Abstract
Background:
The frequency of nonsurgical rhinoplasty has increased in recent years. The occurrence of headaches or migraine symptoms, and their treatment following nonsurgical rhinoplasty, have been scarcely described in the literature. Here, we present a patient who presented with subjective complaints of a new onset headache immediately after nonsurgical rhinoplasty, with subsequent reversal of her symptoms using hylauronidase. Furthermore, a literature review was conducted to establish a possible anatomical pathophysiologic mechanism of these symptoms seen in this patient.
Methods:
A case report of a patient who developed persistent headache symptoms after nonsurgical rhinoplasty, with reversal of symptoms using hylaronidase, is described. A literature review of studies on patients developing headaches or migraine-like symptoms after nonsurgical rhinoplasty was conducted, along with a review of the anatomic causes of migraines.
Results:
Of the 147 relevant citations identified in our search, only 1 individual case report describes a patient who developed a migraine headache after undergoing a nonsurgical rhinoplasty via an injection of hyaluronic acid filler. This was promptly resolved with the utilization of a hyaluronidase injection. The majority of the relevant articles in our search focused on the alarming and most feared complication of vascular compromise of the nasal tissue and intravascular embolization. Within the literature, there was no case series of nonsurgical-rhinoplasty-induced migraines taking into account our inclusion criteria.
Conclusions:
This article demonstrates the paucity of literature regarding nonsurgical-rhinoplasty-induced headaches. Although a causation effect cannot be linked, our study highlights a rare phenomenon associated with this ever-increasing aesthetic procedure.
INTRODUCTION
Nonsurgical rhinoplasty through the off-label use of injectable fillers has increasingly become a popular option in aesthetic surgery. The rise in popularity of this nonsurgical procedure has largely been driven by several factors, which include the minimally invasive nature of the procedure, its short duration, possibility of quick return to work after undergoing the procedure, and lower cost. Hyaluronic Acid (HA) filler injections allow the clinician to sculpt the nasal shape with immediate aesthetic results, along with the benefit of patients returning to their normal activities during the same day.1 However, the long-term results and complications associated with the procedure are sparsely published in the literature.
The most common complication published in the literature regarding nonsurgical rhinoplasty is vascular occlusion causing skin necrosis. The causes of this complication are typically attributed to 2 mechanisms. The first is from a direct intravascular injection, and the second from the compressive effect of the HA filler on local small vessels and the surrounding tissue microvasculature.2 When vascular occlusion is diagnosed, treatment may commonly include the utilization of hyperbaric oxygen therapy, antibiotics, vasodilating therapy, massage, and supportive care. However, the most effective treatment for this complication is reported to be a local hyaluronidase injection to reverse the effects of HA.3,4
One particular complication with nonsurgical rhinoplasty that is not published in the literature is the development of headache or migraine-like symptoms after nonsurgical rhinoplasty. Migraines are one of the leading causes of disability in the United States, affecting more than 10% of the US population.2 Patients with migraines are faced with restricted activity, low productivity, missed work days, and a significantly decreased quality of life.
The correlation between nonsurgical rhinoplasty and the development of headaches or migraine-like symptoms after injections, as well as the lack of published long-term follow-up in this patient population, necessitates our exploration. We present our own case report of a patient who developed subjective headache symptomatology after a nonsurgical rhinoplasty. We provided a retrospective analysis of the data and take a closer look at entrapment neuropathy, and sought to examine the anatomical basis of the development of such debilitating symptoms. We hope that the findings of this study would better elucidate a rare complication regarding this nonsurgical procedure.
PATIENTS AND METHODS
A literature search was conducted in June 2019 through the PUBMED database for articles regarding the complications of nonsurgical rhinoplasty and post-procedural migraines. Key words used in the search included (“Non-surgical Rhinoplasty” or “Migraines after non-surgical rhinoplasty”) AND (“entrapment neuropathy”) AND (“Supratrochlear nerve Rhinoplasty Complications”).
Reference articles were screened manually to obtain relevant studies. A total of 147 citations were identified in the original search. After eliminating duplicate studies and reviewing abstracts of the citations, we further filtered our search regarding predefined inclusion/exclusion criteria. Inclusion criteria comprised studies published in the last 30 years, total number of patients, and complications (including patient post-procedural headache). Exclusion criteria were case reports or series describing <5 patients, letters or editorials, and non-English language articles (Fig. 1).
Fig. 1.
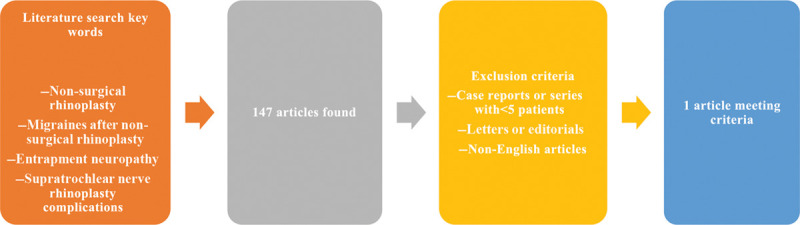
Exclusion criteria diagram for literature search.
We also present our case of a patient who underwent nonsurgical rhinoplasty and developed headache symptoms immediately after injection. These symptoms were alleviated by the use of hylauronidase. Finally, the anatomic basis for migraines was investigated, and articles regarding anatomic course of the nerves involved were identified.
RESULTS
Of the 147 relevant citations identified in our search, only 1 individual case report describes a patient who developed a migraine headache after undergoing a nonsurgical rhinoplasty via an injection of HA filler (Fig. 1). This was promptly resolved with the utilization of hyaluronidase injection. The majority of the relevant articles in our search focused on the most feared complication of vascular occlusion of the nasal tissue and intravascular embolization. Within the literature, there was no case series of nonsurgical-rhinoplasty-induced migraines that take into account our inclusion criteria. We then sought to evaluate studies (Table 1) regarding anatomical variation and topographic relationship between the supratrochlear nerve and locations of HA injection, entrapment neuropathy, as well as supratrochlear neuralgia and migraine headaches.
Table 1.
Journal Articles Found regarding Anatomic Nerves Involved in Migraine Pathology
| Title | Author | Journal and Year | Overview |
|---|---|---|---|
| The supraorbital region revisited: an anatomic exploration of the neuro-vascular bundle with regard to frontal migraine headache | Berchtold et al | Journal of Plastic, Reconstructive and Aesthetic Surgery 2017 | Cadaver study of 22 facial halves with anatomic courses of the supratrochlear and supraorbital nerve |
| Topographic relationship between the supratrochlear nerve and corrugator supercilii muscle—can this anatomical knowledge improve the response to botulinum toxin injections in chronic migraine? | Lee et al | Toxins 2015 | Cadaver study of 58 hemifaces assessing the supratrochlear nerve and its relationship to the corrugator supercilli muscle |
| Supraorbital rim syndrome: definition, surgical treatment, and outcomes for frontal headache | Hagan et al | Plastic and Reconstructive Surgery: Global Open 2016 | Retrospective review of 276 patients who underwent migraine nerve decompression surgery, and explanation of supreorbital rim syndrome |
| Supratrochlear neuralgia: a prospective case series of 15 patients | Pareja et al | Headache 2017 | Prospective study of 15 patients who underwent local injection to the supratrochlear nerve area |
Case Presentation
The patient was a healthy 28-year-old woman with no prior medical history interested in nonsurgical options for rhinoplasty procedure. The patient had no family or personal history of headaches or migraines. She was primarily concerned and self-conscious regarding the appearance of her nasal dorsal hump. The patient agreed to undergo a nonsurgical rhinoplasty procedure and was injected in a strictly midline fashion, with approximately 0.5 cm3 of Juvederm (hyaluronic acid) into her radix, dorsum, and tip. Within 1 hour after the injection, the patient began developing symptoms of a frontal headache, which originated just superior to bilateral medial brows and radiated to the occipital region. Over the following days, the patient attempted to take over-the-counter pain medication, without any relief of pain. She reported subjectively that it felt “like a migraine.” The symptoms of the headache were constant after the injection and no factors were alleviating her symptoms. The patient returned to the clinic approximately 4 days after injection for re-evaluation. She underwent an injection of 20U of Vitrase (hyluronidase ovine) solely into the radix to reverse the filler in this area. Per patient report, the patient had a complete resolution of her symptoms the same day following the injection into the radix. The timeline of the symptomology for the headache, the description regarding bilateral frontal radiating to the occipital region, and quick reversal of the symptoms after hyaluronidase injection are identical to similar self-reported patient complications regarding nonsurgical rhinoplasty that can be found on Real Self and other patient review platforms.
DISCUSSION
When taking into account the potential side effects of vascular occlusion caused by filler injection through the mechanism of compression, one can also infer that there can be potential compression of nervous structures. A proposed alteration in the blood flow of the vasa nervorum of the nerve could also lead to potential irritation of the nerve. The current literature supports entrapment or compression of supratrochlear/supraorbital nerves as an etiology of migraine symptoms; thus, it is highly plausible that fillers in these regions can cause compression, leading to entrapment neuropathy. The areas of filler injection in nonsurgical rhinoplasty have the potential to affect supratrochlear, infratrochlear, supraorbital, and anterior ethomoidal nerves. Compression of these structures, especially the supratrochlear nerve, can lead to migraine symptoms. Furthermore, decompression by removing inciting cause (in this case, the HA filler with hyaluronidase) can potentially alleviate migraine symptoms immediately.
Approved by the FDA in 1950, hyaluronidase is a parenteral enzyme that hydrolyzes HA in the connective tissue, allowing greater permeation of local tissues. Hyaluronidases are enzymes that degrade hyaluronan or HA predominantly. They can be found in bacteria, fungi, and humans. There are 5 structural models for human hyaluronidases.5 The common mechanism is an endolytic random cut pattern of hyaluronidase activity. HA degradation occurs through multiple bites at catalytic points, with each product of degradation reaction becoming substrates for further cleavage. The final end-products of HA digestion are tetrasaccharides. According to a review by Buhren et al, hyaluronidase is the gold standard for the management of complications of HA fillers in terms of vascular compromise or overcorrection, and it should be immediately available at every treatment center.6 In congruity with this conclusion, we propose that hyaluronidase should be considered as immediate treatment when there are new onset migraine-like symptoms after nonsurgical rhinoplasty. Although there exists the possibility of the placebo effect playing a prominent role in the relief of our patient’s symptoms, the literature does provide solid anatomical and pathological rationale for this patient’s onset and resolution of symptoms.
The most recently published literature has explored the concept of nerve entrapment and irritation of the sensory nerves of the head and neck as possible peripheral triggers for migraines.2 Entrapment neuropathy has traditionally been thought of as a disorder of the extremities such as median nerve compression in carpal tunnel syndrome. Entrapment is thought to produce a localized neurogenic inflammatory response with the release of several different neuropeptides.2 Common sites of migraine headaches are located in the frontal region, which is often attributed to sinusitis, and the occipital region, where entrapment of all 3 occipital nerves (greater, lesser, and third) has been described.2 A study by Lee et al describes a total of 6 well-defined migraine headache trigger sites, with some less common minor distal branch sites as a source for chronic headaches.7 Interestingly, the surgical decompression of peripheral sensory nerves at these trigger sites has had a high success rate, with a quoted migraine resolution rate between 35% and 90% and a pain reduction rate between 42% and 97% in the literature.2,7
In efforts to understand the pathogenesis of frontal migraine headache mechanisms, it is important to explore the supraorbital region and revisit accepted neurovascular anatomical variations. One particular study by Berchtold et al describes the relationship of both the supraorbital nerve and supratrochlear nerve into the corrugator supercilii muscle, along with the relationship between the supraorbital artery and supraorbital nerve.8 The supraorbital regions of 22 embalmed facial halves were dissected, and the supratrochlear and supraorbital nerves were identified. The authors discovered possible compression points of the supratrochlear nerve, as it passes through the orbital septum and identified several branches with varying topographies of the supratrochlear nerve.8 The authors describe that the osteofibrous channels vary in shape and that there is a proximity of the supraorbital nerve with the supraorbital artery as well. The authors conclude that the supratrochlear nerve penetrates a dense secession from the trochlea and that this nerve has a higher vulnerability or compression from the surrounding tissue due to its lack of mobility. One may infer that the injecting filler around the supratrochlear nerve may act not only to create a neuroinflammatory mileu, but also act as a compression force for this already vulnerable nerve compression site.
Several other articles have also demonstrated that the entrapment of the supratrochlear nerve within the corrugator supercilii muscle and variations of its branches just above the radix may lead to anatomical problems, which in turn leads to the induction of migraines through compression. Lee et al explored the topographic relationship between the supratrochlear nerve and corrugator supercilii muscle in the forehead as a cause of migraine in 58 hemi-faces from Korean and Thai cadavers.9 The supratrochlear nerve entered the supercilii muscle in every case of their dissection. Both the supratrochlear and supraorbital nerve pass over the roof of the orbit anteromedially and proceed to the frontal belly of the occipitofrontalis muscle to receive sensation of the conjunctiva of the upper eyelid and skin of the lower forehead close to the midsagittal line.9 Branches of these nerves as they cross over are vulnerable to compression by the surrounding soft tissue.
One particular recently published study in Plastic and Reconstructive Surgery Global Open by Hagan et al described a novel term, “supraorbital rim syndrome,” which was used to describe a constellation of frontal peripheral nerve entrapment sites causing frontal headache pain.10 The study was a retrospective review of 276 patients who underwent nerve decompression or neurectomy procedures for frontal or occipital headaches. The study concludes by discussing that the decompression of the peripheral nerves of the supraorbital rim (which include the supraorbital nerve, the supratrochlear nerve, and the zygomaticotemporal nerve) can have varying underlying etiologies and that the decompression of these nerves from the surrounding tissue (whether it includes muscle, bone, fascia, or vessel) results in significant improvement in frontal headaches.1 The study concludes that the understanding of head and neck nerve compression syndromes and the concept of multiple anatomical points of compression guide the treatment strategy for patients whose disabilities are related to frontal and occipital headaches.
A brief communication by the American Headache Society published in the Journal of Headache in 2017 focused on supratrochlear neuralgia through a prospective case series of 15 patients. The article describes the underlying potential anatomical variations between the supraorbital and supratrochlear nerve branches, and that the anatomical variations of the nerve branches make it difficult to pinpoint the origin of pain.11 The authors prospectively identified 15 patients from 2009 to 2016 presenting with pain within the territory of the supratrochlear nerve. Anesthetic blockages were performed in these patients using lidocaine and mepivacaine, and the areas of allodynia and hyperalgesia corresponded to cutaneous distribution of branches of the supratrochlear nerves. Each patient described a distinct continuous or intermittent headache, which was reversed through the anesthetized nerve. The study was able to identify that the most common causes of local nerve damage/irritation were attributed to cranial trauma, surgery, trochlear inflammation, or other idiopathic causes of compression from the surrounding tissue, as branches of the nerve ascend close to the bone under the corrugator and frontalis muscles.11
Although our case cannot prove that that filler injections caused the persistent frontal headache symptoms, reversal of the filler completely resolved our patient’s symptoms. One can infer that local irritation of the supratrochlear nerves near the injection site likely acted as a local irritant, with some compressive effects on the local sensory nerves. This likely contributed to her symptoms and led to her subjective “migraine-like symptoms.” Although this cannot be truly classified as a migraine in our patient, our literature review does show that similar complications may occur on rare occasions.
CONCLUSIONS
The lack of published literature regarding long-term patient follow-up after nonsurgical rhinoplasty, and the development of headache or migraine symptomatology emphasize the need to closely follow these patients after injections. The paucity of published data regarding rare complications such as new onset headaches after nonsurgical rhinoplasty stresses the importance of close follow-ups in patients receiving injectables despite the minimally invasive nature and the benign appearance of the procedure.
Footnotes
Published online 21 December 2020.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
REFERENCES
- 1.Bertossi D, Giampaoli G, Verner I, et al. Complications and management after a nonsurgical rhinoplasty: a literature review. Dermatol Ther. 2019;32:e12978. [DOI] [PubMed] [Google Scholar]
- 2.de Ru JA, Filipovic B, Lans J, et al. Entrapment neuropathy: a concept for pathogenesis and treatment of headaches—A narrative review. Clin Med Insights Ear Nose Throat. 2019;12:1179550619834949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen Q, Liu Y, Fan D. Serious vascular complications after nonsurgical rhinoplasty: a case report. Plast Reconstr Surg Global Open. 2016;4:e683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim DW, Yoon ES, Ji YH, et al. Vascular complications of hyluronic acid fillers and the role of hyaluronidase in management. J Plast Reconstr Aesthet Surg. 2011;64:1590–1595. [DOI] [PubMed] [Google Scholar]
- 5.Stern R, Jedrzejas MJ. Hyaluronidases: their genomics, structures, and mechanisms of action. Chem Rev. 2006;106:818–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buhren BA, Schrumpf H, Hoff NP, et al. Hyaluronidase: from clinical applications to molecular and cellular mechanisms. Eur J Med Res. 2016;21:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee M, Erickson C, Guyuron B. Intranasal pathology in the migraine surgery population: incidence, patterns, and predictors of surgical success. Plast Reconstr Surg. 2017;139:184–189. [DOI] [PubMed] [Google Scholar]
- 8.Berchtold V, Stofferin H, Moriggl B, et al. The supraorbital region revisited: An anatomic exploration of the neuro-vascular bundle with regard to frontal migraine headache. J Plast Reconstr Aesthet Surg. 2017;70:1171–1180. [DOI] [PubMed] [Google Scholar]
- 9.Lee HJ, Choi KS, Won SY, et al. Topographic relationship between the supratrochlear nerve and corrugator supercilii muscle—can this anatomical knowledge improve the response to botulinum toxin injections in chronic migraine? Toxins (Basel). 2015;7:2629–2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hagan RR, Fallucco MA, Janis JE. Supraorbital rim syndrome: definition, surgical treatment, and outcomes for frontal headache. Plast Reconstr Surg Glob Open. 2016;4:e795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pareja JA, López-Ruiz P, Mayo D, et al. Supratrochlear neuralgia: a prospective case series of 15 patients. Headache. 2017;57:1433–1442. [DOI] [PubMed] [Google Scholar]


