Abstract
Nearly 20 years after the year 2000 target for global wild poliovirus eradication, live polioviruses continue to circulate with all three serotypes posing challenges for the polio endgame. We updated a global differential equation-based poliovirus transmission and stochastic risk model to include programmatic and epidemiological experience through October 2019. We used the model to explore the likely dynamics of poliovirus transmission for 2019–2023, which coincides with a new Global Polio Eradication Initiative (GPEI) Strategic Plan. The model stratifies the global population into 72 blocks, each containing 10 subpopulations of approximately 10.7 million people. Exported viruses go into subpopulations within the same block and within groups of blocks that represent large preferentially-mixing geographical areas (e.g., continents). We assign representative World Bank income levels to the blocks along with polio immunization and transmission assumptions, which capture some of the heterogeneity across countries while still focusing on global poliovirus transmission dynamics. We also updated estimates of reintroduction risks using available evidence. The updated model characterizes transmission dynamics and resulting polio cases consistent with the evidence through 2019. Based on recent epidemiological experience and prospective immunization assumptions based on the 2019–2023 Strategic Plan, the updated model does not show successful eradication of serotype 1 wild poliovirus by 2023 or successful cessation of oral poliovirus vaccine (OPV) serotype 2 related viruses.
SOCIAL MEDIA SUMMARY
Risk Analysis modeling shows Global Polio Eradication Initiative not on track to succeed
Keywords: polio, eradication, dynamic modeling, poliovirus vaccine
Introduction
In 2013, the Global Polio Eradication Initiative (GPEI) anticipated ending all transmission of wild polioviruses (WPVs) by 2016 (World Health Organization Global Polio Eradication Initiative, 2013). The GPEI started down a path of globally-coordinating the cessation of oral poliovirus vaccine (OPV) by stopping the use of serotype 2 OPV (OPV2) in April-May 2016 (World Health Organization Global Polio Eradication Initiative, 2013). The GPEI expected to coordinate cessation of OPV serotypes 1 and 3 by 2019 (World Health Organization Global Polio Eradication Initiative, 2013), which it extended to 2020 by aiming to end WPV transmission in 2017 following a midterm review in 2015 (World Health Organization Global Polio Eradication Initiative, 2015). Epidemiological experience to date (as of January 9, 2020 ) demonstrates continued transmission of serotype 1 WPV (WPV1) in a large number of geographic areas in Afghanistan and Pakistan (143 cases) (World Health Organization, 2020). In addition, coordinated cessation of OPV2 did not end all transmission of serotype 2 live polioviruses. Cases caused by circulating vaccine-derived polioviruses (cVDPVs), specifically by serotype 2 (cVDPV2s) (241 to date in 2019), require the ongoing need to use monovalent OPV (mOPV) for serotype 2 (mOPV2) for outbreak response (World Health Organization, 2020). The trend in polio cases in recent years showed increases. Notably, in 2018, the GPEI reported more paralytic cases caused by cVDPV2 (71 cases, with 104 cVDPVs of all 3 serotypes) than by WPV1 (33 cases), and more WPV1 cases than in 2017 (22 cases) (World Health Organization, 2020).
Transmission of live polioviruses requires continued use of OPV and the need to manage the risks of cVDPVs (Duintjer Tebbens et al., 2006; Duintjer Tebbens, Pallansch, Kim, et al., 2013; Duintjer Tebbens, Pallansch, Wassalik, Cochi, & Thompson, 2015). Other polio endgame risks include live polioviruses reintroduced by a very small number of individuals with B-cell-related primary immunodeficiencies (PIDs) who harbor poliovirus infections for relatively long periods (i.e., immunodeficiency-associated VDPVs or iVDPVs) (Duintjer Tebbens, Kalkowska, & Thompson, 2019; Duintjer Tebbens et al., 2006; Duintjer Tebbens, Pallansch, & Thompson, 2015; Duintjer Tebbens, Pallansch, Wassalik, et al., 2015; Duintjer Tebbens & Thompson, 2017) and potential intentional or unintentional release of any live poliovirus (LPV, i.e., WPV, VDPV, OPV, or OPV-related poliovirus) (see appendix A1 for more background, abbreviations, and acronyms) (Duintjer Tebbens, Kalkowsa, & Thompson, 2018; Duintjer Tebbens et al., 2006; Duintjer Tebbens, Pallansch, Wassalik, et al., 2015).
Globally, countries use a wide range of routine immunization (RI) schedules for polio vaccines (Thompson et al., 2013). We stratify the countries broadly according to their 2019 World Bank income level [i.e., low-income (LI), lower middle-income (LMI), upper middle-income (UMI), and high-income (HI)], and RI schedule (World Bank, 2019). Following successful national WPV elimination efforts and in the context of high-performing health systems, HI countries use inactivated poliovirus vaccine (IPV) exclusively (i.e., IPV only) for RI, which eliminates risk for vaccine-associated paralytic polio (VAPP) (Duintjer Tebbens et al., 2006; Duintjer Tebbens, Pallansch, Wassalik, et al., 2015; Thompson & Duintjer Tebbens, 2006). Some middle-income countries (i.e., primarily UMI) use a sequential schedule of IPV followed by OPV for VAPP prevention (i.e., IPV/OPV), while relatively lower-income countries (i.e., primarily LMI and LI) use OPV primarily and deliver a single dose of IPV simultaneous with the third non-birth OPV dose (i.e., OPV+IPV) (Plotkin & Vidor, 2013; World Health Organization, 2014). In 2015, the World Health Organization (WHO) Strategic Advisory Group of Experts on Immunization (SAGE) recommended at least one dose of IPV to protect individuals born after the global cessation of OPV2 from potential paralysis if they became exposed to circulating serotype 2 LPVs (World Health Organization, 2015b). Following this recommendation and in anticipation of global OPV2 cessation, nearly all countries that did not already use IPV initiated its use in their national immunization programs between 2016 and 2018. IPV provides protection from paralysis to vaccine recipients who seroconvert, but it does not significantly help to reduce transmission of LPVs in populations dominated by fecal-oral transmission (Duintjer Tebbens, Pallansch, et al., 2013a; Duintjer Tebbens, Pallansch, et al., 2013b; Thompson & Duintjer Tebbens, 2014; Thompson et al., 2013). Relying on evidence that 2 IPV doses would offer more durable protection than 1 IPV dose, the SAGE recommended in 2017 that all national immunization schedules include at least 2 IPV doses for at least 10 years after cessation of the last OPV serotype (World Health Organization, 2017). Notably, the SAGE made this recommendation without the benefit of any analyses that explored the impact of IPV use on risks, transmission, vaccine supply or costs, and with no timeline for implementation.
With the release of a new GPEI Strategic Plan for 2019–2023 (World Health Organization Global Polio Eradication Initiative, 2019), questions about the trajectory of the polio endgame continue to arise. Models offer the opportunity to explore policy and strategy questions prospectively, and to characterize transmission dynamics and disease incidence as a function of time, which support insights related to surveillance and potential undetected transmission and opportunities for risk management. Recognizing significant polio case underreporting in the 1980s, the WHO estimated burdens of vaccine-preventable diseases using a simple static linear model, which relied on vaccine routine immunization coverage and a fixed rate of polio in unvaccinated children. Using this approach, in 1983, the WHO estimated an incidence of 400,000 polio cases (Henderson, 1985). In 1986 the WHO estimated 275,000 cases of polio for 1985 using the 1985 population and infant death rates for developing countries, reported coverage as of August 1986, vaccine efficacy of 95%, and an assumed paralytic polio rate of 5 per 1,000 unvaccinated surviving infants (World Health Organization, 1986). Subsequent analyses by WHO that used the same approach with updated surviving infant and coverage estimates reported estimated incidence of 200,000 to 250,000 polio cases for 1986, 1987, and 1988, and estimated 350,000 cases of polio prevented in 1988 (Hull, Birmingham, Melgaard, & Lee, 1997; World Health Organization, 1989). WHO reported an estimated incidence of 148,000 polio cases for 1990 despite the additional change for this analysis of assuming a more appropriate, lower vaccine efficacy of 80% (instead of 95%), which would increase the estimated burden in the absence of increased coverage (World Health Organization, 1992). In 1999, the GPEI uses an estimate of 350,000 polio cases occurring in 1988 (Tangermann et al., 1999) based on rounding the 34,617 reported cases (or 35,251 reported cases (Hull et al., 1997)) to 35,000 and applying an assumed reporting efficiency of 10% derived from review of studies that retrospectively surveyed communities to identify lame children. One prior modeling analysis of the health and economic outcomes of the GPEI estimated an underreporting factor of 7 for 1988–1995 and factors of 2 or 1.11 for 1996–2010 depending on the quality of the surveillance based on comparison of its model estimates (which implicitly assume perfect detection by the model) to the reported incidence (Duintjer Tebbens et al., 2011). The analysis highlighted significant improvements in global poliovirus surveillance over time and as polio incidence declined (Duintjer Tebbens et al., 2011). Although uncertainty exists about the true historical incidence of polio, the development and maintenance of highly sensitive global poliovirus surveillance provides high confidence about the successful elimination and absence of transmission globally. In this regard, in October 2019, the Global Certification Commission certified WPV serotype 3 as globally eradicated (World Health Organization, 2019c).
We previously developed a global model that supported the characterization of the dynamic health and economic outcomes for the polio endgame (Duintjer Tebbens, Pallansch, Wassalik, et al., 2015) built on a differential equation-based (DEB) poliovirus transmission and OPV evolution model (Duintjer Tebbens, Pallansch, Kalkowska, et al., 2013; Duintjer Tebbens, Pallansch, Kim, et al., 2013) (see appendix A2 for details about the DEB model and inputs). Given the epidemiological experience from the past 5 years, the GPEI partners are asking new questions about the outlook for the polio endgame and evaluating complex investments choices for which they seek insights from prospective models. We updated our global model to support the characterization of current and prospective transmission dynamics and to consider updated assessments of stochastic re-introduction risks (e.g., breaches in containment) that may occur. This analysis seeks to provide an analytical perspective on the polio endgame prospects and to develop a tool that can support further analysis of the health and economic trade-offs of prospective risk management choices.
Methods
Analytical framework
We previously developed a global model to support prospective analyses of risk management interventions (Duintjer Tebbens, Pallansch, Wassalik, et al., 2015) to support the prior GPEI strategic plan (World Health Organization Global Polio Eradication Initiative, 2013). Briefly, that model divided the global population into approximately even blocks and focused on calibrating the model to 2013 with a relatively simplistic run up to 2013. We applied that model to explore multiple risk management prospective policies, for example related to outbreak response strategies, OPV2 cessation strategies and risks, stockpile choices, vaccine policies with respect to RI and SIAs, etc.) (Duintjer Tebbens, Hampton, & Thompson, 2016; Duintjer Tebbens, Hampton, Wassilak, et al., 2016; Duintjer Tebbens, Pallansch, Wassilak, Cochi, & Thompson, 2016; Duintjer Tebbens & Thompson, 2018; Thompson & Duintjer Tebbens, 2015a, 2015b).
Starting with the prior global model (Duintjer Tebbens, Pallansch, Wassalik, et al., 2015), we reviewed our assumptions with respect to what we anticipated between 2013–2019 compared to what actually occurred. We thus shift the new analytical time horizon for prospective modeling to run from 2019 (T0). However, in contrast to the prior global model (Duintjer Tebbens, Pallansch, Wassalik, et al., 2015), we recognize the opportunity to use the updated model to support retrospective analyses, and we focus on realistic global modeling of poliovirus transmission starting in 1988 (i.e., the year of the launch of the GPEI, TGPEI). Unlike the prior global model (Duintjer Tebbens, Pallansch, Wassalik, et al., 2015), instead of assuming ideal risk management actions by the GPEI and countries from 2013 on, the updated model considers the actual actions taken between TGPEI and T0 and their effectiveness (i.e., for 1988–2019). The model considers the planned prospective actions for prospective analyses. The model uses the 2019 revision of the UN World Population Prospects (Population Division of the Department of Economic and Social Affairs of the United Nations Secretariat, 2019) and the 2019 World Bank list of economies (World Bank, 2019). We use historical population estimates for 1988–2018 and projected (medium variant) population estimates for 2019–2099 (Population Division of the Department of Economic and Social Affairs of the United Nations Secretariat, 2019). With the larger global population (i.e., a global population of 7.7 billion people as of the beginning of 2019) (Population Division of the Department of Economic and Social Affairs of the United Nations Secretariat, 2019) relative to the prior model (Duintjer Tebbens, Pallansch, Wassalik, et al., 2015), we stratify the world into 72 blocks according to the country 2019 income levels (i.e., 6 LI, 28 LMI, 27 UMI, and 11 HI) to characterize some of the global variability in socioeconomic, sanitation, hygiene, and other conditions, costs, and national values and preferences. Similar to the preferential geographic mixing in the prior model (Duintjer Tebbens, Pallansch, Wassalik, et al., 2015), we also group the 72 blocks into 9 preferential mixing areas (PMAs) that correspond to large geographical regions (e.g., Africa, Australasia, Europe) with variable numbers of blocks per PMA (see appendix A3.1). Each block consists of 10 subpopulations, each with approximately 10.7 million people that may represent a country, state, large area within a large country, or a group of well-connected smaller countries of the same income level. The model assumes spatially-homogeneous and age-heterogeneous mixing of the people in the subpopulation.
Global poliovirus transmission model
We use the same DEB model to characterize poliovirus transmission and OPV evolution as prior work, the same generic model inputs (Duintjer Tebbens et al., 2014; Duintjer Tebbens, Pallansch, Kalkowska, et al., 2013) based on an extensive expert review (Duintjer Tebbens, Pallansch, et al., 2013a; Duintjer Tebbens, Pallansch, Kim, et al., 2013) and elicitation process (Duintjer Tebbens, Pallansch, et al., 2013b), and the same model fitting process. The model characterizes each poliovirus (PV) serotype separately (i.e., PV1, PV2, and PV3), and considers both fecal-oral and oropharyngeal transmission (Duintjer Tebbens, Pallansch, Wassalik, et al., 2015). Table 1 summarizes the constant global model inputs (see appendix A2 for full details and equations). Briefly, the DEB model divides the population into 7 age groups (see Table 1) and 8 immunity states (Table 2). The model includes a 5-stage process for waning of protection from infection. The DEB model tracks the flows of people between the immunity states and waning stages as transition events occur (e.g., as people receive a successful vaccination dose or recover from an infection and move into the appropriate recent immunity state, or as some move into the next waning stage with the passage of time since the last event that induced their immunity). Table 2 summarizes the input assumptions for each of the immunity states with respect to the relative susceptibility of individuals in the state to becoming (re)infected and the estimates of the duration and relative infectiousness of their infections for both fecal-oral and oropharyngeal transmission for both the recent immunity stage and those in the last waning stage. We refer to intestinal infection as “fecal infection” since it relates to fecal-oral transmission.
Table 1:
Constant global DEB model inputs
| Model input (unit) (symbol) | Value(s) |
|---|---|
| Demographic data for all situations | Time series 1950–2099 |
| Age groups | 0–2, 3–11 months; 1–4, 5–9, 10–14, 15–39; ≥ 40 years |
| Number of immunity states (ni) | 8 |
| Number of waning stages (nw) | 5 |
| Number of infection stages (r+s) -number of latent stages I (stages 0,1) -number of infectious stages (s) (stages 2,3,4,5) |
6 2 4 |
| Number of OPV reversion stages (h) -Sabin OPV (stage 0) -OPV-related (stages 1–18) -fully-reverted poliovirus (assumed equivalent to homotypic WPV) (stage 19) |
20 1 18 1 |
| Transition rates (days) -average time to full susceptibility for maternally immune infants (ρMI) -average time to develop IPV immunity after successful dose (φ) |
0.25×365 7 |
| Shape of waning function (zw) | 5 |
| Average time to reach last waning stage (ρ, in days) (PV1; PV2; PV3) | 4×365; 4×365; 3×365 |
| Duration of latent period (ξfec or ξoro, in days) | ~ 3a |
| Relative weight of infection stages compared to average weight over the infectious period (θk, k=0, …, r+s-1) (stage 0;1;2;3;4;5) |
0;0;12/17;40/17;12/17;4/17 |
| Average time to reach last OPV reversion stage (ε, in days) (for PV1; PV2; PV3) | 620.5; 408; 620.5 |
| Shape of OPV reversion function with respect to: -R0 (zr) -ln(PIR) (zp) |
1 2.5 |
| Paralysis-to-infection ratios (PV1; PV2; PV3) -for fully susceptible individuals infected with FRPV (PIRh-1) -for fully susceptible individuals infected with OPV (PIR0) |
0.005; 0.0005; 0.001 7.4 ×10–8; 6.2 ×10–7; 1.3 ×10–6 |
| Relative PIR for maternally immune compared to fully susceptible (RPIRMI) | 0.5 |
| Ratio of R0 by serotype in the same setting (PV1:PV2:PV3) | 1:0.9:0.75 |
| Relative R0 of OPV vs. FRPV (τ0) (PV1; PV2; PV3) | 0.37;0.55;0.25 |
| Exportation threshold (E*, i.e., cumulative effective infections needed to trigger a potential exportation from a subpopulation) |
200,000 |
| Proportion of virus exportations -within the same block -in another block within the same preferentially mixing area (PMA) -outside of the PMA |
0.960 0.035 0.005 |
| Relative coverage with birth dose compared to non-birth RI coverage with 3 doses (relbd) -blocks in LI and LMI countries that use OPV+IPV at T0 -all other blocks |
0.5 0 |
| Number of pSIAs -LI, LMI, and UMI countries -HI countries |
Time series NA |
| Average per-dose take rate for IPV (trIPV) -LI and LMI countries -UMI countries -HI countries |
0.63 0.70 0.75 |
| Average time from introduction to potential detection (days) | 10 |
| Time from outbreak detection until the first oSIA (days) -no ongoing outbreak response in block, before OPV2 cessation -no ongoing outbreak response in block, after OPV2 cessation -outbreak response already ongoing in block |
60 45 30 |
| Duration of each oSIA (days) | 5 |
| Number of oSIA rounds -before OPV2 cessation -after OPV2 cessation |
3 2 |
| Time interval between oSIA rounds (days) | 30 |
| Effective infectious proportion below which we assume 0 force-of-infection (transmission threshold EPI*) | 5/1,000,000 |
Abbreviations:DEB = differential equation-based; FRPV = fully-reverted poliovirus; HI, high-income; IPV, inactivated poliovirus vaccine; LI, low-income; LMI, lower middle-income; NA, not applicable; OPV, oral poliovirus vaccine; OPV2, serotype-2 OPV; oSIA, outbreak response SIA; PIR = paralysis-to-infection ratio; PMA, preferentially mixing area; PV(1,2,3) = poliovirus (type 1, 2, or 3, respectively); R0, average annual basic reproduction number; RI, routine immunization; SIA, supplemental immunization activity; T0, beginning of analytical time horizon (i.e., January 1, 2019); UMI, upper middle-income; WPV(1,2,3) = wild poliovirus (type 1, 2, or 3, respectively)
Notes:
Mean estimates obtained from experts and used in the model for the different immunity states, serotypes, and excretion modes vary between 2.85 and 3.37 days
Table 2:
Model inputs for immunity states
| Immunity state | Fully susceptible | Maternally immune | 1 successful IPV | 2 successful IPV | ≥ 3 successful IPV | 1 LPV infection | ≥ 2 LPV infections | IPV and LPV | |
|---|---|---|---|---|---|---|---|---|---|
| Relative susceptibility (σ) of recent immunity states for: | PV1 PV2 PV3 |
1.0 1.0 1.0 |
0.78 0.79 0.77 |
0.91 0.92 0.90 |
0.80 0.80 0.79 |
0.72 0.72 0.71 |
0.42 0.43 0.41 |
0.21 0.22 0.20 |
0.21 0.22 0.20 |
| Duration of fecal infectiousness (γfec, in days) of recent immunity states for: |
PV1 PV2 PV3 |
28.0 27.8 28.3 |
24.6 24.6 24.6 |
24.5 24.4 24.7 |
21.1 20.8 21.3 |
18.0 17.7 18.2 |
11.6 10.5 10.5 |
10.1 8.9 8.9 |
10.1 8.9 8.9 |
| Relative fecal infectiousness (πfec) of recent immunity states for: | PV1 PV2 PV3 |
1.0 1.0 1.0 |
0.96 0.96 0.95 |
0.92 0.92 0.91 |
0.70 0.69 0.68 |
0.61 0.59 0.59 |
0.39 0.43 0.43 |
0.20 0.23 0.23 |
0.20 0.23 0.23 |
| Duration of oropharyngeal infectiousness (γoro, in days) of recent immunity states | 13.4 | 11.9 | 9.9 | 6.6 | 6.1 | 5 | 3.7 | 3.7 | |
| Relative oropharyngeal infectiousness (πoro) of recent immunity states | 1.0 | 0.68 | 0.3 | 0.17 | 0.12 | 0.33 | 0.21 | 0.21 | |
| Relative susceptibility (σ) for last waning stage | NA | NA | 1.0 | 1.0 | 1.0 | 0.8 | 0.7 | 0.7 | |
| Duration of fecal infectiousness (γfec, in days) of last waning stage for: | PV1 PV2 PV3 |
NA | NA | 26.6 26.4 26.9 |
25.2 25.0 25.5 |
23.8 23.6 24.1 |
14.0 13.9 14.1 |
11.4 11.4 11.6 |
11.4 11.4 11.6 |
| Relative fecal infectiousness (πfec) of last waning stage |
NA | NA | 0.95 | 0.9 | 0.85 | 0.5 | 0.3 | 0.3 | |
| Duration of oropharyngeal infectiousness (γoro, in days) of last waning stage |
NA | NA | 11.4 | 6.7 | 6.6 | 6.7 | 4 | 4 | |
| Relative oropharyngeal infectiousness (πoro) of last waning stage | NA | NA | 0.43 | 0.25 | 0.13 | 0.5 | 0.3 | 0.3 |
Abbreviations:IPV, inactivated poliovirus vaccine; LPV = live poliovirus; NA, not applicable; PV(1,2,3) = poliovirus (type 1, 2, or 3, respectively)
The DEB model assumes a 6-stage infection process that includes 2 latent stages and 4 infectious stages, and a 20-stage OPV evolution process. The OPV evolution process characterizes the transition of circulating LPVs from Sabin OPV (stage 0), through progressively more transmissible and neurovirulent OPV-related strains (stages 1–18), and finally to fully-reverted OPV-related viruses (FRPVs, stage 19, for which we assume identical transmission and neurovirulent properties as typical homotypic WPVs) (Duintjer Tebbens et al., 2014; Duintjer Tebbens, Pallansch, Kalkowska, et al., 2013). To characterize processes that occur over multiple stages (m), we define a general function: xm = xy–1 – (xy–1 – x0) × ((y – 1 – m)/(y – 1))z, m = 0, …, y-1 that changes given properties (x) of those processes over the progression from the first stage (m=0) to the last stage (m=y-1), and z controls the shape of the function (linear when z=1, exponential when z<1, and logarithmic when z>1).
For each subpopulation, the transmission model uses several inputs to characterize the variability in conditions relevant to poliovirus transmission: the basic reproduction number (R0), its seasonal amplitude and peak day, the age-group preferential mixing strength, and the proportion of transmissions that occur via the oropharyngeal route vs. fecal-oral. Table 1 summarizes the assumed inputs for R0 for serotypes 2 and 3 relative to serotype 1, and serotype-specific paralysis-to-infection (PIR) ratios for the first (i.e., Sabin OPV or stage 0) and last (i.e., FRPV, WPV, or stage 19) reversion stages. Table 3 shows average values for the transmission inputs for each income level for inputs that vary by block and/or subpopulation (see inputs by subpopulation in appendix A3.2 and A3.3).
Table 3:
Average values for global model inputs stratified by World Bank Income Level that vary by block and/or subpopulation (full distributions in appendix A3.2)
| Input | LI | LMI | UMI | HI |
|---|---|---|---|---|
| Basic reproduction number (R0) | 10.3 | 9.7 | 7 | 4.9 |
| Age-group preferential mixing strength | 0.36 | 0.36 | 0.44 | 0.39 |
| Proportion of transmissions via the oropharyngeal route | 0.3 | 0.36 | 0.61 | 0.83 |
| RI coverage with 3 or more non-birth doses at T0 | 0.77 | 0.83 | 0.94 | 0.95 |
| RI coverage with 1 or 2 non-birth doses at T0 | 0.20 | 0.15 | 0.07 | 0.23 |
| True pSIA coverage | 0.76 | 0.86 | 0.93 | NA |
| pSIA repeatedly missed probability | 0.71 | 0.58 | 0.53 | NA |
| tOPV take rate for PV1; PV2; PV3 | 0.44; 0.69; 0.35 | 0.44; 0.68; 0.35 | 0.58; 0.74; 0.48 | 0.52; 0.71; 0.43 |
| mOPV take rate for PV1; PV2; PV3 | 0.59; 0.69; 0.59 | 0.59; 0.69; 0.58 | 0.82; 0.87; 0.77 | 0.73; 0.80; 0.69 |
| bOPV take rate for PV1; PV3 | 0.53; 0.53 | 0.54; 0.54 | 0.72; 0.72 | 0.65; 0.65 |
| IPV start year | 2015.7 | 2015.4 | 2013.3 | 2002.2 |
| Detection threshold 1970–2018 (AFP case-based) | 1.8 | 1.3 | 1.3 | 1.4 |
| Detection threshold 2019–2024 (AFP case-based) | 3.8 | 3.3 | 3.3 | 5 |
| Detection threshold 2025–2027 (sentinel) | 12.5 | 8.9 | 5.5 | 5 |
| Detection threshold 2028–2058 (event-based) | 30 | 20 | 10 | 5 |
| ES start year (if existing) | 2015.2 | 2015.5 | 2018.9 | 2016.3 |
| ES quality level (if existing) | high to very low | high to very low | high to medium | high to medium |
| ES catchment until 2024 (% of sub-population, if existing) | 4.9 | 5.2 | 2.1 | 1.3 |
| ES catchment 2025–2027 (% of sub-population, if existing) | 3.8 | 4.9 | 1.70.1 | 1.3 |
| ES catchment 2028–2058 (% of sub-population, if existing) | 1.1 | 1.4 | 1.40.1 | 1.3 |
| True oSIA coverage pre-OPV2 cessation | 0.77 | 0.76 | 0.72 | 0.9 |
| oSIA repeatedly missed probability pre-OPV2 cessation | 0.73 | 0.74 | 0.77 | 0.5 |
| True oSIA coverage post-OPV2 cessation | 0.76 | 0.86 | 0.93 | 0.89 |
| oSIA repeatedly missed probability post-OPV2 cessation | 0.71 | 0.58 | 0.53 | 0.58 |
Abbreviations:AFP, acute flaccid paralysis; ES, environmental surveillance; HI, high-income; bOPV, bivalent OPV; IPV, inactivated poliovirus vaccine; LMI, lower middle-income; LI, low-income; mOPV, monovalent OPV; NA, not applicable; OPV, oral poliovirus vaccine; oSIA, outbreak response SIA; pSIA, planned, preventive SIA; PV(1,2,3) = poliovirus (type 1, 2, or 3, respectively); RI, routine immunization; SIA, supplementary immunization activity; T0, beginning of analytical time horizon (i.e., January 1, 2019); tOPV, trivalent OPV; UMI, upper middle-income
We model random interactions and the possibility of spreading an infection between people from different geographical locations by tracking the cumulative number of effective (infectiousness-weighted) infections (CEI) in each subpopulation for each virus reversion stage.(Duintjer Tebbens, Pallansch, Wassalik, et al., 2015) Once the CEI reaches an exportation threshold (E*, see Table 1), the model triggers a potentially effective introduction of virus into another subpopulation and resets the subpopulation CEI value to zero. Next, we randomly determine whether the exportation leads to an effective introduction (i.e., it establishes subpopulation-wide transmission) using a function for the probability of an effective introduction (Duintjer Tebbens, Pallansch, Wassalik, et al., 2015) (see appendix A3.4). We model systematic population movements between blocks using preferential mixing between the subpopulations in an epidemiological block by assuming that 96% of exportations occur between random subpopulations of the same block, while the remaining 4% occur between random subpopulations of other blocks (i.e., inter-block exportations). We assume that 87.5% of all inter-block exportations (3.5% of all exportations) go to a random block in the same PMA and 12.5% of all inter-block exportations (0.5% of all exportations) go to a random block in a different PMA (Duintjer Tebbens, Pallansch, Wassalik, et al., 2015).
Immunization assumptions
In contrast to the prior model (Duintjer Tebbens, Pallansch, Wassalik, et al., 2015), we allowed each subpopulation to use different assumptions for its RI schedule and full coverage, partial RI coverage levels (i.e., 1 or 2 doses, not including a birth dose if given), appropriate OPV take rates, and starting years for IPV introduction into RI (see appendix A3.2). We also changed the assumptions that we used for “burn in” of the model for RI to improve our characterization of WPV prevalence and global immunity levels to support retrospective modeling back to 1988 (see appendix A3.3). Specifically, we introduce RI in LI, LMI, UMI, and HI blocks 8, 8, 18, and 28 years prior to TGPEI, respectively (i.e., sufficiently long to reach equilibrium by 1988 and consistent with actual trends). We assume a linear increase from 0% of the subpopulation-specific RI coverage receiving 3 or more non-birth doses (POL3) up to block-specific relative coverage of the subpopulation-specific POL3 in 1980 in UMI and HI blocks. We use block-specific yearly relative coverage of the subpopulation-specific POL3 from 1980 up to 2017, followed by the subpopulation-specific POL3 for the rest of the time horizon. Table 1 summarizes generic model inputs related to immunization, including relative coverage with a birth dose compared to non-birth RI coverage with 3 doses and average per-dose take rate for IPV varied by income level. Table 3 includes summaries of the immunization inputs that vary by blocks and/or subpopulations (showing the average value per income level, see full inputs by subpopulation in appendix A3.2 and A3.3). We focus on using the model to characterize the baseline or status quo, which we refer to as the reference case (RC). Table 4 summarizes the RC policy inputs related to immunization for the minimum years and number of IPV doses in RI after the cessation of last OPV serotype.
Table 4:
Global model reference case (RC) policy inputs
| Model input (unit) (symbol) | Value(s) |
|---|---|
| Minimum years of IPV in RI after last OPV cessation | 10 |
| Minimum number of doses of IPV in RI after last OPV cessation | 2 |
| Years of mOPV use for oSIAs after homotypic OPV cessation (PV1; PV2; PV3) | 5; 8; 5 |
| Geographical scope of oSIAs -before OPV2 cessation -after homotypic OPV cessation, R0 < 10 -after homotypic OPV cessation, R0 ≥ 10 |
Subpopulation Subpopulation Subpopulation + 4 worst performing neighbors |
| Target age groups for oSIAs -before OPV cessation of the serotype -after OPV cessation of the serotype |
0–2, 3–11 months; 1–4 years Cohorts born since OPV cessation, rounded to next multiple of 5 |
| True IPV oSIA coverage in post-OPV era | 0.50 |
| IPV oSIA repeatedly missed probability in post-OPV era | 0.80 |
| Global Poisson rate for release (1/year) -of unreturned OPV in blocks that use OPV at T0 -within 1st year post serotype-specific cessation -within 2nd years post serotype-specific cessation -of an LPV from an IPV production site -of an LPV from a PEF that does not produce IPV -of an LPV from a facility holding PIMs or any other unintentional release -of an intentional LPV release |
4 1 0.2 0.02 0.004975 0.000025 |
| Serotype-specific cases since OPV cessation to trigger OPV restart | 5,000 |
| Time to restart OPV production after OPV cessation (years) -if licensed OPV manufacturers continue to produce any OPV serotype(s) -if licensed OPV manufacturers maintain an mOPV stockpile for the serotype -if no licensed OPV manufacturers exist for any OPV serotype -if manufacturers already started one OPV serotype for subsequent serotype(s) |
2 3 7 5 |
Abbreviations:IPV, inactivated poliovirus vaccine; LPV = live poliovirus; mOPV, monovalent OPV; OPV, oral poliovirus vaccine; OPV2, serotype-2 OPV; oSIA, outbreak response SIA; PEF, polio essential facility; PIM, potentially infectious material; PV(1,2,3) = poliovirus (type 1, 2, or 3, respectively); R0, average annual basic reproduction number; RI, routine immunization; SIA, supplemental immunization activity; T0, beginning of analytical time horizon (i.e., January 1, 2019)
We also allowed the subpopulations within blocks to use different assumptions for planned preventive supplementary immunization activities (pSIAs), frequency, and coverage, which differed from the prior model (Duintjer Tebbens, Pallansch, Wassalik, et al., 2015). We allow the SIA impact level (sil, characterized by the true coverage and repeated missed probability) and the number of pSIAs to change as a function of conditions and time. We introduce pSIAs in the LI, LMI, and UMI blocks in 1993, 1993, and 1990, respectively, and assume no pSIAs in HI blocks. We updated pSIA schedules, both before and after WPV elimination, with the vaccine choice for each round depending on the time period and RI coverage consistent with actual experience (see Table 3 and appendices A3.2 and A3.3).
Surveillance
Recognizing the continued use of acute flaccid paralysis (AFP) surveillance and the recent introduction and expanded use of environmental surveillance (ES), we significantly changed our characterization of surveillance compared to the prior model (Duintjer Tebbens, Pallansch, Wassalik, et al., 2015). We use a previously developed ES approach (Kalkowska, Duintjer Tebbens, Pallansch, & Thompson, 2019) that determines the probability of detecting poliovirus by any sampling site in a given population as a function of the effective (i.e., infectiousness-weighted) number of infected individuals in the population with ES sites, the total catchment area from all ES sites in that population, and a quality coefficient. We assume values of ES quality coefficients that directly correlate with the AFP detection threshold assumed for 2018, with three quality levels: high, medium, and very low. We assumed ES start years and the number of sampling sites based on the available data (see Table 3 for average values by income level, and appendix A3.2 for subpopulation details). We assume an ES sampling frequency of 1 per month (12 samples/year), ES catchment (% of population) set to 1% per ES site in a subpopulation, and a decrease in ES coverage over time as resources diminish. Given containment requirements (World Health Organization, 2015a), we assume that all subpopulations that include poliovirus essential facilities (PEFs) start ES in 2022 (if not already conducting ES) with 12 samples/year, a 1% catchment, and medium or high quality.
Outbreak response
As with the prior model (Duintjer Tebbens, Pallansch, Wassalik, et al., 2015), we include outbreak response SIAs (oSIAs). After 2005, the model triggers outbreak response in countries that previously stopped indigenous transmission when the cumulative detected incidence of polio cases per 10 million people exceeds the subpopulation-specific AFP detection threshold used to approximate AFP quality (see appendix A3.2) (Duintjer Tebbens, Pallansch, Wassalik, et al., 2015). The model starts to accumulate the incidence in each subpopulation once a block eliminates indigenous WPV. Prior to global OPV cessation of a given serotype, we only accumulate the incidence from effective importations of WPV/cVDPV or indigenous cVDPV emergences. After global OPV cessation we account for any LPV-related incidence (i.e., including cases caused by all OPV-related viruses). The model clears the cumulative detected incidence after each completed outbreak response or every 6 months without any outbreak response.
Although we previously assumed very aggressive outbreak response to minimize the chances of failing to fully control outbreaks and OPV restart for the prior model (Duintjer Tebbens, Pallansch, Wassalik, et al., 2015; Duintjer Tebbens, Pallansch, et al., 2016; Duintjer Tebbens & Thompson, 2018), we modified the outbreak response strategy to reflect the historical delays in response time, and the actual actions taken and planned after OPV2 cessation. Table 1 summarizes generic model inputs related to outbreak response, including the average time from introduction to potential detection, time from outbreak detection until the first oSIA, duration of each oSIA round, number and interval between oSIAs. The bottom of Table 3 includes characteristics of oSIAs that vary by block (i.e., true coverage and repeated missed probabilities, which vary by subpopulation, see appendix A3.2). Table 4 summarizes RC policy inputs related to outbreak response, including the number of years for which mOPV use occurs for oSIAs after OPV cessation of each type, geographical scope and target age groups of oSIAs, and true coverage and repeated missed probability of IPV oSIAs in the post-OPV era. Starting with OPV2 cessation, we assume that oSIAs target all new birth cohorts since the serotype-specific OPV cessation with 2 rounds of the appropriate serotype of mOPV, and we assume the response occurs only in the outbreak subpopulation when R0 < 10 or it occurs within the outbreak subpopulation and its 4 worst-performing neighboring subpopulations within the same block when R0 ≥ 10. The model checks for continued break-through transmission, and schedules 2 additional rounds if the initial oSIAs failed to stop the transmission. The vaccine chosen for the oSIAs depends on timing, vaccine availability, and policy decisions (see Tables 1 and 4). We assume that during outbreaks, oSIAs will displace any pSIAs, and the model adds any missed pSIAs to the regular pSIA schedule once the outbreak ends. We assume that mOPV1, mOPV2, and mOPV3 will be available for outbreak response for 5, 8, and 5 years post-homotypic OPV cessation, respectively. Once mOPV is no longer available for oSIAs, the model uses IPV for oSIAs with lower intensity (see Table 4). Similar to the prior model (Duintjer Tebbens, Pallansch, Wassalik, et al., 2015), we initially run the RC with the assumption of no constraints on vaccine availability to respond to outbreaks (i.e., unlimited stockpiles), which allows us to characterize the potential demands from such stockpiles.
Simulation of post-OPV cessation risks
In contrast to the prior model (Duintjer Tebbens, Pallansch, Wassalik, et al., 2015), which assumed that tOPV intensification leading up to OPV2 cessation would be fully-effective, the updated model approximates the actual experience in the run-up to OPV2 cessation and the experience of the first 3 years after OPV2 cessation. The model uses our understanding of the current implied GPEI plans for SIAs for the run up to globally-coordinated bivalent OPV (bOPV) cessation, which we assume will occur on January 1, 2025 (World Health Organization Global Polio Eradication Initiative, 2019). While bOPV cessation may occur earlier if the world is ready, later if certification of the eradication of all WPVs becomes further delayed, or phased such that OPV3 cessation occurs prior to OPV1 cessation, for purposes of the model the RC (i.e., status quo, baseline) assumes some bOPV SIAs occur throughout 2024 consistent with current GPEI plans (World Health Organization Global Polio Eradication Initiative, 2019).
Given multiple additional years of data to check the performance of our model for iVDPV risks, we compared our assumptions to currently available information. The review led us to update (Kalkowska, Pallansch, & Thompson, 2019) the prior discrete-event simulation (DES) model (Duintjer Tebbens, Pallansch, & Thompson, 2015) used by the prior global model (Duintjer Tebbens, Pallansch, Wassalik, et al., 2015) to characterize long-term iVDPV excreter prevalence until and after OPV cessation of each serotype (appendix A3.5). Specifically, we found new information about the prevalence and survival of individuals with primary immunodeficiencies (PIDs) and additional observations of these individuals excreting iVDPVs (Kalkowska, Pallansch, et al., 2019). Each global model stochastic iteration uses a different stochastic realization of the DES model to generate random introductions of iVDPVs after homotypic OPV cessation (Duintjer Tebbens et al., 2019), which (if successful) enter the general population at reversion stage 10. We also updated from prior work (Duintjer Tebbens et al., 2018; Duintjer Tebbens et al., 2006; Duintjer Tebbens, Pallansch, Wassalik, et al., 2015) the risk estimates related to the small, but non-zero probabilities of unintentional or intentional releases as shown in the bottom of Table 4 assuming current containment policies (appendix A3.6). Consistent with prior modeling (Duintjer Tebbens, Pallansch, Wassalik, et al., 2015), we implicitly ignore releases from OPV production, because we assume that prior to OPV cessation or after an OPV restart of a given OPV serotype the intentional use of large quantities of OPV will overshadow any release (i.e., make it irrelevant within the OPV background).
OPV-restart assumptions
Unlike the prior model, which assumed restart of trivalent OPV (tOPV) for the remainder of the model time horizon after detecting 50,000 cumulative paralytic cases after OPV cessation (Duintjer Tebbens, Pallansch, Wassalik, et al., 2015), the updated model allows for restart of single serotypes of OPV (Thompson & Kalkowska, 2019). The updated model applies a cumulative paralytic case threshold of 5,000 cases following serotype-specific OPV cessation as a trigger to restart homotypic OPV in RI schedules but not in pSIAs. Since the manufacturing and licensing of ready-to-use vaccines post global OPV cessation will vary depending on conditions at the time, we assume different delay times from the OPV restart trigger to the actual restart of OPV vaccine use (Table 4). We assume it takes 7 years to restart OPV for the first serotype(s) restarted if all OPV vaccine production already stopped (i.e., no licensed manufacturers of any OPV serotypes exist at the time of the restart trigger), and we assume an additional 5 years to restart any other OPV serotype(s) after already using the first restarted OPV. In contrast, if OPV vaccine production for any other serotype continues (i.e., at least one other licensed OPV serotype is still in production), then we assume a 3-year delay to restart a second OPV serotype. Finally, we assume that if production of any licensed OPV continues and a sufficient bulk stockpile exists for the needed serotype (e.g., for outbreak response), then we assume it would take only 2 years to restart that OPV serotype.
We assume that the vaccine choice will also depend on the conditions at the time and the global decision to continue or stop OPV cessation as a strategy (Thompson & Kalkowska, 2019). Despite efforts to develop a new OPV2 strain with more favorable properties (Collett et al., 2017; McKinlay et al., 2014; Van Damme et al., 2019), we do not include consideration of this possibility in the RC. We assume that when faced with the need to restart OPV of any serotype, a global decision to stop pursuing OPV cessation as a strategy would lead to the return of tOPV use (Thompson & Kalkowska, 2019). In that case, we assume that blocks that use OPV+IPV at T0 would restart tOPV in their RI schedules and stop any IPV use, while all blocks using IPV/OPV sequential schedule will continue to do so with the use of tOPV in their RI schedules. However, if the decision to restart the use of any OPV serotype(s) after global cessation occurs with a commitment to continue OPV cessation as a strategy, then we assume the use of the appropriate mOPV serotype (i.e., mOPV1, mOPV2, or mOPV3) for the OPV restarted by adding it to the existing schedules in OPV-using blocks (Thompson & Kalkowska, 2019). We assume that current IPV-only blocks will not restart OPV, and that any containment requirements for any restarted OPV serotype(s) would disappear (Thompson & Kalkowska, 2019). The model assumes that any countries experiencing an outbreak would use an available OPV formulation that contains that serotype for oSIAs if they cannot obtain the appropriate mOPV, or they would use IPV.
Simulation
We use numerical integration using Euler’s method with time step of 0.5 day. We coded the model in JAVA™ using Eclipse™. For this analysis we present one deterministic run with one stochastic realization of the long-term reintroduction risks for the RC.
Reported cases, simple linear static model estimates, and underreporting
We use the reported historical cases (World Health Organization, 2019a, 2019b, 2019e) to compare the model estimates to actual experience and prior WHO estimates (Henderson, 1985; Henderson et al., 1988; World Health Organization, 1986, 1989). For 1980–2000, we use the total reported cases (i.e., not by serotype), because serotype testing of the reported cases did not occur consistently at the global level during this time period (World Health Organization, 2019b). From 2001–2018 (after successfully eradicating WPV2 but failing to reach the original target for polio eradication of all three serotypes), the Global Polio Laboratory Network consistently characterized the serotypes for reported cases, and thus for 2001–2018, we use the reported WPV1, WPV3, and cVDPV2 cases (World Health Organization, 2019a, 2019e). For cVDPV2 cases, we further consider the experience before OPV2 cessation, which occurred in late April-May 2016, and after OPV2 cessation. For 2019–2023, we show model estimates by serotype.
To provide context for underreporting and demonstrate the differences between our dynamic model and simple incidence estimates, we also explored the implications of different assumptions similar to those used by WHO to estimate incidence. Specifically, for consistent comparisons, we use the UN World Population Prospects (Population Division of the Department of Economic and Social Affairs of the United Nations Secretariat, 2019) estimated numbers of surviving infants (SI) and WHO/UNICEF Estimates of National Immunization Coverage (WUENIC) (World Health Organization, 2019d) for diphtheria-tetanus-pertussis 3-dose (DTP3) coverage for 200 countries, average take rate (tr) of 95% or 80% for OPV, and either an average PIR (i.e., aPIR of 1/200) or serotype-specific PIRs (i.e., sPIR of 1/200, 1/2000, 1/1000 for serotypes 1, 2, 3 respectively). We also considered the effect of counting or not counting the contribution of cases from countries that achieved WPV elimination (using elimination dates from the model), because people only become paralyzed in countries with transmitting virus, which prior WHO incidence estimates from the 1980s accounted for by focusing on developing countries only. This leads to eight permutations of which we focus on four: aPIR+elim+95%, sPIR+elim+95%, aPIR+elim+80%, and aPIR-elim+95%. We estimate yearly incidence (INC(yr), yr = 1980, …, 1988) using the formula:
where c denotes the index of a country.
Results
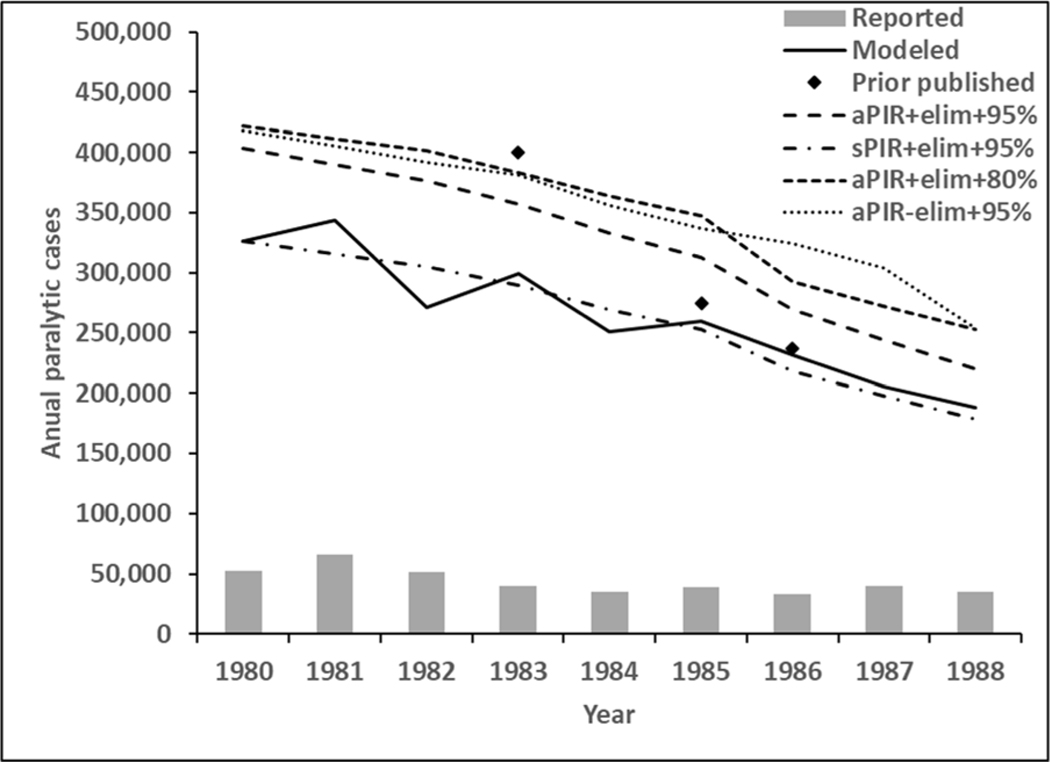
The bars at the bottom of Figure 1 show the reported cases for 1980–1988, and the solid black line shows the estimates from the model. Overlaid on these, Figure 1 includes published point estimates from 1983, 1985, and 1986 for comparison (Henderson, 1985; Henderson et al., 1988; World Health Organization, 1986), and different styles of dashed lines that reflect using different assumptions about simple extrapolations from routine immunization coverage to incidence (i.e., serotype-specific or an average PIR, OPV take rates) The difference between reported cases (bars), the model results (solid line), and the other estimates demonstrates the magnitude of underreporting. Comparison of the aPIR+elim+95% and sPIR+elim+95% curves shows the impact of using serotype-specific PIRs rather than the average PIR over all serotypes. The modeled incidence (solid line) lies closest to the sPIR+elim+95% curve (dotted line), which uses the most similar input assumptions. Comparison of the aPIR+elim+95% and the aPIR+elim+80% lines show the increase in incidence associated with a lower assumed vaccine efficacy. The model varies assumptions for take rates by country and include partial coverage with 1 or 2 doses, which the overlaid simple extrapolations ignore. Finally, the comparison of the curves with and without taking country elimination status into account (i.e., aPIR+elim+95% vs. aPIR-elim+95%) show the importance of focusing only on the fraction of the population affected by transmission.
Figure 1:
Different simple linear estimates of polio incidence and reported cases for 1980–1988
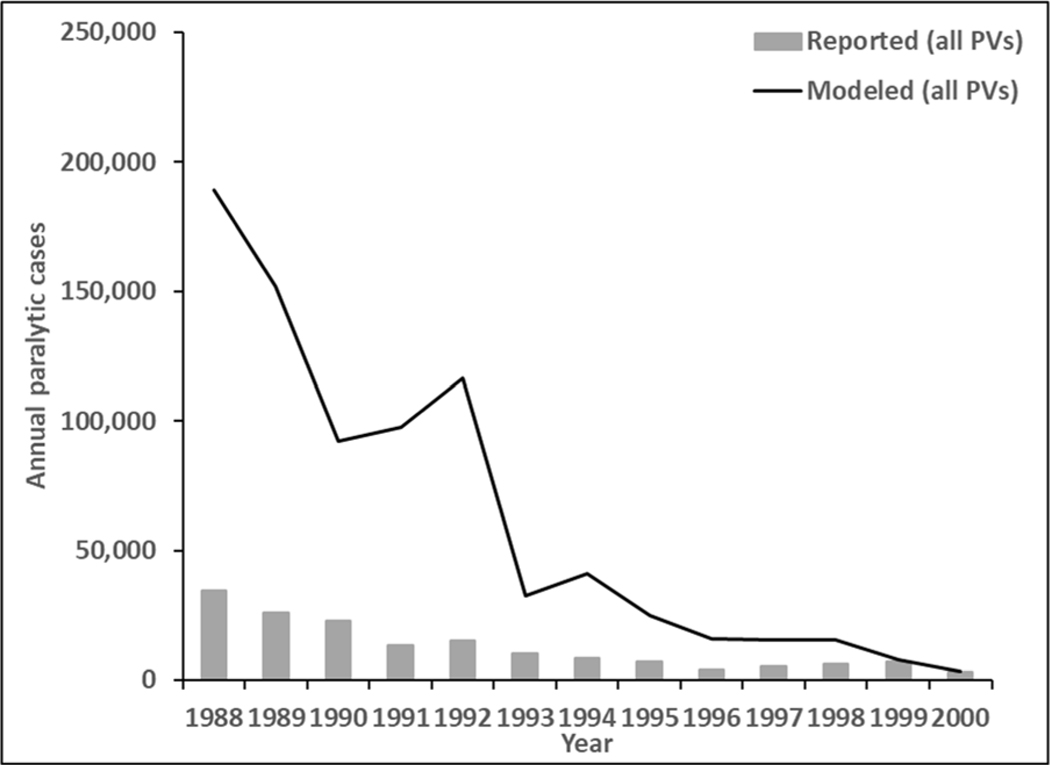
Figure 2 presents the estimated annual incidence of polio cases for 1988–2000 from the global model (solid line) compared to all reported cases (bars). All HI and most UMI blocks eliminate WPV within a few years of introducing RI so their contributions to global paralytic incidence decline significantly before 1988. As shown in Figure 2, the model estimates annual incidence in 1988 at around 169,000 cases per year, which declines as RI coverage increases. With the onset of SIAs in LI and LMI blocks in 1993, the modeled incidence of all serotypes drops further to around 25,000 cases per year in the mid-1990s. Then, within a few years of global pSIAs starting, the remaining UMI and most LI and LMI blocks eliminate all indigenous WPVs.
Figure 2:
Modeled annual all poliovirus (PVs) incidence 1988–2000 compared to reported cases
However, the inclusion of some blocks with preferentially-mixing under-vaccinated subpopulations and with properties similar to the last known WPV reservoirs leads to the last infection of WPV2 in the model in the beginning of 2001 (i.e., complete transmission dies out in the model in 2001 [not shown], consistent with the last reported case in Aligarh, India in 1999). In the model, complete die out of transmission occurs after the last reported case, as the last infected individuals in the population clear their infections. Consistent with Figure 1, the model estimates of cases in Figure 2 significantly exceed the reported cases.
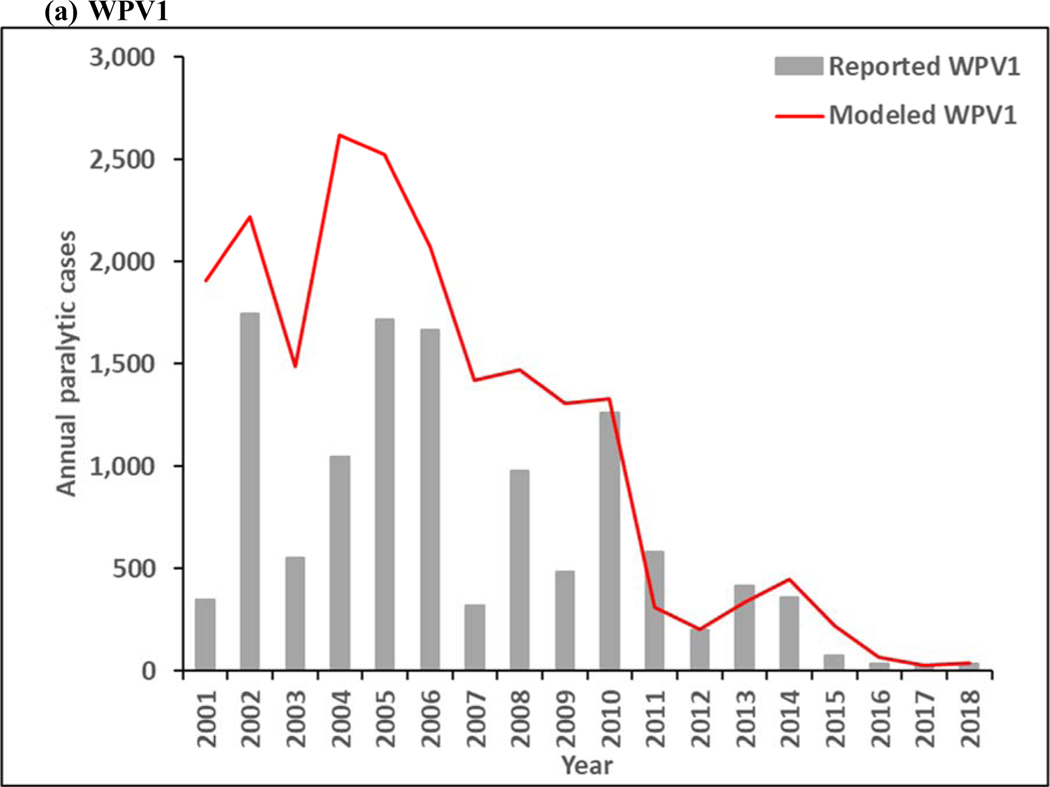
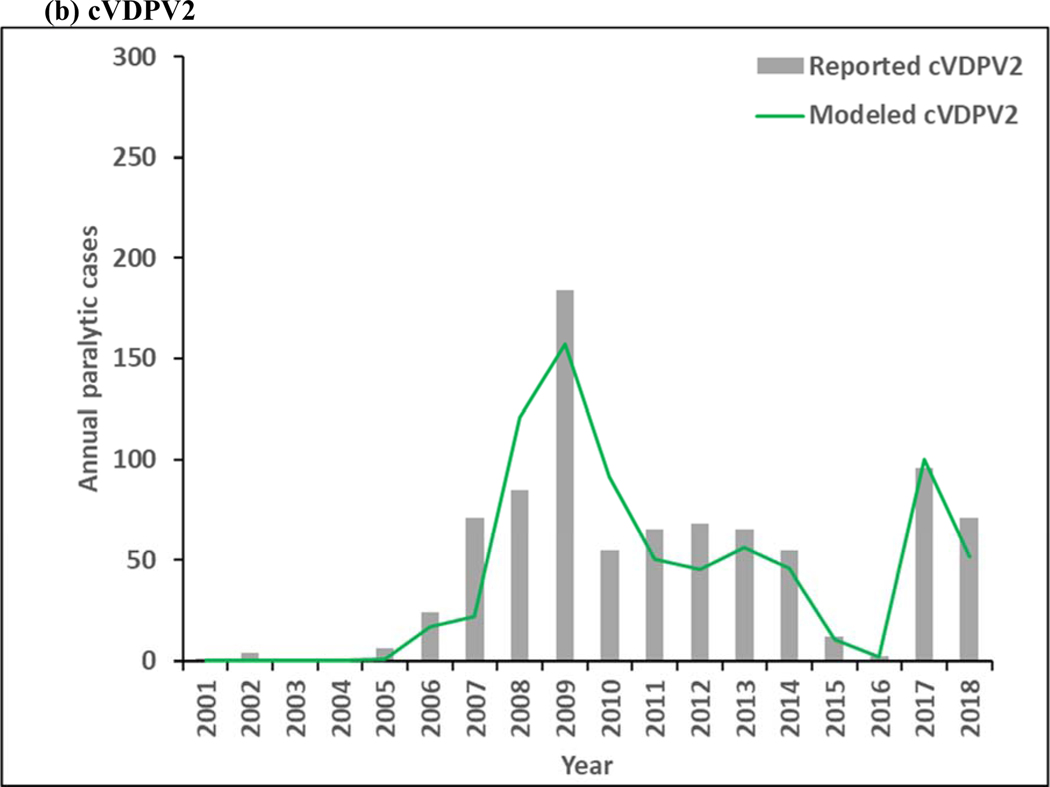
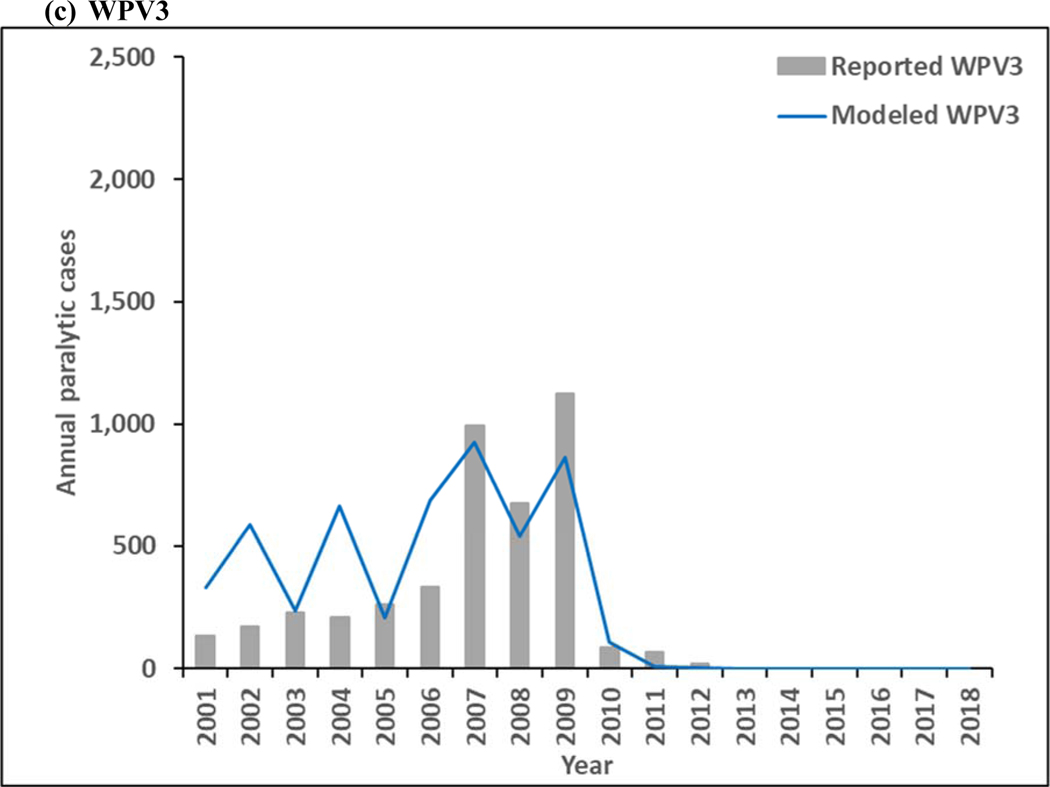
Figure 3 presents estimated annual incidence from the model for 2001–2018 for WPV1 and WPV3 compared to the reported cases by serotype, and estimated incidence of cVDPV2 cases compared to reported cases. The model estimates of incidence follow similar patterns as the reported cases, although the year-to-year serotype-specific distributions vary some, particularly during the time of mOPV use in SIAs between 2005–2010, for which the high level of aggregation in the model does not reproduce all historical nuances. Moreover, Figure 3a includes the effect of WPV1 introductions into the blocks that eliminated all WPVs and then decreased their effort to sustain high population immunity, which led to outbreaks following reintroductions of WPV1 that increased the total WPV1 count. As shown in Figure 3a, WPV1 transmission continues throughout the time period, which implies that WPV1 transmission persists for the RC. Figure 3b shows the emergence and ongoing transmission of cVDPV2 in subpopulations with lowered OPV2 vaccination quality both before and after OPV2 cessation, with the ongoing cVDPV2 transmission at the end of the time period, which similarly implies that cVDPV2 transmission persists for the RC and will lead to an OPV2 restart. WPV3 dies out in the model in 2012 (Figure 3c), which is consistent with the last reported case that occurred in northern Nigeria in November 2012. Now 7 years after the last reported WPV3 case, and with cVDPV3 outbreaks and serotype 3-related VAPP cases occurring, the question of when to globally-coordinate OPV3 cessation remains open, although experience with OPV2 cessation not going as smoothly as hoped appears to temper GPEI willingness to stop OPV3 use in order to prevent these cases.
Figure 3:
Modeled annual poliovirus incidence 2001–2018 by serotype compared to reported cases for (a) serotype 1 wild poliovirus (WPV1), (b) serotype 2 circulating vaccine-derived poliovirus (cVDPV2), and (c) serotype 3 wild poliovirus (WPV3)
Note: Excludes reported cVDPV1 cases that occurred in year (number): 2001 (12), 2002 (12), 2005 (2), 2007 (1), 2008 (4), 2011 (2), 2014 (1), 2015 (20), 2016 (3), and 2018 (27).
Note: Excludes reported cVDPV3 cases that occurred in year (number): 2005 (1), 2006 (1), 2009 (1), 2010 (5), 2012 (3), 2013 (1), and 2018 (7).
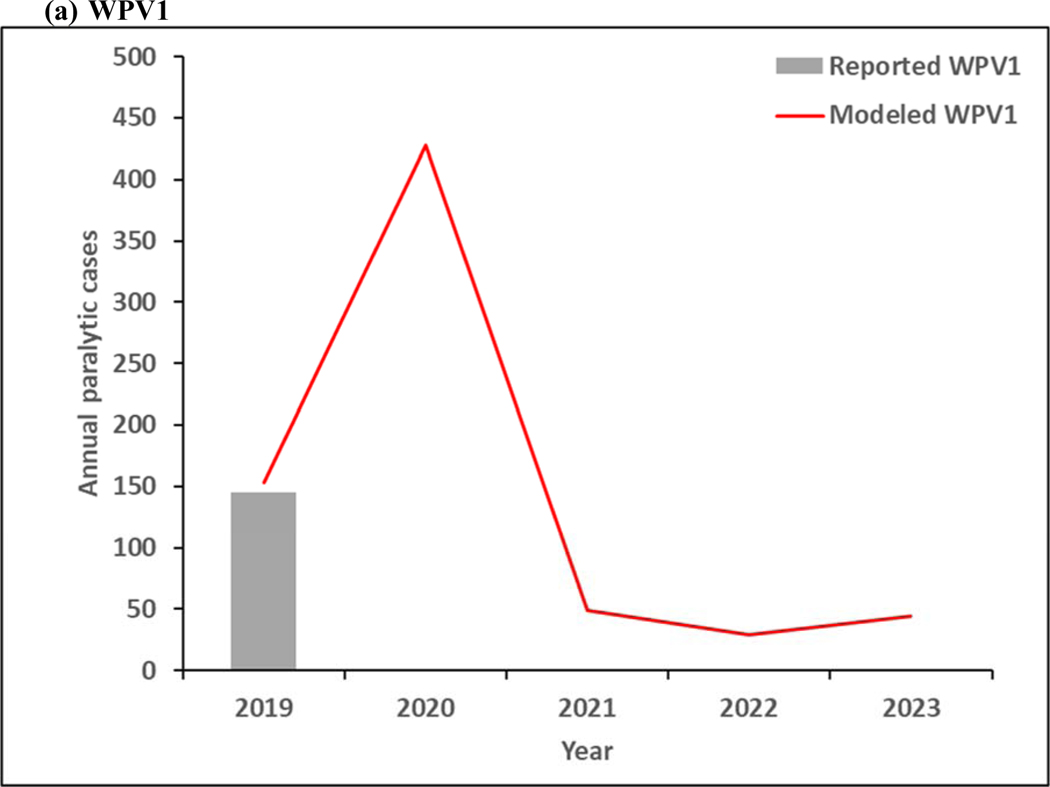
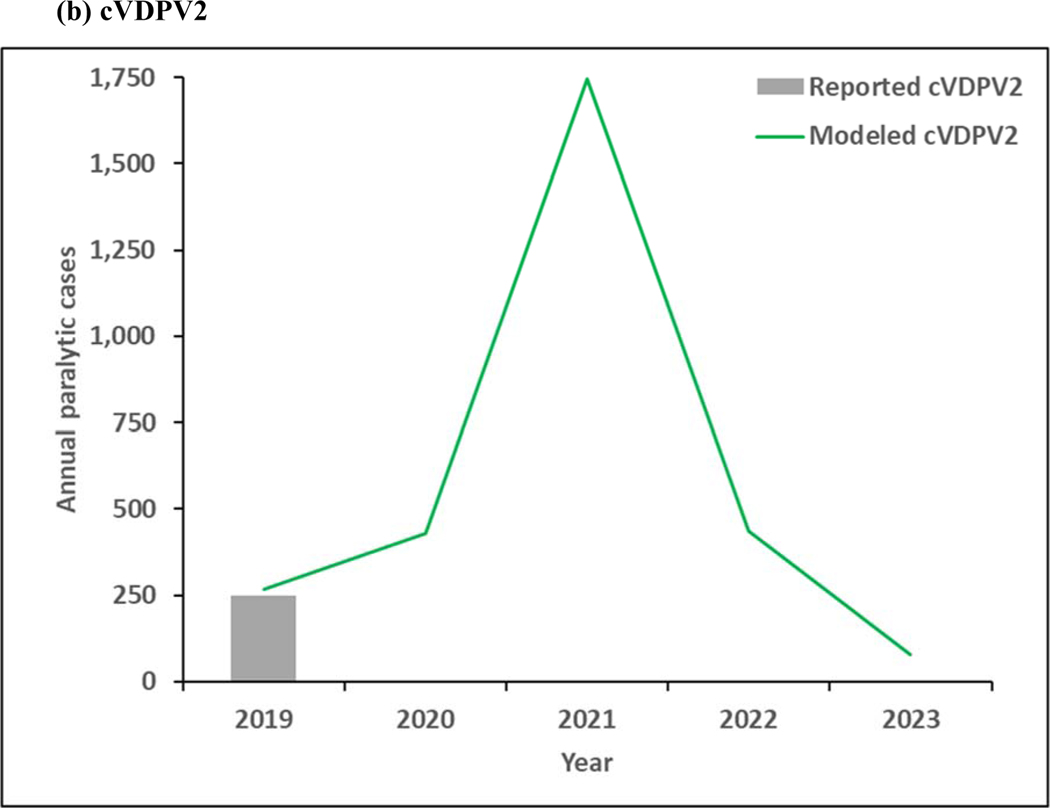
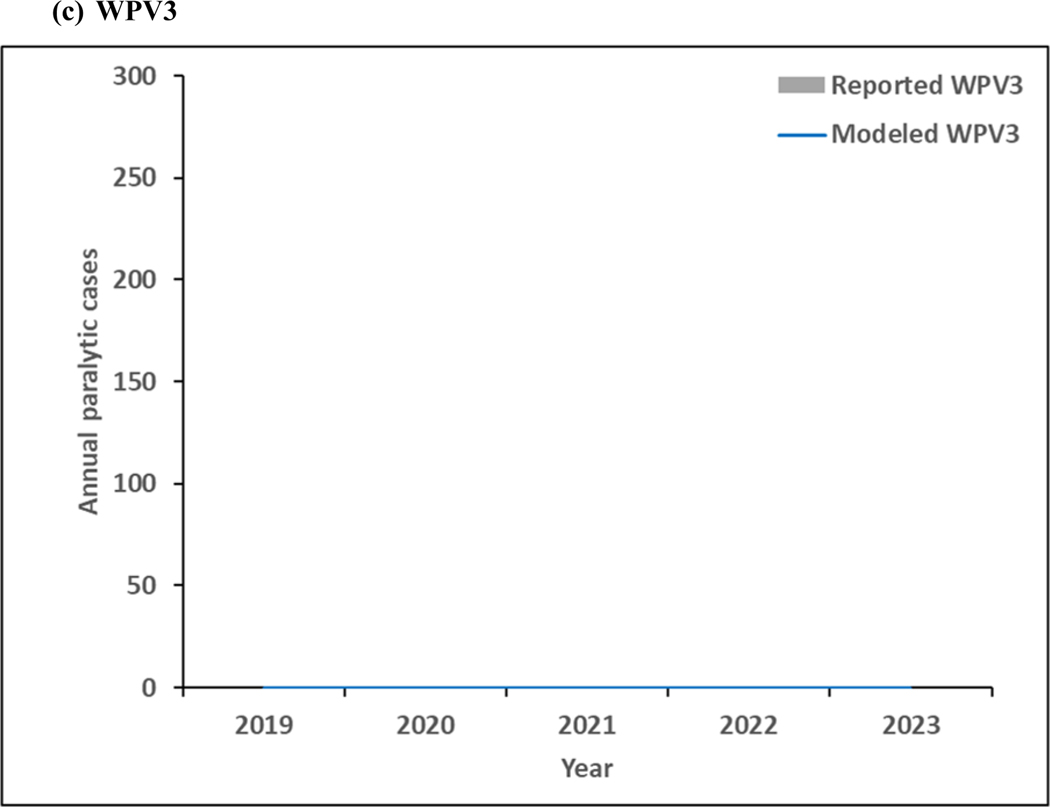
Figure 4 shows the model results for 2019–2023. As shown in Figure 3a, continued transmission of WPV1 through 2023 does not imply successful WPV1 eradication. The limited number of pSIAs in 2019 in the endemic blocks leads to an increase in incidence, consistent with rise of WPV1 cases in Pakistan and Afghanistan in 2019. In the absence of changes in vaccination quality, the prospective model shows further delays in the eradication of all WPVs, which will prevent bOPV cessation. Figure 4b shows continued transmission of cVDPV2s through 2023, resulting from responses to circulation that began before OPV2 cessation and new transmission initiated following the use of mOPV2 for outbreak response in low-quality oSIAs with continued trickling in of mOPV2 vaccine that allows for new cVDPV outbreaks to emerge as population immunity to serotype 2 circulation continues to decline with time. The decline in cVDPV2 cases in Figure 4b at the end of the time horizon for this model iteration reflect model assumptions that allow for outbreak response to occur using mOPV2 from an unlimited supply for up to 8 years after OPV2 cessation (i.e., through mid-2024). These results imply a substantial probability of the need for OPV2 restart, and the need for managing the complex associated logistics (Thompson & Kalkowska, 2019), and these results should not be misinterpreted as implying eventual die-out of the cVDPV2 circulation after the end of 2023. The results in Figure 3c do not show any modeled or reported WPV3 cases from 2012 through 2023, consistent with the certification of WPV3 eradicated that occurred in October 2019 (World Health Organization, 2019c).
Figure 4:
Modeled annual poliovirus incidence 2019–2023 by serotype for (a) serotype 1 wild poliovirus (WPV1), (b) serotype 2 circulating vaccine-derived poliovirus (cVDPV2), and (c) serotype 3 wild poliovirus (WPV3)
Note: 2019 reported WPV1 cases as of January 9, 2020.
Note: 2019 reported cVDPV2 cases as of January 9, 2020.
Note: No 2019 reported WPV3 cases as of January 9, 2020.
Discussion
As of 2019, the GPEI continues to need to respond to population immunity gaps for both serotypes 1 and 2, and our model suggests that the GPEI is not on track to achieve the objectives of the 1988 World Health Assembly resolution of ultimately ending all cases of poliomyelitis (World Health Assembly, 1988). In addition, based on our understanding of the 2019–2023 Strategic Plan, the GPEI is not on track to achieve WPV1 eradication prior to 2024 without improved implementations of that Plan, or to successfully stop the transmission of OPV2-related viruses using current tools. Thus, the current trajectory of the polio endgame indicates significant challenges. This analysis suggests the need for different strategies or changes in the execution of current strategies, and several years at a minimum to achieve the ultimate objective of ending all cases of poliomyelitis. Unless the GPEI gains more access to the last remaining reservoirs of circulating LPVs and increases the quality of OPV vaccination beyond the current and recent past performance, the model suggests no end to WPV1 transmission and the likely need to restart broader use of OPV2.
While the updated model is consistent with observed occurrence of polio over the previous 30 years, the experience with cVDPV2 incidence before and after OPV2 cessation reflects completely different epidemiological situations. Prior to OPV2 cessation, the reported cVDPV2 cases occurred in areas that stopped indigenous WPV2 transmission but failed to maintain high immunization coverage with OPV2, which resulted in insufficient population immunity to transmission to prevent the development and spread of cVDPV2s (Thompson, Kalkowska, & Duintjer Tebbens, 2015). Similar conditions for serotypes 1 and 3 lead to the cVDPV1 and cVDPV3 cases noted at the bottoms in Figures 3a and 3c. Notably, the transmission of cVDPV2 viruses prior to OPV2 cessation predominantly occurred in northern Nigeria, which experienced significant disruptions in tOPV immunization activities in 2003–2004 (i.e., shortly before Nigeria began preferential use of mOPV1 for SIAs in 2005) and which led to multiple independent emergences of cVDPV2 viruses (Burns et al., 2013). Since OPV2 cessation and no use of OPV2 in RI, cVDPV2 circulation resulted from ongoing circulation that began before cessation and new transmission initiated following the use of mOPV2 to respond to post-OPV2 cessation cVDPV2 outbreaks. Initial clinical trials of the novel genetically stable vaccine strains have been encouraging and may create the prospect for use of these nOPV2 vaccines in the near future to stop cVDPV2 transmission by preventing new cVDPV2 emergence (Van Damme et al., 2019). In light of the experience with OPV2, it is worth considering why, despite no detected evidence of WPV3 transmission since November 2012, the GPEI continues to use OPV vaccines that contain serotype 3, and to do so with a decreasing commitment to maintaining high population immunity to transmission for serotype 3 in all polio-free areas.
The insights from this model come with some limitations (see detail in appendices A2.10 and A3.7). As with any model, the assumptions and structure significantly simplify the complexity that actually exists in global poliovirus transmission. We use a model that accounts for many sources of complexity of global transmission, but by design we aggregate populations into manageable sizes for computation without consideration of what happens at a finer scale. In this regard, while the model attempts to mimic actual events, by design it does not model any single event with the level of detail that would occur in the context of a model for only that event (e.g., transmission following introduction of WPV1 in Israel (Kalkowska et al., 2015)). Future studies can use this model to explore the health and economic outcomes of different global policy options using previously demonstrated health economic methods (see appendix A4) and to explore specific populations with different mixing patterns (see appendix A5.1) and the potential for undetected transmission using a stochastic version of the model (see appendix A5.2).
Supplementary Material
Acknowledgments
Funding
This publication was supported by Cooperative Agreement Number 5NU2RGH001913–03-00 funded by the Centers for Disease Control and Prevention. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention or the Department of Health and Human Services. The authors thank Michael Lynch for helpful comments.
References
- Burns CC, Shaw J, Jorba J, Bukbuk D, Adu F, Gumede N, . . . Kew O. (2013). Multiple independent emergences of type 2 vaccine-derived polioviruses during a large outbreak in northern Nigeria. J Virol, 87(9), 4907–4922. doi: 10.1128/jvi.02954-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett MS, Hincks JR, Benschop K, Duizer E, van der Avoort H, Rhoden E, . . . Hartford M. (2017). Antiviral activity of pocapavir in a randomized, blinded, placebo-controlled human oral poliovirus vaccine challenge model. Journal of Infectious Diseases, 215(3), 335–343. doi: 10.1093/infdis/jiw542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duintjer Tebbens RJ, Hampton LM, & Thompson KM (2016). Implementation of coordinated global serotype 2 oral poliovirus vaccine cessation: Risks of potential non-synchronous cessation. BMC Infectious Diseases, 16, 237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duintjer Tebbens RJ, Hampton LM, Wassilak SGF, Pallansch MA, Cochi SL, & Thompson KM (2016). Maintenance and intensification of bivalent oral poliovirus vaccine use prior to its coordinated global cessation. Journal of Vaccines and Vaccination, 7(5), 340. doi: 10.4172/2157-7560.1000340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duintjer Tebbens RJ, Kalkowsa DA, & Thompson KM (2018). Poliovirus containment risks and their management. Future Virology, 13(9), 617–628. doi: 10.2217/fvl-2018-0079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duintjer Tebbens RJ, Kalkowska DA, & Thompson KM (2019). Global certification of wild poliovirus eradication: insights from modelling hard-to-reach subpopulations and confidence about the absence of transmission. BMJ Open, 9(1), e023938. doi: 10.1136/bmjopen-2018-023938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duintjer Tebbens RJ, Kalkowska DA, Wassilak SG, Pallansch MA, Cochi SL, & Thompson KM (2014). The potential impact of expanding target age groups for polio immunization campaigns. BMC Infectious Diseases, 14, 45. doi: 10.1186/1471-2334-14-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duintjer Tebbens RJ, Pallansch MA, Chumakov KM, Halsey NA, Hovi T, Minor PD, . . . Thompson KM (2013a). Expert review on poliovirus immunity and transmission. Risk Analysis, 33(4), 544–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duintjer Tebbens RJ, Pallansch MA, Chumakov KM, Halsey NA, Hovi T, Minor PD, . . . Thompson KM (2013b). Review and assessment of poliovirus immunity and transmission: Synthesis of knowledge gaps and identification of research needs. Risk Analysis, 33(4), 606–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duintjer Tebbens RJ, Pallansch MA, Cochi SL, Wassilak SGF, Linkins J, Sutter RW, . . . Thompson KM (2011). Economic analysis of the Global Polio Eradication Initiative. Vaccine, 29(2), 334–343. [DOI] [PubMed] [Google Scholar]
- Duintjer Tebbens RJ, Pallansch MA, Kalkowska DA, Wassilak SG, Cochi SL, & Thompson KM (2013). Characterizing poliovirus transmission and evolution: Insights from modeling experiences with wild and vaccine-related polioviruses. Risk Analysis, 23(4), 703–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duintjer Tebbens RJ, Pallansch MA, Kew OM, Cáceres VM, Jafari H, Cochi SL, . . . Thompson KM (2006). Risks of paralytic disease due to wild or vaccine-derived poliovirus after eradication. Risk Analysis, 26(6), 1471–1505. [DOI] [PubMed] [Google Scholar]
- Duintjer Tebbens RJ, Pallansch MA, Kim J-H, Burns CC, Kew OM, Oberste MS, . . . Thompson KM (2013). Review: Oral poliovirus vaccine evolution and insights relevant to modeling the risks of circulating vaccine-derived polioviruses (cVDPVs). Risk Analysis, 23(4), 680–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duintjer Tebbens RJ, Pallansch MA, & Thompson KM (2015). Modeling the prevalence of immunodeficiency-associated long-term vaccine-derived poliovirus excretors and the potential benefits of antiviral drugs. BMC Infectious Diseases, 15(379), doi: 10.1186/s12879-12015-11115-12875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duintjer Tebbens RJ, Pallansch MA, Wassalik SGF, Cochi SL, & Thompson KM (2015). An economic analysis of poliovirus risk management policy options for 20132052. BMC Infectious Diseases, 15(389), doi: 10.1186/s12879-12015-11112-12878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duintjer Tebbens RJ, Pallansch MA, Wassilak SGF, Cochi SL, & Thompson KM (2016). Characterization of outbreak response strategies and potential vaccine stockpile needs for the polio endgame. BMC Infectious Diseases, 16, 137. doi: 10.1186/s1287-9016-1465-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duintjer Tebbens RJ, & Thompson KM (2017). Comprehensive screening for immunodeficiency-associated vaccine-derived poliovirus: an essential OPV cessation risk management strategy. Epidemiology & Infection, 145(2), 217–226. doi: 10.1017/S0950268816002302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duintjer Tebbens RJ, & Thompson KM (2018). Polio endgame risks and the possibility of restarting the use of oral poliovirus vaccine. Expert Review of Vaccines, 17(8), 739–751. doi: 10.1080/14760584.2018.1506333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson RH (1985). Vaccine preventable diseases of children. Retrieved from New York: [Google Scholar]
- Henderson RH, Keja J, Hayden G, Galaka A, Clements J, & Chan C. (1988). Immunizing the children of the world: progress and prospects. Bulletin of the World Health Organization, 66(5), 535–543. [PMC free article] [PubMed] [Google Scholar]
- Hull HF, Birmingham ME, Melgaard B, & Lee JW (1997). Progress toward global polio eradication. J Infect Dis, 175 Suppl 1, S4–9. doi: 10.1093/infdis/175.supplement_1.s4 [DOI] [PubMed] [Google Scholar]
- Kalkowska DA, Duintjer Tebbens RJ, Grotto I, Shulman LM, Anis E, Wassilak SGF, . . . Thompson KM (2015). Modeling options to manage type 1 wild poliovirus imported into Israel in 2013. Journal of Infectious Diseases, 211(11), 1800–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkowska DA, Duintjer Tebbens RJ, Pallansch MA, & Thompson KM (2019). Modeling undetected live poliovirus circulation after apparent interruption of transmission: Pakistan and Afghanistan. Risk Analysis, 39(2), 402–413, on-line October 408, 2018. doi: 10.1111/risa.13214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkowska DA, Pallansch MA, & Thompson KM (2019). Updated modelling of the prevalence of immunodeficiency-associated long-term vaccine-derived poliovirus (iVDPV) excreters. Epidemiology and Infection, 147, e295. doi: 10.1017/S095026881900181X [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinlay MA, Collett MS, Hincks JM, Oberste MS, Pallansch MA, Okayasu H, . . . Dowdle WR (2014). Progress in the development of poliovirus antiviral agents and their essential role in reducing risks that threaten eradication. Journal of Infectious Diseases, 210(Suppl 1), S447–453. [DOI] [PubMed] [Google Scholar]
- Plotkin SA, & Vidor E. (2013). Poliovirus vaccine - Inactivated In Plotkin SA, Orenstein WA, & Offit PA (Eds.), Vaccines (4th ed, pp. 573–597). Philadelphia: WB Saunders. [Google Scholar]
- Population Division of the Department of Economic and Social Affairs of the United Nations Secretariat. (2019). World population prospects; The 2019 revision. . Retrieved from https://population.un.org/wpp/ [Google Scholar]
- Tangermann RH, Aylward RB, Hull HF, Nkowane BM, Everts H, & Olive J-M (1999). Progress towards the eradication of poliomyelitis globally and in Africa, January 2000. Médecine tropicale : revue du Corps de santé colonial, 59(4 Pt 2), 475–482. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10901850 [PubMed] [Google Scholar]
- Thompson KM, & Duintjer Tebbens RJ (2006). Retrospective cost-effectiveness analyses for polio vaccination in the United States. Risk Analysis, 26(6), 1423–1440. [DOI] [PubMed] [Google Scholar]
- Thompson KM, & Duintjer Tebbens RJ (2014). National choices related to inactivated poliovirus vaccine, innovation, and the end game of global polio eradication. Expert Review of Vaccines, 13(2), 221–234. [DOI] [PubMed] [Google Scholar]
- Thompson KM, & Duintjer Tebbens RJ (2015a). The differential impact of oral poliovirus vaccine formulation choices on serotype-specific population immunity to poliovirus transmission. BMC Infectious Diseases, 15(376), doi: 10.1186/s12879-12015-11116-12874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson KM, & Duintjer Tebbens RJ (2015b). Health and economic consequences of different options for timing the coordinated global cessation of the three oral poliovirus vaccine serotypes. BMC Infectious Diseases, 15(374), doi: 10.1186/s12879-12015-11113-12877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson KM, & Kalkowska DA (2019). Logistical challenges and assumptions for modeling the failure of global cessation of oral poliovirus vaccine (OPV). Expert Review of Vaccines, 18(7), 725–736. doi: 10.1080/14760584.2019.1635463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson KM, Kalkowska DA, & Duintjer Tebbens RJ (2015). Managing population immunity to reduce or eliminate the risks of circulation following the importation of live polioviruses Vaccine, 33(3), 1568–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson KM, Pallansch MA, Duintjer Tebbens RJ, Wassilak SGF, Kim J-H, & Cochi SL (2013). Preeradication vaccine policy options for poliovirus infection and disease control. Risk Analysis, 33(4), 516–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Damme P, De Coster I, Bandyopadhyay AS, Revets H, Withanage K, De Smedt P, . . . Gast C. (2019). The safety and immunogenicity of two novel live attenuated monovalent (serotype 2) oral poliovirus vaccines in healthy adults: a double-blind, single-centre phase 1 study. Lancet, 394(10193), 148–158. doi: 10.1016/s0140-6736(19)31279-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Bank. (2019). World Bank list of economies (June 2019). Retrieved from http://databank.worldbank.org/data/download/site-content/CLASS.xls
- World Health Assembly. (1988). Global eradication of poliomyelitis by the year 2000 (resolution 41.28). Retrieved from http://www.who.int/csr/ihr/polioresolution4128en.pdf
- World Health Organization. (1986). Expanded Programme on Immunization: EPI global overview (EPI/GAG/86/WP.1). Retrieved from Geneva: [Google Scholar]
- World Health Organization. (1989). Poliomyelitis in 1986, 1987, and 1988 - Part 1. Weekly Epidemiological Record, 64(36), 273–279. [Google Scholar]
- World Health Organization. (1992). Expanded programme on immunization. Poliomyelitis in 1988, 1989, and 1990. Weekly Epidemiological Record, 67(16), 113–117. [PubMed] [Google Scholar]
- World Health Organization. (2014, October 20, 2013). World schedule as of 2013/October/20. Retrieved from http://www.who.int/immunization/monitoring_surveillance/data/en/
- World Health Organization. (2015a). GAP III: WHO Global Action Plan to minimize poliovirus facility-associated risk after type-specific eradication of wild polioviruses and sequential cessation of oral polio vaccine use. Retrieved from http://www.polioeradication.org/Portals/0/Document/Resources/PostEradication/GAPIII_2014.pdf [Google Scholar]
- World Health Organization. (2015b). Meeting of the Strategic Advisory Group of Experts on immunization, October 2015 - conclusions and recommendations. Weekly Epidemiological Record, 90(50), 681–700. [PubMed] [Google Scholar]
- World Health Organization. (2017). Meeting of the Strategic Advisory Group of Experts on immunization, April 2017 - conclusions and recommendations. Weekly Epidemiological Record, 92(22), 301–320.28580777 [Google Scholar]
- World Health Organization. (2019a, May 21, 2019). Circulating vaccine-derived poliovirus. Retrieved from http://polioeradication.org/polio-today/polio-now/this-week/circulating-vaccine-derived-poliovirus/
- World Health Organization. (2019b, May 31, 2019). Polio reported cases. Retrieved from http://apps.who.int/immunization_monitoring/globalsummary/timeseries/tsincidencepoliohtml
- World Health Organization. (2019c). Two out of three wild poliovirus strains eradicated. Retrieved from https://www.who.int/news-room/feature-stories/detail/two-out-of-three-wild-poliovirus-strains-eradicated
- World Health Organization. (2019d). WHO/UNICEF estimated coverage time series. Retrieved from http://www.who.int/immunization/monitoring_surveillance/routine/coverage/en/index4.html
- World Health Organization. (2019e, May 28, 2019). Wild Poliovirus List. Retrieved from http://polioeradication.org/polio-today/polio-now/wild-poliovirus-list/
- World Health Organization. (2020). Polio this week as of 9 January 2020. Retrieved from http://polioeradication.org/polio-today/polio-now/this-week/
- World Health Organization Global Polio Eradication Initiative. (2013). Polio eradication and endgame Strategic Plan (2013–2018). Geneva; 2013. Report No: WHO/POLIO/13.02. Retrieved from http://polioeradication.org/wpcontent/uploads/2016/07/PEESP_EN_A4.pdf [Google Scholar]
- World Health Organization Global Polio Eradication Initiative. (2015). Polio eradication & endgame: Midterm review July 2015. Geneva; 2015. Report No: WHO/POLIO/15.04. Retrieved from http://polioeradication.org/wp-content/uploads/2016/07/GPEI-MTR_July2015.pdf [Google Scholar]
- World Health Organization Global Polio Eradication Initiative. (2019). Polio eradication and endgame strategic plan (2019–2023). Geneva; 2019. Report No: WHO/POLIO/19.04. Retrieved from http://polioeradication.org/wp-content/uploads/2019/05/polio-endgame-strategy-2019-2023.pdf [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.