Abstract
Objective:
Guidelines for appropriate management of chronic opioid therapy are underutilized by primary care physicians (PCPs). The authors hypothesized that developing a multicomponent, team-based opioid management system with electronic health record (EHR) support would allow our clinicians to improve adherence to chronic opioid prescribing and monitoring guidelines.
Design:
This was a retrospective pre-post study.
Setting:
The authors performed this intervention at our large, urban, academic primary care practice.
Patients, participants:
All patients with the diagnosis of “chronic pain, opioid requiring (ICD-10 F11.20)” on their primary care EHR problem lists were included in this study.
Intervention:
The authors implemented a five-pronged strategy to improve our system of opioid prescribing, including (1) a patient registry with regular dissemination of reports to PCPs; (2) standardization of policies regarding opioid prescribing and monitoring; (3) development of a risk-assessment algorithm and risk-stratified monitoring guidelines; (4) a team-based approach to care with physician assistant care managers; and (5) an EHR innovation to facilitate communication and guideline adherence.
Main outcome measures:
The authors measured percent adherence to opioid prescribing guidelines, including annual patient-provider agreements, biannual urine drug screens (UDSs), and prescription monitoring program (PMP) verification.
Results:
Between September 2015 and September 2016, the percentage of patients on chronic opioid therapy with a signed controlled substances agreement within the preceding year increased from 46 to 76 percent (p < 0.0001), while the percentage of patients with a UDS done within the past 6 months rose from 23 to 79 percent (p < 0.0001). The percentage of patients whose state PMPs profile had been checked by a primary care team member in the past year rose from 45 to 97 percent (p < 0.0001).
Conclusion:
A comprehensive strategy to standardize chronic opioid prescribing in our primary care practice coincided with an increase in adherence to opioid management guidelines.
Keywords: chronic opioid management, primary care, electronic health record (EHR)
INTRODUCTION
Opioid-related morbidity and mortality is a public health crisis, leading to intense scrutiny over prescribing practices.1,2 Overdose deaths in the United States due specifically to prescription opioid pain relievers are estimated to have doubled from 2002 to 2016.3 Primary care physicians (PCPs) account for nearly half of all dispensed prescription opioids.1 Education about safe and guideline-driven management of chronic noncancer pain has become a priority of several professional societies, such as the American Pain Society, American Academy of Pain Management, and the Centers for Disease Control and Prevention.4,5 These guidelines include but are not limited to conducting a substance use risk assessment prior to initiating chronic opioid therapy, prescribing the lowest effective dosage with regular reassessment of need for chronic opioid therapy, and periodic urine drug screening (UDS).4,5
Despite broad dissemination efforts, these guidelines are underutilized by PCPs.6-11 Isolated attempts to increase adherence to guidelines, such as provider trainings, clinician feedback, or guideline dissemination, have not achieved desired results.12,13 Time and resource constraints consistently emerge as barriers to safe and effective management of chronic pain.9,14-17
To address this gap in adherence to guidelines for chronic opioid prescribing in primary care, it has been suggested that a system-level overhaul involving electronic health record (EHR) integration is needed.7,18,19 Collaboration with other team members, such as pharmacists, nurse care managers, or nurse practitioners is also well-supported by the literature.20-23 Lastly, greater transparency through clinical dashboards or dissemination of data regarding PCPs’ prescribing practices has been shown to effect change.24-27
We undertook a team-based, systematized initiative to improve guideline-concordant opioid prescribing in our large academic primary care practice using EHR solutions to facilitate team communication, streamline opioid prescribing and monitoring processes, and serve as a repository of resources. We hypothesized that our intervention would increase adherence to chronic opioid prescribing and monitoring guidelines.
METHODS
Setting
This initiative was implemented at a large academic primary care practice affiliated with a tertiary care hospital in downtown Boston serving over 40,000 patients and employing 35 PCPs. There is a diverse payer mix (18 percent state Medicaid, 12 percent Medicare, 74 percent privately insured). At baseline, opioid prescribing guideline adherence rates in the practice were similar to those reported in the literature.18 The practice uses Centricity® (GE Healthcare, Chicago, IL) as its EHR and employs a full-time project manager with an information technology (IT) background who helps to customize the EHR for clinical, operational, quality improvement, and population health initiatives.
Intervention
A multidisciplinary quality improvement team consisting of two physicians, a physician assistant (PA), a social worker, and an IT project manager developed the plan through a literature review of best practices and validated risk assessment tools. The team implemented a five-pronged intervention.
Creating a patient registry: Identification of the target population was necessary to track metrics and enable population health management efforts. A registry of patients was created based on the presence of the diagnosis code for “chronic pain, opioid requiring (ICD-10 F11.20).” PCPs were reminded monthly to add this diagnosis code to the chart for any patient who was regularly prescribed opioids for chronic pain to ensure that all patients were appropriately captured in the registry. Monthly reports were generated for PCPs listing their empaneled patients who were regularly prescribed opioids as well as any overdue monitoring measures for each patient, such as an annual patient-provider agreement, a biannual UDS, or a monthly review of the state prescription monitoring program (PMP). We created a hyperlink in the EHR that clinicians used to access the state PMP, which allowed us to capture clinician adherence to monthly PMP monitoring for each patient.
Standardization of chronic opioid prescribing policies: Standardization of prescribing patterns included 28-day prescriptions to avoid the need for weekend prescriptions and to ensure that prescriptions are due on the same day of the week when the prescriber is in clinic, a regular review of the state PMP by the prescriber or PA team member, and a minimum requirement of biannual UDS. Policy expectations were communicated to patients via explicit annual agreements signed by both prescribers and patients and recorded in our EHR.
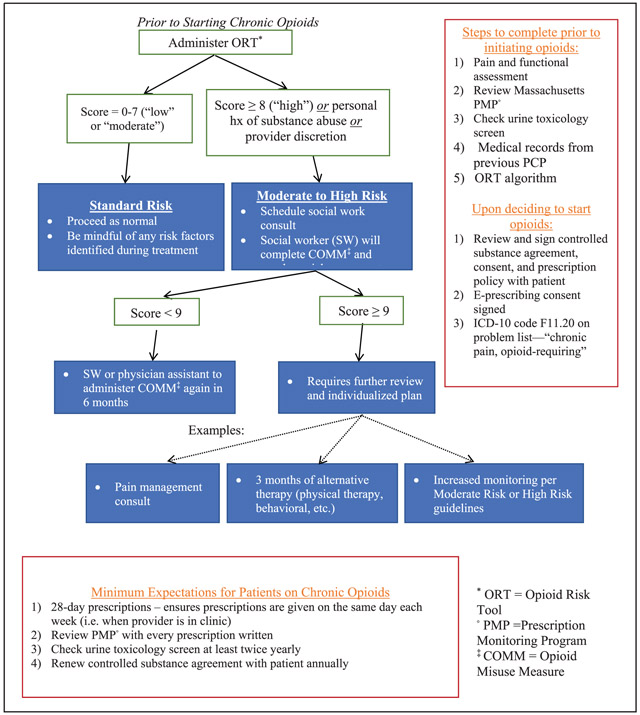
Development of a risk-assessment algorithm: An algorithm to assess risk of opioid misuse was designed using validated screening tools (including the Opioid Risk Tool [ORT] and the Current Opioid Misuse Measure [COMM]) to evaluate patients at the time of opioid initiation or for patients new to our practice (Appendix A).28,29 Patients already established in the practice were risk-stratified according to a schema we developed (Appendix B). All patients were categorized as at standard, moderate, or high risk for future opioid use disorder (as defined by Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5)) based on the presence of specific behavioral patterns regarding their opioid use. Based on the assigned risk category, different monitoring strategies are recommended. Lastly, we defined patient behaviors that were grounds for termination from opioid therapy.
Team-based case management: Two PAs were hired with 0.5 full-time equivalent (FTE) dedicated to serving as population health specialists and case managers for patients prescribed chronic opioids. The PAs regularly reviewed patient panels, appropriateness of opioid prescriptions, and timeliness of UDS monitoring, and discussed any situations requiring attention with the PCP.
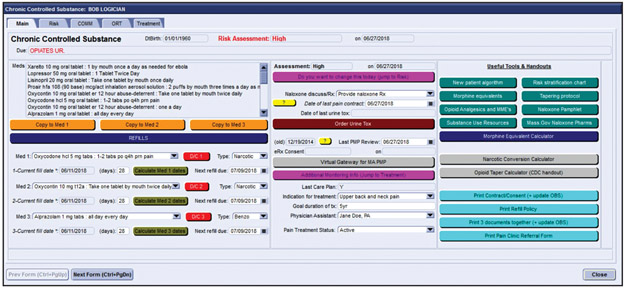
EHR dashboard: A tool, or “component,” in our EHR was designed to centralize all activities related to chronic opioid prescribing (Figure 1). First, this component served as a prescription management tool for controlled substances, both displaying the last prescription date and next due date of 28-day prescriptions as well as allowing for easy writing of prescriptions. Second, it facilitated communication among clinicians with a designated area to document aberrant patient behavior such as losing a prescription, UDS results that were nonconcordant with what a patient was currently being prescribed, or nonadherence to monitoring guidelines. This provided a central, sequential narrative that helped clinicians understand a more complete picture of a patient’s opioid use. Third, the component helped providers adhere to monitoring guidelines by streamlining access to all recommended practices, including one-click access to the state PMP, ordering a UDS, printing the patient-provider controlled substance agreement, and documenting when these procedures were last done. Lastly, it served as a repository for opioid management resources and patient resources, including the ORT, the COMM, and the risk-stratification algorithms. Handouts on naloxone, our practice’s prescription policy, and tapering protocols could be printed from the component with a single mouse click.
Figure 1.
A screenshot of the chronic opioid management component in our EHR. The left-hand side of this screen shows our prescription management center. The middle third of the screen demonstrates some of the monitoring parameters tracked by the component. The right-hand side of the screen shows easily-accessible resources.
These interventions were developed between June and September 2015, with practice-wide implementation achieved by September 2015. An IRB exemption was obtained from the Tufts Medical Center/Tufts University Health Sciences Institutional Review Board.
Measures
Primary outcomes included adherence to recommended guidelines as defined by: (1) percentage of patients with an annual signed controlled substance agreement, (2) percentage of patients with biannual UDS, (3) percentage of patients whose profile in the state PMP had been reviewed by a clinician in the past 60 days, and (4) number of patient visits per year (our guidelines stipulate that patients receiving opioids should have at least four office visits per year). Secondary outcomes included (5) percentage of patients prescribed ≥ 90 morphine milligram equivalents (MMEs) per day and (6) percentage of patients in the practice who were chronically prescribed opioids. We chose percentage of patients prescribed ≥ 90 MMEs per day as an outcome of interest based on the current Centers for Disease Control and Prevention guidelines for opioid prescribing as well as evidence that risks of opioids increase with dosage, with significantly higher risk of overdose for patients prescribed 100 MMEs per day or more.4,30-33
Analyses
Comparisons between pre- and postintervention data were made using a student t-test or a Pearson chi-square test.
RESULTS
The mean age of the cohort was 58.0 ± 12.7 years, 54 percent (282 of 519 patients) were female, and 73 percent (380 of 519 patients) were white. Each PCP cared for a mean of 14.2 ± 12.8 patients (range: 0-50 patients) on long-term opioid therapy (Table 1).
Table 1.
Characteristics of patients on chronic opioid therapy and guideline adherence before and after intervention
| Pre-intervention | Postintervention | p-Value | |
|---|---|---|---|
| Number of patients on chronic opioid therapy | 519 | 480 | N/A |
| Percentage of patients on chronic opioid therapy | 1.6 percent | 1.3 percent | 0.01 |
| Average number of patients on chronic opioid therapy per attending PCP (standard deviation) | 14.2 (12.8) | 13.1 (12.0) | 0.72 |
| Average number of patient visits per year for patients on chronic opioid therapy (standard deviation) | 4.1 (3.6) | 6.0 (4.1) | <0.0001 |
| Percentage of patients on chronic opioid therapy with no visit in the past year | 9 percent | 0.2 percent | <0.0001 |
| Percentage of patients on chronic opioid therapy with a signed controlled substance agreement in past year | 46 percent | 76 percent | <0.0001 |
| Percentage of patients on chronic opioid therapy with no controlled substance agreement on file ever | 13 percent | 3 percent | <0.0001 |
| Percentage of patients with UDS in the past year | 52 percent | 93 percent | <0.0001 |
| Percentage of patients with UDS in the past 6 months | 29 percent | 73 percent | <0.0001 |
| Percentage of patients checked by clinician using the state PMPs in past year | 45 percent | 97 percent | <0.0001 |
| Percentage of patients checked by clinician using the state PMP in last 60 days | 38 percent | 63 percent | <0.0001 |
| Percentage of patients on ≥ 90 MME per day | 32 percent | 29 percent | 0.37 |
Abbreviations: MME, morphine milligram equivalent; N/A, not applicable; PCP, primary care physician; PMP, prescription monitoring program; UDS, urine drug screen.
The percentage of patients chronically prescribed opioids in the practice decreased from 1.6 percent (n = 519) in September 2015 to 1.3 percent (n = 480) in September 2016. Of the patients who stopped receiving prescription opioids from our practice during this time period, the largest proportion (38 percent) had been weaned off due to symptom control via other modalities, patient preference, or resolution of pain. The second largest proportion (21 percent) was terminated due to pain agreement violations. The remaining patients were no longer a patient at our practice (17 percent), were now receiving opioid medication from another provider (7 percent), or were deceased (5 percent) from nonopioid related etiologies.
The percentage of patients on chronic opioid therapy with no primary care visit in the past year decreased from 9 to 0.2 percent (p < 0.0001). The percentage of patients on chronic opioid therapy who had signed a controlled substances agreement in the past year increased from 46 percent at baseline to 76 percent a year after program implementation (p < 0.0001). Similarly, the percentage of patients with a UDS in the past 6 months rose from 29 percent pre-intervention to 73 percent postintervention (p < 0.0001), while patients whose state PMP profile had been checked by a primary care clinical team member in the past year rose from 45 to 97 percent (p < 0.0001). The percentage of patients on chronic opioid therapy who were prescribed ≥ 90 MME per day remained relatively stable from 32 percent in September 2015 to 29 percent in September 2016 (p = 0.37).
DISCUSSION
This team-based chronic opioid management initiative occurred concurrently with an increase in adherence to chronic opioid prescribing guidelines in a large, academic primary care practice. This study adds to the body of evidence showing that interventions at the primary care clinic level may effectively improve adherence to chronic opioid prescribing guidelines.
Three major studies have examined similar multicomponent EHR-based interventions for chronic opioid prescribing. Liebschutz et al.20 randomized PCPs to an intervention involving nurse care management, use of a patient registry, academic detailing, and electronic tools, vs randomization to electronic tools alone.34 They found that after 1 year, patients of PCPs who had been randomized to the intervention arm were more likely to receive guideline-concordant care and more likely to have had their opioid dose reduced or opioids discontinued.20 Our study found similar increases in guideline-concordant care. Similarly, Thakral et al.35 and Losby et al.36 both described multicomponent opioid-prescribing initiatives that included EHR integration, but these studies focused on and were successful in decreasing average daily dose of opioids prescribed.
Our study adds value to the previous reports by showing that in an academic primary care practice, improvements in guideline-concordant opioid prescribing and monitoring are achievable. Our PAs split their time evenly between chronic opioid management (0.5 FTE) and urgent care (0.5 FTE). The clinical revenue generated from the 0.5 FTE that they spent providing clinical care was sufficient to justify their entire salary (1.0 FTE), allowing them to spend half their time as chronic opioid case managers without additional cost to the practice. We acknowledge that there are opportunity costs to using half of our PAs’ time for opioid case management. While not every practice may be able to cover costs in this way, it is a model worth exploring, given the potential benefits of decreasing provider hours spent on non-billable tasks or freeing up provider time for bill-able activities. Our intervention can be replicated at other primary care practices using these elements: data transparency and reporting using a patient registry, standardized risk assessments and monitoring protocols, multidisciplinary collaboration utilizing case managers, and EHR innovations that streamline chronic opioid prescribing and monitoring procedures. These elements match well with those identified in 30 leading primary care clinics nationwide as key to chronic opioid prescribing innovations, with the notable addition of the central EHR tool to facilitate the rest of the initiative.37
A major difference from prior reports is that this study did not find a reduction in daily opioid dose, possibly because it was not the primary focus of this intervention.20,35,36 For example, no reports or EHR alerts were generated or provided to clinicians for patients exceeding 90 MME per day. While not a goal of the initiative, fewer patients were prescribed opioids postintervention. The largest percentage of these patients stopped receiving opioids due to achievement of pain control via other methods, patient preference, or resolution of pain, but many were terminated due to violation of the chronic opioid agreement. It is also possible that the increased scrutiny provided by our more structured program may have played a role in the overall decrease of patients prescribed opioids if physicians were sensitive to being observed.
We acknowledge limitations of our study. The single practice design limits generalizability to practices with several locations. During the course of the intervention, Massachusetts passed a law requiring providers to check the PMP every time opioids were prescribed, and this likely contributed to the increased rate of PMP usage. We therefore cannot assume all changes in opioid prescribing were associated with the systematic changes we implemented. However, there were no concurrent interventions in our practice that may have influenced opioid prescribing practices. Lastly, clinicians may have been adhering to some guidelines pre-intervention, but without a structured field in the EHR to automatically document these practices, they were not uniformly captured beforehand. While this may artificially augment the success of the intervention, it also underscores why the EHR component is crucial for accuracy of data, quality improvement, and population health management purposes.
While adherence to chronic opioid prescribing guidelines has improved in our setting, there is still room for improvement. Next steps for our clinical practice include pursuing a more robust integration of substance use disorder treatment programs into the practice and systematic prescribing of naloxone for all patients on chronic opioid therapy. The ultimate goal is to provide wraparound services to prevent, identify, and treat substance use disorder while also appropriately managing patients’ pain.
ACKNOWLEDGMENT
The authors would like to thank Dr. Saul Weingart for his invaluable advice.
APPENDIX A: INITIATION OF CHRONIC OPIOIDS OR NEW PATIENT ALGORITHM
APPENDIX B: RECOMMENDED MONITORING GUIDELINES BASED ON RISK STRATIFICATION
| Risk stratification | Recommended monitoring |
|---|---|
Standard risk
|
Baseline for everyone:
|
Moderate risk
|
In addition to baseline:
|
High risk
|
In addition to baseline:
|
Termination
|
|
Abbreviations: ED, emergency department; PT, physical therapy; PMP, prescription monitoring program; UDS, urine drug screen.
Contributor Information
Kristin T. L. Huang, Division of Internal Medicine and Adult Primary Care, Tufts Medical Center, Boston, Massachusetts..
Deborah Blazey-Martin, Division of Internal Medicine and Adult Primary Care, Tufts Medical Center, Boston, Massachusetts..
Daniel Chandler, Division of Internal Medicine and Adult Primary Care, Tufts Medical Center, Boston, Massachusetts..
Alysse Wurcel, Division of Geographic Medicine and Infectious Diseases, Tufts Medical Center, Boston, Massachusetts..
Joseph Gillis, Division of Internal Medicine and Adult Primary Care, Tufts Medical Center, Boston, Massachusetts..
Julie Tishler, Division of Internal Medicine and Adult Primary Care, Tufts Medical Center, Boston, Massachusetts..
REFERENCES
- 1.Levy B, Paulozzi L, Mack KA, et al. : Trends in opioid analgesic-prescribing rates by specialty, U.S., 2007-2012. Am J Prev Med. 2015; 49(3): 409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paulozzi LJ, Jones CM, Mack KA, et al. : Vital signs: Overdoses of prescription opioid pain relievers–United States, 1999-2008. Morb Mortal Wkly Rep. 2011; 60(43): 1487–1492. [PubMed] [Google Scholar]
- 3.National Institute on Drug Abuse: Overdose death rates. Available at http://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates. Updated August 2018. Accessed November 24, 2018.
- 4.Dowell D, Haegerich TM, Chou R: CDC guideline for prescribing opioids for chronic pain–United States, 2016. MMWR Recomm Rep. 2016; 65(1): 1–49. [DOI] [PubMed] [Google Scholar]
- 5.Chou R, Fanciullo GJ, Fine PG, et al. : American Pain Society-American Academy of Pain Medicine Opioids Guidelines Panel. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009; 10(2): 113–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salinas GD, Susalka D, Burton BS, et al. : Risk assessment and counseling behaviors of healthcare professionals managing patients with chronic pain: A national multifaceted assessment of physicians, pharmacists, and their patients. J Opioid Manag. 2012; 8(5): 273–284. [DOI] [PubMed] [Google Scholar]
- 7.Starrels JL, Becker WC, Weiner MG, et al. : Low use of opioid risk reduction strategies in primary care even for high risk patients with chronic pain. J Gen Intern Med. 2011; 26(9): 958–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kay C, Wozniak E, Koller S, et al. : Adherence to chronic opioid therapy prescribing guidelines in a primary care clinic. J Opioid Manag. 2016; 12(5): 333–345. [DOI] [PubMed] [Google Scholar]
- 9.Krebs EE, Ramsey DC, Miloshoff JM, et al. : Primary care monitoring of long-term opioid therapy among veterans with chronic pain. Pain Med. 2011; 12(5): 740–746. [DOI] [PubMed] [Google Scholar]
- 10.Lange A, Lasser KE, Xuan Z, et al. : Variability in opioid prescription monitoring and evidence of aberrant medication taking behaviors in urban safety-net clinics. Pain. 2015; 156(2): 335–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lewis M, Herndon CM, Chibnall JT: Patient aberrant drug taking behaviors in a large family medicine residency program: A retrospective chart review of screening practices, incidence, and predictors. J Opioid Manag. 2014; 10(3): 169–175. [DOI] [PubMed] [Google Scholar]
- 12.Chen JH, Horn J, Richman I, et al. : Effect of opioid prescribing guidelines in primary care. Medicine (Baltimore). 2016; 95(35): e4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corson K, Doak MN, Denneson L, et al. : Primary care clinician adherence to guidelines for the management of chronic musculoskeletal pain: Results from the study of the effectiveness of a collaborative approach to pain. Pain Med. 2011; 12(10): 1490–1501. [DOI] [PubMed] [Google Scholar]
- 14.Khodaee M, Deffenbacher B: A look at the burden of opioid management in primary care. J Fam Pract. 2016; 65(12): E1–E6. [PubMed] [Google Scholar]
- 15.Lin DH, Lucas E, Murimi IB, et al. : Physician attitudes and experiences with Maryland’s prescription drug monitoring program (PDMP). Addiction. 2017; 112(2): 311–319. [DOI] [PubMed] [Google Scholar]
- 16.Rutkow L, Turner L, Lucas E, et al. : Most primary care physicians are aware of prescription drug monitoring programs, but many find the data difficult to access. Health Aff (Millwood). 2015; 34(3): 484–492. [DOI] [PubMed] [Google Scholar]
- 17.Irvine JM, Hallvik SE, Hildebran C, et al. : Who uses a prescription drug monitoring program and how?. Insights from a statewide survey of Oregon clinicians. J Pain. 2014; 15(7): 747–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hariharan J, Lamb GC, Neuner JM: Long-term opioid contract use for chronic pain management in primary care practice. A five year experience. J Gen Intern Med. 2007; 22(4): 485–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Porucznik CA, Johnson EM, Rolfs RT, et al. : Opioid prescribing knowledge and practices: Provider survey following promulgation of guidelines–Utah, 2011. J Opioid Manag. 2013; 9(3): 217–224. [DOI] [PubMed] [Google Scholar]
- 20.Liebschutz JM, Xuan Z, Shanahan CW, et al. : Improving adherence to long-term opioid therapy guidelines to reduce opioid misuse in primary care: A cluster-randomized clinical trial. JAMA Intern Med. 2017; 177(9): 1265–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li RM, Franks RH, Dimmitt SG, et al. : Ideas and innovations: Inclusion of pharmacists in chronic pain management services in a primary care practice. J Opioid Manag. 2011; 7(6): 484–487. [DOI] [PubMed] [Google Scholar]
- 22.Jacobs SC, Son EK, Tat C, et al. : Implementing an opioid risk assessment telephone clinic: Outcomes from a pharmacist-led initiative in a large Veterans Health Administration primary care clinic, December 15, 2014-March 31, 2015. Subst Abus. 2016; 37(1): 15–19. [DOI] [PubMed] [Google Scholar]
- 23.Wiedemer NL, Harden PS, Arndt IO, et al. : The opioid renewal clinic: A primary care, managed approach to opioid therapy in chronic pain patients at risk for substance abuse. Pain Med. 2007; 8(7): 573–584. [DOI] [PubMed] [Google Scholar]
- 24.Anderson D, Zlateva I, Khatri K, et al. : Using health information technology to improve adherence to opioid prescribing guidelines in primary care. Clin J Pain. 2015; 31(6): 573–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jamison RN, Scanlan E, Matthews ML, et al. : Attitudes of primary care practitioners in managing chronic pain patients prescribed opioids for pain: A prospective longitudinal controlled trial. Pain Med. 2016; 17(1): 99–113. [DOI] [PubMed] [Google Scholar]
- 26.Lin LA, Bohnert ASB, Kerns RD, et al. : Impact of the opioid safety initiative on opioid-related prescribing in veterans. Pain. 2017; 158(5): 833–839. [DOI] [PubMed] [Google Scholar]
- 27.Penti B, Liebschutz JM, Kopcza B, et al. : Novel peer review method for improving controlled substance prescribing in primary care. J Opioid Manag. 2016; 12(4): 269–279. [DOI] [PubMed] [Google Scholar]
- 28.Webster LR, Webster RM: Predicting aberrant behaviors in opioid-treated patients: Preliminary validation of the opioid risk tool. Pain Med. 2005; 6(6): 432–442. [DOI] [PubMed] [Google Scholar]
- 29.Butler SF, Budman SH, Fernandez KC, et al. : Development and validation of the current opioid misuse measure. Pain. 2007; 130(1-2): 144–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bohnert ASB, Logan JE, Ganoczy D, et al. : A detailed exploration into the association of prescribed opioid dosage and prescription opioid overdose deaths among patients with chronic pain. Med Care. 2016; 54(5): 435–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bohnert AS, Valenstein M, Bair MJ, et al. : Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011; 305(13): 1315–1321. [DOI] [PubMed] [Google Scholar]
- 32.Dunn KM, Saunders KW, Rutter CM, et al. : Opioid prescriptions for chronic pain and overdose: A cohort study. Ann Intern Med. 2010; 152(2): 85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gomes T, Mamdani MM, Dhalla IA, et al. : Opioid dose and drug-related mortality in patients with nonmalignant pain. Ann Intern Med. 2011; 171(7): 686–691. [DOI] [PubMed] [Google Scholar]
- 34.Lasser KE, Shanahan C, Parker V, et al. : A multicomponent intervention to improve primary care provider adherence to chronic opioid therapy guidelines and reduce opioids misuse: A cluster randomized controlled trial protocol. J Subst Abuse Treat. 2016; 60: 101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thakral M, Walker RL, Saunders K, et al. : Impact of opioid dose reduction and risk mitigation initiatives on chronic opioid therapy patients at higher risk for opioid-related adverse outcomes. Pain Med. 2018; 19(12): 2450–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Losby JL, Hyatt JD, Kanter MH, et al. : Safer and more appropriate opioid prescribing: A large healthcare system’s comprehensive approach. J Eval Clin Pract. 2017; 23(6): 1173–1179. [DOI] [PubMed] [Google Scholar]
- 37.Parchman ML, Von Korff M, Baldwin LM, et al. : Primary care clinic re-design for prescription opioid management. J Am Board Fam Med. 2017; 30(1): 44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]