To the Editor:
Drugs have been developed that improve the primary cellular impairment in cystic fibrosis (CF) by increasing functional CFTR (CF transmembrane conductance regulator) protein in epithelial cells (1). Substantial biological and clinical effects were seen in clinical trials of ivacaftor, and more recently with a three-drug combination targeting the F508del mutation (2–4). The concentration of chloride in sweat (SwCl) is a central biomarker that is used to diagnose CF and to assess drug-induced improvement in CFTR function (5). Change in SwCl occurs within days of starting effective CFTR modulator drugs and is a useful predictor of clinical outcomes for groups based on a shared mutation, but has not performed as well for individual study participants (5). Many CF mutations are present in only a handful of people across the globe. This increases the need for alternative or complementary biomarkers that may predict individual clinical responses to drug therapy. For this purpose, cell-based assays of personalized in vitro CFTR function and response to drug exposure are currently being considered (6, 7).
We conducted a proof-of-concept study using an ongoing clinical research program in Dublin, Ireland, to collect primary airway epithelial cells (AECs) from participants treated with ivacaftor. This allowed us to test for a correlation between in vitro ivacaftor-induced CFTR activation and measures of both a change in SwCl and improved lung function (i.e., forced expiratory volume in 1 second (FEV1)% predicted [FEV1pp]) (8). We compared the strength of the correlation between improved lung function (i.e., clinical improvement) and in vitro ivacaftor-induced CFTR function versus the change in SwCl.
Methods
With institutional review board approval and informed consent from 12 adult individuals with either the G551D or R117H mutation, we collected nasal AECs by brushing the inferior nasal turbinate. Cells were placed in PneumaCult EX medium (Stemcell) supplemented with fluconazole (25 μg/ml) and antibiotics targeting CF pathogens (ceftazidime 100 μg/ml, tobramycin 100 μg/ml, and vancomycin 100 μg/ml), and transported warm between Dublin and Seattle, Washington, using vacuum-insulated Thermos containers with HotHands warm packs. Delays during shipping caused most of the cells to arrive ≥3 days after they were collected rather than overnight. Upon arrival in Seattle, each brushing was seeded into a collagen-coated T25 flask and proliferated in submerged conditions until the cells reached 80% confluence (passage 0). One sample was insufficient and another experienced fungal overgrowth during transport. Six of the 10 remaining samples were expanded in submerged culture and passaged into collagen-coated, permeable, 12-mm-diameter Snapwell (Corning) Transwell inserts that were compatible with our Ussing chamber system (Physiologic Instruments, Inc.). They were then differentiated for 21 days at an air–liquid interface in PneumaCult air–liquid interface medium (Stemcell) as previously described (9–11). Differentiated AECs with a transepithelial resistance of ≥300 Ω.cm2 were treated with vx-770 (analog of ivacaftor; 1 μM added apically) during standard Ussing chamber analyses of ion transport. Two additional G551D samples that were cryopreserved at passage 1 were added from the CFF Biorepository to account for unexpected losses from samples collected in Dublin (12). The investigators who cultured the AECs and performed the Ussing assays were blinded to the clinical parameters, including SwCl. CFTR activation in vitro was assessed by measuring the short-circuit current (mean from triplicate wells per cell line) after vx-770 exposure, reflecting drug-induced chloride transport and the relative change in chloride transport. Pearson correlation coefficients were used to analyze the data.
Results
To assess the specificity of chloride conductance measurements in our AEC cultures and Ussing chamber analyses, we compared chemical chloride conductance activation by forskolin (10 μM) plus vx-770 (1 μM) with that by chemical inhibition with I-172 (10 μM). The high correlation found (Pearson r = −0.95; P = 0.0003) supported the validity of the samples and assays.
When we examined clinical outcome measures, we observed a strong correlation between a change in SwCl and in vitro CFTR activation (Figure 1). We also observed a moderately strong correlation between in vitro CFTR activation and a change in FEV1pp (Figure 2). A significant correlation was seen between either SwCl or in vitro CFTR activation and the change in FEV1pp (Figure 3). Changes in SwCl performed better in these analyses, including short- or long-term changes in lung function.
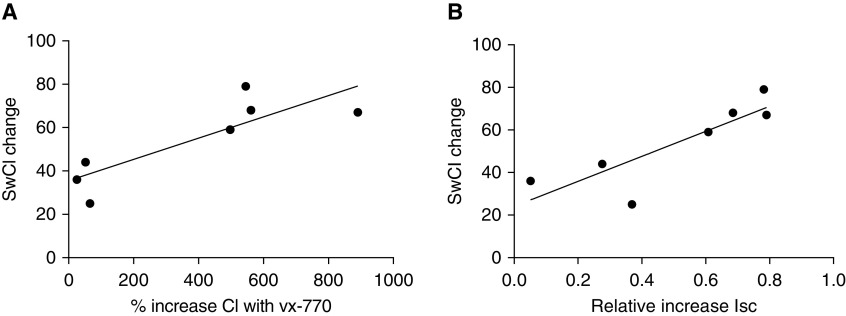
Figure 1.
Cell-based CFTR (cystic fibrosis transmembrane conductance regulator) activation and change in the concentration of chloride in sweat (SwCl). We calculated CFTR activation in two ways: (A) the percent increase in the short-circuit current (Isc) after exposure to vx-770, and (B) the relative increase in Isc with vx-770 versus total chloride conductance (forskolin + vx-770). These data were tested against in vivo CFTR activation measured by the change in SwCl, using the greatest change from baseline measured during the clinical trials. In both cases, a significant correlation was found between in vitro CFTR activation and decrease in sweat chloride in participants after ivacaftor (n = 7; SwCl data were missing for one subject). (A) r = 0.84, R2 = 0.70, and P < 0.02. (B) r = 0.85, R2 = 0.72, and P < 0.02.
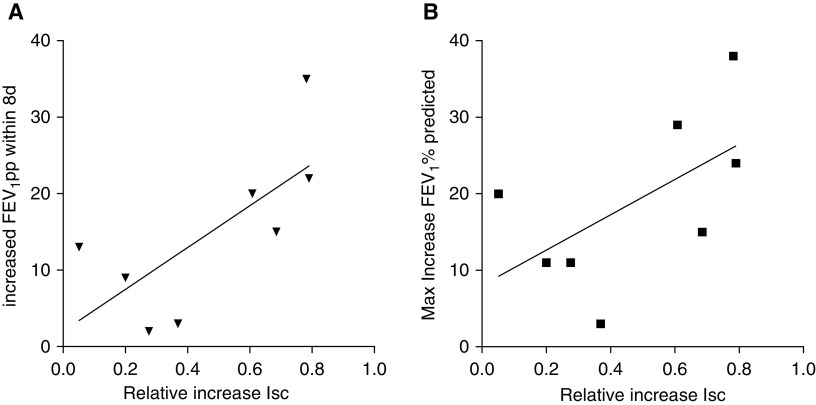
Figure 2.
Cell-based CFTR (cystic fibrosis transmembrane conductance regulator) activation and change in FEV1pp. (A) Improvement in forced expiratory volume in 1 second (FEV1)% predicted (FEV1pp) in the first week after initiating ivacaftor and CFTR activation in the cell model showed a moderately strong correlation (r = 0.71). (B) The correlation was weaker when the maximum increase in FEV1pp during 1 year of clinical follow-up after vx-770 initiation was considered. (A) r = 0.71, R2 = 0.51, and P = 0.05. (B) r = 0.58, R2 = 0.34, and P = 0.13. Isc = short-circuit current; max = maximum.
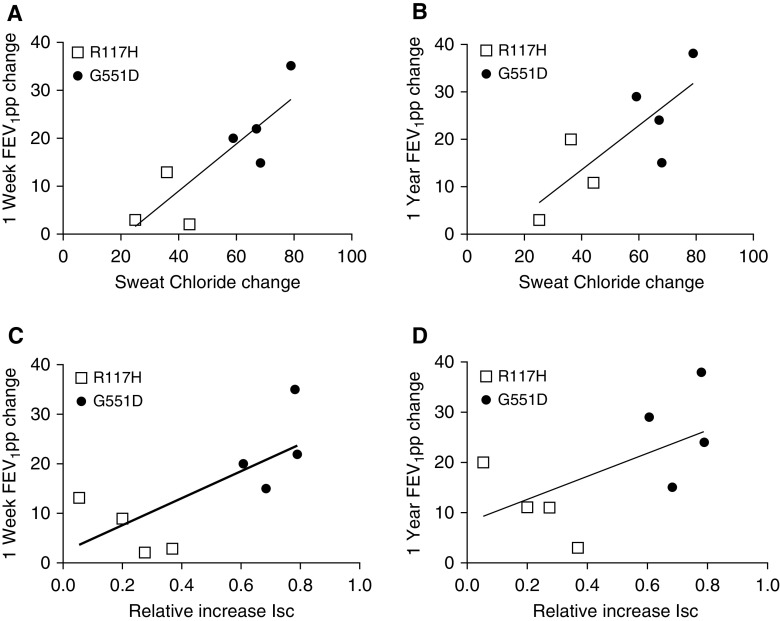
Figure 3.
Correlation between change in forced expiratory volume in 1 second (FEV1)% predicted (FEV1pp) and either sweat chloride (SwCl) or cell-based CFTR (cystic fibrosis transmembrane conductance regulator) activation. (A–D) We compared the strength of the correlation between improved lung function (FEV1pp) and either a change in SwCl (A and B) or a relative increase in chloride conductance in epithelial cells as measured above (C and D). (A and B) Change in SwCl correlated with change in FEV1pp over the first week or first year of ivacaftor use (A: 1 wk r = 0.84, R2 = 0.71, and P < 0.02; B: 1 yr r = 0.78, R2 = 0.60, and P < 0.05). (C and D) In vitro CFTR activation, defined as vx-770/(forskolin + vx-770), correlated with short-term improvement in FEV1pp (C: 1 wk r = 0.71, R2 = 0.51, and P < 0.05; D: 1 yr r = 0.58, R2 = 0.34, and P = 0.13). In both assays, participants with G551D (solid circles) versus R117H (open squares) mutations appeared to segregate by response to ivacaftor, but there was overlap in FEV1pp change.
Discussion
This proof-of-concept study included relatively few cell samples. Despite this important limitation, the results support the concept that nasal AECs can be interrogated to measure increased chloride transport afforded by CFTR modulator drugs, and that these results may associate with important clinical or in vivo biological outcome measures. Certainly, studies with larger numbers of samples representing more diverse CFTR mutations will be critical to better understand this potential. We observed a much stronger correlation between changes in SwCl and FEV1pp than has been reported (12, 13). The reason for this is uncertain, but it may relate to the early assessments (1 wk) and our use of the greatest change in SwCl across multiple time points. We observed a significant but modestly weaker correlation between the in vitro AEC response to vx-770 and change in FEV1pp. FEV1 is affected by multiple factors that are not directly related to chloride transport, including airway microbiology, variable individual immune/inflammatory responses, and other therapies, all of which may weaken the association between SwCl and AECs’ response to vx-770 and lung function over time.
Apart from these promising early results, several key lessons were learned from this proof-of-concept study. International transportation of nonsterile, temperature-controlled, live airway cell samples is challenging, and methods should allow for unanticipated delays. This is particularly important when the primary sample has microbial contamination and the samples are maintained warm rather than cold or frozen. Our group has had extensive experience over the past decade in culturing and differentiating primary AECs (9–11, 14). Our early attempts to transport primary AECs from the point of collection to the laboratory for initiation of cultures failed when AECs were cooled (unpublished results), leading to our longstanding successful approach of transporting primary AEC brushings in culture media at 37°C. Simulated conditions indicated that this approach would work well for at least 24 hours, but we did not test longer periods of time. In addition, alternative definitions for clinical and in vitro outcome measurements may be useful. We chose to focus on relative rather than absolute chloride conductance with vx-770, accounting for forskolin-induced responses. In our hands, this improved the correlation with SwCl changes. We also identified the greatest improvement in FEV1pp over shorter (1 wk) and longer (1 yr) timespans than are typically used in modulator drug trials. Short-term changes in FEV1pp are more closely associated with changes in chloride conductance and may be less susceptible to stochastic events.
Large studies are underway to assess the predictive potential of alternative epithelial models (i.e., rectal organoids) (15). Our data support continued work with nasal or other respiratory samples, which may be cultured as spheroids or planar differentiated cultures (16). Ideally, these data should be directly compared in future studies to best determine the value, limitations, and complementary roles of each model.
Supplementary Material
Footnotes
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Cuyx S, De Boeck K. Treating the underlying cystic fibrosis transmembrane conductance regulator defect in patients with cystic fibrosis. Semin Respir Crit Care Med. 2019;40:762–774. doi: 10.1055/s-0039-1696664. [DOI] [PubMed] [Google Scholar]
- 2.Ramsey BW, Davies J, McElvaney NG, Tullis E, Bell SC, Dřevínek P, et al. VX08-770-102 Study Group. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011;365:1663–1672. doi: 10.1056/NEJMoa1105185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heijerman HGM, McKone EF, Downey DG, Van Braeckel E, Rowe SM, Tullis E, et al. VX17-445-103 Trial Group. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomised, phase 3 trial. Lancet. 2019;394:1940–1948. doi: 10.1016/S0140-6736(19)32597-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Middleton PG, Mall MA, Dřevínek P, Lands LC, McKone EF, Polineni D, et al. VX17-445-102 Study Group. Elexacaftor-tezacaftor-ivacaftor for cystic fibrosis with a single Phe508del allele. N Engl J Med. 2019;381:1809–1819. doi: 10.1056/NEJMoa1908639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fidler MC, Beusmans J, Panorchan P, Van Goor F. Correlation of sweat chloride and percent predicted FEV1 in cystic fibrosis patients treated with ivacaftor. J Cyst Fibros. 2017;16:41–44. doi: 10.1016/j.jcf.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Dekkers JF, Wiegerinck CL, de Jonge HR, Bronsveld I, Janssens HM, de Winter-de Groot KM, et al. A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nat Med. 2013;19:939–945. doi: 10.1038/nm.3201. [DOI] [PubMed] [Google Scholar]
- 7.Pranke IM, Hatton A, Simonin J, Jais JP, Le Pimpec-Barthes F, Carsin A, et al. Correction of CFTR function in nasal epithelial cells from cystic fibrosis patients predicts improvement of respiratory function by CFTR modulators. Sci Rep. 2017;7:7375. doi: 10.1038/s41598-017-07504-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hisert KB, Heltshe SL, Pope C, Jorth P, Wu X, Edwards RM, et al. Restoring cystic fibrosis transmembrane conductance regulator function reduces airway bacteria and inflammation in people with cystic fibrosis and chronic lung infections. Am J Respir Crit Care Med. 2017;195:1617–1628. doi: 10.1164/rccm.201609-1954OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reeves SR, Barrow KA, White MP, Rich LM, Naushab M, Debley JS. Stability of gene expression by primary bronchial epithelial cells over increasing passage number. BMC Pulm Med. 2018;18:91. doi: 10.1186/s12890-018-0652-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lopez-Guisa JM, Powers C, File D, Cochrane E, Jimenez N, Debley JS. Airway epithelial cells from asthmatic children differentially express proremodeling factors. J Allergy Clin Immunol. 2012;129:990–997.e6. doi: 10.1016/j.jaci.2011.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.James RG, Reeves SR, Barrow KA, White MP, Glukhova VA, Haghighi C, et al. Deficient follistatin-like 3 secretion by asthmatic airway epithelium impairs fibroblast regulation and fibroblast-to-myofibroblast transition. Am J Respir Cell Mol Biol. 2018;59:104–113. doi: 10.1165/rcmb.2017-0025OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rowe SM, Heltshe SL, Gonska T, Donaldson SH, Borowitz D, Gelfond D, et al. GOAL Investigators of the Cystic Fibrosis Foundation Therapeutics Development Network. Clinical mechanism of the cystic fibrosis transmembrane conductance regulator potentiator ivacaftor in G551D-mediated cystic fibrosis. Am J Respir Crit Care Med. 2014;190:175–184. doi: 10.1164/rccm.201404-0703OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Durmowicz AG, Witzmann KA, Rosebraugh CJ, Chowdhury BA. Change in sweat chloride as a clinical end point in cystic fibrosis clinical trials: the ivacaftor experience. Chest. 2013;143:14–18. doi: 10.1378/chest.12-1430. [DOI] [PubMed] [Google Scholar]
- 14.Altman MC, Reeves SR, Parker AR, Whalen E, Misura KM, Barrow KA, et al. Interferon response to respiratory syncytial virus by bronchial epithelium from children with asthma is inversely correlated with pulmonary function. J Allergy Clin Immunol. 2018;142:451–459. doi: 10.1016/j.jaci.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berkers G, van Mourik P, Vonk AM, Kruisselbrink E, Dekkers JF, de Winter-de Groot KM, et al. Rectal organoids enable personalized treatment of cystic fibrosis. Cell Rep. 2019;26:1701–1708.e3. doi: 10.1016/j.celrep.2019.01.068. [DOI] [PubMed] [Google Scholar]
- 16.Brewington JJ, Filbrandt ET, LaRosa FJ, III, Ostmann AJ, Strecker LM, Szczesniak RD, et al. Detection of CFTR function and modulation in primary human nasal cell spheroids. J Cyst Fibros. 2018;17:26–33. doi: 10.1016/j.jcf.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.