Abstract
Background
COVID-19 community mitigation measures (e.g., stay-at-home orders) may worsen mental health and substance use-related harms such as opioid use disorder and overdose and limit access to medications for these conditions. We used nationally-representative data to assess dispensing of select substance use and mental health medications during the pandemic in the U.S.
Methods
IQVIA Total Patient Tracker data were used to calculate U.S. monthly numbers of unique patients dispensed buprenorphine, extended-release (ER) intramuscular naltrexone, naloxone, selective serotonin or serotonin-norepinephrine reuptake inhibitors, benzodiazepines, and for comparison, HMG-CoA reductase inhibitors (statins) and angiotensin receptor blockers (ARBs) between January 2019-May 2020. Forecasted estimates of number of unique patients dispensed medications, generated by exponential smoothing statistical forecasting, were compared to actual numbers of patients by month to examine access during mitigation measures (March 2020-May 2020).
Results
Between March 2020-May 2020, numbers of unique patients dispensed buprenorphine and numbers dispensed naloxone were within forecasted estimates. Numbers dispensed ER intramuscular naltrexone were significantly below forecasted estimates in March 2020 (-1039; 95 %CI:-1528 to -550), April 2020 (-2139; 95 %CI:-2629 to -1650), and May 2020 (-2498; 95 %CI:-2987 to -2009). Numbers dispensed antidepressants and benzodiazepines were significantly above forecasted estimates in March 2020 (977,063; 95 %CI:351,384 to 1,602,743 and 450,074; 95 % CI:189,999 to 710,149 additional patients, respectively), but were within forecasted estimates in April 2020-May 2020. Dispensing patterns for statins and ARBs were similar to those for antidepressants and benzodiazepines.
Conclusions
Ongoing concerns about the impact of the COVID-19 pandemic on substance use and mental health underscore the need for innovative strategies to facilitate continued access to treatment.
Keywords: Substance use, Overdose, Opioid use disorder, Mental health, Depression, Anxiety
1. Introduction
Implementation of community mitigation measures such as stay-at-home orders to slow the spread of SARS-CoV-2, the virus that causes the 2019 novel coronavirus disease (COVID-19), has been widespread in the United States (Gostin and Wiley, 2020). Collateral consequences of these mitigation measures (e.g., economic stress, social isolation), coupled with fear of virus transmission, have raised concerns about worsening mental health and substance use-related harms such as opioid use disorder and overdose (Henry et al., 2020; Volkow, 2020). A recent survey found that 1-in-7 U.S. adults reported serious psychological distress in April 2020, during peak use of community mitigation measures (McGinty et al., 2020). A subsequent survey of adults in the U.S. reported that 13.3 % of adults had started or increased substance use to cope with pandemic-related stress or emotions, 30.9 % had symptoms of anxiety or depressive disorders, 26.3 % had symptoms of a trauma and stress-related disorder, and 10.7 % had seriously considered suicide in the past 30 days (Czeisler et al., 2020). In addition, emerging data indicate that drug overdoses have increased during the same time period as peak community mitigation measures (Alter and Yeager, 2020).
Further, as a result of community mitigation measures, access to medical treatment, including medications used to treat opioid use disorder, opioid overdose, and mental health conditions, may have been limited due to clinician office closures, discontinuation of in-person treatment and recovery support services, and delays in seeking care due to concerns about exposure to COVID-19 during medical visits (Henry et al., 2020; Volkow, 2020). Analysis of pharmacy dispensing data is one approach to examine if access to medications changed during community mitigation measures; yet, to date, such analyses are lacking. To address this research gap, this study used nationally representative data to assess dispensing patterns of selected substance use and mental health medications from January 2019 through May 2020 in the United States.
2. Materials and methods
2.1. Data source and measures
In this time series analysis, data from the IQVIA Total Patient Tracker database, which captures 92 % of prescriptions dispensed from U.S. retail pharmacies, were used to calculate the number of unique patients (all ages) dispensed the following medications by month from January 2019 to May 2020: medications for opioid use disorder treatment, buprenorphine (single entity and buprenorphine-naloxone combinations), extended-release (ER) intramuscular naltrexone; the overdose-reversal medication naloxone, including those issued under a standing order in retail pharmacies; selective serotonin reuptake inhibitor or serotonin-norepinephrine reuptake inhibitor antidepressants; benzodiazepines; and for comparison purposes, two chronic disease medications, HMG-CoA reductase inhibitors (statins) used in the treatment of hyperlipidemia and angiotensin receptor blockers (ARBs) used in the treatment of hypertension and other cardiovascular conditions. Buprenorphine formulations approved for the treatment of pain (i.e., Butrans, Belbuca, Buprenex) were excluded from the analysis. In addition, oral naltrexone formulations were not included as they are not generally recommended in the treatment of opioid use disorder (American Society of Addiction Medicine, 2020; Substance Use and Mental Health Services Administration, 2020a).
2.2. Data analysis
To assess changes in the number of unique patients dispensed medications during the time of COVID-19 mitigation measures, March 2020 to May 2020, we used the exponential triple smoothing statistical forecasting function in Microsoft Excel (Seattle, Washington) to generate monthly forecasted estimates and 95 % confidence intervals (CIs) for each drug or drug class examined. This statistical forecasting method predicts future values based on historical data by utilizing the additive error, additive trend, and additive seasonality (AAA) exponential triple smoothing algorithm; a method well suited for data with seasonality or other cyclical patterns over time (Microsoft, 2015; Makridakis et al., 1998). Once forecasted estimates and 95 % CIs were estimated, we then compared the forecasted estimates with the actual number of unique patients dispensed each medication by month for March 2020 to May 2020. The actual number of unique patients dispensed the medication were considered statistically significantly different than forecasted estimates if the actual number of unique patients in a particular month fell outside the forecasted estimates and associated 95 % CIs. When this occurred, the actual number of unique patients dispensed the medication was considered to be higher or lower than what would have been expected based on prior trends in the data for that medication. All analyses were conducted with Microsoft Excel (Seattle, Washington) and Stata V15.1 (College Station, Texas). This study was a secondary analysis of de-identified data and was therefore exempt from institutional review board approval.
3. Results
3.1. Mental health medications and chronic disease medications
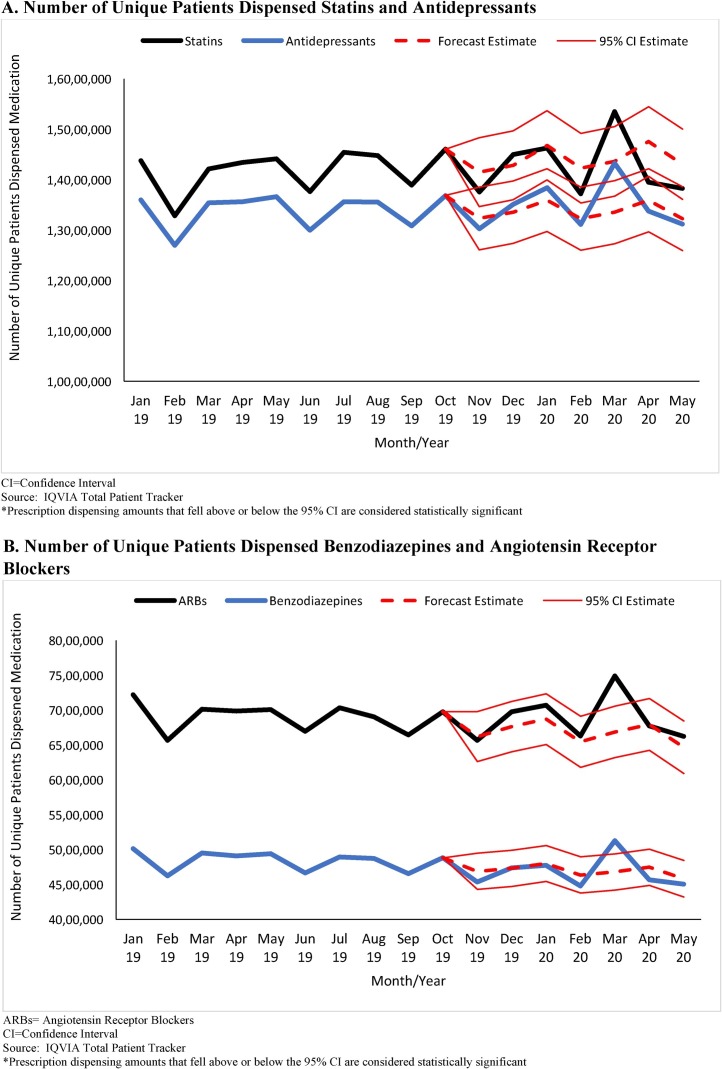
From January 2019 to February 2020, the monthly number of unique patients dispensed antidepressants (mean = 13,888,901 [SD = 337,357]; range = 12,696,855 to 13,844,201) as well as benzodiazepines (mean = 4,781,043 [SD = 166,850]; range = 4,478,448 to 5,011,279) was relatively stable; however in March 2020, the number of unique patients dispensed antidepressants (14,330,662) and benzodiazepines (5,128,721) was statistically significantly higher than forecasted estimates (Fig. 1 ). In March 2020, an estimated additional 977,063 (95 % CI: 351,384 to 1,602,743) unique patients and 450,074 (95 % CI:189,999 to 710,149) unique patients were dispensed antidepressants and benzodiazepines, respectively, compared to forecasted estimates. The numbers of unique patients dispensed antidepressants and benzodiazepines in April 2020 and May 2020 were within forecasted estimates. The patterns of dispensing in March 2020 to May 2020 for the two comparison chronic disease medications, statins and ARBs, were similar to those for antidepressants and benzodiazepines during this time.
Fig. 1.
A/B. Number of Unique Patients Dispensed Select Antidepressants, Benzodiazepines, and Chronic Disease Medications, United States, January 2019 – May 2020*.
A. Number of Unique Patients Dispensed Statins and Antidepressants
CI = Confidence Interval
Source: IQVIA Total Patient Tracker
*Prescription dispensing amounts that fell above or below the 95 % CI are considered statistically significant
B. Number of Unique Patients Dispensed Benzodiazepines and Angiotensin Receptor Blockers
ARBs = Angiotensin Receptor Blockers
CI = Confidence Interval
Source: IQVIA Total Patient Tracker
*Prescription dispensing amounts that fell above or below the 95 % CI are considered statistically significant
3.2. Medications for opioid use disorder treatment and opioid overdose reversal
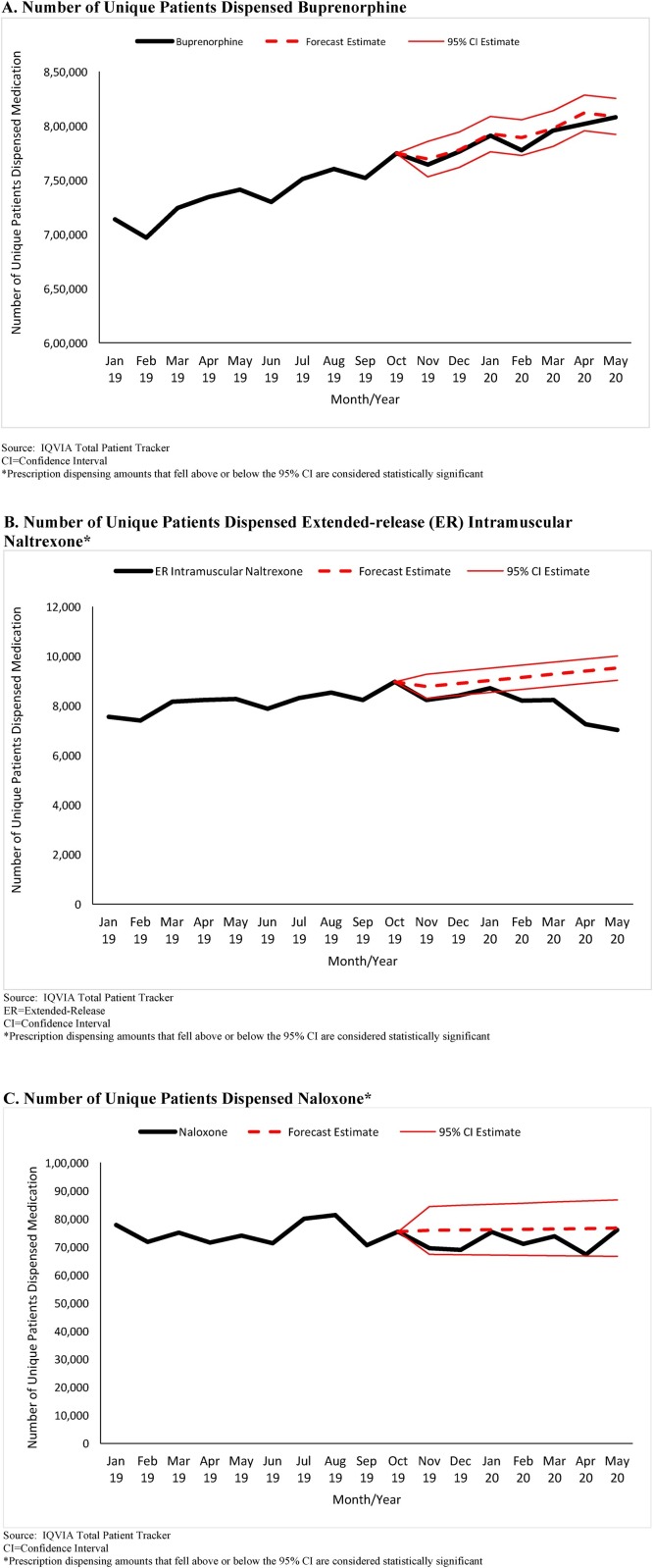
The number of unique patients dispensed buprenorphine products approved to treat opioid use disorder increased from 713,778 in January 2019 to 814,019 in May 2020 (Fig. 2 A). Between March 2020 to May 2020, the number of unique patients dispensed buprenorphine products was within forecasted estimates. The number of unique patients dispensed ER intramuscular naltrexone fell statistically significantly below forecasted estimates in each month, March 2020 to May 2020. In March 2020, an estimated -1039 (95 % CI: -1528 to -550) fewer patients were dispensed ER intramuscular naltrexone compared to forecasted estimates, followed by -2139 (95 %CI: -2629 to -1650) fewer patients in April 2020, and -2498 (95 % CI: -2987 to -2009) fewer patients in May 2020 (Fig. 2B). The numbers of unique patients dispensed naloxone fluctuated during the study period (mean = 73,573 [SD = 3768]; range 67,294 to 81,323) but were within forecasted estimates for March 2020 to May 2020.
Fig. 2.
A/B/C. Number of Unique Patients Dispensed Buprenorphine, Extended-Release Naltrexone, and Naloxone, United States, January 2019 – May 2020*.
A. Number of Unique Patients Dispensed Buprenorphine
Source: IQVIA Total Patient Tracker
CI = Confidence Interval
*Prescription dispensing amounts that fell above or below the 95 % CI are considered statistically significant
B. Number of Unique Patients Dispensed Extended-release (ER) Intramuscular Naltrexone*
Source: IQVIA Total Patient Tracker
ER = Extended-Release
CI = Confidence Interval
*Prescription dispensing amounts that fell above or below the 95 % CI are considered statistically significant
C. Number of Unique Patients Dispensed Naloxone*
Source: IQVIA Total Patient Tracker
CI = Confidence Interval
*Prescription dispensing amounts that fell above or below the 95 % CI are considered statistically significant
4. Discussion
Emerging research suggests indicators of mental health, substance use, and overdose have worsened during the peak of COVID-19 community mitigation measures in the U.S. (McGinty et al., 2020; Alter and Yeager, 2020). Our findings show the number of unique patients dispensed medications used to treat anxiety, depression, and other chronic diseases increased above forecasted levels in March 2020, potentially reflecting public health recommendations to increase medication supplies on hand (Centers for Disease Control and Prevention, 2020), and were within forecasted levels in April 2020 and May 2020.
The number of unique patients dispensed buprenorphine products indicated for opioid use disorder treatment and naloxone for overdose reversal did not experience a similar increase in March 2020 and were within forecasted estimates for March 2020 to May 2020. In contrast, during March 2020 to May 2020, the number of unique patients dispensed ER intramuscular naltrexone was significantly lower than forecasted estimates each month, suggesting patients may have experienced difficulties obtaining this medication, possibly due to healthcare provider office closures and the need for a healthcare provider to administer the medication.
During the COVID-19 pandemic, a number of steps have been taken at the federal, state, and local levels to facilitate continued access to medications for substance use and mental health treatment, including deeming pharmacies essential services during stay-at-home orders (Cybersecurity and Infrastructure Security Agency, 2020), expanding the use of and increasing reimbursement for telemedicine (Centers for Medicare and Medicaid Services, 2020), allowing the remote prescribing of buprenorphine for new patients without first conducting an in-person physical examination (Drug Enforcement Administration, 2020), and relaxing policies related to take-home medications for opioid use disorder treatment from opioid treatment programs (Substance Abuse and Mental Health Services Administration, 2020b). Although our analysis did not specifically examine the impacts of these policies, our findings suggest that, with the exception of ER intramuscular naltrexone, there was not a significant drop in dispensing of several critical substance use and mental health medications, suggesting that steps taken to date may have facilitated continued access to medications. However, given data suggesting worsening mental health (McGinty et al., 2020) and increases in overdoses (Alter and Yeager, 2020) during the study timeframe, it is also possible that our lack of finding a significant increase in dispensing of buprenorphine for OUD treatment and naloxone for overdose reversal reflects challenges in accessing care and treatment for OUD and harm reduction interventions during the time of community mitigation measures. Future research should assess specific policy changes related to mental health and substance use treatment implemented during COVID-19, including differential impacts on continuity of care for people already receiving treatment prior to COVID-19 and access and ability to receive care among those that sought incident treatment during COVID-19 community mitigation measures, in order to better characterize their intended and potential unintended consequences.
4.1. Limitations
This study is subject to limitations. First, our data do not include information on whether prescriptions were used by patients, the patient-specific clinical indication for the medication, or whether these were new prescriptions or refills. Second, sociodemographic characteristics that could affect treatment access were not included in the data. Third, although our data reflect unique patients receiving medications at given points in time, we were not able to examine individual patients over time. Fourth, naloxone administered by emergency medical services personnel or first responders and naloxone distributed by community-based organizations outside of retail pharmacies are not included in the IQVIA data used in this study. Fifth, our data did not include geographic identifiers; thus we were not able to assess differential impacts on unique patients dispensed medications across geographic areas. Finally, we were not able to assess use of non-medication-based treatments for mental health or substance use conditions or receipt of medications outside of U.S. retail pharmacies, including methadone for opioid use disorder treatment provided through opioid treatment programs as well as extended-release naltrexone provided through clinician offices.
4.2. Conlusions
This study is the first to provide national estimates of access to select addiction, overdose reversal, and mental health medications during the COVID-19 pandemic and time of peak community mitigation measures. Importantly, we found that prescription dispensing for most medications examined fell above or within forecasted levels. However, ongoing concerns about the prolonged effects of the COVID-19 pandemic and related stressors on mental health and substance use (Henry et al., 2020; McGinty et al., 2020; Alter and Yeager, 2020; Volkow, 2020) underscore the importance of implementing innovative strategies to facilitate continued access to treatment, recovery, and harm reduction services.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention/the Agency for Toxic Substances and Disease Registry.
Contributors
Drs. Jones and Guy conceived of the study. Drs. Jones and Guy conducted the data analysis and Dr. Jones drafted the manuscript. Drs. Guy and Board provided critical review and revisions to the manuscript.
Declaration of Competing Interest
The authors report no declarations of interest.
References
- Alter A., Yeager C. 2020. The Consequences of COVID-19 on the Overdose Epidemic: Overdoses Are Increasing.http://odmap.org/Content/docs/news/2020/ODMAP-Report-May-2020.pdf Available at: [Google Scholar]
- American Society of Addiction Medicine . 2020. The ASAM National Practice Guideline for the Treatment of Opioid Use Disorder.https://www.asam.org/docs/default-source/quality-science/npg-jam-supplement.pdf?sfvrsn=a00a52c2_2 Focused Update. Available at: [Google Scholar]
- Centers for Disease Control and Prevention . 2020. Coronavirus Disease (COVID-19): People With Certain Medical Conditions.https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html [updated July 17, 2020]. Available from: [Google Scholar]
- Centers for Medicare and Medicaid Services . 2020. Centers for Medicare & Medicaid Services (CMS) and Substance Abuse and Mental Health Services Administration (SAMHSA): Leveraging Existing Health and Disease Management Programs to Provide Mental Health and Substance Use Disorder Resources During the COVID-19 Public Health Emergency (PHE)https://www.cms.gov/CCIIO/Programs-and-Initiatives/Health-Insurance-Marketplaces/Downloads/Mental-Health-Substance-Use-Disorder-Resources-COVID-19.pdf Available at: [Google Scholar]
- Cybersecurity and Infrastructure Security Agency . 2020. Identifying Critical Infrastructure During COVID-19.https://www.cisa.gov/identifying-critical-infrastructure-during-covid-19 Available at: [Google Scholar]
- Czeisler M.E., Lane R.I., Petrosky E., Wiley J.F., Christensen A., Njai R., et al. Mental health, substance use, and suicidal ideation during the COVID-19 pandemic – United States, June 24-30, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;14(August (32)):1049–1057. doi: 10.15585/mmwr.mm6932a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drug Enforcement Administration . 2020. Use of Telephone Evaluations to Initiate Buprenorphine Prescribing.https://www.deadiversion.usdoj.gov/GDP/(DEA-DC-022)(DEA068)%20DEA%20SAMHSA%20buprenorphine%20telemedicine%20%20(Final)%20+Esign.pdf Available at: [Google Scholar]
- Gostin L.O., Wiley L.F.J.J. Governmental public health powers during the COVID-19 pandemic: stay-at-home orders, business closures, and travel restrictions. JAMA. 2020;(April 2) doi: 10.1001/jama.2020.5460. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- Henry B.F., Mandavia A.D., Paschen-Wolff M.M., Hunt T., Humensky J.L., Wu E., et al. COVID-19, mental health, and opioid use disorder: old and new public health crises intertwine. Psychol. Trauma. 2020;(June 18) doi: 10.1037/tra0000660. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makridakis S., Wheelwright S., Hyndman R. 3rd ed. Wiley; Hoboken, NJ: 1998. Forecasting, Methods and Applications. [Google Scholar]
- McGinty E.E., Presskreischer R., Han H., Barry C.L. Psychological distress and loneliness reported by US adults in 2018 and April 2020. JAMA. 2020;324(1):93–94. doi: 10.1001/jama.2020.9740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Microsoft . 2015. Using Exponential Smoothing for Forecasting in Excel.https://support.microsoft.com/en-us/office/forecast-ets-function-15389b8b-677e-4fbd-bd95-21d464333f41 Available at: [Google Scholar]
- Substance Abuse and Mental Health Services Administration . Publication No. PEP20-02-01-006; Rockville, MD: 2020. Medications for Opioid Use Disorder. Treatment Improvement Protocol (TIP) Series 63. [Google Scholar]
- Substance Abuse and Mental Health Services Administration . 2020. Opioid Treatment Program (OTP): Guidance.https://www.samhsa.gov/sites/default/files/otp-guidance-20200316.pdf (March 16, 2020). Available at: [Google Scholar]
- Volkow N.D. Collision of the COVID-19 and addiction epidemics. Ann. Int. Med. 2020;173(July (1)):61–62. doi: 10.7326/M20-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]