Abstract
Aims -
Annually variable, but synchronous production of large seed crops (‘masting’) is a widespread phenomenon in temperate trees. Mounting concerns about the impacts of anthropogenic climate change (ACC) on plant reproduction, gives urgency to our need to understand better the role of climate on tree reproduction, and in particular, mast events. Unlike our understanding of reproductive phenology however, there is little consensus regarding how climate affects plant reproductive effort, or indeed the actual environmental triggers that underpin masting behaviour.
Methods -
We used a 27-year record of acorn yield from a population of 12 Quercus robur trees located in southern England to compare masting frequency and post-dispersal acorn yield each year for each tree, with long-term weather data over the same period. We focussed on discrete or sequential climate cues (temperature, precipitation, and frost days) as likely predictors of oak reproduction.
Important Findings -
Annual post-dispersal acorn crop varied greatly; i.e. no acorns in 14 of the 27 years, but there was no sequential pattern of crop versus non-crop years indicating that weather, rather than resource limitation alone, dictated the timing of reproduction. Crop years were instead most closely associated with relatively cool late summer conditions in the preceding year, followed by anomalous summer warmth within crop year. Acorn yield increased following dry April and above average May and June temperatures within crop year. Although our results support a general association between warm late spring and summer conditions, and crop frequency and yield respectively, the influence of cooler later summer conditions in the year prior to masting highlights how a combination of weather cues may dictate the occurrence of mast years. Consequently, our results corroborate not only the hypothesis that temperature differentials between consecutive years, not absolute temperatures, may be the better predictor of mast seeding events, but lend support also to the suggestion that reproductive failure and resource accumulation resulting from a climate-linked environmental veto, drives future reproductive synchronization in temperate tree species.
Keywords: Anthropogenic climate change, Environmental veto, Moran effect, Quercus robur, Reproductive effort
INTRODUCTION
The synchronous production of seed crops by trees (‘masting’), whereby plants display variable between-, but similar within-year high seed yield, is described widely (Salisbury 1942; Kelly 1994; Kelly and Sork 2002; Pearse et al. 2016). Its occurrence in nature has been ascribed to the adaptive benefit of economies of scale; i.e. plants investing heavily in reproduction in years when con-specifics do the same incur lower costs per surviving offspring (Norton and Kelly 1988, Kelly 1994). These so-called ‘Ultimate-level’ causes (Pearse et al. 2016) include predator satiation, whereby infrequent and unpredictable seed production limits putative predator populations (Janzen 1971); increased pollination efficiency, a consequence of synchronised flowering (Smith et al. 1990); and environmental prediction, common where large-scale disturbances (fire, hurricanes etc.) generate spatio-temporally limited recruitment opportunity (Kelly 1994). Of these, predator satiation is perhaps the most widely supported (Pearse et al. 2016), but whatever adaptive benefit underpins its evolution, successful seedling recruitment and community persistence is, for many long-lived tree species, limited to ‘mast’ years (Salisbury 1942; Tapper 1992; Crawley and Long 1995). Fluctuations in seed yield also have important cascading effects on ecosystem interactions (Ostfield and Keesing 2000; Pesendorfer and Koenig 2016; Lichti et al. 2017). Consequently, an understanding of the causes and consequences of reproductive behaviour is important from the perspectives of tree population biology, forest management, and conservation.
While the actual environmental triggers and plant physiological processes underpinning masting remain unresolved, there is much evidence that climate is the key proximate cue (Koenig and Knops 2005; Pearse et al. 2016). Variations in temperature or precipitation do not necessarily signal better conditions for plant growth or development, but by virtue of a regional influence, synchronise individual reproductive activity within the wider population (Pearse et al. 2016). Consequently, and although reproductive allocation is often moderated by internal resource budgets (Pearse et al. 2016; Bogdziewicz et al. 2018), various climate triggers catalyse different developmental processes such as flower formation, pollen release, and/or fruit development that then confer adaptive benefits upon the individual and its progeny (Pearse et al. 2016).
Although there may be some phylogenetic conservation across species (Koenig et al. 2016), even with a single genus (e.g. Quercus), crop synchronicity has been associated with a variety of climate-triggers. For temperate oak species these include; cool summer temperatures (Q. macrocarpa), or warm spring temperatures 2 years prior to acorn maturity (Q. rubra) (Koenig and Knops 2014); cool, wet conditions in the early autumn (Q. robur) preceding the event (Crawley and Long 1995); and warm spring temperatures (Q. robur) within the crop year (Askeyev et al. 2005). For the Mediterranean species, Quercus ilex however, acorn crop was more closely associated with rainfall (Perez-Ramos et al. 2010) highlighting likely variation between climate triggers in temperate versus Mediterranean-climate regions. Indeed, rather than a single proximate cause, there is consensus that for most plant species, seed crop yield most likely corresponds with a combination or sequence of climate cues (see Allen et al. 2014; Buechling et al. 2016). Kelly et al. (2013) for instance report that for a wide variety of native New Zealand plants (i.e. 15 species from five families), reproductive effort was driven by a difference in temperatures between successive years rather than the absolute temperature within a particular year. Although there is mixed support for this so-called ‘Δt’ hypothesis (e.g. Koenig and Knops 2014; Pearse et al. 2014; Koenig et al. 2015; Moreira et al. 2015), climate nonetheless likely provides the main proximate cue for synchrony in reproductive timing and effort (Monks et al. 2016; Pearse et al. 2016).
Notwithstanding the view that climate variation does not explain the ultimate biological (adaptive) reasons for its evolution (Kelly 1994; Pearse et al. 2016), the strong link between climate and reproductive effort has important implications at a time of Anthropogenic Climate Change (ACC). A combination of a gradual increase in global temperatures and shifts in precipitation patterns, coupled with an increased incidence of extreme weather events (IPCC 2014), have been implicated widely as causes of observed individual tree mortality and forest dieback (Allen et al. 2010; Matusick et al. 2013). Although temporal (ontogenetic) ACC-linked mortality patterns are difficult to resolve (Allen et al. 2010), the fact that for most plant species the regeneration stage represents the most vulnerable life history phase (Fenner and Thompson 2005) suggests that any climate-linked impacts on reproduction are critical in understanding tree response to ACC. Despite a wealth of recent studies documenting the impact of ACC on plant ecophysiology, distributions, phenology, and plant community responses however, there remains a paucity of information on the influence of ACC on plant regeneration, and in particular, reproductive effort (Parmesan and Hanley 2015).
Given the importance of masting events for tree population persistence and the likelihood that climate extremes will intensify and increase in frequency over coming decades (IPCC, 2014), an understanding of the link between climate and key demographic processes like crop yield would seem to be particularly pressing (Clarke et al. 2011; Allen et al. 2014). Fenner (1991) was one of the first to draw attention to the importance of keeping long-term records of reproduction in trees to provide a simple bioassay of the effects of climate change. Remarkably, relatively few studies (see Allen et al. 2014; Richardson et al. 2015; Buechling et al. 2016; Gaignard et al. 2017) have however, looked at how tree crop yields respond to contemporary climate shifts as a basis to predict how future ACC scenarios will likely affect these events into coming decades. We use a 27-year long record of post-dispersal annual acorn yield (1989–2015) in an even-aged population of 12 Quercus robur L. trees located in southern England to explore how climate variability affects oak reproductive behaviour. Specifically, we examine whether mast years (defined here as years where acorn yield exceeded pre-and post-dispersal seed predation) is associated with any specific individual, combination, or sequence of proximate climate cues.
METHODS
Study Population and annual assessment of acorn yield
In October 1989, twelve mature oaks growing on Southampton Common, Hampshire (50.9262°N, 1.4092°W) were chosen to record variation in annual acorn production. All were isolated specimens with an even branching structure, selected for their uniform size (mean dbh = 341 cm (± 10.9 cm 1SE)) and ease with which the acorns could be observed and collected from the ground. While the age of the trees was not determined, they most likely date from the early 19th century when grazing ceased on the Common.
Recording occurred each year in the last week of October (21st to 26th), by which time the trees had shed the bulk of their acorns. Crop samples from each tree were taken from four 50cm-wide transects following compass bearings north, south, east and west of the base of the trunk, extending as the canopy edge (mean length 8.28 m (±0.22)). On each sampling occasion, the acorns and other debris were raked together and collected in bags before samples were cleaned to leave only the acorns (including a small minority infested by gall-wasps). The fresh weight of these samples was recorded and corresponding dry weight calculated by oven-drying sub-samples overnight at 105°C. We then used transect length to calculate acorn yield (Kg) per unit area (m2). By the end of the 27 years of the study, three trees were unusable, either through bramble incursion at the base, or because of branch loss (supplementary Table S1).
We did not attempt to exclude post-dispersal seed predators from our samples for two reasons; first, our study site precluded pan traps commonly used in isolated forests (Allen et al. 2014; Richardson et al. 2015; Buechling et al. 2016). Second we assumed that as the most likely ‘ultimate cause’ (Pearse et al. 2016), true mast years are by definition, those where acorn crop exceeded predator consumption. Although this meant we could not quantify acorn crops in the non-mast years, by definition these must have been low-acorn years, as even if acorns were produced, seed predators were able to remove the whole crop before collection. Moreover, even where previous studies have visually estimated pre-dispersal cone (Moreira et al. 2015; Zamorano et al. 2017) or seed numbers (Koenig and Knops 2014; Koenig et al. 2015), they are unable to draw strong conclusions regarding tree crop yield and post-dispersal predator satiation.
Acorn-Climate Analyses
To investigate climate influences on the occurrence and productivity of acorn mast years, we used monthly spring to autumn (i.e. nine months from February to October corresponding with the growth/reproductive season) observations of maximum and minimum temperatures, frost days, and rainfall. Climate data were obtained from a meteorological station located at 50.8997°N, 1.39556°W (www.southamptonweather.co.uk/sotonhist.php), 3 km from our oak population. For reproductive occurrence, we compared climate data between prior and current crop (n=13) and non-crop (n=14) years using a two-tailed Student’s t-test. Since we define ‘mast years’ as those where acorn crop satiated post-dispersal predation (i.e. acorns were left on the ground); all other events were considered to be zero crop years.
For reproductive effort, we compared monthly climate data against acorn yield (‘mast’ years only) using Spearman’s rank correlations. We recognize that the comparison of oak reproductive behavior with multiple climate variables, increases the likelihood of committing type I error. Nonetheless, we chose not to make the a priori assumption that weather for periods known to be strongly associated with reproductive effort in other species and studies, would be the sole drivers of acorn production in our oaks. Rather, we sought to eliminate all possibilities outside of the recognized pollination and seed maturation times and then corroborate post-priori any significant (P < 0.05) relationships from the literature. Having done this, we then developed a linear regression model to predict acorn crop as a function of seasonal average climate.
RESULTS
Climate and Oak Reproductive Trends
Mast years (thirteen of the total 27 years of observation) occurred synchronously across all trees, except during 2001 when two failed to produce acorns (supplementary Table S1). The number of trees we were able to observe reduced over time: 12 trees from 1989–1998 (4 events), 11 trees from 1999–2005 (4 events), 10 trees from 2006–2014 (5 events), and nine in 2015 (a crop year). Mean acorn production (Figure 1) ranged between a minimum of 0.018 kg m−2 in 2001 to a maximum of 0.446 kg m−2 in 1995. Only in 2001–2003 and 2010 and 2011 did we record consecutive acorn years. Years 2001 and 2002 (0.053 kg m−2) were the two lowest yields recorded, and 2010 (0.124 kg m−2) was the sixth lowest, all below the mean yield calculated across all trees and mast years (0.196 kg m−2).
Figure 1.
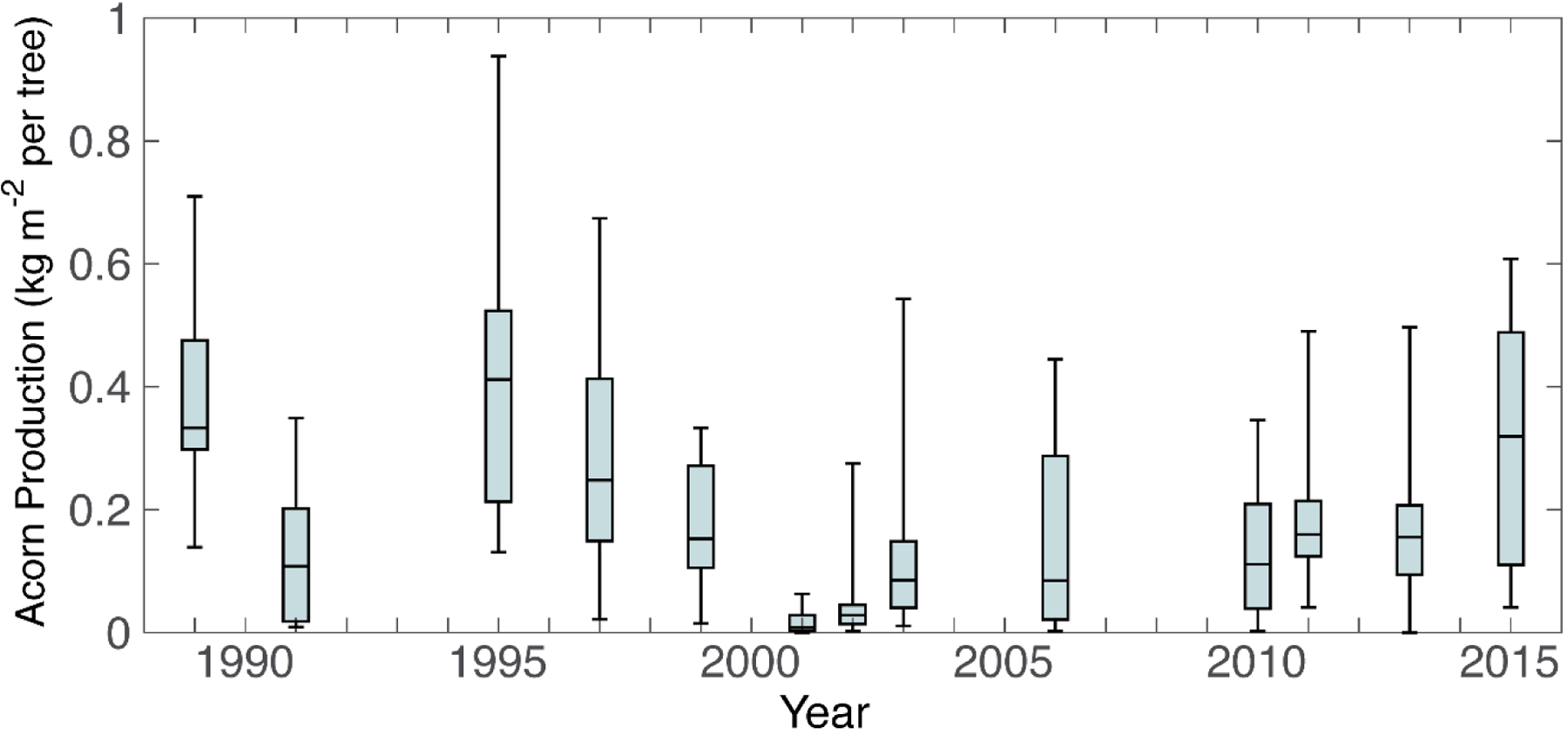
Acorn production (kg m−2) across individual trees during mast seeding events recorded from 1989 to 2015 for a population of Quercus robur trees growing in Southampton, southern England. Years without a box = zero acorns post-predation.
Within cropping years, there was a large spread in productivity across trees. For individual events, the largest range occurred in 1995 (0.807 kg m−2 difference between least and most productive trees) and the smallest in 2001 (0.063 kg m−2 difference between least and most productive trees). Since seed size is generally aplastic (Fenner and Thompson 2005), and for Q. robur in particular unlikely to vary by more than a factor of three (Brookes and Wigston 1979; Nikolić and Orlović 2002), we conclude that observed variation in crop yield was most closely associated with change in acorn number rather than individual size.
Climate Influence on Mast Year Occurrence
The likelihood of masting occurrence in any given year depends on both favourable environmental conditions (e.g., climate) and internal resource dynamics (Pearse et al. 2016; Bogdziewicz et al. 2018). If the latter were the sole mechanism, crop and non-crop years would be expected to alternate, as a high reproductive effort in one year would exhaust resources such that a high acorn crop would be unlikely in the following year even if environmental conditions were favourable. To test whether masting/nonmasting years alternate more than would be predicted from random, we conducted a Wald–Wolfowitz runs test by converting the masting data to a sequence of ones (masting, n=13) and zeros (non-masting, n=14). A ‘runs test’ evaluates the probability that a given sequence of events occurs randomly against the alternative hypotheses that events either tend to cluster together or alternate from one trial (year of observation) to the next. Results indicated that the null hypothesis that the sequencing of masting/nonmasting events is random cannot be rejected (z = 1.5796, P = 0.11), suggesting that internal resource limitation was not the sole driver of mast events.
To investigate possible climate triggers for masting, we compared climate between crop- and non-crop years. Although temperature in the year prior to masting had no discernible influence, July conditions were on average, warmer during the actual mast year (Figure 2: left and centre columns). When recalculated as the difference between the current and previous year, however, the strongest potential triggers were maximum and mean temperatures during July (Tmax P = 0.028; Tmean P = 0.0364) and August (Tmax P = 0.038; Tmean P = 0.007) (Figure 2: right column). This suggests mast years tend to occur when cold summer conditions during previous year, were followed by anomalous warmth in the same months in the mast year. We found less association between mast seeding occurrence and either the number of frost days or rainfall (Figure 3), the only significant anomalous occurrence being April rainfall (P = 0.001) where mast years were more common in drier conditions.
Figure 2.
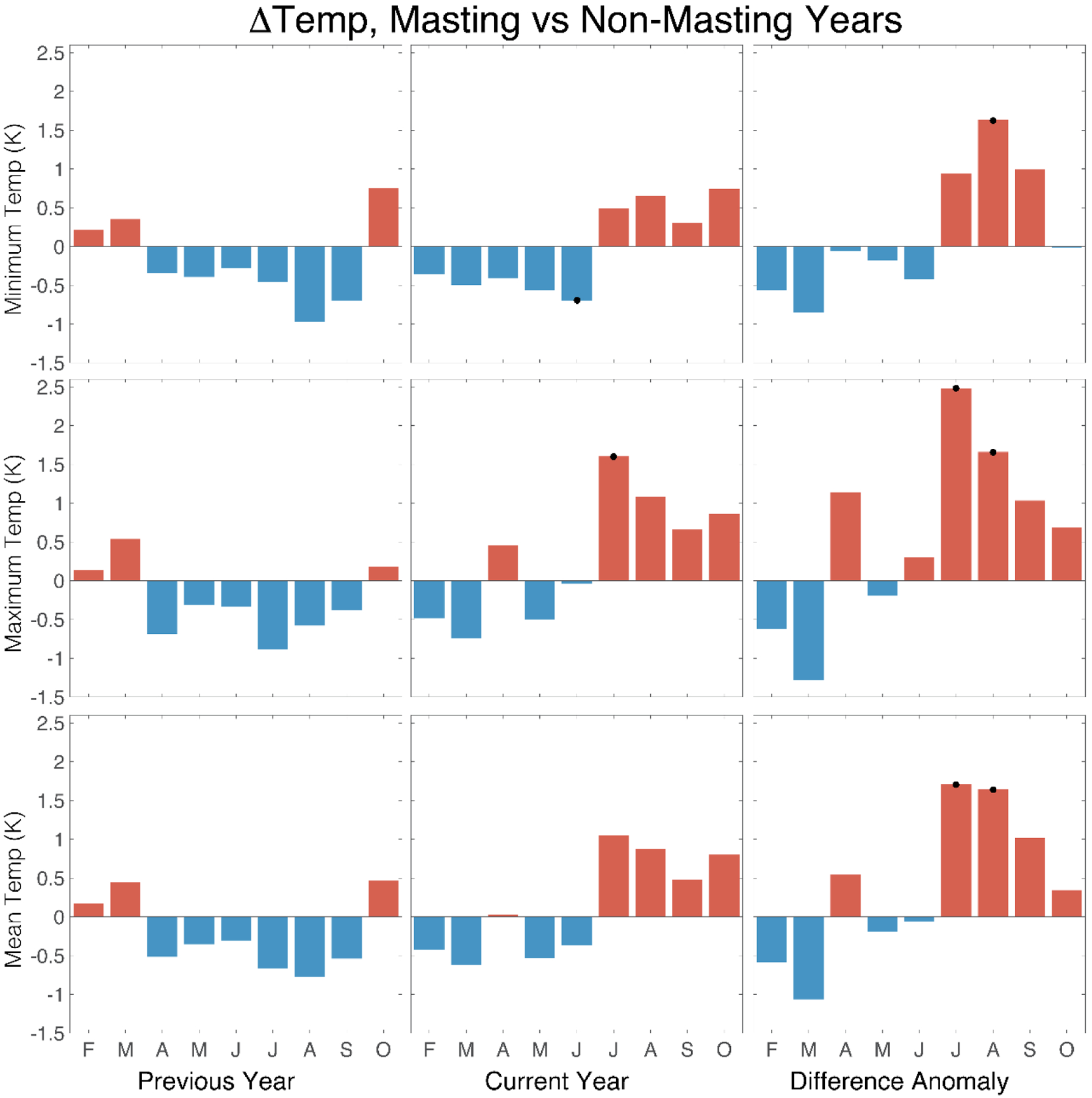
Monthly minimum, maximum, and mean temperature anomalies (significance assessed with a two-sided Student’ t-test and indicated by black (P <0.05) dots) associated with mast and non-years for a population of Quercus robur trees growing in Southampton, southern England. Results are shown for years prior to the mast year (left column); the mast year (centre column); the difference between the two (right column).
Figure 3.
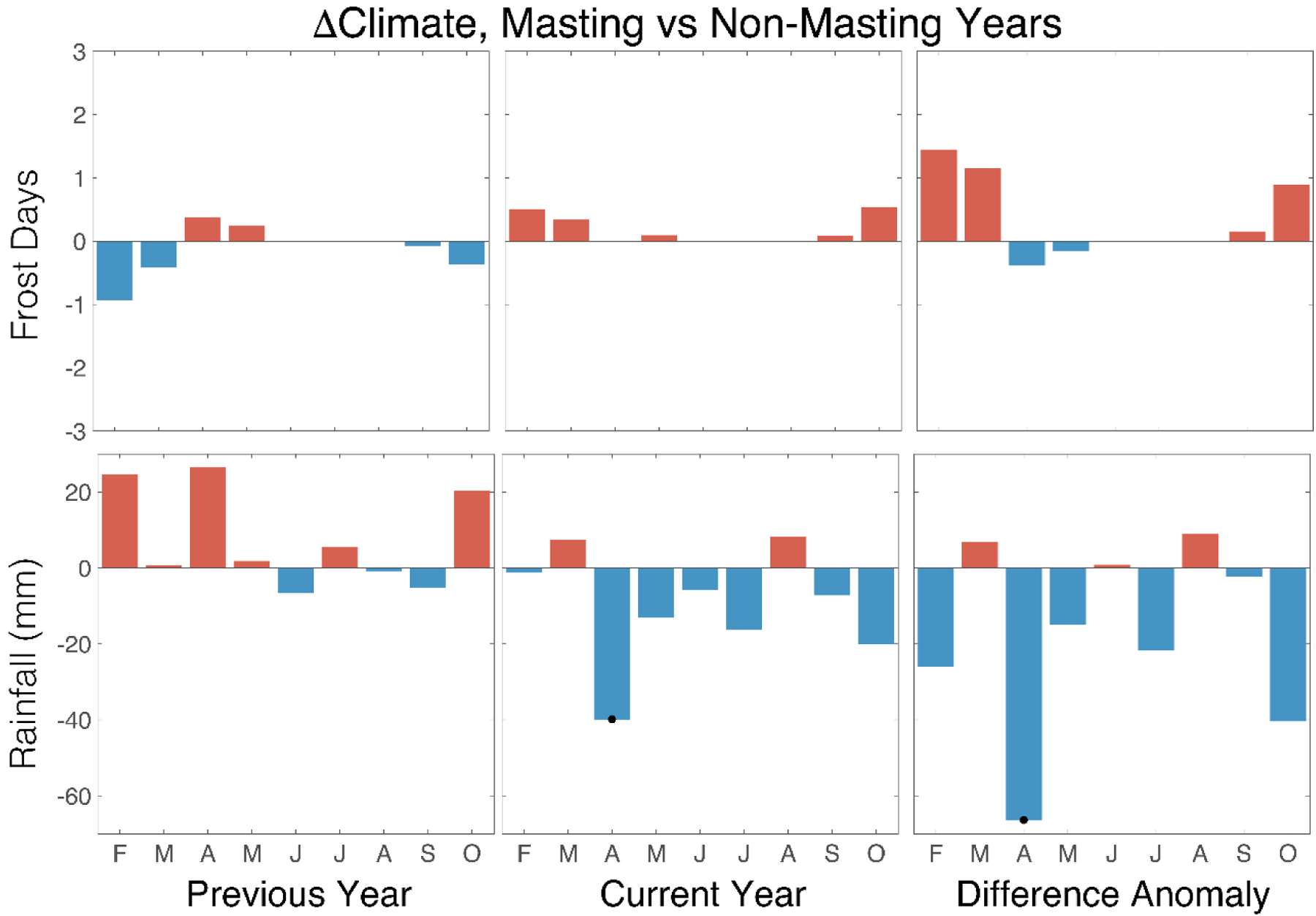
Anomalies (assessed with a two-sided Student’ t-test and indicated by black (P <0.05) dots) in frost days and rainfall associated with mast and non-mast years for a population of Quercus robur trees growing in Southampton, southern England. Results are shown for years prior to the mast year (left column); the mast year (centre column); the difference between the two (right column).
Climate Influence on Acorn Yields
To estimate climate effects on acorn production while accounting for changes in the number of trees observed, we estimated an average per tree acorn yield in each mast year. Of all climate variables, concurrent year monthly mean temperatures were most strongly related to total acorn production. The strongest correlations were with May (Spearman’s r = 0.66; P = 0.01) and June (r = 0.75; P = 0.003) temperatures, indicating that warm conditions during these months were associated with larger acorn crops. Averaging mean temperatures together for May and June, we developed a simple linear regression model for acorn yield per tree (Figure 4). The regression was highly significant with large explanatory power (P = 0.002, r2 = 0.61) and corresponds with the peak pollination period for oak in southern England (Grime et al. 2007). From the slope of the regression, we estimate that acorn yield per tree increases by about 0.11 kg m−2 per °C of warming.
Figure 4.
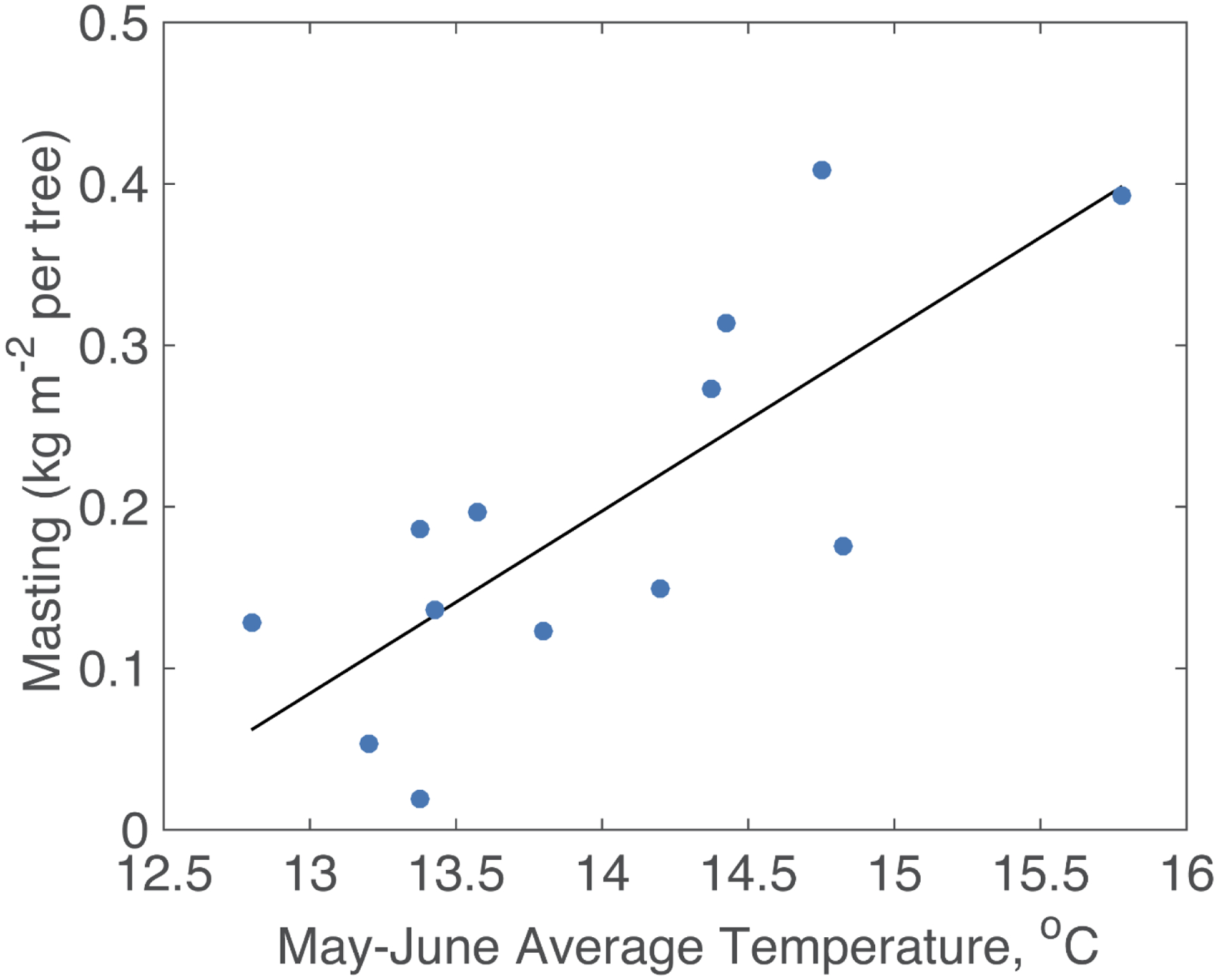
Relationship between average acorn yield (kg m−2) and local May-June average temperature for a population of Quercus robur trees growing in Southampton, southern England. Each dot represents one mast year.
DISCUSSION
Despite observing the relatively large between-tree variation in acorn crops reported elsewhere (Herrera et al. 1998; Crawley and Long 1995; Koenig and Knops 2000; Zamorano et al. 2017), our oaks exhibited uniform variation in seed crop yield across the population. For example, eight of the 13 mast years, including 2015, were followed by non-mast years (i.e. no acorns survived post-dispersal predation) for all individuals. Nonetheless, we found no evidence that high reproductive allocation in one year was followed sequentially by a low acorn crop the next. Although at face value this may seem to contradict the view that internal resource dynamics prevent individuals from producing sequential mast crops (Herrera et al. 1998; Kelly and Sork 2002; Crone and Rapp 2014), a combination of internal resource dynamics and climate may nonetheless, together trigger masting behaviour.
Aside from dry April conditions, precipitation and the number of frost days had no detectable influence on acorn production; both phenomena can be relatively localised and consequently thought not to impose the uniform regional climate cue needed to ensure regional synchronisation (Norton and Kelly 1988; Kelly et al. 2013). Temperature signals in either the previous, or current, years alone were only weakly related to masting occurrence. Masting was, however, strongly associated with a combination of temperature cues across these years; specifically relatively cool conditions in the summer of the year before masting when followed by relatively warmer temperatures during the period of the actual mast year, together promoted mast occurrence. In addition, warm, dry May and June weather within the mast year were associated with relatively high acorn crops, results corroborating studies that associate warm late spring/early summer conditions to high tree seed yield. Askeyev et al. (2005), Bogdziewicz et al. (2017) and Caignard et al. (2017) for example, reported that ‘within-year’ warm spring and summer conditions promoted increased acorn production in Russian, Polish, and French Q. robur populations respectively. Similar relationships were described for the Californian Q. lobata (Koenig et al. 2015), and Scandinavian Picea abies and Betula species (Zamorano et al. 2017). Consequently, it seems likely that our analyses identified bona fide biological relationships between reproductive effort and monthly weather data, rather than generating ‘significant’ correlations due simply to type I error (see Cabin & Mitchell 2000).
The long-accepted explanation for the link between warm, dry spring conditions and high reproductive output in temperate oak species is that this weather promotes pollen transfer in anemophilous trees (Norton and Kelly 1988; Smith, et al. 1990). This explanation underpins the Moran effect hypothesis; i.e. pollination success and thus reproductive output, is decoupled from mechanisms affecting flower production but is instead, associated with environmental conditions during flowering (Koenig 2012; Pearse et al. 2016; Bogdziewicz et al. 2017). In addition to warm dry conditions favouring pollination, warm early summer growing conditions in July and August may also promote increased photosynthesis and so increase the resources available for reproductive allocation (Norton and Kelly 1988; Kelly and Sork 2002).
The apparent influence of the summer temperature difference anomaly on acorn crops, also highlights however, the potential importance of pollen coupling; i.e. pollination success and seed yield are functions of environmental and/or resource constraints that dictate the parent tree’s ability to produce flowers (Satake and Iwasa 2000; Kelly et al. 2001; Monks et al. 2016). Bogdziewicz et al. (2018) developed this idea further by suggesting that the environment has a ‘veto’ effect on reproductive allocation. Put simply, reproductive failure brought about by poor environmental conditions in one year, facilitates the accumulation of resources that plants can then allocate to subsequent (increased) reproductive output. Consequently, masting may not simply arise from the occurrence of favourable environmental conditions. Instead Bogdziewicz et al. (2018) argue that masting it is a combination of unfavourable environmental conditions (that limit reproduction), subsequent resource accumulation (more to spend on reproduction), and the environmental triggering of resource release to reproduction when weather conditions are favourable. The cool late summer conditions the year prior to mast (likely reducing seed development and maturation) may have facilitated resource accumulation and high reproductive output when coincidental with above-average summer conditions in mast year. Since a good summer does not predictably follow a bad one, resource accumulation alone cannot dictate acorn yield, and so explain why we failed to detect any sequential pattern of crop/non-crop years. Certainly, our results corroborate a growing consensus that the initiation of masting results from a combination or sequence of climate cues (Allen et al. 2014; Buechling et al. 2016).
Given the close association between oak masting (occurrence and amount), and spring and summer weather we elucidate here, the likely increased temperatures and shifts in precipitation associated with ACC would be expected to affect greatly reproductive timing and output. Our data and analysis suggest a 0.11 kg m−2 per tree increase in acorn yield per °C of warming; we recognise however, that a scenario of continual increased masting is highly unlikely to unfold. As Buechling et al. (2016) point out; positive climate/masting relationships must eventually be constrained by inherent physiological limitations. Indeed, there is an emerging consensus that in order to best understand masting behaviour, predictive models based on environmental cues for flowering, pollination success and acorn production, must incorporate also a term for internal parental resource budgets (Koenig et al 2015; Pesnedorfer et al. 2016; Bogdziewicz et al. 2017, 2018). In addition, changes in the abundance and activity of seed and seedling predators, and extrinsic factors affecting germination and seedling dormancy (Newbold and Goldsmith 1981), are additional complexities that serve to highlight the fact that we still understand remarkably little regarding the impacts of ACC on plant regeneration biology (Parmesan and Hanley 2015).
Supplementary Material
ACKNOWLEDGEMENTS
We thank two anonymous referees for their comments on earlier drafts of this manuscript.
Footnotes
SUPPLEMENTARY MATERIAL
Supplementary material (Table S1) is available at Journal of Plant Ecology online.
REFERENCES
- Allen CD, Macalady AK, Chenchouni H, et al. (2010) A global overview of drought and heat-induced tree mortality reveals emerging climate risks for forests. Forest Ecology and Management 259: 660–684. [Google Scholar]
- Allen RB, Hurst JM, Portier J, Richardson SJ (2014) Elevation-dependent responses of tree mast seeding to climate change over 45 years. Ecology and Evolution 4: 3525–3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askeyev OV, Tischin D, Sparks TH, Askeyev IV (2005) The effects of climate on the phenology, acorn crop and radial increment of pendulate oak (Quercus robur) in the middle Volga region, Tararstan, Russia. International Journal of Biometeorology 49: 262–266. [DOI] [PubMed] [Google Scholar]
- Ballardie RT, Whelan RJ (1986) Masting, seed dispersal and seed predation in the cycad Macrozmia communis. Oecologia 70: 100–105. [DOI] [PubMed] [Google Scholar]
- Bogdziewicz M, Szymkowiak J, Kasprzyk I, et al. (2017) Masting in wind-pollinated trees: system-specific roles of weather and pollination dynamics in driving seed production. Ecology 98: 2615–2625. [DOI] [PubMed] [Google Scholar]
- Bogdziewicz M, Fernández-Martínez M, Bonal R, Belmonte J, Espelta JM. (2017) The Moran effect and environmental vetoes: phenological synchrony and drought drive seed production in a Mediterranean oak. Proceedings of the Royal Society B: Biological Sciences 284: 20171784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdziewicz M, Steele MA, Marino S, Crone EE. (2018) Correlated seed failure as an environmental veto to synchronize reproduction of masting plants. New Phytologist 219: 98–108. [DOI] [PubMed] [Google Scholar]
- Brookes PC, Wigston DL (1979) Variation of morphological and chemical characteristics of acorns from populations of Quercus petraea (Matt.) LiebI., Q. robur L. and their hybrids. Watsonia 12: 315–324. [Google Scholar]
- Buechling A, Martin PH, Canham CD, Shepperd WD, Battaglia MA (2016) Climate drivers of seed production in Picea engelmannii and response to warming temperatures in the southern Rocky Mountains. Journal of Ecology 104: 1051–1062. [Google Scholar]
- Cabin RJ, Mitchell RJ. (2000) To Bonferroni or not to Bonferroni: when and how are the questions. Bulletin of the Ecological Society of America 81: 246–248. [Google Scholar]
- Clark JS, Bell DM, Hersh MH, Nichols L (2011) Climate change vulnerability of forest biodiversity: climate and competition tracking of demographic rates. Global Change Biology 17: 1834–1849. [Google Scholar]
- Crawley MJ, Long CR (1995) Alternate bearing, predator satiation and seedling recruitment in Quercus robur L. Journal of Ecology 83: 683–696. [Google Scholar]
- Crone EE, Rapp JM (2014) Resource depletion, pollen coupling, and the ecology of mast seeding. Annals of the New York Academy of Sciences 1322: 21–34. [DOI] [PubMed] [Google Scholar]
- Fenner M (1991) Irregular seed crops in forest trees. Quarterly Journal of Forestry 85: 166–172. [Google Scholar]
- Fenner M, Thompson K (2005) The Ecology of Seeds. Cambridge University Press, Cambridge UK. [Google Scholar]
- Grime JP, Hodgson JG, Hunt R (2007) Comparative Plant Ecology 2nd Edn - Castlepoint Press, Dalbeattie, UK. [Google Scholar]
- Hallett TB, Coulson T, Pilkington JG, et al. (2004) Why large-scale climate indices seem to predict ecological processes better than local weather. Nature 430: 71–75. [DOI] [PubMed] [Google Scholar]
- Herrera CM Jordano P, Guitián J, et al. (1998) Annual variability in seed production by woody plants and the masting concept: reassessment of principles and relationship to pollination and seed dispersal. American Naturalist 152: 576–594. [DOI] [PubMed] [Google Scholar]
- IPCC (2014) Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part B: Regional Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change [eds Barros VR, Field CB, Dokken DJ, Mastrandrea MD, Mach KJ, Bilir TE, Chatterjee M, Ebi KL, Estrada YO, Genova RC, Girma B, Kissel ES, Levy AN, MacCracken S, Mastrandrea PR & White LL]. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, pp. 688. [Google Scholar]
- Janzen DH (1971) Seed predation by animals. Annual Review of Ecology and Systematics 2: 465–492. [Google Scholar]
- Jones EW (1959) Biological floral of the British Isles: Quercus robur L. Journal of Ecology 47: 169–216. [Google Scholar]
- Kelly D (1994) The evolutionary ecology of mast seeding. Trends in Ecology and Evolution 9: 465–470. [DOI] [PubMed] [Google Scholar]
- Kelly D, Sork VL (2002) Mast seeding in perennial plants: why, how, where? Annual Review of Ecology and Systematics 33: 427–447. [Google Scholar]
- Kelly D, Hart DE, Allen RB (2011) Evaluating the wind pollination benefits of mast seeding. Ecology 82: 117–126. [Google Scholar]
- Kelly D Geldenjuis A, James A et al. (2013) Of mast and mean: differential temperature cue makes mast seeding insensitive to climate change. Ecology Letters 16: 90–98. [DOI] [PubMed] [Google Scholar]
- Koenig WD (2012) Global patterns of environmental synchrony and the Moran effect. Ecography 25: 283–288. [Google Scholar]
- Koenig WD, Knops JMH (2000) Patterns of annual seed production by northern hemisphere trees: a global perspective. American Naturalist 155: 59–69. [DOI] [PubMed] [Google Scholar]
- Koenig WD, Knops JMH (2005) The mystery of masting in trees. American Scientific 93: 340–347. [Google Scholar]
- Koenig WD, Knops JMH (2014) Environmental correlates of acorn production by four species of Minnesota oaks. Population Ecology 56: 63–71. [Google Scholar]
- Koenig WD, Knops JMH, Carmen WJ, Pearse IS (2105) What drives masting? The phenological synchrony hypothesis. Ecology 96: 184–192. [DOI] [PubMed] [Google Scholar]
- Koenig WD, Alejano R, Carbonero MD, et al. (2016) Is the relationship between mast-seeding and weather in oaks related to their life-history or phylogeny? Ecology 97: 2603–2615. [DOI] [PubMed] [Google Scholar]
- Lichti NI, Steele MA, Swihart RK (2017) Seed fate and decision-making processes in scatter-hoarding rodents. Biological Reviews 92: 474–504. [DOI] [PubMed] [Google Scholar]
- Masaka K, Maguchi S. (2001) Modelling the masting behaviour of Betula platyphylla var. japonica using the resource budget model. Annals of Botany 88: 1049–1055. [Google Scholar]
- Matusick G, Ruthrof KX, Brouwers NC, Dell B, Hardy GSJ (2013) Sudden forest canopy collapse corresponding with extreme drought and heat in a Mediterranean-type eucalypt forest in southwestern Australia. European Journal of Forest Research 13: 497–510. [Google Scholar]
- Monks A, Monks JM, Tanentzap AJ. (2016) Resource limitation underlying multiple masting models makes mast seeding sensitive to future climate change. New Phytologist 210: 419–430. [DOI] [PubMed] [Google Scholar]
- Moreiera X, Abdala-Roberts L, Linhart YB, Mooney KA (2015) Effects of climate on reproductive investment in a masting species: assessment of climatic predictors and underlying mechanisms. Journal of Ecology 103: 1317–1324. [Google Scholar]
- Murphy JM, Booth BB, Collins M, Harris GR, Sexton DM, Webb MJ (2007) A methodology for probabilistic predictions of regional climate change from perturbed physics ensembles. Philosophical Transactions of the Royal Society London series A 365: 1993–2028. [DOI] [PubMed] [Google Scholar]
- Newbold AJ, Goldsmith FB (1981) Discussion papers on conservation 33: The regeneration of oak and beech; a literature review (with an addendum on birch by Harding JS). University College London, London. [Google Scholar]
- Nikolić N, Orlović S (2002) Genotypic variability of morphological characteristics of English oak (Quercus robur L.) acorn. Proceedings for Natural Sciences, Matica Srpska Novi Sad 102: 53–58. [Google Scholar]
- Norton DA, Kelly D (1988) Mast seeding over 33 years by Dacrydium cupressum Lamb. (rimu) (Podocarpaceae) in New Zealand: the importance of economies of scale. Functional Ecology 2: 399–408. [Google Scholar]
- Ostfield RS, Keesing F (2000) Pulsed resources and community dynamics of consumers in terrestrial ecosystems. Trends in Ecology and Evolution 15: 232–237. [DOI] [PubMed] [Google Scholar]
- Parmesan C, Hanley ME (2015) Plants and climate change: complexities and surprises. Annals of Botany 115: 849–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse IS, Koenig WD, Kelly D (2016) Mechanisms of mast seeding: resources, weather, cues and selection. New Phytologist 212: 546–562. [DOI] [PubMed] [Google Scholar]
- Pérez-Ramos IM, Ourcival JM, Limousin JM, Rambal S (2010) Mast seeding under increasing drought: results from a long-term data set and from a rainfall exclusion experiment. Ecology 91: 3057–3068. [DOI] [PubMed] [Google Scholar]
- Pesendorfer MB, Koenig WD (2016) The effect of within-year variation in acorn crop size on seed harvesting by avian hoarders. Oecologia 181: 97–106. [DOI] [PubMed] [Google Scholar]
- Pesnedorfer MB, Koenig WD, Pearse IS, Knops JM, Funk KA (2016) Individual resource-limitation combined with population-wide pollen availability drives masting in the valley oak (Quercus lobata). Journal of Ecology 104: 637–645. [Google Scholar]
- Richardson SJ, Allen RB, Whitehead D, et al. (2005) Climate and net carbon availability both determine seed production in a temperate tree species. Ecology 86: 972–981. [Google Scholar]
- Salisbury EJ (1942) The Reproductive Capacity of Plants. Bell and Sons, London. [Google Scholar]
- Satake A, Iwasa Y (2000) Pollen coupling of forest trees: forming synchronized and periodic reproduction out of chaos. Journal of Theoretical Biology 203: 63–84. [DOI] [PubMed] [Google Scholar]
- Smith CC, Hamrick JL, Kramer CL (1990) The advantage of mast years for wind pollination. American Naturalist 136: 154–166. [Google Scholar]
- Tapper P-G (1992) Irregular fruiting in Fraxinus excelsior. Journal of Vegetation Science 3: 41–46. [Google Scholar]
- Wesołowski T, Rowiński P, Maziarz M (2015) Interannual variation in tree seed production in a primeval temperate forest: does masting prevail? European Journal of Forest Research 134: 99–112. [Google Scholar]
- Zamorano JG, Hokkanen T, Lehikoinen A (2017) Climate driven synchrony in seed production of masting deciduous and conifer tree species. Journal of Plant Ecology 11: 180–188. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


