;44:1487–1489. [DOI] [PubMed] [Google Scholar]
- 17.Criswell SR, Warden MN, Searles Nielsen S, et al. Selective D2 receptor PET in manganese-exposed workers. Neurology. 2018;91:e1022–e1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fischl B FreeSurfer. Neuroimage. 2012;62:774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hershey T, Black KJ, Carl JL, McGee-Minnich L, Snyder AZ, Perlmutter JS. Long term treatment and disease severity change brain responses to levodopa in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2003;74:844–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab. 1996;16:834–840. [DOI] [PubMed] [Google Scholar]
- 21.Hobson AJ, Sterling DA, Emo B, et al. Validity and reliability of an occupational exposure questionnaire for parkinsonism in welders. J Occup Environ Hyg. 2009;6:324–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Racette BA, Criswell SR, Lundin JI, et al. Increased risk of parkinsonism associated with welding exposure. Neurotoxicology. 2012;33:1356–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hobson A, Seixas N, Sterling D, Racette BA. Estimation of particulate mass and manganese exposure levels among welders. Ann Occup Hyg. 2011;55:113–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bohnen NI, Albin RL, Koeppe RA, et al. Positron emission tomography of monoaminergic vesicular binding in aging and Parkinson disease. J Cereb Blood Flow Metab. 2006;26:1198–1212. [DOI] [PubMed] [Google Scholar]
- 25.de la Fuente-Fernandez R, Schulzer M, Kuramoto L, et al. Age-specific progression of nigrostriatal dysfunction in Parkinson’s disease. Ann Neurol. 2011;69:803–810. [DOI] [PubMed] [Google Scholar]
- 26.Occupational Safety and Health Administration (OSHA). Permissible Exposure Limits, OSHA Annotated Table Z-1. Washington, DC: Occupational Safety and Health Administration; 2018. [Google Scholar]
- 27.Li SJ, Jiang L, Fu X, et al. Pallidal index as biomarker of manganese brain accumulation and associated with manganese levels in blood: a meta-analysis. PLoS One. 2014;9:e93900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pavese N, Rivero-Bosch M, Lewis SJ, Whone AL, Brooks DJ. Progression of monoaminergic dysfunction in Parkinson’s disease: a longitudinal 18F-dopa PET study. Neuroimage. 2011;56:1463–1468. [DOI] [PubMed] [Google Scholar]
- 29.Sun J, Xu J, Cairns NJ, Perlmutter JS, Mach RH. Dopamine D1, D2, D3 receptors, vesicular monoamine transporter type-2 (VMAT2) and dopamine transporter (DAT) densities in aged human brain. PLoS One. 2012;7:e49483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henderson JM, Carpenter K, Cartwright H, Halliday GM. Degeneration of the centre median-parafascicular complex in Parkinson’s disease. Ann Neurol. 2000;47:345–352. [PubMed] [Google Scholar]
- 31.Blesa J, Trigo-Damas I, Dileone M, Del Rey NL, Hernandez LF, Obeso JA. Compensatory mechanisms in Parkinson’s disease: circuits adaptations and role in disease modification. Exp Neurol. 2017;298:148–161. [DOI] [PubMed] [Google Scholar]
- 32.Ma RE, Ward EJ, Yeh CL, et al. Thalamic GABA levels and occupational manganese neurotoxicity: association with exposure levels and brain MRI. Neurotoxicology. 2018;64:30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dlamini WW, Nelson G, Nielsen SS, Racette BA. Manganese exposure, parkinsonian signs, and quality of life in South African mine workers. Am J Ind Med. 2020;63:36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meyer-Baron M, Knapp G, Schaper M, van Thriel C. Performance alterations associated with occupational exposure to manganese–a meta-analysis. Neurotoxicology. 2009;30:487–496. [DOI] [PubMed] [Google Scholar]
- 35.American Conference of Governmental Industrial H. Annual Reports for the Year 2012: Committees on Threshold Limit Values (TLVs) and Biological Exposure Indices (BEIs). Cincinnati, OH: ACGIH; 2013. [Google Scholar]
- 36.Hoffman BJ, Hansson SR, Mezey E, Palkovits M. Localization and dynamic regulation of biogenic amine transporters in the mammalian central nervous system. Front Neuroendocrinol. 1998;19:187–231. [DOI] [PubMed] [Google Scholar]
- 37.Wilson JM, Levey AI, Rajput A, et al. Differential changes in neurochemical markers of striatal dopamine nerve terminals in idiopathic Parkinson’s disease. Neurology. 1996;47:718–726. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


