Abstract
Background
The increasing prevalence of overweight and obesity among the worldwide population has been associated with a range of adverse health consequences such as Type 2 diabetes and cardiovascular diseases. The metabolic syndrome (MetS) is a cluster of cardiometabolic abnormalities that occur more commonly in overweight individuals. Time-restricted feeding (TRF) is a dietary approach used for weight loss and overall health. TRF may be an option for those subjects who struggle with extreme restriction diets with foods that generally do not belong to an individual's habits.
Objective
The purpose of this study was to determine the effect of TRF on body composition and the association of weight loss with metabolic and cardiovascular risks in obese middle-aged women.
Methods
A non-randomized controlled clinical trial was performed over 3 months in obese women (TRF group, n = 20, BMI 32.53 ± 1.13 vs. Control n = 12, BMI 34.55 ± 1.20). The TRF protocol adopted was 16 h without any energy intake followed by 8 h of normal food intake.
Main outcomes and measures
Anthropometric measurements, body composition, blood biomarkers, cardiovascular risk in 30 years (CVDRisk30y), and quality of life were evaluated at baseline and after the 3 months.
Results
TRF was effective in reducing weight (~ 4 kg), BMI, % of body fat (%BF), waist circumference from baseline without changes in blood biomarkers associated with MetS. TRF promoted a reduction in CVDRisk30y (12%) wich was moderately correlated with %BF (r = 0.62, n = 64, p < 0.001) and %MM (r = − 0.74, n = 64, p < 0.001).
Conclusions
TRF protocol reduces body weight without changes in biomarkers related to MetS. In addition, the anthropometric evaluation that predicts %BF and %MM could be used as an approach to follow individuals engaged in the TRF regimen since they correlate with cardiovascular risk.
Keywords: Time-restricted feeding, Intermittent fasting, Weight loss, Metabolic syndrome, Anthropometry, Obesity, Cardiovascular risk
Background
Obesity is the major risk factor in the development of type 2 diabetes mellitus (T2DM), with approximately 90% of the population with T2DM being overweight or obese [1]. Another concern of obesity is the increased risk of cardiovascular disease (CVD), such as high blood pressure, atherosclerosis, acute myocardial infarction, heart failure [2]. Abdominal circumference above 102 cm in the case of men and above 88 cm in the case of women qualifies as central obesity and involves increased cardiovascular risk [3]. Both T2DM and CVD are the outcomes of a complex organic dysfunction known as metabolic syndrome (MetS) [4].
Weight loss by only 5–10% is known to improve metabolic outcomes such as glycaemic, lipid profile, and blood pressure [5]. In addition, weight loss interventions promote the reduction in all-cause premature mortality in adults with obesity [6]. Time-restricted feeding (TRF), defined by periods of severe or complete energy restriction followed by periods of habitual eating, has been suggested as a potential strategy of weight loss because it offers a reduced burden of dietary restriction and may, therefore, be more acceptable by some individuals [7]. In fact, adherence to daily energy restriction decreases after 1 month and continues to decline thereafter [8].
Although TRF is a well-known strategy to lose weight [9, 10], improvement in insulin sensitivity, blood pressure, and oxidative stress are seen even without weight loss in human studies [11]. Several randomized controlled trials (RCTs) have reported that weight loss reduces mortality in obese individuals [6]. Therefore, there is an inconsistency of whether or not weight loss is associated with changes in classical blood biomarkers such as glycemia and lipid profile. Since obesity has multifactorial factors, it is feasible to consider that blood biomarkers should be linked with body composition evaluation to follow the process of weight loss.
Despite growing bodies of studies, TRF is still a matter of debate among clinicians due to different approaches and the uncertainties of its impact on whole-body function, i.e., kidney, liver, lipid profile, hypophysis-thyroid axis, among others. Herein, weight loss was associated with blood markers of metabolic syndrome and cardiovascular risk.
It is known that the extended morning fasting period observed in TRF does not cause compensatory intake during an ad libitum lunch nor does it increase appetite during the afternoon [12]. Therefore TRF could be an alternative to those individuals who struggle with restrictive diets which changes substantially their daily habits. The purpose of this study was to determine the effect of TRF on body composition and the association of weight loss with metabolic and cardiovascular risks in obese middle-aged women.
Methods
Subjects
Eligibity criteria for participation
Obese women were included (BMI ≥ 30 kg/m2) in this study. Physically inactive women were excluded (less than 150 min of moderate or less than 75 min of intense physical activity per week). Women with non-communicable diseases other than T2DM and hypertension were excluded and individuals who were using medications other than birth control pill were also excluded.
A non-randomized controlled clinical trial on TRF was performed over 3 months in obese women. Volunteers were recruited by social networks of people inserted in the community and instant messaging applications. Outcomes were assessed at baseline and after 3 months. The control group was recruited by social media as well but participants were informed that they would be engaged in diet habit research. Participants in the TRF group were asked to continue their regular nutritional habits during the non-fasting hours while the control group was instructed to maintain their habitual nutrition throughout the whole period. The protocol was approved by the Committee of Ethical Research from the Federal University of Fronteira Sul (protocol number 2.819.932), and written informed consent was obtained from all participants. Trial registration: ensaiosclinicos.gov.br (10051). Registered 16 June 2020—retrospectively registered.
Time-restricted feeding
TRF is based on the manipulation of timing (fasting) that aims the energy intake abstention [9]. The TRF protocol adopted here was a fasting period (no energy intake whatsoever) of 16 h (8 pm to 12 pm) and ad libitum feeding for 8 h (12 pm to 8 pm). The protocol was performed 7 days per week for 3 months. All participants received daily a reminder through instant messaging that informed the time to stop eating and the time in which food was allowed. In addition, periodic meetings were promoted (one every 15 days) for participants to share their experience and receive support from physicians. The control group was instructed to maintain the same dietary and living habits.
Main outcomes and measurements
The primary outcome was the effect of intermittent fasting on body weight and composition after 3 months compared to baseline values. Secondary outcomes were the characterization of metabolic risk factors and their association with weight loss. We also measured the cardiovascular risk of the participants at baseline and post 3 months.
Anthropometric measures and body composition
Weight was collected using the digital weight balance (Urano, PS 180). Height was measured by the wall stadiometer and waist circumference (upper edge of the iliac crest) with fine metric (Sanny, fiberglass tape). Body mass index (BMI) values were calculated from these measurements. For body composition analysis, we used anthropometric prediction equations, which were validated by the National Health and Nutrition Examination Survey (NHANES). We used the equation of Lee et al. [13] [SE (R2) = 2.44 (0.93)] and Heymsfied et al. [14] [SE (R2) = 1.6 (0.87)] with weight, height, waist circumference (WC) and race/ethnicity data to calculate total body fat mass (FM) and total skeletal muscle mass (MM), respectively. The values of total FM were divided by the total body weight and height2, to obtain the body fat percentage (%BF) and fat mass index (FMI), respectively. The values of MM were divided by total body weight, BMI and height2, to obtain the body skeletal muscle percentage (%MM), muscle mass related to BMI (MM/BMI), and muscle mass index related to height (SMIheight), respectively. All anthropometric measurements were performed thrice by one subject. If an error greater than 1% among the measurements was detected, a new measurement was taken.
Blood pressure measurement
Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured using a mercury sphygmomanometer (Kenz, 600) by a single evaluator according to the European Society of Cardiology/European Society of Hypertension checklist criteria [15].
Blood collection and analysis
Blood samples taken from the antecubital vein at baseline and after 3 months were collected in BD Vacutainers Tubes (SST™ II Advance, REF 367953). Samples were centrifuged (4000 RPM at 4 °C using centrifuge J6-MC by Beckman), and the resultant serum was aliquoted and stored at − 80 °C.
Acid uric, albumin, bilirubin, total cholesterol, high-density lipoprotein cholesterol (HDL-c), low‐density lipoprotein cholesterol (LDL), creatinine, alkaline phosphatase, gamma-glutamyl transferase (GGT), fasting glucose, Na+, K+, reactive C protein (PCR), alanine transaminase (ALT), aspartate aminotransferase (AST), triglycerides, and urea were evaluated by AU480 Chemistry Analyzer (Beckman Coulter) following the instruction provided by the manufacturer. Estradiol, insulin, free thyroxine (T4), and Thyroid-stimulating hormone (TSH) were analyzed by UniCel DxI 800 Access Immunoassay System (Beckman Coulter) following the instructions and protocols provided by the manufacturer.
The homeostatic model assessment (HOMA) index was calculated by the formula: HOMA-IR = fasting plasma insulin (µU/mL) × fasting plasma glucose (mmol/L)/22.5 [16].
Metabolic syndrome
To evaluate whether or not women presented MetS, we followed the criteria from Alberti et al. [4]. Participants were considered to have MetS when they presented three or more of the following components: WC > 80 cm (reference value for Ethnic Central and South American) [17]; triglycerides > 150 mg/dL or drug treatment for triglycerides reduction; HDL-C < 50 mg/dL or drug treatment for reduced HDL-C; SBP > 130 mmHg and/or DBP > 85 mmHg or antihypertensive drug treatment and fasting glucose > 100 mg/dL or drug treatment for elevated glucose. Participants were classified in 0–5 score according to the MetS criteria.
30-year cardiovascular disease risk evaluation
We have used the Framingham Heart Study, which provided an estimation of the 30-year CVD risk (CVDRisk30y) for each individual [18]. Framingham risk scores (FRS) for CVD covers the full spectrum of CVD, including coronary heart disease, peripheral vascular disease, stroke, and heart failure [19]. The online FRS calculator is user-friendly and free of cost (https://framinghamheartstudy.org/fhs-risk-functions/cardiovascular-disease-30-year-risk). The online calculation requires information on the age, gender, systolic blood pressure (at the time of the interview), treatment for hypertension (yes/no), diabetes (yes/no), smoking (yes/no), and BMI (kg/m2).
Quality of Life and Mini-Mental State Examination
The Quality of Life (QOL) assessed by WHOQOL-bref questionnaire has been translated and validated in Brazil [20]. The abbreviated WHOQOL-bref provides scores for four domains related to QOL: physical health, psychological, social relationships, and environment. Also, a self-perception of quality of life is measured by this questionnaire [21]. The WHOQOL-bref consists of 26 items rated on a 5-point Likert scale. The response options range from 1 (very dissatisfied/very poor) to 5 (very satisfied/very good) [20]. The scores are transformed and vary from 0 to 100, with higher scores representing better QOL [22]. The Mini-Mental State Examination (MMSE) evaluates the cognition health and it has been translated and validated in Brazil [23]. The test provides a score of 0–30. Given the low levels of education among older adults in Brazil, specific cut-off points are used based on the schooling level of the older adults: 13 for illiterate people, 18 for those with 1–11 years of schooling, and 26 for those with more than 11 years of schooling [23]. This questionnaire was used as a tool to characterize the participants and assess whether or not they would fully understand the protocol in order to follow it.
Statistical analysis
This study was conducted as an exploratory pilot study with the recruitment of women who were engaged in performing the protocol. Therefore, a sample size calculation did not seem possible. Results are presented as mean ± standard error of mean (SEM). Shapiro–Wilk’s W test was conducted to assess the normality. In order to reduce the influence of within-group variability, a univariate test of significance (ANCOVA) was performed. Levene’s test of homogeneity for equal variances was performed. For multiple comparisons (sensitivity analyses) Bonferroni correction of p-values was used. We fixed as dependent variable the post value of the clinical exams/anthropometric measurements for each group and the baseline values of the outcomes were adopted as a covariate. TRF group vs. Control group were assumed as categorical predictors. A paired Student t-test was performed between baseline and 3 months for each group. A Chi‐square test was used to compare the difference in MetS between groups. A post hoc power was performed using effect size Cohen’s d = 0.5. We calculated that this study had a power of 75%. The correlation CVDRisk30y and %BF, and CVDRisk30y and %MM were performed by Pearson´s correlation. P < 0.05 was adopted as significant differences. Statistical analyses were made with the statistical software package Statistical Package for the Social Science (SPSS), version 26.0.
Results
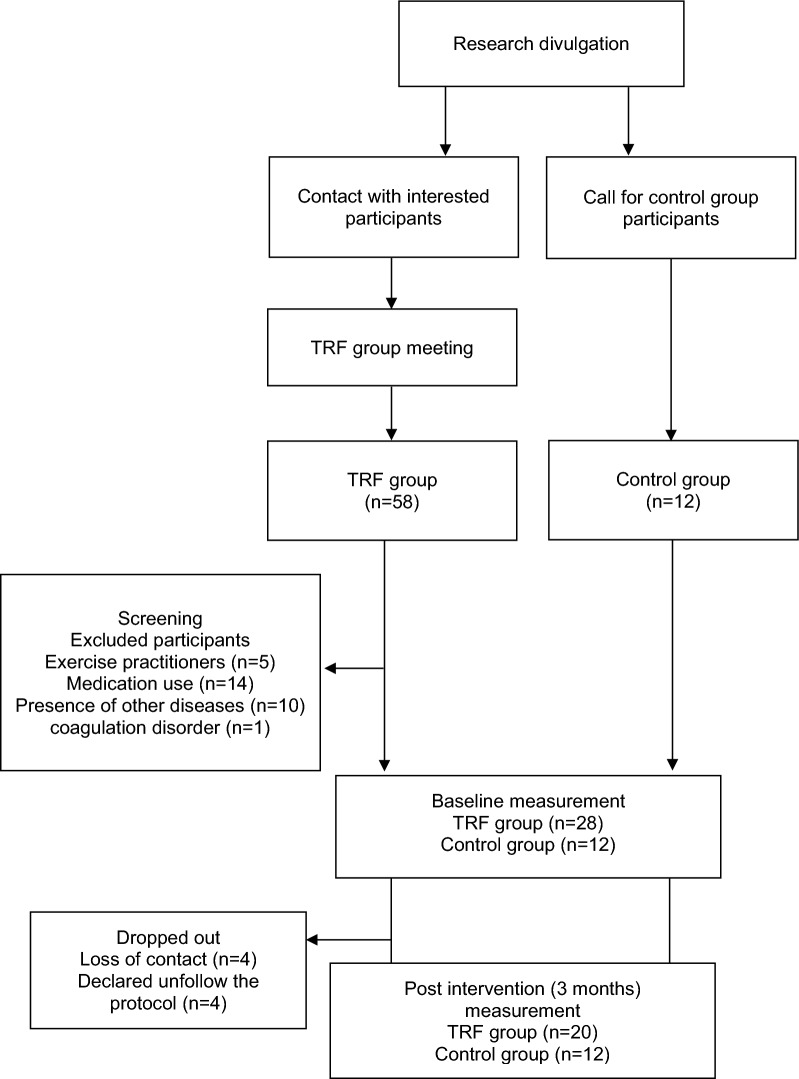
Fifty-eight interested subjects contacted our staff willing to engage in the intermittent fasting protocol, twenty-eight subjects after screening met the eligibility criteria, and one subject had to be excluded due to a coagulation disorder (Fig. 1). A total of twenty obese women (n = 20) voluntarily adopted the fasting protocol, while twelve obese women (n = 12) were enrolled in the control group. All women who were enrolled in this study attended the following criteria: sedentarily, non-communicable diseases, and physically inactive. Figure 1 shows the flowchart of the study with the dates that each event occurred.
Fig. 1.
Flow chart from the study design
Sample characteristic
Participant’s baseline characteristics are provided in Table 1. No significant between-group differences were observed at baseline in any measurement performed. Baseline values for participants of both groups reflect middle-aged women with obesity with no cognition impairment.
Table 1.
Subject characteristics at baseline
| TRF group (n = 20) | Control Group (n = 12) | P-value | |
|---|---|---|---|
| Baseline | Baseline | ||
| Age | 36.6 ± 1.6 | 42.3 ± 3.5 | 0.10 |
| Height (cm) | 159.8 ± 1.6 | 158.8 ± 1.3 | 0.66 |
| Weight (kg) | 83.62 ± 3.95 | 87.14 ± 3.25 | 0.54 |
| BMI (kg/m2) | 32.53 ± 1.13 | 34.55 ± 1.20 | 0.25 |
| Fat mass (kg) | 36.91 ± 2.30 | 39.74 ± 2.00 | 0.41 |
| WC (cm) | 101.13 ± 2.42 | 105.68 ± 3.11 | 0.26 |
| %BF | 43.61 ± 0.69 | 45.38 ± 0.69 | 0.10 |
| FMI (kg/m2) | 14.33 ± 0.72 | 15.71 ± 0.76 | 0.22 |
| MM (kg) | 21.59 ± 0.93 | 21.59 ± 0.70 | 0.99 |
| %MM | 26 ± 0.00 | 25 ± 0.00 | 0.01 |
| SMIheight (kg/m2) | 8.40 ± 0.25 | 8.51 ± 0.23 | 0.75 |
| MM/BMI (kg/kg/m2) | 0.66 ± 0.02 | 0.63 ± 0.02 | 0.15 |
| Insulin (mU/L) | 9.2 ± 0.9 | 10.3 ± 1.8 | 0.54 |
| Fasting glucose (mg/dL) | 86.2 ± 1.2 | 91.6 ± 3.0 | 0.07 |
| HOMA-IR | 1.88 ± 1.91 | 2.35 ± 0.44 | 0.19 |
| SBP (mmHg) | 126.7 ± 3.4 | 134.5 ± 7.3 | 0.28 |
| DBP (mmHg) | 86.9 ± 2.1 | 91.3 ± 4.1 | 0.28 |
| MMSE | 27.0 ± 0.4 | 26.5 ± 1.0 | 0.60 |
| CVDRisk30y (%) | 32.1 ± 7.3 | 15.6 ± 1.8 | < 0.001 |
Results are presented as mean ± SEM
%BF body fat percentage, %MM body skeletal muscle percentage, BMI body mass index, CVDRisk30y 30 year CVD risk, DBP diastolic blood pressure, FMI fat mass index, MAP median arterial pressure MetS metabolic syndrome, MM muscle mass, MM/BMI muscle mass related to BMI, MMSE mini-mental state examination, SBP systolic blood pressure, SMI skeletal muscle index, WC waist circumference
Outcome measurements
Table 2 summarizes the body weight and composition outcomes. The TRF group exhibited a decrease in body weight, BMI, FM, WC, %BF, FMI, MM, SMIheight, SBP, and DBP. Despite changes in body weight and body composition, there were no significant changes in blood biomarkers associated with metabolic and cardiovascular risk.
Table 2.
Results of anthropometric analysis and blood biomarkers in the time-restricted-feeding and control group at baseline and after 3 months
| TRF group (n = 20) | Control group (n = 12) | ∆ P-value | ||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 3 months | Δ TRF t test | Baseline | 3 months | Δ Control t test | ∆ (TRF-control) | ||
| Weight (kg) | 83.62 ± 3.95 | 80.24 ± 3.87 | < 0.001 | 87.14 ± 3.25 | 88.49 ± 3.04 | 0.007 | − 4.856 | < 0.001 |
| BMI (kg/m2) | 32.53 ± 1.13 | 31.19 ± 1.11 | < 0.001 | 34.55 ± 1.20 | 35.00 ± 1.10 | 0.078 | − 1.886 | < 0.001 |
| Fat mass (kg) | 36.91 ± 2.30 | 34.74 ± 2.24 | < 0.001 | 39.74 ± 2.00 | 40.59 ± 1.95 | 0.050 | − 3.125 | < 0.001 |
| WC (cm) | 101.13 ± 2.42 | 97.15 ± 2.21 | 0.001 | 105.68 ± 3.11 | 106.95 ± 2.81 | 0.412 | − 6.041 | < 0.001 |
| %BF | 43.61 ± 0.69 | 42.71 ± 0.73 | < 0.001 | 45.38 ± 0.69 | 45.73 ± 0.67 | 0.079 | − 1.218 | < 0.001 |
| FMI (kg/m2) | 14.33 ± 0.72 | 13.47 ± 0.71 | < 0.001 | 15.71 ± 0.76 | 16.10 ± 0.76 | 0.060 | − 1.301 | < 0.001 |
| MM (kg) | 21.59 ± 0.93 | 20.91 ± 0.92 | < 0.001 | 21.59 ± 0.70 | 21.78 ± 0.68 | 0.121 | − 0.901 | < 0.001 |
| %MM | 25.9 ± 1.1 | 26.2 ± 1.2 | 0.11 | 24.8 ± 1.3 | 24.7 ± 1.3 | 0.349 | 0.397 | 0.11 |
| SMI (kg/m2) | 8.40 ± 0.25 | 8.13 ± 0.24 | < 0.001 | 8.51 ± 0.23 | 8.63 ± 0.23 | 0.128 | − 0.394 | < 0.001 |
| MM/BMI (kg/kg/m2) | 0.66 ± 0.02 | 0.67 ± 0.02 | 0.09 | 0.63 ± 0.02 | 0.62 ± 0.02 | 0.220 | 0.011 | 0.05 |
| Insulin (mU/L) | 9.2 ± 0.9 | 9.1 ± 1.0 | 0.86 | 10.3 ± 1.8 | 13.8 ± 3.2 | 0.133 | − 3.494 | 0.07 |
| Glucose (mg/dL) | 86.2 ± 1.2 | 88.0 ± 1.4 | 0.25 | 91.6 ± 3.0 | 93.6 ± 4.5 | 0.458 | − 0.336 | 0.91 |
| HOMA-IR | 1.88 ± 1.91 | 1.90 ± 0.22 | 0.88 | 2.35 ± 0.44 | 3.27 ± 0.86 | 0.117 | − 0.717 | 0.13 |
| SBP (mmHg) | 126.7 ± 3.4 | 121.3 ± 3.1 | 0.03 | 134.5 ± 7.3 | 128.0 ± 4.0 | 0.227 | − 2.542 | 0.48 |
| DBP (mmHg) | 86.9 ± 2.1 | 83.5 ± 2.1 | 0.06 | 91.3 ± 4.1 | 86.5 ± 4.1 | 0.167 | 0.111 | 0.97 |
| Na+ (mEq/L) | 141.5 ± 0.7 | 144.0 ± 0.8 | 0.02 | 142.7 ± 0.6 | 140.5 ± 1.8 | 0.336 | 3.268 | 0.10 |
| K+ (mEq/L) | 4.3 ± 0.08 | 4.3 ± 0.10 | 1.000 | 4.3 ± 0.07 | 4.3 ± 0.17 | 0.769 | 0.045 | 0.80 |
| Frutosamine (µmol/L) | 240.1 ± 5.6 | 242.5 ± 3.4 | 0.52 | 236.5 ± 5.2 | 240.3 ± 6.2 | 0.260 | 0.128 | 0.10 |
| TC (mg/dL) | 205.9 ± 8.3 | 214.7 ± 9.7 | 0.18 | 195.1 ± 11.3 | 188.5 ± 13.3 | 0.307 | 15.818 | 0.11 |
| HDL-C (mg/dL) | 60.5 ± 2.1 | 61.1 ± 2.3 | 0.73 | 56.6 ± 5.1 | 53.7 ± 4.8 | 0.201 | 4.177 | 0.14 |
| LDL-C (mg/dL) | 120.0 ± 6.5 | 127.4 ± 7.8 | 0.20 | 112.8 ± 7.3 | 111.1 ± 9.1 | 0.715 | 9.468 | 0.26 |
| Cr (mg/dL) | 0.70 ± 0.03 | 0.69 ± 0.02 | 0.79 | 0.67 ± 0.18 | 0.69 ± 0.03 | 0.475 | − 0.007 | 0.82 |
| Triglycerides (mg/dL) | 144.0 ± 18.0 | 131.9 ± 17.6 | 0.18 | 148.1 ± 28.9 | 130.9 ± 24.8 | 0.228 | 4.307 | 0.77 |
| CRP (mg/L) | 6.3 ± 1.5 | 6.6 ± 1.4 | 0.86 | 9.1 ± 2.8 | 9.4 ± 2.2 | 0.889 | − 1.599 | 0.47 |
| Urea (mg/dL) | 27.8 ± 1.4 | 27.6 ± 2.1 | 0.90 | 32.8 ± 1.4 | 32.3 ± 2.4 | 0.801 | − 1.259 | 0.70 |
| Total Bilirubin (mg/dL) | 0.46 ± 0.07 | 0.42 ± 0.06 | 0.35 | 0.35 ± 0.03 | 0.35 ± 0.02 | 0.902 | − 0.008 | 0.88 |
| Indirect bilirubin (mg/dL) | 0.37 ± 0.06 | 0.34 ± 0.05 | 0.38 | 0.28 ± 0.03 | 0.28 ± 0.02 | 0.922 | − 0.002 | 0.97 |
| Direct bilirubin (mg/dL) | 0.09 ± 0.01 | 0.08 ± 0.01 | 0.30 | 0.07 ± 0.007 | 0.07 ± 0.005 | 0.461 | − 0.005 | 0.65 |
| ALP (U/L) | 69.8 ± 4.9 | 65.5 ± 3.9 | 0.26 | 81.6 ± 9.5 | 69.9 ± 8.1 | 0.340 | − 0.946 | 0.90 |
| GOT (U/L) | 19.7 ± 1.1 | 17.7 ± 1.6 | 0.14 | 18.2 ± 1.5 | 18.0 ± 1.3 | 0.916 | − 1.307 | 0.53 |
| ALT (U/L) | 18.6 ± 2.0 | 15.0 ± 1.9 | 0.002 | 16.8 ± 2.1 | 17.1 ± 1.7 | 0.919 | − 3.219 | 0.12 |
| GGT (U/L) | 23.0 ± 2.9 | 19.4 ± 2.1 | 0.06 | 29.3 ± 8.7 | 27.8 ± 5.4 | 0.751 | − 4.818 | 0.05 |
| Uric Acid (mg/dL) | 4.7 ± 0.2 | 4.7 ± 0.2 | 0.93 | 5.0 ± 0.4 | 5.3 ± 0.4 | 0.358 | − 0.398 | 0.27 |
| TSH (µUI/mL) | 3.06 ± 0.89 | 2.51 ± 0.39 | 0.09 | 2.65 ± 0.46 | 2.04 ± 0.21 | 0.130 | 0.126 | 0.71 |
| T4 (ng/dL) | 0.82 ± 0.03 | 0.86 ± 0.02 | 0.15 | 0.81 ± 0.02 | 0.83 ± 0.04 | 0.426 | 0.027 | 0.41 |
| Total Protein (g/dL) | 7.3 ± 0.1 | 7.3 ± 0.1 | 0.84 | 7.3 ± 0.1 | 7.0 ± 0.2 | 0.251 | 0.335 | 0.12 |
| Albumin (g/dL) | 4.2 ± 0.1 | 4.3 ± 0.1 | 0.70 | 4.2 ± 0.1 | 4.0 ± 0.2 | 0.195 | 0.307 | 0.18 |
| Globulin (g/dL) | 3.1 ± 0.1 | 3.0 ± 0.1 | 0.77 | 3.1 ± 0.1 | 3.0 ± 0.1 | 0.336 | 0.045 | 0.79 |
| Estradiol (pg/mL) | 128.2 ± 18.1 | 98.6 ± 15.7 | 0.17 | 67.9 ± 18.4 | 59.4 ± 10.6 | 0.565 | 24.190 | 0.31 |
Results are presented as mean ± SEM. A paired t-test was performed between baseline and post-intervention in each group. ANCOVA was performed to detect changes in effect between groups
ns not significant, %BF body fat percentage, %MM body skeletal muscle percentage, ALP alkaline phosphatase, ALT alanine aminotransferase, AST aspartate aminotransferase, BMI body mass index, CRP C-reactive protein, DBP diastolic blood pressure, FMI fat mass index, GGT gamma-glutamyl transferase, HDL-C high-density lipoprotein-cholesterol; K+ blood potassium, LDL-C low-density lipoprotein-cholesterol, MM muscle mass, MM/BMI muscle mass related to BMI, Na+ plasmatic sodium, SBP systolic blood pressure, SMI skeletal muscle index, TC total cholesterol, TSH thyroid-stimulating hormone, T4 free thyroxin, WC waist circumference
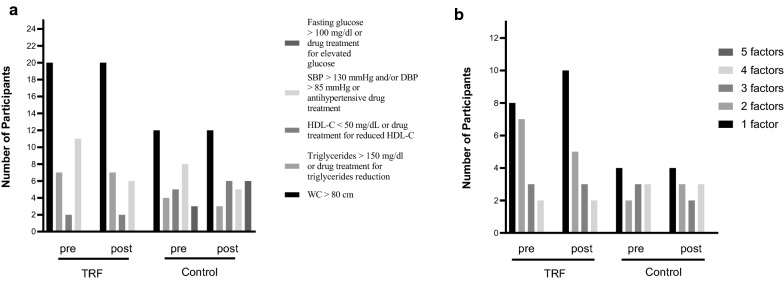
Figure 2a shows the criteria and the number of participants with the respective risk of MetS at baseline and post-intervention. According to Alberti et al. [4] criteria, the number of individuals with metabolic syndrome remained the same in both groups after the period of the study. Figure 2b shows the number of women who presented 1, 2, 3, 4, or 5 risk factors at baseline (pre) and post-intervention.
Fig. 2.
a Metabolic Syndrome risk factors and the number of individuals that have each factor in TRF and control groups in pre and post-intervention. b The number of participants who have 1, 2, 3, 4, or 5 risk factors
CVDRisk30y and its correlation to body fat and muscle mass
We have conducted an analysis of CVDRisk30y based on The Framingham Heart Study. In the TRF group, there was a ~ 12% reduction (%, 15.6 ± 1.8 pre vs. 13.8 ± 1.8 post-intervention, P < 0.05) in the probability of general cardiovascular events to happen. There was no change in CVDRisk30y in the control group (%, 32.1 ± 7.2 pre vs. 31.1 ± 6.7 post-intervention). To evaluate anthropometric changes with cardiovascular risk other than the ones predict in the Framingham Heart Study, we correlated all anthropometric indexes to CVDRisk30y (Additional file 1: Table S1). The best predictors of CVDRisk30y were %BF (r = 0.62, n = 64, p < 0.001) and %MM (r = − 0.74, n = 64, p < 0.001), and they are shown in Fig. 3.
Fig. 3.
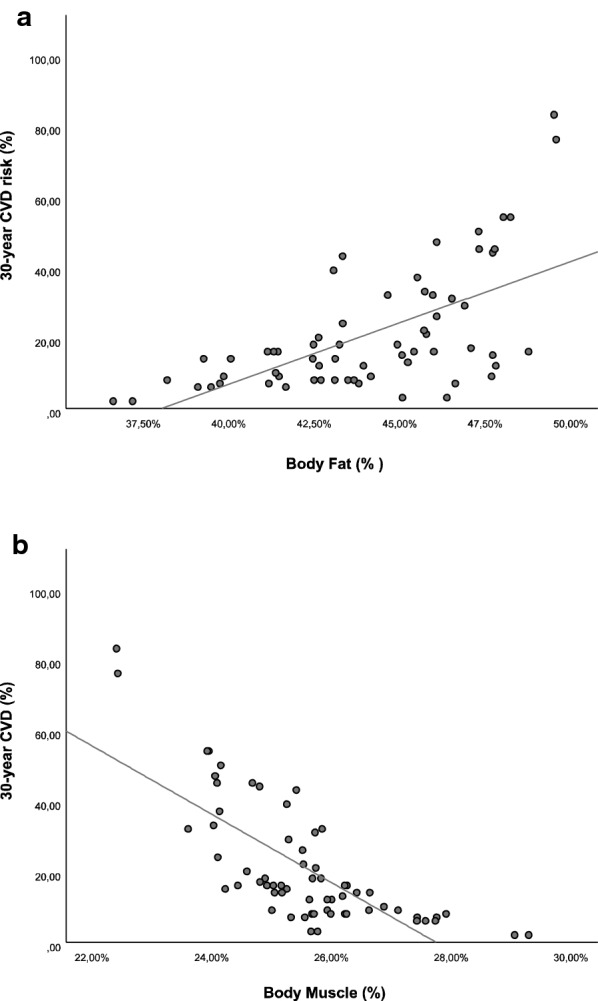
Correlation between percentual of body fat (%BF) and the risk of cardiovascular disease in 30 years (CVRisk30y) and the percentage of body skeletal muscle mass (%MM) and the risk of cardiovascular disease in 30 years (CVRisk30y)
Quality of Life Questionnaire
The total score for WHOQOL-bref questionnaire and the scores by subdomain are represented in Fig. 4a, b, respectively. Participants after TRF protocol obtained a higher score when compared to baseline values, and this fact was due to a self-perception of a better quality of life, as seen in Fig. 4b.
Fig. 4.
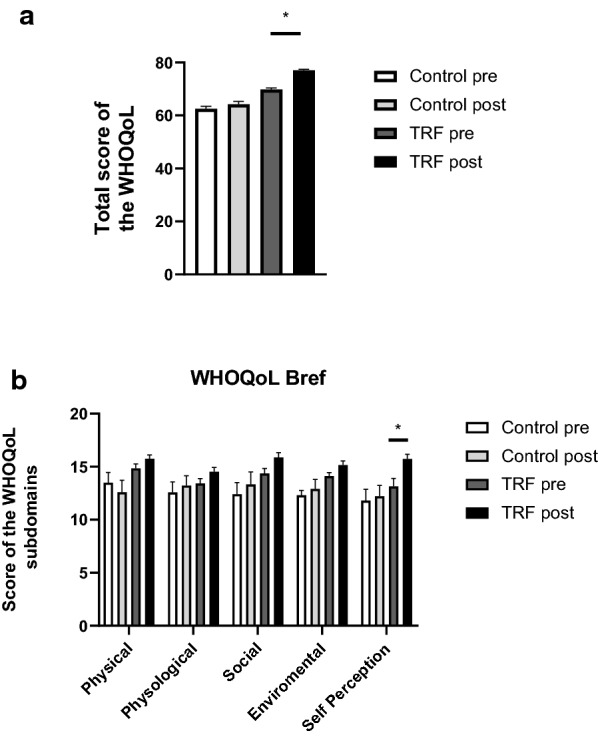
Total (a) and subdomains (b) scores of the WHOQoL. Values represent mean ± SEM and are represented as a percentage. (pre refers before intervention and post after intermittent fasting protocol). *P < 0.05 vs. IF pre
Discussion
In this study, the short-term effects of TRF on metabolic, hormonal, and anthropometric parameters were evaluated. TRF has shown to be an effective protocol to promote weight loss, anthropometric, and body composition changes, but did not show significant changes in blood biomarkers associated with metabolic and cardiovascular risk. Our findings differ from previous results in which TRF promotes changes in blood exams and metabolic parameters (glycemia, HDL, LDL, cholesterol, among others) [11, 24]. The TREAT Randomized Clinical Trial reported similar findings with no changes in fasting insulin and glycemia in a 16:8-h time-restricted eating protocol in overweighted adults [25].
It is widely accepted that obesity is associated with all-cause mortality and the development of cardiovascular events in mid-age adults [26, 27]. Also, overweight per se, without the presence of MetS, is an independent factor related to increased mortality and cardiovascular events [28–30]. In our study, 75% of obese women in the TRF group did not exhibit MetS at baseline measurements (presence of 3 or more risk factors, according to Fig. 2b). Therefore they were considered to have obesity since their BMI was greater than 30 kg/m2 without any change in metabolic biomarkers. This aspect is extremely relevant once any significant change in blood exams (glucose, triglycerides, HDL-c, etc.) may not appear before three months, confirming that obesity first-line treatment should aim weight-loss strategies. Evidence in humans suggests that the benefits of TRF are due mostly or only to weight loss [8, 31–33]. The variation among participants with distinct risk factors may be taken into account for different responses to the TRF protocol. Further studies are necessary to evaluate whether or not these factors impact on individual responses to TRF.
Except for weight and height, WC was the only direct anthropometric measurement performed in this study and it was reduced with TRF. It is well-known that WC is an indicator of visceral adiposity and a predictor of morbidity and mortality [34]. RCTs reveal that reduction in WC promoted by lifestyle change is associated with a significant decrease in cardiometabolic risk independently from gender or age [35, 36]. In our study, a significant positive correlation was seen between WC and cardiovascular risk, WC and %BF, and a significant negative correlation between WC and %MM. Therefore, WC measurement and %BF and%MM calculation by the prediction methods used here are valuable approaches for monitoring the results of a TRF protocol.
Our study has shown that TRF is a benefic dietary intervention that leads to an increase in self-reported quality of life, which can be explained by weight loss [37, 38]. Besides, obese women often report dissatisfaction concerning their bodies when compared to subjects with normal weight [39]. Since TRF promoted a reduction in weight and waist circumference, the improvement in the quality of life seen in these women may be indeed attributed to a self-perception of a better body image [40].
Clinical relevance
Obesity is characterized by the accumulation of adipose tissue and an increase in body mass, which develops under a chronic positive energy balance. Therefore the reduction of this excess of adipose mass is the main goal of the clinical approach to treat obesity [41–43]. TRF can be an efficient strategy to promote weight loss in those subjects who cannot adhere to diets that alter the whole nutritional pattern of the individual’s daily food ingestion.
Most of the subjects in our study are obese but did classic biomarkers are not altered, corroborating data from populational studies [44]. Nonetheless, these individuals are targets of the deleterious effects of excess adipose tissue that will trigger MetS at any time. This study points out the importance of a more comprehensive evaluation of overweight or obese subjects, which includes anthropometric measurements.
Limitations
This study was not a randomized controlled trial and does not include detailed nutritional aspects of the subject´s diet since we found conflicting data reported by individuals, such as an incomplete description of the amount of food ingested, the frequency of meals, and the type of foods (e.g.: what kind of rice was ingested). In addition, the dietary intake self-report can differ or underestimate the real value, offering an inconclusive and misleading analysis [45, 46]. Energy restriction is the main factor that leads to weight loss independently of the type of diet [9]. Therefore, it is feasible to assume that a reduction in total energy consumed was achieved, considering that energy expenditure has maintained constant.
A recent meta-analysis has shown a dose–response between weight loss and reduction in energy intake [47]. Therefore, one can assume that weight loss was higher in those women who had more energy balance deficit. Another concern is the protocol adherence, which can interfere in the outcomes. Since we are not able to control whether or not the subjects followed the TRF strictly for three months without any gap, explaining differences in weight loss among subjects is a hard task. Herein, 45% of the women in the TRF group did not lose more than 4% of the weight. Since it is well established that negative energy balance promotes weight loss, we have stratified our analysis to avoid misinterpretation of what event was due to protocol adherence or the individual’s response to the protocol.
Conclusion
TRF is an effective dietary strategy to promote weight loss and to decrease WC with no remarkable changes in blood biomarkers. This can be explained by the considerable number of obese women without MetS, in which they have an excess of weight and WC, but not always altered blood biomarkers. Also, anthropometric prediction of %BF and %MM can be used as an approach to guide health professionals to evaluate and follow individuals engaged in TRF protocol since those measurements correlate with cardiovascular risk.
Supplementary Information
Additional file 1: Table S1. Two-variable correlation. Pearson correlation, p-value (Sig.), and the number of cases (n) are presented in the table.
Acknowledgements
We would like to thank the staff of the Physiology department of UNOCHAPECÓ for providing the ambulatory space for blood collection and anthropometric measurements studies.
Abbreviations
- %BF
Body fat percentage
- %MM
Body skeletal muscle percentage
- ALT
Alanine transaminase
- BMI
Body mass index
- CVD
Cardiovascular disease
- CVDRisk30y
Estimation of the 30-year CVD risk
- DBP
Diastolic blood pressure
- FM
Fat mass
- FMI
Fat mass index
- FRS
Framingham risk scores
- GGT
Gamma-glutamyl transferase
- HDL-c
High-density lipoprotein cholesterol
- HOMA
Homeostatic model assessment
- LDL
Low‐density lipoprotein cholesterol
- MAP
Mean arterial pressure
- MetS
Metabolic syndrome
- MHO
Metabolically healthy obesity
- MM/BMI
Muscle mass related to BMI
- MM
Muscle mass
- MMSE
Mini-Mental State Examination
- NHANES
National Health and Nutrition Examination Survey
- PCR
Reactive C protein
- QOL
Quality of Life Questionnaire
- SBP
Systolic blood pressure
- SE
Standard error
- SEM
Standard error of mean
- SMIheight
Muscle mass index related to height
- SPSS
Statistical package for the social science
- T2DM
Type 2 diabetes mellitus
- T4
Thyroxine
- TRF
Time-restricted feeding
- TSH
Thyroid-stimulating hormone
Authors’ contributions
JDS participated in the research divulgation, design of the experiments, blood collection, questionnaire application, data synthesis. HF participated in the design of the study, anthropometric measurements, data extraction, interpreted data, and drafted the manuscript. AM participated in blood collection, blood analysis, and data extraction. DZ participated in blood collection, blood analysis, and extraction of data. TO participated in the orientation of the participants. CASA participated in the anthropometric data collection. AMC participated in blood collection, blood analysis, and extraction of data. LHM participated in designing and coordinating the study, analyzed and interpreted all data, performed the statistical analysis, and drafted the manuscript. All authors read and approved the final manuscript.
Funding
This work was support by Federal University of Fronteira Sul (480/GR/UFFS/2018; 1010/GR/UFFS/2018), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior‐CAPES, and Conselho Nacional de Desenvolvimento Científico e Tecnológico‐CNPq.
Availability of data and materials
We may provide all raw data to the Journal upon request. Relevant data may be provided to readers upon request.
Ethics approval and consent to participate
The protocol was approved by the Committee of Ethical Research from the Federal University of Fronteira Sul (Protocol Number 2.819.932), and written informed consent was obtained from all participants.
Consent for publication
All participants accepted in the written informed consent that the results obtained in the research could be used to scientific publication and divulgation as long as their name was not revealed.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jéssica D. Schroder and Hugo Falqueto contributed equally to this work
Supplementary Information
The online version contains supplementary material available at 10.1186/s12967-020-02687-0.
References
- 1.Dixon JB, Browne JL, Mosely KG, Rice TL, Jones KM, Pouwer F, et al. Severe obesity and diabetes self-care attitudes, behaviours and burden: implications for weight management from a matched case-controlled study. Results from Diabetes MILES–Australia. Diabet Med. 2014;31:232–240. doi: 10.1111/dme.12306. [DOI] [PubMed] [Google Scholar]
- 2.Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, et al. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113:898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. [DOI] [PubMed] [Google Scholar]
- 3.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 4.Alberti KGMM, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 5.Wing RR, Lang W, Wadden TA, Safford M, Knowler WC, Bertoni AG, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34:1481–1486. doi: 10.2337/dc10-2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma C, Avenell A, Bolland M, Hudson J, Stewart F, Robertson C, et al. Effects of weight loss interventions for adults who are obese on mortality, cardiovascular disease, and cancer: systematic review and meta-analysis. BMJ. 2017;359:j4849. doi: 10.1136/bmj.j4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnstone A. Fasting for weight loss: an effective strategy or latest dieting trend? Int J Obes. 2005;2015(39):727–733. doi: 10.1038/ijo.2014.214. [DOI] [PubMed] [Google Scholar]
- 8.Trepanowski JF, Kroeger CM, Barnosky A, Klempel MC, Bhutani S, Hoddy KK, et al. Effect of alternate-day fasting on weight loss, weight maintenance, and cardioprotection among metabolically healthy obese adults: a randomized clinical trial. JAMA Intern Med. 2017;177:930–938. doi: 10.1001/jamainternmed.2017.0936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freire R. Scientific evidence of diets for weight loss: different macronutrient composition, intermittent fasting, and popular diets. Nutrition. 2020;69:110549. doi: 10.1016/j.nut.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Cienfuegos S, Gabel K, Kalam F, Ezpeleta M, Wiseman E, Pavlou V, et al. Effects of 4- and 6-h time-restricted feeding on weight and cardiometabolic health: a randomized controlled trial in adults with obesity. Cell Metab. 2020;32(366–378):e3. doi: 10.1016/j.cmet.2020.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, Peterson CM. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. 2018;27:1212.e3–1221.e3. doi: 10.1016/j.cmet.2018.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chowdhury EA, Richardson JD, Tsintzas K, Thompson D, Betts JA. Effect of extended morning fasting upon ad libitum lunch intake and associated metabolic and hormonal responses in obese adults. Int J Obes. 2005;2016(40):305–311. doi: 10.1038/ijo.2015.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee DH, Keum N, Hu FB, Orav EJ, Rimm EB, Sun Q, et al. Development and validation of anthropometric prediction equations for lean body mass, fat mass and percent fat in adults using the National Health and Nutrition Examination Survey (NHANES) 1999–2006. Br J Nutr. 2017;118:858–866. doi: 10.1017/S0007114517002665. [DOI] [PubMed] [Google Scholar]
- 14.Heymsfield SB, Stanley A, Pietrobelli A, Heo M. Simple skeletal muscle mass estimation formulas: what we can learn from them. Front Endocrinol. 2020;11:31. doi: 10.3389/fendo.2020.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: the Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J hypertens. 2018;36:1953–2041. doi: 10.1097/HJH.0000000000001940. [DOI] [PubMed] [Google Scholar]
- 16.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 17.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 18.Pencina MJ, D’Agostino RB, Larson MG, Massaro JM, Vasan RS. Predicting the 30-year risk of cardiovascular disease: the framingham heart study. Circulation. 2009;119:3078–3084. doi: 10.1161/CIRCULATIONAHA.108.816694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D’Agostino RB, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care: the Framingham heart study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 20.Fleck MP, Louzada S, Xavier M, Chachamovich E, Vieira G, Santos L, et al. Application of the Portuguese version of the abbreviated instrument of quality life WHOQOL-bref. Rev Saúde Pública. Faculdade de Saúde Pública da Universidade de São Paulo; 2000;34:178–83. [DOI] [PubMed]
- 21.The WHOQOL Group Development of the World Health Organization WHOQOL-BREF quality of life assessment. Psychol Med. 1998;28:551–558. doi: 10.1017/S0033291798006667. [DOI] [PubMed] [Google Scholar]
- 22.Fleck MP, Chachamovich E, Trentini C. Development and validation of the Portuguese version of the WHOQOL-OLD module. Rev Saúde Pública. Faculdade de Saúde Pública da Universidade de São Paulo; 2006;40:785–91. [DOI] [PubMed]
- 23.Bertolucci PH, Brucki SM, Campacci SR, Juliano Y. The mini-mental state examination in a general population: impact of educational status. Arq Neuropsiquiatr. 1994;52:1–7. doi: 10.1590/S0004-282X1994000100001. [DOI] [PubMed] [Google Scholar]
- 24.Wilkinson MJ, Manoogian ENC, Zadourian A, Lo H, Fakhouri S, Shoghi A, et al. Ten-hour time-restricted eating reduces weight, blood pressure, and atherogenic lipids in patients with metabolic syndrome. Cell Metab. 2020;31:92–104. doi: 10.1016/j.cmet.2019.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lowe DA, Wu N, Rohdin-Bibby L, Moore AH, Kelly N, Liu YE, et al. Effects of time-restricted eating on weight loss and other metabolic parameters in women and men with overweight and obesity: the TREAT randomized clinical trial. JAMA Intern Med. 2020;180(11):1491–1499. doi: 10.1001/jamainternmed.2020.4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun Y, Liu B, Snetselaar LG, Wallace RB, Caan BJ, Rohan TE, et al. Association of normal-weight central obesity with all-cause and cause-specific mortality among postmenopausal women. JAMA Netw Open. 2019;2:e197337. doi: 10.1001/jamanetworkopen.2019.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Global BMI Mortality Collaboration. Di Angelantonio E, Bhupathiraju S, Wormser D, Gao P, Kaptoge S, et al. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet. 2016;388:776–786. doi: 10.1016/S0140-6736(16)30175-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stefan N, Häring H-U, Hu FB, Schulze MB. Metabolically healthy obesity: epidemiology, mechanisms, and clinical implications. Lancet Diabetes Endocrinol. 2013;1:152–162. doi: 10.1016/S2213-8587(13)70062-7. [DOI] [PubMed] [Google Scholar]
- 29.Lin L, Zhang J, Jiang L, Du R, Hu C, Lu J, et al. Transition of metabolic phenotypes and risk of subclinical atherosclerosis according to BMI: a prospective study. Diabetologia. 2020 doi: 10.1007/s00125-020-05116-5. [DOI] [PubMed] [Google Scholar]
- 30.Hsueh Y, Yeh T-L, Lin C-Y, Tsai S-Y, Liu S-J, Lin C-M, et al. Association of metabolically healthy obesity and elevated risk of coronary artery calcification: a systematic review and meta-analysis. PeerJ. 2020;8:e8815. doi: 10.7717/peerj.8815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soeters MR, Lammers NM, Dubbelhuis PF, Ackermans M, Jonkers-Schuitema CF, Fliers E, et al. Intermittent fasting does not affect whole-body glucose, lipid, or protein metabolism. Am J Clin Nutr. 2009;90:1244–1251. doi: 10.3945/ajcn.2008.27327. [DOI] [PubMed] [Google Scholar]
- 32.Harvie M, Howell A. Potential benefits and harms of intermittent energy restriction and intermittent fasting amongst obese, overweight and normal weight subjects—a narrative review of human and animal evidence. Behav Sci Basel. 2017;7:4. doi: 10.3390/bs7010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Halberg N, Henriksen M, Söderhamn N, Stallknecht B, Ploug T, Schjerling P, et al. Effect of intermittent fasting and refeeding on insulin action in healthy men. J Appl Physiol. 1985;2005(99):2128–2136. doi: 10.1152/japplphysiol.00683.2005. [DOI] [PubMed] [Google Scholar]
- 34.Ross R, Neeland IJ, Yamashita S, Shai I, Seidell J, Magni P, et al. Waist circumference as a vital sign in clinical practice: a consensus statement from the IAS and ICCR Working Group on visceral obesity. Nat Rev Endocrinol. 2020;16:177–189. doi: 10.1038/s41574-019-0310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Church TS, Earnest CP, Skinner JS, Blair SN. Effects of different doses of physical activity on cardiorespiratory fitness among sedentary, overweight or obese postmenopausal women with elevated blood pressure: a randomized controlled trial. JAMA. 2007;297:2081–2091. doi: 10.1001/jama.297.19.2081. [DOI] [PubMed] [Google Scholar]
- 36.Ross R, Dagnone D, Jones PJ, Smith H, Paddags A, Hudson R, et al. Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men. A randomized, controlled trial. Ann Intern Med. 2000;133:92–103. doi: 10.7326/0003-4819-133-2-200007180-00008. [DOI] [PubMed] [Google Scholar]
- 37.Kolotkin RL, Crosby RD, Williams GR, Hartley GG, Nicol S. The relationship between health-related quality of life and weight loss. Obes Res. 2001;9:564–571. doi: 10.1038/oby.2001.73. [DOI] [PubMed] [Google Scholar]
- 38.Vasiljevic N, Ralevic S, Kolotkin RL, Marinkovic J, Jorga J. The relationship between weight loss and health-related quality of life in a serbian population. Eur Eat Disord Rev. 2012;20:162–168. doi: 10.1002/erv.1114. [DOI] [PubMed] [Google Scholar]
- 39.Weinberger N-A, Kersting A, Riedel-Heller SG, Luck-Sikorski C. Body dissatisfaction in individuals with obesity compared to normal-weight individuals: a systematic review and meta-analysis. Obes Facts. 2017;9:424–441. doi: 10.1159/000454837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Legenbauer T, Müller A, de Zwaan M, Herpertz S. Body image and body avoidance nine years after bariatric surgery and conventional weight loss treatment. Front Psychiatry. 2020;10:945. doi: 10.3389/fpsyt.2019.00945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heymsfield SB, Wadden TA. Mechanisms, pathophysiology, and management of obesity. N Engl J Med. 2017;376:1492. doi: 10.1056/NEJMra1514009. [DOI] [PubMed] [Google Scholar]
- 42.Gadde KM, Martin CK, Berthoud H-R, Heymsfield SB. Obesity: pathophysiology and management. J Am Coll Cardiol. 2018;71:69–84. doi: 10.1016/j.jacc.2017.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lean MEJ. Management of obesity and overweight. Medicine. 2019;47:175–183. doi: 10.1016/j.mpmed.2018.12.008. [DOI] [Google Scholar]
- 44.Caleyachetty R, Thomas GN, Toulis KA, Mohammed N, Gokhale KM, Balachandran K, et al. Metabolically healthy obese and incident cardiovascular disease events among 3.5 million men and women. J Am Coll Cardiol. 2017;70:1429–1437. doi: 10.1016/j.jacc.2017.07.763. [DOI] [PubMed] [Google Scholar]
- 45.Schoeller DA, Thomas D, Archer E, Heymsfield SB, Blair SN, Goran MI, et al. Self-report-based estimates of energy intake offer an inadequate basis for scientific conclusions. Am J Clin Nutr. 2013;97:1413–1415. doi: 10.3945/ajcn.113.062125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dhurandhar NV, Schoeller D, Brown AW, Heymsfield SB, Thomas D, Sørensen TIA, et al. Energy balance measurement: when something is not better than nothing. Int J Obes. 2005;2015(39):1109–1113. doi: 10.1038/ijo.2014.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kloecker DE, Zaccardi F, Baldry E, Davies MJ, Khunti K, Webb DR. Efficacy of low- and very-low-energy diets in people with type 2 diabetes mellitus: a systematic review and meta-analysis of interventional studies. Diabetes Obes Metab. 2019;21:1695–1705. doi: 10.1111/dom.13727. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Two-variable correlation. Pearson correlation, p-value (Sig.), and the number of cases (n) are presented in the table.
Data Availability Statement
We may provide all raw data to the Journal upon request. Relevant data may be provided to readers upon request.