Abstract
Although common evolutionary principles drive the growth of cancer cells regardless of the tissue of origin, the microenvironment in which tumours arise substantially differs across various organ sites. Recent studies have established that, in addition to cell-intrinsic effects, tumour growth regulation also depends on local cues driven by tissue environmental factors. In this Review, we discuss how tissue-specific determinants might influence tumour development and argue that unravelling the tissue-specific contribution to tumour immunity should help the development of precise immunotherapeutic strategies for patients with cancer.
Immunotherapy has led to remarkable recent achievements in cancer treatment with the induction of strong and durable clinical responses in cancers traditionally thought to have poor outcomes. In particular, blockade of the T cell inhibitory checkpoint molecules cytotoxic T lymphocyte antigen 4 (CTLA4) and programmed cell death 1 (PD1) — or its ligand, PD1 ligand 1 (PDL1) — can unleash antitumour T cell activity and lead to long-term clinical responses in patients with advanced cancer who had not responded to traditional therapies1. In the past decade, this has led to US Food and Drug Administration (FDA) and European Medicines Agency (EMA) approval of antibodies targeting CTLA4 and/or the PD1-PDL1 axis in melanoma, non-small-cell lung cancer (NSCLC), renal cell carcinoma (RCC), Hodgkin lymphoma, bladder cancer, head and neck squamous cell carcinoma (HNSCC), Merkel cell carcinoma, microsatellite instability (MSI)-high tumours, gastro-oesophageal cancer, hepatocellular carcinoma (HCC) and, more recently, cervical cancer and small-cell lung cancer (SCLC)1–4.
However, the clinical benefit of immune checkpoint therapies remains limited to a subset of patients, with overall response rates varying widely across cancer types. Patients with pancreatic cancer, prostate cancer or colorectal cancer (CRC) lesions rarely benefit from current immune checkpoint blockade regimens, reflecting either tumour cell-inherent resistance mechanisms or extrinsic factors restraining antitumour immunity. Uncovering the mechanisms of response and resistance to PD1 immunotherapy is at the forefront of cancer research. One of the most established tumour factors associated with increased response to immune checkpoint blockade is the presence of high tumour mutational burden (TMB)5–8. High TMB is thought to increase the occurrence of mutation-derived tumour-specific epitopes (also called neoantigens), thereby promoting immune recognition of tumour cells and leading to enhanced therapeutic activity9.
While tumour-intrinsic features have been shown to affect immune responses across cancer types (as reviewed elsewhere10), emerging evidence suggests a possible contribution of host tissue determinants in shaping tumour immunity. For example, first, cancers that are poorly responsive to PD1 blockade, such as ovarian or prostate cancers, can still retain a substantial mutational load11, suggesting that adaptive immunity to tumours is not determined by TMB alone. Second, unfavourable gut microbiome signatures12,13 are associated with poor tumour response to immune checkpoint blockade, highlighting the role of tumour-extrinsic factors in controlling cancer response to immunotherapy. Third, although T cell infiltration in tumour lesions correlates with improved tumour response to immune checkpoint blockade, the presence of T cells in ovarian14 and colorectal15 cancers does not correlate with response to immunotherapy, suggesting that additional features of these tissues may contribute to tumour outcome. Fourth, studies of ‘seeds’ (cancer cells) versus ‘soil’ (the invaded host organ) have revealed that the immune contexture can strongly differ between primary and metastatic tumour lesions16,17. Accordingly, in mice, the same melanoma cells implanted in the skin or the lung tissue have distinct tumour-associated macrophage (TAM) infiltrates18, supporting a role of the soil in regulating antitumour responses.
The tumour microenvironment (TME) is composed of a heterogeneous mixture of tissue-resident immune cells, fibroblasts, endothelial cells (ECs) and neurons that predate tumour formation, together with blood-derived cells that are recruited to the tumour site upon cancer progression19. Each of these cellular components can be co-opted by the tumour and contribute to cancer progression20. However, there are major differences in the composition and spatial organization of the tissue microenvironment across cancer types, including differences in tissue vascularization and innervation and tissue-resident immune and stromal cellular networks that may impact local immunosurveillance and the induction of tumour immunity regardless of the inherent tumour immunogenicity (FIG. 1).
Fig. 1 |. The cellular and architectural heterogeneity of the tumour microenvironment at distinct cancer sites.
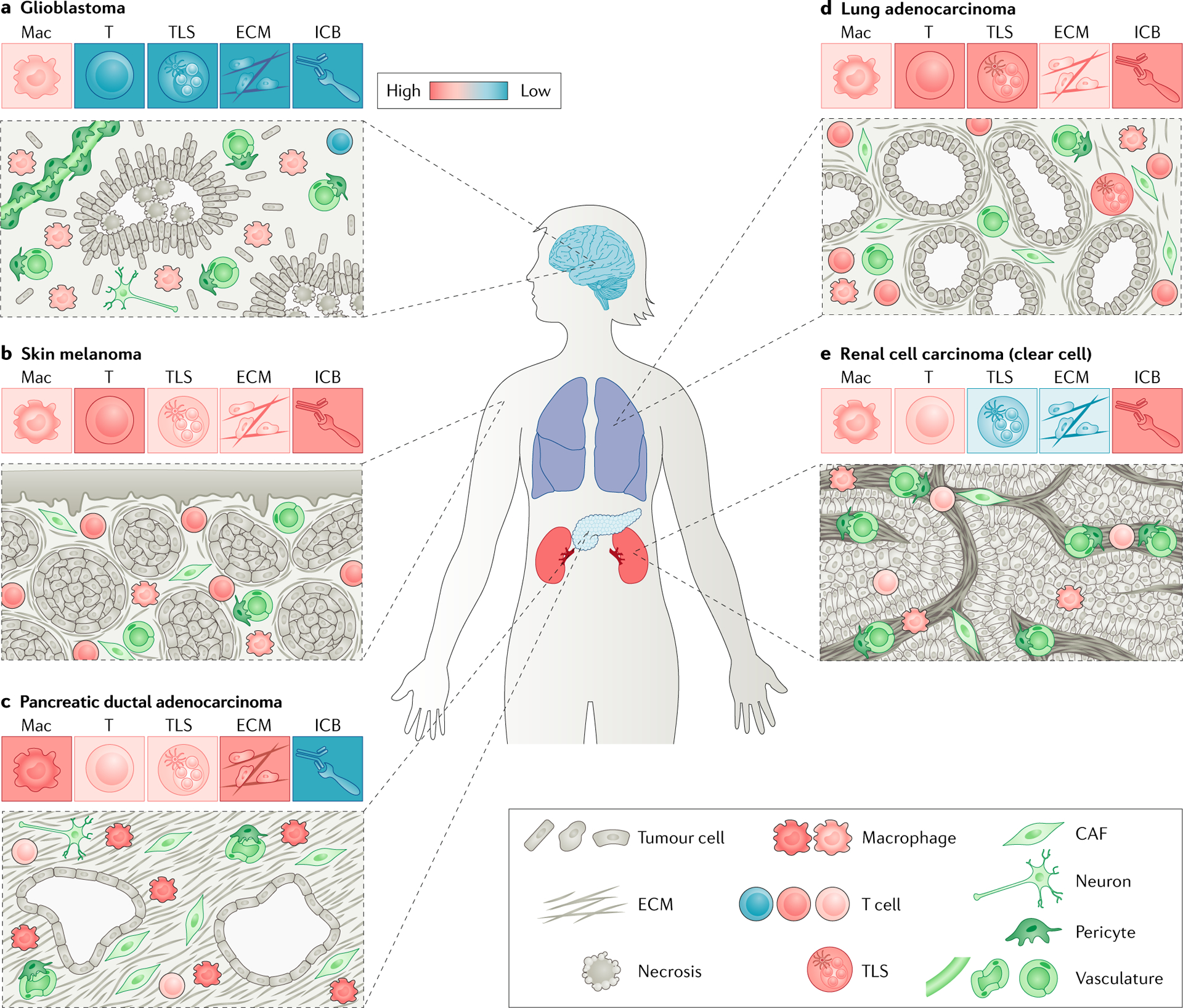
Schematics of representative histological patterns of glioblastoma (part a), skin melanoma (part b), pancreatic ductal adenocarcinoma (part c), lung adenocarcinoma (part d) and clear-cell renal cell carcinoma (part e) are shown. Tumour lesions at distinct tissue sites display different tumour mass organization, stroma to tumour ratios and levels of fibrotic reaction. In addition to neoplastic cells, each tumour microenvironment contains cells derived from both circulating cells and local cells such as fibroblasts, pericytes and endothelial cells that may differentially impact antitumour immune responses across cancer sites. For each tumour type, a colour-coded heatmap (red: high; blue: low) shows the level of dominance of macrophage or lymphocyte infiltrate, presence of tertiary lymphoid structures (TLSs), matrix deposition and response to immune checkpoint blockade (ICB). CAF, cancer-associated fibroblast; ECM, extracellular matrix;Mac, macrophage; T, T cell.
In this Review, we provide an overview of recent insights into the role of host tissue cellular and molecular cues in regulating local immunity and of how these tissue determinants may influence the antitumour immune response across disease sites. We also discuss potential implications for future research and clinical therapeutic strategies.
Tissue-specific immune microenvironment
Distinct features of tumour immune cell infiltration across tumour types.
Immune cells form a major cellular compartment of tumour lesions. Although in most cancer types, the immune microenvironment is often dominated by monocytes and/or macrophages and T lymphocytes21–27 accumulating at the invasive margin and in the peritumoural stroma8,28,29, the cellular composition and functional state of tumour-infiltrating immune cells vary considerably across tumours.
Transcriptional analysis of 10,000 tumours comprising 33 different cancer types performed by The Cancer Genome Atlas (TCGA) consortium identified several immune expression signatures that interestingly spanned anatomical location yet substantially varied in their proportion across cancers, with some immune signature modules dominating specific tumour types30. Uveal melanoma and brain tumours have the lowest immune cell infiltration, which is strongly dominated by macrophages over lymphocytes and natural killer (NK) cells24,30,31, which likely contributes to their limited response to immune checkpoint blockade32. Conversely, tumours containing the highest immune cell fraction include malignancies most responsive to immunotherapy, such as lung carcinoma, HNSCC and skin melanoma30. Mass cytometry and single-cell RNA sequencing analyses of these tumours have revealed a high frequency of T cells in the immune compartment, often dominating the myeloid cell pool26,33,34. Pancreatic cancer lesions contain a strong immune infiltrate frequently dominated by macrophages35. The myeloid-inflamed stroma in pancreatic ductal adenocarcinoma (PDAC) has been shown to be associated with restricted T cell functionality in both human and mouse lesions22,36, which may contribute to the poor clinical responses to immunotherapy observed in patients with PDAC2,22,37 (Fig. 1).
The strong variations in lymphocytic infiltration across cancers may be driven by the distinct molecular compositions and states of antigen-presenting cells (APCs) found between tissues. Non-lymphoid tissue APCs present in sterile tissues such as the pancreas and brain, filtering sites such as the kidney and liver, and environmental interfaces such as the skin, lung and gut differ from each other38. Those distinct locations expose APCs to different levels of signals from microorganisms or proliferating cells (for example, the epithelium), which can imprint their composition and immune function on an organ site, which in turn likely shapes tumour T cell infiltrates in tumour lesions.
Tertiary lymphoid structures across cancer types.
Similar to most chronically inflamed lesions, tumour lesions frequently display ectopic lymphoid-like structures called tertiary lymphoid structures (TLSs), preferentially found at the invasive margin, that contain large numbers of B cells, CD4+ T cells and mature dendritic cells (DCs)39–44. More than 60% of NSCLC, CRC, PDAC, ovarian cancer and breast cancer lesions and more than 45% of HCCs contain TLSs, whereas RCCs, uveal melanomas and brain tumours are almost devoid of such structures45–48. Interestingly, the B16-OVA melanoma cell line, when injected either subcutaneously or intraperitoneally in mice, induced TLS formation around peripheral node addressin (PNAd)+ vasculature in all intraperitoneal tumours but not in subcutaneous tumours49, supporting the idea that the local environment of the skin poorly supports TLS formation. However, in human melanoma resection samples, approximately 10–30% of primary lesions or cutaneous metastases contain TLSs40,50,51. Thus, it is currently unclear whether the local environment of the skin is poorly permissive to TLS development40,49,52 and/or whether other intrinsic host tissue factors might be involved.
The molecular mechanism s leading to lymphoid neogenesis are only partially understood53. They appear to be similar to those that lead to the development of new secondary lymphoid organs (SLOs), which are orchestrated by haematopoietic cells (the lymphoid tissue inducer (LTi) cells) and stromal cells (the lymphoid tissue organizer (LTo) cells), which differentiate into follicular dendritic cells and fibroblastic reticular cells54.
TLS neogenesis is driven by inflammatory molecules and cell types that may differ across tumour tissues. Similar to SLOs, immune cells, fibroblasts and lymphotoxin-α (LTα) and LTβ signalling are required for TLS form ation53. Several studies have reported that resident stromal cells can become LTo cells under inflammatory conditions and are able to initiate TLS form ation55, whereas activated lymphocytes substitute for LTi cells56. Cytokines (such as interleukin-17 (IL-17), IL-22, IL-23 and IL-36), chemokines (such as CXC-chemokine ligand 12 (CXCL12), CXCL13, CC-chemokine ligand 19 (CCL19) and CCL21), adhesion molecules (such as mucosal addressin cell adhesion molecule 1 (MAdCAM1), vascular cell adhesion molecule 1 (VCAM1) and intercellular cell adhesion molecule 1 (ICAM1)) and high endothelial venule neogenesis seem to play an important role in TLS development49,57,58. Conversely, regulatory T (Treg) cells negatively regulate TLS neogenesis59,60.
Thus, differences in stromal cells, immune infiltration and molecular composition at the tissue site could dramatically influence TLS formation. In the lung, TLSs called bronchus-associated lymphoid tissues develop spontaneously in multiple conditions of infection, cancer and chronic inflammation in both mice and humans61–64. A recent mouse study showed that type I interferons induce platelet-derived growth factor-α (PDGFRa)+ lung fibroblasts to secrete CXCL13 upon influenza virus infection, leading to B cell recruitment and germinal centre formation65. The strong ability of lung tissue stromal cells to respond to inflammatory mediators could contribute to the high frequency of TLSs observed in tumour lesions from patients with lung cancer41,64. The reduced stromal compartment and lack of pro-TLS-forming chemokines and cytokines may explain the reduced TLS number in RCC53 and brain tumour lesions — both glioblastoma and lower-grade glioma17. Further investigation is needed to better understand the different TLS frequencies observed across anatomical sites.
The tumour immune microenvironment: impact on clinical outcomes.
The impact of the immune infiltrate on patient survival also differs between tumour types. A worldwide task force including 14 different centres recently validated the benefit of CD3+ and CD8+ T cell quantification in tumours (Immunoscore) to stratify prognosis of patients with colon cancer independently of the tumour-node-metastasis (TNM) stage66. In melanoma, HNSCC, NSCLC and breast, bladder, urothelial and ovarian cancers, high densities of tumour-infiltrating cytotoxic and memory T cells and skewing towards a T helper 1 (TH1) cell immune response are also associated with improved overall survival and disease-free survival47. Surprisingly, in RCC and prostate cancer, high CD8+ T cell densities are associated with poor overall survival15,67. In RCC, the adverse microenvironment characterized by an increased level of angiogenesis, immature DCs, Treg cells and high PDL1 expression might explain this negative association with prognosis68. In line with this, CD8+ T cells purified from tumour lesions of patients with RCC have been shown to be polyclonal and poorly cytotoxic and display phenotypic profiles similar to the poorly activated adjacent kidney-infiltrating T cells69.
Tissue-resident bona-fide DCs are the most immunogenic APCs and are uniquely able to prime efficient effector immune responses against tumour cells. Cross-presenting tissue-resident DCs (also called DC1s, which are characterized in mice by the expression of the integrin CD103) excel at driving antitumour CD8+ T cell immunity70–72. DC1s are required for tumour response to PD1-PDL1 blockade, as shown in mouse melanoma tumours71,73. Importantly, mice lacking DC1s have reduced infiltration of endogenous CD8+ interferon-γ (IFNγ)+ T cells in lung cancer lesions (M.M., unpublished observations). While reduced tumour T cell infiltration in DC1-deficient animals is likely due to defective priming in the draining lymph node, in vivo imaging studies revealed that proliferating CD8+ T cells were closely interacting with DC1s in the TME74. These results suggest that, in addition to their role in the draining lymph node, DC1s may also contribute to the reactivation and expansion of T cells in the TME. Given that the presence of tumour-infiltrating T cells correlates with tumour response to immune checkpoint blockade75,76, densities of tumour-infiltrating DC1s may provide a potential biomarker of tumour response to immune checkpoint blockade77. Their abundance has been shown to predict responses to anti-PD1 therapy in human metastatic melanoma78. Interestingly, we found that lung tissue is one of the most enriched organs for cross-presenting DCs (M.M., unpublished observations), which may contribute to shaping lung tumour T cell content at baseline.
The accumulation of immune cells positive for CD68 — a pan-macrophage marker — is associated with increased survival in colorectal or prostate cancers but poor clinical outcome in melanoma, RCC, HNSCC and breast, bladder and pancreatic cancers47. These contradictory prognoses may result from the imprecision of this marker — which labels a wide diversity of macrophages in addition to DCs — as well as differences in origin, activation levels, response to stimuli79,80 or specific functional attributes that are deleterious to some tumour lesions but not all. For example, the surfactant clearance function of alveolar macrophages may impact their metabolic ability and potentially their immunogenicity. Better characterization of macrophage populations — beyond the simple expression of CD68 or CD163 markers — through the use of high-dimensional profiling is critically needed to foster our understanding of the role of TAMs in cancer progression.
NK cell infiltration correlates with increased overall survival in clear-cell RCC81, CRC82 and melanoma78. In addition to direct NK cell antitumour effects — through secretion of perforin and granzyme and expression of tumour necrosis factor (TNF)-related apoptosis-inducing ligand receptor (TRAILR; also known as TNFRSF10)83–85 — the antitumour impact of NK cells has also been attributed to their secretion of the chemokines CCL5 and XCL1 and the DC growth factor FMS-like tyrosine kinase 3 ligand (FLT3L), which regulate tumour DC recruitment and abundance, as recently shown in mouse melanoma lesions78,86. Conversely, the infiltration of NK cells has no impact on clinical outcome in NSCLC, where their cytotoxic functions have been shown to be impaired by a strong reduction in NK cell activating receptors including NKp30, NKp80 and DNAM1 (REF27). This altered phenotype was specifically observed in the tumour and not the distant lung tissue, indicating that the NSCLC TME locally impairs NK cells27. Of note, NK cells were found in only the peritumoural stroma of human NSCLC sam ples27,87, whereas they were observed throughout the tumour in RCC, emphasizing the need to consider immune cell spatial distribution in addition to global infiltration in tumour lesions88. Interestingly, some tumour types such as HNSCC are strongly infiltrated by NK cells and may benefit from NK cell-targeting immunotherapies, as recently shown with an NKG2A-blocking antibody promoting the antitumour activity of both NK cells and CD8+ T cells89.
Stromal cell contribution to cancer immunity
Most solid tumours comprise two distinct compartments that include the tumour parenchyma and a surrounding stroma. Depending on the cellular origin of the malignant cell — epithelial, melanocyte or mesenchymal — the tumour mass differs in its organization, cellular cohesion and tumour-stroma boundaries, which impacts on immune cell distribution and function (BOX 1). This section primarily focuses on the nonhaematopoietic stromal compartment, a connective tissue comprising fibroblasts, pericytes and lymphatic and blood vessels as well as nerve cells all embedded in extracellular matrix (ECM) components such as collagen and fibronectin fibres90. Beyond immune cells, the dominant cellular components of the tumour stroma are blood ECs and cancer-associated fibroblasts (CAFs), both of which have been shown to strongly influence the antitumour immune response. Nerve cells, the microbiota and tissue-resident macrophages (Tr-macs) are also discussed as host tissue cellular components impacting tumour immunity (FIG. 2).
Box 1 |. Heterogeneity of the tumour architecture.
Epithelial tissue, which lines all body cavities, is a laterally extending layer of connected cells resting on a basement membrane, with apical-basal polarity that separates the organ from the external environment. Carcinomas — cancers that derive from the epithelium — are commonly organized as multiple distinct islets of tumour cells. Within carcinomas, a basal lamina can separate the tumour mass from its surrounding tissue, although it is often disrupted after tumour cells gain invasive capabilities191,192. Before undergoing dedifferentiation and, in some cases, epithelial-to-mesenchymal transition, carcinoma cells often maintain expression of cell-cell junctions such as E-cadherin and tight junctions, which provide cellular cohesion to the tumour nests193–195. This cohesive cellular structure likely constitutes a physical barrier that limits the entry of immune cells such as lymphocytes and dendritic cells, which do not use protease degradation for their migration in tissues and must physically migrate through intercellular spaces29,196.
In contrast to other solid tumours, carcinomas often overexpress mucins, which are transmembrane or secreted glycosylated proteins produced by epithelial cells182. In addition to protecting interface tissues against pathogens, mucins also restrict the activation of potentially damaging inflammatory responses at the epithelial barrier182,197. In tumours, mucins have been shown to mask surface antigens on target tumour cells, limiting antibody binding198 and tumour cell killing by cytotoxic lymphocytes199. Of note, the transmembrane mucin MUC1 is also aberrantly expressed on malignant haematopoietic cells200, suggesting that other cancers could also exploit the immunoregulatory function of mucins to promote growth.
A different architecture is observed in non-carcinoma tumours such as glioblastoma, the most common malignant primary brain cancer lesion in adults, and is characterized by lower cell cohesion and poorly defined tumour margins201. In melanoma, it is also not uncommon to observe small clusters or individual tumour cells interspersed with extracellular matrix molecules in the tumour mass, a feature that is particularly striking in desmoplastic melanoma, which, together with a high tumour mutational burden, may contribute to the increased intratumoural T cell infiltration and increased response to immune checkpoint inhibitors observed in this subtype of melanoma202.
Fig. 2 |. Cellular contributors to tissue-specific antitumour responses.
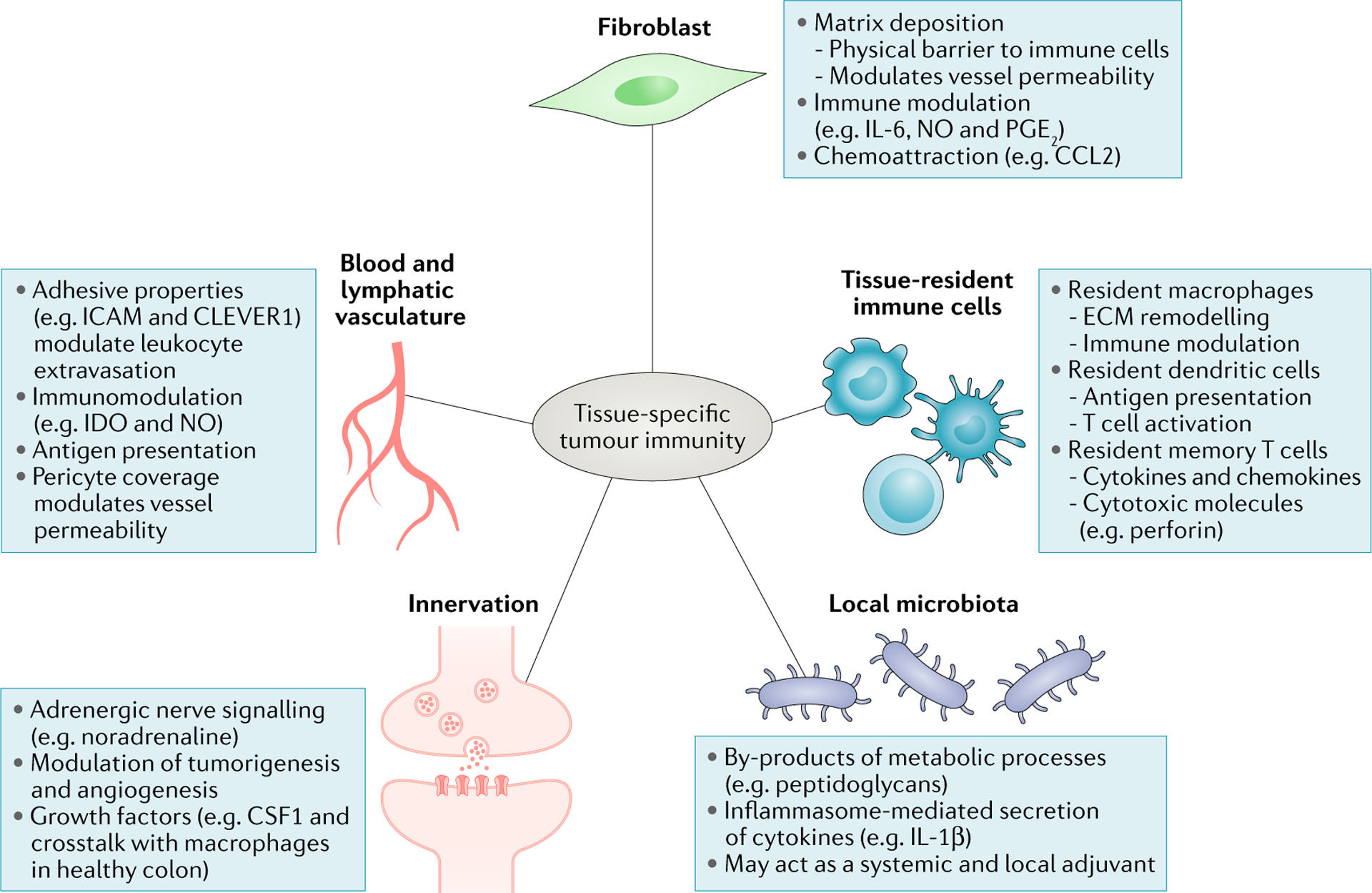
Blood vessels across anatomical sites can differentially control tumour immune infiltrate through distinct levels of expression of adhesion molecules, cohesiveness and pericyte coverage, regulating immune cell extravasation. While the differential impact of lymphatic vessels on the immune infiltrate across cancer types is poorly understood, it is now well established that lymphatics help shape immune responses by promoting tolerance to self-antigens, archiving antigen for later presentation and dampening effector immune responses214–217. Cancer-associated fibroblasts modulate antitumour immune responses by secreting chemokines, cytokines, growth factors and reactive oxygen species, as well as producing and remodelling the stromal matrix that serves both a guiding and a barrier function for immune cells. Emerging evidence shows that commensal bacteria can set the tone of antitumour immune responses both systemically and locally, for example, by stimulating immune cells to secrete inflammatory cytokines. Tissue-resident cells, which include macrophages, dendritic cells and memory T cells, contribute to shaping antitumour immune responses by acting directly on tumour cells or modulating infiltrating immune cells. Nerve cells, whose presence has been reported in a limited number of cancer types, influence tumour cell survival, angiogenesis and the function of immune cells such as macrophages by releasing neurotransmitters and growth factors. CCL2, CC-chemokine 1igand2; CLEVER1, common lymphatic endothelial and vascular endothelial receptor 1; CSF1, colony-stimulating factor 1; ECM, extracellular matrix; ICAM, intercellular cell adhesion molecule; IDO, indoleamine 2,3-dioxygenase; IL, interleukin; NO, nitric oxide; PG E2, prostaglandin E2.
Tissue-specific vascular features.
The vascular system is highly specialized and adapted to the needs of different organs, with differences in cohesiveness, pericyte coverage and expression of adhesion molecules (for example, P-selectin and ICAM1), all of which regulate vessel permeability and immune cell extravasation. For example, the blood vessels of the liver are lined by discontinuous ECs favouring cellular trafficking, whereas the retina and brain endothelia are characterized by numerous tight junctions linking their ECs and limiting immune cell ingress91. Accordingly, ECs isolated from various anatomic sites present distinct gene expression profiles92–94.
In the tumour context, new blood vessels — that primarily sprout from the host organ vasculature95 — are generally altered and characterized by structural abnormalities and increased permeability96. However, several lines of evidence support the presence of tissue-specific vascular features in the TME. The tumour vasculature is sparse and leaky in PDAC lesions, which is thought to be primarily explained by the extensive deposits of ECM molecules in the TM E resulting in interstitial hypertension and vascular compression97 (FIG. 1). By contrast, glioblastomas and RCCs are characterized by high microvascular proliferation68,98 (FIG. 1). In line with the important contribution of host tissue determinants to site-specific tumour vascular features, multiple studies have shown that the tumour vasculature differs substantially between similar tumours growing in different host organs99,100. For instance, human RCC xenografts implanted subcutaneously into nude mice displayed low numbers of vessels, whereas the same tumours injected into the kidney were highly vascularized101. This can be attributed to non-tumour-cell-derived local microenvironment signals, such as vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) by fibroblasts or macrophages101,102, as well as intrinsic site-specific properties of ECs91,92. ECs from distinct tissues exhibit different responses to tissue damage and inflammatory signals93,103, partly owing to the differential expression of pattern recognition receptors as well as receptors for cytokines and growth factors (for example, TNF receptor (TNFR) and VEGF receptor (VEGFR))93,104, which contributes to differences between EC phenotypes across cancers. A mouse study showed that RCC metastases to the liver are more reliant on VEGFR1 to induce angiogenesis than the same metastases to the lung owing to differential activity of VEGFRs on liver versus lung ECs105.
The tumour vasculature contributes to regulating the composition of the immune infiltrate through structural features that control immune cell extravasation and the homing of specific immune cell subsets. For example, hepatic sinusoidal ECs in the inflamed human liver and HCC express high levels of the receptor common lymphatic endothelial and vascular endothelial receptor 1 (CLEVER1; also known as stabilin 1), as well as vascular adhesion protein 1 (VAP1), which have been shown in vitro to favour the selective extravasation of Treg cells106. A similar observation was made with PDAC ECs through the expression of another combination of addressins, including E-selectin and MAdCAM1 (REF107). In vivo studies will be needed to further investigate whether these distinct molecules provide organ-specific signals for Treg cell recruitment to HCC and PDAC lesions. Tumour ECs also shape the tumour immune infiltrate through direct immunosuppression by expressing immunomodulatory molecules such as PDL1 (REF108) or FAS ligand (FASL)90,109. A study analysing tissue microarrays of more than 600 samples of human renal, breast, ovarian, colon, bladder and prostate cancers detected expression of FASL on tumour ECs predominantly in CRC and ovarian cancer lesions, with less frequent expression on RCC and breast tumour ECs109. Further mechanistic analysis showed that FASL-expressing ECs from human ovarian tumours favour immune tolerance by killing effector T cells109.
Cancer-associated fibroblast heterogeneity across tumour types.
Fibroblasts are mesenchymal cells that play a critical role in shaping the structure of organs in homeostasis, through synthesis and remodelling of the tissue ECM110, and in secreting cytokines, chemokines and growth factors, thereby maintaining the homeostasis of adjacent cells and orchestrating the inflammatory response upon tissue damage. Although bone marrow mesenchymal cells contribute to the generation of CAFs in mice111–113, most studies including analysis of bone marrow chimaeras and parabiosis experiments have shown that CAFs derive mainly from local stromal precursors90,112,114,115. Interestingly, transcriptome analyses have emphasized the heterogeneity of the fibroblast network across organs at baseline116–119 and revealed tissue-specific fibroblast programmes, including transcription factors, guidance molecules and growth factors, as well as ECM components and enzymes for ECM remodelling116,118,119. Accordingly, a recent proteomics study that compared ECM molecules from 11 porcine tissues including skin, lung, pancreas and brain revealed major differences in matrix composition and relative abundance across tissue types120.
Beyond the ability of CAFs to modulate tumour cells directly, CAFs also control tumour progression through regulation of tumour immunity90. CXCL12 has been shown to be expressed by CAFs in a broad range of tumour tissues yet linked to different functions, that is, Treg cell attraction as shown in vitro with primary human breast cancer cells121 and T cell exclusion as shown in mouse pancreatic and lung tumour models122. The abundance of CAFs also underlies strong stromal differences across cancers. Breast, colorectal, gastric and pancreatic tumour lesions generally contain a CAF-rich stroma, whereas fibroblasts are found in lower numbers in RCC and brain tumours31,121,123–125. Given the key role of fibroblasts in producing ECM molecules in the TME, the relative CAF abundance can have a strong impact on the immune infiltrate across tumour sites. The stroma represents a high fraction of the tumour mass in desmoplastic cancers such as PDAC, where the abundant CAFs secrete high amounts of ECM molecules, creating a physical barrier of dense fibres that limit immune cell infiltration126. Dynamic imaging of T cells deposited onto fresh human lung tumour slices revealed that in addition to their density, the orientation of stromal fibres parallel to the tumour-stroma boundary controls T cell motility and restrains them from contacting cancer cells29 (FIG. 1).
Compared with solid tumours, haematological tumour lesions localized in the bone marrow or lymph nodes are often less fibrotic, and their looser architecture is thought to facilitate immune cell dynamics and their interactions with neoplastic cells127. A better understanding of CAF regulation of T cell access to tumour sites across cancers is crucial to improve chimeric antigen receptor (CAR) T cell accumulation in solid tumours and to increase the accumulation of endogenous tumour-specific T cells in cancer lesions, which correlates with increased response to immune checkpoint inhibition8,75,128.
We are just starting to unravel the heterogeneity of the CAF compartment33,115,121,129, and further investigation is needed to better decipher CAF differences between tumour types. Several studies in animal models have started to provide insight into the contribution of both tissue and tumour cues in shaping the CAF compartment126,130–133. Data comparing CAFs in skin and cervical tumours driven by the same oncogene showed that despite similarities in oncogene drivers, CAF transcriptional identity differed between the two tissue sites130. Skin CAFs expressed a pro-inflammatory gene signature including genes encoding the two cytokines IL-1β and IL-6 and two chemoattractants for neutrophils and macrophages, CXCL1 and CXCL2, whereas cervical CAFs did not, supporting an important role for the host tissue in regulating CAF phenotype. Accordingly, a comprehensive study of ECM proteins in mouse primary breast tumours and at two metastatic sites — lung and lymph node — showed organ-specific changes in matrix composition131. Importantly, tissue-specific matrix differences have been shown to impact immune cell behaviour, including T cell motility as well as macrophage morphology and polarization29,120,134. However, studies have also revealed similarities in the fibrotic stroma of primary and metastatic lesions of PDAC126,133, which in addition to a direct impact of cancer cells on the host stroma may partly be explained by the fact that tumour cells might travel with stromal cells — bringing their own ‘soil’ — to the metastatic tissue135. Altogether, these data support the dual contribution of both tumour-intrinsic features and external cues mediated by the local environment in shaping the CAF compartment and hence the stromal architecture and immunomodulatory functions across tumour types (FiG. 1).
Other host factors shaping tumour tissue immune cell infiltrates.
The presence of nerves within tumour lesions has been reported in various cancer types, particularly in prostate136, gastrointestinal137,138 and pancreatic139 cancer, with the density of nerve fibres generally associated with poor clinical outcomes136. Several studies have attributed the pro-tumoural effect of innervation to direct neuronal stimulation of tumour cell survival, proliferation and dissemination through release of neurotransmitters such as catecholamines and acetylcholine138–141. More recently, adrenergic nerve-derived noradrenaline has been shown to promote tumour-associated angiogenesis by regulating oxidative metabolism in tumour ECs142. While poorly studied in the TME, nerve-immune cell interactions are well described in healthy and inflamed tissues143, in particular, the crosstalk between neurons and macrophages in gut tissue143–145, where neurotransmitters mainly exert immunosuppressive functions144,146. Mouse studies suggest that targeting nerve fibres or neurotrophic factors may prove useful in the treatment of highly innervated cancer lesions138,139,147. In support of this, it has been suggested that β-blockers, traditionally used for cardiovascular disorders, could increase the survival of patients with cancer, as observed for patients with PDAC and prostate tumours139,148. More studies are needed to investigate the contribution of nerve-immune cell interactions to these effects of β-blockers and to identify novel specific components of the neuro-immune crosstalk to target.
Whereas immune cells in tissues are typically thought to derive from adult haematopoiesis in the bone marrow and be recruited into tissues mostly during tissue injuries, recent studies have revealed that some immune cell populations can remain in tissues for very prolonged periods of time and contribute to tissue homeostasis and integrity independently of blood circulation80 (BOX 2). The most striking example of tissue-resident immune cells are Tr-macs. Most Tr-macs arise from embryonic precursors that are recruited to tissues before birth and are self-renewing independent of adult haematopoiesis149–153. Consistent with their life cycle, Tr-macs are heavily imprinted by tissue-specific cues and express unique transcriptional and enhancer programmes depending on the tissues in which they reside79. In addition to their role in tissue immunity, Tr-macs play a key role in tissue homeostasis. For example, in the gut, Tr-macs respond to microbial cues to enhance Treg cell function154 and to promote intestinal transit through the regulation of enteric neuronal function145. Most tumours produce myeloid cytokines that contribute to the recruitment of circulating monocytes and their differentiation into macrophage-like cells. Although monocyte-derived macrophages and Tr-macs share some phenotypic markers, they have a distinct molecular programme and likely play distinct roles in tumour outcome80. Fate mapping studies from our group and others revealed that TAMs result from a mixture of the two macrophage lineages (Tr-macs that precede tumour formation and monocyte-derived TAMs that are recruited to the tissue upon tumour progression)155 (M.M, unpublished observations). These two TAM lineages likely contribute differently to tumour outcome. For example, as we recently showed in breast cancer lesions, Tr-macs shape early tumour progression and early metastasis156. Fate mapping studies of macrophages in PDAC lesions revealed that embryonically derived Tr-macs exhibited a unique pro-fibrotic transcriptional profile distinct from that of their bone marrow-derived counterparts, which instead exhibited a programme enriched in antigen presentation155. However, the development of TAM-specific targets has proved extremely difficult. This is in large part because we still do not have a complete understanding of the functional diversity of the TAM compartment. This is particularly true for human macrophages, whose biology has primarily been studied using human monocyte-derived macrophages generated in vitro, with limited knowledge of human Tr-macs. We believe that understanding the functional programme of Tr-macs in each type of tumour lesion may help unravel the tissue-specific influence on tumour outcome.
Box 2 |. Tissue-resident immune cells.
Tissue-resident macrophages80, γδ T cells203, memory T (TRM) cells204 and innate lymphocytes205 including natural killer (NK) cells206,207 and mast cells207 can persist in tissues for prolonged periods of time and contribute to shaping the tissue immune tone. The role of tissue-resident CD8+ TRM cells has been particularly studied in the skin208–210 and their accumulation is associated with enhanced antitumour immunity in various human cancer types211–213.
In response to threat from pathogens and injuries and likely also within tumour lesions, tissue-resident immune cells are activated locally and release inflammatory molecules. In the tumour context, these secreted inflammatory molecules may affect early tumour outcome and promote the recruitment of effector immune cells, thus contributing to shaping the microenvironment of tumour lesions.
Recent reports suggest the potential key contribution of the gut microbiome to the modulation of antitumour immunity12,13,157. In these studies, the gut microbiome is thought to modulate tumour immunity through its systemic effect on the immune system158,159. Multiple anatomic sites beyond the gastrointestinal tract are in close association with commensal bacteria. Analyses of microbiota from the gut, skin, lung, vagina and oral cavity have revealed site-specific commensal communities that shape the tissue immune tone160 by acting as local adjuvants setting the threshold of effector cell activation. Several studies demonstrate the role of the local tissue microbiome: in the skin, commensals shape T cell function independently of the gut microorganism s161, and the lung tissue microbiota contributes to respiratory health162, whereas gut microorganisms contribute to Treg cell function and intestinal peristalsis145. A recent mouse study shed light on the role of the local microbiota in shaping tumour-associated immune responses, showing that the lung microbiome promotes lung cancer development by stimulating IL-1β and IL-23 production from myeloid cells, which in turn activate lung-resident γδ T cells163. Thus, in addition to studies of the gut microbiota, further analysis of the local microbiome and its dysbiosis especially in interface tissues such as skin and lung may uncover novel immunoregulatory pathways at the tumour site.
Therapeutic implications
With the growing list of US FDA approvals for antibodies targeting immune checkpoints, it would be tempting from a therapeutic standpoint to dismiss differences described above in the immune microenvironments of these various tissues as minor. However, while revolutionizing cancer treatment, these novel immunotherapies work in only a subset of patients, and overall response rates vary widely on the basis of tissue and stage, suggesting that, while there is some intrinsic tumour susceptibility to the mechanisms of action of these drugs, response is also based on lymphocytic infiltration and presence of immunomodulatory cells or molecules, among some of the commonly proposed biomarkers predicting response8,164. Moreover, whether indolent or aggressive, prevalent diseases like hormone-sensitive breast cancer, prostate cancer or microsatellite-stable (MSS) CRC rarely appear to benefit from current immune checkpoint blockade regimens165,166. Therefore, to maximize the impact of novel immunotherapies as well as conventional treatments on patient care, researchers in the field have honed their efforts on biomarker discovery, in particular, on tissue-based immune monitoring that encompasses immunopheno-typing, functional immune assays, immunogenomics and immunopathology. FIGURE 3 summarizes the tissue specificity of immune, stromal and tumour components and mechanisms and their potential therapeutic implications.
Fig. 3 |. Therapeutic implications of tumour cell-intrinsic and tumour cell-extrinsic factors dependent on tissue specificity.
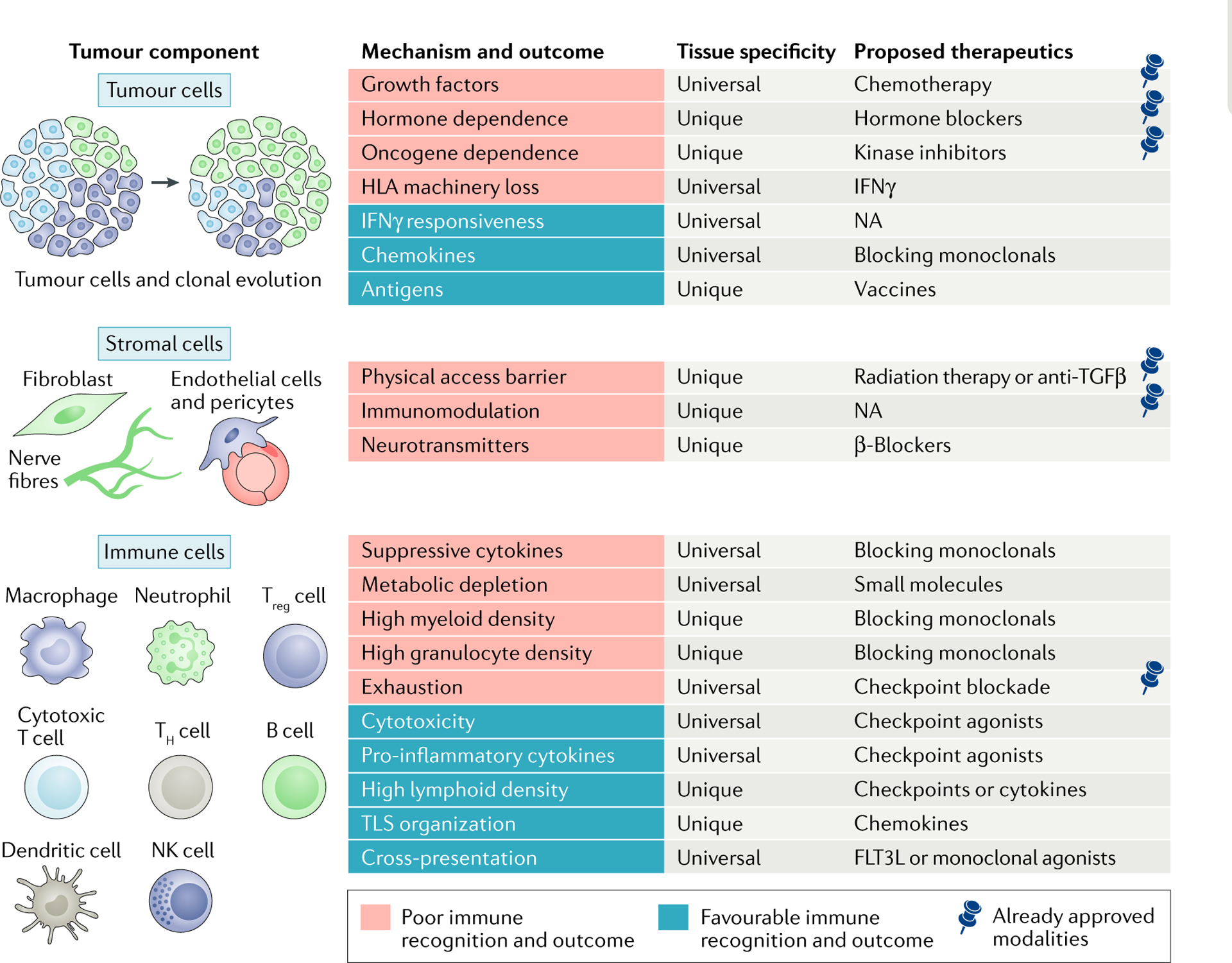
Each tumour component has characteristics leading to poor or favourable immune recognition and outcome. Presence of the pin symbol in the column for proposed therapeutics indicates already approved modalities. FLT3L, FMS-like tyrosine kinase 3 ligand; HLA, human leukocyte antigen; IFNγ, interferon-γ; NK, natural killer; NA, not available; TGFβ, transforming growth factor-β; TH cell, T helper cell; TLS, tertiary lymphoid structure; Treg cell, regulatory T cell.
Improving the accessibility of tissues to increase immunotherapeutic efficacy.
One line of evidence to explain differential immunotherapeutic potential across different solid tumours may simply reside in immune cell accessibility to tissues. For tumour types with relatively poorer lymphocytic infiltration, such as glioblastoma, or for cancers where vast differences are seen from patient to patient, an attractive approach to improve therapeutic effectiveness is to alter the tumour capacity for immune infiltration, in particular, to convert so-called immune deserts or T cell-excluded tumours into highly infiltrated ones. So far, cell-based therapies such as CAR T cells have met the greatest success in haematological malignancies (acute lymphocytic leukaemia, diffuse large B cell lymphoma and multiple myeloma), likely owing to the loosely structured environment of the bone marrow and blood that may help maximize interactions between effectors and their targets (in addition to the prevalence of tissue-restricted surface antigens such as CD19 or B cell maturation antigen (BCMA; also known as TNFRSF17) targeted by these treatments). By contrast, compartmentalized compact solid tumours have not fared as well with adoptive T cell transfer (ACT) alone167,168. In addition to a paucity of truly tumour-specific surface antigens to target, it is likely that transferred effectors may fail to penetrate the tumour either owing to tight stromal packaging and limited vascularization physically limiting ingress or owing to tissue-resident immunomodulators, such as dysregulated chemokine production from myeloid cells or active suppression by Treg cells. Responsiveness to immune checkpoint blockade, which ultimately relies on adaptive immunity locally at the tumour site, provides hope that ACT may eventually work in solid malignancies if the transferred T cells can navigate their way in sufficient numbers to the tumour site and if appropriate antigens can be safely targeted.
Strategies have been explored to bypass the stromal cell and ECM barrier in CAF-rich solid tumours. Unexpectedly, CAF depletion promoted tumour progression in two studies using mouse models of pancreatic cancer, which was attributed to increased angiogenesis and Treg cell infiltration169,170. More encouraging are strategies to reduce stromal ECM97,126,134 or reprogramme CAFs8,132. Promising results were obtained in preclinical studies with drugs targeting matrix molecules, such as enzymatic agents degrading hyaluronan97,126, or CAR T cells expressing the enzyme heparanase, which degrades heparan sulfate proteoglycans, major ECM com ponents134. Lack of response to immune checkpoint blockade has been associated with a transforming growth factor-β (TGFβ) signature in CAFs of several human tumour types including bladder cancer and melanoma8,171, and blocking TGFβ in mouse models of breast cancer increased T cell penetration into the tumour parenchyma, improving tumour response to PDL1 blockade8. A challenge for future development of these types of therapy is to better understand CAF biology, in particular, features and gene expression signatures that distinguish tumour-associated fibroblasts from normal fibroblasts, to specifically target CAFs and minimize adverse events172. Of note, preconditioning strategies that include total body irradiation or preparative chemotherapy regimens have improved clinical efficacy of ACT173. In addition to expansion of transferred cells in the lymphodepleted host and reduction in Treg cells, alteration of the tissue architecture might also contribute to the effect by maximizing T cell infiltration at the tumour site.
Tissue-specific antigenic profiles and response to immune checkpoint blockade.
Antigens known to be recognized by immune effectors include those that are uniquely specific to individual tumours (random passenger mutation-derived neoantigens), those that are shared across various tumour types but distinct from normal tissue (cancer testis, stem cell, endogenous retroviral and oncofetal antigens), those that are broadly expressed tissue-specific targets (differentiation antigens) and those with viral aetiology (for example, human papillomavirus and hepatitis C virus). Each category has proved successful in acting as rejection antigens from case reports of patients with established cancers following ACT and vaccine strategies, thereby establishing proof-of-principle that many different tumour antigens are valid to pursue. The likelihood of a mutation generating a neoepitope increases with a higher number of mutations, and tumours with a carcinogen aetiology (such as ultraviolet light exposure in melanoma and smoking in lung and bladder cancer) are also among the most responsive to immune checkpoint blockade therapy5–7.
Antigenic content could therefore be partially responsible for the differential response to immune checkpoint blockade, with evidence that colon, breast and prostate tumours have less expression of cancer testis antigens than do melanoma, lung or ovarian tumours174. Furthermore, many common cancers where PD1 intervention is futile do not have a lesser mutational burden and fewer potential neoepitopes, as these are within the range of numbers seen in approved tumour types such as bladder and renal cancer or HCC. Tissue-driven antigen content and resulting immunogenicity based on the cell of origin may therefore be more important predictors of lack of response to immune checkpoints than simply the number of mutations. In support of this, a recent paper indicates that MAGEA antigens are negative predictors of CTLA4 but not PD1 response in patients with melanoma independent of TMB175. The underlying mechanism suggested in this study is MAGEA protein-associated suppression of autophagy, a process known to induce potent immune responses by cytokine-mediated priming of effector T cells176. Thus, tumour antigen content and composition will need to be treated in a tissue-specific manner to maximize the rational design of immune-mediated treatments.
Implications of tissue-specific tumour immunity for combination therapies.
The presence of immune effectors in tumours should logically be dependent on antigenicity, as a tumour cell-intrinsic characteristic. Nevertheless, evidence is accumulating that immune infiltration and antigenicity are not always correlated, with similar predicted epitope frequency seen in somatic mutations, differentiation antigens and cancer testis antigens in inflamed and non-inflamed tumours, such as melanoma or myeloma177–179. In colon cancer, a high Immunoscore is of good prognostic value in tumours with both high and low mutational burden, even if patients with MSI-high disease generally have more immune infiltration180. Despite having tumour-infiltrating lymphocytes, adequate antigenicity and susceptible pathways, tumours where PD1 treatment is unsuccessful either fail to have enough of these factors occurring simultaneously or have other dominant characteristics preventing them from responding more frequently. These could either be driven by the tumour itself, such as intrinsic mutations leading to defects or losses in the antigen presentation machinery or in the capacity to attract cross-presenting DCs, or be related to non-tumour idiosyncrasies in the tissue of origin. For example, hormone addiction in prostate and breast tumours driving oncogenic growth181, mucinous layers preventing physical access of T cells in ovarian cancer182 and/or microbiome interactions thwarting proper anti-tumour immune priming in CRC183 should be further explored as potential confounders for the lack of efficacy of immune checkpoint inhibitors.
Patients resistant to or relapsing after immune checkpoint blockade may still benefit from tissue-specific modulation strategies. Targeting of Tr-macs with monoclonal antibodies or small molecules to chemokine receptors or cytokines184 is a promising avenue that could eventually have a clinical impact on tumours in organs with specific myeloid cell prevalence or unique characteristics, such as microglia for glioblastoma or brain metastases.
As discussed earlier in this Review, the combination of EC-intrinsic features and the integration of signals from tumour cells and the TME triggers immunomodulatory pathways in ECs that shape the outcome of anti-tumour immune responses differently across cancer types, which should be considered for the development of therapeutic strategies. RCC, characterized by aberrant angiogenic signalling and a strongly immune tolerogenic environment, could particularly benefit from the combination of vessel modulation and immune checkpoint blockade, as suggested by recent clinical trials185.
Immunotherapies based on T cell activation should in theory be tumour agnostic because they do not directly target the tumour itself. Therefore, even in hormone-sensitive tumours and MSS colon cancer, which rarely benefit from current immune checkpoint blockade regimens, it may be that the right combination of predictive biomarkers is required to select those very rare patients most likely to benefit. The corollary of this hypothesis is that tailored combinatorial interventions that modulate or induce these predictive biomarkers could rescue the lack of activity with immune checkpoints alone in the remaining patients.
In a given tumour, cancer therapies such as chemotherapy186,187, radiotherapy188 or immunotherapy189,190 can remodel the immune contexture by acting directly or indirectly on different components of the tumour ecosystem such as cancer cells, immune or stromal cells or tumour vasculature. Therefore, a global evaluation of inter-patient (between and within given tumour types) and intratumour (spatial) variability will be needed to optimize the choice of interventional approach. Ultimately, therapeutic targeting of solid tumours is most likely to benefit from a biomarker-based combinatorial but tailored use of strategies to drive immune infiltration (targeting tumour-associated fibroblasts or vessels), prevent immunosuppressive mechanisms (immunomodulators) and minimize immune escape (multivalent vaccines).
Conclusions and future perspectives
In this Review, we have presented evidence that the strength of antitumour immunity is influenced not only by the tumour genetics but also by the tissue in which the tumour evolves. Most of our understanding of the human TME stems from low-throughput tissue imaging using immunohistochemistry, immunofluorescence imaging or transcriptional profiling of tumour lesions in bulk (such as TCGA). These methods are unable to capture the complexity of the tumour immune infiltrates, which consist of dozens of cell subtypes whose activation states are highly dependent on tissue location, environmental triggers and host genetics. Recent advances in high-throughput single-cell sequencing and cell profiling technologies are now enabling the cellular and molecular complexity of tumour lesions to be deciphered with unprecedented granularity. This Review emphasizes the need for building comprehensive tumour cell atlases for each cancer type and site at diagnosis, during treatment and upon relapse. The challenge for the near future is to stratify patients on the basis of the molecular and cellular states of the tumour cells and their cellular microenvironment. Deep and dynamic knowledge of the cellular network that constitutes tumour lesions beyond the tumour cell should help inform the mechanisms of resistance to treatment and transform the rational design of combination therapy trials.
Uveal melanoma
The most common primary cancer of the eye in adults, arising from melanocytes located in the uvea (which comprises the choroid, the ciliary body and the iris).
Peripheral node addressin
(PNAd). A sulfated protein at the surface of high endothelial venules in secondary and tertiary lymphoid tissues that is crucial for the homing of naive and central memory T cells.
Lymphoid neogenesis
The de novo formation of ectopic lymphoid structures within peripheral tissues during chronic inflammation.
Lymphotoxin
A cytokine expressed by lymphoid tissue inducer cells and lymphocytes. in lymphoid neogenesis, lymphotoxin-α(LTα) forms a complex with LTβ to bind to the LTβ receptor on stromal cells, leading to nuclear factor-κB (NF-κB) signalling, which promotes the production of chemokines necessary for T cell and B cell recruitment.
Parabiosis
The surgical union of two organisms leading to the sharing of blood circulation, enabling the assessment of the recruitment and contribution of blood circulating cells to a cellular compartment in a tissue or lesion.
β-blockers
Drugs blocking β-adrenergic signalling on nerve cells, causing blood vessels to relax and dilate; these are commonly used to treat high blood pressure and other cardiac conditions.
Tumour agnostic
Compatible with any tumour type.
Multivalent vaccines
Vaccines designed to immunize against two or more antigens.
Acknowledgements
The laboratory of M.M. is supported by funding from R01 CA154947, R01 CA190400, R01 AI113221, U24 AI118644, U19 AI117873 and U19 AI128949. S.G. is supported by R01 CA224319 and CA190174 grants. The authors thank G. Akturk, A. O. Kamphorst and J. Martin for helpful comments, and S. Maskey for her help with generating figures.
Footnotes
Competing interests
H.S. receives research funding from Takeda and Genentech. R.R. is an employee of Innate Pharma. S.G. reports consultancy and/or advisory roles for Merck, Neon Therapeutics and OncoMed and research funding from Bristol-Myers Squibb, Genentech, Immune Design, Agenus, Janssen R&D and Pfizer. M.M. receives funding from Regeneron, Takeda, Genentech and Boehringer Ingelheim.
References
- 1.Ribas A & Wolchok JD Cancer immunotherapy using checkpoint blockade. Science 359, 1350–1355 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brahmer JR et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med 366, 2455–2465 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hodi FS et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med 363, 711–723 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zou W, Wolchok JD & Chen L PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci. Transl Med 8, 328rv324 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rizvi NA et al. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 348, 124–128 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Snyder A et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N. Engl. J. Med 371, 2189–2199 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Allen EM et al. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science 350, 207–211 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mariathasan S et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 554, 544–548 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le DT et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 357, 409–413 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spranger S & Gajewski TF Impact of oncogenic pathways on evasion of antitumour immune responses. Nat. Rev. Cancer 18, 139–147 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alexandrov LB et al. Signatures of mutational processes in human cancer. Nature 500, 415–421 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gopalakrishnan V et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 359, 97–103 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Routy B et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 359, 91–97 (2018). [DOI] [PubMed] [Google Scholar]
- 14.Zhang L et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N. Engl. J. Med 348, 203–213 (2003). [DOI] [PubMed] [Google Scholar]
- 15.Naito Y et al. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 58, 3491–3494 (1998). [PubMed] [Google Scholar]
- 16.Jimenez-Sanchez A et al. Heterogeneous tumor-immune microenvironments among differentially growing metastases in an ovarian cancer patient. Cell 170, 927–938 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; This is a case report showing that different tumour immune microenvironments can coexist in a single patient at primary and metastatic sites.
- 17.Lee M et al. Presence of tertiary lymphoid structures determines the level of tumor-infiltrating lymphocytes in primary breast cancer and metastasis. Mod. Pathol 32, 70–80 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Lehmann B et al. Tumor location determines tissue-specific recruitment of tumor-associated macrophages and antibody-dependent immunotherapy response. Sci. Immunol 2, eaah6413 (2017). [DOI] [PubMed] [Google Scholar]
- 19.Balkwill FR, Capasso M & Hagemann T The tumor microenvironment at a glance. J. Cell Sci 125, 5591–5596 (2012). [DOI] [PubMed] [Google Scholar]
- 20.Quail DF & Joyce JA Microenvironmental regulation of tumor progression and metastasis. Nat. Med 19, 1423–1437 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kargl J et al. Neutrophils dominate the immune cell composition in non-small cell lung cancer. Nat. Commun 8, 14381 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsujikawa T et al. Quantitative multiplex immunohistochemistry reveals myeloid-inflamed tumor-immune complexity associated with poor prognosis. Cell Rep. 19, 203–217 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ali HR, Chlon L, Pharoah PD, Markowetz F & Caldas C Patterns of immune infiltration in breast cancer and their clinical implications: a gene-expression-based retrospective study. PLOS Med. 13, e1002194 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gentles AJ et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat. Med 21, 938–945 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study highlights the diversity of the TME across tumour types and the impact of tumour heterogeneity on patient outcomes.
- 25.Biton J et al. TP53, STK11 and EGFR mutations predict tumor immune profile and the response to anti-PD-1 in lung adenocarcinoma. Clin. Cancer Res 24, 5710–5723 (2018). [DOI] [PubMed] [Google Scholar]
- 26.Lavin Y et al. Innate immune landscape in early lung adenocarcinoma by paired single-cell analyses. Cell 169, 750–765 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Platonova S et al. Profound coordinated alterations of intratumoral NK cell phenotype and function in lung carcinoma. Cancer Res. 71, 5412–5422 (2011). [DOI] [PubMed] [Google Scholar]
- 28.Bindea G et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity 39, 782–795 (2013). [DOI] [PubMed] [Google Scholar]
- 29.Salmon H et al. Matrix architecture defines the preferential localization and migration of T cells into the stroma of human lung tumors. J. Clin. Invest 122, 899–910 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thorsson V et al. The immune landscape of cancer. Immunity 48, 812–830 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; This is an extensive immunogenomic analysis of over 10,000 patients with 33 different cancer types that is accomplished by utilizing data compiled by TCGA. Also visit CRI iAtlas.
- 31.Becht E et al. Estimating the population abundance of tissue-infiltrating immune and stromal cell populations using gene expression. Genome Biol. 17, 218 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heppt MV et al. Immune checkpoint blockade for unresectable or metastatic uveal melanoma:a systematic review. Cancer Treat. Rev 60, 44–52 (2017). [DOI] [PubMed] [Google Scholar]
- 33.Puram SV et al. Single-cell Transcriptomic analysis of primary and metastatic tumor ecosystems in head and neck cancer. Cell 171, 1611–1624 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tirosh I et al. Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science 352, 189–196 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Komura T et al. Inflammatory features of pancreatic cancer highlighted by monocytes/macrophages and CD4+ T cells with clinical impact. Cancer Sci. 106, 672–686 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beatty GL et al. Exclusion of T cells from pancreatic carcinomas in mice is regulated by Ly6Clow F4/80+ extratumoral macrophages. Gastroenterology 149, 201–210 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Royal RE et al. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J. Immunother 33, 828–833 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Merad M & Manz MG Dendritic cell homeostasis. Blood 113, 3418–3427 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bell D et al. In breast carcinoma tissue, immature dendritic cells reside within the tumor, whereas mature dendritic cells are located in peritumoral areas. J. Exp. Med 190, 1417–1426 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cipponi A et al. Neogenesis of lymphoid structures and antibody responses occur in human melanoma metastases. Cancer Res. 72, 3997–4007 (2012). [DOI] [PubMed] [Google Scholar]
- 41.Dieu-Nosjean MC et al. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J. Clin. Oncol 26, 4410–4417 (2008). [DOI] [PubMed] [Google Scholar]
- 42.Germain C et al. Presence of B cells in tertiary lymphoid structures is associated with a protective immunity in patients with lung cancer. Am. J. Respir. Crit. Care Med 189, 832–844 (2014). [DOI] [PubMed] [Google Scholar]
- 43.Mlecnik B et al. Integrative analyses of colorectal cancer show Immunoscore is a stronger predictor of patient survival than microsatellite instability. Immunity 44, 698–711 (2016). [DOI] [PubMed] [Google Scholar]
- 44.Montfort A et al. A strong B cell response is part of the immune landscape in human high-grade serous ovarian metastases. Clin. Cancer Res 23, 250–262 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buisseret L et al. Tumor-infiltrating lymphocyte composition, organization and PD-1/PD-L1 expression are linked in breast cancer. Oncoimmunology 6, e1257452 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Calderaro J et al. Intra-tumoral tertiary lymphoid structures are associated with a low risk of early recurrence of hepatocellular carcinoma. J. Hepatol 70, 58–65 (2019). [DOI] [PubMed] [Google Scholar]
- 47.Fridman WH, Zitvogel L, Sautes-Fridman C & Kroemer G The immune contexture in cancer prognosis and treatment. Nat. Rev. Clin. Oncol 14, 717–734 (2017). [DOI] [PubMed] [Google Scholar]
- 48.Hiraoka N et al. Intratumoral tertiary lymphoid organ is a favourable prognosticator in patients with pancreatic cancer. Br. J. Cancer 112, 1782–1790 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Engelhard VH et al. Immune cell infiltration and tertiary lymphoid structures as determinants of antitumor immunity. J. Immunol 200, 432–442 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ladanyi A et al. Prognostic impact of B cell density in cutaneous melanoma. Cancer Immunol. Immunother 60, 1729–1738 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martinet L et al. High endothelial venules (HEVs) in human melanoma lesions: major gateways for tumor-infiltrating lymphocytes. Oncoimmunology 1, 829–839 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Baren N, Baurain JF & Coulie PG Lymphoid neogenesis in melanoma: what does it tell us? Oncoimmunology 2, e22505 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barone F et al. Stromal fibroblasts in tertiary lymphoid structures: a novel target in chronic inflammation. Front. Immunol 7, 477(2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aloisi F & Pujol-Borrell R Lymphoid neogenesis in chronic inflammatory diseases. Nat. Rev. Immunol 6, 205–217 (2006). [DOI] [PubMed] [Google Scholar]
- 55.Buckley CD, Barone F, Nayar S, Benezech C & Caamano J Stromal cells in chronic inflammation and tertiary lymphoid organ formation. Annu. Rev. Immunol 33, 715–745 (2015). [DOI] [PubMed] [Google Scholar]
- 56.Marinkovic T et al. Interaction of mature CD3+CD4+T cells with dendritic cells triggers the development of tertiary lymphoid structures in the thyroid. J. Clin. Invest 116, 2622–2632 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sautes-Fridman C et al. Tertiary lymphoid structures in cancers: prognostic value, regulation, and manipulation for therapeutic intervention. Front. Immunol 7, 407 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weinstein AM & Storkus WJ Therapeutic lymphoid organogenesis in the tumor microenvironment. Adv. Cancer Res 128, 197–233 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Colbeck EJ et al. Treg depletion licensesT cell-driven HEV neogenesis and promotes tumor destruction. Cancer Immunol. Res 5, 1005–1015 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kocks JR, Davalos-Misslitz AC, Hintzen G,Ohl L & Forster R Regulatory T cells interfere with the development of bronchus-associated lymphoid tissue. J. Exp. Med 204, 723–734 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.de Chaisemartin L et al. Characterization of chemokines and adhesion molecules associated with T cell presence in tertiary lymphoid structures in human lung cancer. Cancer Res. 71, 6391–6399 (2011). [DOI] [PubMed] [Google Scholar]
- 62.Fleige H et al. IL-17-induced CXCL12 recruits B cells and induces follicle formation in BALT in the absence of differentiated FDCs. J. Exp. Med 211, 643–651 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kuroda E et al. Inhaled fine particles induce alveolar macrophage death and interleukin-1 a release to promote inducible bronchus-associated lymphoid tissue formation. Immunity 45, 1299–1310 (2016). [DOI] [PubMed] [Google Scholar]
- 64.Silina K et al. Germinal centers determine the prognostic relevance of tertiary lymphoid structures and are impaired by corticosteroids in lung squamous cell carcinoma. Cancer Res. 78, 1308–1320(2018). [DOI] [PubMed] [Google Scholar]
- 65.Denton AE et al. Type I interferon induces CXCL13 to support ectopic germinal center formation. J. Exp. Med 10.1084/JEM.20181216 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pages F et al. International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet 391, 2128–2139 (2018). [DOI] [PubMed] [Google Scholar]; This paper provides international validation of the Immunoscore as a predictive tool for recurrence of disease in CRC.
- 67.Petitprez F et al. PD-L1 expression and CD8+ T cell infiltrate are associated with clinical progression in patients with node positive prostate cancer. Eur. Urol. Focus https://doi.org/10.1016Zj.euf.2017.05.013 (2017). [DOI] [PubMed] [Google Scholar]
- 68.Giraldo NA et al. Orchestration and prognostic significance of immune checkpoints in the microenvironment of primary and metastatic renal cell cancer. Clin. Cancer Res 21, 3031–3040 (2015). [DOI] [PubMed] [Google Scholar]
- 69.Giraldo NA et al. Tumor-infiltrating and peripheral blood T cell immunophenotypes predict early relapse in localized clear cell renal cell carcinoma. Clin. Cancer Res 23, 4416–4428 (2017). [DOI] [PubMed] [Google Scholar]
- 70.Roberts EW et al. Critical role for CD103+/CD141 + dendritic cells bearing CCR7 for tumor antigen trafficking and priming of T cell immunity in melanoma. Cancer Cell 30, 324–336 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Salmon H et al. Expansion and activation of CD103+ dendritic cell progenitors at the tumor site enhances tumor responses to therapeutic PD-L1 and BRAF inhibition. Immunity 44, 924–938 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Spranger S, Dai D, Horton B & Gajewski TF Tumor-residing Batf3 dendritic cells are required for effector T cell trafficking and adoptive T cell therapy. Cancer Cell 31, 711–723 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sanchez-Paulete AR et al. Cancer immunotherapy with immunomodulatory anti-CD137 and anti-PD-1 monoclonal antibodies requires BATF3-dependent dendritic cells. Cancer Discov. 6, 71–79 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Broz ML et al. Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell 26, 938 (2014). [DOI] [PubMed] [Google Scholar]
- 75.Herbst RS et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515, 563–567 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tumeh PC et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515, 568–571 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Remark R et al. In-depth tissue profiling using multiplexed immunohistochemical consecutive staining on single slide. Sci. Immunol 1, aaf6925 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Barry KC et al. A natural killer-dendritic cell axis defines checkpoint therapy-responsive tumor microenvironments. Nat. Med 24, 1178–1191 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; This is an elegant study showing the importance of the crosstalk between DCs and NK cells in enhancing the antitumour T cell response during immunotherapy.
- 79.Lavin Y et al. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell 159, 1312–1326 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the first study to establish the epigenetic profile of Tr-macs and to show that most of their transcriptional programme is specific to the tissue of residence.
- 80.Lavin Y, Mortha A, Rahman A & Merad M Regulation of macrophage development and function in peripheral tissues. Nat. Rev. Immunol 15, 731–744 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Eckl J et al. Transcript signature predicts tissue NK cell content and defines renal cell carcinoma subgroups independent of TNM staging. J. Mol. Med 90, 55–66 (2012). [DOI] [PubMed] [Google Scholar]
- 82.Coca S et al. The prognostic significance of intratumoral natural killer cells in patients with colorectal carcinoma. Cancer 79, 2320–2328 (1997). [DOI] [PubMed] [Google Scholar]
- 83.Chiossone L, Dumas PY, Vienne M & Vivier E Natural killer cells and other innate lymphoid cells in cancer. Nat. Rev. Immunol 18, 671–688 (2018). [DOI] [PubMed] [Google Scholar]
- 84.Finnberg N, Klein-Szanto AJ & El-Deiry WS TRAIL-R deficiency in mice promotes susceptibility to chronic inflammation and tumorigenesis. J. Clin. Invest 118, 111–123 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Halfteck GG et al. Enhanced in vivo growth of lymphoma tumors in the absence of the NK-activating receptor NKp46/NCR1. J. Immunol 182,2221–2230 (2009). [DOI] [PubMed] [Google Scholar]
- 86.Bottcher JP et al. NK cells stimulate recruitment of cDC1 into the tumor microenvironment promoting cancer immune control. Cell 172, 1022–1037 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Carrega P et al. Natural killer cells infiltrating human nonsmall-cell lung cancer are enriched in CD56 bright CD16(-) cells and display an impaired capability to kill tumor cells. Cancer 112, 863–875 (2008). [DOI] [PubMed] [Google Scholar]
- 88.Keren L et al. A structured tumor-immune microenvironment in triple negative breast cancer revealed by multiplexed ion beam imaging. Cell 174, 1373–1387 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Andre P et al. Anti-NKG2A mAb is a checkpoint inhibitor that promotes anti-tumor immunity by unleashing both T and NK cells. Cell 175, 1731–1743 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Turley SJ, Cremasco V & Astarita JL Immunological hallmarks of stromal cells in the tumour microenvironment. Nat. Rev. Immunol 15, 669–682 (2015). [DOI] [PubMed] [Google Scholar]
- 91.Aird WC Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ. Res 100, 158–173 (2007). [DOI] [PubMed] [Google Scholar]
- 92.Chi JT et al. Endothelial cell diversity revealed by global expression profiling. Proc. Natl Acad. Sci. USA 100, 10623–10628 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nolan DJ et al. Molecular signatures of tissue-specific microvascular endothelial cell heterogeneity in organ maintenance and regeneration. Dev. Cell 26, 204–219 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Seaman S et al. Genes that distinguish physiological and pathological angiogenesis. Cancer Cell 11, 539–554 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Carmeliet P & Jain RK Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat. Rev. Drug Discov 10, 417–427 (2011). [DOI] [PubMed] [Google Scholar]
- 96.Jain RK Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 307, 58–62 (2005). [DOI] [PubMed] [Google Scholar]
- 97.Jacobetz MA et al. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut 62, 112–120 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Brem S The role of vascular proliferation in the growth of brain tumors. Clin. Neurosurg 23, 440–453 (1976). [DOI] [PubMed] [Google Scholar]
- 99.Fidler IJ Angiogenic heterogeneity: regulation of neoplastic angiogenesis by the organ microenvironment. J. Natl Cancer Inst 93, 1040–1041 (2001). [DOI] [PubMed] [Google Scholar]
- 100.Roberts WG et al. Host microvasculature influence on tumor vascular morphology and endothelial gene expression. Am. J. Pathol 153, 1239–1248 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Singh RK et al. Organ site-dependent expression of basic fibroblast growth factor in human renal cell carcinoma cells. Am. J. Pathol 145, 365–374 (1994). [PMC free article] [PubMed] [Google Scholar]
- 102.Fukumura D et al. Tumor induction of VEGF promoter activity in stromal cells. Cell 94, 715–725 (1998). [DOI] [PubMed] [Google Scholar]
- 103.Eppihimer MJ, Wolitzky B, Anderson DC, Labow MA & Granger DN Heterogeneity of expression of E- and P-selectins in vivo. Circ. Res 79, 560–569 (1996). [DOI] [PubMed] [Google Scholar]
- 104.Rafii S, Butler JM & Ding BS Angiocrine functions of organ-specific endothelial cells. Nature 529, 316–325 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lee YJ et al. Differential effects of VEGFR-1 and VEGFR-2 inhibition on tumor metastases based on host organ environment. Cancer Res. 70, 8357–8367 (2010) [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 106.Shetty S et al. Common lymphatic endothelial and vascular endothelial receptor-1 mediates the transmigration of regulatory T cells across human hepatic sinusoidal endothelium. J. Immunol 186, 4147–4155 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nummer D et al. Role of tumor endothelium in CD4+ CD25+ regulatory T cell infiltration of human pancreatic carcinoma. J. Natl Cancer Inst 99, 1188–1199 (2007). [DOI] [PubMed] [Google Scholar]
- 108.Lee SS, Bindokas VP & Kron SJ Multiplex three-dimensional optical mapping of tumor immune microenvironment. Sci. Rep 7, 17031 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Motz GT et al. Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nat. Med 20, 607–615 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kalluri R The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 16, 582–598 (2016). [DOI] [PubMed] [Google Scholar]
- 111.Ishii G et al. Bone-marrow-derived myofibroblasts contribute to the cancer-induced stromal reaction. Biochem. Biophys. Res. Commun 309, 232–240 (2003). [DOI] [PubMed] [Google Scholar]
- 112.Quante M et al. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell 19, 257–272 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Raz Y et al. Bone marrow-derived fibroblasts are a functionally distinct stromal cell population in breast cancer. J. Exp. Med 215, 3075–3093 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Arina A et al. Tumor-associated fibroblasts predominantly come from local and not circulating precursors. Proc. Natl Acad. Sci. USA 113, 7551–7556 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study uses bone marrow chimaeras and parabiotic mouse experiments to show that CAFs derive mainly from cell precursors present in the local tissue microenvironment.
- 115.Ohlund D et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J. Exp. Med 214, 579–596 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Buchholz M et al. Transcriptome analysis of human hepatic and pancreatic stellate cells: organ-specific variations of a common transcriptional phenotype. J. Mol. Med 83, 795–805 (2005). [DOI] [PubMed] [Google Scholar]
- 117.Chang HY et al. Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc. Natl Acad. Sci. USA 99, 12877–12882 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Higuchi Y et al. Gastrointestinal fibroblasts have specialized, diverse transcriptional phenotypes: a comprehensive gene expression analysis of human fibroblasts. PLOSONE 10, e0129241 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rinn JL, Bondre C, Gladstone HB, Brown PO & Chang HY Anatomic demarcation by positional variation in fibroblast gene expression programs. PLOS Genet. 2, e119 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Beachley VZ et al. Tissue matrix arrays for high-throughput screening and systems analysis of cell function. Nat. Methods 12, 1197–1204 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper presents a proteomic analysis of ECM molecules purified from 11 porcine tissues, highlighting tissue-specific macrophage responses This paper presents a proteomic analysis of ECM molecules purified from to specific matrix microenvironments.
- 121.Costa A et al. Fibroblast heterogeneity and immunosuppressive environment in human breast cancer. Cancer Cell 33, 463–479 (2018). [DOI] [PubMed] [Google Scholar]
- 122.Feig C et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc. Natl Acad. Sci. USA 110, 20212–20217 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Calon A et al. Stromal gene expression defines poor-prognosis subtypes in colorectal cancer. Nat. Genet 47, 320–329 (2015). [DOI] [PubMed] [Google Scholar]
- 124.Darmanis S et al. Single-cell RNA-seq analysis of infiltrating neoplastic cells at the migrating front of human glioblastoma. Cell Rep. 21, 1399–1410 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fujita H et al. a-Smooth muscle actin expressing stroma promotes an aggressive tumor biology in pancreatic ductal adenocarcinoma. Pancreas 39, 1254–1262 (2010). [DOI] [PubMed] [Google Scholar]
- 126.Provenzano PP et al. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell 21, 418–429 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.D’Aloia MM, Zizzari IG, Sacchetti B, Pierelli L & Alimandi M CAR-T cells: the long and winding road to solid tumors. Cell Death Dis 9, 282 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chen DS & Mellman I Elements of cancer immunity and the cancer-immune set point. Nature 541, 321–330 (2017). [DOI] [PubMed] [Google Scholar]
- 129.Lambrechts D et al. Phenotype molding of stromal cells in the lung tumor microenvironment. Nat. Med 24, 1277–1289 (2018). [DOI] [PubMed] [Google Scholar]
- 130.Erez N, Truitt M, Olson P, Arron ST& Hanahan D Cancer-associated fibroblasts are activated in incipient neoplasia to orchestrate tumor-promoting inflammation in an NF-κB-dependent manner. Cancer Cell 17, 135–147 (2010). [DOI] [PubMed] [Google Scholar]
- 131.Mayorca-Guiliani AE et al. ISDoT: in situ decellularization of tissues for high-resolution imaging and proteomic analysis of native extracellular matrix. Nat. Med 23, 890–898 (2017). [DOI] [PubMed] [Google Scholar]
- 132.Tauriello DVF et al. TGFβ drives immune evasionin genetically reconstituted colon cancer metastasis. Nature 554, 538–543 (2018). [DOI] [PubMed] [Google Scholar]
- 133.Whatcott CJ et al. Desmoplasia in primary tumors and metastatic lesions of pancreatic cancer. Clin. Cancer Res 21, 3561–3568 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Caruana I et al. Heparanase promotes tumor infiltration and antitumor activity of CAR-redirected T lymphocytes. Nat. Med 21, 524–529 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Duda DG et al. Malignant cells facilitate lung metastasis by bringing their own soil. Proc. Natl Acad. Sci. USA 107, 21677–21682 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Magnon C et al. Autonomic nerve development contributes to prostate cancer progression. Science 341, 1236361 (2013). [DOI] [PubMed] [Google Scholar]
- 137.Liebl F et al. The severity of neural invasion is associated with shortened survival in colon cancer. Clin. Cancer Res 19, 50–61 (2013). [DOI] [PubMed] [Google Scholar]
- 138.Zhao CM et al. Denervation suppresses gastric tumorigenesis. Sci. Transl Med 6, 250ra115 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Renz BW et al. β2 adrenergic-neurotrophin feedforward loop promotes pancreatic cancer. Cancer Cell 33, 75–90 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gross ER et al. Neuronal serotonin regulates growth of the intestinal mucosa in mice. Gastroenterology 143, 408–417 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Venkatesh HS et al. Neuronal activity promotes glioma growth through neuroligin-3 secretion. Cell 161, 803–816 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zahalka AH et al. Adrenergic nerves activate an angio-metabolic switch in prostate cancer. Science 358, 321–326 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ley S, Weigert A & Brune B Neuromediators in inflammation—a macrophage/nerve connection. Immunobiology 215, 674–684 (2010). [DOI] [PubMed] [Google Scholar]
- 144.Gabanyi I et al. Neuro-immune interactions drive tissue programming in intestinal macrophages. Cell 164, 378–391 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Muller PA et al. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell 158, 1210 (2014). [DOI] [PubMed] [Google Scholar]
- 146.Wang H et al. Nicotinic acetylcholine receptor a7 subunit is an essential regulator of inflammation. Nature 421, 384–388 (2003). [DOI] [PubMed] [Google Scholar]
- 147.Jobling P et al. Nerve-cancer cell cross-talk: a novel promoter of tumor progression. Cancer Res. 75, 1777–1781 (2015). [DOI] [PubMed] [Google Scholar]
- 148.Grytli HH, Fagerland MW, Fossa SD& Tasken KA Association between use of beta-blockers and prostate cancer-specific survival: a cohort study of 3561 prostate cancer patientswith high-risk or metastatic disease. Eur. Urol 65, 635–641 (2014). [DOI] [PubMed] [Google Scholar]
- 149.Ginhoux F et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330, 841–845 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Guilliams M et al. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. J. Exp. Med 210, 1977–1992 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Schulz C et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 336, 86–90 (2012). [DOI] [PubMed] [Google Scholar]
- 152.Yona S et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 38, 79–91 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hashimoto D et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity 38, 792–804 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mortha A et al. Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science 343, 1249288 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhu Y et al. Tissue-resident macrophages in pancreatic ductal adenocarcinoma originate from embryonic hematopoiesis and promote tumor progression. Immunity 47, 597 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Linde N et al. Macrophages orchestrate breast cancer early dissemination and metastasis. Nat. Commun 9, 21 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Matson V et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science 359, 104–108 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Paulos CM et al. Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling. J. Clin. Invest 117, 2197–2204 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Viaud S et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 342, 971–976 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Costello EK et al. Bacterial community variation in human body habitats across space and time. Science 326, 1694–1697 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Naik S et al. Commensal-dendritic-cell interaction specifies a unique protective skin immune signature. Nature 520, 104–108 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Man WH, de Steenhuijsen Piters WA & Bogaert D The microbiota of the respiratory tract: gatekeeper to respiratory health. Nat. Rev. Microbiol 15, 259–270 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Jin C et al. Commensal microbiota promote lung cancer development via γδ T cells. Cell 10.1016/j.cell.2018.12.040 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; This mouse study shows the impact of the local lung microbiota on lung tumour-associated immune responses.
- 164.Cristescu R et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science 362, eaar3593 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Attalla K, Sfakianos JP & Galsky MD Current role of checkpoint inhibitors in urologic cancers. Cancer Treat. Res 175, 241–258 (2018). [DOI] [PubMed] [Google Scholar]
- 166.Solinas C et al. Targeting immune checkpoints in breast cancer: an update of early results. ESMO Open 2, e000255 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Garber K Driving T cell immunotherapy to solid tumors. Nat. Biotechnol 36, 215–219 (2018). [DOI] [PubMed] [Google Scholar]
- 168.Long KB et al. CAR T cell therapy of nonhematopoietic malignancies: detours on the road to clinical success. Front. Immunol 9, 2740 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ozdemir BC et al. The molecular signature of the stroma response in prostate cancer-induced osteoblastic bone metastasis highlights expansion of hematopoietic and prostate epithelial stem cell niches. PLOSONE 9, e114530 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Rhim AD et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell 25, 735–747 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Chakravarthy A, Khan L, Bensler NP, Bose P & De Carvalho DD TGF-β-associated extracellular matrix genes link cancer-associated fibroblaststo immune evasion and immunotherapy failure. Nat. Commun 9, 4692 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Roberts EW et al. Depletion of stromal cells expressing fibroblast activation protein-alpha from skeletal muscle and bone marrow results in cachexia and anemia. J. Exp. Med 210, 1137–1151 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Yao X et al. Levels of peripheral CD4+FoxP3+ regulatory T cells are negatively associated with clinical response to adoptive immunotherapy of human cancer. Blood 119, 5688–5696 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Gnjatic S et al. NY-ESO-1: review of an immunogenic tumor antigen. Adv. Cancer Res 95, 1–30 (2006). [DOI] [PubMed] [Google Scholar]
- 175.Shukla SA et al. Cancer-germline antigen expression discriminates clinical outcome to CTLA-4 blockade. Cell 173, 624–633 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows that MAGEA expression and ensuing autophagy suppression in metastatic melanoma predict resistance to CTLA4 blockade, but not PD1 blockade, independently of neoantigen load.
- 176.Ghiringhelli F et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1β-dependent adaptive immunity against tumors. Nat. Med 15, 1170–1178 (2009). [DOI] [PubMed] [Google Scholar]
- 177.Spranger S et al. Density of immunogenic antigens does not explain the presence or absence of the T cell-inflamed tumor microenvironment in melanoma. Proc. Natl Acad. Sci. USA 113, E7759–E7768 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows that melanomas with or without T cell signatures have similar numbers of predicted neoantigens owing to point mutations, suggesting that other factors may dictate spontaneous T cell infiltration.
- 178.Braga WM et al. Is there any relationship between gene expression of tumor antigens and CD4+ T cells in multiple myeloma? Immunotherapy 6, 569–575 (2014). [DOI] [PubMed] [Google Scholar]
- 179.Rooney MS, Shukla SA, Wu CJ, Getz G & Hacohen N Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell 160, 48–61 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Maby P et al. Correlation between density of CD8+T cell infiltrate in microsatellite unstable colorectal cancers and frameshift mutations: a rationale for personalized immunotherapy. Cancer Res. 75, 3446–3455 (2015). [DOI] [PubMed] [Google Scholar]
- 181.Coutinho I, Day TK, Tilley WD & Selth LA Androgen receptor signaling in castration-resistant prostate cancer: a lesson in persistence. Endocr. Relat. Cancer 23, T179–T197 (2016). [DOI] [PubMed] [Google Scholar]
- 182.Kufe DW Mucins in cancer: function, prognosis and therapy. Nat. Rev. Cancer 9, 874–885 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zitvogel L, Ayyoub M, Routy B & Kroemer G Microbiome and anticancer immunosurveillance. Cell 165, 276–287 (2016). [DOI] [PubMed] [Google Scholar]
- 184.Molon B et al. Chemokine nitration prevents intratumoral infiltration of antigen-specific T cells. J. Exp. Med 208, 1949–1962(2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Gao X & McDermott DF Combinations of bevacizumab with immune checkpoint inhibitors in renal cell carcinoma. Cancer J. 24, 171–179 (2018). [DOI] [PubMed] [Google Scholar]
- 186.Zitvogel L et al. The anticancer immune response: indispensable for therapeutic success? J. Clin. Invest 118, 1991–2001 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Tsuda N et al. Taxol increases the amount and T cell activating ability of self-immune stimulatory multimolecular complexes found in ovarian cancer cells. Cancer Res. 67, 8378–8387 (2007). [DOI] [PubMed] [Google Scholar]
- 188.Jiang W, Chan CK, Weissman IL, Kim BYS & Hahn SM Immune priming of the tumor microenvironment by radiation. Trends Cancer 2, 638–645 (2016). [DOI] [PubMed] [Google Scholar]
- 189.Riaz N et al. Tumor and microenvironment evolution during immunotherapy with nivolumab. Cell 171, 934–949 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Smyth MJ, Ngiow SF, Ribas A & Teng MW Combination cancer immunotherapies tailored to the tumour microenvironment. Nat. Rev. Clin. Oncol 13, 143–158 (2016). [DOI] [PubMed] [Google Scholar]
- 191.Bosman FT, Havenith M & Cleutjens JP Basement membranes in cancer. Ultrastruct. Pathol 8, 291–304 (1985). [DOI] [PubMed] [Google Scholar]
- 192.Liotta LA et al. Metastatic potential correlates with enzymatic degradation of basement membrane collagen. Nature 284, 67–68 (1980). [DOI] [PubMed] [Google Scholar]
- 193.Dorudi S, Sheffield JP, Poulsom R, Northover JM & Hart IR E-Cadherin expression in colorectal cancer. An immunocytochemical and in situ hybridization study. Am. J. Pathol 142, 981–986 (1993). [PMC free article] [PubMed] [Google Scholar]
- 194.Hoover KB, Liao SY & Bryant PJ Loss of the tight junction MAGUK ZO-1 in breast cancer: relationship to glandular differentiation and loss of heterozygosity. Am. J. Pathol 153, 1767–1773 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Pignatelli M et al. Loss of membranous E-cadherin expression in pancreatic cancer: correlation with lymph node metastasis, high grade, and advanced stage. J. Pathol 174, 243–248 (1994). [DOI] [PubMed] [Google Scholar]
- 196.Wolf K, Muller R, Borgmann S, Brocker EB & Friedl P Amoeboid shape change and contact guidance: T-lymphocyte crawling through fibrillar collagen is independent of matrix remodeling by MMPs and other proteases. Blood 102, 3262–3269 (2003). [DOI] [PubMed] [Google Scholar]
- 197.Shan M et al. Mucus enhances gut homeostasis and oral tolerance by delivering immunoregulatory signals. Science 342, 447–453 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Price-Schiavi SA et al. Rat Muc4 (sialomucin complex) reduces binding of anti-ErbB2 antibodies to tumor cell surfaces, a potential mechanism for herceptin resistance. Int. J. Cancer 99, 783–791 (2002). [DOI] [PubMed] [Google Scholar]
- 199.Komatsu M, Yee L & Carraway KL Overexpression of sialomucin complex, a rat homologue of MUC4, inhibits tumor killing by lymphokine-activated killer cells. Cancer Res. 59, 2229–2236 (1999). [PubMed] [Google Scholar]
- 200.Brossart P et al. The epithelial tumor antigen MUC1 is expressed in hematological malignancies and is recognized by MUC1-specific cytotoxic T-lymphocytes. Cancer Res. 61, 6846–6850 (2001). [PubMed] [Google Scholar]
- 201.Claes A, Idema AJ & Wesseling P Diffuse glioma growth: a guerilla war. Acta Neuropathol. 114, 443–458 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Eroglu Z et al. High response rate to PD-1 blockade in desmoplastic melanomas. Nature 553, 347–350 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Havran WL & Jameson JM Epidermal T cells and wound healing. J. Immunol 184, 5423–5428 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Clark RA et al. Skin effector memory T cells do not recirculate and provide immune protection in alemtuzumab-treated CTCL patients. Sci. Transl Med 4, 117ra117 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Gasteiger G, Fan X, Dikiy S, Lee SY & Rudensky AY Tissue residency of innate lymphoid cells in lymphoid and nonlymphoid organs. Science 350, 981–985 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Peng H et al. Liver-resident NK cells confer adaptive immunity in skin-contact inflammation. J. Clin. Invest 123, 1444–1456 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kumamoto T et al. Hair follicles serve as local reservoirs of skin mast cell precursors. Blood 102, 1654–1660 (2003). [DOI] [PubMed] [Google Scholar]
- 208.Enamorado M et al. Enhanced anti-tumour immunity requires the interplay between resident and circulating memory CD8+ T cells. Nat. Commun 8, 16073 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Malik BT et al. Resident memory T cells in the skin mediate durable immunity to melanoma. Sci. Immunol 2, eaam6346 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Park SL et al. Tissue-resident memory CD8+ T cells promote melanoma-immune equilibrium in skin. Nature 565, 366–371 (2019). [DOI] [PubMed] [Google Scholar]
- 211.Djenidi F et al. CD8+CD103+ tumor-infiltrating lymphocytes are tumor-specific tissue-resident memory T cells and a prognostic factor for survival in lung cancer patients. J. Immunol 194, 3475–3486 (2015). [DOI] [PubMed] [Google Scholar]
- 212.Edwards J et al. CD103+ tumor-resident CD8+ T cells are associated with improved survival in immunotherapy-naive melanoma patients and expand significantly during anti-PD-1 treatment. Clin. Cancer Res 24, 3036–3045 (2018). [DOI] [PubMed] [Google Scholar]
- 213.Webb JR, Milne K, Watson P, Deleeuw RJ & Nelson BH Tumor-infiltrating lymphocytes expressing the tissue resident memory marker CD103 are associated with increased survival in high-grade serous ovarian cancer. Clin. Cancer Res 20, 434–444 (2014). [DOI] [PubMed] [Google Scholar]
- 214.Bordry N et al. Lymphatic vessel density is associated with CD8+ T cell infiltration and immunosuppressive factors in human melanoma. Oncoimmunology 7, e1462878 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Lund AW et al. Lymphatic vessels regulate immune microenvironments in human and murine melanoma. J. Clin. Invest 126, 3389–3402 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Podgrabinska S et al. Inflamed lymphatic endothelium suppresses dendritic cell maturation and function via Mac-1/ICAM-1-dependent mechanism. J. Immunol 183, 1767–1779 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Tamburini BA, Burchill MA & Kedl RM Antigen capture and archiving by lymphatic endothelial cells following vaccination or viral infection. Nat. Commun 5, 3989 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]


