Abstract
Antipsychotic drugs (APD) have clinically important, adverse effects on metabolism that limit their therapeutic utility. Pancreatic beta cells produce dopamine and express the D2 dopamine receptor (D2R). As D2R antagonists, APDs alter glucose-stimulated insulin secretion, indicating that dopamine likely plays a role in APD-induced metabolic dysfunction. Insulin secretion from beta cells is also modulated by the circadian clock. Disturbed circadian rhythms cause metabolic disturbances similar to those observed in APD-treated subjects. Given the importance of dopamine and circadian rhythms for beta cells, we hypothesized that the beta cell dopamine system and circadian clock interact and dually regulate insulin secretion, and that circadian manipulations may alter the metabolic impact of APDs. We measured circadian rhythms, insulin release, and the impact of dopamine upon these processes in beta cells using bioluminescent reporters. We then assessed the impact of circadian timing on weight gain and metabolic outcomes in mice treated with the APD sulpiride at the onset of light or dark. We found that molecular components of the dopamine system were rhythmically expressed in beta cells. D2R stimulation by endogenous dopamine or the agonist bromocriptine reduced circadian rhythm amplitude, and altered the temporal profile of insulin secretion. Sulpiride caused greater weight gain and hyperinsulinemia in mice when given in the dark phase compared to the light phase. D2R-acting drugs affect circadian-dopamine interactions and modulate beta cell metabolic function. These findings identify circadian timing as a novel and important mechanism underlying APD-induced metabolic dysfunction, offering new possibilities for therapeutic interventions
Keywords: Dopamine, Circadian rhythm, Antipsychotic, Insulin, Obesity, Diabetes
1. Introduction
Antipsychotic drugs (APDs) are effective treatments for schizophrenia, bipolar disorder, and major depression. Despite important differences, nearly all APDs produce significant metabolic side effects including weight gain, and insulin resistance (Fleischhacker et al., 2013; Freyberg et al., 2017; Rajkumar et al., 2017; Vazquez-Bourgon et al., 2018). These metabolic disturbances place patients at risk for cardiac disease and type 2 diabetes (T2D), causing excess morbidity and reducing life span (Goldstein et al., 2015; Khan et al., 2013; Laursen et al., 2007).
The mechanisms underlying APD-induced metabolic dysfunction are incompletely understood, but may involve antagonism of dopamine D2 receptors (D2R). D2R is expressed in pancreatic beta cells where it functions as a negative modulator of glucose-stimulated insulin secretion (GSIS) (Farino et al., 2019; Simpson et al., 2012). By blocking D2R, APDs prolong the duration of insulin release and increase target tissue insulin resistance (Ballon et al., 2014, 2018; Farino et al., 2019). Conversely, D2R stimulation may improve metabolic function. Bromocriptine, a D2R agonist, improves insulin sensitivity, and promotes weight loss in T2D (Cincotta and Meier, 1996; Kok et al., 2006).
Recent work has characterized dopamine’s role in regulating GSIS (Farino et al., 2019; Simpson et al., 2012; Ustione et al., 2013). Like neurons, beta cells express the genes necessary for dopamine biosynthesis, catabolism and signaling (Farino et al., 2019; Simpson et al., 2012). However, unlike neurons, beta cell dopamine synthesis relies upon glucose-sensitive uptake of L-DOPA and its conversion by L-DOPA decarboxylase (DDC) into dopamine (Farino et al., 2019; Korner et al., 2019). Autocrine/paracrine dopamine release in beta cells diminishes insulin secretion and maintains glucose homeostasis (Rubi et al., 2005; Simpson et al., 2012). Disruption of beta cell D2R signaling either pharmacologically or genetically results in metabolic disturbances including elevated insulin levels.
Insulin release and glucose sensitivity follow circadian rhythms that are independent of feeding (Merl et al., 2004; Polonsky et al., 1988; Schulz et al., 1983). Since feeding behaviors are coordinated with activity and sleep (Acosta-Rodriguez et al., 2017), and beta cell dopamine synthesis is coupled to dietary precursor intake (Farino et al., 2019; Goldstein et al., 2003), dopamine’s functions in the beta cell may also be subject to regulation by the circadian clock to anticipate the expected changes in glucose and amino acids that follow feeding and fasting (Freyberg and McCarthy, 2017). Levels of L-DOPA, glucose and dopamine change over the day depending on feeding status and metabolic state (Goldstein et al., 2003). Therefore, changes in beta cell capacity to synthesize dopamine from L-DOPA and to terminate dopamine signaling may provide cells with an important means of controlling insulin secretion. Such temporal control of glucose metabolism, insulin release and dopamine signaling likely requires coordination with the circadian clock.
Disrupted circadian rhythms have been identified as a risk factor for weight gain and insulin resistance (Perelis et al., 2016; Stenvers et al., 2019). In animals, disruptions of circadian rhythms cause obesity and hyperglycemia both in systemic (Marcheva et al., 2010; Turek et al., 2005) and tissue-specific clock gene knockouts restricted to adipose (Paschos et al., 2012), liver (Lamia et al., 2008) or beta cells (Perelis et al., 2015; Saini et al., 2016). Therefore, one mechanism by which D2R could affect metabolism is by altering the circadian rhythm of insulin release. In the brain, key genetic components of the dopamine system are regulated by the circadian clock (Chung et al., 2014; Ozburn et al., 2015). Recent work suggests beta cells express a variety of rhythmic transcripts, including components of monoaminergic signaling systems (Petrenko et al., 2017). However, the degree to which beta cell dopamine metabolism is rhythmic or how it affects the response to APDs remains unknown.
Presently, we hypothesize that APDs cause some of their adverse metabolic effects by altering beta cell dopamine signals and circadian rhythms, thereby disrupting the rhythmic release of insulin. To test this idea, we examined circadian-dopamine interactions using INS-1E beta cells (Farino et al., 2016; Merglen et al., 2004) to first determine the extent to which the dopamine system is temporally regulated by the circadian clock; and then to determine whether dopaminergic drugs exert effects on insulin by way of the circadian clock. Finally, we have investigated whether the daily timing of APD dosing in mice affects the weight gain and metabolic impact of APDs. We find that both D2R agonists and antagonists modify beta cell clock function and rhythmic insulin secretion. Moreover, we find that the timing of APD administration plays a crucial role in determining the metabolic impact of these drugs. These data suggest a fundamentally new and important mechanism by which these drugs act on peripheral targets to alter metabolism in vivo. Our results may have implications for understanding the mechanisms by which APDs cause metabolic side effects through dopamine-circadian clock interactions and may offer new opportunities to mitigate the metabolic liability of APDs via novel circadian interventions.
2. Methods and materials
2.1. Drugs
L-DOPA, bromocriptine mesylate and (S)-(−)-sulpiride were purchased from Tocris (Minneapolis, MN). Drugs were dissolved in DMSO (Sigma-Aldrich, St. Louis, MO).
2.2. Cell culture
Rat INS-1E beta cells (Dr. Pierre Maechler, Université de Genève) were maintained in a humidified 37 °C incubator with 5 % CO2. Cells were cultured with RPMI 1640 medium (Life Technologies Corp., Norwalk, CT) supplemented with 5 % (v/v) heat inactivated fetal bovine serum, 2 mM glutamate, 10 mM HEPES, 1 mM sodium pyruvate, 100 units/mL penicillin, 100 μg/mL streptomycin, and 50 μM 2-mercaptoethanol.
2.3. Gene expression analyses
For gene expression studies, cellular rhythms were synchronized with a media change. Starting three hours later, duplicate samples were collected for RNA analysis at 6 h intervals over 24 h. RNA was isolated using an RNeasy kit (Qiagen, Valencia, CA) according to the manufacturer’s protocol. cDNA (∼750 ng) was then obtained using a cDNA reverse transcription kit (Applied Biosystems, Foster City, CA). Gene expression was estimated by quantitative real-time PCR (qRT-PCR) using a CFX384 thermocycler (Bio-Rad, Hercules, CA). Pre-validated Taqman primers (Thermo Fisher Scientific, Waltham, MA) were used to measure expression of Bmal1 (Arntl), Cry1, Per2, Maob, Comt, Th, Ddc, Drd2, and Adra2c. Expression of each gene was normalized to a housekeeping gene, Gapdh (experimentally verified as non-rhythmic in INS1 cells). Target gene expression at a given time was estimated by calculating 2ΔCt where ΔCt is the difference in cycle threshold between Gapdh and the target gene. Expression at a given time was then normalized as a percent value of the mean expression level across all time points. Two to three biological replicates were prepared for each gene and run in technical triplicates.
2.4. Luminometry
INS-1E cells expressing the Per2-luc bioluminescent circadian reporter were generated using lentivirus (Liu et al., 2007). Cells were cultured under blasticidin selection to maintain stable expression of Per2-luc. Per2-luc-expressing cells were grown in 24-well plates at a density of 2 × 105 cells/well. Using a media change, 1 mM luciferin (Biosynth International, Itasca, IL) was added to the cultures and rhythms were measured in a luminometer (Actimetrics, Wilmette, IL) over 4–7 days (McCarthy et al., 2013). The media change was sufficient to synchronize the beta cell cellular rhythms. When indicated, drugs were added to the recording media at the time of the media change and remained present throughout the full duration of the recordings. Samples were batch processed and included each relevant treatment on the same plate, thereby controlling for run to run variability across experiments.
2.5. Insulin secretion analyses
The nLuc-insulin reporter was generated as reported previously (Burns et al., 2015). Luciferase was inserted into mouse insulin C-peptide encoded from Ins2, resulting in a bioluminescent signal upon enzymatic processing of the insulin pro-peptide. INS-1E cells were transduced with a lentiviral nLuc-insulin reporter to generate nLuc-insulin-expressing cells plated into a 24-well format. Insulin release was estimated as reported previously (Farino et al., 2016) with modifications for longer-term studies, including the use of Enduren (Promega, Madison, WI), a coelenterazine substrate optimized for long term studies. Luciferase activity was measured for 3 days in a manner similar to that described for Per2-luc.
2.6. Rhythm determinations and analysis
Rhythm determinations of candidate gene qRT-PCR data were analyzed using a Fourier-curve cosinor analyses. A forward linear harmonic regression model was tested against a fitted horizontal line corresponding to the mean expression level (Circwave, version 1.4, https://www.euclock.org). Significant rhythms were determined to be present when the corresponding F-value generated for each PCR data set resulted in α <0.05. For bioluminescence assays, photoemissions from Per2-luc were recorded every 10 min and logged automatically. To isolate the rhythmic component of the data, the 12 h moving average of the total counts were subtracted yielding a detrended measure of the rhythm. These baseline-subtracted data were then fit to a damped sine wave using the least squares method. Per2-luc rhythm parameters (period i.e. the time between cycles of a best fit sine wave, and amplitude i.e. the difference in signal intensity between minimum/maximum points of a best fit, damped sine wave) were calculated using the Per2-luc signal analyzed over a 4-day window using commercial software (LumiCycle analysis, Actimetrics, Wilmette, IL). For nLuc-insulin, circadian oscillations were confirmed as for Per2-luc and by fast Fourier transform (FFT) using LumiCycle analysis. Detrended values were obtained by subtracting a 12 h moving average from the total expression level. Detrended values were then averaged across replicates for each data point. Amplitude of the insulin oscillation was then calculated as the normalized mean difference between the minimum 4 hr and maximum 4 hr of baseline subtracted reporter activity over a single cycle.
2.7. Animal studies
All procedures are approved by UCSD IACUC committee. 20–22-week-old female C57BL/6 mice were divided into 2 groups and acclimated for 3 weeks to a 12 h light/dark cycle (ZT0 at 8AM, ZT12 at 8 P M = “Light group”) or reverse dark/light cycle (ZT0 at 8 P M, ZT12 at 8AM = “Dark group”).
Three days before the start of the study, mice were singly housed and switched from normal chow to high fat diet (45 % calories from fat, Research Diets, D09092903, New Brunswick, NJ) and fed ad libitum. During this 3-day period, mice were also habituated to daily injections of saline (s.c.). Upon study start, mice were injected subcutaneously (s.c.) with sulpiride (20 mg/kg) or vehicle daily for 12 days at either ZT0 or ZT12. The sulpiride dose was chosen based on previous literature showing that it induces weight gain and hyperphagia in rodent models (Baptista et al., 2002, 1987). Body weight and food intake was measured daily for the first seven days and again on days 10–12. Glucose tolerance was assessed in mice on day 13 after a 12 h fast and one hour after sulpiride (20 mg/kg, s.c) injection (i.e. ZT1 or ZT13). One hour prior to the start of glucose tolerance test mice were injected with sulpiride and baseline blood was sampled from the tail. Mice were then injected with 1 mg/kg dextrose (i.p.) and blood was sampled from the tail vein and blood glucose measured at time points indicated. Insulin was measured by ELISA (Alpco, Salem, NH) from blood collected at baseline and after 15 min.
2.8. Statistical analysis
All statistical analyses (GraphPad Prism Software version 5.03 San Diego, CA) defined significance as α < 0.05. Two group analyses were performed via a two-tailed t-test. Analyses of three or more conditions were performed using one-way ANOVA or two-way ANOVAs as indicated. ANOVA analyses were followed by post-hoc t-tests to compare between-group differences. Error bars indicate standard error of the mean (SEM).
3. Results
3.1. Beta cell dopamine system components are rhythmically expressed
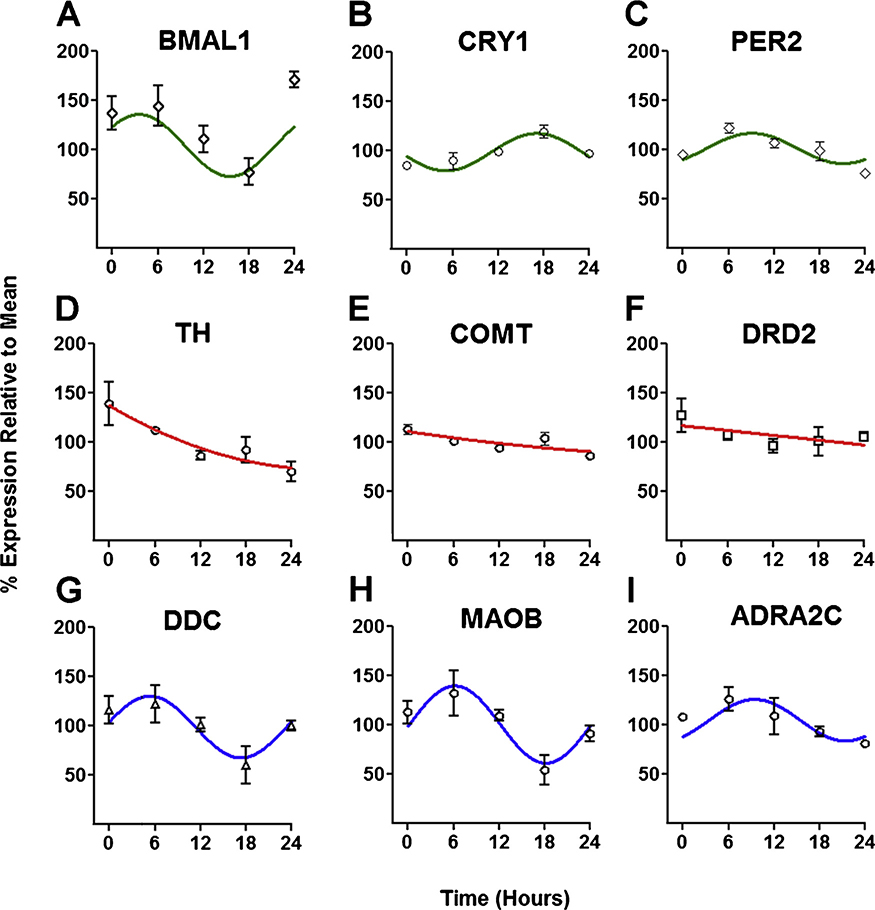
Since dopamine modulates GSIS (Farino et al., 2019, 2016), and insulin secretion is rhythmic, we examined the temporal expression patterns of genes with known functions in dopamine metabolism in INS-1E cells. As phase markers, we first measured expression of three core clock genes. As expected, Bmal1, Cry1 and Per2 each revealed distinct temporal expression patterns (Fig. 1A–C) and were determined to be rhythmic as indicated by cosinor analyses (Bmal1 r2 = 0.51, p < 0.005; Cry1 r2 = 0.72, p<0.01, Per2 r2 = 0.58, p < 0.05). These expression patterns indicate INS-1E cells rhythmically express at least some core clock genes (Fig. 1A–C).
Fig. 1.
Key elements of the dopamine system are rhythmically expressed in INS-1E cells. Expression of the core circadian clock genes A) Bmal1 B) Cry1 and C) Per2 is rhythmic in INS-1E cells (top row). Expression of the dopamine system-related genes D) Th E) Drd2 and F) Comt is readily detectable, but not rhythmic (middle row); whereas expression of the monoamine pathway genes G) Ddc, H) Maob and I) Adra2c is rhythmic (lower row). Rhythmicity was determined by cosinor analysis and plotted using the resulting, best fitting function (top and middle rows). Non-rhythmic genes (lower row) were plotted using the best fit second order polynomial. Data represent replicates of INS-1E cells collected at 6 h intervals (n = 6–9/time point). Data for each transcript are normalized to the mean expression level of each geneover the course of 24 h. Error bars indicate standard error of the mean (SEM).
We then examined expression of key genes in the dopamine system. Both non-rhythmic (Fig. 1D–F) and rhythmic (Fig. 1G–I) component genes were identified. Expression of Th which encodes tyrosine hydroxylase (TH), was confirmed in INS-1E cells, but was not rhythmic. In contrast, DDC (encoded by Ddc) was rhythmically expressed (r2 = 0.69, p <0.02). INS-1E cells also express the enzymes responsible for dopamine catabolism, catechol-O-methyltransferase and monoamine oxidase B (Farino et al., 2019), encoded by Comt and Maob, respectively. Maob expression was strongly rhythmic (r2 = 0.77, p< 0.005), while Comt expression was not. Interestingly, Ddc and Maob genes were expressed in phase with each other (r = 0.96, p <0.01). Both Ddc and Maob showed a weaker nominal correlation with Bmal1 (r = 0.72 for Ddc and r = 0.57 for Maob), indicating a possible phase relationship among these genes. These findings suggest that expression of key elements of the beta cell dopamine system are temporally coordinated, both with each other and with the circadian clock.
Previous work showed that INS-1E cells express D2R (encoded by Drd2) (Farino et al., 2019; Rubi et al., 2005; Simpson et al., 2012). We confirmed Drd2 expression, but found it was not rhythmically expressed. Beta cells also express other catecholamine receptors including the α2C adrenergic receptor (encoded by Adra2c) (Amisten et al., 2013). Adra2c expression was found to be rhythmic (r2 = 0.68, p< 0.05) with a temporal expression profile similar to Per2 (r = 0.79) indicating a possible phase relationship between genes.
3.2. Beta cells exhibit circadian rhythms
To study rhythms at higher resolution and over a longer time course, we used Per2-luc to examine INS-1E cell rhythms. While there was run to run variability across experiments, we found rhythms that persisted for 3–4 days, with the expected circadian period, ranging from 23.0 to 28.2 h (mean ±SEM: 25.9 ±0.2 h). There was no period difference in cells that transiently expressed Per2-luc compared to a clonal cell line that stably expressed Per2-luc (p > 0.05). Therefore, to standardize experiments, we used the stably-expressing Per2-luc cell line for subsequent experiments.
3.3. D2R agonists modulate rhythm amplitude
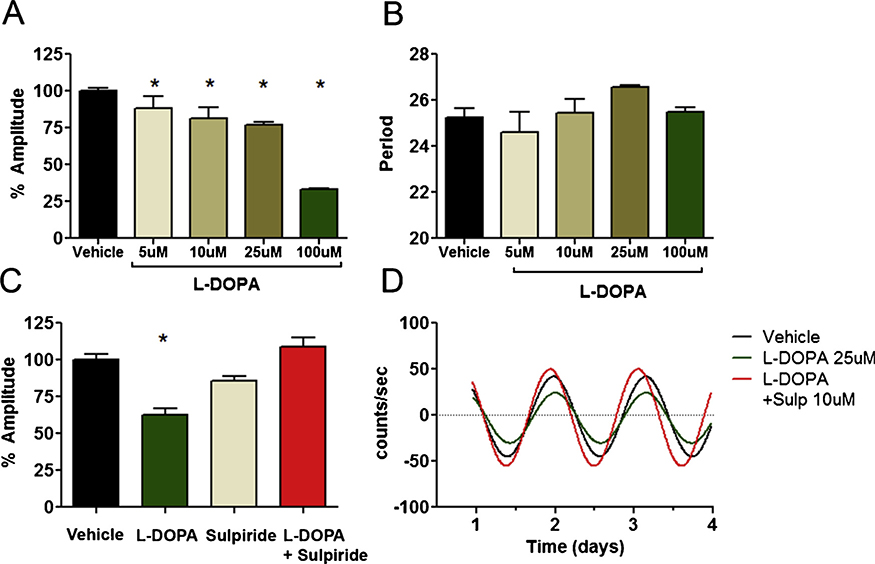
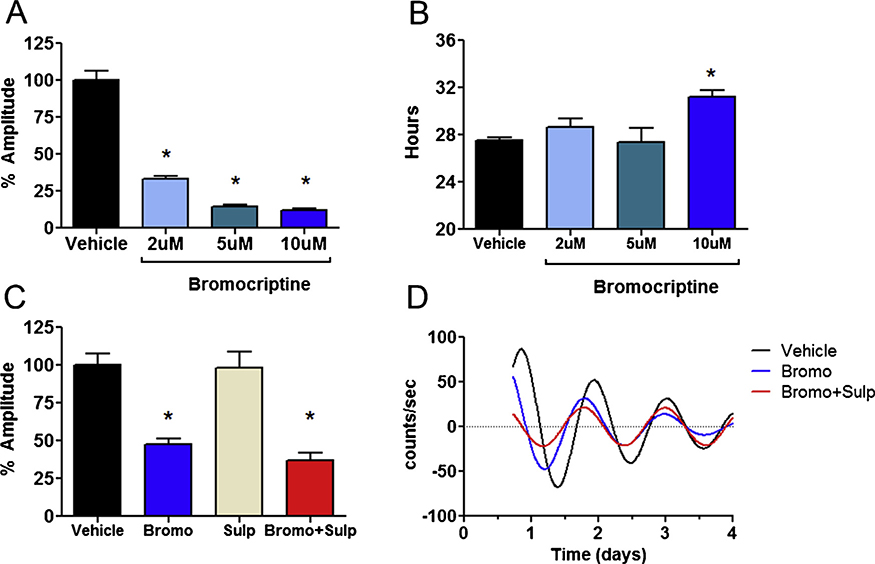
We next examined whether D2R antagonists or agonists affect beta cell rhythms. Since exogenous uptake of L-DOPA is a major mechanism for dopamine biosynthesis and release in INS-1E cells (Farino et al., 2019), we tested whether L-DOPA affected rhythms. L-DOPA treatment significantly reduced amplitude in a concentration-dependent manner across a range of concentrations (5−100 μM), but had no significant effect on period (Fig. 2A,B). Co-administration of L-DOPA with sulpiride, a D2R antagonist, reversed the decrease in amplitude produced by L-DOPA treatment, and restored amplitude to the levels of the vehicle treated cells (Fig. 2C,D, S2). These results indicated that D2R modulates effects of L-DOPA on amplitude. Sulpiride alone had a nominal amplitude decreasing effect that was not statistically significant (Fig. 2A). There was no significant effect of sulpiride on period (Fig. 2B). We next investigated whether the D2R stimulation has effects on rhythms using the D2R agonist bromocriptine. Consistent with our L-DOPA findings, bromocriptine potently diminished rhythm amplitude in a concentration-dependent manner, reducing amplitude by 50–90 % (Fig. 3A). At the highest concentration (10 μM), bromocriptine also induced a phase advance of 4.8 h and lengthened period (Fig. 3B). In contrast to L-DOPA, co-administration of sulpiride with bromocriptine had no effect on bromocriptine-induced changes in rhythms (Fig. 3C,D, S3). The effects of bromocriptine were not explained by cytotoxicity. Treatment with 10 μM bromocriptine for 3 days had no adverse effect on viability over 48 h, and caused only a modest 20 % decrease in cell count at 72 h, too small a decrease to account for the large and immediate effect of bromocriptine observed on amplitude (Figure S1).
Fig. 2.
Effects of L-DOPA on beta cell Per2 rhythms. A) L-DOPA reduces the amplitude of circadian rhythms in a concentration-dependent manner in INS-1E cells. Amplitude has been normalized to vehicle treated control. * indicates p < 0.05 by one-way ANOVA compared to vehicle control (n=15 vehicle, for L-DOPA n=6/9/6/3 for 5,10,25 and 100 μM respectively). B) L-DOPA had no significant effect on period (p >0.05). C) The effect of L-DOPA (25 μM) on rhythm amplitude is reversed by the D2R antagonist sulpiride (10 μM), indicating that stimulation of endogenous dopamine by L-DOPA contributed to amplitude modulation. D) Representative traces are shown of INS-1E cell rhythms at baseline, and after treatment with L-DOPA, sulpiride or both drugs together.
Fig. 3.
Effects of bromocriptine on beta cell circadian Per2 rhythms. A) Bromocriptine reduces the amplitude of circadian rhythms in a concentration dependen-tmanner in INS-1E cells. Amplitude has been normalized to vehicle-treated control. * indicates p < 0.05 by one-way ANOVA compared to vehicle control (n=3–7 per group). B) Bromocriptine also significantly lengthened the period at the highest concentration (10 μM). * indicates p < 0.05 by one-way ANOVA compared to vehicle control (n=3–7 per group). C) The effect of Bromocriptine (10μM) is not reversed by the D2R antagonist, sulpiride (10μM). N = 4 per group. D) Representative traces are shown of INS-1E cell rhythms at baseline, after treatment with bromocriptine, and bromocriptine + sulpiride.
3.4. D2R modulates temporal patterns of insulin release
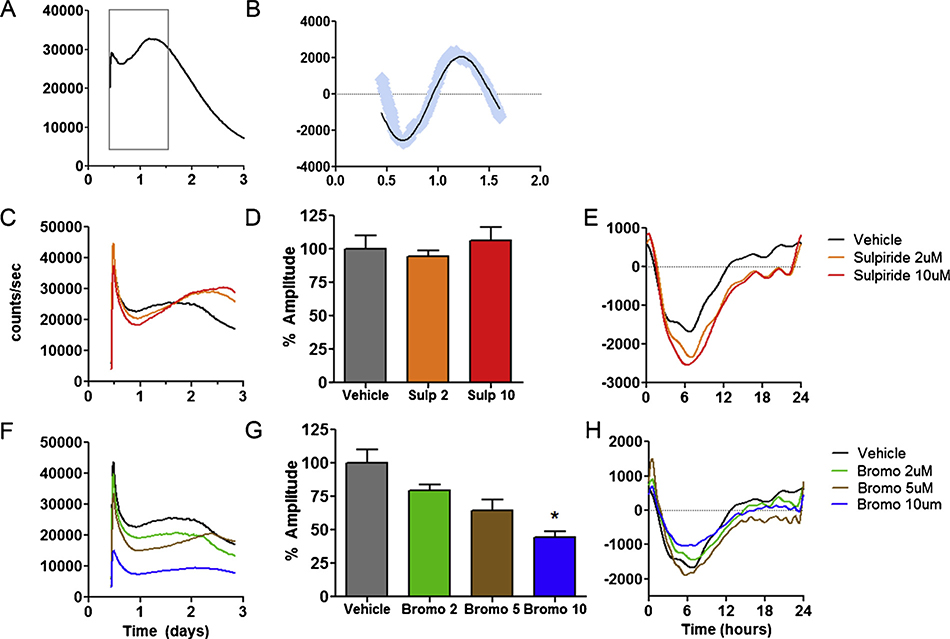
Rhythmic insulin release is an important output of the circadian clock in vivo (Perelis et al., 2015). Using nLuc-insulin, a bioluminescent reporter of insulin secretion (Burns et al., 2015), we measured temporal patterns of insulin release over several days to determine if cellular insulin rhythms could be measured in INS-1E cells. The nLuc-insulin reporter showed a pattern of early oscillation, followed by a gradual, linear damping over the subsequent three days (Fig. 4A). While only observed over a single cycle, FFT analysis indicated the presence of a transient rhythmic component, showing an oscillation with peak spectral power in the circadian range that accounted for the majority of the variance (mean ± SEM: 25.9 ± 0.02 h, mean r2 = 0.81, Fig. 4B). D2R blockade by sulpiride produced complex, concentration-dependent effects on insulin secretion (Fig. 4C). Over the first 36 h, sulpiride reduced total insulin release compared to vehicle-treated cells at both concentrations (2 μM and 10 μM), but did not significantly alter the circadian component (Fig. 4C–E). However, after 36 h, we observed increased insulin release (Fig. 4C). This pattern indicates that sulpiride altered the temporal pattern of release with a significant drug × time interaction. In contrast, D2R agonism by bromocriptine strongly reduced total insulin release in a concentration-dependent fashion that was apparent immediately and persisted for 3 days (Fig. 4F), and significantly reduced the amplitude of the circadian component (Fig. 4G,H).
Fig. 4.
Longitudinal analyses of D2R modulation of insulin release. A) Using the nLuc-Insulin bioluminescent reporter, insulin release in INS-1E cells followed an oscillatory pattern consistent with a circadian rhythm the first 1.5 days (gray box). Afterwards, the rhythm damped and signal declined linearly. B) After baseline subtraction, the early circadian component of the release pattern was isolated. Blue lines indicate raw counts while the black line indicates the best fitting curve as determined by rhythm analysis. C) D2R blockade by sulpiride had concentration dependent effects on insulin release, altering both the quantity and temporal characteristics of release. Sulpiride (2 and 10 μM) decreased overall insulin release for the first 1.5 days, followed by increased release thereafter. 2-way ANOVA indicated that there was no mean difference insulin release in sulpiride treated beta cells, but there was a significant effect of time (p <0.0001), and a significant time x drug interaction (p < 0.0001) indicating the pattern of insulin release was altered by sulpiride. D) Analysis of detrended data over a 24 h period indicate that sulpiride alone has no effects on circadian amplitude (n = 3–6 per group). E) Representative traces of baseline detrended data used for circadian analyses of sulpiride. Y-axis has been re-scaled to correspond to the 24 h time between day 0.5–1.5 on Panel A. F–G) Bromocriptine had concentration-dependent effects on insulin release, altering both the quantity and temporal characteristics of release. Bromocriptine decreased F) overall insulin release and G) circadian amplitude in a concentration-dependent manner. * indicates p <0.05 one-way ANOVA, (n=3–6 per group). H) Representative traces of baseline detrended data used for circadian analyses of bromocriptine. Y-axis has been re-scaled, corresponding to the 24 h between day 0.5–1.5 on Panel A.
3.5. Time-dependent metabolic effects of sulpiride in vivo
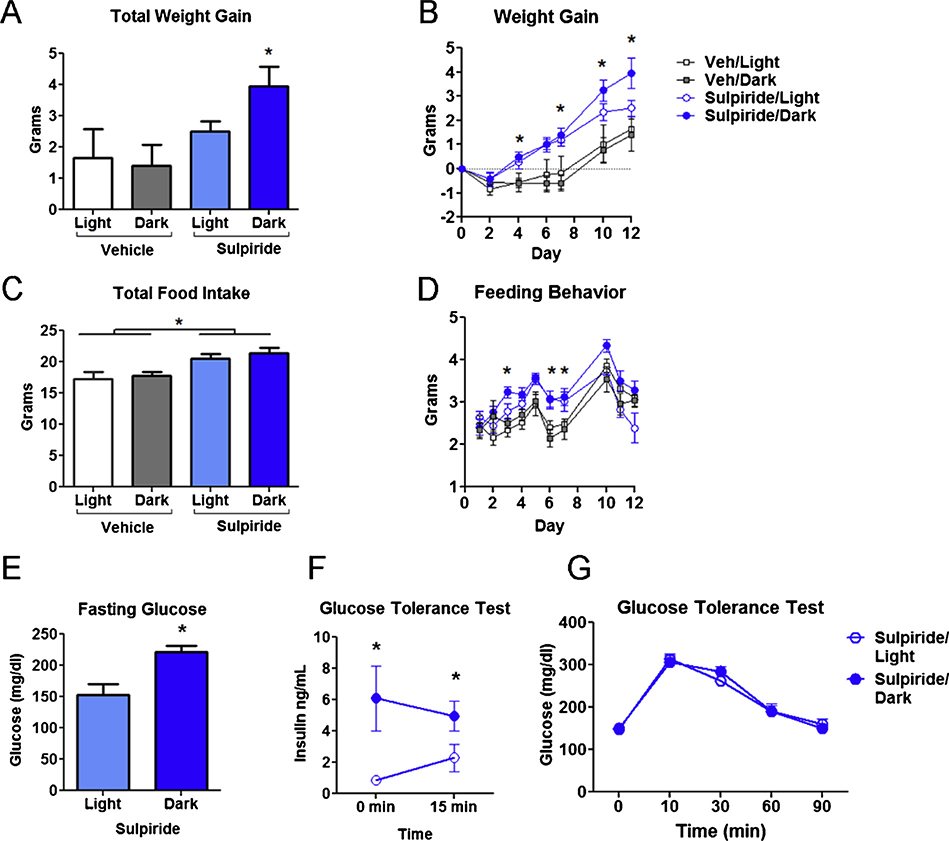
Based on our beta cell results showing APD effects on long-term insulin secretion, we reasoned that APD effects on metabolism in vivo may differ according to the time of administration. Patients treated with APDs gain weight due in part to increasing the proportion of dietary fat (McCreadie et al., 1998; Teasdale et al., 2018). To model this observation, mice were fed a high fat diet at the start of treatment with sulpiride, similar to previous mouse studies of APD induced weight gain (Lord et al., 2017; Morgan et al., 2014; Perez-Gomez et al., 2018). Consistent with earlier work showing that sulpiride treatment induces hyperphagia and weight gain (Baptista et al., 2002, 1987), sulpiride-treated mice gained significantly more weight than controls (Fig. 5A,B). Interestingly, starting at day 10 mice receiving sulpiride at ZT12 (sulpiride/dark) gained significantly more weight than mice treated at ZT0 (sulpiride/light) [Fig. 5A,B Mean ±SEM cumulative weight gain (g) for vehicle: 2.76 ±0.17 (light)/2.73 ±0.13 (dark) vs. sulpiride: 2.94 ±0.14 (light)/3.25 ±0.16 (dark)]. Though increased daily feeding behavior accounted for some of the weight gain, it was increased similarly in both sulpiride-treated groups compared to the controls (Fig. 5C,D), suggesting that increased feeding was not the main reason for the differential effect of time.
Fig. 5.
Time dependent effects of sulpiride on weight gain and insulin metabolism. A) Female mice fed with 45 % high fat diet all gained weight over 12 days (significant effect of time 2-way ANOVA p < 0.0001). There was also a statistically significant effect of drug indicating that the sulpiride treated animals gained significantly more weight than controls (2-way ANOVA p < 0.0001). However, post-hoc T-tests indicated that the sulpiride/dark animals were significantly heavier than the vehicle treated controls (p < 0.05), whereas the sulpiride/light while nominally heavier, did not differ significantly from controld (2-way ANOVA with Bonferroni post-test). B) Cumulative weight gain on day 7. A significant difference between sulpiride/dark vs controls is indicated by * symbol. C) Average daily food intake over the 12-day experiment is shown. Significant group differences from sulpiride vs control treatments are indicated by * symbol (p <0.05 using 2-way ANOVA with Bonferroni post-hoc test, n = 5–8 per group). D) Cumulative food intake over the first seven days increased significantly in both sulpiride-treated groups compared to controls (One-way ANOVA p <0.01). There was no significant difference in food intake between sulpiride/light and sulpiride/dark groups. E) Blood glucose after 12 h fast was higher in sulpiride/dark vs sulpiride/light groups after 12 days on high fat diet (mean glucose: 153.0 ± 17.3 mg/dl sulpiride/light vs. 220.9 ±11.0 mg/dl sulpiride/dark, Student’s t-test p< 0.005). F) Compared to sulpiride/light (n = 6), blood insulin was higher in the sulpiride/dark group (n = 7), both after 12 h fast and 15 min after dextrose infusion (2-way ANOVA of dosing schedule × time indicated an effect of dose schedule p< 0.05. G) Following dextrose infusion, there was no significant difference in blood glucose over a 90 min period in sulpiride/dark vs. sulpiride/light animals.
Since hyperinsulinemia is a hallmark of APD-induced metabolic disturbances and has been implicated as a critical driver for the weight gain produced by these medications (Ballon et al., 2014; Ebdrup et al., 2014), we investigated whether the timing of sulpiride administration impacts changes in blood glucose and insulin levels in response to drug. Indeed, consistent with our weight gain data, the sulpiride/dark group also had elevated 12 h fasting blood glucose levels compared to the sulpiride/light group (Fig. 5E). Moreover, blood insulin levels were significantly higher in the sulpiride/dark group compared the sulpiride/light group, both at baseline after fasting and 10 min after a dextrose challenge (Fig. 5F). However, both groups responded similarly to a glucose challenge (Fig. 5G). These data suggest that the time-dependent effects of sulpiride are driven by actions on beta cells to elevate blood insulin, disturb the glucose homeostasis and promote weight gain.
4. Discussion
D2R antagonism is essential for the therapeutic activity of APDs and may also play a critical role in APD-induced metabolic disturbances (Farino et al., 2019; Freyberg and McCarthy, 2017). Conversely, D2R agonists like bromocriptine, cabergoline and amantadine improve metabolic function (Bahar et al., 2016; Gaziano et al., 2012; Graham et al., 2005; Shivaprasad and Kalra, 2011). Therefore, developing an improved understanding of how dopamine affects metabolism may be critical to mitigating the adverse metabolic effects of APDs. Circadian regulation of dopamine may be one critical dimension that warrants special attention.
Our previous work demonstrated the role of dopamine in regulating GSIS in beta cells, and that beta cell-selective D2R knockout impairs regulation of insulin secretion in vivo leading to hyperinsulinemia (Farino et al., 2019). We now extend this work, demonstrating circadian-dopamine interactions in beta cells, and showing the influence of D2R acting drugs on beta cell rhythms and the long-term dynamics of insulin release. Our work shows that timing may play an important role in determining the metabolic side effects caused by APD administration in vivo, suggesting that some of these adverse effects may be modifiable by coordinating APD actions with insulin rhythms.
We found several examples of circadian modulation of the dopamine system in beta cells. However, not all studies of beta cells have identified rhythmic expression of all of these genes (Lawlor et al., 2017; Perelis et al., 2015; Petrenko et al., 2017). The INS-1E beta cell may therefore differ from primary beta cells in important ways, and additional differences across species may affect the role of dopamine across different models. Nonetheless, our data reveal some notable differences between the dopamine system in beta cells and the brain. Unlike midbrain dopamine neurons where Th expression is rate-limiting and rhythmic (Chung et al., 2014; Sidor et al., 2015), Th in INS-1E cells was only weakly expressed and non-rhythmic. In contrast, Ddc was abundant and rhythmically expressed in phase with Bmal1. This is consistent with our recent work showing negligible intracellular dopamine stores in beta cells in the absence of L-DOPA, but rapidly increasing dopamine synthesis and release following glucose-stimulated L-DOPA uptake (Farino et al., 2019). These data suggest that in beta cells DDC may play a larger role than TH in regulating dopamine synthesis, perhaps explaining its tighter regulation and closer coordination with the circadian clock. Feeding is rhythmic and greatly affects the exposure of beta cells to dietary dopamine precursors such as L-DOPA (Farino et al., 2019; Korner et al., 2019; Maffei et al., 2015). Therefore, in beta cells regulation of DDC may require coordination with feeding in a way that is absent in neurons, perhaps leading to distinct circadian regulation of L-DOPA across these cell types. MAO-B expression was also strongly rhythmic. Along with COMT, MAO-B is part of the catabolic system involved in the termination of dopamine signaling, both in the brain and beta cells (Farino et al., 2019). Our data suggest that there is circadian control over dopamine catabolism by regulation of Maob expression, but not over Comt. Notably, D2R does not appear to be rhythmically expressed, unlike in the brain where Drd2 is expressed with a diurnal rhythm in the striatum (Ozburn et al., 2015). Altogether, our data imply that, through rhythmic oscillations in Ddc and Maob expression, dopamine’s control over GSIS may vary over time, stemming both from oscillations in the conversion of L-DOPA to dopamine and in the ability of MAO-B to terminate dopamine signals, whereas D2R expression is relatively constant. How rhythms in Ddc and Maob expression relate to enzyme activity, dopamine signaling and the coordination of rhythmic feeding behaviors and insulin secretion in vivo is presently unknown, and remains to be studied in future investigations. If the importance of dopamine rhythms for metabolism is confirmed in vivo, then dietary or drug-mediated manipulations of the dopamine system may have distinct effects on beta cells depending on the time of day and affect the corresponding sensitivity of the system to perturbations (Figure S4A).
We found evidence for reciprocal actions of dopamine on the beta cell circadian clock, both on the core circadian oscillator and on rhythmic insulin release. By stimulating the synthesis and release of endogenous dopamine, L-DOPA reduced circadian amplitude. Sulpiride attenuated the actions of L-DOPA, identifying a link between dopamine and beta cell circadian rhythms mediated through the D2R. While sulpiride alone had only modest effects on Per2-luc in vitro, our L-DOPA findings suggest that D2R antagonists may have important effects on beta cell rhythms in vivo by disrupting and/or blocking endogenous dopamine signaling to the core circadian oscillator and its negative feedback roles on GSIS (Figure S4B). Sulpiride also affected the long-term temporal profile of insulin release. Sulpiride decreased insulin release early before increasing the amount of insulin release over the longer-term. We were unable to detect an effect of sulpiride on the rhythmic aspect of insulin secretion, but our nLuc-insulin assay was able to detect a circadian-like secretion pattern for only a single cycle, and had limited resolution in this regard. The long-term enhancement of insulin release by sulpiride is consistent with earlier data showing that D2R blockade by APDs or genetic D2R knockout resulted in elevated insulin levels (Farino et al., 2019), and that the APD clozapine increased insulin secretion over the circadian cycle in humans (Sun et al., 2016). This suggests a model whereby APDs cause prolonged hyperinsulinemia which desensitizes tissues and contributes to insulin resistance. The mechanism by which APDs increase long-term insulin secretion is unknown. Our data suggest it could be related to disinhibition from the effects of endogenous dopamine which tends to flatten circadian oscillations of insulin, but could also involve compensatory changes in D2R expression or sensitivity that are known to occur in the CNS after long-term exposure to sulpiride (Stefanini et al., 1991).
The D2R agonist bromocriptine also had effects on circadian rhythms. Like L-DOPA, bromocriptine reduced the amplitude of Per2-luc expression. Interestingly, while L-DOPA’s circadian effects could be reversed by sulpiride, the effects of bromocriptine on rhythms were not reversed by D2R antagonism. This latter finding suggests that bromocriptine’s effects may be mediated by non-D2R receptors. Alpha2 adrenergic receptors are a possible candidate, as they have been proposed as an additional target of bromocriptine (de Leeuw van Weenen et al., 2010), and are abundantly expressed in beta cells (Amisten et al., 2013). Alternatively, bromocriptine affinity for D2R may be too high to allow for sulpiride to effectively compete for binding at the receptor. With respect to insulin, bromocriptine reduced circadian rhythm amplitude and altered the temporal profile of its release, reducing the amount of total insulin release in a concentration-dependent manner. Considered together, both D2R agonists and antagonists alter the temporal patters of insulin release in generally opposite ways, but with complex profiles.
We propose that the AMP (cAMP) network which includes adenylyl cyclase (AC), and protein kinase A (PKA) is a plausible candidate pathway linking the dopamine, D2R and the circadian systems. The cAMP pathway lies at the intersection of the dopamine and circadian pathways and modulates circadian rhythms as demonstrated by AC activators such as forskolin that synchronize cellular rhythms through phosphorylation of CREB and upregulation of Per1 (Motzkus et al., 2000). Previous studies have shown that AC/PKA/calcium-mediated insulin secretion is attenuated in arrhythmic mutant mice lacking BMAL1 (Perelis et al., 2015), implying close coordination of the clock with the cAMP system in beta cells, and underscoring the potential importance of this overlap. However, the role of dopamine was not investigated by this study, and other pathways may contribute to the dopamine-mediated effects on beta cell rhythms. Further study is required to identify the exact molecular coupling mechanisms.
Since physiological insulin release and glucose sensitivity both follow circadian rhythms (Perelis et al., 2015; Polonsky et al., 1988; Schulz et al., 1983), APD-induced alterations in circadian rhythms may impact physiology in beta cells and systemically. In particular, by changing rhythms in beta cells, APDs may alter the amount of insulin to which target tissues are exposed across cycles of feeding and fasting. Our data indicate this difference in insulin signaling could be either quantitative (i.e., total exposure), qualitative (i.e., time of exposure) or both. Our experimental data in mice indicate that APD at ZT12 (the onset of peak insulin secretion in mice) causes greater weight gain and hyperinsulinemia compared to APD at ZT0, onset of the light phase. However, we did not have a vehicle-treated control for the GTT study and insulin measurements. Previous studies of insulin levels in mice indicate ∼50 % increases during the dark vs. light phase consistent with diurnal oscillation (Basse et al., 2018; Marcheva et al., 2010; Qian et al., 2015). In the same studies, diurnal changes in glucose were typically smaller, varying from 0 to 10%. In our experiment we found fold changes in insulin of 2–6x and 40 % increases in fasting glucose in the sulpiride/dark vs. sulpiride/light treated mice, indicating the differential effect of APD timing was not explained by diurnal oscillations in insulin and glucose and likely reflects effects from the drug and schedule. We hypothesized previously that APDs dosed at night in humans may be more detrimental than during the day, primarily because they may increase fasting insulin levels during the trough phase (Chipchura et al., 2018; Freyberg and McCarthy, 2017). However, because the insulin rhythm in nocturnal mice and diurnal humans is oppositely phased, our mouse data do not necessarily lend unambiguous support to our original model. Greater weight gain in the mice dosed in darkness suggests that further increasing peak insulin levels during the active period underlies the time-dependent differential weight gain effects of sulpiride, i.e. not increased insulin during the inactive, insulin trough phase. However, APD effects on several important physiological variables were not examined, including motor activity and other important rhythmic processes including hepatic gluconeogenesis (Kapse et al., 2017), or brain processes underlying sedation, anxiety, appetite regulation and feeding (Perez-Gomez et al., 2018). Moreover, there may be other differences in nocturnal and diurnal species that limit the generalizability of our mouse data to human subjects receiving APDs. For instance, exogenous melatonin mitigates the metabolic effects of APDs in humans (Agahi et al., 2018) and cardiovascular effects of olanzapine in rats (Romo-Nava et al., 2017). In humans, endogenous melatonin is rhythmically produced and secreted overnight. In contrast, most laboratory mice (including C57BL/6J) do not make melatonin (Roseboom et al., 1998). These important species differences remain to be examined in future studies of APD-induced weight gain.
Our findings may have important implications for how APDs are used clinically and suggest that careful timing of APD dosing may be helpful under some conditions, most obviously with short half-life drugs with time-limited pharmacological actions. However, many commonly used APD have long half-lives (> 24 h) and the role of timing is less immediately obvious. Importantly, even with these long half-life drugs, the onset of peak drug levels is relatively rapid and time-limited (Turrone et al., 2003). Moreover, many APD target receptors (Ozburn et al., 2015) or enzymatic elimination pathways (Matsunaga et al., 2012; Takiguchi et al., 2007) show diurnal expression patterns. Therefore, drug levels and tissue sensitivity are dynamic across the day, even under mean steady state conditions, indicating APD timing may be an important consideration under some circumstances, even with longer-acting APDs. In a retrospective study of aripiprazole (a long half-life drug), we found that chronic patients who took the APD at night for 1 year had worse lipid profiles compared to those who took the APD in the morning (Chipchura et al., 2018). This suggests that the possibility that easily implementable strategies such as adjusting dosing schedules to optimize circadian alignment may mitigate metabolic risk of APDs. Other recent work has shown time-restricted feeding (TRF) can mitigate the effects of high-fat diet in mice (Hatori et al., 2012) and glucose sensitivity in humans with T2D (Hutchison et al., 2019). TRF could therefore also conceivably be incorporated into an optimized APD dosing schedule. However, APDs vary considerably in terms of metabolic impact, pharmacological mechanisms, and pharmacokinetic profiles, and time considerations may not be uniform across drugs. Further research is required to determine which APDs are amenable to time-based dosing strategies to mitigate metabolic impact, and which schedules are optimal for each drug. If confirmed, the timing of dopamine-based pharmacological interventions may be a critical determinant of their effects and ultimately lead to therapies with greater efficacy and/or better metabolic profiles.
Supplementary Material
Acknowledgements and disclosures
This study was supported by the U.S. Department of Veterans Affairs, VA Merit Award BX003431 (MM), the Dept of Defense PRMRP Investigator Initiated Award PR141292, the John F. and Nancy A. Emmerling Fund of The Pittsburgh Foundation (ZF) and National Institute of Health1R01DK117872–01 (OO) and the Triton Research and Experiential Learning Scholarship (ML-V). The authors report no relevant biomedical financial interests or potential conflicts of interest.
Footnotes
Conflict of interest statement
None of the authors have financial conflicts of interest with the work presented.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi: https://doi.org/10.1016/j.psyneuen.2019.104551.
References
- Acosta-Rodriguez VA, de Groot MHM, Rijo-Ferreira F, Green CB, Takahashi JS, 2017. Mice under caloric restriction self-impose a temporal restriction of food intake as revealed by an automated feeder system. Cell Metab. 26, 267–277 e262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agahi M, Akasheh N, Ahmadvand A, Akbari H, Izadpanah F, 2018. Effect of melatonin in reducing second-generation antipsychotic metabolic effects: a double blind controlled clinical trial. Diabetes Metab. Syndr 12, 9–15. [DOI] [PubMed] [Google Scholar]
- Amisten S, Salehi A, Rorsman P, Jones PM, Persaud SJ, 2013. An atlas and functional analysis of G-protein coupled receptors in human islets of Langerhans. Pharmacol. Ther 139, 359–391. [DOI] [PubMed] [Google Scholar]
- Bahar A, Kashi Z, Daneshpour E, Akha O, Ala S, 2016. Effects of cabergoline on blood glucose levels in type 2 diabetic patients: a double-blind controlled clinical trial. Medicine (Baltimore) 95, e4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballon JS, Pajvani U, Freyberg Z, Leibel RL, Lieberman JA, 2014. Molecular pathophysiology of metabolic effects of antipsychotic medications. Trends Endocrinol. Metab. 25, 593–600. [DOI] [PubMed] [Google Scholar]
- Ballon JS, Pajvani UB, Mayer LE, Freyberg Z, Freyberg R, Contreras I, Rosenbaum M, Leibel RL, Lieberman JA, 2018. Pathophysiology of drug induced weight and metabolic effects: findings from an RCT in healthy volunteers treated with olanzapine, iloperidone, or placebo. J Psychopharmacol. 32, 533–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baptista T, Araujo de Baptista E, Ying Kin NM, Beaulieu S, Walker D, Joober R, Lalonde J, Richard D, 2002. Comparative effects of the antipsychotics sulpiride or risperidone in rats. I: bodyweight, food intake, body composition, hormones and glucose tolerance. Brain Res. 957, 144–151. [DOI] [PubMed] [Google Scholar]
- Baptista T, Parada M, Hernandez L, 1987. Long term administration of some antipsychotic drugs increases body weight and feeding in rats. Are D2 dopamine receptors involved? Pharmacol. Biochem. Behav 27, 399–405. [DOI] [PubMed] [Google Scholar]
- Basse AL, Dalbram E, Larsson L, Gerhart-Hines Z, Zierath JR, Treebak JT, 2018. Skeletal muscle insulin sensitivity show circadian rhythmicity which is independent of exercise training status. Front. Physiol 9, 1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns SM, Vetere A, Walpita D, Dancik V, Khodier C, Perez J, Clemons PA, Wagner BK, Altshuler D, 2015. High-throughput luminescent reporter of insulin secretion for discovering regulators of pancreatic Beta-cell function. Cell Metab. 21, 126–137. [DOI] [PubMed] [Google Scholar]
- Chipchura DA, Freyberg Z, Edwards C, Leckband SG, McCarthy MJ, 2018. Does the time of drug administration alter the metabolic risk of aripiprazole? Front. Psychiatry 9, 494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S, Lee EJ, Yun S, Choe HK, Park SB, Son HJ, Kim KS, Dluzen DE, Lee I, Hwang O, Son GH, Kim K, 2014. Impact of circadian nuclear receptor REV-ERBalpha on midbrain dopamine production and mood regulation. Cell 157, 858–868. [DOI] [PubMed] [Google Scholar]
- Cincotta AH, Meier AH, 1996. Bromocriptine (Ergoset) reduces body weight and improves glucose tolerance in obese subjects. Diabetes Care 19, 667–670. [DOI] [PubMed] [Google Scholar]
- de Leeuw van Weenen JE, Parlevliet ET, Maechler P, Havekes LM, Romijn JA, Ouwens DM, Pijl H, Guigas B, 2010. The dopamine receptor D2 agonist bromocriptine inhibits glucose-stimulated insulin secretion by direct activation of the alpha2-adrenergic receptors in beta cells. Biochem. Pharmacol 79, 1827–1836. [DOI] [PubMed] [Google Scholar]
- Ebdrup BH, Knop FK, Madsen A, Mortensen HB, Sogaard B, Holst JJ, Szecsi PB, Lublin H, 2014. Glucometabolic hormones and cardiovascular risk markers in antipsychotic-treated patients. J. Clin. Psychiatry 75, e899–905. [DOI] [PubMed] [Google Scholar]
- Farino ZJ, Morgenstern TJ, Maffei A, Quick M, De Solis AJ, Wiriyasermkul P, Freyberg RJ, Aslanoglou D, Sorisio D, Inbar BP, Free RB, Donthamsetti P, Mosharov EV, Kellendonk C, Schwartz GJ, Sibley DR, Schmauss C, Zeltser LM, Moore H, Harris PE, Javitch JA, Freyberg Z, 2019. New roles for dopamine D2 and D3 receptors in pancreatic beta cell insulin secretion. Mol. Psychiatry [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farino ZJ, Morgenstern TJ, Vallaghe J, Gregor N, Donthamsetti P, Harris PE, Pierre N, Freyberg R, Charrier-Savournin F, Javitch JA, Freyberg Z, 2016. Development of a rapid insulin assay by homogenous time-resolved fluorescence. PLoS One 11, e0148684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischhacker WW, Siu CO, Boden R, Pappadopulos E, Karayal ON, Kahn RS, 2013. Metabolic risk factors in first-episode schizophrenia: baseline prevalence and course analysed from the European First-Episode Schizophrenia Trial. Int. J. Neuropsychopharmacol 16, 987–995. [DOI] [PubMed] [Google Scholar]
- Freyberg Z, Aslanoglou D, Shah R, Ballon JS, 2017. Intrinsic and antipsychotic drug-induced metabolic dysfunction in schizophrenia. Front. Neurosci. 11, 432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freyberg Z, McCarthy MJ, 2017. Dopamine D2 receptors and the circadian clock reciprocally mediate antipsychotic drug-induced metabolic disturbances. NPJ Schizophr. 3, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaziano JM, Cincotta AH, Vinik A, Blonde L, Bohannon N, Scranton R, 2012. Effect of bromocriptine-QR (a quick-release formulation of bromocriptine mesylate) on major adverse cardiovascular events in type 2 diabetes subjects. J. Am. Heart Assoc 1, e002279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein BI, Schaffer A, Wang S, Blanco C, 2015. Excessive and premature new-onset cardiovascular disease among adults with bipolar disorder in the US NESARC cohort. J. Clin. Psychiatry 76, 163–169. [DOI] [PubMed] [Google Scholar]
- Goldstein DS, Eisenhofer G, Kopin IJ, 2003. Sources and significance of plasma levels of catechols and their metabolites in humans. J. Pharmacol. Exp. Ther 305, 800–811. [DOI] [PubMed] [Google Scholar]
- Graham KA, Gu H, Lieberman JA, Harp JB, Perkins DO, 2005. Double-blind, placebo-controlled investigation of amantadine for weight loss in subjects who gained weight with olanzapine. Am. J. Psychiatry 162, 1744–1746. [DOI] [PubMed] [Google Scholar]
- Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong EA, Gill S, Leblanc M, Chaix A, Joens M, Fitzpatrick JA, Ellisman MH, Panda S, 2012. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 15, 848–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison AT, Regmi P, Manoogian ENC, Fleischer JG, Wittert GA, Panda S, Heilbronn LK, 2019. Time-restricted feeding improves glucose tolerance in men at risk for type 2 diabetes: a randomized crossover trial. Obesity (Silver Spring) 27, 724–732. [DOI] [PubMed] [Google Scholar]
- Kapse S, Ando H, Fujiwara Y, Suzuki C, Ushijima K, Kitamura H, Hosohata K, Kotani K, Shimba S, Fujimura A, 2017. Effect of a dosing-time on quetiapine-induced acute hyperglycemia in mice. J. Pharmacol. Sci 133, 139–145. [DOI] [PubMed] [Google Scholar]
- Khan A, Faucett J, Morrison S, Brown WA, 2013. Comparative mortality risk in adult patients with schizophrenia, depression, bipolar disorder, anxiety disorders, and attention-deficit/hyperactivity disorder participating in psychopharmacology clinical trials. JAMA Psychiatry 70, 1091–1099. [DOI] [PubMed] [Google Scholar]
- Kok P, Roelfsema F, Frolich M, van Pelt J, Stokkel MP, Meinders AE, Pijl H, 2006. Activation of dopamine D2 receptors simultaneously ameliorates various metabolic features of obese women. Am. J. Physiol. Endocrinol. Metab 291, E1038–1043. [DOI] [PubMed] [Google Scholar]
- Korner J, Cline GW, Slifstein M, Barba P, Rayat GR, Febres G, Leibel RL, Maffei A, Harris PE, 2019. A role for foregut tyrosine metabolism in glucose tolerance. Mol. Metab 23, 37–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamia KA, Storch KF, Weitz CJ, 2008. Physiological significance of a peripheral tissue circadian clock. Proc Natl Acad Sci U S A 105, 15172–15177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laursen TM, Munk-Olsen T, Nordentoft M, Mortensen PB, 2007. Increased mortality among patients admitted with major psychiatric disorders: a register-based study comparing mortality in unipolar depressive disorder, bipolar affective disorder, schizoaffective disorder, and schizophrenia. J. Clin. Psychiatry 68, 899–907. [DOI] [PubMed] [Google Scholar]
- Lawlor N, George J, Bolisetty M, Kursawe R, Sun L, Sivakamasundari V, Kycia I, Robson P, Stitzel ML, 2017. Single-cell transcriptomes identify human islet cell signatures and reveal cell-type-specific expression changes in type 2 diabetes. Genome Res. 27, 208–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu AC, Welsh DK, Ko CH, Tran HG, Zhang EE, Priest AA, Buhr ED, Singer O, Meeker K, Verma IM, Doyle FJ 3rd, Takahashi JS, Kay SA, 2007. Intercellular coupling confers robustness against mutations in the SCN circadian clock network. Cell 129, 605–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord CC, Wyler SC, Wan R, Castorena CM, Ahmed N, Mathew D, Lee S, Liu C, Elmquist JK, 2017. The atypical antipsychotic olanzapine causes weight gain by targeting serotonin receptor 2C. J. Clin. Invest 127, 3402–3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei A, Segal AM, Alvarez-Perez JC, Garcia-Ocana A, Harris PE, 2015. Anti-incretin, anti-proliferative action of dopamine on beta-cells. Mol. Endocrinol 29, 542–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcheva B, Ramsey KM, Buhr ED, Kobayashi Y, Su H, Ko CH, Ivanova G, Omura C, Mo S, Vitaterna MH, Lopez JP, Philipson LH, Bradfield CA, Crosby SD, JeBailey L, Wang X, Takahashi JS, Bass J, 2010. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature 466, 627–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga N, Inoue M, Kusunose N, Kakimoto K, Hamamura K, Hanada Y, Toi A, Yoshiyama Y, Sato F, Fujimoto K, Koyanagi S, Ohdo S, 2012. Time-dependent interaction between differentiated embryo chondrocyte-2 and CCAAT/enhancer-binding protein alpha underlies the circadian expression of CYP2D6 in serum-shocked HepG2 cells. Mol. Pharmacol. 81, 739–747. [DOI] [PubMed] [Google Scholar]
- McCarthy MJ, Wei H, Marnoy Z, Darvish RM, McPhie DL, Cohen BM, Welsh DK, 2013. Genetic and clinical factors predict lithium‘s effects on PER2 gene expression rhythms in cells from bipolar disorder patients. Transl. Psychiatry 3, e318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCreadie R, Macdonald E, Blacklock C, Tilak-Singh D, Wiles D, Halliday J, Paterson J, 1998. Dietary intake of schizophrenic patients in Nithsdale, Scotland: case-control study. BMJ 317, 784–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merglen A, Theander S, Rubi B, Chaffard G, Wollheim CB, Maechler P, 2004. Glucose sensitivity and metabolism-secretion coupling studied during two-year continuous culture in INS-1E insulinoma cells. Endocrinology 145, 667–678. [DOI] [PubMed] [Google Scholar]
- Merl V, Peters A, Oltmanns KM, Kern W, Hubold C, Hallschmid M, Born J, Fehm HL, Schultes B, 2004. Preserved circadian rhythm of serum insulin concentration at low plasma glucose during fasting in lean and overweight humans. Metabolism 53, 1449–1453. [DOI] [PubMed] [Google Scholar]
- Morgan AP, Crowley JJ, Nonneman RJ, Quackenbush CR, Miller CN, Ryan AK, Bogue MA, Paredes SH, Yourstone S, Carroll IM, Kawula TH, Bower MA, Sartor RB, Sullivan PF, 2014. The antipsychotic olanzapine interacts with the gut microbiome to cause weight gain in mouse. PLoS One 9, e115225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzkus D, Maronde E, Grunenberg U, Lee CC, Forssmann W, Albrecht U, 2000. The human PER1 gene is transcriptionally regulated by multiple signaling pathways. FEBS Lett. 486, 315–319. [DOI] [PubMed] [Google Scholar]
- Ozburn AR, Falcon E, Twaddle A, Nugent AL, Gillman AG, Spencer SM, Arey RN, Mukherjee S, Lyons-Weiler J, Self DW, McClung CA, 2015. Direct regulation of diurnal Drd3 expression and cocaine reward by NPAS2. Biol. Psychiatry 77, 425–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschos GK, Ibrahim S, Song WL, Kunieda T, Grant G, Reyes TM, Bradfield CA, Vaughan CH, Eiden M, Masoodi M, Griffin JL, Wang F, Lawson JA, Fitzgerald GA, 2012. Obesity in mice with adipocyte-specific deletion of clock component Arntl. Nat. Med 18, 1768–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perelis M, Marcheva B, Ramsey KM, Schipma MJ, Hutchison AL, Taguchi A, Peek CB, Hong H, Huang W, Omura C, Allred AL, Bradfield CA, Dinner AR, Barish GD, Bass J, 2015. Pancreatic beta cell enhancers regulate rhythmic transcription of genes controlling insulin secretion. Science 350, aac4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perelis M, Ramsey KM, Marcheva B, Bass J, 2016. Circadian transcription from Beta cell function to diabetes pathophysiology. J. Biol. Rhythms 31, 323–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Gomez A, Carretero M, Weber N, Peterka V, To A, Titova V, Solis G, Osborn O, Petrascheck M, 2018. A phenotypic Caenorhabditis elegans screen identifies a selective suppressor of antipsychotic-induced hyperphagia. Nat. Commun 9, 5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrenko V, Saini C, Giovannoni L, Gobet C, Sage D, Unser M, Heddad Masson M, Gu G, Bosco D, Gachon F, Philippe J, Dibner C, 2017. Pancreatic alpha- and beta-cellular clocks have distinct molecular properties and impact on islet hormone secretion and gene expression. Genes Dev. 31, 383–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polonsky KS, Given BD, Hirsch LJ, Tillil H, Shapiro ET, Beebe C, Frank BH, Galloway JA, Van Cauter E, 1988. Abnormal patterns of insulin secretion in non-insulin-dependent diabetes mellitus. N. Engl. J. Med 318, 1231–1239. [DOI] [PubMed] [Google Scholar]
- Qian J, Yeh B, Rakshit K, Colwell CS, Matveyenko AV, 2015. Circadian disruption and diet-induced obesity synergize to promote development of beta-cell failure and diabetes in male rats. Endocrinology 156, 4426–4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkumar AP, Horsdal HT, Wimberley T, Cohen D, Mors O, Borglum AD, Gasse C, 2017. Endogenous and antipsychotic-related risks for diabetes mellitus in young people with schizophrenia: a danish population-based cohort study. Am. J. Psychiatry 174, 686–694. [DOI] [PubMed] [Google Scholar]
- Romo-Nava F, Buijs FN, Valdes-Tovar M, Benitez-King G, Basualdo M, Perusquia M, Heinze G, Escobar C, Buijs RM, 2017. Olanzapine-induced early cardiovascular effects are mediated by the biological clock and prevented by melatonin. J. Pineal Res 62. [DOI] [PubMed] [Google Scholar]
- Roseboom PH, Namboodiri MA, Zimonjic DB, Popescu NC, Rodriguez IR, Gastel JA, Klein DC, 1998. Natural melatonin‘ knockdown‘ in C57BL/6J mice: rare mechanism truncates serotonin N-acetyltransferase. Brain Res. Mol. Brain Res 63, 189–197. [DOI] [PubMed] [Google Scholar]
- Rubi B, Ljubicic S, Pournourmohammadi S, Carobbio S, Armanet M, Bartley C, Maechler P, 2005. Dopamine D2-like receptors are expressed in pancreatic beta cells and mediate inhibition of insulin secretion. J. Biol. Chem 280, 36824–36832. [DOI] [PubMed] [Google Scholar]
- Saini C, Petrenko V, Pulimeno P, Giovannoni L, Berney T, Hebrok M, Howald C, Dermitzakis ET, Dibner C, 2016. A functional circadian clock is required for proper insulin secretion by human pancreatic islet cells. Diabetes Obes. Metab 18, 355–365. [DOI] [PubMed] [Google Scholar]
- Schulz B, Ratzmann KP, Albrecht G, Bibergeil H, 1983. Diurnal rhythm of insulin sensitivity in subjects with normal and impaired glucose tolerance. Exp. Clin. Endocrinol 81, 263–272. [DOI] [PubMed] [Google Scholar]
- Shivaprasad C, Kalra S, 2011. Bromocriptine in type 2 diabetes mellitus. Indian J. Endocrinol. Metab 15, S17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidor MM, Spencer SM, Dzirasa K, Parekh PK, Tye KM, Warden MR, Arey RN, Enwright JF 3rd, Jacobsen JP, Kumar S, Remillard EM, Caron MG, Deisseroth K, McClung CA, 2015. Daytime spikes in dopaminergic activity drive rapid mood-cycling in mice. Mol. Psychiatry 20, 1406–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson N, Maffei A, Freeby M, Burroughs S, Freyberg Z, Javitch J, Leibel RL, Harris PE, 2012. Dopamine-mediated autocrine inhibitory circuit regulating human insulin secretion in vitro. Mol. Endocrinol 26, 1757–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanini E, Frau M, Gessa GL, 1991. Increase in D2 dopamine receptors in the substantia nigra after chronic (−)-sulpiride treatment. Brain Res. 555, 340–342. [DOI] [PubMed] [Google Scholar]
- Stenvers DJ, Scheer F, Schrauwen P, la Fleur SE, Kalsbeek A, 2019. Circadian clocks and insulin resistance. Nat. Rev. Endocrinol 15, 75–89. [DOI] [PubMed] [Google Scholar]
- Sun HQ, Li SX, Chen FB, Zhang Y, Li P, Jin M, Sun Y, Wang F, Mi WF, Shi L, Yue JL, Yang FD, Lu L, 2016. Diurnal neurobiological alterations after exposure to clozapine in first-episode schizophrenia patients. Psychoneuroendocrinology 64, 108–116. [DOI] [PubMed] [Google Scholar]
- Takiguchi T, Tomita M, Matsunaga N, Nakagawa H, Koyanagi S, Ohdo S, 2007. Molecular basis for rhythmic expression of CYP3A4 in serum-shocked HepG2 cells. Pharmacogenet. Genomics 17, 1047–1056. [DOI] [PubMed] [Google Scholar]
- Teasdale SB, Ward PB, Jarman R, Wade T, Rossimel E, Curtis J, Lappin J, Watkins A, Samaras K, 2018. Is obesity in young people with psychosis a foregone conclusion? Markedly excessive energy intake is evident soon after antipsychotic initiation. Front. Psychiatry 9, 725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, Eckel RH, Takahashi JS, Bass J, 2005. Obesity and metabolic syndrome in circadian Clock mutant mice. Science 308, 1043–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrone P, Remington G, Kapur S, Nobrega JN, 2003. Differential effects of within-day continuous vs. transient dopamine D2 receptor occupancy in the development of vacuous chewing movements (VCMs) in rats. Neuropsychopharmacology 28, 1433–1439. [DOI] [PubMed] [Google Scholar]
- Ustione A, Piston DW, Harris PE, 2013. Minireview: dopaminergic regulation of insulin secretion from the pancreatic islet. Mol. Endocrinol 27, 1198–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Bourgon J, Perez-Iglesias R, Ortiz-Garcia de la Foz V, Suarez Pinilla P, Diaz Martinez A, Crespo-Facorro B, 2018. Long-term metabolic effects of aripiprazole, ziprasidone and quetiapine: a pragmatic clinical trial in drug-naive patients with a first-episode of non-affective psychosis. Psychopharmacology (Berl.) 235, 245–255. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.