Abstract
Modulation of cognitive control by emotion and motivation has become a major topic in cognition research; however, characterizing the extent to which these influences may dissociate has proved challenging. Here, I examine recent advances in this literature, focusing on: (1) neuromodulator mechanisms underlying positive affect and reward motivation effects on cognitive control; (2) contingency and associative learning in interactions between affect/reward and cognitive control; (3) aspects of task design, unrelated to affect/reward, that may have acted as confounding influences on cognitive control in prior work. I suggest that positive affect and reward should not be considered singular in their effects on cognitive control, but instead varying on multiple parameters and interacting with task demands, to determine goal-directed, adaptive behavior.
Keywords: positive affect, Reward, cognitive control, neuromodulators, associative learning
Introduction
While integral to goal-directed behavior, cognitive control has traditionally been characterized in terms of “cold” cognitive processes, without consideration of the role of emotion or motivation. This has begun to change in recent years, with burgeoning evidence that cognitive control is modulated by affective influences, including mood inductions, emotional stimuli, and reward and punishment incentives (1–7). Many studies have characterized these influences on cognitive control in terms of the balance between proactive control, engaged in a preparatory or anticipatory fashion; and reactive control, engaged as-needed in response to changing environmental demands; this perspective, termed the Dual Mechanisms Framework (8,9), has proved useful in characterizing variability in control processes and performance across a variety of contexts. With the growth of this literature, so has the need to characterize emotional and motivational influences, and the mechanisms by which they shape performance, with greater nuance.
Ten years ago, my colleagues and I advocated for a systematic characterization of positive affect versus reward1 influences on cognitive control, to clarify the extent to which these influences should be considered to operate via common or dissociable mechanisms (10). Specifically, drawing on prior accounts linking positive affect to decreased proactive control (and related behavioral outcomes, including increased cognitive flexibility and distractibility; (11,12)2) in contrast to observed increases in proactive control and cognitive stability with reward (2,13), we directly compared within-subjects manipulations of positive affect and reward motivation on cognitive control (3). To our surprise, we observed that both reward and positive affect increased proactive control, relative to baseline. Along with this observation, additional studies comparing positive affect and reward effects on cognitive control have yielded mixed findings, including the previously-predicted result of decreased proactive control with positive affect and increased proactive control with reward incentive (4); decreased proactive control with performance non-contingent reward and increased proactive control with performance-contingent reward (14); no changes in cognitive control with positive affective stimuli, and increases in both proactive and reactive control with reward incentive (15); and similar to our findings, increases in children’s proactive control with both positive affect and reward (16). With such inconsistencies in recent empirical work, the extent to which positive affect and reward motivation should be considered overlapping versus separable influences on cognitive control remains debated.
The present review highlights the following three research dimensions (summarized in Figure 1) explored in recent work on affect/reward and cognitive control: (1) the role of neuromodulators beyond dopamine; (2) potential contributions of contingency and associative learning; (3) aspects of task design, unrelated to affect/reward, that may have influenced performance in prior studies. I argue that advancing understanding of these factors is necessary for clarifying the complexity and variability of positive affect and reward influences on cognitive control, and ultimately, progressing towards a more comprehensive account of goal-directed behavior.
Figure 1.
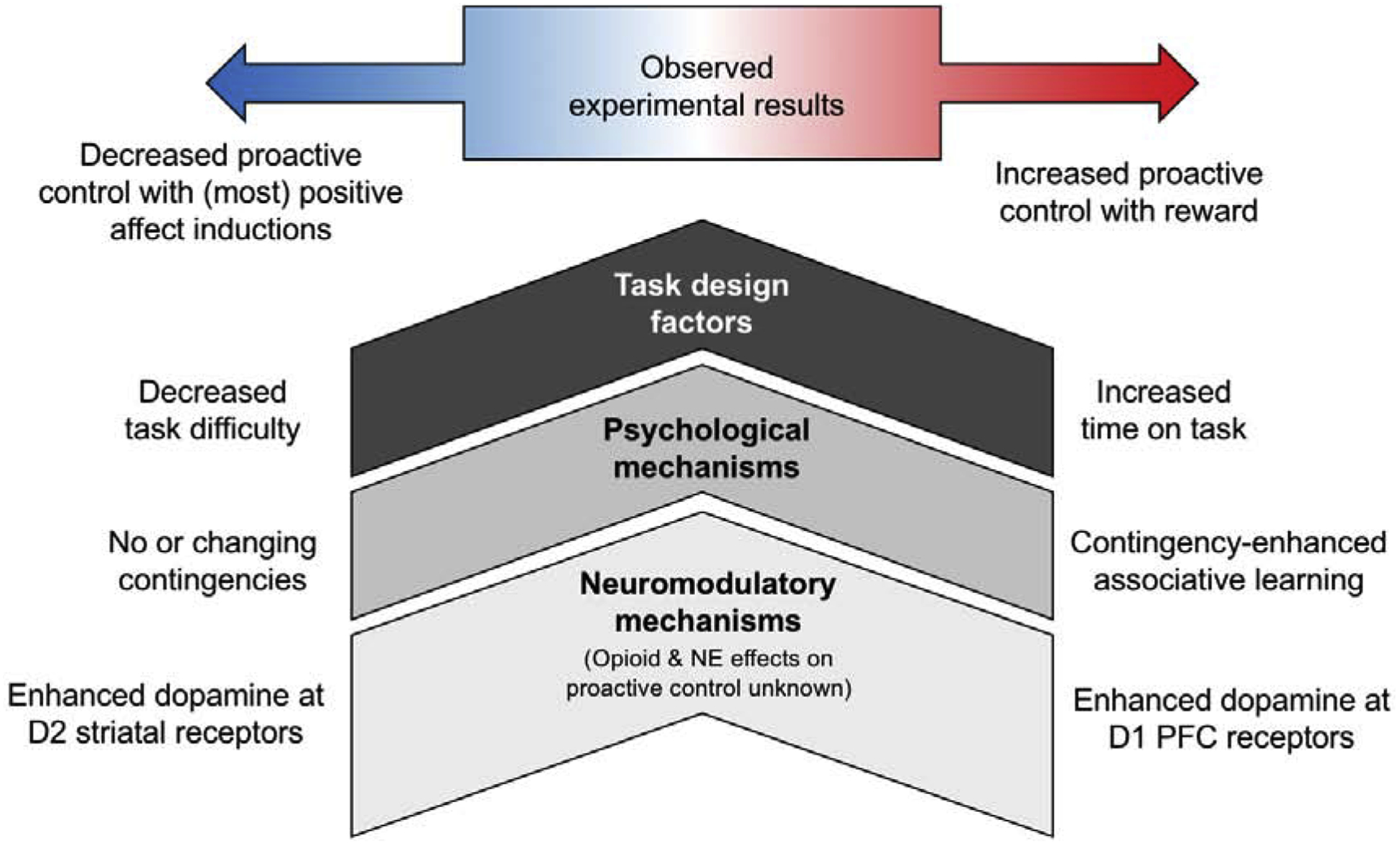
Summary of putative neuromodulatory mechanisms, psychological mechanisms, and task design factors that may contribute to effects of positive affect and reward on cognitive control (specifically, increasing or decreasing proactive control) and that, when not controlled for, may have acted as potential sources of variability in previously observed results.
Neuromodulators and affectively-driven control: going beyond dopamine
Neuromodulator activity has been implicated as playing a key role in emotional and motivational processes as well as the influences of those processes on cognitive performance. In particular, the role of mesolimbic dopamine (DA) in reward and positive affect has been extensively studied. Decades of research indicates that DA is integral to incentive salience and reward processing (17,18): building on this work, the dopamine theory of positive affect (19) posited that positive affect is associated with enhanced DA activity, leading to its observed effects on cognition. While evidence for DA’s role in positive affect and mood has been inconsistent (20–22), robust evidence indicates that DA activity underlies reward modulation of cognitive control (1,23–25). In particular, DA activity at D1 receptors in prefrontal cortex might support cognitive stability and proactive control, while activity at striatal D2 receptors might support cognitive flexibility (26).
Despite these observations, many open questions remain regarding neuromodulator influences on cognitive control. Characterizing these influences has been challenging given both the complexity of potential effects neuromodulators can exert, as well as the diverse methodological approaches used (including pharmacological, imaging, and genetics investigations, in both human and animal models). Further, in contrast to dopamine, limited research has examined the potential contributions of other neuromodulator systems, such as the opioid system and the norepinephrine system, to interactions between positive affect or reward and cognitive control. Despite the fact that the opioid system is well-known to mediate hedonic experiences of pleasure and pain integral to affect (27,28), only recently has manipulation of the opioid system and its effect on human cognitive control been investigated (29,30). In line with recent assertions that aversive emotional arousal can trigger adaptive cognitive control and that such arousal may be counteracted by opioids (31), blocking the opioid system with naltrexone increased cognitive control (specifically, increased post-error slowing) in a Stroop-like task (29). Many open questions remain when interpreting these results in the context of prior findings comparing positive affect and reward effects on proactive and reactive control, especially given that naltrexone did not significantly alter self-reported affect in this study, and given that the paradigm used was not explicitly designed to separate proactive and reactive control processes. However, given involvement of opioids in modulating both hedonic experience and cognitive control, characterizing the effect of the opioid system in interactions between cognitive control and affect remains an important direction for future work.
Additionally, norepinephrine (NE) has been implicated in cognitive performance, both in terms of modulating neural gain and response selection during cognitive control performance (32,33), as well as in terms of modulating emotional arousal and arousal-related effects on perception and memory: in particular, the prioritization of highly-salient stimuli (34,35)3. A growing literature suggests that NE and DA systems overlap and interact (36,37) and a recently proposed theory argues for distinct and interacting roles for NE and DA systems in shaping affective influences on memory (38). To my knowledge, characterizations of positive affect/reward influences on cognitive control have primarily been considered in the context of DA activity, without accounting for potential interactions with NE. Additionally, it has been suggested that the balance between cognitive flexibility and stability might differ under low- versus high-arousal emotion (39), but the role of arousal in affectively-driven control has yet to be systematically investigated. Given NE’s multiple influences on affect, arousal, and cognitive performance, as well as potential interactions with DA, clarifying the potential contributions of this neuromodulator system may help elucidate the neural mechanisms by which emotion and motivation modulate cognitive control performance.
Contingency and associative learning in affectively-driven control
An important point that has emerged from recent empirical evidence is that the effects of reward manipulations on cognitive control may depend on their performance contingency (i.e., whether reward receipt is contingent on performance success or not). Studies that have compared the effects of contingent and non-contingent reward on cognitive performance suggest that contingent rewards may increase proactive control, while non-contingent rewards have been associated with unchanged performance as well as reduced proactive control (4,14,40). It has been argued that in the absence of performance contingency, reward manipulations are largely analogous to positive affect manipulations and lead to similar outcomes (i.e., reduced proactive control; (14)).
What accounts for this effect of contingency? Arguably, previously-observed shifts towards enhanced proactive control under performance-contingent reward may be understood as a product of learning processes. While associative learning was originally conceptualized in terms of stimulus-response (S-R) relationships, Abrahamse and colleagues recently suggested that associations can also be learned between stimuli and higher-level cognitive features, such as goal representations and control settings, and that such learning is critical to successful controlled performance (41). In support of this proposal, associative and reinforcement learning models have been shown to account for cognitive control performance and its flexible adjustment in response to control demands, such as manipulated proportions of conflicting versus non-conflicting trials (42–44). Contingent reward reinforces S-R associations (45) and, it is suggested, may likewise reinforce associations between stimuli and control demands in cognitive tasks (41). Complementary to this account, the Expected Value of Control (EVC) framework (46) proposes that cognitive control is allocated based on evaluation of the anticipated rewards (versus costs) of control implementation, itself argued to be partly determined by the outcomes of reinforcement learning. Many characterizations of proactive/reactive control and its modulation by affect and reward use tasks where performance is optimized by a reliance on proactive control (i.e., cue-probe paradigms such as the AX Continuous Performance Task [AX-CPT], where cue-based preparation typically enhances performance). Observed shifts towards proactive control with contingent reward in such tasks might reflect enhanced associative learning of control demands and increased allocation of control, given the expected rewards of that increased control, within the task context. Learning accounts might also account for the finding that, in addition to increasing proactive control and cognitive stability, reward incentives have also been linked to cognitive flexibility but specifically under circumstances where reward prospects are changing (47,48). Such flexibility might support adaptive performance in uncertain environments (49); like other aspects of task context, uncertainty might be learned and used to shape controlled performance. Despite these findings, as well as suggestions that both incidental and contingency-based affect may influence learning rate and other parameters in modeling the expected value of control (50), reward and affective influences on cognitive control are, to my knowledge, as of yet largely uncharacterized through formal associative learning models. This represents an important direction for future research.
Importantly, not all effects of reward motivation on cognition should be attributed to contingency and related learning. Manohar and colleagues recently demonstrated that both contingent and non-contingent rewards led to heightened motivational vigor in an oculomotor task (51), and argued that increased motivation with non-contingent reward reflected an increase in expected reward rate, occurring independently from contingency effects. Given that non-contingent reward has been suggested as largely analogous to positive affect in its effect on controlled performance, investigating whether other manipulations of positive affect (e.g., mood inductions, emotional stimuli) also increase expected reward rate may also help clarify the mechanisms by which such affective influences take their effect.
Aspects of task design influencing observed cognitive control performance
In addition to being modulated by emotional and motivational influences, the balance between proactive and reactive control can vary as a function of multiple task-level, individual-level, and population-level factors (9). These sources of potential variability may have contributed to inconsistent findings across studies regarding effects of positive affect and reward on cognitive performance. In particular, recent work has begun to characterize how aspects of task design in studies examining emotional and motivational influences on cognitive control may have acted as confounds impacting performance. I highlight some of these potential variables here.
Isolating the effect of emotional or motivational influences on cognitive control typically requires a comparison condition, ideally within-subjects, that does not manipulate emotion or motivation. However, this has led to designs where performance with and without the affective manipulation of interest are measured in separate blocks; when task blocks are not counterbalanced, different affective conditions may vary as a function of time on task. My colleagues and I utilized this approach in our own studies (2,3,52), where a baseline task block preceded a task block with either a positive affect or reward motivation manipulation (thus, our baseline and affect/reward task conditions differed in time on task). Recently, Hefer and Dreisbach (53) investigated whether increasing time on task was associated with increased proactive control in the AX-CPT, in three conditions: positive affect induction, neutral affect induction, and alternating positive and neutral affect. They observed that in all three conditions, participants became more proactive with increasing time on task. Self-report measures verified that this shift in cognitive performance was independent of possible changes in affect over time (i.e., decreasing efficacy of the positive affect induction over time, due to fatigue or boredom). Hefer and Dreisbach also suggest that increases in proactive control with increasing time on task may indicate participants’ learning over time that a proactive control strategy is intrinsically rewarding in this task context. Arguably, this prediction could be investigated using associative learning models that, as discussed previously, formally quantify the learning of stimulus-control mode relationships over time and relate such learning to performance. In the meantime, time on task should be kept in mind as a potential moderator when considering inconsistencies between observed results in this literature.
In addition to time on task effects, different cognitive control paradigms used across studies may vary in task difficulty, which may potentially interact with emotional or motivational influences. Many studies examining the effects of positive affect and reward motivation on proactive/reactive control have used the AX-CPT, but this paradigm itself can be modified to vary its difficulty. Classic studies using the AX-CPT manipulated the delay between cue and probe from <1 second to as much as 8 seconds (54): by increasing the time that cue information must be maintained in working memory on each trial before probe and participant response, proactive control demands of the task also increase (9). Across six recent studies using the AX-CPT to examine positive affect and reward influences on cognitive control (3,4,12,14,15,53), cue-probe intervals have generally been consistent in time length (typically between 1 and 2 seconds), but some studies presented distracter stimuli during that interval (4,14,53), which arguably increase proactive control demands. Interestingly, these studies employing distractors during cue maintenance observed decreases in proactive control under positive affect, while other studies without distractors reported increased proactive control or no significant changes in performance under positive affect (3,12,15). Although proactive control is often thought of as a high-effort strategy (9), it is possible that participants may tend more strongly towards its use in contexts where a proactive mode both supports successful performance (such as the AX-CPT), and where it is not too difficult to employ (i.e., where working memory demands are not too high to begin with), potentially altering or lessening the impact of affective manipulations promoting reactive control on performance. To my knowledge, potential interactions between cognitive control task difficulty and affective/motivational influences have yet to be investigated in this fashion.
Conclusions
Recognizing and accounting for the role of emotional and motivational influences in cognitive control has represented an important advancement in our characterizations of controlled processes and their contributions to adaptive behavior. However, findings within this literature have been inconsistent – in particular, the effect of positive affect on cognitive control and the extent to which it should be considered similar to or diverging from the effect of reward motivation has proven challenging to pin down. In the present review, I highlighted three important factors that may act as potential sources of variability in determining the effects of affect and reward on cognitive control. While not an exhaustive list, these factors illustrate the complexity inherent in characterizing affective influences on cognitive control: both affect and cognitive control may vary on many potential parameters, and performance outcomes may differ as a product of these parameters interacting with one another. Beyond the factors outlined here, additional sources of variation, such as individual differences in response to affective and motivational inductions, may also contribute to variation in subsequent cognitive outcomes (55) and should be explored further. Taken together, while growing evidence indicates that positive affect and reward motivation can have diverging effects on the balance between proactive and reactive cognitive control, several important dimensions of these interactions have yet to be systematically characterized. Further, accounting for these dimensions might help explain previously-observed inconsistencies in performance outcomes. This interplay between affective and cognitive processes is key to goal-directed, adaptive behavior: deepening our understanding of it promises to offer new insights into goal-directed behavior in educational and occupational contexts, as well as offering potential intervention targets when these processes are disrupted, as in psychopathology (56).
Highlights.
Inconsistencies in observed positive affect/reward effects on cognitive control
Multiple neuromodulators may be involved in affectively-driven cognitive control
Reward contingency may influence associative learning of control modes
Task design confounds may have led to inconsistencies in prior results
Future work should account for these and other potential sources of variability
Acknowledgements
This work was supported by the National Institute of Mental Health (R15MH117690) and the Brain & Behavior Research Foundation. I gratefully thank Holly Bowen for helpful comments on this article.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
Nothing declared.
While conceptualizations of affect and reward remain debated in affective science, the “working definition” distinction between positive affect and reward used here (following Chiew & Braver, 2011) considers positive affect to refer to a subjectively-experienced, psychological state that is positively valenced, while reward refers to the receipt of an outcome of value, that may or may not be the product of operational action (i.e., performance contingent) and that may lead to an affective response, but does not necessarily do so.
The Dual Mechanisms Framework’s distinction between proactive versus reactive control modes is highly analogous to, but not completely overlapping with, the theoretical distinction that has been proposed between cognitive stability and flexibility. Both proactive control and cognitive stability have been associated with increased goal maintenance, maintained over time, while both reactive control and cognitive flexibility have been associated with reduced goal maintenance, facilitating the ability to switch actions and thoughts (i.e., in response to changing task demands). Arguably, however, the ability to engage and switch between proactive and reactive control modes could be considered a form of cognitive flexibility in and of itself, an idea being increasingly explored in accounts of meta-control (e.g., Dreisbach & Frober, 2019). While a full exploration of potential overlaps and distinctions between proactive/reactive control and cognitive stability/flexibility is beyond the scope of the present manuscript, these nuances should be kept in mind.
Note that NE-related increases in neural gain and arousal sometimes co-occur, but not always; a recent study reported increases in neural gain and arousal co-occurring in younger, but not older, adults. (34).
References
* of special interest
** of outstanding interest
- 1.Botvinick M, Braver T. Motivation and cognitive control: from behavior to neural mechanism. Annu Rev Psychol [Internet]. 2014/09/25. 2015;66:83–113. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25251491 [DOI] [PubMed] [Google Scholar]
- 2.Chiew KS, Braver TS. Temporal dynamics of motivation-cognitive control interactions revealed by high-resolution pupillometry. Front Psychol [Internet]. 2013;4:15 Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=23372557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiew KS, Braver TS. Dissociable influences of reward motivation and positive emotion on cognitive control. Cogn Affect Behav Neurosci [Internet]. 2014;14(2):509–29. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=24733296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frober K, Dreisbach G. The differential influences of positive affect, random reward, and performance-contingent reward on cognitive control. Cogn Affect Behav Neurosci [Internet]. 2014/03/25. 2014;14(2):530–47. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24659000 [DOI] [PubMed] [Google Scholar]
- 5.Martin EA, Kerns JG. The influence of positive mood on different aspects of cognitive control. Cogn Emot. 2011;25(2):265–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schuch S, Koch I. Mood states influence cognitive control: The case of conflict adaptation. Psychol Res. 2015;79(5):759–72. [DOI] [PubMed] [Google Scholar]
- 7.Cubillo A, Makwana AB, Hare TA. Differential modulation of cognitive control networks by monetary reward and punishment. Soc Cogn Affect Neurosci. 2019;14(3):305–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braver TS. The variable nature of cognitive control: a dual mechanisms framework. Trends Cogn Sci [Internet]. 2012/01/17. 2012;16(2):106–13. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22245618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiew KS, Braver TS. Context processing and cognitive control: From gating models to dual mechanisms. Wiley Handb Cogn Control. 2017;143–66. [Google Scholar]
- 10.Chiew KS, Braver TS. Positive affect versus reward: emotional versus motivational influences on cognitive control. Front Psychol. 2011;2(279):doi: 10.3389/fpsyg.2011.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dreisbach G, Goschke T. How positive affect modulates cognitive control: reduced perseveration at the cost of increased distractibility. J Exp Psychol Learn Mem Cogn [Internet]. 2004;30(2):343–53. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=14979809 [DOI] [PubMed] [Google Scholar]
- 12.van Wouwe NC, Band GP, Ridderinkhof KR. Positive affect modulates flexibility and evaluative control. J Cogn Neurosci [Internet]. 2011;23(3):524–39. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19925199 [DOI] [PubMed] [Google Scholar]
- 13.Braver TS, Paxton JL, Locke HS, Barch DM. Flexible neural mechanisms of cognitive control within human prefrontal cortex. Proc Natl Acad Sci U S A [Internet]. 2009;106(18):7351–6. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19380750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fröber K, Dreisbach G. How performance (non-) contingent reward modulates cognitive control. Acta Psychol (Amst). 2016;168:65–77. [DOI] [PubMed] [Google Scholar]
- 15.Chaillou A-C, Giersch A, Hoonakker M, Capa RL, Bonnefond A. Differentiating motivational from affective influence of performance-contingent reward on cognitive control: the wanting component enhances both proactive and reactive control. Biol Psychol. 2017;125:146–53. [DOI] [PubMed] [Google Scholar]
- 16.Jin X, Auyeung B, Chevalier N. External rewards and positive stimuli promote different cognitive control engagement strategies in children. Dev Cogn Neurosci. 2020;100806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wise RA, Rompre PP. Brain dopamine and reward. Annu Rev Psychol [Internet]. 1989;40:191–225. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=2648975 [DOI] [PubMed] [Google Scholar]
- 18.Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Rev [Internet]. 1998. [cited 2017 Apr 13];28(3):309–69. Available from: http://www.sciencedirect.com/science/article/pii/S0165017398000198 [DOI] [PubMed] [Google Scholar]
- 19.Ashby FG, Isen AM, Turken AU. A neuropsychological theory of positive affect and its influence on cognition. Psychol Rev [Internet]. 1999;106(3):529–50. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10467897 [DOI] [PubMed] [Google Scholar]
- 20.Liggins J, Pihl RO, Benkelfat C, Leyton M. The dopamine augmenter L-DOPA does not affect positive mood in healthy human volunteers. PLoS One [Internet]. 2012;7(1):e28370 Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=22238577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ridderinkhof KR, Van Wouwe NC, Band GPH, Wylie SA, Van der Stigchel S, van Hees P, et al. A tribute to Charlie Chaplin: induced positive affect improves reward-based decision-learning in Parkinson’s disease. Front Psychol. 2012;3:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goschke T, Bolte A. Emotional modulation of control dilemmas: the role of positive affect, reward, and dopamine in cognitive stability and flexibility. Neuropsychologia [Internet]. 2014/07/30. 2014;62:403–23. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25068705 [DOI] [PubMed] [Google Scholar]
- 23.Aarts E, Roelofs A, Franke B, Rijpkema M, Fernandez G, Helmich RC, et al. Striatal dopamine mediates the interface between motivational and cognitive control in humans: evidence from genetic imaging. Neuropsychopharmacology [Internet]. 2010/05/14. 2010;35(9):1943–51. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20463658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jonasson LS, Axelsson J, Riklund K, Braver TS, Ogren M, Backman L, et al. Dopamine release in nucleus accumbens during rewarded task switching measured by [(1)(1)C]raclopride. Neuroimage [Internet]. 2014/05/28. 2014;99:357–64. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24862078 [DOI] [PubMed] [Google Scholar]
- 25.Westbrook A, van den Bosch R, Määttä JI, Hofmans L, Papadopetraki D, Cools R, et al. Dopamine promotes cognitive effort by biasing the benefits versus costs of cognitive work. Science (80- ). 2020;367(6484):1362–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cools R The costs and benefits of brain dopamine for cognitive control. Wiley Interdiscip Rev Cogn Sci. 2016;7(5):317–29. [DOI] [PubMed] [Google Scholar]
- 27.Berridge KC, Kringelbach ML. Affective neuroscience of pleasure: reward in humans and animals. Psychopharmacol [Internet]. 2008;199(3):457–80. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18311558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leknes S, Tracey I. A common neurobiology for pain and pleasure. Nat Rev Neurosci. 2008;9(4):314–20. [DOI] [PubMed] [Google Scholar]
- 29.van Steenbergen H, Weissman DH, Stein DJ, Malcolm-Smith S, van Honk J. More pain, more gain: Blocking the opioid system boosts adaptive cognitive control. Psychoneuroendocrinology. 2017;80:99–103. [DOI] [PubMed] [Google Scholar]
- 30.**.van Steenbergen H, Eikemo M, Leknes S. The role of the opioid system in decision making and cognitive control: A review. Cogn Affect Behav Neurosci. 2019;19(3):435–58. [DOI] [PMC free article] [PubMed] [Google Scholar]; A recent review discussing evidence that the mu-opioid system can influence decision making and cognitive control by increasing the subjective value of reward and reducing aversive arousal.
- 31.van Steenbergen H Affective modulation of cognitive control: A biobehavioral perspective In: Handbook of biobehavioral approaches to self-regulation. Springer; 2015. p. 89–107. [Google Scholar]
- 32.Eldar E, Cohen JD, Niv Y. The effects of neural gain on attention and learning. Nat Neurosci. 2013;16(8):1146–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mückschel M, Chmielewski W, Ziemssen T, Beste C. The norepinephrine system shows information-content specific properties during cognitive control–Evidence from EEG and pupillary responses. Neuroimage. 2017;149:44–52. [DOI] [PubMed] [Google Scholar]
- 34.Lee T-H, Greening SG, Ueno T, Clewett D, Ponzio A, Sakaki M, et al. Arousal increases neural gain via the locus coeruleus–noradrenaline system in younger adults but not in older adults. Nat Hum Behav. 2018;2(5):356–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mather M The locus coeruleus-norepinephrine system role in cognition and how it changes with aging. Cogn Neurosci. 2020;91–101. [Google Scholar]
- 36.Xing B, Li Y-C, Gao W-J. Norepinephrine versus dopamine and their interaction in modulating synaptic function in the prefrontal cortex. Brain Res. 2016;1641:217–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ranjbar-Slamloo Y, Fazlali Z. Dopamine and Noradrenaline in the Brain; Overlapping or Dissociate Functions? Front Mol Neurosci. 2020;12:334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.*.Clewett D, Murty VP. Echoes of emotions past: How neuromodulators determine what we recollect. Eneuro. 2019;6(2). [DOI] [PMC free article] [PubMed] [Google Scholar]; A theoretical framework arguing that memory representations of emotional events may be influenced by relative engagement of and interactions between noradrenergic and dopaminergic systems, as opposed to emotional valence, at time of encoding.
- 39.Frober K, Dreisbach G. How positive affect modulates proactive control: reduced usage of informative cues under positive affect with low arousal. Front Psychol [Internet]. 2012;3:265 Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=22866047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.*.Dreisbach G, Fröber K. On how to be flexible (or not): Modulation of the stability-flexibility balance. Curr Dir Psychol Sci. 2019;28(1):3–9. [Google Scholar]; A recent review of research arguing that positive affect, reward prospect, and task context can modulate the balance between cognitive stability and flexibility by multiple possible working memory-related mechanisms.
- 41.Abrahamse E, Braem S, Notebaert W, Verguts T. Grounding cognitive control in associative learning. Psychol Bull. 2016;142(7):693. [DOI] [PubMed] [Google Scholar]
- 42.Chiu Y-C, Jiang J, Egner T. The caudate nucleus mediates learning of stimulus–control state associations. J Neurosci. 2017;37(4):1028–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.**.Jiang J, Bramão I, Khazenzon A, Wang S-F, Johansson M, Wagner AD. Temporal Dynamics of Memory-guided Cognitive Control and Generalization of Control via Overlapping Associative Memories. J Neurosci. 2020;40(11):2343–56. [DOI] [PMC free article] [PubMed] [Google Scholar]; A study using EEG and reinforcement learning models to demonstrate that associative encoding links cognitive control demands to triggering stimuli, and that such demands can be generalized to related stimuli, via changes in alpha and theta power.
- 44.Jiang J, Beck J, Heller K, Egner T. An insula-frontostriatal network mediates flexible cognitive control by adaptively predicting changing control demands. Nat Commun. 2015;6(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Balleine BW, Dickinson A. Goal-directed instrumental action: contingency and incentive learning and their cortical substrates. Neuropharmacology. 1998;37(4–5):407–19. [DOI] [PubMed] [Google Scholar]
- 46.Shenhav A, Botvinick MM, Cohen JD. The expected value of control: an integrative theory of anterior cingulate cortex function. Neuron. 2013;79(2):217–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shen YJ, Chun MM. Increases in rewards promote flexible behavior. Attention, Perception, Psychophys. 2011;73(3):938–52. [DOI] [PubMed] [Google Scholar]
- 48.Fröber K, Pfister R, Dreisbach G. Increasing reward prospect promotes cognitive flexibility: Direct evidence from voluntary task switching with double registration. Q J Exp Psychol. 2019;72(8):1926–44. [DOI] [PubMed] [Google Scholar]
- 49.Gershman SJ. Uncertainty and exploration. Decision. 2019;6(3):277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grahek I, Musslick S, Shenhav A. A computational perspective on the roles of affect in cognitive control. Int J Psychophysiol. 2020;151:25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.*.Manohar SG, Finzi RD, Drew D, Husain M. Distinct motivational effects of contingent and noncontingent rewards. Psychol Sci. 2017;28(7):1016–26. [DOI] [PMC free article] [PubMed] [Google Scholar]; A study using eye movements and pupil dilation to identify distinct, independent influences of performance-contingent and non-performance-contingent rewards on performance, with performance-contingent rewards leading to larger effects.
- 52.Chiew KS, Braver TS. Reward favors the prepared: Incentive and task-informative cues interact to enhance attentional control. J Exp Psychol Hum Percept Perform [Internet]. 2015/09/01. 2016;42(1):52–66. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26322689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.**.Hefer C, Dreisbach G. The volatile nature of positive affect effects: Opposite effects of positive affect and time on task on proactive control. Psychol Res. 2020;84(3):774–83. [DOI] [PubMed] [Google Scholar]; A behavioral study demonstrating that positive affect is associated with decreased proactive control in the AX-CPT paradigm, but that this effect is counteracted by increasing proactive control as a function of increasing time on task.
- 54.Braver TS, Barch DM, Cohen JD. The role of the prefrontal cortex in normal and disordered cognitive control: a cognitive neuroscience perspective In: Stuss DT, Knight RT, editors. Principles of Frontal Lobe Function. Oxford: Oxford University Press; 2002. p. 428–47. [Google Scholar]
- 55.Muller J, Dreisbach G, Goschke T, Hensch T, Lesch KP, Brocke B. Dopamine and cognitive control: the prospect of monetary gains influences the balance between flexibility and stability in a set-shifting paradigm. Eur J Neurosci [Internet]. 2007;26(12):3661–8. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18088285 [DOI] [PubMed] [Google Scholar]
- 56.Joormann J Is the glass half empty or half full and does it even matter? Cognition, emotion, and psychopathology. Cogn Emot. 2019;33(1):133–8. [DOI] [PubMed] [Google Scholar]


