Abstract
Objective
While many seroprevalence studies of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have been performed, few are demographically representative. This investigation focused on defining the nature and frequency of symptomatic and asymptomatic SARS-CoV-2 infection in a representative, cross-sectional sample of communities in Louisiana, USA.
Methods
A sample of 4778 adults from New Orleans and Baton Rouge, Louisiana were given a survey of symptoms and co-morbidities, nasopharyngeal swab to test for active infection (PCR), and blood draw to test for past infection (IgG). Odds ratios, cluster analysis, quantification of virus and antibody, and linear modelling were used to understand whether certain symptoms were associated with a positive test, how symptoms grouped together, whether virus or antibody varied by symptom status, and whether being symptomatic was different across the age span.
Results
Reported anosmia/ageusia was strongly associated with a positive test; 40.6% (93/229) tested positive versus 4.8% (218/4549) positivity in those who did not report anosmia/ageusia (OR 13.6, 95% CI 10.1–18.3). Of the people who tested positive, 47.3% (147/311) were completely asymptomatic. Symptom presentation clustered into three groups; low/no symptoms (0.4 ± 0.9, mean ± SD), highly symptomatic (7.5 ± 1.9) or moderately symptomatic (4.0 ± 1.5). Quantity of virus was lower in the asymptomatic versus symptomatic group (cycle number 23.3 ± 8.3 versus 17.3 ± 9.0; p < 0.001). Modelling the probability of symptoms showed changes with age; the highest probability of reporting symptoms was 64.6% (95% CI 50.4–76.5) at age 29 years, which decreased to a probability of 49.3% (95% CI 36.6–62.0) at age 60 years and only 25.1% (95% CI 5.0–68.1) at age 80 years.
Conclusion
Anosmia/ageusia can be used to differentiate SARS-CoV-2 infection from other illnesses, and, given the high ratio of asymptomatic individuals, contact tracing should include those without symptoms. Regular testing in congregant settings of those over age 60 years may help mitigate asymptomatic spread.
Keywords: Ageusia, Anosmia, Asymptomatic infection, Severe acute respiratory syndrome coronavirus 2 prevalence, Symptom incidence
Graphical abstract
Introduction
Prevalence studies have been conducted worldwide to understand the true spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). There are several strategies for surveillance, but the quickest way to survey the population is to obtain blood remnants from laboratories or blood banks that collect a high volume of samples, and test for antibodies [[1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11]]. This method offers a quick turnaround at a low cost with minimal data, usually sex, age and region of collection. However, this sample skews heavily toward (a) people who regularly interact with health care or (b) those who are healthy enough to give blood. This method gives no information regarding symptoms or demographics, which may be important to understand how variable the presentation and spread of SARS-CoV-2 have been.
Prospective enrolment of individuals adds complexity. Although many studies attempt to recruit a representative sample, most do not end up analysing one [[12], [13], [14], [15], [16], [17], [18]]. Those that do, involve an extensive effort; two Italian studies tested nearly the entire population of Vo [19] and Lombardy [20], and a nationwide Spanish study tested over 60 000 residents [21]. A study in Atlanta, Georgia was able to closely represent the population but had to send staff door-to-door to enrol based on US Census blocks [22]. Other European and Brazilian studies used representative households to similarly approach participants, but response rates even with this level of effort can still be low [[23], [24], [25], [26], [27]].
For this study, we developed a novel recruitment system with Public Democracy (Arlington, VA, USA) to ensure a representative sample through a stratified random selection process. We administered a survey of symptoms and co-morbidities, collected a nasopharyngeal (NP) swab to assess active viral infection, and collected blood to assess IgG. We distinguished significant racial disparities and ZIP code-level variation in spread of the virus [28,29]. The objective of this report is to tabulate the nature and frequency of symptoms in community presentation of SARS-CoV-2 infection in Louisiana using odds ratios to estimate odds of positivity by symptom, cluster analysis to see how symptoms tend to group together, quantification of virus by symptom status and disease stage to determine differences, and a logistic regression model to understand how symptoms are reported by age. We hypothesize that similarly to emergency department and hospital presentation [30], community presentation of SARS-CoV-2 infection will be heterogeneous.
Materials and methods
This study was approved by the Ochsner Clinic Foundation Institutional Review Board #2020.163. To be eligible, participants had to be adult residents of the greater New Orleans or Baton Rouge areas and willing to undergo both an NP swab and blood draw. Participants could not have previously tested positive for the virus so that prevalence projections from the study [28,29] could be added to state-reported numbers to estimate true spread.
A system developed by Public Democracy was used to recruit a sample that met a priori goals for a demographically representative sample of Greater New Orleans (Orleans and Jefferson parishes) and Greater Baton Rouge (Ascension, East Baton Rouge, West Baton Rouge and Livingston parishes). More than 50 characteristics, including social determinants of health and US Census population data, were used to establish a representative pool of potential participants, from which a random subset was targeted through dynamic, cross-device digital advertisements. Those who affirmed their interest in participating were stratified based on Census designations to account for different response rates between groups and were randomly issued text invitations to enrol. Details are summarized in the Supplementary material (Fig. S1, Appendix S1). Those without access to the internet were able to call a hotline to register. Invitations were adjusted daily based on enrolment (e.g. over-invite groups that had low response rates). Participants were offered a free rideshare service.
Testing was completed over 6 days (9 May and 11–15 May 2020) in New Orleans and 2 weeks (15–31 July 2020) in Baton Rouge (for community testing context, see ldh.la.gov/Coronavirus/). Participants completed survey questions, a blood draw and NP swab. All study materials were in English, Spanish and Vietnamese, and translators were onsite or available by phone. Specimens were sent to the clinical laboratory at Ochsner Health for testing.
Abbott instrumentation was used for both nucleic acid (NP swabs) and antibody (serum) tests for SARS-CoV-2 infection (Abbott Laboratories, Abbott Park, IL, USA). Real-time reverse transcription PCR tests (NP swabs) were performed on the Abbott m2000 RealTime system (100 copies/mL limit of detection), and cycle number (CN) was collected by the laboratory. Qualitative IgG blood tests were performed on the ARCHITECT i2000SR (99.63% specificity and 100% sensitivity, nucleocapsid target), and sample to calibrator ratio (S/C) was collected by the laboratory. Both tests are US Food and Drug Administration-Emergency Use Authorization approved, and the antibody test meets the criteria described by the CDC to yield high positive predictive value [6]. No cross-reaction with other coronaviruses and common respiratory viruses is reported.
The number reporting symptoms and co-morbidities in the total sample and those who tested positive are tabulated in Table 1 . Prevalence for each factor was calculated as the number with a positive test divided by the total reporting each factor. To determine which symptoms and co-morbidities were associated with a positive test, we carried out a Cochran–Mantel–Haenszel analysis and estimated common odds ratios across cities. Validity of the combined city approach was confirmed by negligible observed city-level variation in multilevel models with a random city effect. City-specific and common odds ratios from the Cochran–Mantel–Haenszel analysis are displayed with 95% CI in the Supplementary material (Fig. S3). Corresponding p values from homogeneity tests are presented for each. Virus and IgG quantity was averaged by probable disease stage (PCR+/IgG–, contagious; PCR+/IgG+, early recovery; and PCR–/IgG+, recovered) and symptom status within that disease stage to determine whether the amount of virus or antibody was different among these groups. Binary (1/0) symptom data for all patients were hierarchically clustered and plotted using the Ward method and Gower distance in R (hclust, dendextend packages) to determine whether distinct, patient-reported symptom presentations were present. Demographic and co-morbidity data were compared by symptom cluster using exact confidence intervals and p values from the exact χ2 homogeneity test for categorical variables, and analysis of variance with unequal variances or a Kruskal–Wallis scores test for continuous or ordinal variables. To determine whether symptomatic or asymptomatic presentation was different across the age span, modelling of the probability of reporting symptoms by age was performed using a logistic regression with cubic splines and four nodes. Statistical analyses were carried out in SAS STAT 14.2 (SAS, Cary, NC, USA).
Table 1.
Frequency of symptoms and co-morbidities in the total sample and in those testing positive for current or past infection with severe acute respiratory syndrome coronavirus 2 in Louisiana
| Number reporting |
Any positive test |
Prevalence by factora |
|||
|---|---|---|---|---|---|
|
n = 4778 |
n = 311 (6.5%) |
||||
| n | % | n | % | % | |
| New Orleans (May 2020) | 2640 | 55.3% | 183 | 58.8% | 6.9% |
| Baton Rouge (July 2020) | 2138 | 44.7% | 128 | 41.2% | 6.0% |
| Any symptoms | 1223 | 25.6% | 164 | 52.7% | 13.4% |
| Fever | 471 | 9.9% | 84 | 27.0% | 17.8% |
| Fatigue | 658 | 13.8% | 95 | 30.5% | 14.4% |
| Dry cough | 568 | 11.9% | 82 | 26.4% | 14.4% |
| Myalgia | 519 | 10.9% | 83 | 26.7% | 16.0% |
| Nasal congestion | 456 | 9.5% | 68 | 21.9% | 14.9% |
| Runny nose | 327 | 6.8% | 45 | 14.5% | 13.8% |
| Sore throat | 488 | 10.2% | 60 | 19.3% | 12.3% |
| Diarrhoea | 245 | 5.1% | 33 | 10.6% | 13.5% |
| Anosmia/dysgeusia | 229 | 4.8% | 93 | 29.9% | 40.6% |
| Chills | 337 | 7.1% | 54 | 17.4% | 16.0% |
| Headache | 522 | 10.9% | 74 | 23.8% | 14.2% |
| Other | 194 | 4.1% | 16 | 5.1% | 8.2% |
| No symptoms | 3555 | 74.4% | 147 | 47.3% | 4.1% |
| Any co-morbidities | 2167 | 45.4% | 139 | 44.7% | 6.4% |
| Diabetes | 444 | 9.3% | 30 | 9.6% | 6.8% |
| High blood pressure | 1367 | 28.6% | 90 | 28.9% | 6.6% |
| Elevated cholesterol | 797 | 16.7% | 53 | 17.0% | 6.6% |
| Heart disease | 170 | 3.6% | 12 | 3.9% | 7.1% |
| Kidney disease | 51 | 1.1% | 6 | 1.9% | 11.8% |
| COPD | 50 | 1.0% | 3 | 1.0% | 6.0% |
| History of cancer | 318 | 6.7% | 13 | 4.2% | 4.1% |
| HIV | 23 | 0.5% | 0 | 0.0% | 0.0% |
| Organ Tx | 19 | 0.4% | 1 | 0.3% | 5.3% |
| Weakened immune system | 155 | 3.2% | 9 | 2.9% | 5.8% |
| Other | 216 | 4.5% | 9 | 2.9% | 4.2% |
| No co-morbidities | 2611 | 54.6% | 172 | 55.3% | 6.6% |
Abbreviations: COPD, chronic obstructive pulmonary disease; HIV, human immunodeficiency virus; Tx, transplant.
Prevalence by factor is calculated by dividing the number of positives over the total number in the sample reporting that factor.
Results
A total of 4778 individuals were analysed: 2640 from New Orleans and 2138 from Baton Rouge. The sample population was 63.5% (3036/4778) female, 65.3% (3121/4778) white and 28.2% (1349/4778) black, with an average age of 49.8 years (SD 15.1) and average household size of 2.7 people. Three hundred and eleven participants tested positive on one or both PCR and IgG tests (6.5%, 311/4778), and details of prevalence are published elsewhere [28,29].
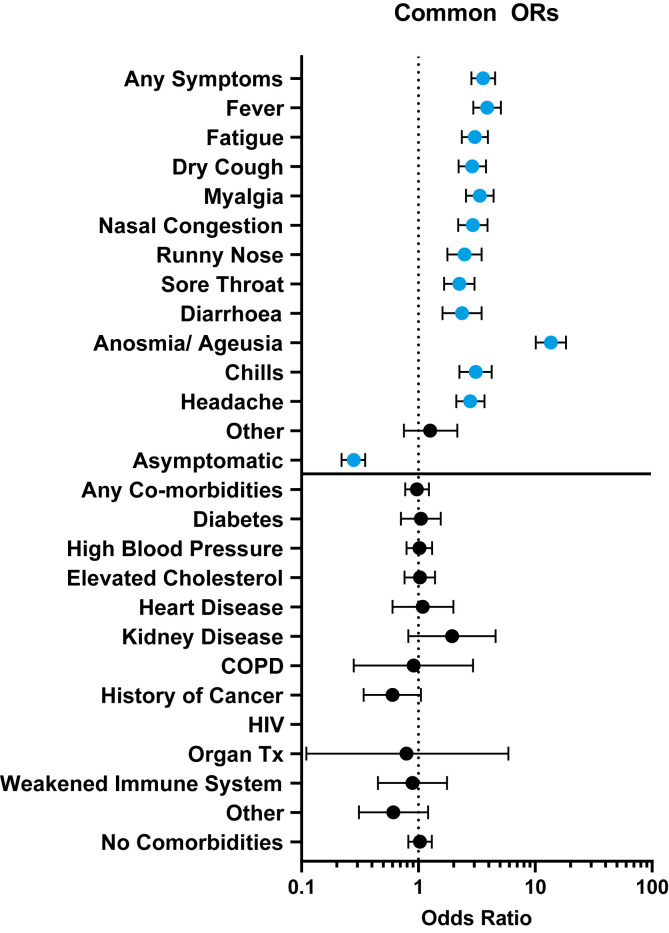
Symptoms and comorbidities captured in the survey are listed in Table 1 and the Supplementary material (Fig. S2), and the common odds ratios with 95% CI are reported in Fig. 1 . Overall, 25.6% (1223/4778) of participants reported symptoms, and over half of all positive participants were symptomatic (52.7%, 164/311) Of those who were symptomatic, prevalence was 13.4% (164/1223). Anosmia/ageusia was reported by only 4.8% (229/4778) of the sample but was present in 29.9% (93/311) of positive cases with a prevalence of 40.6% (93/229), making it a standalone symptom with 13.6 times higher odds of testing positive versus those who did not report anosmia/ageusia. Other symptom ORs ranged from 2.2 (95% CI 1.66–3.02) for sore throat to 3.9 (95% CI 2.95–5.09) for fever. ORs were homogeneous between cities for most factors, except for anosmia (p 0.0290), co-morbidity group Other (p 0.0193) and No Co-morbidities (p 0.0232) (see Supplementary material, Fig. S3). The overall proportion of asymptomatic subjects in the sample was 74.4% (3555/4778) and of those who tested positive, 47.3% (147/311) were without symptoms. Prevalence among asymptomatic participants was lower than among symptomatic participants (4.1%, 147/3555 versus 13.4%, 164/1223), and the odds of testing positive were lower than in those with symptoms (OR 0.28, 95% CI 0.22–0.35). No co-morbidity was associated with higher or lower odds of testing positive.
Fig. 1.
Odds ratios of SARS-CoV-2 infections by symptoms and co-morbidities in New Orleans and Baton Rouge, LA. Odds ratios from Cochran–Mantel–Haenszel analysis are shown with 95% CI. Symptoms of any kind increased the odds of testing positive, but co-morbidities were not associated with infection. No one reporting HIV or history of tuberculosis tested positive. Abbreviations: COPD, chronic obstructive pulmonary disease; HIV, human immunodeficiency viruses; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TX, transplant.
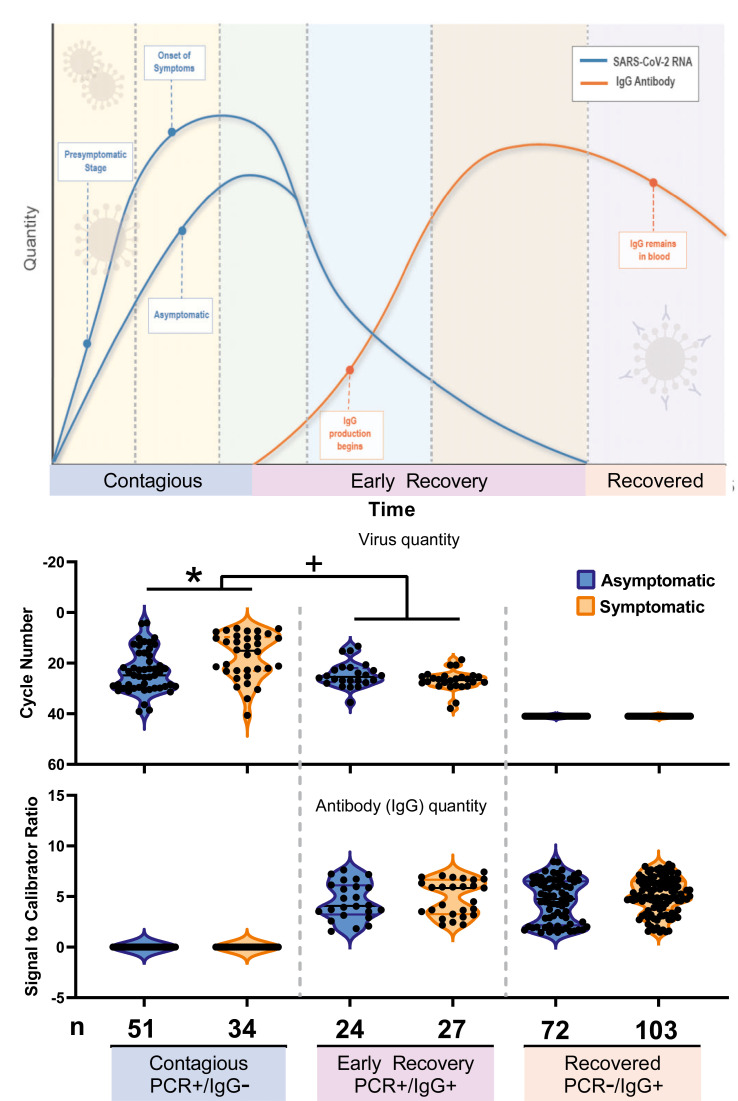
Operating under the assumption that PCR + alone indicates the most contagious, acute infection, PCR+/IgG + indicates early recovery, and PCR–/IgG + indicates convalescence, the number of participants at each stage and the proportion who were asymptomatic are listed under the schematic in Fig. 2 . The percentages who tested positive but did not have symptoms were 60.0% (51/85) when PCR+/IgG–, 47.1% (24/51) when PCR+/IgG+ and 41.1% (72/175) when PCR–/IgG+.
Fig. 2.
Quantity of virus and antibody by disease stage and symptom status. The top panel is a schematic of disease progression. Cycle number (CN) and signal-to-calibrator ratio (S/C) were averaged by disease stage to assess differences in CN or S/C by symptom status, using a Wilcoxon–Mann–Whitney test. The symptomatic group had lower mean CN versus the asymptomatic group during the contagious phase of disease. Antibody levels did not differ by symptom or disease stage. CN is inversely related to viral quantity, so the axis is reversed for clarity. ∗p < 0.001, + p 0.004.
CN and S/C were used to approximate the quantity of virus and IgG, respectively, in each phase of disease by symptom status (Fig. 2). A lower quantity of virus was present in asymptomatic versus symptomatic individuals in the contagious phase of disease (CN 23.3 ± 8.3 versus 17.3 ± 9.0, mean ± SD) (p < 0.001). In the early recovery stage, CN was similar by symptom status (24.6 ± 5.0 asymptomatic versus 26.9 ± 4.0 symptomatic). IgG was not significantly different by symptom status or across early to late recovery.
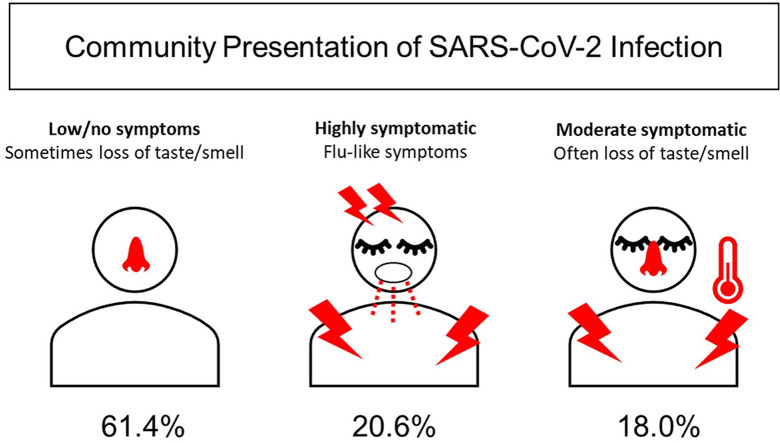
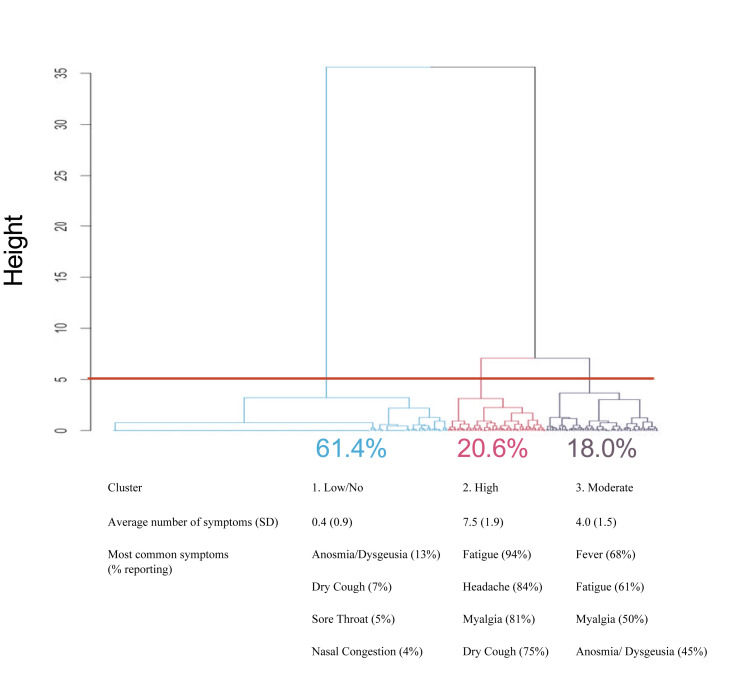
A symptom cluster analysis was performed to explore patterns in patient-reported symptom presentation (Fig. 3 ). The two primary branches indicate clear separation of apparent symptomatic (38.6%, 120/311) and asymptomatic (61.4%, 191/311) groups. A cut at height = 5 separates the symptomatic branch into two clusters (cluster 2 20.6%, 64/311; cluster 3 18.0%, 56/311), varying most clearly in number of reported symptoms. These clusters were not different by demographics or other variables with the following exceptions: a higher proportion of Orleans Parish in the high-symptom cluster (cluster 2) versus other clusters, a higher proportion of Hispanic individuals in the moderate-symptom cluster (cluster 3) versus the high-symptom cluster, larger average household size in the moderate-symptom cluster versus other clusters, and a higher proportion of people who thought they had coronavirus disease 2019 (COVID-19) in the two symptomatic clusters (2 and 3) versus the low-symptom cluster (see Supplementary material, Table S1). The supplemental table also shows the frequency of each symptom by cluster.
Fig. 3.
Cluster analysis of symptom presentation in the community. Symptom data were hierarchically clustered and plotted using the Ward method with the hclust and dendextend packages in R to explore patterns in patient-reported symptom presentation. The above plot of patient-reported symptoms of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection with cut at height = 5 generated three symptomatic clusters. Clusters varied in mean (SD) number of symptoms as well as specific symptom prevalence. The top four most frequently reported symptoms are listed. Frequency of other symptoms in each cluster are listed in the Supplementary material (Table S1).
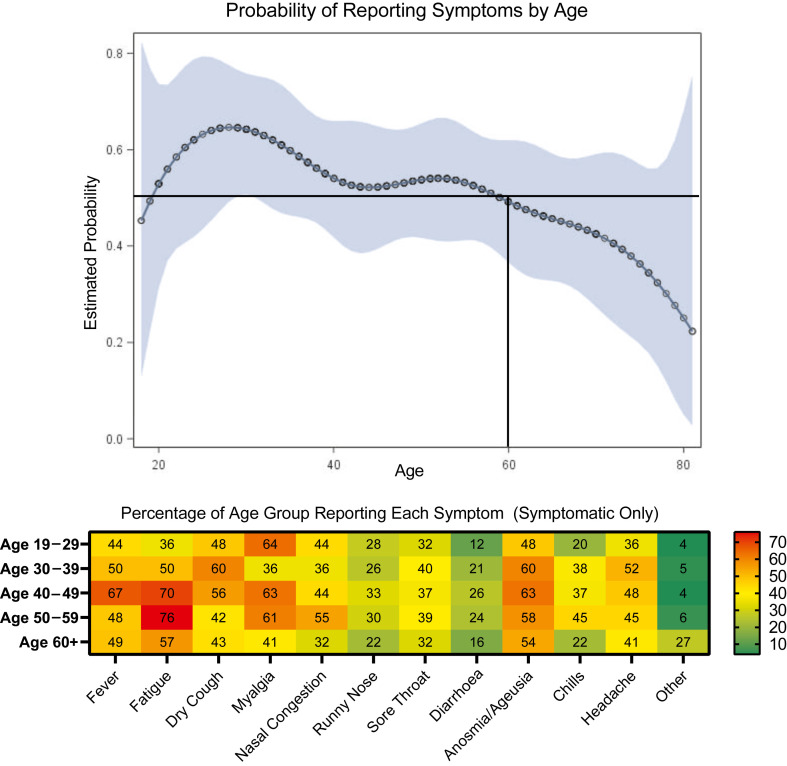
Finally, we generated a model of the probability of reporting symptoms by age (Fig. 4 ) that indicates variable probability of reporting symptoms with ages 20–59 years having >50% probability of reporting symptoms and ages 60+ having <50% probability of reporting symptoms. The highest probability of reporting symptoms was 64.6% (50.4%–76.5%) at age 29 years, which decreased to a probability of 49.3% (36.6%–62.0%) at age 60 and only 25.1% (5.0%–68.1%) at age 80. Anosmia/ageusia was one of the top two reported symptoms for those aged over 60 years.
Fig. 4.
Reported symptoms of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection by age. (Top) Probability and confidence band of reporting symptoms by age for individuals testing positive for active or past SARS-CoV-2 infection in New Orleans and Baton Rouge, Louisiana. The probability of reporting symptoms by age was modelled using a logistic regression with cubic splines and four nodes. Older individuals (>60 years) had decreasing probability of reporting symptoms as age increased. The highest probability of reporting symptoms was 64.6% (50.4%–76.5%) at age 29 years which decreased to a probability of 49.3% (36.6%–62.0%) at age 60 and only 25.1% (5.0%–68.1%) at age 80. (Bottom) Percentage of symptomatic individuals reporting each symptom by age group with an overlaid heatmap to indicate the relative detectability of each symptom in each age group.
Discussion
By using representative sampling, a survey of symptoms, and antibody testing with paired PCR, we were able to determine the prevalence of symptomatic and asymptomatic SARS-CoV-2 infection infections in the community. This study found that 47.3% (147/311) of infections were asymptomatic, 29.9% (93/311) of positive cases reported anosmia/ageusia (Table 1), and those who reported anosmia/ageusia had higher odds of testing positive versus any other symptomatic group (Fig. 1). A Spanish study conducted nationwide found that 33.8% of infections were asymptomatic and 43% of all positive people reported losing their sense of taste and smell [21]. In Vo, Italy and Sao Paulo City, Brazil, 42.5% [19] and 45.3% [26] reported no symptoms, respectively. Atlanta, USA has similar population characteristics to New Orleans, USA and found 50% asymptomatic infections and 28.2% of positive people reporting anosmia/ageusia [22]. A study of individuals in London, UK with a loss of smell and/or taste found that antibody prevalence was even higher (78%) if testing was solely focused on anosmia/ageusia, which underscores the specificity of this symptom for SARS-CoV-2 infection despite methodological differences between these studies [31]. Our results align with other major studies and suggest that across geographies, a large proportion of infections occur with few or no symptoms.
We observed proxy measures of virus and antibody quantity by symptom status and disease stage and found that those with symptoms in the contagious phase of infection had a greater quantity of virus. Most prevalence studies exclusively used antibody testing, but in Vo, Italy, PCR testing of nearly the whole population revealed that the quantity of virus in symptomatic and asymptomatic groups was similar, but did not separate early-stage (PCR+/IgG–) and late-stage (PCR+/IgG+) infections [19]. In our study, the group with late-stage infections (PCR+/IgG+) did not have differences in viral or antibody quantity by symptom status, suggesting that after the initial contagious phase, viral load decreases as antibody increases. A longitudinal study of a small cohort of PCR + individuals found that asymptomatic individuals shed virus for longer than symptomatic individuals, and asymptomatic disease caused lower IgG levels, which dropped off or disappeared more robustly than in symptomatic disease [32]. There is a chance that in our cross-sectional study, the people with the lowest viral quantity in the asymptomatic group were presymptomatic and would have gone on to experience symptoms as the virus replicated. Similarly, although we found no differences in IgG quantity by symptom status or disease stage, it is likely that longitudinal sampling would have found IgG changes within an individual or within groups over time.
Presentation of SARS-CoV-2 infection fits into three distinct clusters in our sample (Fig. 3), which was reflective of community appearance of the virus and not severe illness with hospitalization. We found that a large proportion of positive cases in the community were asymptomatic or low-symptomatic individuals, with anosmia/ageusia still detectable in 13% (25/191) of this cohort. In cluster 2, fatigue, headaches and myalgia were nearly ubiquitous. Unfortunately, these symptoms are not specific to COVID-19, making it difficult to identify someone in this group as uniquely COVID-positive. The third cluster commonly reported fatigue and myalgia, but anosmia/ageusia and fever were also highly present. Nearly half of this cluster could have been prioritized for SARS-CoV-2 testing because of anosmia/ageusia, or perhaps in lieu of other testing (e.g. influenza) in resource-limited settings.
Finally, the non-linear relationship between age and probability of reporting symptoms was surprising. Anecdotally, younger individuals were thought to be asymptomatic more often, but our model demonstrates the opposite. Individuals who were 60 years of age and older had a lower probability of reporting symptoms, which could be a public health concern in nursing homes and assisted living facilities, where the disease may silently spread. In those who were symptomatic, anosmia/ageusia was highly detectable.
This study relies on self-report data and there are many unknowns about biological factors that could impact antibody development, which could impact our results. This report does not include those who were critically ill, living in nursing homes, or children and excludes symptom presentation in emergency departments or hospitals. There is a chance that someone reporting symptoms from a non-COVID-19 illness actually had an asymptomatic infection, received an IgG + result and was incorrectly classified as symptomatic, or that someone had a low symptomatic response and forgot, causing them to report no symptoms. Additionally, there is a chance that those in the initial stages of disease were presymptomatic and went on to have symptoms later.
In conclusion, anosmia/ageusia seems to be a hallmark of COVID-19 even in the cluster of symptoms that represents low or no symptoms. Given the high likelihood of someone with anosmia/ageusia testing positive, people experiencing this symptom should be encouraged to quarantine and/or receive a test, and screening for this symptom should be implemented for those at high risk. Given the high proportion of asymptomatic infections, long-term outcomes should be studied to determine if there are associated lasting risks. Contact tracing should include testing people who do not have symptoms, especially those over age 60, as part of a public health strategy.
Transparency declaration
The authors report no conflicts of interest. This work was supported by ReNOLA, which funded the recruitment effort by Public Democracy, and by Ochsner Health, which funded the rest of the New Orleans portion of the study. ReNOLA was not involved in the study design, execution, or analysis. Except for Eric Sapp, all authors are employed by Ochsner Health. Therefore, Ochsner Health was involved in the design, execution, analysis and writing of this paper. The Baton Rouge study was funded by the Baton Rouge Area Foundation, Louisiana COVID-19 Health Equity Task Force, and The Humana Foundation, with additional support from The Blue Cross and Blue Shield of Louisiana Foundation, Healthy Blue, the Huey and Angelina Wilson Foundation, and the Irene W. and C.B. Pennington Foundation. These funders were not involved in study design, execution, or analysis. The authors have not been paid to write this article beyond regular employment contracts. Amy K Feehan had full access to the data and takes final responsibility for the decision to submit for publication.
Acknowledgements
The authors thank all study participants. We would like to especially thank the laboratories at the Ochsner Medical Center Jefferson Highway Campus for expedient testing and keeping track of research samples; Dan Nichols, marketing technologist at Public Democracy; George Hutter, Benjamin Cappiello and ReNOLA for their strategic support; Sarah Roberts, Gina Mmahat, Susan Green, Charlene Ho, Lena Hooper, Patty Kline and Candace Melancon for clinical site management; Samantha Bright, Lyndsey Buckner-Baiamonte, Johanna Veal, Ansley Hammons and Ashley LaRoche for research site management; Emily Arata and Christy Reeves for liaising with public leaders; and countless research coordinators, clinical staff, marketing personnel, medical students and Epic and IT staff for making site testing possible. We would like to thank Barbara Siede for designing the graphic in Fig. 2. The authors thank Kathleen McFadden for her thorough editing. We would like to thank the Ochsner Language Services Department for helping to increase inclusivity. We would also like to acknowledge the New Orleans Mayor's Office, City of New Orleans Office of Public Health, the New Orleans City Council, the Jefferson Parish President and Parish Council, the East Baton Rouge Mayor-President Sharon Weston Broome, and Pennington Biomedical Research Center for their collaboration and support of this project.
Editor: L Leibovici
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2020.12.029.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Flow diagram of recruitment, enrolment and analysis.
Relevant survey questions from severe acute respiratory virus 2 prevalence testing in New Orleans and Baton Rouge Louisiana, 2020.
Cochran–Mantel–Haenszel analysis and odds ratios by city with p values estimating homogeneity.
Demographic and comorbidity data comparison.
References
- 1.Amorim Filho L., Szwarcwald C.L., Mateos S.O.G., Leon A., Medronho R.A., Veloso V.G. Seroprevalence of anti-SARS-CoV-2 among blood donors in Rio de Janeiro, Brazil. Rev Saude Publica. 2020;54:69. doi: 10.11606/s1518-8787.2020054002643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Emmenegger M., De Cecco E., Lamparter D., Jacquat R.P.B., Ebner D., Schneider M.M. Early plateau of SARS-CoV-2 seroprevalence identified by tripartite immunoassay in a large population. medRxiv. 2020:2020. 05.31.20118554. [Google Scholar]
- 3.Erikstrup C., Hother C.E., Pedersen O.B.V., Mølbak K., Skov R.L., Holm D.K. Estimation of SARS-CoV-2 infection fatality rate by real-time antibody screening of blood donors. medRxiv. 2020:2020. doi: 10.1093/cid/ciaa849. 04.24.20075291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fiore J.R., Centra M., de Carlo A., Granato M., Rosa A., de Feo L. Far away from herd immunity to SARS-CoV-2: results from a survey in healthy blood donors in South Eastern Italy. medRxiv. 2020:2020. doi: 10.1002/jmv.26425. 06.17.20133678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ng D.L., Goldgof G.M., Shy B.R., Levine A.G., Balcerek J., Bapat S.P. SARS-CoV-2 seroprevalence and neutralizing activity in donor and patient blood. Nat Commun. 2020;11:4698. doi: 10.1038/s41467-020-18468-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valenti L., Bergna A., Pelusi S., Facciotti F., Lai A., Tarkowski M. SARS-CoV-2 seroprevalence trends in healthy blood donors during the COVID-19 Milan outbreak. medRxiv. 2020:2020. doi: 10.2450/2021.0324-20. 05.11.20098442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sughayer M.A., Mansour A., Al Nuirat A., Souan L., Ghanem M., Siag M. Covid-19 Seroprevalence rate in healthy blood donors from a community under strict lockdown measures. medRxiv. 2020:2020. 06.06.20123919. [Google Scholar]
- 8.Fischer B., Knabbe C., Vollmer T. SARS-CoV-2 IgG seroprevalence in blood donors located in three different federal states, Germany, March to June 2020. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.28.2001285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doi A., Iwata K., Kuroda H., Hasuike T., Nasu S., Kanda A. Estimation of seroprevalence of novel coronavirus disease (COVID-19) using preserved serum at an outpatient setting in Kobe, Japan: a cross-sectional study. medRxiv. 2020:2020. doi: 10.1016/j.cegh.2021.100747. 04.26.20079822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stadlbauer D., Tan J., Jiang K., Hernandez M.M., Fabre S., Amanat F. Repeated cross-sectional sero-monitoring of SARS-CoV-2 in New York City. Nature. 2020 doi: 10.1038/s41586-020-2912-6. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 11.Havers F.P., Reed C., Lim T., Montgomery J.M., Klena J.D., Hall A.J. Seroprevalence of antibodies to SARS-CoV-2 in 10 sites in the United States, march 23–may 12, 2020. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.4130. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 12.Hallal P.C., Hartwig F.P., Horta B.L., Silveira M.F., Struchiner C.J., Vidaletti L.P. SARS-CoV-2 antibody prevalence in Brazil: results from two successive nationwide serological household surveys. Lancet Glob Health. 2020;8:e1390–e1398. doi: 10.1016/S2214-109X(20)30387-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shakiba M., Hashemi Nazari S.S., Mehrabian F., Rezvani S.M., Ghasempour Z., Heidarzadeh A. Seroprevalence of COVID-19 virus infection in Guilan province, Iran. medRxiv. 2020:2020. doi: 10.3201/eid2702.201960. 04.26.20079244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Streeck H., Schulte B., Kuemmerer B., Richter E., Hoeller T., Fuhrmann C. Infection fatality rate of SARS-CoV-2 infection in a German community with a super-spreading event. medRxiv. 2020:2020. doi: 10.1038/s41467-020-19509-y. 05.04.20090076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sood N., Simon P., Ebner P., Eichner D., Reynolds J., Bendavid E. Seroprevalence of SARS-CoV-2-specific antibodies among adults in Los Angeles county, California. JAMA. 2020 doi: 10.1001/jama.2020.8279. on April 10–11, 2020. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gomes C.C., Cerutti C., Zandonade E., Maciel E.L.N., de Alencar F.E.C., Almada G.L. A population-based study of the prevalence of COVID-19 infection in Espirito Santo, Brazil: methodology and results of the first stage. medRxiv. 2020:2020. 06.13.20130559. [Google Scholar]
- 17.Bryan A., Pepper G., Wener M.H., Fink S.L., Morishima C., Chaudhary A. Performance characteristics of the Abbott architect SARS-CoV-2 IgG assay and seroprevalence in Boise, Idaho. J Clin Microbiol. 2020;58 doi: 10.1128/JCM.00941-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bendavid E., Mulaney B., Sood N., Shah S., Ling E., Bromley-Dulfano R. COVID-19 antibody seroprevalence in Santa Clara county, California. medRxiv. 2020:2020. doi: 10.1093/ije/dyab010. 04.14.20062463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lavezzo E., Franchin E., Ciavarella C., Cuomo-Dannenburg G., Barzon L., Del Vecchio C. Suppression of a SARS-CoV-2 outbreak in the Italian municipality of Vo. Nature. 2020 doi: 10.1038/s41586-020-2488-1. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 20.Pagani G., Conti F., Giacomelli A., Bernacchia D., Rondanin R., Prina A. Seroprevalence of SARS-CoV-2 significantly varies with age: preliminary results from a mass population screening. J Infect. 2020 doi: 10.1016/j.jinf.2020.09.021. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pollan M., Perez-Gomez B., Pastor-Barriuso R., Oteo J., Hernan M.A., Perez-Olmeda M. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet. 2020 doi: 10.1016/S0140-6736(20)31483-5. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Biggs H.M., Harris J.B., Breakwell L., Dahlgren F.S., Abedi G.R., Szablewski C.M. Estimated community seroprevalence of SARS-CoV-2 antibodies – two Georgia counties, April 28–May 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:965–970. doi: 10.15585/mmwr.mm6929e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perez-Saez J., Lauer S.A., Kaiser L., Regard S., Delaporte E., Guessous I. Serology-informed estimates of SARS-CoV-2 infection fatality risk in Geneva, Switzerland. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30584-3. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silveira M.F., Barros A.J.D., Horta B.L., Pellanda L.C., Victora G.D., Dellagostin O.A. Population-based surveys of antibodies against SARS-CoV-2 in Southern Brazil. Nat Med. 2020;26:1196–1199. doi: 10.1038/s41591-020-0992-3. [DOI] [PubMed] [Google Scholar]
- 25.Stringhini S., Wisniak A., Piumatti G., Azman A.S., Lauer S.A., Baysson H. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Geneva, Switzerland (SEROCoV-POP): a population-based study. Lancet. 2020;396:313–319. doi: 10.1016/S0140-6736(20)31304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tess B.H., Granato C.F.H., Alves M.C.G.P., Pintao M.C., Rizzatti E., Nunes M.C. SARS-CoV-2 seroprevalence in the municipality of Sao Paulo, Brazil, ten weeks after the first reported case. medRxiv. 2020:2020. 6.29.20142331. [Google Scholar]
- 27.Maver Vodičar P., Oštrbenk Valenčak A., Zupan B., Avšič Županc T., Kurdija S., Korva M. Low prevalence of active COVID-19 in Slovenia: a nationwide population study of a probability-based sample. Clin Microbiol Infect. 2020;26:1514–1519. doi: 10.1016/j.cmi.2020.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feehan A.K., Fort D., Garcia-Diaz J., Price-Haywood E., Velasco C., Sapp E. Seroprevalence of SARS-CoV-2 and infection fatality ratio, Orleans and Jefferson Parishes, Louisiana, USA, May 2020. Emerg Infect Dis. 2020;26 doi: 10.3201/eid2611.203029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feehan A.K., Velasco C., Fort D., Burton J.H., Price-Haywood E.G., Katzmarzyk P.T. Racial and workplace disparities in seroprevalence of SARS-CoV-2, Baton Rouge, Louisiana, USA. Emerg Infect Dis. 2020;27 doi: 10.3201/eid2701.203808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borges do Nascimento I.J., von Groote T.C., O'Mathuna D.P., Abdulazeem H.M., Henderson C., Jayarajah U. Clinical, laboratory and radiological characteristics and outcomes of novel coronavirus (SARS-CoV-2) infection in humans: a systematic review and series of meta-analyses. PLoS One. 2020;15 doi: 10.1371/journal.pone.0239235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Makaronidis J., Mok J., Balogun N., Magee C.G., Omar R.Z., Carnemolla A. Seroprevalence of SARS-CoV-2 antibodies in people with an acute loss in their sense of smell and/or taste in a community-based population in London, UK: an observational cohort study. PLoS Med. 2020;17 doi: 10.1371/journal.pmed.1003358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Long Q.X., Tang X.J., Shi Q.L., Li Q., Deng H.J., Yuan J. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;26:1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Flow diagram of recruitment, enrolment and analysis.
Relevant survey questions from severe acute respiratory virus 2 prevalence testing in New Orleans and Baton Rouge Louisiana, 2020.
Cochran–Mantel–Haenszel analysis and odds ratios by city with p values estimating homogeneity.
Demographic and comorbidity data comparison.