Abstract
Background
Identifying factors associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection among health care workers (HCWs) may help health systems optimize SARS-CoV-2 infection control strategies.
Methods
We conducted a cross-sectional analysis of baseline data from the Northwestern HCW SARS-CoV-2 Serology Cohort Study. We used the Abbott Architect Nucleocapsid IgG assay to determine seropositivity. Logistic regression models (adjusted for demographics and self-reported community exposure to coronavirus disease 2019 [COVID-19]) were fit to quantify the associations between occupation group, health care delivery tasks, and community exposure and seropositive status.
Results
A total of 6510 HCWs, including 1794 nurses and 904 non-patient-facing administrators, participated. The majority were women (79.6%), 74.9% were White, 9.7% were Asian, 7.3% were Hispanic, and 3.1% were non-Hispanic Black. The crude prevalence of seropositivity was 4.8% (95% CI, 4.6%–5.2%). Seropositivity varied by race/ethnicity as well as age, ranging from 4.2% to 9.6%. Out-of-hospital exposure to COVID-19 occurred in 9.3% of HCWs, 15.0% (95% CI, 12.2%–18.1%) of whom were seropositive; those with family members diagnosed with COVID-19 had a seropositivity rate of 54% (95% CI, 44.2%–65.2%). Support service workers (10.4%; 95% CI, 4.6%–19.4%), medical assistants (10.1%; 95% CI, 5.5%–16.6%), and nurses (7.6%; 95% CI, 6.4%–9.0%) had significantly higher seropositivity rates than administrators (referent; 3.3%; 95% CI, 2.3%–4.4%). However, after adjustment, nursing was the only occupation group with a significantly higher odds (odds ratio, 1.9; 95% CI, 1.3–2.9) of seropositivity. Exposure to patients receiving high-flow oxygen therapy and hemodialysis was significantly associated with 45% and 57% higher odds for seropositive status, respectively.
Conclusions
HCWs are at risk for SARS-CoV-2 infection from longer-duration exposures to people infected with SARS-CoV-2 within health care settings and their communities of residence.
Keywords: COVID-19, health care workers, SARS-CoV-2, serology
Health care workers (HCWs) have provided essential front-line care for patients throughout the coronavirus disease 2019 (COVID-19) pandemic at considerable personal risk. Data from the Centers for Disease Control and Prevention (CDC) found that 11% of the total number of reported COVID-19 cases in the United States were HCWs [1]. As of November 2020, there have been 797 deaths among HCWs in the United States, for a mortality rate of 0.39% of those with known infection [2]. Thus, it remains a high priority to identify factors associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in health care settings, so that we may protect the essential workforce that delivers care.
Studies of active and past infection, defined as presence of immunoglobulin G (IgG) antibodies to SARS-CoV-2, report that risks to HCWs come from work-related exposures (patients and coworkers), as well as from the communities in which they live [3]. An ongoing review of HCW infections and impact by Chou et al. (2020) found seroprevalence rates ranging from <2% to >25% depending on timing in the pandemic, location, and type of HCW included [4–7]. The contribution of specific tasks (eg, intubation, bronchoscopy) performed in the care of patients to SARS-CoV-2 infection risk is less clear due to a smaller number of studies that characterized specific occupational tasks and mixed results across studies [7]. There is also growing evidence that community exposure is a strong determinant of HCW COVID-19 risk. For example, in a study from Minnesota, the highest rates of HCW infection (12.5%) were related to an exposure from a household or social setting, whereas high-risk exposures from other HCWs and patients were associated with a 3.5% and 1.3% HCW infection rate, respectively [8].
The city of Chicago experienced an early, prolonged surge in COVID-19 cases and was second only to the Northeastern tri-state region in cases, hospitalizations, and deaths through the end of June 2020 [9]. Coordination and planning ensured that hospital bed capacity was not exceeded and personal protective equipment (PPE) supplies were not exhausted. To date, there have been no reported seroprevalence studies from Chicago that examine the rates of seropositivity in HCWs and their correlates during the first wave of the pandemic in the context of adequate PPE in the patient care environment.
We established the Northwestern Healthcare Worker SARS-CoV-2 Serology Study Cohort in May 2020 to determine the prevalence and correlates of anti-SARS-CoV-2 IgG status. The objective of our study was to describe the prevalence of SARS-CoV-2 seropositivity and correlates by occupational categories, clinical tasks, and sociodemographic characteristics. We collected information about community and household exposures to describe the relative contribution of out-of-hospital SARS-CoV-2 exposures to seropositivity among HCWs. In this manuscript, we report the cross-sectional baseline findings from the cohort study. We hypothesized that HCWs that participated in aerosolizing procedures, those with high COVID-19 patient exposure, and those with self-reported out-of-hospital exposures would have higher prevalence rates of anti-SARS-CoV-2 antibodies than those without these exposures.
METHODS
Study Design and Setting
This investigation is part of an ongoing prospective cohort study of SARS-CoV-2 in patient-facing and non-patient-facing HCWs from a large, tertiary academic health care system that includes 10 hospitals, 18 immediate care centers, and 325 outpatient practices in the Chicago area and surrounding Illinois suburbs. The largest hospital in the health system is located in downtown Chicago, whereas the other 9 regional centers are in the west, northwest, and north suburbs of Chicago. Affiliated outpatient practices and immediate care centers are located in downtown Chicago and the near suburbs.
From May 28, 2020, through June 30, 2020, 38 127 Northwestern Medicine (NM) HCWs (employees and physician members of affiliated outpatient practices) were invited to participate in an employer-sponsored benefit of free SARS-CoV-2 serology assessment and this cohort study. All institutional HCWs were eligible for participation in the benefit (Table 1; Supplementary Table 1). Participation in the research study was not required to receive serology testing results. This study was approved by the Northwestern University Institutional Review Board, and all participants gave written informed consent. Outreach consisted of existing methods of health care system communication including emails and information banners embedded in the health system clinical information website. The email invitation specified 41 locations across Chicago and suburban areas where HCWs could obtain serological testing for SARS-CoV-2 and included information about the cohort study and an electronic link to consent and enroll. Testing was available through July 8, 2020. Due to low enrollment from environmental services, food service, and patient transportation groups, research team members (J.T.W., C.T.E.) conducted 1 in-person recruitment briefing with this group. Twenty-one individuals in these occupation groups subsequently enrolled in the study.
Table 1.
Characteristics of Participants by Occupation Group
| Characteristics | Overall | RN | MD | Administrative Role | Other Occupationsa | |
|---|---|---|---|---|---|---|
| No. | 6510 | 1794 | 1260 | 904 | 2552 | |
| Age category, No. (%) | 18–29 y | 1304 (20.0) | 557 (31.0) | 128 (10.2) | 84 (9.3) | 535 (21.0) |
| 30–39 y | 2208 (33.9) | 519 (28.9) | 527 (41.8) | 296 (32.7) | 866 (33.9) | |
| 40–49 y | 1368 (21.0) | 326 (18.2) | 310 (24.6) | 197 (21.8) | 535 (21.0) | |
| 50–59 y | 1042 (16.0) | 245 (13.7) | 177 (14.0) | 225 (24.9) | 395 (15.5) | |
| 60+ y | 588 (9.0) | 147 (8.2) | 118 (9.4) | 102 (11.3) | 221 (8.7) | |
| Gender, No. (%) | Femaleb | 5180 (79.6) | 1701 (94.8) | 682 (54.1) | 699 (77.3) | 2098 (82.2) |
| Male | 1330 (20.4) | 93 (5.2) | 578 (45.9) | 205 (22.7) | 454 (17.8) | |
| Race, No. (%) | Asian | 634 (9.7) | 125 (7.0) | 283 (22.5) | 50 (5.5) | 176 (6.9) |
| Hispanic/Latino | 477 (7.3) | 105 (5.9) | 49 (3.9) | 63 (7.0) | 260 (10.2) | |
| Multiracial | 136 (2.1) | 23 (1.3) | 33 (2.6) | 20 (2.2) | 60 (2.4) | |
| Non-Hispanic Black | 201 (3.1) | 22 (1.2) | 25 (2.0) | 36 (4.0) | 118 (4.6) | |
| Non-Hispanic White | 4877 (74.9) | 1496 (83.4) | 843 (66.9) | 717 (79.3) | 1821 (71.4) | |
| Other/NA | 185 (2.8) | 23 (1.3) | 27 (2.1) | 18 (2.0) | 117 (4.6) | |
| Diabetes, No. (%) | Yes | 191 (2.9) | 49 (2.7) | 22 (1.7) | 28 (3.1) | 92 (3.6) |
| No | 6189 (95.1) | 1731 (96.5) | 1229 (97.5) | 868 (96.0) | 2361 (92.5) | |
| NA | 130 (2.0) | 14 (0.8) | 9 (0.7) | 8 (0.9) | 99 (3.9) | |
| Obesity, No. (%) | Yes | 982 (15.1) | 283 (15.8) | 89 (7.1) | 171 (18.9) | 439 (17.2) |
| No | 5382 (82.7) | 1492 (83.2) | 1163 (92.3) | 725 (80.2) | 2002 (78.4) | |
| NA | 146 (2.2) | 19 (1.1) | 8 (0.6) | 8 (0.9) | 111 (4.3) | |
| High blood pressure, No. (%) | Yes | 800 (12.3) | 197 (11.0) | 122 (9.7) | 151 (16.7) | 330 (12.9) |
| No | 5581 (85.7) | 1575 (87.8) | 1130 (89.7) | 749 (82.9) | 2127 (83.3) | |
| NA | 129 (2.0) | 22 (1.2) | 8 (0.6) | 4 (0.4) | 95 (3.7) |
Abbreviation: NA, not answered.
aFor complete list of occupational groups included in the “other occupations” group, please see Supplementary Table 1.
bIncludes <10 who did not self-identify.
A total of 38 127 NM HCWs received email invitations to participate in the employee benefit to have serology checked: 18 985 (49.8%) participated in the employer-sponsored serology benefit. Among the latter group, 6714 (35.4%) enrolled in the cohort study. After exclusions for withdrawal of consent, no baseline survey data completed, or inability of the research team to verify the identity of the participant or view serology results (n = 204), 6510 participants comprised the final study sample (Supplementary Figure 1).
SARS-CoV-2 IgG Assay Testing
Blood samples were collected by a trained phlebotomist. The SARS-CoV-2 IgG assay on the high-throughput ARCHITECT i2000SR Immunoassay System from Abbott Laboratories (Abbott Park, IL, USA) was used. The SARS-CoV-2 IgG assay is a qualitative, chemiluminescent microparticle immunoassay that identifies whether human serum or plasma has IgG antibodies to SARS-CoV-2 nucleocapsid antigen. Performance characteristics for this assay are reported to be 100% positive agreement at ≥14 days post–symptom onset in those with confirmed COVID-19 and 99.6% negative agreement in those without COVID-19 [10].
Health System Infection Control Procedures
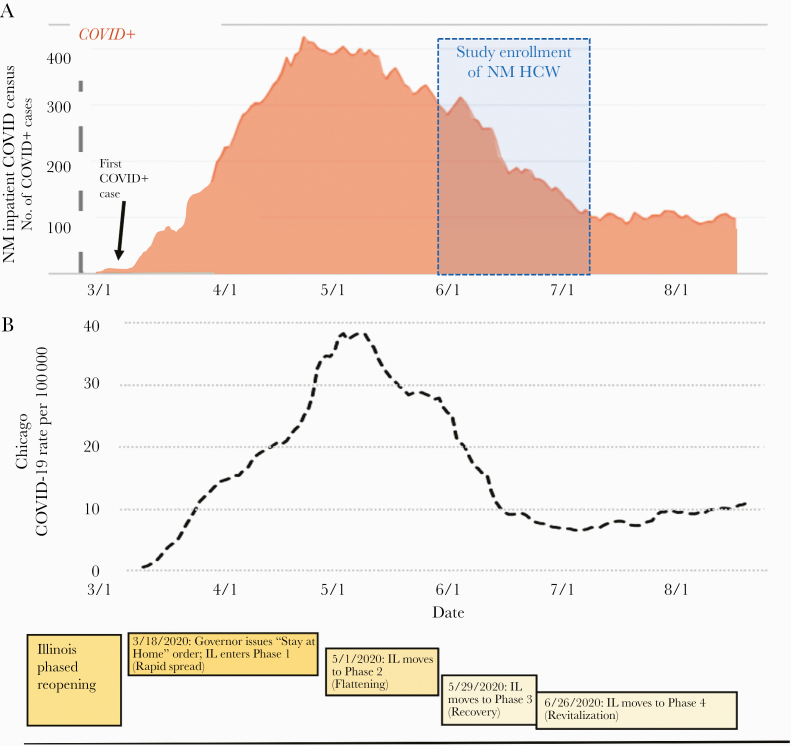
Since January 2020, droplet isolation precautions have been used on all patients at NM with known or suspected COVID-19. N95 respirators were recommended for aerosol-generating procedures. Universal masking was initiated in late March. NM had adequate PPE available for use by all staff at all times. In early March, COVID-19 inpatients were cared for in COVID-19 floors and intensive care units. Remote working was mandated whenever possible for all HCWs. NM inpatient COVID-19 census and Chicago cases are shown in Figure 1.
Figure 1.
Timeline of Northwestern Medicine COVID-19 Inpatient Census, Chicago case rate, and state government response during the local accelerated phase of the pandemic. A, Northwestern Medicine COVID-19 in-patient admitted patients from 3/1/2020 through 8/6/2020. B, Chicago COVID-19 case rate by date. Cases are presented as case/100 000 population. Abbreviations: COVID-19, coronavirus disease 2019; NM HCW, Northwestern Medicine health care worker.
Measures
The baseline survey collected self-reported data on demographics (age, gender, race/ethnicity, job position, and home zip code); medical history and comorbidities; history of COVID symptoms; history of SARS-CoV-2 testing and diagnosis of COVID-19; health care and non–health care exposures to COVID-19; work-related tasks; whether the respondent cared for COVID-19 patients; the use of PPE during exposures (select survey questions available in Supplementary Figure 2). Participants were categorized into 4 broad occupational classes: (1) physicians; (2) nurses; (3) administrators; and (4) other occupations (Supplementary Table 1). The survey was developed using adapted questions from the World Health Organization COVID-19 HCW and seroprevalence protocols [11].
Statistical Analysis
We estimated the demographics of the sampling frame by creating a weighted average of demographic data provided by the NM Human Resources Department (NM employees) and McGaw Medical Center (NM residents and fellows). Due to under-representation of Hispanic and Non-Hispanic Black participants in our cohort, we used inverse probability weighting to estimate the prevalence of IgG-positive serologic status within NM HCWs. The prevalence of IgG seropositivity for all NM HCWs who had serology evaluated in the spring (inclusive of cohort participants) was identified to determine if the overall prevalence differed from those who participated. No other data on nonenrollees were available for comparison.
To create stable estimates and statistical models and preserve participant anonymity, several occupation groups with <50 participants were pooled together based on the research team’s perceived likelihood of SARS-CoV-2 exposure at work for each group (ie, similarity in degree of patient contact and tasks performed) (Supplementary Table 1).
Up to 2.2% of survey participants had at least 1 incomplete question for demographics, occupation group, and patient care–related tasks, and 4% were missing responses for symptoms. We excluded participants with missing data when the missing variable was the primary exposure of interest in a given model.
The prevalence rates and 95% CIs of HCWs with positive IgG antibodies to SARS-CoV-2 were calculated using exact binomial methods and described by age, gender, race/ethnicity, job position, patient care tasks, and COVID-19 exposures. Administrators were included as the referent group in occupation analyses to reflect exposure consistent with non-HCWs. To assess the independent associations between each of the covariates and seropositivity, we calculated odds ratios (ORs) and 95% CIs from logistic regression models adjusted for age, sex, race/ethnicity, and self-reported out-of-hospital exposure to COVID-19. Age, sex, and race/ethnicity were included as adjustment factors because of the known association between these variables and COVID-19 and occupation group. We used Holm’s procedures to adjust for multiple testing in the 14 patient care task groups and symptom questions [12]. The influence of variability in community spread was investigated by mapping employees’ residential address zip code with Illinois Department of Public Health COVID-19 case reporting data from June 15, 2020. All analyses were conducted using R, version 3.6.0 (R Core Team).
RESULTS
The cohort included 79.6% women and 74.9% non-Hispanic White, 9.7% Asian, 7.3% Hispanic, and 3.1% non-Hispanic Black participants; the mean (SD) age was 40.6 (12.0) years. The largest occupation groups sampled were nurses (n = 1794), physicians (n = 1260), and administrators (n = 904). The demographics of occupation groups are shown in Table 1 and Supplementary Table 1.
The crude overall prevalence rate of anti-SARS CoV-2 IgG-positive status was 4.8% (95% CI, 4.6%–5.2%). The inverse probability weighted value (adjusted for the race/ethnicity of the sampling frame) was 5.3% (95% CI, 4.8%–5.9%). The crude seropositivity rate was 3.6% among all NM employees who had their serology checked in the spring of 2020.
Sociodemographics by Seropositivity
Participants between 18 and 29 years old had higher seropositive rates than older age groups (7.4%; 95% CI, 6.1%–9.0%; vs 4.2%; 95% CI, 3.7%–4.8%) (Table 2). Hispanic and non-Hispanic Black participants had the highest IgG+ prevalence rates, 9.6% (95% CI, 7.1%–12.7%) and 8.5% (95% CI, 5%–13.2%), respectively. Asian and White HCWs had prevalence rates of 4.6% (95% CI, 3.1%–6.5%) and 4.3% (95% CI, 3.8%–5.0%), respectively. There were no significant differences in the seropositive rates across gender or self-reported history of diabetes, hypertension, and obesity.
Table 2.
Participant Characteristics by Seropositive Group (n = 6510)
| Characteristics | IgG Positive (%, 95% CI) | Total, No. (%) |
|---|---|---|
| Age category | ||
| No. | 316 | 6510 |
| 18–29 y | 97 (7.4, 6.1–9) | 1304 (20.0) |
| 30–39 y | 97 (4.4, 3.6–5.3) | 2208 (33.9) |
| 40–49 y | 60 (4.4, 3.4–5.6) | 1368 (21.0) |
| 50–59 y | 47 (4.5, 3.3–6) | 1042 (16.0) |
| 60+ y | 15 (2.6, 1.4–4.2) | 588 (9.0) |
| Gender | ||
| No. | 316 | 6510 |
| Femalea | 256 (4.9, 4.4–5.6) | 5180 (79.6) |
| Male | 60 (4.5, 3.5–5.8) | 1330 (20.4) |
| Race/ethnicity | ||
| No. | 316 | 6510 |
| Asian | 29 (4.6, 3.1–6.5) | 634 (9.7) |
| Hispanic/Latino | 46 (9.6, 7.1–12.7) | 477 (7.3) |
| Non-Hispanic Black | 17 (8.5, 5–13.2) | 201 (3.1) |
| Non-Hispanic White | 212 (4.3, 3.8–5) | 4877 (74.9) |
| Other/multiracial/NA | 12 (3.7, 1.9–6.4) | 321 (4.9) |
| Obesity | ||
| No | 271 (5.0, 4.5–5.7) | 5382 (84.6) |
| Yes | 43 (4.4, 3.2–5.9) | 982 (15.4) |
| High blood pressure | ||
| No. | 312 | 6381 |
| No | 285 (5.1, 4.5–5.7) | 5581 (87.5) |
| Yes | 27 (3.4, 2.2–4.9) | 800 (12.5) |
| Diabetes | ||
| No. | 315 | 6380 |
| No | 301 (4.9, 4.3–5.4) | 6189 (97.0) |
| Yes | 14 (7.3, 4.1–12) | 191 (3.0) |
| COVID-19 patient exposure | ||
| No. | 314 | 6404 |
| No | 59 (2.8, 2.2–3.6) | 2092 (32.7) |
| Yes, I think so | 25 (3.4, 2.2–5) | 731 (11.4) |
| Unsure | 50 (4.3, 3.2–5.6) | 1162 (18.1) |
| Yes, definitely | 180 (7.4, 6.4–8.6) | 2419 (37.8) |
| Nonhospital COVID-19 exposure | ||
| No. | 314 | 6402 |
| No | 117 (3.4, 2.8–4.1) | 3436 (53.7) |
| Unsure | 70 (3.8, 3–4.8) | 1846 (28.8) |
| Yes, I think so | 38 (7.2, 5.2–9.8) | 526 (8.2) |
| Yes, definitely | 89 (15.0, 12.2–18.1) | 594 (9.3) |
| Family member tested for COVID-19 | ||
| No. | 314 | 6402 |
| No | 248 (4.0, 3.5–4.5) | 6186 (96.6) |
| Yes | 66 (30.6, 24.5–37.2) | 216 (3.4) |
| COVID-19 family test result | ||
| No. | 314 | 6402 |
| Got no test | 248 (4.0, 3.5–4.5) | 6186 (96.6) |
| Negative | 15 (12.2, 7–19.3) | 123 (1.9) |
| Positive | 51 (54.8, 44.2–65.2) | 93 (1.5) |
| Self-reporting on COVID-19 status | ||
| No. | 314 | 6385 |
| I believe I had COVID-19 because I had a compatible illness but tested negative | 18 (6.6, 4–10.2) | 273 (4.3) |
| I believe I had COVID-19 because I had a compatible illness but was not tested | 65 (10.2, 8–12.8) | 636 (10.0) |
| I do not believe I have had COVID-19 | 76 (1.4, 1.1–1.8) | 5298 (83.0) |
| I know I had COVID-19 because I tested positive | 155 (87.1, 81.2–91.6) | 178 (2.8) |
| Self-reporting recent illness | ||
| No. | 314 | 6385 |
| No | 169 (3.3, 2.8–3.8) | 5139 (80.5) |
| Yes | 145 (11.6, 9.9–13.5) | 1246 (19.5) |
Abbreviations: COVID-19, coronavirus disease 2019; IgG, immunoglobulin G; NA, not answered.
aIncludes <10 who did not self-identify.
Out-of-Hospital Exposure
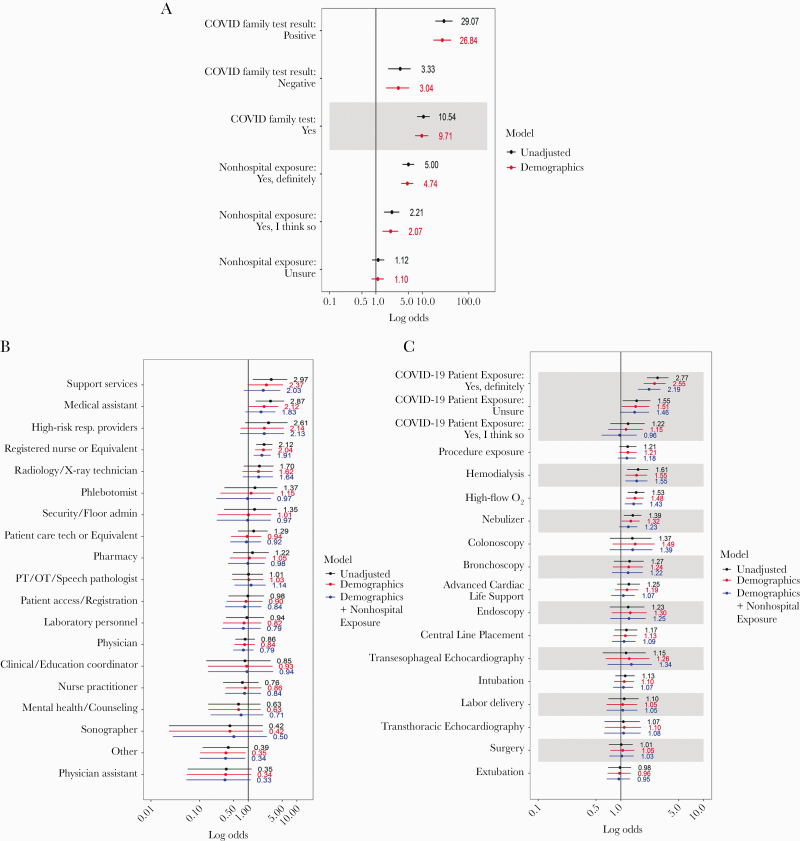
Participants who reported a known out-of-hospital exposure (9.3%) had a seropositive rate of 15.0% (95% CI, 12.2%–18.1%). Those who reported having a family member in their home residence who tested positive for COVID-19 (n = 93) had seropositive rates of 54.8% (95% CI, 44.2%–65.2%). After demographic adjustment, the adjusted OR for seropositive status of participants with a known out-of-hospital exposure was 4.7 (95% CI, 3.5–6.4) when compared with those without. Participants with a family member who tested positive for COVID-19 had a demographic-adjusted OR of 26.8 (95% CI, 17.3–41.8) when compared with those without a positive family member.
Occupation Categories
Across occupation groups, we observed crude prevalence rates of 10.4% (95% CI, 4.6%–19.4%) in support service HCWs (ie, Environmental Services, Food Services, and Patient Transporters) and 10.1% (95% CI, 5.5%–16.6%) in medical assistants. Nurses and respiratory technicians had crude seropositive rates of 7.6% (95% CI, 6.4%–9.0%) and 9.3% (95% CI, 3.1%–20.3%), respectively. Administrators had crude seropositive rates of 3.8% (95% CI, 2.6%–5.2%), and physicians had rates of 3.3% (95% CI, 2.3%–4.4%).
In unadjusted models, support services, medical assistants, and nurses had higher ORs for being seropositive (as compared with administrators) of 3.0 (95% CI, 1.2–6.4), 2.9 (95% CI, 1.4–5.5), and 2.12 (95% CI, 1.5–3.2), respectively (Figure 2). After adjustment for demographics and self-reported out-of-hospital exposure to someone with COVID-19, the association remained significant for nurses (OR, 1.9; 95% CI, 1.3–2.9) but was no longer significant for all other occupation groups.
Figure 2.
Unadjusted and multivariable adjusteda logistic regression models of the association between anti-SARS-CoV-2-seropositive status and (A) out-of-hospital exposures,b (B) occupation group,c and (C) clinical care tasks.d aMultivariable adjustment including age, race/ethnicity, and gender (red) or age, race/ethnicity, gender, and variable for nonhospital exposure (blue). bOut-of-hospital exposures: For the question on whether the participant reported a family member having a COVID-19 test, the reference group is family did not have a test. For the question on whether the participant reported an exposure to COVID-19 outside the hospital, no reported nonhospital exposure is the reference. cThe reference group for occupations is administrators. dFor the question on whether the participant reported exposure to a patient with COVID-19, the reference is no exposure to a patient with COVID-19. For the question on whether a participant conducted a procedure or a specific procedure, the reference is no or not that specific procedure. Abbreviations: CLP, central line placement; COVID-19, coronavirus disease 2019; PT/OT, physical therapy/occupational therapy; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TEE, transesophageal echocardiography; TTE, transthoracic echocardiography.
Among physician specialties, the seropositive prevalence rate was 6.4% (95% CI, 3.1%–11.5%) for surgeons, 6.0% (95% CI, 1.7%–14.6%) for anesthesiologists, 4.3% (95% CI, 0.9%–12.2%) for Emergency Medicine, 2.9% (95% CI, 1.6%–5%) for Medicine and Family Medicine, and 0.5% (95% CI, 0%–2.6%) for Pediatrics (Supplementary Table 2). Among Medicine subspecialties, the seropositivity rate for Pulmonary/Critical Care (n = 34) was 0% (95% CI, 0%–10.3%).
Occupational Tasks
Significantly higher crude rates for IgG+ were seen in HCWs who reported being exposed to COVID-19 patients (n = 2419) than those who did not (7.4%; 95% CI, 6.4%–8.6%; vs 2.8%; 95% CI, 2.2%–3.6%). Among HCWs who were involved in overall patient care, those exposed to patients receiving high-flow oxygen (n = 1842) and nebulizer therapy (n = 1653) had higher rates of seropositive status (6.4%; 95% CI, 5.3%–7.6%; vs 4.2%; 95% CI, 3.7%–4.9%; and 6.1%; 95% CI, 5.0%–7.4%; vs 4.4%; 95% CI, 3.9%–5.1%, respectively) than those who were not. Exposure to patients receiving hemodialysis (n = 807) was also associated with higher crude seropositive status rates (7.2%; 95% CI, 5.5–9.2; vs 4.5%; 95% CI, 4.0%–5.1%). Intubation, bronchoscopy, and surgery were not significantly associated with seropositivity.
In demographic- and out-of-hospital-adjusted models, participating in the care of COVID-19 patients remained associated with higher seropositivity (OR, 2.19; 95% CI, 1.61–3.01) when compared with those who did not report participating in the care of COVID-19 patients. Being exposed to patients receiving high-flow oxygen therapy and hemodialysis also remained significantly associated with 45% and 57% higher odds for seropositive status, respectively. Participation in transesophageal echocardiography (n = 214), intubation (n = 1360), and bronchoscopy (n = 431) was not significantly associated with seropositive status when compared with participants who did not participate in those procedures.
Community Variation in Seropositivity
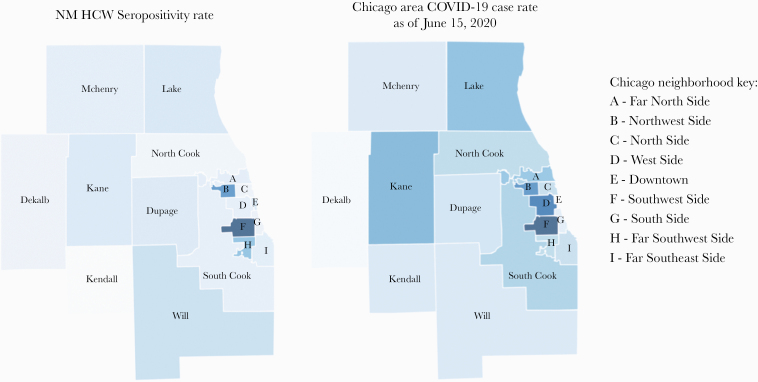
The percent seropositive status by Chicago neighborhood is shown in Figure 3. The highest case rates were in the Southwest and Northwest Side neighborhoods and lower case rates on the North Side and Near-North suburbs. The neighborhood of residence of study participants and COVID-19 seropositivity mirror COVID-19 case rates in the Chicagoland area.
Figure 3.
Heat map of Chicago neighborhoods and surrounding counties by seropositive rate for NM HCW and Chicagoland COVID-19 case rate data. Range of % positive IgG across neighborhoods is on the left. COVID-19 case rates from the Illinois Department of Public Health as of June 15, 2020, is on the right. Darker colors represent higher IgG/case % rates, and lighter represent lower IgG/case + rates. Abbreviations: COVID-19, coronavirus disease 2019; IgG, immunoglobulin G; NM HCW, Northwestern Medicine health care worker.
Reporting on Previous Infection and Impact on Health
Participants who reported that they did not believe that they had been infected with COVID-19 (n = 5298, 83%) had an IgG seropositive rate of 1.4% (95% CI, 1.1%–1.8%; n = 76). These 76 participants represented 24% of all seropositive participants in the study. Participants who thought they might have been infected with COVID-19 but tested polymerase chain reaction (PCR) negative, or were not tested for virus, had seropositive rates of 6.6% (95% CI, 4.0%–10.2%) and 10.2% (95% CI, 8.0%–12.8%), respectively. Participants who reported that they knew they had COVID-19 because they had a positive PCR test had a seroprevalence rate of 87.1% (95% CI, 81.2%–91.6%).
Among all seropositive participants in the study, 145 (46.2%) reported having a decline in their health. Seropositivity varied by symptoms, with loss of smell or taste (OR, 13.2; 95% CI, 9.8–17.8) having the strongest association with positivity (Supplementary Figure 2).
DISCUSSION
Within a single large health system serving Northeastern Illinois, we observed substantial variability in seropositivity rates by occupational class and tasks. However, despite these clear risks within the health care setting, out-of-hospital (community and home) exposure had the largest association with seropositive status.
Nurses, medical assistants, and support services workers were at high risk for SARS-CoV-2 infection. However, of those groups, only nurses had higher odds ratios for seropositive status after adjustment for differences in demographics and rates of out-of-hospital exposure between occupation groups. Thus, the rates observed in nurses are more likely due to exposures that occur in the course of performing their jobs, whereas the higher rates seen in medical assistants and support service workers in our sample may be more strongly driven by exposures that occurred outside of the hospital/clinical context.
The higher work-related risks observed in nurses are likely a function of nurses’ essential role on the care team that relies on frequent and close contact with patients [13]. Socialization between HCWs, particularly localized groups like nurses, is another plausible vehicle for transmission, which may lead to “clusters” of infected HCWs within specific occupation groups that co-locate for meals or face-to-face meetings [14]. Our sample was 80% female and similar to US health care worker statistics [15]. Although no difference in gender was identified in seropositivity, it is important to note that HCWs, and especially nurses, are overwhelmingly women, thus the burden of SARS-CoV-2 infection will be mostly borne by female HCWs.
Risk factors that found differences based on exposure to specific patient care tasks in the health care setting were largely seen earlier in the epidemic, with inadequate use of PPE being a significant factor. For example, in a follow-up from an exposure to a patient before they were discovered to have COVID-19, higher risks were seen with aerosol-generating procedures and longer exposure [16]. Celebi from Turkey looked at risk factors for 703 HCWs (7.1% positive by PCR) and found significant associations with inadequate PPE use and socializing or eating with other HCWs without a medical mask, but not intubation or other aerosol-generating procedures (obtaining respiratory samples), although the numbers were small [17]. The health system under study in this analysis was not overwhelmed by COVID-19 cases early in the pandemic. Thus, PPE was readily available to all HCWs during the first COVID-19 surge. Nevertheless, exposure to patients receiving hemodialysis and exposure to patients receiving high-flow oxygen therapy were strong predictors of seropositive status, which may, in part, be because these are both sustained exposures for HCWs. Thus, differences by occupation group in exposure risk in health care settings may be due to risk for aerosolization and the duration of exposure to a patient with COVID-19 [18]. This suggests that availability and appropriate use of PPE and diligent infection control procedures can keep HCWs safe during brief exposures, while more work is needed on how to sustain protection over longer-term exposures.
Approximately 1 in 5 participants who were seropositive did not think they had COVID-19, which is consistent with prior estimates of asymptomatic rates of COVID-19 infection that have ranged from 20% to 40% in the general population and among HCWs [19]. Many factors associated with COVID-19 infection in community surveillance studies were correlated with HCW seropositive status. For example, we observed higher rates in Hispanic and non-Hispanic Black HCW cohort participants. In Chicago, COVID-19 case rates are higher, on average, in neighborhoods with a higher proportion of Black and Hispanic residents [9, 20]. Detailed study of the socioeconomic characteristics, modifiable behaviors, and community events that facilitate virus transmission in these neighborhoods needs to be undertaken.
There are some important limitations to this study. First, these data represent a single large health system that maintained adequate PPE throughout the crisis and launched infection control policies early on. Thus, the findings may not be generalizable to hospital systems working in communities where the burden exceeded the health system capacity. Second, while the seroprevalence reporting by race and ethnicity is consistent with national reports describing higher rates of infection in Black and Hispanic adults, HCWs in those groups were under-represented in our sample. Thus, our estimates of seropositivity in these groups may be unstable. In addition, the participation rate of 35% may have biased the results if those who had higher or lower rates of seropositivity chose not to participate. Notably, seropositive rates between all NM employees and those who chose to join our cohort are modestly different (1.2%), suggesting a selection bias that favored enrolling HCWs at higher risk for COVID-19. Third, our data on occupation group and work-related behaviors come from survey data, which may be susceptible to recall bias, particularly in participants who received their serologic testing results before filling out their surveys. We did not, however, see different directions of associations between participant characteristics (demographic and occupational) and seropositive status when we stratified the cohort by the relative timing of serologic testing and questionnaire completion, suggesting that recall bias does not explain the reported associations between work type, symptoms, and beliefs about COVID-19 infection and serologic status. Fourth, the performance of all currently available assays for IgG detection has not been rigorously validated in community-based studies with consistent reference standard samples. Further, some individuals infected with SARS-CoV-2 may not develop a detectable antibody response, and/or their serum antibody presence may be transient [21]. Thus, the reported prevalence estimates could be under- or overestimated if the accuracy and precision of the assays were lower than initially reported. However, the relative differences that we observed across groups would not be systematically biased by assay performance alone.
In conclusion, HCWs in this study were at modestly lower risk for SARS-CoV-2 infection as compared with other studies of HCWs from the New York area and Spain, and similar seropositive rates as reported in Denmark [4, 22, 23]. Across occupation groups, nurses were at the highest-level risk from work-related exposures. Given the exposure that HCWs face in the direct care of patients with known and unknown COVID-19 status, these data support the effectiveness of PPE and infection control policies to keep HCWs safe. In the setting of a well-resourced health system not overwhelmed by hospitalized COVID-19 patients, the majority of risk for SARS-CoV-2 infection was associated with community transmission, suggesting that persistent infection control within the workplace will require adequate PPE supplies, refined infection control policies, and sustained vigilance in and out of the hospital.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We would like to thank our colleagues at Northwestern Medicine, who helped coordinate the serologic testing effort for NM employees and data extraction. In particular, we would like to thank Jay Anderson, Julia Lynch, Anne Cunningham, F. Andy Eichler, Kenneth Hedley, Kristina Hedley, Jen Steinmetz, and Tracey Woods. We thank our informatics colleagues Quan Mai, Daniel Schneider, Theresa Walunas, and Firas Wehbe. We would like to thank the Illinois Department of Public Health for providing files of the public data, the Northwestern All of Us Research Program for providing staff, and Dr. Donald M. Lloyd-Jones for his editing of this manuscript. We are grateful to the NM employees who volunteered to participate in this project.
Financial support. We would like to thank the Northwestern University Clinical and Translational Sciences Institute and the Northwestern Memorial Foundation for their financial support of this research effort.
Potential conflicts of interest. Research funding support was received from Novo Nordisk, Eli Lily, United Healthcare Group (A.W.); consulting fees were received from BioK+ (C.T.E.). All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Participant consent. This study was approved by the Northwestern University Institutional Review Board, and all participants gave written informed consent.
References
- 1.CDC COVID-19 Response Team. Characteristics of health care personnel with COVID-19 - United States, February 12-April 9, 2020. MMWR Morb Mortal Wkly Rep 2020; 69:477–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. CDC. COVID Data Tracker Cases and Deaths Among Healthcare Personnel CDC. Atlanta: Centers for Disease Control and Prevention; 2020. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. Accessed 7 November 2020. [Google Scholar]
- 3. Sikkema RS, Pas SD, Nieuwenhuijse DF, et al. COVID-19 in health-care workers in three hospitals in the south of the Netherlands: a cross-sectional study. Lancet Infect Dis 2020; 20:1273–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moscola J, Sembajwe G, Jarrett M, et al. ; Northwell Health COVID-19 Research Consortium Prevalence of SARS-CoV-2 antibodies in health care personnel in the New York City area. JAMA 2020; 324:893–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hunter BR, Dbeibo L, Weaver CS, et al. Seroprevalence of severe acute respiratory coronavirus virus 2 (SARS-CoV-2) antibodies among healthcare workers with differing levels of coronavirus disease 2019 (COVID-19) patient exposure. Infect Control Hosp Epidemiol 2020; 41:1441–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Steensels D, Oris E, Coninx L, et al. Hospital-wide SARS-CoV-2 antibody screening in 3056 staff in a tertiary center in Belgium. JAMA 2020; 324:195–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chou R, Dana T, Buckley DI, et al. Update alert 5: epidemiology of and risk factors for coronavirus infection in health care workers. Ann Intern Med 2020; 173:W154–W55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fell A, Beaudoin A, D’Heilly P, et al. ; Minnesota Department of Health COVID-19 HCW Monitoring Response Team; Minnesota Department of Health COVID-19 Response Task Force SARS-CoV-2 exposure and infection among health care personnel - Minnesota, March 6-July 11, 2020. MMWR Morb Mortal Wkly Rep 2020; 69:1605–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chicago Department of Public Health Chicago COVID-19 update. 2020. https://www.chicago.gov/content/dam/city/sites/covid/reports/2020-08-11/Chicago_COVID-19_Update_V8_8.11.2020.pdf. Accessed 11 August 2020.
- 10. Bryan A, Pepper G, Wener MH, et al. Performance characteristics of the Abbott Architect SARS-CoV-2 IgG assay and seroprevalence in Boise, Idaho. J Clin Microbiol 2020; 58:e00941–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. Protocol for assessment of potential risk factors for 2019-novel coronavirus (2019-nCoV) infection among health care workers in a health care setting 2020. https://www.who.int/docs/default-source/inaugural-who-partners-forum/20200131-2019-ncov-health-care-worker-protocol-v1.pdf?sfvrsn=a2b876f8_0. Accessed 12 August 2020.
- 12. Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat 1979; 6:65–70. [Google Scholar]
- 13. Gregory L, Weston LE, Harrod M, et al. Understanding nurses’ workflow: batching care and potential opportunities for transmission of infectious organisms, a pilot study. Am J Infect Control 2019; 47:1213–8. [DOI] [PubMed] [Google Scholar]
- 14. Wang X, Zhou Q, He Y, et al. Nosocomial outbreak of COVID-19 pneumonia in Wuhan, China. Eur Respir J 2020; 55:2000544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. US Department of Health and Human Services, Health Resources and Services Administration, National Center for Health Workforce Analysis. Sex, Race, and Ethnic Diversity of U.S. Health Occupations (2011–2015). Rockville, MD: U.S. Department of Health and Human Services; 2017. [Google Scholar]
- 16. Heinzerling A, Stuckey MJ, Scheuer T, et al. Transmission of COVID-19 to health care personnel during exposures to a hospitalized patient - Solano County, California, February 2020. MMWR Morb Mortal Wkly Rep 2020; 69:472–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Çelebi G, Pişkin N, Çelik Bekleviç A, et al. Specific risk factors for SARS-CoV-2 transmission among health care workers in a university hospital. Am J Infect Control 2020; 48:1225–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Somsen GA, van Rijn C, Kooij S, et al. Small droplet aerosols in poorly ventilated spaces and SARS-CoV-2 transmission. Lancet Respir Med 2020; 8:658–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rivett L, Sridhar S, Sparkes D, et al. Screening of healthcare workers for SARS-CoV-2 highlights the role of asymptomatic carriage in COVID-19 transmission. Elife 2020; 9:e58728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Illinois Department of Health. COVID-19 statistics 2020. https://www.dph.illinois.gov/covid19/covid19-statistics. Accessed 12 August 2020.
- 21. Wu F, Liu M, Wang A, et al. Evaluating the association of clinical characteristics with neutralizing antibody levels in patients who have recovered from mild COVID-19 in Shanghai, China. JAMA Intern Med 2020; 180:1356–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pollán M, Pérez-Gómez B, Pastor-Barriuso R, et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet 2020; 396:535–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Iversen K, Bundgaard H, Hasselbalch RB, et al. Risk of COVID-19 in health-care workers in Denmark: an observational cohort study. Lancet Infect Dis 2020; 20:1401–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.