Abstract
PURPOSE:
The purpose of the study was to assess the treatment protocol of topical 2.5% Povidone-Iodine (PovI) and 0.1% fluorometholone (FluM) for Adenoviral Epidemic keratoconjunctivitis (EKC) in reducing the duration and severity of the disease as compared to conventional treatment. This was a retrospective case–control study.
MATERIALS AND METHODS:
Cases were defined as patients with EKC receiving the treatment protocol and conjunctival swab taken for polymerase chain reaction. Controls were defined as similar patients receiving conventional treatment protocol. Forty-one cases and 35 controls were identified for analysis. Cases were treated with a protocol using 2.5% povidone-iodine eye drops and 0.1% FluM eye drops. Controls received conventional treatment until resolution of signs and symptoms. Both the groups were followed up for 1 month. Data collected were analyzed for effect of the two treatment protocols on the duration of EKC, rate of recovery, and incidence of complications.
RESULTS:
The treatment protocol was significantly better than conventional treatment protocol in achieving cure (P = 0.002) with large effect size. The proportion of cases achieving cure was significantly higher with treatment protocol (64% vs. 11% at 5 days, P < 0.001) by 5 days. There was a significant reduction of the subepithelial infiltrates (SEI) incidence group (10% vs. 57%, P < 0.001). There were no SEI at 1 month in the treatment group (0% vs. 31%).
CONCLUSION:
Treatment protocol used in our study can significantly reduce the severity and duration of EKC. It can prevent chronic keratitis in majority of cases. Since povidone-iodine is nonspecific and virucidal for adenovirus, this therapy can be used for other types of adenoviral conjunctivitis.
Keywords: Adenovirus, epidemic keratoconjunctivitis, povidone-iodine
Introduction
The most common virus causing conjunctivitis is human adenovirus (HAdV).[1,2,3] Epidemic keratoconjunctivitis (EKC) is the most severe form of HAdV conjunctivitis. HAdV serotypes 4, 8, 19, 37, 51, and 54 are known to cause EKC.[4,5,6,7] HAdV is highly contagious and spreads rapidly within close contacts. The clinical features and diagnosis of EKC have been discussed previously.[8] HAdV is highly resistant to regular disinfectants and desiccation resulting in epidemics in various environments including ophthalmology outpatient department.[8,9,10] While the diagnosis of adenoviral conjunctivitis by clinical findings can be difficult, a typical case of EKC can be diagnosed clinically alone based on history and signs.[8,11,12] The treatment of EKC is primarily supportive, the agents used having no effect on either infective agent or the outcome of the disease except for topical steroids. Steroids have been shown to reduce the symptoms but prolong the infectivity of the case.[11,12] Polymerase chain reaction (PCR) test is 93% sensitive and 100% specific for HAdV.[13,14] Other methods have been developed for rapid diagnosis of HAdV infection in the clinics. AdenoPlus is one such test which uses the principle of lateral flow immunochemistry.[15,16]
We noticed an epidemic of EKC beginning in March 2015, which continues till date in the region served by our hospital. During initial few months, cases were managed with conventional treatment. Literature review revealed that various antiviral drugs have been tried for the treatment of adenoviral conjunctivitis, of which povidone-iodine was reported to be effective to some extent.[17,18,19,20,21,22,23] We started treating patients with 2.5% Povidone-Iodine (PovI) eye drops in 0.25% proparacaine (PovI) to relieve the initial stinging sensation. Proparacaine has a similar pH as PovI aqueous solution which we thought can stabilize the PovI on dilution to 2.5%.[24] Addition of a mild steroid Fluorometholone (FluM) 0.1% available as commercial preparation, four times daily, helped control symptoms faster. Toward the end of 2015, we shifted to treatment with PovI + FluM protocol for all age groups and severity of adenoviral conjunctivitis including EKC cases.
Rationale behind the treatment protocol
Various factors including initial concentration, duration of exposure, diffusion into tissues, and pH of tear film affect the action of PovI on HAdV. Monnerat et al. demonstrated that exposure to PovI at concentration of 0.8% for 10 min renders the extracellular viral particles inactive.[25] They also concluded that exposure to PovI for up to 6 h causes little cytotoxicity in healthy cells.
The lacrimal pump removes 16% of tear volume from conjunctival sac every minute.[26] Thus, beginning at 2.5% concentration, to reach 0.8%, it requires about 6–7 min at normal blink rate. Pels and Vrensen have demonstrated that 2% PovI solution is safe for stromal histiocytes, whereas 5% or more can damage the histiocytes at 10 min of immersion of intact globe.[27] Hence, a concentration of 2.5% which was safely used in previous studies was decided upon in the present treatment.[22] At 2.5% concentration, PovI drops have marked stinging effect. To reduce the stinging effect, we added the least toxic of topical anesthetics at minimum anesthetic concentration of 0.25% making the combination drops more tolerable to the patients.[28,29] Overuse of topical anesthetics has been recorded to cause various toxic effects on the corneal epithelium, stroma, and endothelium.[30] However, most of the recorded cases had psychiatric disorder or occurred in specific work setting. We counseled the patients about possibility of toxicity due to combination of eye drops and made it a point to follow up the patients strictly at 5 days or less.
Pelletier et al. in their study of PovI treatment of adenoviral conjunctivitis have noticed a clinical improvement by 5 days.[31] This was set as the follow-up time to look for effect of the current protocol. The comparison between this treatment protocol and conventional treatment is presented in this study.
Methods
The study was in adherence to the tenets of the Declaration of Helsinki. This study was approved by the institutional ethics committee (IEC 394/2016). For this retrospective case–control study, records of patients seen between March 2015 and June 2016 were reviewed. Patients diagnosed clinically as EKC in one or both eyes were included for the study. Informed consent was obtained for either of treatment protocols. In case of minors, one/both of the parents had given informed consent for the treatment. All the patients treated with 2.5% povidone-iodine in 0.25% proparacaine eye drops (PovI drops) with 0.1% FluM eye drops were considered as “cases” in the “PovI group.” Patients treated with standard treatment protocol such as artificial tears, antihistamine or nonsteroidal eye drops, and steroid eye drops when necessary were considered to be on conventional treatment protocol and grouped together as the “control group.”
Composite factor
A “composite factor (CF)” was created for grading severity of disease at the time of diagnosis as follows:
All the signs and symptoms were recorded on a 3-point severity scale (1 – mild, 2 – moderate, and 3 – severe). Sum of two signs and two symptoms with the highest average severity at the first visit was calculated. This value was divided by 4 to arrive at a “CF.” The highest score of CF would be 3. This CF represents both subjective and objective perceptions of disease severity together. The CF was calculated for each follow-up data using the same set of signs and symptoms as of the first visit. This was compared to previous score to assess response to treatment.
CF value of < 1 was considered as indicative of clinical cure.
PovI group
A patient record with EKC was included in this group if one of these criteria was fulfilled:
Any new case of clinically diagnosed EKC, who had undergone PCR test for adenovirus. Clinical diagnosis was defined as the presence of follicular conjunctivitis with preauricular lymphadenopathy or membranes at presentation
Any severe follicular conjunctivitis with CF >2 and PCR positive for adenovirus. This definition was set to include patients without preauricular lymphadenopathy or membranes
Any family member of diagnosed case of EKC presenting with severe follicular conjunctivitis.
These cases additionally had to fulfill the following criteria:
A new EKC case started on 2.5% PovI + 0.1% FluM eye drops at diagnosis
Such a case should have completed the follow-up of 15 days.
The followings were exclusion criteria:
Prior treatment
Patients with known allergy to iodine
Patients unable to tolerate PovI eye drops
Pregnant patients
Patients with thyroid abnormalities.
The eye with more severe signs was used to take conjunctival swab for adenoviral real-time transcription PCR test.
The patient was counseled and informed consent was obtained before starting PovI protocol. PovI eye drops and FluM eye drops were dispensed in separate bottles. The dosage was scheduled every 4–6 h in waking period. The follow-up was scheduled on the 5th day after each visit. If a patient was improving, the typical consultations were on initial visit, day 5, day 10, and day 15. A follow-up visit after 1 month was scheduled to look for any corneal involvement. Data collected on these days were used for analysis.
During each visit, the presence of the following factors was recorded for each eye: time from onset in days, pain, stiffness/puffiness, matting of lids, discomfort, photophobia, blurred vision, lid edema, conjunctival congestion, follicles, membranes, mucopurulent discharge, epithelial haze, epithelial edema, subepithelial infiltrates, anterior chamber reaction, visual acuity, preauricular lymphadenopathy, systemic symptoms, irritation to PovI drops, and allergy to povidone-iodine. The severity of observations was recorded on a scale of 1–3.
The incidence of complications during treatment was calculated excluding the first visit. Any fresh recording of membranes, SEI, subconjunctival scarring, preauricular lymphadenopathy, or allergy to PovI was considered a new incidence.
The irritation of PovI eye drops was graded between 0 and 3 as per patient perception.
The followings were set as endpoints in the PovI group:
Clinical cure defined as CF <1
A patient not able to tolerate PovI eye drops. In this case, the patient was shifted to conventional treatment, and the record was excluded from the study
Incomplete data or if a patient desired to stop medications before 15 days of treatment for any reason. This patient was excluded from the study.
The followings were set as outcome measures:
Clinical cure as defined above
Time taken for clinical cure.
Control group
The present-day treatment of EKC is mainly supportive with artificial tears, nonsteroidal anti-inflammatory drugs, or antihistamines used to relieve symptoms. Topical steroids are indicted only for membranes or corneal involvement.[11,12] This is a conventional treatment protocol. All the patients started on conventional treatment protocol were considered as controls. Any severe follicular conjunctivitis with preauricular lymphadenopathy with or without membrane formation was diagnosed as EKC. The controls were given symptomatic treatment as has been mentioned previously and were followed up for as long as required.[8] Any patient with incomplete treatment or treated previously was excluded from this group. At each visit, all the factors mentioned in PovI cases were recorded.
The patients were followed every 2–3 days till clinical improvement and then every 5 days. For patients developing SEIs, follow-up was scheduled every week till resolution of all the SEIs and tapering of steroids.
Data from both the groups were collected at 0, 5, 10, 15, and 30 days. For both the groups, when both eyes were affected, worse affected eye was included in the study. This was considered as one case. The findings were tabulated and analyzed.
Topical anesthetic toxicity
Since the use of topical anesthetic multiple times up to 2 weeks can be potentially epitheliotoxic, we observed for significant epitheliopathy in the PovI group. Fresh incidence of epithelial haze and epithelial edema after starting treatment was considered together as “epitheliopathy” and recorded for both the groups. This was compared between the PovI and control groups to look for epithelial toxicity in the PovI group.
Statistical analysis
All statistical analyses were performed using SPSS version 20 (IBM, Chicago, IL, USA). The normality of variables was tested using the Kolmogorov–Smirnov test and Q–Q plots. A mixed analysis of variance (ANOVA) test was performed to find the difference between the responses to the two treatment protocols during the course of the treatment. The setup was as follows: CF was considered as a dependent factor, its change over time was considered as an independent factor within the groups, and the treatment protocol was considered as an independent between-group factor. Box's test of equality of covariance matrices (P > 0.001) was performed to compare variation in samples. Mauchly's test of sphericity (P > 0.05) was used for interpreting tests of within-group effects. Homogeneity of variance was checked using Levene's test while interpreting between-group effect. P < 0.05 was considered as evidence of a significant effect and P < 0.001 as very significant for both within-group and between-group effects. To compare the outcome between the study group, the control group with steroid treatment, and the control group without steroid treatment, a mixed ANOVA analysis was carried out between these three groups using a similar setup as the two group analyses.
Mixed ANOVA analysis was performed similarly for the PovI group keeping dependent factor and within-group factors same, but between-group factor was PCR result (positive/negative). If mixed ANOVA outcome was found significant, the data were further analyzed using independent samples t-test to compare the change in CF between the groups at different time periods.
The incidence of various complications was compared between the groups using independent samples t-test. The fresh incidence of epitheliopathy during treatment was compared between the PovI and control groups using independent samples t-test. Cure rates at follow-up were recorded as percentages and compared using Chi-square test.
results
In this retrospective case–control study, 45 cases and 42 controls had undergone treatment during the study period. After applying exclusion criteria, 41 cases and 35 controls were found to fulfill inclusion criteria and were analyzed. The study population in both the groups was comparable in terms of age and sex distribution, eyes involved, severity of the disease at presentation, and the duration of the clinical features at presentation [Table 1]. While PCR was sent for all the patients in the PovI group, only 71% turned out positive. Figure 1 presents the severity of signs and symptoms at presentation.
Table 1.
Baseline characters and complications during treatment of the two study groups
| Value | Group | |||||
|---|---|---|---|---|---|---|
| PovI group (n=41) | Control group (n=35) | |||||
| Mean | Minimum | Maximum | Mean | Minimum | Maximum | |
| Age (years) | 32.12±15.3 | 5.0 | 76.0 | 32.34±17.7 | 2.0 | 62.0 |
| Sex (male: female) | 26:15 | 18:17 | ||||
| PCR (positive: negative) | 71:29 | Not sent | ||||
| Time since onset (days) | 4.4±2.5 | 2.0 | 12.0 | 4.9±3.1 | 2.0 | 14.0 |
| Eye involved (BE:RE:LE)* | 29:4:8 | 23:7:5 | ||||
| Stiffness/puffiness | 1.5±0.6 | 1.0 | 3.0 | 1.7±0.6 | 1.0 | 3.0 |
| Discomfort | 1.5±0.7 | 0.0 | 3.0 | 1.9±0.6 | 1.0 | 3.0 |
| Congestion | 2.3±0.6 | 1.0 | 3.0 | 2.2±0.8 | 0.0 | 3.0 |
| Follicles | 2.2±0.6 | 1.0 | 3.0 | 1.9±0.8 | 0.0 | 3.0 |
| Composite factor | 1.87±0.5 | 1.3 | 2.8 | 1.94±0.5 | 0.8 | 3.0 |
| Epitheliopathy incidence (%)† | 51 | 71 | ||||
| PovI irritation score | 0.57±0.56 | 0 | 2 | Not applicable | ||
| SEI incidence (%)† | 9.8 | 57.1 | ||||
| Membrane incidence (%)† | 34.1 | 37.1 | ||||
| SEI at one month (%) | 0.0 | 31 | ||||
| Subconjunctival scarring (%) | 7 | 43 | ||||
*BE=Both eyes, RE=Right eye, LE=Left eye,†Fresh incidence after starting treatment, PovI=Povidone Iodine, PCR=Polymerase chain reaction, SEI= subepithelial infiltrates
Figure 1.
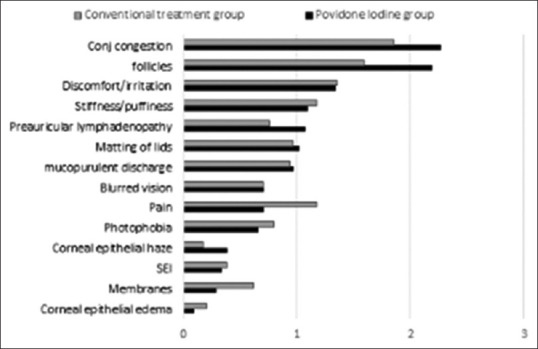
Average scores of various symptoms and signs at baseline. The most severe two symptoms and two signs at baseline were used to calculate composite factor at each visit
Two most severe symptoms in both the groups were similar: discomfort/irritation and stiffness/puffiness of eye. Two most severe signs also were similar in both the groups: conjunctival congestion and follicles. The mean scores of these symptoms and signs at initial diagnosis are presented in Table 1. Initial scores of CF of symptoms and signs were 1.87 + 0.5 and 1.94 + 0.5 for the PovI and control groups, respectively.
The criteria set for analysis in mixed ANOVA were fulfilled in both of the test scenarios (PovI group vs. control group and PCR positive vs. PCR negative).
Comparison of treatment protocols in the PovI and control groups
In the PovI group versus control group analysis, the main effect of treatment protocol was significant, F (1, 74) =31, P < 0.001, partial Eta squared = 0.297. Thus, there was a difference in response to treatment between the groups, and the effect size was large. A significant main effect for change in CF was obtained, F (3, 74) =240, P < 0.001, partial Eta squared = 0.764. This effect size was also large. Thus, there was a significant reduction in mean CF over the treatment period in both the groups. A significant main effect of change in CF and Group (CF * Group) was also seen, F (1, 74) =10, P = 0.002, partial Eta squared = 0.12. The mean CF in the PovI group reduced at every follow-up and attained definition of cure (<1) by 5 days, whereas the control group attained this level later than 10 days [Figure 2].
Figure 2.
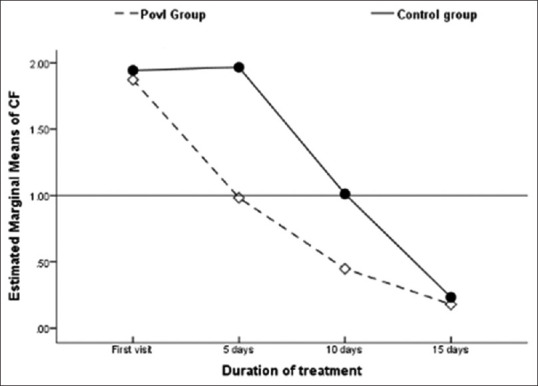
Between-group comparison of response to treatment over time for the PovI and control groups. The mean composite factor value was <1 for the PovI group by day 5, whereas it took >10 days for the control group
Independent t-test comparison between the groups at 0, 5, 10, and 15 days showed that there was a significant difference in CF values between the two groups (P < 0.001) at 5 and 10 days, but by day 15, the difference was insignificant (P > 0.05). Thus, there was a reduction in clinical features in both the treatment groups in successive follow-ups, and the PovI protocol achieved cure in half the time taken by conventional treatment. Figure 3 shows cure rates at different time intervals after starting treatment.
Figure 3.
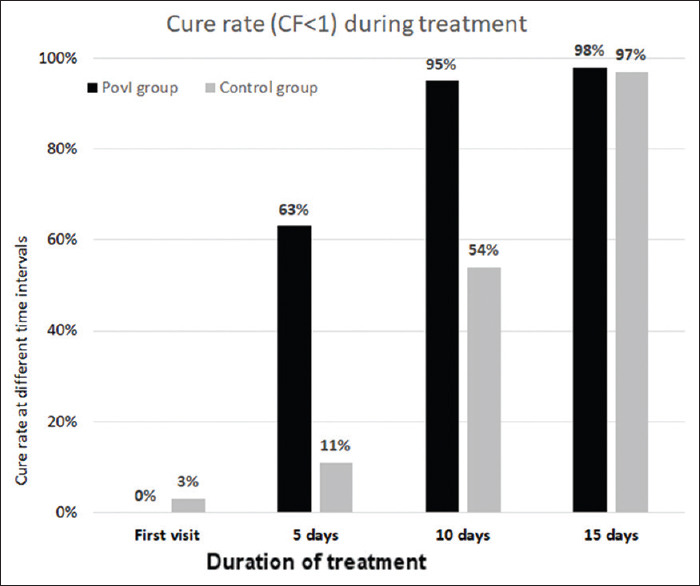
Cure rate (composite factor <1) during treatment. While >60% were cured by day 5 in the PovI group, <15% were cured in the control group. By 10 days, this was 95% and 54% in the PovI and control groups, respectively
At day 5, 64% were cured in the PovI group, whereas only 11% were cured in the control group. At day 10, the cure rate was 95% and 54%, respectively, whereas at day 15, it was 98% and 97%, respectively [Figure 3].
Comparison of outcomes between PovI group, control group on steroid treatment, and control group without steroid treatment
The main effect of treatment protocol was significant, F (2, 32) =12.5, P < 0.001, partial Eta squared = 0.438. Thus, there was a difference in response to treatment between the three groups, and the effect size was large. A significant main effect for change in CF was obtained, F (3, 32) =44, P < 0.001, partial Eta squared = 0.579. This effect size was also large, suggesting that there was a significant reduction in mean CF over the treatment period in all the three groups. A significant main effect of change in CF and Group (CF * Group) was also seen, F (2, 32) =8.33, P = 0.001, partial Eta squared = 0.343. The mean CF in the PovI group reduced at every follow-up and attained definition of cure (<1) by 5 days, whereas both the with-and-without steroid control groups attained this level later than 10 days [Figure 4]. Post hoc test using independent samples t-test revealed a significant difference in change in CF between the PovI and control groups on steroids (P < 0.001) and the PovI group and the control group not on steroids (P < 0.001). However, the change in CF over the study period between the control groups with and without steroid treatment was not significant (P > 0.05).
Figure 4.
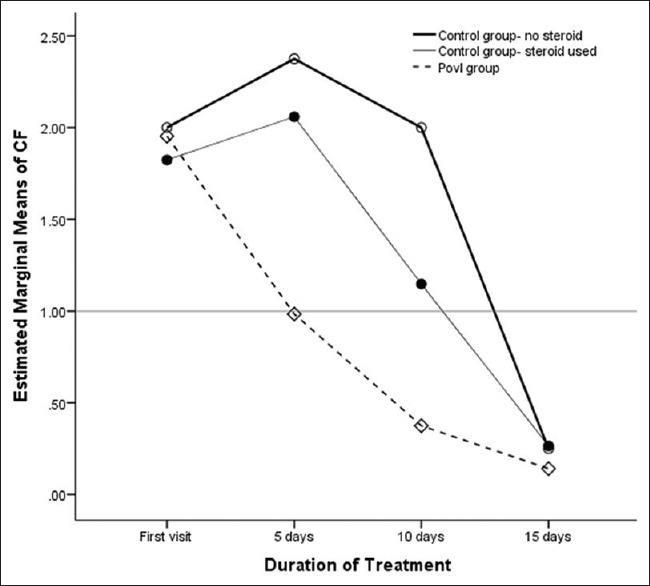
Between-group comparison of response to treatment over time for the PovI group and control group with steroid treatment and control group without steroid treatment. The mean composite factor value was <1 for the PovI group by day 5, whereas it took >10 days for both the control groups. The change in composite factor between two control groups was not significant at each follow-up
Effect of topical anesthetic on the cornea
The difference in incidence of epitheliopathy between the PovI and control groups was not significant (51% vs. 71%, P = 0.07) [Table 1], indicating that the toxicity of topical anesthetics was either masked by effects of EKC or other medications in PovI treatment protocol, or the epithelial toxicity of topical anesthetics in the protocol used for the PovI group was clinically insignificant.
Comparison of outcomes between polymerase chain reaction-positive and polymerase chain reaction-negative patients
The analysis of the PovI group to compare the response of PCR-positive/PCR-negative cases to PovI protocol showed no significant difference between the groups in any of the analyzed factors (P > 0.05), indicating that clinical diagnosis of EKC is sufficient to start the treatment [Figure 5].
Figure 5.
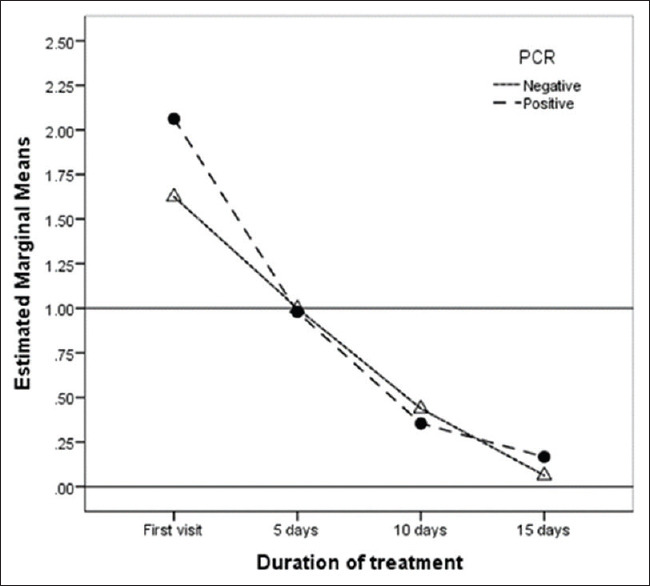
Between-group comparison of polymerase chain reaction positive/polymerase chain reaction negative within the PovI group over time. The mean composite factor was <1 for both the groups by day 5, indicating that treatment was equally effective in polymerase chain reaction confirmed and clinically diagnosed cases
Comparison of complications
When the complication rates were compared between the two groups, there was a marked reduction of SEI incidence over the treatment period in the PovI group as compared to the control group (10% vs. 57%, P < 0.001). However, the incidence of membranes was similar (34% vs. 37%, P = 0.79). By 30 days, there were no cases of SEI in the PovI group, whereas 31% of the control group patients still had SEI.
Almost all the patients could tolerate the PovI + proparacaine eye drops with mild-to-moderate irritation (mean score = 0.57) with only one patient discontinuing the drops due to excessive irritation. This patient was excluded from the final analysis. None of the treated patients developed allergy to PovI eye drops.
Discussion
The present-day management of EKC mainly is aimed at supportive medications to relieve symptoms and prevention of spread. Keratitis following acute conjunctivitis may run a chronic course and affect the quality of life for a prolonged period.
Results in our study indicate that the protocol we used is effective in treatment of the most severe form of adenoviral conjunctivitis, the EKC. In addition to control of conjunctivitis, there is an added advantage of minimal fresh incidence of SEI and earlier resolution of existing SEI with this treatment protocol [Table 1].
As compared to conventional treatment with artificial tears, the PovI cases recovered faster. Similarly, PovI cases had a better response than controls treated with steroid drops demonstrating overall better efficacy of our treatment protocol.
In the studies involving combination formulation of 0.4% to 1.0% PovI and 0.1% dexamethasone for adenoviral conjunctivitis, the severity of conjunctivitis was varied and the efficacy of the above conventional therapy has varied from no difference to significantly better for the treatment period of 5–7 days.[31,32,33,34] A better outcome in our study could be due to higher initial concentration of PovI and use of the two therapeutic agents as separate drops. The pH for optimum efficacy is 3–5 for proparacaine and PovI but 6–8 for steroids which was maintained in this protocol.[24,35]
PovI at concentration 2% diffuses into the corneal stroma through the intact epithelium, and this concentration is not toxic to stromal fibroblasts up to 10 min.[27] About 2.5% PovI eye drop can achieve a sufficient concentration in the epithelium, Bowman's layer, and anterior stroma if it remains in conjunctival sac for 5–7 min. This would explain the low incidence (10% vs. 57%) of SEIs in the PovI group over the control group as the PovI penetrating up to the anterior stroma may neutralize virus particles spreading to this area. The epithelium and stroma can also act as reservoirs for PovI which slowly release free iodine. This would also explain why four times dosage in waking hours can effectively control the viral infection.
The PCR results of our study are comparable to those of another study from India (71% positive in our study vs. 60%).[36] There was no significant difference between PCR-positive and PCR-negative cases treated in the study group [Figure 4]. Thus, clinical confirmation of EKC seems to be adequate for starting this treatment protocol.
Mechanism of action of the protocol
Keeping our study results as clinical evidence, we would like to propose the following mechanism of action of 2.5% PovI in 0.25% proparacaine eye drops with low-potency steroid eye drops in adenoviral EKC.
PovI at 0.8% or more neutralizes free viral particles of adenovirus in the conjunctival sac and cornea when exposed for 10 min[25]
At 2.5% initial concentration, PovI can penetrate into the epithelium, Bowman's membrane, and anterior corneal stroma to achieve therapeutic concentration with a single dosage.[27] It can also remain at concentrations above 0.8% for nearly 10 min in conjunctival sac if a patient keeps eyes closed for initial 3–5 min[26]
These two factors reduce infective load in the conjunctival sac as well as in the cornea
Cell-mediated immunity working concurrently in response to viral infection destroys the infected cells, further reducing production and release of free viral particles
Repetition of these factors reduces stimulus for inflammation as well as risk of transmission of disease
If a mild topical steroid is used with PovI, it suppresses excessive inflammation. Along with reduced stimulus for inflammation, this leads to rapid reduction in severity of clinical features and reduces the risk of complications
Within a short span of 5 days (our study), the infective load disappears resulting in clinical cure.
Few subepithelial keratocytes are partially or totally rendered nonfunctional by the povidone-iodine diffusing into the superficial stroma.[27] This may prevent the antigen-presenting activity and interfere with excessive immune reaction to adenoviral particles in the corneal stroma, thus reducing the incidence of chronic keratitis (0% in our study)
As free viral particles are physically altered within minutes by the free iodine, there is a low chance for virus-induced immune reaction in the cornea. Hence, corneal subepithelial infiltrates are milder and resolve within 1 month
Although not confirmed, our observation suggests that the presence of proparacaine along with PovI in the same solution can maintain or enhance the efficacy of PovI over the 15-day treatment period.
Topical anesthetic toxicity and abuse potential
There are numerous reports of topical anesthetic toxicity due to misuse by a patient. The damage can be limited to epithelium in the form of punctate epitheliopathy and delayed healing, or it can be more serious like stromal infiltrates.[30] In our PovI group, though we used the combination drops four times a day for 10–15 days, the epitheliopathy in comparison to the control group was insignificant. This can be due to following reasons: the concentration of anesthetic at 0.25% was lesser, dilution of the preservative of the anesthetic drop reducing its toxicity, and some protective effect of steroid drops used along with the combination drops. Despite this, we strongly advise cautious use of this protocol if the patient compliance is doubtful.
Strengths of this study
In this study, patients' data were collected every 5 days, which is the shortest time required for cure as demonstrated by Pelletier et al.[31] The CF represents both subjective and objective perceptions of disease severity. This is important because, in case of conjunctivitis, the patient's perception of cure is much earlier than clinician's observation of cure as demonstrated in Isenberg et al. study of PovI treatment for infective conjunctivitis in children.[37]
Limitations of this study
The present study also has some limitations. We could not get serotyping of adenovirus in our cases. Different serotypes have been used in vitro to study the effect of PovI on adenovirus, and it has been found to be effective against all the strains.[25] Since the action of PovI is not dependent on specific antigen on viral surface, serotyping for treatment thus is not necessary in routine practice. Hence, our treatment protocol can be used in all the serotypes of EKC.
Controls were not confirmed with PCR test in this study. The diagnosis of EKC is mainly clinical. The treatment protocol for conventional treatment does not require PCR. However, for inclusion in the study, all the important symptoms and signs of EKC had to be present to minimize the possibility of other viral and chlamydial conjunctivitis. For the PovI group, PCR was done mainly to confirm the diagnosis before starting the unconventional treatment with PovI. We also could not assess the toxicity of topical anesthetic used as an adjunct in the PovI group since EKC itself has various epithelial manifestations. Setting up the control group with similar dosage of the proparacaine drops would reveal any possible effects of the prolonged use of this medication.
Further study using randomized controlled trials is required to confirm these observations, possibly using different strengths of PovI with proparacaine and mild steroid in separate bottles. Another question to be considered is the frequency of instillation of PovI drops. Although our study demonstrates the efficacy and safety of four times daily dosage, possibility of the same effect with lesser frequency or better efficacy with increased frequency of dosage has to be evaluated.
The treatment regimen had to be discontinued in one case in the PovI group due to excessive irritation of PovI drops. The patient had followed PovI treatment protocol for 3 days. He was treated with conventional treatment of lubricant drops and cold compresses and excluded from the analysis. He recovered without corneal involvement.
One of the excluded cases in the PovI group is a 16-year-old female developed geographic ulcer in the right eye on the 3rd day of treatment. This can be due to viral infection as it is a known complication of EKC.
Systemic effect of topical povidone-iodine
Considering maximum dosage of four drops in each eye for 15 days and 15 drops per ml, maximum amount of elemental iodine entering the patient's body is about 20 mg in 2 weeks. This amount of iodine is much less than maximum recommended dose for this duration and does not affect thyroid function in normal individuals at any age.[38] Thus, 2.5% povidone-iodine eye drops appear to be safe for 10–15 days of use in the age group of this study (5–76 years).
Conclusion
Although PovI has virucidal action on adenovirus, the concentration required for its clinical efficacy seems to be 2.5%. Simultaneous use of a mild steroid is helpful but should be a separate medication. The protocol presented here can be used as a first-line treatment for EKC cases.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors declare that there are no conflicts of interests of this paper.
References
- 1.Azari AA, Barney NP. Conjunctivitis: A systematic review of diagnosis and treatment. JAMA. 2013;310:1721–9. doi: 10.1001/jama.2013.280318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O'Brien TP, Jeng BH, McDonald M, Raizman MB. Acute conjunctivitis: Truth and misconceptions. Curr Med Res Opin. 2009;25:1953–61. doi: 10.1185/03007990903038269. [DOI] [PubMed] [Google Scholar]
- 3.Sambursky RP, Fram N, Cohen EJ. The prevalence of adenoviral conjunctivitis at the wills eye hospital emergency room. Optometry. 2007;78:236–9. doi: 10.1016/j.optm.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 4.Ghebremedhin B. Human adenovirus: Viral pathogen with increasing importance. Eur J Microbiol Immunol (Bp) 2014;4:26–33. doi: 10.1556/EuJMI.4.2014.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buckwalter SP, Teo R, Espy MJ, Sloan LM, Smith TF, Pritt BS. Real-time qualitative PCR for 57 human adenovirus types from multiple specimen sources. J Clin Microbiol. 2012;50:766–71. doi: 10.1128/JCM.05629-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aoki K, Tagawa Y. A twenty-one year surveillance of adenoviral conjunctivitis in Sapporo, Japan. Int Ophthalmol Clin. 2002;42:49–54. doi: 10.1097/00004397-200201000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Kaneko H, Suzutani T, Aoki K, Kitaichi N, Ishida S, Ishiko H, et al. Epidemiological and virological features of epidemic keratoconjunctivitis due to new human adenovirus type 54 in Japan. Br J Ophthalmol. 2011;95:32–6. doi: 10.1136/bjo.2009.178772. [DOI] [PubMed] [Google Scholar]
- 8.Reed K. Epidemic viral keratoconjunctivitis diagnosis and management. J Am Optom Assoc. 1983;54:141–4. [PubMed] [Google Scholar]
- 9.Gordon YJ, Gordon RY, Romanowski E, Araullo-Cruz TP. Prolonged recovery of desiccated adenoviral serotypes 5, 8, and 19 from plastic and metal surfaces in vitro . Ophthalmology. 1993;100:1835–9. doi: 10.1016/s0161-6420(93)31389-8. [DOI] [PubMed] [Google Scholar]
- 10.Dart JK, El-Amir AN, Maddison T, Desai P, Verma S, Hughes A, et al. Identification and control of nosocomial adenovirus keratoconjunctivitis in an ophthalmic department. Br J Ophthalmol. 2009;93:18–20. doi: 10.1136/bjo.2007.130112. [DOI] [PubMed] [Google Scholar]
- 11.Jhanji V, Chan TC, Li EY, Agarwal K, Vajpayee RB. Adenoviral keratoconjunctivitis. Surv Ophthalmol. 2015;60:435–43. doi: 10.1016/j.survophthal.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 12.American Academy of Ophthalmology. Basic and Clinical Science Course Section 8: External Disease and Cornea. Vol. 8. San Francisco, CA: American Academy of Ophthalmology; 2018. Basic and Clinical Science Course Section 8: External Disease and Cornea. [Google Scholar]
- 13.Cooper RJ, Yeo AC, Bailey AS, Tullo AB. Adenovirus polymerase chain reaction assay for rapid diagnosis of conjunctivitis. Invest Ophthalmol Vis Sci. 1999;40:90–5. [PubMed] [Google Scholar]
- 14.El-Sayed Zaki M, Abd-El Fatah GA. Rapid detection of oculopathogenic adenovirus in conjunctivitis. Curr Microbiol. 2008;56:105–9. doi: 10.1007/s00284-007-9054-z. [DOI] [PubMed] [Google Scholar]
- 15.Sambursky R, Trattler W, Tauber S, Starr C, Friedberg M, Boland T, et al. Sensitivity and specificity of the AdenoPlus test for diagnosing adenoviral conjunctivitis. JAMA Ophthalmol. 2013;131:17–22. doi: 10.1001/2013.jamaophthalmol.513. [DOI] [PubMed] [Google Scholar]
- 16.Kam KY, Ong HS, Bunce C, Ogunbowale L, Verma S. Sensitivity and specificity of the AdenoPlus point-of-care system in detecting adenovirus in conjunctivitis patients at an ophthalmic emergency department: A diagnostic accuracy study. Br J Ophthalmol. 2015;99:1186–9. doi: 10.1136/bjophthalmol-2014-306508. [DOI] [PubMed] [Google Scholar]
- 17.Hillenkamp J, Reinhard T, Ross RS, Böhringer D, Cartsburg O, Roggendorf M, et al. The effects of cidofovir 1% with and without cyclosporin a 1% as a topical treatment of acute adenoviral keratoconjunctivitis: A controlled clinical pilot study. Ophthalmology. 2002;109:845–50. doi: 10.1016/s0161-6420(02)00992-2. [DOI] [PubMed] [Google Scholar]
- 18.Trousdale MD, Goldschmidt PL, Nóbrega R. Activity of ganciclovir against human adenovirus type-5 infection in cell culture and cotton rat eyes. Cornea. 1994;13:435–9. doi: 10.1097/00003226-199409000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Ward JB, Siojo LG, Waller SG. A prospective, masked clinical trial of trifluridine, dexamethasone, and artificial tears in the treatment of epidemic keratoconjunctivitis. Cornea. 1993;12:216–21. doi: 10.1097/00003226-199305000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Uchio E, Inoue H, Kadonosono K. Antiadenoviral effects of N-chlorotaurine in vitro confirmed by quantitative polymerase chain reaction methods. Clin Ophthalmol. 2010;4:1325–9. doi: 10.2147/OPTH.S14282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uchio E, Inoue H, Fuchigami A, Kadonosono K. Anti-adenoviral effect of interferon-β and interferon-γ in serotypes that cause acute keratoconjunctivitis. Clin Exp Ophthalmol. 2011;39:358–63. doi: 10.1111/j.1442-9071.2010.02457.x. [DOI] [PubMed] [Google Scholar]
- 22.Isenberg SJ, Apt L, Wood M. A controlled trial of povidone-iodine as prophylaxis against ophthalmia neonatorum. N Engl J Med. 1995;332:562–6. doi: 10.1056/NEJM199503023320903. [DOI] [PubMed] [Google Scholar]
- 23.Özen Tunay Z, Ozdemir O, Petricli IS. Povidone iodine in the treatment of adenoviral conjunctivitis in infants. Cutan Ocul Toxicol. 2015;34:12–5. doi: 10.3109/15569527.2014.888077. [DOI] [PubMed] [Google Scholar]
- 24.Lim LT, Ah-Kee EY, Collins CE. Common eye drops and their implications for pH measurements in the management of chemical eye injuries. Int J Ophthalmol. 2014;7:1067–8. doi: 10.3980/j.issn.2222-3959.2014.06.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monnerat N, Bossart W, Thiel MA. Povidone-iodine for treatment of adenoviral conjunctivitis: An in vitro study. Klin Monbl Augenheilkd. 2006;223:349–52. doi: 10.1055/s-2006-926633. [DOI] [PubMed] [Google Scholar]
- 26.Puffer MJ, Neault RW, Brubaker RF. Basal precorneal tear turnover in the human eye. Am J Ophthalmol. 1980;89:369–76. doi: 10.1016/0002-9394(80)90006-9. [DOI] [PubMed] [Google Scholar]
- 27.Pels E, Vrensen GF. Microbial decontamination of human donor eyes with povidone-iodine: Penetration, toxicity, and effectiveness. Br J Ophthalmol. 1999;83:1019–26. doi: 10.1136/bjo.83.9.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grant RL, Acosta D. Comparative toxicity of tetracaine, proparacaine and cocaine evaluated with primary cultures of rabbit corneal epithelial cells. Exp Eye Res. 1994;58:469–78. doi: 10.1006/exer.1994.1040. [DOI] [PubMed] [Google Scholar]
- 29.Shahinian L, Jr, Jain S, Jager RD, Lin DT, Sanislo SS, Miller JF, et al. Dilute topical proparacaine for pain relief after photorefractive keratectomy. Ophthalmology. 1997;104:1327–32. doi: 10.1016/s0161-6420(97)30139-0. [DOI] [PubMed] [Google Scholar]
- 30.Patel M, Fraunfelder FW. Toxicity of topical ophthalmic anesthetics. Expert Opin Drug Metab Toxicol. 2013;9:983–8. doi: 10.1517/17425255.2013.794219. [DOI] [PubMed] [Google Scholar]
- 31.Pelletier JS, Stewart K, Trattler W, Ritterband DC, Braverman S, Samson CM, et al. A combination povidone-iodine 04%/dexamethasone 01% ophthalmic suspension in the treatment of adenoviral conjunctivitis. Adv Ther. 2009;26:776–83. doi: 10.1007/s12325-009-0062-1. [DOI] [PubMed] [Google Scholar]
- 32.Pinto RD, Lira RP, Abe RY, Zacchia RS, Felix JP, Pereira AV, et al. Dexamethasone/povidone eye drops versus artificial tears for treatment of presumed viral conjunctivitis: A randomized clinical trial. Curr Eye Res. 2015;40:870–7. doi: 10.3109/02713683.2014.964419. [DOI] [PubMed] [Google Scholar]
- 33.Pepose JS, Ahuja A, Liu W, Narvekar A, Haque R. Randomized, controlled, phase 2 trial of povidone-iodine/Dexamethasone ophthalmic suspension for treatment of adenoviral conjunctivitis. Am J Ophthalmol. 2018;194:7–15. doi: 10.1016/j.ajo.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 34.Kovalyuk N, Kaiserman I, Mimouni M, Cohen O, Levartovsky S, Sherbany H, et al. Treatment of adenoviral keratoconjunctivitis with a combination of povidone-iodine 1.0% and dexamethasone 0.1% drops. A clinical prospective controlled randomized study. Acta Ophthalmol. 2017;95:e686–92. doi: 10.1111/aos.13416. [DOI] [PubMed] [Google Scholar]
- 35.Garcia-Valldecabres M, López-Alemany A, Refojo MF. PH stability of ophthalmic solutions. Optometry. 2004;75:161–8. doi: 10.1016/s1529-1839(04)70035-4. [DOI] [PubMed] [Google Scholar]
- 36.Gopalkrishna V, Ganorkar NN, Patil PR. Identification and molecular characterization of adenovirus types (HAdV-8, HAdV-37, HAdV-4, HAdV-3) in an epidemic of keratoconjunctivitis occurred in Pune, Maharashtra, Western India. J Med Virol. 2016;88:2100–5. doi: 10.1002/jmv.24565. [DOI] [PubMed] [Google Scholar]
- 37.Isenberg SJ, Apt L, Valenton M, Del Signore M, Cubillan L, Labrador MA, et al. A controlled trial of povidone-iodine to treat infectious conjunctivitis in children. Am J Ophthalmol. 2002;134:681–8. doi: 10.1016/s0002-9394(02)01701-4. [DOI] [PubMed] [Google Scholar]
- 38.Backer H, Hollowell J. Use of iodine for water disinfection: Iodine toxicity and maximum recommended dose. Environ Health Perspect. 2000;108:679–84. doi: 10.1289/ehp.00108679. [DOI] [PMC free article] [PubMed] [Google Scholar]


