Dear Editor,
Hemolytic anemia (HA) is a common condition in intensive care units (ICU) responsible for life-threatening organ failure in severe cases [1]. HA needs urgent treatment initiation, but its diagnosis remains challenging as none of the biological diagnostic parameters, including bilirubin, LDH and haptoglobin, are specific. During hemolysis, free hemoglobin released by red blood cells is catabolized by heme-oxygenase 1, leading to formation of iron, biliverdin and carbon monoxide [2]. Next, carbon monoxide binds to free hemoglobin to form carboxyhemoglobin. Carboxyhemoglobin is routinely measured in ICU and available within a few minutes by CO-oximetry, a point of care testing (GEM® Premier™ 4000, Werfen, Le Pré-Saint-Gervais, France) [3]. Our objective was to evaluate carboxyhemoglobin as a diagnostic tool for HA in adult patients admitted in ICU.
Methods and statistical analysis
We retrospectively analyzed data from patients hospitalized for HA and non-HA in our ICU during an 8-year and 1-year period, respectively. An adjudication committee consisting of three senior experts (from hematology, internal medicine and critical care departments) confirmed final HA and non-HA diagnosis with all available clinical and biological data. Carboxyhemoglobin was measured at ICU admission on arterial blood.
Differences between groups were compared using the Student's t test or Wilcoxon’s test. Correlations were computed using Pearson’s formula. Discrimination performances were assessed by using area under the receiver operating characteristic curve (ROC). Statistical analyses were performed using R software (v 2.12.0; http://cran.r-project.org).
Results
Overall, 187 patients were included, 94 patients with HA and 93 with non-HA (Table 1). Among patients with HA, 50 (54%) had thrombotic micro-angiopathy, 25 (26%) had auto-immune hemolytic anemia, and 19 (20%) had sickle cell disease.
Table 1.
Characteristics of patients with and without hemolytic anemia
| Hemolytic anemia (94) | Non-hemolytic anemia (93) | p | |
|---|---|---|---|
| General characteristics | |||
| Age (years) | 47 (32–62) | 69 (59–77) | 0.0004 |
| Women (%) | 51 | 33 | 0.004 |
| Smokers (%) | 34 | 37 | 0.9 |
| SOFA at admission | 6 (3–7) | 6 (4–9) | 0.004 |
| Sepsis (%) | 18 | 27 | 0.1 |
| Mechanical ventilation (%) | 17 | 57 | < 0.0001 |
| Catecholamines (norepinephrine) (%) | 17 | 54 | < 0.0001 |
| Extra-renal epuration (%) | 17 | 13 | 0.43 |
| Length of stay in ICU (days) | 6 (3–12) | 7 (4–12) | 0.54 |
| Mortality (%) | 16 | 32 | 0.24 |
| Cause of anemia (%) | |||
| Hemolytic anemia (94) | |||
| AIHA | 26 | ||
| SCD | 20 | ||
| TMA | 54 | ||
| Non-hemolytic anemia (93) | |||
| Bleeding | 37 | ||
| Chronic renal failure | 15 | ||
| Cytopenia | 23 | ||
| Splenomegaly | 10 | ||
| Other | 15 | ||
| Biological marker (blood) | |||
| Hemoglobin (g/dL) | 7.8 (6.2–9.1) | 7.3 (6.5–8.1) | 0.14 |
| White blood cells (× 109/L) | 10.6 (7.8–17.9) | 11.3 (6.7–16.5) | 0.2 |
| Platelets (10 3/mm3) | 76 (33.2–228) | 141 (48–237) | 0.09 |
| Mean corpuscular volume (fL) | 88 (83–93) | 88 (82–94) | 0.47 |
| Reticulocytes (G/L) | 134 (77–211) | 55 (29–77) | 0.0001 |
| LDH (UI/L) | 1361 (866–2121) | 536 (420–786) | 0.46 |
| Unconjugated bilirubin (μmol/L) | 27 (18–45) | 15 (10–20) | < 0.0001 |
| Prothrombin time (%) | 76 (62–89) | 70 (57–80) | 0.009 |
| Activated partial thromboplastin time ratio | 1.07 (0.94–1.3) | 1.17 (1.01–1.51) | 0.055 |
| Creatinine (μmol/L) | 102 (71–160) | 122 (81–206) | 0.47 |
| pH | 7.44 (7.4–7.48) | 7.44 (7.34–7.48) | 0.25 |
| pO2 (mmHg) | 87 (70–113) | 81 (67–107) | 0.92 |
| pCO2 (mmHg) | 34 (31–43) | 35 (30–42) | 0.93 |
| Lactate (mmol/L) | 1.3 (0.9–2.5) | 1.25 (0.9–2.3) | 0.88 |
| Carboxyhemoglobin (%) | 3.0 (2.3–3.9) | 1.6 (1.0–2.1) | < 0.0001 |
| Methemoglobin (%) | 1.5 (1.2–1.8) | 1 (0.7–1.4) | < 0.0001 |
Data are expressed as number, percentage or median and interquartile ranges (IQRs)
SOFA sepsis-related organ failure assessment, AIHA auto-immune hemolytic anemia, TMA thrombotic micro-angiopathy, SCD sickle cell disease, LDH lactate dehydrogenase
Carboxyhemoglobin levels were twofold higher in patients with HA in comparison with patients with non-HA (3.0 [2.3–3.9] vs 1.6 [1.0–2.1] %, p < 0.0001). Carboxyhemoglobin level at admission was an accurate diagnostic tool for HA as the area under the curve (AUC) was 0.93 (CI 95% [0.89–0.96]), higher than LDH (AUC = 0.80, CI 95% [0.73–0.86]), unconjugated bilirubin (AUC = 0.77, CI 95% [0.71–0.84]) and methemoglobin (AUC = 0.71, CI 95% [0.64–0.79]) (Fig. 1). A threshold of carboxyhemoglobin of 2.0% for detection of hemolysis, yielded a sensitivity of 85% (CI 95% [77–90]) and specificity of 86% (CI 95% [80–90]). Specificity of carboxyhemoglobin for hemolysis detection was ≥ 99% for levels ≥ 2.7%. Using a logistic regression, we adjusted the analysis of carboxyhemoglobin for age and SOFA and we found that carboxyhemoglobin remained strongly associated with hemolysis: crude OR 74 (CI 95% [19–281]), adjusted OR 53 (CI 95% [12–240], p < 0.001 (both Wald and LR test). Focusing on patients with HA, we found that carboxyhemoglobin levels inversely correlated with hemoglobin levels (r = 0.42, p < 0.0001).
Fig. 1.
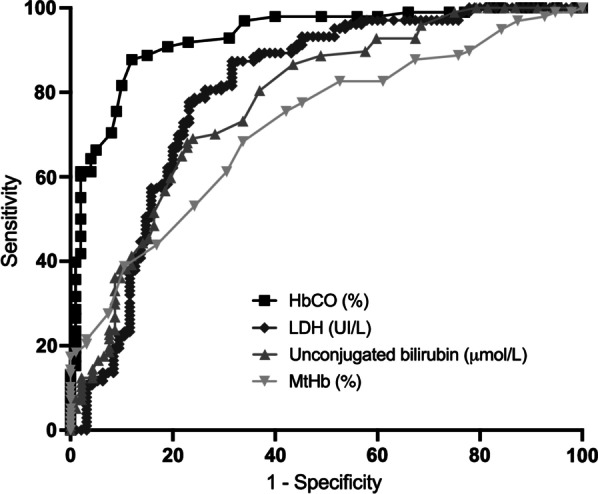
Receiver operator characteristic curves for hemolysis biomarkers: carboxyhemoglobin (HbCO) (black square), AUC = 0.93 (CI 95% [0.89–0.96]); lactate dehydrogenase (gray diamond), AUC = 0.80 (CI 95% [0.73–0.86]), unconjugated bilirubin (light gray triangle), AUC = 0.77, (CI 95% [0.71–0.84]) and methemoglobin (reversed light gray triangle), AUC = 0.71 (CI 95% [0.64–0.79])
Discussion
In anemic adult patients admitted in ICU, we found that carboxyhemoglobin was a reliable diagnostic biomarker of hemolysis. Diagnostic accuracy of HA was better using carboxyhemoglobin than LDH and unconjugated bilirubin, when an optimal threshold of 2.0% was used. A similar threshold of carboxyhemoglobin at 2.2% has recently been reported in a cohort of term newborns [4]. In critically ill patients with comorbidities and multiple organ failures, classical hemolysis biomarkers as LDH and unconjugated bilirubin may lack specificity [5]. Haptoglobin is another biomarker for hemolysis, but its level may change in several critical conditions including sepsis or red blood cell transfusion [6]. Unfortunately, in our study, haptoglobin was not available in patients with non-HA. We also found a significant relationship between plasma carboxyhemoglobin and hemoglobin levels, meaning that the higher the carboxyhemoglobin, the more severe the hemolytic anemia. Carboxyhemoglobin has to be analyzed after evaluation of confounding factors that potentially increase (heavy smoker, sepsis, carbon monoxide chronic sub-intoxication) or decrease (hyperoxia) its levels.
Acknowledgements
Not applicable.
Authors’ contributions
All authors helped in study concept and design. K.H.P., G.H. and H.A.O acquired the data. G.H., K.H.P, H.A.O. drafted the manuscript. All authors critically revised the manuscript. G.H. and H.A.O statistically analyzed. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable for retrospective monocenter study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Geoffroy Hariri, Email: geoffroy.hariri@aphp.fr.
Kyann Hodjat Panah, Email: kyannhp@gmail.com.
Bénédicte Beneteau-Burnat, Email: benedicte.beneteau-burnat@aphp.fr.
Michael Chaquin, Email: michael.chaquin@aphp.fr.
Arsene Mekinian, Email: arsene.mekinian@aphp.fr.
Hafid Ait-Oufella, Email: hafid.aitoufella@aphp.fr.
References
- 1.Dhaliwal G, Cornett PA, Tierney LM., Jr Hemolytic anemia. Am Fam Phys. 2004;11:2599–2606. [PubMed] [Google Scholar]
- 2.Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol. 1997;37:517–554. doi: 10.1146/annurev.pharmtox.37.1.517. [DOI] [PubMed] [Google Scholar]
- 3.Beneteau-Burnat B, Pernet P, Pilon A, Latour D, Goujon S, Feuillu A, Vaubourdolle M. Evaluation of the GEM Premier 4000: a compact blood gas CO-Oximeter and electrolyte analyzer for point-of-care and laboratory testing. Clin Chem Lab Med. 2008;2:271–279. doi: 10.1515/CCLM.2008.043. [DOI] [PubMed] [Google Scholar]
- 4.Karabulut B, Arcagok BC. A neglected and promising predictor of severe hyperbilirubinemia due to hemolysis: carboxyhemoglobin. Fetal Pediatr Pathol. 2020;2:124–131. doi: 10.1080/15513815.2019.1641862. [DOI] [PubMed] [Google Scholar]
- 5.Cui J, Xiong J, Zhang Y, Peng T, Huang M, Lin Y, Guo Y, et al. Serum lactate dehydrogenase is predictive of persistent organ failure in acute pancreatitis. J Crit Care. 2017;41:161–165. doi: 10.1016/j.jcrc.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Kormoczi GF, Saemann MD, Buchta C, Peck-Radosavljevic M, Mayr WR, Schwartz DW, Dunkler D, et al. Influence of clinical factors on the haemolysis marker haptoglobin. Eur J Clin Investig. 2006;3:202–209. doi: 10.1111/j.1365-2362.2006.01617.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


